- 1Clinical Laboratory, Huzhou Central Hospital, Affiliated Central Hospital of Huzhou University, Huzhou, Zhejiang, China
- 2Clinical Laboratory, Huzhou Maternity and Child Health Care Hospital, Huzhou, Zhejiang, China
Background: The prognosis of several malignancies has been influenced by the systemic immune-inflammation index (SII); however, its association with the prognostic outcome of ovarian cancer (OC) remains controversial. The present meta-analysis focused on the systemic and comprehensive identification of the role of SII in predicting OC prognosis.
Methods: We searched the Web of Science, PubMed, Cochrane Library, Embase, and China National Knowledge Infrastructure (CNKI) from inception until March 6, 2023. To predict the prognostic value of SII for overall survival (OS) and progression-free survival (PFS) in patients with OC, we calculated pooled hazard ratios (HRs) and corresponding 95% confidence intervals (CIs).
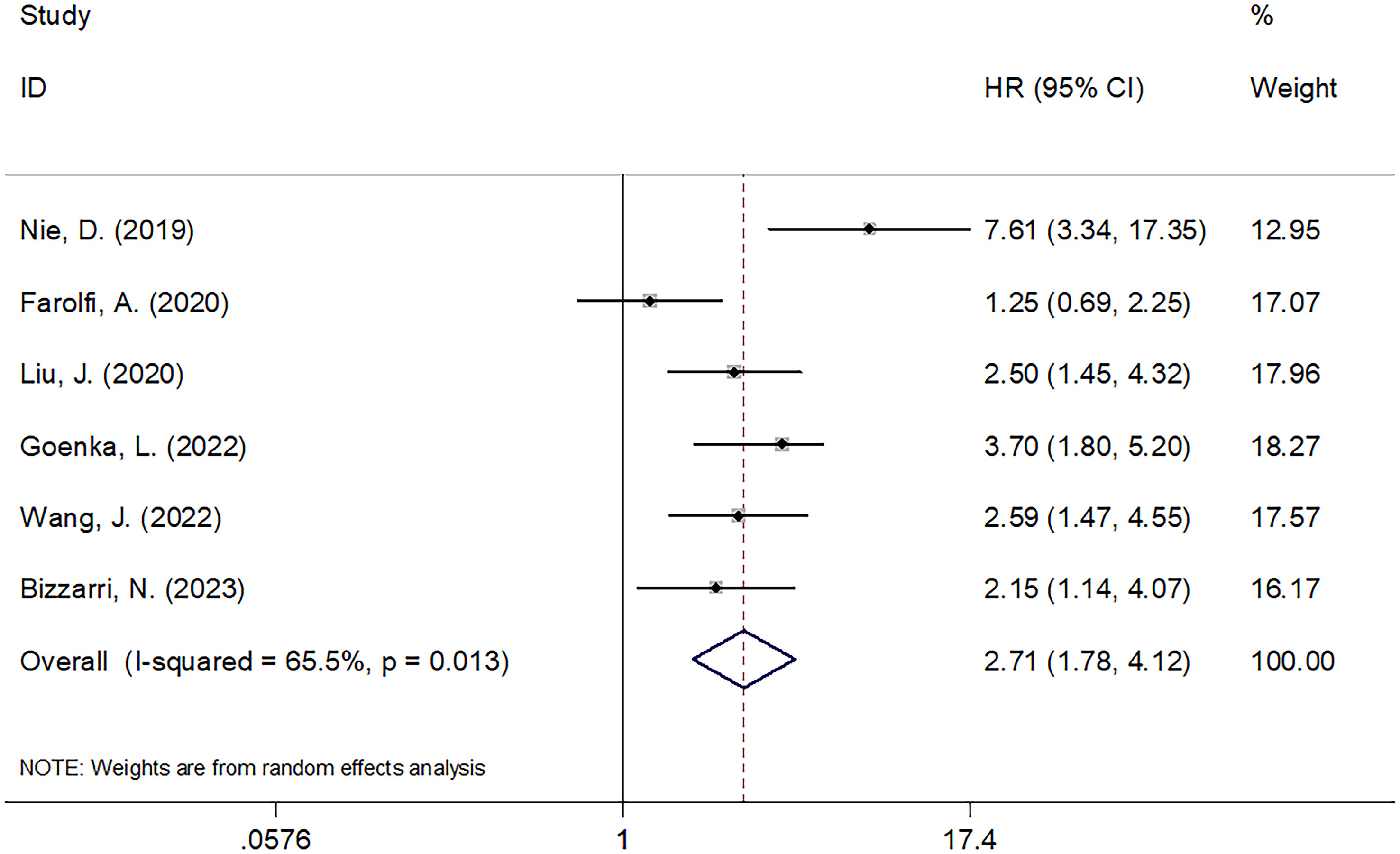
Results: The meta-analysis included six studies involving 1546 patients. The combined results showed that a high SII was significantly associated with poor OS (HR=2.70, 95% CI=1.98–3.67, p<0.001) and poor PFS (HR=2.71, 95% CI=1.78–4.12, p<0.001) in OC patients. These results were confirmed using subgroup and sensitivity analyses.
Conclusion: Our results concluded that a high SII significantly predicted poor OS and PFS in patients with OC. Therefore, it can be speculated that the SII may have an independent effect on the prognosis of OC.
Introduction
There are several histopathological subtypes of ovarian neoplasms, with epithelial ovarian cancer (OC) accounting for the majority (about 90%) of them (1). Globally, OC is ranked 7th and 8th in terms of cancer morbidity and mortality in women, respectively (2). According to GLOBOCAN estimates, there were 313 959 newly diagnosed cases of OC and 207 252 deaths due to OC in 2020 worldwide (3). Approximately 60% of patients with OC are at an advanced stage or have widespread intra-abdominal metastatic disease at the time of initial diagnosis because there are no specific early symptoms (4). The treatments for OC include surgical resection, radiotherapy, chemotherapy, immunotherapy, and targeted therapy (5, 6). Although systemic treatment of OC is becoming increasingly advanced, there has been no significant improvement in the prognosis of OC patients. The five-year survival rate of patients with early-stage OC is 95%, whereas it is less than 30% in patients with advanced OC (stage III or IV) (7). The poor prognosis of OC is partially due to a lack of effective prognostic markers (8). Consequently, it is important to identify new and accurate biomarkers for predicting OC.
Recently, inflammation was found to exert a critical effect on tumor development, recurrence, and metastasis (9). Existing studies have found that systemic inflammation-related indicators can adequately evaluate and predict tumor development and prognosis. These inflammatory response markers include the neutrophil-to-lymphocyte ratio (NLR) (10), platelet-to-lymphocyte ratio (PLR) (11), lymphocyte-to-monocyte ratio (LMR) (12), and the modified Glasgow prognostic score (mGPS) (13). The systemic immune inflammation index (SII) is a blood test-based inflammatory marker that is determined as follows: SII = neutrophil count × platelet count/lymphocyte count. The SII is valuable for predicting cancer prognosis, including colorectal cancer (CRC) (14), esophageal cancer (15), nasopharyngeal carcinoma (NPC) (16), bladder cancer (17), gastric cancer (GC) (18), and neuroblastoma (19). For example, an elevated SII index could predict the diagnosis of postoperative infectious complications and the long-term prognosis of patients with CRC (14). Preoperative SII could predict the survival of patients with thymoma who underwent radical resection (20). A recent study showed that in patients with NPC, SII is a promising indicator for predicting survival, especially the risk of uncontrolled recurrence (16). Some existing studies have analyzed the role of SII in predicting OC prognosis. However, their results remain conflicting (21–26). Few studies have identified high SII as a significant prognostic factor for survival in OC (21, 25, 26). In contrast, other studies have found an insignificant relationship between the SII and prognostic outcomes of OC (22). Consequently, we conducted a comprehensive literature review to identify the precise role of SII in the prognosis of patients with OC.
Materials and methods
Study guideline
This work was conducted and reported in line with the guidelines from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (27).
Search strategy
This study comprehensively and thoroughly searched PubMed, Embase, Web of Science, the China National Knowledge Infrastructure (CNKI), and Cochrane Library databases from inception until May 10, 2023. T The search terms were as follows: (systemic immune-inflammation index or systemic immune-inflammatory index or SII), and (ovarian cancer or ovarian carcinoma or carcinoma of the ovary or ovarian neoplasm or ovary cancer or ovary tumor). There were no restrictions on publication language. Reference lists of the collected articles were manually searched for additional candidate articles.
Selection criteria
We developed the selection criteria based on previous literature (28–30). Studies that met the following criteria were included in the analysis (1): the diagnosis of OC was histologically or pathologically confirmed; (2) studies reporting the hazard ratios (HRs) together with corresponding 95% confidence intervals (CIs) concerning SII before treatment, as well as survival outcomes in OC; (3) the threshold SII was identified; and (4) studies published in Chinese or English. The exclusion criteria were: (1) reviews, case reports, letters, meeting abstracts, or comments; (2) studies with overlapping patients; and (3) animal studies.
Data collection
Eligible studies were selected, and the relevant information was collected independently by two researchers (HM and FY). Any differences between the authors were resolved through mutual negotiation until a consensus was reached. The following information was collected: first author, publication year, country, sample size, study period, age, FIGO stage, treatment, survival outcomes, threshold SII, study center, follow-up, and HRs with 95% CIs.
Quality evaluation
Methodological quality was scored using the Newcastle-Ottawa Scale (NOS) by two independent investigators (HM and FY) (31). The NOS covers three domains: selection quality (0–4 points), comparability (0–2 points), outcome evaluation, and adequacy of follow-up (0–3 points), with a total score ranging between 0 and 9. The scores > 6 indicated high-quality studies.
Statistical analysis
We computed pooled HRs and corresponding 95% CIs to estimate the significance of SII in predicting overall survival (OS) progression-free survival (PFS) in patients with OC. Cochran’s Q test (32) and I2 test (33) were used to assess statistical heterogeneity across studies. As I2 > 50% and p < 0.10 represent substantial heterogeneity, the random-effects model (REM) was applied. Otherwise, the fixed-effects model (FEM) was adopted. Sources of heterogeneity were detected using subgroup analysis. Furthermore, each study was eliminated in sequence during the sensitivity analysis to detect any influence on the pooled results. Publication bias was quantified using Begg’s and Egger’s tests. Statistical significance was set at p < 0.05. All statistical analyses were performed using Stats software (version 12.0; StataCorp LP, College Station, TX, USA).
Ethnics statement
This investigation was based on data from previously published studies. Therefore, ethical approval was not required.
Results
Process of literature selection
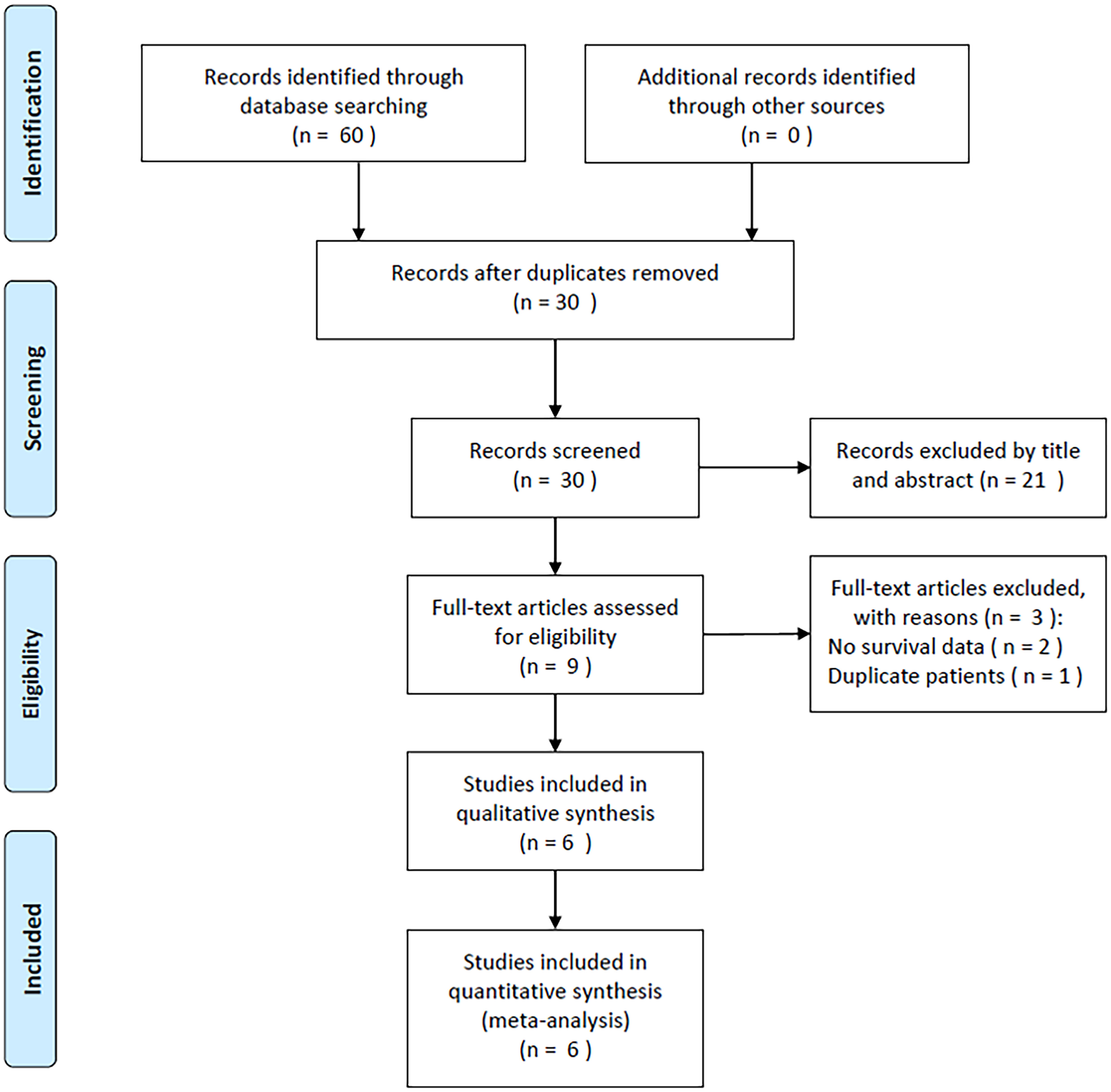
In total, 60 studies were obtained through a preliminary search, and duplicates were removed to obtain 30 studies (Figure 1). After title and abstract reviews, 21 irrelevant studies were excluded. Nine studies were assessed by reading the full texts. Three studies were subsequently eliminated owing to the unavailability of survival data (n=2) and overlapping patient recruitment (n=1). Finally, the meta-analysis included six studies involving 1546 patients (21–26) (Figure 1).
Study characteristics
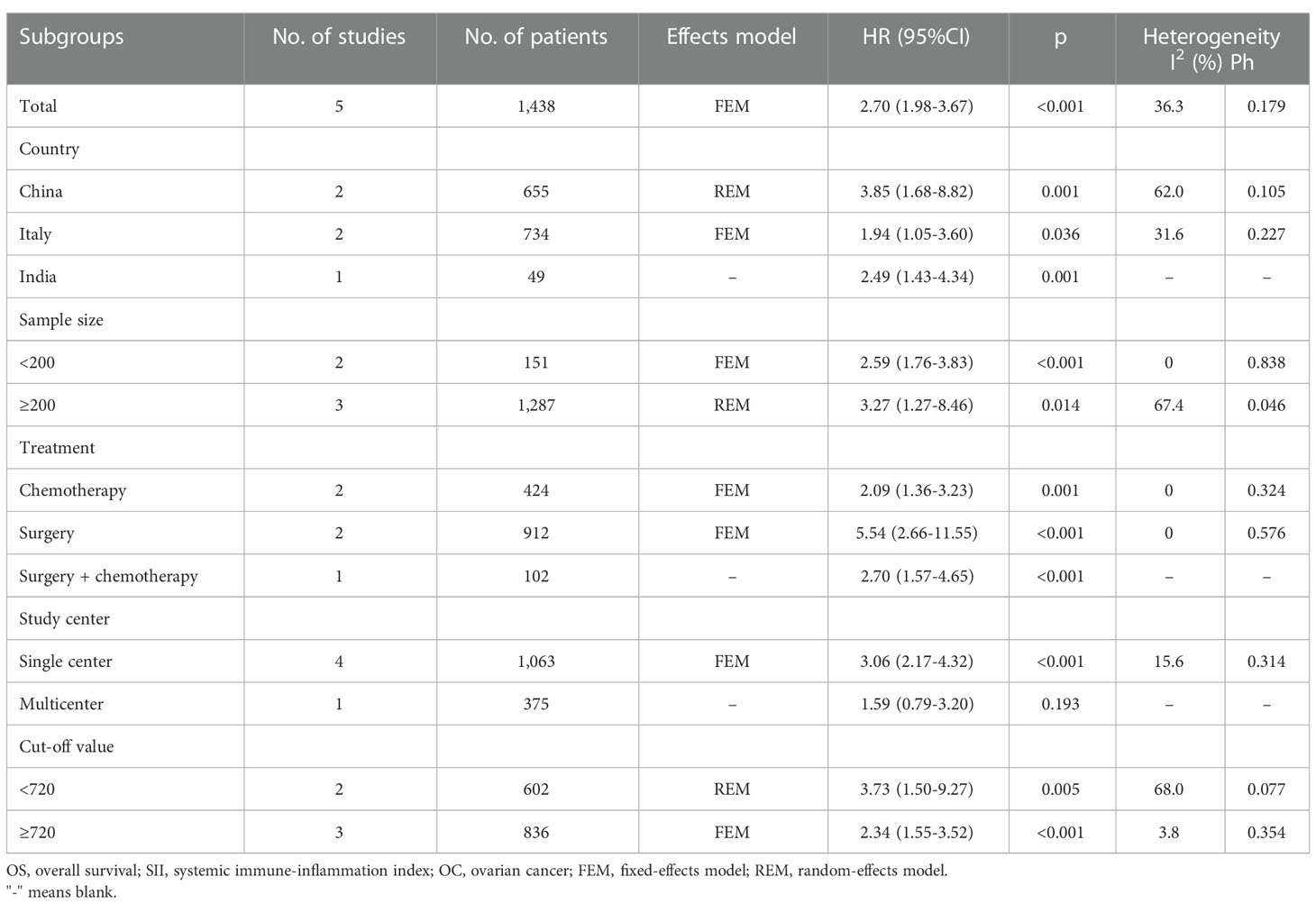
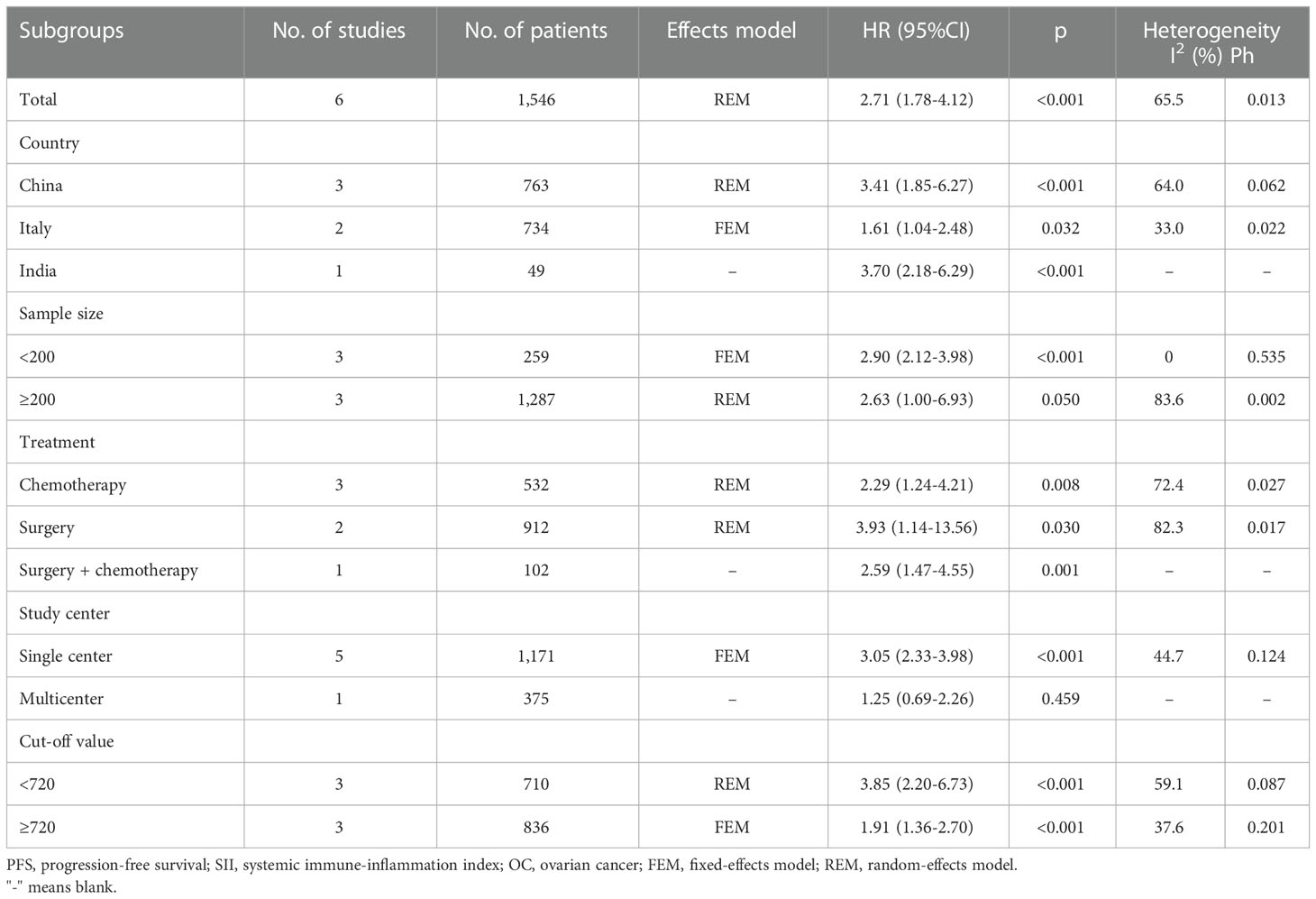
Table 1 summarizes the basic features of the selected studies. The publication years of the enrolled articles, which had a retrospective design, were between 2019 and 2023. Five articles were published in English (21, 22, 24–26) and one in Chinese (23). Three studies were conducted in China (21, 23, 25), two in Italy (22, 26), and one in India (24). The sample sizes were 49–553 (median, 233.5). Three studies included patients with OC who underwent chemotherapy (22–24), two studies included patients treated surgically (21, 26), and one study included patients treated using surgery and chemotherapy (25). The SII threshold was 612–1000 (median: 715.5). Five studies, which included 1438 patients, reported a relationship between SII and OS (21, 22, 24–26). All six studies involving 1546 patients confirmed the role of SII in PFS prediction (21–26). Five studies were performed at a single center (21, 23–26) and one was a multicenter study (22). Moreover, the NOS scores were 6–9, indicating their high quality (Table 1).
SII and OS
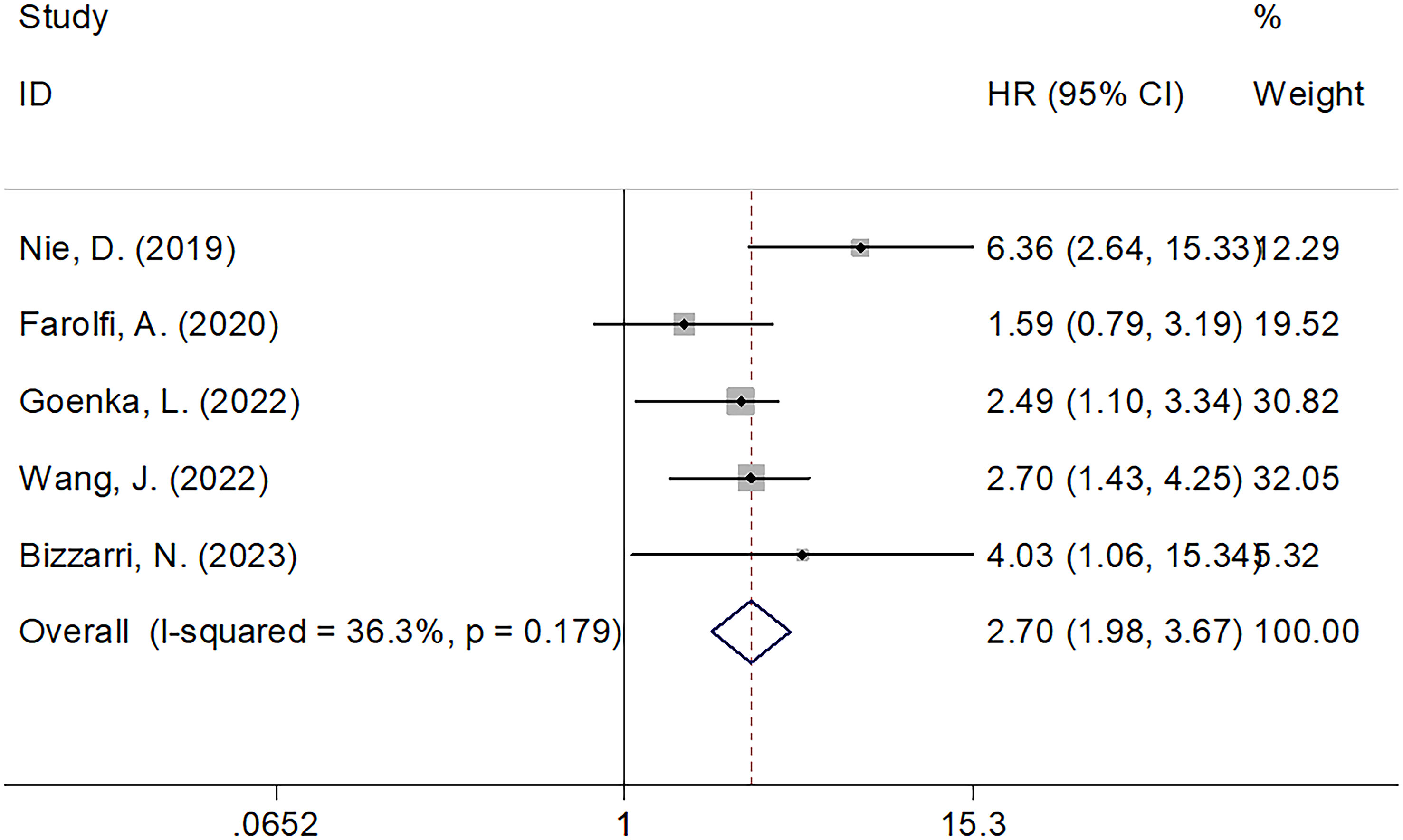
Five studies comprising 1438 patients (21, 22, 24–26) mentioned SII’s performance in predicting the OS of patients with OC. The FEM was used because of the non-obvious heterogeneity (I2 = 36.3%, p=0.179). According to Figure 2; Table 2, pooled HR=2.70, 95% CI=1.98–3.67, p<0.001 were obtained. These suggested that an increased SII remarkably predicted a poor OS. Subgroup analyses were performed based on country, sample size, treatment, study center, and threshold values. As shown in Table 2, the role of SII in predicting OS remained unchanged by country, sample size, treatment, or study center (p<0.05). Moreover, elevated SII still significantly predicted OS among single-center studies (HR=3.06, 95% CI=2.17–4.32, p<0.001; Table 2).
SII and PFS
The relationship between the SII and PFS was investigated in all six studies involving 1546 patients (21–26). We used REM because of its significant heterogeneity (I2 = 65.5%, p=0.013). Pooled HR=2.71, 95% CI=1.78–4.12, p<0.001 were obtained, suggesting the obvious relation between increased pretreatment SII and poor PFS of OC (Table 3; Figure 3). As suggested by the subgroup analysis, an increased SII remarkably predicted poor PFS, regardless of the country, sample size, or threshold (p<0.05; Table 3). Furthermore, as shown in Table 3, an elevated SII still predicted shortened PFS in studies with a sample size <200 (p<0.001) and in single-center studies (p<0.001).
Sensitivity analysis
We determined whether an individual study affected the overall results by performing a sensitivity analysis. None of the articles significantly affected the magnitude of the combined effects on OS and PFS after deletion, indicating that the results were reliable (Figure 4).
Publication bias
Begg’s test and Egger’s test were used to test publication bias. The funnel plots in Figure 5 show that OS and PFS were symmetric. The OS and PFS obtained using Begg’s and Egger’s tests were p=0.806 and 0.424, and p=0.851 and 0.388, respectively. These suggested the pooled outcomes were due to the absence of publication bias (Figure 5).
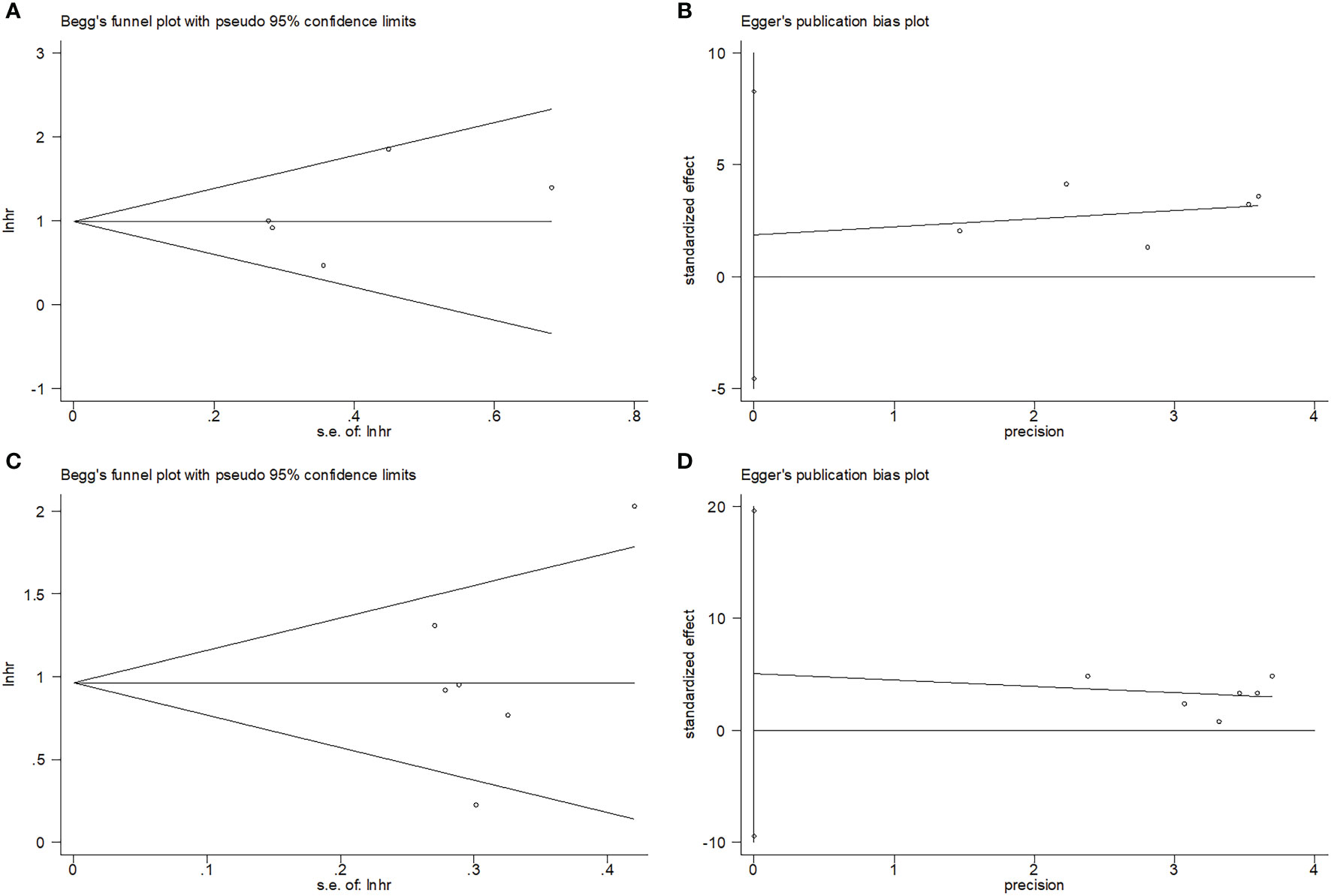
Figure 5 Publication test by Begg’ test and Egger’s test. (A) Begg’s test for OS, p=0.806; (B) Egger’s test for OS, p=0.424; (C) Begg’s test for PFS, p=0.851; and (D) Egger’s test for PFS, p=0.388.
Discussion
The role of SII in predicting OC prognosis remains controversial in existing investigations (21–26). This study collected information from six studies involving 1546 patients and quantitatively identified the role of SII in predicting OS and PFS in patients with OC. Based on the pooled data, an increased SII markedly predicted OS and PFS. Additionally, the SII achieved creditable performance in predicting prognosis, as suggested by sensitivity analysis and publication bias. The SII is a blood-test-derived biomarker and is cheap and simple because neutrophil, lymphocyte, and platelet counts are routinely examined in clinical settings. Therefore, the SII may be an effective inflammatory index to estimate the short- and long-term survival of patients with OC owing to its good discriminatory value. To our knowledge, the present work is the first meta-analysis to investigate the role of SII in predicting OC prognosis.
There is growing evidence that immune cells play an essential role in inflammation, contributing to the generation of chemokines and cytokines that promote tumorigenesis, development, metastasis, and angiogenesis (34, 35). Simultaneously, SII is an inflammatory index derived from neutrophil, lymphocyte, and platelet counts. Therefore, a higher SII may result from higher neutrophil, platelet, and/or lower lymphocyte counts. Moreover, the significant connection between SII and the poor prognosis of OC is interpreted below. First, neutrophils are the first line of natural immune defense that is mobilized by the body when an infection occurs. Previous studies have established that neutrophils regulate tumor cell growth and metastasis by generating different inflammatory factors, such as OSM, TGF-β, HGF, and CXCL8 (36). Neutrophils can help construct an immune-privileged tumor microenvironment, which facilitates cancer cells to escape immune surveillance (37). Second, adenine and adenylate are released by platelets to protect circulatory cancer cells, inducing epithelial-mesenchymal transformation (EMT) while favoring tumor cell colonization and invasion (38). In addition, platelets may promote distal metastasis by activating the NF-κB and Smad pathways, which protect tumor cells against lysis induced by natural killer cells (39, 40). Third, lymphocytes have an important effect on cellular immunity, tumor immune editing, and immune surveillance. An increase in lymphocytes indicates immune pathway activation (41). Tumor-infiltrating lymphocytes (TILs) enhance the suppression of cancer cell growth, invasion, and cytotoxic cell death, thereby having an important effect on cancer defense (42). Impaired innate immunity may result from lymphocytopenia and suppression of lymphocyte activity induced by the systemic immune response (43). As a result, patients with a higher SII had higher neutrophil/platelet counts and lower lymphocyte counts, which are factors that predict a poor prognosis.
Notably, the subgroup analysis demonstrated that the prognostic role of the SII for OS and PFS was not influenced by the treatment methods (Tables 2, 3). These results have important implications for clinical practice. Treatments for OC include systemic therapies such as surgery, chemotherapy, radiotherapy, targeted therapy, poly (ADP-ribose) polymerase (PARP) inhibitors, and immunotherapy (44). Patients with OC may receive one or more treatments at different disease stages (6). Therefore, SII may be an effective prognostic marker for patients with OC undergoing various therapeutic strategies. The disease stages in the included studies were also not uniform (21–26). One study examined early-stage OC (25), whereas the other four studies examined recurrent/relapsed OC (21–24). We combined these studies for the following reasons: first, all eligible studies were selected based on the same inclusion and exclusion criteria; therefore, they were comparable. Second, the subgroup analysis demonstrated that the prognostic value of the SII was not affected by the various treatment methods.
The SII was calculated as SII = neutrophil count × platelet count/lymphocyte count. Therefore, SII can be considered a combination of NLR and PLR. Moreover, SII is more sensitive than NLR or PLR because it uses the product of neutrophil and platelet count as the numerator. SII is also cost-effective and easily available as other blood test-related markers such as NLR, PLR, LMR, and mGPS. We noticed that the HRs and 95% CIs of Nie’s study (21) were higher than those of the other included studies (22–26). These observations could be attributed to several factors. First, the cut-off value in Nie’s study was the lowest among the included studies (Table 1). Second, the patients in Nie’s study underwent surgery, and their survival may have been longer than that of patients undergoing chemotherapy. Therefore, the HRs and 95% CIs in Nie’s study could be higher than those in other studies.
Recently, numerous meta-analyses have analyzed SII’s performance in predicting various cancer types (28, 30, 45–48). In a meta-analysis of 1768 patients, Wang et al. reported that in breast cancer cases (BC), poor OS and disease-free survival (DFS) were considerably correlated with increased SII (48). As discovered by Fu et al. in their meta-analysis of 6925 patients, an increased SII remarkably predicted poor OS and worse DFS in GC (49). According to a recent meta-analysis of 2132 patients, a higher pretreatment SII score predicted poor OS and low cancer-specific survival (CSS), DFS, and PFS in pancreatic cancer (28). In the meta-analysis involving eight studies carried out by Zhou et al., the higher SII remarkably predicted poor OS and extensive-stage among patients with small cell lung cancer (SCLC) (30). As shown by Cao et al. in a meta-analysis of 11 studies, an increased SII before treatment remarkably predicted dismal OS, CSS, PFS, and recurrence-free survival (RFS) inpatients with bladder cancer (50).
However, this study had some limitations. First, there were variations in the treatment of patients with OC in the enrolled studies, possibly affecting survival and causing heterogeneity. Different chemotherapy treatments were administered to 532 patients (22–25), of whom 108 patients received bevacizumab (23), and 423 patients received platinum-based drugs (22, 24, 25). More studies are needed to investigate the prognostic role of SII in patients with OC who received bevacizumab. Neoadjuvant chemotherapy (NACT) is commonly used to treat OC. The prognostic value of SII in patients with OC treated with NACT + surgery needs to be verified. Second, although the SII was markedly related to OS and PFS, the studies had small sample sizes and few publications. Third, retrospective articles were included in the meta-analysis, which may have induced a selection bias. Therefore, large prospective multiregional studies should be conducted to validate our findings.
Conclusions
In conclusion, the present meta-analysis showed that an increased SII significantly predicted worse OS and PFS in patients with OC. Consequently, we can speculate that SII may have an independent effect on the prognosis of OC.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author contributions
HM and FY contributed to the study design and data collection. Statistical analyses and interpretation of results were performed by HM and FY. HM drafted the manuscript. FY revised the manuscript and edited the language. All authors made the critical revision of the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
SII, systemic immune-inflammation index; OC, ovarian cancer; CNKI, China National Knowledge Infrastructure; OS, overall survival; PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; mGPS, modified Glasgow prognostic score; CRC, colorectal cancer; NPC, nasopharyngeal carcinoma; GC, gastric cancer; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; NOS, Newcastle-Ottawa Scale; REM, random-effects model; FEM, fixed-effects model; TIL, tumor-infiltrating lymphocyte; BC, breast cancer; DFS, disease-free survival; CSS, cancer-specific survival; SCLC, small cell lung cancer; RFS, recurrence-free survival.
References
1. Gaona-Luviano P, Medina-Gaona LA, Magaña-Pérez K. Epidemiology of ovarian cancer. Chin Clin Oncol (2020) 9(4):47. doi: 10.21037/cco-20-34
2. Momenimovahed Z, Tiznobaik A, Taheri S, Salehiniya H. Ovarian cancer in the world: epidemiology and risk factors. Int J Womens Health (2019) 11:287–99. doi: 10.2147/ijwh.S197604
3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
4. Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, et al. Ovarian cancer statistics, 2018. CA: Cancer J Clin (2018) 68(4):284–96. doi: 10.3322/caac.21456
5. Morand S, Devanaboyina M, Staats H, Stanbery L, Nemunaitis J. Ovarian cancer immunotherapy and personalized medicine. Int J Mol Sci (2021) 22(12):6532. doi: 10.3390/ijms22126532
6. Kuroki L, Guntupalli SR. Treatment of epithelial ovarian cancer. BMJ (Clinical Res ed) (2020) 371:m3773. doi: 10.1136/bmj.m3773
7. Prat J. Pathology of borderline and invasive cancers. Best Pract Res Clin obstetrics Gynaecol (2017) 41:15–30. doi: 10.1016/j.bpobgyn.2016.08.007
8. Atallah GA, Abd Aziz NH, Teik CK, Shafiee MN, Kampan NC. New predictive biomarkers for ovarian cancer. Diagnost (Basel Switzerland) (2021) 11(3):465. doi: 10.3390/diagnostics11030465
9. Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature (2017) 541(7637):321–30. doi: 10.1038/nature21349
10. Miao Y, Yan Q, Li S, Li B, Feng Y. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio are predictive of chemotherapeutic response and prognosis in epithelial ovarian cancer patients treated with platinum-based chemotherapy. Cancer Biomark: Section A Dis Markers (2016) 17(1):33–40. doi: 10.3233/cbm-160614
11. Hu D, Lin Y, Liu F, Zeng L, Ouyang X, Wang K, et al. Elevated preoperative platelet to lymphocyte ratio indicates poor survival in patients with resected high-grade serous ovarian carcinoma. Clin Lab (2016) 62(8):1443–9. doi: 10.7754/Clin.Lab.2016.151137
12. Xiang J, Zhou L, Li X, Bao W, Chen T, Xi X, et al. Preoperative monocyte-to-Lymphocyte ratio in peripheral blood predicts stages, metastasis, and histological grades in patients with ovarian cancer. Transl Oncol (2017) 10(1):33–9. doi: 10.1016/j.tranon.2016.10.006
13. Roncolato FT, Berton-Rigaud D, O’Connell R, Lanceley A, Sehouli J, Buizen L, et al. Validation of the modified Glasgow prognostic score (mGPS) in recurrent ovarian cancer (ROC) - analysis of patients enrolled in the GCIG symptom benefit study (SBS). Gynecol Oncol (2018) 148(1):36–41. doi: 10.1016/j.ygyno.2017.10.019
14. Feng L, Xu R, Lin L, Liao X. Effect of the systemic immune-inflammation index on postoperative complications and the long-term prognosis of patients with colorectal cancer: a retrospective cohort study. J Gastrointest Oncol (2022) 13(5):2333–9. doi: 10.21037/jgo-22-716
15. Han W, Weng K, Zhang P, Hong Z. Predictive value of systemic immune-inflammation index for pathological complete response in patients receiving neoadjuvant immunochemotherapy for locally advanced esophageal cancer. Front Surg (2022) 9:1091601:. doi: 10.3389/fsurg.2022.1091601
16. Yuan X, Feng H, Huang H, Li J, Wu S, Yuan Y, et al. Systemic immune-inflammation index during treatment predicts prognosis and guides clinical treatment in patients with nasopharyngeal carcinoma. J Cancer Res Clin Oncol (2023) 149(1):191–202. doi: 10.1007/s00432-022-04506-z
17. Zhang S, Du J, Zhong X, Tan P, Xu H, Zhang J, et al. The prognostic value of the systemic immune-inflammation index for patients with bladder cancer after radical cystectomy. Front Immunol (2022) 13:1072433:. doi: 10.3389/fimmu.2022.1072433
18. Uzunoglu H, Kaya S. Does systemic immune inflammation index have predictive value in gastric cancer prognosis? North Clin Istanb (2023) 10(1):24–32. doi: 10.14744/nci.2021.71324
19. Cesur IB, Özçelik Z. Systemic immune-inflammation index may predict mortality in neuroblastoma. Cureus (2023) 15(3):e35705. doi: 10.7759/cureus.35705
20. Li Q, Pu Y, Gong Z, Yu Y, Sun W, Cheng Z, et al. Preoperative systemic immune-inflammation index for predicting the prognosis of thymoma with radical resection. Thorac Cancer (2023) 14(13):1192–200. doi: 10.1111/1759-7714.14854
21. Nie D, Gong H, Mao X, Li Z. Systemic immune-inflammation index predicts prognosis in patients with epithelial ovarian cancer: a retrospective study. Gynecol Oncol (2019) 152(2):259–64. doi: 10.1016/j.ygyno.2018.11.034
22. Farolfi A, Scarpi E, Greco F, Bergamini A, Longo L, Pignata S, et al. Inflammatory indexes as predictive factors for platinum sensitivity and as prognostic factors in recurrent epithelial ovarian cancer patients: a MITO24 retrospective study. Sci Rep (2020) 10(1):18190. doi: 10.1038/s41598-020-75316-x
23. Liu J, Tu J, Yang J, Huang X, Pang Z, Wu J, et al. Correlation between systemic immune inflammation index and treatment effect of bevacizumab in patients with recurrent ovarian cancer. Mil Med J S Chin (2020) 34(08):535–8 + 42. doi: 10.13730/j.issn.1009-2595.2020.08.003
24. Goenka L, Thejeswar N, Dubashi B, Kayal S, Ganesan P. Systemic immune-inflammation index predicts outcomes in platinum-resistant relapsed ovarian cancer. Indian J Med Paediatric Oncol (2022) 43. doi: 10.1055/s-0042-1749399
25. Wang J, Yin S, Chen K. Predictive value of the systemic immune-inflammation index for the efficacy of neoadjuvant chemotherapy and prognosis in patients with stage III ovarian cancer-a retrospective cohort study. Gland Surg (2022) 11(10):1639–46. doi: 10.21037/gs-22-459
26. Bizzarri N, D’Indinosante M, Marchetti C, Tudisco R, Turchiano F, Scambia G, et al. The prognostic role of systemic inflammatory markers in apparent early-stage ovarian cancer. Int J Clin Oncol (2023) 28(2):314–20. doi: 10.1007/s10147-022-02272-z
27. Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Internal Med (2009) 151(4):264–W64. doi: 10.7326/0003-4819-151-4-200908180-00135
28. Li M, Li Z, Wang Z, Yue C, Hu W, Lu H. Prognostic value of systemic immune-inflammation index in patients with pancreatic cancer: a meta-analysis. Clin Exp Med (2022) 22(4):637–46. doi: 10.1007/s10238-021-00785-x
29. Qiu Y, Zhang Z, Chen Y. Prognostic value of pretreatment systemic immune-inflammation index in gastric cancer: a meta-analysis. Front Oncol (2021) 11::537140. doi: 10.3389/fonc.2021.537140
30. Zhou Y, Dai M, Zhang Z. Prognostic significance of the systemic immune-inflammation index (SII) in patients with small cell lung cancer: a meta-analysis. Front Oncol (2022) 12:814727. doi: 10.3389/fonc.2022.814727
31. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
32. Cochran W. The combination of estimates from different experiments. Biometrics (1954) 10:101–29. doi: 10.2307/3001666
33. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21(11):1539–58. doi: 10.1002/sim.1186
34. Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer (2013) 13(11):759–71. doi: 10.1038/nrc3611
35. Cantiello F, Russo GI, Vartolomei MD, Farhan ARA, Terracciano D, Musi G, et al. Systemic inflammatory markers and oncologic outcomes in patients with high-risk non-muscle-invasive urothelial bladder cancer. Eur Urol Oncol (2018) 1(5):403–10. doi: 10.1016/j.euo.2018.06.006
36. Tecchio C, Scapini P, Pizzolo G, Cassatella MA. On the cytokines produced by human neutrophils in tumors. Semin Cancer Biol (2013) 23(3):159–70. doi: 10.1016/j.semcancer.2013.02.004
37. Houghton AM, Rzymkiewicz DM, Ji H, Gregory AD, Egea EE, Metz HE, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med (2010) 16(2):219–23. doi: 10.1038/nm.2084
38. Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis (2003) 6(4):283–7. doi: 10.1023/B:AGEN.0000029415.62384.ba
39. Haemmerle M, Stone RL, Menter DG, Afshar-Kharghan V, Sood AK. The platelet lifeline to cancer: challenges and opportunities. Cancer Cell (2018) 33(6):965–83. doi: 10.1016/j.ccell.2018.03.002
40. Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemostasis: JTH (2011) 9(2):237–49. doi: 10.1111/j.1538-7836.2010.04131.x
41. Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer (2011) 105(1):93–103. doi: 10.1038/bjc.2011.189
42. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science (2011) 331(6024):1565–70. doi: 10.1126/science.1203486
43. Schalk E, Zeremski V, Fischer T. Impact of lymphopenia on prognosis of patients with primary central nervous system lymphoma. Eur J Cancer (Oxford England: 1990) (2017) 75:280–3. doi: 10.1016/j.ejca.2016.11.035
44. Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA: Cancer J Clin (2019) 69(4):280–304. doi: 10.3322/caac.21559
45. Yang C, Hu BW, Tang F, Zhang Q, Quan W, Wang J, et al. Prognostic value of systemic immune-inflammation index (SII) in patients with glioblastoma: a comprehensive study based on meta-analysis and retrospective single-center analysis. J Clin Med (2022) 11(24):7514. doi: 10.3390/jcm11247514
46. Liu XC, Jiang YP, Sun XG, Zhao JJ, Zhang LY, Jing X. Prognostic significance of the systemic immune-inflammation index in patients with cholangiocarcinoma: a meta-analysis. Front Oncol (2022) 12:938549:. doi: 10.3389/fonc.2022.938549
47. Li X, Zhang S, Lu J, Li C, Li N. The prognostic value of systemic immune-inflammation index in surgical esophageal cancer patients: an updated meta-analysis. Front Surg (2022) 9:922595. doi: 10.3389/fsurg.2022.922595
48. Wang Y, Cong ZJ, Li ZG, Huang GL. Clinicopathological and prognostic importance of systemic immune inflammation index (SII) in breast cancer patients: a meta-analysis. Int J Clin Exp Med (2020) 13(8):5527–49.
49. Fu S, Yan J, Tan Y, Liu D. Prognostic value of systemic immune-inflammatory index in survival outcome in gastric cancer: a meta-analysis. J Gastrointest Oncol (2021) 12(2):344–54. doi: 10.21037/jgo-20-252
Keywords: SII, ovarian cancer, meta-analysis, prognosis, survival
Citation: Mao H and Yang F (2023) Prognostic significance of systemic immune-inflammation index in patients with ovarian cancer: a meta-analysis. Front. Oncol. 13:1193962. doi: 10.3389/fonc.2023.1193962
Received: 26 March 2023; Accepted: 22 May 2023;
Published: 05 June 2023.
Edited by:
Yinu Wang, Northwestern University, United StatesCopyright © 2023 Mao and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fan Yang, eWFuZ3lhbmd5YW5nODk3NTdAMTYzLmNvbQ==
 Huaying Mao1
Huaying Mao1 Fan Yang
Fan Yang