- 1School of Nursing, Hexi University, Zhangye, China
- 2Peking University First Hospital Ningxia Women and Children’s Hospital (Ningxia Hui Autonomous Region Maternal and Child Health Hospital), Nursing Department, Yinchuan, Ningxia Hui Autonomous Region, China
- 3The First Ward of the Department of Gynecology, The First Hospital of Lanzhou University, Lanzhou, China
Objectives: To evaluate the efficacy of Difluoromethylornithine (DFMO) chemoprevention in the high-risk population for colorectal cancer (CRC).
Methods: Meta-analysis was conducted to assess the caliber of the included literature by searching five databases for randomized controlled trials of DFMO chemoprevention in the high-risk population of CRC, with RevMan 5.4, Stata 15.0 and TSA 0.9.5.10 employed to statistically analyze the extracted data. Grade profiler 3.6 was employed for grading the evidence for the outcome indicators (disease progression and adenoma incidence).
Results: Six trials were finally included in this research, with the collective data indicating that the DFMO combination therapy was efficacious in lowering the incidence of recurrent adenomas in patients who had experienced advanced CRC [RR 0.34, 95% CI 0.14 - 0.83, P < 0.05]. Meta-analysis showed that DFMO combined therapy had no statistical difference in disease progression in patients with familial adenomatous polyposis[RR 0.52, 95% CI 0.14 - 1.86, P > 0.05]; Trial Sequential Analysis reveals that the combination therapy of DFMO effectively diminishes the occurrence of recurrent adenomas in patients with a history of advanced colorectal tumors, displaying a Risk Ratio (RR) of 0.33 with a 95% Confidence Interval (CI) of 0.12 - 0.90 and a significance level of P < 0.05. This combination exhibits a statistically significant difference. Subgroup analysis demonstrates that, depending on the drug treatment regimen (DFMO+ Aspirin/DFMO+ Sulindac), the combination of DFMO and aspirin exhibits an effect comparable to a placebo in diminishing the occurrence of new adenomas in patients with a history of advanced colorectal tumors. However, the combination of DFMO and sulindac significantly mitigates the incidence of recurrent adenomas in this patient population.
Conclusion: This meta-analysis indicates that the existing randomized controlled trials are adequate to ascertain the efficacy of DFMO combination therapy in diminishing the incidence of recurrent adenomas in patients who have previously encountered advanced colorectal tumors. However, further clinical trials need to be conducted to evaluate the optimum dosage and treatment course of prophylactic implementation of DFMO combination therapy in high-risk populations.
1 Introduction
Colorectal cancer (CRC) is a highly prevalent and fatal disease worldwide (1, 2). Globally, CRC causes approximately 1.8 million new cases with 900,000 deaths reported each year. It ranks as the third most prevalent malignancy and the second most common cause of cancer death, estimated by the International Agency for Research on Cancer (IARC) in 2018 (3). Most CRCs develop from adenomas, which, if left untreated, carry a lifetime risk of CRC of up to 100% (4, 5), whereas removal of adenomas can reduce the risk of CRC. Although the incidence of CRC can be reduced by prophylactic resection of adenomatous polyps found at screening, chemoprophylactic therapy is gaining attention from clinicians because of its characteristic of low toxicity, cost-effectiveness, and high efficiency.
Chemopreventive drugs enhance the management of colorectal adenoma by delaying or avoiding the development of advanced colorectal adenomas. Additionally, the ideal chemopreventive drug should have a biologically plausible mechanism of action, be safe and easily tolerated over a longer treatment period, and produce long-lasting and clinically significant effects (6). Among the latest chemopreventive agents for CRC, difluoromethylornithine (DFMO) is considered to be the most promising agent. DFMO is an irreversible inhibitor of ornithine decarboxylase(ODC) (7), and it possesses cyto-inhibitory properties. Importantly, its threshold concentration for toxicity is also much higher than that required to inhibit ODC activity (8), and it does not inhibit any other polyamine-metabolizing enzymes. Therefore, DFMO has received widespread attention for its advantages such as low toxicity and significant preventive effects on certain tumors. This effect was noted especially in solid tumors of epithelial origin, with DFMO being included in several clinical trials for the prevention and treatment of malignancies.
Recently, several studies on the efficacy of DFMO chemoprevention for CRC have been published, but conflicting results were noted (9–14). In 2022, a report of a network meta-analysis involving 13 chemistries for the prevention of colorectal adenoma incidence (15) reported that DFMO combined with sulindac was optimally effective for the chemoprevention of colorectal adenoma. However, the study included only one randomized controlled trial (RCT), and the included number of samples was quite small. In addition, the results of studies on the efficacy of DFMO alone in treating patients with CRC are lacking, and the impact on a high-risk population with CRC needs further clarification. Hence, the primary objective of this meta-analysis is to evaluate the influence of DFMO on the recurrence of adenomas in populations at elevated risk for colorectal cancer. The secondary objective is to assess the advancements in research pertaining to the disease and to conduct subgroup analyses based on the following predefined drug treatment regimens (DFMO+ Aspirin/DFMO+ Sulindac).
2 Methods
2.1 Search strategy
This study was systematically reviewed, meta-analyzed, and reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (16). Up to July 2023, PubMed, Embase, Web of Science, OVID, and the Cochrane Library were assessed for relevant research on DFMO for chemoprevention in the high-risk population of CRC. The study utilized subject terms combined with free words as search criteria (MeSH in PubMed and Emtree in Embase) using Population, Intervention, Comparison, Outcomes, and Study (PICOS) principles. There was no restriction on the language of publication. References from relevant reviews and meta-analyses were also reviewed to recognize eligible studies for this study. The strategy utilized for this particular search is described in Appendix 1. Moreover, the manual search was performed complementally in this study to discover pertinent literature.
2.2 Study selection
The inclusion criteria were: (a) study population: the population with increased risk of CRC (17) which includes familial adenomatous polyposis, previous history of colorectal adenomatosis, history of CRC, associated inflammatory bowel disease such as Crohn’s disease or ulcerative colitis; (b) intervention method: use of DFMO in combination with other drugs or DFMO alone for the treatment of colorectal disease; (c) control group: use of placebo or other similar drugs. (d) Main outcome: Incidence of recurrent colorectal adenomas (advanced adenomas and any adenomas); Secondary outcome: disease progression. Disease progression was defined as requiring surgery, advanced adenomas requiring endoscopic resection, the occurrence of high dysplasia, or stage progression of duodenal polyposis (10); (e) Study design: RCT study.
The exclusion criteria were: (a) review, individual cases, commentary, or case report; (b) animal experiments; (c) incomplete reported data; (d) duplicate publications or single articles published in multiple languages, and duplicate data publication (overlapping samples from several studies), the most informative articles were included to circumvent duplication of data.
2.3 Data collection and quality assessment
Two authors (WY and YLF) independently conducted study selection based on predetermined inclusion and exclusion criteria. Any Disagreements were resolved by thorough discussion. WY and WXY independently collected data from the included trials. The following information was extracted from each included article: first author, year of publication, region, drug type, sample size, dose, and outcome indicators. The authors of the included studies were contacted for additional information when needed.
This study evaluated the risk of bias according to the guidelines outlined in the Cochrane Handbook for the Evaluation of Intervention Systems (5.1.0) (18). HSS and WXY scrutinized all studies and designated “high,” “low,” or “unclear” values to the following categories: random sequence generation; allocation concealment, blinding of participant and investigator; blinding of outcome measures; incomplete outcome data; selective reporting; and other biases.
2.4 Statistical analysis
Relative risk ratios (RRs) were calculated for dichotomous outcomes at a 95% confidence interval (CI). A random-effects model was utilized for the meta-analysis of clinical heterogeneity. All analyses were conducted on an intention-to-treat basis. Heterogeneity was reported using I2 statistics, with I2 > 50% indicating significant heterogeneity (19).
Subgroup analyses were executed based on predefined drug treatment regimens (DFMO+ Aspirin/DFMO+ Sulindac), with sensitivity analyses conducted for all outcomes.
Egger’s test was used to evaluate publication bias (20), and P < 0.05 were considered a statistically significant difference. All statistical analyses were conducted employing Stata 15.0 (Stata Corporation, TX, USA) and RevMan 5.4 (Nordic Cochrane Centre, Denmark).
2.5 Trial sequential analysis
To address the primary outcomes, Trial Sequential Analysis (TSA) was undertaken utilizing TSA software. This approach mitigates the risks associated with inadequate sample sizes and successive updates of studies incorporated into the meta-analysis, which could potentially escalate the probability of random errors (21). The meta-analysis adjusted the Required Information Size (RIS) and computed the trial sequential monitoring boundaries to ascertain the reliability of the evidence within our meta-analysis (22).
2.6 Grading the quality of evidence
GRADE (23) was used to assess the overall quality of the evidence and the strength of the recommendations. The standard classifies evidence quality into four categories: very low, low, medium and high.
3 Results
3.1 Trial selection
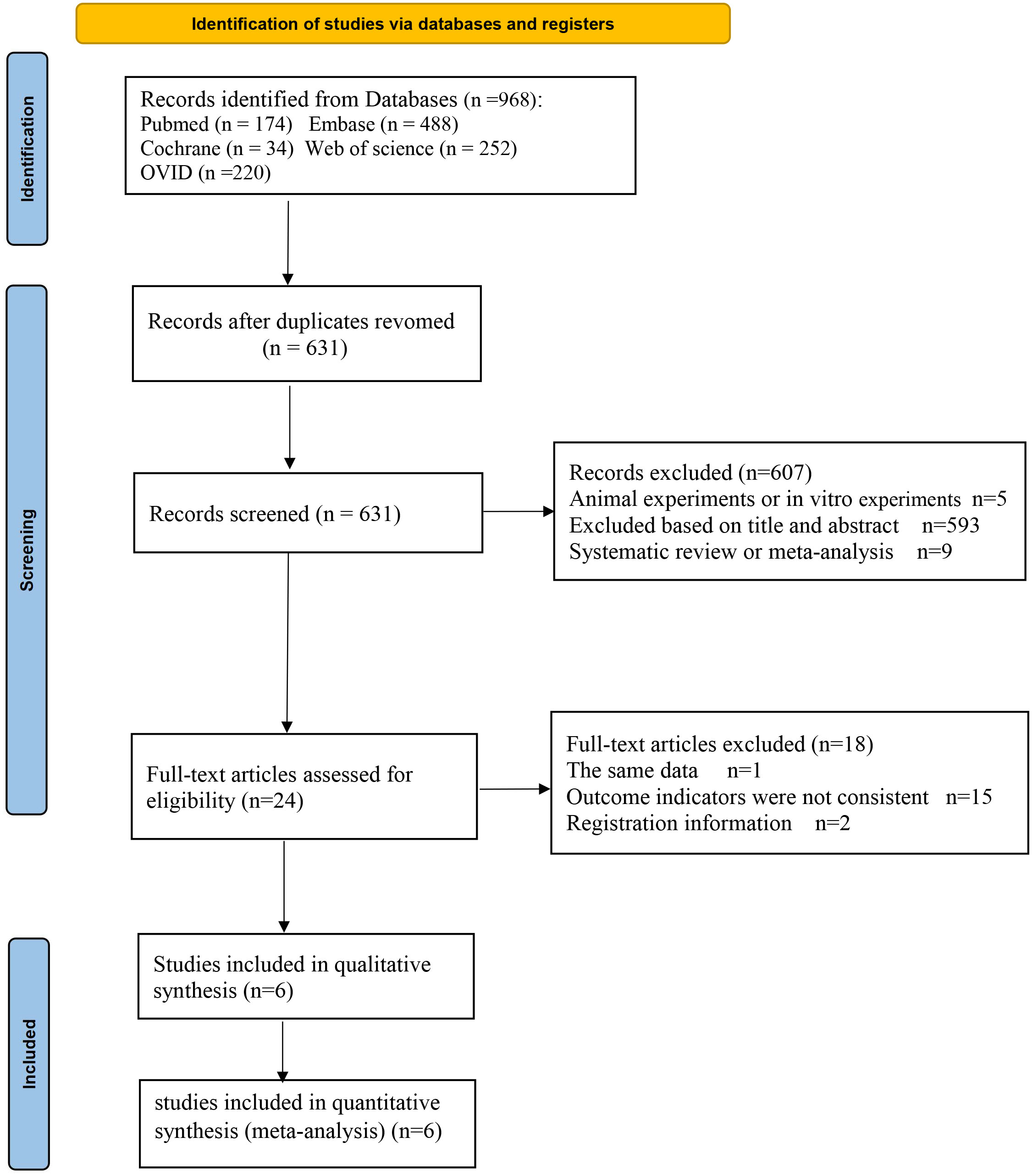
A total of 968 articles were identified from the online database. After removing duplicates, 631 articles remained for further consideration based on their titles and abstracts. The 593 articles that were not relevant to the topic were excluded, nine review articles and five animal experiments were eliminated as well, with 24 articles were being selected for full-text review. Among these, one article was excluded due to duplication of data, 2 articles are registration information, and 15 articles were excluded because the outcome indicators were not met. Six trials (9–14) were finally included (Figure 1).
3.2 Trial characteristics
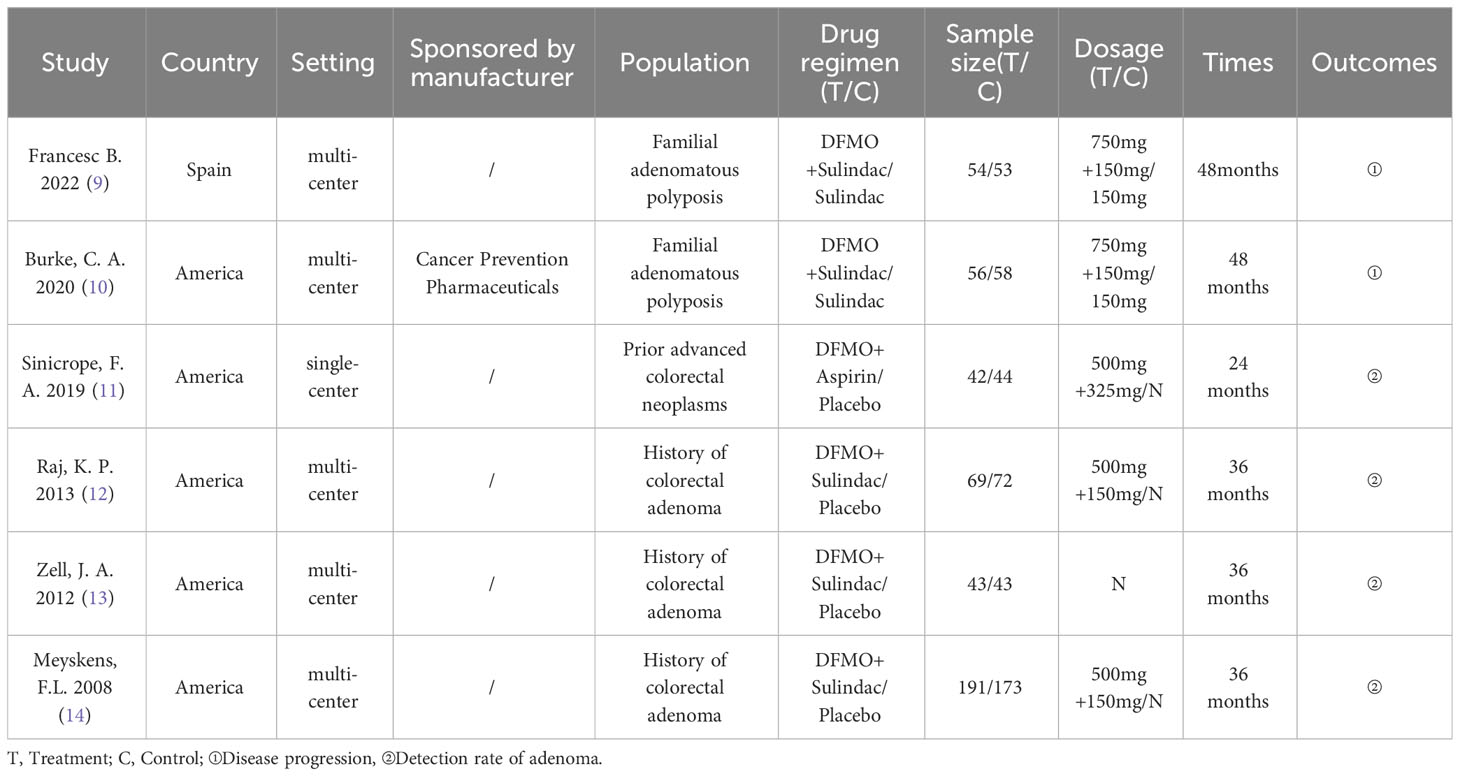
The primary features of the six included RCTs are demonstrated in Table 1. These studies were published between 2008 and 2022. Among the six included studies, five were carried out in the United States (10–14) and one in Spain (9). Five RCTs used multicenter studies (9, 10, 12–14). Five RCTs used Eflornithine + Sulindac as treatment regimen (9, 10, 12–14), one RCT used Eflornithine + Aspirin as treatment regimen (11). Furthermore, two studies used Sulindac as a control (9, 10), and four studies used placebo as a control (11–14). Sample sizes for the six RCTs ranged from 86 to 375. In two studies the treatment period was two years (9, 10), in one study, the treatment period was one year (11), and in three studies, treatment period was three years (12–14). Four trials reported the incidence of adenomas in patients with advanced colorectal neoplasms (11–14), and two studies reported on the outcome of the progression of disease in patients with familial adenomatous polyposis (9, 10). One study (10) reported that the research was funded by the manufacturer.
3.3 Risk bias evaluation
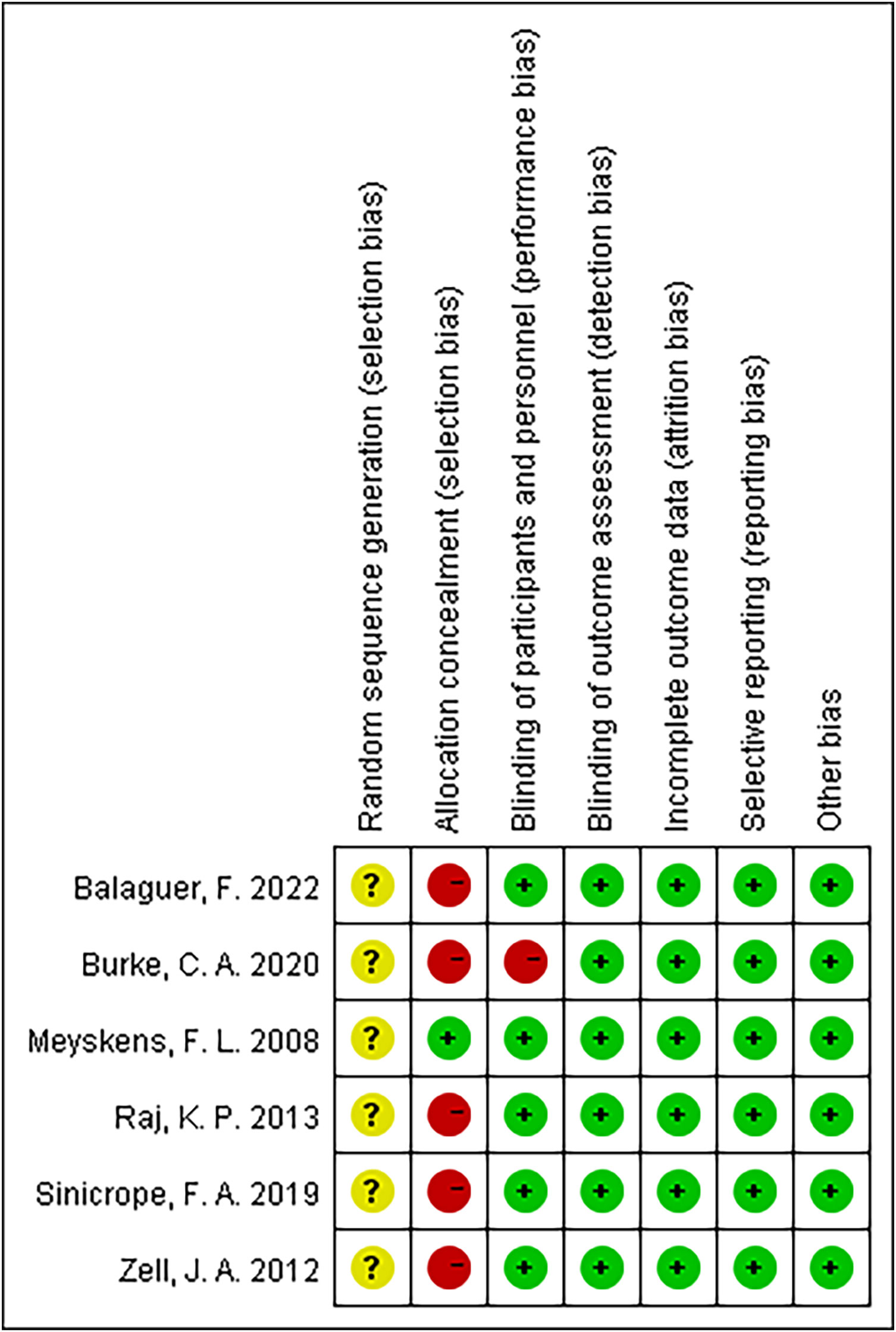
Figure 2 summarizes the details of the risk of bias assessment. Six trials produced unclear randomized sequences (9–14), stating that only randomization was used without elaborating on the specific manner. One study used correct allocation concealment (14), and five studies did not mention random allocation concealment (9–13). Five studies were double-blinded (9, 11–14), and one study did not mention the use of the blinding method (10).
3.4 Outcome
3.4.1 Disease progression
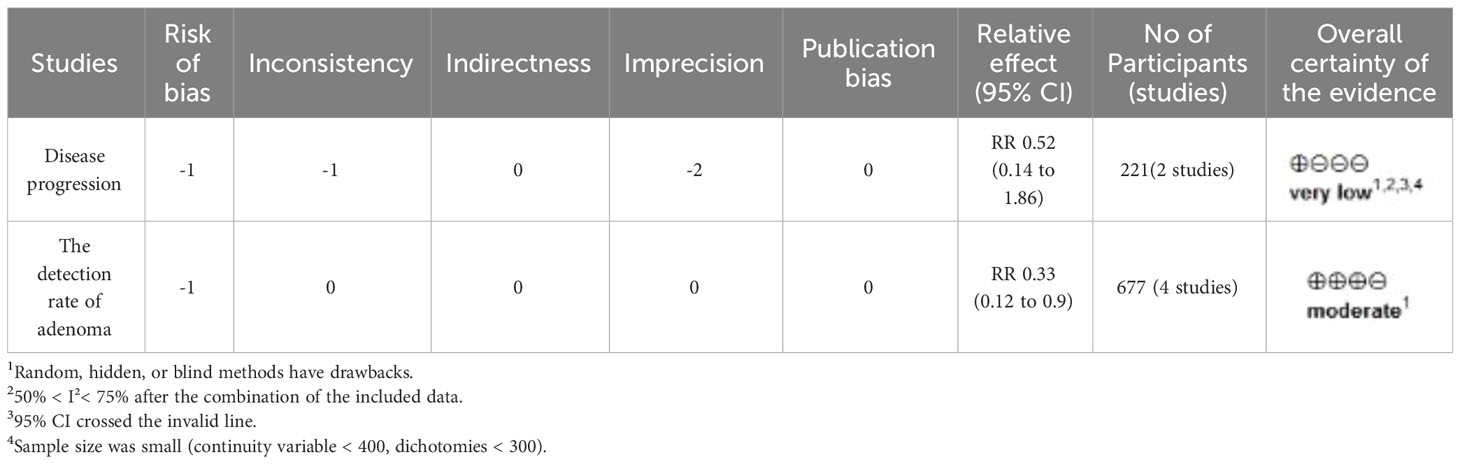
Two trials, including 221 participants, reported the effect of DFMO combination therapy on the progression of disease in patients with familial adenomatous polyposis. The data indicated that DFMO combination therapy had no impact on the reduction of disease progression in such patients relative to the control sulindac (RR 0.52, 95% CI 0.14 - 1.86, P > 0.05; I2 = 65%; Figure 3A). The sensitivity analysis showed no change in results after changing the effect model, indicating robust results.
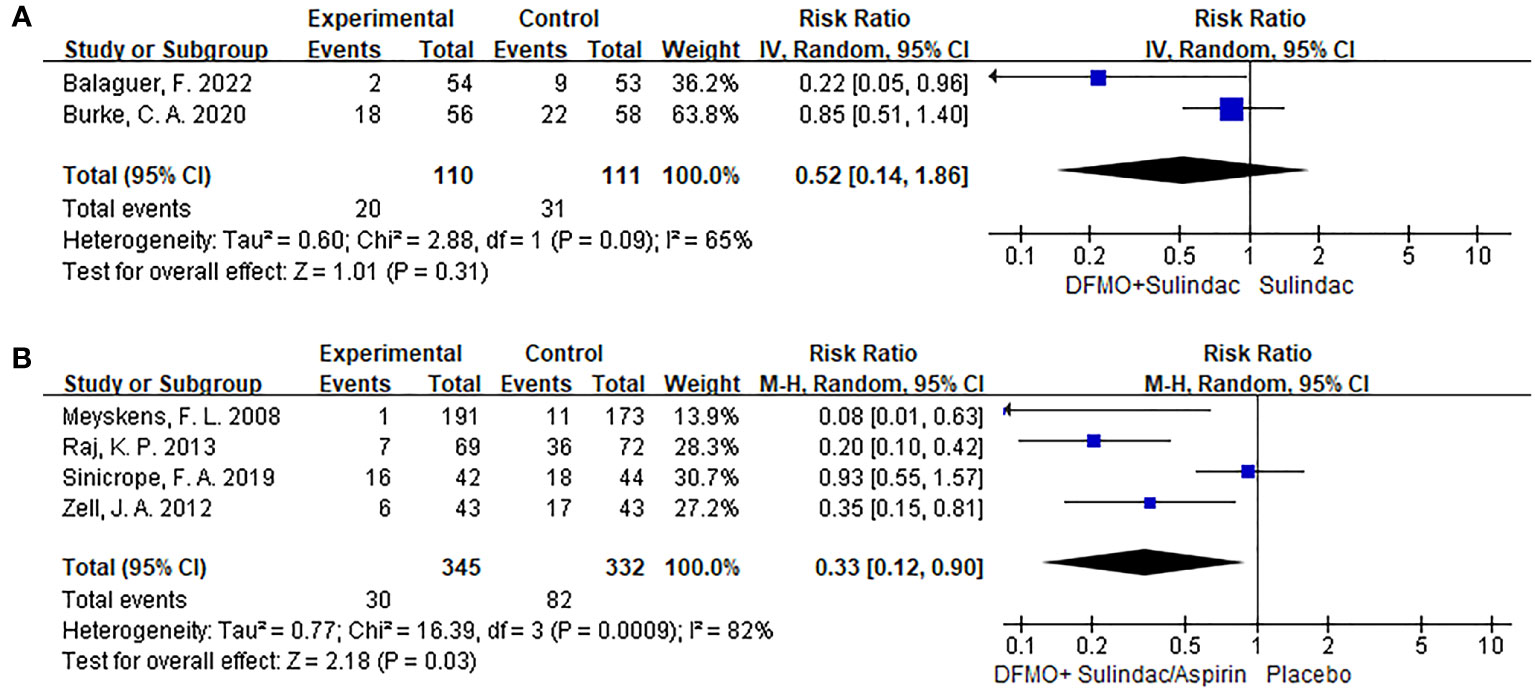
Figure 3 Forest plots of studies included comparing the disease progression and the detection recurrent rate of adenoma. A random-effects model was utilized for the meta-analysis of clinical heterogeneity. (A) Forest plot for disease progression in patients with colorectal cancer compared with DFMO combination therapy and sulindac; (B) Forest plot for the detection rate of recurrent adenoma in patients with colorectal cancer compared with DFMO combination therapy and placebo.
3.4.2 Incidence of recurrent colorectal adenomas (advanced adenomas and any adenomas)
In the analysis, four trials involving a total of 677 participants provided data on the impact of DFMO combinations on the incidence of recurrent adenomas in individuals who had previously experienced CRC. The DFMO combination therapy significantly reduced the incidence of recurrent adenomas in patients with previously advanced CRC in comparison to the control placebo group (RR 0.33, 95% CI 0.12 - 0.90, P < 0.05; I2 = 82%; Figure 3B). Sensitivity analysis exhibited no variance in the results after changing the effect model, which reflects the robustness of the results.
3.5 Subgroup analysis
Subgroup analysis revealed that DFMO combined with aspirin was comparable to placebo in the incidence of recurrent adenomas in patients with previous advanced CRC. Nevertheless, DFMO combined with sulindac significantly reduced the incidence of new adenomas in patients with previous advanced CRC. Sensitivity analysis showed no change in the results after changing the effect model, which reflects the robustness of the results. (Show in Figure 4)
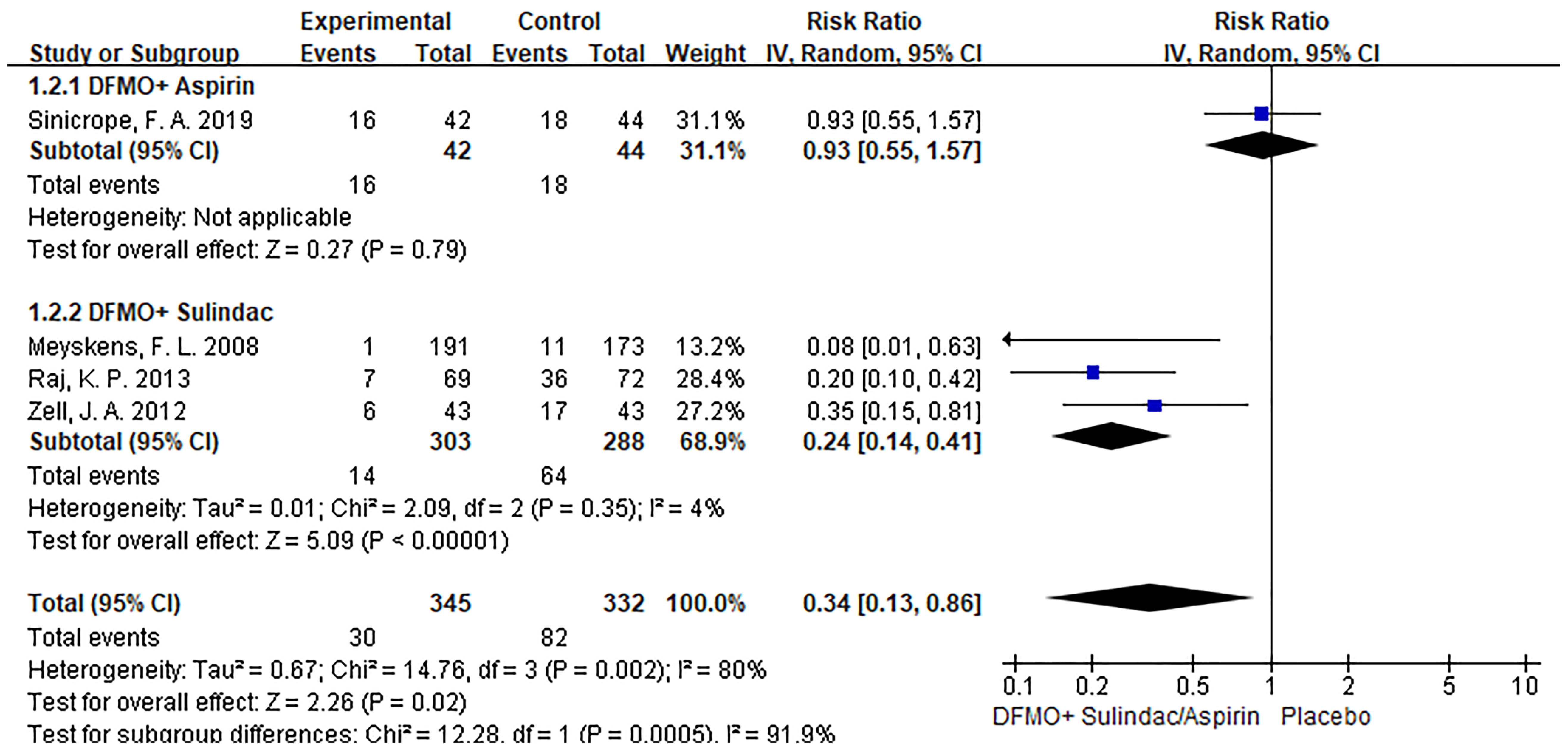
Figure 4 Subgroup analysis of detection rate of colorectal cancer adenoma with DFMO combined with drug therapy (DFMO+Aspirin/DFMO+ sulindac).
3.6 Publication bias
The assessment of publication bias using Egger’s test revealed no evidence of bias in the detection rates of adenomas. (Egger’s test, P = 0.384, Figure 5).
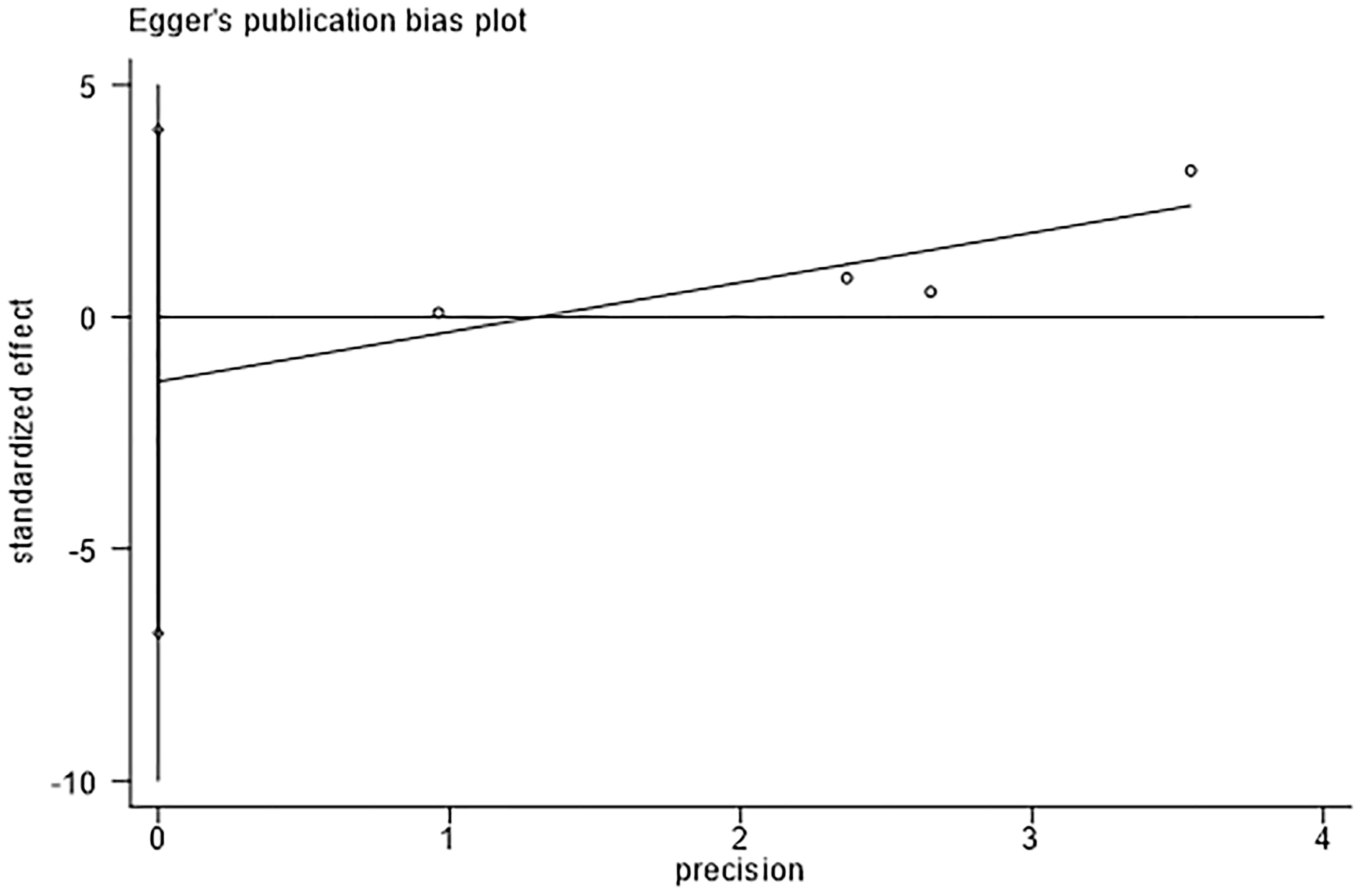
Figure 5 Egger’s test for detection rate of adenoma indicates that there was no substantial publication bias.
3.7 Trial sequential analysis
Trial Sequential Analysis (TSA) addresses the limitations inherent in traditional meta-analysis, rendering the results of statistical analysis more robus. Additionally, TSA can estimate the Required Information Size (RIS) that a meta-analysis needs to achieve a stable conclusion, thereby offering a termination criterion for the sample size of clinical trials (21). In this study, TSA was applied to four studies focusing on the incidence of new adenomas, with type 1 error set at 0.05, power at 0.80, and the RIS designated as the requisite sample size for the meta-analysis (24).
The trial sequential analysis for DFMO combination therapy regarding the incidence of recurrent adenomas revealed that the estimated required information size was 1159, while the organized information size fell short of this value, standing at 677. The findings indicate that the Z-curve concurrently crosses both the traditional boundary and the TSA boundary. This suggests that, even though the accumulated information size has yet to meet the anticipated value, no additional trials are necessary, and early confirmation can be achieved. This denotes that the current data is ample to ascertain the efficacy of DFMO combination therapy in addressing the incidence of recurrent colorectal adenomas, as illustrated in Figure 6A. The curve surpassing the traditional boundary post-penalty further substantiates this conclusion, as depicted in Figure 6B.
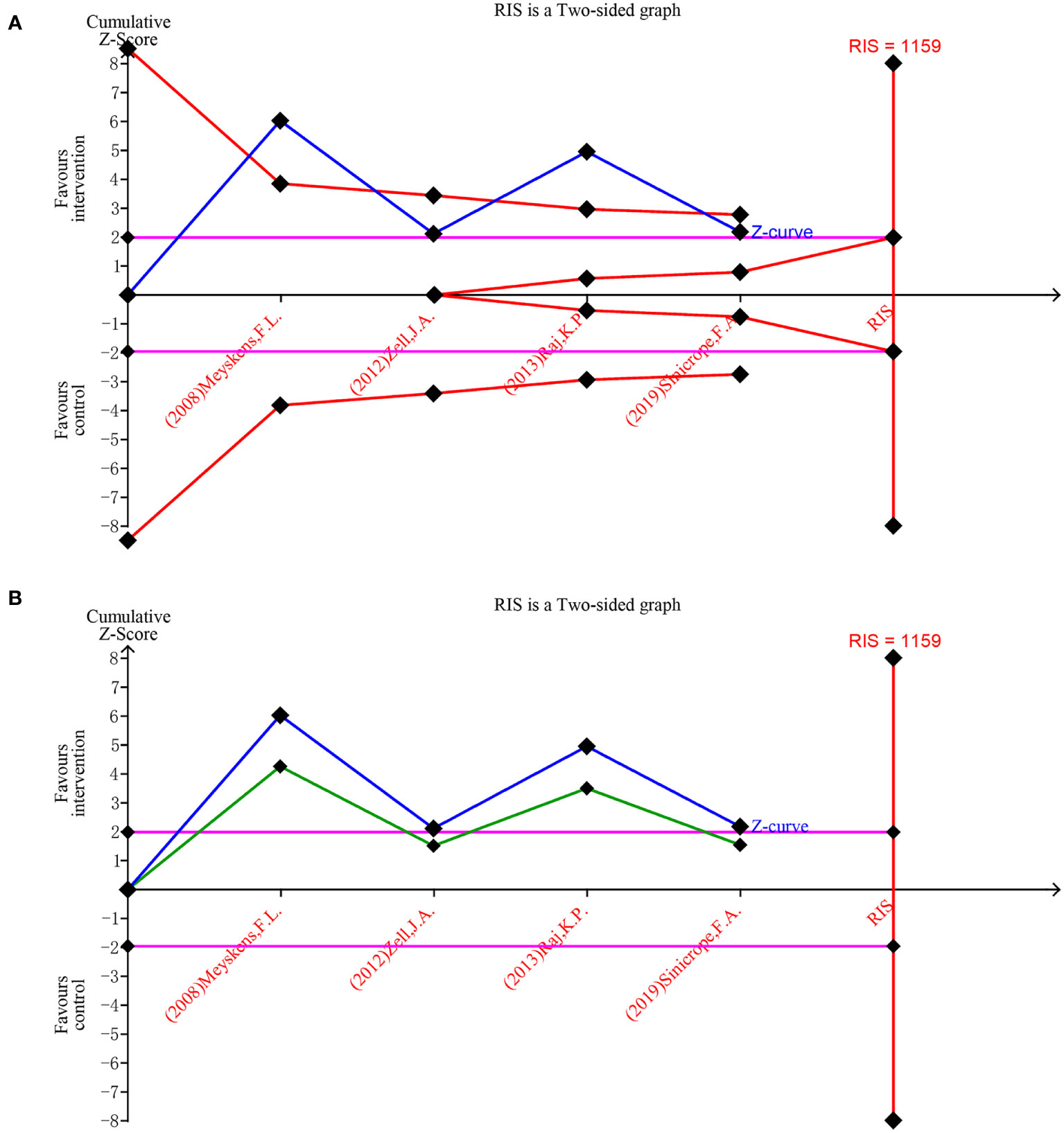
Figure 6 Forest plot for the detection rate of recurrent adenoma in patients with colorectal cancer compared with DFMO combination therapy and placebo. (A) Random effects model of the TSA of detection rate of recurrent adenoma. A diversity-adjusted information size of 1159 participants was calculated on the basis of using α=5% (two-sided), β=20% (power 80%), and I2 = 82%. The solid blue line represents the cumulative Z-curve, which crossed the futility boundary (solid red line); (B) Penalty statistic analysis of the incidence of recurrent adenoma with DFMO combination. This conclusion is further confirmed by the fact that the penalty curve (solid green line) exceeds the traditional threshold value after inclusion of the first study (solid purple line, Z = 1.96).
3.8 GRADE evidence evaluation
The GRADE evidence profile for the primary outcome is shown in Table 2. The GRADE working group graded the evidence for recurrent adenoma detection rate as intermediate and the evidence for disease progression as very low. Indirectness and publication bias were not detected.
4 Discussion
This study comprehensively and systematically reviewed the literature on the efficacy of DFMO chemoprevention in a high-risk population for CRC. The RCT data shows that DFMO combination significantly reduced the incidence of adenomas in patients with recurrent colorectal neoplasms, quality of assessment evidence based on GRADE methodology “medium”. There was no difference in the control of disease progression in familial adenomatous polyposis with DFMO combination therapy. This study demonstrated robust results through sensitivity analysis.
The combined outcome of the two studies demonstrated no difference in the control of the progression of disease in familial adenomatous polyposis between the DFMO combination with sulindac. However, one study confirmed (25) that sulindac was effective in delaying colonic polyposis progression in patients with familial adenomatous polyposis. The reason for the non-significant combined result may be that the sulindac-treated group (9) showed a lower observed event rate of disease progression than the expected 70%, thus affecting the final results. Future investigation is expected to authenticate the contribution of DFMO in combination or alone in controlling the progression of disease in familial adenomatous polyposis.
The recurrence of adenoma is an important factor that prompts the need for re-treatment of CRC. The current dilemma of a high recurrence rate after resection of colorectal adenoma and early CRC lesions exists. Existing studies suggest that the detection rate of colorectal adenoma by repeat colonoscopy in the first year after surgery is as high as 36%-61% (26–29). Hence, there is an urgent need to explore effective programs to prevent the recurrence of CRC. As demonstrated by this study, the combined results of four studies suggest that the use of DFMO in combination with NSAIDs is efficacious in lowering the incidence of new adenomas in patients with previous CRC. Subgroup analysis exhibited that DFMO combined with aspirin did not significantly reduce adenoma recurrence, but the combination of drugs remarkably decreased the number of abnormal rectal crypt foci, consistent with chemoprevention, according to the results of the study (11). Janakiram, N. B. et al. (30) used low-dose DFMO in combination with rosuvastain in 8-week-old CRC rats and found that the combination therapy was effective in inhibiting the proliferation rate of CRC by 76%. Additionally, Patlolla, J. M. R. et al. (31) used DFMO in combination with sulindac in 7-week-old CRC rats. They showed that the combination therapy effectively reduced the incidence of colorectal adenomas by 42%, especially for adenocarcinomas > 0.5 mm. In addition, the combination therapy also significantly enhanced the expression of p21WAF1/CIP1, caspase 3 cleavage, and down-regulated the expression of bcl-xL, bcl-2, and surviving in rat colon tumor tissues. The sequential analysis shows that although the sample size is not reached, the reliability of the data is satisfied.
Increased ODC activity accompanied by tumor transformation, and elevated polyamine concentrations further promoted tumor development (32). Therefore, several strategies to reduce intracellular polyamine levels have been investigated to study their efficacy in cancer prevention. One successful strategy is the use of DFMO in combination with NSAIDs to reduce polyamine levels to achieve tumor suppressive effects. DFMO reduces the number and size of colonic adenomas and significantly decreases tumor cell proliferation by inhibiting ODC and promoting apoptosis pathways to lower polyamine levels (31). NSAIDs combat CRC by promoting polyamine export through the activation of spermidine/Spermine -N1-acetyltransferase (33–35). Polyamines are elevated in adenomatous colon polyps and CRC compared with normal mucosa, and related studies have shown that DFMO combined with sulindac significantly reduces polyamine production in rectal mucosa (36–38) and inhibits the growth and activity of human CRC cells (39), with good synergistic effects (40). In animal models of colon cancer, DFMO, promoted a significant reduction in intestinal tumors compared with DFMO alone (11, 41). The low-dose combination therapy contributed to a significant downregulation of the inflammatory markers IL-6, stat3, and COX-2, along with the proliferation markers β-catenin and cyclin D1, in rat tumors compared with low-dose resulvastatin or DFMO alone (30). Thus, this combination of DFMO and sulindac or aspirin has been identified as an effective inhibitor for the prevention of CRC (42).
It is important to note that DFMO is labeled as pregnancy category C and should be used with caution in pregnant women because the drug has been found to reduce fetal weight and may cause skeletal variation (43). While evidence (44–47) points to DFMO inducing clinical ototoxicity, evident through symptoms like tinnitus, hearing impairment, and vertigo (32, 48). concurrent research underscores that this ototoxicity is dose-responsive. Notably, these symptoms tend to subside within three months of discontinuing the medication (32). Additionally, the usual therapeutic dose (500 mg/m2 per day) did not cause clinically detectable ototoxicity (49–51). In addition, compared with many other oncology treatments, DFMO is administered in an oral form, which is easy to take and can improve the quality of patient survival. The chemical synthesis of the drug is mature and stable for long-term storage and is relatively cheaper, which can benefit low-income patients (51). Good compliance is also an important reason for the recommendation of this drug, as previous phase III clinical trials have shown that patients have a 95% compliance rate with this drug (49). Therefore, DFMO, in combination with NSAIDs, is recommended as a combination therapy for the chemoprevention of CRC.
This study was evaluated by the Grade evidence, which showed moderate evidence that the DFMO combination was effective in reducing the incidence of adenomas in patients with previous colorectal neoplasms and could provide some reference for clinical practitioners. The other two outcome indicator had extremely low outcome indicator evidence, most notably related to imprecision, i.e., due to small sample size and wide confidence intervals. It is anticipated that future research endeavors focusing on chemoprevention studies involving DFMO in combination with NSAIDs, will have the opportunity to delve deeper into determining the optimal dosage and regimen for preventing colorectal tumors in high-risk patient groups. These findings may serve as valuable references for clinical practitioners.
4.1 Strengths and limitations
The strengths of this meta-analysis lie in the conformity with the PRISMA statement and the certainty of applying the GRADE approach to evaluate the evidence. We verified the reliability of our findings through Trial Sequential Analysis (TSA), thereby affirming the authenticity of the results prematurely and circumventing the unnecessary expenditure of clinical resources. Our meta-analysis has some shortcomings that may influence the interpretation of the outcome. First, it is difficult to completely rule out the presence of publication bias, as this meta-analysis included only six trials. Second, data limitations prevented further subgroup analyses to explore the effects of different doses and follow-up period of drugs on outcome indicators.
5 Conclusions
In conclusion, this meta-analysis indicates that the presently available randomized controlled trials are adequate to conclusively ascertain the effectiveness of DFMO combination therapy, particularly in reducing the incidence of recurrent adenomas. Based on the assessment of evidence quality, the quality of evidence from previous randomized controlled trials is deemed “moderate,” making it a viable reference.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
LY: Conceptualization, Data curation, Methodology, Resources, Visualization, Writing – original draft, Writing – review & editing. YW: Writing – original draft, Writing – review & editing. SH: Conceptualization, Data curation, Software, Writing – original draft, Writing – review & editing. XW: Formal Analysis, Investigation, Software, Supervision, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the First Hospital of Lanzhou University (No.2007002002302), The President’s Youth Fund of Hexi University (No.QN2022011), Hexi University Curriculum Ideological and Political Teaching Research Project (HXXYJY-2021-24) and the Innovation Fund project of Gansu Provincial Department of Education (No.2022B-192) are supported.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet (London England) (2014) 383(9927):1490–502. doi: 10.1016/s0140-6736(13)61649-9
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
3. Cancer IAfRo. Globocan 2018: Cancer Fact Sheets — Crc. Iarc (2018). Available at: http://gco.iarc.fr/today/data/factsheets/cancers/10_8_9-Colorectum-fact-sheet.pdf.
4. Waller A, Findeis S, Lee MJ. Familial adenomatous polyposis. J Pediatr Genet (2016) 5(2):78–83. doi: 10.1055/s-0036-1579760
5. Kennedy RD, Potter DD, Moir CR, El-Youssef M. The natural history of familial adenomatous polyposis syndrome: A 24 year review of a single center experience in screening, diagnosis, and outcomes. J Pediatr Surg (2014) 49(1):82–6. doi: 10.1016/j.jpedsurg.2013.09.033
6. Kemp Bohan PM, Mankaney G, Vreeland TJ, Chick RC, Hale DF, Cindass JL, et al. Chemoprevention in familial adenomatous polyposis: past, present and future. Fam Cancer (2021) 20(1):23–33. doi: 10.1007/s10689-020-00189-y
7. Half E, Arber N. Chemoprevention of gastrointestinal neoplasia. Curr Gastroenterol Rep (2013) 15(5):320. doi: 10.1007/s11894-013-0320-x
8. Raul F. Revival of 2-(Difluoromethyl)Ornithine (Dfmo), an inhibitor of polyamine biosynthesis, as a cancer chemopreventive agent. Biochem Soc Trans (2007) 35(Pt 2):353–5. doi: 10.1042/bst0350353
9. Balaguer F, Stoffel EM, Burke CA, Dekker E, Samadder NJ, Van Cutsem E, et al. Combination of sulindac and eflornithine delays the need for lower gastrointestinal surgery in patients with familial adenomatous polyposis: post hoc analysis of a randomized clinical trial. Dis colon rectum (2022) 65(4):536–45. doi: 10.1097/dcr.0000000000002095
10. Burke CA, Dekker E, Lynch P, Samadder NJ, Balaguer F, Hüneburg R, et al. Eflornithine plus sulindac for prevention of progression in familial adenomatous polyposis. New Engl J Med (2020) 383(11):1028–39. doi: 10.1056/NEJMoa1916063
11. Sinicrope FA, Velamala PR, Song L, Viggiano TR, Bruining DH, Rajan E, et al. Efficacy of difluoromethylornithine and aspirin for treatment of adenomas and aberrant crypt foci in patients with prior advanced colorectal neoplasms. Cancer Prev Res (Philadelphia Pa) (2019) 12(11):821–30. doi: 10.1158/1940-6207.Capr-19-0167
12. Raj KP, Zell JA, Rock CL, McLaren CE, Zoumas-Morse C, Gerner EW, et al. Role of dietary polyamines in a phase iii clinical trial of difluoromethylornithine (Dfmo) and sulindac for prevention of sporadic colorectal adenomas. Br J Cancer (2013) 108(3):512–8. doi: 10.1038/bjc.2013.15
13. Zell JA, Lin BS, Madson N, McLaren CE, Gerner EW, Meyskens FL. Role of obesity in a randomized placebo-controlled trial of difluoromethylornithine (Dfmo) + sulindac for the prevention of sporadic colorectal adenomas. Cancer Causes Control (2012) 23(10):1739–44. doi: 10.1007/s10552-012-0051-6
14. Meyskens FL Jr., McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: A randomized placebo-controlled, double-blind trial. Cancer Prev Res (Philadelphia Pa) (2008) 1(1):32–8. doi: 10.1158/1940-6207.Capr-08-0042
15. Heer E, Ruan Y, Mah B, Nguyen T, Lyons H, Poirier A, et al. The efficacy of chemopreventive agents on the incidence of colorectal adenomas: A systematic review and network meta-analysis. Prev Med (2022) 162:107169. doi: 10.1016/j.ypmed.2022.107169
16. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. BMJ (Clinical Res ed) (2009) 339:b2535. doi: 10.1136/bmj.b2535
17. Geneve N, Kairys D, Bean B, Provost T, Mathew R, Taheri N. Colorectal cancer screening. Primary Care (2019) 46(1):135–48. doi: 10.1016/j.pop.2018.11.001
18. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clinical Res ed) (2011) 343:d5928. doi: 10.1136/bmj.d5928
19. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21(11):1539–58. doi: 10.1002/sim.1186
20. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Res ed) (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
21. Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta-analyses may be inconclusive–trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. Int J Epidemiol (2009) 38(1):287–98. doi: 10.1093/ije/dyn188
22. Takwoingi Y, Hopewell S, Tovey D, Sutton AJ. A multicomponent decision tool for prioritising the updating of systematic reviews. Bmj (2013) 347:f7191. doi: 10.1136/bmj.f7191
23. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. Grade: an emerging consensus on rating quality of evidence and strength of recommendations. Bmj (2008) 336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD
24. Hong W, Sheng LI, Xian-Tao Z, Shan-Shan WU, Qing L, Xing-Huan W. Application of the trial sequential analysis soft ware for meta-analysis. Chin J Evidence-Based Med (2016) 16(5):604–11. doi: 10.7507/1672-2531.20160093
25. Kim B, Giardiello FM. Chemoprevention in familial adenomatous polyposis. Best Pract Res Clin Gastroenterol (2011) 25(4-5):607–22. doi: 10.1016/j.bpg.2011.08.002
26. Chen YX, Gao QY, Zou TH, Wang BM, Liu SD, Sheng JQ, et al. Berberine versus placebo for the prevention of recurrence of colorectal adenoma: A multicentre, double-blinded, randomised controlled study. Lancet Gastroenterol Hepatol (2020) 5(3):267–75. doi: 10.1016/s2468-1253(19)30409-1
27. Gao QY, Chen HM, Sheng JQ, Zheng P, Yu CG, Jiang B, et al. The first year follow-up after colorectal adenoma polypectomy is important: A multiple-center study in symptomatic hospital-based individuals in China. Front Med China (2010) 4(4):436–42. doi: 10.1007/s11684-010-0200-9
28. Hull MA, Sprange K, Hepburn T, Tan W, Shafayat A, Rees CJ, et al. Eicosapentaenoic acid and aspirin, alone and in combination, for the prevention of colorectal adenomas (Seafood polyp prevention trial): A multicentre, randomised, double-blind, placebo-controlled, 2×2 factorial trial. Lancet (2018) 392(10164):2583–94. doi: 10.1016/s0140-6736(18)31775-6
29. Passarelli MN, Barry EL, Rees JR, Mott LA, Zhang D, Ahnen DJ, et al. Folic acid supplementation and risk of colorectal neoplasia during long-term follow-up of a randomized clinical trial. Am J Clin Nutr (2019) 110(4):903–11. doi: 10.1093/ajcn/nqz160
30. Janakiram NB, Mohammed A, Bryant T, Zhang Y, Brewer M, Duff A, et al. Clinically relevant low dose combination of rosuvastatin and difluoromethylornithine provides effective chemopreventive efficacy against aom-induced colon cancers in F344 rats. Cancer Res (2013) 73(8):186–. doi: 10.1158/1538-7445.AM2013-186
31. Madka V, Patlolla JMR, Venkatachalam K, Zhang Y, Pathuri G, Stratton N, et al. Chemoprevention of colon cancer by DFMO, Sulindac, and NO-Sulindac administered individually or in combinations in F344 Rats. Cancers (Basel) (2023) 15(5):4001. doi: 10.3390/cancers15154001
32. Laukaitis CM, Gerner EW. Dfmo: targeted risk reduction therapy for colorectal neoplasia. Best Pract Res Clin Gastroenterol (2011) 25(4-5):495–506. doi: 10.1016/j.bpg.2011.09.007
33. Turchanowa L, Dauletbaev N, Milovic V, Stein J. Nonsteroidal anti-inflammatory drugs stimulate spermidine/spermine acetyltransferase and deplete polyamine content in colon cancer cells. Eur J Clin Invest (2001) 31(10):887–93. doi: 10.1046/j.1365-2362.2001.00901.x
34. Babbar N, Gerner EW, Casero RA Jr. Induction of spermidine/spermine N1-acetyltransferase (Ssat) by aspirin in caco-2 colon cancer cells. Biochem J (2006) 394(Pt 1):317–24. doi: 10.1042/bj20051298
35. Babbar N, Gerner EW. Polyamines as modifiers of genetic risk factors in human intestinal cancers. Biochem Soc Trans (2003) 31(2):388–92. doi: 10.1042/bst0310388
36. Thompson PA, Wertheim BC, Zell JA, Chen WP, McLaren CE, LaFleur BJ, et al. Levels of rectal mucosal polyamines and prostaglandin E2 predict ability of dfmo and sulindac to prevent colorectal adenoma. Gastroenterology (2010) 139(3):797–805. doi: 10.1053/j.gastro.2010.06.005
37. Cong Y, Zhe G. Application of difuloromethylornithine in solid tumors. Chin J Cancer Prev Treat (2016) 23(16):1124–8. doi: 10.3969/jissn.1673-5269.2016.16.016
38. Zell JA, McLaren CE, Chen WP, Thompson PA, Gerner EW, Meyskens FL. Ornithine decarboxylase-1 polymorphism, chemoprevention with eflornithine and sulindac, and outcomes among colorectal adenoma patients. J Natl Cancer Inst (2010) 102(19):1513–6. doi: 10.1093/jnci/djq325
39. Lawson KR, Ignatenko NA, Piazza GA, Cui H, Gerner EW. Influence of K-ras activation on the survival responses of caco-2 cells to the chemopreventive agents sulindac and difluoromethylornithine. Cancer epidemiol Biomarkers Prev Publ Am Assoc Cancer Res cosponsored by Am Soc Prev Oncol (2000) 9(11):1155–62.
40. Gerner EW, Bruckheimer E, Cohen A. Cancer pharmacoprevention: targeting polyamine metabolism to manage risk factors for colon cancer. J Biol Chem (2018) 293(48):18770–8. doi: 10.1074/jbc.TM118.003343
41. Ignatenko NA, Besselsen DG, Stringer DE, Blohm-Mangone KA, Cui H, Gerner EW. Combination chemoprevention of intestinal carcinogenesis in a murine model of familial adenomatous polyposis. Nutr Cancer (2008) 60 Suppl 1:30–5. doi: 10.1080/01635580802401317
42. Gerner EW, Meyskens FL Jr., Goldschmid S, Lance P, Pelot D. Rationale for, and Design of, a Clinical Trial Targeting Polyamine Metabolism for Colon Cancer Chemoprevention. Amino Acids (2007) 33(2):189–95. doi: 10.1007/s00726-007-0515-2
43. Smith KJ, Skelton HG. A-difluoromethylornithine, a polyamine inhibitor: its potential role in controlling hair growth and in cancer treatment and chemo-prevention. Int J Dermatol (2006) 45(4):337–44. doi: 10.1111/j.1365-4632.2006.01231.x
44. Croghan MK, Aickin MG, Meyskens FL. Dose-related alpha-difluoromethylornithine ototoxicity. Am J Clin Oncol (1991) 14(4):331–5. doi: 10.1097/00000421-199108000-00012
45. Croghan MK, Booth A, Meyskens FL Jr. A phase I trial of recombinant interferon-alpha and alpha-difluoromethylornithine in metastatic melanoma. J Biol Response Mod (1988) 7(4):409–15.
46. Meyskens FL, Kingsley EM, Glattke T, Loescher L, Booth A. A phase ii study of alpha-difluoromethylornithine (Dfmo) for the treatment of metastatic melanoma. Invest New Drugs (1986) 4(3):257–62. doi: 10.1007/bf00179593
47. Abeloff MD, Rosen ST, Luk GD, Baylin SB, Zeltzman M, Sjoerdsma A. Phase ii trials of alpha-difluoromethylornithine, an inhibitor of polyamine synthesis, in advanced small cell lung cancer and colon cancer. Cancer Treat Rep (1986) 70(7):843–5.
48. Saulnier Sholler GL, Gerner EW, Bergendahl G, MacArthur RB, VanderWerff A, Ashikaga T, et al. A phase I trial of dfmo targeting polyamine addiction in patients with relapsed/refractory neuroblastoma. PloS One (2015) 10(5):e0127246. doi: 10.1371/journal.pone.0127246
49. Bailey HH, Kim K, Verma AK, Sielaff K, Larson PO, Snow S, et al. A randomized, double-blind, placebo-controlled phase 3 skin cancer prevention study of {Alpha}-difluoromethylornithine in subjects with previous history of skin cancer. Cancer Prev Res (Philadelphia Pa) (2010) 3(1):35–47. doi: 10.1158/1940-6207.Capr-09-0096
50. Meyskens FL Jr., Gerner EW. Development of difluoromethylornithine (Dfmo) as a chemoprevention agent. Clin Cancer Res (1999) 5(5):945–51.
Keywords: Eflornithine, colorectal cancer, high-risk group, chemoprevention, meta-analysis, trial sequential analysis
Citation: Yang L, Wang Y, Hu S and Wang X (2023) Eflornithine for chemoprevention in the high-risk population of colorectal cancer: a systematic review and meta-analysis with trial sequential analysis. Front. Oncol. 13:1281844. doi: 10.3389/fonc.2023.1281844
Received: 23 August 2023; Accepted: 31 October 2023;
Published: 15 November 2023.
Edited by:
Yi-Chiang Hsu, I-Shou University, TaiwanReviewed by:
Luz Maria Rodriguez, National Cancer Institute (NIH), United StatesChung-chi Hsu, I-Shou University, Taiwan
Copyright © 2023 Yang, Wang, Hu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shasha Hu, aHNzNTQ2MjYzOTlAMTYzLmNvbQ==; Xiaoyan Wang, ODE0MDYwMDUyQHFxLmNvbQ==
 Lifeng Yang1
Lifeng Yang1 Yan Wang
Yan Wang Xiaoyan Wang
Xiaoyan Wang