- 1Organ Transplant Center, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
- 2Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou, China
- 3Guangdong Provincial Key Laboratory of Organ Donation and Transplant Immunology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
- 4Guangdong Provincial International Cooperation Base of Science and Technology (Organ Transplantation), The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
- 5Department of Biliary-Pancreatic Surgery, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China
Three-dimensional cell culture technology (3DCC) sits between two-dimensional cell culture (2DCC) and animal models and is widely used in oncology research. Compared to 2DCC, 3DCC allows cells to grow in a three-dimensional space, better simulating the in vivo growth environment of tumors, including hypoxia, nutrient concentration gradients, micro angiogenesis mimicism, and the interaction between tumor cells and the tumor microenvironment matrix. 3DCC has unparalleled advantages when compared to animal models, being more controllable, operable, and convenient. This review summarizes the comparison between 2DCC and 3DCC, as well as recent advances in different methods to obtain 3D models and their respective advantages and disadvantages.
1 Introduction
Despite significant advances in human research on tumor staging, diagnosis and treatment, tumors remain one of the leading causes of death (1). Cancer cells can grow and metastasize rapidly, which is largely attributed to the ability of cancer cells to create a tumor microenvironment (TME) for themselves and progressively modulate it from an anti-tumor response to a tumor-friendly one (2).
Therefore, establishing an experimental model system that accurately mimics the complexity of the TME is essential. Traditional in vitro two-dimensional cell culture systems (2DCC) (on planar scaffold) and animal models have been widely used for cancer research. However, 2DCC systems do not mimic natural TME due to a lack of cell-cell communication and interactions of cell-cell and cell-matrix (3), while in vivo animal models are expensive, ethically problematic, and challenging to set up as they show difficulties in tracking tumor growth and drug screening (4). To address these limitations, the three-dimensional cell culture system (3DCC) is increasingly developed in research and is now crucial for oncology studies due to its ability to accurately maintain TME without any additional manipulation. In this review, we summarize the comparison between 2DCC and 3DCC, as well as recent advances in different methods to obtain 3D models and their advantages and disadvantages.
2 Introduction of the tumor microenvironment
TME refers to the cellular environment in which tumor or cancer stem cells reside which has its own unique characteristics compared to the microenvironment of the normal one. These characteristics are important for the tumor immune escape, growth, survival, and metastasis which include hypoxia, acidic environment, inflammatory microenvironment, specific vascularization (Figure 1). TME consists of the extracellular matrix (ECM) and various tumor-associated cells such as cancer-associated fibroblasts (CAFs), endothelial cells, adipocytes, and immune cells (5, 6). These cells are located around tumor cells and are energized by the vascular network (7). CAFs can be simply defined as fibroblasts (non-epithelial, non-cancerous, non-endothelial, and non-immune cells) located within or adjacent to a tumor and are the major producer of ECM and various other cytokines in the TME. CAFs have functions of immunosuppression, promoting angiogenesis, producing enzymes that degrade ECM (such as matrix metalloproteinases), and promoting tumor growth and metastasis. However, some CAFs have been shown to inhibit tumor activity (8). Immune cells (T cells, neutrophils, macrophages, etc) play an important role in tumor growth, migration, and immune escape. The pro-tumor inflammation feature within the TME promotes tumor growth by blocking anti-tumor immunity and influent the composition of immune cells within it. Result to the activation of transcription factors in tumor cells, leading to increased inflammation and the production of inflammatory microenvironments adapted to tumor cell growth. Tumor-associated macrophages (TAM) usually divided into M1 type, which mediates antibody-dependent cytotoxic effects (ADCC) to kill tumor cells, and M2 type, which promotes tumor growth, invasion, metastasis and drug resistance. These two cell types can be interconverted (9). Angiogenesis is essential for tumors. Neovascularization provides oxygen and nutrients to the tumor and promotes tumor metastasis. Tumor vascular endothelial cells (TEC) are involved in the metastasis of cancer cells to the neovascular lumen, help generate CAFs, and mediate tumor invasion and metastasis (10). Tumor cells and cancer stem cells (CSCs) secrete molecules that induce a tumor-promoting phenotype, polarizing macrophages to M2 subtype, fibroblasts to CAF, and ECs to TEC (11). ECM is generally defined as the non-cellular component of a tissue that provides metabolic and structural support to its cellular components. Its main components are collagen, proteoglycan, laminin and fibronectin. In the process of tumor progression, a large number of enzymes such as MMP are produced, leading to active remodeling of the extracellular matrix, and changes in collagen degradation or deposition result in loss of ECM homeostasis, which ultimately interferes with cell-cell adhesion, cell polarity and increases growth factor signaling to promote tumor metastasis (12, 13).
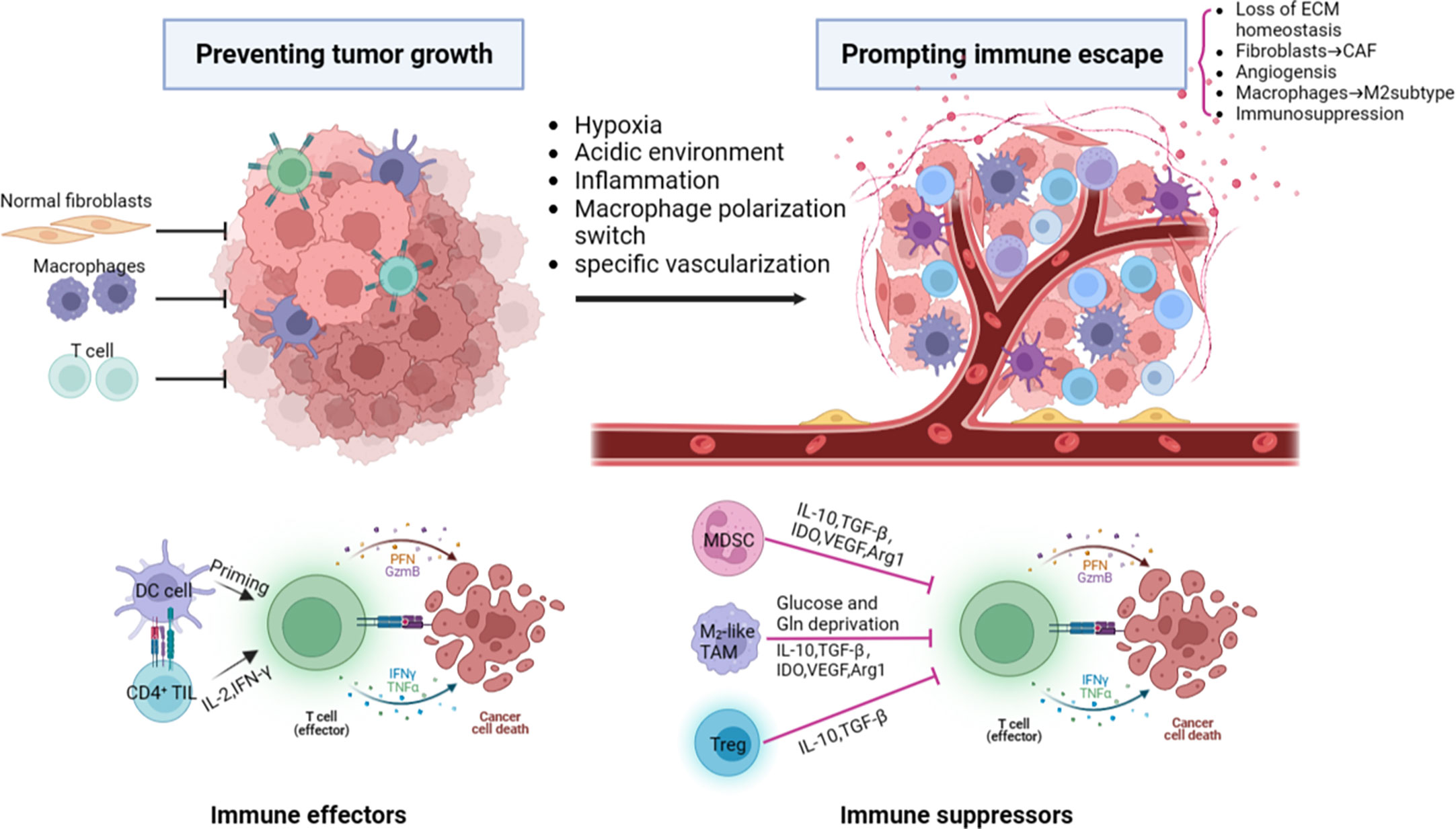
Figure 1 The major components and characteristics related to tumor progression within the tumor microenvironment. ECM, extracellular matrix; CAF, cancer-associated fibroblast; DC, dendritic cells; PFN, perforin; GzmB, granzyme B; IFNγ, interferon γ; TNFα, tumor necrosis factor α; TGF-β, transforming growth factor-β;MDSC, myeloid-derived suppressor cell; IDO, Indoleamine 2,3-dioxygenase; VEGF, vascular endothelial growth factor; Arg1, arginase 1; Gln, glutamine; Treg, regulatory T cell.
3 2D vs 3D: Introduction of 3DCC model and its advantages
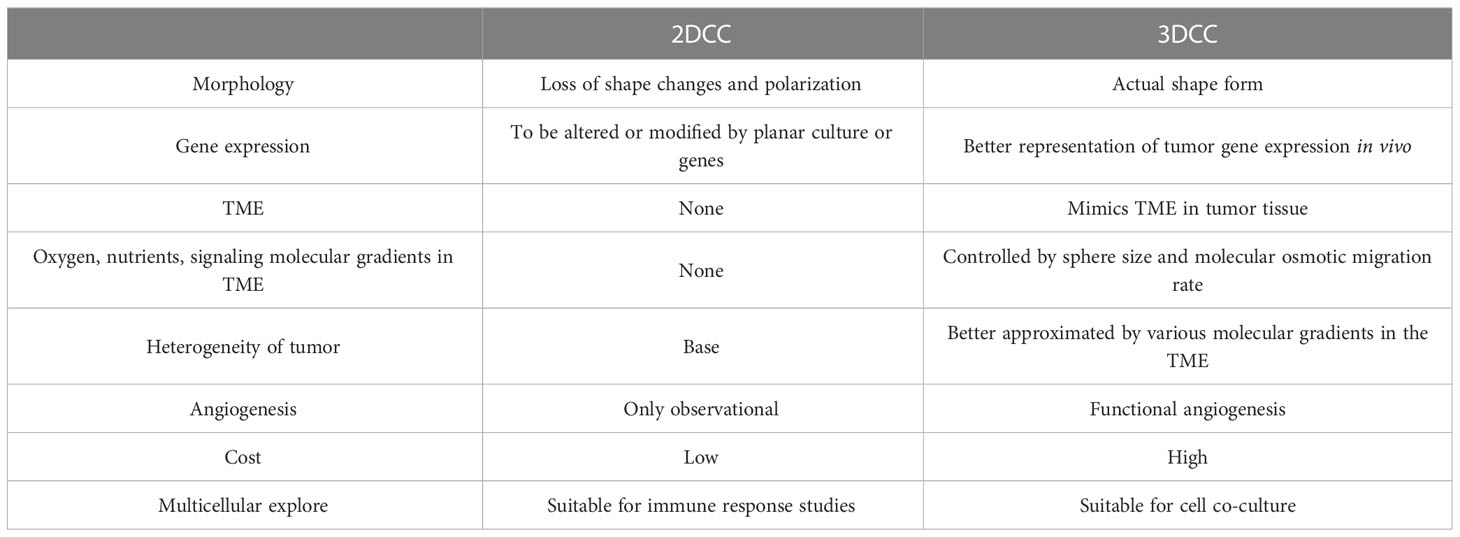
Whether the in vitro culture model can effectively mimic TME has become an important basis to investigate its practical value. As a traditional in vitro cell culture system, 2DCC has long been used in cancer research. However, 2DCC does not mimic the complexity of 3D tissues in vivo, nor does it mimic the interaction between tumor cells and TME. Gradients of nutrient and oxygen concentrations are common in TME (14), but cannot be reproduced in 2DCC (15, 16). To address these limitations, 3D cell culture (3DCC) was developed. The 3D tumor sphere model can narrow the gap between 2DCC and in vivo tumor model, making the model closer to the real tumor tissue (17) (Figure 2 and Table 1). At present, 3D sphere models can be divided into four types: multicellular tumor sphere (MCTS), neoplastic sphere, tissue-derived tumor sphere (TDTS), and organotypic multicellular sphere (OMS) (20). The cultural methods and biological characteristics of the different types of models are different. MCTS were produced in single-cell suspension cultures in conventional FBS supplemented media without the supply of exogenous ECM. But not all cell lines are capable of producing compact MCTS (20). Tumor spheres were established as amplification models of CSCs in a serum-free medium supplemented with growth factors. It was used to enrich CSCs and cells with stem cell-related characteristics (21). TDTS and OMS were obtained from the tumor tissue department. TDTS were observed in an in vitro study of colon cancer cell lines (22). The histological features of OMS are very similar to those of tumors in vivo, and capillaries can be maintained for up to 6 weeks (23). MCTS is one of the most commonly used models because it is relatively easy to assemble, possesses reproducibility and ability to mimic tumor cell heterogeneity (24). For the study of tumor initiation, smaller, well-oxygenated spheres (optimal diameter of about 200μm) can be used. In contrast, for studies related to tumor expansion, larger spheres are preferred to mimic the hypoxic and necrotic regions observed in hypovascularized tumors (14). The following is a detailed description of how 3DCC is constructed.
4 3DCC and tumor initiating cells
Tumor initiating cells play a huge role in tumor malignancy and chemotherapy resistance. The niche of cancer stem cells in vitro differs significantly from that in vivo. One important aspect of the niche is to maintain stem cells in a quiescent state while simultaneously driving a sufficient number of stem cells into proliferation and differentiation pathways to maintain organs’ function (25). Many signaling pathways that mediate the interaction of normal stem cells with their niche are also involved in the interaction between cancer stem cells and their niches and can promote tumorigenesis and cancer proliferation. Cancer stem cells tend to be quiescent in the body’s milieu interieur, but they exhibit greater proliferative activity in vitro than non-cancer stem cells (26).
Currently, the most reliable model for studying cancer stem cells is a 3D assay using an ECM-rich Matrigel, which maintains the growth of heterogeneous layered cancer stem cell cultures. The addition of ECM group stratified adhesins to serum-free medium increases tumor cell growth, self-renewal, and tumorigenic characteristics of glioma cancer stem cells (27). Therefore, the use of 3DCC to study its biological behavior and role in tumors is also a hot topic today. Now some new models are built using 3DCC to study the self-renewing cancer population in depth. For instance, Hubert et al. (28) established CSC cultures derived from the hyperoxia, vegetatively high regions and mixed regions of chronic hypoxia and necrosis regions derived from human glioblastoma. Li et al. (27) constructed a three-dimensional spheroid model of non-small cell lung cancer and used A549 and SK-MES-1 to assess cell growth, migration, drug resistance and other phenomena. In the three-dimensional spheroid model, the commonly used drug tadalafil showed a more pronounced inhibitory effect. Fibrin deposition in the matrix of CRCs proved to be the cause of tumor development. Zhang M et al. (29) used salmon fibrin gel to provide 3D ECM for colon cancer cells and found that 90 Pascal (Pa) fibrin gel was the most effective in isolating and enriching tumor colonies compared to rigid 420 Pa and 1,050 Pa gels. The size and number of colony formations are inversely correlated with gel hardness.
5 3D model construction methods for cancer research and its recent progress
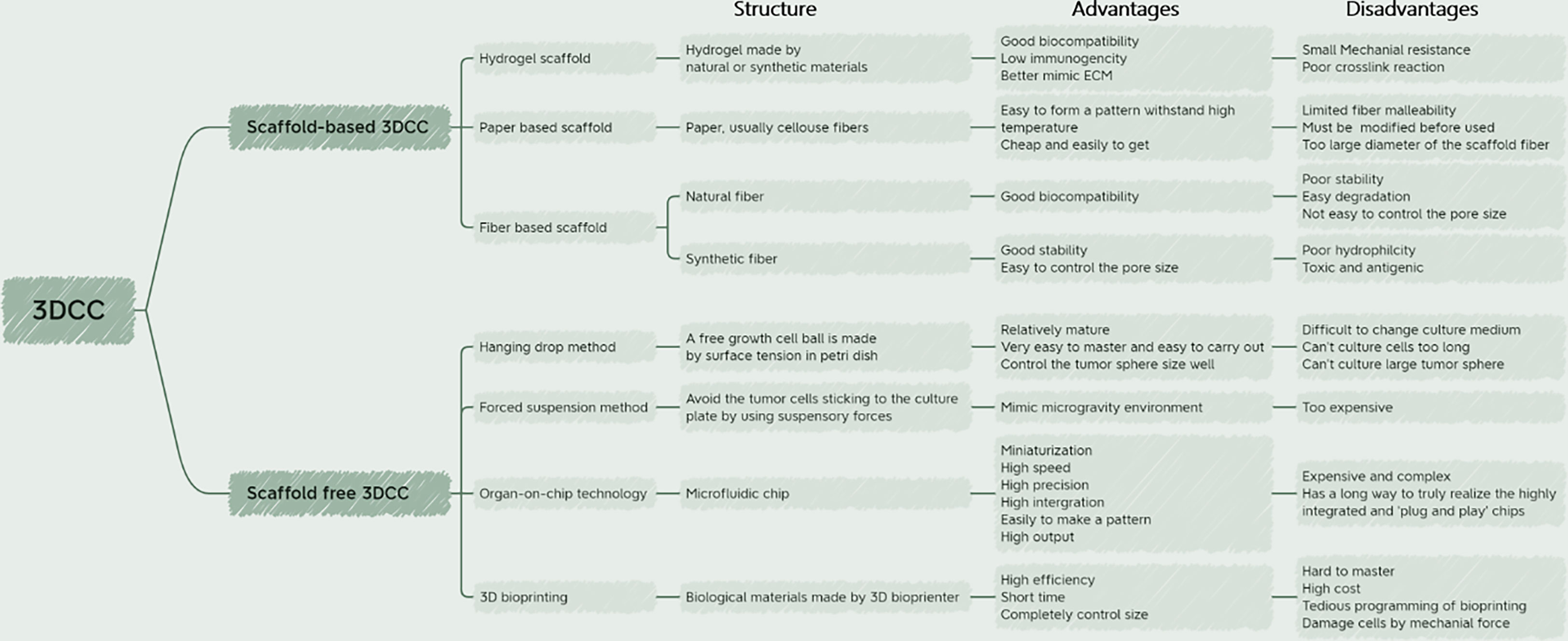
In general, 3DCC construction methods can be divided into scaffold-based and scaffold-free models, each with its own set of advantages and disadvantages (30). The following sections will discuss these two methods individually, and provide an overview of the latest advancements in each.
5.1 Scaffold-based 3DCC model
3D scaffolds can influence tumor cell-cell and cell-TME interactions by affecting mechanical and biochemical signaling and mimicking the conditions of hypoxia and nutrient deprivation in TME (17). Various forms of materials have been used to construct scaffolds for 3DCC. Depending on the materials, scaffold-based 3DCC can be further classified into hydrogel scaffolds, paper-based scaffolds, and fiber-based scaffolds (15). The support method is simple to operate and easy to disassemble and assemble. However, the disadvantage is that some of the scaffold materials are expensive.
5.1.1 Hydrogel scaffold
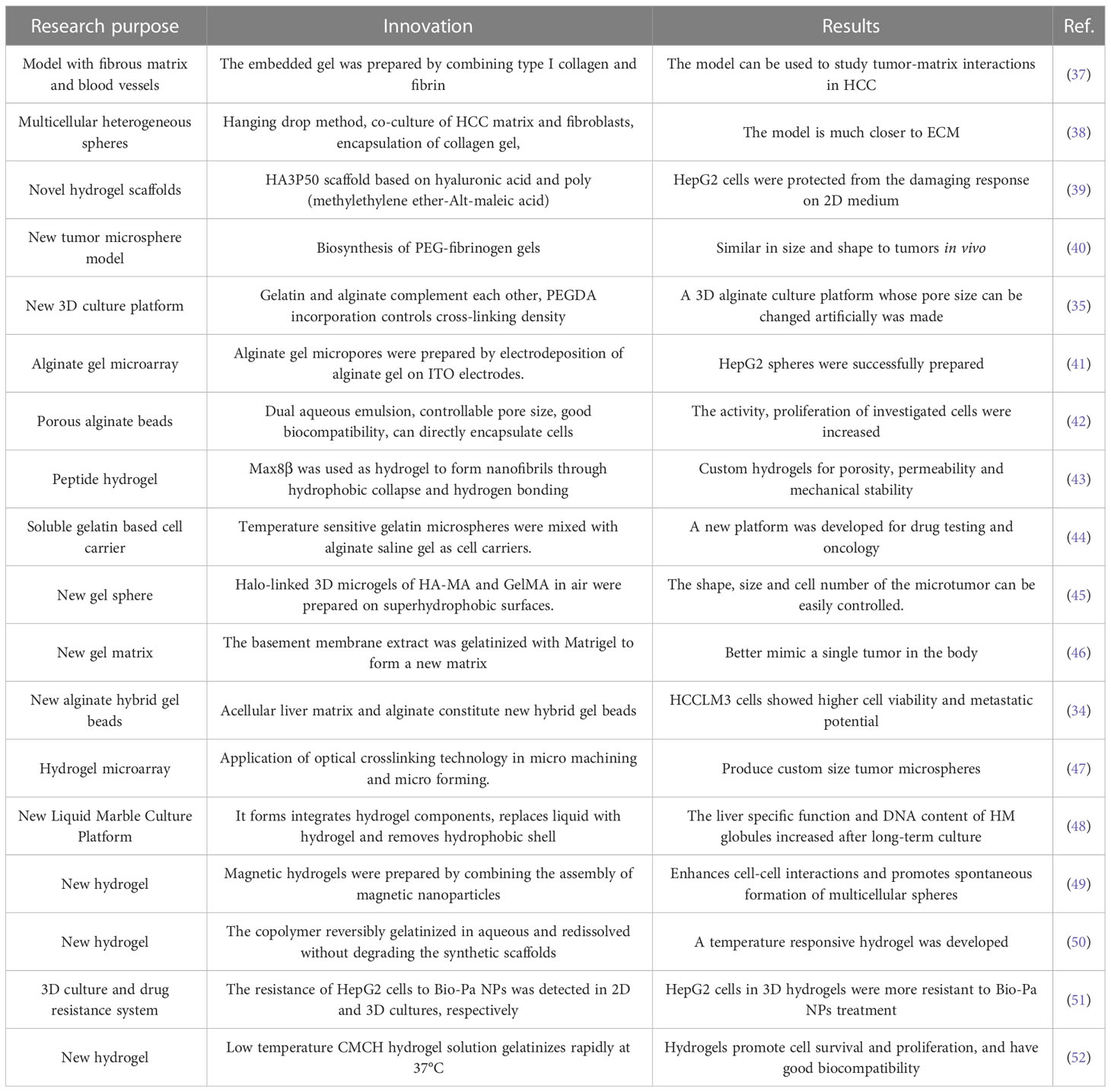
Hydrogels consist of one or more different hydrophilic polymers, their unique polymerization mode allows for the free movement of cells and molecules through their pores (31). In the human body, most mammalian cells rely on the extracellular matrix (ECM) for support to carry out life activities. Hydrogels are special because they allow cytokines and growth factors to cross tissue-like gels. Although they contain 95% water, they do provide the solid-liquid level required for cell culture (32). Hydrogels can better replicate the ECM in vivo, as they are usually composed of hydrated proteins. They exhibit good biocompatibility and low immunogenicity, but have poor mechanical resistance and crosslinking reactions (17). Hydrogels can be created using natural materials such as collagen, fibrin, hyaluronic acid, and alginate, or synthetic materials like polyethylene glycol (33). The properties of hydrogels, including hydration, porosity, and stiffness, can be fine-tuned by adjusting the components of the material. However, some collagen hydrogels are expensive and have poor renewability (16). Alginate-based hydrogels, like alginate gel beads (ALG beads), are popular 3D substrates for medical applications due to their mild gelation process, biocompatibility, and structural similarity to native tissues (34). Alginate can be cross-linked in the presence of calcium ions and can also be used to degrade scaffolds with sodium citrate to recover cells (35). However, alginate itself does not possess cell adhesion properties due to the lack of interaction with integrins. It also lacks matrix receptors similar to those found in native times, which are important for cell adhesion (34). The pores formed by alginate in the presence of calcium ions are dense and difficult to control accurately, weakening the migration of cells and other biomolecules. Therefore, many improvements have been made to alginate saline gels. Synthetic peptide hydrogels are also a hot research topic. The physicochemical properties of gels can be easily modified by adding or subtracting amino acids or modifying the side chains of amino acid residues (36). There are also many new developments in hydrogel scaffolds of other materials. Table 2 provides an overview of the latest progress of hydrogel scaffolds.
5.1.2 Paper base scaffold
Paper is produced by pressing wet cellulose fibers together. Paper-based scaffolds are rigid and can withstand high temperatures, yet they possess some deformability and can be folded into complex geometries, providing pores for cell growth (15, 53). These scaffolds are also hydrophilic with capillary adsorption capacity (54), making them a convenient and cost-effective option for mass production and utilization (53). A convenient 3D culture environment can be created by combining paper-based scaffolds with hydrogel-simulated ECM (55). The gradient of hypoxia and biomolecules in TME can be imitated by stacking paper-based scaffolds. Disassembling the paper-based scaffold facilitates cell harvesting and analysis of the structure and function of cells in the paper-based scaffold without histological sections. The paper platform is used to culture primary cells, tumor cells, patient biopsies, stem cells, fibroblasts, osteoblasts, immune cells, bacteria, fungi, and plant cells. These platforms are compatible with standard analytical assays commonly used to monitor cell behavior. Due to its thickness and porosity, there is no mass transfer limitation to and from cells in the paper scaffold (56). However, paper-based scaffolds have some limitations, such as limited fiber malleability and the need for physical and chemical modification before use in cell culture (54). Furthermore, the diameter of the scaffold fiber is much larger than that of body fibrils (about 500nm), with a minimum diameter of 1mm (15).
5.1.3 Fiber base scaffold
Man-made fiber structures date back thousands of years, and they are used as clothing and decoration in the form of textiles (57). Fiber products are also widely used in filtration, cell culture, composite materials and other processes. Fiber-based scaffolds can be constructed using either natural fibers such as collagen, chitosan, and hyaluronic acid, or synthetic fibers such as polylactic acid, polyglycolic acid, and other degradable polyester polymers. Under the premise of ensuring the porosity of hydrogel and the normal growth of cultured cells, the use of fiber materials to build a platform can act as a scaffold to compensate for the lack of structural rigidity of hydrogels. Adding carbon nanotubes to a hydrogel is a good try (58). Natural fiber scaffolds are known for their good biocompatibility and ability to interact with cell-ECM receptors, which facilitates cell growth. However, these scaffolds have poor stability, are easily degradable, and have a limited ability to control the size of the fiber pore (31). Some fiber production processes, such as electrospinning, use solvents that denature natural fibers (59). In contrast, synthetic fiber scaffolds are stable over a wide range of temperatures and in solution (31). Synthetic fibers are easier to control the pore size and can also be used to mimic the porous structure of ECM (60). However, these fibers may be less hydrophilic, and some may be toxic and antigenic, which can damage cells. Due to these limitations, efforts are being made to enhance their properties while preserving their respective benefits.
In terms of natural materials, Mahmoudzadeh et al. (61) developed collagen-chitosan nanoscaffolds and utilized them to culture 4T1 tumor cells, allowing for the construction of a 3D microenvironment as the tumor cells infiltrated the scaffolds. Koh et al. (62) presented a comprehensive protocol for studying live cell microscopy and immunohistochemistry to quantitatively assess physiological cell-cell contact dynamics. Decellularized natural tissues have also emerged as a source of fibrous scaffolds for cancer research (63), such as decellularized lung scaffolds, which retain the ECM arrangement of the original tissue and allow for better simulation of cell-ECM interactions (64). Tissue engineering can also be employed to construct tumors in vitro, as demonstrated by Lu et al. (65), who utilized Tris-trypsin-Triton to treat tumor tissues in multiple steps, creating 3D scaffolds with the ideal spatial arrangement, biomechanical properties, and biocompatibility - a promising approach for modeling the TME.
In terms of synthetic materials, Girard (66) et al. developed the “3P” scaffold, which is produced by electrospinning the block copolymers of poly (lactate-coglycolic acid) (PLGA), polylactic acid (PLA) and mono-methoxy polyethylene glycol (mPEG). Fischbach (67) et al. used polylactide to fabricate fiber scaffolds. Both types of scaffolds are non-toxic to tumor cells and can be produced on a large scale. Additionally, tumor cells grown on these scaffolds exhibit invasion and metastasis characteristics that better replicate the in vivo tumor microenvironment. Mazzini (68) et al. and Murakami (69) et al. have both developed 3D tissue culture systems using silicon as a raw material. Mazzini’s team utilized silicon microprocessing technology to produce 3D microarrays for the study of tumor cell invasion. Meanwhile, Murakami’s “Cellbed” culture system is composed of a fibrous polymer made of ultra-fine silica fibers, mimicking the loose connective tissue structure of living organisms. Cancer cells can easily migrate and form 3D structures in this system.
All of the above-mentioned scaffolds are based on natural or artificial materials and are further developed to have more applications as fiber scaffolds.
5.2 Scaffold-free 3DCC model
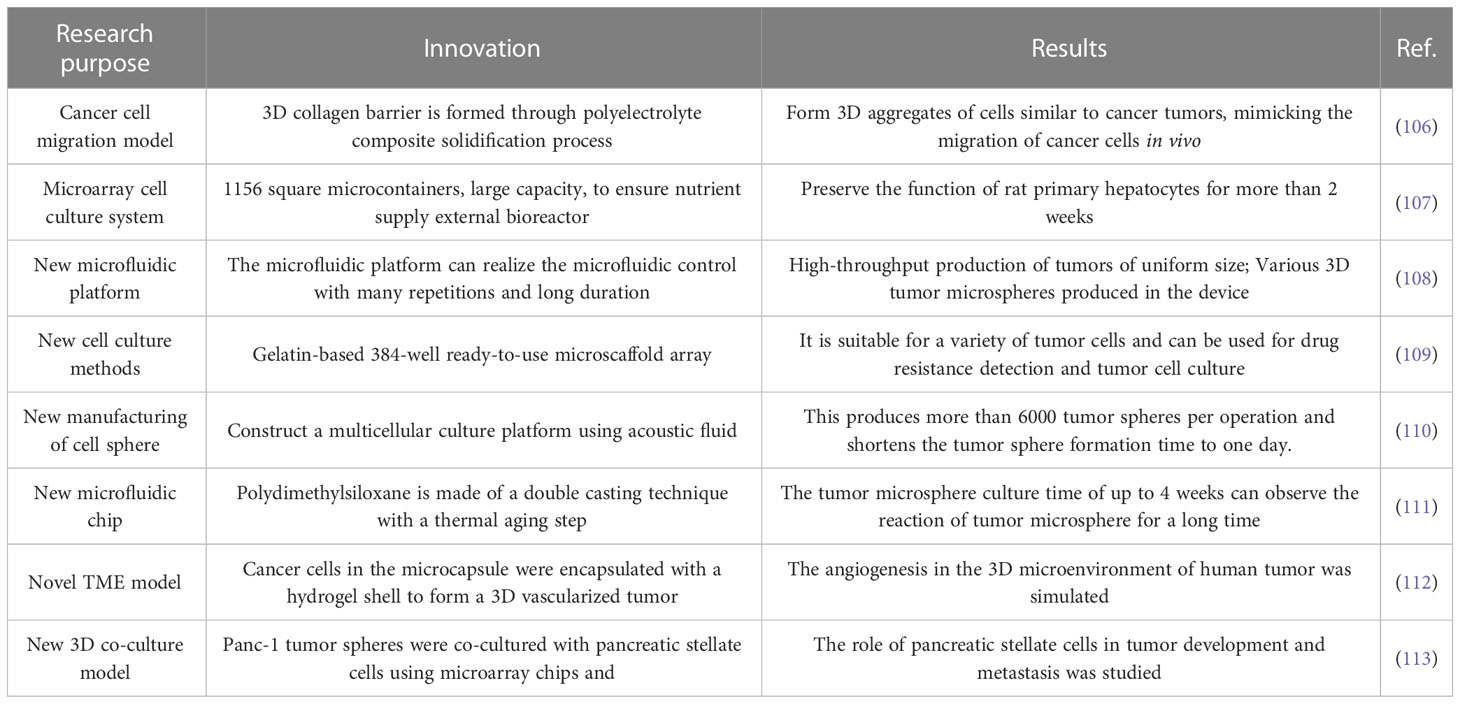
3DCC without scaffolds mainly uses various methods to prevent cell adherent growth and aggregate tumor pellets in culture medium (24). These methods include magnetic force, agitation and rotation, hanging drops, low-adhesion culture plates, and advanced technologies such as microfluidic chips and 3D printing. While scaffold-free 3DCC is suitable for only a limited number of cell types and is initially expensive, it allows spontaneously aggregated cells to form their own ECM (24). Scaffold-free 3DCC does not involve vasculogenesis and can restore the heterogeneity of tumors in vivo, thus more closely resembling solid tumors in vivo. It is not affected by the shear force of scaffold assembly, nor is it limited by the pore size of scaffold fibers, and can produce controllable size tumor microspheres (70). T Below, we introduce the main methods and recent advances in scaffold-free 3DCC formation.
5.2.1 Hanging drop method
The hanging drop method is a relatively simple technique. Initially, cells are cultured in two dimensions and allowed to adhere to the wall before being digested into monolayer cells. The resulting digested cell culture liquid is then dropped onto the lid of a Petri dish, which is subsequently inverted. The liquid was drooped to form hanging drops through the action of surface tension, and the desired tumor pellets could be formed in the hanging drops. It usually forms in spheres or sheets within 24 hours but may take longer. The length of time required depends on the type of cells (71). This method is well-established, requiring no specialized equipment and is easy to master. The resulting tumor spheres are easy to control in terms of size, with only one sphere formed per drop. Mesenchymal stem cells cultured by the hanging drop system can secrete a large number of potent anti-inflammatory and antitumor factors (72). However, it is difficult to change the culture medium of the traditional hanging drop method, and the culture time of the cells should not be too long. Large tumor spheres cannot be cultivated due to nutrient supply effects (73). Ratnayaka (74) et al. invented a PDMS platform combining the hanging drop method and polydimethylsiloxane (PDMS) scaffold. By using this method, HepG2 cells could be grown to the level of millimeter, which was much higher than the volume of tumor microspheres obtained by the ordinary hanging drop method. Although the pendant method does not mimic tumor angiogenesis and makes it difficult to grow tumors to the size of advanced tumors in vivo, it is still possible to obtain larger tumor microspheres with this method, which provides convenience for tumor research.
5.2.2 Forced suspension method
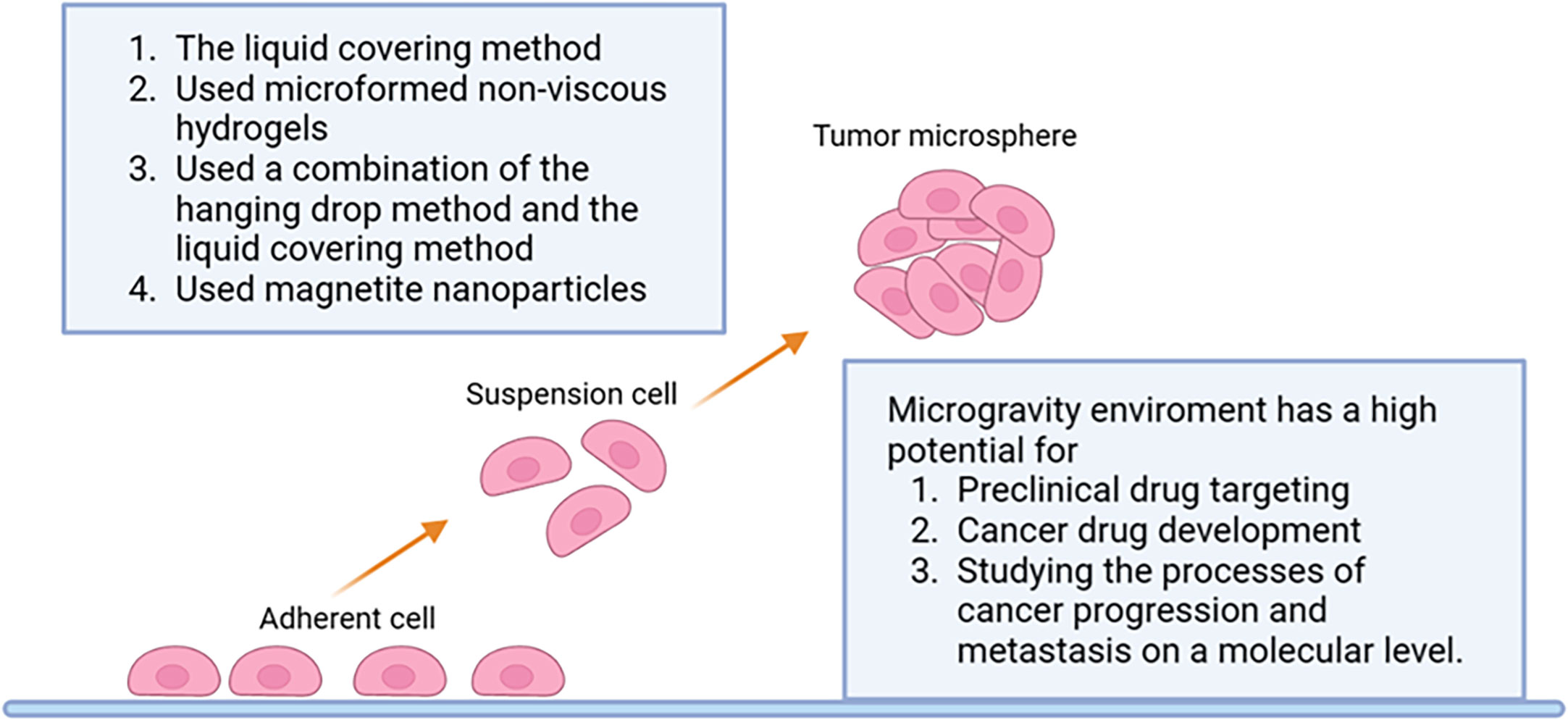
The forced suspension method is to prevent tumor cells from sticking to the culture plate and forming tumor microspheres by forced suspension. The commonly used method is the liquid covering method, which precoats the surface of the culture plate with low adhesion material in advance to form an ultra-low adhesion culture plate. Tumor cells cannot adhere to the culture plate, so they spontaneously suspend to form tumor microspheres (70). The most commonly used ultra-low adhesion culture plate is a 96-well polystyrene culture plate (70). This method is simple and convenient, and most tumor cells can form tumor microspheres by this method. However, this method cannot control the size and homogeneity of the tumor microspheres formed. Napolitano et al. (75) used microformed non-viscous hydrogels to conduct forced suspension cell culture, and cells spontaneously self-assembled and reached A structural balance controlled by cell-cell interactions. Shao (76) et al. constructed a novel tumor microsphere model by co-culturing melanoma cells and cancer-associated fibroblasts (CSF). In this model, tumor ECM is completely controlled by CSF, which facilitates the study of the interaction between tumor cells and TME. Beheshti (77) et al. used a combination of the hanging drop method and the liquid covering method to form 3D multicellular spheres to test the anticancer effect of Ipomoea purpurea. In addition to these new materials and methods, physical means such as magnetic force and rotation can also be used to achieve the purpose of forced suspension. Magnetic cell suspension is an emerging spheroid-forming technique. To generate spheroids, cells are preloaded with magnetic nanoparticles and then float towards the air/liquid interface within the low-adhesion plate using an externally applied magnetic field to promote cell-cell aggregation and spheroid formation. Glauco R Souza et al. reported a magnetic levitation cell culture model. By controlling the magnetic field, the geometry of the cell can be changed (78). Okochi (79) et al. used magnetite nanoparticles to make cells suspended and gathered in the center of the culture pore through the effect of magnetic force, thus realizing the suspension culture of cells. The rotating cell culture system simulates the microgravity environment by producing laminar flow (80), minimizing the mechanical stress of cell aggregation, making cells grow in suspension and preventing them from sticking to the wall and forming cell spheres. Human mesenchymal stem cells cultured in simulated microgravity have osteogenesis and enhanced adipoiesis (81). This method has been recommended by NASA as an effective tool for modeling microgravity (82). Numerous studies have illustrated the impact of short- and long-term exposure to real and simulated µg on various processes, including differentiation, growth behavior, migration, proliferation, survival, apoptosis, and adhesion, all of which are pertinent to cancer research (83). Thus, the µg-environment facilitates the creation of in vitro 3D tumor models, such as multicellular spheroids and organoids, that offer significant potential for preclinical drug targeting, cancer drug development, and the study of cancer progression and metastasis on a molecular level. In conclusion, as depicted in Figure 3, the forced suspension method is a widely utilized and well-established technique.
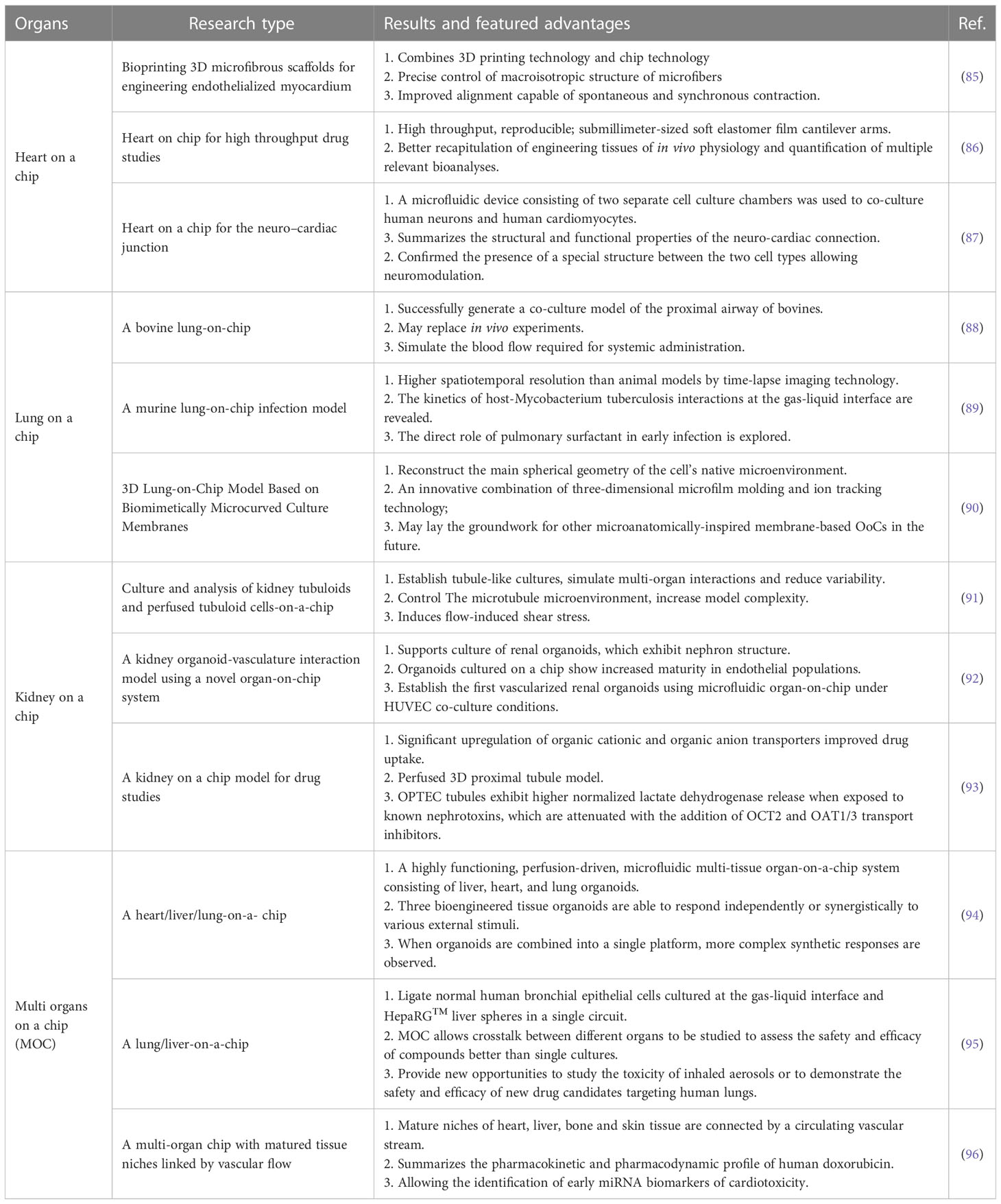
5.2.3 Organ on chip technologies
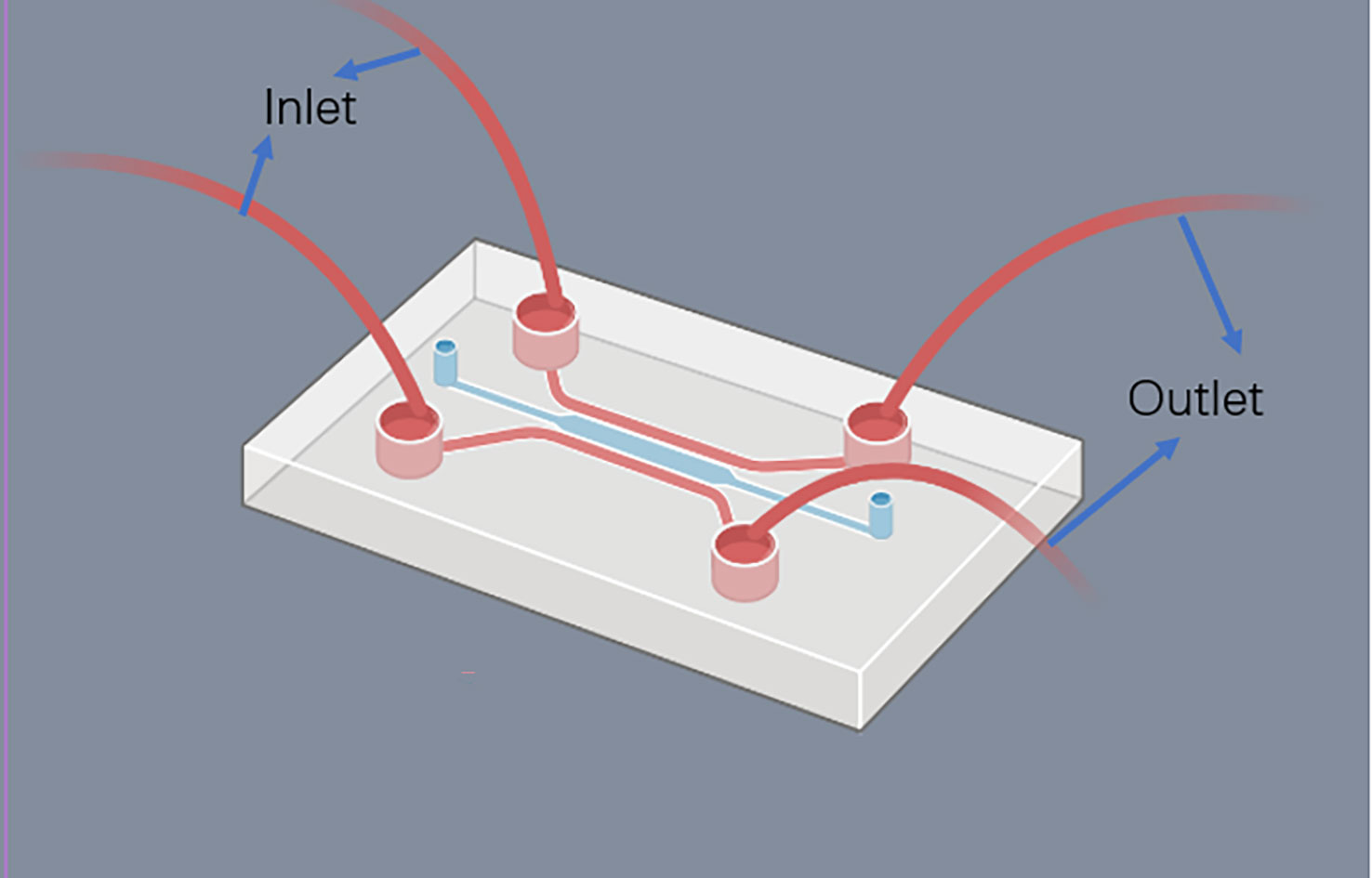
FDA has recently agreed to assess organ on a chip technology, which has the potential to replace animal models altogether. Cancer on-chip technology typically offers the following solutions (84): (1) 2D chips. Single or multi-chamber chips with controlled substance concentration gradients to study the impact of concentration gradients on cancer metastasis; (2) Lumen chips. Lumen consisting of a patterned 3D matrix, suitable for vascular studies of tumors; (3) Partition chip. Chips are divided into several cells with a separator, capable of culturing different types of cells, making them versatile; (4) Y-type chip. Similar to partition chips but with parallel-matrix compartments operating in co-flow mode; (5) Membrane chip. Capable of creating numerous microchannels with porous membranes, facilitating solute gradients at channel interfaces, cell culture, and transfer observation. Summary of the major organ on chip technologies is now illustrated in Table 3. The use of microfluidic chips is an essential component of this technology (Figure 4). Being selected as one of the World Economic Forum’s Top 10 Emerging Technologies, Microfluidics integrates sample preparation, reaction, separation, detection, and basic operational units such as cell culture, sorting, and cell lysis (97).
Microfluidics is the science of precisely manipulating fluids and particles in sizes ranging from microns to submicrons (98, 99). The use of Polydimethylsiloxane (PDMS) as a basic material for microfluidic chips is popular due to its low cost, good biocompatibility, high oxygen permeability, light transmittance, and convenience (99, 100). Glass and silicon, which are comparatively expensive and difficult to work with, have been gradually replaced by PDMS. Moreover, hydrogels and paper can also be employed to produce microfluidic chips (101). By integrating cell culture, cell separation, cell detection, and other procedures into a small chip, microfluidic technology offers the benefits of miniaturization, high precision, and high integration. Compared with traditional laboratory techniques in the past, the microfluidic platform has the advantages of requiring fewer samples, high sensitivity, rapid control and so on (102).
The microfluidic chip is the main platform of microfluidic technology. It takes a micropipe network as its main structural feature and microfluidics technology to control the flow of liquid in the micropipe as its working principle. Microtubules are filled with living cells, and in this way organs or tissues in vitro are constructed to study their physiology and pathophysiology mechanisms (103). Due to the structure of the micron scale, the fluid exhibits and produces special properties in it that are different from those of the macroscopic scale. Hence the development of unique analysis of the resulting performance. Using a microfluidic chip to culture tumor cells can rapidly produce tumor microspheres of controllable size and can also be used for high-throughput analysis of tumor cells at any time. It can be used to simulate the tumor microenvironment, study the invasion and metastasis of cancer cells, simulate tumor angiogenesis, and conduct high-throughput tumor detection (99, 100). Microfluidic chips have the potential to surpass tumor xenograft modeling, which can lead to a significant reduction in animal experimentation and make tumor modeling and research more efficient (104). Despite this, the design and production of microfluidic chips are rather intricate, and there is still a long road ahead before achieving fully integrated and “plug and play” microfluidic chips without costly external auxiliary equipment (105). Table 4 provides an overview of some of the latest microfluidic chips developed.
5.2.4 3D bioprinting
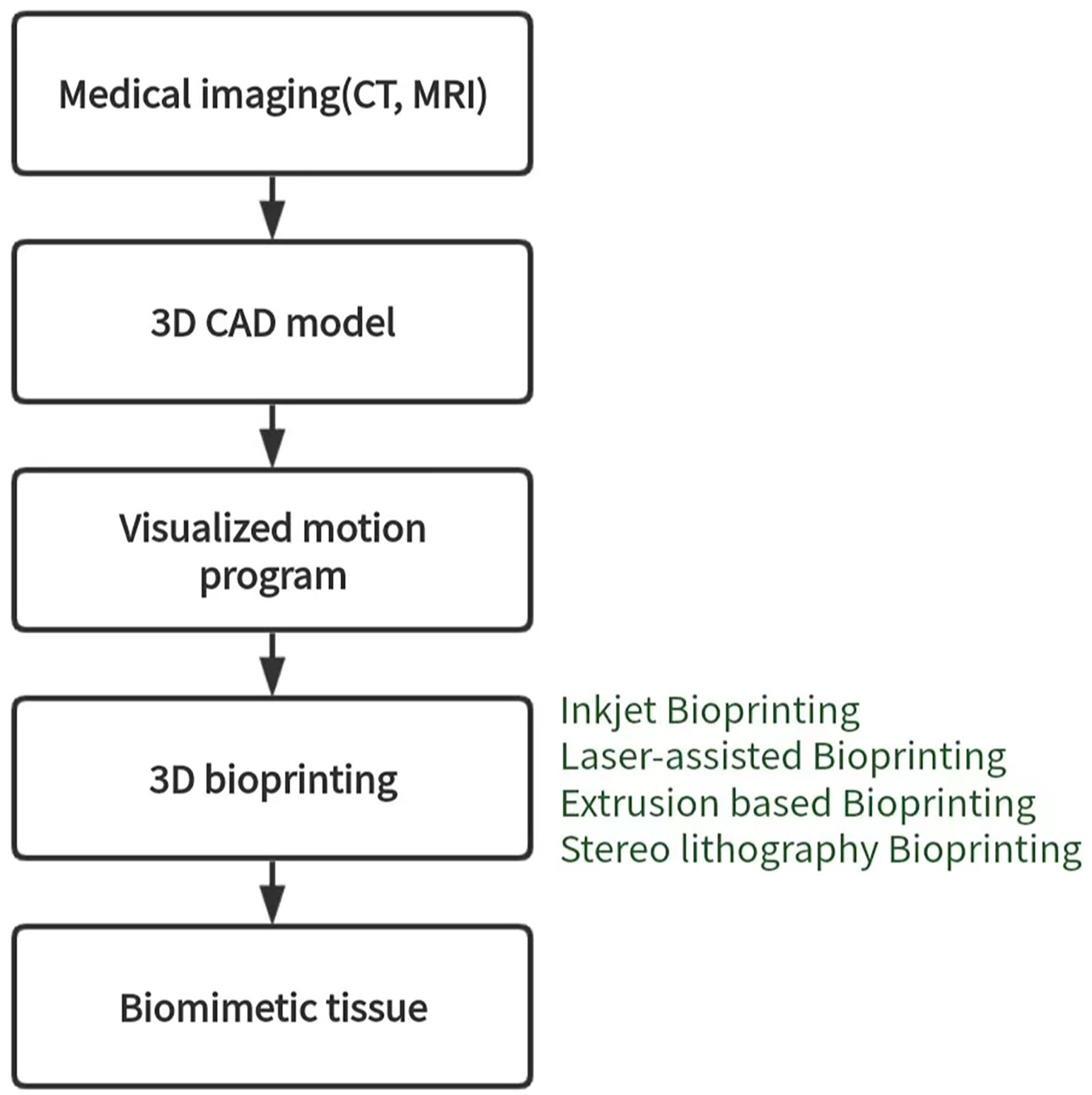
3D bioprinting is a manufacturing technology that accurately distributes biological materials containing cells to construct three-dimensional living tissues and human organs using a 3D printer. Currently, there are four types of 3D bioprinting technology, including inkjet, laser-assisted, extrusion, and stereo lithography, each with its advantages and disadvantages (114) (Figure 5). To construct 3D tumor models in vitro, tumors or tumor cells can be combined with TME as printing materials, and bioinks are essential for building effective 3D tumor models. Bioinks are typically biocompatible hydrogels and living cells of interest and play an important role in providing printability of samples (115, 116). Alginate and gelatin are the most commonly used substrates for bioprinting due to their good biocompatibility and mechanical properties. Bioprinting is a new research field. Compared with other cell culture technologies, the biggest advantage of the bioprinting method is that it can form tumor microsphere model with controllable size and shape in a short time, which can be used for various tumor research. However, this method is difficult to master because of its complicated technology, high cost and tedious programming of bioprinting. Inkjet 3D printing is limited due to clogged nozzles, which limits the steady flow of ink, as well as reduced cell viability (72). The mechanical pressure of extrusion printers can also damage cultured cells. Jiang (117) et al. combined alginate and gelatin to form a composite hydrogel similar to a natural tumor matrix. Chen (118) et al. cocultured primary HepG2 human hepatocytes and hepatic stellate cells (HSC) to form spherules. Then the spheres were bioprinted into liver tissue constructs using a Regenova bioprinter to construct a new liver cancer model. Bhattacharjee (119) et al. used packaged granular microgels to make liquid-like solid materials. The material is locally and temporarily fluidized under concentrated applied stress and spontaneously solidifies after the applied stress is removed, facilitating the transport of biomolecules and 3D printing of multicellular structures. In summary, 3D bioprinted cancer models can be valuable as invasion models and serve as an excellent tool to study cancer progression and visualize EMT and metastasis in real-time. Nonetheless, there are still several challenges and limitations that need to be addressed. These include the development of a perfusable, vascularized 3D bioprinted construct, achieving large organ reconstruction in vitro, long-term in vitro culture, and other related issues.
6 Conclusion
3DCC is increasingly important in oncology research as it better mimics solid tumor conditions in vivo with minimal use of animal models. Although 3DCC has obvious advantages over 2DCC, 3DCC cannot completely replace 2DCC (120) at present because the monolayer culture equipment is easy to manufacture, the production cost is low and the technology is easy. As summarized in Figure 2, 3DCC models have unparalleled advantages in simulating the tumor microenvironment. Although tumor-like 3D cultures allow the expansion of the tumor epithelium, they often lack non-epithelial stromal cells from tumors of origin, limiting their usefulness in addressing therapeutic strategies targeting this compartment (121). With the maturation and development of 3DCC technology, its application in the study of tumor metastasis mechanism, tumor microenvironment, tumor cell-ECM interaction and anti-cancer drug screening will be more extensive and in-depth, making it a promising technology for the future.
Author contributions
LZ, XL and YM contributed to the manuscript’s composition, literature review, and drafting and finalization of the manuscript. SC, WL and YC contributed to the literature review and search. PC contributed to the manuscript’s drafting and critical review. XL and YM contributed to the approval of the final version of the manuscript.
Funding
This study was supported by grants from the Natural Science Foundation of Guangdong Province (2023A1515011805 and 2022A1515011052), the National Natural Science Foundation of China (81873591), the Science and Technology Planning Project of Guangdong Province (2018A050506030), the Science and Technology Program of Guangzhou (201704020073), the Guangdong Provincial Key Laboratory Construction Projection on Organ Donation and Transplant Immunology (2013A061401007, 2017B030314018, and 2020B1212060026), and the Guangdong Provincial International Cooperation Base of Science and Technology (Organ Transplantation) (2015B050501002 and 2020A0505020003).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hulvat MC. Cancer incidence and trends. Surg Clin North Am (2020) 100:469. doi: 10.1016/j.suc.2020.01.002
2. Witz IP. The cross talk between cancer cells and their microenvironments. Biochem Biophys Res Commun (2022) 633:59. doi: 10.1016/j.bbrc.2022.09.066
3. Atat OE, Farzaneh Z, Pourhamzeh M, Taki F, Abi-Habib R, Vosough M, et al. 3D modeling in cancer studies. Hum Cell (2022) 35:23. doi: 10.1007/s13577-021-00642-9
4. Robinson NB, Krieger K, Khan FM, Huffman W, Chang M, Naik A. The current state of animal models in research: A review. Int J Surg (2019) 72:9. doi: 10.1016/j.ijsu.2019.10.015
5. Côté MF, Turcotte A, Doillon C, Gobeil S. Three-dimensional culture assay to explore cancer cell invasiveness and satellite tumor formation. J Vis Exp (2016) (114):54322. doi: 10.3791/54322
6. Gencoglu MF, Barney LE, Hall CL, Brooks EA, Schwartz AD, Corbett DC, et al. Comparative study of multicellular tumor spheroid formation methods and implications for drug screening. ACS Biomater Sci Eng (2018) 4:410. doi: 10.1021/acsbiomaterials.7b00069
7. Wu T, Dai Y. Tumor microenvironment and therapeutic response. Cancer Lett (2017) 387:61. doi: 10.1016/j.canlet.2016.01.043
8. Chen Y, McAndrews KM, Kalluri R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol (2021) 18:792. doi: 10.1038/s41571-021-00546-5
9. Pan Y, Yu Y, Wang X, Zhang T. Tumor-associated macrophages in tumor immunity. Front Immunol (2020) 11:583084. doi: 10.3389/fimmu.2020.583084
10. Sobierajska K, Ciszewski WM, Sacewicz-Hofman I, Niewiarowska J. Endothelial cells in the tumor microenvironment. Adv Exp Med Biol (2020) 1234:71. doi: 10.1007/978-3-030-37184-5_6
11. Colombo E, Cattaneo MG. Multicellular 3D models to study tumour-stroma interactions. Int J Mol Sci (2021) 22(4):1633. doi: 10.3390/ijms22041633
12. Walker C, Mojares E, Del Río Hernández A. Role of extracellular matrix in development and cancer progression. Int J Mol Sci (2018) 19(10):3028. doi: 10.3390/ijms19103028
13. Shannon AE, Boos CE, Hummon AB. Co-Culturing multicellular tumor models: Modeling the tumor microenvironment and analysis techniques. Proteomics (2021) 21:e2000103. doi: 10.1002/pmic.202000103
14. Thoma CR, Zimmermann M, Agarkova I, Kelm JM, Krek W. 3D cell culture systems modeling tumor growth determinants in cancer target discovery. Adv Drug Delivery Rev (2014) 29:69–70. doi: 10.1016/j.addr.2014.03.001
15. Barbosa M, Xavier C, Pereira RF, Petrikaitė V, Vasconcelos MH. 3D cell culture models as recapitulators of the tumor microenvironment for the screening of anti-cancer drugs. Cancers (Basel) 14 (2021) 14(1):190. doi: 10.3390/cancers14010190
16. Habanjar O, Diab-Assaf M, Caldefie-Chezet F, Delort L. 3D cell culture systems: Tumor application, advantages, and disadvantages. Int J Mol Sci (2021) 22(22):12200. doi: 10.3390/ijms222212200
17. Unnikrishnan K, Thomas LV, Ram KR. Advancement of scaffold-based 3D cellular models in cancer tissue engineering: An update. Front Oncol (2021) 11:733652. doi: 10.3389/fonc.2021.733652
18. Hoarau-Véchot J, Rafii A, Touboul C, Pasquier J. Halfway between 2D and animal models: Are 3D cultures the ideal tool to study cancer-microenvironment interactions? Int J Mol Sci (2018) 19(1):181. doi: 10.3390/ijms19010181
19. Wang C, Tang Z, Zhao Y, Yao R, Li L, Sun W, et al. Three-dimensional in vitro cancer models: a short review. Biofabrication (2014) 6:22001. doi: 10.1088/1758-5082/6/2/022001
20. Weiswald LB, Bellet D, Dangles-Marie V. Spherical cancer models in tumor biology. Neoplasia (2015) 17:1. doi: 10.1016/j.neo.2014.12.004
21. Ishiguro T, Ohata H, Sato A, Yamawaki K, Enomoto T, Okamoto K, et al. Tumor-derived spheroids: Relevance to cancer stem cells and clinical applications. Cancer Sci (2017) 108:283. doi: 10.1111/cas.13155
22. Weiswald LB, Richon S, Validire P, Briffod M, Lai-Kuen R, Cordelières FP, et al. Newly characterised ex vivo colospheres as a three-dimensional colon cancer cell model of tumour aggressiveness. Br J Cancer (2009) 101:473. doi: 10.1038/sj.bjc.6605173
23. Kaaijk P, Troost D, Das PK, Leenstra S, Bosch DA. Long-term culture of organotypic multicellular glioma spheroids: a good culture model for studying gliomas. Neuropathol Appl Neurobiol (1995) 21:386. doi: 10.1111/j.1365-2990.1995.tb01075.x
24. Ferreira LP, Gaspar VM, Mano JF. Design of spherically structured 3D in vitro tumor models -advances and prospects. Acta Biomater (2018) 75:11. doi: 10.1016/j.actbio.2018.05.034
25. Iwasaki H, Suda T. Cancer stem cells and their niche. Cancer Sci (2009) 100:1166. doi: 10.1111/j.1349-7006.2009.01177.x
26. Bielecka ZF, Maliszewska-Olejniczak K, Safir IJ, Szczylik C, Czarnecka AM. Three-dimensional cell culture model utilization in cancer stem cell research. Biol Rev Camb Philos Soc (2017) 92:1505. doi: 10.1111/brv.12293
27. Pollard SM, Yoshikawa K, Clarke ID, Danovi D, Stricker S, Russell R, et al. Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell (2009) 4:568. doi: 10.1016/j.stem.2009.03.014
28. Hubert CG, Rivera M, Spangler Wu LC Q, Mack SC, Prager BC, et al. A three-dimensional organoid culture system derived from human glioblastomas recapitulates the hypoxic gradients and cancer stem cell heterogeneity of tumors found In vivo. Cancer Res (2016) 76:2465. doi: 10.1158/0008-5472.CAN-15-2402
29. Zhang M, Xu C, Wang HZ, Peng YN, Li HO, Zhou YJ, et al. Soft fibrin matrix downregulates DAB2IP to promote nanog-dependent growth of colon tumor-repopulating cells. Cell Death Dis (2019) 10:151. doi: 10.1038/s41419-019-1309-7
30. Saglam-Metiner P, Gulce-Iz S, Biray-Avci C. Bioengineering-inspired three-dimensional culture systems: Organoids to create tumor microenvironment. Gene (2019) 686:203. doi: 10.1016/j.gene.2018.11.058
31. Castiaux AD, Spence DM, Martin RS. Review of 3D cell culture with analysis in microfluidic systems. Anal Methods (2019) 11:4220. doi: 10.1039/C9AY01328H
32. Langhans SA. Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front Pharmacol (2018) 9:6. doi: 10.3389/fphar.2018.00006
33. Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng (2009) 103:655. doi: 10.1002/bit.22361
34. Sun D, Liu Y, Wang H, Deng F, Zhang Y, Zhao S, et al. Novel decellularized liver matrix-alginate hybrid gel beads for the 3D culture of hepatocellular carcinoma cells. Int J Biol Macromol (2018) 109:1154. doi: 10.1016/j.ijbiomac.2017.11.103
35. De S, Joshi A, Tripathi DM, Kaur S, Singh N. Alginate based 3D micro-scaffolds mimicking tumor architecture as in vitro cell culture platform. Mater Sci Eng C Mater Biol Appl (2021) 128:112344. doi: 10.1016/j.msec.2021.112344
36. Worthington P, Pochan DJ, Langhans SA. Peptide hydrogels - versatile matrices for 3D cell culture in cancer medicine. Front Oncol (2015) 5:92. doi: 10.3389/fonc.2015.00092
37. Calitz C, Pavlović N, Rosenquist J, Zagami C, Samanta A Heindryckx F, et al. A biomimetic model for liver cancer to study tumor-stroma interactions in a 3D environment with tunable bio-physical properties. J Vis Exp (2020) (162):10.3791/61606. doi: 10.3791/61606
38. Yip D, Cho CH. A multicellular 3D heterospheroid model of liver tumor and stromal cells in collagen gel for anti-cancer drug testing. Biochem Biophys Res Commun (2013) 433:327. doi: 10.1016/j.bbrc.2013.03.008
39. Turtoi M, Anghelache M, Bucatariu SM, Deleanu M, Voicu G, Safciuc F, et al. A novel platform for drug testing: Biomimetic three-dimensional hyaluronic acid-based scaffold seeded with human hepatocarcinoma cells. Int J Biol Macromol (2021) 185:604. doi: 10.1016/j.ijbiomac.2021.06.174
40. Pradhan S, Clary JM, Seliktar D, Lipke EA. A three-dimensional spheroidal cancer model based on PEG-fibrinogen hydrogel microspheres. Biomaterials (2017) 115:141. doi: 10.1016/j.biomaterials.2016.10.052
41. Ozawa F, Ino K, Arai T, Ramón-Azcón J, Takahashi Y, Shiku H, et al. Alginate gel microwell arrays using electrodeposition for three-dimensional cell culture. Lab chip (2013) 13:3128. doi: 10.1039/c3lc50455g
42. Liu T, Yi S, Liu G, Hao X, Du T, Chen J, et al. Aqueous two-phase emulsions-templated tailorable porous alginate beads for 3D cell culture. Carbohyd Polym (2021) 258:117702. doi: 10.1016/j.carbpol.2021.117702
43. Worthington P, Drake KM, Li Z, Napper AD, Pochan DJ, Langhans SA, et al. Beta-hairpin hydrogels as scaffolds for high-throughput drug discovery in three-dimensional cell culture. Anal Biochem (2017) 535:25. doi: 10.1016/j.ab.2017.07.024
44. Leong W, Kremer A, Wang DA. Development of size-customized hepatocarcinoma spheroids as a potential drug testing platform using a sacrificial gelatin microsphere system. Mater Sci Eng C Mater Biol Appl (2016) 63:644. doi: 10.1016/j.msec.2016.03.046
45. Antunes J, Gaspar VM, Ferreira L, Monteiro M, Henrique R, Jerónimo C, et al. In-air production of 3D co-culture tumor spheroid hydrogels for expedited drug screening. Acta Biomater (2019) 94:392. doi: 10.1016/j.actbio.2019.06.012
46. Benton G, Arnaoutova I, George J, Kleinman HK, Koblinski J. Matrigel: from discovery and ECM mimicry to assays and models for cancer research. Adv Drug Delivery Rev (2014) 3:79–80. doi: 10.1016/j.addr.2014.06.005
47. Singh M, Close DA, Mukundan S, Johnston PA, Sant S. Production of uniform 3D microtumors in hydrogel microwell arrays for measurement of viability, morphology, and signaling pathway activation. Assay Drug Dev Technol (2015) 13:570. doi: 10.1089/adt.2015.662
48. Ramadhan W, Ohama Y, Minamihata K, Moriyama K, Wakabayashi R, Goto M, et al. Redox-responsive functionalized hydrogel marble for the generation of cellular spheroids. J Biosci BIOENG (2020) 130:416. doi: 10.1016/j.jbiosc.2020.05.010
49. Hu K, Zhou N, Li Y, Ma S, Guo Z, Cao M, et al. Sliced magnetic polyacrylamide hydrogel with cell-adhesive microarray interface: A novel multicellular spheroid culturing platform. ACS Appl Mater Interfaces (2016) 8:15113. doi: 10.1021/acsami.6b04112
50. Heffernan JM, Overstreet DJ, Srinivasan S, Le LD, Vernon BL, Sirianni RW, et al. Temperature responsive hydrogels enable transient three-dimensional tumor cultures via rapid cell recovery. J BioMed Mater Res A (2016) 104:17. doi: 10.1002/jbm.a.35534
51. Chen H, Wei X, Chen H, Wei H, Wang Y, Nan W, et al. The study of establishment of an in vivo tumor model by three-dimensional cells culture systems methods and evaluation of antitumor effect of biotin-conjugated pullulan acetate nanoparticles. Artif Cells Nanomed Biotechnol (2019) 47:123. doi: 10.1080/21691401.2018.1544142
52. Liu H, Liu J, Qi C, Fang Y, Zhang L, Zhuo R, et al. Thermosensitive injectable in-situ forming carboxymethyl chitin hydrogel for three-dimensional cell culture. Acta Biomater (2016) 35:228. doi: 10.1016/j.actbio.2016.02.028
53. Mahadeva SK, Walus K, Stoeber B. Paper as a platform for sensing applications and other devices: a review. ACS Appl Mater Interfaces (2015) 7:8345. doi: 10.1021/acsami.5b00373
54. Ng K, Gao B, Yong K, Li Y, Shi M, Zhao X, et al. Paper-based cell culture platform and its emerging biomedical applications. Mater Today (2017) 20:32. doi: 10.1016/j.mattod.2016.07.001
55. Derda R, Laromaine A, Mammoto A, Tang SK, Mammoto T, Ingber DE, et al. Paper-supported 3D cell culture for tissue-based bioassays. Proc Natl Acad Sci U.S.A. (2009) 106:18457. doi: 10.1073/pnas.0910666106
56. Lantigua D, Kelly YN, Unal B, Camci-Unal G. Engineered paper-based cell culture platforms. Adv Healthc Mater (2017) 6(22):10.1002/adhm.201700619. doi: 10.1002/adhm.201700619
57. Tamayol A, Akbari M, Annabi N, Paul A, Khademhosseini A, Juncker D, et al. Fiber-based tissue engineering: Progress, challenges, and opportunities. Biotechnol Adv (2013) 31:669. doi: 10.1016/j.biotechadv.2012.11.007
58. Shin SR, Bae H, Cha JM, Mun JY, Chen YC, Tekin H, et al. Carbon nanotube reinforced hybrid microgels as scaffold materials for cell encapsulation. ACS Nano (2012) 6:362. doi: 10.1021/nn203711s
59. Liu T, Teng WK, Chan BP, Chew SY. Photochemical crosslinked electrospun collagen nanofibers: synthesis, characterization and neural stem cell interactions. J BioMed Mater Res A (2010) 95:276. doi: 10.1002/jbm.a.32831
60. Xue J, Xie J, Liu W, Xia Y. Electrospun nanofibers: New concepts, materials, and applications. Acc Chem Res (2017) 50:1976. doi: 10.1021/acs.accounts.7b00218
61. Mahmoudzadeh A, Mohammadpour H. Tumor cell culture on collagen-chitosan scaffolds as three-dimensional tumor model: A suitable model for tumor studies. J Food Drug Anal (2016) 24:620. doi: 10.1016/j.jfda.2016.02.008
62. Koh WH, Zayats R, Lopez P, Murooka TT. Visualizing cellular dynamics and protein localization in 3D collagen. Star Protoc (2020) 1:100203. doi: 10.1016/j.xpro.2020.100203
63. Li W, Hu X, Yang S, Wang S, Zhang C, Wang H, et al. A novel tissue-engineered 3D tumor model for anti-cancer drug discovery. Biofabrication (2018) 11:15004. doi: 10.1088/1758-5090/aae270
64. Stabler CT, Lecht S, Mondrinos MJ, Goulart E, Lazarovici P, Lelkes PI, et al. Revascularization of decellularized lung scaffolds: principles and progress. Am J Physiol Lung Cell Mol Physiol (2015) 309:L1273. doi: 10.1152/ajplung.00237.2015
65. Lü WD, Zhang L, Wu CL, Liu ZG, Lei GY, Liu J, et al. Development of an acellular tumor extracellular matrix as a three-dimensional scaffold for tumor engineering. PloS One (2014) 9:e103672. doi: 10.1371/journal.pone.0103672
66. Girard YK, Wang C, Ravi S, Howell MC, Mallela J, Alibrahim M, et al. A 3D fibrous scaffold inducing tumoroids: a platform for anticancer drug development. PloS One (2013) 8:e75345. doi: 10.1371/journal.pone.0075345
67. Fischbach C, Chen R, Matsumoto T, Schmelzle T, Brugge JS, Polverini PJ, et al. Engineering tumors with 3D scaffolds. Nat Methods (2007) 4:855. doi: 10.1038/nmeth1085
68. Mazzini G, Carpignano F, Surdo S, Aredia F, Panini N, Torchio M, et al. 3D silicon microstructures: A new tool for evaluating biological aggressiveness of tumor cells. IEEE Trans Nanobioscience (2015) 14:797. doi: 10.1109/TNB.2015.2476351
69. Murakami S, Tanaka H, Nakayama T, Taniura N, Miyake T, Tani M, et al. Similarities and differences in metabolites of tongue cancer cells among two- and three-dimensional cultures and xenografts. Cancer Sci (2021) 112:918. doi: 10.1111/cas.14749
70. Amaral R, Miranda M, Marcato PD, Swiech K. Comparative analysis of 3D bladder tumor spheroids obtained by forced floating and hanging drop methods for drug screening. Front Physiol (2017) 8:605. doi: 10.3389/fphys.2017.00605
71. Foty R. A simple hanging drop cell culture protocol for generation of 3D spheroids. J Vis Exp (2011) (51):2720. doi: 10.3791/2720-v
72. Khunmanee S, Park H. Three-dimensional culture for In vitro folliculogenesis in the aspect of methods and materials. Tissue Eng Part B Rev (2022) 28:1242. doi: 10.1089/ten.teb.2021.0229
73. Huang SW, Tzeng SC, Chen JK, Sun JS, Lin F-H. A dynamic hanging-drop system for mesenchymal stem cell culture. Int J Mol Sci (2020) 21(12):4298. doi: 10.3390/ijms21124298
74. Ratnayaka SH, Hillburn TE, Forouzan O, Shevkoplyas SS, Khismatullin DB. PDMS well platform for culturing millimeter-size tumor spheroids. Biotechnol Prog (2013) 29:1265. doi: 10.1002/btpr.1764
75. Napolitano AP, Dean DM, Man AJ, Youssef J, Ho DN, Rago AP, et al. Scaffold-free three-dimensional cell culture utilizing micromolded nonadhesive hydrogels. Biotechniques (2007) 43:494, 496. doi: 10.2144/000112591
76. Shao H, Moller M, Wang D, Ting A, Boulina M, Liu ZJ, et al. A novel stromal fibroblast-modulated 3D tumor spheroid model for studying tumor-stroma interaction and drug discovery. J Vis Exp (2020) (156):10.3791/60660. doi: 10.3791/60660
77. Beheshti F, Shabani AA, AkbariEidgahi MR, Kookhaei P, Vazirian M, Safavi M, et al. Anticancer activity of ipomoea purpurea leaves extracts in monolayer and three-dimensional cell culture. Evid-Based Compl Alt (2021) 2021:6666567. doi: 10.1155/2021/6666567
78. Souza GR, Molina JR, Raphael RM, Ozawa MG, Stark DJ, Levin CS, et al. Three-dimensional tissue culture based on magnetic cell levitation. Nat Nanotechnol (2010) 5:291. doi: 10.1038/nnano.2010.23
79. Okochi M, Takano S, Isaji Y, Senga T, Hamaguchi M, Honda H, et al. Three-dimensional cell culture array using magnetic force-based cell patterning for analysis of invasive capacity of BALB/3T3/v-src. Lab Chip (2009) 9:3378. doi: 10.1039/b909304d
80. Ryu NE, Lee SH, Park H. Spheroid culture system methods and applications for mesenchymal stem cells. Cells-Basel (2019) 8(12):1620. doi: 10.3390/cells8121620
81. Meyers VE, Zayzafoon M, Douglas JT, McDonald JM. RhoA and cytoskeletal disruption mediate reduced osteoblastogenesis and enhanced adipogenesis of human mesenchymal stem cells in modeled microgravity. J Bone Miner Res (2005) 20:1858. doi: 10.1359/JBMR.050611
82. Yu B, Yu D, Cao L, Zhao X, Long T, Liu G, et al. Simulated microgravity using a rotary cell culture system promotes chondrogenesis of human adipose-derived mesenchymal stem cells via the p38 MAPK pathway. Biochem Biophys Res Commun (2011) 414:412. doi: 10.1016/j.bbrc.2011.09.103
83. Grimm D, Schulz H, Krüger M, Cortés-Sánchez JL, Egli M, Kraus A, et al. The fight against cancer by microgravity: The multicellular spheroid as a metastasis model. Int J Mol Sci (2022) 23(6):3073. doi: 10.3390/ijms23063073
84. Sleeboom J, Eslami AH, Nair P, Sahlgren CM, den Toonder J. Metastasis in context: modeling the tumor microenvironment with cancer-on-a-chip approaches. Dis Model Mech (2018) 11(3):dmm033100. doi: 10.1242/dmm.033100
85. Zhang YS, Arneri A, Bersini S, Shin SR, Zhu K, Goli-Malekabadi Z, et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. BIOMATERIALS (2016) 110:45. doi: 10.1016/j.biomaterials.2016.09.003
86. Park J, Wu Z, Steiner PR, Zhu B, Zhang J. Heart-on-Chip for combined cellular dynamics measurements and computational modeling towards clinical applications. Ann BioMed Eng (2022) 50:111. doi: 10.1007/s10439-022-02902-7
87. Bernardin AA, Shabani AA, AkbariEidgahi MR, Kookhaei P, Vazirian M, Safavi M, et al. Impact of neurons on patient-derived cardiomyocytes using organ-On-A-Chip and iPSC biotechnologies. Cells-Basel (2022) 11(23):3764. doi: 10.3390/cells11233764
88. Ferrari E, Rasponi M. Liver-heart on chip models for drug safety. APL Bioeng (2021) 5:31505. doi: 10.1063/5.0048986
89. Thacker VV, Dhar N, Sharma K, Barrile R, Karalis K, McKinney JD, et al. A lung-on-chip model of early mycobacterium tuberculosis infection reveals an essential role for alveolar epithelial cells in controlling bacterial growth. Elife (2020) 9:e59961. doi: 10.7554/eLife.59961
90. Baptista D, Moreira TeixeiraL , Barata D, Tahmasebi BirganiZ , King J, vanRiet S, et al. 3D lung-on-Chip model based on biomimetically microcurved culture membranes. ACS Biomater Sci Eng (2022) 8:2684. doi: 10.1021/acsbiomaterials.1c01463
91. Gijzen L, Yousef YFA, Schutgens F, Vormann MK, Ammerlaan CME, Nicolas A, et al. Culture and analysis of kidney tubuloids and perfused tubuloid cells-on-a-chip. Nat Protoc (2021) 16:2023. doi: 10.1038/s41596-020-00479-w
92. Bas-Cristóbal MA, Du Z, vandenBosch TPP, Othman A, Gaio N, Silvestri C. Creating a kidney organoid-vasculature interaction model using a novel organ-on-chip system. Sci Rep (2022) 12:20699. doi: 10.1038/s41598-022-24945-5
93. Aceves JO, Heja S, Kobayashi K, Robinson SS, Miyoshi T, Matsumoto T, et al. 3D proximal tubule-on-chip model derived from kidney organoids with improved drug uptake. Sci Rep (2022) 12:14997. doi: 10.1038/s41598-022-19293-3
94. Skardal A, Murphy SV, Devarasetty M, Mead I, Kang HW, Seol YJ, et al. Multi-tissue interactions in an integrated three-tissue organ-on-a-chip platform. Sci Rep (2017) 7:8837. doi: 10.1038/s41598-017-08879-x
95. Bovard D, Sandoz A, Luettich K, Frentzel S, Iskandar A, Marescotti D, et al. A lung/liver-on-a-chip platform for acute and chronic toxicity studies. Lab Chip (2018) 18:3814. doi: 10.1039/C8LC01029C
96. Ronaldson-Bouchard K, Teles D, Yeager K, Tavakol DN, Zhao Y, Chramiec A, et al. A multi-organ chip with matured tissue niches linked by vascular flow. Nat BioMed Eng (2022) 6:351. doi: 10.1038/s41551-022-00882-6
97. Wu Q, Liu J, Wang X, Feng L, Wu J, Zhu X, et al. Organ-on-a-chip: recent breakthroughs and future prospects. BioMed Eng Online (2020) 19:9. doi: 10.1186/s12938-020-0752-0
98. Ying L, Wang Q. Microfluidic chip-based technologies: emerging platforms for cancer diagnosis. BMC Biotechnol (2013) 13:76. doi: 10.1186/1472-6750-13-76
99. Lin Z, Luo G, DuW , Kong T, Liu C, Liu Z, et al. Recent advances in microfluidic platforms applied in cancer metastasis: Circulating tumor cells' (CTCs) isolation and tumor-On-A-Chip. Small (2020) 16:e1903899. doi: 10.1002/smll.201903899
100. Fetah KL, DiPardo BJ, Kongadzem EM, Tomlinson JS, Elzagheid A, Elmusrati M, et al. Cancer modeling-on-a-Chip with future artificial intelligence integration. Small (2019) 15:e1901985. doi: 10.1002/smll.201901985
101. Regmi S, Poudel C, Adhikari R, Luo KQ. Applications of microfluidics and organ-on-a-Chip in cancer research. Biosensors (Basel) (2022) 12(7):459. doi: 10.3390/bios12070459
102. Cho HY, Choi JH, Lim J, Lee SN, Choi JW. Microfluidic chip-based cancer diagnosis and prediction of relapse by detecting circulating tumor cells and circulating cancer stem cells. Cancers (Basel) (2021) 13(6):1385. doi: 10.3390/cancers13061385
103. Sontheimer-Phelps A, Hassell BA, Ingber DE. Modelling cancer in microfluidic human organs-on-chips. Nat Rev Cancer (2019) 19:65. doi: 10.1038/s41568-018-0104-6
104. Komen J, van Neerven SM, van den Berg A, Vermeulen L, van der Meer AD. Mimicking and surpassing the xenograft model with cancer-on-chip technology. Ebiomedicine (2021) 66:103303. doi: 10.1016/j.ebiom.2021.103303
105. Yeo LY, Chang HC, Chan PP, Friend JR. Microfluidic devices for bioapplications. SMALL (2011) 7:12. doi: 10.1002/smll.201000946
106. Toh YC, Raja A, Yu H, van Noort D. A 3D microfluidic model to recapitulate cancer cell migration and invasion. Bioengineering (Basel) (2018) 5(2):29. doi: 10.3390/bioengineering5020029
107. Gottwald E, Lahni B, Thiele D, Giselbrecht S, Welle A, Weibezahn KF, et al. Chip-based three-dimensional cell culture in perfused micro-bioreactors. J Vis Exp (2008) (15):564. doi: 10.3791/564-v
108. Liu W, Tian C, Yan M, Zhao L, Ma C, Li T, et al. Heterotypic 3D tumor culture in a reusable platform using pneumatic microfluidics. Lab Chip (2016) 16:4106. doi: 10.1039/C6LC00996D
109. Yan X, Zhou L, Wu Z, Wang X, Chen X, Yang F, et al. High throughput scaffold-based 3D micro-tumor array for efficient drug screening and chemosensitivity testing. Biomaterials (2019) 198:167. doi: 10.1016/j.biomaterials.2018.05.020
110. Chen B, Wu Y, Ao Z, Cai H, Nunez A, Liu Y, et al. High-throughput acoustofluidic fabrication of tumor spheroids. Lab Chip (2019) 19:1755. doi: 10.1039/C9LC00135B
111. Ziółkowska K, Stelmachowska A, Kwapiszewski R, Chudy M, Dybko A, Brzózka Z, et al. Long-term three-dimensional cell culture and anticancer drug activity evaluation in a microfluidic chip. Biosens Bioelectron (2013) 40:68. doi: 10.1016/j.bios.2012.06.017
112. Agarwal P, Wang H, Sun M, Xu J, Zhao S, Liu Z, et al. Microfluidics enabled bottom-up engineering of 3D vascularized tumor for drug discovery. ACS Nano (2017) 11:6691. doi: 10.1021/acsnano.7b00824
113. Hwang HJ, Oh MS, Lee DW, Kuh HJ. Multiplex quantitative analysis of stroma-mediated cancer cell invasion, matrix remodeling, and drug response in a 3D co-culture model of pancreatic tumor spheroids and stellate cells. J Exp Clin Cancer Res (2019) 38:258. doi: 10.1186/s13046-019-1225-9
114. Mandrycky C, Wang Z, Kim K, Kim DH. 3D bioprinting for engineering complex tissues. Biotechnol Adv (2016) 34:422. doi: 10.1016/j.biotechadv.2015.12.011
115. Tiwari AP, Thorat ND, Pricl S, Patil RM, Rohiwal S, Townley H, et al. Bioink: a 3D-bioprinting tool for anticancer drug discovery and cancer management. Drug Discovery Today (2021) 26:1574. doi: 10.1016/j.drudis.2021.03.010
116. Xing JL, Wang YX, Du SD. Application and research progress of in vitro liver cancer cell culture models. World Chin J Digestol (2021) 29:563. doi: 10.11569/wcjd.v29.i11.563
117. Jiang T, Munguia-Lopez J, Flores-Torres S, Grant J, Vijayakumar S, DeLeon-Rodriguez A, et al. Bioprintable Alginate/Gelatin hydrogel 3D In vitro model systems induce cell spheroid formation. J Vis Exp (2018) (137):57826. doi: 10.3791/57826-v
118. Chen AM, Zhang W, Smith LJ, Walsh J, Sterner J, Gramelspacher E, et al. Hepatocellular carcinoma 3D bioprinted model with 7 days of sorafenib perfusion: Modeling the cancer microenvironment's chemotherapy response. Transplantation (2021) 105:14. doi: 10.1097/01.tp.0000789500.50801.c7
119. Bhattacharjee T, Gil CJ, Marshall SL, Urueña JM. Liquid-like solids support cells in 3D. ACS Biomater Sci Eng (2016) 2:1787. doi: 10.1021/acsbiomaterials.6b00218
120. Altmann B, Welle A, Giselbrecht S, Truckenmüller R, Gottwald E. The famous versus the inconvenient - or the dawn and the rise of 3D-culture systems. World J Stem Cells (2009) 1:43. doi: 10.4252/wjsc.v1.i1.43
121. Cianciosi D, Ansary J, Forbes-Hernandez TY, Regolo L, Quinzi D, Gracia Villar S, et al. The molecular basis of different approaches for the study of cancer stem cells and the advantages and disadvantages of a three-dimensional culture. MOLECULES (2021) 26(9):2615. doi: 10.3390/molecules26092615
Keywords: tumor microenvironment, tumor cells, the three dimensional, cell culture, the two dimensional
Citation: Zhang L, Liao W, Chen S, Chen Y, Cheng P, Lu X and Ma Y (2023) Towards a New 3Rs Era in the construction of 3D cell culture models simulating tumor microenvironment. Front. Oncol. 13:1146477. doi: 10.3389/fonc.2023.1146477
Received: 17 January 2023; Accepted: 22 March 2023;
Published: 03 April 2023.
Edited by:
Christopher R. Cederroth, Swiss 3R Competence Centre, SwitzerlandReviewed by:
Farhan Chowdhury, Southern Illinois University Carbondale, United StatesFrancesca Della Sala, Institute of Polymers, National Research Council (CNR), Italy
Copyright © 2023 Zhang, Liao, Chen, Chen, Cheng, Lu and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinjun Lu, bHV4ajMzQG1haWwuc3lzdS5lZHUuY24=; Yi Ma, YW5odWltYXlpMjAwMkAxNjMuY29t
 Long Zhang
Long Zhang Weiqi Liao2
Weiqi Liao2 Yi Ma
Yi Ma