- 1The Department of Blood Purification, First Affiliated Hospital, Guangxi Medical University, Guangxi, China
- 2The Department of Oncology, First Affiliated Hospital, Guangxi Medical University, Guangxi, China
- 3The Department of Nursing, First Affiliated Hospital, Guangxi Medical University, Guangxi, China
Background: The geriatric nutritional risk index (GNRI) has been wildly used to predict the prognosis of patients with solid cancer, but it’s value in postoperative complications remains unclear. The aim of our study was to systematically explore the value of the GNRI in postoperative complications in patients with solid cancer.
Method: The study conducted a systematic literature search using electronic databases to investigate the influence of the GNRI on postoperative complications in patients with solid cancer. The search covered articles published up until May 2023. The odds ratio (OR) with a 95% confidence interval (CI) was employed to assess the effect of GNRI on postoperative complications.
Result: A total of 11 studies with 11,002 patients were enrolled in our meta-analysis. The results suggested that patients with a low GNRI have a higher risk of experiencing postoperative complications (OR=2.51, 95%CI 2.05–3.02, z=9.86, p<0.001), a higher risk of suffering Clavien-Dindo (CD) grades≥2 complications(OR=2.24, 95%CI 1.84–2.73, z=8.01, p<0.001), a higher risk of suffering infection (OR=1.85, 95%CI 1.18–2.88, z=2.70, p=0.007) and a higher risk of suffering respiratory complications(OR = 2.94, 95%CI: 1.56-5.55, z=3.31, p=0.001).
Conclusion: Based on existing evidence, the GNRI was a valuable predictor of postoperative complications in patients with solid cancer.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=434299, identifier CRD42023434299.
1 Introduction
Cancer is a major public health problem worldwide and it has been the second leading cause of death in the United States. According to the 2023 edition of Cancer Statistics, There are 1,958,310 new cases of cancer and 609,820 cancer deaths are projected to take place in the United States (1). It is well known that surgical resection of the lesion is one of the important means to treat early tumor (2), However, the incidence of postoperative complications was high, and the common complications included intestinal obstruction, postoperative bleeding and anastomotic fistula, which significantly affected the prognosis and subsequent treatment of patients (3–5). Studies have shown that the incidence of malnutrition in cancer patients is 20% ~ 70%, and preoperative malnutrition is one of the important factors affecting the occurrence of postoperative complications (6). Malnutrition will not only lead to the increase of postoperative complications, but also prolong the hospital stay, resulting in poor treatment effect and increased mortality (7, 8). Effective nutritional evaluation holds significant importance in managing postoperative complications in tumor patients.
Several nutritional scoring systems have been proposed in previous studies to assess the nutritional status of people with different clinical conditions, such as the dystrophic inflammation score (9), the subjective global assessment (10) and the mini-nutritional assessment (11). However, these parameters are not widely used in clinical practice due to their instability and large differences in baseline values (12). Recently, the Geriatric Nutrition Risk Index (GNRI), a comprehensive malnutrition index that incorporates height, weight, and serum albumin levels, has shown associations with the prognosis and postoperative complications in various types of solid tumors. Such as colorectal cancer (13), esophageal cancer (14) and so on. While several previous meta-analyses have demonstrated the prognostic value of the GNRI in cancer patients, there is a lack of discussion regarding the association between GNRI and postoperative complications in solid tumor patients. This absence of discussion raises certain limitations in the existing literature (15, 16). Therefore, this meta-analysis is based on existing evidence to verify the role of GNRI in postoperative complications in patients with solid tumors.
2 Materials and methods
2.1 Search strategy
Our meta-analysis adhered to the Preferred Reporting Project for Systematic Review and Meta-Analysis (PRISMA) guidelines. We systematically searched the Web of Science, PubMed, Embase databases, and Cochrane Library to identify relevant literature on the assessment of GNRI in evaluating postoperative complications in patients with solid tumors. The search was conducted up until May 1, 2023. The search was also restricted to English language publications. The search terms and keywords employed included: “GNRI”, “geriatric nutritional risk index”, as well as “cancer”, “carcinoma”, “neoplasm”, and “complication”. In addition, the reference lists of the search literature was reviewed to identify additional potential studies.
2.2 Study inclusion and exclusion criteria
The inclusion criteria are shown below (1): Study on GNRI and postoperative complications in elderly cancer patients (2); Cohort or case-control studies (3); In cases where multiple studies by the same author or with overlapping data exist, the study with the largest sample size or the most recent publication date is selected.
2.3 Data withdraw and quality assessment
We extracted various variables from each included study, which encompassed the first author’s name, country and year of publication, patient characteristics, study type, sample size, age range, sex ratio, GNRI cut-off point, duration of follow-up, and assessed outcomes. The main endpoint included postoperative complications. The other endpoints included CD grades ≥2, infection, ileus, leakage and respiratory complications.
To evaluate the quality of the eligible studies, two independent investigators utilized the Newcastle-Ottawa Scale (NOS). In instances where there was a disagreement, a third investigator was involved, and consensus was reached through discussion. In our study, a NOS score higher than 7 indicated a high methodological quality.
2.4 Statistical analysis
The extracted data were pooled for analysis. An OR > 1 indicated an increased risk of postoperative complications in patients with a low GNRI. Statistical heterogeneity among the included studies was assessed using the I2 statistics. A random-effects model was employed in cases of significant heterogeneity. Subgroup analysis was conducted to evaluate the impact of each subgroup on the combined effect. Sensitivity analysis was performed to assess the stability of the results. Potential publication bias was assessed using Begg’s test and Egger’s test. A significance level of P<0.05 was considered statistically significant. All statistical analyses were conducted using Stata 17.0.
3 Results
3.1 Literature search
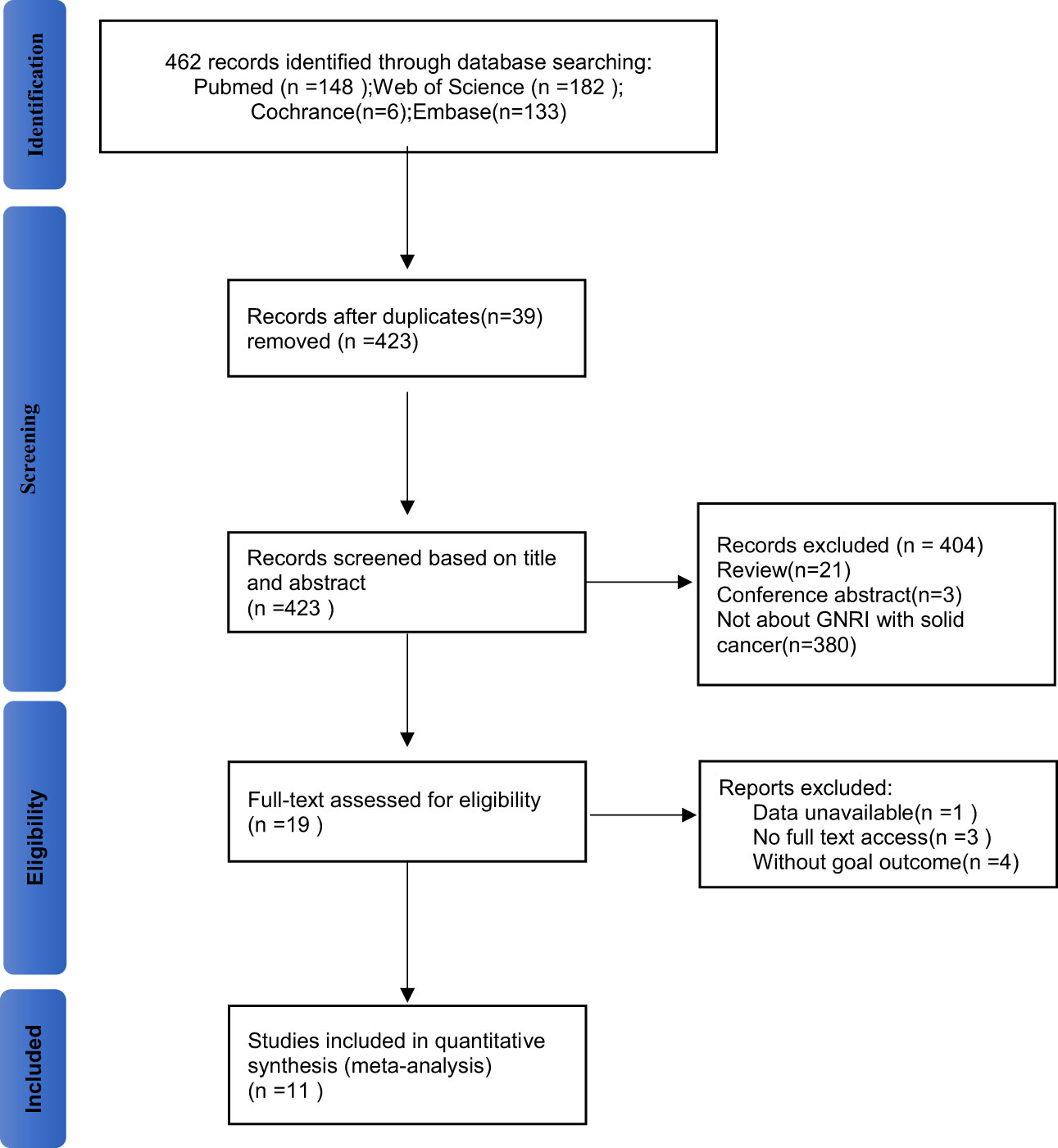
Figure 1 shows the literature screening process of this study. A total of 462 studies were retrieved from the databases according to the search strategy. 39 duplicate studies were removed before screening, leaving 423 studies for further screening. After thorough review of the titles and abstracts, 21 reviews, 3 conference abstracts, and 380 studies that did not focus on GNRI as a predictor of complications in solid cancer patients were excluded. Then, the full texts of the 19 included studies were evaluated. Three of these studies were not able to get the full text; meanwhile, the one with incomplete data was excluded. Four studies were lacked relevant outcomes. Therefore, our meta-analysis included 11 studies involving 11,002patients (8, 14, 17–25).
3.2 Study characteristics
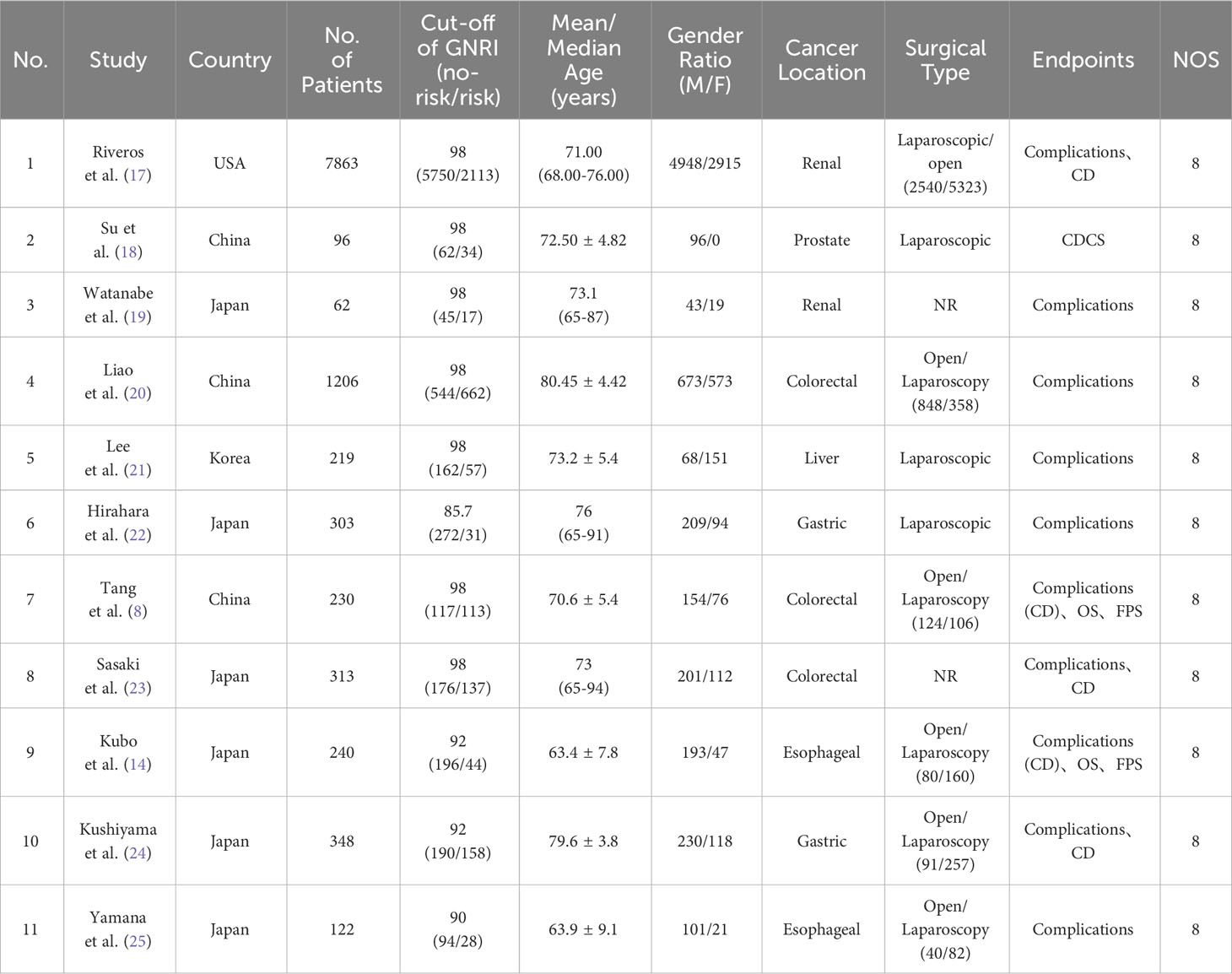
The baseline information of the 11 eligible studies is presented in Table 1. Out of these studies, 10 were single-center retrospective studies (8, 14, 18–27), while one study was based on a retrospective analysis of a database (17). All of the included studies in this analysis were published between 2015 and 2023. The sample sizes of these studies ranged from 62 to 7863 participants. Six studies were from Japan (14, 19, 22–25), three studies were from China (8, 18, 20), one study from USA (17) and one study from Korea (21). Three studies reported colorectal cancer (8, 20, 23), two studies reported Esophageal cancer (14, 25), two studies reported Gastric cancer (22, 24), two studies reported renal cancer (17, 19), one study reported liver cancer (21) and one study reported prostate cancer (18). In addition, 10 studies reported postoperative complications, six studies reported CD grades. The NOS score for all studies were 8.
3.3 GNRI and postoperative complication
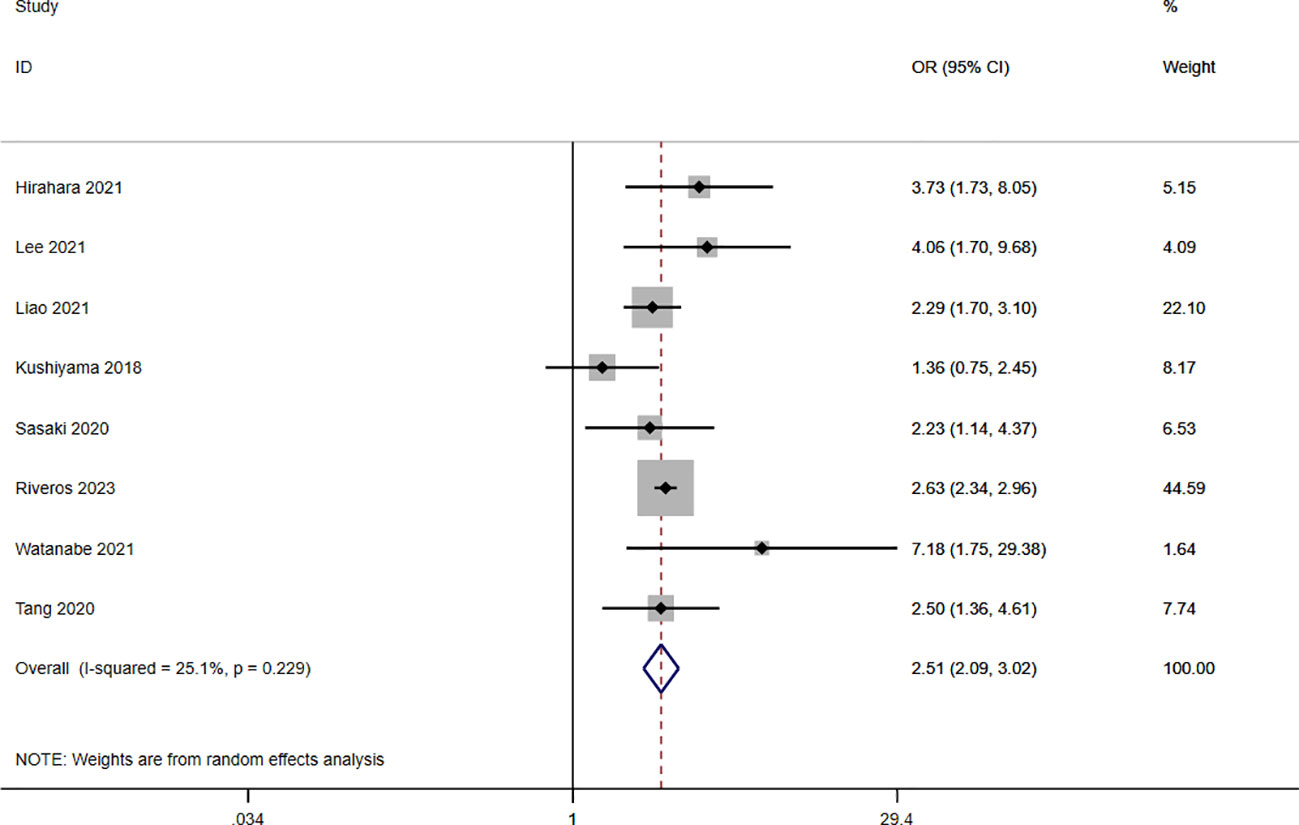
A total of nine studies involving 10,544 patients reported a relationship between the GNRI and postoperative complications in patients with solid cancer. Based on a random effects model (I2 = 25.1%, p = 0.229), the GNRI was significantly relevant to postoperative complication (OR=2.51, 95%CI 2.05–3.02, z=9.86, p<0.001) (Figure 2). These findings suggest that patients with a low GNRI have a higher risk of experiencing postoperative complications compared to those with a high GNRI. We performed a further subgroup analysis based on cancer site (Figure 3A), country (Figure 3B), sample size (Figure 3C), and cut off value of GNRI (Figure 3D). The results revealed that a low GNRI was an independent risk factor affecting postoperative complications in all subgroups. Meanwhile, GNRI was a predictor in liver, colorectal and renal cancer, but have no statistic significant in gastric cancer.
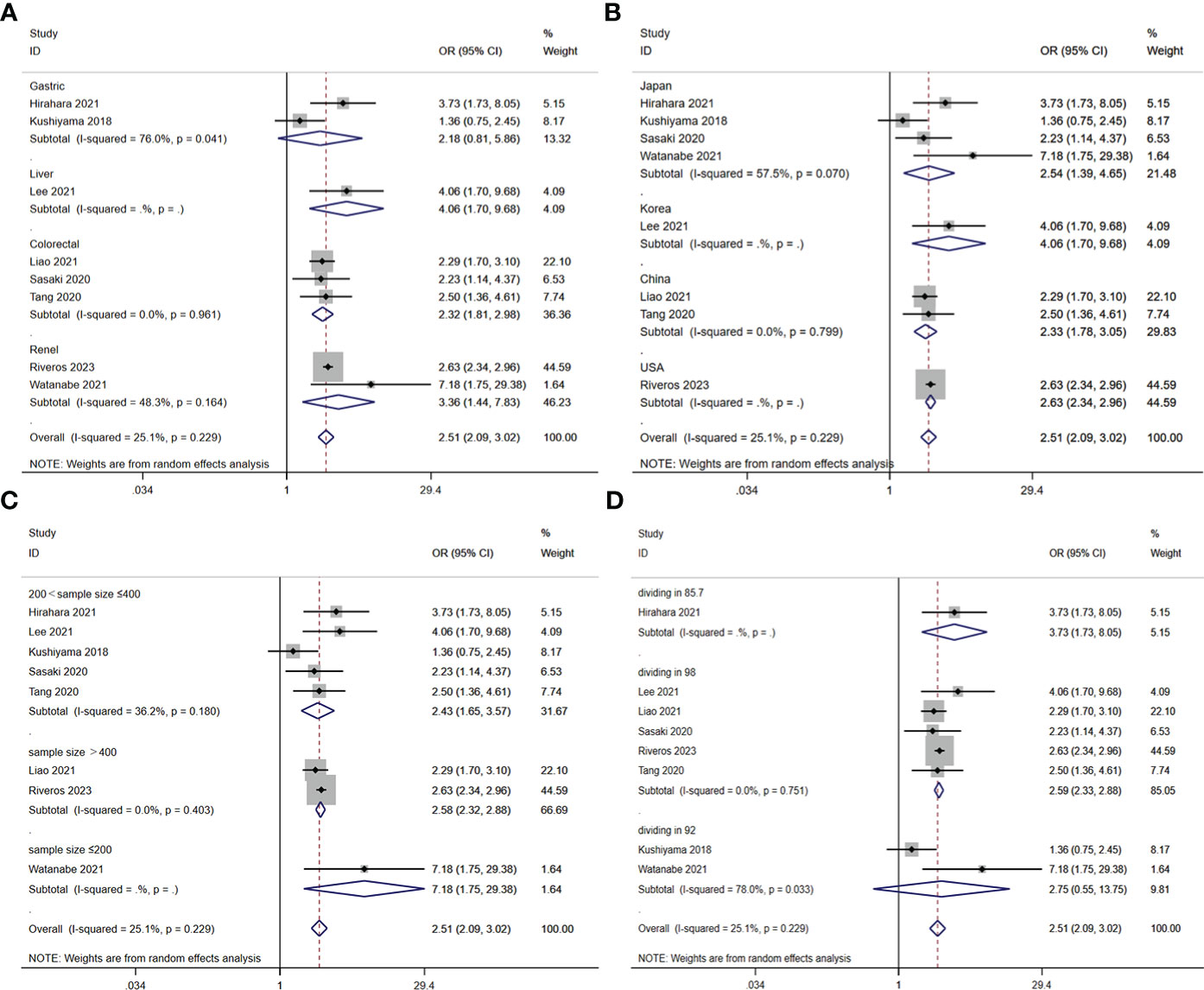
Figure 3 Stratified analysis for the meta-analysis with postoperative complication by cancer site (A), country (B), sample size (C), and cut off value of GNRI (D).
3.4 GNRI and CD grades
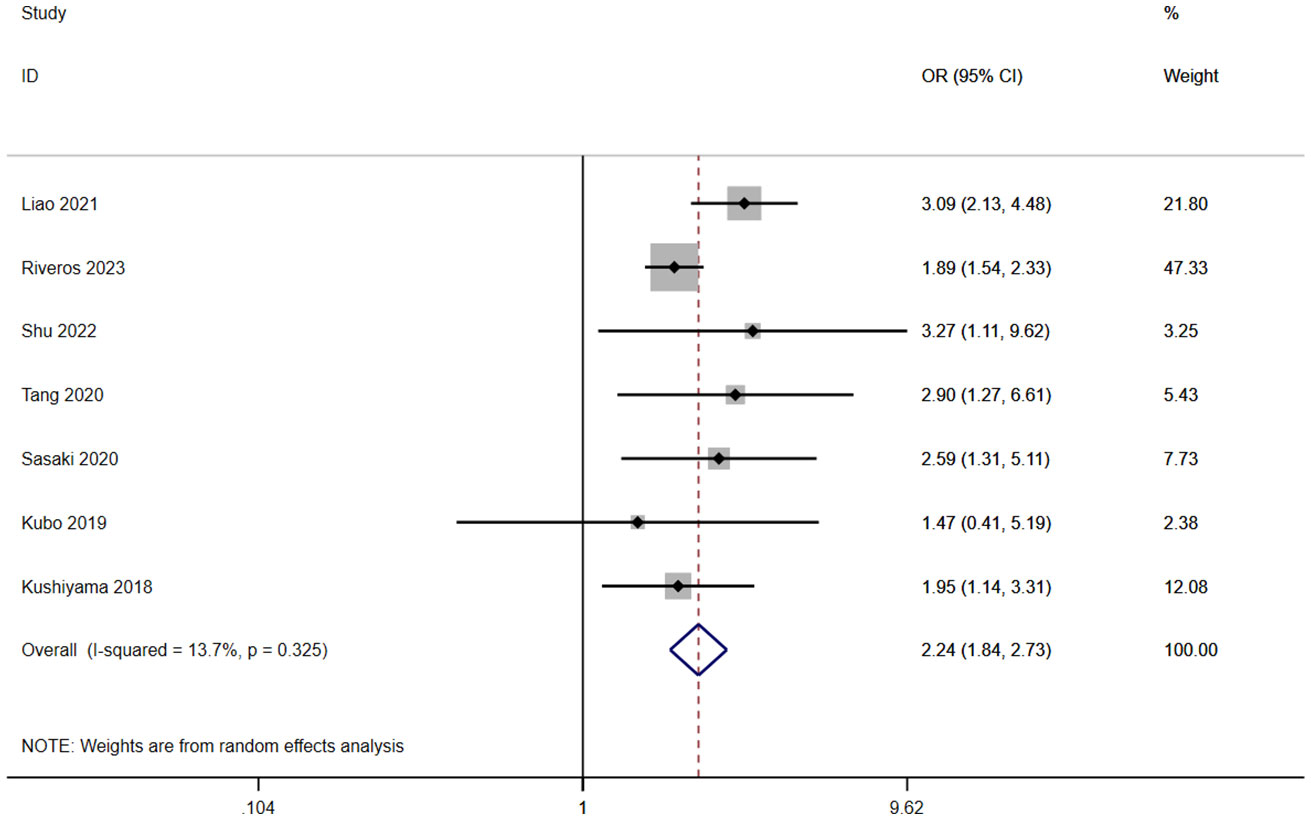
A total of seven studies involving 10,255 patients reported a relationship between the GNRI and CD grades in patients with solid cancer. According to a random effects model (I2 = 13.7%, p = 0.325), the GNRI was also significantly relevant to CD grades (OR=2.24, 95%CI 1.84–2.73, z=8.01, p<0.001) (Figure 4). It indicated that the patients with a low GNRI had a higher risk of suffering CD grades≥2 complications than those with a high GNRI.
3.5 GNRI and infection
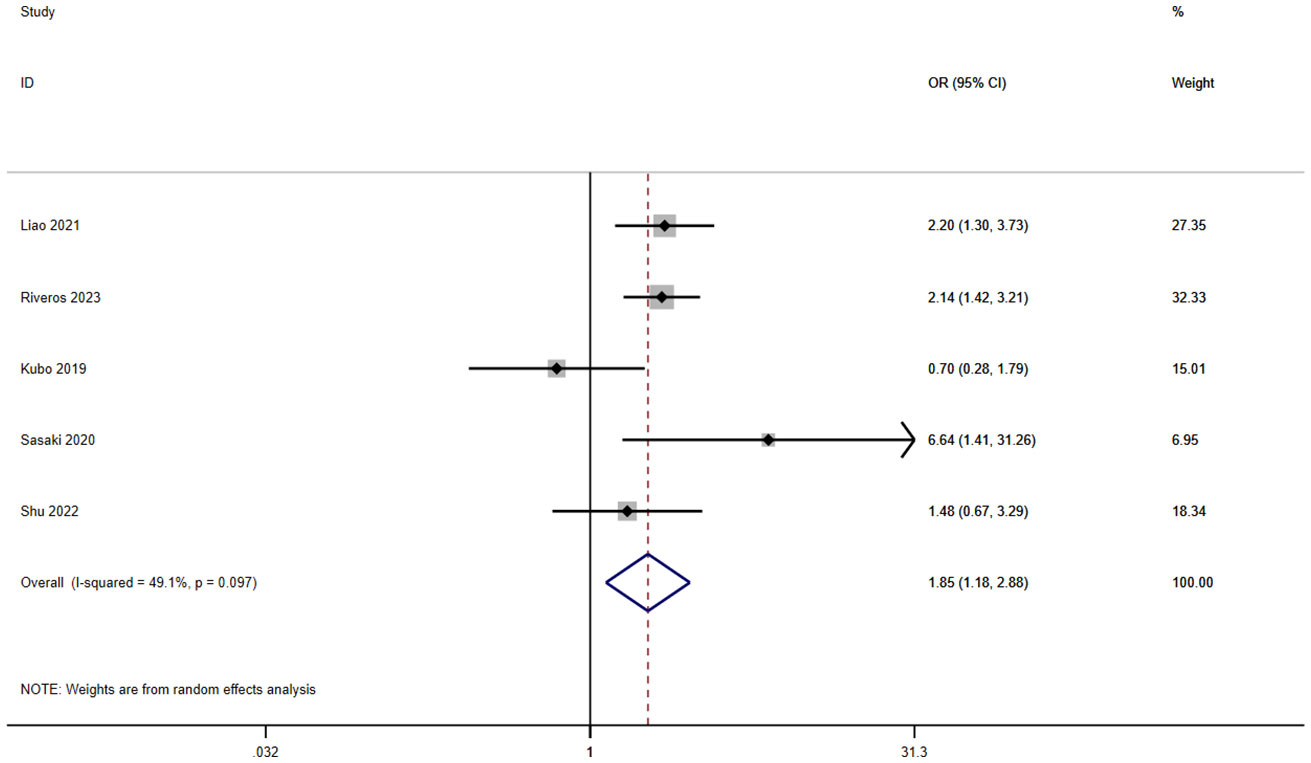
A total of six studies involving 9,718 patients reported a relationship between the GNRI and infection in patients with solid cancer. According to a random effects model (I2 = 49.1%, p = 0.097), the GNRI was also significantly relevant to infection (OR=1.85, 95%CI 1.18–2.88, z=2.70, p=0.007) (Figure 5). It shows that the patients with a low GNRI had a higher risk of suffering infection than those with a high GNRI.
3.6 GNRI and ileus
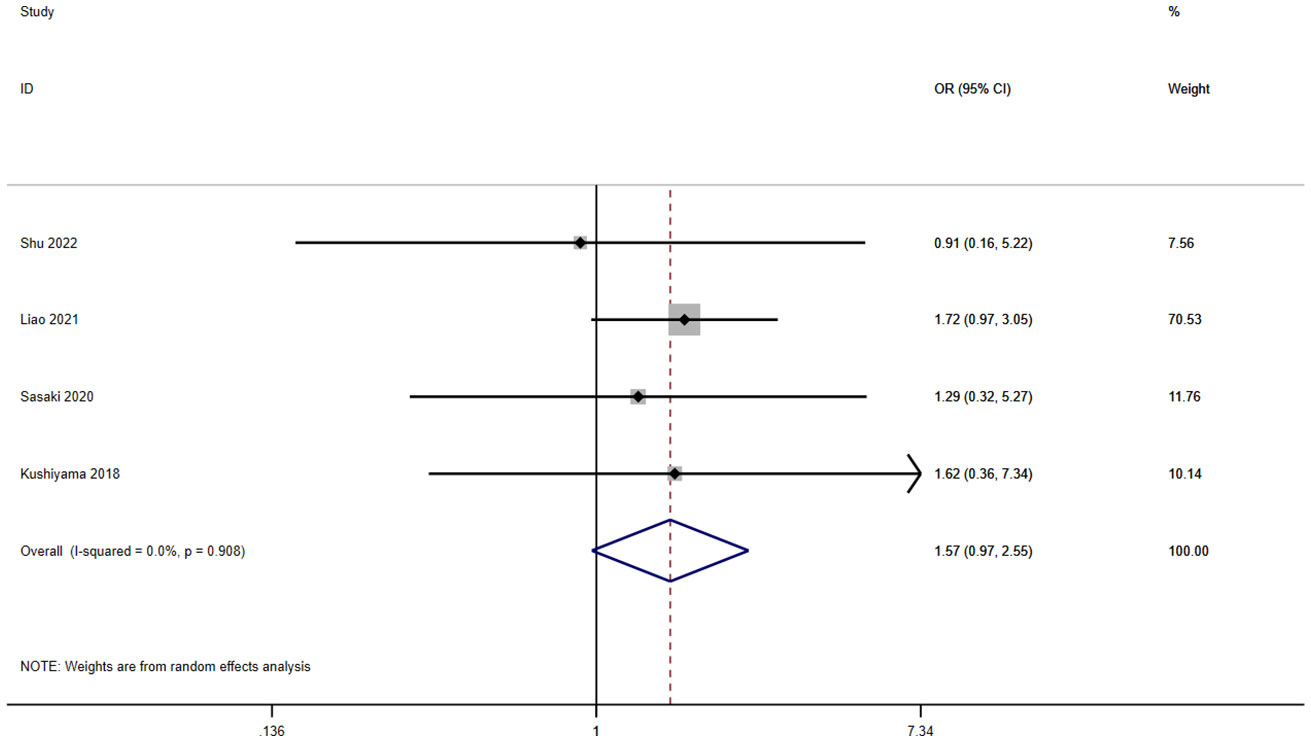
A total of five studies involving 1,963 patients reported a relationship between the GNRI and ileus in patients with solid cancer. According to a random effects model (I2 = 0.0%, p = 0.908), the GNRI had no relevant to ileus (OR=1.57, 95%CI 0.97–2.55, z=1.85, p=0.065) (Figure 6).
3.7 GNRI and leakage
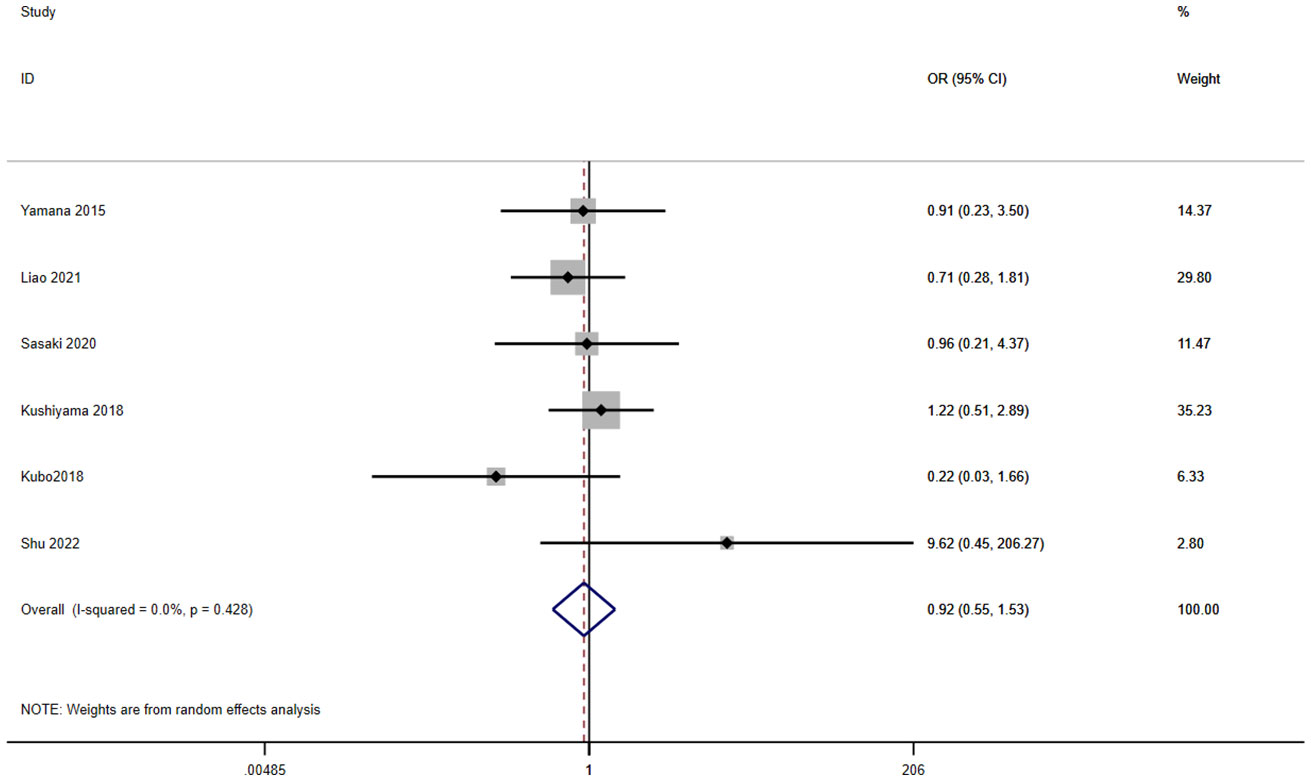
A total of eight studies involving 2,325 patients reported a relationship between the GNRI and leakage in patients with solid cancer. According to a random effects model (I2 = 0.0%, p = 0.428), the GNRI had no relevant to leakage (OR=0.92, 95%CI 0.55–1.53, z=0.33, p=0.741) (Figure 7).
3.8 GNRI and respiratory complications
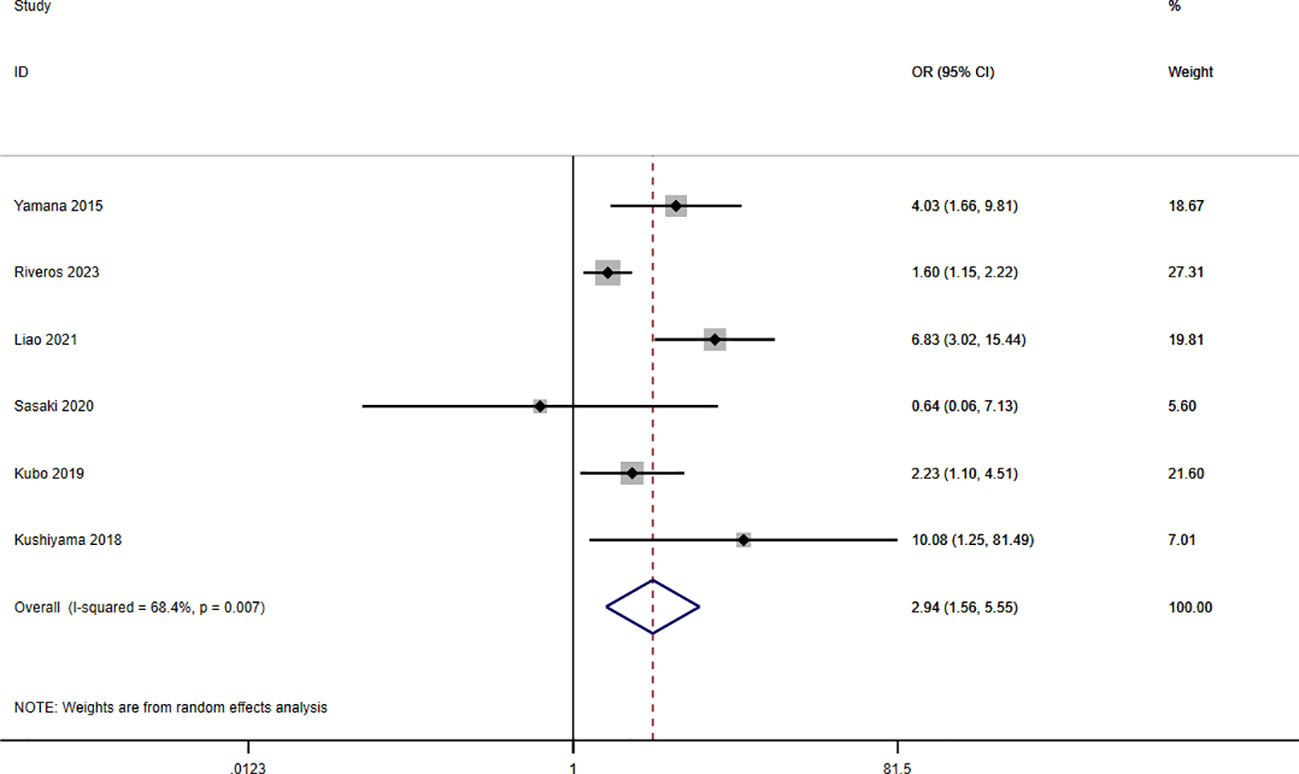
A total of seven studies involving 10,092 patients reported a relationship between the GNRI and respiratory complications in patients with solid cancer. Meta-analysis results from a random effects model (I2 = 68.4%, p = 0.007) evinced that GNRI was also significantly correlated with respiratory complications (OR = 2.94, 95%CI: 1.56-5.55, z=3.31, p=0.001) (Figure 8). That is, patients with low GNRI had a higher risk of suffered from respiratory complications than those with a high GNRI. We performed a further subgroup analysis based on sample size (Figure 9A), country (Figure 9B), cut off value of GNRI (Figure 9C), and cancer site (Figure 9D). The results revealed that the heterogeneity mainly from the sample size, cut off value and cancer site. It quite stable in country.
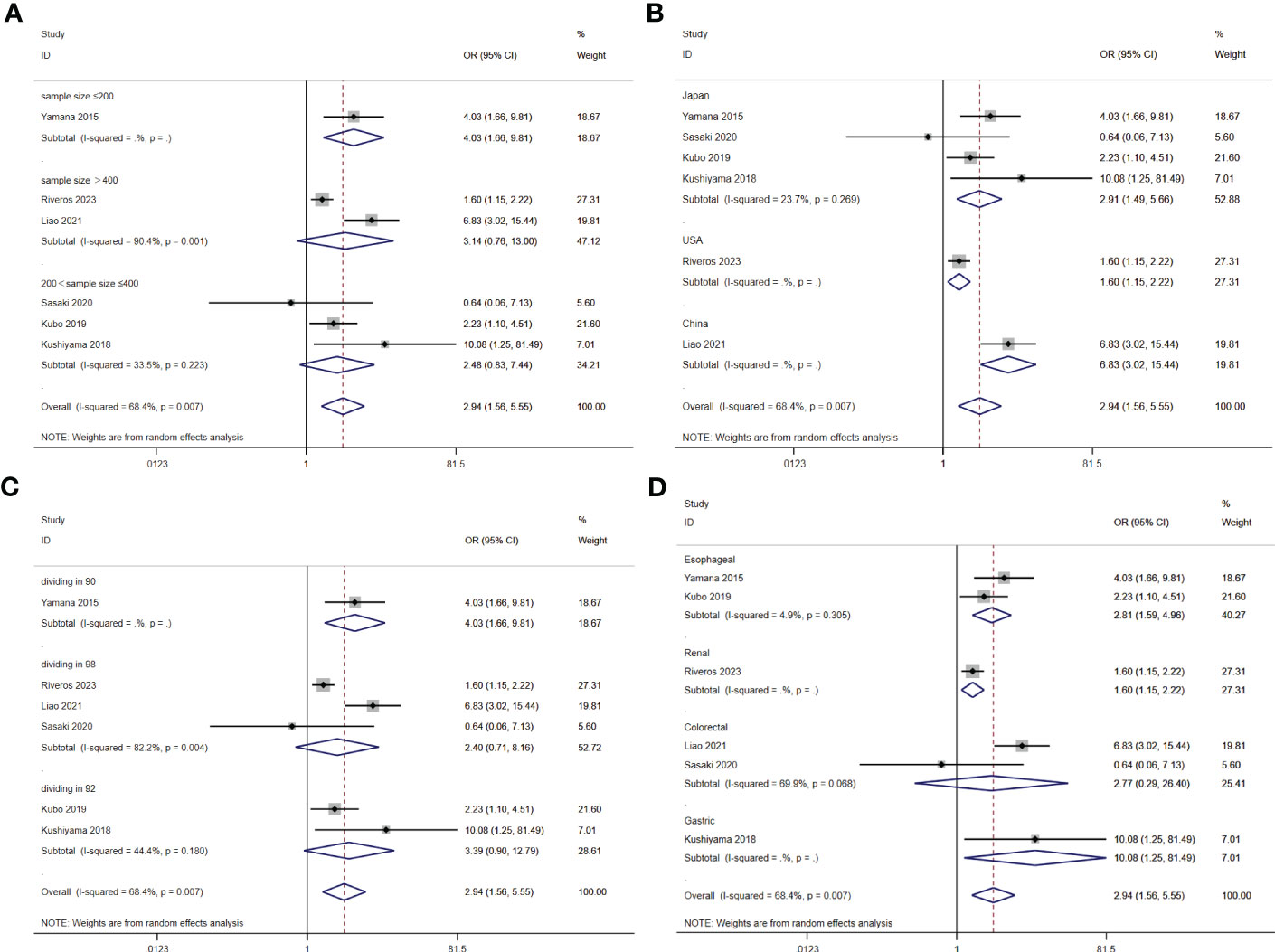
Figure 9 Stratified analysis for the meta-analysis with respiratory complication by sample size (A), country (B), cut off value of GNRI (C), and cancer site (D).
3.9 Sensitivity analyses
Sensitivity analysis was performed to analysis the robustness of our results by removing each individual included study. Omitting any of the included studies did not change the combined meta-analysis effect of GNRI on the ORs for postoperative complication, CD grades≥2, infection or respiratory complications (Figures 10A–D. That is to say, our findings were robust across sensitivity analyses.
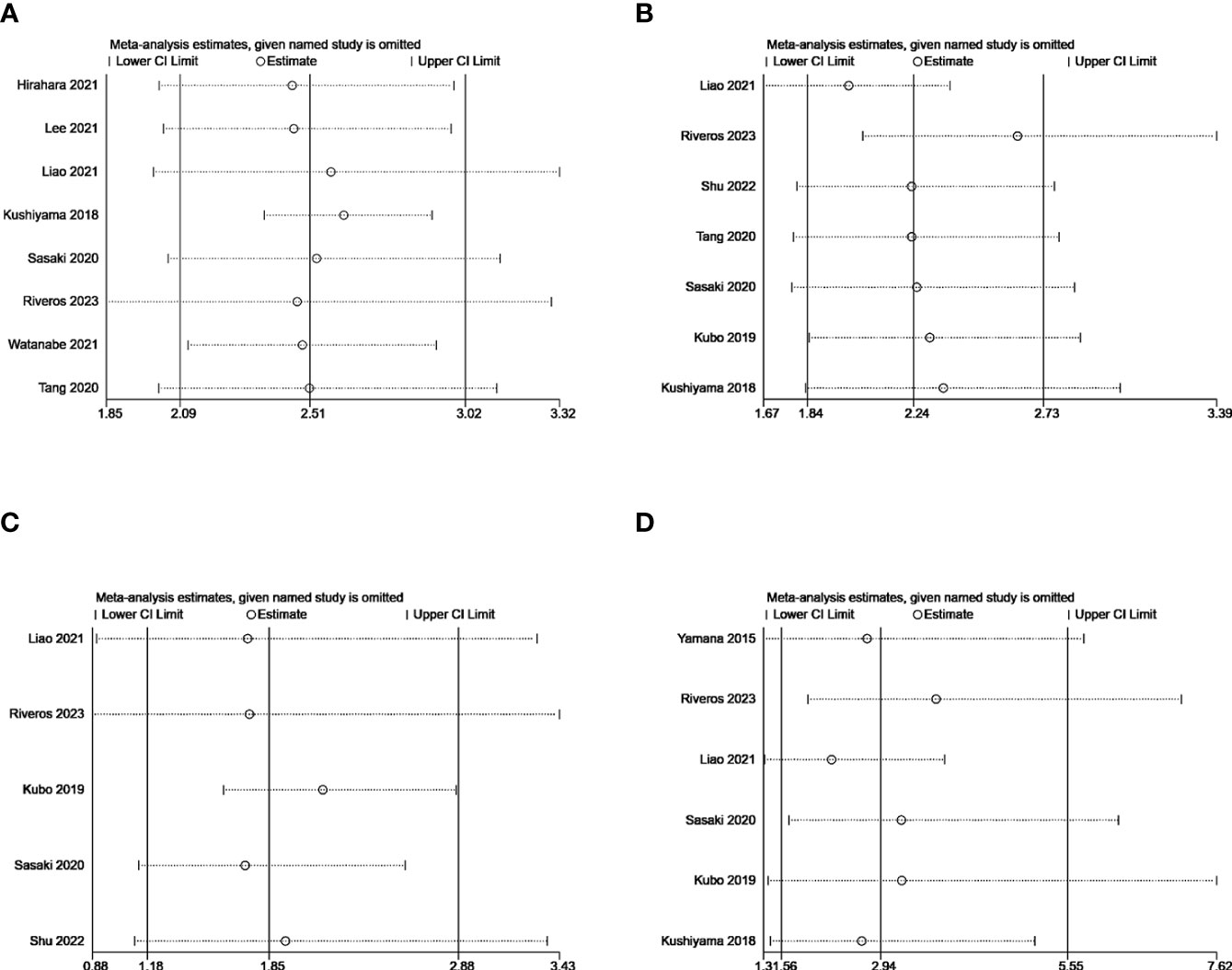
Figure 10 Sensitivity analysis for the correlation of GNRI with postoperative complication (A), CD grades≥2 (B), infection (C), and respiratory complications (D).
3.10 Publication bias
In the meta-analysis for postoperative complication, no publication bias was found by Begg’s test (p = 0.063), or by Egger’s test (p =0.861). Publication bias was not examined in the other meta-analysis, since the included study number was less than ten.
4 Discussion
Geriatric Nutritional Risk Index (GNRI), a novel indicator for evaluating the nutritional risk of elderly medical patients, was first introduced by Bouillanne et al. in 2005 and has been widely adopted since then. The GNRI is a concise and precise screening instrument that relies solely on objective components, such as serum albumin levels, height, and weight measurements. These parameters are readily obtained from laboratory data on a regular basis. The GNRI calculation formula is expressed as follows: GNRI= (1.489 × serum albumin level, g/L) + (41.7 × actual body weight/ideal body weight, kg). GNRI has been proposed as a prognostic factor for various cancers (26). Further studies in oncology have demonstrated that GNRI can also serve as an effective prognostic index for patients with diverse malignancies, not limited to the elderly population (27), but the predictive value of GNRI remains controversial and inconsistent across studies (28, 29). The current literature on the association between GNRI and postoperative complications in solid tumor patients is limited, and the predictive accuracy of GNRI for such complications remains uncertain. Therefore, the objective of this meta-analysis is to investigate the influence of GNRI on postoperative complications in patients with solid tumors. To our knowledge, no previous meta-analysis has been conducted on this subject.
In this study, we included a total of 13 studies involving 12,170 patients with solid cancer. Our findings revealed that GNRI is an independent factor that influences the occurrence of complications in solid cancer patients. Through a stratified meta-analysis, we observed a significant association between low GNRI and postoperative complications, despite variations in country, sample size, GNRI cut-off value, and cancer site among different groups. The consistent results obtained from sensitivity and subgroup analyses underscore the reliability and robustness of our findings. Moreover, there was no evidence of publication bias in the meta-analysis of postoperative complications. Furthermore, we conducted additional investigations to explore the relationship between GNRI and major complications. The results demonstrated that GNRI independently influenced the occurrence of CD grads ≥2, infection, ileus, and respiratory complications in patients with solid cancer. However, no statistical association was observed between low GNRI and leakage.
Based on our study findings, we can conclude that GNRI holds significant clinical value as a practical indicator for predicting postoperative complications in patients with solid cancer. The consistent association between low GNRI and complications such as CD grades ≥2, infection, ileus, and respiratory complications suggests that GNRI can be an effective tool in clinical practice for assessing and managing the risk of postoperative complications in this patient population. Malnutrition is commonly observed in cancer patients and has been linked to both the onset and progression of the disease. Moreover, elderly patients are particularly susceptible to nutritional deficiencies, which can lead to suboptimal treatment outcomes and reduced survival rates (27, 30, 31). Malnutrition can increase the incidence of postoperative complications in cancer patients and have a negative impact on long-term survival. In a study conducted by Shinsuke Kanekiyo et al. (32), it was demonstrated that malnutrition in patients with esophageal cancer undergoing esophagectomy could lead to heightened postoperative complications and mortality. As an index for assessing the nutritional risk of elderly patients, GNRI is primarily linked to body weight and serum albumin levels, which may impact the occurrence of postoperative complications in cancer patients. On one hand, it is well-established that body weight is closely associated with the occurrence of postoperative complications in cancer patients (8). It is well known that our body needs sufficient energy and nutrients to heal after surgery, and inadequate nutrition can weaken the immune system and impair wound healing. However, malnutrition and even cachexia with weight loss as the main manifestation often appear in cancer patients, resulting in poor postoperative prognosis (33). Shu Aoyama et al.’s study suggested that maintaining weight during neoadjuvant chemotherapy may help reduce the risk of postoperative infectious complications (24).This highlights keeping the weight by proper nutrition and support are crucial during cancer treatment to help manage symptoms and improve outcomes. Meredith C Mason et al. found that preoperative weight loss could increase the risk of postoperative complications, including anastomotic leakage (33). Hence, it is obvious that weight loss is an independent factor for adverse prognosis of cancer patients after surgery, which has been proved by O Hynes et al (34) and Bowen Liu et al (35). For another hand, serum albumin level is also strongly correlated with the incidence of postoperative complications (36). Albumin is a protein produced by the liver and is important for maintaining blood volume and transporting various substances in the body, including medications. Numerous studies have demonstrated a positive correlation between perioperative serum albumin levels and patient outcomes. For instance, preoperative hypoalbuminemia is proved to be a predictor of survival (37),or mortality (38).Low preoperative serum albumin levels have been linked to a higher risk of postoperative infections (30), difficulties in wound healing, and prolonged hospital stays (32). Furthermore, Yong Wang et al.’s study discovered that albumin was an independent risk factor for severe complications among colorectal cancer patients following surgery (33). Hence, both weight and albumin levels have a significant influence on the postoperative prognosis of cancer patients. This demonstrates that the GNRI, which is calculated based on body weight and albumin levels, holds predictive value for the occurrence of postoperative complications in solid tumor patients.
In addition to GNRI, commonly used nutritional screening tools for elderly hospitalized patients currently include Subjective Global Assessment (SGA), Mini Nutritional Assessment (MNA), MNA-short form(MNA-SF), Malnutrition Universal Screening Tool (MUST), Simplified Nutritional Appetite Questionnaire (SNAQ), and others (39). SGA is a multidimensional nutritional assessment tool that does not use a numerical scoring system and relies on subjective judgments from professionals, making it less accurate due to variations in different evaluators’ subjectivity (40). MNA has poor specificity for evaluating the nutritional status of hospitalized elderly patients (41, 42) and contains subjective questions that are more suitable for community-living elderly patients (43), potentially leading to overdiagnosis of malnutrition in frail elderly patients and lacking the ability to predict future malnutrition (39). Compared to MNA, MNA-SF requires less than 5 minutes for evaluation and has higher sensitivity and specificity (44). MUST has similar reliability to MNA in screening nutritional risk in elderly populations, with less subjectivity, but research shows that it cannot predict any postoperative clinical outcomes (45). SNAQ is a reliable and effective tool for evaluating appetite loss and weight loss in elderly patients with liver cirrhosis and can be widely used to identify patients at risk of poor appetite and weight loss, but its evaluation parameters focus on appetite (46). Despite controversy surrounding the prognostic ability of albumin, weight, and height used in calculating GNRI, which can be affected by measurement errors, fluid retention, and acute-phase inflammatory reactions (47, 48), GNRI has the advantage of being specifically designed and cross-validated for predicting disease incidence and mortality in elderly patients. It has good predictive validity in design studies and is therefore more suitable for classifying the nutritional status of hospitalized elderly patients and identifying nutrition-related complications (49).
However, it is important to acknowledge the limitations of this study. Firstly, only one included study was a multicenter retrospective study, and the overall number of samples and studies was relatively small. Further investigation through prospective randomized controlled trials is necessary to explore and assess the effectiveness of the GNRI in predicting postoperative complications among patients with solid cancers. Additionally, since the enrolled studies were primarily from Asian countries, the applicability and value of GNRI in other countries still require investigation. Despite these limitations, the available evidence from this meta-analysis confirms the significant predictive value of GNRI for postoperative complications in patients with solid cancers.
Author contributions
WL: Writing – original draft, Writing – review & editing. ML: Writing – review & editing. SL: Investigation, Writing – original draft. XH: Formal analysis, Investigation, Writing – original draft. YL: Conceptualization, Funding acquisition, Methodology, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by Nursing Clinical Research “Climbing” Program of the First Affiliated Hospital of Guangxi Medical University (YYZS2020026). Excellent nursing talents No.202203.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA: Cancer J Clin (2023) 73(1):17–48. doi: 10.3322/caac.21763
2. Villet R. The surgery and surgeons of tomorrow in the treatment of cancer. J visceral Surg (2021) 158(6):459–61. doi: 10.1016/j.jviscsurg.2021.11.011
3. Warps AK, Tollenaar R, Tanis PJ, Dekker JWT. Postoperative complications after colorectal cancer surgery and the association with long-term survival. Eur J Surg Oncol (2022) 48(4):873–82. doi: 10.1016/j.ejso.2021.10.035
4. Gonçalves D, Henriques R, Santos LL, Costa RS. On the predictability of postoperative complications for cancer patients: A Portuguese cohort study. BMC Med Inf decision making (2021) 21(1):200. doi: 10.1186/s12911-021-01562-2
5. Ojima T, Hayata K, Kitadani J, Takeuchi A, Yamaue H. Risk factors of postoperative intra-abdominal infectious complications after robotic gastrectomy for gastric cancer. Oncology (2022) 100(11):583–90. doi: 10.1159/000526920
6. Xie H, Tang S, Wei L, Gan J. Geriatric nutritional risk index as a predictor of complications and long-term outcomes in patients with gastrointestinal Malignancy: A systematic review and meta-analysis. Cancer Cell Int (2020) 20(1):530. doi: 10.1186/s12935-020-01628-7
7. Fukuda Y, Yamamoto K, Hirao M, Nishikawa K, Maeda S, Haraguchi N, et al. Prevalence of malnutrition among gastric cancer patients undergoing gastrectomy and optimal preoperative nutritional support for preventing surgical site infections. Ann Surg Oncol (2015) 22(Suppl 3):S778–85. doi: 10.1245/s10434-015-4820-9
8. Tang S, Xie H, Kuang J, Gao F, Gan J, Ou H. The value of geriatric nutritional risk index in evaluating postoperative complication risk and long-term prognosis in elderly colorectal cancer patients. Cancer Manage Res (2020) 12:165–75. doi: 10.2147/cmar.S234688
9. Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis (2001) 38(6):1251–63. doi: 10.1053/ajkd.2001.29222
10. Steiber AL, Kalantar-Zadeh K, Secker D, McCarthy M, Sehgal A, McCann L. Subjective global assessment in chronic kidney disease: A review. J Renal Nutr (2004) 14(4):191–200.
11. Valentini A, Federici M, Cianfarani MA, Tarantino U, Bertoli A. Frailty and nutritional status in older people: the mini nutritional assessment as a screening tool for the identification of frail subjects. Clin Interventions Aging (2018) 13:1237–44. doi: 10.2147/cia.S164174
12. Lu W, Shen J, Zou D, Li P, Liu X, Jian Y. Predictive role of preoperative geriatric nutritional risk index for clinical outcomes in surgical gastric cancer patients: A meta-analysis. Front Surg (2022) 9:1020482. doi: 10.3389/fsurg.2022.1020482
13. Hayama T, Hashiguchi Y, Ozawa T, Watanabe M, Fukushima Y, Shimada R, et al. The preoperative geriatric nutritional risk index (Gnri) is an independent prognostic factor in elderly patients underwent curative resection for colorectal cancer. Sci Rep (2022) 12(1):3682. doi: 10.1038/s41598-022-07540-6
14. Kubo N, Sakurai K, Tamura T, Toyokawa T, Tanaka H, Muguruma K, et al. The impact of geriatric nutritional risk index on surgical outcomes after esophagectomy in patients with esophageal cancer. Esophagus (2019) 16(2):147–54. doi: 10.1007/s10388-018-0644-6
15. Zhang Q, Zhang L, Jin Q, He Y, Wu M, Peng H, et al. The prognostic value of the Gnri in patients with stomach cancer undergoing surgery. J Pers Med (2023) 13(1):155. doi: 10.3390/jpm13010155
16. Shen F, Ma Y, Guo W, Li F. Prognostic value of geriatric nutritional risk index for patients with non-small cell lung cancer: A systematic review and meta-analysis. Lung (2022) 200(5):661–9. doi: 10.1007/s00408-022-00567-6
17. Riveros C, Chalfant V, Bazargani S, Bandyk M, Balaji KC. The geriatric nutritional risk index predicts complications after nephrectomy for renal cancer. Int Braz J urol (2023) 49(1):97–109. doi: 10.1590/S1677-5538.IBJU.2022.0380
18. Shu W, Tao W, Chunyan H, Jie F, Yuan L, Yan X, et al. Preoperative nutritional evaluation of prostate cancer patients undergoing laparoscopic radical prostatectomy. PloS One (2022) 17(2):e0262630. doi: 10.1371/journal.pone.0262630
19. Watanabe D, Miura K, Yamashita A, Minowa T, Uehara Y, Mizushima S, et al. A comparison of the predictive role of the geriatric nutritional risk index and immunonutritional parameters for postoperative complications in elderly patients with renal cell carcinoma. J Invest Surg (2021) 34(10):1072–7. doi: 10.1080/08941939.2020.1762808
20. Liao CK, Chern YJ, Hsu YJ, Lin YC, Yu YL, Chiang JM, et al. The clinical utility of the geriatric nutritional risk index in predicting postoperative complications and long-term survival in elderly patients with colorectal cancer after curative surgery. Cancers (2021) 13(22):5852. doi: 10.3390/cancers13225852
21. Lee B, Cho JY, Han H-S, Yoon Y-S, Lee HW, Lee JS, et al. A scoring system to predict the risk of postoperative complications after laparoscopic liver resection in elderly hepatocellular carcinoma patients. Ann hepato-biliary-pancreatic Surg (2021) 25(Suppl 1):S73–S. doi: 10.14701/ahbps.LV-OP-4-7
22. Hirahara N, Tajima Y, Fujii Y, Kaji S, Kawabata Y, Hyakudomi R, et al. Prediction of postoperative complications and survival after laparoscopic gastrectomy using preoperative geriatric nutritional risk index in elderly gastric cancer patients. Surg Endosc (2021) 35(3):1202–9. doi: 10.1007/s00464-020-07487-7
23. Sasaki M, Miyoshi N, Fujino S, Ogino T, Takahashi H, Uemura M, et al. The geriatric nutritional risk index predicts postoperative complications and prognosis in elderly patients with colorectal cancer after curative surgery. Sci Rep (2020) 10(1):10744. doi: 10.1038/s41598-020-67285-y
24. Kushiyama S, Sakurai K, Kubo N, Tamamori Y, Nishii T, Tachimori A, et al. The preoperative geriatric nutritional risk index predicts postoperative complications in elderly patients with gastric cancer undergoing gastrectomy. In Vivo (2018) 32(6):1667–72. doi: 10.21873/INVIVO.11430
25. Yamana I, Takeno S, Shibata R, Shiwaku H, Maki K, Hashimoto T, et al. Is the geriatric nutritional risk index a significant predictor of postoperative complications in patients with esophageal cancer undergoing esophagectomy? Eur Surg Res (2015) 55(1-2):35–42. doi: 10.1159/000376610
26. Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, et al. Geriatric nutritional risk index: A new index for evaluating at-risk elderly medical patients. Am J Clin Nutr (2005) 82(4):777–83. doi: 10.1093/ajcn/82.4.777
27. Lv GY, An L, Sun DW. Geriatric nutritional risk index predicts adverse outcomes in human Malignancy: A meta-analysis. Dis Markers (2019) 2019:4796598. doi: 10.1155/2019/4796598
28. Yan D, Shen Z, Zhang S, Hu L, Sun Q, Xu K, et al. Prognostic values of geriatric nutritional risk index (Gnri) and prognostic nutritional index (Pni) in elderly patients with diffuse large B-cell lymphoma. J Cancer (2021) 12(23):7010–7. doi: 10.7150/jca.62340
29. Ruan GT, Zhang Q, Zhang X, Tang M, Song MM, Zhang XW, et al. Geriatric nutrition risk index: prognostic factor related to inflammation in elderly patients with cancer cachexia. J cachexia sarcopenia Muscle (2021) 12(6):1969–82. doi: 10.1002/jcsm.12800
30. Hong H, Wang Q, Li J, Liu H, Meng X, Zhang H. Aging, cancer and immunity. J Cancer (2019) 10(13):3021–7. doi: 10.7150/jca.30723
31. Durán Alert P, Milà Villarroel R, Formiga F, Virgili Casas N, Vilarasau Farré C. Assessing risk screening methods of malnutrition in geriatric patients: mini nutritional assessment (Mna) versus geriatric nutritional risk index (Gnri). Nutricion hospitalaria (2012) 27(2):590–8. doi: 10.1590/s0212-16112012000200036
32. Kanekiyo S, Takeda S, Iida M, Nishiyama M, Kitahara M, Shindo Y, et al. Efficacy of perioperative immunonutrition in esophageal cancer patients undergoing esophagectomy. Nutrition (2019) 59:96–102. doi: 10.1016/j.nut.2018.08.006
33. Mason MC, Garcia JM, Sansgiry S, Walder A, Berger DH, Anaya DA. Preoperative cancer cachexia and short-term outcomes following surgery. J Surg Res (2016) 205(2):398–406. doi: 10.1016/j.jss.2016.06.076
34. Hynes O, Anandavadivelan P, Gossage J, Johar AM, Lagergren J, Lagergren P. The impact of pre- and post-operative weight loss and body mass index on prognosis in patients with oesophageal cancer. Eur J Surg Oncol (2017) 43(8):1559–65. doi: 10.1016/j.ejso.2017.05.023
35. Liu B, Cheng B, Wang C, Chen P, Cheng Y. The prognostic significance of metabolic syndrome and weight loss in esophageal squamous cell carcinoma. Sci Rep (2018) 8(1):10101. doi: 10.1038/s41598-018-28268-2
36. Motamed C, Mariani L, Suria S, Weil G. Serum albumin kinetics in major ovarian, gastrointestinal, and Cervico facial cancer surgery. Int J Environ Res Public Health (2022) 19(6):3394. doi: 10.3390/ijerph19063394
37. Tang Y, Liu Z, Liang J, Zhang R, Wu K, Zou Z, et al. Early post-operative serum albumin level predicts survival after curative nephrectomy for kidney cancer: A retrospective study. BMC Urol (2018) 18(1):111. doi: 10.1186/s12894-018-0427-3
38. Larsen PB, Liest S, Hannani D, Jørgensen HL, Sørensen LT, Jørgensen LN. Preoperative hypoalbuminemia predicts early mortality following open abdominal surgery in patients above 60 Years of age. Scandinavian J Surg (2021) 110(1):29–36. doi: 10.1177/1457496919888598
39. Dent E, Hoogendijk EO, Visvanathan R, Wright ORL. Malnutrition screening and assessment in hospitalised older people: a review. J Nutr Health Aging (2019) 23(5):431–41. doi: 10.1007/s12603-019-1176-z
40. Marshall S, Craven D, Kelly J, Isenring E. A systematic review and meta-analysis of the criterion validity of nutrition assessment tools for diagnosing protein-energy malnutrition in the older community setting (the MACRo study). Clin Nutr (2018) 37(6 Pt A):1902–12. doi: 10.1016/j.clnu.2017.09.022
41. Ranhoff AH, Gjøen AU, Mowé M. Screening for malnutrition in elderly acute medical patients: the usefulness of MNA-SF. J Nutr Health Aging (2005) 9(4):221–5.
42. Neelemaat F, Meijers J, Kruizenga H, van Ballegooijen H, van Bokhorst-de van der Schueren M. Comparison of five malnutrition screening tools in one hospital inpatient sample. J Clin Nurs (2011) 20(15-16):2144–52. doi: 10.1111/j.1365-2702.2010.03667.x
43. Rasmussen HH, Holst M, Kondrup J. Measuring nutritional risk in hospitals. Clin Epidemiol (2010) 2:209–16. doi: 10.2147/CLEP.S11265
44. Dent E, Chapman I, Piantadosi C, Visvanathan R. Screening for malnutrition in hospitalised older people: Comparison of the Mini Nutritional Assessment with its short-form versions. Australas J Ageing (2017) 36(2):E8–E13. doi: 10.1111/ajag.12402
45. Koren-Hakim T, Weiss A, Hershkovitz A, Otzrateni I, Anbar R, Gross Nevo RF, et al. Comparing the adequacy of the MNA-SF, NRS-2002 and MUST nutritional tools in assessing malnutrition in hip fracture operated elderly patients. Clin Nutr (2016) 35(5):1053–8. doi: 10.1016/j.clnu.2015.07.014
46. Wang T, Shen J. Usefulness of simplified nutritional appetite questionnaire (SNAQ) in appetite assessment in elder patients with liver cirrhosis. J Nutr Health Aging (2018) 22(8):911–5. doi: 10.1007/s12603-018-1086-5
47. Li ZE, Lu SB, Kong C, Sun WZ, Wang P, Zhang ST. A prospective comparative study of the MNA-SF and GNRI nutritional screening tools in predicting infectious complications among elderly patients over 70 years undergoing posterior lumbar arthrodesis. Aging Clin Exp Res (2021) 33(7):1947–53. doi: 10.1007/s40520-020-01725-7
48. Hébuterne X, Lemarié E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. JPEN J Parenter Enteral Nutr (2014) 38(2):196–204. doi: 10.1177/0148607113502674
Keywords: geriatric nutritional risk index, postoperative complications, solid cancer, CD grades, meta-analysis
Citation: Liu W, Li M, Lian S, Hou X and Ling Y (2024) Geriatric nutritional risk index as a predictor for postoperative complications in patients with solid cancers: a meta-analysis. Front. Oncol. 14:1266291. doi: 10.3389/fonc.2024.1266291
Received: 24 July 2023; Accepted: 18 January 2024;
Published: 07 February 2024.
Edited by:
Luca Nespoli, University of Milano-Bicocca, ItalyReviewed by:
Stefano Turi, San Raffaele Hospital (IRCCS), ItalyBen Liu, University of Kansas, United States
Copyright © 2024 Liu, Li, Lian, Hou and Ling. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Ling, bGluZ3lpbmcxOTgzQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Weichen Liu
Weichen Liu Ming Li2†
Ming Li2†