- 1Oncology Department, Shenzhen Second People’s Hospital, the First Affiliated Hospital of Shenzhen University, Shenzhen, China
- 2Department of Medical Imaging, the First Dongguan Affiliated Hospital of Guangdong Medical University, Dongguan, China
Background: Statin may confer anticancer efficacy, while the studies evaluating the influence of statin on survival of patients with renal cell cancer (RCC) yielded inconsistent results. A systematic review and meta-analysis was performed to investigate the association between statin use and survival of patients with RCC.
Materials and Methods: Cohort studies were identified by search of PubMed, Embase, and Web of Science databases according to the objective of the meta-analysis. A random-effect model incorporating the possible between-study heterogeneity was used for meta-analysis. Subgroup analyses according to study characteristics were also performed.
Results: Seventeen cohort studies involving 42528 patients with RCC were available for the meta-analysis. Results showed that statin use was associated with a better overall survival (OS, hazard ratio [HR]: 0.73, 95% confidence interval [CI]: 0.65 to 0.84, p < 0.001; I2 = 40%), progression progression-free survival (PFS, HR: 0.82, 95% CI: 0.68 to 0.98, p = 0.03; I2 = 52%), and cancer-specific survival (CSS, HR: 0.76, 95% CI: 0.59 to 0.99, p = 0.04; I2 = 38%). Besides, for the outcome of OS and PFS, subgroup analyses showed similar results in patients with surgical and non-surgical anticancer treatments, and in patients with stage I-III and stage IV RCC (p values for subgroup difference all > 0.05).
Conclusions: Statin use may be associated with improved survival outcomes in patients with RCC. Although prospective clinical studies should be considered to validate these results, these findings suggest that statins may be potential adjuvant therapy for patients with RCC.
Introduction
Renal cell cancer (RCC) is among the most common malignancy of the urinary system (1, 2). Globally, approximately 400,000 cases of RCC were diagnosed annually, and about 175,000 people died from RCC, according to the statistics in 2018 (3, 4). Besides, it could be estimated that RCC will continuously be a serious threat to the health of the global population because the worldwide incidence of RCC has been reported to be increasing continuously within the recent decades (5). While imaging techniques for cancer screening have advanced, about 30% of patients with RCC are diagnosed at an advanced stage, which may be an underlying cause to the overall poor prognosis of patients with RCC (6). Therefore, efforts are still needed to identify novel treatment options which may improve the survival of patients with RCC (7).
The statin family is a class of lipid-lowering drugs that inhibit the 3-hydroxy-3-methylglutarylcoenzyme-A reductase, a key enzyme involved in cholesterol synthesis (8). Moreover, further studies have confirmed the anti-inflammatory, anti-proliferative, pro-apoptotic, immunomodulatory, and anti-metastasizing properties of statins, suggesting that statins may inhibit the pathogenesis and progression of tumor (9–11). Accumulating evidence has suggested that statin use may be related to improved prognosis of patients with certain cancers, such as those with pancreatic cancer (12), lung cancer (13), endometrial cancer (14), and colorectal cancer (15). However, previous studies evaluating the influence of statin on survival of patients with RCC yielded inconsistent results (16). Some studies suggested that statin use may be associated with improved survival in patients with RCC (17–25), while others did not show consistent results (26–33). Therefore, in this study, we performed a systematic review and meta-analysis to comprehensively investigate the association between statin use and survival outcomes in patients with RCC.
Materials and methods
We followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) Statement (34, 35) in this study. The analytic methods were in accordance with the instructions of the Cochrane’s Handbook for Systematic Review and Meta-analysis (36).
Database search
We systematically searched the electronic databases of PubMed, Embase, and Web of Science using combined search terms including (1) “statin” OR “3-hydroxy-3-methyl-glutarylCoA reductase inhibitor” OR “CS-514” OR “simvastatin” OR “atorvastatin” OR “fluvastatin” OR “lovastatin” OR “rosuvastatin” OR “pravastatin” OR “pitavastatin”; (2) “renal” OR “kidney”; (3) “cancer” OR “tumor” OR “carcinoma” OR “neoplasm” OR “adenoma” OR “malignancy”; and (4) “recurrence” OR “death” OR “mortality” OR “survival” OR “prognosis” OR “deaths” OR “remission” OR “collapse” OR “progression” OR “metastasis”. We used filters to limit the searches to studies in humans. No restriction was applied to the language of publication. As a supplementation, we manually screened the reference lists of the related literatures for possible relevant studies. The final database search was performed on October 23, 2022.
Study inclusion
The PICOS criteria were followed during the determination of the inclusion criteria.
P (patients): adult patients with confirmed diagnosis of RCC;
I (exposure): patients with statin use as defined by the original studies;
C (control): patients without statin use as defined by the original studies;
O (outcomes): relative risks for the incidence of overall survival (OS), progression-free survival (PFS), or cancer-specific survival (CSS) between users versus non-users of statin during follow-up durations. Specifically, OS was defined as time from diagnosis to death from any cause, PFS was defined as time from diagnosis to disease progression or relapse, unplanned re-treatment after initial management, or death from any cause, and CSS was defined as time from diagnosis to death from RCC.
S (study design): cohort studies, including the retrospective and prospective studies.
We only considered studies published as full-length articles in peer-reviewed journals. For studies with overlapped patient population, the one with the largest sample size was included. Reviews, preclinical studies, cross-sectional studies, studies did not evaluate statin use as exposure, studies including non-RCC patients, or studies did not report the survival outcomes were excluded from the meta-analysis.
Data extracting and quality evaluation
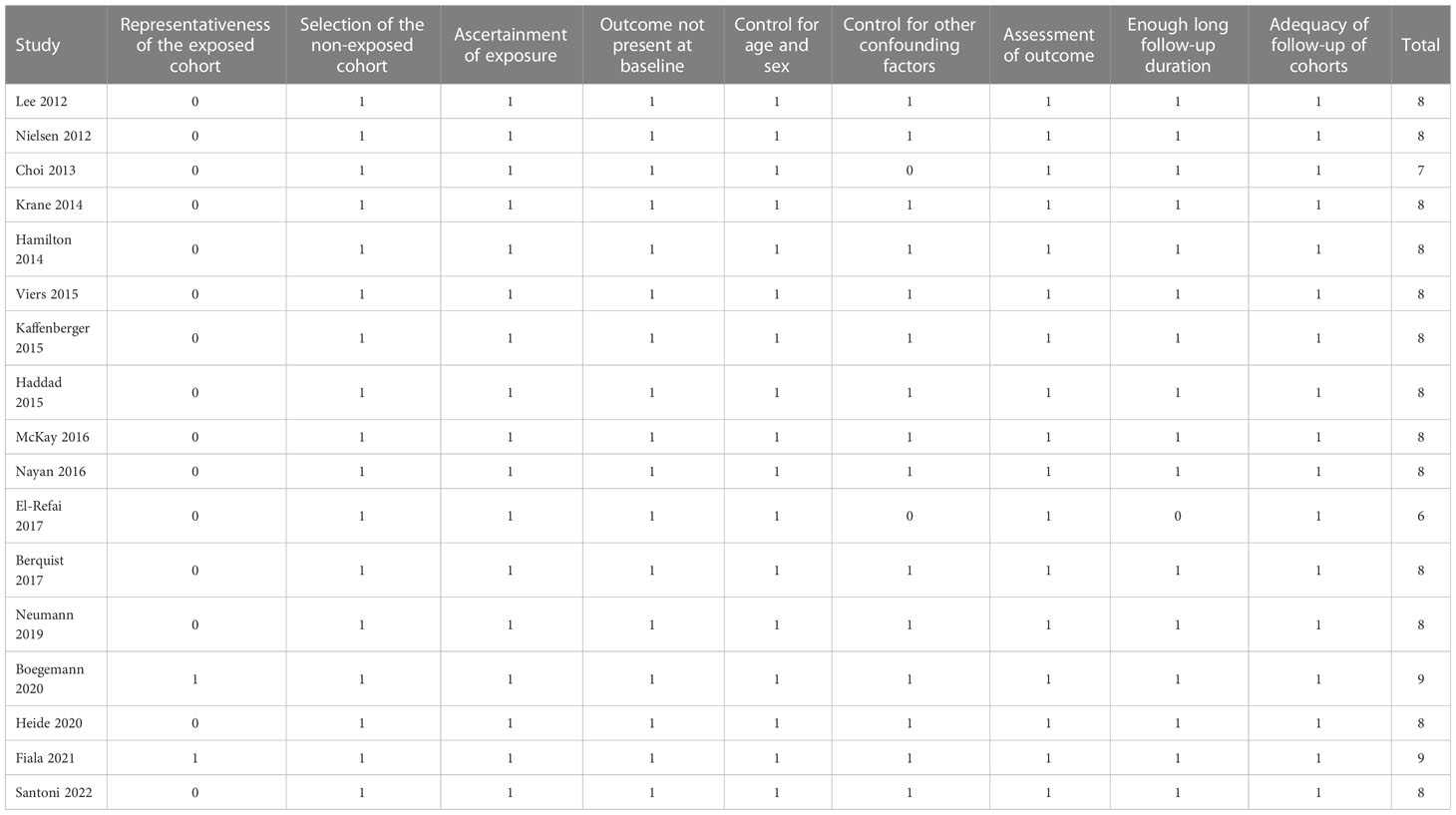
Two authors implemented database search, data extraction, and study quality assessment separately. If disagreements occurred, they were discussed with the third author for consensus. Data regarding the study information, patient characteristics, definition of statin use, follow-up durations, and outcomes reported were collected by the two independent authors using a predefined data extraction table. The Newcastle-Ottawa Scale (NOS) (37) was used for study quality evaluation. This scale is rated from 1 to 9 stars and reflected the quality of the study by aspects of participant selection, comparability between groups, and outcome validation.
Statistical analyses
The relative risk for the incidence of survival outcomes between users and non-users of statins were presented as hazard ratio (HR) and the corresponding 95% confidence interval (CI). For studies reported multiple HRs according to different models of multivariate regression analyses, the most adequately adjusted HR from each study was extracted and combined in this meta-analysis. Then, standard errors (SEs) of HRs were estimated from the 95% CIs or P values. For normalization of their distribution, HRs were logarithmically transformed and combined (36). Heterogeneity within the included cohort studies was tested via Cochrane’s Q test, as well as the estimation of I2 statistic (38). An I2 > 50% suggests significant level of heterogeneity. A random-effect model was chosen to combine the HRs by incorporating the potential heterogeneity within studies (36). Predefined subgroup analyses were conducted to explore the possible influences of study characteristics on the outcomes, including main anticancer treatment (surgical versus non-surgical), and the clinical stages of the tumor. Funnel plots were constructed, and were used for the assessment of publication bias (36). Visually asymmetrical funnel plots implied potential publication bias, which could be further validated by the Egger’s regression asymmetry test. The RevMan (Version 5.1; Cochrane Collaboration, Oxford, UK) and Stata (version 12.0; Stata Corporation, College Station, TX) software was used for the statistical analyses.
Results
Literature search
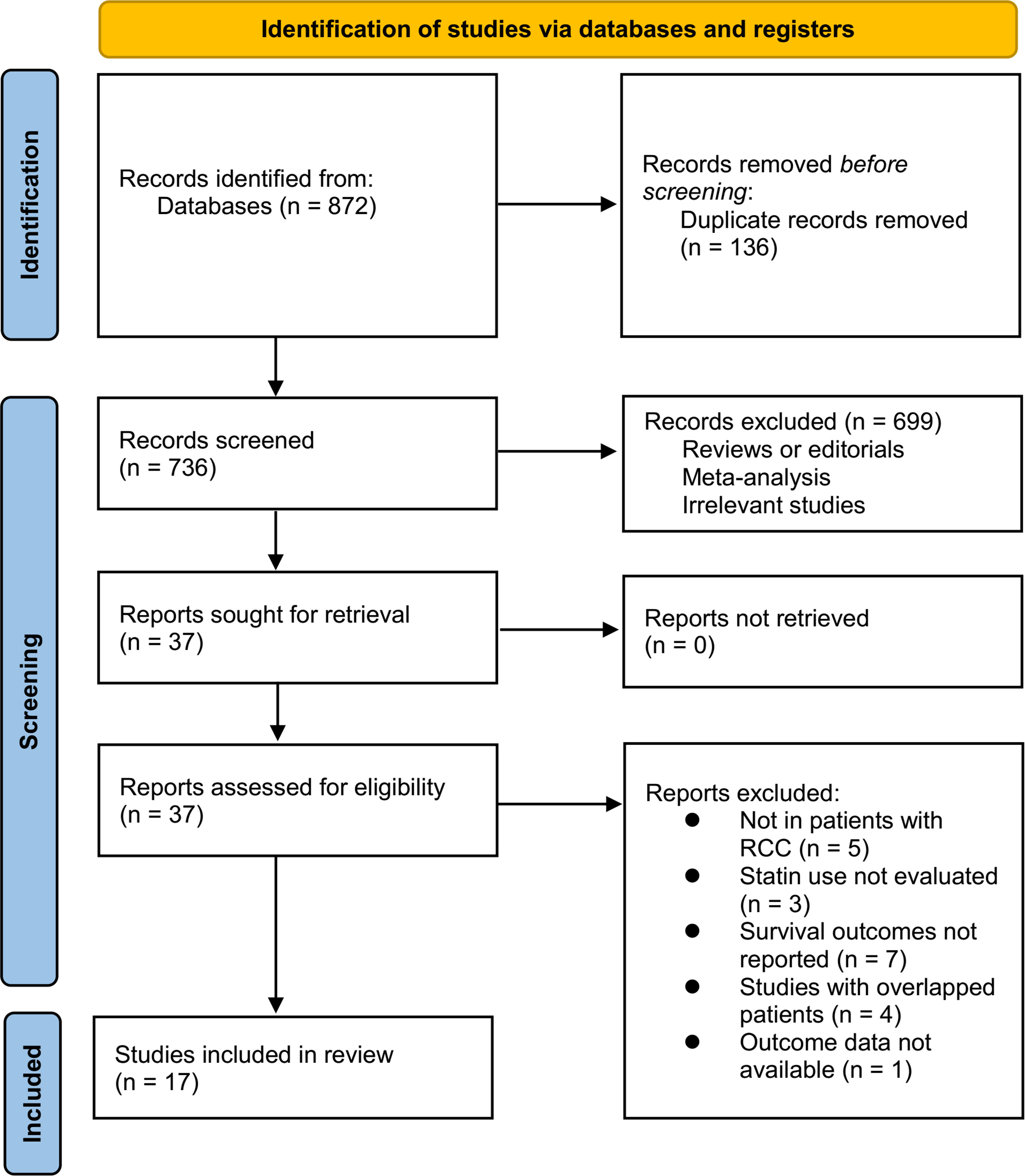
Figure 1 summarizes the process of literature search. In brief, 872 articles were retrieved in initial database search, and 736 articles were obtained after excluding the duplications. Then, 37 articles were considered to be potentially relevant after excluding 699 irrelevant articles in title and abstract screening. Through full-text review, anther 20 studies were further excluded because of the reasons listed in Figure 1. Finally, 17 cohort studies were included in the meta-analysis (17–33).
Summary of study characteristics
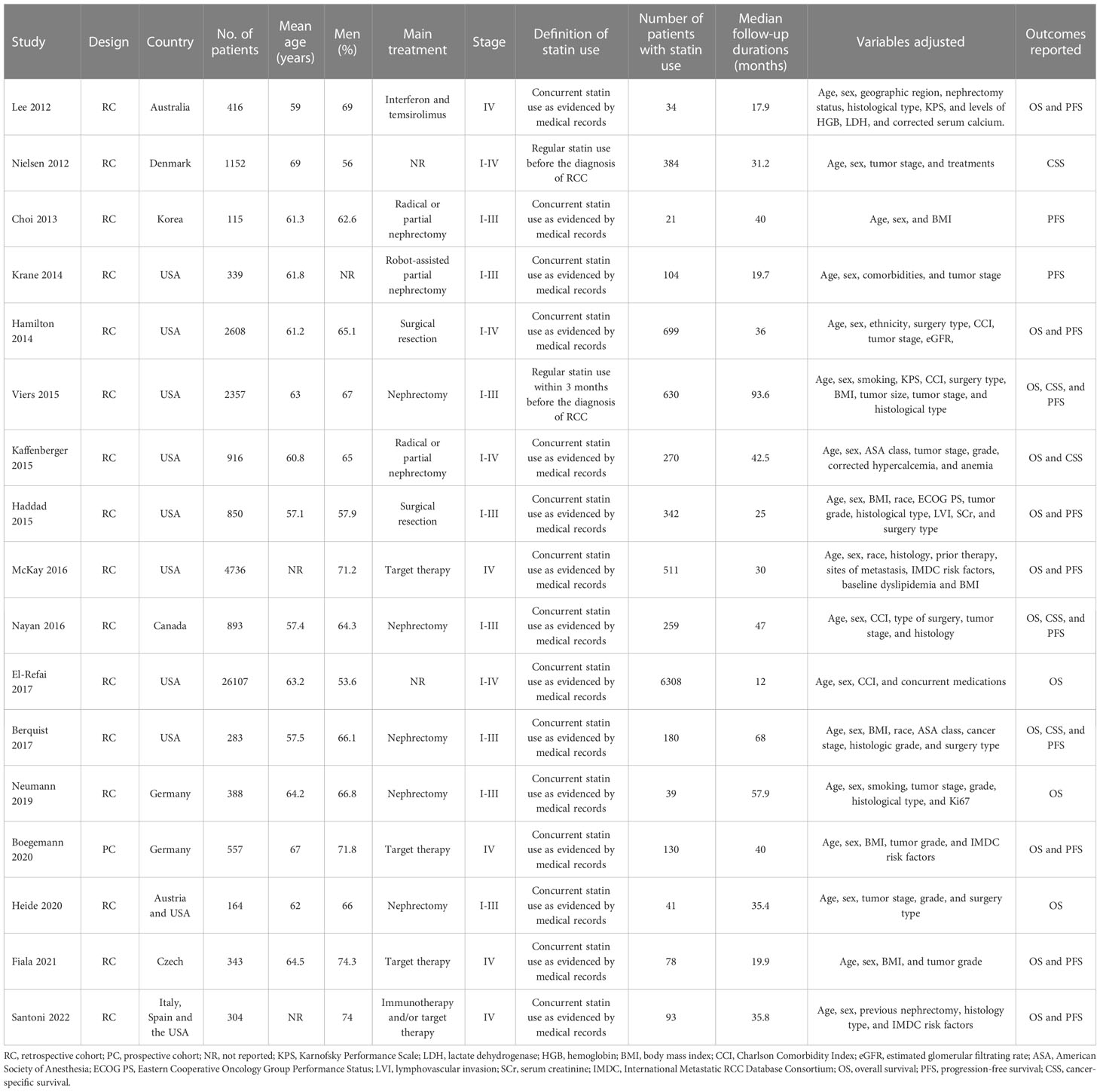
Seventeen cohort studies (17–33) involving 42528 patients with RCC were available for the meta-analysis. The characteristics of the included studies are shown in Table 1. Briefly, these studies were published between 2012 and 2022, and performed in Australia, Denmark, Korea, the United States, Canada, Germany, Austria, Czech, Italy, and Spain. All of the studies were retrospective cohort studies except for one study, which was a prospective study (31). All the patients were diagnosed with RCC. The mean ages of the patients were 57 to 67 years, and the proportions of men were 56 to 75%. In ten of the included studies (18–21, 24, 27–30, 32), surgeries such as nephrectomy were performed, while in five studies (17, 22, 25, 31, 33), non-surgical therapies such as interferon, temsirolimus, Immunotherapy, and target drugs were applied. In 15 of the included studies, statin use was defined as confirmed concurrent use of statin via medical charts at the diagnosis of RCC (17–25, 27, 29–33), while for the other two studies (26, 28), stain use was defined as regular statin use before the diagnosis of RCC. Accordingly, 10123 (23.8%) patients were statin users. The mean follow-up durations varied from 12 to 94 months, and confounding factors such as age, sex, tumor stage, performance status, and comorbidities etc. were controlled in the multivariate analyses. The NOS for the studies varied between six and nine, indicating good study quality (Table 2).
Association between statin use and OS of patients with RCC
Pooled results of 14 studies (17, 18, 20–25, 28–33) showed that statin use was associated with a better OS (HR: 0.73, 95% CI: 0.65 to 0.84, p < 0.001; I2 = 40%; Figure 2A) of patients with RCC. Subgroup analyses showed similar results in patients with surgical (HR: 0.71, 95% CI: 0.58 to 0.87, p < 0.001; I2 = 48%) and non-surgical anticancer treatments (HR: 0.74, 95% CI: 0.65 to 0.85, p < 0.001; I2 = 41%; p for subgroup difference = 0.57; Figure 2B), and in patients with stage I-III (HR: 0.66, 95% CI: 0.49 to 0.89, p = 0.006; I2 = 53%) and stage IV RCC (HR: 0.77, 95% CI: 0.62 to 0.96, p < 0.02; I2 = 39%; p for subgroup difference = 0.40; Figure 2C).
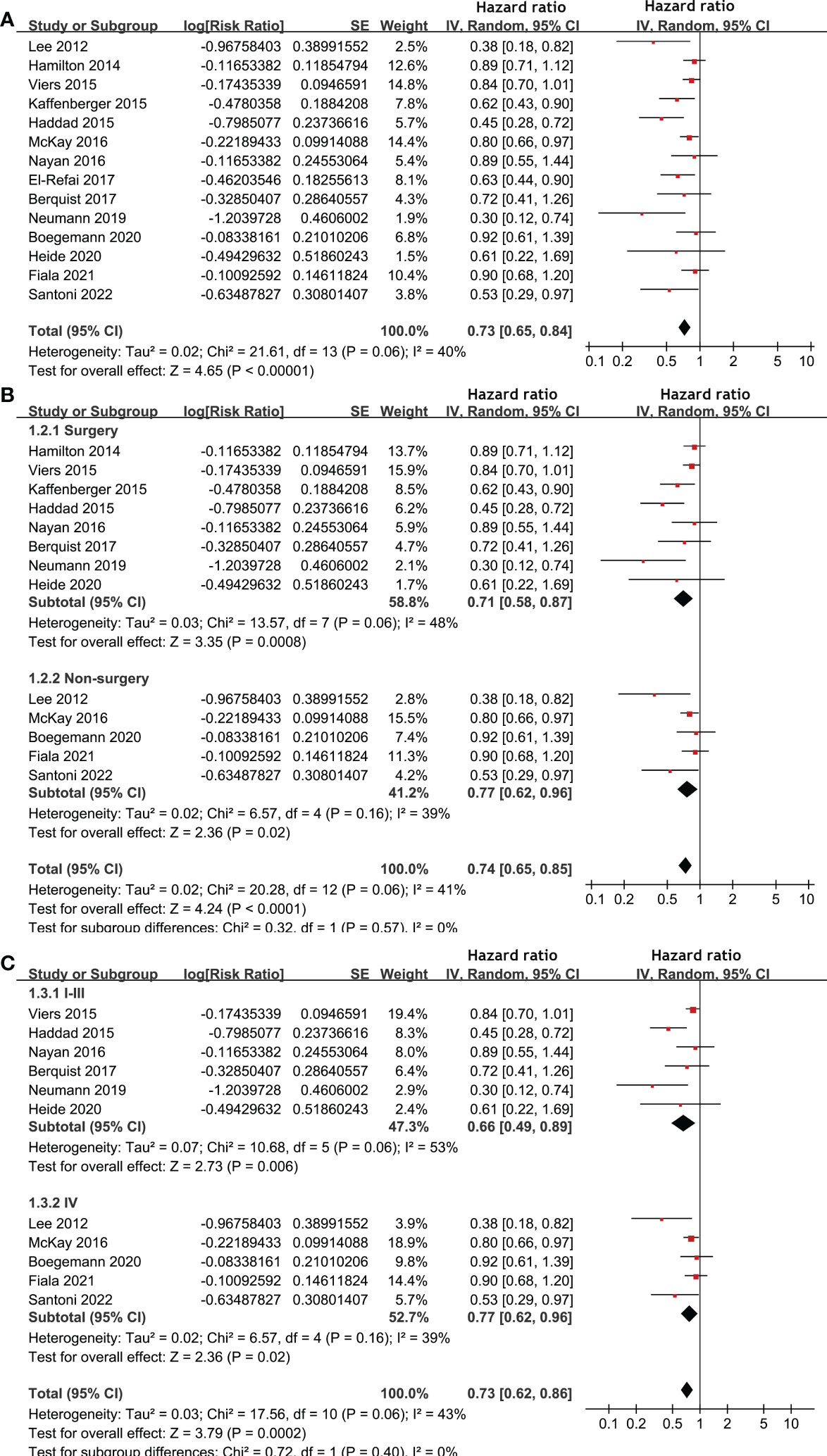
Figure 2 Forest plots for the meta-analysis of the association between statin use and OS of patients with RCC. (A), overall meta-analysis; (B), subgroup analysis according to the main treatment; and (C), subgroup analysis according to the tumor stage.
Influence of statin use on PFS and CCS in patients with RCC
Meta-analysis of 12 studies (17–20, 22, 25, 27–31, 33) indicated that statin use was associated with an improved PFS of patients with RCC (HR: 0.82, 95% CI: 0.68 to 0.98, p = 0.03; I2 = 52%; Figure 3A). Subgroup analyses showed similar results in patients with surgical and non-surgical anticancer treatments (p for subgroup difference = 0.98, Figure 3B), and in patients with stage I-III and stage IV RCC (p for subgroup difference = 0.83, Figure 3C). In addition, pooling the results of five studies (21, 26, 28–30) suggested that statin use was associated with an improved CSS (HR: 0.76, 95% CI: 0.59 to 0.99, p = 0.04; I2 = 38%; Figure 4) in patients with RCC.
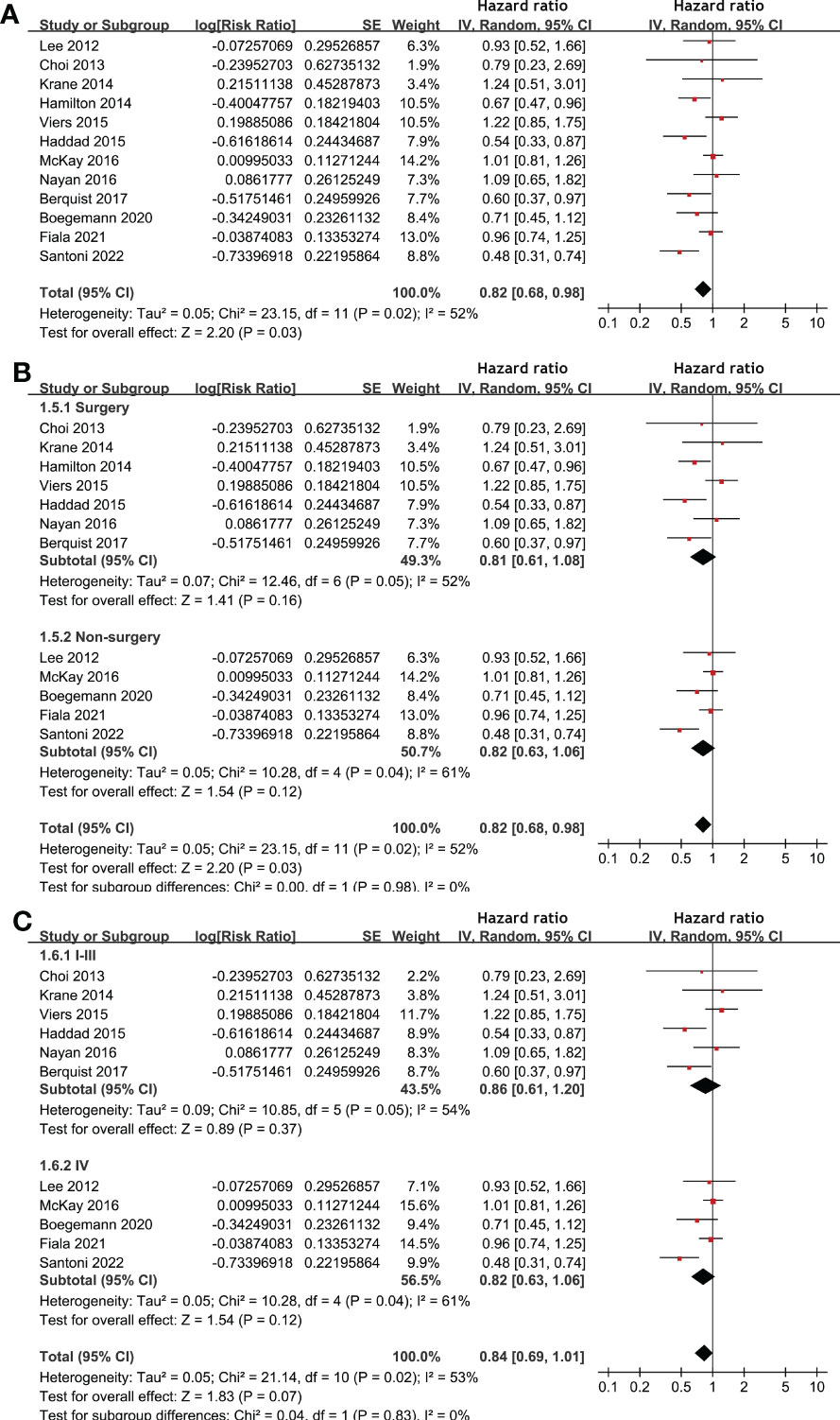
Figure 3 Forest plots for the meta-analysis of the association between statin use and PFS of patients with RCC. (A), overall meta-analysis; (B), subgroup analysis according to the main treatment; and (C), subgroup analysis according to the tumor stage.
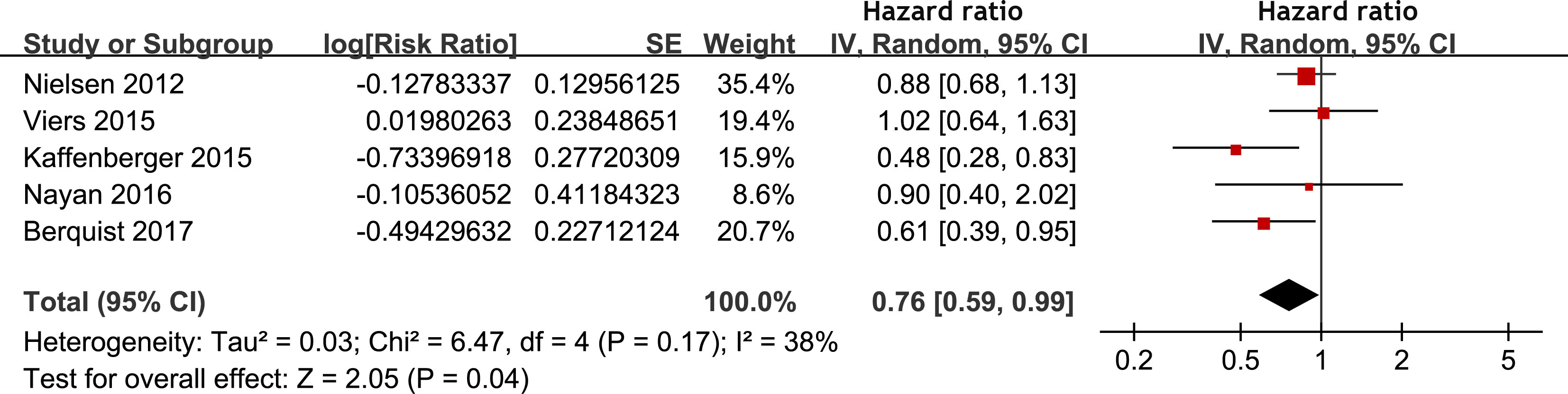
Figure 4 Forest plots for the meta-analysis of the association between statin use and CSS of patients with RCC.
Publication bias
Funnel plots for the meta-analyses of OS and PFS were symmetrical on visual examination (Figures 5A, B), suggesting low risk of publication biases. Egger’s regression tests showed consistent results (p = 0.17 and 0.33, respectively). The publication bias for the meta-analysis of CSS was unable to determine because only five studies were included for the outcome.
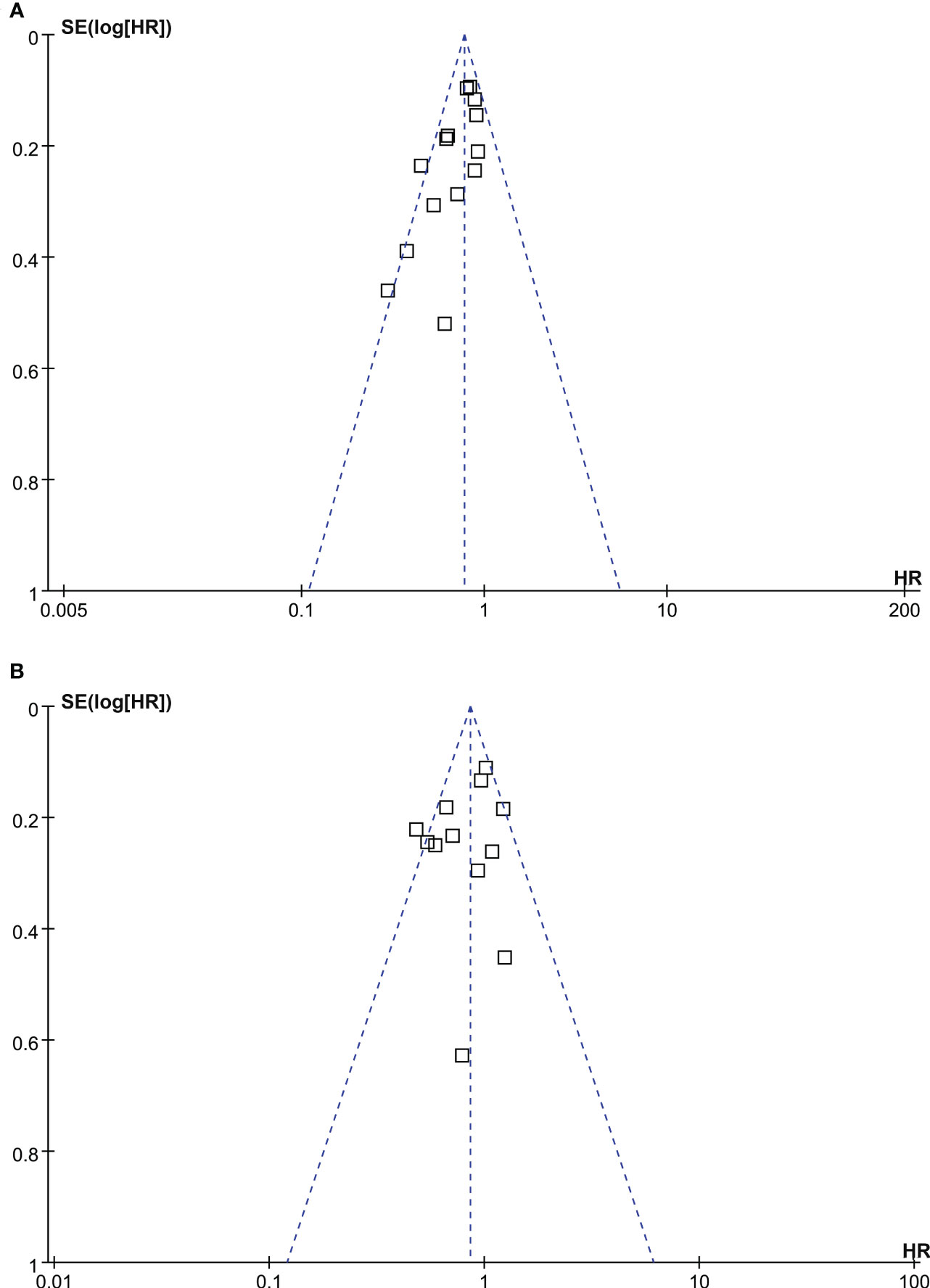
Figure 5 Funnel plots for the meta-analyses; (A), funnel plots for the association between statin use and OS of patients with RCC; and (B), funnel plots for the association between statin use and PFS of patients with RCC.
Discussion
In this study, by pooling the results of 17 available cohort studies, results of the meta-analysis showed that compared non-users of statin, RCC patients who used statin were associated with improved survival outcomes, including OS, PFS, and CSS. In addition, subgroup analyses showed consistent results in patients who were treated surgically and non-surgically, and in patients with stage I-III and stage IV RCC. Collectively, these findings suggest that statin use may be associated with an improved survival in patients with RCC.
To the best of our knowledge, there are three meta-analyses which evaluated the association between statin use and prognosis in patients with RCC. An early meta-analysis published in 2015 showed that statin use may improve the OS in patients with RCC, while other outcomes, such as PFS or CSS were not significantly affected (39). The authors therefore concluded that although a benefit of statin on survival was suggested, this may not be related to the anticancer efficacy of statin because outcomes related to the tumor progression was not significantly affected (39). However, only four cohort studies were included in the meta-analysis, which made the results less convincing (39). A subsequent meta-analysis in 2017 with 12 studies suggested that statin use in patients with RCC was associated with improved OS and CSS, but not PFS. However, for the outcome of PFS, only two studies were available, which made the results also less reliable (40). A recent meta-analysis with literatures by July 2019 showed that statin use in patients with RCC was not associated with improved OS (41). However, the process of literature searching in this meta-analysis may have flaw because only 5 studies were included, and considerable eligible studies were not enrolled (41). Collectively, the influence of statin on survival of patients with RCC remains not fully determined to date. Our meta-analysis has several strengths in methodology as compared to the previous ones. Firstly, we performed updated literature search in three commonly used electronic databases, and retrieved 17 up-to-date eligible studies. Among them, six were published recently and not included in the previous meta-analyses (23–25, 31–33). Secondly, only cohort studies were included, which could suggest a longitudinal relationship between statin use and improved survival in patients with RCC. Also, multivariate analyses were performed in all available studies when the association between statin and survival of patients with RCC was estimated, which minimized the potential influence of confounding factors. Moreover, three commonly used survival outcomes including OS, PFS, and CSS were all analyzed in this study, and the consistent results confirmed the robustness of the findings that statin may attenuate the progression of RCC. Finally, the relative large number of included studies enabled us to perform subgroup analyses according to the anticancer treatments and tumor stages of RCC. The consistent results of these subgroup analyses further validated the stability of the findings. Taken together, results of this meta-analysis indicated that statin use may be associated with improved survival outcomes in patients with RCC, which were independent of the anticancer treatment and the stage of the tumor.
The potential mechanisms for the improved survival of statin users with RCC may be multifactorial. Early in vitro studies showed that by causing cell cycle arrest and apoptosis, simvastatin inhibited the growth of RCC cells in a dose- and time-dependent manner, when cholesterol was depleted and prenylation-associated mechanisms were involved (42). In addition, fluvastatin was demonstrated to enhance the phosphorylation of AKT, mammalian target of rapamycin, and extracellular signal-regulated kinase, resulting in a reduction in the movement of RCC cells in vitro (43). In addition, in combination with sorafenib, a vascular endothelial growth factor inhibitor, lovastatin showed synergistic effects against RCC cell lines proliferation (44). Finally, a recent study suggested that simvastatin could inhibit RCC cell viability, migration, invasion, and regulated the cell cycle and induced apoptosis, which were associated with the restoration of the abnormal expression of DDX5/DUSP5 in RCC. Further studies are needed to determine the major molecular mechanisms and signaling pathways underlying the potential anticancer efficacy of statins for RCC.
Our study has limitations. Firstly, most of the included studies are retrospective, which may be associated with the risks of recall and selection biases. Therefore, results of the study should be validated in large-scale prospective studies. In addition, due to the insufficient data of the included studies, we were unable to determine if some study characteristics may affect the outcomes, such as the histological type of RCC, ethnicity and sex of the patients, and type, dose, and treatment duration of statins. These factors may lead to the between study heterogeneity of the meta-analysis. Moreover, although multivariate analysis was applied among the included studies when the associations between statin use and survival outcomes of patients with RCC were estimated, we could not exclude the possibility that there were still residual factors which may confound the results, such as the cholesterol levels of the patients. Finally, a causative relationship between statin use and improved survival of patients with RCC could not be established on the basis of our finding, because this meta-analysis was based on the results of observational studies. Clinical trials should be performed to evaluate the role of statins as adjuvant treatment for patients with RCC.
In conclusion, statin use may be associated with improved survival outcomes in patients with RCC. Although prospective clinical studies should be considered to validate these results, these findings suggest that statins may be potential adjuvant therapy for patients with RCC.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
WL conceived the study. WL and YP performed database search, literature review, study selection, quality evaluation, and data collection. WL, AL, YH, and YZ performed data statistics and interpreted the results. WL drafted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by GuangDong Basic and Applied Basic Research Foundation (2021A1515011426).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kanesvaran R, Porta C, Wong A, Powles T, Ng QS, Schmidinger M, et al. Pan-Asian adapted ESMO clinical practice guidelines for the diagnosis, treatment and follow-up of patients with renal cell carcinoma. ESMO Open (2021) 6(6):100304. doi: 10.1016/j.esmoop.2021.100304
2. Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin (2022) 72(5):409–36. doi: 10.3322/caac.21731
3. Capitanio U, Bensalah K, Bex A, Boorjian SA, Bray F, Coleman J, et al. Epidemiology of renal cell carcinoma. Eur Urol (2019) 75(1):74–84. doi: 10.1016/j.eururo.2018.08.036
4. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
5. Gluba-Brzozka A, Rysz J, Lawinski J, Franczyk B. Renal cell cancer and obesity. Int J Mol Sci (2022) 23(6). doi: 10.3390/ijms23063404
6. Chowdhury N, Drake CG. Kidney cancer: An overview of current therapeutic approaches. Urol Clin North Am (2020) 47(4):419–31. doi: 10.1016/j.ucl.2020.07.009
7. Barata PC, Rini BI. Treatment of renal cell carcinoma: Current status and future directions. CA Cancer J Clin (2017) 67(6):507–24. doi: 10.3322/caac.21411
8. Yu D, Liao JK. Emerging views of statin pleiotropy and cholesterol lowering. Cardiovasc Res (2022) 118(2):413–23. doi: 10.1093/cvr/cvab032
9. Zhang Q, Dong J, Yu Z. Pleiotropic use of statins as non-lipid-lowering drugs. Int J Biol Sci (2020) 16(14):2704–11. doi: 10.7150/ijbs.42965
10. Jiang W, Hu JW, He XR, Jin WL, He XY. Statins: a repurposed drug to fight cancer. J Exp Clin Cancer Res (2021) 40(1):241. doi: 10.1186/s13046-021-02041-2
11. Fatehi Hassanabad A. Current perspectives on statins as potential anti-cancer therapeutics: clinical outcomes and underlying molecular mechanisms. Transl Lung Cancer Res (2019) 8(5):692–9. doi: 10.21037/tlcr.2019.09.08
12. Jian-Yu E, Graber JM, Lu SE, Lin Y, Lu-Yao G, Tan XL. Effect of metformin and statin use on survival in pancreatic cancer patients: a systematic literature review and meta-analysis. Curr Med Chem (2018) 25(22):2595–607. doi: 10.2174/0929867324666170412145232
13. Chen Y, Li X, Zhang R, Xia Y, Shao Z, Mei Z. Effects of statin exposure and lung cancer survival: A meta-analysis of observational studies. Pharmacol Res (2019) 141:357–65. doi: 10.1016/j.phrs.2019.01.016
14. Li J, Liu R, Sun Z, Tang S, Wang L, Liu C, et al. The association between statin use and endometrial cancer survival outcome: A meta-analysis. Medicine (2018) 97(47):e13264. doi: 10.1097/MD.0000000000013264
15. Gray RT, Coleman HG, Hughes C, Murray LJ, Cardwell CR. Statin use and survival in colorectal cancer: Results from a population-based cohort study and an updated systematic review and meta-analysis. Cancer Epidemiol (2016) 45:71–81. doi: 10.1016/j.canep.2016.10.004
16. Santoni M, Monteiro FSM, Massari F, Abahssain H, Aurilio G, Molina-Cerrillo J, et al. Statins and renal cell carcinoma: Antitumor activity and influence on cancer risk and survival. Crit Rev Oncol Hematol (2022) 176:103731. doi: 10.1016/j.critrevonc.2022.103731
17. Lee CK, Marschner IC, Simes RJ, Voysey M, Egleston B, Hudes G, et al. Increase in cholesterol predicts survival advantage in renal cell carcinoma patients treated with temsirolimus. London UK. Clin Cancer Res (2012) 18(11):3188–96. doi: 10.1158/1078-0432.CCR-11-3137
18. Hamilton RJ, Morilla D, Cabrera F, Leapman M, Chen LY, Bernstein M, et al. The association between statin medication and progression after surgery for localized renal cell carcinoma. J Urol. (2014) 191(4):914–9. doi: 10.1016/j.juro.2013.10.141
19. Krane LS, Sandberg JM, Rague JT, Hemal AK. Do statin medications impact renal functional or oncologic outcomes for robot-assisted partial nephrectomy? J Endourol (2014) 28(11):1308–12. doi: 10.1089/end.2014.0276
20. Haddad AQ, Jiang L, Cadeddu JA, Lotan Y, Gahan JC, Hynan LS, et al. Statin use and serum lipid levels are associated with survival outcomes after surgery for renal cell carcinoma. Urology. (2015) 86(6):1146–52. doi: 10.1016/j.urology.2015.09.015
21. Kaffenberger SD, Lin-Tsai O, Stratton KL, Morgan TM, Barocas DA, Chang SS, et al. Statin use is associated with improved survival in patients undergoing surgery for renal cell carcinoma. Urol Oncol (2015) 33(1):21.e11–7. doi: 10.1016/j.urolonc.2014.10.007
22. McKay RR, Lin X, Albiges L, Fay AP, Kaymakcalan MD, Mickey SS, et al. Statins and survival outcomes in patients with metastatic renal cell carcinoma. Eur J Cancer. (2016) 52:155–62. doi: 10.1016/j.ejca.2015.10.008
23. El-Refai SM, Brown JD, Arnold SM, Black EP, Leggas M, Talbert JC. Epidemiologic analysis along the mevalonate pathway reveals improved cancer survival in patients who receive statins alone and in combination with bisphosphonates. JCO Clin Cancer Inform. (2017) 1:1–12. doi: 10.1200/CCI.17.00010
24. Neumann E, Klaiber P, Freitag K, Schwab M, Schaeffeler E, Hennenlotter J, et al. Assessment of concomitant non-oncologic medication in patients with surgically treated renal cell carcinoma: impact on prognosis, cell-cycle progression and proliferation. J Cancer Res Clin Oncol (2019) 145(7):1835–43. doi: 10.1007/s00432-019-02914-2
25. Santoni M, Massari F, Matrana MR, Basso U, De Giorgi U, Aurilio G, et al. Statin use improves the efficacy of nivolumab in patients with advanced renal cell carcinoma. Eur J Cancer. (2022) 172:191–8. doi: 10.1016/j.ejca.2022.04.035
26. Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med (2012) 367(19):1792–802. doi: 10.1056/NEJMoa1201735
27. Choi SK, Min GE, Jeon SH, Lee HL, Chang SG, Yoo KH. Effects of statins on the prognosis of local and locally advanced renal cell carcinoma following nephrectomy. Mol Clin Oncol (2013) 1(2):365–8. doi: 10.3892/mco.2012.55
28. Viers BR, Houston Thompson R, Psutka SP, Lohse CM, Cheville JC, Leibovich BC, et al. The association of statin therapy with clinicopathologic outcomes and survival among patients with localized renal cell carcinoma undergoing nephrectomy. Urol Oncol (2015) 33(9):388.e11–8.
29. Nayan M, Finelli A, Jewett MAS, Juurlink DN, Austin PC, Kulkarni GS, et al. Statin use and kidney cancer outcomes: A propensity score analysis. Urol Oncol (2016) 34(11):487.e1– e6. doi: 10.1016/j.urolonc.2016.06.007
30. Berquist SW, Lee HJ, Hamilton Z, Bagrodia A, Hassan AE, Beksac AT, et al. Statin utilization improves oncologic and survival outcomes in patients with dyslipidemia and surgically treated renal cell carcinoma. Minerva Urol Nefrol. (2017) 69(5):501–8. doi: 10.23736/S0393-2249.17.02788-6
31. Boegemann M, Schlack K, Rink M, Bernhardt S, Moran M, Hubbe M, et al. Effect of comorbidities/comedications on sunitinib outcomes for metastatic renal cell carcinoma: the STAR-TOR registry. Future Oncol (2020) 16(35):2939–48. doi: 10.2217/fon-2020-0548
32. Heide J, Ribback S, Klatte T, Shariat S, Burchardt M, Dombrowski F, et al. Evaluation of the prognostic role of co-morbidities on disease outcome in renal cell carcinoma patients. World J Urol (2020) 38(6):1525–33. doi: 10.1007/s00345-019-02930-4
33. Fiala O, Ostasov P, Rozsypalova A, Hora M, Sorejs O, Sustr J, et al. Impact of concomitant cardiovascular medication on survival of metastatic renal cell carcinoma patients treated with sunitinib or pazopanib in the first line. Target Oncol (2021) 16(5):643–52. doi: 10.1007/s11523-021-00829-y
34. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (2021) 372:n71.
35. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ (2021) 372:n160. doi: 10.1136/bmj.n160
36. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane handbook for systematic reviews of interventions version 6.2. (London UK:The Cochrane Collaboration) (2021). Available at: www.training.cochrane.org/handbook.
37. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses (2010). Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
38. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21(11):1539–58. doi: 10.1002/sim.1186
39. Luo Y, She DL, Xiong H, Fu SJ, Yang L. The prognostic effect of statin use on urologic cancers: An updated meta-analysis of 35 observational studies. Medicine (2015) 94(36):e1523. doi: 10.1097/MD.0000000000001523
40. Nayan M, Punjani N, Juurlink DN, Finelli A, Austin PC, Kulkarni GS, et al. Statin use and kidney cancer survival outcomes: A systematic review and meta-analysis. Cancer Treat Rev (2017) 52:105–16. doi: 10.1016/j.ctrv.2016.11.009
41. Wu P, Xiang T, Wang J, Lv R, Zhuang Y, Wu G. Statin use and the overall survival of renal cell carcinoma: A meta-analysis. Clin Invest Med (2020) 43(4):E17–23. doi: 10.25011/cim.v43i4.34908
42. Woodard J, Sassano A, Hay N, Platanias LC. Statin-dependent suppression of the akt/mammalian target of rapamycin signaling cascade and programmed cell death 4 up-regulation in renal cell carcinoma. Clin Cancer Res (2008) 14(14):4640–9. doi: 10.1158/1078-0432.CCR-07-5232
43. Okubo K, Isono M, Miyai K, Asano T, Sato A. Fluvastatin potentiates anticancer activity of vorinostat in renal cancer cells. Cancer Sci (2020) 111(1):112–26. doi: 10.1111/cas.14225
Keywords: statin, renal cell cancer, survival, prognosis, meta-analysis
Citation: Liang W, Pan Y, Liu A, He Y and Zhu Y (2023) Influence of statin use on prognosis of patients with renal cell cancer: a meta-analysis. Front. Oncol. 13:1132177. doi: 10.3389/fonc.2023.1132177
Received: 27 December 2022; Accepted: 14 March 2023;
Published: 13 July 2023.
Edited by:
Robert Figlin, Cedars Sinai Medical Center, United StatesReviewed by:
Xun Lin, Pfizer, United StatesJun-Jun Yeh, Ditmanson Medical Foundation Chia-Yi Christian Hospital, Taiwan
Copyright © 2023 Liang, Pan, Liu, He and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenli Liang, d2VubGlfbGlhbmc5MzZAMjFjbi5jb20=
 Wenli Liang
Wenli Liang Yongmei Pan2
Yongmei Pan2