- 1The Department of Urology Surgery, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 2National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin, China
Purpose: Observational studies have revealed that serum minerals and vitamins are associated with cancer. However, the causal relationships between serum minerals and vitamins and renal malignancies remain unclear.
Methods: Mendelian randomization (MR) was used for causal estimation. Single nucleotide polymorphisms (SNPs) for serum minerals and vitamins were obtained from published genome-wide association studies (GWAS). GWAS for malignant kidney neoplasm was obtained from the FinnGen consortium. Methods of inverse variance weighted (IVW), MR-Egger, and weighted median were carried out for causal inference. F-statistic was calculated to ensure a robust instrumental variable. Cochran’s Q statistics was applied to calculate heterogeneity. MR-Egger regression, MR-pleiotropy residual sum and outlier methods (MR-PRESSO) methods were used to perform pleiotropy analysis. Meanwhile, confounding factors were considered to determine whether causal inference would be biased.
Results: Eight different micronutrients were included (zinc, iron, magnesium, calcium, copper, selenium, phosphate, and vitamin B12). After MR analysis, we found a protective effect of serum zinc against malignant kidney neoplasm (IVW: odds ratios (ORs), 0.86; 95% confidence interval (95% CI), 0.78–0.94; p, 0.0016; MR-Egger: OR, 0.80; 95% CI, 0.64–0.97; p, 0.052; weighted median: OR, 0.85; 95% CI, 0.75–0.96; p, 0.011). Causal relationships between other micronutrients and malignant kidney neoplasm were not obtained. No heterogeneity and pleiotropy were detected, while causality was not biased by confounding factors.
Conclusion: We considered that serum zinc exerted a protective effect against malignant kidney neoplasm. In clinical practice, for people with high malignant kidney neoplasm risk, an oral zinc supplementation might play a role in a potential therapeutic target.
Background
In 2020, there are 431,288 new cases of kidney cancer and 179,368 new cancer deaths in 185 countries (1). In Europe, the number of deaths from kidney cancer exceeds 50,000 in 2018 (2). Screening and early detection had been identified as a top priority in kidney cancer research (3). Significant correlation was found between early diagnosis of kidney cancer and patient survival. Cancer-specific 5-year survival rates for stage I and IV kidney cancer patients were 83% and 6%, respectively. However, approximately 20% of patients with kidney cancer demonstrated signs of metastasis in Europe (4, 5).
In spite of the current advanced medical technology, the incidence of kidney cancer is still increasing. Modifiable risk factors for kidney cancer, such as lifestyle habits (smoking, obesity, hypertension, excessive alcohol intake, and diet), were increasingly being emphasized (6). Targeting modifiable risk factors has become a strategy to reduce the incidence of cancer (7). Associations between dietary nutrients and cancer have been reported in numerous studies. A randomized controlled trial by François et al. concluded that men with standard PSA who received the supplement (zinc, selenium, beta-carotene, vitamin E, and vitamin C) demonstrated a statistically significant decline in prostate cancer incidence and were statistically significant (hazard ratio, 0.52; 95% CI, 0.29–0.92) (8). Ray et al. examined zinc levels in hair to explore its role in esophageal cancer and concluded that zinc deficiency led to an increased risk in developing esophageal cancer (9). A study by Khoshdel et al. founded that there was a lower plasma zinc concentration in the colorectal cancer group compared to the control group (10). Association between serum micronutrients and renal malignancies was also revealed. Serum folate and vitamin B12 levels were associated with decreased risk of renal cell carcinoma (11) Research by Panaiyadiyan et al. found a significantly higher serum concentration of arsenic, copper, manganese, cadmium, lead, and mercury while a lower serum concentration of selenium in patients with renal cell carcinoma compared to controls (12). Xu et al. revealed that low vitamin D status was associated with an increased risk of renal cell carcinoma (13). However, it is uncertain whether there is a causal relationship between serum micronutrients and renal malignancies.
With the increasing number of large-scale genome-wide association studies (GWAS), Mendelian randomization (MR) has been used to make causal inference in different phenotypes. By using genetic variants as instrumental variables, MR can overcome the interference of reverse causality and minimize confounding factors, but still suffer from residual confounding (14). The MR assumptions are presented in Figure 1.
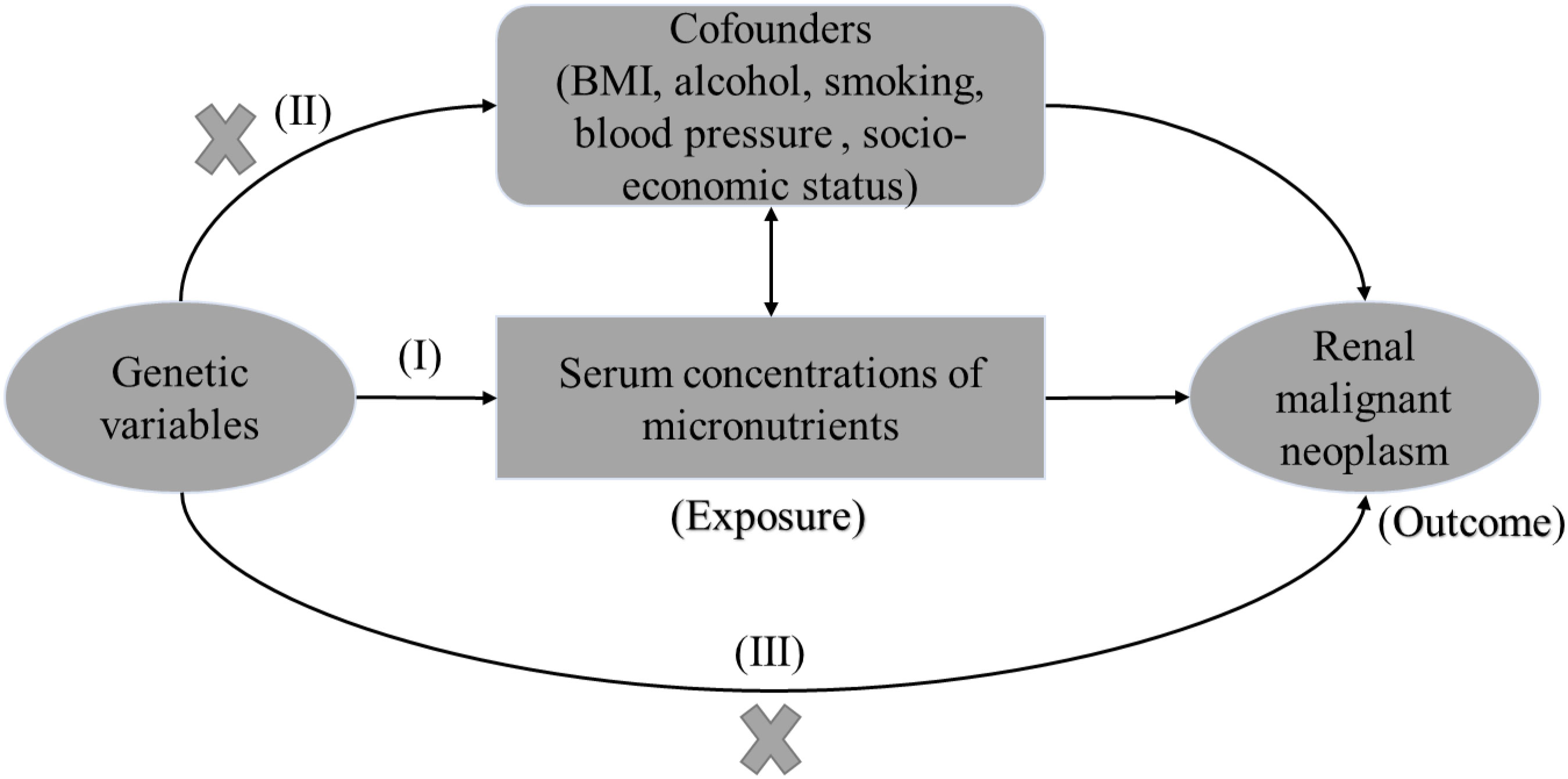
Figure 1 The three mendelian assumptions in the study. The instrumental variables should: (I) be robustly associated with serum micronutrients (exposure of interest); (II) be independent of BMI, alcohol, smoking, blood pressure (confounders); and (III) have no direct association with the renal malignant neoplasm (outcome of interest), but affect outcome exclusively through serum micronutrients. BMI, body mass index.
In the present study, we used genetically proxied serum micronutrients and malignant kidney neoplasm to explore their causal relationship using a two-sample MR approach. Meanwhile, we evaluated whether potential confounders induced bias in causal inference. As mentioned earlier, smoking, alcohol abuse, obesity, and blood pressure are modifiable factors for renal malignancy. Meanwhile, serum micronutrients are related to those factors (15–18). Therefore, we took smoking, alcohol abuse, body mass index (BMI), and blood pressure as confounding factors. Generally, adherence to a healthier lifestyle is associated with higher educational and socio-economic level of the family. Thus, household socio-economic status is also a potential confounding factor. Within our knowledge, studies on the causal relationship between serum micronutrients and renal malignancies are still limited, and we comprehensively elucidate the causal relationship through MR analysis on our research.
Methods
The acquisition of exposure GWAS data
GWAS data for serum zinc and copper were derived from Ng et al.; micronutrients were assayed in a cohort of 949 individuals using mass spectrometry. DNA samples were genotyped on Infinium Omni Express magnetic bead microarrays and extrapolated from 1000 Genome Project reference plates (19). We performed further analyses for the original data because the possibility of linkage disequilibrium (LD) of individual single nucleotide polymorphism (SNP) loci was not considered; LD of r2 = 0.001 and physical distance kb=10,000 were set to minimize linkage disequilibrium. GWAS data for serum iron, magnesium, calcium, selenium, phosphate, and vitamin B12 were obtained from Tsilidis et al. This study was conducted with strict quality control to avoid the presence of collision bias. SNPs that were related to the concentrations of serum micronutrients at a genome-wide significance level (p<5×10−8) and were not in linkage disequilibrium (LD of r2 = 0.01) were selected (20).
The retrieval of the outcome GWAS data
GWAS data for malignant kidney neoplasm was obtained from the FinnGen consortium (https://www.finngen.fi/fi), consisting of 1,631 patients and 307,523 controls. The diagnosis criteria for of kidney cancer were ICD-O-3 code. We extracted exposure-related SNPs in the outcome, and if SNPs that were significantly associated with serum micronutrients were also related to renal malignancy (p<0.05) were excluded.
The obtainment of GWAS data on confounding factors
When performing MR analysis, the causal relationship between exposure and outcome cannot be mediated by confounders; therefore, a causal relationship between exposure and confounders must be excluded. Smoking, alcohol abuse, BMI, blood pressure (systolic, diastolic), and socio-economic status (average total household income before tax, full-time or part-time education) were common confounders. The GWAS data for these confounders were obtained from OpenGWAS database (21), and the specific information is presented in Table 1.
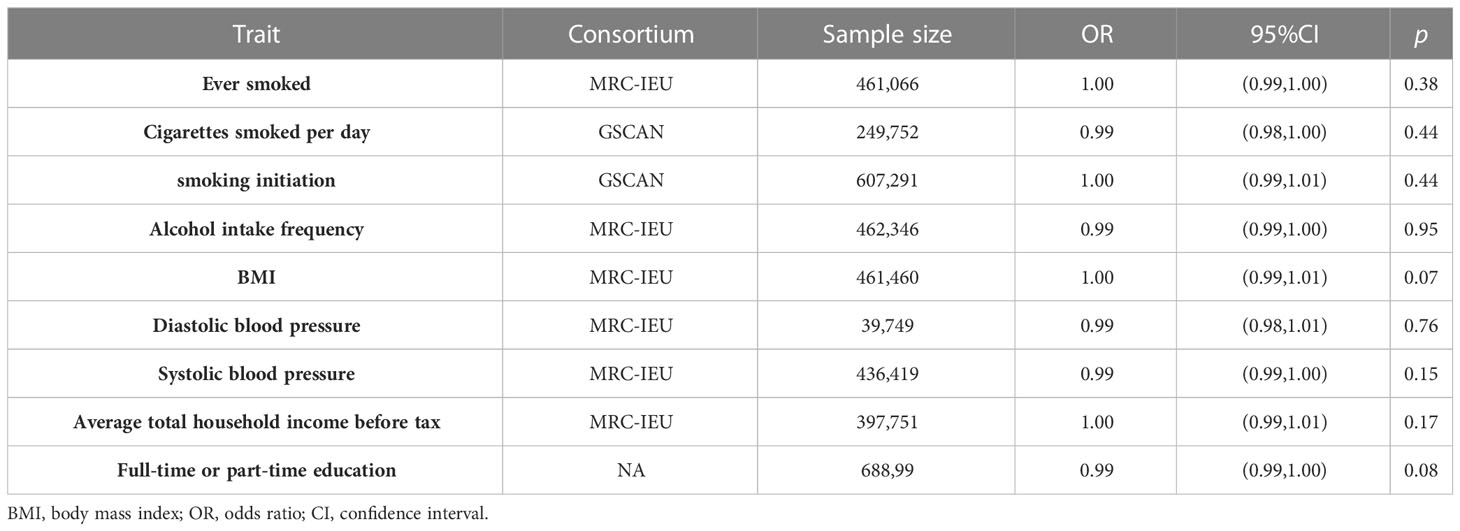
Table 1 Details of confounders and casual inference between genetically predicted serum zinc and cofounders.
Statistical analysis
In our study, we used the inverse variance weighted (IVW), MR-Egger, and weighted median methods for causal inference. Different methods were used because these methods had different conditional assumptions. The IVW approach could be performed either by random effects model or fixed effects model. p<0.05 of Cochran’s Q statistics was used as a threshold to decide whether a random effects model or a fixed effects model was chosen (22). The IVW approach, which performed a meta-analysis of the Wald ratios of individual SNPs, assumed that the genetic variants could only influence the outcomes through the exposure of interest and not through any other pathways (23). MR-Egger and weighted median could complement the IVW approach in a broader scenario. In the sensitivity analysis, we used Cochran’s Q statistics to detect heterogeneity. MR-Egger regression, MR-pleiotropy residual sum and outlier methods (MR-PRESSO) were employed to detect pleiotropy, which referred to the fact that proxied SNPs led to the occurrence of outcome by other pathways. The intercept in MR-Egger regression approach was considered as a sign of the presence of pleiotropy, but p<0.05 was taken as a statistical criterion. MR-PRESSO could detect the presence of outlier SNPs and filter them out, and provide detection of statistical differences in causal assessments before and after the removal of outliers. The leave-one-out method was adopted to examine whether the causal assessment would be biased by a particular SNP. R2 was taken to evaluate the proportion of variance explained by SNPs (R2 = 2 × (1 − MAF) × MAF × (β)2). The F-statistic was adopted to calculate the strength of the instrumental variable (F = β2/se2). F>10 was usually considered as a strong instrumental variable. β was the coefficient for the SNP, MAF was the minor allele frequency, and se was the standard error of coefficient. All MR analyses were performed in R (version 4.2.1) with the help of the R packages “TwoSampleMR” (21) and “MRPRESSO” (24).
Results
Causal effect from serum zinc on malignant kidney neoplasm
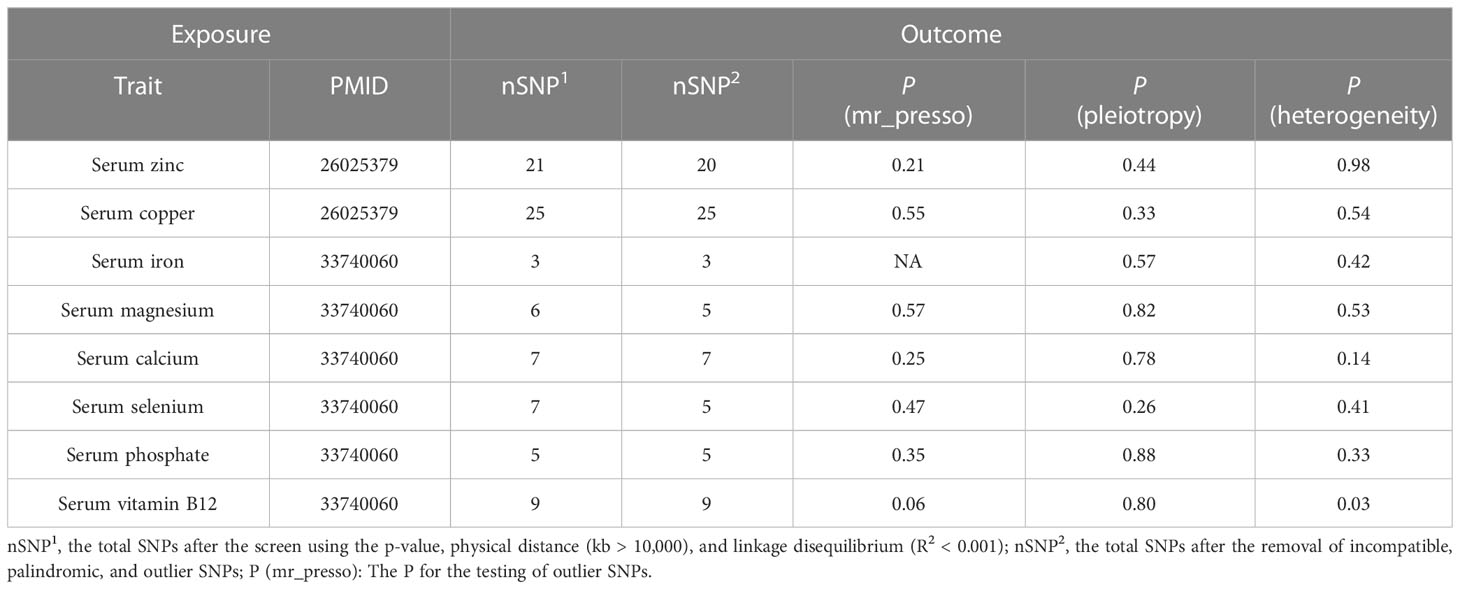
A total of 21 SNPs associated with serum zinc were included for causal estimation. The SNPs explained 2.0%–2.7% of the variance in circulating zinc concentration. The mean value of F-statistic was 21.7, ranged from 19.53 to 27.77 (Supplementary Table S1). In the merge process, rs2635551 was not found in the outcome. In MR-PRESSO, no outliers were detected, and the p-value for Global Test was 0.979. A total of 20 SNPs were finally included in the final MR causal estimation. Using the IVW approach, we detected a statistically significant causal relationship between serum zinc and malignant kidney neoplasm (OR, 0.86; 95% CI, 0.78–0.94; p, 0.0016). The result was also remarkable in weighted median (OR, 0.85; 95% CI, 0.75–0.96; p, 0.011). However, to our disappointment, the method of MR-Egger did not produce a causal relationship (OR, 0.80; 95% CI, 0.64–0.97; p, 0.052) (Figure 2). Heterogeneity was not detected with methods of IVW, and the p-value of Cochran’s Q statistics were 0.98. In the test of pleiotropy, the intercept was 0.024, but it was not statistically significant (p, 0.44) (Table 2). MR regression slope is displayed in Figure 3A. The result of leave-one-out also indicated that none of the SNPs had a distinct effect on causal inference, and this relationship exhibited robustness (Figure 3B). Forest plot could display the effect of individual SNP on causal assessment (Figure 3C). Funnel plot demonstrated that the distribution of SNPs tended to be balanced (Figure 3D).
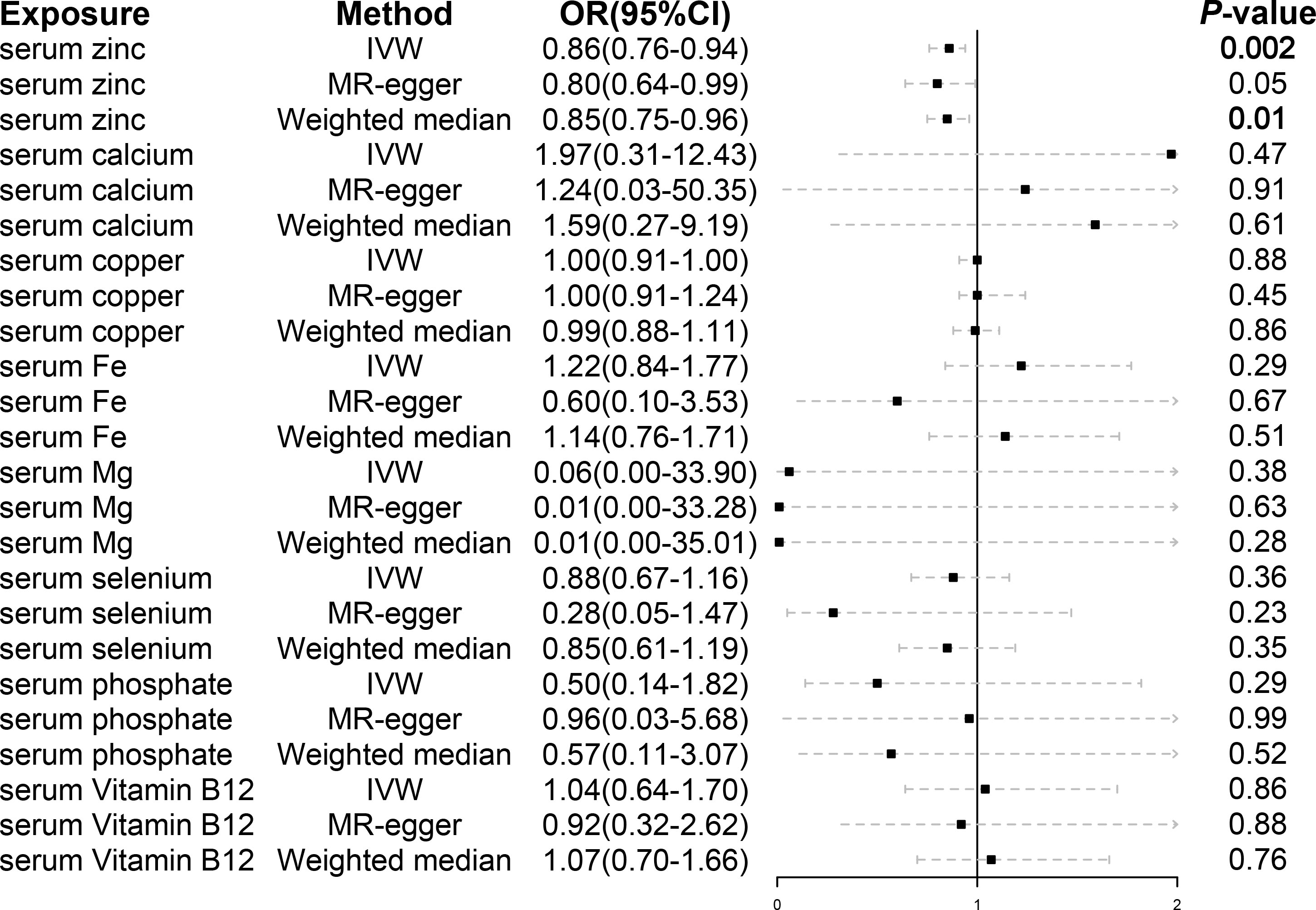
Figure 2 The casual inference of serum micronutrients on malignant kidney neoplasm using IVW, MR-Egger, and weighted median methods. IVW, inverse variance weighted; OR, odds ratio; CI, confidence interval.
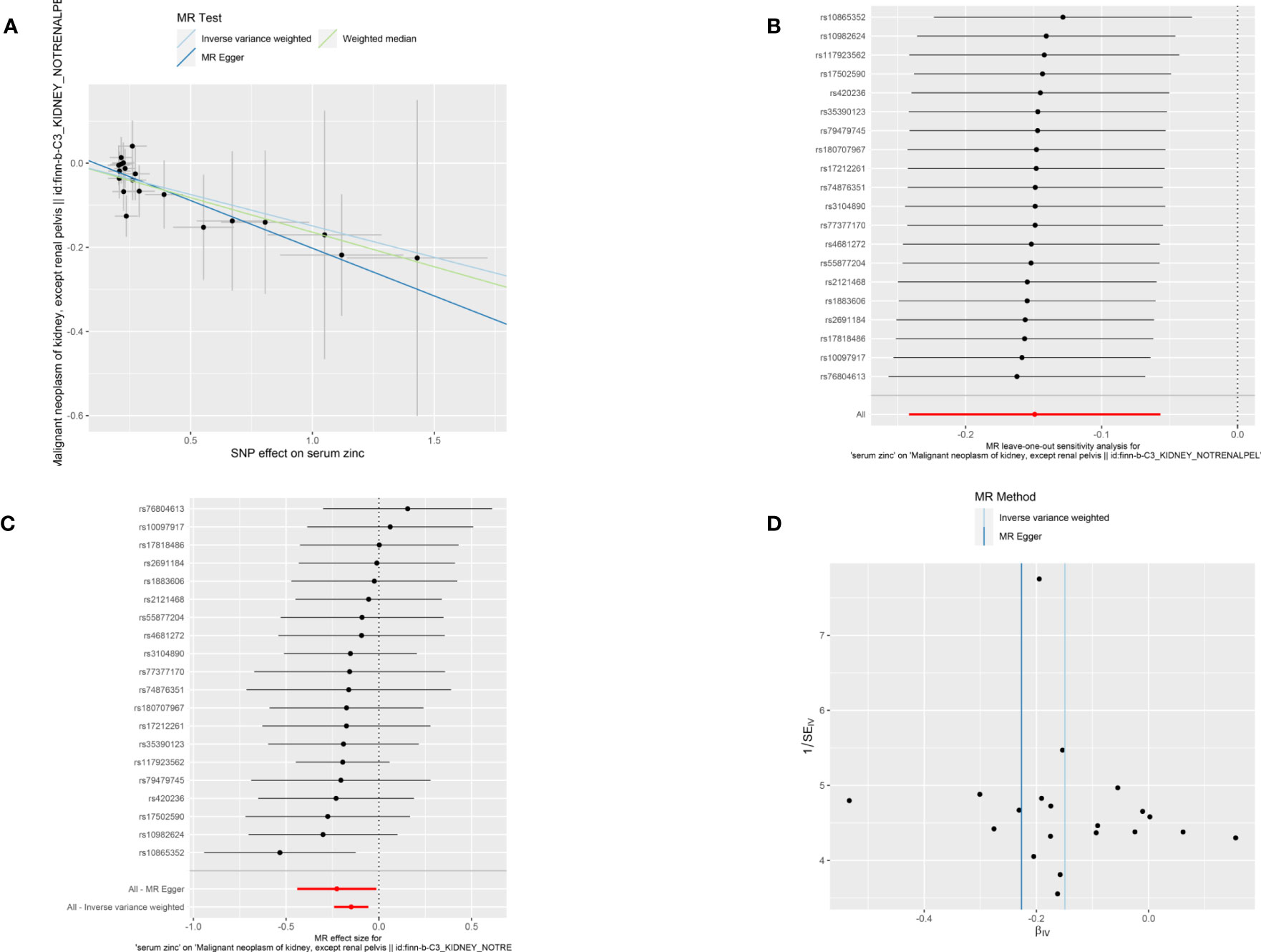
Figure 3 Visualization of causal inference from serum zinc on malignant kidney neoplasm. (A) MR-Egger regression slope. (B) Leave one out analysis. (C) Forest plot. (D) The funnel plot.
Causal effect from other serum micronutrients on malignant kidney neoplasm
A total of 9, 7, 25, 3, 6, 5, and 7 SNPs of serum vitamin B12, calcium, copper, iron, magnesium, phosphate, and selenium, respectively, were included for causal estimation. In the merge process, the genetically proxied rs7965584 for magnesium and rs6586282 and rs1789953 for selenium were not found in outcome. All the SNPs were robust genetic variants. In MR-PRESSO, no outliers were detected. After MR analysis, none of the above serum micronutrients had causal associations with malignant kidney neoplasm (Figure 2). Pleiotropy was not found (Table 2).
Causal effect from serum zinc on potential cofounding factors
To exclude the bias of confounders in the causal inference from serum zinc on malignant kidney neoplasm, we evaluated potential confounders such as smoking, alcohol consumption, obesity, and blood pressure. We obtained GWAS data for risk factors: smoking (ever smoked, smoking initiation, and cigarettes smoked per day), alcohol abuse (alcohol intake frequency), blood pressure (systolic blood pressure and diastolic blood pressure), obesity (BMI), and socio-economic status (average total household income before tax and full-time or part-time education). The IVW approach was taken to assess the causal relationship between serum zinc and cofounding factors. As shown in the Table 1, causal relationships were not observed.
Discussion
In the present study, we used published GWAS data to infer the causal relationship between serum micronutrients and malignant kidney neoplasm. We found that serum zinc reduced the risk of malignant kidney neoplasm causally. Furthermore, the causal inference was not biased by potential confounding factors. However, we did not obtain a causal relationship between other serum micronutrients and renal malignancies.
As a common antioxidant, the anticancer effect of zinc has been widely studied. Many observational studies have explored the relationship between zinc and kidney cancer, but no consensus has been reached. Wang et al. reveal that zinc played an important role in promoting the development of kidney cancer (25). Zhang et al. suggested that an above median zinc was associated with a reduced risk of kidney tumors (26). We assumed that different geographical locations and various dietary habits led to distinct food sources of zinc, so we could not judge whether other substances in food would influence the results. In addition, the adjustment for confounding factors varied. For example, high dietary intake of zinc increased the risk of stone (27), and renal stone was a risk factor for malignant kidney neoplasm (28); therefore, it was possible that the presence of renal stones also mediated the association between zinc and malignant kidney neoplasm.
The anticancer effect of zinc was mainly attributed to its antioxidant properties. Under homeostatic conditions, the body produced reactive oxygen species (ROS), which were involved in many biological processes (cell differentiation, apoptosis) (29, 30). Due to the high metabolic demand of tumors, tumor tissues produced large amounts of ROS, which promoted tumor growth by causing DNA damage and inducing gene mutations (31, 32). The antioxidant effect of zinc then exerted a very good role in inhibiting tumor growth. The antioxidant of zinc was primarily due to its ability to act as a component of superoxide dismutase, preventing the production of free radicals and their derivatives (33, 34). In addition, zinc acted as an antagonist of iron and copper involved in lipid peroxidation, thus reducing the production of free radicals (35, 36).
Meanwhile, zinc could also have an impact on the immune system. The impact of zinc on the immune system was primarily through the Th lymph nodes. Zinc deficiency led to an imbalance of Th1 and Th2, which contributed to a dysfunctional immune response (37, 38). Zinc supplementation slowed this imbalance by increasing IFN-γ (Th1 inducer). Additionally, IFN-γ itself had immunomodulatory and antitumor effects (39). Zinc deficiency impaired granulocyte recruitment, ROS production, chemotaxis, and phagocytosis (40, 41). Adherence of mononuclear cells to epithelial cells also occurred in a zinc-dependent manner (42).
Since zinc got involved in the formation of zinc fingers, the latter could perform a significant role in regulating DNA replication, transcription, transformation, and repair due to its high selectivity and capacity to bind to DNA, RNA, and proteins (43, 44). Furthermore, zinc ensured the clearance of mutated or damaged forms that could lead to cancer by participating in the regulation of apoptosis (45). Endogenous zinc was also indispensable in the induction of autophagy under conditions of oxidative stress.
A couple of advantages were present in our study. First, we observed a protective effect of serum zinc concentration against malignant kidney neoplasm, so that zinc supplementation might be a potential clinical option for clinically high-risk population. However, more studies are needed to verify the conclusion. Second, our study used publicly available GWAS data to make causal inferences, and the statistical strength was higher due to the large sample size. Several drawbacks were also present in our study. First, we were not able to perform subtype analysis of malignant kidney neoplasm. The MR analysis was performed using summary GWAS data, and individual-level data were not available. The cancer subtype in each individual were also unknown. Second, our analysis was limited to an ancestral European sample, and it remained to be verified whether the same results would be obtained in other populations. Third, our study was only a basic theoretical study, and more animal experiments and cohort studies were needed to confirm the conclusions, before applied into clinical practice. Fourth, a causal relationship between other serum malignancies and renal malignancies were not obtained. Possible reasons were as follows: (i) the study population in this study was mainly of European origin, and it was likely that the sensitivity of renal malignancies to serum micronutrients differed in different populations; (ii) causal associations were probably not present between other micronutrients and renal malignancies; it was probable that previous observational studies have not controlled for confounding factors better; and (iii) phenotypes were defined differently in different studies.
Conclusion
In conclusion, we obtained that genetically proxied serum zinc reduced the risk of malignant kidney neoplasm causally. Therefore, zinc might play a role in a potential therapeutic target for renal malignancies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Acknowledgments
We acknowledge UK Biobank and FinnGen for providing high-quality GWAS resources for researchers.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1191825/full#supplementary-material
Additional file 1 | Detailed information of the proxied SNPs of serum zinc concentration.
Abbreviations
MR, Mendelian randomization; GWAS, genome-wide association studies; SNPs, single nucleotide polymorphisms; OR, odds ratios; CI, confidence interval; IVW, inverse variance weighted; LD, linkage disequilibrium; BMI, body mass index; ROS, reactive oxygen species.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Rossi SH, Fielding A, Blick C, Handforth C, Brown JE, Stewart GD. Setting research priorities in partnership with patients to provide patient-centred urological cancer care. Eur Urol (2019) 75:891–3. doi: 10.1016/j.eururo.2019.03.008
3. Motzer RJ. Perspective: What next for treatment? Nature (2016) 537(7620):S111. doi: 10.1038/537S111a
4. Innos K, Sepp T, Baburin A, Kotsar A, Lang K, Padrik P, et al. Increasing kidney cancer incidence and survival in Estonia: role of age and stage. Acta Oncol (2019) 58:21–8. doi: 10.1080/0284186X.2018.1512158
5. Thorstenson A, Harmenberg U, Lindblad P, Holmström B, Lundstam S, Ljungberg B. Cancer characteristics and current treatments of patients with renal cell carcinoma in Sweden. BioMed Res Int (2015) 2015:456040. doi: 10.1155/2015/456040
6. Tahbaz R, Schmid M, Merseburger AS. Prevention of kidney cancer incidence and recurrence: lifestyle, medication and nutrition. Curr Opin Urol (2018) 28:62–79. doi: 10.1097/MOU.0000000000000454
7. Gelfond J, Al-Bayati O, Kabra A, Iffrig K, Kaushik D, Liss MA. Modifiable risk factors to reduce renal cell carcinoma incidence: Insight from the PLCO trial. Urol Oncol (2018) 36:340.e1–6. doi: 10.1016/j.urolonc.2018.04.011
8. Meyer F, Galan P, Douville P, Bairati I, Kegle P, Bertrais S, et al. Antioxidant vitamin and mineral supplementation and prostate cancer prevention in the SU.VI.MAX trial. Int J Cancer (2005) 116:182–6. doi: 10.1002/ijc.21058
9. Ray SS, Das D, Ghosh T, Ghosh AK. The levels of zinc and molybdenum in hair and food grain in areas of high and low incidence of esophageal cancer: a comparative study. Glob J Health Sci (2012) 4:168–75. doi: 10.5539/gjhs.v4n4p168
10. Khoshdel Z, Naghibalhossaini F, Abdollahi K, Shojaei S, Moradi M, Malekzadeh M. Serum copper and zinc levels among Iranian colorectal cancer patients. Biol Trace Elem Res (2016) 170:294–9. doi: 10.1007/s12011-015-0483-4
11. Huang CY, Chen WJ, Lee HL, Lin YC, Huang YL, Shiue HS, et al. Possible combined effects of plasma folate levels, global DNA methylation, and blood cadmium concentrations on renal cell carcinoma. Nutrients (2023) 15:937. doi: 10.3390/nu15040937
12. Panaiyadiyan S, Quadri JA, Nayak B, Pandit S, Singh P, Seth A, et al. Association of heavy metals and trace elements in renal cell carcinoma: A case-controlled study. Urologic Oncol (2022) 40:111.e11–8. doi: 10.1016/j.urolonc.2021.11.017
13. Xu S, Song J, Zhang ZH, Fu L, Gao L, Xie DD, et al. The Vitamin D status is associated with serum C-reactive protein and adhesion molecules in patients with renal cell carcinoma. Sci Rep (2019) 9:16719. doi: 10.1038/s41598-019-53395-9
14. Haycock PC, Burgess S, Wade KH, Bowden J, Relton C, Davey Smith G. Best (but oft-forgotten) practices: the design, analysis, and interpretation of Mendelian randomization studies. Am J Clin Nutr (2016) 103:965–78. doi: 10.3945/ajcn.115.118216
15. Zhang Y, Lin S, Li J, Song X, Chen G, Pei L. Interaction of passive smoking and diet habits on vitamin D deficiency among women of reproductive age in rural central China. Nutrients (2022) 15:126. doi: 10.3390/nu15010126
16. Maguire D, Burns A, Talwar D, Catchpole A, Stefanowicz F, Ross DP, et al. Randomised trial of intravenous thiamine and/or magnesium sulphate administration on erythrocyte transketolase activity, lactate concentrations and alcohol withdrawal scores. Sci Rep (2022) 12:6941. doi: 10.1038/s41598-022-10970-x
17. Zhao J, Fu S, Chen Q. Association between the serum vitamin D level and prevalence of obesity/abdominal obesity in women with infertility: a cross-sectional study of the National Health and Nutrition Examination Survey data. Gynecological Endocrinol (2023) 39:2217251. doi: 10.1080/09513590.2023.2217251
18. Pei X, Yao J, Ran S, Lu H, Yang S, Zhang Y, et al. Association of serum water-soluble vitamin exposures with the risk of metabolic syndrome: results from NHANES 2003-2006. Front Endocrinol (2023) 14:1167317. doi: 10.3389/fendo.2023.1167317
19. Ng E, Lind PM, Lindgren C, Ingelsson E, Mahajan A, Morris A, et al. Genome-wide association study of toxic metals and trace elements reveals novel associations. Hum Mol Genet (2015) 24:4739–45. doi: 10.1093/hmg/ddv190
20. Tsilidis KK, Papadimitriou N, Dimou N, Gill D, Lewis SJ, Martin RM, et al. Genetically predicted circulating concentrations of micronutrients and risk of colorectal cancer among individuals of European descent: a Mendelian randomization study. Am J Clin Nutr (2021) 113:1490–502. doi: 10.1093/ajcn/nqab003
21. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife (2018) 7:e34408. doi: 10.7554/eLife.34408
22. Burgess S, Davey Smith G, Davies NM, Dudbridge F, Gill D, Glymour MM, et al. Guidelines for performing Mendelian randomization investigations. Wellcome Open Res (2020) 4:186. doi: 10.12688/wellcomeopenres.15555.2
23. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol (2015) 44:512–25. doi: 10.1093/ije/dyv080
24. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
25. Wang Y, Jafar TH, Jin A, Yuan JM, Koh WP. Dietary intakes of trace elements and the risk of kidney cancer: the Singapore Chinese health study. Nutr Cancer (2021) 73:239–45. doi: 10.1080/01635581.2020.1743870
26. Zhang H, Xu Z, Zhang J, Wei D, Liu K, Hu W, et al. Disordered serum essential element levels are associated with increased risk of kidney tumors. Environ Sci pollut Res Int (2022) 29:31675–85. doi: 10.1007/s11356-021-18201-y
27. Tang J, McFann K, Chonchol M. Dietary zinc intake and kidney stone formation: evaluation of NHANES III. Am J Nephrol (2012) 36:549–53. doi: 10.1159/000345550
28. Mihalopoulos M, Yaghoubian A, Razdan S, Khusid JA, Mehrazin R, Badani KK, et al. Understanding the link between kidney stones and cancers of the upper urinary tract and bladder. Am J Clin Exp Urol (2022) 10:277–98.
29. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol (2007) 39:44–84. doi: 10.1016/j.biocel.2006.07.001
30. Zheng L, Lin Y, Zhong S. ROS signaling-mediated novel biological targets: brf1 and RNA pol III genes. Oxid Med Cell Longevity (2021) 2021:5888432. doi: 10.1155/2021/5888432
31. Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discovery (2013) 12:931–47. doi: 10.1038/nrd4002
32. Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer (2011) 11:85–95. doi: 10.1038/nrc2981
33. Baek Y, Woo TG, Ahn J, Lee D, Kwon Y, Park BJ, et al. Structural analysis of the overoxidized Cu/Zn-superoxide dismutase in ROS-induced ALS filament formation. Commun Biol (2022) 5:1085. doi: 10.1038/s42003-022-04017-0
34. Higashi Y, Takechi K, Takano H, Takio S. Involvement of microRNA in copper deficiency-induced repression of chloroplastic CuZn-superoxide dismutase genes in the moss Physcomitrella patens. Plant Cell Physiol (2013) 54:1345–55. doi: 10.1093/pcp/pct084
35. D’Egidio F, Castelli V, Cimini A, d’Angelo M. Cell rearrangement and oxidant/antioxidant imbalance in huntington’s disease. Antioxidants (Basel Switzerland) (2023) 12:571. doi: 10.3390/antiox12030571
36. Koppenol WH, Hider RH. Iron and redox cycling. Do’s and don’ts. Free Radical Biol Med (2019) 133:3–10. doi: 10.1016/j.freeradbiomed.2018.09.022
37. Baltaci AK, Mogulkoc R. Leptin and zinc relation: In regulation of food intake and immunity. Indian J Endocrinol Metab (2012) 16:S611–6. doi: 10.4103/2230-8210.105579
38. Koyasu S, Moro K. Type 2 innate immune responses and the natural helper cell. Immunology (2011) 132:475–81. doi: 10.1111/j.1365-2567.2011.03413.x
39. Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol (2004) 75:163–89. doi: 10.1189/jlb.0603252
40. Oteiza PI. Zinc and the modulation of redox homeostasis. Free Radic Biol Med (2012) 53:1748–59. doi: 10.1016/j.freeradbiomed.2012.08.568
41. Hasan R, Rink L, Haase H. Chelation of free zn²+ Impairs chemotaxis, phagocytosis, oxidative burst, degranulation, and cytokine production by neutrophil granulocytes. Biol Trace Elem Res (2016) 171:79–88. doi: 10.1007/s12011-015-0515-0
42. Lee S, Eskin SG, Shah AK, Schildmeyer LA, McIntire LV. Effect of zinc and nitric oxide on monocyte adhesion to endothelial cells under shear stress. Ann BioMed Eng (2012) 40:697–706. doi: 10.1007/s10439-011-0434-y
43. Pomorski A, Drozd A, Kocyła A, Krężel A. From methodological limitations to the function of metallothioneins - a guide to approaches for determining weak, moderate, and tight affinity zinc sites. Metallomics: integrated biometal Sci (2023) 15(5):mfad027. doi: 10.1093/mtomcs/mfad027
44. Bollier N, Gonzalez N, Chevalier C, Hernould M. Zinc Finger-Homeodomain and Mini Zinc Finger proteins are key players in plant growth and responses to environmental stresses. J Exp Bot (2022) 73(14):4662–73. doi: 10.1093/jxb/erac194
Keywords: micronutrients, causal estimation, cancer prevention, Mendelian randomization analysis, kidney neoplasms
Citation: Qiao P and Tian Z (2023) The causal effect of serum micronutrients on malignant kidney neoplasm in European descent. Front. Oncol. 13:1191825. doi: 10.3389/fonc.2023.1191825
Received: 22 March 2023; Accepted: 28 July 2023;
Published: 16 August 2023.
Edited by:
Yinan Zheng, Northwestern University, United StatesReviewed by:
Kaifa Tang, Guizhou University of Traditional Chinese Medicine, ChinaMartina Maggi, Sapienza University of Rome, Italy
Copyright © 2023 Qiao and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhentao Tian, emhlbnRhb21haWxAMTI2LmNvbQ==
 Pengfei Qiao1,2
Pengfei Qiao1,2 Zhentao Tian
Zhentao Tian