- In-Patient Ultrasound Department, Second Affiliated Hospital of Harbin Medical University, Surgeons’ Hall, Harbin, Heilongjiang, China
Background: While an elevated risk of second primary cancers (SPCs) has been observed in many other cancers, risk of SPCs has not been quantified in patients with rectal neuroendocrine neoplasms (NENs).
Methods: Survivors of primary rectal NENs diagnosed between 2000 and 2018 were identified from the Surveillance, Epidemiology, and End Results (SEER)-18 registries. Relative risk of SPCs was estimated as the standardized incidence ratio (SIR), which was calculated using SEER*Stat software.
Results: Between 2000 and 2018, a total of 15836 patients diagnosed with rectal NENs, of whom 1436 (9.1%) received diagnosis of SPCs (SIR: 1.19, 95%CI: 1.13-1.26). The majority of patients were aged 50-69 and had their first cancer diagnosed at the localized stage. Male survivors had a higher propensity for developing SPCs overall, while female survivors exhibited higher risks of specific SPCs. Age at diagnosis of rectal NENs influenced the risk of SPCs, with younger patients having greater risks. A statistically significant increase in the incidence of SPCs was observed among patients aged 30-64 years. Black patients had higher relative risks of certain SPCs, while White patients had a lower risk of subsequent melanoma. Trend analysis revealed that the highest excess burden of SPCs was observed in the years 2000 to 2002. Risk of SPCs remained elevated within the first four years post-diagnosis for survivors of rectal NENs, but diminished thereafter.
Conclusion: The study revealed that individuals who survived rectal NENs were at an elevated risk of developing SPCs compared to the general population. Our results hold important implications for the formulation of lifelong surveillance recommendations for cancer survivors.
Introduction
Neuroendocrine neoplasms (NENs) are a heterogeneous group of uncommon diseases with varied clinical characteristics and biological behaviors which arising from neuroendocrine cells throughout the diffuse endocrine system (1, 2). Due to advances in diagnostic technologies, there has been a significant increase in the detection of NENs both in the United States and globally (3–5). Rectal NENs are one of the main subtypes of NENs in the gastrointestinal tract (6, 7). Despite the typically indolent nature of rectal NENs and the improving outcomes in cancer management, survivors of these tumors still remain at a higher risk of developing second primary cancers (SPCs) during their cancer survivorship (8, 9). The heightened risk of SPCs may be partially attributed to genetic susceptibility and shared risk factors between NENs and secondary malignancies. Furthermore, regular medical surveillance for cancer survivors often leads to the frequent detection of SPCs.
In order to mitigate the relative risk of SPCs, it is imperative for individuals who have been diagnosed with rectal NENs to undergo regular follow-up visits and screenings as recommended by their healthcare providers. While previous studies have assessed the relative risk of SPCs in patients with various other cancers, there is a lack of research specifically examining the risk of developing a secondary malignancy among rectal NENs survivors in comparison to the general population in the United States. Therefore, in this present study, we attempted to comprehensively evaluate the relative risk of SPCs among patients with a history of rectal NENs using data from the SEER-18 program and to demonstrate the need for the development of appropriate surveillance protocols for this high-risk patient population.
Materials and methods
Patients who were histologically diagnosed with rectal NENs were extracted from the Surveillance, Epidemiology, and End Results (SEER)-18 registries, which includes nearly 28% of the US population from 2000 to 2018. This database includes incidence and population data stratified by race, sex, year of diagnosis, geographic area, and age. An at least 2-month latency between first rectal NENs diagnosis and SPCs was used to exclude synchronous primary malignancies. The study protocol was approved by the Institutional Review Board (IRB) of Second Affiliated Hospital of Harbin Medical University, and the requirement for written informed consent was waived due to the study’s design.
Statistical analysis
Standardized incidence ratios (SIRs) and absolute excess rate (AER) per 10,000 person-years were calculated to estimate the relative risk of a SPCs among rectal NENs survivors relative to the year 2000 US general population. Poisson regression models were used to calculated the corresponding 95% confidence intervals (CIs). All analyses were conducted by the SEER*Stat software.
Results
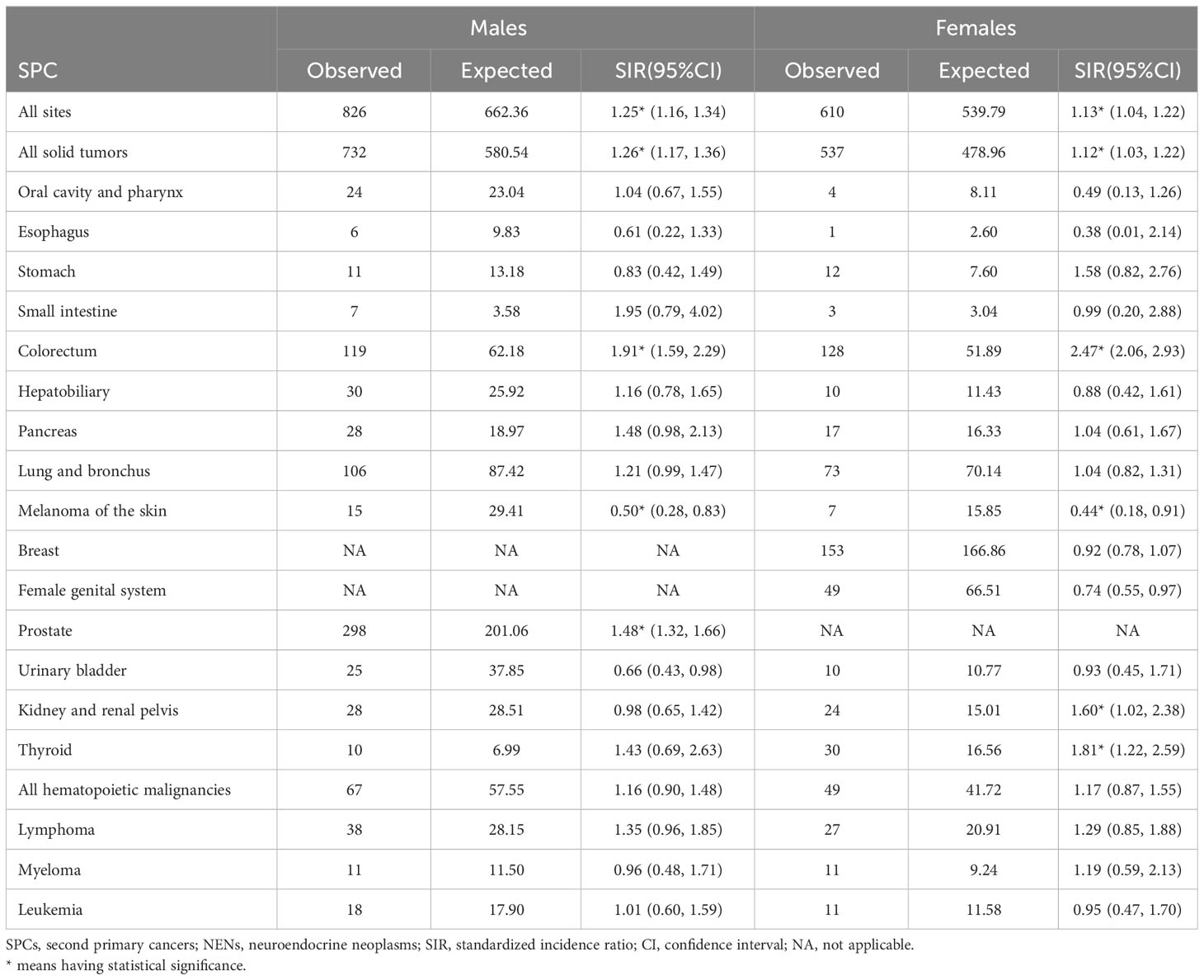
Between 2000 and 2018, we identified 15836 patients diagnosed with rectal NENs, of whom 1436 (9.1%) received diagnosis of a second primary cancer. The detailed demographics and characteristics of the entire study population are presented in Table 1. The majority of patients (66.7%) were between the ages of 50 and 69, and 78.3% had their first cancer diagnosed at the localized stage. More than half of the patients were of white ethnicity (55.6%) and were married (52.4%). The majority of patients (81.7%) underwent surgical intervention as the primary treatment for their initial malignancy, while only a small minority (2.9%) received chemotherapy. Male patients demonstrated a higher propensity for developing subsequent malignancies compared to female patients. Likewise, among those who received a diagnosis of a SPC, the majority had their initial malignancy identified at the localized stage.
Risk and burden of SPC
Figure 1 displays the risk and burden of SPCs overall and by patient characteristics. Among the study population, a total of 1436 patients developed a SPC, surpassing the expected cases if these patients had the same cancer risk as the general population. The SIR of developing a SPC was 1.19 (95%CI: 1.13-1.26) and the EAR was 21.51 cases per 10000 person-years. Among all patients with rectal NENs in the SEER database, there was a notable increase in the risk of developing four specific subsequent primary cancers: colorectum, prostate, thyroid, and lymphoma (Figure 2). The relative risk was highest for the occurrence of second colorectal cancers (SIR: 2.34, 95%CI: 2.08-2.63). Additionally, patients with rectal NENs exhibited significantly lower incidence rates of certain cancers compared to the general US population, including melanoma of the skin and female genital system cancers. Rectal NENs survivors who had their disease diagnosed at the unknown stage or at the localized stage exhibited a higher risk of developing SPCs, with SIRs of 1.24 (95%CI: 1.07-1.43) and 1.18 (95%CI: 1.12-1.25), respectively. Furthermore, patients who underwent surgery or chemotherapy for their initial malignancy had a more pronounced relative risk of developing subsequent cancers compared to those who did not receive these treatments.
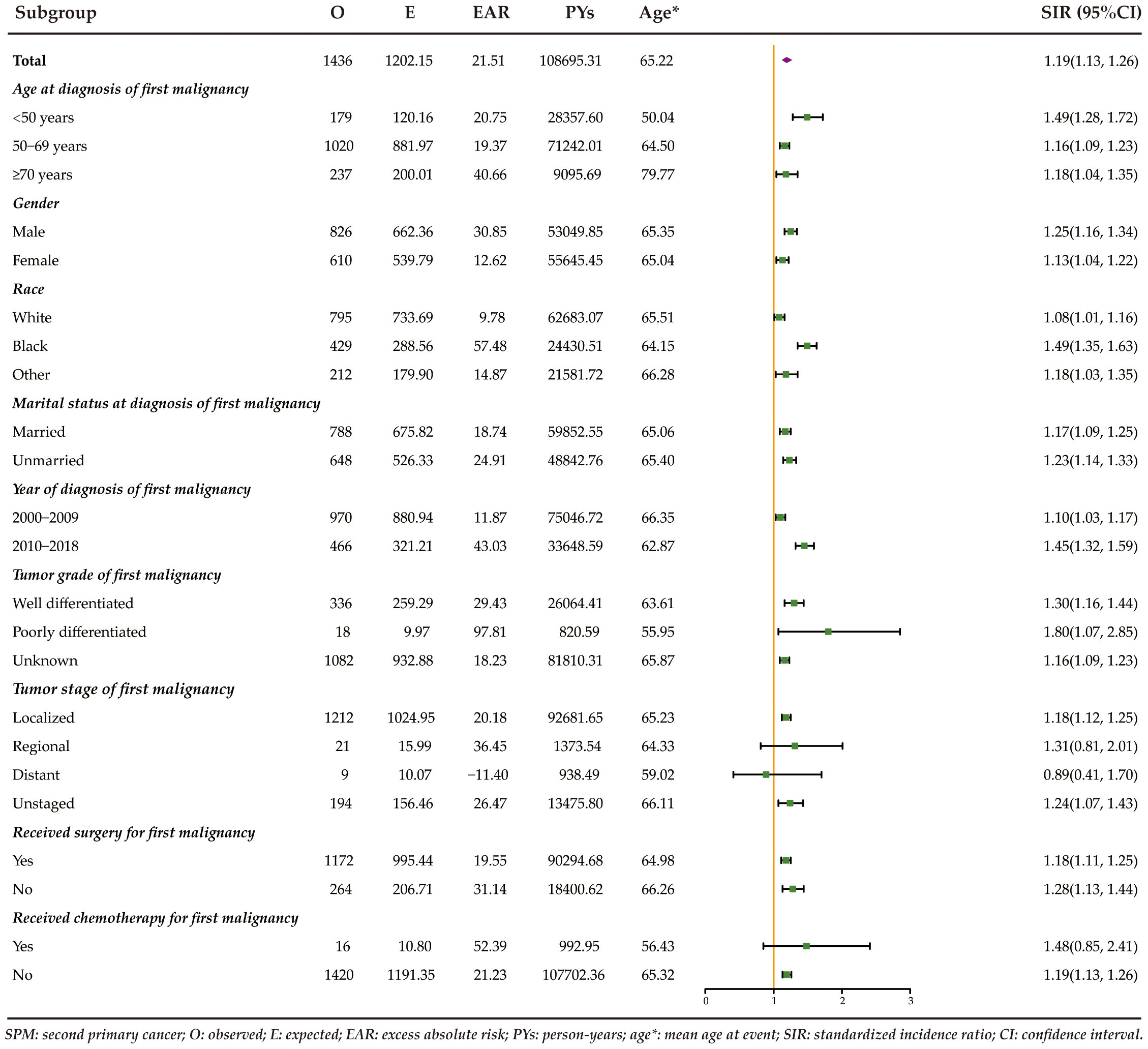
Figure 1 Risk of developing SPCs stratified by patient characteristics of rectal NENs in the United States between 2000 and 2018.
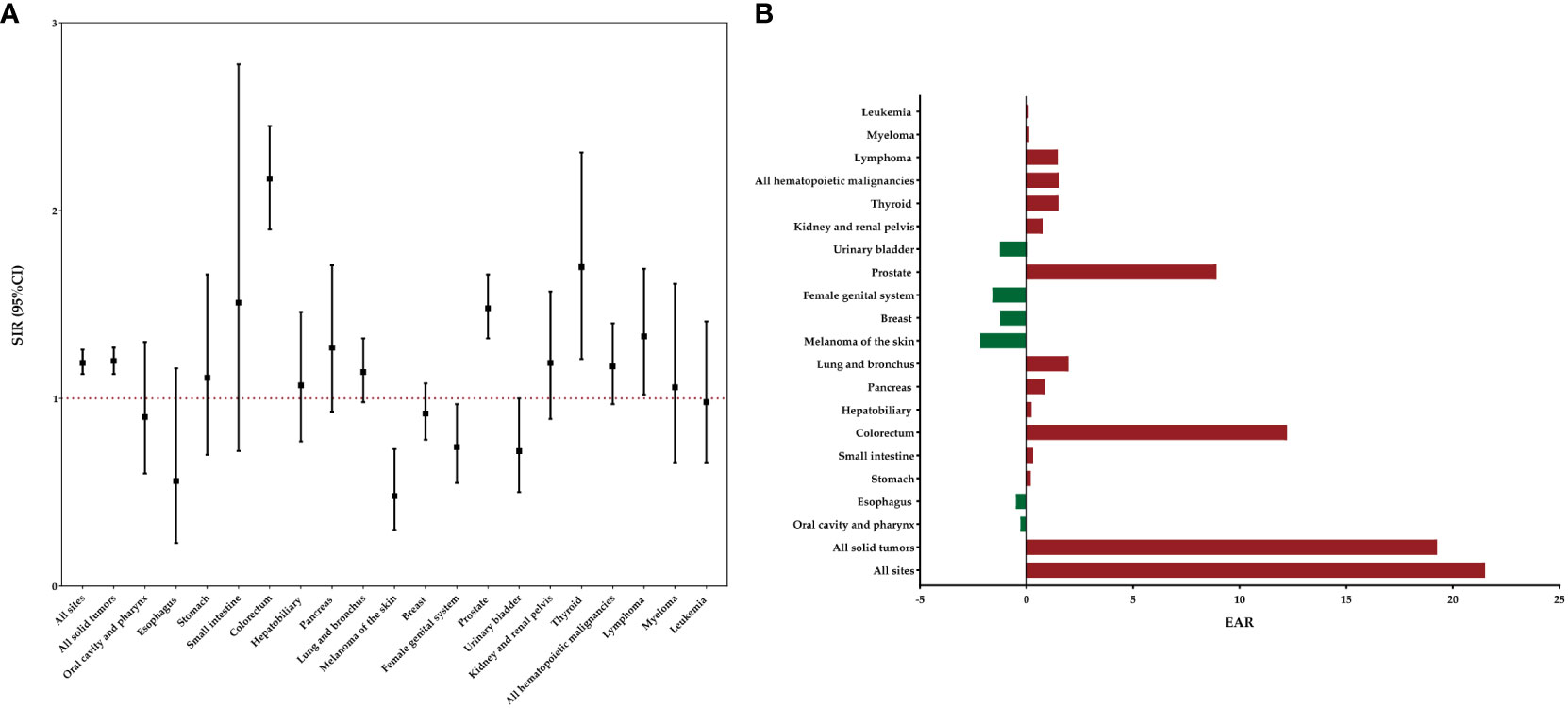
Figure 2 Risk of developing specific SPCs after rectal NENs in the overall study population. (A) SIRs with corresponding 95% CIs. (B) EAR per 10,000 person-years.
For male survivors, a 25% increased relative risk was observed for SPCs overall (Table 2). Similarly, female survivors exhibited a 13% increased relative risk for SPCs overall. Further analysis by sex revealed that female patients had higher relative risks of developing SPCs in the colorectum, kidney and renal pelvis, and thyroid. However, male survivors only displayed an elevated risk of second primary colorectal and prostate cancers compared to the general population. Both of them showed a reduced risk of developing second melanoma of the skin relative to the age-matched US population.
The impact of age at diagnosis of first primary rectal NENs on the risk of SPCs was examined in our study (Table 3). Rectal NENs patients diagnosed at less than 50 years old had a significantly elevated risk of developing SPCs compared to the general population (SIR: 1.49, 95%CI: 1.28-1.72). Similarly, patients aged 50-69 and ≥70 years exhibited overall relative risks that were 16% and 18% higher, respectively. When examining specific SPCs, significantly increased relative risks were observed for colorectal cancer and pancreatic cancer among survivors younger than 50 years old at the time of their first cancer diagnosis. For individuals aged 50-69 years, the risks of developing second colorectal cancer, prostate cancer, and second thyroid cancer were significantly higher compared to the general population. Patients older than 70 years were associated with a higher risk of developing second cancers of the kidney and renal pelvis, as well as lymphoma.
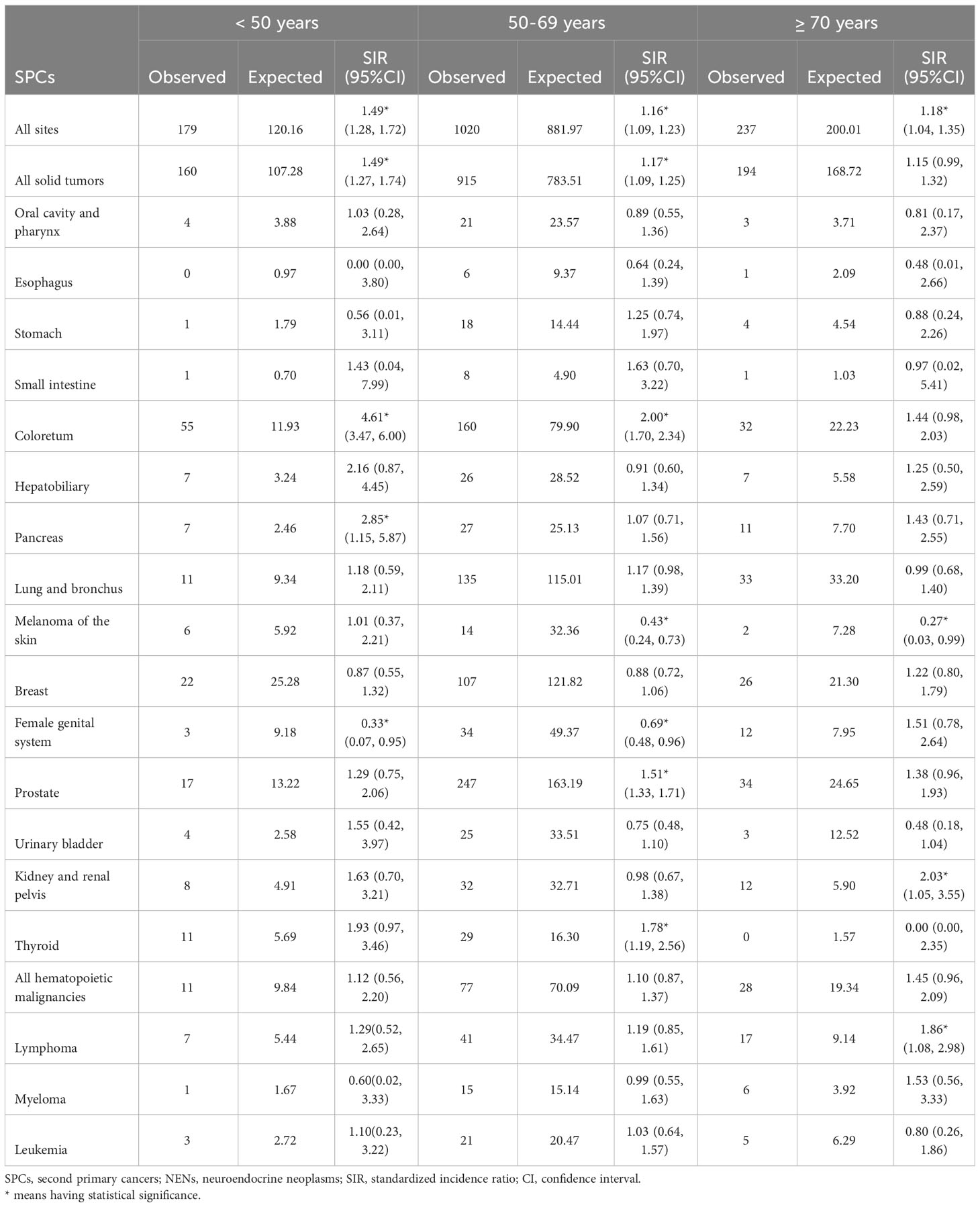
Table 3 Relative risk of selected SPCs after first erctal NENs by age at diagnosis in US from 2000 through 2018.
Race-specific analyses reveal that Black patients had a significantly higher relative risk of developing secondary cancers of colorectum, lung and bronchus, prostate, and thyroid compared to White patients (Table 4). It is noteworthy that White patients were found to have a significantly lower risk of subsequent melanoma of the skin (SIR: 0.51, 95%CI: 0.32-0.78).
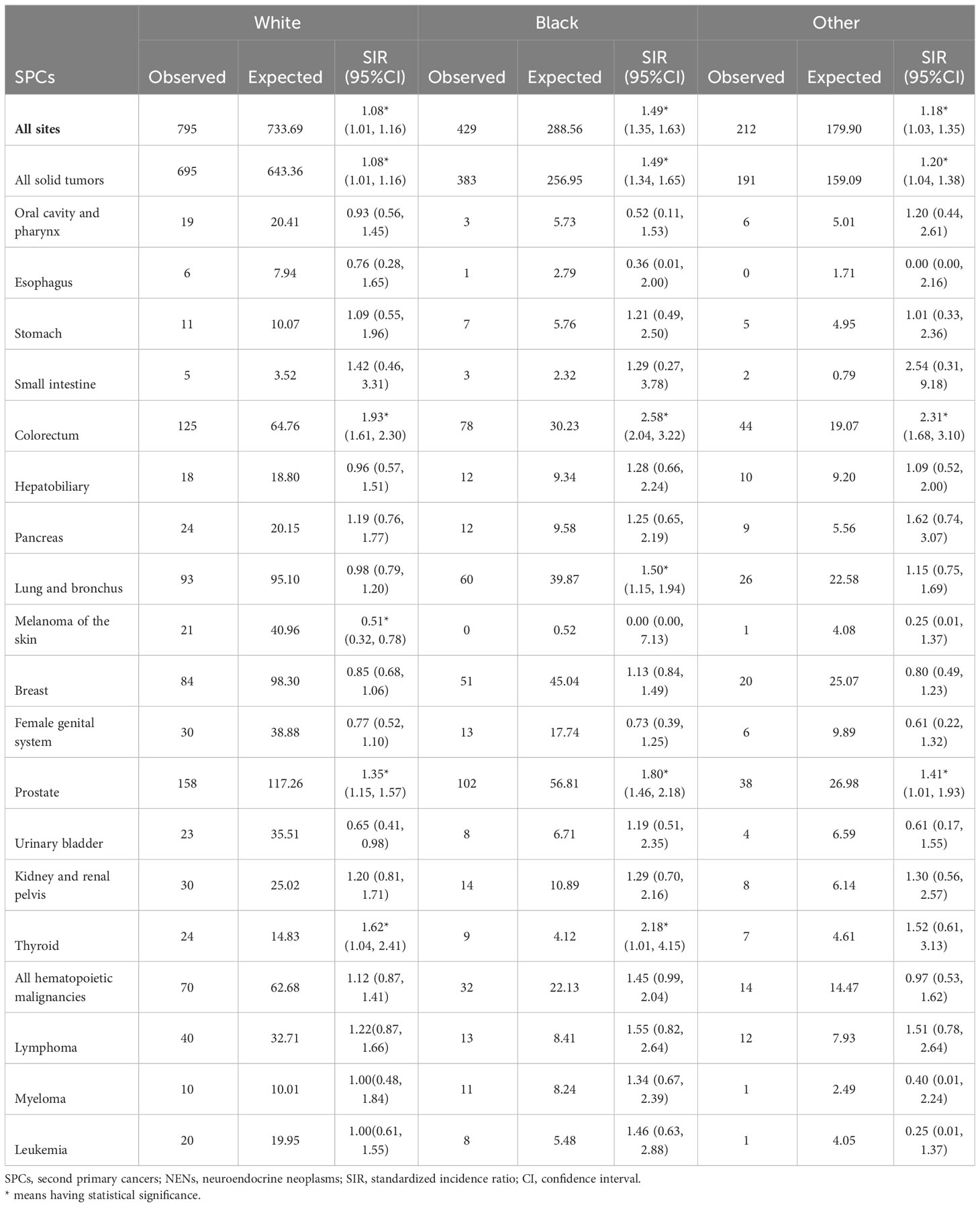
Table 4 Relative risk of selected SPCs after first erctal NENs by race in US from 2000 through 2018.
Patients diagnosed with poorly differentiated rectal NENs exhibited a statistically significant increase in the SIR for developing SPCs at all sites (SIR: 1.80, 95% CI: 1.07, 2.85) (Table 5). Among patients with well-differentiated tumors, prostate cancer was the most common subsequent primary cancer, showing a 74% increase compared to the general population.
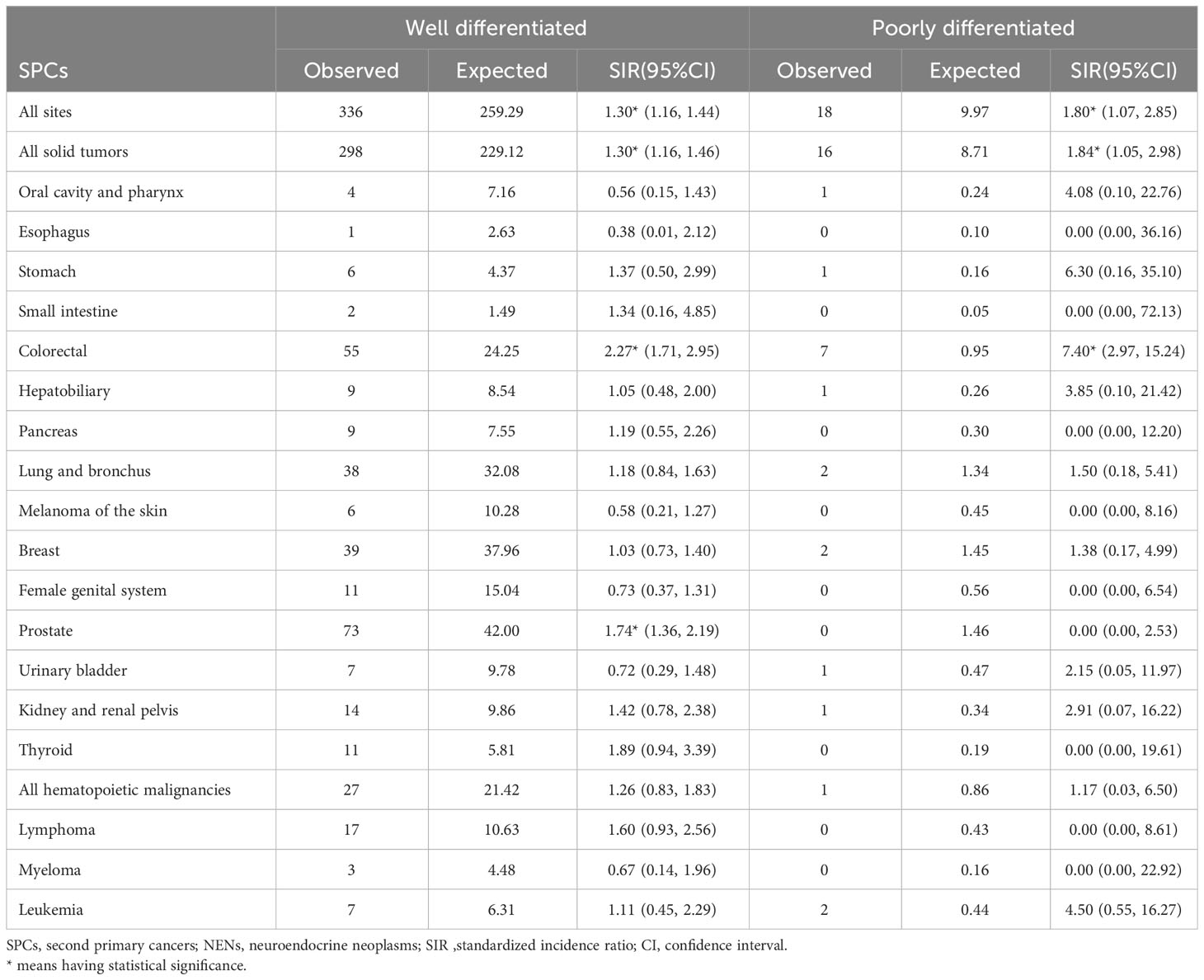
Table 5 Relative risk of selected SPCs after first rectal NENs by tumor differentiation in US from 2000 through 2018.
Stage-specific risk analyses indicate that patients with localized rectal NENs had a higher likelihood of developing SPCs, with a SIR of 1.18 between 2000 and 2018. Notably, patients with regional disease had a significantly elevated risk of developing second hepatobiliary cancers compared to the general US population (SIR: 5.91, 95% CI: 1.22-17.27), whereas this increased risk was not observed for patients with distant rectal NENs (Table 6).
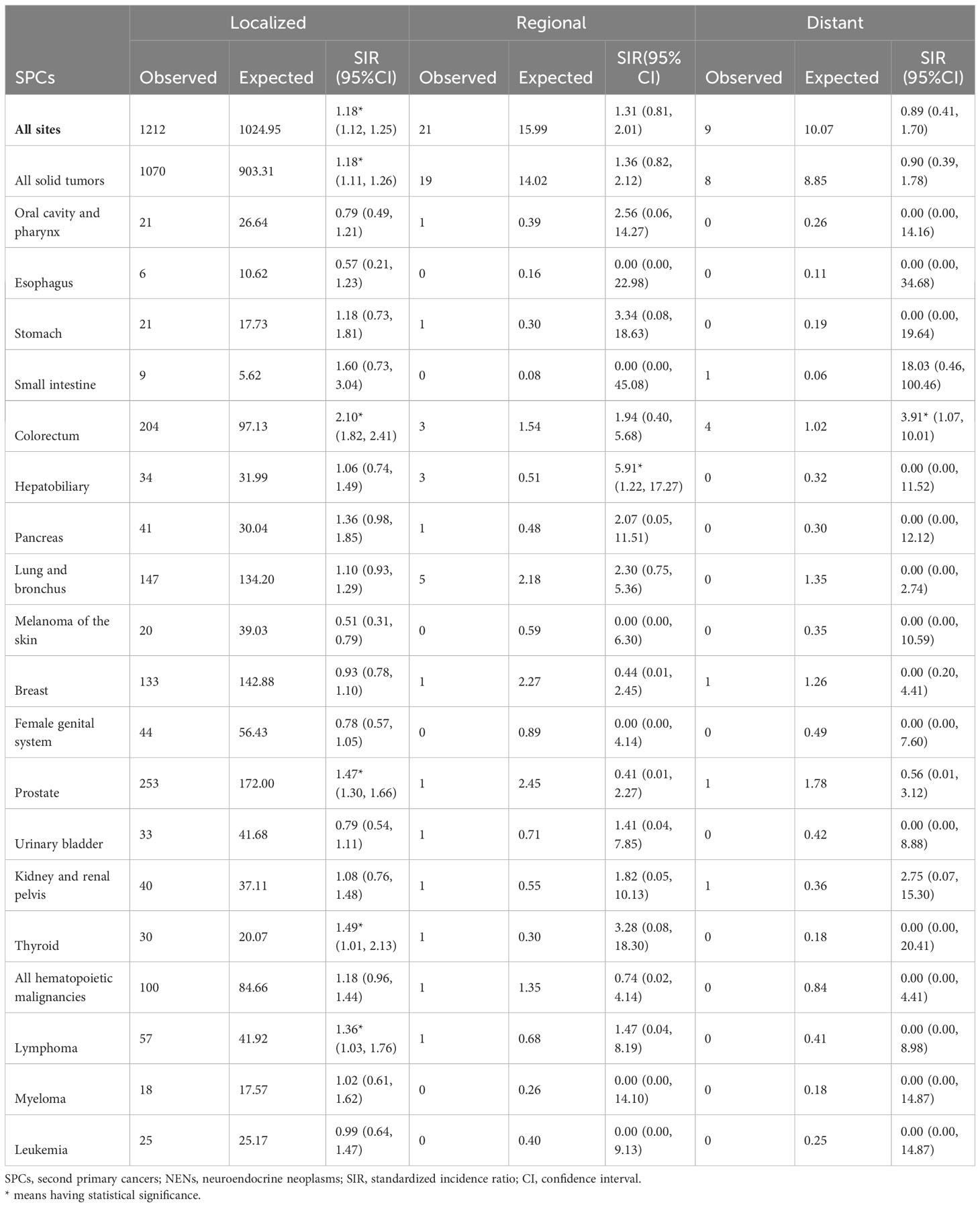
Table 6 Relative risk of selected SPCs after first rectal NENs by tumor stage in US from 2000 through 2018.
Trend in excess burden of SPC
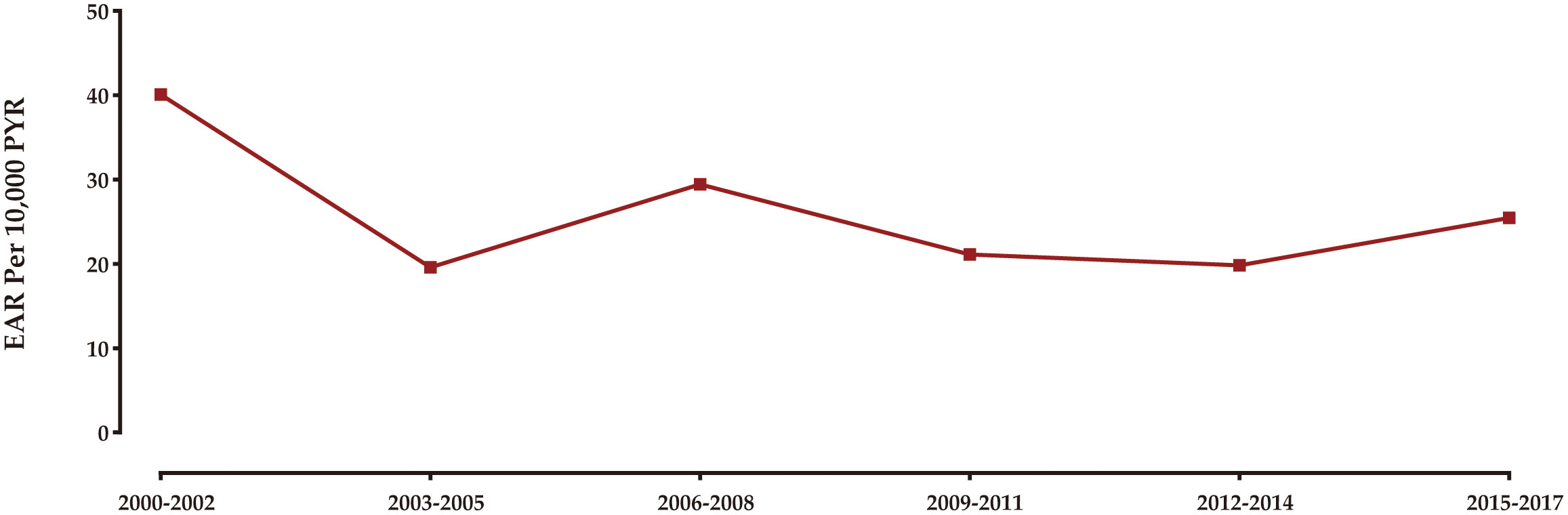
Figure 3 illustrates the temporal pattern of the burden of SPCs among rectal NENs survivors in the United States from 2000 to 2018. The analysis reveals that the highest excess burden of SPCs was observed in the years 2000 to 2002. Subsequently, there was a gradual decrease in the burden until 2003-2005. From 2005 onwards, the excess burden of SPCs remained relatively stable, with minor fluctuations.
Risk of SPC by time latency
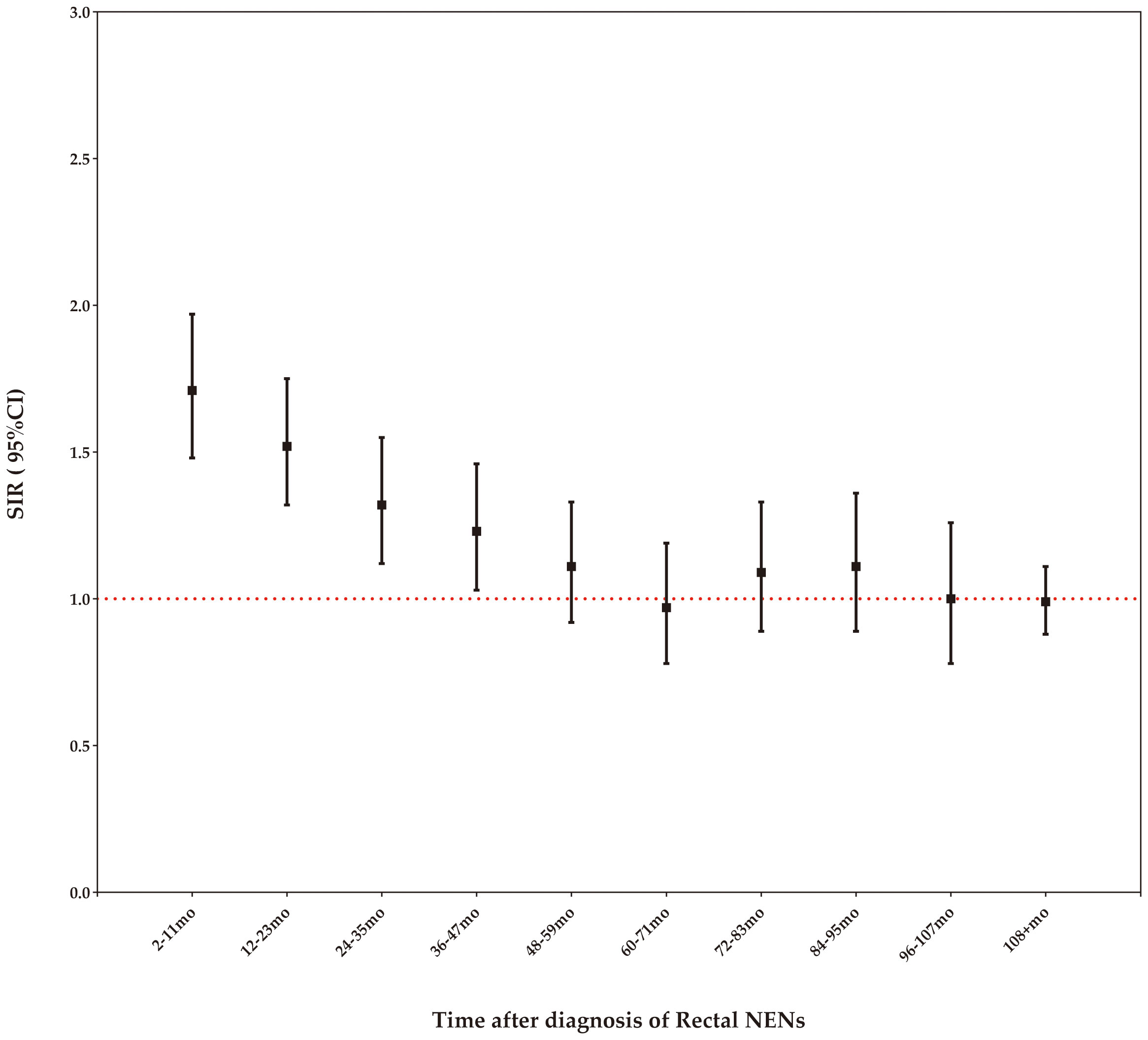
Our study demonstrates that the risk of SPCs remains elevated compared to the age-matched general US population within the first four years following a rectal NENs diagnosis, but this increased risk diminishes after four years (Figure 4).
Risk of SPC by age at rectal NENs diagnosis
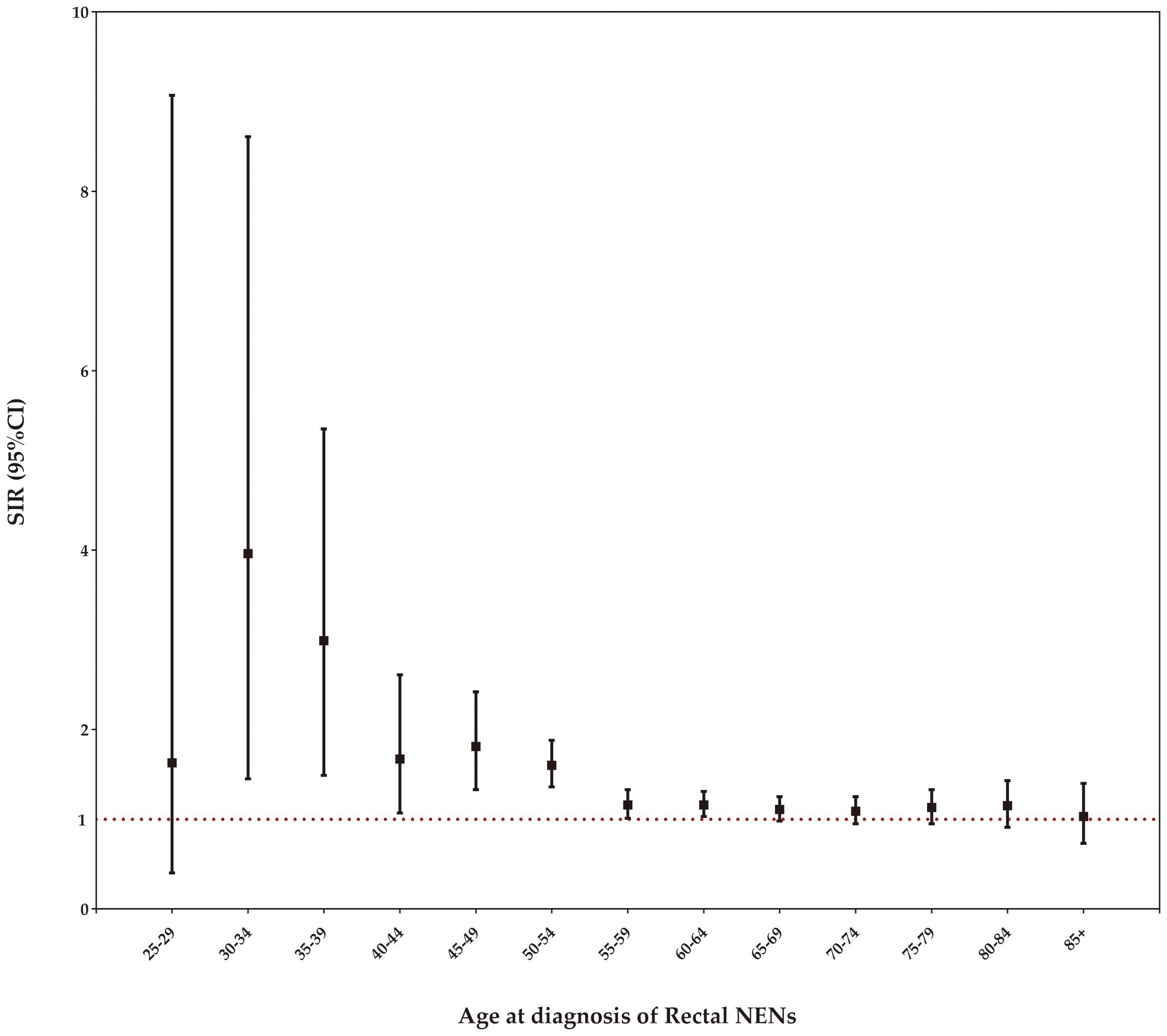
Stratifying the SIRs by age at diagnosis of rectal NENs, we observed a statistically significant increase in the incidence of SPCs among patients aged 30-64 years. As age increased within this range, the SIR gradually declined, while still maintaining statistical significance, as demonstrated in Figure 5.
Discussion
As a result of advancements in cancer detection and management, the extended survivorship of cancer patients may have led to an increased risk of developing SPCs (10, 11). A better understanding of the relative risk in this population is crucial for improving lifelong surveillance, especially in diseases where therapeutic innovations have significantly improved the survival from the first primary cancer. This is true for rectal NENs as well, where recent advances in treatments have shown promising results in improving patient outcomes for rectal NENs, including those with high-risk disease (12–15). The occurrence of SPCs may be influenced by shared etiological factors, environmental exposures, and prior cancer treatments. In this study, we assessed the risks of SPCs among patients with a history of rectal NENs in the United States. Our findings revealed an increased relative risk of SPCs among rectal NENs patients (SIR: 1.19, 95%CI: 1.13-1.26, EAR: 21.51 cases per 10000 person-years). This study demonstrates a significant and persistent risk of SPCs among rectal NENs survivors, particularly within the first four years after their cancer diagnosis. These results contribute to the growing body of evidence highlighting the elevated risk of SPCs in cancer survivors. To the best of our knowledge, it is the first study to investigate the relative risk of second cancer development in survivors of rectal NENs based on a large cohort of study participants in the United States.
Our study addresses the question of how to effectively identify rectal NENs patients at a higher risk of developing SPCs. Through analyses of SIR by patient characteristics, we identified significant associations between SPCs and a prior diagnosis of rectal NENs. Male survivors were found to have a higher risk of SPCs diagnosis compared to their female counterparts (SIR: 1.25 versus 1.13). Notably, female patients had a significantly increased risk of developing second thyroid cancer relative to the general US population. Our results also showed that the age at diagnosis of first rectal NENs was a significant factor in the risk of developing SPCs among this population. For individuals aged less than 50 years old, the relative risk of SPCs was significantly elevated by 49% for all cancers combined, compared to matched peers in the general US population. Specially, survivors under the age of 50 at initial cancer diagnosis had a significantly increased risk of subsequent colorectal and pancreatic cancers, but a decreased risk of female genital system cancer. On the other hand, individuals older than 70 years had significantly higher risks of subsequent kidney and renal pelvis cancer, as well as lymphoma, compared to the general population. As the number of younger cancer patients surviving for several decades increases, it becomes crucial to understand the impact of specific SPCs diagnoses on survival, which is essential for tailoring age-specific prevention, screening, and treatment strategies. However, the underlying reasons for this heightened risk still remain unclear, and further studies are needed to better comprehend the diverse age-related risks associated with SPCs.
When developing screening strategies for rectal NENs survivors, it is crucial to consider the time gap between the initial cancer diagnosis and SPCs. Our findings indicate that the risk of SPCs remains elevated compared to the age-matched general population within the first four years following a rectal NENs diagnosis, but this increased risk diminishes after four years. This suggests that the higher incidence of SPCs may be influenced by individual genetic predisposition (16–19). Therefore, implementing focused surveillance during the initial four years is important for promptly detecting potential subsequent cancers in this cohort.
Our study has several limitations that need to be considered when interpreting the results. Firstly, we lacked detailed information on genetic or environmental risk factors and treatment, which may influence the outcomes. Additionally, a 2-month latency period between the initial cancer diagnosis and the identification of subsequent malignancies may have resulted in an overestimation of the true incidence of SPCs. Despite these limitations, our study stands out by examining the association between a first diagnosis of rectal NENs and subsequent malignancies using the largest and most recent cohort from the United States. Furthermore, we provided risk stratification based on patient demographics and characteristics, which enhances awareness regarding SPCs in this specific patient population.
In conclusion, rectal NENs survivors were found to have an increased risk of SPCs compared with the age-matched general US population, especially within the first four years following their initial cancer diagnosis. Furthermore, our study expands upon existing data by demonstrating long-term trends in SPC incidence among rectal NENs survivors, based on a larger patient sample. These findings have the potential to inform further studies on the etiology of SPCs and aid in shaping improved survivorship strategies for patients with rectal NENs. With advancements in cancer treatment and improved survival rates, it is crucial for healthcare practitioners and cancer survivors to be aware of the heightened risk of subsequent primary malignancies. Our findings provide valuable insights that can guide healthcare providers in customizing survivorship care plans to meet individual patient needs and address associated risks. Given the complexities of cancer management, there is a pressing need for research focused on identifying the most appropriate and feasible strategies.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: SEER programs.
Author contributions
XZ contributed to the conception. MW performed statistical analysis and wrote the manuscript. JW and ZJ collected the data. WG edited the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. La Rosa S, Uccella S. Classification of neuroendocrine neoplasms: lights and shadows. Rev Endoc Metab Disord (2021) 22(3):527–38. doi: 10.1007/s11154-020-09612-2
2. Rizen EN, Phan AT. Neuroendocrine tumors: a relevant clinical update. Curr Oncol Rep (2022) 24(6):703–14. doi: 10.1007/s11912-022-01217-z
3. Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol (2017) 3(10):1335–42. doi: 10.1001/jamaoncol.2017.0589
4. Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol (2008) 26(18):3063–72. doi: 10.1200/JCO.2007.15.4377
5. Das S, Dasari A. Epidemiology, incidence, and prevalence of neuroendocrine neoplasms: are there global differences? Curr Oncol Rep (2021) 23(4):43. doi: 10.1007/s11912-021-01029-7
6. Lee MR, Harris C, Baeg KJ, Aronson A, Wisnivesky JP, Kim MK. Incidence trends of gastroenteropancreatic neuroendocrine tumors in the United States. Clin Gastroenterol Hepatol (2019) 17(11):2212–2217.e2211. doi: 10.1016/j.cgh.2018.12.017
7. Xu Z, Wang L, Dai S, Chen M, Li F, Sun J, et al. Epidemiologic trends of and factors associated with overall survival for patients with gastroenteropancreatic neuroendocrine tumors in the United States. JAMA network Open (2021) 4(9):e2124750. doi: 10.1001/jamanetworkopen.2021.24750
8. Clift AK, Drymousis P, Al-Nahhas A, Wasan H, Martin J, Holm S, et al. Incidence of second primary Malignancies in patients with neuroendocrine tumours. Neuroendocrinology (2015) 102(1-2):26–32. doi: 10.1159/000381716
9. Kamp K, Damhuis RA, Feelders RA, de Herder WW. Occurrence of second primary Malignancies in patients with neuroendocrine tumors of the digestive tract and pancreas. Endocrine-related Canc (2012) 19(1):95–9. doi: 10.1530/ERC-11-0315
10. Sung H, Hyun N, Leach CR, Yabroff KR, Jemal A. Association of first primary cancer with risk of subsequent primary cancer among survivors of adult-onset cancers in the United States. Jama (2020) 324(24):2521–35. doi: 10.1001/jama.2020.23130
11. Sung H, Siegel RL, Hyun N, Miller KD, Yabroff KR, Jemal A. Subsequent primary cancer risk among 5-year survivors of adolescent and young adult cancers. J Natl Cancer Institute. (2022) 114(8):1095–108. doi: 10.1093/jnci/djac091
12. Gallo C, Rossi RE, Cavalcoli F, Barbaro F, Boškoski I, Invernizzi P, et al. Rectal neuroendocrine tumors: Current advances in management, treatment, and surveillance. World J Gastroenterol (2022) 28(11):1123–38. doi: 10.3748/wjg.v28.i11.1123
13. Maione F, Chini A, Milone M, Gennarelli N, Manigrasso M, Maione R, et al. Diagnosis and management of rectal neuroendocrine tumors (NETs). Diagnost (Basel Switzerland). (2021) 11(5):771. doi: 10.3390/diagnostics11050771
14. Basuroy R, Haji A, Ramage JK, Quaglia A, Srirajaskanthan R. Review article: the investigation and management of rectal neuroendocrine tumours. Alimentary Pharmacol Ther (2016) 44(4):332–45. doi: 10.1111/apt.13697
15. Bertani E, Ravizza D, Milione M, Massironi S, Grana CM, Zerini D, et al. Neuroendocrine neoplasms of rectum: A management update. Cancer Treat Rev (2018) 66:45–55. doi: 10.1016/j.ctrv.2018.04.003
16. Travis LB, Rabkin CS, Brown LM, Allan JM, Alter BP, Ambrosone CB, et al. Cancer survivorship–genetic susceptibility and second primary cancers: research strategies and recommendations. J Natl Cancer Institute. (2006) 98(1):15–25. doi: 10.1093/jnci/djj001
17. Travis LB, Demark Wahnefried W, Allan JM, Wood ME, Ng AK. Aetiology, genetics and prevention of secondary neoplasms in adult cancer survivors. Nat Rev Clin Oncol (2013) 10(5):289–301. doi: 10.1038/nrclinonc.2013.41
18. Oeffinger KC, Baxi SS, Novetsky Friedman D, Moskowitz CS. Solid tumor second primary neoplasms: who is at risk, what can we do? Semin Oncol (2013) 40(6):676–89. doi: 10.1053/j.seminoncol.2013.09.012
Keywords: rectal NENs, second primary cancer, Sir, Ear, SEER
Citation: Wan M, Wu J, Jiang Z, Gong W and Zhou X (2023) Risk of second primary cancers in patients with rectal neuroendocrine neoplasms: a surveillance, epidemiology, and end results analysis. Front. Oncol. 13:1248268. doi: 10.3389/fonc.2023.1248268
Received: 02 August 2023; Accepted: 21 August 2023;
Published: 13 September 2023.
Edited by:
Zequn Li, The Affiliated Hospital of Qingdao University, ChinaReviewed by:
Chuncheng Yang, Shandong University, ChinaFu Gang Wang, Qingdao Municipal Hospital, China
Copyright © 2023 Wan, Wu, Jiang, Gong and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianli Zhou, aHJiemhvdXhsQDE2My5jb20=
 Ming Wan
Ming Wan Jiaqi Wu
Jiaqi Wu