- 1Department of Gastrointestinal, Bariatric and Metabolic Surgery, Research Center for Nutrition, Metabolism and Food Safety, West China-PUMC C.C. Chen Institute of Health, West China School of Public Health and West China Fourth Hospital, Sichuan University, Chengdu, China
- 2Department of Immunology, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences, Beijing, China
- 3School of Basic Medicine, Peking Union Medical College, Beijing, China
- 4Department of Nutrition, Food Hygiene, and Toxicology, West China School of Public Health and West China Fourth Hospital, Sichuan University, Chengdu, China
- 5Department of Oncology and Department of Biomedical and Clinical Sciences, Linköping University, Linköping, Sweden
Background: Previous epidemiological studies have yielded inconsistent results regarding the effects of dietary tomato, tomato products, and lycopene on the incidence of colorectal cancer (CRC), possibly due to variations in sample sizes and study designs.
Methods: The current study used multivariable Cox regression, subgroup analyses, and restricted cubic spline functions to investigate correlations between CRC incidence and mortality and raw tomato, tomato salsa, tomato juice, tomato catsup, and lycopene intake, as well as effect modifiers and nonlinear dose-response relationships in 101,680 US adults from the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial.
Results: During follow-up 1100 CRC cases and 443 CRC-specific deaths occurred. After adjustment for confounding variables, high consumption of tomato salsa was significantly associated with a reduced risk of CRC incidence (hazard ratio comparing the highest category with the lowest category 0.8, 95% confidence interval 0.65–0.99, p for trend = 0.039), but not with a reduced risk of CRC mortality. Raw tomatoes, tomato juice, tomato catsup, and lycopene consumption were not significantly associated with CRC incidence or CRC mortality. No potential effect modifiers or nonlinear associations were detected, indicating the robustness of the results.
Conclusion: In the general US population a higher intake of tomato salsa is associated with a lower CRC incidence, suggesting that tomato salsa consumption has beneficial effects in terms of cancer prevention, but caution is warranted when interpreting these findings. Further prospective studies are needed to evaluate its potential effects in other populations.
1 Introduction
Colorectal cancer (CRC) is a significant global public health challenge, and the third most prevalent cancer in the United States with an estimated 147,950 new cases and 53,200 deaths in 2020 (1). Unhealthy lifestyle factors including heavy alcohol consumption, cigarette smoking, physical inactivity, excess body weight, and dietary choices may contribute to nearly half of CRC cases (2). Emerging evidence suggests that high consumption of red or processed meat (3, 4), trans-fatty acids (5) and dietary supplements containing aristolochic acid (6) may increase the risk of CRC, whereas consumption of calcium (7, 8), whole grains and fiber (9), fruit and vegetables (10), and dairy products (11) may decrease the risk. It would therefore be beneficial to establish a primary prevention strategy after clarifying associations between different dietary components and CRC incidence.
Tomatoes and tomato products are recognized as a component of a healthy diet (12). Epidemiological studies have shown that higher intake of tomato, tomato products, and/or lycopene may reduce the risk of various cancers, including hepatocellular carcinoma (13), prostate cancer (14), pancreatic cancer (15), gastric cancer (16), and ovarian cancer (17). Nevertheless, associations between tomato/tomato product intake and CRC risk remain unclear due to limited participant sizes and inconsistent study results (18, 19). Meta-analyses have yielded conflicting results with regard to associations between lycopene intake and the incidence of CRC (20, 21). Notably, these studies did not differentiate between raw and processed tomatoes, which may have different effects on CRC risk. Dose-response relationships between tomato or lycopene intake and mortality have not been investigated. A recent study investigated relationships between the intake of raw tomatoes, tomato catsup, or lycopene and all-cause and cause-specific mortality, but no such analysis has been done to examine their relationship with CRC (22).
To provide evidence to fill this gap, we conducted a comprehensive, prospective cohort study using data from the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial, which was a multicenter randomized controlled study involving approximately 155,000 participants. The aim of the current study was to investigate potential correlations between the risk of CRC incidence and mortality and the consumption of tomatoes, tomato products, and lycopene. Additionally, we sought to examine the possible dose-response relationships and nonlinear associations between the intake of tomato products/lycopene and CRC risk. We also aimed to enhance the generalizability of our findings by conducting subgroup analyses.
2 Materials and methods
2.1 Data source and study population
The PLCO study design and methodology have been previously described (23). Briefly, it was a multicenter randomized controlled trial aimed at determining whether specific screening examinations reduce mortality from PLCO cancers. Approximately 155,000 participants aged 55–74 years were recruited between 1993 and 2001 via ten screening centers across the United States, and were randomly assigned to either a control group or an intervention group upon entry, in accordance with a detailed plan. The study was approved by the NCI’s Institutional Review Boards and each study center, and all enrolled participants signed informed consent forms.
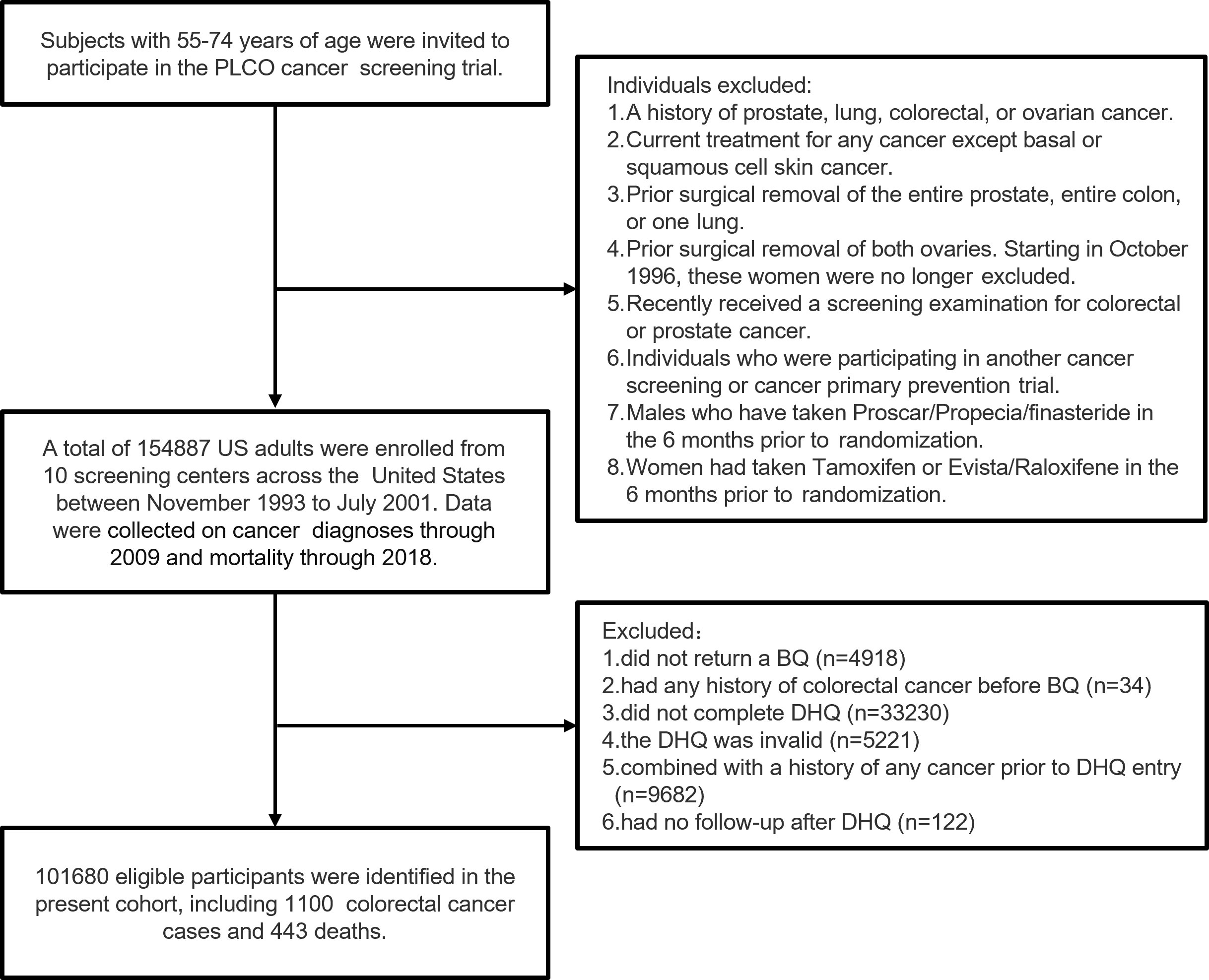
Participants were excluded if they (1) did not return the baseline questionnaire (n = 4918) or had any history of CRC before the baseline questionnaire (n = 34) (2); had an incomplete dietary history questionnaire (DHQ) (n = 33,230) or an invalid DHQ that was missing the completion date, was completed before the date of death, had ≥ 8 missing frequency responses, or indicated extremely high or low calorie intake (i.e., top 1% or bottom 1%) (n = 5,221) (3); had a history of any cancer before DHQ entry (n = 9,682); or (4) no follow-up time after the DHQ (n = 122). Ultimately 101,680 eligible participants were included in our cohort (Figure 1).
2.2 Data collection and dietary assessment
All participants completed a baseline questionnaire in which they self-reported information on demographics and medical history, including sex, race, trial arm, body mass index (BMI), educational level, marital status, aspirin use, cigarette smoking, family history of CRC, history of colon comorbidities, history of colorectal polyps, and diabetes history. Dietary data were collected using a self-administered DHQ. The DHQ included the serving size and response frequency of 124 food items and supplement use over the past year, such as red meat, processed meat, fruit, vegetables, whole grain, dairy, added sugars, dietary fiber, protein, total fat, carbohydrate, glycemic load, glycemic index, calcium, folate, magnesium, iron, vitamin D, and olive oil. The 1994-96 Continuing Survey of Food Intakes by Individuals, available from the USDA Food Surveys Research Group, and the Nutrition Data Systems for Research from the University of Minnesota were used to calculate the daily intake of all nutrients in the database (24). The DHQ has been validated and has shown good or better performance in estimating dietary intake compared to other commonly used food frequency questionnaires (25). Five independent exposures were included in the current analysis; tomato juice, raw tomato, tomato salsa, tomato catsup, and lycopene. Due to a lack of data on total tomato consumption, the overall relationship between total tomato and CRC risk could not be investigated.
2.3 Outcome ascertainment
The primary endpoint of the study was the incidence of CRC, which was determined via annual medical record reviews that updated participants’ cancer diagnosis status, including the date of detection and the site of the cancer. The secondary endpoint was mortality related to CRC. Information regarding deaths was obtained through various sources, including Annual Study Update questionnaires, reports from relatives, friends, or physicians, and National Death Index Plus searches. Upon notification, PLCO Screening Centers made efforts to obtain a death certificate for each death that occurred on or before 31 December 2018. The trial database recorded and coded information from the death certificate, and the underlying cause of death was determined using rules established by the National Center for Health Statistics. To ensure a more accurate assessment of trial endpoints a death review process was conducted, and medical records were reviewed for all deaths that may have been related to prostate, lung, colorectal, and ovarian cancers. The DRP cause of death was considered authoritative and was used in statistical analyses of the primary endpoints. The follow-up duration was calculated from the date of completion of the DHQ to the first occurrence of CRC diagnosis, participant dropout, CRC-related death, or the end of follow-up through to 31 December 2009 for incidence, and through to 31 December 2018 for mortality.
2.4 Statistical analyses
Dietary exposures were adjusted for total energy from the diet using the residual method (26). Energy-adjusted dietary tomato, tomato products, and lycopene intakes were then divided equally into quintiles, with the lowest quintile serving as the referent group. Continuous variables are expressed as medians and interquartile ranges (IQRs), and categorical variables are presented as numbers and percentages. Kruskal-Wallis H tests and chi-squared tests were used to compare between-group variance if appropriate. Multivariable Cox regression analyses were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). Schoenfeld residuals were used to verify the proportional hazard assumption of baseline covariates (all p > 0.05) (27). Due to the abnormal distribution of the five exposures, a Log2 transformation was performed. The linear trend of each quintile of energy-adjusted dietary tomato, tomato products, and lycopene intakes were also analyzed by entering the median value as a continuous variable in the models. Model 2 was fully adjusted for age, sex, race, trial arm, BMI, educational level, marital status, aspirin use, cigarette smoking, alcohol consumption, family history of CRC, history of colon comorbidities, history of colorectal polyps, diabetes history, and dietary energy intake. In addition, the five exposures were mutually adjusted to assess individual contributions to the risk of CRC.
Subgroup analyses were conducted in several prespecified subgroups, including age group, sex, trial arm, BMI group, aspirin use, cigarette smoking, alcohol consumption, family history of CRC, history of colon comorbidities, colorectal polyps, and diabetes. The interaction effect on each stratum was compared using likelihood-ratio tests. Restricted cubic spline functions with four knots (5th, 35th, 65th, and 95th percentiles) were used to investigate non-linear associations between dietary tomato, tomato product, and lycopene intakes and the incidence and CRC mortality. Notably subjects with energy-adjusted dietary tomato/lycopene intakes < 1st or > 90th percentile were excluded to reduce potential bias for extreme values in the dose-response analyses.
Several sensitivity analyses were conducted as follows: (1) Excluding events within the first 2 years of follow-up (1636 participants excluded); (2) excluding events involving extreme energy intake (< 800/> 4000 kcal/day for men and < 500/> 3500 kcal/day for women) (2886 participants excluded); (3) additional adjustment for the factors listed in the fully-adjusted model (model 2; Table 1), and processed meat (g/day), red meat (g/day), vegetables (g/day), fruit (g/day), whole grain (servings/day), sugar (tsp/day), dairy (servings/day), and dietary fiber (g/day); (4) additional adjustment for glycemic load, glycemic index, protein (% energy), total fat (% energy), and carbohydrate (% energy) in model 2; (5) additional adjustment for total calcium (mg/day), folate (mg/day), magnesium (mg/day), iron (mg/day), and vitamin D (µg/day) in model 3; (6) additional adjustment for olive oil (g/day) in model 4. Additional analyses to investigate associations between the five dietary exposures and CRC mortality were also conducted. All analyses were performed using R statistical software (http://www.R-project.org, R Foundation) and the Free Statistics analysis platform (28). All tests were two-tailed, and the significance level was set at 0.05.
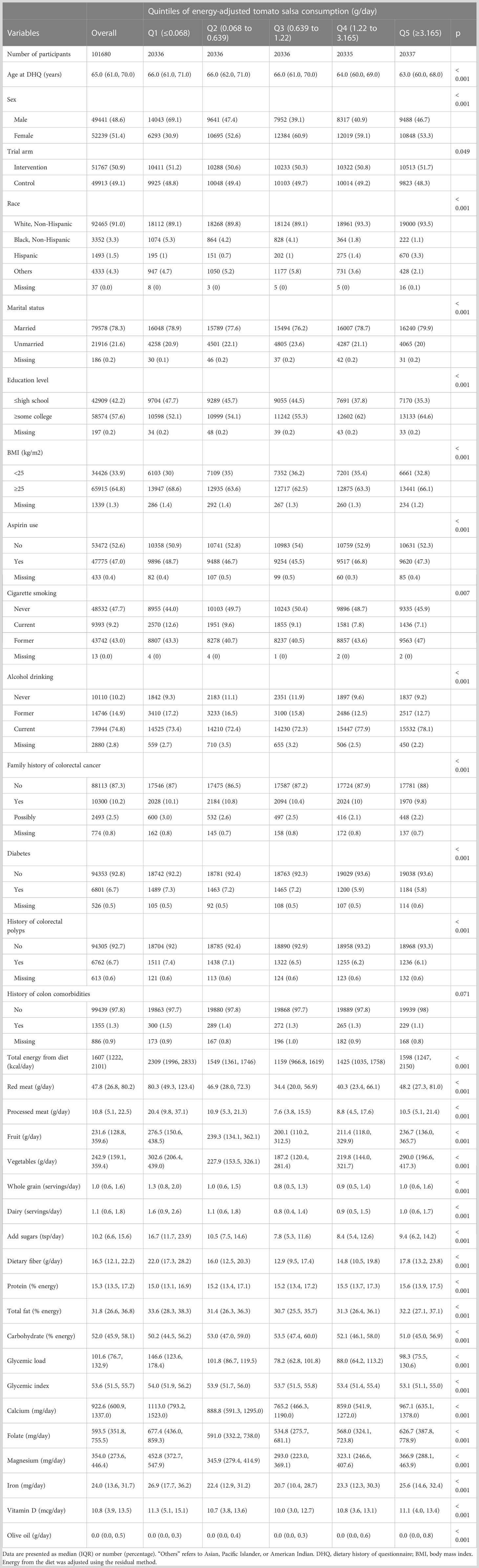
Table 1 Baseline characteristics of study population according to quintiles of energy-adjusted tomato salsa consumption in 101680 participants.
3 Results
3.1 Participant characteristics
The cohort included 101,680 participants with a median follow-up of 9.54 years, corresponding to 908,801 person-years. During this period 1100 cases of CRC were reported, corresponding to an incidence rate of 12.10 per 10,000 person-years. Within a median follow-up of 14.5 years (corresponding to 1,353,326 person-years) 443 CRC-specific deaths were recorded. The average age of participants at baseline was 65.0 years. The median intakes of the five dietary items of primary interest were raw tomato 13.58 g/day, tomato salsa 0.90 g/day, tomato juice 12.76 g/day, tomato catsup 1.41 g/day, and lycopene 5.26 mg/day. The baseline characteristics of the study population according to the quintiles of the five exposure variables are summarized in Table 1 and Supplementary Tables S1–S4. Compared to the lowest quintile of energy-adjusted tomato salsa consumption, participants in the highest quintile were more likely to be young (median age 64 years), Caucasian, more highly educated, have a history of diabetes, have a lower glycemic load, and have lower total dietary energy intake. On average they consumed less red meat, processed meat, and added sugars, and they were less likely to be current smokers. In the Q1 category (representing the lowest consumption of tomato salsa), 69.1% of participants were male. Overall, the distribution was similar however, with 48.6% being male and 51.4% being female. There were also similar trends in the consumption of other tomato products, including raw tomato, tomato juice, tomato ketchup, and lycopene (Supplementary Tables S1–S4).
3.2 Associations between CRC incidence and tomato, tomato product, and lycopene intakes
There was an inverse association between CRC incidence and the moderate and the highest dietary intake of tomato salsa and in the crude model (Q4 vs. Q1: HR 0.81, 95% CI 0.67–0.97; Q5 vs. Q1: HR 0.64, 95% CI 0.53–0.79) (Table 2). Similar results on the association between CRC incidence and the highest intake of tomato salsa were obtained in adjusted models (model 1, Q5 vs. Q1: HR 0.77, 95% CI 0.63–0.94, p trend = 0.016; model 2, HR Q5 vs. Q1: HR 0.80, 95% CI 0.65–0.99, p trend = 0.028). There were no significant associations between raw tomato, tomato juice, tomato catsup, or lycopene intake and CRC incidence. With respect to individual contributions to CRC incidence assessed after mutual adjustments for each of the five exposure variables, comparing tomato salsa Q5 and Q1 the HR for CRC incidence was 0.79 (95% CI 0.64–0.99, p = 0.037, p for trend = 0.030); thus tomato salsa intake remained a significant predictor of CRC risk even after adjustment for the other tomato variables and covariates.

Table 2 Association between energy-adjusted tomato-related products/lycopene intakes and colorectal cancer incidence in the PLCO cancer screening trial.
There were no significant interactions between tomato salsa intake and CRC incidence in any subgroups including age, sex, trial arm, BMI group, aspirin use, cigarette smoking, alcohol drinking, family history of CRC, history of colon comorbidities, colorectal polyps, and diabetes (Supplementary Table S5, p for interaction > 0.05). Given the distinct distribution of tomato salsa intake between males and females within the Q1 category, we also performed subgroup analyses to assess the association between tomato salsa intake (as quintiles) and CRC risk by sex. There was a negative association between tomato salsa intake and CRC incidence in women (Q5 vs. Q1 HR 0.60, 95% CI 0.41–0.87, p = 0.007). In men there was only a tendency towards a negative association. There was no significant interaction effect between sex and salsa intake on CRC incidence (p for interaction = 0.703).
Sensitivity analyses were performed to examine the robustness of the correlation between tomato salsa intake and CRC incidence. The analyses included the exclusion of events ascertained within 2 years, the exclusion of subjects with extreme energy intakes, and the use of additional models. In those analyses the correlation between tomato salsa intake and CRC incidence remained robust (Supplementary Table S6). Smooth curve-fitting plots did not provide any evidence of nonlinear dose-response associations between energy-adjusted tomato salsa consumption and CRC incidence after full adjustment (Supplementary Figure S1; p for nonlinearity > 0.05).
3.3 Associations between CRC-specific mortality and tomato, tomato product, and lycopene intakes
Consumption of tomato salsa was significantly associated with lower CRC-specific mortality in the crude model (Table 3, p trend = 0.024). After adjustment for confounding variables in models 1 and 2 however, there were no significant associations (Table 3, p trend > 0.05). There were no significant associations between CRC-specific mortality and the intake of raw tomato, tomato juice, tomato ketchup, or lycopene. When the five exposures were mutually adjusted, comparing tomato salsa Q5 and Q1 yielded an HR for CRC mortality of 0.96 (95% CI 0.7–1.32, p = 0.807, p for trend = 0.942). Thus, there was no significant association between tomato salsa intake and CRC mortality.

Table 3 Association between energy-adjusted tomato-related products/lycopene intakes and colorectal cancer mortality in the PLCO cancer screening trial.
In subgroup analyses there were no significant effect modifiers in the prespecified groups when the exposures were treated as categorical variables (quintiles) (Supplementary Table S7; p for interaction > 0.05). In sensitivity analyses there was also a lack of an association between dietary tomato salsa intake and CRC mortality (Supplementary Table S8). In dose-response analyses there was no non-linear relationship between tomato salsa intake and CRC mortality (Supplementary Figures S2; p for non-linearity > 0.05).
4 Discussion
In this prospective cohort study of 101,680 US adults’ higher consumption of tomato salsa was associated with a 20% lower risk of CRC incidence after adjustment for potential confounders. There were no significant associations between the consumption of raw tomato, tomato juice, tomato catsup, or lycopene and the risk of CRC incidence or mortality. These results were robust in a series of analyses. No effect modifiers or no non-linear relationships were observed. To our knowledge this is the first study to report a protective effect of tomato salsa against CRC risk. In contrast, a previous study did not observe a significant association between bladder cancer risk and tomato salsa consumption after adjustment for confounders in the PLCO cohort (29). These results suggest that the protective effect of tomato salsa against cancer risk is heterogenous among different cancers. To further assess the individual contribution of tomato-related dietary intake and CRC risk, we conducted a series of analyses including mutual adjustment for the five primary dietary factors of interest, and additional adjustment for other foods and nutrients, and the association between tomato salsa and CRC incidence remained robust. Although intake of tomato and/or lycopene has been associated with reduced risk of several cancers such as hepatocellular carcinoma (13), prostate cancer (14), pancreatic cancer (15), gastric cancer (16), ovarian cancer (17), and CRC (18, 19), in this large PLCO study CRC risk was not significantly associated with consumption of raw tomato, tomato juice, or tomato catsup. That was consistent with a previous study investigating bladder cancer (29), but inconsistent with a previous case-control study conducted in a CRC population in Italy, in which there was a protective association between a higher intake of tomato and the incidence of CRC (18), and sub-sites of CRC stratified by cancer site (19). These differences may be due to the retrospective nature of the previous studies on this topic, which only examined associations with total tomato intake. The current study investigated the effects of specific types of tomato products (i.e., raw tomato and tomato catsup) on the incidence of CRC, which may have differential effects on health outcomes (29). Similarly, selection and recall bias due to the retrospective designs and residual confounders in previous studies may also have led the inconsistent results.
Although we did not specifically investigate the mechanisms underlying associations identified in the study, several potential explanations could be explored. It has been proposed that the cancer-preventing effects of high tomato consumption may be attributed to lycopene. This powerful antioxidant not only neutralizes harmful free radicals but also potentially mitigates oxidative stress, a condition associated with cellular damage and implicated in various types of cancer (30–32). Processed and concentrated tomato products such as salsa (9.28 mg/100 g) and tomato juice (7.83 mg/100 mg) contain higher levels of lycopene than raw tomatoes (3.1–7.74 mg/100 g) (33), which may contribute to their cancer-protective effects (34). However, we did not observe a significant association between CRC risk and dietary intake of lycopene after adjusting for confounders, which was similar to previously reported results (21, 35, 36). Notably our study only investigated the link between dietary lycopene intake and CRC risk, and did not directly measure serum lycopene levels. Because the estimated dietary lycopene absorption rate in humans ranges from 10%–30% (37), dietary intake may not fully reflect serum lycopene levels. Therefore, the observed correlational coefficient of 0.46 between dietary and serum lycopene levels could be influenced by various factors (14). In addition, the method of cooking and chopping can affect the bioavailability of lycopene, and certain food preparation techniques may enhance absorption (38, 39).
Tomato salsa (sofrito) is a traditional Mediterranean diet preparation comprised of a mix of foods characteristic of the Mediterranean diet such as tomato, onion, garlic, and extra virgin olive oil, and it contains many bioactive phenolic compounds and carotenoids (40, 41). The inverse association between salsa and CRC in our study may be attributable to the presence of unique additives such as olive oil. Olive oil is known to contain a variety of substances, including monounsaturated free fatty acids (such as oleic acid), hydrocarbon squalene, tocopherols, aroma components, and phenolic compounds. Although olive oil quality can affect biological/nutritional actions (42), these components have been associated with anticancer properties (43, 44). Therefore, the addition of olive oil to tomato sauces may have positive health outcomes. Furthermore, the potential health benefits may be related to the method of tomato salsa processing. Evidence from a prospective randomized, cross-over intervention study suggested that the plasma concentration and urinary excretion of naringenin glucuronide were both significantly higher after the consumption of tomato sauce than after the consumption of raw tomatoes. It was suggested that mechanical and thermal treatments during tomato sauce manufacture may help to deliver these potentially bioactive phenolics from the food matrix more effectively than the addition of an oil component, thus increasing their bioavailability (45). Moreover, tomato salsa, characterized by its intricate mixture of ingredients, should be considered for the potential synergistic interactions among its bioactive compounds. It is not only a rich source of antioxidant lycopene, but also a treasure trove of other bioactive components, including phenolic acids, flavonoids, and ascorbic acid (46). The interaction among these ingredients yields synergistic effects that amplify the health benefits of tomato salsa. For instance, the bioavailability of lycopene can be significantly boosted by the presence of fats, such as those found in avocados, olive oil - a common ingredient in salsa recipes (47). The assortment of antioxidants in salsa promises more robust protection against oxidative stress and inflammation than any single compound could offer (42). Lastly, participants with a higher intake of salsa often reported other healthy dietary habits at baseline, such as a higher intake of vegetables and fruits and a lower intake of red or processed meat, which may provide additional protective effects against CRC. To better understand the potential association between tomato and CRC risk, future studies should investigate the sources, bioavailability, and serum concentration of lycopene in tomato salsa, ideally with a longer follow-up period.
The strengths of this study included its prospective design based on a large and well-established cohort (the PLCO trial), which ensured reliable data, a large sample size, and a comprehensive assessment of dietary intake of various tomato products and lycopene. The data enabled investigation of dose-response relationships, as well as long-term follow-up with a high follow-up rate to minimize reverse causality and selection bias. The study also had several limitations. Firstly, due to the observational nature of the study there may have been residual confounders that we could not control for. Secondly, the data were derived from a dietary questionnaire, and may thus have been subject to recall bias and misclassification errors. Thirdly, we only had baseline dietary information, which limited our ability to examine dynamic changes between nutrients and cancer risk. Fourthly, serum assessment of nutrients was lacking, which prevented a more detailed evaluation. Fifthly, the study population was limited to the US, which may limit the generalizability of the results to other countries with different dietary patterns. Further studies with larger sample sizes and longer follow-up periods, as well as more detailed assessments of dietary intake and serum nutrient levels are warranted to confirm our findings and better understand potential associations between dietary factors and cancer risk.
5 Conclusions
The current study indicates that high amounts of tomato salsa may be a beneficial addition to a healthy diet, and may contribute to CRC prevention in the adult population in the US. However, more prospective studies that involve more detailed assessments of tomato salsa intake are necessary to assess its potential effects in other populations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
As this study used data from the PLCO trial, which had obtained ethical permission, no additional ethical permit was required for our analysis. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization: ZJ and HC. Methodology: ZJ and HC. Formal analysis: ZJ, WW, and ML. Original draft preparation: ZJ, HC, and CF. Supervision and project administration: ML, CF, and FL. Acquisition of data: ZJ and FL. Review and editing: ZJ, FL, and CF. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Natural Science Foundation of Sichuan Province, China (Grant No. 2022NSFSC0764) and the Fundamental Research Funds for the Central Universities (Grant No.2022SCU12025 and 2022SCU12020).
Acknowledgments
The authors thank the National Cancer Institute for access to NCI’s data collected by the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1220270/full#supplementary-material
References
1. Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal Cancer Statistics, 2020. CA Cancer J Clin (2020) 70(3):145–64. doi: 10.3322/caac.21601
2. Islami F, Goding Sauer A, Miller KD, Siegel RL, Fedewa SA, Jacobs EJ, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin (2018) 68(1):31–54. doi: 10.3322/caac.21440
3. Farvid MS, Sidahmed E, Spence ND, Mante Angua K, Rosner BA, Barnett JB. Consumption of red meat and processed meat and cancer incidence: A systematic review and meta-analysis of prospective studies. Eur J Epidemiol (2021) 36(9):937–51. doi: 10.1007/s10654-021-00741-9
4. Bradbury KE, Murphy N, Key TJ. Diet and colorectal cancer in Uk biobank: A prospective study. Int J Epidemiol (2020) 49(1):246–58. doi: 10.1093/ije/dyz064
5. Michels N, Specht IO, Heitmann BL, Chajès V, Huybrechts I. Dietary trans-fatty acid intake in relation to cancer risk: A systematic review and meta-analysis. Nutr Rev (2021) 79(7):758–76. doi: 10.1093/nutrit/nuaa061
6. Bara T Jr., Gurzu S, Sugimura H, Bara T, Beleaua MA, Jung I. A systematic review of the possible carcinogenic role of the aristolochic acid. ROmanian J morphology embryology = Rev roumaine morphologie embryologie (2017) 58(1):41–4.
7. Garland C, Shekelle RB, Barrett-Connor E, Criqui MH, Rossof AH, Paul O. Dietary vitamin D and calcium and risk of colorectal cancer: A 19-year prospective study in men. Lancet (1985) 1(8424):307–9. doi: 10.1016/s0140-6736(85)91082-7
8. Park SY, Murphy SP, Wilkens LR, Nomura AM, Henderson BE, Kolonel LN. Calcium and vitamin D intake and risk of colorectal cancer: the multiethnic cohort study. Am J Epidemiol (2007) 165(7):784–93. doi: 10.1093/aje/kwk069
9. Aune D, Chan DS, Lau R, Vieira R, Greenwood DC, Kampman E, et al. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. Bmj (2011) 343:d6617. doi: 10.1136/bmj.d6617
10. van Duijnhoven FJ, Bueno-De-Mesquita HB, Ferrari P, Jenab M, Boshuizen HC, Ros MM, et al. Fruit, vegetables, and colorectal cancer risk: the european prospective investigation into cancer and nutrition. Am J Clin Nutr (2009) 89(5):1441–52. doi: 10.3945/ajcn.2008.27120
11. Barrubés L, Babio N, Becerra-Tomás N, Rosique-Esteban N, Salas-Salvadó J. Association between dairy product consumption and colorectal cancer risk in adults: A systematic review and meta-analysis of epidemiologic studies. Adv Nutr (2019) 10(suppl_2):S190–s211. doi: 10.1093/advances/nmy114
12. Schulpen M, Peeters PH, van den Brandt PA. Mediterranean diet adherence and risk of esophageal and gastric cancer subtypes in the Netherlands cohort study. Gastric Cancer (2019) 22(4):663–74. doi: 10.1007/s10120-019-00927-x
13. Thomas CE, Luu HN, Wang R, Adams-Haduch J, Jin A, Koh WP, et al. Association between dietary tomato intake and the risk of hepatocellular carcinoma: the Singapore Chinese health study. Cancer Epidemiol Biomarkers Prev (2020) 29(7):1430–5. doi: 10.1158/1055-9965.Epi-20-0051
14. Giovannucci E, Rimm EB, Liu Y, Stampfer MJ, Willett WC. A prospective study of tomato products, lycopene, and prostate cancer risk. J Natl Cancer Inst (2002) 94(5):391–8. doi: 10.1093/jnci/94.5.391
15. Jansen RJ, Robinson DP, Stolzenberg-Solomon RZ, Bamlet WR, de Andrade M, Oberg AL, et al. Fruit and vegetable consumption is inversely associated with having pancreatic cancer. Cancer Causes Control: CCC (2011) 22(12):1613–25. doi: 10.1007/s10552-011-9838-0
16. Yang T, Yang X, Wang X, Wang Y, Song Z. The role of tomato products and lycopene in the prevention of gastric cancer: A meta-analysis of epidemiologic studies. Med Hypotheses (2013) 80(4):383–8. doi: 10.1016/j.mehy.2013.01.005
17. Kiani F, Knutsen S, Singh P, Ursin G, Fraser G. Dietary risk factors for ovarian cancer: the adventist health study (United States). Cancer Causes Control: CCC (2006) 17(2):137–46. doi: 10.1007/s10552-005-5383-z
18. La Vecchia C. Tomatoes, lycopene intake, and digestive tract and female hormone-related neoplasms. Exp Biol Med (Maywood) (2002) 227(10):860–3. doi: 10.1177/153537020222701004
19. Franceschi S, Bidoli E, La Vecchia C, Talamini R, D’Avanzo B, Negri E. Tomatoes and risk of digestive-tract cancers. Int J Cancer (1994) 59(2):181–4. doi: 10.1002/ijc.2910590207
20. Wang X, Yang HH, Liu Y, Zhou Q, Chen ZH. Lycopene consumption and risk of colorectal cancer: A meta-analysis of observational studies. Nutr Cancer (2016) 68(7):1083–96. doi: 10.1080/01635581.2016.1206579
21. Männistö S, Yaun SS, Hunter DJ, Spiegelman D, Adami HO, Albanes D, et al. Dietary carotenoids and risk of colorectal cancer in a pooled analysis of 11 cohort studies. Am J Epidemiol (2007) 165(3):246–55. doi: 10.1093/aje/kwk009
22. Xu X, Li S, Zhu Y. Dietary intake of tomato and lycopene and risk of all-cause and cause-specific mortality: results from a prospective study. Front Nutr (2021) 8:684859. doi: 10.3389/fnut.2021.684859
23. Prorok PC, Andriole GL, Bresalier RS, Buys SS, Chia D, Crawford ED, et al. Design of the prostate, lung, colorectal and ovarian (Plco) cancer screening trial. Controlled Clin Trials (2000) 21(6 Suppl):273s–309s. doi: 10.1016/s0197-2456(00)00098-2
24. Subar AF, Midthune D, Kulldorff M, Brown CC, Thompson FE, Kipnis V, et al. Evaluation of alternative approaches to assign nutrient values to food groups in food frequency questionnaires. Am J Epidemiol (2000) 152(3):279–86. doi: 10.1093/aje/152.3.279
25. Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, et al. Comparative validation of the block, willett, and national cancer institute food frequency questionnaires : the eating at America's table study. Am J Epidemiol (2001) 154(12):1089–99. doi: 10.1093/aje/154.12.1089
26. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr (1997) 65(4 Suppl):1220S–8S; discussion 9S-31S. doi: 10.1093/ajcn/65.4.1220S
27. Tahir MR, Tran QX, Nikulin MS. Comparison of hypertabastic survival model with other unimodal hazard rate functions using a goodness-of-fit test. Stat Med (2017) 36(12):1936–45. doi: 10.1002/sim.7244
28. Su NJ, Huang CY, Liu J, Kang DY, Wang SL, Liao LJ, et al. Association between baseline lh/fsh and live-birth rate after fresh-embryo transfer in polycystic ovary syndrome women. Sci Rep (2021) 11(1):20490. doi: 10.1038/s41598-021-99850-4
29. Xu X, Xie B, Li S, Wang S, Xia D, Meng H. Association of dietary tomato intake with bladder cancer risk in a prospective cohort of 101,683 individuals with 12.5 years of follow-up. Aging (2021) 13(13):17629–37. doi: 10.18632/aging.203252
30. Amorim A, Vasconcelos AG, Souza J, Oliveira A, Gullón B, de Souza de Almeida Leite JR, et al. Bio-availability, anticancer potential, and chemical data of lycopene: an overview and technological prospecting. Antioxidants (Basel Switzerland) (2022) 11(2):360. doi: 10.3390/antiox11020360
31. Song X, Luo Y, Ma L, Hu X, Simal-Gandara J, Wang LS, et al. Recent trends and advances in the epidemiology, synergism, and delivery system of lycopene as an anti-cancer agent. Semin Cancer Biol (2021) 73:331–46. doi: 10.1016/j.semcancer.2021.03.028
32. Ali D, Ali H, Alifiri S, Alkahtani S, Alkahtane AA, Huasain SA. Detection of oxidative stress and DNA damage in freshwater snail lymnea leuteola exposed to profenofos. Front Environ Sci Eng (2018) 12(5):1. doi: 10.1007/s11783-018-1039-6
33. Singh P, Goyal GK. Dietary lycopene: its properties and anticarcinogenic effects. Compr Rev Food Sci Food Saf (2008) 7(3):255–70. doi: 10.1111/j.1541-4337.2008.00044.x
34. Gärtner C, Stahl W, Sies H. Lycopene is more bioavailable from tomato paste than from fresh tomatoes. Am J Clin Nutr (1997) 66(1):116–22. doi: 10.1093/ajcn/66.1.116
35. Malila N, Virtamo J, Virtanen M, Pietinen P, Albanes D, Teppo L. Dietary and serum alpha-tocopherol, beta-carotene and retinol, and risk for colorectal cancer in male smokers. Eur J Clin Nutr (2002) 56(7):615–21. doi: 10.1038/sj.ejcn.1601366
36. Slattery ML, Benson J, Curtin K, Ma KN, Schaeffer D, Potter JD. Carotenoids and colon cancer. Am J Clin Nutr (2000) 71(2):575–82. doi: 10.1093/ajcn/71.2.575
37. Stahl W, Sies H. Uptake of lycopene and its geometrical isomers is greater from heat-processed than from unprocessed tomato juice in humans. J Nutr (1992) 122(11):2161–6. doi: 10.1093/jn/122.11.2161
38. Shi J, Le Maguer M. Lycopene in tomatoes: chemical and physical properties affected by food processing. Crit Rev Food Sci Nutr (2000) 40(1):1–42. doi: 10.1080/10408690091189275
39. Agarwal A, Shen H, Agarwal S, Rao AV. Lycopene content of tomato products: its stability, bioavailability and in vivo antioxidant properties. J Med Food (2001) 4(1):9–15. doi: 10.1089/10966200152053668
40. Storniolo CE, Sacanella I, Lamuela-Raventos RM, Moreno JJ. Bioactive compounds of mediterranean cooked tomato sauce (Sofrito) modulate intestinal epithelial cancer cell growth through oxidative stress/arachidonic acid cascade regulation. ACS Omega (2020) 5(28):17071–7. doi: 10.1021/acsomega.9b04329
41. Storniolo CE, Sacanella I, Mitjavila MT, Lamuela-Raventos RM, Moreno JJ. Bioactive compounds of cooked tomato sauce modulate oxidative stress and arachidonic acid cascade induced by oxidized Ldl in macrophage cultures. Nutrients (2019) 11(8):1880. doi: 10.3390/nu11081880
42. Storniolo CE, Cabral M, Busquets MA, Martin-Venegas R, Moreno JJ. Dual behavior of long-chain fatty acids and their cyclooxygenase/lipoxygenase metabolites on human intestinal caco-2 cell growth. Front Pharmacol (2020) 11:529976. doi: 10.3389/fphar.2020.529976
43. Hashim YZ, Eng M, Gill CI, McGlynn H, Rowland IR. Components of olive oil and chemoprevention of colorectal cancer. Nutr Rev (2005) 63(11):374–86. doi: 10.1111/j.1753-4887.2005.tb00374.x
44. Casaburi I, Puoci F, Chimento A, Sirianni R, Ruggiero C, Avena P, et al. Potential of olive oil phenols as chemopreventive and therapeutic agents against cancer: A review of in vitro studies. Mol Nutr Food Res (2013) 57(1):71–83. doi: 10.1002/mnfr.201200503
45. Martínez-Huélamo M, Tulipani S, Estruch R, Escribano E, Illán M, Corella D, et al. The tomato sauce making process affects the bioaccessibility and bioavailability of tomato phenolics: A pharmacokinetic study. Food Chem (2015) 173:864–72. doi: 10.1016/j.foodchem.2014.09.156
46. Zacarias-Garcia J, Perez-Traves L, Gil JV, Rodrigo MJ, Zacarias L. Bioactive compounds, nutritional quality and antioxidant capacity of the red-fleshed kirkwood navel and ruby valencia oranges. Antioxidants (Basel Switzerland) (2022) 11(10):1905. doi: 10.3390/antiox11101905
Keywords: cohort, colorectal cancer, dietary nutrients, LYCOPENE, PLCO, tomato
Citation: Jiang Z, Chen H, Li M, Wang W, Long F and Fan C (2023) Associations between colorectal cancer risk and dietary intake of tomato, tomato products, and lycopene: evidence from a prospective study of 101,680 US adults. Front. Oncol. 13:1220270. doi: 10.3389/fonc.2023.1220270
Received: 10 May 2023; Accepted: 24 July 2023;
Published: 11 August 2023.
Edited by:
Simona Gurzu, George Emil Palade University of Medicine, Pharmacy, Sciences and Technology of Târgu Mureş, RomaniaReviewed by:
Zhihong Gong, University at Buffalo, United StatesDaoud Ali, King Saud University, Saudi Arabia
Copyright © 2023 Jiang, Chen, Li, Wang, Long and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feiwu Long, bG9uZ2Z3MTk3OEBzaW5hLmNvbQ==
 Zongze Jiang
Zongze Jiang Huilin Chen2,3
Huilin Chen2,3 Feiwu Long
Feiwu Long Chuanwen Fan
Chuanwen Fan