- 1Department of General Surgery (Hepatopancreatobiliary Surgery), The Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China
- 2Department of Vascular Surgery, The Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China
Background: Biliary tract cancer (BTC) is a malignancy associated with unfavorable outcomes. Advanced BTC patients have a propensity to experience compromised immune and nutritional status as a result of obstructive jaundice and biliary inflammation. Currently, there is a lack of consensus on the impact of the Controlling Nutritional Status (CONUT) score in the context of BTC prognosis. The purpose of this study is to conduct a meta-analysis on the association between CONUT and the prognosis of patients suffering from BTC.
Methods: A defined search strategy was implemented to search the PubMed, Embase, and Web of Science databases for eligible studies published until March 2023, with a focus on overall survival (OS), relapse-free survival/recurrence-free survival(RFS), and relevant clinical characteristics. The prognostic potential of the CONUT score was evaluated using hazard ratios (HRs) or odds ratios (ORs) with 95% confidence intervals (CIs).
Results: In this meta-analysis, a total of 1409 patients from China and Japan were involved in 9 studies. The results indicated that the CONUT score was significantly correlated with worse OS (HR=2.13, 95% CI 1.61-2.82, P<0.0001) and RFS (HR=1.83, 95% CI 1.44–2.31, P<0.0001) in patients with BTC. And, the analysis showed that a high CONUT score was significantly associated with clinical characteristics such as jaundice (OR=1.60, 95% CI=1.14–2.25, P=0.006), poorly differentiated tumor (OR=1.43, 95% CI=1.03–1.99, P=0.03), pT3 and 4 stage of the tumor (OR=1.87, 95% CI=1.30–2.68, P=0.0007), and complications of Clavien-Dindo classification grade IIIa or higher (OR=1.79, 95% CI=1.03–3.12, P=0.04).
Conclusion: This meta-analysis indicates that a high CONUT score can serve as a significant prognostic indicator for survival outcomes among patients diagnosed with BTC.
1 Introduction
Biliary tract cancer (BTC) including cholangiocarcinoma, gallbladder cancer, and ampulla of Vater cancer (1), represents a significant challenge in clinical practice. BTC is a rare global occurrence, exhibiting an extremely unfavorable prognosis, with a substantially higher incidence observed in low-income countries compared to their high-income counterparts (2, 3). Currently, there is a widespread consensus that surgical intervention is the primary therapeutic modality for patients diagnosed with BTC. The prognosis for BTC patients, however, is notably unfavorable and the 5-year overall survival (OS) rate is estimated to be less than 20% when all stages of the disease are considered. Patients with advanced BTC often exhibit declining immune-nutritional status due to pathophysiological changes induced by obstructive jaundice and inflammation of the biliary tract (4, 5). Thus, the utilization of immune nutritional markers can facilitate precise risk stratification and forecast the optimal surgical intervention and treatment for BTC patients, proving to be an invaluable approach.
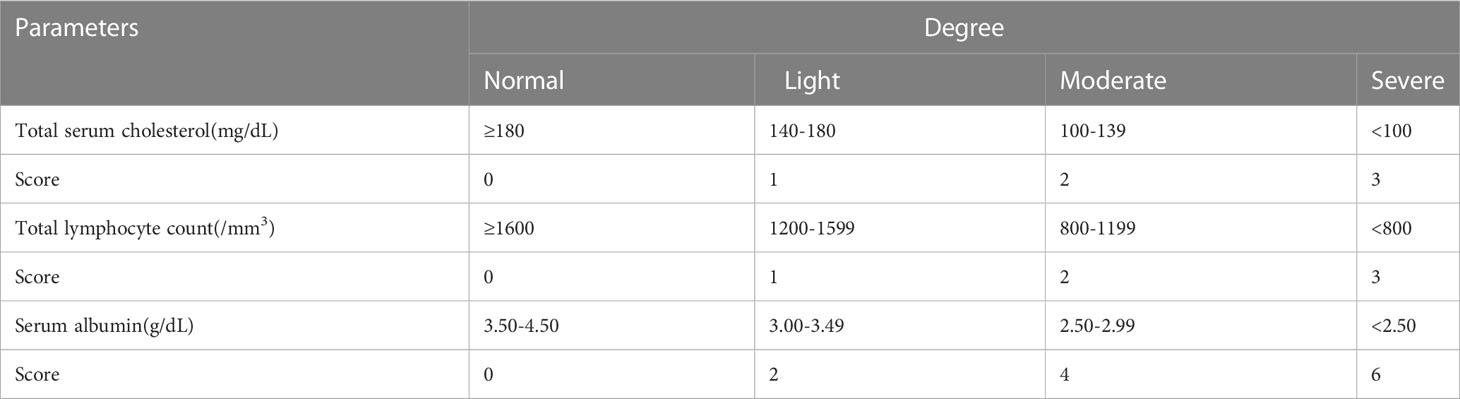
The Controlling Nutritional Status (CONUT) score is a self-sufficient tool for nutritional assessment, which was first established by Ignacio et al. (6). It is computed by analyzing three variables: serum albumin concentration, cholesterol level, and peripheral lymphocyte count. The CONUT score is then categorized into four graded levels based on total points, which include normal (0-1 points), mild (2-4 points), moderate (5-8 points), and severe (9-12 points) (Table 1)
In recent years, several studies have demonstrated the prognostic significance of the CONUT score in patients with malignant tumors (7–10). More recently, a multitude of investigations have investigated the relationship between the CONUT score and prognosis in individuals with BTC. Many of these studies have found that the CONUT score is an independent prognostic factor in patients with BTC. However, there remains a lack of consensus regarding the prognostic value of the CONUT score in this patient population. So, this meta-analysis’s objective was to comprehensively evaluate the associations between the CONUT score and clinical outcomes in individuals with BTC, drawing from all relevant available research.
2 Method
2.1 Literature search
The search was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Systematic searches were conducted in the PubMed, Embase, and Web of Science databases to identify all relevant studies published before March 2023 to evaluate the prognostic value of the CONUT score in BTC. using the following search items: “Controlling Nutritional Status”, “CONUT”, “CONUT score”, “biliary tract cancer”, “bile duct cancer”, “bile duct neoplasms”, “cholangiocarcinoma”, “gallbladder cancer”, “Vater ampullary carcinoma” and “Ampulla of Vater”. All searches were performed using a combination of MeSH terms and free-text words. The publication language was limited to English. References within the identified articles were manually examined to identify other potentially eligible studies. This meta-analysis has been registered in PROSPERO (http://www.crd.york.ac.uk/PROSPERO) with registration number CRD42023424382.
2.2 Inclusion and exclusion criteria
After retrieving relevant articles using specified search terms, articles were screened based on the following selection criteria: (i) articles specifically addressing the predictive significance of the CONUT score in patients with BTC; (ii) patients diagnosed with BTC and divided into two groups; (iii) availability of hazard ratios (HRs) and their corresponding 95% confidence intervals (CIs) and P-values for OS, relapse-free survival/recurrence-free survival(RFS) or other relevant effect metrics; (iv) articles published in full text. In addition, retrieved articles meeting any of the following criteria were excluded: previous reviews, letters, case reports, conference abstracts, comments, meta-analyses, books or documents, and unpublished articles. Two authors (ZL and YZ) independently assessed the eligibility of studies based on the aforementioned criteria, with any disagreements resolved through consultation with a third author (HZ).
2.3 Quality assessment and data extraction
The selected studies were subject to data extraction of crucial information, such as the first author’s name, publication year, duration, country, sample size, tumor type, study design, treatment method, CONUT score cut-off, study endpoints, and survival data, which includes outcome type, analysis method, HRs, and corresponding 95% CIs. Given the superior accuracy of multivariate analysis compared to univariate analysis, we opted to extract the HRs and corresponding 95% CIs from multivariate analysis. Furthermore, the quality of the included studies was assessed using the Newcastle-Ottawa quality assessment scale (NOS). This evaluation serves to enhance the credibility and reliability of the findings presented in this academic paper. Data collection for each article were conducted independently by two authors (ZL and HZ). In the event of any disagreements, a third author (YZ) was consulted to resolve any discrepancies.
2.4 Statistical analysis
The results of the multivariate analysis, which include HRs and 95% CIs, were used to evaluate the prognostic effect of the CONUT score on the OS and RFS of patients with BTC. The Cochran’s Q test and I2 statistics were employed to evaluate the heterogeneity among studies. In cases where heterogeneity was significant (I2 > 50% and P < 0.10), a random effects model was utilized to combine the HRs and 95% CIs. If heterogeneity was insignificant, a fixed effects model was chosen. Subgroup analysis was performed to identify the sources of heterogeneity. Odds ratios (ORs) and corresponding 95% CIs were employed to determine the association between CONUT score and clinical characteristics. The statistical analyses were conducted using Stata software version 15.1 (Stata Corporation, College Station, TX, USA).
3 Results
3.1 Study characteristics
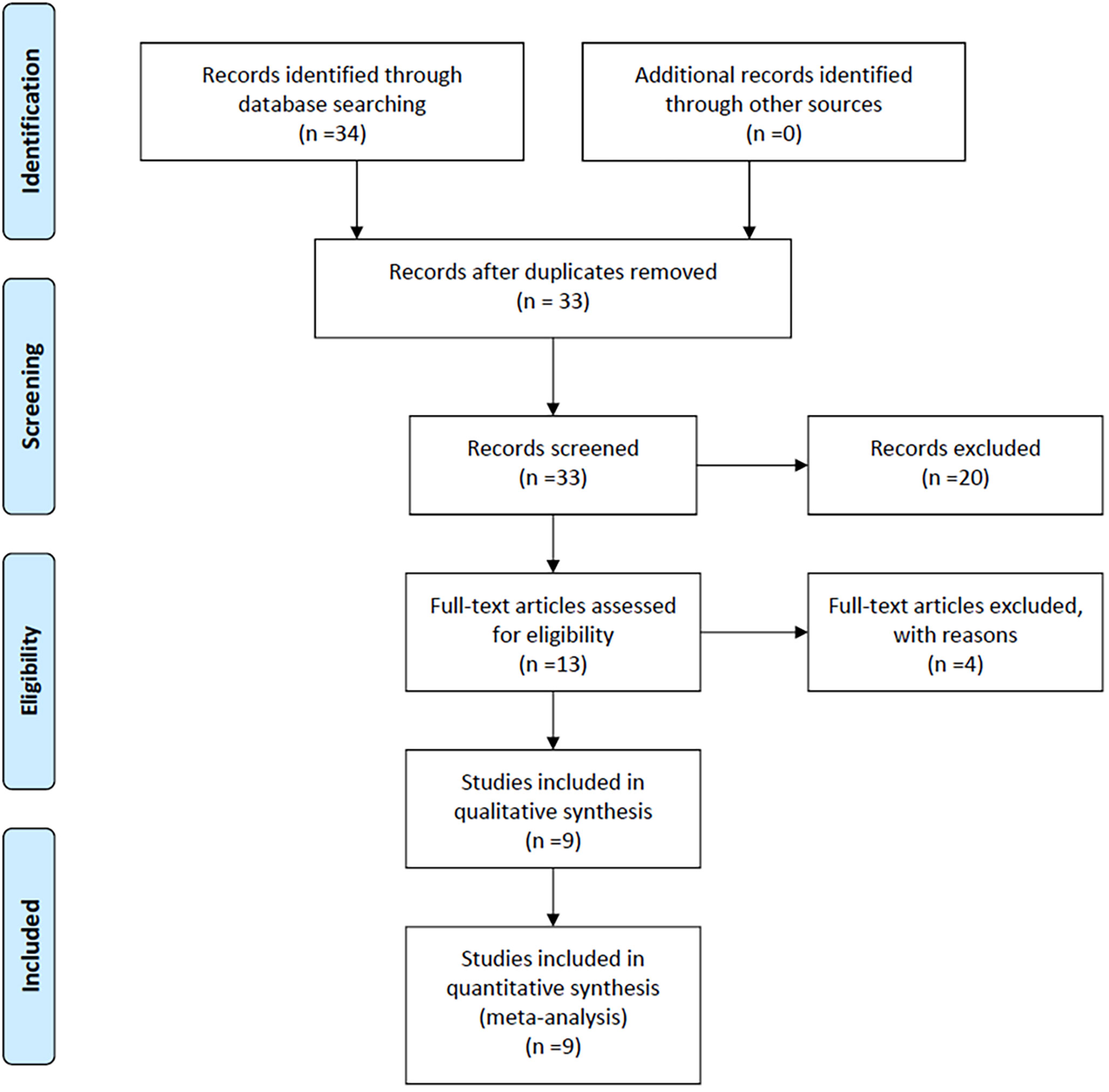
Figure 1 illustrates that the initial literature search yielded 34 studies. After removing duplicate entries and excluding 20 studies that were not relevant to BTC and CONUT score, or comprised of posters, abstracts, or editorials, the titles and abstracts of the remaining studies were scrutinized. Subsequently, 13 studies were included for further screening. Among them, the studies by He (11) and Miyamoto (12) lacked survival outcome information related to the CONUT score. The study by Utsumi did not provide HRs data associated with the CONUT score (13). Additionally, Utsumi’s study included a subset of non-biliary cancer patients (14). Therefore, we excluded these 4 studies. Finally, the inclusion criteria were met by the remaining 9 studies (15–23).
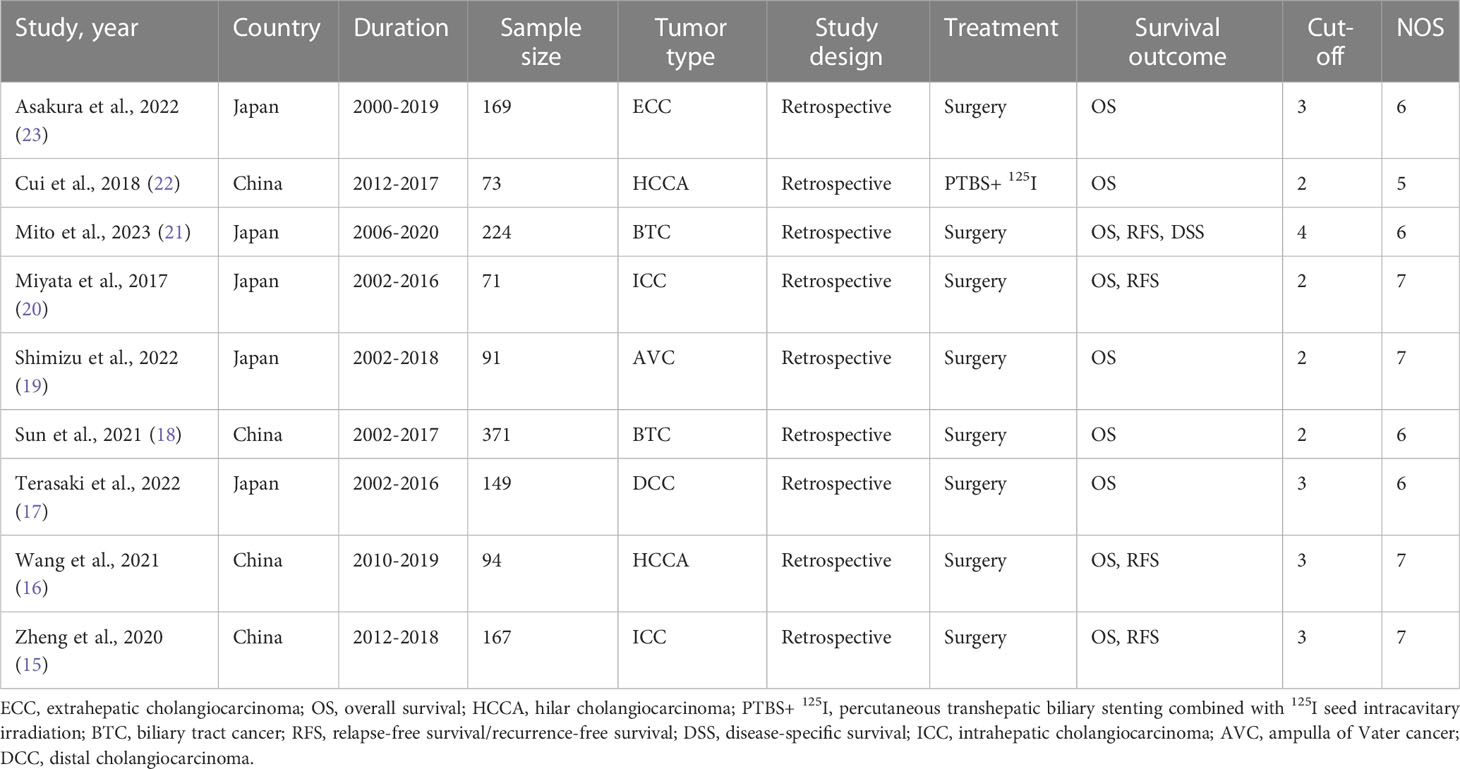
The main clinical characteristics of the included studies are summarized in Table 2. The nine studies included 1409 patients from China and Japan who met the inclusion criteria. Five studies were conducted in Japan (17, 19–21, 23), and four studies were performed in China (15, 16, 18, 22). All of these studies were retrospective study. Except for patients in Cui et al. who received treatment by percutaneous transhepatic biliary stenting combined with 125I seed intracavitary irradiation (22), patients in other studies received surgery. Eight studies have reported the independent prognostic value of the CONUT score in predicting OS (16–23), and four studies have reported its independent prognostic value in predicting RFS (15, 16, 20, 21). The cut-off value of the CONUT score ranged from 2 to 4. The NOS scores ranged from 5 to 7.
3.2 CONUT score and OS
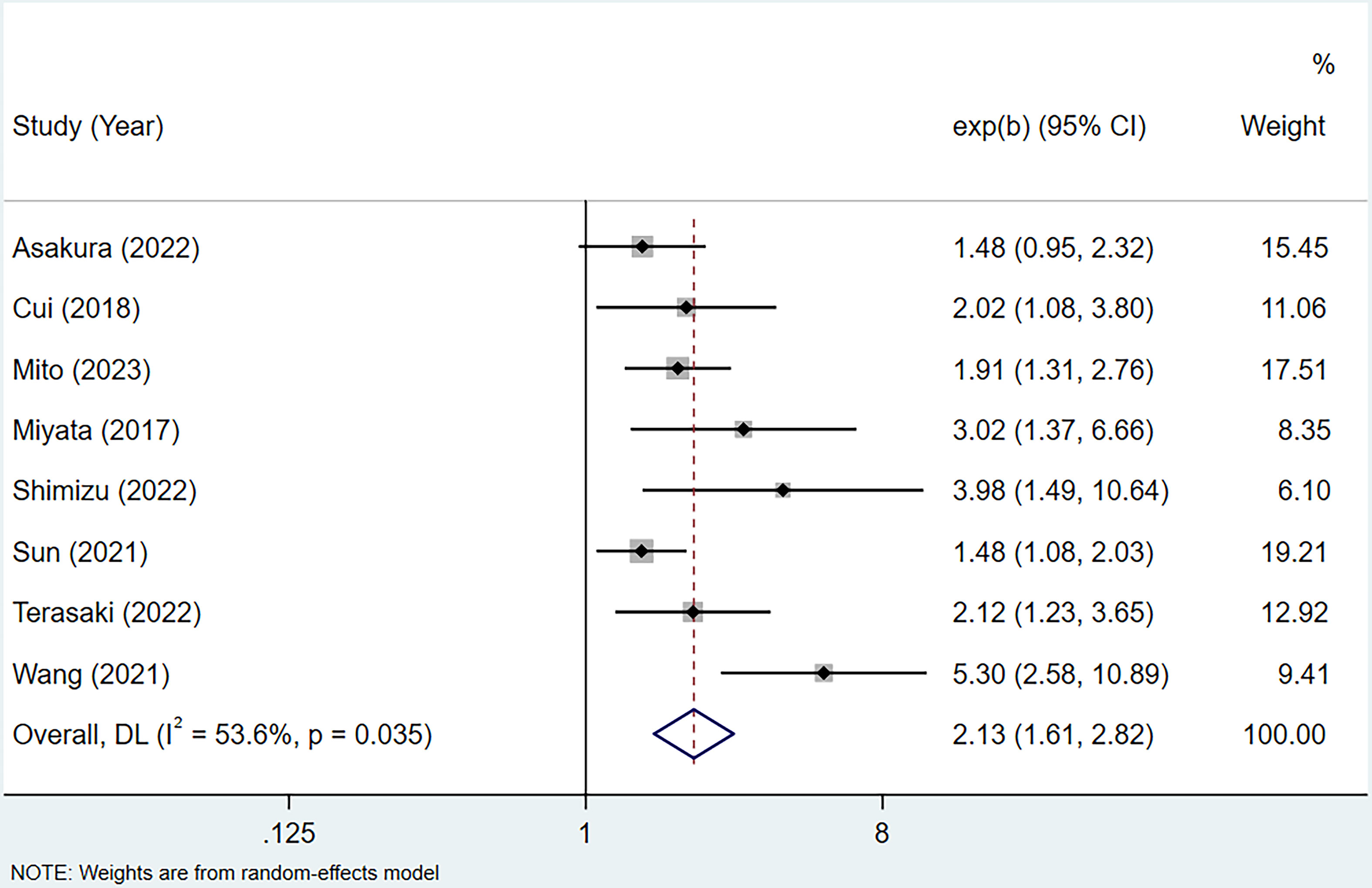
8 studies (16–23), involving a total of 1242 patients diagnosed with BTC, reported the HRs and 95%CIs for OS. The study by Zheng et al. was excluded because only univariate analysis result was reported (15). The results of the heterogeneity test, characterized by an I² value of 53.6 and a P-value of 0.035, indicated significant differences among the studies included. Therefore, a random-effects model was employed. Based on a summary estimate of the HR, the high CONUT score was found to be a significant risk factor for patients with BTC (HR=2.13, 95% CI 1.61-2.82, P<0.0001) (Figure 2).
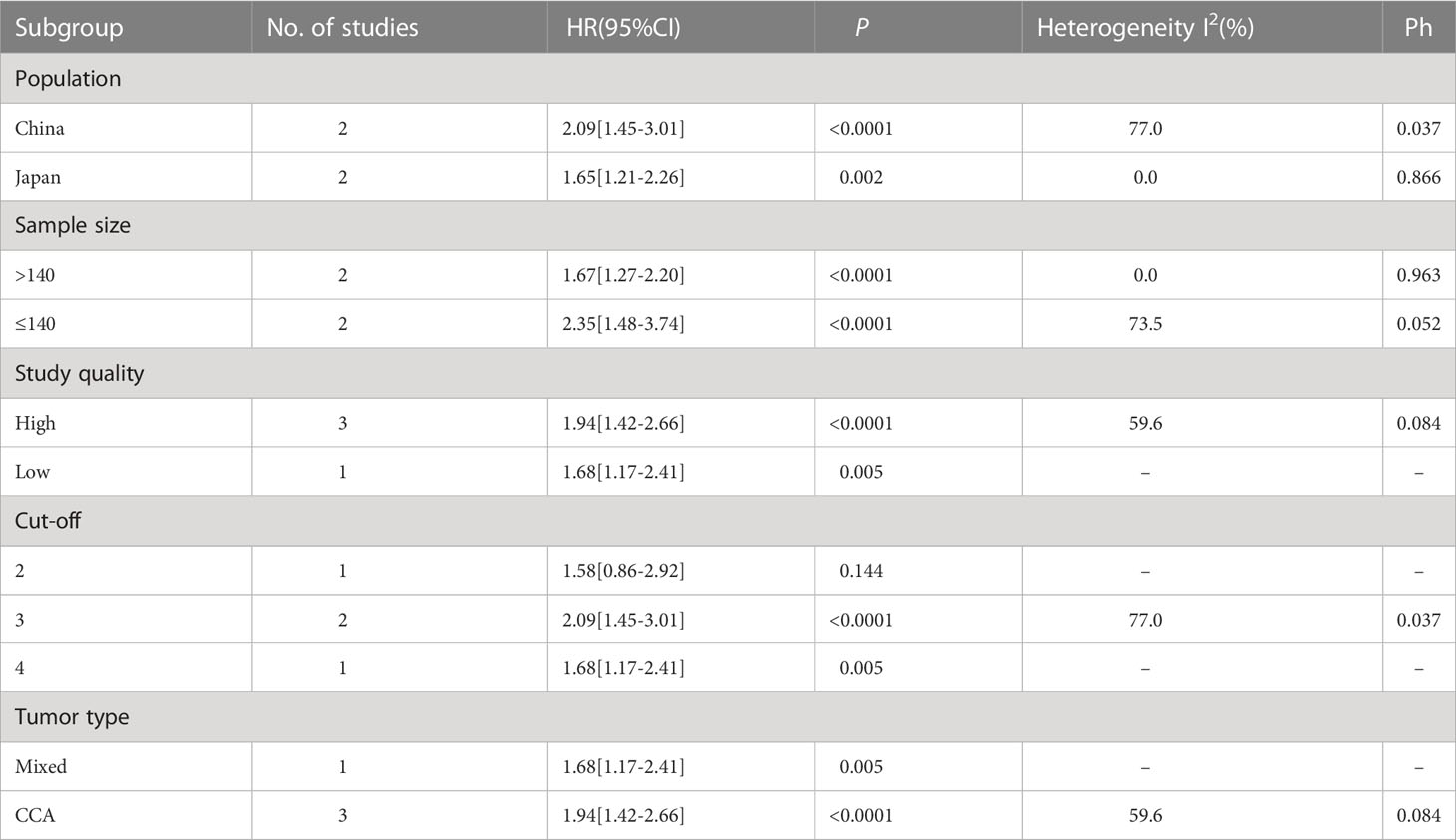
To account for the heterogeneity of OS, subgroup analyses were performed according to patients’ nationality, sample size, study quality, treatment method, tumor type, and CONUT score’s cut-off. As presented in Table 3, stratification by sample size revealed an HR of 1.67 (95% CI 1.37-2.03, P < 0.0001, I²=0%) for study with high sample size and an HR of 3.23 (95% CI 2.07-5.06, P < 0.0001, I²=27.3%) for study with low sample size. Stratification by study quality yielded an HR of 4.08 (95% CI 2.55-6.51, P < 0.0001, I²=0%) for high-quality studies and an HR of 1.70 (95% CI 1.40-2.05, P < 0.0001, I²=0%) for low-quality studies. These findings suggest that the sources of heterogeneity can be attributed to differential sample sizes and varying levels of study quality. Furthermore, significant correlations were observed between CONUT scores and OS within different subgroups.
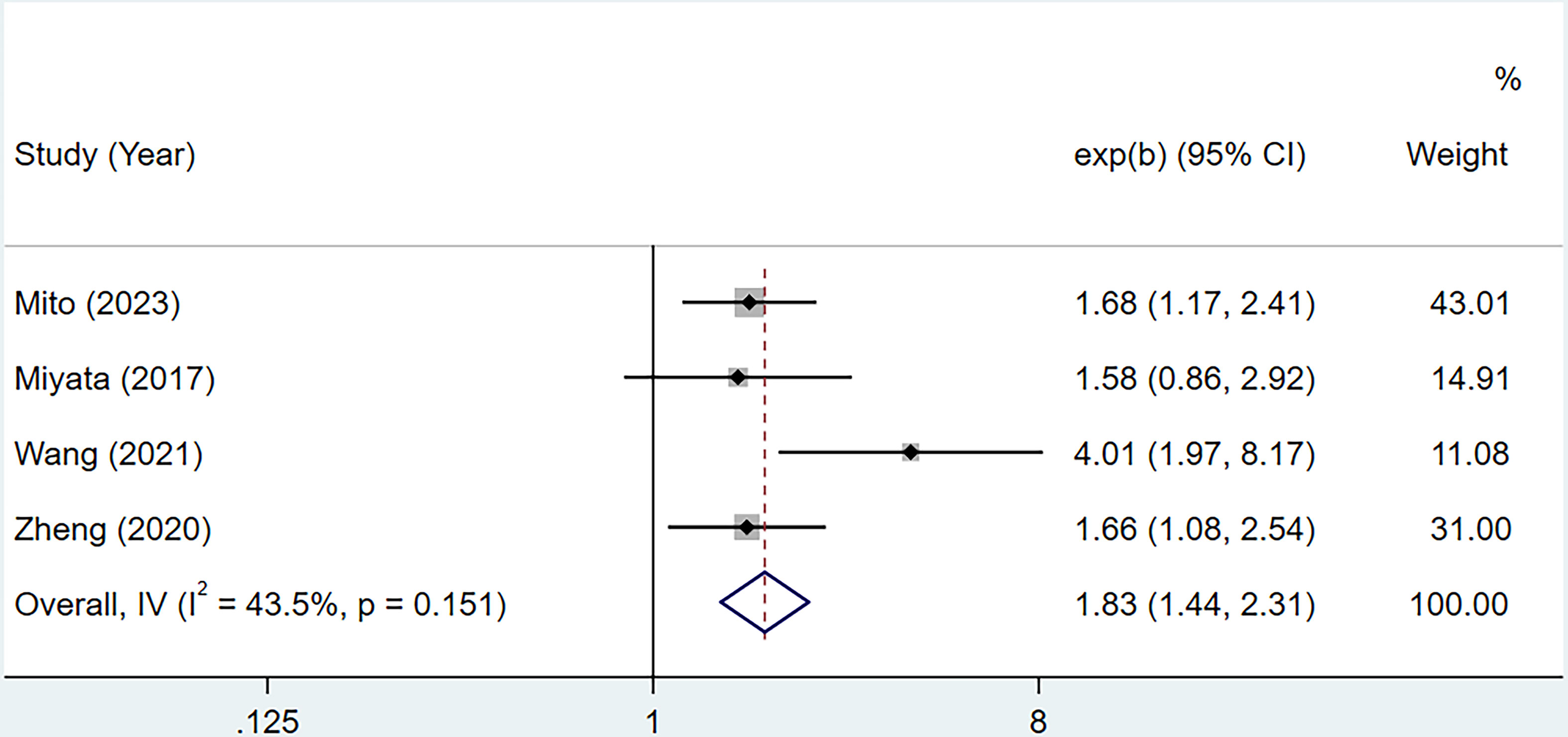
3.3 CONUT score and RFS
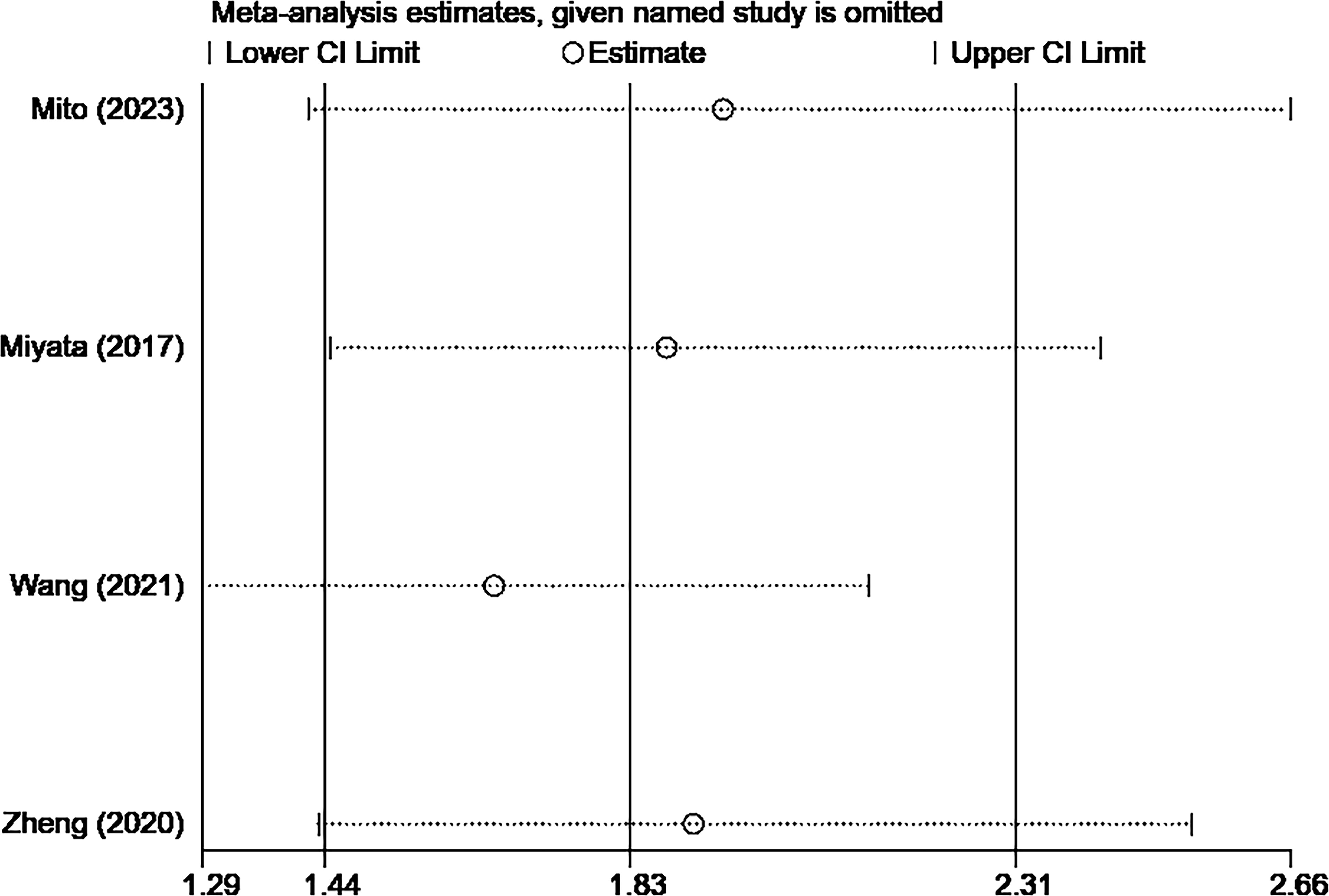
A total of 4 studies with 556 cases investigated the relationship between CONUT score and RFS in BTC (15, 16, 20, 21). Based on the analysis depicted in Figure 3, the pooled HR was found to be 1.83 (95% CI: 1.44-2.31, P<0.0001) without statistically significant heterogeneity (I2 = 43.5%, P=0.151). These findings provide evidence that the CONUT score is significantly associated with RFS in BTC. After conducting a subgroup analysis, it was found that the relationship between a high CONUT score and poor RFS was not influenced by population, sample size, study quality, and tumor type. However, when using a cut-off value of 2 for the CONUT score, there was no significant correlation between a high CONUT score and poor RFS (HR:1.58, 95% CI: 0.86-2.92, P=0.144) (Table 4). We speculate that this lack of correlation may be due to the small number of studies included in this subgroup analysis.
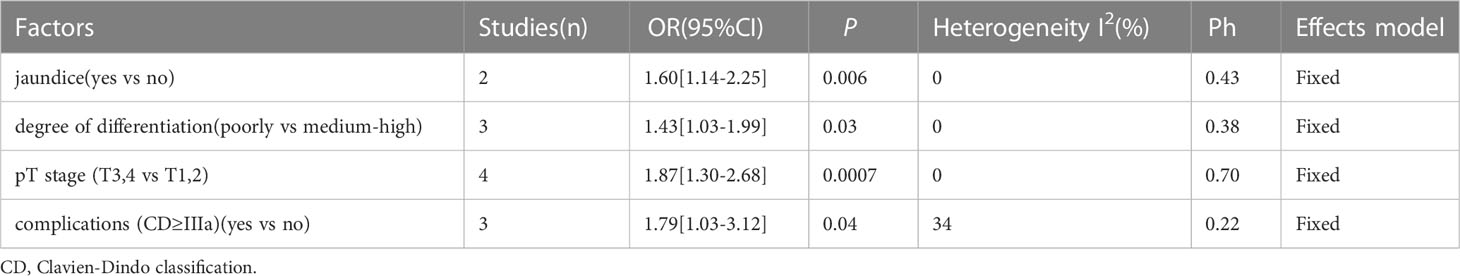
3.4 The association between CONUT and clinical characteristics
Based on data from 7 studies (15–21), this study investigated the association between CONUT score and various clinical characteristics. The results in Table 5 indicate a significant association between CONUT score and jaundice (OR=1.60, 95% CI=1.14–2.25, P=0.006), poorly differentiated tumor (OR=1.43, 95% CI=1.03–1.99, P=0.03), pT3 and 4 stage of the tumor (OR=1.87, 95% CI=1.30–2.68, P=0.0007), and complications of Clavien-Dindo classification grade IIIa or higher (OR=1.79, 95% CI=1.03–3.12, P=0.04).
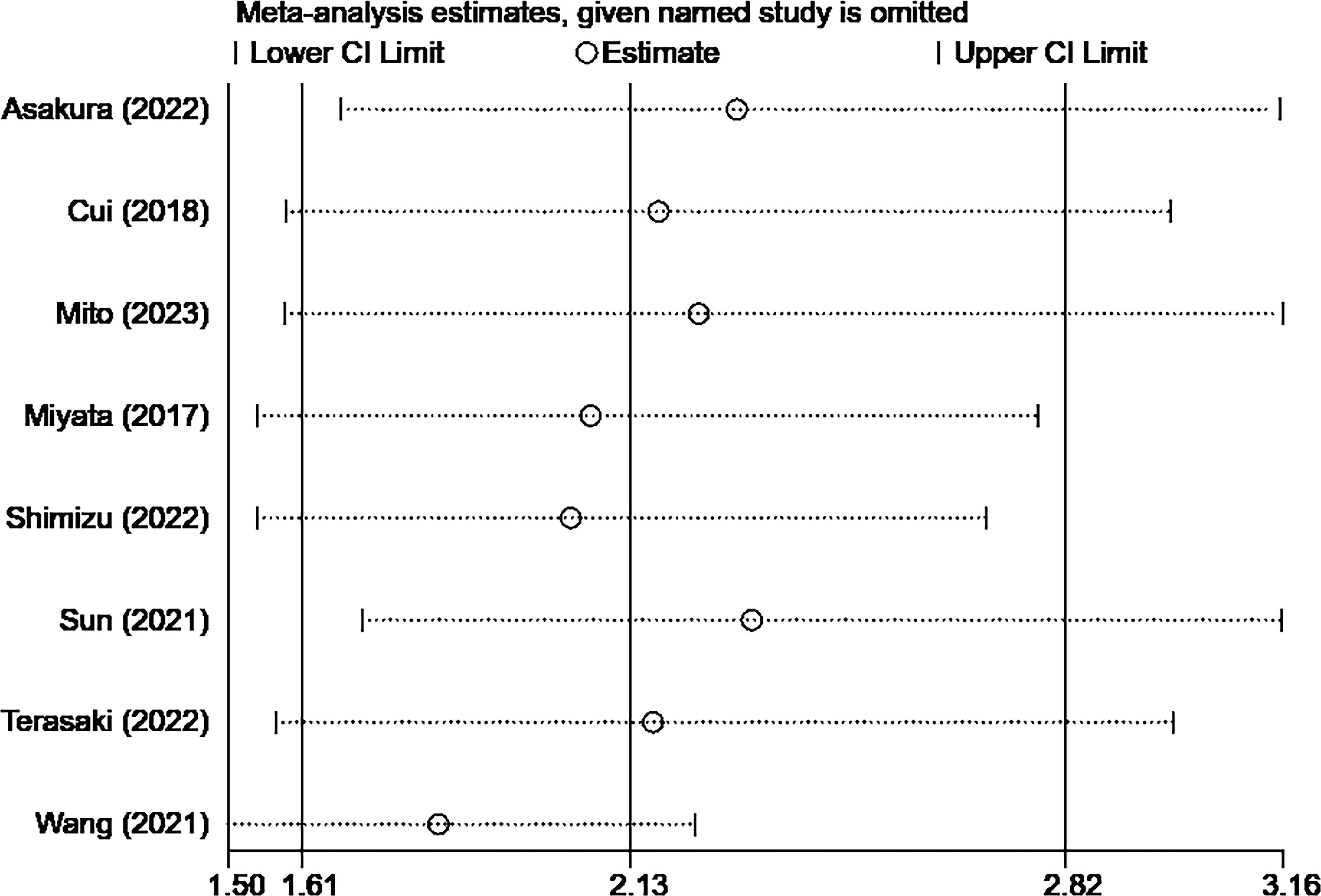
3.5 Sensitivity analysis
The sensitivity analysis showed that the overall results remained stable and reliable even when one study was omitted, indicating no significant changes (Figures 4, 5).
4 Discussion
In recent years, researchers have conducted extensive investigations into the potential prognostic impact of inflammatory and nutritional markers in cancer patients. In this study, our objective was to examine the prognostic value of the CONUT score in patients diagnosed with BTC. However, given the inconsistent and contradictory findings from the existing studies, we employed the meta-analysis approach to elucidate the potential role of the CONUT score in predicting prognosis in BTC patients.
Previous meta-analyses, which were limited in terms of literature coverage and solely focused on cholangiocarcinoma, have omitted to elucidate the association between the CONUT score and BTC outcomes in both short and long terms, thereby casting doubt on the credibility of their findings. In a meta-analysis by Takagi and colleagues (24), no correlation was observed between the CONUT score and postoperative complications in cases of hepatobiliary pancreatic malignancies. Our study reveals a significant correlation between a high CONUT score and postoperative complications of BTC (OR = 1.79, 95% CI: 1.03-3.12, P = 0.04). We further demonstrate that the CONUT score serves as an independent prognostic factor for OS and RFS in patients with BTC. Specifically, our findings suggest that the CONUT score may be associated with preoperative jaundice, postoperative tumor differentiation, pT stage, and complications in these patients. Moreover, through a sensitivity analysis, we obtained stable and reliable meta-analytical results. Our study represents the first to systematically investigate the prognostic significance of the CONUT score in BTC.
The CONUT score, which encompasses serum albumin, cholesterol, and peripheral blood lymphocyte counts, is employed as a prognostic tool for patients with BTC. However, the underlying mechanism through which it influences patient prognosis remains inadequately understood. A wealth of pertinent literature suggests that tumor development is inextricably linked to both immune function and nutritional status (25, 26), Research findings suggest that decreased levels of albumin are linked to unfavorable outcomes in malignant conditions (27, 28), decreased levels of albumin may impair the immune function of the body, leading to a reduction in the immune response against cancer cells and promoting tumor development (29, 30). Cholesterol is involved in the fundamental construction of cell membranes and maintains cellular physiological functions through intracellular signal transduction. When cholesterol levels decrease, it indicates insufficient energy storage and metabolic imbalance (31). The activity of low-density lipoprotein receptors is reported to be elevated in cholangiocarcinoma cells, indicating that hypocholesteremia may result from excessive uptake of cholesterol by tumor cells (32). Lymphocytes, as a crucial immune component, have been shown to inhibit the proliferation, migration, and invasion of cancer cells. Consequently, a higher CONUT score is associated with decreased patient survival (33).
Several meta-analyses have previously investigated the prognostic value of the CONUT score in malignant tumors. Kosei et al. conducted a meta-analysis that included 2601 patients, revealing a significant association between high CONUT score and poor prognosis in patients with colorectal cancer (10). A meta-analysis involving eight studies has demonstrated the correlation between high CONUT score and OS, cancer-specific survival, and RFS in patients with urothelial carcinoma who have undergone systematic treatment (34). A meta-analysis conducted by Zhang et al. showed that the preoperative CONUT score can serve as an independent prognostic factor for long-term survival in patients with gastrointestinal tumors, which can assist in predicting their postoperative survival status (35). Our meta-analysis demonstrates that the CONUT score has consistent prognostic value with other cancer types in terms of OS and RFS in patients with BTC. Additionally, we found a significant correlation between higher CONUT score and clinical and pathological characteristics of BTC. Based on these findings, the CONUT score can provide important evaluation for the treatment of patients with BTC, helping clinicians to develop more personalized treatment plans. Therefore, caution should be taken in the treatment strategy for BTC patients with high CONUT score.
This meta-analysis has several limitations. Firstly, all included studies were retrospective and the relatively small sample size necessitates further improvement of the quality of evidence. Secondly, the study only included patients from Asia, which may introduce region-based biases. Thirdly, there was inconsistency in the critical values of the CONUT score used in different studies. Finally, more multi-center, large-scale, prospective studies are required to validate these findings due to limited research reports on disease-specific survival and related areas.
5 Conclusion
The CONUT score is a reliable, simple, easily obtainable, and cost-effective index for predicting the prognosis of patients with BTC. It is an independent prognostic factor for OS and RFS in this patient population and has correlations with preoperative jaundice, postoperative tumor differentiation, pT stage, and complications. The European Society for Clinical Nutrition and Metabolism suggests employing a range of tools, including nutritional risk screening tool 2002, for the periodic evaluation of nutritional status and repeat assessment during cancer diagnosis (36). However, the use of current nutritional screening tools has not reached a consensus. The CONUT score can complement the selection of nutrition screening tools. Preoperative use of the CONUT score for nutritional risk stratification and individualized treatment based on different nutritional statuses can potentially improve treatment outcomes, particularly in individuals who are elderly and feeble, who may be at greater risk, and who have certain chronic diseases. A significant inference might also be generated by current research trends. The emergence of novel biomarkers that have caught the attention of researchers and use of these biomarkers to determine different treatment options for patients holds promise as a potential future therapeutic target. Although there is a lack of research on how improvements in CONUT score values relate to differences in disease prognosis, it is undeniable that the CONUT score reflects nutritional deficiency and inflammatory responses in the body. As a potential tool for nutritional risk stratification, further research is still needed for validation.
Author contributions
ZL and JL designed the study. ZL, HZ, and YZ established the process of literature selection and screened the abstracts and articles. MY, YC and HZ analyzed data and wrote the main manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Sichuan Provincial Science and Technology Plan Joint Innovation Special Project under Grant number 2022YFS0626-C1.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nagino M, HIrano S, Yoshitomi H, Aoki T, Uesaka K, Unno M, et al. Clinical practice guidelines for the management of biliary tract cancers 2019: the 3rd english edition. J Hepato-biliary-pancreatic Sci (2021) 28(1):26–54. doi: 10.1002/jhbp.870
2. MIranda-Filho A, Piñeros M, Ferreccio C, Adsay V, Soerjomataram I, Bray F, et al. Gallbladder and extrahepatic bile duct cancers in the americas: incidence and mortality patterns and trends. Int J Cancer (2020) 147(4):978–89. doi: 10.1002/ijc.32863
3. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA: Cancer J Clin (2023) 73(1):17–48. doi: 10.3322/caac.21763
4. Lamarca A, Edeline J, Goyal L. How I treat biliary tract cancer. ESMO Open (2022) 7(1):100378. doi: 10.1016/j.esmoop.2021.100378
5. Yao J, Kong Y, Wang C, Wei Y, Li H, Liu C. Endobiliary ablation combined with immune nutrition improves quality of life: A preliminary clinical study in patients with advanced Malignant obstructive jaundice. Med Sci Monitor: Int Med J Exp Clin Res (2022) 28:e936863. doi: 10.12659/msm.936863
6. Niu Z, Yan B. Prognostic and clinicopathological impacts of controlling nutritional status (Conut) score on patients with gynecological cancer: A meta-analysis. Nutr J (2023) 22(1):33. doi: 10.1186/s12937-023-00863-8
7. Li W, Li M, Wang T, Ma G, Deng Y, Pu D, et al. Controlling nutritional status (Conut) score is a prognostic factor in patients with resected breast cancer. Sci Rep (2020) 10(1):6633. doi: 10.1038/s41598-020-63610-7
8. Xue W, Zhang Y, Wang H, Zhang Y, Hu X. Multicenter study of controlling nutritional status (Conut) score as a prognostic factor in patients with hiv-related renal cell carcinoma. Front Immunol (2021) 12:778746. doi: 10.3389/fimmu.2021.778746
9. Kheirouri S, Alizadeh M. Prognostic potential of the preoperative controlling nutritional status (Conut) score in predicting survival of patients with cancer: A systematic review. Adv Nutr (Bethesda Md) (2021) 12(1):234–50. doi: 10.1093/advances/nmaa102
10. Takagi K, Buettner S, Ijzermans JNM. Prognostic significance of the controlling nutritional status (Conut) score in patients with colorectal cancer: A systematic review and meta-analysis. Int J Surg (London England) (2020) 78:91–6. doi: 10.1016/j.ijsu.2020.04.046
11. He Y, Liu H, Ma Y, Li J, Zhang J, Ren Y, et al. Preoperative prognostic nutritional index predicts short-term complications after radical resection of distal cholangiocarcinoma. Front Surg (2022) 9:1091534. doi: 10.3389/fsurg.2022.1091534
12. Miyamoto R, Takahashi A, Ogura T, Kitamura K, Ishida H, Matsudaira S, et al. Transduodenal ampullectomy for early ampullary cancer: clinical management, histopathological findings and long-term outcomes at a single center. Surgery (2023) 173(4):912–9. doi: 10.1016/j.surg.2022.12.005
13. Utsumi M, Aoki H, Nishimura S, Une Y, Kashima H, Kimura Y, et al. Safety of surgical treatment for elderly patients with gallbladder carcinoma. Acta Med Okayama (2019) 73(3):241–6. doi: 10.18926/amo/56867
14. Utsumi M, Aoki H, Nagahisa S, Nishimura S, Une Y, Kimura Y, et al. Preoperative predictive factors of pancreatic fistula after pancreaticoduodenectomy: usefulness of the conut score. Ann Surg Treat Res (2020) 99(1):18–25. doi: 10.4174/astr.2020.99.1.18
15. Zheng Y, Wu F, Rong W, Liu Y, Siqin T, Wang L, et al. Prognostic value of the controlling nutritional status (Conut) score in intrahepatic cholangiocarcinoma patients especially who had long-time alcohol consumption. J Clin Biochem Nutr (2020) 67(3):323–31. doi: 10.3164/jcbn.20-27
16. Wang A, He Z, Cong P, Qu Y, Hu T, Cai Y, et al. Controlling nutritional status (Conut) score as a new indicator of prognosis in patients with hilar cholangiocarcinoma is superior to nlr and pni: A single-center retrospective study. Front Oncol (2020) 10:593452. doi: 10.3389/fonc.2020.593452
17. Terasaki F, Sugiura T, Okamura Y, Ito T, Yamamoto Y, Ashida R, et al. Use of preoperative controlling nutritional status (Conut) score as a better prognostic marker for distal cholangiocarcinoma after pancreatoduodenectomy. Surg Today (2021) 51(3):358–65. doi: 10.1007/s00595-020-02098-0
18. Sun L, Su S, Xiong J, Hu W, Liu L, Xu H, et al. Controlling nutritional status score as a prognostic marker to predict overall survival in resected biliary tract cancers. Ann Trans Med (2021) 9(8):644. doi: 10.21037/atm-20-6770
19. Shimizu Y, Ashida R, Sugiura T, Okamura Y, Ito T, Yamamoto Y, et al. Prognostic impact of indicators of systemic inflammation and the nutritional status of patients with resected carcinoma of the ampulla of vater: A single-center retrospective study. World J Surg (2022) 46(1):246–58. doi: 10.1007/s00268-021-06346-3
20. Miyata T, Yamashita YI, Higashi T, Taki K, Izumi D, Kosumi K, et al. The prognostic impact of controlling nutritional status (Conut) in intrahepatic cholangiocarcinoma following curative hepatectomy: A retrospective single institution study. World J Surg (2018) 42(4):1085–91. doi: 10.1007/s00268-017-4214-1
21. Mito M, Sakata J, Hirose Y, Abe S, Saito S, Miura Y, et al. Preoperative controlling nutritional status score predicts systemic disease recurrence in patients with resectable biliary tract cancer. Eur J Surg Oncol (2023) 49(2):399–409. doi: 10.1016/j.ejso.2022.11.003
22. Cui P, Pang Q, Wang Y, Qian Z, Hu X, Wang W, et al. Nutritional prognostic scores in patients with hilar cholangiocarcinoma treated by percutaneous transhepatic biliary stenting combined with 125i seed intracavitary irradiation: A retrospective observational study. Medicine (2018) 97(22):e11000. doi: 10.1097/md.0000000000011000
23. Asakura R, Yanagimoto H, Ajiki T, Tsugawa D, Mizumoto T, So S, et al. Prognostic impact of inflammation-based scores for extrahepatic cholangiocarcinoma. Dig Surg (2022) 39(2-3):65–74. doi: 10.1159/000521969
24. Takagi K, Domagala P, Polak WG, Buettner S, Ijzermans JNM. The controlling nutritional status score and postoperative complication risk in gastrointestinal and hepatopancreatobiliary surgical oncology: A systematic review and meta-analysis. Ann Nutr Metab (2019) 74(4):303–12. doi: 10.1159/000500233
25. Qian S, Golubnitschaja O, Zhan X. Chronic inflammation: key player and biomarker-set to predict and prevent cancer development and progression based on individualized patient profiles. EPMA J (2019) 10(4):365–81. doi: 10.1007/s13167-019-00194-x
26. Tanriverdi O. A discussion of serum albumin level in advanced-stage hepatocellular carcinoma: A medical oncologist’s perspective. Med Oncol (Northwood London England) (2014) 31(11):282. doi: 10.1007/s12032-014-0282-3
27. Kao HK, Löfstrand J, Loh CY, Lao WW, Yi JS, Chang YL, et al. Nomogram based on albumin and neutrophil-to-lymphocyte ratio for predicting the prognosis of patients with oral cavity squamous cell carcinoma. Sci Rep (2018) 8(1):13081. doi: 10.1038/s41598-018-31498-z
28. Loftus TJ, Brown MP, Slish JH, Rosenthal MD. Serum levels of prealbumin and albumin for preoperative risk stratification. Nutr Clin Pract (2019) 34(3):340–8. doi: 10.1002/ncp.10271
29. Güç ZG, Alacacıoğlu A, Kalender ME, Oflazoğlu U, Ünal S, Yıldız Y, et al. Halp score and gnri: simple and easily accessible indexes for predicting prognosis in advanced stage nsclc patients. The izmir oncology group (Izog) study. Front Nutr (2022) 9:905292. doi: 10.3389/fnut.2022.905292
30. Wang L, Li Q, Zhang J, Lu J. A novel prognostic scoring model based on albumin and Γ-glutamyltransferase for hepatocellular carcinoma prognosis. Cancer Manage Res (2019) 11:10685–94. doi: 10.2147/cmar.S232073
31. Ma K, Wang H, Jiang X, Fang C, Ma J. Prognostic value of combination of controlling nutritional status and tumor marker in patients with radical non-small-cell lung cancer. Dis Markers (2022) 2022:4764609. doi: 10.1155/2022/4764609
32. Terai K, Jiang M, Tokuyama W, Murano T, Takada N, Fujimura K, et al. Levels of soluble lr11/sorla are highly increased in the bile of patients with biliary tract and pancreatic cancers. Clinica Chim Acta (2016) 457:130–6. doi: 10.1016/j.cca.2016.04.010
33. Li B, Huang D, Zheng H, Cai Q, Guo Z, Wang S. Preoperative serum total cholesterol is a predictor of prognosis in patients with renal cell carcinoma: A meta- analysis of observational studies. Int Braz J Urol (2020) 46(2):158–68. doi: 10.1590/s1677-5538.Ibju.2019.0560
34. Peng L, Du C, Meng C, Li J, You C, Li X, et al. Controlling nutritional status score before receiving treatment as a prognostic indicator for patients with urothelial cancer: an exploration evaluation methods. Front Oncol (2021) 11:702908. doi: 10.3389/fonc.2021.702908
35. Zhang Y, Zhang X. Controlling nutritional status score, a promising prognostic marker in patients with gastrointestinal cancers after surgery: A systematic review and meta-analysis. Int J Surg (London England) (2018) 55:39–45. doi: 10.1016/j.ijsu.2018.05.018
Keywords: meta-analysis, biliary tract cancer, prognosis, controlling nutritional status score, clinical use
Citation: Liu Z, Zhou H, Zhou Y, Yu M, Cheng Y and Li J (2023) Prognostic impact of the Controlling Nutritional Status Score in patients with biliary tract cancer: a systematic review and meta-analysis. Front. Oncol. 13:1240008. doi: 10.3389/fonc.2023.1240008
Received: 14 June 2023; Accepted: 31 July 2023;
Published: 17 August 2023.
Edited by:
Stefano Francesco Crinò, University of Verona, ItalyReviewed by:
Sonia Tewani Orcutt, University of Arkansas for Medical Sciences, United StatesJulissa Luvián-Morales, National Institute of Cancerology (INCAN), Mexico
Copyright © 2023 Liu, Zhou, Zhou, Yu, Cheng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Li, bGlqaW5nXzMxMDc2MjNAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Zhuoran Liu
Zhuoran Liu Haoge Zhou2†
Haoge Zhou2† Menglin Yu
Menglin Yu