- Department of Oncology, The Third People’s Hospital of Zhengzhou, Zhengzhou, China
Background: Gastric cancer is a common cancer worldwide and is responsible for over one million new cases in 2020 and an estimated 769,000 deaths, ranking fifth for incidence and fourth for mortality globally. Incidence rates are highest in Eastern Asia and Eastern Europe. Gastric cancer is highly heterogeneous and progresses rapidly. The prognosis of gastric cancer with liver metastases is poor, and clinical treatment remains challenging. Human epidermal growth factor receptor 2 (HER2) positivity is correlated to a bad prognosis for gastric cancer. Trastuzumab combined with systemic chemotherapy is the preferred treatment for HER2-positive advanced gastric cancer. However, intravenous chemotherapy has severe systemic toxicity, which reduces the local drug concentration and tumor uptake rate, and the effect is unsatisfactory.
Case summary: We reported a 66-year-old patient with HER2-positive advanced gastric cancer with jaundice due to multiple liver metastases, after 6 cycles of trastuzumab combined with hepatic arterial infusion chemotherapy (HAIC), the tumor retracted significantly, the jaundice subsided, and the patient recovered well. The patient achieved disease control with an intensive regimen followed by less toxic maintenance therapy. Trastuzumab combined with capecitabine maintenance therapy followed up for more than 16 months.
Conclusion: HAIC plus trastuzumab may be a tolerable treatment option for patients with severe liver metastases from HER2-positive gastric cancer to achieve local control and prolong survival.
Introduction
Gastric cancer (GC) is the fifth most common malignancy in the world, with a high mortality rate ranking fourth (1). The liver is one of the most frequently metastasized organs in advanced gastric cancer, with approximately 30% of patients experiencing blood metastasis through portal vein circulation (2–5). Usually, the first-line chemotherapy regimen is based on fluorouracil drugs and combined with platinum and/or paclitaxel drugs to form a two or three-drug chemotherapy regimen. Although the three-drug regimen DCF (Docetaxel, Cisplatin, 5-FU) met the study endpoint in the phase III study, its high toxicity limited its clinical use. In China, a combination of fluorouracil and platinum drugs is recommended. Due to better patient tolerance and the current status of real-world clinical treatment applications in China, platinum drugs are more recommended as oxaliplatin (6–15). Approximately 10-22% of GC patients have HER2 overexpression. The ToGA trial suggests that trastuzumab and chemotherapy prolonged overall survival (OS) for 2.7 months and improved objective response rate (ORR) in gastric cancer patients with HER2-positive compared with chemotherapy alone, therefore trastuzumab has been added to conventional chemotherapy in patients with advanced gastric cancer (16). The 5-year survival rate of gastric cancer with multiple liver metastases (GCLM) was as low as 6% to 13.1% (17). Meanwhile, intravenous chemotherapy has severe systemic toxicity, which reduces the local drug concentration and tumor uptake rate, and the effect is unsatisfactory.
HAIC is a kind of interventional therapy that can increase the concentration of liver tumor drugs and improve the anti-tumor effect by injecting chemotherapy drugs through the hepatic artery. By using the trans-arterial route of administration, drug concentrations in the liver metastases can be significantly increased compared with the intravenous route, resulting in a 3- to 5-fold increase in the response rate of 5-FU and oxaliplatin (18). The feasibility, safety, and efficacy of hepatic artery infusion chemotherapy have been confirmed in colorectal cancer patients (19, 20). Therefore, researchers have turned their attention to the application of HAIC in GCLM. Wang et al. reviewed five GCLM patients who received HAI oxaliplatin and S-1 oral treatment, the objective response rate was 40%, the disease control rate was 80%, and HAI chemotherapy had better local control of liver metastasis, with a median PFS of 8.8 months (21). Ojima et al. retrospectively evaluated the efficacy of HAIC for synchronous GCLM, ORR of HAIC was 83%, there were no serious side effects, and the median survival time (MST) of the HAIC group was 19.2 months (22). In the currently published studies on the treatment of gastric cancer with liver metastases (GCLM), there are no reports of HAIC combined with trastuzumab. We report a HER2-positive GCLM patient who received this combination approach in first-line treatment, which resulted in tumor control, improved quality of life, and improved prognosis.
Case presentation
A 66-year-old male had abdominal distension, sour regurgitation, and heartburn, aggravated by full eating and spicy food for 1 month, but did not receive treatment for his symptoms, came to our hospital in April 2022. The patient was previously a taxi driver with an irregular diet and a history of Helicobacter pylori infection and did not receive eradication treatment. He had a history of hypertension, and arrhythmia, and taken oral valsartan, hydrochlorothiazide, and metoprolol medications, and no family history related to tumors. His Eastern Cooperative Oncology Group (ECOG) performance status (PS) was 2. Physical examination: no palpable enlargement of superficial lymph nodes throughout the body, no tenderness or rebound tenderness in the abdomen, and no palpation under the hepatic subcostals. His laboratory findings, including AST, 100 U/L; ALT, 127U/L; and total bilirubin (T. Bil), 75.4μmol/L, indirect bilirubin (I. Bil), 55.1μmol/L, suggested severe hepatic injury. Laboratory tests suggested that his kidney function was normal. Tumor marker detection indicated CEA>2000ng/ml, CA50 105.66U/ml, CA199>1000U/ml, CA72-4 251.88U/ml, and CA24-2 57.36U/ml. Cardiac ultrasound indicated that the left ventricular ejection fraction (LVEF) was normal. Gastroscopy revealed an ulcerating mass in lesser gastric curvature near the gastric antrum (Figure 1A). Gastroscopic biopsies showed moderately differentiated adenocarcinoma, as shown in Figure 1B. Immunohistochemistry assay showed HER2 positive staining, microsatellite stability, PD-L1 CPS 0, and Ki-67 (80%). Abdominal computed tomography (CT) revealed a lump in the gastric antrum, accompanied by enlarged lymph nodes in the hepatogastric space; Multiple enlarged nodules were observed in the liver, as shown in Figures 1C, D. Thoracic CT showed no signs of lung metastasis. Bone scintigraphy showed no evidence of bone metastasis. Head magnetic resonance imaging (MRI) indicated no signs of brain metastasis.
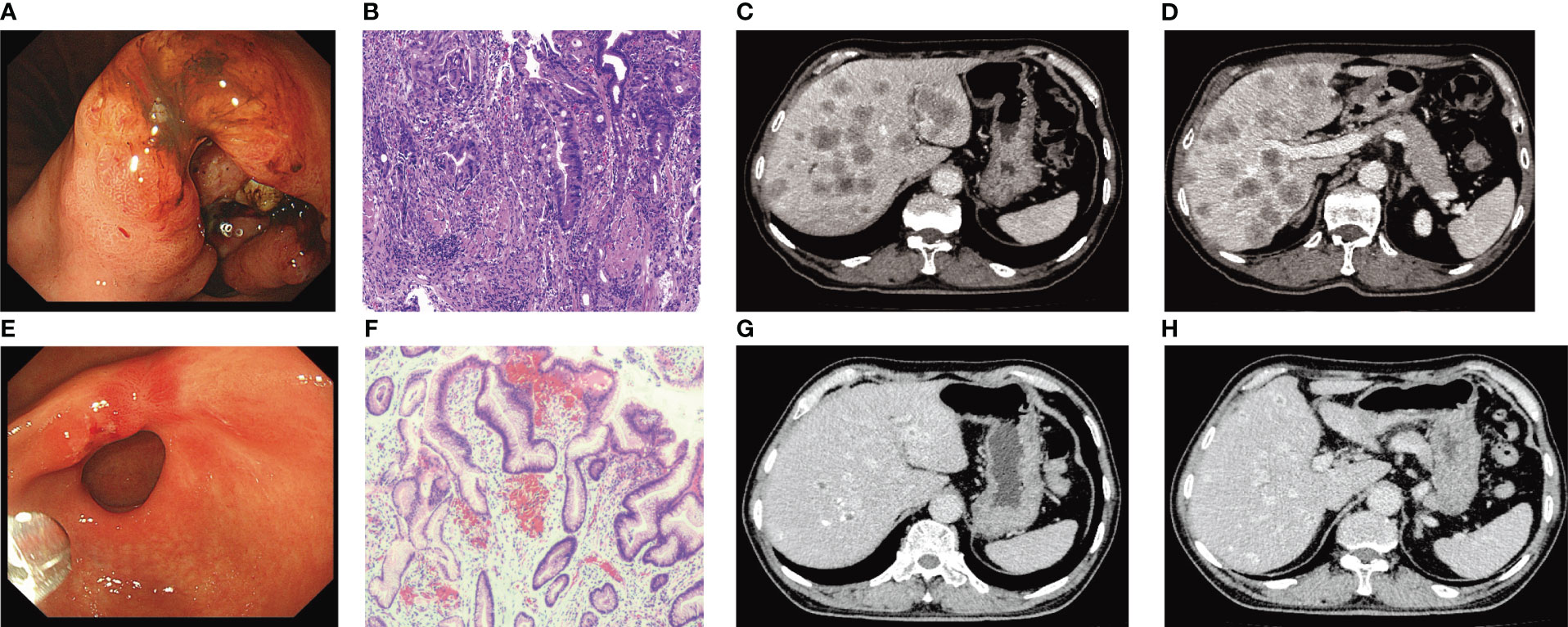
Figure 1 (A) Gastroscopy before treatment. (B) Pathological findings of gastroscopic biopsy (HE×200) (suggesting moderately differentiated adenocarcinoma). (C, D) Abdominal CT before treatment. (E) Results of gastroscopy examination after 6 cycles of treatment (disappearance of lesions before treatment). (F) Pathological examination results after 6 cycles of treatment (HE×200). (G, H) Evaluation of Partial response (PR) after 6 cycles of trastuzumab combined with HAIC treatment.
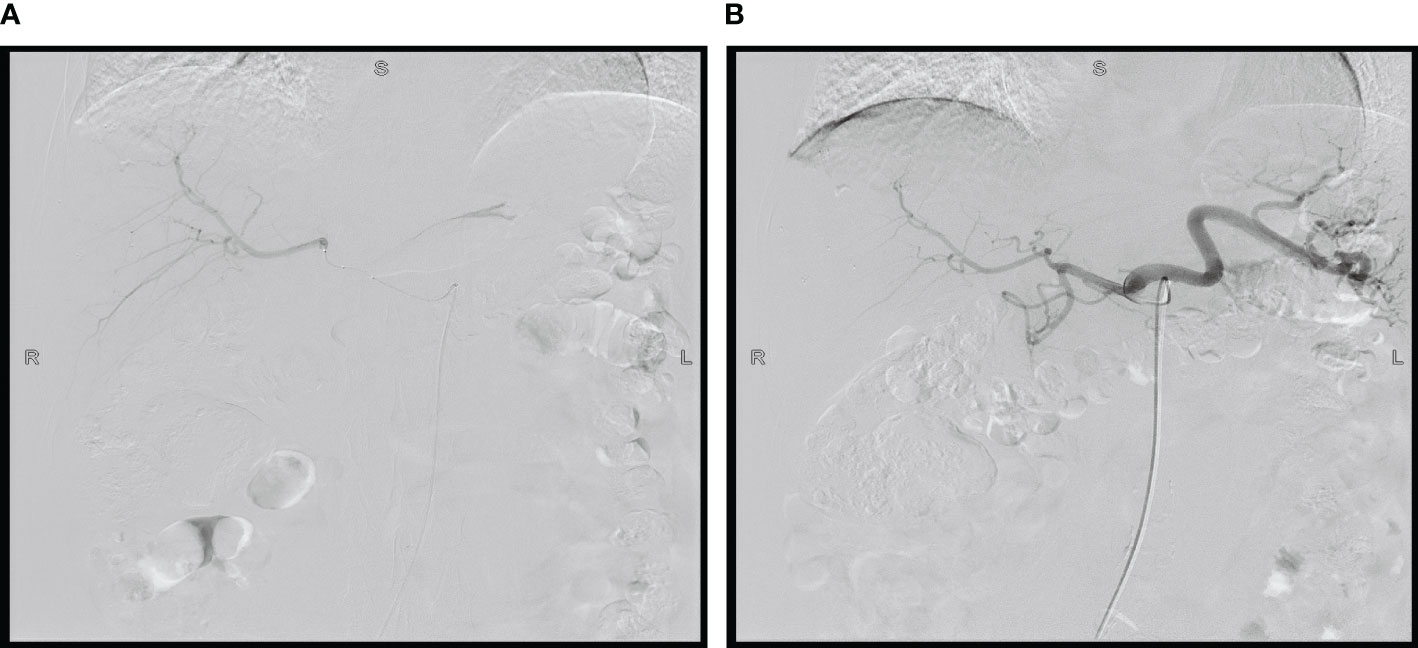
Considering the large tumor burden and liver metastases, we devised a therapeutic regimen consisting of targeted drugs and HAIC. The treatment regimen included intravenous Trastuzumab, hepatic arterial infusion of oxaliplatin, and fluorouracil, and the treatment was repeated after one cycle (21 days). The initial load dose of trastuzumab was 8 mg/kg, followed by 6 mg/kg every 3 weeks. During the regular treatment of trastuzumab, we monitored the patient’s cardiac enzymes, electrocardiogram, and cardiac color ultrasound, and did not reduce or discontinue the drug due to adverse reactions. For HAIC, after ultrasonic positioning, the Seldinger method was used to puncture the femoral artery, and after successful insertion of 5F arterial sheath, the catheter guide wire was sent along the sheath, selective insertion of abdominal trunk arteriography, the catheter with a drug delivery device and insert the tip into the common hepatic artery after imaging confirmation. Oxaliplatin (80 mg/m2 for 2 hours) and fluorouracil (2,600 mg/m2 for 72 hours) were administered through the port catheter system (Figure 2). After one cycle of HAIC combined with intravenous trastuzumab, jaundice quickly subsided and total bilirubin decreased to normal. The level of tumor markers significantly decreased. Partial response was observed after 1 month. After receiving six cycles of treatment, the patient achieved maximum efficacy (Figures 1G, H). Gastroscopy showed no mass and ulcer (Figure 1E); Pathological biopsy showed no tumor cells (Figure 1F). The patient’s ECOG score returned to a level of 1. The significant adverse reactions during HAIC were fatigue, neuropathy, and mild nausea, which improved after symptomatic treatment, with no grade 3-4 adverse effects.
To improve the patient’s quality of life and prolong his survival time, maintenance therapy is recommended. The optimal mode of maintenance therapy in the first-line advanced gastric cancer has not been established. Targeted therapy combined with monochemotherapy as a maintenance regimen may be the choice in clinical practice. We recommend the combination of intravenous trastuzumab and capecitabine for maintenance treatment, with a specific dose of 6mg/Kg of trastuzumab; Capecitabine 1000mg/m2, taken orally for 2 weeks, stopped for 1 week, every 3 weeks. During the oral administration of capecitabine, the patient experienced nausea, acid reflux, and neuropathy, and capecitabine was reduced by 20%. At present, the tumor markers of the patient are normal (Figure 3A), and the imaging assessment is stable (Figures 3B, C). In the follow-up maintenance treatment of trastuzumab combined with capecitabine, the patients have been followed up for more than 16 months.
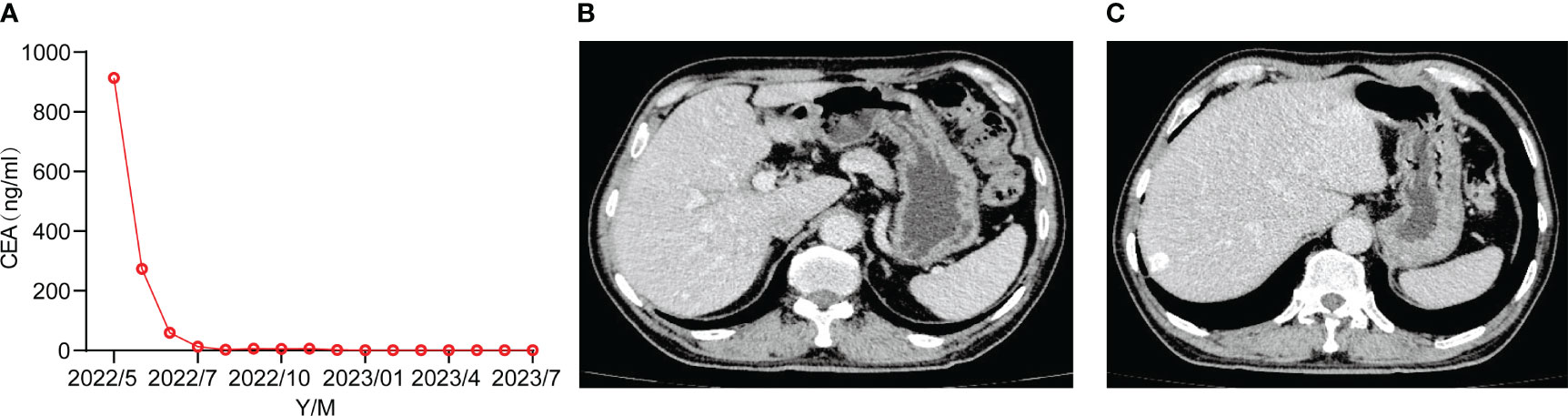
Figure 3 (A) Tumor markers returned to normal. (B, C) Follow-up CT in July 2023 assessed the stability.
Discussion
The treatment of liver metastases from gastric cancer remains debatable. The median overall survival of intravenous fluorouracil combined with platinum chemotherapy is 10.5-11.6 months. With the development of precision medicine, molecular-targeted therapy has become the main treatment method for gastric cancer. HER2 plays an important role in the biological behavior and pathogenesis of advanced GC and is also an important target for the systemic treatment of advanced GC at present (23). About 15%-20% of patients have abnormal activation or overexpression of HER2, which is significantly associated with poor prognosis of patients (24). Trastuzumab plus systemic chemotherapy has become the standard first-line treatment for HER2-positive advanced gastric cancer, with a median overall survival of 13.8 months. However, for gastric cancer patients with jaundice due to extensive liver metastases, the effect of systemic chemotherapy is unsatisfactory, while the patients often experience serious adverse reactions, their quality of life is affected, the compliance decreases, and their prognosis is poor (25). Since one of the key factors determining prognosis is liver metastases, local control is considered to be very important (22).
HAIC is a treatment that continuously infuses chemotherapy drugs into tumors through hepatic arteries. Hepatic metastases derive their blood supply mainly from the hepatic artery, whereas portal circulation mainly supplies normal hepatic tissues (26). HAIC increases drug concentration in local lesions, prolongs drug action time, directly leads to tumor cell death, and inhibits tumor proliferation. In addition to HAIC, there are other minimally invasive treatment methods, such as transcatheter arterial chemoembolization (TACE), radiofrequency ablation (RFA), etc. But compared to HAIC, TACE mainly focuses on embolism, leading to more severe liver function damage, and affecting treatment compliance (27). HAIC is effective for both detectable liver lesions and intrahepatic micrometastases, whereas the therapeutic efficacy of RFA was reduced for large tumors, and the presence of as many as four or five lesions was considered suitable. HAIC has been used to increase the local drug concentration of liver metastases, thereby increasing liver disease control and the resectability of colorectal cancer with liver metastasis (28). The efficacy of HAIC in gastric cancer with liver metastasis is still uncertain.
Most of the HAI data on liver metastases from gastric cancer involve Asian patients. The largest analysis was a phase II study that included 88 patients, 55 of whom had not received any prior chemotherapy. It evaluates regimen 5-FU/epirubicin/mitomycin C for HAI administration. In 63 evaluable patients, the response rate was 55.6%. Toxicity was mild, with an incidence of 30% nausea -vomiting (2.5%, grade 3-4) and so on (29).
Qiang et al. analyzed 21 patients with gastric cancer with extensive liver metastases who were treated with oral S-1 and HAI oxaliplatin plus floxuridine (FUDR), the overall response rate was 76.2% (9.5% complete response). Intrahepatic and extrahepatic median progression-free survival times were 9.5 and 5.2 months, respectively. MST was 12.3 months. None of the patients experienced grade 4 adverse effects. Grade 3 toxic effects included bone marrow suppression (14.3%) and diarrhea (9.5%) (30). A study of Western patients showed that seven patients received HAIC with a median duration of six cycles. The treatment was feasible and safe, and no grade 3-4 adverse effects had been observed. One patient had stable liver metastases within 7 months (29).
Ojima et al. reported one case that survived for more than 5 years without any signs of recurrence after treatment with HAIC (20). Toyokawa et al. reported that a patient with gastric cancer with liver metastases was disease-free after more than 12 years of HAIC without further chemotherapy (31). Although the sample size is small, it also provides some references for clinical practice.
We report a case of HAIC combined with trastuzumab in a patient with HER2-positive gastric cancer with liver metastases. The patient was 66 years old, but with jaundice due to extensive liver metastases, his general condition was not good. His ECOG score was 2. Jatoi et al. analyzed 367 patients with gastrointestinal tumors, and a total of 154 patients (42%) were aged ≥ 65 years old. The result showed that patients who were ≥65 years old had worse performance scores (32). For this patient, Trastuzumab plus intravenous chemotherapy is the effective option, but it may not adequately control local symptoms, and toxicity tends to be high. We chose HAIC combined with trastuzumab therapy, the patient’s jaundice subsided, symptoms improved, tumor markers decreased, and tumors shrank significantly. The patient’s ECOG score returned to a level of 1. The patient achieved disease control with an intensive regimen followed by less toxic maintenance therapy to delay disease progression.
The choice of maintenance therapy should be based on the patient’s age, physical condition, concomitant diseases, previous treatment, patient preference, economic status, clinical practice bias, and drug accessibility. Li et al. published a prospective observational study that compared maintenance with trastuzumab alone versus the combination of trastuzumab plus mono-chemo-agent (capecitabine or S1) derived from the initial chemotherapy. There were no significant differences in OS (16.5 vs 20.0 months, HR 0.71 P = 0.169) and PFS (7.9 vs 11.0 months, HR 1.06, P = 0.892) between the two groups, although the addition of a chemo-agent to trastuzumab led to a 29% reduction in mortality risk. The safety profile was also similar for both arms (33).
After 6 cycles of intensive therapy, the patient’s liver metastases were locally controlled. He benefited from this therapy. According to the different studies available (33, 34), we recommend the combination of trastuzumab and capecitabine for maintenance treatment to delay disease progression. Trastuzumab combined with capecitabine maintenance therapy followed up for more than 16 months. With only one patient, the limitations of the analysis were obvious. Firstly, since there is only one case, we cannot cover all adverse events. Secondly, the patient’s follow-up time is still short, and the results require long-term follow-up and validation. Thirdly, this protocol did not routinely use the combination of HAIC and trastuzumab, suggesting that efficacy evaluation must be further objectified and standardized through prospective multicenter clinical trials.
In conclusion, HAIC may be an effective treatment, and it may be a traditional tolerable treatment option for patients with major liver metastases from cancer who do not tolerate more intense chemotherapy regimens. This case indicates that HAIC combined with trastuzumab may be a safe and effective treatment selection for extensive GCLM who refused or cannot tolerate first-line intravenous chemotherapy, and may achieve a high-local response and help prolong patient survival.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Zhengzhou Third People’s Hospital Ethics Review Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HL: Writing – original draft, Data curation. QW: Formal Analysis, Writing – review & editing. LZ: Investigation, Writing – review & editing. JL: Project administration, Writing – review & editing. YW: Methodology, Writing – review & editing. LW: Resources, Writing – review & editing. LY: Writing – review & editing, Supervision.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study obtained the joint construction project of Henan Provincial Health Department in 2021 (No.LHGJ20210737).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Schlansky B, Sonnenberg A. Epidemiology of noncardiac gastric adenocarcinoma in the United States. Am J Gastroenterol (2011) 106(11):1978–85. doi: 10.1038/ajg.2011.213
3. Lee JH, Kim HI, Kim MG, Ha TK, Jung MS, Kwon SJ. Recurrence of gastric cancer in patients who are disease-free for more than 5 years after primary resection. Surgery (2016) 159:1090–8. doi: 10.1016/j.surg.2015.11.002
4. Sun Z, Zheng H, Yu J, Huang W, Li T, Chen H, et al. Liver metastases in newly diagnosed gastric cancer: A population-based study from SEER. J Cancer (2019) 10:2991–3005. doi: 10.7150/jca.30821
5. Song JC, Ding XL, Zhang Y, Zhang X, Sun XH. Prospective and prognostic factors for hepatic metastasis of gastric carcinoma: A retrospective analysis. J Cancer Res Ther (2019) 15:298–304. doi: 10.4103/jcrt.JCRT_576_17
6. Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: A randomised phase III noninferiority trial. Ann Oncol (2009) 20(4):666–73. doi: 10.1093/annonc/mdn717
7. Kouzumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): A phase III trial. Lancet Oncol (2008) 9:215–21. doi: 10.1016/S1470-2045(08)70035-4
8. AL-Batran SE, Hartmann JT, Probst S, Schmalenberg H, Hollerbach S, Hofheinz R, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: A study of the Arbeitsgermeinschaft Intemistische Onkologie. J Clin Oncol (2008) 26(9):1435–42. doi: 10.1200/JCO.2007.13.9378
9. Luo HY, Xu RH, Wang F, Qiu MZ, Li YH, Li FH, et al. Phase II trial of XELOX as first-line treatment for patients with advanced gastric cancer. Chemotherapy (2010) 56(2):94–100. doi: 10.1159/000305256
10. Hall PS, Swinson D, Waters JS, Wadsley J, Falk S, Roy R, et al. Optimizing chemotherapy for frail and elderly patients (pts) with advanced gastroesophageal cancer (aGOAC): The GO2 phase III trial. J Clin Oncol (2019) 37(Suppl 15):4006. doi: 10.1200/JCO.2019.37.15suppl.4006
11. Yamaday Y, Higuchi K, Nishikawak K, Gotoh M, Fuse N, Sugimoto N, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naive patients with advanced gastric cancer. Ann Oncol (2015) 26(1):141–8. doi: 10.1093/annonc/mdu472
12. Xu R, Wang ZQ, Shen L, Wang W, Lu JW, Dai GH, et al. S-1 plus oxaliplatin versus S-1 plus cisplatin as first-line treatment for advanced diffuse-type or mixed-type gastric/gastroesophageal junction adenocarcinoma: A randomized, phase 3 trial. J Clin Oncol (2019) 37(Suppl 15):4017.
13. Lu Z, Zhang X, Liu W, Liu T, Hu B, Li W, et al. A multicenter, randomized trial comparing efficacy and safety of paclitaxel/capecitabine and cisplatin/capecitabine in advanced gastric cancer. Gastric Cancer (2018) 21(5):782–91. doi: 10.1007/s10120-018-0809-y
14. Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 Study Group. J Clin Oncol (2006) 24(31):4991–7. doi: 10.1200/JCO.2006.06.8429
15. Wang J, Xu R, Li J, Bai Y, Liu T, Jiao S, et al. Randomized multicenter phase III study of a modified docetaxel and cisplatin plus fluorouracil regimen compared with cisplatin and fluorouracil as first-line therapy for advanced or locally recurrent gastric cancer. Gastric Cancer (2016) 19(1):234–44. doi: 10.1007/s10120-015-0457-4
16. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomized controlled trial. Lancet (2010) 376(9742):687–97. doi: 10.1016/S0140-6736(10)61121-X
17. Cui JK, Liu M, Shang XK. Hepatectomy for liver metastasis of gastric cancer: a meta-analysis. Surg Innov (2019) 26:692–7. doi: 10.1177/1553350619856491
18. Lim A, Le Sourd S, Senellart H, Luet D, Douane F, Perret C, et al. Hepatic arterial infusion chemotherapy for unresectable liver metastases of colorectal cancer: A multicenter retrospective study. Clin Colorectal Cancer (2017) 16:308–15. doi: 10.1016/j.clcc.2017.03.003
19. Kemeny N. Management of liver metastases from colorectal cancer. Oncology (2006) 20(10):1161–76.
20. Kemeny NE, Niedzwiecki D, Hollis DR, Lenz HJ, Warren RS, Naughton MJ, et al. Hepatic arterial infusion vs. systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J Clin Oncol (2006) 24(9):1395–403. doi: 10.1200/JCO.2005.03.8166
21. Wang K, Zhang X, Wei J, Xu Y, Liu Q, Xie J, et al. Hepatic arterial infusion oxaliplatin plus oral S-1 chemotherapy in gastric cancer with unresectable liver metastases: A case series and literature review. Cancer Manag Res (2020) 7:12. doi: 10.2147/CMAR.S233123
22. Ojima H, Ootake S, Yokobori T, Mochida Y, Hosouchi Y, Nishida Y, et al. Treatment of multiple liver metastasis from gastric carcinoma. World J Surg Oncol (2007) 5(1):70. doi: 10.1186/1477-7819-5-70
23. London M, Gallo E. Epidermal growth factor receptor (EGFR) involvement in epithelial-derived cancers and its current antibody-based immunotherapies. Cell Biol Int (2020) 44:1267–82. doi: 10.1002/cbin.11340
24. Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol (2008) 19:1523–9. doi: 10.1093/annonc/mdn169
25. Moriya A, Hyodo I, Nishina T, Imaoka H, Imagawa A, Doi T, et al. Extensive liver metastasis of gastric cancer effectively treated by hepatic arterial infusion of 5-fluorouracil/cisplatin. Gastric Cancer (2000) 3(2):110–5. doi: 10.1007/pl00011695
26. Breedis C, Young G. The blood supply of neoplasms in the liver. Am J Pathol (1954) 30(5):969–77.
27. Chen J, Zhang Y, Cai H, Yang Y, Fei Duan Y. Comparison of the effects of postoperative prophylactic transcatheter arterial chemoembolization (TACE) and transhepatic arterial infusion (TAI) after hepatectomy for primary liver cancer. J BUON (2018) 23:629–34.
28. Kemeny NE, Gonen M. Hepatic arterial infusion after liver resection. N Engl J Med (2005) 352:734–5. doi: 10.1056/NEJM200502173520723
29. Flörcken A, Schaefer C, Bichev D, Breithaupt K, Dogan Y, Schumacher G, et al. Hepatic arterial infusion chemotherapy for liver metastases from gastric cancer: an analysis in western patients. Tumori (2011) 97(1):19–24. doi: 10.1177/030089161109700104
30. Qiang W, Shi H, Wu J, Ji M, Wu C. Hepatic arterial infusion combined with systemic chemotherapy for patients with extensive liver metastases from gastric cancer. Cancer Manag Res (2020) 12:2911–6. doi: 10.2147/CMAR.S245697
31. Toyokawa T, Ohira M, Sakurai K, Amano R, Kubo N, Tanaka H, et al. Long-term survival with complete remission after hepatic arterial infusion chemotherapy for liver metastasis from gastric cancer: a case report. World J Surg Oncol (2015) 13:268. doi: 10.1186/s12957-015-0686-3
32. Jatoi A, Foster NR, Egner JR, Burch PA, Stella PJ, Rubin J, et al. Older versus younger patients with metastatic adenocarcinoma of the esophagus, gastroesophageal junction, and stomach: A pooled analysis of eight consecutive North Central Cancer Treatment Group (NCCTG) trials. Int J Oncol (2010) 36(3):601–6. doi: 10.3892/ijo_00000535
33. Roviello G, Rodriquenz MG, Aprile G, D'Angelo A, Roviello F, Nobili S, et al. Maintenance in gastric cancer: New life for an old issue? Crit Rev Oncol Hematol (2021) 160:103307. doi: 10.1016/j.critrevonc.2021.103307
Keywords: gastric cancer, liver metastases, hepatic arterial infusion chemotherapy, trastuzumab, human epidermal growth factor receptor 2
Citation: Li H-q, Wang Q, Zhang L-y, Li J-y, Wang Y-j, Wei L and Yao L-g (2023) Hepatic arterial infusion chemotherapy and trastuzumab in gastric cancer with liver metastases: a case report. Front. Oncol. 13:1283274. doi: 10.3389/fonc.2023.1283274
Received: 25 August 2023; Accepted: 04 December 2023;
Published: 21 December 2023.
Edited by:
Qun Zhang, Nanjing Medical University, ChinaReviewed by:
Kangshuai Li, Shandong University, ChinaEsther Una Cidon, University Hospitals Dorset NHS Foundation Trust, United Kingdom
Copyright © 2023 Li, Wang, Zhang, Li, Wang, Wei and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li-ge Yao, dG9uZ3NoaTE5ODBAMTI2LmNvbQ==
 Hui-qin Li
Hui-qin Li Qin Wang
Qin Wang