- 1Department of Radiotherapy, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 2Department of Chemotherapy, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 3Department of Oncology, The Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 4Department of Radiotherapy, Radiotherapy Clinical Medical Research Center of Shaanxi Province, Xi’an, China
Introduction: The occurrence of metastasis is a threat to patients with colon cancer (CC), and the liver is the most common metastasis organ. However, the role of the extrahepatic organs in patients with liver metastasis (LM) has not been distinctly demonstrated. Therefore, this research aimed to explore the prognostic value of extrahepatic metastases (EHMs).
Methods: In this retrospective study, a total of 13,662 colon patients with LM between 2010 and 2015 were selected from the Surveillance, Epidemiology, and End Results database (SEER). Fine and Gray’s analysis and K–M survival analysis were utilized to explore the impacts of the number of sites of EHMs and different sites of EHMs on prognosis. Finally, a prognostic nomogram model based on the number of sites of EHMs was constructed, and a string of validation methods was conducted, including concordance index (C-index), receiver operating characteristic curves (ROC), and decision curve analysis (DCA).
Results: Patients without EHMs had better prognoses in cancer-specific survival (CSS) and overall survival (OS) than patients with EHMs (p < 0.001). Varied EHM sites of patients had different characteristics of primary location site, grade, and histology. Cumulative incidence rates for CSS surpassed that for other causes in patients with 0, 1, 2, ≥ 3 EHMs, and the patients with more numbers of sites of EHMs revealed worse prognosis in CSS (p < 0.001). However, patients with different EHM sites had a minor difference in cumulative incidence rates for CSS (p = 0.106). Finally, a nomogram was constructed to predict the survival probability of patients with EHMs, which is based on the number of sites of EHMs and has been proven an excellent predictive ability.
Conclusion: The number of sites of EHMs was a significant prognostic factor of CC patients with LM. However, the sites of EHMs showed limited impact on survival. Furthermore, a nomogram based on the number of sites of EHMs was constructed to predict the OS of patients with EHMs accurately.
Introduction
Colorectal cancer (CRC) leads to a vast health program in the world, with an incidence of 10.2% and a mortality of 9.2% all over the world in 2018, which is the third most common cancer and the second leading cause of cancer-related mortality (1). Moreover, the incidence and deaths are increasing for both males and females in most countries, especially in developing countries (2). CRC can be divided into colon cancer (CC), and rectum cancer. CC, the most common of these subtypes, was responsible for 59.46% of new cases and 61.68% of fatalities globally in 2020. Additionally, among all malignancies, CC alone ranks seventh in terms of new cases and fatalities (2, 3).
The occurrence of metastasis is a threat for CRC patients and may be fatal (4). At the time of first diagnosis, 20% of CRC patients had metastases, and this percentage has remained constant over the past two decades (5). The prognosis for CRC patients with distant metastases is substantially worse, and the 5-year survival rates for CRC patients with or without localized tumors are 91 and 14%, respectively (6). The liver is the most common organ for distant metastasis of CRC with a relatively poor prognosis (7). Population-based research reported that 24.7% of patients with CRC developed liver metastasis (LM) during the disease. About 71% of patients with LM were found at first diagnosis (8). The 5-year survival rate is lower than 5% for these patients with only palliative intent (9). However, in patients who have received successful radical resection of LM, a 5-year survival rate can achieve to 30–57% (10–12).
Great effort has been made to predict how CRC patients with LM would do. AJCC stage system is a reliable criterion for evaluating patients’ prognoses. However, it is unsuitable for patients with distant metastasis (Stage IV), especially patients with more than one metastasis organ. Some population-based research constructed a prognostic model for CRC with distant metastasis (13, 14). Nevertheless, the number of sites of extrahepatic metastases (EHMs) was not involved in these prognostic analyses, which may be an efficient indicator for the survival of patients with LM. To our best research, the role of the number of extrahepatic organs in LM has not been clearly demonstrated. Additionally, a certain extrahepatic organ metastasis may result in a particular prognosis. It may be efficient to improve the prognostic value of these models when considering the information on the number of sites of EHMs and metastatic sites.
Thus, in this research, we mainly investigated the prognostic values of the number of sites of EHMs and explored the prognostic features among different EHM sites. Moreover, in order to estimate survival time, we also created a unique risk framework, and the nomogram was validated in a validation cohort.
Methods
Data acquisition and eligibility criteria
This was a retrospective research employing information from the Surveillance, Epidemiology, and End Results (SEER) database (https://seer.cancer.gov/data/), which is a public cancer statistic database founded by the National Cancer Institute (NCI), providing a string of clinical and pathological data including treatment, metastasis, and survival time of many tumors. In this present study, data from patients were extracted by utilizing SEER*Stat (version 8.4.0). The requirement of the Declaration of Helsinki was honored in this research.
In this study, individuals with LM of the colon who were diagnosed between 2010 and 2015 were included after meeting certain inclusion and exclusion criteria. The inclusion criteria were as follows: (a) malignancies that originated in colon without rectum, (b) patients with confirmed pathological diagnosis (biopsy or surgical samples), and (c) patients with confirmed LM from CC. Exclusion criteria were as follows: (a) patients with unknown metastasis information, (b) evidence of other coexisting malignancies, and (c) patients without complete records for American Joint Committee on Cancer (AJCC)-TNM staging, treatment, overall survival (OS), and cancer-specific survival (CSS).
Variable extraction and cohort identification
Clinical variables were extracted from this dataset, including demographic features, location site of the primary lesion, grade, T stage, N stage, M stage, detailed information about metastasis, histology, anticancer treatment (surgery, radiotherapy, and chemotherapy), survival status, and survival time (CSS and OS). The location of the primary lesion was further divided into the right-sided colon and left-sided colon. The histology was classified into four subtypes: adenocarcinoma, mucinous adenocarcinoma, signet ring cell carcinoma, and other. Furthermore, T stage fell into two categories: “T1-2” and “T3-4.” In this research, all eligible patients were randomly divided into a training cohort and a validation cohort according to a ratio of 7:3 before constructing our nomogram.
Statistical analysis
All categorical variables were summarized as count and percentage, and Chi-square test and Fisher’s exact test were utilized for categorized data. The clinical and demographic features of eligible patients with involvement of 0, 1, 2, or 3 extrahepatic organs were compiled using descriptive statistics. Kaplan–Meier analysis was performed by utilizing the “survival” and “survmine” packages in R software, and a Log-Rank test was used to assess how the curves differed from one another. By using the R software’s “Survival” package, univariate and multivariate regression analyses were used to pinpoint OS and CSS prognostic risk variables. Using the “cmprsk” package in R, the Fine and Gray’s model was adjusted to forecast the cumulative incidence function (CIF) of mortality from CSS and other causes. The “rms” R program was used to create the nomogram for our model. Moreover, a string of validation measurements, including concordance index (Cindex), receiver operating characteristic curves (ROC), and calibration curves, were conducted to evaluate the discrimination ability and calibration ability of our nomogram by utilizing “riskRegression,” “timeROC,” “pec,” “cmprsk,” and “survival” R packages. In this study, R software (version 4.1.2) was adopted for all statistical analyses. P value < 0.05 was considered statistically significant, and all statistical tests were two-sided.
Results
Clinical characteristics of eligible patients
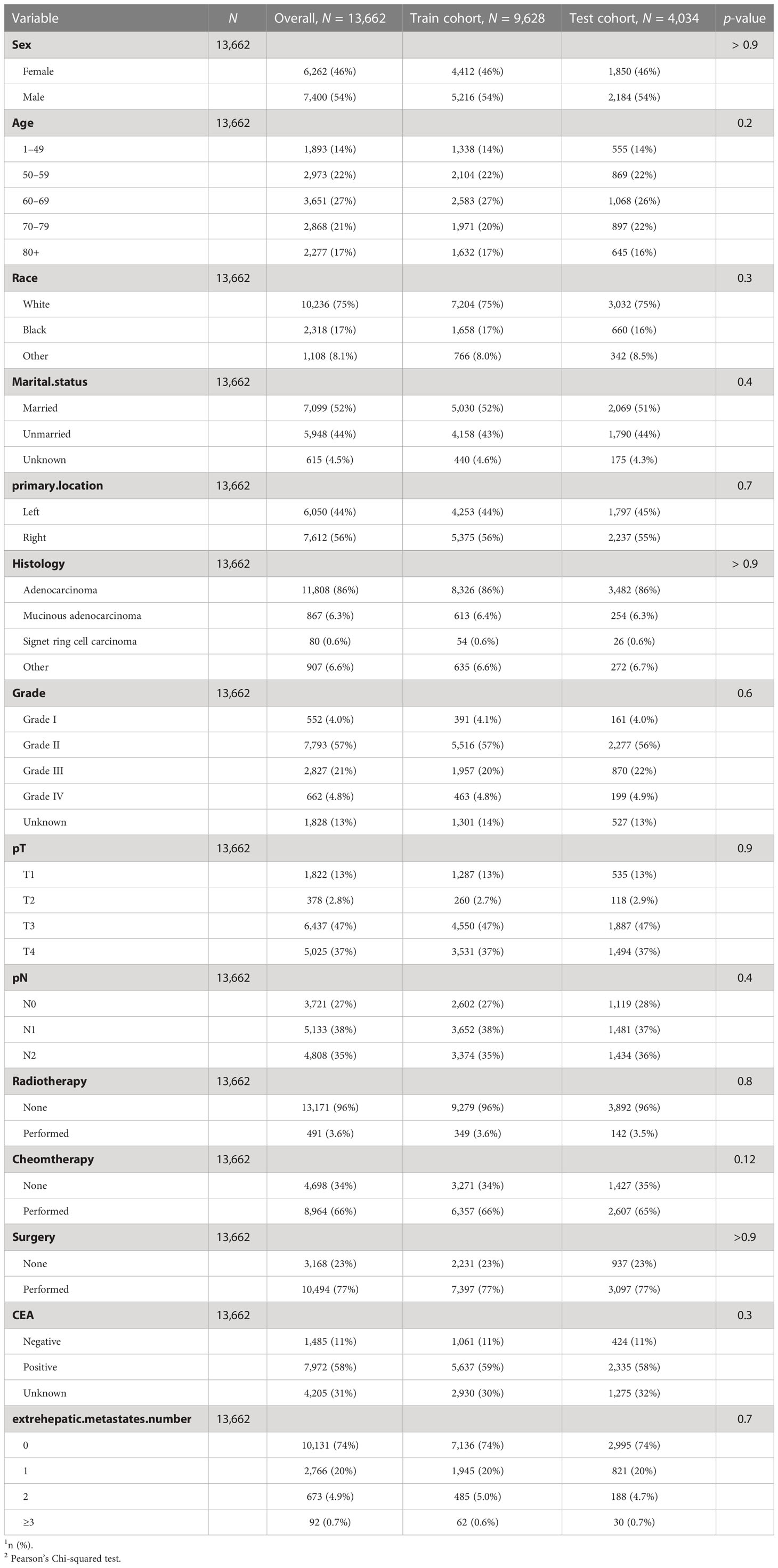
A total of 13,662 CC patients with LM were enrolled in our research, among whom 6,262 were women and 7,400 were men. For the distribution of the number of EHM in our overall cohort, 10,131 (74%) patients had zero involved extrahepatic organs; 2,766 (20%) patients had one involved extrahepatic organ; 673 (4.9%) patients had two involved extrahepatic organs, and 92 (0.7%) patients had three or more than three involved extrahepatic organs. Detailed information about other clinical features of the overall cohort was summarized in Table 1.
In our research, we also divided all patients into train-cohort and test-cohort randomly according to a ratio of 7:3 for our prognostic model’s construction and validation subsequently. Moreover, we found no significant statistical difference among clinicopathological characteristics between these two cohorts via the Chi-square test (Table 1).
The correlation between clinicopathological characteristics and the number of extrahepatic metastasis sites
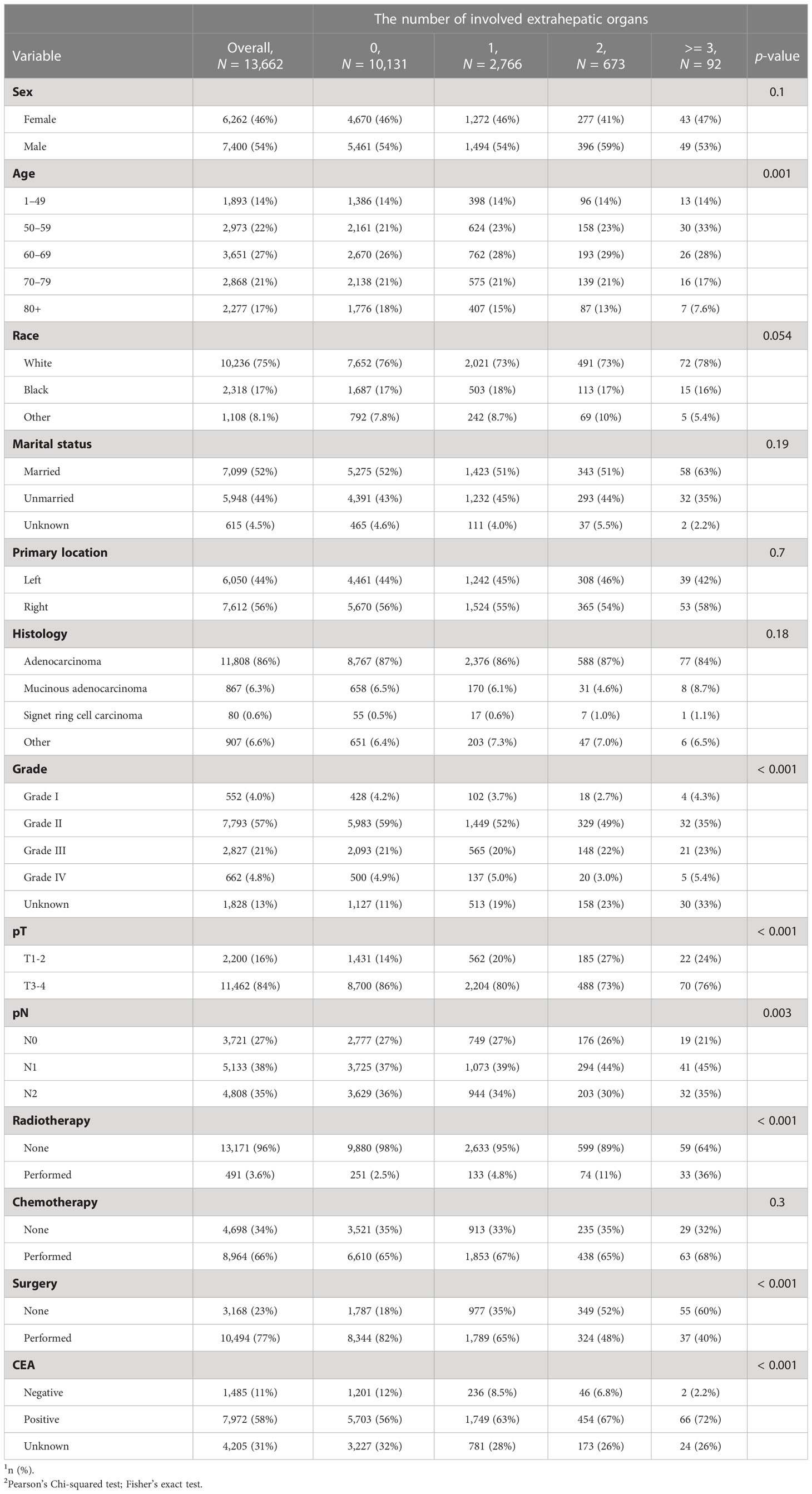
As shown in Table 2, CC patients with different numbers of EHMs presented a significant difference in some clinicopathological features, including age, grade, T stage, N stage, CEA, radiotherapy, and surgery (all p < 0.05). However, other characteristics did not present an obvious association with the number of EHM. It is worth noting that patients with a larger number of EHMs were more likely to have a higher rate of N1 stage (the percentages of patients with N1 stage in patients with none EHM site vs. patients with 1 EHM site vs. patients with 2 EHM sites vs. patients with ≥3 EHM sites: 37% vs. 39% vs. 44% vs. 45%, p < 0.001), nevertheless, a lower rate of N0 stage (the percentages of patients with N0 stage in patients with none EHM site vs. patients with 1 EHM site vs. patients with 2 EHM sites vs. patients with ≥ 3 EHM sites: 27% vs. 27% vs. 26% vs. 21%, p < 0.001). Meanwhile, we also found an interesting phenomenon that patients with a more significant number of EHM sites were more likely to present a positive result of CEA (the percentages of patients with positive CEA in patients with none EHM site vs. patients with 1 EHM site vs. patients with 2 EHM sites vs. patients with ≥ 3 EHM sites: 56% vs. 63% vs. 67% vs. 72%, p < 0.001); however, the opposite result was presented in CEA negative (12% vs. 8.5% vs. 6.8% vs. 2.2%, p < 0.001). For the therapy aspect, patients with a smaller number of EHMs were more likely to accept surgery and radiotherapy. Nevertheless, there were no differences in the amount of ECM across chemotherapy patients.
Extrahepatic metastasis sites
In our cohort, the most and least frequent EHM sites were the lung (57.54%) and brain (2.31%), respectively. Moreover, the frequent EHM sites of distant lymph nodes (dLNs) and bone were 11.6 and 35.46%, respectively. Then, we selected patients with only one EHM site to explore whether different EHM sites presented various patterns of some clinical features, including sex, primary location site, grade, and histology. As Figure 1 shown, patients with various EHM sites presented different distributed characteristics significantly for primary location site, grade, and histology. We easily found that the primary location on the right accounted for the highest rate (88%) of brain metastasis, and the rate was higher than other metastasis sites. Patients with lung metastasis had the highest rate of adenocarcinoma (90%). Nevertheless, patients with bone metastasis had the lowest (75%).
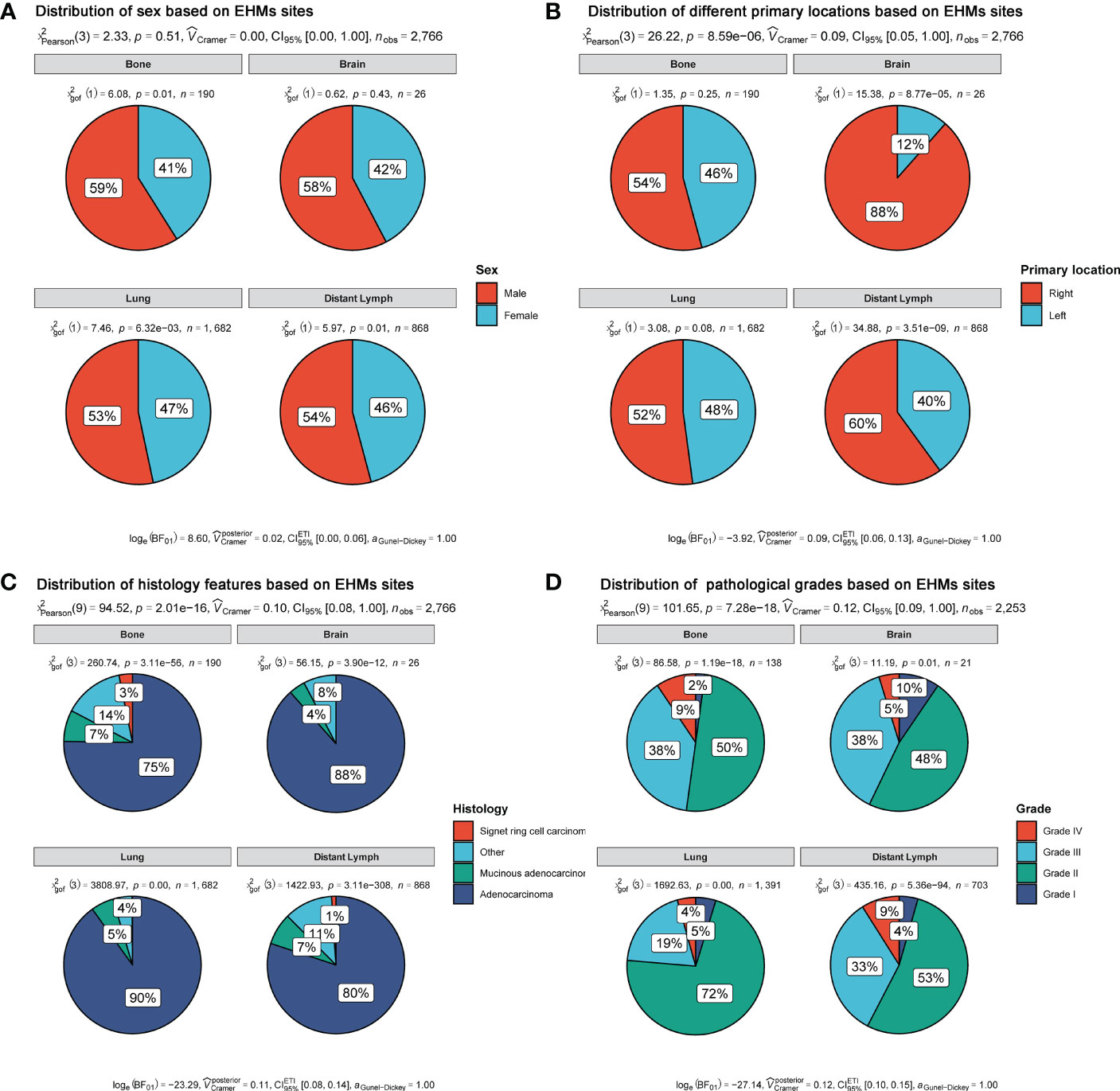
Figure 1 Distribution of clinical features based on EHM sites. (A) Sex feature. (B) Primary location. (C) Histology feature. (D) Pathological grades.
Moreover, it is notable that patients of Grade II accounted for the most prominent rate in all different metastases sites, rather than patients of Grade IV or III. Meanwhile, patients with lung metastasis had different frequencies for grade compared with patients with others metastasis. Grade III+IV accounted for only 23% of patients with lung metastasis. However, the rates of grade III+IV were 47, 43, and 42% in patients with metastases of bone, brain, and dLNs, respectively.
The prognostics value of the number of sites of extrahepatic metastases
We first explored whether the presence of EHMs had an impact on prognosis. As shown in Figures 2A, B, patients without EHMs had better prognosis both in CSS and OS than patients with others who had EHMs [median CSS: 20 months (without EHMs) vs. 11 months (with EHMs), p < 0.001; median OS: 18 months (without EHMs) vs. 11 months (with EHMs), p < 0.001].
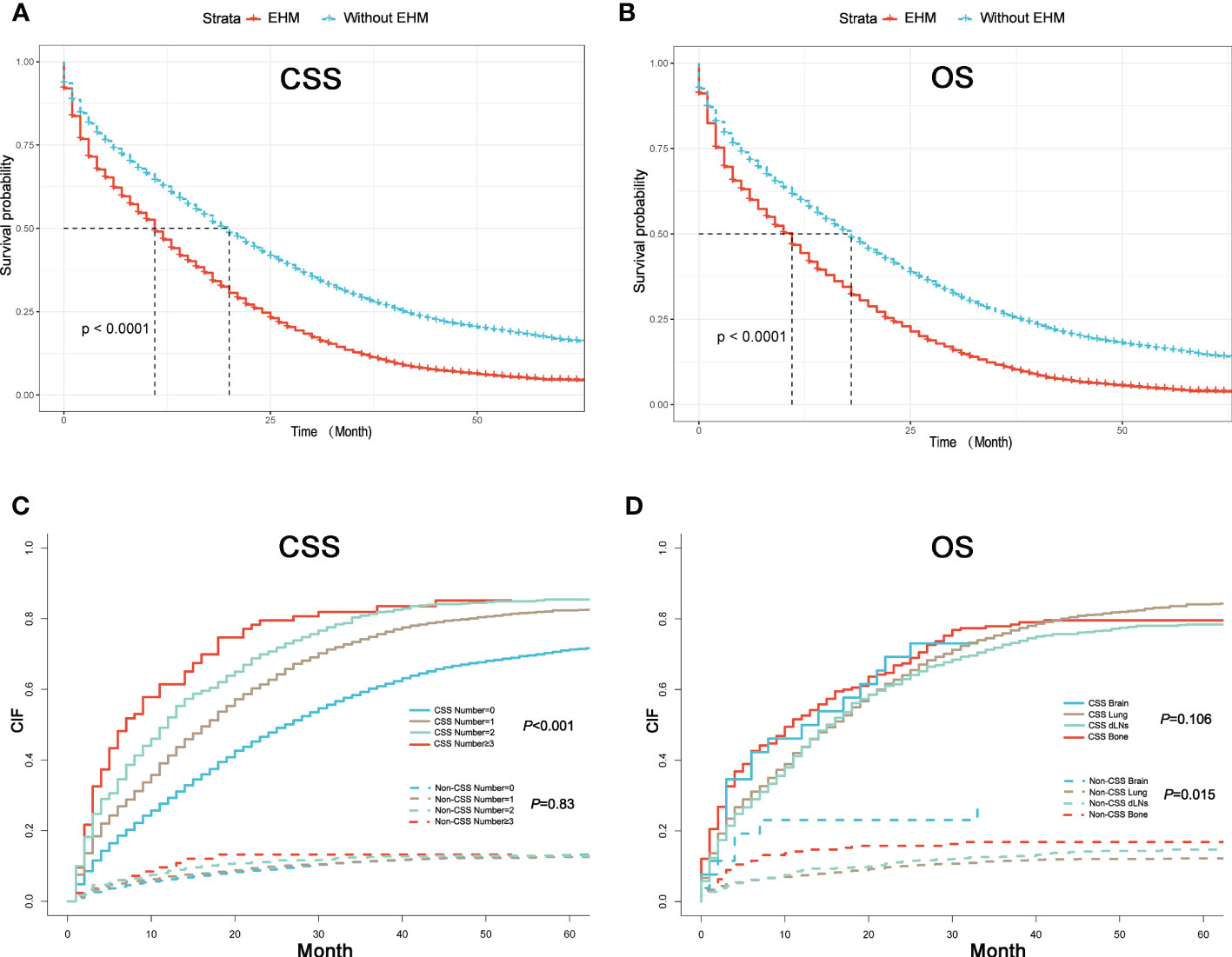
Figure 2 (A) K–M curves of CSS for patients with or without extrahepatic metastases (EHMs). (B) K–M curves of OS for patients with or without EHMs. (C) Cumulative incidence function curves of CSS and non-CSS cause according to the number of sites of EHMs. (D) Cumulative incidence function curves of CSS and non-CSS cause according to different sites of EHMs.
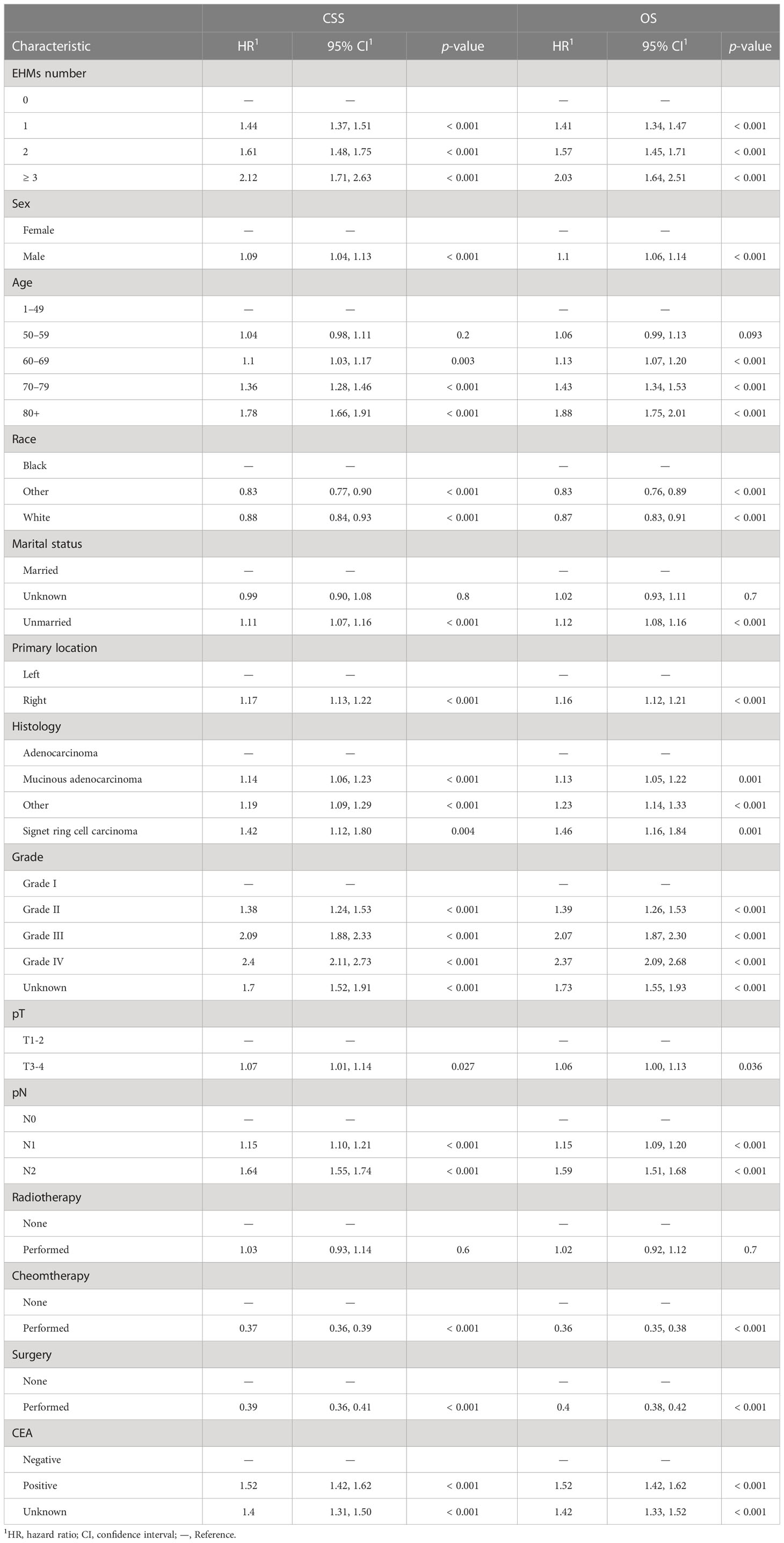
The cumulative incidence rates for CSS outnumbered those for other causes in patients with 0, 1, 2, or ≥ 3 EHMs, and cumulative incidence rates for CSS were increased gradually with time since initial (Figure 2C). In addition, the patients with a larger number of EHMs revealed worse prognosis in CSS (p < 0.001); however, it is not different in other causes (p = 0.83). Moreover, we found that the CSS and OS were correlated with the number of sites of EHMs significantly [median CSS: 20 months vs. 12 months vs. 8 months vs. 5 months (EHMs number: 0 vs. 1 vs. 2 vs. ≥3), p < 0.001; median CSS: 18 months vs. 11 months vs. 8 months vs. 5 months (EHMs number: 0 vs. 1 vs. 2 vs. ≥3)] by utilizing K-M analysis for survival rates (Figures 3A, B). Univariate cox regression analysis was also conducted to explore the prognostic value of the number EHMs. As shown in Figures 4A, B, patients with a larger number of EHMs presented a higher hazard ratio (HR) for CSS and OS [CSS-HR: 1.6(1 vs. 0), 2.1(2 vs. 0), 2.8 (≥3 vs. 0); OS-HR: 1.5(1 vs. 0), 2.0(2 vs. 0), 2.6 (≥ 3 vs. 0), all p < 0.001]. In addition, multivariate cox regression analysis showed a consistent result after adjustment for age, race, gender, histology, marital status, grade, CEA, and stage N and T, and treatment [CSS-HR: 1.44(1 vs. 0), 1.61(2 vs. 0), 2.12 (≥ 3 vs. 0); OS-HR: 1.41(1 vs. 0), 1.57(2 vs. 0), 2.03 (≥3 vs. 0), all p < 0.001] (Table 3).

Figure 3 (A) K–M curves of CSS for patients with different numbers of extrahepatic metastases (EHMs). (B) K–M curves of OS for patients with different numbers of EHMs. (C) K–M curves of CSS for patients with different sites of EHMs. (D) K–M curves of OS for patients with different sites of EHMs.
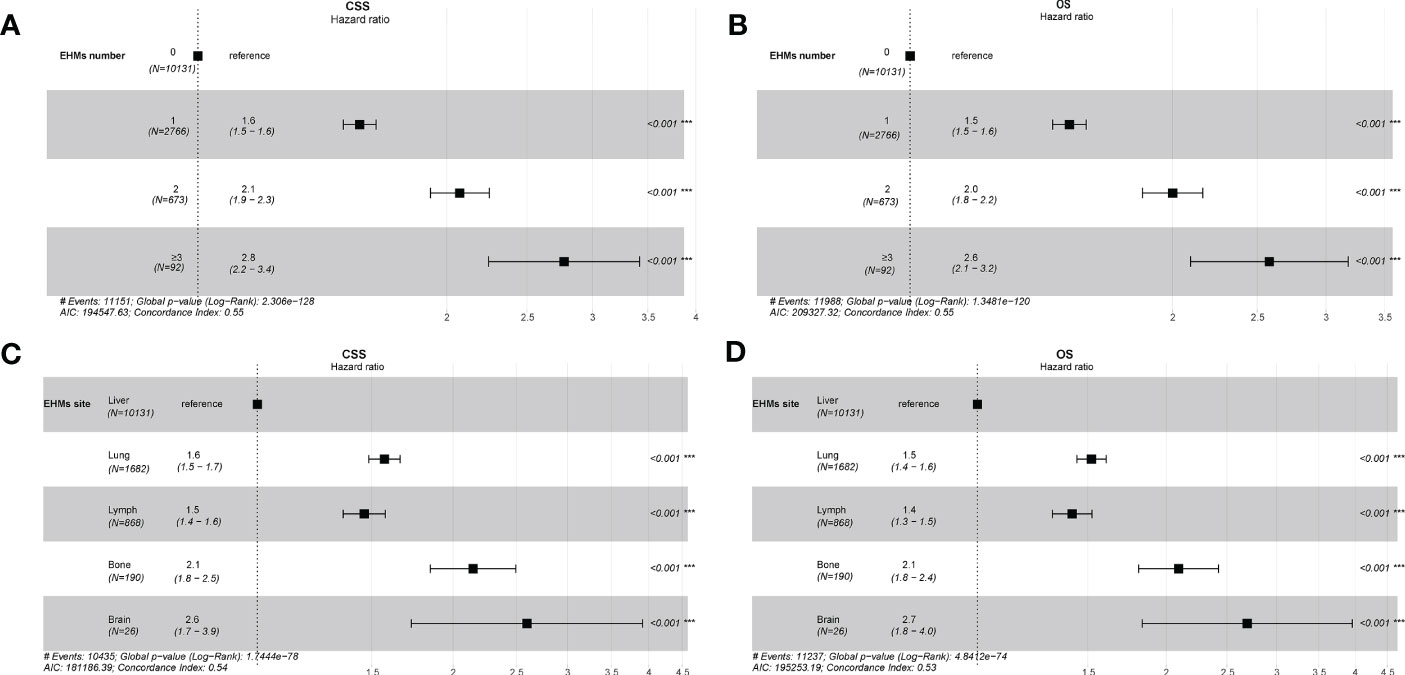
Figure 4 (A) Univariate cox regression analysis of CSS for different numbers of extrahepatic metastases (EHMs). (B) Univariate cox regression analysis of overall survival (OS) for different numbers of EHMs. (C) Univariate cox regression analysis of CSS for different sites of EHMs. (D) Univariate cox regression analysis of OS for different sites of EHMs.
The prognostic difference among variant extrahepatic metastasis sites
To further understand whether different EHM sites impacted survival time, we also compared CSS and OS in patients with bong, lung, dLNs, and brain as the only EHMs organ. As Figure 2B shown, patients with different EHM sites had a more negligible difference in cumulative incidence rates for CSS (p = 0.106). However, patients with brain metastasis presented the highest cumulative incidence rate for other causes (p = 0.015). In addition, as shown in Figures 3C, D, we found that brain metastasis and bone metastasis brought worse prognosis than lung metastasis and dLNs [CSS: 6 months vs. 13 months vs. 12 months (bone vs. brain vs. lung vs. dLNs), p > 0.05(bone vs. brain; lung vs. dLNs) other p < 0.05; OS: 5.5 months vs. 4 vs. 12 months vs. 12 months (bone vs. brain vs. lung vs. dLNs), p > 0.05(Bone vs. brain; lung vs. dLNs) other p < 0.05]. Moreover, Univariate cox regression analysis showed that EHMs presented a higher hazard ratio (HR) for CSS and OS (Figures 4C, D), and HRs of bone and brain were more severe that lung and dLNs (p < 0.001), which was consisted with our other results above.
Construction and validation of nomogram for patients with extrahepatic metastases
Finally, we constructed a nomogram to predict the survival probability of patients with EHMs (Figure 5A). EHMs number, age, primary location, histology, grade, CEA, stage N, and treatments were involved in this nomogram, which were employed to predict the total point of each patient, thus predicting the 6-, 12-, 18-, 24-, and 36-month OS probability of these patients. In addition, we calculated the C-index of this nomogram to estimate its predictive power, suggesting the model had excellent performance in predicting the OS of CC patients with EHMs (training cohort: 0.738; validation cohort: 0.739). Moreover, ROC curves and calibration curves were generated of our nomogram. As shown in Figure 5B, the area under the curve (AUC) of our nomogram were 0.863, 0.833, 0.815, 0.8, and 0.784 in predicting 6-, 12-, 18-, 24-, and 36- month OS, respectively, of patients in training cohort. Moreover, the AUCs of validation cohort were 0.862, 0.833, 0.817, 0.807, and 0.792, respectively (Figure 5C). Additionally, we displayed the calibration curves, which showed excellent concordance between the actual and projected OS in the training cohort and validation cohort (Figures 5D, E). All of these findings showed how well our nomogram predicted the likelihood that patients with EHMs would survive.
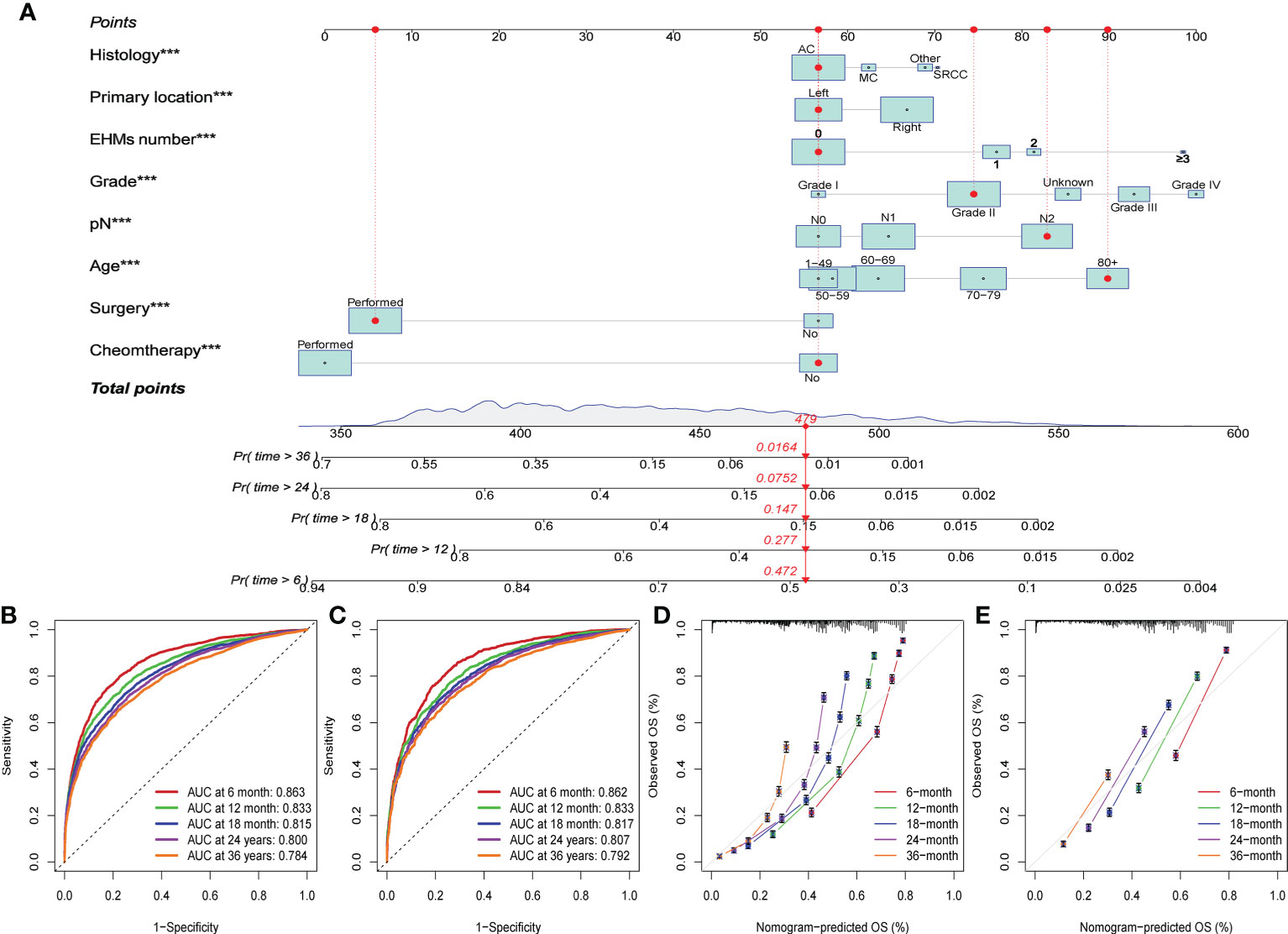
Figure 5 (A) The constructed nomogram for predicting 6-, 12-, 18-, 24-, and 36-month overall survival (OS) of patients with EHMs. ***: p≤0.001. (B) The ROC curves for predicting 6-, 12-, 18-, 24-, and 36-month OS in the training cohort. (C) The ROC curves for predicting 6-, 12-, 18-, 24-, and 36-month OS in the validation cohort. (D) The calibration curves for predicting 6-, 12-, 18-, 24-, and 36-month OS in the training cohort. (E) The calibration curves for predicting 6-, 12-, 18-, 24-, and 36-month OS in the validation cohort.
Discussion
The liver is the most common distant metastatic organ in patients with CC and threaten patients’ survival time (15–17). Our results also revelated that CC patients with LM presented high cancer mortality. Some clinicopathological features affect patients’ prognosis, including TNM stage, age, TP53, KRAS, MSI, and so on (18–21). However, the way of EHMs impacts the prognosis of CC is still unclearly.
We found that the number of sites of EHMs was a significant prognostic factor for CC patients with LM. In our research, the patient with no EHMs had a better prognosis, while a patient with 1, 2, ≥ 3 EHMs presented a worse survival. It might result from that patient with EHMs would not receive liver resection. A study of 1,600 patients demonstrated that 5-year survival rates of patients who received liver resection could achieve above 50%, compared with only 5% for patients treated with palliative (10). Moreover, the survival time decreased as the number of EHMs increased, which may be attributed to the limited effectiveness of systemic treatment for patients with multiple metastases. A similar result was obtained by other research. Wang et al. found that more numbers of sites of EHMs were associated with poor prognosis in NSCLC patients with brain metastasis (22). Meanwhile, the results of cox regression analysis also implied that the number of sites of EHMs caused different hazard ratios significantly, which implied that it might be a suitable prognostic indicator for patients with EHMs.
Moreover, we also explored the impact of sites of EHMs on survival. In our research, patients with different EHM sites had a smaller difference in cumulative incidence rates for CSS, which indicted that the site of EHMs could not be a suitable index for prediction of survival time. Also, patient with brain metastasis and bone metastasis tended to present a worse prognosis than with lung metastasis and dLNs, which was consistent with other research. Tokore had reported that the median survival time of CC patient with brain metastasis was only 2.8 months (23). Moreover, the median survival time of bone metastasis was similar to brain metastasis, which was 5–7 months (24). Compared with the worse prognosis of brain and bone metastasis, the median survival time of lung and dLNs were 10 months and 8 months, respectively (4, 25). However, there were no difference in survival time between brain metastasis and bone metastasis, which was revelated again that EHM site was not a suitable prognostic indicator for patients with EHMs.
In this present research, the lung was the most common EHM site, and the brain was the least common site, which implied that it is essential to consider lung metastasis when patients with CC have LM. We also found an interesting result that the primary location of the right tended to have EHMs than of the left, and it accounted for the highest rate (88%) of brain metastasis, which was higher than other metastasis sites. The difference might be induced by the different features between right-sided CC and left-side CC, including molecular, embryological, biological, and anatomical characteristics (26). Research also demonstrated that the sidedness of CC not only has an essential role in the metastatic setting but also is a predictive marker of response to anti-EGFR drugs (26, 27). Previous studies also found that right-sided CC has a more advanced stage at diagnosis (7, 28). In addition, Price et al. speculated that it was induced by the delay in diagnosis for right-sided cancer, which could lead to more metastasis, resulting in worse survival in patients with right-sided cancer (29). Moreover, it is notable that patients with lung metastasis had different frequencies for grade compared with patients with others metastasis. Grade III+IV accounted for only 23% of patients with lung metastasis. However, the rates of grade III+IV were 47, 43, and 42% in patients with metastases of bone, brain, and dLNs, respectively. This result indicated that the occurrence of lung metastasis for patients with LM might not be associated with the level of pathological grade. Li et al. also found that CC patients with lung metastasis were mainly presented as a well-differentiated grade rather than poorly differentiated grade, which is in line with our result (30). However, the detailed mechanism is not revealed, and a string of research is still being urged for this issue.
Based on our results, we concluded that the number of sites of EHMs was a significant predictive factor for CC patients with LM, while the sites of EHMs showed limited impact on survival. Therefore, we constructed a nomogram to predict the survival probability of patients with EHMs. Some clinical features, especially the EHMs number, were involved in this nomogram. Additionally, our nomogram demonstrated strong predictive capacity for the clinical outcome of patients with EHMs in both the training group and the validation cohort. To our best research, it was the first study that aimed to construct a nomogram based on EHMs number. In addition, compared with other research which constructed a prognostic model for CC patients with stage IV, our model also had an obvious advantage. Deng et al. constructed a nomogram model to predict survival time in patients with CRC hepato-pulmonary metastasis. The AUC values of 1 and 3 years were 0.802, 0.759 in the training cohort; 0.814, 0.769 in the validation cohort (31). Han et al. also constructed a model, and the AUC for 1 year was 0.705, for 2 years was 0.675, and for 3 year was 0.648 (32). All these were not excellent as our model, based on that AUC were 0.863, 0.833, 0.815, 0.8, and 0.784 in predicting 6-, 12-, 18-, 24-, and 36- month OS in the training cohort, and 0.862, 0.833, 0.817, 0.807, and 0.792 in a validation cohort.
Conclusion
In conclusion, the number of sites of EHMs was a significant prognostic factor of CC patients with LM. Patients with zero or one involved extrahepatic organ exhibited better survival compared with patients with two or more EHM sites. Patients with a more significant number of ECMs presented a higher cumulative incidence rate of CSS. Moreover, patients with various EHM sites had different impacts on survival and presented variant distribution features of primary location site, grade, and histology. Finally, a nomogram based on the number of sites of EHMs was constructed that can accurately predict the OS of patients with EHMs.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
JR supervised the study; SB conceived the study. JR, SB, LC, GZ, YLY, WX, WaL, WeL, FH, NL, MC, and YPY analyzed data; SB wrote the manuscript; JR and SB made manuscript revisions. All authors have read and approved the final version of this submission.
Funding
This manuscript is supported by the Scientific and Technological Research Foundation of Shaan’xi Province, Key Research and Development Project, General project (JR, 2023-YBSF-666); supported by the Shaanxi Provincial Key Research and Development Plan Project(Key support projects Category A) (JR, 2021A011); supported by the Basic and Clinical Integration Innovation Project of Xi’an Jiao tong University, (JR,YXJLRH2022006).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Hossain MS, Karuniawati H, Jairoun AA, Urbi Z, Ooi J, John A, et al. Colorectal cancer: a review of carcinogenesis, global epidemiology, current challenges, risk factors, preventive and treatment strategies. Cancers (Basel) (2022) 14. doi: 10.3390/cancers14071732
3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
4. Riihimaki M, Hemminki A, Sundquist J, Hemminki K. Patterns of metastasis in colon and rectal cancer. Sci Rep (2016) 6:29765. doi: 10.1038/srep29765
5. van der Geest LG, Lam-Boer J, Koopman M, Verhoef C, Elferink MA, de Wilt JH. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin Exp Metastasis (2015) 32:457–65. doi: 10.1007/s10585-015-9719-0
6. Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics. CA Cancer J Clin (2017) 67:177–93. doi: 10.3322/caac.21395
7. Engstrand J, Nilsson H, Stromberg C, Jonas E, Freedman J. Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer (2018) 18:78. doi: 10.1186/s12885-017-3925-x
8. Hackl C, Neumann P, Gerken M, Loss M, Klinkhammer-Schalke M, Schlitt HJ. Treatment of colorectal liver metastases in Germany: a ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer (2014) 14:810. doi: 10.1186/1471-2407-14-810
9. Noren A, Eriksson HG, Olsson LI. Selection for surgery and survival of synchronous colorectal liver metastases; a nationwide study. Eur J Cancer (2016) 53:105–14. doi: 10.1016/j.ejca.2015.10.055
10. House MG, Ito H, Gonen M, Fong Y, Allen PJ, DeMatteo RP, et al. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg (2010) 210:744–52. doi: 10.1016/j.jamcollsurg.2009.12.040
11. Noren A, Sandstrom P, Gunnarsdottir K, Ardnor B, Isaksson B, Lindell G, et al. Identification of inequalities in the selection of liver surgery for colorectal liver metastases in Sweden. Scand J Surg (2018) 107:294–301. doi: 10.1177/1457496918766706
12. Ren L, Zhu D, Benson AB, Nordlinger B, Koehne CH, Delaney CP, et al. Shanghai international consensus on diagnosis and comprehensive treatment of colorectal liver metastases (version 2019). Eur J Surg Oncol (2020) 46:955–66. doi: 10.1016/j.ejso.2020.02.019
13. Liu Z, Xu Y, Xu G, Baklaushev VP, Chekhonin VP, Peltzer K, et al. Nomogram for predicting overall survival in colorectal cancer with distant metastasis. BMC Gastroenterol (2021) 21:103. doi: 10.1186/s12876-021-01692-x
14. Tang M, Wang H, Cao Y, Zeng Z, Shan X, Wang L. Nomogram for predicting occurrence and prognosis of liver metastasis in colorectal cancer: a population-based study. Int J Colorectal Dis (2021) 36:271–82. doi: 10.1007/s00384-020-03722-8
15. Bhullar DS, Barriuso J, Mullamitha S, Saunders MP, O'Dwyer ST, Aziz O. Biomarker concordance between primary colorectal cancer and its metastases. EBioMedicine (2019) 40:363–74. doi: 10.1016/j.ebiom.2019.01.050
16. Czauderna C, Luley K, von Bubnoff N, Marquardt JU. Tailored systemic therapy for colorectal cancer liver metastases. Int J Mol Sci (2021) 22. doi: 10.3390/ijms222111780
17. Saad AM, Abdel-Rahman O. Initial systemic chemotherapeutic and targeted therapy strategies for the treatment of colorectal cancer patients with liver metastases. Expert Opin Pharmacother (2019) 20:1767–75. doi: 10.1080/14656566.2019.1642324
18. Lee JH, Jung S, Park WS, Choe EK, Kim E, Shin R, et al. Prognostic nomogram of hypoxia-related genes predicting overall survival of colorectal cancer-analysis of TCGA database. Sci Rep (2019) 9:1803. doi: 10.1038/s41598-018-38116-y
19. Pietrantonio F, Di Nicolantonio F, Schrock AB, Lee J, Tejpar S, Sartore-Bianchi A, et al. ALK, ROS1, and NTRK rearrangements in metastatic colorectal cancer. J Natl Cancer Inst (2017) 109. doi: 10.1093/jnci/djx089
20. Torshizi Esfahani A, Seyedna SY, Nazemalhosseini Mojarad E, Majd A, Asadzadeh Aghdaei H. MSI-L/EMAST is a predictive biomarker for metastasis in colorectal cancer patients. J Cell Physiol (2019) 234:13128–36. doi: 10.1002/jcp.27983
21. Tsilimigras DI, Ntanasis-Stathopoulos I, Bagante F, Moris D, Cloyd J, Spartalis E, et al. Clinical significance and prognostic relevance of KRAS, BRAF, PI3K and TP53 genetic mutation analysis for resectable and unresectable colorectal liver metastases: a systematic review of the current evidence. Surg Oncol (2018) 27:280–8. doi: 10.1016/j.suronc.2018.05.012
22. Wang M, Wu Q, Zhang J, Qin G, Yang T, Liu Y, et al. Prognostic impacts of extracranial metastasis on non-small cell lung cancer with brain metastasis: a retrospective study based on surveillance, epidemiology, and end results database. Cancer Med (2021) 10:471–82. doi: 10.1002/cam4.3562
23. Tokoro T, Okuno K, Hida JC, Ueda K, Yoshifuji T, Daito K, et al. Prognostic factors for patients with advanced colorectal cancer and symptomatic brain metastases. Clin Colorectal Cancer (2014) 13:226–31. doi: 10.1016/j.clcc.2014.09.008
24. Khattak MA, Martin HL, Beeke C, Price T, Carruthers S, Kim S, et al. Survival differences in patients with metastatic colorectal cancer and with single site metastatic disease at initial presentation: results from south Australian clinical registry for advanced colorectal cancer. Clin Colorectal Cancer (2012) 11:247–54. doi: 10.1016/j.clcc.2012.06.004
25. Ge Y, Lei S, Cai B, Gao X, Wang G, Wang L, et al. Incidence and prognosis of pulmonary metastasis in colorectal cancer: a population-based study. Int J Colorectal Dis (2020) 35:223–32. doi: 10.1007/s00384-019-03434-8
26. Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet (2019) 394:1467–80. doi: 10.1016/S0140-6736(19)32319-0
27. Loree JM, Pereira AAL, Lam M, Willauer AN, Raghav K, Dasari A, et al. Classifying colorectal cancer by tumor location rather than sidedness highlights a continuum in mutation profiles and consensus molecular subtypes. Clin Cancer Res (2018) 24:1062–72. doi: 10.1158/1078-0432.CCR-17-2484
28. Meguid RA, Slidell MB, Wolfgang CL, Chang DC, Ahuja N. Is there a difference in survival between right- versus left-sided colon cancers? Ann Surg Oncol (2008) 15:2388–94. doi: 10.1245/s10434-008-0015-y
29. Price TJ, Beeke C, Ullah S, Padbury R, Maddern G, Roder D, et al. Does the primary site of colorectal cancer impact outcomes for patients with metastatic disease? Cancer (2015) 121:830–5. doi: 10.1002/cncr.29129
30. Li X, Hu W, Sun H, Gou H. Survival outcome and prognostic factors for colorectal cancer with synchronous bone metastasis: a population-based study. Clin Exp Metastasis (2021) 38:89–95. doi: 10.1007/s10585-020-10069-5
31. Deng S, Jiang Z, Cao Y, Gu J, Mao F, Xue Y, et al. Development and validation of a prognostic scoring system for patients with colorectal cancer hepato-pulmonary metastasis: a retrospective study. BMC Cancer (2022) 22:643. doi: 10.1186/s12885-022-09738-3
Keywords: colorectal cancer, competing risk analysis, extrahepatic metastasis, prognostic model, SEER, risk factor
Citation: Bai S, Chen L, Zhu G, Xuan W, Hu F, Liu W, Li W, Lan N, Chen M, Yan Y, Li R, Yang Y and Ren J (2023) Prognostic value of extrahepatic metastasis on colon cancer with liver metastasis: a retrospective cohort study. Front. Oncol. 13:1172670. doi: 10.3389/fonc.2023.1172670
Received: 23 March 2023; Accepted: 10 May 2023;
Published: 06 June 2023.
Edited by:
Zheng Wang, Shanghai Jiao Tong University, ChinaReviewed by:
Sonia Tewani Orcutt, University of Arkansas for Medical Sciences, United StatesBelgin Sever, Anadolu University, Türkiye
Copyright © 2023 Bai, Chen, Zhu, Xuan, Hu, Liu, Li, Lan, Chen, Yan, Li, Yang and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Ren, ODY5NDkxNTMzQHFxLmNvbQ==
 Shuheng Bai
Shuheng Bai Ling Chen2
Ling Chen2 Wang Xuan
Wang Xuan Fengyuan Hu
Fengyuan Hu Ning Lan
Ning Lan Yanli Yan
Yanli Yan Juan Ren
Juan Ren