- 1Department of General Surgery, Yan’an Hospital Affiliated to Kunming Medical University, Kunming, China
- 2Key Laboratory of Tumor Immunological Prevention and Treatment in Yunnan Province, Yan’an Hospital Affiliated to Kunming Medical University, Kunming, China
- 3Department of Pathology, Yan’an Hospital Affiliated to Kunming Medical University, Kunming, China
Background: Expansion and activation of cytotoxic T lymphocytes (CTLs) in vitro represents a promising immunotherapeutic strategy, and CTLs can be primed by dendritic cells (DCs) loaded with tumor-associated antigens (TAAs) transformed by recombinant adeno-associated virus (rAAV). This study aimed to explore the impact of rAAV-DC-induced CTLs on prognosis of CRC and to explore factors associated with prognosis.
Methods: This prospective observational study included patients operated for CRC at Yan’an Hospital Affiliated to Kunming Medical University between 2016 and 2019. The primary outcome was progression-free survival (PFS), secondary outcomes were overall survival (OS) and adverse events. Totally 49 cases were included, with 29 and 20 administered rAAV-DC-induced CTL and chemotherapy, respectively.
Results: After 37-69 months of follow-up (median, 54 months), OS (P=0.0596) and PFS (P=0.0788) were comparable between two groups. Mild fever occurred in 2 (6.9%) patients administered CTL infusion. All the chemotherapy group experienced mild-to-moderate adverse effects, including vasculitis (n=20, 100%), vomiting (n=5, 25%), nausea (n=17, 85%) and fatigue (n=17, 85%).
Conclusions: Lymphatic metastasis (hazard ratio [HR]=4.498, 95% confidence interval [CI]: 1.290-15.676; P=0.018) and lower HLA-I expression (HR=0.294, 95%CI: 0.089-0.965; P=0.044) were associated with poor OS in the CTL group. CTLs induced by rAAV-DCs might achieve comparable effectiveness in CRC patients compare to chemotherapy, cases with high tumor-associated HLA-I expression and no lymphatic metastasis were more likely to benefit from CTLs.
Introduction
Colorectal cancer (CRC) is a commonly diagnosed malignancy with high mortality worldwide (1). Conventional therapeutic options include surgery, chemotherapy, and radiotherapy (2, 3). In CRC, postoperative adjuvant chemotherapy has been shown to be effective in improving survival and reducing disease recurrence (4). Recommended regimens include fluorouracil and capecitabine, with or without oxaliplatin (5). Despite the obvious benefits of chemotherapy, the prognosis of patients with late-stage or metastatic CRC remains poor. In addition, patients administered chemotherapy experience a variety of adverse effects, including neutropenia, diarrhea, vomiting, and neurotoxicity (5, 6). Therefore, effective treatment strategies with lower toxicity are urgently required.
With the current understanding of the pathophysiology and immunology of cancer, immunotherapy, with the main features of specificity and low toxicity, has become the most promising treatment strategy for patients with CRC (7, 8). To date, immunotherapeutic strategies used in CRC include immune checkpoint blockade, specific antibody therapy, cellular immunotherapy, cancer vaccine therapy, and other combination strategies such as antiangiogenic agents combined with chemotherapy (7, 9). Cellular immunotherapy refers primarily to adoptive cell therapy, which often involves collecting T cells from a patient’s tumor, lymph nodes, or peripheral blood, expanding and activating them in vitro, and transferring them to the patient (10). Several clinical trials of CRC have used chimeric antigen receptor T (CAR-T) cell therapy (11), tumor-infiltrating lymphocyte (TIL) therapy (12), and natural killer (NK) cell therapy (13), with variable responses.
In adoptive cell immunotherapy, it is crucial to activate or modify T lymphocytes to specifically kill tumor cells. To this end, antigen-presenting cells (APCs) can be loaded with tumor-associated antigens (TAAs) as an effective approach to prime T lymphocytes. Dendritic cells (DCs), which are considered professional APCs, have been shown to be capable of generating antitumor cytotoxic T lymphocytes (CTLs) (14). There are generally two strategies to load DC cells with TAAs. One is to use tumor lysate containing both identified and unidentified antigens to directly stimulate DC cells (15–19), and the other is to use genetic engineering methods to introduce DNA or mRNA of defined TAAs into DC cells (20, 21).Studies have confirmed that the latter is more effective (21, 22). Recombinant adeno-associated virus (rAAV) is considered to be an ideal vector for genetic engineering because of its non-integration into host genome and no residue (23). rAAV has been used to transform DCs and activate CTLs with tumor associated antigen in several studies, and its safety and effectiveness have been confirmed (23, 24). CTLs induced by rAAV-DC have been used to treat CRC in a phase I clinical trial, and the safety of this approach has been demonstrated (25).
However, the impact of CTLs induced by rAAV-DC in the treatment of CRC remains unclear. Furthermore, no study has identified factors associated with prognosis of CTLs therapy in CRC. Therefore, this study aimed to explore the impact of rAAV-DC-induced CTLs on prognosis of CRC and to explore factors associated with prognosis.
Patients and methods
Study design and patients
This prospective observational study included resectable CRC patients in General Surgery Department, Yan’an Hospital Affiliated to Kunming Medical University between February 2016 and 30 September 2019. Inclusion criteria were: 1) age between 18 and 80 years; 2) resectable colorectal tumor confirmed by postoperative histopathology; 3) Karnofsky performance score (KPS) ≥70 before treatment with adequate hematologic, hepatic, and renal functions (i.e., hemoglobin ≥8 g/dL, platelet count ≥100,000/μL, white blood cell count >3000/μL, serum blood urea nitrogen <25 mg/dL, serum creatinine <1.8 mg/dL, and serum ALT, AST, and TBIL levels ≤1.5-times of the respective upper limits of normal [ULNs]). Exclusion criteria were: 1) autoimmune, uncontrolled cardiovascular or lung diseases; 2) pregnancy or lactation (women); 3) treatment with immunosuppressants.
All patients provided written informed consent, and the study was performed in accordance with the Declaration of Helsinki. The ethics committee of Yan’an Hospital affiliated with Kunming Medical University approved the study protocol (approval no. 2015-049-01).
The patients were divided into CTL group and chemotherapy group according to their treatment strategy. In the CTL group, tumor-associated antigens (TAAs) and human leukocyte antigen (HLA) class I protein were required to be positive (weakly positive, positive, or strongly positive) in specimen by an immunohistochemical assay (26).
Procedures
Patients in CTL group received treatment by infusion with CTLs generated by transfection with DCs harboring rAAV containing TAAs (25).
Preparation of dendritic cells
Peripheral blood samples (60–70 mL) were collected and subjected to heparin-anticoagulation in each patient receiving immunotherapy. These samples were added to an equal volume of normal saline. Peripheral blood mononuclear cells (PBMCs) separated by Ficoll gradient centrifugation were cultured in 10-cm plates (4.5×107 cells/plate) in AIM-V medium (Invitrogen Co. Carlsbad, CA) at 37°C in a 6% CO2 incubator for 3–4 h. Non-adherent cells (lymphocytes) were obtained by gentle shaking and transferred to a centrifuge tube to generate T cells. Several types of rAAVs carrying specific TAA-cDNA (1.0×108/mL, Immuclin Biomed, Inc. Shenzhen, China) were mixed according to immunohistochemical data. Washed adherent cells were added to mixed rAAVs in AIM-V medium containing GM-CSF (800 IU/mL, Amoytop Biotech Co. Ltd. Xiamen, China) and incubated at 37°C in a 6% CO2 incubator for more than 8 h. The medium containing the rAAVs was then removed. AIM-V medium containing GM-CSF (800 IU/mL, Amoytop Biotech Co. Ltd.) and IL-4 (1000 IU/mL, R&D Systems, Inc. Minneapolis, MN) was used to induce monocyte differentiation into DCs at 37°C in a 5% CO2 incubator. The cytokine-containing medium was refreshed on days 3 and 5, and TNF-α (20 ng/mL, R&D Systems) was added on day 5. Finally, mature DCs were harvested on day 6.
Preparation of T lymphocytes
Non-adherent PBMCs (lymphocytes) were transferred to T75 culture flasks and cultured in AIM-V medium at 37°C in a 6% CO2 incubator. IL-2 (20 IU/mL, R&D Systems) was added to activate T lymphocytes. The medium was refreshed every other day, and activated T lymphocytes were harvested on day 6.
Preparation of cytotoxic T lymphocytes
On day 6, activated T lymphocytes and mature DCs were mixed at a 20:1 ratio and co-cultured in 10-cm culture plates in AIM-V medium supplemented with IL-2 (20 IU/mL) and IL-7 (20 ng/mL, R&D Systems) at 37°C in a 6% CO2 incubator. The medium containing IL-2 and IL-7 was refreshed every other day. Finally, cells were harvested on day 15 and washed three times by centrifugation (1200 rpm/min, 12 min) with normal saline. The suspension volume was adjusted to 200 mL. After counting, the CTL suspension was filtered through a cell sieve (100 mesh) once and transferred to a sterile infusion bag or bottle for infusion.
Transfusion procedure
After evaluation and testing of the specimen, peripheral blood was collected within 4 weeks after surgery to generate CTLs as described above. The harvested CTLs were then infused for 30 minutes intravenously. Subsequent generation and infusion of CTLs was performed every 15 days for a total of 5 times. After each administration, patients were observed for at least 6 h to identify potential adverse effects.
A modified FOLFOX6 regimen was administered to patients who selected chemotherapy. Dosage and schedule were as follows: oxaliplatin at 100 mg/m2, ivgtt (2 h) on day 1; 5-flurouracil at 500 mg/m2, ivgtt on days 1-5; calcium folinate at 200 mg/m2, ivgtt on days 1-5. The first chemotherapy was performed 3–4 weeks after surgery and was repeated every 4 weeks, for 6 times in total.
Demographic (age and sex) and clinical (tumor subtype, lymphatic metastasis and stage at diagnosis) features were also recorded. Patients were staged according to the 8th AJCC CRC pTNM staging system (27).
Outcomes
The primary outcome was progression-free survival (PFS), defined as the time interval between the date of surgery and the date of progression or death from the disease. Secondary outcomes included overall survival (OS), adverse events, including fever, hypoleukocytemia, liver dysfunction, renal dysfunction, nausea and vomiting. OS was defined as the time interval between the date of surgery and the date of death from any cause. Disability and adverse events leading to prolonged hospitalization or affecting the ability to work were defined as serious adverse events.
Follow up
The patients were continuously followed up. Chest and abdomen computed tomography (CT) scans were performed every 3 months. Peripheral blood cell counts, liver function, and renal function were tested after each transfusion. In patients administered CTL transfusion, the immunophenotype of peripheral blood cells was also assessed by flow cytometry after each infusion. Any symptoms considered to be related to treatment were recorded. Follow-up ended in September 2021.
Statistical analysis
SPSS 22.0 (IBM Corp., Armonk, NY, USA) for Windows and GraphPad prism 8 (GraphPad Software Inc., San Diego, California, USA) were used for statistical analysis. Normally and skewed distributed continuous data were expressed as mean ± SD and median (interquartile range), respectively, and compared by the t-test and Mann Whitney U test, respectively. Categorical data were expressed as number (percentage), and compared by the χ2 test.
PFS and OS were estimated by the Kaplan-Meier method and compared by the log rank test. In CTL transfusion group, factors associated with prognosis were defined by Cox proportional hazard regression analysis. HLA-I and CEA levels were dichotomized into high (2+ to 3+) and low (+), and TNM stage was dichotomized into early (I/II) and late (III/IV). The status of lymphatic metastasis was marked as positive or negative. Two-sided P<0.05 was considered statistically significant.
Results
A total of 49 CRC patients were enrolled in this study, including 29 and 20 in the CTL and chemotherapy groups, respectively. There was no case of drop out or lost to follow up. There were no significant differences in demographic and baseline clinical characteristics between the two groups (Table 1).
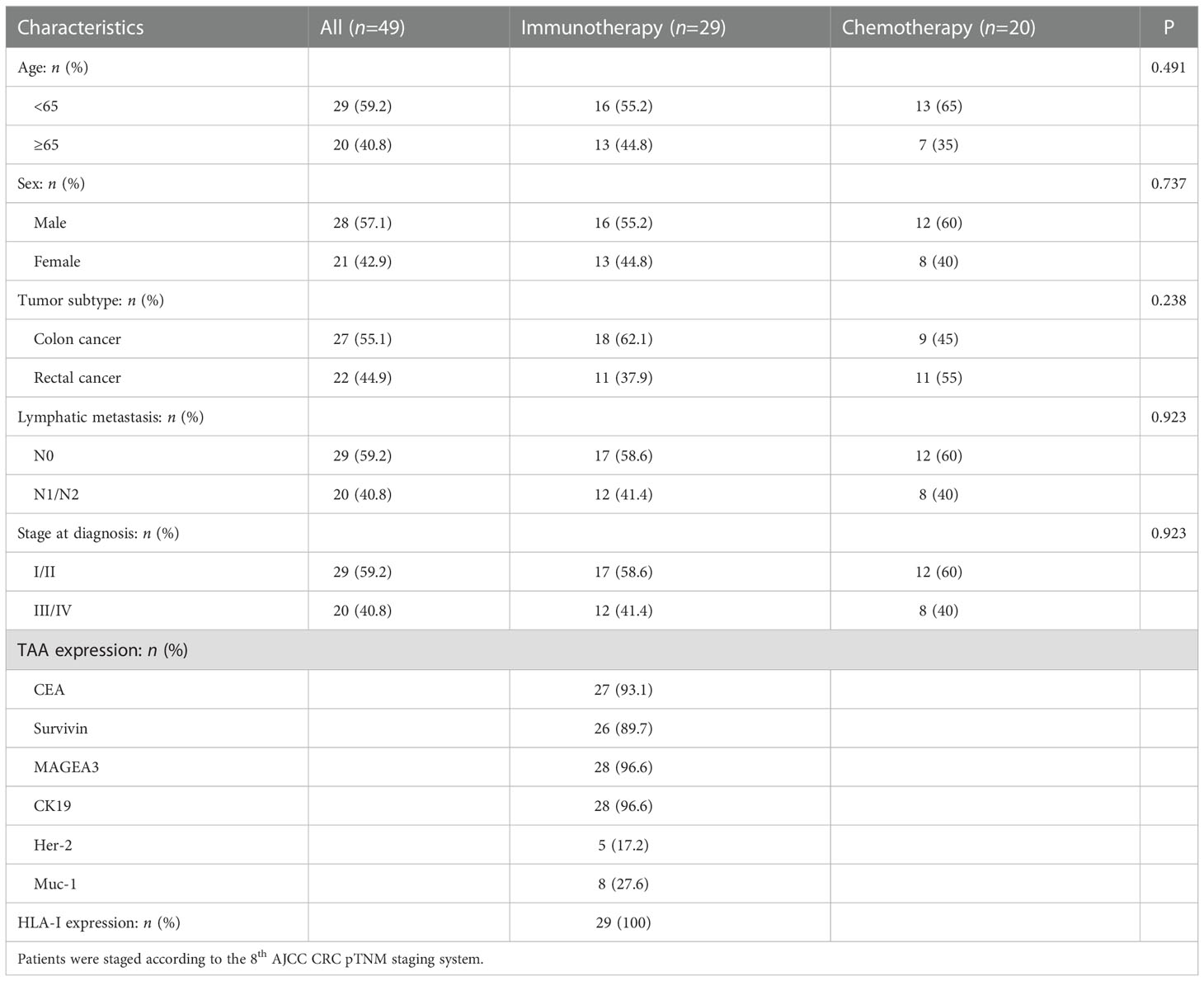
After culture, the total average amounts of transfused cells were 3.04 ± 0.25 × 108 cells. The average percentages of CD3+, CD3+CD4+ and CD3+CD8+ lymphocytes were 76.83 ± 2.29%, 37.90 ± 3.84% and 29.72 ± 2.84%, respectively. CD80, CD86 and HLA-DR were upregulated in CTLs after induced by rAAV-DC (Figure 1), indicating that the CTLs were indeed activated.
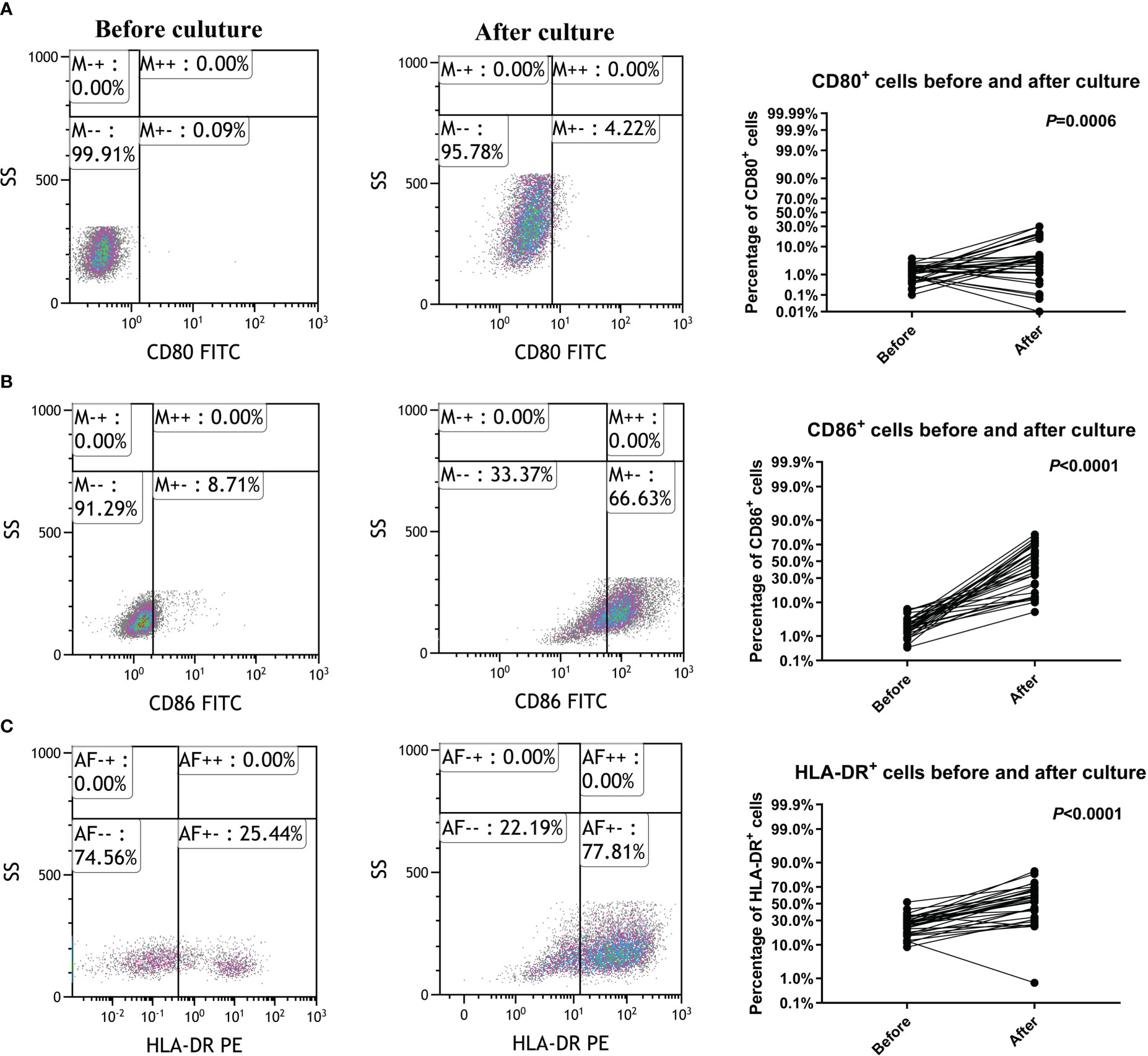
Figure 1 Cytotoxic T lymphocyte activation by culture and induction. (A) CD80 was upregulated in Cytotoxic T lymphocytes (CTLs). (B) CD86 was upregulated in CTLs. (C) HLA-DR was upregulated in CTLs.
No serious adverse events occurred in either group. Fever occurred in 2 patients administered infusion therapy with CTLs, with temperatures of 38.2°C and 38.7°C, respectively. Both fevers resolved within 6 hours after indomethacin administration. However, all 20 patients administered chemotherapy experienced adverse events, including vasculitis (20 cases, 100%), vomiting (5 cases, 25%), nausea (17 cases, 85%) and fatigue (17 cases, 85%).
The patients were followed up for 37 to 69 months, with a median follow-up duration of 54 months. One sample from the CTL group was excluded due to hemolysis. Twenty-eight pairs of PBMC samples were collected from CTL group patients and analyzed by flow cytometry before and after CTL transfusion. There were no significant differences in the percentages of CD3+, CD3+CD8+, CD11c+, CD80+ and CD86+ cells (Figure 2).
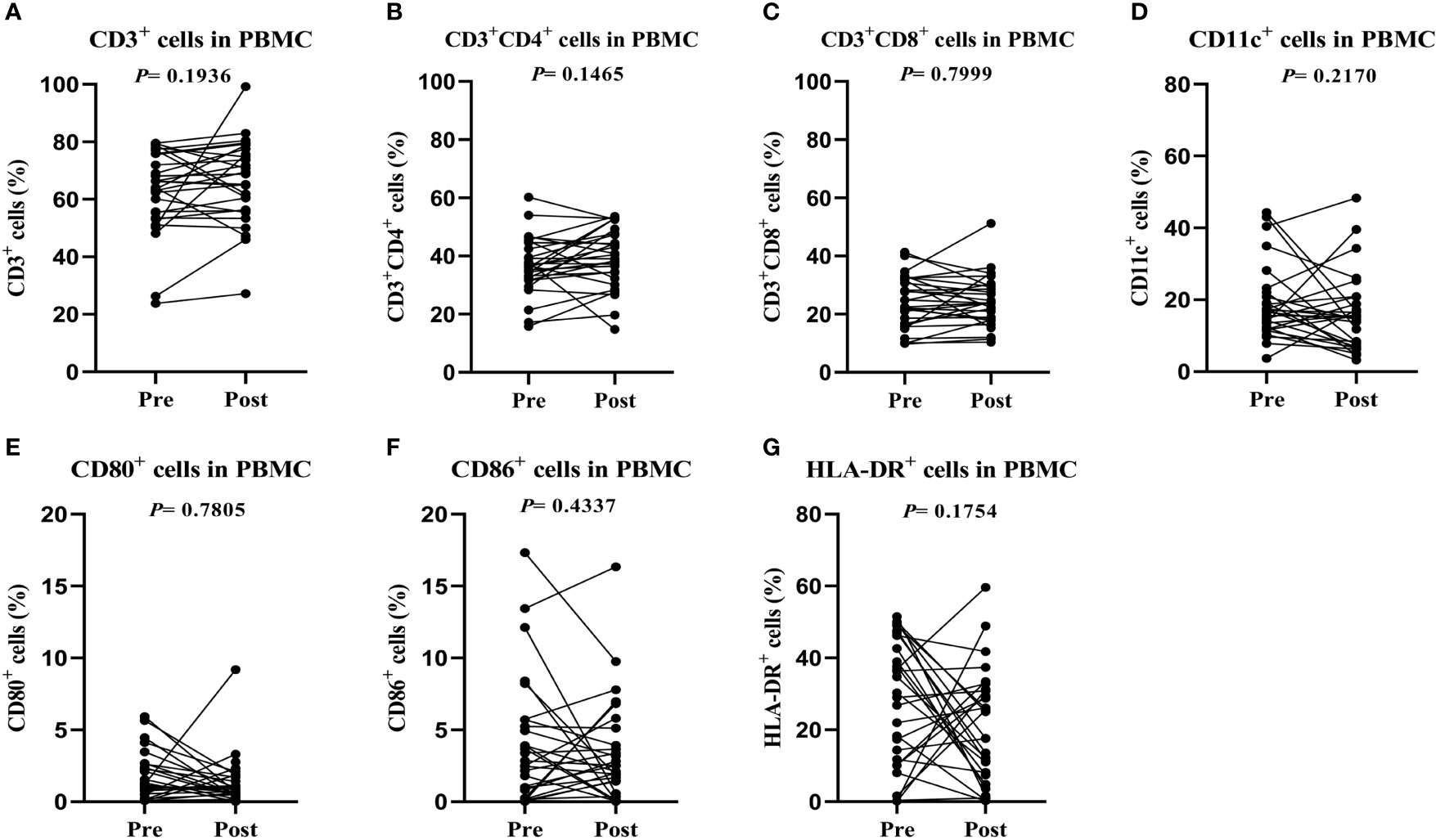
Figure 2 Peripheral blood samples have similar phenotypes before and after cytotoxic T lymphocyte infusion. (A) T lymphocyte proportions in peripheral blood. (B) Cytotoxic T lymphocyte proportions in peripheral blood. (C) Dendritic cell proportions in peripheral blood. (D–G) Activated T lymphocyte proportions in peripheral blood. Immunophenotypes were determined by flow cytometry.
Median PFS in the CTL transfusion group was 42 months; the median PFS of the chemotherapy group has not been reached. The 1-, 3- and 5-year PFS rates were 75.9%, 55.0% and 49.5%, respectively, in the CTL group, versus 90%, 74.0% and 64.7%, respectively, in the chemotherapy group. OS rates in the CTL infusion and chemotherapy groups were 58.6% (17/29) and 80.0% (16/20), respectively. The median OS of both groups has not been reached. The 1-, 3-, and 5-year OS rates were 65.5%, 58.2% and 58.2%, respectively, in the CTL group, versus 95%, 90% and 75.9%, respectively, in the chemotherapy group. There were no significant differences in OS (P=0.0596) and PFS (P=0.0788) between two groups (Figure 3).
Figure 3 Cytotoxic T lymphocytes induced by rAAVs are effective and well-tolerated. (A) Overall survival (OS) and (B) progression-free survival (PFS) estimated by the Kaplan-Meier method. (C) Adverse events in both group.
In CTL transfusion group, Kaplan-Meier method showed patients with high tumor HLA-I expression had better OS (P=0.0115) and PFS (P=0.0251) compared with those with low tumor HLA-I expression (Figures 4A, B), and patients with lymphatic metastasis had worse OS (P=0.0081) and PFS (P=0.0346) compared with those without lymphatic metastasis (Figures 4C, D). Cox proportional hazard regression indicated lymphatic metastasis (hazard ratio [HR]=4.498, 95% confidence interval [CI]: 1.290-15.676; P=0.018) and lower HLA-I expression (HR=0.294, 95%CI: 0.089-0.965; P=0.044) were associated with poor OS in the CTL group (Table 2) (Figures 4E, F).
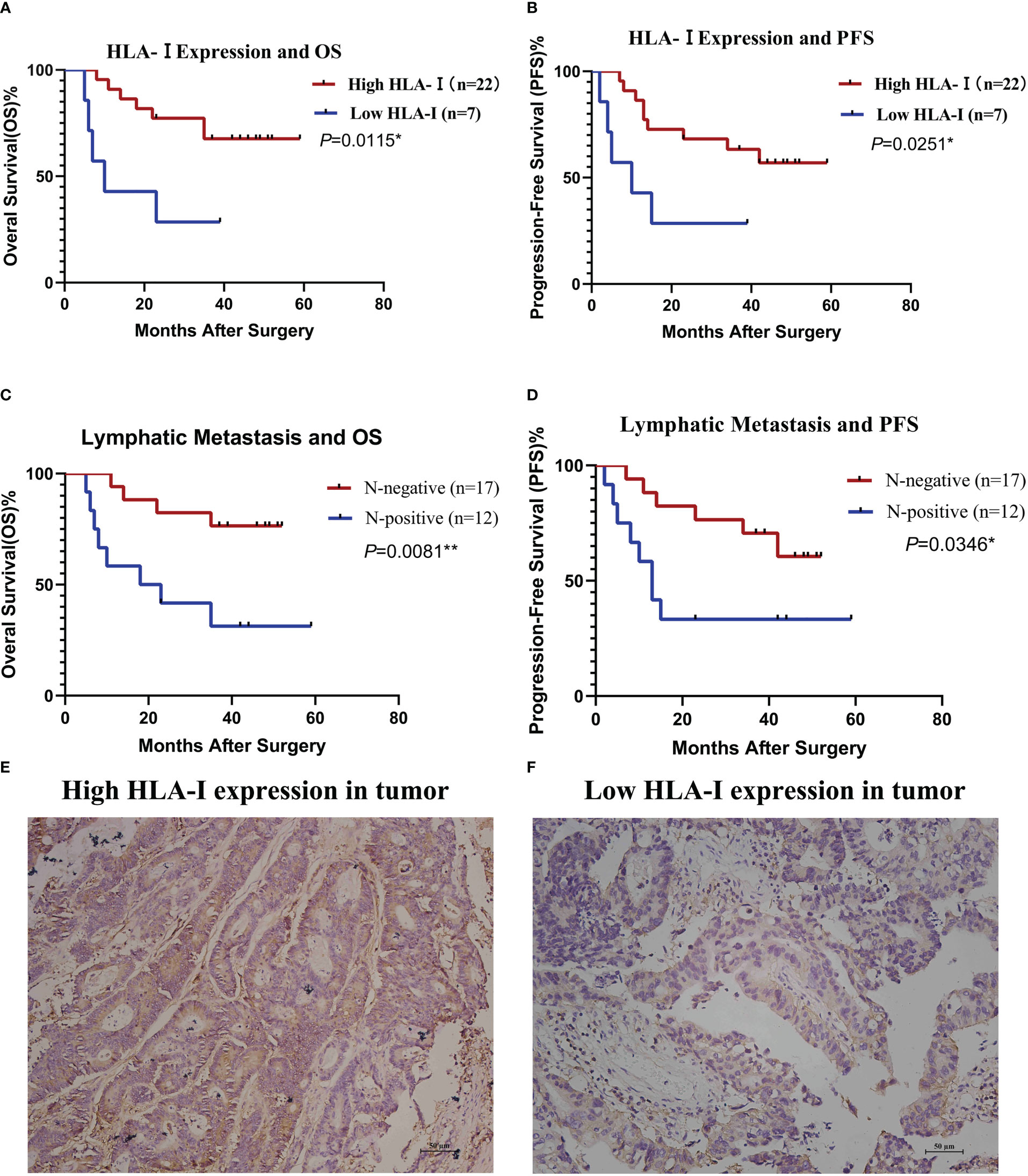
Figure 4 HLA-I expression and lymphatic metastasis are associated with the clinical outcome of cytotoxic T lymphocyte (CTL) infusion therapy. Stratified analysis of overall survival (OS, A) and progression-free survival (PFS, B) based on HLA-I expression. Stratified analysis of OS (C) and PFS (D) based on lymphatic metastasis status. OS and PFS were estimated by the Kaplan-Meier method. *P<0.05, **P<0.01. (E) Immunohistochemistry of a colon cancer tissue immunostained with anti- HLA-I antibodies showing high HLA-I expression. (F) immunohistochemistry of a colon cancer tissue immunostained with anti- HLA-I antibodies showing low HLA-I expression.
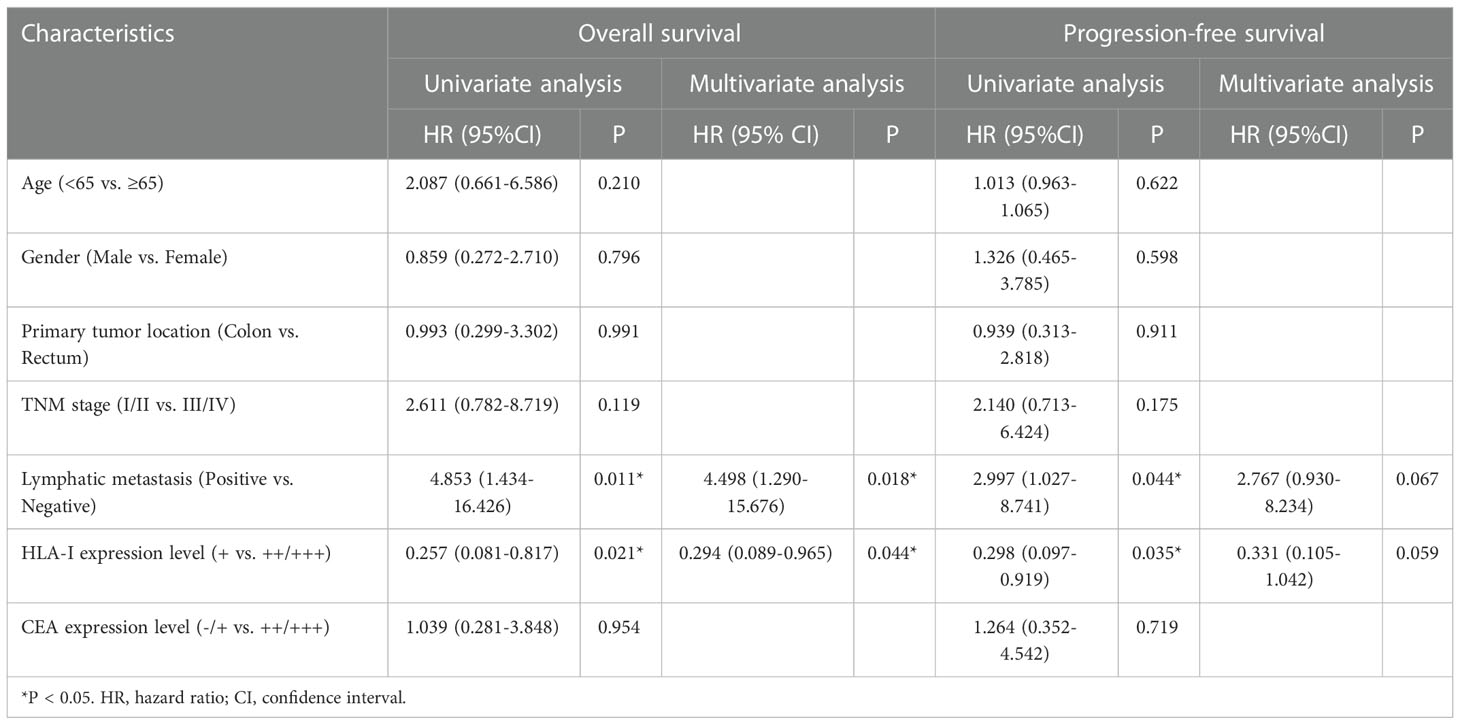
Table 2 Univariate and multivariate analyses of overall survival and progression-free survival in patients administered CTL infusion.
Discussion
In this study, rAAV was used to load DCs with defined TAAs according to the immunohistochemical test results of tumor specimens. These rAAV-DCs were further induced to mature cells capable of expressing and presenting TAAs through culture with GM-CSF and IL-4. Then expanded T lymphocytes were activated by rAAV-DCs in vitro. After transfusion, T lymphocytes as effector cells could kill tumor cells in vivo, while rAAV-DCs could act as tumor vaccine. The results showed there was no significant differences in OS and FPS between CTL transfusion group and chemotherapy group. But the occurrence rates of adverse events were lower in CTL transfusion group. This study also showed that lymphatic metastasis and tumor HLA-I expression were independent prognostic factors in CRC patients administered CTL immunotherapy. The result might provide evidence for application of CTL immunotherapy. Previous studies focused on loading DCs with TAAs to make tumor vaccines, but did not expand and activate T lymphocytes in vitro (15, 16, 18–20). Our study focused on the preparation of effector T lymphocytes to maximize tumor killing effect. In addition, we have followed up the patients for more than three years and have a relatively objective evaluation on the therapeutic effect of rAAV-DC-induced CTLs, which is an important supplement to the previous phase I clinical trial focusing on safety research (25).
CRC, a heterogeneous disease caused by many genetic and epigenetic alterations, can be categorized into hypermutated tumors (mutation rates of >12 per 106 base pair) and non-hypermutated tumors based on exome sequencing (28). Since hypermutated tumors may present more neoantigens to the host immune system, they are more sensitive to immunotherapy (29). CRC can also be divided into tumors with mismatch-repair (MMR) deficiency or high levels of microsatellite instability (dMMR-MSI-H) and those with MMR proficiency or low levels of microsatellite instability (pMMR-MSI-L) (28). CRC patients with dMMR-MSI-H do not benefit from fluorouracil-based adjuvant chemotherapy (30). Conversely, such cases are sensitive to immune checkpoint inhibition therapy (31, 32). Due to the limited sample population, the number of cases enrolled are generally small, with the proportion of hypermutated tumor amounting to only approximately 16% of all cases (28). Therefore, this study did not perform sample sequencing or case grouping. Nonetheless, this study showed no significant differences in PFS between the immunotherapy and chemotherapy groups. Further case grouping would have revealed some differences.
CTLs are effector cells of the adaptive immune response. The degree of T lymphocyte infiltration, especially CD8+ T lymphocyte infiltration in tumors, is associated with prognosis in patients with CRC (33). Therefore, many immunotherapeutic strategies focus on improving the functional status of CTLs, which are mostly effective (7). In agreement, the present study found no significant differences in OS and PFS between the CTL and chemotherapy groups. In addition to effectiveness, safety must be considered for clinical application. Immunotherapy can cause a spectrum of adverse events called immune-related adverse events (irAEs) due to the activation of the immune system, which could damage healthy tissues of organs; the incidence of irAEs for immune check point inhibitors is between 23% and 83% (34). In CAR-T cell therapy, a systemic inflammatory response termed the cytokine release syndrome (CRS) is the most common severe irAE; neurological toxicity can also be caused by CAR-T cells (35). Compared with other strategies, there are fewer irAEs following exposure to CTLs induced by rAAV-DCs. DCs and CTLs are both derived from PBMCs collected from patients or their immediate family members; thus, the possibility of immune rejection is very low. rAAV has been approved by the US Food and Drug Administration for clinical use, with proven safety. Activated CTLs kill tumor cells specifically and to a limited extent, with no evidence of a CRS. In this trial, only 2 cases (6.9%) experienced a mild fever. In another previous phase I clinical trial evaluating rAAV-DC-induced CTLs for the treatment of CRC, 27 patients were administered CTL infusion, with cases experiencing fever and fatigue; the overall incidence of irAE was 18.5% (25). The current study and the latter trial demonstrated that rAAV-DC-induced CTL is a safe treatment strategy for CRC patients postoperatively. No significant changes were found in the immunophenotype of PBMCs from the treated patients, which may be due to the dilution of the infused CTLs.
To kill tumor cells, CTLs must distinguish them from normal cells firstly through the binding of T cell receptors (TCRs) on T cells to TAA peptide complexes with tumor cell-specific HLA class I molecules presented on the cell surface. Tumor cells can alter the expression of HLA class I to escape immune surveillance through a variety of mechanisms, e.g., deficient synthesis of β2-microglobulin, loss of genes encoding heavy chains of HLA antigens, mutations that inhibit HLA antigen transcription, and defects in one or more components of antigen processing machinery components (APM) (36). Tumor HLA class I expression is essential for treatment response in patients receiving immunotherapy with CTLs. This study showed that high HLA-I expression was associated with better PFS in CRC patients treated postoperatively with rAAV-DC induced CTLs. Previously, tumor patients with high HLA- I expression were shown to benefit from CTL-based immunotherapy after assessing the responses of tumor cells with different levels of HLA- I expression to CTLs (26). The above data provide evidence confirming this hypothesis in a clinical setting.
Lymph node metastasis was independently associated with worse PFS in CRC patients treated with CTLs in this study. It is well-known that regional lymph nodes are the sites where DCs present tumor-derived antigens to naïve T cells to generate CTLs (37). The theoretical function of lymph nodes is to inhibit metastasis growth, although recent investigation has reported that tumor cell infiltration into lymph nodes induces a certain level of T cell expansion (38). To escape immune surveillance, the tumor can produce a variety of regulatory molecules to downregulate the immune function of lymph nodes (37). In other words, lymphatic metastasis indicates that tumor cells are resistant to CTLs in certain circumstances. This explains why CRC patients with lymphatic metastasis do not benefit from CTL-based immunotherapy.
Conclusion
This study had several limitations. First, this was a single-center observational study without randomization, which could result in bias. In addition, the sample size was relatively limited, which precluded subgroup analysis. For example, loss of HLA-I is known to induce resistance to immunotherapy, and HLA-I genotype’s diversity may also affect the efficiency of immunotherapy (39). However, due to the limited sample size, we did not perform further HLA typing. Finally, follow-up was relatively short in this study, the OS and PFS was not reached. Therefore, long-term follow-up and large multicenter, randomized controlled studies are needed to verify the effectiveness and safety of CTLs induced by rAAV-DCs. Further analyses based on genotyping are also needed to explore the ideal predictors of response to treatment.
In conclusion, CTLs induced by rAAV-DCs might achieve comparable effectiveness in CRC patients compare to chemotherapy, cases with high tumor-associated HLA-I expression and no lymphatic metastasis were more likely to benefit from CTLs.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Kunming Yan’an Hospital (Approval No. 2015-049-01). The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization: RL, JT. Methodology: YYZ, MM, WW, FY, HG. Formal analysis and investigation: ZH, WW, LL, AG, RW, HX. Writing - original draft preparation: YKZ, MM. Funding acquisition: RL. Resources: JT. Writing - review and editing and supervision: RL, JT. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by International Science and Technology Cooperation Project (No. 2015IA034) and Central Funds Guiding the local Science and Technology Development (No. 202207AB110017).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet (London England) (2019) 394(10207):1467–80. doi: 10.1016/s0140-6736(19)32319-0
3. Akagi T, Inomata M. Essential advances in surgical and adjuvant therapies for colorectal cancer 2018-2019. Ann Gastroenterol Surg (2020) 4(1):39–46. doi: 10.1002/ags3.12307
4. Simillis C, Singh H, Afxentiou T, Mills S, Warren OJ, Smith JJ, et al. Postoperative chemotherapy improves survival in patients with resected high-risk stage ii colorectal cancer: Results of a systematic review and meta-analysis. Colorectal Dis (2020) 22(10):1231–44. doi: 10.1111/codi.14994
5. Gelibter AJ, Caponnetto S, Urbano F, Emiliani A, Scagnoli S, Sirgiovanni G, et al. Adjuvant chemotherapy in resected colon cancer: When, how and how long? Surg Oncol (2019) 30:100–7. doi: 10.1016/j.suronc.2019.06.003
6. Raphael MJ, Fischer HD, Fung K, Austin PC, Anderson GM, Booth CM, et al. Neurotoxicity outcomes in a population-based cohort of elderly patients treated with adjuvant oxaliplatin for colorectal cancer. Clin Colorectal Cancer (2017) 16(4):397–404 e1. doi: 10.1016/j.clcc.2017.03.013
7. Ganesh K, Stadler ZK, Cercek A, Mendelsohn RB, Shia J, Segal NH, et al. Immunotherapy in colorectal cancer: Rationale, challenges and potential. Nat Rev Gastroenterol Hepatol (2019) 16(6):361–75. doi: 10.1038/s41575-019-0126-x
8. Tintelnot J, Stein A. Immunotherapy in colorectal cancer: Available clinical evidence, challenges and novel approaches. World J Gastroenterol (2019) 25(29):3920–8. doi: 10.3748/wjg.v25.i29.3920
9. Bashir B, Snook AE. Immunotherapy regimens for metastatic colorectal carcinomas. Hum Vaccin Immunother (2018) 14(2):250–4. doi: 10.1080/21645515.2017.1397244
10. Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science (2015) 348(6230):62–8. doi: 10.1126/science.aaa4967
11. Zhang C, Wang Z, Yang Z, Wang M, Li S, Li Y, et al. Phase I escalating-dose trial of car-T therapy targeting cea(+) metastatic colorectal cancers. Mol Ther (2017) 25(5):1248–58. doi: 10.1016/j.ymthe.2017.03.010
12. Tran E, Robbins PF, Lu YC, Prickett TD, Gartner JJ, Jia L, et al. T-Cell transfer therapy targeting mutant kras in cancer. N Engl J Med (2016) 375(23):2255–62. doi: 10.1056/NEJMoa1609279
13. Ishikawa T, Okayama T, Sakamoto N, Ideno M, Oka K, Enoki T, et al. Phase I clinical trial of adoptive transfer of expanded natural killer cells in combination with Igg1 antibody in patients with gastric or colorectal cancer. Int J Cancer (2018) 142(12):2599–609. doi: 10.1002/ijc.31285
14. Goyvaerts C, Breckpot K. The journey of in vivo virus engineered dendritic cells from bench to bedside: A bumpy road. Front Immunol (2018) 9:2052. doi: 10.3389/fimmu.2018.02052
15. Barth RJ Jr., Fisher DA, Wallace PK, Channon JY, Noelle RJ, Gui J, et al. A randomized trial of ex vivo Cd40l activation of a dendritic cell vaccine in colorectal cancer patients: Tumor-specific immune responses are associated with improved survival. Clin Cancer Res (2010) 16(22):5548–56. doi: 10.1158/1078-0432.CCR-10-2138
16. Hunyadi J, Andras C, Szabo I, Szanto J, Szluha K, Sipka S, et al. Autologous dendritic cell based adoptive immunotherapy of patients with colorectal cancer-a phase I-ii study. Pathol Oncol Res (2014) 20(2):357–65. doi: 10.1007/s12253-013-9704-3
17. Zhu H, Yang X, Li J, Ren Y, Zhang T, Zhang C, et al. Immune response, safety, and survival and quality of life outcomes for advanced colorectal cancer patients treated with dendritic cell vaccine and cytokine-induced killer cell therapy. BioMed Res Int (2014) 2014:603871. doi: 10.1155/2014/603871
18. Caballero-Banos M, Benitez-Ribas D, Tabera J, Varea S, Vilana R, Bianchi L, et al. Phase ii randomised trial of autologous tumour lysate dendritic cell plus best supportive care compared with best supportive care in pre-treated advanced colorectal cancer patients. Eur J Cancer (2016) 64:167–74. doi: 10.1016/j.ejca.2016.06.008
19. Liu KJ, Chao TY, Chang JY, Cheng AL, Ch’ang HJ, Kao WY, et al. A phase I clinical study of immunotherapy for advanced colorectal cancers using carcinoembryonic antigen-pulsed dendritic cells mixed with tetanus toxoid and subsequent il-2 treatment. J BioMed Sci (2016) 23(1):64. doi: 10.1186/s12929-016-0279-7
20. Morse MA, Niedzwiecki D, Marshall JL, Garrett C, Chang DZ, Aklilu M, et al. A randomized phase ii study of immunization with dendritic cells modified with poxvectors encoding cea and Muc1 compared with the same poxvectors plus gm-csf for resected metastatic colorectal cancer. Ann Surg (2013) 258(6):879–86. doi: 10.1097/SLA.0b013e318292919e
21. Wu L, Zhang H, Jiang Y, Gallo RC, Cheng H. Induction of antitumor cytotoxic lymphocytes using engineered human primary blood dendritic cells. Proc Natl Acad Sci U.S.A. (2018) 115(19):E4453–E62. doi: 10.1073/pnas.1800550115
22. Kurilin V, Kulikova E, Shevchenko J, Lopatnikova J, Obleukhova I, Khantakova J, et al. Dendritic cells transfected with a polyepitope DNA construct stimulate an antitumor cytotoxic response in various tumors. Mol Clin Oncol (2022) 17(5):155. doi: 10.3892/mco.2022.2588
23. Wang WH, Zhou CH, Ding J, Zhang YX, Zheng LL, Chen SF, et al. Immune effect and safety evaluation of vaccine prepared by dendritic cells modified by raav-carrying Bcsg1 gene. Gene Ther (2016) 23(12):839–45. doi: 10.1038/gt.2016.63
24. Sui CG, Wu D, Meng FD, Yang MH, Jiang YH. Anti-prostate cancer effects of ctl cell induction in vitro by recombinant adenovirus mediated Psma/4-1bbl dendritic cells: An immunotherapy study. Genet Mol Res (2015) 14(2):7208–17. doi: 10.4238/2015.June.29.14
25. Di L, Zhu Y, Jia J, Yu J, Song G, Zhang J, et al. Clinical safety of induced ctl infusion through recombinant adeno-associated virus-transfected dendritic cell vaccination in Chinese cancer patients. Clin Transl Oncol (2012) 14(9):675–81. doi: 10.1007/s12094-012-0854-7
26. Akazawa Y, Nobuoka D, Takahashi M, Yoshikawa T, Shimomura M, Mizuno S, et al. Higher human lymphocyte antigen class I expression in early-stage cancer cells leads to high sensitivity for cytotoxic T lymphocytes. Cancer Sci (2019) 110(6):1842–52. doi: 10.1111/cas.14022
27. Weiser MR. Ajcc 8th edition: Colorectal cancer. Ann Surg Oncol (2018) 25(6):1454–5. doi: 10.1245/s10434-018-6462-1
28. The Cancer Genome Atlas NetworkComprehensive molecular characterization of human colon and rectal cancer. Nature (2012) 487(7407):330–7. doi: 10.1038/nature11252
29. Goodman AM, Sokol ES, Frampton GM, Lippman SM, Kurzrock R. Microsatellite-stable tumors with high mutational burden benefit from immunotherapy. Cancer Immunol Res (2019) 7(10):1570–3. doi: 10.1158/2326-6066.CIR-19-0149
30. Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med (2003) 349(3):247–57. doi: 10.1056/NEJMoa022289
31. Loupakis F, Depetris I, Biason P, Intini R, Prete AA, Leone F, et al. Prediction of benefit from checkpoint inhibitors in mismatch repair deficient metastatic colorectal cancer: Role of tumor infiltrating lymphocytes. oncol (2020) 25(6):481–7. doi: 10.1634/theoncologist.2019-0611
32. Chu JN, Choi J, Ostvar S, Torchia JA, Reynolds KL, Tramontano A, et al. Cost-effectiveness of immune checkpoint inhibitors for microsatellite instability-High/Mismatch repair-deficient metastatic colorectal cancer. Cancer (2019) 125(2):278–89. doi: 10.1002/cncr.31795
33. Noh BJ, Kwak JY, Eom DW. Immune classification for the pd-L1 expression and tumour-infiltrating lymphocytes in colorectal adenocarcinoma. BMC Cancer (2020) 20(1):58. doi: 10.1186/s12885-020-6553-9
34. Almutairi AR, McBride A, Slack M, Erstad BL, Abraham I. Potential immune-related adverse events associated with monotherapy and combination therapy of ipilimumab, nivolumab, and pembrolizumab for advanced melanoma: A systematic review and meta-analysis. Front Oncol (2020) 10:91. doi: 10.3389/fonc.2020.00091
35. Brudno JN, Kochenderfer JN. Recent advances in car T-cell toxicity: Mechanisms, manifestations and management. Blood Rev (2019) 34:45–55. doi: 10.1016/j.blre.2018.11.002
36. Ozcan M, Janikovits J, von Knebel Doeberitz M, Kloor M. Complex pattern of immune evasion in msi colorectal cancer. Oncoimmunology (2018) 7(7):e1445453. doi: 10.1080/2162402X.2018.1445453
37. Cochran AJ, Huang RR, Lee J, Itakura E, Leong SP, Essner R. Tumour-induced immune modulation of sentinel lymph nodes. Nat Rev Immunol (2006) 6(9):659–70. doi: 10.1038/nri1919
38. Matsuda T, Miyauchi E, Hsu YW, Nagayama S, Kiyotani K, Zewde M, et al. Tcr sequencing analysis of cancer tissues and tumor draining lymph nodes in colorectal cancer patients. Oncoimmunology (2019) 8(6):e1588085. doi: 10.1080/2162402X.2019.1588085
Keywords: colorectal neoplasm, adoptive immunotherapy, T-lymphocyte, histocompatibility antigens class Ⅰ, lymphatic metastasis
Citation: Zhu Y, Meng M, Hou Z, Wang W, Li L, Guan A, Wang R, Tang W, Yang F, Zhao Y, Gao H, Xie H, Li R and Tan J (2023) Impact of cytotoxic T lymphocytes immunotherapy on prognosis of colorectal cancer patients. Front. Oncol. 13:1122669. doi: 10.3389/fonc.2023.1122669
Received: 13 December 2022; Accepted: 02 January 2023;
Published: 16 January 2023.
Edited by:
Xiong Wang, Huazhong University of Science and Technology, ChinaReviewed by:
Guihua Wang, Huazhong University of Science and Technology, ChinaYongbin Chen, Kunming Institute of Zoology, Chinese Academy of Sciences (CAS), China
Weiyi Fang, Southern Medical University, China
Copyright © 2023 Zhu, Meng, Hou, Wang, Li, Guan, Wang, Tang, Yang, Zhao, Gao, Xie, Li and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruhong Li, THJoMjcyQDEyNi5jb20=; Jing Tan, a210amluZ0BzaW5hLmNvbQ==
†These authors have contributed equally to this work
 Yankun Zhu1†
Yankun Zhu1† Zongliu Hou
Zongliu Hou Wenju Wang
Wenju Wang Lin Li
Lin Li Jing Tan
Jing Tan