- Department of Orthopaedic Oncology, Spinal Tumor Center, Shanghai Changzheng Hospital, Naval Medical University, Shanghai, China
Objective: Spinal osteosarcoma is a rare osseous neoplasm. The aim of this study is to make a comprehensive analysis of the demographic features, clinicopathologic characteristics and factors affecting prognosis of spinal osteosarcoma using the Surveillance, Epidemiology and End Results (SEER) database.
Methods: SEER data were reviewed to identify patients diagnosed with spinal osteosarcoma between 1975 and 2016 and determine their overall survival (OS) and disease-specifc survival (DSS). Univariate and multivariate analyses were performed using the Cox-regression proportional hazards model and Kaplan-Meier method.
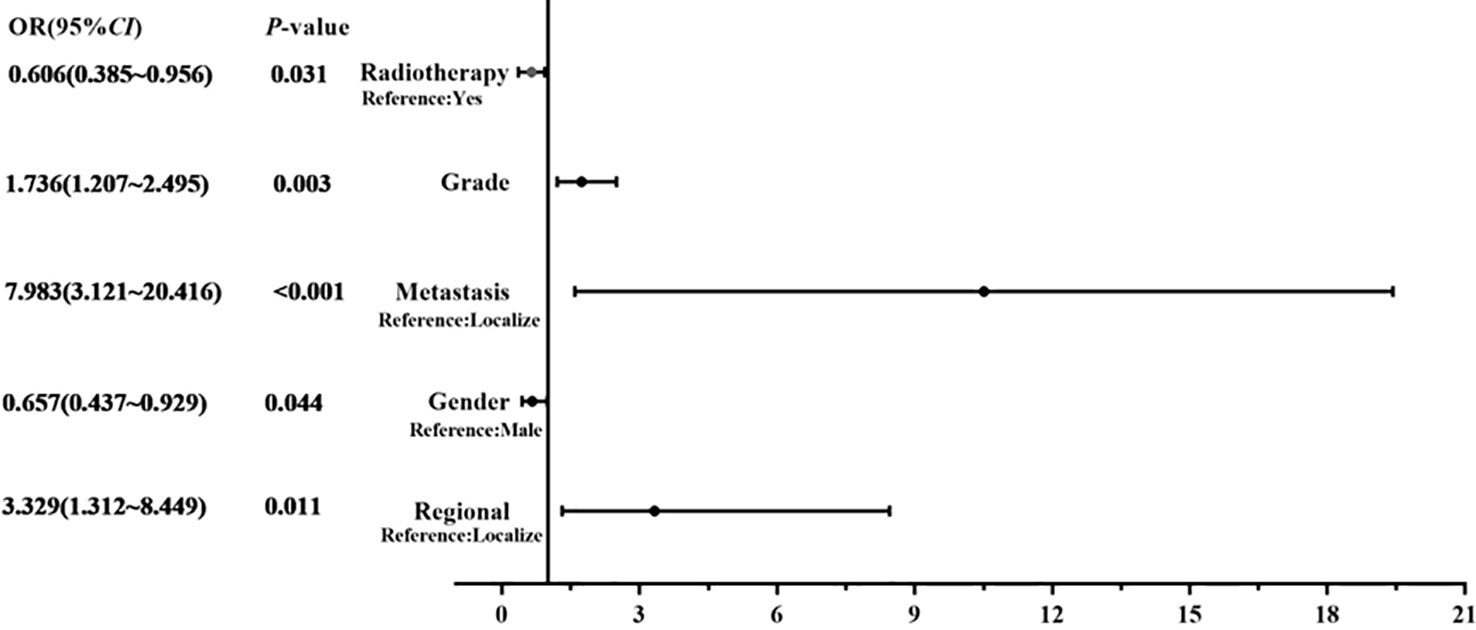
Results: A total of 668 patients (53.1% males) with spinal osteosarcoma were identified. The mean age at diagnosis was 45.2 years, including 67.5% patients younger than 60 years. The median OS of these patients was 15 months, and the 5-year OS was 16.8%. Multivariate analysis showed that age ≥60 year (HR=2.271, p = 0.008), high grade (HR=1.323, p = 0.008), regional stage (HR=1.658, p = 0.017), metastasis stage (HR=3.045, p < 0.001) and no-surgery treatment (HR=1.761, p < 0.001) were adversely associated with OS; gender (HR=0.657, p = 0.044), tumor grade (HR=1.616, p = 0.006), tumor stage (HR=3.329, p = 0.011; HR=7.983, p < 0.001) and radiotherapy (HR=0.606, p = 0.031) were independent prognostic factors affecting DSS.
Conclusion : Based on SEER data analysis, male, high tumor grade, regional stage, metastasis stage and radiotherapy are independent predictors of poor survival of patients with spinal osteosarcoma. The clinical treatment of spinal osteosarcoma still faces serious challenges. Future research should focus on the clinical impact and survival outcomes of the emerging targeted and immune therapies for the sake of improving the survival stalemate of spinal osteosarcoma.
1 Introduction
Osteosarcoma is the most common malignant bone sarcoma, accounting for 0.5% of all malignant tumors (1, 2). Osteosarcoma derives from mesenchymal stem cells, characterized by the proliferation of neoplastic cells and the formation of immature bone or osteoid tissues (3). While, spinal osteosarcoma is relatively rare, accounting for 3-5% of all spinal malignancies (4, 5). It mainly affects the sacral region, followed by the lumbar and thoracic segments (6). Given the complex anatomic sites and the severe neurological lesions, clinical treatment of spinal osteosarcoma has always been a challenge, with relatively a worse prognosis as compared with limb osteosarcoma (7, 8).
The current standard treatment for osteosarcoma includes surgical resection of the primary tumor after neoadjuvant chemotherapy, surgical resection of all clinically significant metastases, and postoperative adjuvant chemotherapy. By this way, the 5-year overall survival (OS) rate of patients with osteosarcoma has increased gradually from 60% to 80% (9, 10). Despite the great success achieved in the treatment of osteosarcoma, the treatment of spinal osteosarcoma still faces considerable challenges due to high recurrence, metastasis susceptibility and a mortality rate (11, 12). Due to its rarity, most studies on spinal osteosarcoma are limited to small case reports from individual institutions (13). More research based on larger populations is required to estimate the survival of patients with spinal osteosarcoma and identify factors affecting their survival prognosis.
In 1973, the surveillance, epidemiology and end results (SEER) registration maintained by the National Cancer Institute (NCI) began collecting cancer-related information, and since then it has covered 30% of the total US population and become a comprehensive source of population-level cancer data (14). The sample size of the SEER is larger than that of any single institution and most multi-institutions. To the best of our knowledge, so far there is no multivariate regression analysis on the treatment, confounding factors and independent prognostic factors affecting the prognosis of spinal osteosarcoma. The aim of the present study is to make a comprehensive analysis of the demographic features, clinicopathologic characteristics and factors affecting prognosis of spinal osteosarcoma using the Surveillance, Epidemiology and End Results (SEER) database, and propose treatment strategies for the treatment of this malignancy.
2 Materials and methods
2.1 Ethics approval
A population-based search was made of patients diagnosed with spinal osteosarcoma by using the case-listing session protocol from the NCI’s SEER 18 database [www.seer.cancer.gov]. The database is publicly available online, and shows no personal identification, so it does not need the approval from the internal review board approval.
2.2 Research population
Data of the patients diagnosed with spinal osteosarcoma between 1975 and 2016 were reviewed, and this is the widest date range available in the latest version of the software. Firstly, the primary tumors on the spine, C41.2 (vertebral column) and C41.4 (pelvic bones, sacrum, coccyx, and associated joints), were identified by site-specific codes. Secondly, the Histologic International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) was used to identify the patients with osteosarcoma (ICD-O-3 histologic type: 9180-9187, 9192-9195), including osteosarcoma NOS, chondroblastic osteosarcoma, fibroblastic osteosarcoma, telangiectatic osteosarcoma, osteosarcoma in Paget’s disease, small cell osteosarcoma, central osteosarcoma, intraosseous well-differentiated osteosarcoma, periosteal osteosarcoma, high grade surface osteosarcoma, and parosteal osteosarcoma. Exclusion criteria were patients who were diagnosed solely based on the clinical findings or imaging, confirmed by the death certificate or autopsy, received unknown treatment method, and/or lacked survival information or survival time missing less than 1 month. Finally, 668 patients diagnosed with spinal osteosarcoma were selected and included in this work.
2.3 Analytic variables
The assessed predictor variables from the SEER database included demographics, disease stage, histologic subtype, tumor grade, tumor size, surgical treatment, radiotherapy, chemotherapy, survival months, and cause of death of the patient. The primary outcome was defined as the time (in months) of OS from diagnosis to death with any cause and the time from diagnosis to death with disease-specific survival (DSS) specific to a cancer-related diagnosis.
2.4 Statistical analysis
Epidemiological description and survival statistics were given to all variables. All statistical analyses were performed by SPSS software (ver. 21.0; SPSS Inc., Chicago, IL, USA). Median survival and OS were calculated by Kaplan-Meier method. Variables with p < 0.2 in univariate analysis were subjected to multivariate analysis. The independent predictors of OS and DSS obtained from multivariate analysis were verified by Cox proportional hazards regression model. The hazard ratio (HR) and the corresponding 95% confidence interval (CI) were used to indicate the impact of each factor on OS and DSS. p < 0.05 was considered statistical significance.
3 Results
Finally, 668 patients diagnosed with spinal osteosarcoma were selected and included in this work from the database from 1975 to 2016 (Table 1), in whom 451 patients (67.5%) were younger than 60 years. Demographically, 53.1% patients were males, and 81.1% patients were White. In addition, 67.7% of patients were diagnosed after 2000. Histologically, the most common tumor grade of differentiation was grade III–IV (51.4%) and the most common histological subtype was osteosarcoma NOS (68.7%), followed by chondroblastic osteosarcoma (18.7%), fibroblastic osteosarcoma (4.5%), osteosarcoma with Paget disease (4.3%), telangiectatic osteosarcoma (1.6%), small cell osteosarcoma (1%), and other rare histological subtypes. The tumor stage was known in 68.9% cases, and most cases presented with regional invasive diseases (29.5%). Tumor size information was available in 48.6% cases, with three groups divided. As for the treatment methods for spinal osteosarcoma, local surgery was applied in more than one-third (39.4%) of the patients, chemotherapy in about two-thirds (67.4%) of the patients, and radiotherapy in 208 patients (31.1%). Ultimately, 339 patients (50.7%) died of osteosarcoma.
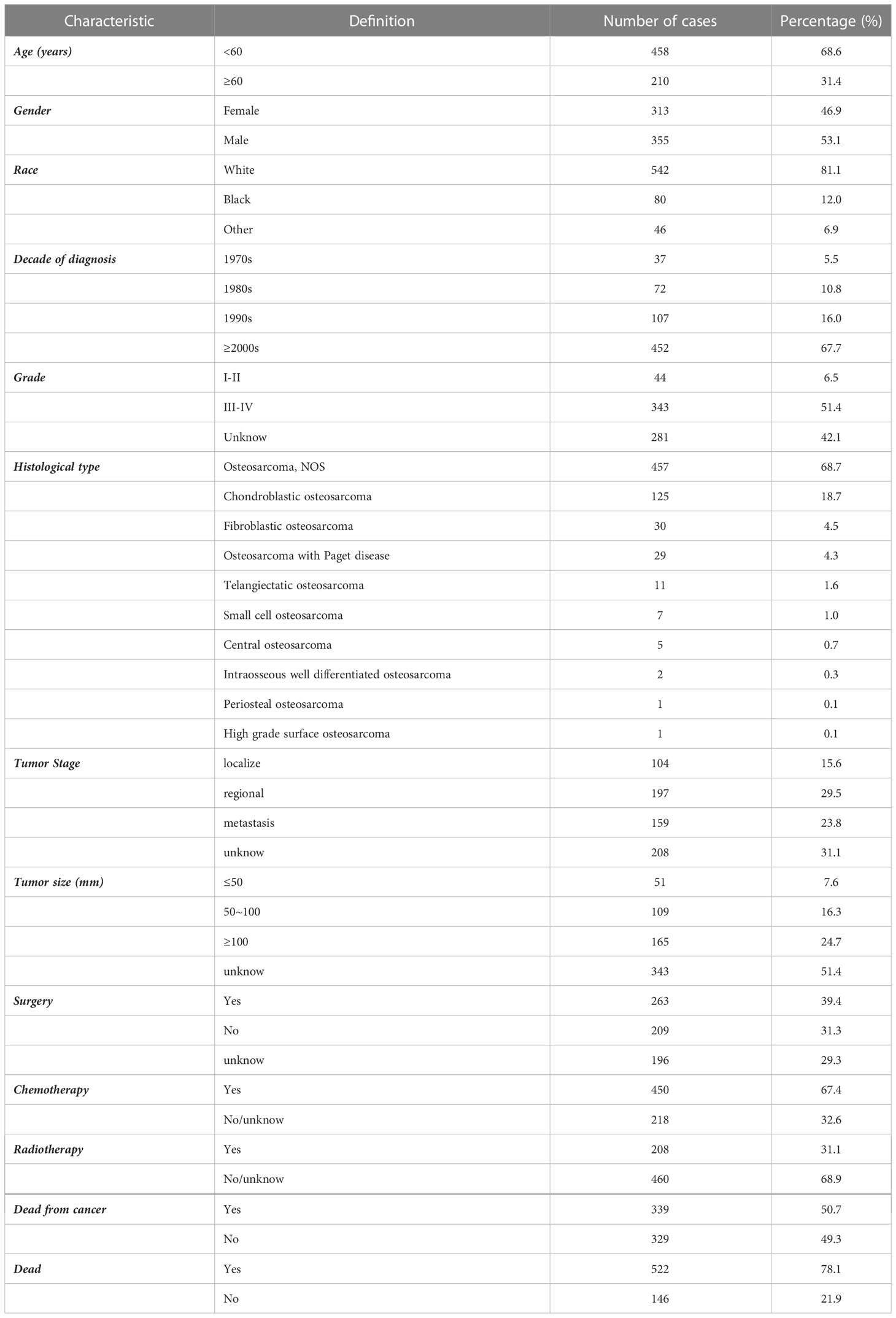
Table 1 Demographic and clinical characteristics of spinal osteosarcoma patients identified in the SEER database from 1975 to 2016.
The results of univariate analysis in variables associated with survival of the patients with spinal osteosarcoma are shown in Tables 2, 3, indicating that patients over 60 years of age had significantly worse prognosis than those under 60 years of age (HR = 2.550, p < 0.001; HR = 1.719, p < 0.001). Gender and race had no significant correlation with OS or DSS. A more recent decade of diagnosis was associated with the improved DSS (HR = 0.985, p < 0.001) for spinal osteosarcoma. Both OS and DSS varied with different tumor grades, and a higher tumor grade predicted a worse prognosis (HR = 1.458, p < 0.001; HR = 1.447, p < 0.001). OS and DSS also varied with different tumor stages, and metastasis predicted a worst prognosis. Similarly, a larger tumor size was associated with worse OS and DSS (HR = 1.215, p = 0.033; HR = 1.325, p = 0.012). OS and DSS in patients who received surgical treatment were significantly better than those in non-surgical patients (HR = 2.651, p < 0.001; HR = 2.285, p < 0.001). Chemotherapy was significantly associated with OS (HR = 0.827, p = 0.043) but not with DSS. However, patients who received radiotherapy had worse OS and DSS than those who did not (HR = 0.615, p < 0.001; HR = 0.542, p < 0.001).
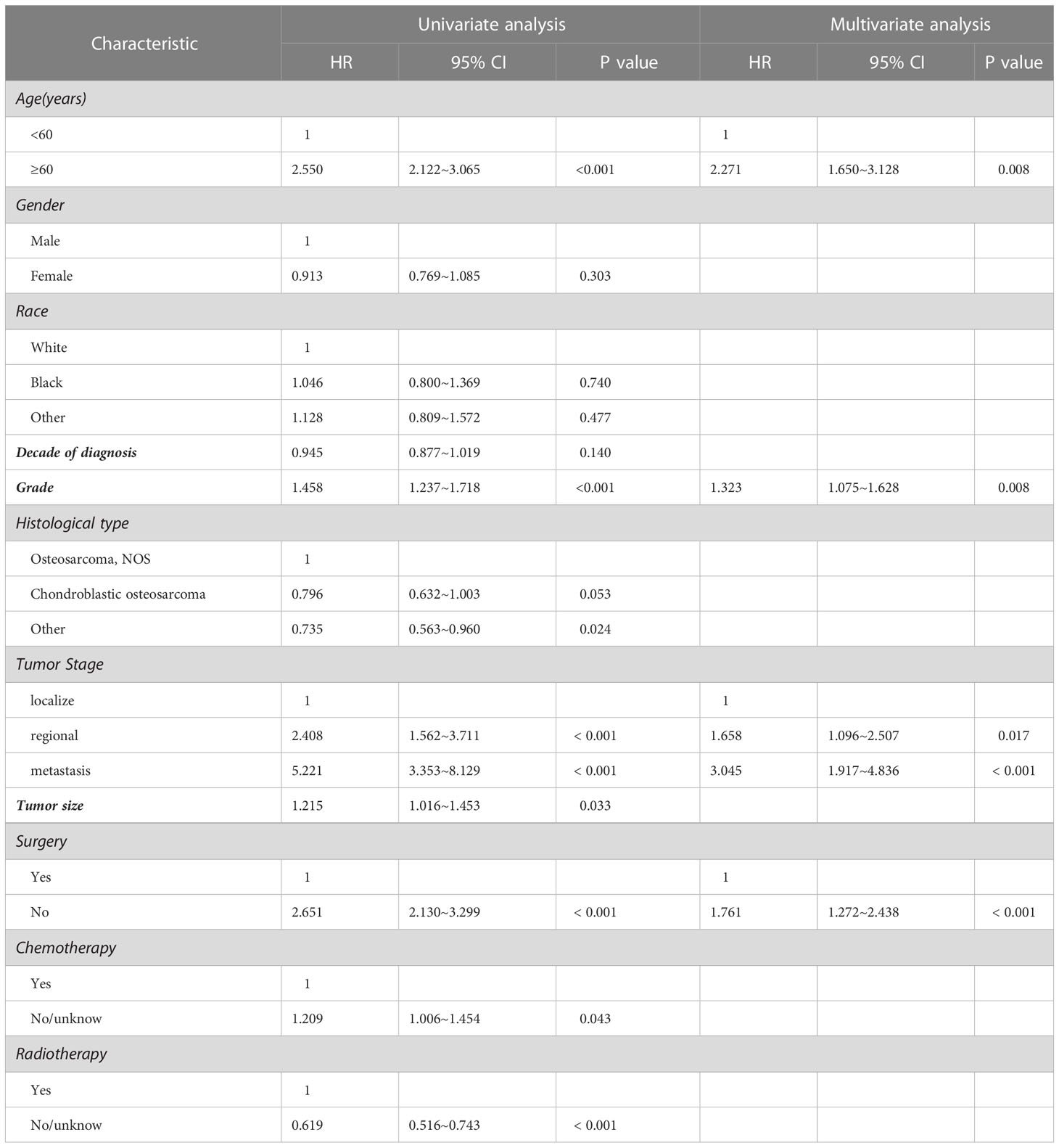
Table 2 Univariate and multivariate analysis for clinical factors associated with OS of spinal osteosarcoma patients.
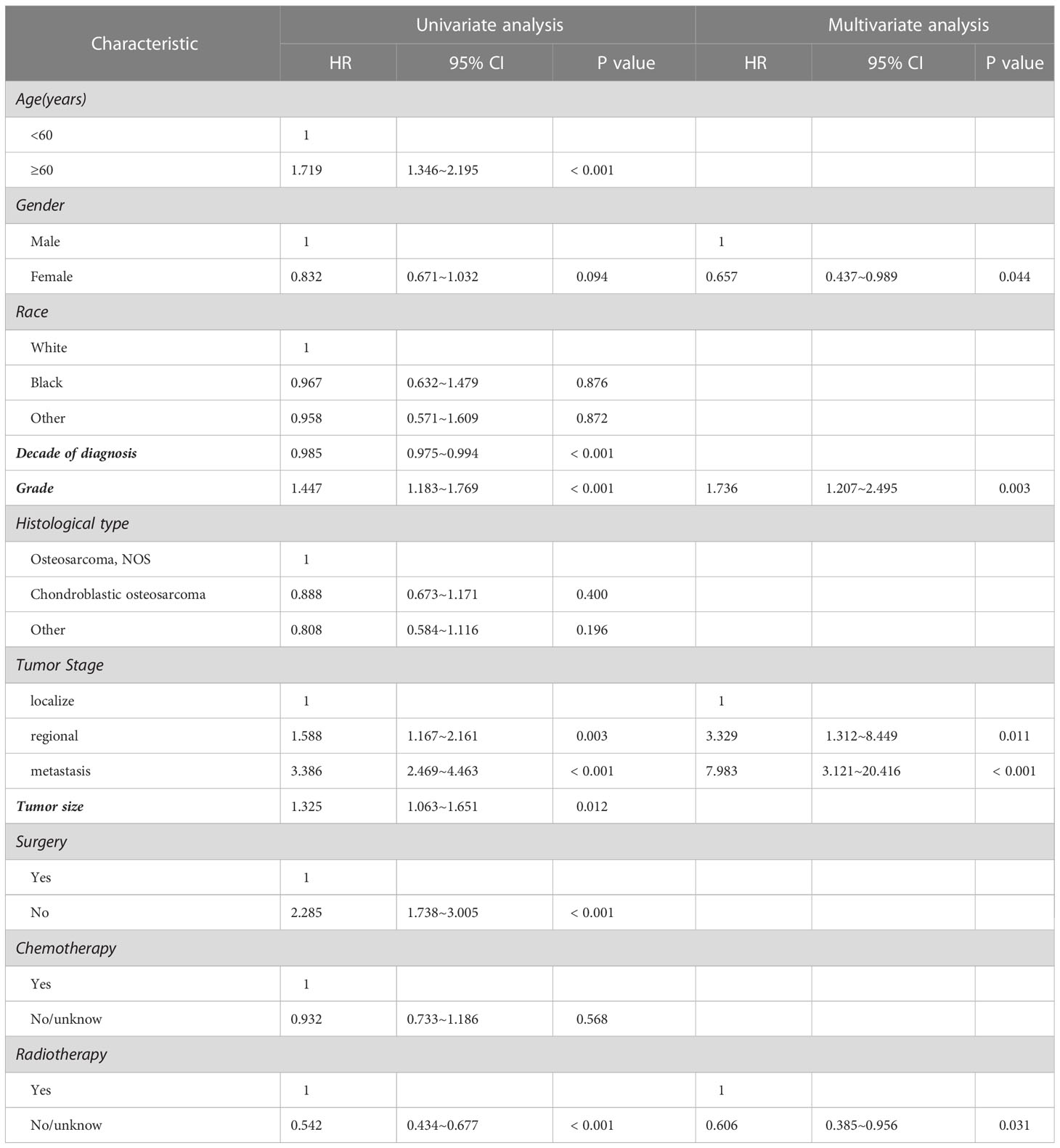
Table 3 Univariate and multivariate analysis for clinical factors associated with DSS of spinal osteosarcoma patients.
Considering the limitations of univariate Cox analysis, multivariable Cox analysis was performed to investigate the independent prognostic factors associated with OS and DSS (Tables 2, 3). As indicated by multivariate analysis of all spinal osteosarcoma patients, there was a negative correlation between OS and age ≥ 60 (HR = 2.271, p = 0.008), high grade (HR = 1.323, p = 0.008), regional stage (HR = 1.658, p = 0.017), metastasis stage (HR = 3.045, p < 0.001), and no-surgery treatment (HR = 1.761, p < 0.001). In terms of DSS, gender (HR = 0.657, p = 0.044), tumor grade (HR = 1.736, p = 0.003), tumor stage (HR = 3.329, p = 0.011; HR = 7.983, p < 0.001), and radiotherapy (HR = 0.606, p = 0.031) all were independent prognostic factors (Figures 1, 2).
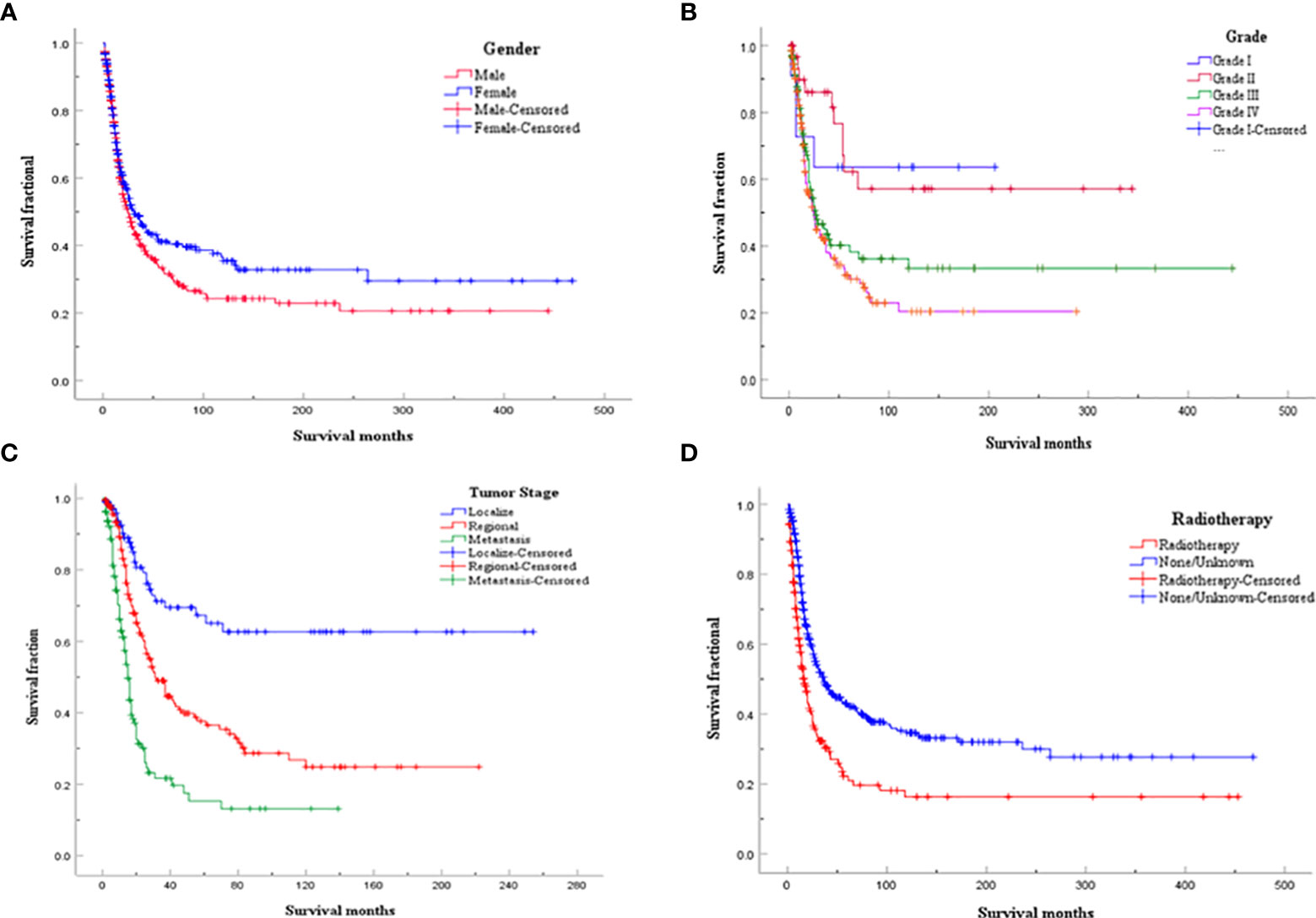
Figure 2 Kaplan-Meier analysis for DDS of spinal osteosarcoma according to (A) gender; (B) Tumor grade; (C) Tumor stage; (D) Radiotherapy.
4 Discussion
Spinal osteosarcoma is a rare malignant tumor with susceptibility of invasive destruction and systemic metastasis, accounting for 3-5% of all cases of osteosarcoma and about 10% of primary spine tumors (15, 16). Although extensive resection and neoadjuvant chemotherapy have greatly improved the prognosis of patients with limb osteosarcoma (17, 18), there are still few large population-based studies to estimate survival and determine prognostic factors of patients with spinal osteosarcoma due to rarity of the disease. Therefore, identification of risk and prognostic factors of spinal osteosarcoma is of great clinical significance in early diagnosis, treatment and prognostic prediction of the disease.
To further understand spinal osteosarcoma, we conducted a comprehensive analysis by using the SEER database, aiming to estimate the survival of patients with spinal osteosarcoma, identify prognostic factors, and propose a standard treatment strategy. To the best of our knowledge, the current research is the largest retrospective research on spinal osteosarcoma involving a total of 668 cases diagnosed between 1975 and 2016.
It was found in this work that the mean age of these patients with spinal osteosarcoma was 45.2 years at diagnosis. There was no significant gender preference in patients with spinal osteosarcoma (M/F = 1.13:1), although male predilection was reported in some case reports in the previous literature (19). Regarding survival outcomes, the median OS of the patients with spinal osteosarcoma was about 15 months and the 5-year OS was about 16.8%, which are both worse than those of patients with limb osteosarcoma. This may be caused by the following reasons. First, spinal osteosarcoma is more susceptible to metastasis at diagnosis than limb osteosarcoma, probably due to the delayed diagnosis or the age-specific differences in tumor biology (20). Second, it is usually difficult to detect spinal osteosarcoma in the early stage because local pain is the first or even the only symptom, which is often mistaken for the symptom caused by some benign diseases, resulting in delayed diagnosis and high risk of metastasis (21). Third, although en-block resection is the most effective surgical method for the treatment of spinal tumors, radical surgery is more difficult for spinal osteosarcoma because of the complex anatomical structures and associated complications.
It is worth noting that patients above 60 years had median OS of 8 months and 5-year OS of 4.6%. Ours multivariate analysis showed that age was not a prognostic factor in DSS, but OS was decreased significantly in patients older than 60 years, which is consistent with the previous reports. This trend is understandable, given the aggressive treatment for such diseases. Like other literature reports, we also identified the male gender as an independent risk factor for worse DSS in patients with spinal osteosarcoma, which may be attributed to the aggressiveness of the tumor and/or poor response to treatment (13). Although advances in medical technologies have to some extent improved the overall clinical outcome of spinal osteosarcoma, our multivariate analysis showed no significant improvement in DSS over the past decade. Besides, our research identified osteosarcoma NOS as the most common histological type in spinal osteosarcoma, which is similar to previous studies on all osteosarcomas (13). Tumor grade and stage are generally recognized as the independent prognostic indicators of both OS and DSS in patients with spinal osteosarcoma. A high grade (low differentiation or differentiation) is known as the risk factor of mortality and is associated with higher rates of metastasis and recurrence as compared with the low grade (high or moderate differentiation) (22). Many other studies have reported that tumor size above 10 cm is associated with poorer prognosis and decreased survival in osteosarcoma patients (23–25). However, our study showed that tumor size had no significant value in predicting OS and DSS of patients with spinal osteosarcoma.
The multivariate analysis indicated that definitive surgery had a positive effect on OS in patients with spinal osteosarcoma, while chemotherapy did not seem to significantly affect either OS or DSS. Other studies also found that chemotherapy could not prolong the survival of osteosarcoma patients (26). Interestingly, our study discovered that radiotherapy tended to decrease OS and DSS, while there was no statistical difference as for OS in the multivariate analysis, which is not consistent with the previous research (27). Surgical resection and neoadjuvant chemotherapy are considered the standard treatments for osteosarcoma, but the efficacy of radiotherapy is still controversial. Radiotherapy does not seem to bring about significant benefits to patients with spinal osteosarcomas due to radiotherapy resistance and the risk of radiation-induced spinal cord injury and paralysis in most cases (28). Therefore, radiotherapy may be more commonly used for local control, or as a last resort in inoperable cases (29–31). In addition, as indicated by many studies (32–34), radiation can induce aggressive behavior in osteosarcoma, and therefore radiotherapy should be used prudently in these patients.
The limitations of this work need to be considered, and this is inherent to registration-based databases. Although the SEER is an excellent resource for the longitudinal and population data analysis and the surgical intervention condition report, there are still limitations in its ability to retrospectively analyze other surgical variables, such as surgery type, margin status, surgical resection extent, and postoperative tumor recurrence. Besides, the SEER database does not contain sufficient information on specific chemotherapy regimens, radiation therapy dose, and molecular pathological characteristics, which may affect the prognosis of patients, and these variables may be an effective complement to this work. Furthermore, it is inevitable that data of some patients may be lost, due to the retrospective characteristic of this work. This may reduce the number of eligible cases. Despite these limitations, to our knowledge, the current research represents the largest analysis evaluating the important associations and predictors of spinal osteosarcoma.
5 Conclusion
In this population-based research, we made a comprehensive analysis of the clinical characteristics of spinal osteosarcoma, aiming to improve the current awareness and diagnosis of this malignant tumor. The male gender, a high tumor grade, regional stage and metastasis stage, and radiotherapy are independent predictors of poor survival of patients with spinal osteosarcoma. Treatment of spinal osteosarcoma remains a clinical challenge at present. In addition to the conventional treatments, future research should devote more efforts to gaining a better understanding about the pathogenesis of spinal osteosarcoma and explore the clinical impact and survival outcomes of the emerging targeted and immune therapies for this rare and aggressive disease.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
T-lL: conceptualization, supervision, and methodology. JW: formal analysis, investigation, and writing-original draft preparation. X-zN: data curation and writing- reviewing and editing. M-lY: data curation and investigation. S-mH: investigation. XH: investigation. CP: investigation. J-sC: investigation. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Natural Science Foundation of China (82172779 and 81972506).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ritter J, Bielack SS. Osteosarcoma. Ann Oncol (2010) 21 Suppl 7:vii320–5. doi: 10.1093/annonc/mdq276
2. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin (2013) 63(1):11–30. doi: 10.3322/caac.21166
3. Gumbel D, Gelbrich N, Weiss M, Napp M, Daeschlein G, Sckell A, et al. New treatment options for osteosarcoma – inactivation of osteosarcoma cells by cold atmospheric plasma. Anticancer Res (2016) 36(11):5915–22. doi: 10.21873/anticanres.11178
4. Katonis P, Datsis G, Karantanas A, Kampouroglou A, Lianoudakis S, Licoudis S, et al. Spinal osteosarcoma. Clin Med Insights Oncol (2013) 7:199–208. doi: 10.4137/CMO.S10099
5. Mathkour M, Garces J, Beard B, Bartholomew A, Sulaiman OA, Ware ML. Primary high–grade osteosarcoma of the clivus: A case report and literature review. World Neurosurg (2016) 89:730.e9–e13. doi: 10.1016/j.wneu.2016.01.054
6. Ciftdemir M, Kaya M, Selcuk E, Yalniz E. Tumors of the spine. World J Orthop (2016) 7(2):109–16. doi: 10.5312/wjo.v7.i2.109
7. Green R, Saifuddin A, Cannon S. Pictorial review: Imaging of primary osteosarcoma of the spine. Clin Radiol (1996) 51(5):325–9. doi: 10.1016/S0009-9260(96)80108-5
8. Bielack SS, Wulff B, Delling G, Gobel U, Kotz R, Ritter J, et al. Osteosarcoma of the trunk treated by multimodal therapy: experience of the cooperative osteosarcoma study group (COSS). Med Pediatr Oncol (1995) 24(1):6–12. doi: 10.1002/mpo.2950240103
9. Gill J, Gorlick R. Advancing therapy for osteosarcoma. Nat Rev Clin Oncol (2021) 18(10):609–24. doi: 10.1038/s41571-021-00519-8
10. Wu J, Sun H, Li J, Guo Y, Zhang K, Lang C, et al. Increased survival of patients aged 0–29 years with osteosarcoma: A period analysis, 1984–2013. Cancer Med (2018) 7(8):3652–61. doi: 10.1002/cam4.1659
11. Groves ML, Zadnik PL, Kaloostian P, Sui J, Goodwin CR, Wolinsky JP, et al. Epidemiologic, functional, and oncologic outcome analysis of spinal sarcomas treated surgically at a single institution over 10 years. Spine J (2015) 15(1):110–4. doi: 10.1016/j.spinee.2014.07.005
12. Duffaud F, Digue L, Baciuchka–Palmaro M, Volot F, Perles–Daniel C, Garbe L, et al. Osteosarcomas of flat bones in adolescents and adults. Cancer. (2000) 88(2):324–32. doi: 10.1002/(SICI)1097-0142(20000115)88:2<324::AID-CNCR12>3.0.CO;2-E
13. Duchman KR, Gao Y, Miller BJ. Prognostic factors for survival in patients with high–grade osteosarcoma using the surveillance, epidemiology, and end results (SEER) program database. Cancer Epidemiol (2015) 39(4):593–9. doi: 10.1016/j.canep.2015.05.001
14. Scosyrev E, Messing J, Noyes K, Veazie P, Messing E. Surveillance epidemiology and end results (SEER) program and population–based research in urologic oncology: an overview. Urol Oncol (2012) 30(2):126–32. doi: 10.1016/j.urolonc.2009.11.005
15. Kelley SP, Ashford RU, Rao AS, Dickson RA. Primary bone tumours of the spine: a 42–year survey from the Leeds regional bone tumour registry. Eur Spine J (2007) 16(3):405–9. doi: 10.1007/s00586-006-0188-7
16. Sciubba DM, Okuno SH, Dekutoski MB, Gokaslan ZL. Ewing And osteogenic sarcoma: evidence for multidisciplinary management. Spine (2009) 34(22 Suppl):S58–68. doi: 10.1097/BRS.0b013e3181ba6436
17. Anwar MA, El–Baba C, Elnaggar MH, Elkholy YO, Mottawea M, Johar D, et al. Novel therapeutic strategies for spinal osteosarcomas. Semin Cancer Biol (2020) 64:83–92. doi: 10.1016/j.semcancer.2019.05.018
18. Camuzard O, Santucci–Darmanin S, Carle GF, Pierrefite–Carle V. Role of autophagy in osteosarcoma. J Bone Oncol (2019) 16:100235. doi: 10.1016/j.jbo.2019.100235
19. Smeland S, Muller C, Alvegard TA, Wiklund T, Wiebe T, Bjork O, et al. Scandinavian Sarcoma group osteosarcoma study SSG VIII: prognostic factors for outcome and the role of replacement salvage chemotherapy for poor histological responders. Eur J Cancer (2003) 39(4):488–94. doi: 10.1016/S0959-8049(02)00747-5
20. Miller BJ, Cram P, Lynch CF, Buckwalter JA. Risk factors for metastatic disease at presentation with osteosarcoma: an analysis of the SEER database. J Bone Joint Surg Am (2013) 95(13):e89. doi: 10.2106/JBJS.L.01189
21. Sundaresan N, Rosen G, Boriani S. Primary malignant tumors of the spine. Orthop Clin North Am (2009) 40(1):21–36v. doi: 10.1016/j.ocl.2008.10.004
22. Hung GY, Yen HJ, Yen CC, Wu PK, Chen CF, Chen PC, et al. Improvement in high–grade osteosarcoma survival: Results from 202 patients treated at a single institution in Taiwan. Medicine (2016) 95(15):e3420. doi: 10.1097/MD.0000000000003420
23. Anderson ME. Update on survival in osteosarcoma. Orthop Clin North Am (2016) 47(1):283–92. doi: 10.1016/j.ocl.2015.08.022
24. Colding–Rasmussen T, Thorn AP, Horstmann P, Rechnitzer C, Hjalgrim LL, Krarup–Hansen A, et al. Survival and prognostic factors at time of diagnosis in high–grade appendicular osteosarcoma: a 21 year single institution evaluation from east Denmark. Acta Oncol (2018) 57(3):420–5. doi: 10.1080/0284186X.2017.1351620
25. Parry MC, Laitinen M, Albergo J, Jeys L, Carter S, Gaston CL, et al. Osteosarcoma of the pelvis. Bone Joint J (2016) 98–B(4):555–63. doi: 10.1302/0301-620X.98B4.36583
26. Iwata S, Ishii T, Kawai A, Hiruma T, Yonemoto T, Kamoda H, et al. Prognostic factors in elderly osteosarcoma patients: a multi–institutional retrospective study of 86 cases. Ann Surg Oncol (2014) 21(1):263–8. doi: 10.1245/s10434-013-3210-4
27. Schwarz R, Bruland O, Cassoni A, Schomberg P, Bielack S. The role of radiotherapy in oseosarcoma. Cancer Treat Res (2009) 152:147–64. doi: 10.1007/978-1-4419-0284-9_7
28. Sole CV, Calvo FA, Alvarez E, Cambeiro M, Cuervo M, San Julian M, et al. Adjuvant radiation therapy in resected high–grade localized skeletal osteosarcomas treated with neoadjuvant chemotherapy: Long–term outcomes. Radiother Oncol (2016) 119(1):30–4. doi: 10.1016/j.radonc.2016.02.029
29. Schoenfeld AJ, Hornicek FJ, Pedlow FX, Kobayashi W, Garcia RT, DeLaney TF, et al. Osteosarcoma of the spine: experience in 26 patients treated at the Massachusetts general hospital. Spine J (2010) 10(8):708–14. doi: 10.1016/j.spinee.2010.05.017
30. Zhang W, Tanaka M, Sugimoto Y, Takigawa T, Ozaki T. Carbon–ion radiotherapy of spinal osteosarcoma with long–term follow. Eur Spine J (2016) 25 Suppl 1:113–7. doi: 10.1007/s00586-015-4202-9
31. Blattmann C, Oertel S, Schulz–Ertner D, Rieken S, Haufe S, Ewerbeck V, et al. Non–randomized therapy trial to determine the safety and efficacy of heavy ion radiotherapy in patients with non–resectable osteosarcoma. BMC Cancer (2010) 10:96. doi: 10.1186/1471-2407-10-96
32. Liao LQ, Yan HH, Mai JH, Liu WW, Li H, Guo ZM, et al. Radiation–induced osteosarcoma of the maxilla and mandible after radiotherapy for nasopharyngeal carcinoma. Chin J Cancer (2016) 35(1):89. doi: 10.1186/s40880-016-0153-8
33. Puhaindran ME, Hamilton K, Schlumbohm S, Rich M, Mitchell D, Steensma M. Radiation–induced osteosarcoma of the hand: case report. J Handb Surg Am (2014) 39(6):1151–4. doi: 10.1016/j.jhsa.2014.03.010
Keywords: spinal osteosarcoma, epidemiology, SEER, survival, prognosis
Citation: Wang J, Ni X-z, Yang M-l, Huang X, Hou S-m, Peng C, Cao J-s and Liu T-L (2023) Prognostic factors and treatment outcomes of spinal osteosarcoma: Surveillance, epidemiology, and end results database analysis. Front. Oncol. 13:1083776. doi: 10.3389/fonc.2023.1083776
Received: 29 October 2022; Accepted: 10 February 2023;
Published: 01 March 2023.
Edited by:
Xiaodong Tang, Peking University People’s Hospital, ChinaReviewed by:
Feng Wei, Peking University Third Hospital, ChinaHaicong Chen, Affiliated Hospital of Guangdong Medical University, China
Paolo Palmisciano, University of Cincinnati, United States
Copyright © 2023 Wang, Ni, Yang, Huang, Hou, Peng, Cao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tie-Long Liu, Y3p5eWx0bEAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Jing Wang
Jing Wang Xiang-zhi Ni†
Xiang-zhi Ni† Shu-ming Hou
Shu-ming Hou Tie-Long Liu
Tie-Long Liu