- 1Clinical Laboratory, Huzhou Central Hospital, Affiliated Central Hospital of Huzhou University, Huzhou, Zhejiang, China
- 2Clinical Laboratory, Huzhou Maternity and Child Health Care Hospital, Huzhou, Zhejiang, China
Background: Whether the albumin-to-globulin ratio (AGR) predicts the prognosis of renal cell carcinoma (RCC) remains controversial. Herein, we performed a meta-analysis to critically evaluate the relationship between the AGR and RCC prognosis, as well as the association between the AGR and the clinicopathological characteristics of RCC.
Methods: The PubMed, Web of Science, Embase, and Cochrane Library databases were thoroughly and comprehensively searched from their inception until 24 June 2023. To determine the predictive significance of the AGR, hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) were calculated from the pooled data. The relationship between the AGR and the clinicopathological features of RCC was evaluated by estimating odds ratios (ORs) and 95% CIs in subgroup analyses.
Results: The meta-analysis included nine articles involving 5,671 RCC cases. A low AGR significantly correlated with worse overall survival (OS) (HR = 1.82, 95% CI = 1.37–2.41, p <0.001) and progression-free survival (PFS) (HR = 2.44, 95% CI = 1.61–3.70, p <0.001). Analysis of the pooled data also revealed significant associations between a low AGR and the following: female sex (OR = 1.48, 95% CI = 1.31–1.67, p <0.001), pT stage T3–T4 (OR = 4.12, 95% CI = 2.93–5.79, p <0.001), pN stage N1 (OR = 3.99, 95% CI = 2.40–6.64, p <0.001), tumor necrosis (OR = 3.83, 95% CI = 2.23–6.59, p <0.001), and Fuhrman grade 3–4 (OR = 1.82, 95% CI = 1.34–2.42, p <0.001). The AGR was not related to histology (OR = 0.83, 95% CI = 0.60–1.15, p = 0.267).
Conclusion: In patients with RCC, a low AGR strongly predicted poor OS and PFS and significantly correlated with clinicopathological features indicative of disease progression.
Introduction
Renal cell carcinoma (RCC) accounts for approximately 2.2% of all cancer cases, and the median age at diagnosis age is 64 years (1, 2). Most (approximately 90%) malignant solid lesions in the kidney are RCCs (3). Globally, there were 431,288 new cases of RCC and 179,368 deaths from RCC in 2020 (1). Since 2012, the number of people developing RCC worldwide has increased by 22% according to the World Cancer Research Fund International (4).
RCC has three major subtypes: clear cell (approximately 80% of cases), papillary (approximately 15%), and chromophobe (approximately 5%) (5, 6). Despite significant advances in therapeutic strategies, RCC still has a poor prognosis. The overall 5-year survival rate is 8–59% (7); for advanced disease, it is <20%, and for metastatic RCC (mRCC), the median overall survival (OS) time is 10 months (8, 9). These poor outcomes may partly reflect the lack of powerful prognostic indicators (10). Consequently, identifying novel and effective biomarkers for prognosis prediction in patients with RCC in clinical settings is imperative.
Current evidence indicates that systemic chronic inflammation and malnutrition contribute to carcinogenesis and tumor progression (11, 12). Albumin (ALB) and globulin (GLB) are two major serum proteins that reflect nutritional and inflammatory status. The ALB-to-GLB ratio (AGR) is an established marker in oncology; it is calculated as follows: AGR = serum ALB/(total serum protein - serum ALB) (13). A low AGR has been widely associated with poor outcomes in various cancers, such as non-small-cell lung cancer (14), esophageal cancer (15), cervical cancer (16), multiple myeloma (17), and pancreatic cancer (18).
Previous studies have explored the prognostic significance of the AGR in patients with RCC (19–27). However, inconsistent results were obtained: in some studies, a low AGR significantly predicted worse survival (23, 26, 27), whereas in others, the AGR was unrelated to prognosis (21, 24, 25). To provide resolution, we performed a meta-analysis that evaluated the relationship between the AGR and RCC prognosis, as well as between the AGR and the clinicopathological characteristics of RCC.
Materials and methods
Study guideline
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (28).
Ethics statement
This study used data from previous articles, and approval from an ethics committee or institutional review board was therefore waived.
Search strategy
We thoroughly and comprehensively searched the PubMed, Web of Science, Embase, and Cochrane Library databases from their inception until 24 June 2023. Only English publications were selected. The following combinations of Medical Subject Headings and other terms were used: (albumin to globulin ratio, albumin/globulin ratio, AGR, or albumin-globulin ratio) and (renal cancer, kidney neoplasm, kidney cancer, renal cell carcinoma, renal carcinoma, or RCC). The references of the retrieved studies were manually examined to identify additional relevant studies.
Study eligibility criteria
We included English-language articles that reported the following: (1) the association between the AGR and survival outcome [e.g., OS, recurrence-free survival, cancer-specific survival (CSS), and progression-free survival (PFS)]; (2) hazard ratios (HRs) with 95% confidence intervals (CIs) or data that allowed their calculation; and (3) the threshold used to stratify a high/low AGR. Additional inclusion criteria were a pathological diagnosis of RCC and measurement of serum ALB and GLB levels before treatment. Meeting abstracts, reviews, case reports, letters, comments, studies with overlapping patients, and animal studies were excluded.
Data collection and quality evaluation
Two researchers (HM and FY) independently screened the retrieved articles and extracted and crosschecked the data. Disagreements between the two investigators were settled through negotiation until a consensus was reached. The data collected in this study included first author, country, publication year, sample size, study period, patient age and sex, number of study centers (single or multiple), metastatic status, treatment, follow-up period, AGR threshold, survival endpoints, type of survival analysis (univariate or multivariate), and HRs with 95% CIs. We used the Newcastle-Ottawa Scale (NOS) (29) to evaluate the quality of the study in three domains: selection (0–4 points), comparability (0–2 points), and outcome assessment (0–3 points), yielding a total score of 0–9. Articles with NOS scores ≥6 were considered high-quality.
Statistical analysis
HRs with 95% CIs were calculated to determine whether the AGR significantly predicted survival outcome. Inter-study heterogeneity was assessed using Higgin’s I2 statistic (30) and Cochran’s Q test (31). P values <0.10 and I2 values >50% indicated heterogeneity. Random-effects and fixed-effects models are used for heterogeneous and non-heterogeneous data, respectively.
To further explore the ability of the AGR to predict RCC prognosis, subgroup analyses stratified by country, sample size, study center number, metastatic status, treatment, AGR threshold, and type of survival analysis were performed. Associations between the AGR and the clinicopathological features of RCC were evaluated by determining odds ratios (ORs) with 95% CIs. Begg’s test (32) and Egger’s test (33) were used to assess publication bias. Statistical analyses were performed using Stata software, version 12.0 (Stata Corporation, College Station, TX, USA). P values <0.05 indicated statistical significance.
Results
The literature selection process
A total of 155 studies were retrieved in the preliminary search; 37 duplicates were eliminated, leaving 118 studies (Figure 1). An additional 97 studies were excluded owing to irrelevance as determined upon title and abstract screening. Among the remaining 21 studies, 12 were discarded following full-text assessment: six did not analyze AGRs, four lacked survival data, one was a meeting abstract, and one had overlapping patients. Finally, nine articles involving 5,671 cases (19–27) were included in the present study.
Study features
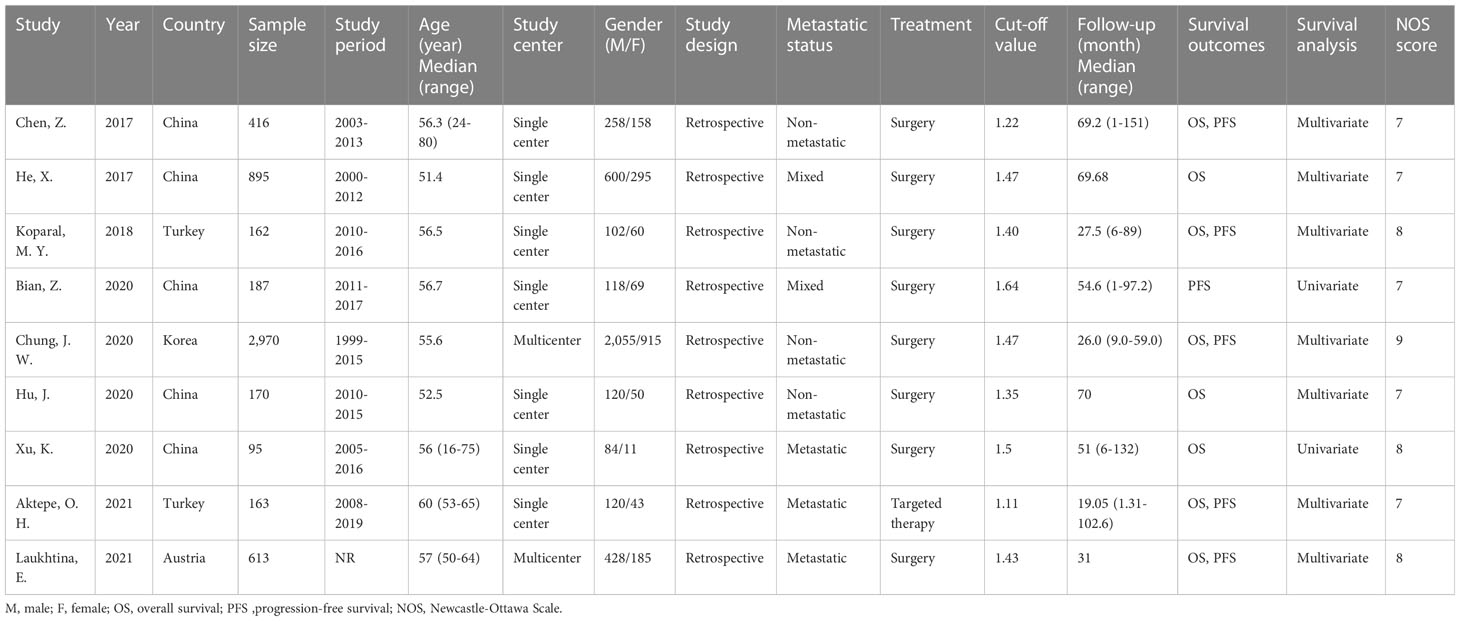
Table 1 lists the basic features of the nine included studies. All studies were published between 2017 and 2021; five were performed in China (19, 20, 22, 24, 25), two in Turkey (21, 26), and one each in Korea (23) and Austria (27). All studies were retrospective (19–27), with sample sizes of 95–2,970 (median, 187). Seven studies were single-center (19–22, 24–26) and two were multicenter (23, 27). Four studies examined patients with non-mRCC (19, 21, 23, 24), three examined patients with mRCC (25–27), and two included patients at multiple stages (20, 22). Surgery was performed in eight studies (19–25, 27) and targeted therapy in one study (26). The threshold AGR was 1.11–1.64 (median, 1.43). Eight (19–21, 23–27) and six (19, 21–23, 26, 27) studies assessed the significance of the AGR in predicting OS and PFS, respectively. Multivariate regression analyses with HRs and CIs were performed in seven studies (19–21, 23, 24, 26, 27) and univariate analyses in two (22, 25). The NOS scores for the included articles ranged from 7 to 9, which indicated high quality.
AGR and OS
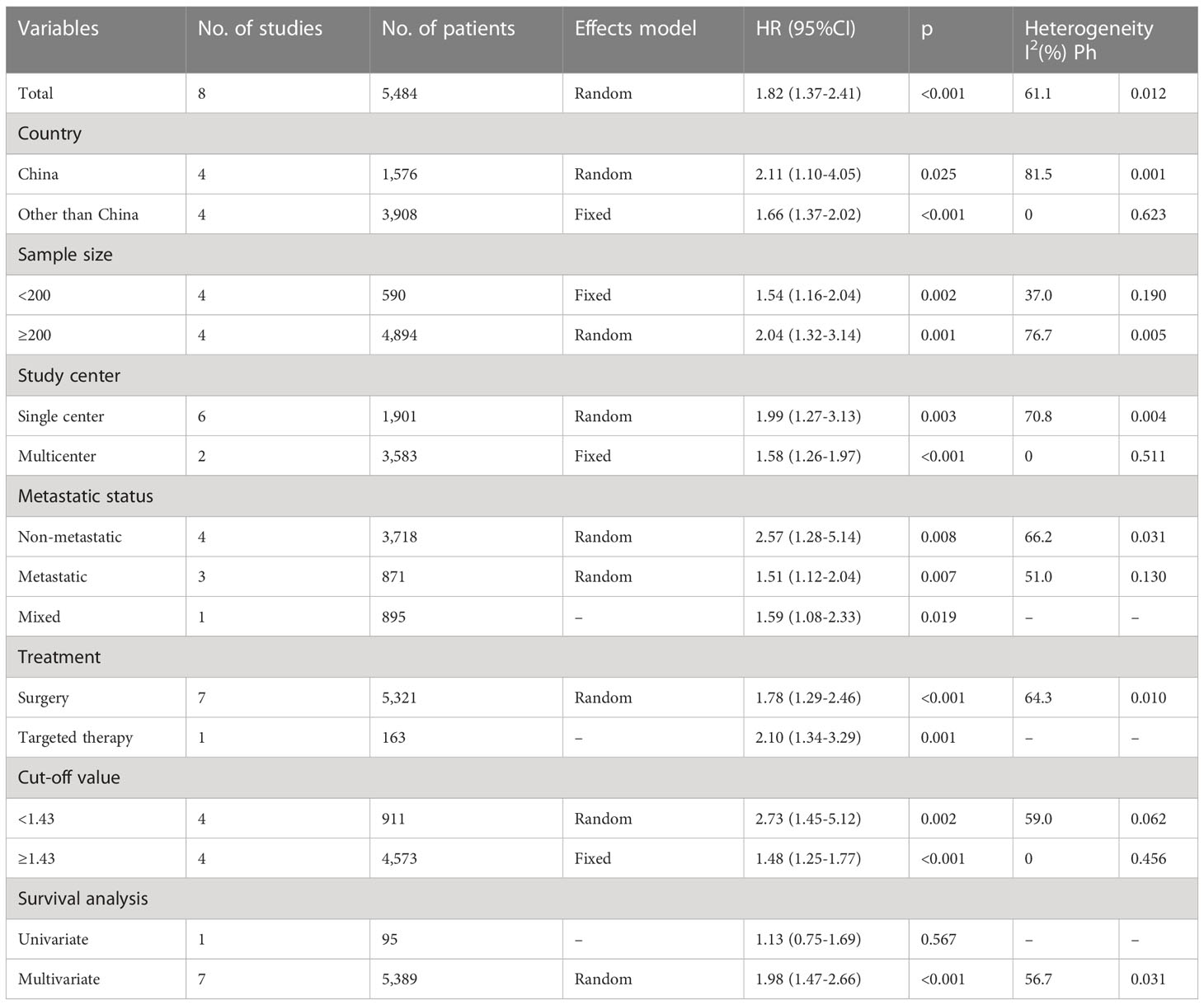
Eight studies with 5,484 patients provided information regarding the relationship between the pretreatment AGR and OS (19–21, 23–27). Owing to obvious heterogeneity (I2 = 61.1%, p = 0.012), we used a random-effects model to collectively analyze the data in these studies. We found that a low AGR remarkably predicted worse OS (HR = 1.82, 95% CI = 1.37–2.41, p <0.001; Table 2 and Figure 2). In subgroup analyses, the ability of the AGR to predict OS was unaffected by sample size, country, study center number, metastatic status, treatment, or AGR threshold (Table 2). Moreover, a low AGR significantly predicted poor OS in a multivariate analysis (HR = 1.98, 95% CI = 1.47–2.66, p <0.001; Table 2).
AGR and PFS
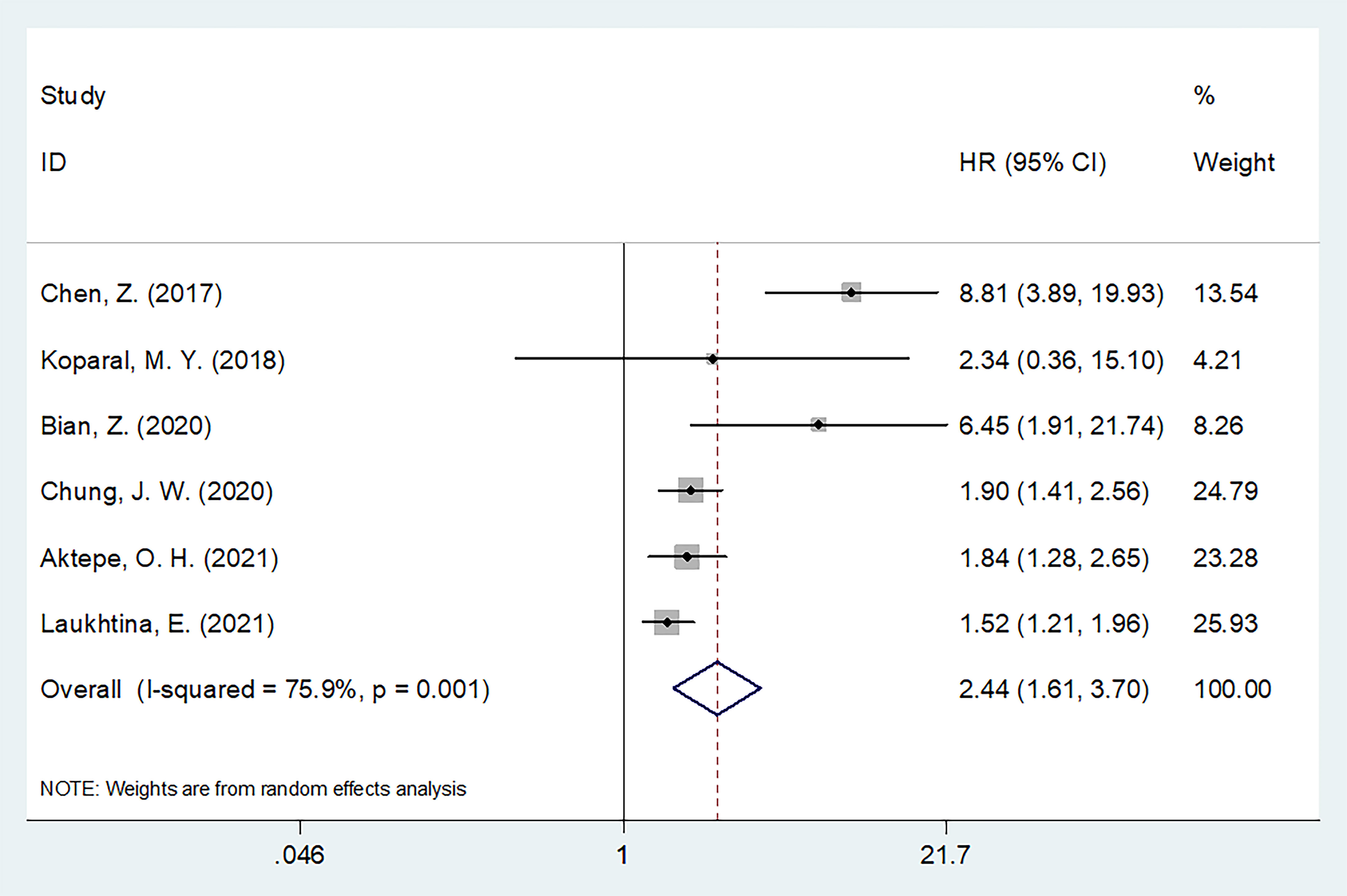
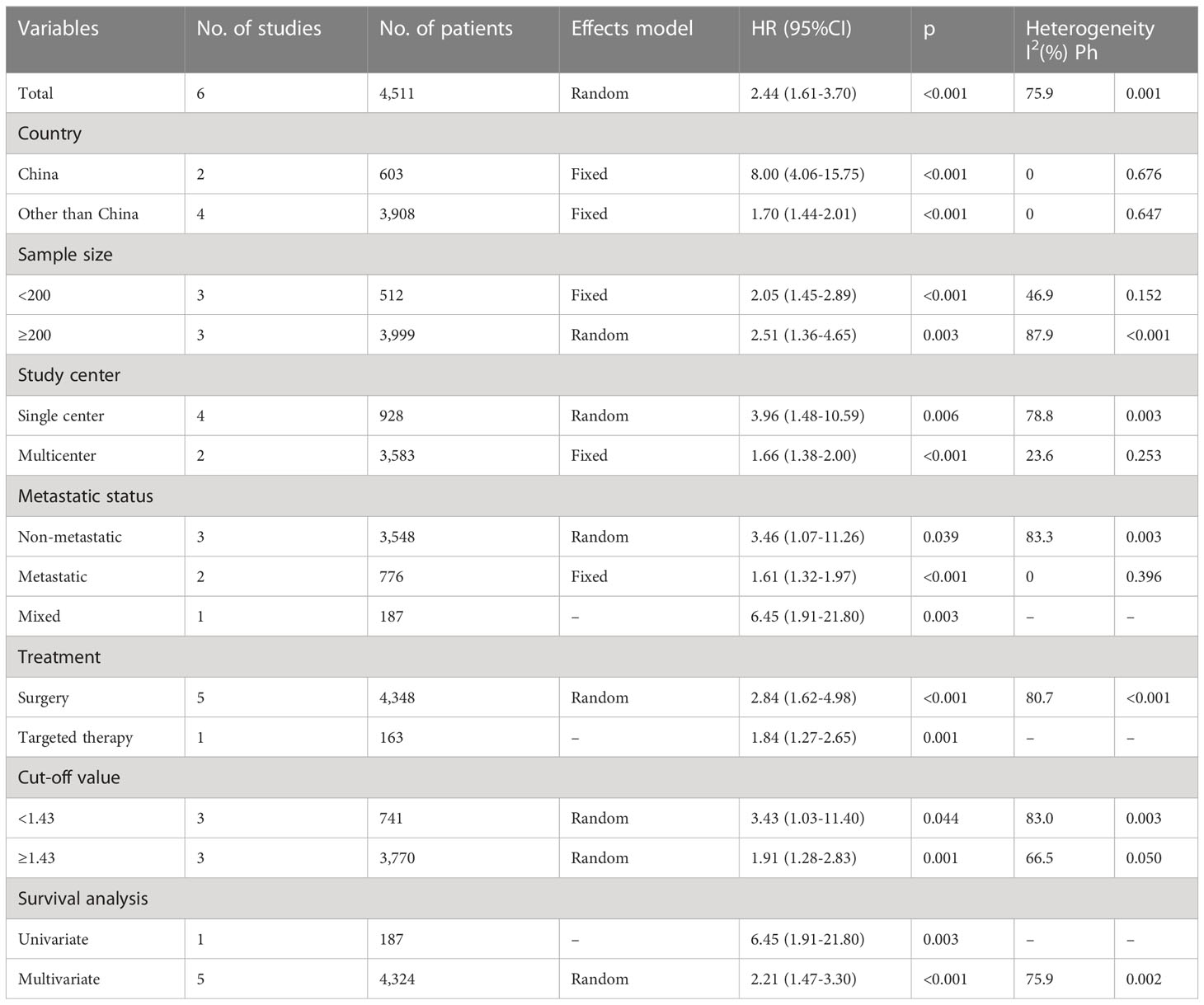
Six articles with 4,511 patients examined the relationship between the pretreatment AGR and PFS (19, 21–23, 26, 27). Owing to obvious heterogeneity (I2 = 75.9%, p = 0.001), we used a random-effects model to collectively analyze the data in these articles. We found that a low AGR strongly predicted worse PFS (HR = 2.44, 95% CI = 1.61–3.70, p <0.001; Figure 3 and Table 3). In subgroup analyses, a low AGR significantly predicted poor PFS regardless of country, sample size, study center number, metastatic status, treatment, cut-off value, or type of survival analysis (Table 3).
Relationship between the AGR and clinicopathological features
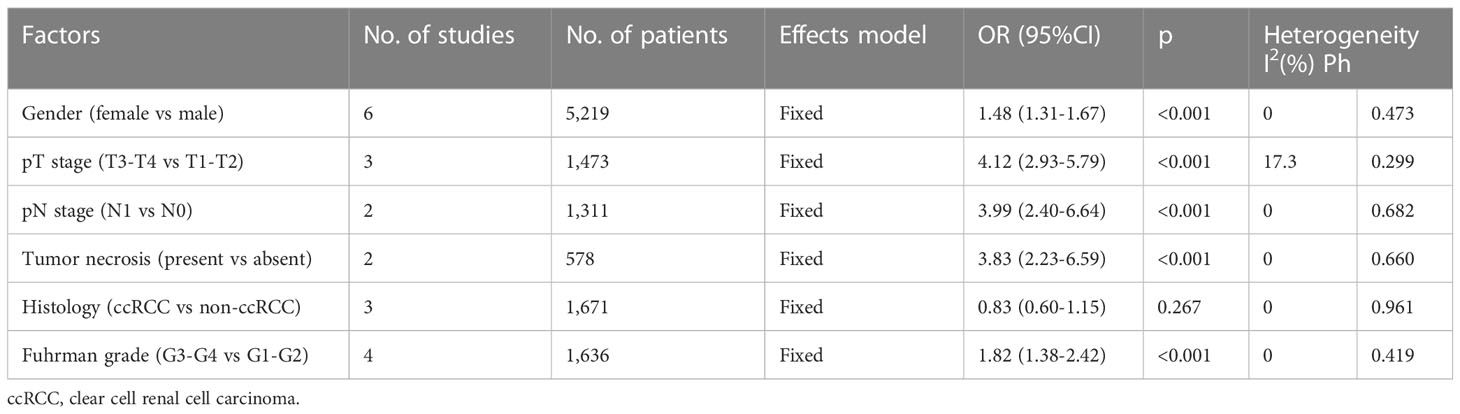
The relationship between the pretreatment AGR and the clinicopathological features of RCC was analyzed using data from six studies with 5,219 patients (19–21, 23, 26, 27). The clinicopathological characteristics examined were as follows: sex (female vs male), pT stage (T3–T4 vs T1–T2), pN stage (N1 vs N0), tumor necrosis (present vs absent), histology (clear cell RCC vs non-clear cell RCC), and Fuhrman grade (3–4 vs 1–2). As shown in Table 4 and Figure 4, a low AGR closely correlated with the female sex (OR = 1.48, 95% CI = 1.31–1.67, p <0.001), pT stage T3–T4 (OR = 4.12, 95% CI = 2.93–5.79, p <0.001), pN stage N1 (OR = 3.99, 95% CI = 2.40–6.64, p <0.001), presence of tumor necrosis (OR = 3.83, 95% CI = 2.23–6.59, p <0.001), and Fuhrman grade 3–4 (OR = 1.82, 95% CI = 1.34–2.42, p <0.001). The AGR was not related to histology (OR = 0.83, 95% CI = 0.60–1.15, p = 0.267).
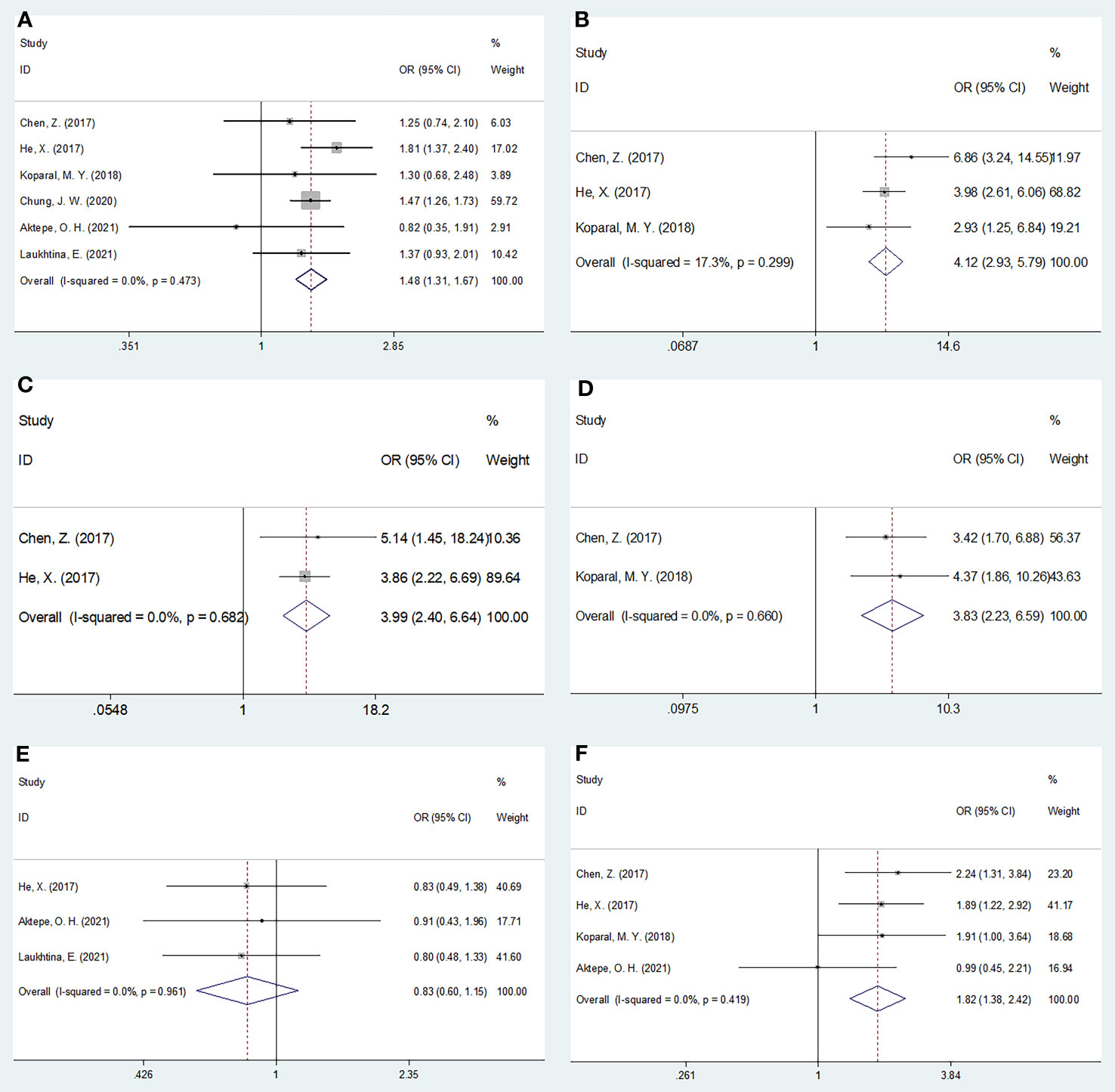
Figure 4 Forest plots evaluating the association between AGR and clinicopathological features in RCC. (A) Gender (female vs male); (B) pT stage (T3-T4 vs T1-T2); (C) pN stage (N1 vs N0); (D) Tumor necrosis (present vs absent); (E) Histology (ccRCC vs non-ccRCC); and (F) Fuhrman grade (G3-G4 vs G1-G2).
Publication bias
Begg’s and Egger’s tests were used to assess publication bias. Funnel plots revealed rough symmetry regarding the distribution of many of the included articles, indicating the absence of obvious publication bias for OS (p = 0.266 and 0.236 for Begg’s and Egger’s tests, respectively) and PFS (p = 0.260 and 0.087, respectively; Figure 5).
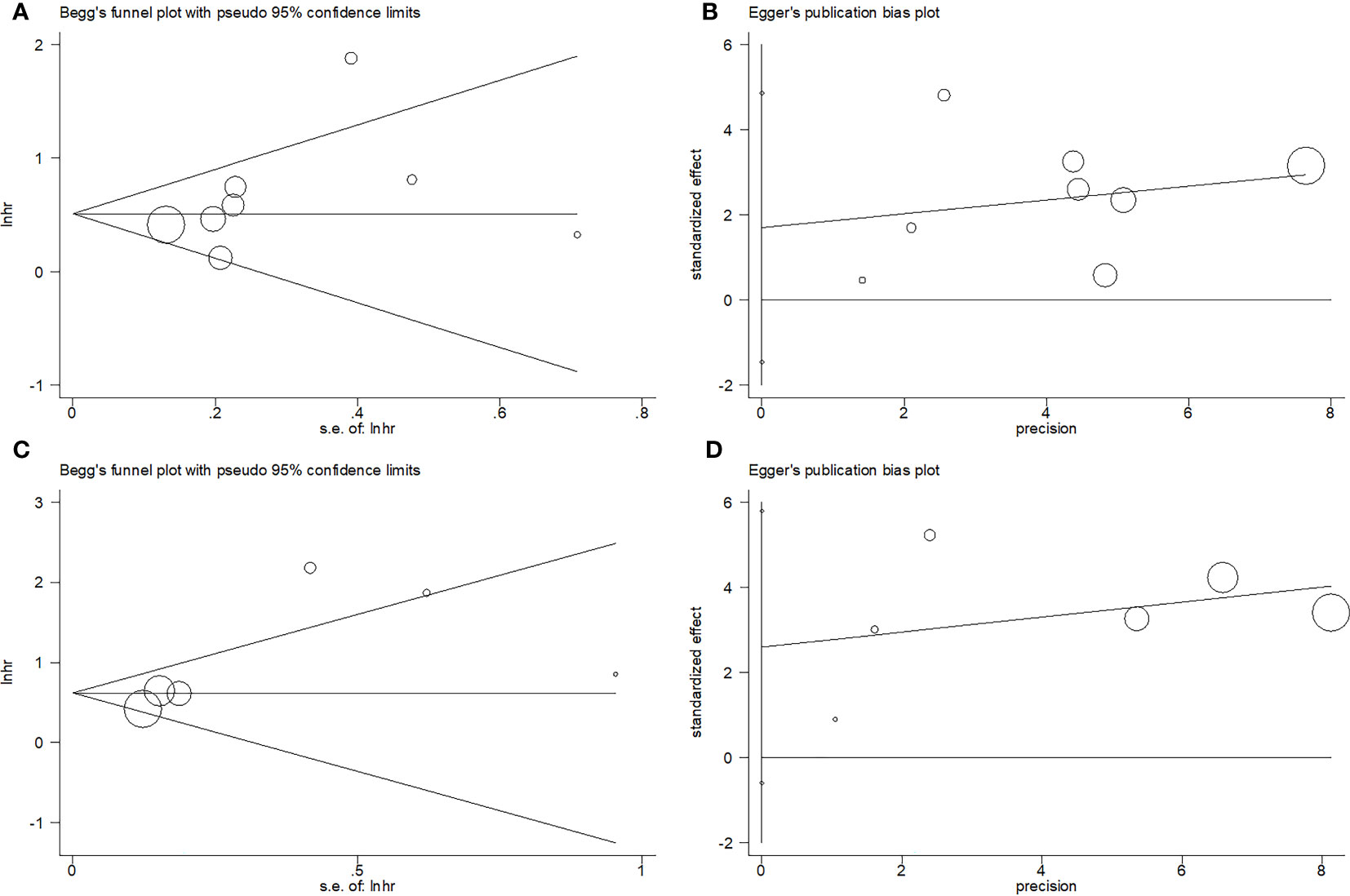
Figure 5 Publication test by Begg’ test and Egger’s test. (A) Begg’s test for OS, p=0.266; (B) Egger’s test for OS, p=0.236; (C) Begg’s test for PFS, p=0.260; and (D) Egger’s test for PFS, p=0.087.
Discussion
Whether the AGR predicts RCC prognosis is unclear, with previous reports providing conflicting results (19–27). The present meta-analysis, which synthesized data from nine studies with 5,671 RCC cases, revealed a robust association between a low pretreatment AGR and shorter OS and PFS times. As a reflection of the aggressive nature of RCC, a low AGR also correlated with pT stage T3–T4, pN stage N1, tumor necrosis, and Fuhrman grade 3–4. Hence, it might serve as a stable predictor of the short- and long-term prognosis of patients with RCC; notably, patients with low AGRs tended to experience tumor progression and metastasis. To our knowledge, this is the first reported meta-analysis of the significance of the AGR in predicting the prognosis and clinicopathological features of RCC.
The AGR compares ALB and GLB levels, with a low AGR indicating a low ALB and/or high GLB level. The potential mechanisms underlying the correspondence between a low AGR and poor RCC prognosis reflect the anti-oncogenic and pro-oncogenic actions of ALB and GLB, respectively. Serum ALB, a liver-generated soluble protein, maintains capillary osmotic pressure, removes free radicals from the blood (13), inhibits systemic inflammatory reactions, and serves as a marker of nutritional status (34). Malnutrition and inflammation impede its synthesis, as does interleukin-6 during the acute phase of inflammation in hepatocytes (35). ALB levels have been useful for predicting the outcomes of various cancers (36). The GLB component of the AGR includes diverse proinflammatory proteins, such as immunoglobulins, C-reactive protein, complement components (37), α-2 macroglobulin, fibrinogen, prothrombin, and serum amyloid A (38, 39). Because immunoglobulins are primarily metabolized in the liver, their clearance is impaired in patients with severe hepatic dysfunction, resulting in hyperglobulinemia (40, 41). In support of a potential role of GLB in apoptosis and carcinogenesis, a previous study associated increases in immunoglobulin levels with variations in the gene encoding tumor necrosis factor receptor 13B (42). Therefore, the AGR, which considers both ALB and GLB levels, is a reasonable and reliable prognostic marker.
Notably, a recent large-scale multicenter real-world retrospective study used pretreatment clinical characteristics to identify patients with muscle-invasive bladder cancer (MIBC) who could benefit from neoadjuvant combination therapy (43). Based on the combined prognostic efficiency of four hematological indexes [platelet-to-lymphocyte ratio (PLR) and hemoglobin, globulin, and platelet levels], a new indicator, the PLR.GHR (PLR*Globulin/Hemoglobin), was developed for MIBC (43). This finding has important implications in terms of the findings of our meta-analysis on RCC
Previous studies have evaluated various hematological parameters as predictors of RCC outcomes. In the report by De Giorgi et al., the systemic immune inflammation index and body mass index independently predicted OS in RCC patients treated with nivolumab (44). In studies of mRCC, a low pretreatment prognostic nutritional index was a potential risk factor after first-line therapy with tyrosine kinase inhibitors (45), and the PLR was an independent indicator of survival in a large cohort (n = 921) (46). Additionally, a recent meta-analysis associated a high pretreatment neutrophil-to-lymphocyte ratio and PLR with progression and mortality in mRCC patients treated with immune checkpoint inhibitors. Collectively, these studies implicate multiple hematological parameters in RCC prognosis (44–47).
Both units of the AGR (ALB and GLB) are important components of the tumor microenvironment. Therefore, the prognostic capability of the AGR can be influenced by tumor immune markers, such as branched chain aminotransferase 2 (BCAT2) (48), 5 methylcytosine (5mC) (49), and Siglec15 (50), all of which have been shown to shape the tumor microenvironment (48–50). The relationship between the AGR and these markers should be investigated in future studies.
Several meta-analyses suggest that a low pretreatment AGR can predict the prognosis of various cancers (51–55). In a meta-analysis of 3,211 patients with head and neck cancer, a low AGR significantly correlated with poor disease-free survival (DFS), distant metastasis-free survival, OS, T3–T4 status, lymph node metastasis, and stage III–IV disease (51). In a meta-analysis of 7,211 patients with metastatic prostate cancer, the AGR independently predicted PFS and CSS (52). Furthermore, in a meta-analysis of 8,397 patients with colorectal cancer, a low AGR robustly predicted poor OS and DFS/PFS (53). In additional meta-analyses, a low AGR remarkably predicted poor OS and DFS in patients (n = 3,496) with lung cancer (54) and was closely associated with poor OS and DFS/PFS in patients with gastric cancer (12 articles) (55).
The present study had some limitations. First, because all included articles were retrospective, inherent heterogeneity existed. However, the appropriate model for analysis of heterogeneous data (the random-effects model) was used. Second, the sample size was relatively small. Although we searched several databases, only nine relevant studies were retrieved. Third, most of the eligible studies were conducted in Asia. Consequently, our findings might be more applicable to Asian vs non-Asian patients with RCC. Owing to these limitations, larger, multi-arm prospective studies are needed to validate the prognostic significance of the pretreatment AGR in patients with RCC.
In conclusion, a low AGR markedly predicted poor OS and PFS in patients with RCC and significantly correlated with clinicopathological features indicative of disease progression. Use of the AGR will aid the identification of high-risk individuals and expedite the development of effective therapeutic strategies for RCC.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author contributions
HM and FY contributed to conception and design of the study. HM and FY were in charge of data collection. HM and FY performed the statistical analysis. HM wrote the first draft of the manuscript. FY edited the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AGR, albumin-to-globulin ratio; RCC, renal cell carcinoma; HR, hazard ratio; CI, confidence interval; OR, odds ratio; OS, overall survival; PFS, progression-free survival; ALB, albumin; GLB, globulin; NSCLC, non-small-cell lung cancer; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RFS, recurrence-free survival; CSS, cancer-specific survival; NOS, Newcastle-Ottawa Scale; DFS, disease-free survival; DMFS, distant metastasis-free survival.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Scelo G, Larose TL. Epidemiology and risk factors for kidney cancer. J Clin Oncol (2018) 36(36):Jco2018791905. doi: 10.1200/jco.2018.79.1905
3. Ljungberg B, Albiges L, Abu-Ghanem Y, Bedke J, Capitanio U, Dabestani S, et al. European association of urology guidelines on renal cell carcinoma: the 2022 update. Eur Urol (2022) 82(4):399–410. doi: 10.1016/j.eururo.2022.03.006
4. International WCRF. Kidney cancer statistics (2020). Available at: https://wwwwcrforg/cancer-trends/kidney-cancer-statistics/.
5. Hsieh JJ, Purdue MP, Signoretti S, Swanton C, Albiges L, Schmidinger M, et al. Renal cell carcinoma. Nat Rev Dis Primers (2017) 3:17009. doi: 10.1038/nrdp.2017.9
6. Singh D. Current updates and future perspectives on the management of renal cell carcinoma. Life Sci (2021) 264:118632. doi: 10.1016/j.lfs.2020.118632
7. Park JY, Tae BS, Jeong CW, Song C, Seo SI, Hong SK, et al. Development of the clinical calculator for mortality of patients with metastatic clear cell type renal cell carcinoma: An analysis of patients from Korean Renal Cancer Study Group database. Invest Clin Urol (2020) 61(3):260–8. doi: 10.4111/icu.2020.61.3.260
8. Culp SH, Karam JA, Wood CG. Population-based analysis of factors associated with survival in patients undergoing cytoreductive nephrectomy in the targeted therapy era. Urologic Oncol (2014) 32(5):561–8. doi: 10.1016/j.urolonc.2013.12.003
9. Internò V, De Santis P, Stucci LS, Rudà R, Tucci M, Soffietti R, et al. Prognostic factors and current treatment strategies for renal cell carcinoma metastatic to the brain: an overview. Cancers (Basel) (2021) 13(9):2114. doi: 10.3390/cancers13092114
10. Gulati S, Vogelzang NJ. Biomarkers in renal cell carcinoma: Are we there yet? Asian J Urol (2021) 8(4):362–75. doi: 10.1016/j.ajur.2021.05.013
11. Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell (2009) 15(2):79–80. doi: 10.1016/j.ccr.2009.01.009
12. McMillan DC, Watson WS, O'Gorman P, Preston T, Scott HR, McArdle CS. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer (2001) 39(2):210–3. doi: 10.1207/S15327914nc392_8
13. Song WJ, Li NC, Gao J, Xu ZP, Liu JY, Long Z, et al. The predictive significance of prognostic nutritional index and serum albumin/globulin ratio on the overall survival of penile cancer patients undergoing penectomy. Curr Oncol (Toronto Ont) (2022) 29(10):7569–78. doi: 10.3390/curroncol29100596
14. Lu P, Ma Y, Wei S, Liang X. A low albumin-to-globulin ratio predicts a poor prognosis in patients with metastatic non-small-cell lung cancer. Front Med (2021) 8:621592. doi: 10.3389/fmed.2021.621592
15. Atsumi Y, Kawahara S, Kakuta S, Onodera A, Hara K, Kazama K, et al. Low preoperative albumin-to-globulin ratio is a marker of poor prognosis in patients with esophageal cancer. In Vivo (2021) 35(6):3555–61. doi: 10.21873/invivo.12658
16. Kawata A, Taguchi A, Baba S, Miyamoto Y, Tanikawa M, Sone K, et al. A low preoperative albumin-to-globulin ratio is a negative prognostic factor in patients with surgically treated cervical cancer. Int J Clin Oncol (2021) 26(5):980–5. doi: 10.1007/s10147-021-01861-8
17. Cai Y, Zhao Y, Dai Q, Xu M, Xu X, Xia W. Prognostic value of the albumin-globulin ratio and albumin-globulin score in patients with multiple myeloma. J Int Med Res (2021) 49(3):300060521997736. doi: 10.1177/0300060521997736
18. Hayashi M, Kobayashi D, Takami H, Inokawa Y, Tanaka N, Kurimoto K, et al. Albumin-globulin ratio indicates the survival outcome of pancreatic cancer cases who underwent preoperative treatment and curative surgical resection. Nutr Cancer (2023) 75(5):1330–39. doi: 10.1080/01635581.2023.2191384
19. Chen Z, Shao Y, Yao H, Zhuang Q, Wang K, Xing Z, et al. Preoperative albumin to globulin ratio predicts survival in clear cell renal cell carcinoma patients. Oncotarget (2017) 8(29):48291–302. doi: 10.18632/oncotarget.15162
20. He X, Guo S, Chen D, Yang G, Chen X, Zhang Y, et al. Preoperative albumin to globulin ratio (AGR) as prognostic factor in renal cell carcinoma. J Cancer (2017) 8(2):258–65. doi: 10.7150/jca.16525
21. Koparal MY, Polat F, Çetin S, Bulut EC, Sözen TS. Prognostic role of preoperative albumin to globulin ratio in predicting survival of clear cell renal cell carcinoma. Int Braz J urol (2018) 44(5):933–46. doi: 10.1590/s1677-5538.Ibju.2018.0012
22. Bian Z, Meng J, Niu Q, Jin X, Wang J, Feng X, et al. Prognostic role of prothrombin time activity, prothrombin time, albumin/globulin ratio, platelets, sex, and fibrinogen in predicting recurrence-free survival time of renal cancer. Cancer Manag Res (2020) 12:8481–90. doi: 10.2147/cmar.S264856
23. Chung JW, Park DJ, Chun SY, Choi SH, Lee JN, Kim BS, et al. The prognostic role of preoperative serum albumin/globulin ratio in patients with non-metastatic renal cell carcinoma undergoing partial or radical nephrectomy. Sci Rep (2020) 10(1):11999. doi: 10.1038/s41598-020-68975-3
24. Hu J, Chen J, Li H, He T, Deng H, Gong G, et al. A preoperative nomogram predicting the pseudocapsule status in localized renal cell carcinoma. Transl Androl Urol (2020) 9(2):462–72. doi: 10.21037/tau.2020.01.26
25. Xu K, Li J, Hu M, Zhang H, Yang J, Gong H, et al. Prognostic significance of preoperative inflammatory biomarkers and traditional clinical parameters in patients with spinal metastasis from clear cell renal cell carcinoma: A retrospective study of 95 patients in a single center. Cancer Manag Res (2020) 12:59–70. doi: 10.2147/cmar.S228570
26. Aktepe OH, Güner G, Güven DC, Taban H, Yıldırım H, Şahin TK, et al. Impact of albumin to globulin ratio on survival outcomes of patients with metastatic renal cell carcinoma. Turkish J Urol (2021) 47(2):113–9. doi: 10.5152/tud.2021.20377
27. Laukhtina E, Pradere B, D'Andrea D, Rosiello G, Luzzago S, Pecoraro A, et al. Prognostic effect of preoperative serum albumin to globulin ratio in patients treated with cytoreductive nephrectomy for metastatic renal cell carcinoma. Transl Androl Urol (2021) 10(2):609–19. doi: 10.21037/tau-20-1101
28. Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol (2009) 62(10):1006–12. doi: 10.1016/j.jclinepi.2009.06.005
29. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
30. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21(11):1539–58. doi: 10.1002/sim.1186
31. Cochran W. The combination of estimates from different experiments. Biometrics (1954) 10:101–29.
32. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics (1994) 50(4):1088–101. doi: 10.2307/2533446
33. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj-British Med J (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
34. Doweiko JP, Nompleggi DJ. The role of albumin in human physiology and pathophysiology, Part III: Albumin and disease states. JPEN J parenteral enteral Nutr (1991) 15(4):476–83. doi: 10.1177/0148607191015004476
35. Xu C, Wu X, Hack BK, Bao L, Cunningham PN. TNF causes changes in glomerular endothelial permeability and morphology through a Rho and myosin light chain kinase-dependent mechanism. Physiol Rep (2015) 3(12):e12636. doi: 10.14814/phy2.12636
36. Tang LQ, Li CF, Chen QY, Zhang L, Lai XP, He Y, et al. High-sensitivity C-reactive protein complements plasma Epstein-Barr virus deoxyribonucleic acid prognostication in nasopharyngeal carcinoma: a large-scale retrospective and prospective cohort study. Int J Radiat oncology biology Phys (2015) 91(2):325–36. doi: 10.1016/j.ijrobp.2014.10.005
37. Afshar-Kharghan V. The role of the complement system in cancer. J Clin Invest (2017) 127(3):780–9. doi: 10.1172/jci90962
38. Deng Y, Pang Q, Miao RC, Chen W, Zhou YY, Bi JB, et al. Prognostic significance of pretreatment albumin/globulin ratio in patients with hepatocellular carcinoma. Onco Targets Ther (2016) 9:5317–28. doi: 10.2147/ott.S109736
39. Xing Y, Guo ZN, Yan S, Jin H, Wang S, Yang Y. Increased globulin and its association with hemorrhagic transformation in patients receiving intra-arterial thrombolysis therapy. Neurosci Bull (2014) 30(3):469–76. doi: 10.1007/s12264-013-1440-x
40. Doi H, Hayashi E, Arai J, Tojo M, Morikawa K, Eguchi J, et al. Enhanced B-cell differentiation driven by advanced cirrhosis resulting in hyperglobulinemia. J Gastroenterol Hepatol (2018). doi: 10.1111/jgh.14123
41. Tanaka S, Okamoto Y, Yamazaki M, Mitani N, Nakqjima Y, Fukui H. Significance of hyperglobulinemia in severe chronic liver diseases–with special reference to the correlation between serum globulin/IgG level and ICG clearance. Hepato-gastroenterology (2007) 54(80):2301–5.
42. Osman W, Okada Y, Kamatani Y, Kubo M, Matsuda K, Nakamura Y. Association of common variants in TNFRSF13B, TNFSF13, and ANXA3 with serum levels of non-albumin protein and immunoglobulin isotypes in Japanese. PloS One (2012) 7(4):e32683. doi: 10.1371/journal.pone.0032683
43. Hu J, Chen J, Ou Z, Chen H, Liu Z, Chen M, et al. Neoadjuvant immunotherapy, chemotherapy, and combination therapy in muscle-invasive bladder cancer: A multi-center real-world retrospective study. Cell Rep Med (2022) 3(11):100785. doi: 10.1016/j.xcrm.2022.100785
44. De Giorgi U, Procopio G, Giannarelli D, Sabbatini R, Bearz A, Buti S, et al. Association of systemic inflammation index and body mass index with survival in patients with renal cell cancer treated with nivolumab. Clin Cancer Res (2019) 25(13):3839–46. doi: 10.1158/1078-0432.Ccr-18-3661
45. Cai W, Zhong H, Kong W, Dong B, Chen Y, Zhou L, et al. Significance of preoperative prognostic nutrition index as prognostic predictors in patients with metastatic renal cell carcinoma with tyrosine kinase inhibitors as first-line target therapy. Int Urol Nephrol (2017) 49(11):1955–63. doi: 10.1007/s11255-017-1693-9
46. Yuk HD, Kang M, Hwang EC, Park JY, Jeong CW, Song C, et al. The platelet-to-lymphocyte ratio as a significant prognostic factor to predict survival outcomes in patients with synchronous metastatic renal cell carcinoma. Invest Clin Urol (2020) 61(5):475–81. doi: 10.4111/icu.20200002
47. Yanagisawa T, Mori K, Katayama S, Mostafaei H, Quhal F, Laukhtina E, et al. Hematological prognosticators in metastatic renal cell cancer treated with immune checkpoint inhibitors: a meta-analysis. Immunotherapy (2022) 14(9):709–25. doi: 10.2217/imt-2021-0207
48. Cai Z, Chen J, Yu Z, Li H, Liu Z, Deng D, et al. BCAT2 shapes a noninflamed tumor microenvironment and induces resistance to anti-PD-1/PD-L1 immunotherapy by negatively regulating proinflammatory chemokines and anticancer immunity. Adv Sci (Weinh) (2023) 10(8):e2207155. doi: 10.1002/advs.202207155
49. Hu J, Othmane B, Yu A, Li H, Cai Z, Chen X, et al. 5mC regulator-mediated molecular subtypes depict the hallmarks of the tumor microenvironment and guide precision medicine in bladder cancer. BMC Med (2021) 19(1):289. doi: 10.1186/s12916-021-02163-6
50. Hu J, Yu A, Othmane B, Qiu D, Li H, Li C, et al. Siglec15 shapes a non-inflamed tumor microenvironment and predicts the molecular subtype in bladder cancer. Theranostics (2021) 11(7):3089–108. doi: 10.7150/thno.53649
51. Wang YT, Kuo LT, Lai CH, Tsai YH, Lee YC, Hsu CM, et al. Low pretreatment albumin-to-globulin ratios predict poor survival outcomes in patients with head and neck cancer: A systematic review and meta-analysis. J Cancer (2023) 14(2):281–9. doi: 10.7150/jca.80955
52. Salciccia S, Frisenda M, Bevilacqua G, Viscuso P, Casale P, De Berardinis E, et al. Prognostic value of albumin to globulin ratio in non-metastatic and metastatic prostate cancer patients: A meta-analysis and systematic review. Int J Mol Sci (2022) 23(19):11501. doi: 10.3390/ijms231911501
53. Quan L, Jiang X, Jia X, Cheng F. Prognostic value of the albumin-to-globulin ratio in patients with colorectal cancer: A meta-analysis. Nutr Cancer (2022) 74(9):3329–39. doi: 10.1080/01635581.2022.2076890
54. Li J, Wang Y, Wu Y, Li J, Che G. Prognostic value of pretreatment albumin to globulin ratio in lung cancer: A meta-analysis. Nutr Cancer (2021) 73(1):75–82. doi: 10.1080/01635581.2020.1737155
Keywords: albumin-to-globulin ratio, renal cell carcinoma, meta-analysis, prognosis, blood test
Citation: Mao H and Yang F (2023) Prognostic significance of albumin-to-globulin ratio in patients with renal cell carcinoma: a meta-analysis. Front. Oncol. 13:1210451. doi: 10.3389/fonc.2023.1210451
Received: 22 April 2023; Accepted: 03 July 2023;
Published: 19 July 2023.
Edited by:
Wen-Hao Xu, Fudan University, ChinaReviewed by:
Fahad Quhal, King Fahad Specialist Hospital Dammam, Saudi ArabiaJiao Hu, Central South University, China
Copyright © 2023 Mao and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fan Yang, eWFuZ3lhbmd5YW5nODk3NTdAMTYzLmNvbQ==
 Huaying Mao1
Huaying Mao1 Fan Yang
Fan Yang