- 1Shandong Provincial Qianfoshan Hospital, Shandong University, Jinan, China
- 2Department of Gastrointestinal Surgery, Shandong Provincial Third Hospital, Shandong University, Jinan, China
- 3Department of General Surgery, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Jinan, China
Hepatocellular carcinoma is a rather common malignant tumor. Most patients with hepatocellular carcinoma receive their diagnosis at an advanced stage, at which surgical resection is no longer appropriate. A growing body of research has demonstrated the value of convention therapy for patients with intermediate-stage hepatocellular carcinoma, while specific application protocols and treatment guidelines are not well developed. Emerging clinical researches suggest that a tyrosine kinase inhibitor in combination with an immune checkpoint inhibitor is a reasonable strategy for unresectable hepatocellular carcinoma. However, there are relatively few reports on the efficacy of apatinib and camrelizumab in the treatment of hepatocellular carcinoma. We were able to successfully remove one patient’s hepatocellular carcinoma after 8 cycles of conversion therapy with apatinib (250 mg orally every day) and camrelizumab (200 mg intravenously every 2 weeks). The patient continued to receive the same dose of 16 cycles of apatinib and camrelizumab after hepatectomy. By the time of this study, the patient has completed 18 months of follow-up, and no tumor recurrence or metastasis was found in tumor markers and imaging examinations. Apatinib in combination with camrelizumab is an effective therapy for the treatment of advanced hepatocellular carcinoma, and surgical resection after this conversion therapy may provide patients with long-term oncological benefits. However, this requires more samples to validate the conclusion.
1 Introduction
One of the most prevalent malignant tumors in the world is primary liver cancer (1, 2), of which the most prevalent type is hepatocellular carcinoma (HCC) (3). With a 5-year survival rate of more than 70%, surgery is the most efficient curative therapy for HCC in its early stage (4–7). Unfortunately, the majority of patients have advanced disease when they first appear (8, 9), making them unsuitable for surgery and giving them a terrible prognosis with a 5-year survival rate of only 10%–20% (10). A significant medical need is the creation of novel medicines for the efficient management of advanced HCC. As a result, the idea of conversion therapy—which refers to turning an HCC that cannot be surgically removed into one that can—was created (11). Conversion therapy has a number of options, including systemic and localized treatments. On the one hand, systemic therapy can be used alone to treat HCC, and the most popular form of treatment is the admixture of two medications: tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs). On the other hand, local therapy usually refers to the treatment of hepatocellular carcinoma using radiation therapy, transarterial chemoembolization (TACE), hepatic arterial infusion chemotherapy (HAIC), and selective internal radiation therapy (SIRT), and it is often used in conjunction with one or both of the TKIs and ICIs. Although conversion therapy is of great interest in the treatment of HCC, its application and treatment guidelines are not well developed. As we know, apatinib is a highly selective small-molecule tyrosine kinase inhibitor of vascular endothelial growth factor receptor 2 (VEGFR-2). And results from phase II clinical trials have shown that apatinib as a first-line treatment has potential survival benefits for Chinese patients with advanced HCC (12). Camrelizumab is a humanized PD-1 monoclonal antibody that has a high affinity with PD-1, high occupancy of circulating T-lymphocyte receptors (85% at a dose of 200 mg) (13), and a good tolerance and curative effect on a variety of solid tumors, including HCC (14). Currently, more and more studies have proved the clinical value of TKIs plus ICIs in patients with unresectable HCC, while apatinib combined with camrelizumab in patients with advanced HCC, only a few studies report this issue. Thus, we reported a case that apatinib and camrelizumab were used to treat a patient with unresectable HCC. The results show that conversion therapy with apatinib and camrelizumab is a safe and efficient method for resecting originally unresectable HCC, which may improve the patient’s long-term survival.
2 Case presentation
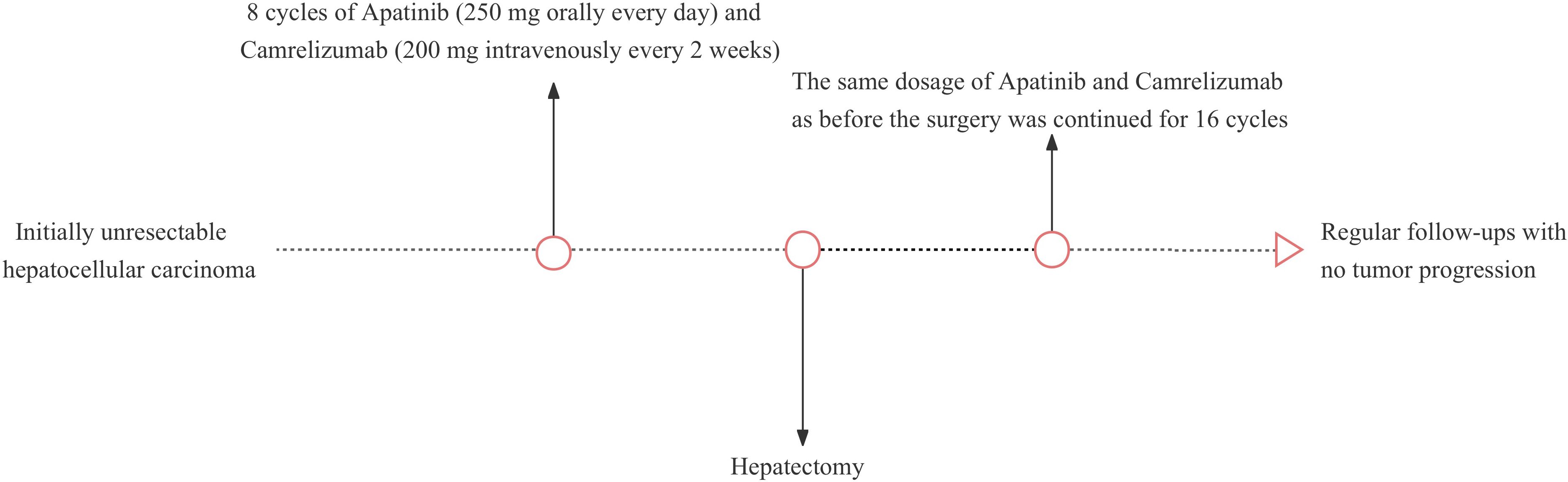
An adult patient with right upper abdominal pain was admitted to our hospital. The patient did not undergo antiviral therapy despite having a history of liver cirrhosis caused by the hepatitis B virus for more than 2 years. The albumin concentration was 47.2 (baseline range, 35-50) g/L. The findings of the tumor marker test revealed an AFP level of 21.32 (baseline range, 0-7) ng/ml. A dynamic contrast-enhanced abdominal magnetic resonance imaging (MRI) scan revealed a lesion of approximately 19.1x14.6x10.4cm in size was seen in the right lobe of the liver. Besides, the distal end of the right hepatic vein and the right branch of the portal vein are poorly visualized, which is consistent with invasion by HCC (Figures 1A, B). The patient’s HCC was stage IIIA according to the China Liver Cancer staging (CNLC), equivalent to Barcelona Cancer Liver Clinic staging (BCLC) stage C. It is advised that conversion treatment be carried out after the multidisciplinary team (MDT) discussion. As a result, the patient had 8 cycles of conversion therapy with apatinib (250 mg orally every day) and camrelizumab (200 mg intravenously every 2 weeks). The patient also underwent routine hepatoprotective and anti-hepatitis B virus treatments at the same time. We thoroughly examined the patient after the final conversion therapy before the operation. The Eastern Cooperative Oncology Group- Performance Status (ECOG-PS) rating was zero. A liver function test revealed a 38.3 g/L albumin level and a 10.1 (baseline range, 3.42-17.1) umol/l total bilirubin level. Prothrombin time (PT) was 11.8 (baseline range, 11-13) seconds. Thus, the patient’s cirrhosis was categorized as Child-Pugh class A. Following the final conversion treatment, the abdominal contrast-enhanced computed tomography (CT) revealed tumor in the right lobe of the liver shrinks to 8.6x6.9cm (Figure 1C) and CT angiography (CTA) showed approximately normal hepatic artery blood flow (Figure 1D). No severe adverse effects were noticed during the conversion therapy. These findings led to the conclusion that the HCC had been downstaged to CNLC stage IB, equivalent to BCLC stage A. After the MDT meeting, we decided to operate on this patient and asked that the patient stopped taking medications for four weeks. Preoperative laboratory findings for the patient revealed a level of albumin of 40.2 g/L, a total bilirubin of 8.8 umol/l, and a PT of 11s. The ECOG-PS rating was zero. As a result, the cirrhosis was still given the Child-Pugh class A diagnosis. We further verified by abdominal contrast-enhanced CT that there was no appreciable difference between the preoperative and post-conversion therapy abdominal CTs. This patient also had the indocyanine green retention rate at 15 min (ICG-R15) and the three-dimensional reconstruction of the liver done. ICG-R15 = 7.6%. The liver’s three-dimensional reconstruction (Figures 1E, F) revealed a future liver remnant (FLR)/standard liver volume (SLV)=49.6%. The patient’s condition met the requirements for surgery. So, following a preoperative discussion, we conducted a hepatectomy on this patient. The postoperative pathology revealed lymphocyte infiltration, hyperplasia of the surrounding fibrous tissue, and coagulative necrosis of all target lesions (Figure 1G). Following surgery, the patient recovered without incident, and no evident problems were discovered. The patient continued to receive the same dose of 16 cycles of apatinib together with camrelizumab 4 weeks after the hepatectomy. By the time of this study, the patient has completed 18 months of follow-up, and no tumor recurrence or metastasis was found in tumor markers and imaging examinations. The patient’s latest follow-up dynamic contrast-enhanced abdominal MRI showed that encapsulated fluid seen at the site of liver surgery with no significant enhancement (Figures 1H, I). The treatment history of this patient is detailed in Figure 2.
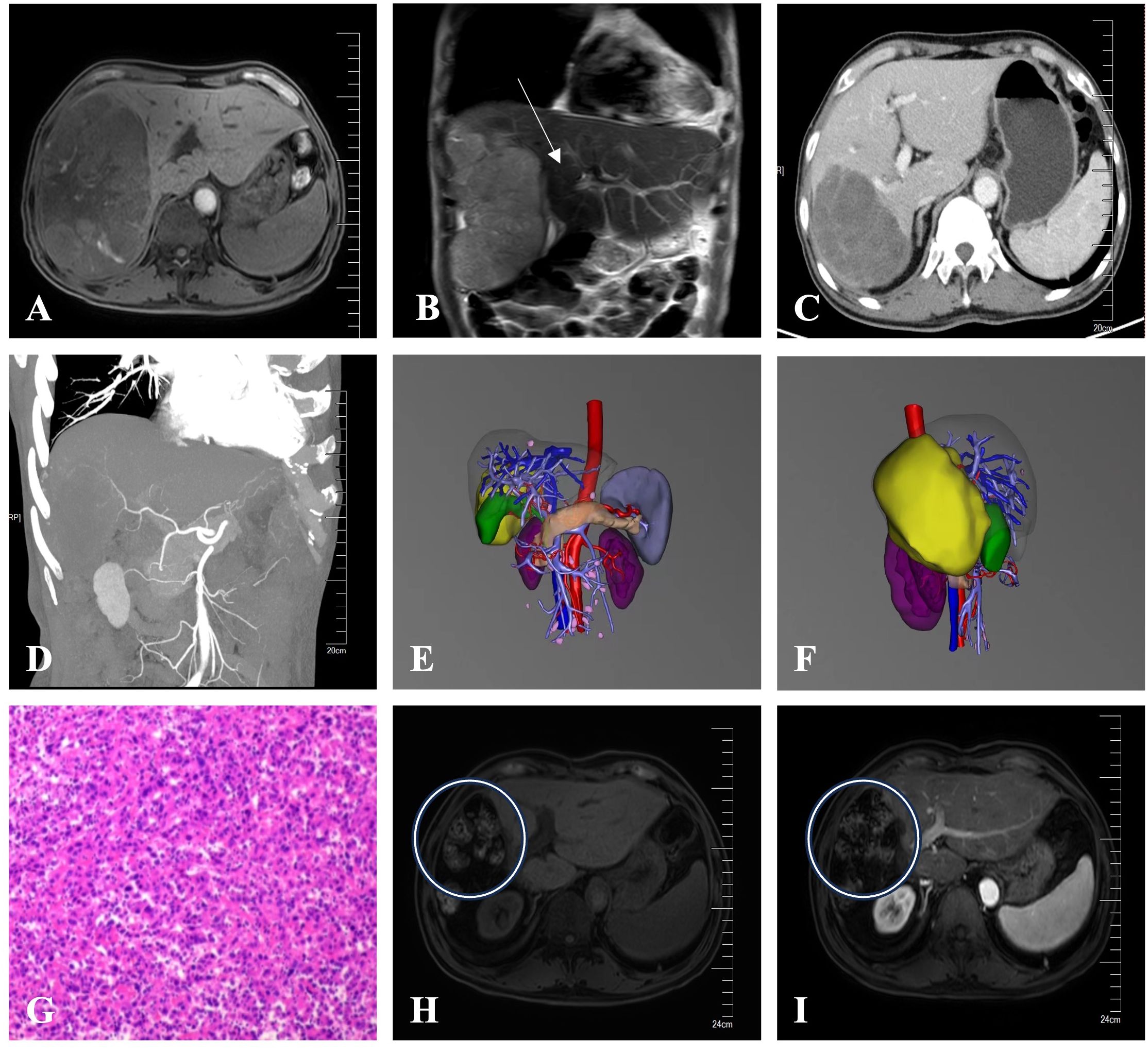
Figure 1 (A) Before conversion therapy, a dynamic contrast-enhanced abdominal MRI showed a tumor in the right lobe of the liver that was roughly 19.1x14.6x10.4cm in size. (B) Before conversion therapy, an abdominal coronal MRI showed the distal end of the right branch of the portal vein are poorly visualized, which is consistent with invasion by HCC (white arrow). (C) The tumor in the right lobe of the liver shrank to 8.6x6.9cm in size after conversion therapy, according to an abdominal contrast-enhanced CT scan. (D) CTA revealed roughly normal blood flow across the hepatic arteries. (E, F) The liver’s three-dimensional reconstruction from the positive and right-side views showed the location of the tumor (the yellow part). (G) The target lesions had coagulative necrosis, hyperplasia of the surrounding fibrous tissue, and lymphocyte infiltration, according to the postoperative pathology (HE staining). (H, I) The patient’s latest dynamic contrast-enhanced abdominal MRI revealed encapsulated fluid (white circles) with no discernible enhancement at the site of liver operation after the hepatectomy.
3 Discussion
3.1 The possibility of apatinib combined with camrelizumab as a conversion therapy
Recently, TKIs and ICIs in patients with unresectable HCC have demonstrated a specific effectiveness profile. It has been reported that systemic therapy, particularly anti-angiogenic drugs combined with immunotherapy, can achieve an objective response rate (ORR) of about 30% and have a median survival time of up to 20 months for patients with advanced or irresectable HCC (15–17). The combination of apatinib and camrelizumab also showed encouraging anticancer activity and a tolerable safety profile in advanced HCC in a phase I study that was begun in October 2016 (18). The combination of apatinib and camrelizumab is effective for the following reasons.
Highly selective tyrosine kinase inhibitor apatinib and program-death receptor 1 (PD-1) inhibitor camrelizumab both work by inhibiting vascular endothelial growth factor 2 (VEGF-2) (19). What’s more, by restoring normalcy to the tumor blood vessels, TKIs are intended to change the hypoxic and immunosuppressive tumor microenvironment (TME) (18). Interferon Gamma (IFN g) + Type 1 T helper (Th1) cells are the predominant populations associated with tumor vascular normalization, and ICIs may increase tumor vascular normalization and activate these cells (20). “Vascular normalization” has the potential to improve therapeutic medication delivery and, more importantly, to reverse immunosuppressive TME by promoting impacted T-cell infiltration into TME, maturation of antigen-presenting cells, and a decrease in immunosuppressive factors (20, 21). TKIs, therefore, improve the effectiveness of ICIs by normalizing the vascular system.
3.2 Methods for evaluating the feasibility of surgery
Morphological and functional examinations were undertaken in order to estimate the therapeutic effect before surgery which consists of the overall assessment, the tumor response to conversion therapy, and major side events. First, the ECOG-PS score can be used to evaluate the whole assessment. The patient in our case complied with the following standards: ECOG-PS rating was zero. Second, the modified response evaluation criteria in solid tumors (mRECIST) standard was created to more accurately assess the response of liver lesions because it has advantages in determining the level of pathological response (22, 23). Therefore, in our report, we evaluated the tumor response to therapy using mRECIST criteria. The patient’s tumor target lesion(s), as seen in the abdomen dynamic contrast-enhanced MRI, was lessened. The patient was determined to have a partial response (PR) based on the mRECIST criteria assessment. Last but not least, potential adverse events were assessed primarily based on the patient’s primary complaint, in addition to an electrocardiogram, chest X-ray film, liver and renal function test, cardiac enzymes, regular blood test, standard coagulation test, and other biochemical indicators (24). No severe adverse effects were noticed during the conversion therapy except palmar-plantar erythrodysesthesia and hypertension (Grade 2). In summary, these are the reasons why we consider apatinib in combination with camrelizumab to be effective.
3.3 Timing of surgery
Giving patients the chance for radical treatment is the significance of conversion therapy. Our opinion is that through postoperative pathological analysis, surgical resection can not only eradicate any residual tumor cells but also direct adjuvant therapy (25). Based on this, we carefully monitored how the patient’s condition changed before performing drastic surgery on him. Active antiviral therapy and hepatoprotective therapy were provided to the patient during the conversion therapy to meet the surgical requirements (26). As a result, the question of when to undergo surgical treatment has emerged for us to ponder.
Few studies have highlighted the best timing to halt medication therapy before surgery for systemic treatments (27). To assure the safety of a hepatectomy, it is typically required to cease using bevacizumab for 4-6 weeks before surgery based on the experience of treating colorectal liver metastases. According to certain literature findings, HCC conversion surgery should be carried out within four weeks following the conclusion of the previous medication cycle (28). Following a thorough examination of the literature, we discovered that PD-1 inhibitors and small-molecule targeted medications should be discontinued at least two weeks before surgery (25). Therefore, we mandated that the patient stop taking their medications four weeks before surgery. In addition, it is well known that a safe hepatectomy should be performed in patients with normal liver function (Child-Pugh A, ICG-R15 retention rate <10%) and that in patients with chronic liver disease or liver parenchymal damage (including liver cirrhosis, severely fatty liver, and chemotherapy-related liver damage), the sufficient FLR/SLV should be >40% (29). The patient’s cirrhosis was determined to be Child-Pugh class A, and his ICG-R15 was 7.6% before surgery. The three-dimensional reconstruction of the liver can be carried out with the use of various digital imaging tools, which is useful for precisely quantifying the remaining functional liver volume (30). After a comprehensive evaluation, we successfully operated on this patient following preoperative consultations.
3.4 Maintenance of postoperative antitumor therapy
It is advised that postoperative adjuvant therapy after R0 resection last for longer than 6 months, with vigilant monitoring occurring every 2-3 months (31). The pharmaceutical dose should be lowered or stopped if severe adverse events or treatment intolerance develop. If there is no recurrence or metastasis in two consecutive imaging tests and tumor markers stay normal, drug discontinuation may be considered (25). As a result, the patient underwent additional 16 cycles of apatinib and camrelizumab following the surgery, during which time he was routinely checked at a nearby hospital every three months. The medication was stopped because there was no evidence of metastasis or recurrence.
4 Conclusion
According to the outcomes of our case, the combination of apatinib and camrelizumab may be a successful conversion therapy for HCC that was initially unresectable. Subsequent surgery after conversion therapy with apatinib and camrelizumab may provide patients with long-term oncological benefits. However, subject to sample size, this finding requires more prospective clinical trials to provide higher-level evidence.
5 Patient perspective
The doctors and nurses in the hospital are kind to patients, communicate with patients fully, protect patients’ privacy, give proper examinations, and perform proper procedures.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
This study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Shandong First Medical University (Shandong Provincial Qianfoshan Hospital) [Number: YXLL-KY-2022(064)]. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
X-LL: Writing – original draft. HT: Supervision, Writing – review & editing. X-ZL: Writing – original draft. Y-FC: Writing – original draft. FL: Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by Jinan Science and Technology Bureau (202225060).
Acknowledgments
The authors thank the patient and his family, and all the clinical technicians’ support for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. (2018) 391:1301–14. doi: 10.1016/S0140-6736(18)30010-2
3. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. (2019) 16:589–604. doi: 10.1038/s41575-019-0186-y
4. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. (2018) 68:723–50. doi: 10.1002/hep.29913
5. Zhang ZM, Lai EC, Zhang C, Yu HW, Liu Z, Wan BJ, et al. The strategies for treating primary hepatocellular carcinoma with portal vein tumor thrombus. Int J Surg. (2015) 20:8–16. doi: 10.1016/j.ijsu.2015.05.009
6. Pons F, Varela M, Llovet JM. Staging systems in hepatocellular carcinoma. HPB (Oxford). (2005) 7:35–41. doi: 10.1080/13651820410024058
7. Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. (1999) 19:329–38. doi: 10.1055/s-2007-1007122
8. Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, et al. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. (2018) 6:e555–e67. doi: 10.1016/S2214-109X(18)30127-X
9. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. (2020) 70:7–30. doi: 10.3322/caac.21590
10. Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. (2015) 385:977–1010. doi: 10.1016/S0140-6736(14)62038-9
11. Hermann RE, Lonsdale D. Chemotherapy, radiotherapy, and hepatic lobectomy for hepatoblastoma in an infant: report of a survival. Surgery. (1970) 68:383–8.
12. Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. (2016) 34:1448–54. doi: 10.1200/JCO.2015.63.5995
13. Markham A, Keam SJ. Camrelizumab: first global approval. Drugs. (2019) 79:1355–61. doi: 10.1007/s40265-019-01167-0
14. Mo H, Huang J, Xu J, Chen X, Wu D, Qu D, et al. Safety, anti-tumour activity, and pharmacokinetics of fixed-dose SHR-1210, an anti-PD-1 antibody in advanced solid tumours: a dose-escalation, phase 1 study. Br J Cancer. (2018) 119:538–45. doi: 10.1038/s41416-018-0100-3
15. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. (2020) 38:2960–70. doi: 10.1200/JCO.20.00808
16. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. (2020) 382:1894–905. doi: 10.1056/NEJMoa1915745
17. Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): A nonrandomized, open-label, phase II trial. Clin Cancer Res. (2021) 27:1003–11. doi: 10.1158/1078-0432.CCR-20-2571
18. Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the checkMate 040 randomized clinical trial. JAMA Oncol. (2020) 6:e204564. doi: 10.1001/jamaoncol.2020.4564
19. Shigeta K, Datta M, Hato T, Kitahara S, Chen IX, Matsui A, et al. Dual programmed death receptor-1 and vascular endothelial growth factor receptor-2 blockade promotes vascular normalization and enhances antitumor immune responses in hepatocellular carcinoma. Hepatology. (2020) 71:1247–61. doi: 10.1002/hep.30889
20. Tian L, Goldstein A, Wang H, Ching Lo H, Sun Kim I, Welte T, et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature. (2017) 544:250–4. doi: 10.1038/nature21724
21. Danaher P, Warren S, Dennis L, D’Amico L, White A, Disis ML, et al. Gene expression markers of Tumor Infiltrating Leukocytes. J Immunother Cancer. (2017) 5:18. doi: 10.1186/s40425-017-0215-8
22. Tang H, Cao Y, Jian Y, Li X, Li J, Zhang W, et al. Conversion therapy with an immune checkpoint inhibitor and an antiangiogenic drug for advanced hepatocellular carcinoma: A review. Biosci Trends. (2022) 16:130–41. doi: 10.5582/bst.2022.01019
23. Edeline J, Boucher E, Rolland Y, Vauléon E, Pracht M, Perrin C, et al. Comparison of tumor response by Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST in patients treated with sorafenib for hepatocellular carcinoma. Cancer. (2012) 118:147–56. doi: 10.1002/cncr.26255
24. Thompson JA, Schneider BJ, Brahmer J, Achufusi A, Armand P, Berkenstock MK, et al. Management of immunotherapy-related toxicities, version 1.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2022) 20:387–405. doi: 10.6004/jnccn.2022.0020
25. Sun HC, Zhou J, Wang Z, Liu X, Xie Q, Jia W, et al. Chinese expert consensus on conversion therapy for hepatocellular carcinoma (2021 edition). Hepatobil Surg Nutr. (2022) 11:227–52. doi: 10.21037/hbsn-21-328
26. Huang G, Li PP, Lau WY, Pan ZY, Zhao LH, Wang ZG, et al. Antiviral therapy reduces hepatocellular carcinoma recurrence in patients with low HBV-DNA levels: A randomized controlled trial. Ann Surg. (2018) 268:943–54. doi: 10.1097/SLA.0000000000002727
27. Bertacco A, Vitale A, Mescoli C, Cillo U. Sorafenib treatment has the potential to downstage advanced hepatocellular carcinoma before liver resection. Per Med. (2020) 17:83–7. doi: 10.2217/pme-2018-0114
28. Kaseb AO, Vence L, Blando J, Yadav SS, Ikoma N, Pestana RC, et al. Immunologic correlates of pathologic complete response to preoperative immunotherapy in hepatocellular carcinoma. Cancer Immunol Res. (2019) 7:1390–5. doi: 10.1158/2326-6066.CIR-18-0605
29. Oldhafer KJ, Stavrou GA, van Gulik TM. ALPPS–where do we stand, where do we go?: eight recommendations from the first international expert meeting. Ann Surg. (2016) 263:839–41. doi: 10.1097/SLA.0000000000001633
30. Olthof PB, van Dam R, Jovine E, Campos RR, de Santibañes E, Oldhafer K, et al. Accuracy of estimated total liver volume formulas before liver resection. Surgery. (2019) 166:247–53. doi: 10.1016/j.surg.2019.05.003
Keywords: hepatocellular carcinoma, conversion therapy, apatinib, camrelizumab, tyrosine kinase inhibitors, immune checkpoint inhibitors, surgical resection
Citation: Liu X-L, Li X-Z, Chu Y-F, Liu F and Tian H (2024) Resection of unresectable hepatocellular carcinoma after conversion therapy with apatinib and camrelizumab: a case report and literature review. Front. Oncol. 14:1280805. doi: 10.3389/fonc.2024.1280805
Received: 21 August 2023; Accepted: 18 March 2024;
Published: 27 March 2024.
Edited by:
Simona Kranjc Brezar, Institute of Oncology Ljubljana, SloveniaReviewed by:
Antonella Argentiero, National Cancer Institute Foundation (IRCCS), ItalyJohn Gibbs, Hackensack Meridian Health, United States
Copyright © 2024 Liu, Li, Chu, Liu and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hu Tian, dGlhbmh1NjU4NUAxNjMuY29t
 Xin-Liang Liu
Xin-Liang Liu Xiang-Ze Li
Xiang-Ze Li Yi-Fu Chu3
Yi-Fu Chu3 Hu Tian
Hu Tian