- 1Department of Oncology, Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2Department of Graduate School, Beijing University of Chinese Medicine, Beijing, China
Background and aim: Chinese herbal injection (CHI) is a widely used preparation for advanced non-small cell lung cancer (NSCLC) treatment to alleviate the adverse drug reactions and enhance the clinical efficacy of chemotherapy. However, its efficacy and safety in combination with platinum-based chemotherapy (PBC) remain poorly understood owing to the lack of high-level evidence in the face of a wide variety of CHIs. Therefore, in this study, we aimed to explore the efficacy and safety of CHIs in combination with PBC regimens in the treatment of mid- and advanced NSCLC.
Methods: Systematic evaluation and meta-analysis were conducted as per the Preferred Reporting Project for Systematic Evaluation and Meta-Analysis Protocols (PRISMA-P). Seven databases were comprehensively searched for relevant randomized controlled trials (RCTs) through August 1, 2022. The quality of each study was evaluated based on the Cochrane Handbook for Systematic Reviews of Interventions. Statistical analysis was performed using Revman 5.3, with dichotomies expressed as risk ratio (RR) and 95% confidence interval (CI). Objective response rate (ORR) and disease control rate (DCR) were selected as the primary outcomes, with quality of life (QoL) and toxic side effects as secondary outcomes.
Results: A total of 140 RCTs were included in this study. The results of the meta-analysis suggested that, compared with PBC alone, PBC combined with CHIs significantly improved the ORR (RR=1.35, 95% CI: 1.30–1.41, P<0.001), DCR (RR=1.15, 95% CI: 1.13–1.18, P<0.001) and QoL (RR=1.29, 95% CI: 1.24–1.33, P<0.001). Moreover, the combination treatment reduced chemotherapy-induced leukopenia (RR=0.69, 95% CI: 0.64–0.75, P<0.001), anemia (RR=0.70, 95% CI: 0.62–0.79, P<0.001), thrombocytopenia (RR=0.68, 95% CI: 0.62–0.75, P<0.001), nausea and vomiting (RR=0.69, 95% CI: 0.63–0.76, P<0.001), diarrhea (RR=0.59, 95% CI: 0.48–0.73, P<0.001), and constipation (RR=0.68, 95% CI: 0.54–0.86, P=0.001).
Conclusion: According to the available evidence, CHIs in combination with PBC can improve clinical efficacy and reduce the toxic side effects in the treatment of advanced NSCLC. However, considering the study’s limitations, more rigorous and high-quality studies are needed to further confirm the results.
Systematic review registration: https://inplasy.com/inplasy-2022-1-0104/, identifier INPLASY202210104.
1 Introduction
The 2020 Global Cancer Report showed 2.1 million new cases and 1.8 million deaths from lung cancer in 2018. Lung cancer remains the leading cause of cancer deaths, accounting for approximately one-fifth of all cancer deaths (1). Non-small cell lung cancer (NSCLC) accounts for approximately 85% of lung cancers. Most patients with NSCLC are at an advanced stage at the time of discovery, losing optimal timing for surgical treatment (2). Platinum-based chemotherapy (PBC) remains the preferred treatment for patients with advanced NSCLC who are not driven by a positive gene. PBC is based on cisplatin or carboplatin and is often used in combination with pemetrexed, docetaxel, gemcitabine, paclitaxel, and vincristine. Previous clinical studies found that the median progression-free survival (PFS) of pemetrexed plus cisplatin or carboplatin in the treatment of advanced NSCLC was 6 months and 4.7 months, respectively, and the 1-year survival rates were 45.7% and 39.2% (3), respectively. The median survival time (overall survival, OS) of gemcitabine plus cisplatin or carboplatin in the treatment of advanced NSCLC was 8.2 months (4), which has been proven clinically effective. Therefore, the efficacy of PBC in treating middle and advanced NSCLC is considerable; moreover, OS and health-related quality of life (QoL) do not exhibit differences between carboplatin- and cisplatin-based chemotherapy (5, 6). However, although PBC kills cancer cells, it also damages the human body, causing toxic side effects such as bone marrow suppression, nausea and vomiting, and liver and kidney toxicity, adversely affecting the smooth chemotherapy cycle and severely reducing the QoL of patients (7). Therefore, searching for effective NSCLC complementary alternative therapies remains one of the research hotspots.
Traditional Chinese medicine (TCM) has long been used as a supplement and alternative therapy in NSCLC treatment. Previous studies have shown that TCM can inhibit tumor growth, modulate immune function, increase sensitivity to chemotherapy when combined with it, reduce toxic side effects, and improve the QoL of patients (8, 9). Chinese herbal injection (CHI) is the combination of modern pharmaceutical technology and traditional Chinese prescription, where a sterile preparation is extracted from Chinese herbal medicine. It has the advantages of convenient application, immediate effect, and clear indication and has been widely used in clinical practice. Moreover, TCM has proved to be effective in treating advanced NSCLC. Various CHIs have been developed based on TCMs such as ginseng, Sophora flavescens, coix seed, and toad cake. Such CHIs include Compound Kushen injection, Aidi injection, Kanglaite injection, and Shenqifuzheng injection. Previous studies have shown that these CHIs when combined with PBC can positively increase efficacy and reduce chemotherapy-associated toxicity (10–12).
However, despite the wide clinical application of CHIs, their efficacy and safety in combination with PBC need to be further evaluated given the lack of high-level evidence or clinical guidelines in the face of a wide variety of CHIs. Therefore, in this study, we systematically evaluate and meta-analyze the overall efficacy and safety of CHIs in combination with PBC for advanced NSCLC, providing evidence and guidance for clinical use. A graphic summary of the meta-analysis is shown in Figure 1.
Figure 1 Graphical abstract of the meta-analysis. Meta-analytic process of clinical efficacy and safety of Chinese herbal injections in combination with platinum-based chemotherapy for advanced non-small cell lung cancer.
2 Methods
In this study, systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Project for Systematic Evaluation and Meta-Analysis Protocols (PRISMA-P) (13). The protocol has been registered with the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY) under registration number: INPLASY202210104 (https://inplasy.com/inplasy-2022-1-0104/). Because all studies are published articles, ethical approval was not required.
2.1 Inclusion criteria
2.1.1 Type of study
Only randomized controlled studies (RCTs) were included in this study to compare the efficacy and safety of CHI–first-line platinum-based chemotherapy combination (treatment group) and first-line platinum-based chemotherapy alone (control group) in the treatment of NSCLC.
2.1.2 Types of participants
All patients were cytologically or pathologically confirmed cases of NSCLC and belonged to Stage III or IV according to American Joint Committee on Cancer Staging System (8th edition).
2.1.3 Types of interventions
The control group was treated with PBC. The treatment group was treated with at most one CHI plus PBC regimen. The chemotherapy regimen was the same in the treatment and control groups, and no restrictions were placed on the type, dose, and duration of chemotherapy drugs or CHI. CHIs included Aidi injection (drug approval number: Z52020236); Compound Kushen injection (drug approval number: Z14021231); Huachansu injection (drug approval number: Z34020274); Javanica oil emulsion injection (drug approval number: Z44021325); Kanglaite injection (drug approval number: Z10970091); Kangai injection (drug approval number: Z20026868); Lanxiangxi injection (drug approval number: H20110114); Lentinan injection (drug approval number: H20030131); Shenfu injection (drug approval number: Z51020664); Shenmai injection (drug approval number: Z13020888); Shenqifuzheng injection (drug approval number: Z19990065); Xiaoaiping injection (drug approval number: Z20025868); Huangqi injection (drug approval number: Z23020782); and Chansu injection (drug approval number: Z34020604). These CHIs have been approved by China’s State Food and Drug Administration for cancer treatment (14). PBC included NP (vinorelbine plus cisplatin), PP (paclitaxel/albumin paclitaxel/paclitaxel liposome plus cisplatin), PC (paclitaxel/albumin paclitaxel liposome plus carboplatin), GP (gemcitabine plus cisplatin), GC (gemcitabine plus carboplatin), DP (docetaxel plus cisplatin), DC (docetaxel plus carboplatin), AP (pemetrexed plus cisplatin), and AC (pemetrexed plus carboplatin).
2.1.4 Types of outcome measures
According to World Health Organization (WHO) (15) guidelines for solid tumor responses or Response Evaluation Criteria in Solid Tumors (RECIST) (16), tumors were evaluated as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The primary outcomes were objective response rate (ORR) and disease control rate (DCR). ORR refers to the proportion of patients with CR or PR. DCR refers to the proportion of patients with CR, PR, or SD. Secondary outcomes were QoL and toxic side effects. QoL was assessed according to the Karnofsky performance scale (KPS) (17). An increase or decrease of 10 points was considered an improvement in QoL (18). The safety indexes were myelosuppression and digestive tract reaction. Standard Classification of WHO or National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) was used to measure the incidence of leukopenia, decreased hemoglobin, thrombocytopenia, nausea and vomiting, diarrhea, and constipation. The involved studies included at least one primary outcome.
2.2 Exclusion criteria
The studies 1) which included animal experiments, literature reviews, case reports, and other unrelated studies; 2) in which patients received multiple CHIs or non-PBC regimens simultaneously; 3) in which ORR or DCR was not evaluated in the literature; 4) that included single-arm trials; 5) for which data were not available and those for which data were still not available after contacting the author by email; and 6) where no references to specific randomization methods or to “high risk” assessments of risk bias were made were excluded from this meta-analysis.
2.3 Information sources
PubMed, EMBASE, Cochrane, China National Knowledge Infrastructure (CNKI), Wanfang Data, VIP Database for Chinese Technical Periodicals (VIP), and SinoMed were searched systematically from their inception until July 1, 2023. The language of RCTs was limited to Chinese and English.
2.4 Search strategy
The literature search was conducted by combining subject words and free words. To ensure a comprehensive search, the search terms of CHIs and NSCLC were mainly formulated as follows (1) NSCLC, including “Carcinoma,” “non-small-cell Lung,” “Lung Carcinoma,” “Non-Small-Cell,” “Non-Small-Cell Lung Carcinomas,” “Non-small Cell Lung Carcinoma,” “Non-Small Cell Lung Cancer,” and “NSCLC.” (2) CHIs not only include the overall name, such as “Chinese herbal injection,” “Chinese medicine injection,” “injection of TCM,” but also contains the name of the specific injection, such as “Aidi,” “Chansu,” “Compound Kushen,” “Huachansu,” “Xiaoaiping,” “Kanglaite,” “Javanica oil emulsion,” “Shenqifuzheng,” “Kangai,” “Shenfu,” “Huangqi,” “Lentinan,” “Shenmai,” and “Lanxiangxi.” More detailed search terms and search policies are provided in Supplementary Materials.
2.5 Study selection
The retrieved literature were imported into Notexpress (version 3.0) for literature management. Two researchers (QC and WZ) independently screened the literature according to inclusion and exclusion criteria. First, literature management software deleted duplicate works of literature, and the literature that evidently did not meet the inclusion criteria were deleted by reading the title and abstract of the remaining literature. The literature obtained after the preliminary screening were downloaded and read. The final screening was conducted according to the type of research, type of patients, intervention measures, and outcome indicators. In case of any disagreement in the screening process, a consensus was reached through discussion with Hou Wei.
2.6 Data extraction
Two researchers (DW and CQ) used Microsoft Excel 2019 to independently extract data, including 1) publication year, first author, publishing country, and region; 2) the random model and implementation method and the blind method; 3) number of subjects, age, sex, and pathological type; and 4) specific drugs, dosage, course of treatment and duration of treatment.
2.7 Risk of bias and quality assessment
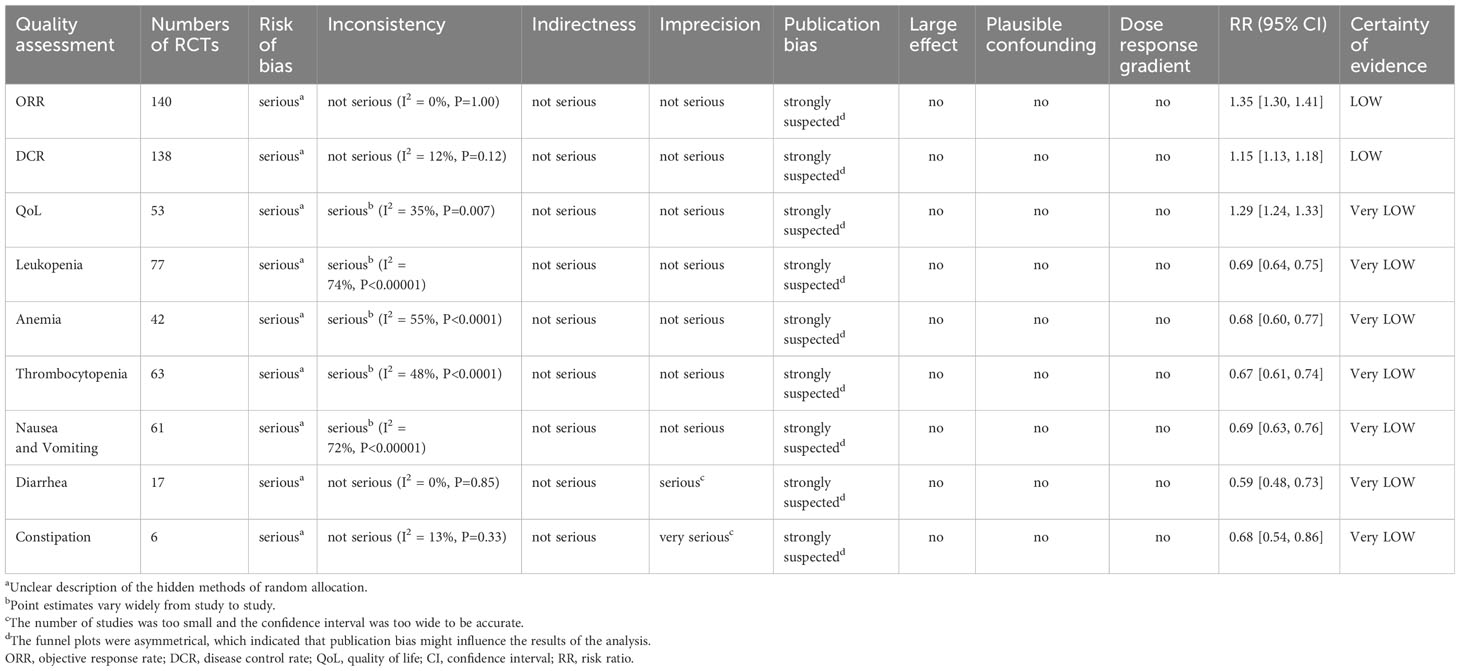
Two researchers (KC and SH) independently evaluated literature quality using the “Risk of Bias Assessment Tool” in the Cochrane Handbook for Randomized Controlled Trials (19). The evaluation mainly focused on seven aspects of randomization, assignment concealment, patient or investigator blindness, outcome evaluator blindness, outcome data integrity, selective outcome reporting, and other bias, and the evaluation results were categorized into “low risk,” “unclear,” or “high risk.” In case of any disagreement, the study team discussed it with HW to decide the result. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) was used to evaluate the quality of evidence, focusing on five aspects of downgrade factors (risk of bias, inconsistency, indirectness, imprecision, and publication bias) and three elements of upgrade factors (large effect, plausible confounding, and dose-response gradient). The evaluation results of downgrade factors were categorized into “not serious,” “serious,” and “very serious.” The evaluation results of upgrade factors were categorized into “no,” “large,” and “very large.” The two factors were combined to evaluate the quality of evidence as high, medium, low, or very low (20).
2.8 Statistical methods and analysis
In this study, Review Manager software (version 5.4) was used for meta-analysis. The dichotomous variables were expressed by risk ratio (RR) with 95% confidence intervals (CIs). A P-value < 0.05 was considered statistically significant.
Considering the potential heterogeneity between the trials, I2 and P-values were used to I2 was used to quantitate the heterogeneity; if studies or subgroups with P>0.1, and I2<50% were included, a meta-analysis using fixed effect models showed little to no heterogeneity between studies, and if studies or subgroups with P<0.1 and I2>50%, respectively, were included, it was indicative of a large heterogeneity among the included studies. By eliminating the studies one by one, we determined the literature with high heterogeneity and analyzed the causes of heterogeneity. In cases where heterogeneity of the literatures with high heterogeneity was significantly reduced after removal and the evaluation results were not affected, the literature was retained, and the random effects model were used for analysis (21). Descriptive analysis was performed if significant clinical heterogeneity was observed even after removal. Simultaneously, we conducted subgroup analysis according to the type of CHIs, and only descriptive analysis was performed when there were less than three included studies. In case of more than ten studies, a funnel plot was used to assess publication bias (22).
2.9 Sensitivity analysis
To ensure the stability of the meta-analysis results, we performed sensitivity analyses of the primary outcome measures by excluding articles by screening for sample size, duration of treatment for CHIs, and year of publication.
2.10 Trial sequential analysis
TSA software version 0.9 was used for sequential analysis of the primary outcome indicators, ORR and DCR, to reduce false positive results caused by random error, estimate the amount of information needed for meta-analysis, and further improve the credibility of this study.
3 Results
3.1 Study selection
A total of 14037 articles were retrieved from the database. First, 7626 duplicate articles were removed, and the titles and abstracts of the remaining 6411 articles were scanned. Of these, 4798 were removed, because they did not meet the inclusion and exclusion criteria. Further, the remaining 1613 articles were further screened and evaluated, and 993 articles were excluded. Finally, owing to a large number of clinical studies in this field, we excluded low-quality literature with large risk of bias in order to ensure the quality of evidence of meta-analysis. Through quality evaluation of the remaining 620 articles, 480 studies that did not mention specific randomized methods and literature quality evaluation as “high risk” were excluded. A total of 140 studies were included in the meta-analysis (23–162). The detailed literature screening process is shown in Figure 2.
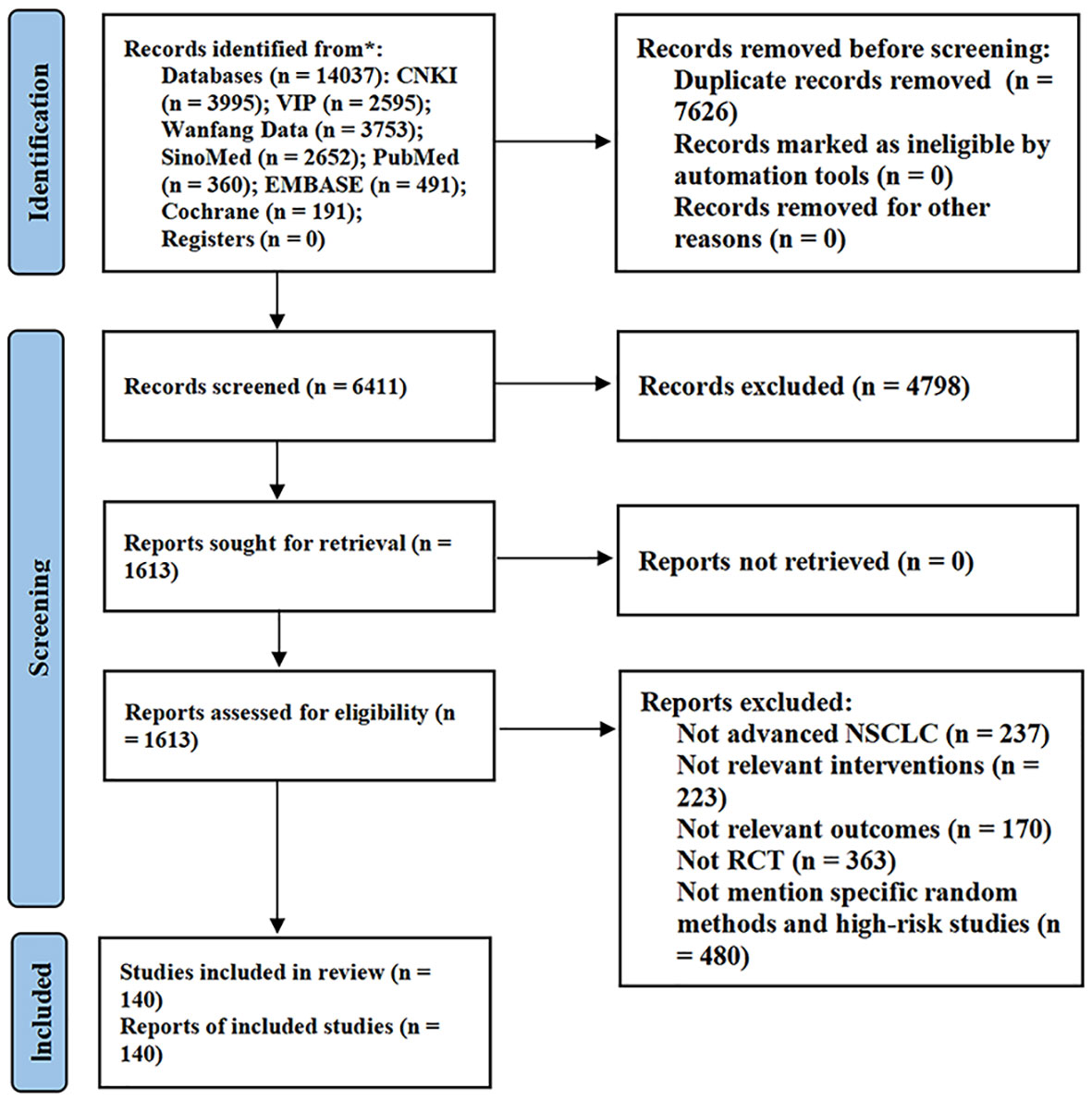
Figure 2 Flow diagram of study selection. CNKI, China National Knowledge Infrastructure; VIP, Chinese Scientific Journal Database.
3.2 Study characteristics
The baseline characteristics of the included studies are detailed in Supplementary Table 1. The 140 studies had a total of 12053 patients, including 6083 in the treatment group, 5970 in the control group, and 3912, 4976, and 837 in squamous cell carcinoma, adenocarcinoma, and other NSCLC pathological types, respectively; the number of subjects in each study ranged from 24 to 96, and the age range varied from 25 to 87.
All RCTs were conducted in China, and the specific numbers of the 12 CHIs included were as follows: Aidi injection was used in 31 trials (40, 43, 50, 53, 55, 58–60, 72, 76, 79, 85, 87–90, 93, 96, 101, 106, 108, 121, 125–127, 131, 141, 143, 151, 159, 161); Compound Kushen injection was used in 16 trials (44, 47, 54, 75, 97, 98, 113, 116, 130, 132, 142, 144, 148, 150, 152, 160); Huachansu injection was used in 5 trials (25, 27, 42, 65, 92); Javanica oil emulsion injection was used in 7 trials (29, 80, 95, 109, 112, 114, 137); Kanglaite injection was used in 21 trials (30, 33, 34, 36, 56, 63, 66, 67, 70, 94, 104, 105, 110, 118, 124, 133, 134, 136, 146, 147, 158); Kangai injection was used in 14 trials (31, 35, 48, 49, 51, 64, 71, 119, 120, 138, 140, 145, 149, 162); Lanxiangxi injection was used in 5 trials (39, 77, 103, 128, 157); Lentinan injection was used in 2 trials (86, 155); Shenfu injection was used in 2 trials (83, 117); Shenmai injection was used in 10 trials (26, 28, 32, 68, 74, 99, 102, 107, 129, 153); Shenqifuzheng injection was used in 18 trials (24, 38, 40, 45, 46, 57, 69, 81, 82, 84, 111, 115, 122, 123, 135, 139, 154, 156); and Xiaoaiping injection was used in 9 trials (23, 37, 52, 61, 62, 73, 78, 91, 100).
3.3 Quality evaluation
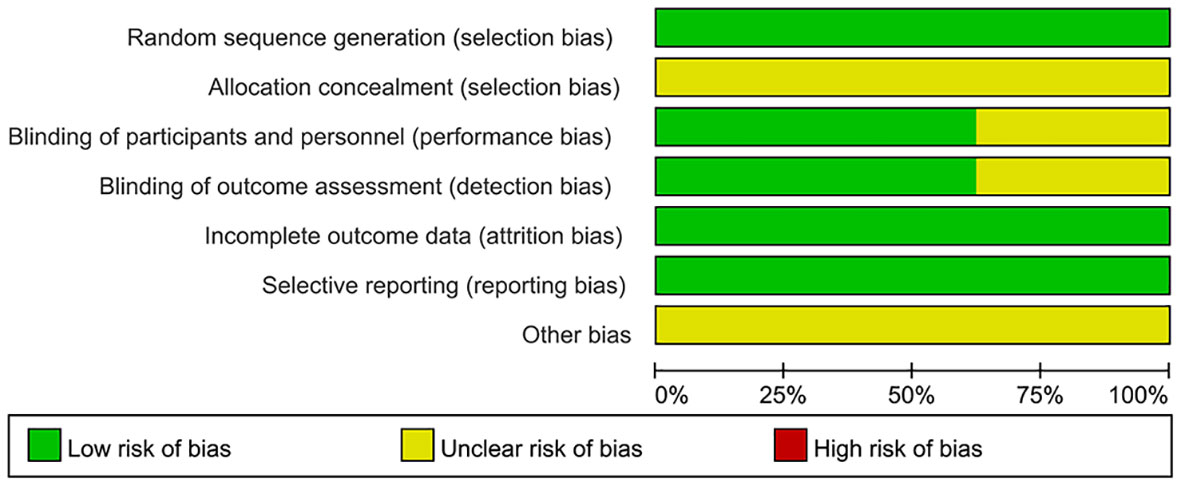
The methodological quality assessment of the included literature is detailed in Figure 3 and Supplementary Figure 1. In terms of randomization methods, all 140 studies in the pooled analysis were rated as “low risk” owing to the exclusion of literature that did not mention specific randomization methods. All studies were classified as “unclear,” because they did not mention allocation concealment. All 53 studies that did not mention blinding and did not use a placebo were rated as “unclear,” because subjective outcome measures, including QoL, would be affected by blinding; however, 87 studies had objective outcome measures such as ORR and DCR and did not include subjective outcome measures. Clinical discrimination would not be affected by blinding and was rated as “low risk.” Concerning the completeness of outcome data, because the data for outcome measures were consistent with those assigned to randomization, the risk of incomplete data was low, and all studies were rated as “low risk”.
In the selective assessment, all studies were rated as “low risk,” because they reported outcomes as described in the methodology. Finally, other biases were not mentioned in all the studies and were evaluated as “unclear.” (163) The GRADE assessment results of this study and the reasons for the downgrade are listed in Table 1.
3.4 Effectiveness and safety
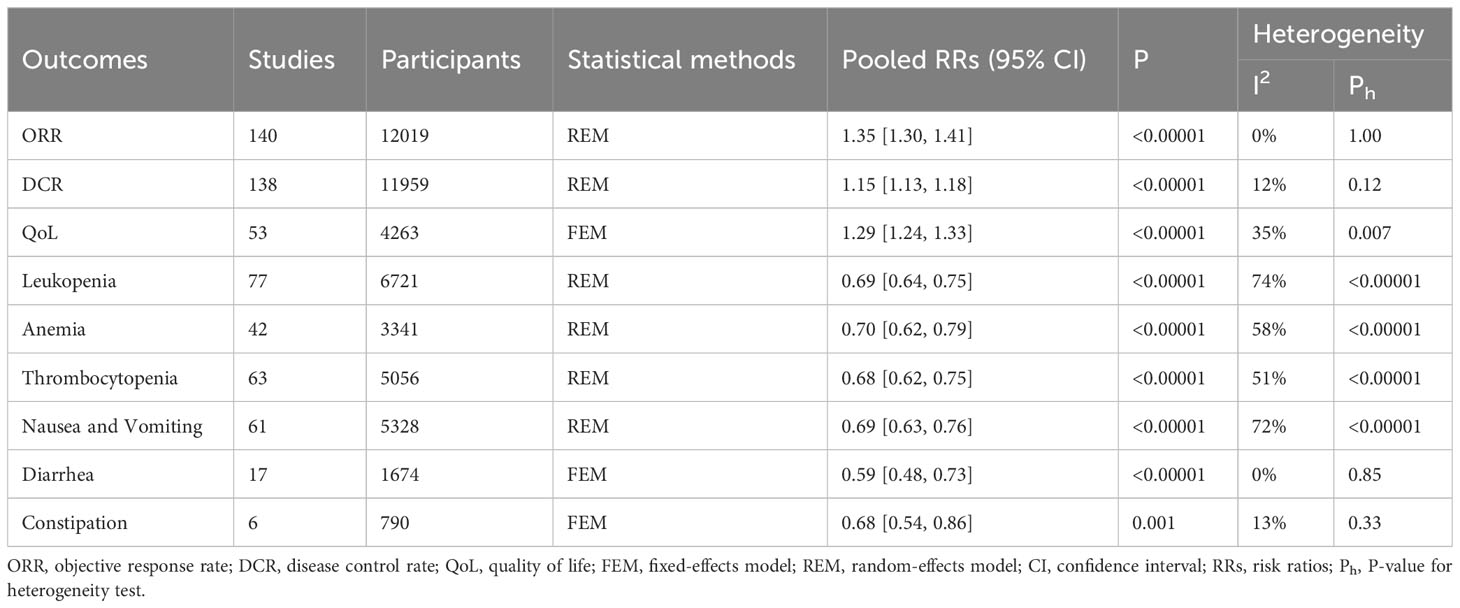
The overall meta-analysis results are detailed in Table 2, and the subgroup analyses by type of CHIs are detailed in Table 3.
3.4.1 Objective response rate
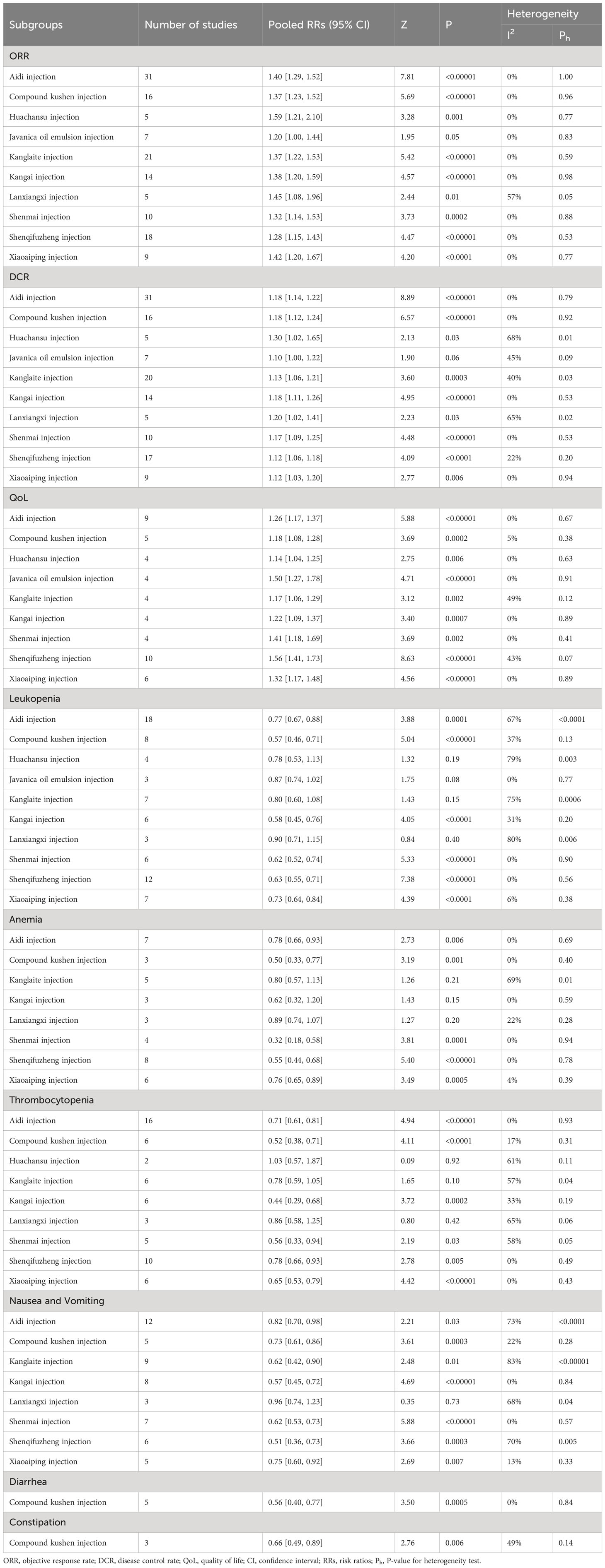
A total of 140 RCTs including 12 CHIs reported ORR outcomes. No statistical heterogeneity was found after pooling (I2=0%, P=1.00); however, the heterogeneity of Lanxiangxi injection subgroup was high (I2=57%, P=0.05). Therefore, the random effect model was used for meta-analysis. The results showed that the ORR of the treatment group was significantly higher than that of the control group (RR=1.35, 95% CI: 1.30–1.41, P<0.001; Figure 4). According to the results of subgroup analysis of CHIs type, Aidi injection subgroup (RR=1.40, 95% CI: 1.29–1.52, P<0.001; I2=0%), Compound Kushen injection subgroup (RR=1.37, 95% CI: 1.23–1.52, P<0.001; I2=0%), Huachansu injection subgroup (RR=1.59, 95% CI: 1.21–2.10, P=0.001; I2=0%, Kanglaite injection subgroup (RR=1.37, 95% CI: 1.22–1.53, P<0.001; I2=0%), Kangai injection subgroup (RR=1.38, 95% CI: 1.20–1.59, P<0.001; I2=0%), Lanxiangxi injection subgroup (RR=1.45, 95% CI: 1.08–1.96, P=0.01; I2=57%), Shenmai injection subgroup (RR=1.32, 95% CI: 1.14–1.53, P<0.001; I2=0%), Shenqifuzheng injection subgroup (RR=1.28, 95% CI: 1.15–1.43, P<0.001; I2=0%), Xiaoaiping injection subgroup (RR=1.42, 95% CI: 1.20–1.67, P<0.001; I2=0%) showed that the ORR of the treatment group was significantly higher than that of the control group. However, Javanica oil emulsion injection subgroup (RR=1.20, 95% CI: 1.00–1.44, P=0.05; I2=0%) exhibited no significant difference compared to the control group in terms of ORR. After individually selecting studies, it was found that the heterogeneity of Lanxiangxi injection subgroup mainly arose from one trial (128), which could be attributed to the low dose of Lanxiangxi injection in this study (400 mg/d, 7 days/course, 4 courses). After excluding this trial, there was no significant difference between the merged statistical values and the original results (RR=1.61, 95% CI: 1.23–2.11, P=0.001; I2=0%). Less than two articles were retrieved for the Lentinan and Shenfu injection subgroups, so only descriptive analysis was performed without merging. Overall, Lentinan and Shenfu injection subgroups did not exhibit any significant advantage in terms of ORR.
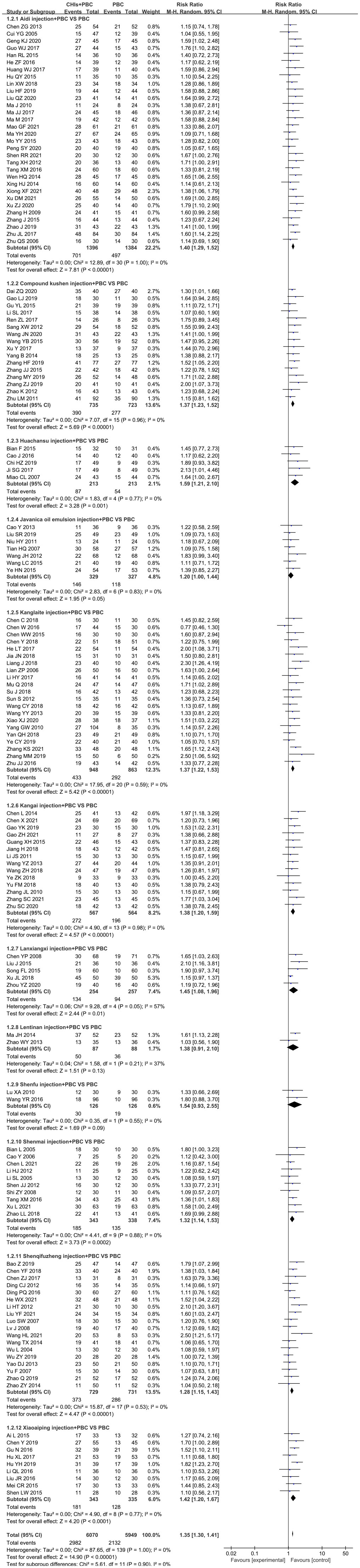
Figure 4 Forest plot of ORR in PBC versus PBC plus CHIs. The meta-analysis results, stratified by the type of CHIs, showed differences in ORR between PBC and PBC plus CHIs. Objective response rate ORR=(CR+PR)/total cases×100%; CHIs, Chinese herbal injections; PBC, platinum-based chemotherapy.
3.4.2 Disease control rate
A total of 138 RCTs covering 12 CHIs reported the outcomes of DCR. No significant heterogeneity was found in the combined analysis (I2=12%, P=0.12). However, the heterogeneity of the Huachansu injection subgroup (I2=68%, P=0.01) and Lanxiangxi injection subgroup (I2=65%, P=0.02) was relatively high. Therefore, the random effect model was used for meta-analysis. The results showed that the DCR of the treatment group was significantly higher than that of the control group (RR=1.15, 95% CI: 1.13–1.18, P<0.001; Figure 5). Subgroup analysis according to the type of CHIs showed that the DCR value of Aidi injection subgroup (RR=1.18, 95% CI: 1.14–1.22, P<0.001; I2=0%), Compound Kushen injection subgroup (RR=1.18, 95% CI: 1.12–1.24, P<0.001; I2=0%), Huachansu injection subgroup (RR=1.30, 95% CI: 1.02–1.65, P=0.03; I2=68%), Kanglaite injection subgroup (RR=1.13, 95% CI: 1.06–1.21, P<0.001; I2=40%), Kangai injection subgroup (RR=1.18, 95% CI: 1.11–1.26, P<0.001; I2=0%), Lanxiangxi injection subgroup (RR=1.20, 95% CI: 1.02–1.41, P=0.01; I2=65%), Shenmai injection subgroup (RR=1.17, 95% CI: 1.09–1.25, P<0.001; I2=0%), Shenqifuzheng injection subgroup (RR=1.12, 95% CI: 1.06–1.25, P<0.001; I2=22%), and Xiaoaiping injection subgroup (RR=1.12, 95% CI: 1.03–1.20, P=0.01; I2=0%) showed that the DCR of the treatment group was significantly higher than that of the control group. However, Javanica oil emulsion injection subgroup (RR=1.10, 95% CI: 1.00–1.22, P=0.06; I2=45%) exhibited no significant difference compared to the control group in terms of DCR. The heterogeneity of the Huachansu and Lanxiangxi injection subgroups arose from two trials (Miao CL, 2017 (128) respectively). The heterogeneity may be due to the lower dosage of CHIs compared with that in other studies (Huachansu injection: 20 mL/d, 5 days/course, 3–6 courses; Lanxiangxi injection: 400 mg/d, 7 days/course, 4 courses). After excluding the literature of Miao CL (2017) and (128), the statistical values were as follows: Huachansu injection subgroup, RR=1.39, 95% CI: 1.16–1.67, P<0.001; I2=4%; Lanxiangxi injection subgroup, RR=1.24, 95% CI: 1.08–1.42, P=0.002; I2=15%. No significant differences were observed with respect to the original results. Less than two articles were retrieved on the Lentinan and Shenfu injection subgroups; hence, only descriptive analysis was performed without merging. Altogether, Shenfu injection subgroup had a positive effect on DCR. However, the Lentinan injection subgroup exhibited no obvious advantage in terms of DCR.
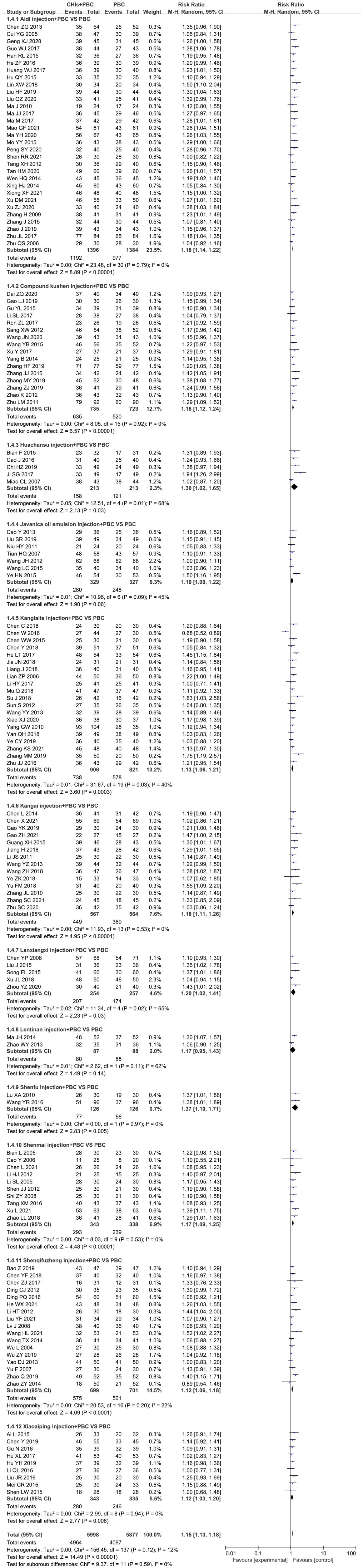
Figure 5 Forest plot of DCR in PBC versus PBC plus CHIs. The meta-analysis results, stratified by the type of CHIs, showed differences in DCR between PBC and PBC plus CHIs. Disease control rate DCR=(CR+PR+SD)/total cases×100%; CHIs, Chinese herbal injections; PBC, platinum-based chemotherapy.
3.4.3 Quality of life
A total of 52 studies determined QoL and included 11 CHIs, with low heterogeneity after combined analysis (I2=35%, P=0.01). Therefore, the fixed effects model was used for meta-analysis. The results showed that the QoL of the treatment group was significantly higher than that of the control group (RR=1.29, 95% CI: 1.24–1.33, P<0.001; Figure 6). Additionally, the subgroup analysis showed that QoL of Aidi injection subgroup (RR=1.26, 95% CI: 1.17–1.37, P<0.001; I2=0%), Compound Kushen injection subgroup (RR=1.18, 95% CI: 1.09–1.28, P<0.001; I2=0%), Huachansu injection subgroup (RR=1.14, 95% CI: 1.04–1.25, P=0.01; I2=0%), Javanica oil emulsion injection subgroup (RR=1.50, 95% CI: 1.27–1.78, P<0.001; I2=0%), Kanglaite injection subgroup (RR=1.42, 95% CI: 1.27–1.59, P=0.002; I2=49%), Kangai injection subgroup (RR=1.22, 95% CI: 1.09–1.37, P=0.001; I2=0%), Shenmai injection subgroup (RR=1.56, 95% CI: 1.19– 2.03, P=0.001; I2=0%), Shenqifuzheng injection subgroup (RR=1.56, 95% CI: 1.41–1.73, P<0.001; I2=43%), and Xiaoaiping injection subgroup (RR=1.32, 95% CI: 1.17–1.48, P<0.001; I2=0%) were increased significantly. The literature search of the Lanxiangxi injection subgroup yielded less than two articles; hence, only descriptive analysis was carried out without merging. Overall, Lanxiangxi injection subgroup exhibited no obvious advantage in terms of QoL.
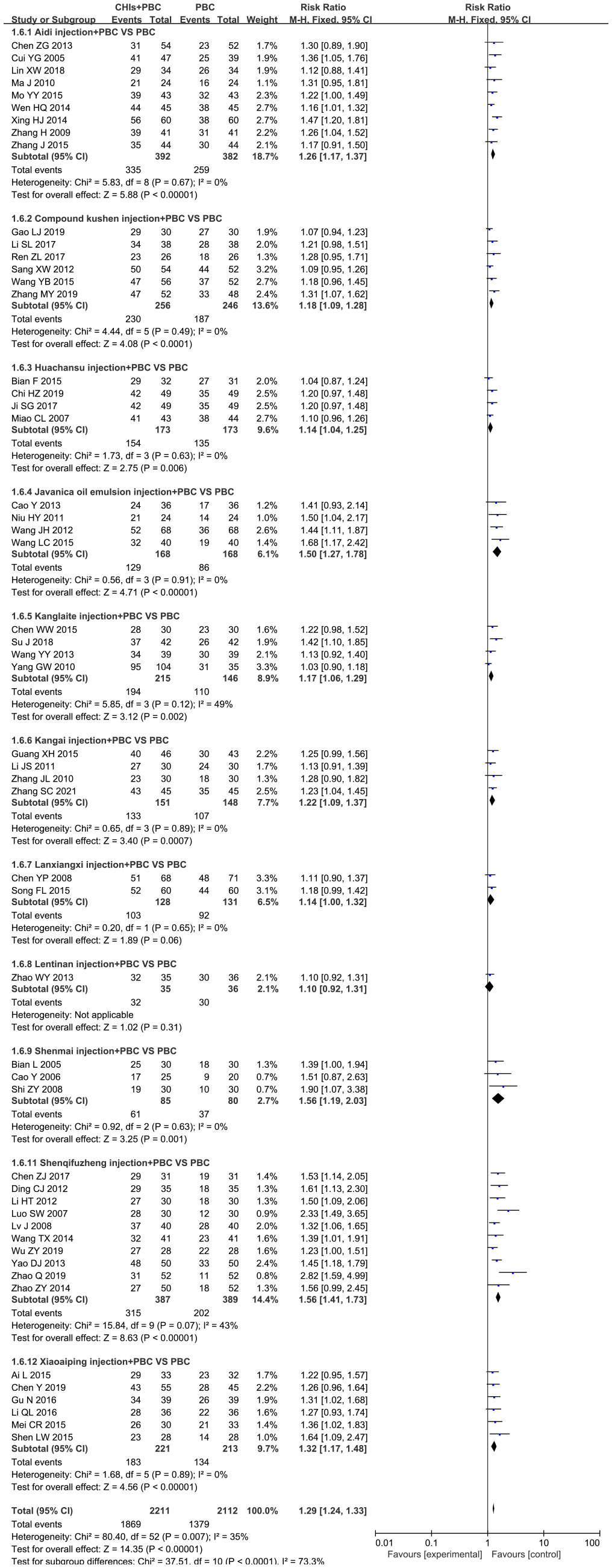
Figure 6 Forest plot of QoL in PBC versus PBC plus CHIs. The meta-analysis results, stratified by the type of CHIs, showed differences in QoL between PBC and PBC plus CHIs. QoL, quality of life; CHIs, Chinese herbal injections; PBC, platinum-based chemotherapy.
3.4.4 Leukopenia
A total of 77 studies including 12 CHIs reported the occurrence of leukopenia. The heterogeneity of the combined analysis was high (I2=75%, P<0.001). Therefore, a random-effects model was used for meta-analysis. The results showed that the incidence of leukopenia in the treatment group was significantly lower than that in the control group (RR=0.69, 95% CI: 0.64–0.75, P<0.001). By individually eliminating the literature, it was found that the heterogeneity mainly arose from (39, 43, 65, 103) Tan XM (2020) (125, 158, 160, 161) and was significantly reduced after exclusion (I2 = 13%, P=0.19). The heterogeneity may be related to the drug toxicity, intervention dose, course of treatment, and chemotherapy regimen of different CHIs. After excluding the literature with large heterogeneity, the pooled analysis was performed, and the statistical values were not significantly different from the original results (RR=0.68, 95% CI: 0.64–0.72, P<0.001). Further subgroup analysis showed that the incidence of leukopenia of Aidi injection subgroup (RR=0.77, 95% CI: 0.67–0.88, P<0.001; I2=67%), Compound Kushen injection subgroup (RR=0.57, 95% CI: 0.46–0.71, P<0.001; I2=37%), Kangai injection subgroup (RR=0.58, 95% CI: 0.45–0.76, P<0.001; I2=31%), Shenmai injection subgroup (RR=0.62, 95% CI: 0.52–0.74, P<0.001; I2=0%), Shenqifuzheng injection subgroup (RR=0.63, 95% CI: 0.55–0.71, P<0.001; I2=0%), and Xiaoaiping injection subgroup (RR=0.73, 95% CI: 0.64–0.84, P<0.001; I2=6%) was significantly decreased. However, Huachansu injection subgroup (RR=0.78, 95% CI: 0.53–1.13, P=0.19; I2=79%), Javanica oil emulsion injection subgroup (RR=0.87, 95% CI: 0.74–1.02, P=0.08; I2=0%), Kanglaite injection subgroup (RR=0.80, 95% CI: 0.60–1.08, P=0.15; I2=75%), and Lanxiangxi injection subgroup (RR=0.90, 95% CI: 0.71–1.15, P=0.40; I2=80%) exhibited no obvious advantage in terms of the incidence of leukopenia. The subgroup analysis showed that the heterogeneity of the Aidi injection subgroup was derived from (43), Tan XM (2020) (125), and (161) and that of the Huachansu injection subgroup was derived from (65). The heterogeneity of the Kanglaite injection subgroup was derived from (158) and that of the Lanxiangxi injection subgroup was derived from (157). The analysis found that the heterogeneity of the Aidi injection subgroup was derived from (43, 125), used NP chemotherapy regimen and did not specify the pathological type of the enrolled population (161), used a DP chemotherapy regimen, and Tan XM (2020) used a shorter intervention time for Aidi injection, with only 10 days for each course of treatment. The heterogeneity of the Huachansu injection subgroup was attributed to the small proportion of squamous cell carcinoma and adenocarcinoma in (65). The heterogeneity of the Kanglaite injection subgroup was derived from (158), in which the Kanglaite injection intervention duration was 10 days, which was relatively short. The heterogeneity of the Lanxiangxi injection subgroup was attributed to the pathological type of the included population in (157), which included only adenocarcinoma. After excluding the literature with large heterogeneity, the combined statistical values were as follows: Aidi injection subgroup, RR=0.68, 95% CI: 0.59–0.78, P<0.001; I2=12; Lanxiangxi injection subgroup, RR=0.99, 95% CI: 0.92–1.06, P=0.70; I2=0%. No significant differences were observed with respect to the original results. However, the Huachansu injection subgroup (RR=0.66, 95% CI: 0.54–0.80, P<0.001; I2=0%) excluded (65), while the Kanglaite injection subgroup (RR=0.74, 95% CI: 0.58–0.94, P=0.01; I2=36%) excluded (158). After excluding the above studies, the combined analysis showed that the value differed from the original result, and the literature heterogeneity had a certain effect on the meta-analysis results. After excluding heterogeneity, it was confirmed that the Huachansu and Kanglaite injections when combined with PBC were effective than the PBC alone in the alleviation of the incidence of leukopenia. The number of articles retrieved for the Shenfu injection subgroup was less than two; hence, only descriptive analysis was performed without merging. Overall, the Shenfu injection could play a positive role in the alleviation of leukopenia. Forest plot of leukopenia is provided in the Supplementary Figure 2.
3.4.5 Anemia
A total of 42 studies including 8 CHIs reported the occurrence of anemia. The heterogeneity of the combined analysis was high (I2=58%, P<0.001); hence, the random-effects model was used for the meta-analysis. The results showed that the incidence of anemia in the treatment group was significantly lower than that in the control group (RR=0.70, 95% CI: 0.62–0.79, P<0.00001). By paying attention to the exclusion literature, it was found that the heterogeneity mainly arose from the literature of (39, 65, 73, 103, 118, 158) and was significantly reduced after exclusion (I2=0%, P=0.58). The intervention measures of these studies were found to be different, which may be related to the drug toxicity, intervention dose, course of treatment, and chemotherapy regimen of different CHIs. After excluding studies with large heterogeneity, the statistical values were not significantly different from the original results (RR=0.65, 95% CI: 0.59–0.71, P<0.001; I2=0%). Further subgroup analysis showed that The incidence of anemia of the Aidi injection subgroup (RR=0.78, 95% CI: 0.66–0.93, P=0.01; I2=0%), Compound Kushen injection subgroup (RR=0.50, 95% CI: 0.33–0.77, P<0.001; I2=0%), Shenmai injection subgroup (RR=0.32, 95% CI: 0.18–0.58, P<0.001; I2=0%), Shenqifuzheng injection subgroup (RR=0.55, 95% CI: 0.44–0.68, P<0.001; I2=0%), and Xiaoaiping injection subgroup (RR=0.76, 95% CI: 0.65–0.89, P=0.001; I2=4%) was significantly reduced. However, Kanglaite injection subgroup (RR=0.80, 95% CI: 0.57–1.13, P=0.21; I2=69%), Kangai injection subgroup (RR=0.62, 95% CI: 0.32–1.20, P=0.15; I2=0%), and Lanxiangxi injection subgroup (RR=0.89, 95% CI: 0.74–1.07, P=0.20; I2=22%) exhibited no evident advantage in terms of the incidence of anemia. The heterogeneity of the Kanglaite injection subgroup was derived from (146), a study which did not specifically describe CHI and the cycle of chemotherapy and was considered as the main source of heterogeneity. The pooled statistics by excluding this literature showed no significant difference from the original results (RR=0.92, 95% CI: 0.72–1.16, P=0.46; I2=44%). Forest plot of anemia is provided in the Supplementary Figure 3.
3.4.6 Thrombocytopenia
The occurrence of thrombocytopenia was reported in 63 studies including 8 CHIs. The heterogeneity was high in the pooled analysis (I2=51%, P<0.001); hence, the random-effects model was used for the meta-analysis. The results showed that the incidence of anemia in the treatment group was significantly lower than that in the control group (RR=0.68, 95% CI: 0.62–0.75, P<0.001). By individually excluding studies, we found that the heterogeneity mainly arose from (103, 117, 158, 161). After excluding these studies, the heterogeneity was significantly reduced (I2=19%, P=0.11). This may be related to the different toxicity, dose, intervention course, and chemotherapy regimens of Lanxiangxi, Shenfu, Kanglaite, and Aidi injections. After excluding studies with large heterogeneity, the pooled statistical values were not significantly different from the original results (RR=0.70, 95% CI: 0.64–0.76, P<0.001; I2=19%). Subgroup analysis showed that the incidence of thrombocytopenia of the Aidi injection subgroup (RR=0.71, 95% CI: 0.61–0.81, P<0.00001; I2=0%), Compound Kushen injection subgroup (RR=0.52, 95% CI: 0.38–0.71, P<0.0001; I2=17%), Kangai injection subgroup (RR=0.44, 95% CI: 0.29–0.68, P=0.0002; I2=33%), Shenmai injection subgroup (RR=0.56, 95% CI: 0.33–0.94, P=0.03; I2=58%), Shenqifuzheng injection subgroup (RR=0.78, 95% CI: 0.66–0.93, P=0.005; I2=0%), and Xiaoaiping injection subgroup (RR=0.65, 95% CI: 0.53–0.79, P<0.00001; I2=0%) was significantly reduced. However, Kanglaite injection subgroup (RR=0.78, 95% CI: 0.59–1.05, P=0.10; I2=57%) and Lanxiangxi injection subgroup (RR=0.86, 95% CI: 0.58–1.25, P=0.42; I2=65%) exhibited no obvious advantage in terms of the incidence of thrombocytopenia. In the subgroup analysis, the heterogeneity of the Shenmai injection subgroup was derived from (107) and that of the Kanglaite injection subgroup was derived from (146). The heterogeneity of the Lanxiangxi injection subgroup arose from (157). Through analysis, it was found that the heterogeneity of the Shenmai injection subgroup was due to the only pathological type of adenocarcinoma in the population included in (107). The heterogeneity of the Kanglaite injection subgroup was because (146) did not specifically describe CHI and the cycle of chemotherapy. The heterogeneity of the Lanxiangxi injection subgroup arose from the fact that the pathological type of the included population in (157) was only adenocarcinoma. After excluding the literature with large heterogeneity, the combined statistical values were as follows: Shenmai injection subgroup, RR=0.47, 95% CI: 0.27–0.84, P=0.05; I2=28%; Kanglaite injection subgroup, RR=0.86, 95% CI: 0.72–1.04, P=0.12; I2=24%; and Lanxiangxi injection subgroup, RR=0.93, 95% CI: 0.82–1.06, P=0.29; I2=0%. Literature heterogeneity within subgroups had no significant effect on the study results. Less than two articles were retrieved for the Shenfu injection subgroup; hence, only descriptive analysis was performed without pooling. Overall, the Shenfu injection subgroup could play a positive role in thrombocytopenia. Forest plot of thrombocytopenia is provided in the Supplementary Figure 4.
3.4.7 Nausea and vomiting
A total of 61 RCTs including 12 CHIs reported on nausea and vomiting. The pooled heterogeneity was high (I2=72%, P<0.001); hence, the random-effects model was used for the meta-analysis. The results showed that the incidence of nausea and vomiting in the treatment group was significantly lower than that in the control group (RR=0.69, 95% CI: 0.63–0.76, P<0.001). The heterogeneity of the literature may be due to the different side effects of nausea and vomiting caused by different CHIs and chemotherapy regimens. By excluding (39, 43, 82, 103) Tan XM (2020), and (125), the heterogeneity of (158) and (161) was significantly reduced (I2=20%, P=0.11; RR=0.67, 95% CI: 0.62–0.72, P<0.001). The heterogeneity of the literature had little effect on the meta-analysis, and no significant difference was observed with respect to the original results. Subgroup analysis by CHIs type showed that the incidence of nausea and vomiting of the Aidi injection subgroup (RR=0.82, 95% CI: 0.70–0.98, P=0.03; I2=73%), Compound Kushen injection subgroup (RR=0.73, 95% CI: 0.61–0.86, P=0.003; I2=22%), Kanglaite injection subgroup (RR=0.62, 95% CI: 0.42–0.90, P=0.01; I2=83%), Kangai injection subgroup (RR=0.57, 95% CI: 0.45–0.72, P<0.001; I2=0%), Shenmai injection subgroup (RR=0.62, 95% CI: 0.53–0.73, P<0.001; I2=0%), Shenqifuzheng injection subgroup (RR=0.51, 95% CI: 0.36–0.73, P<0.001; I2=70%), and Xiaoaiping injection subgroup (RR=0.75, 95% CI: 0.06–0.92, P=0.01; I2=13%) were significantly reduced. However, Lanxiangxi injection subgroup (RR=0.96, 95% CI: 0.74–1.23, P=0.73; I2=68%) exhibited no obvious advantage in terms of the incidence of nausea and vomiting. Subgroup analysis showed that the heterogeneity of Aidi injection subgroup was derived from Ma JJ (2017) (89) and (121), that of the heterogeneity of Kanglaite injection subgroup was derived from (118, 158) the Shenqifuzheng injection subgroup was derived from (82) and (111), and that of the Lanxiangxi injection subgroup was derived from (157). According to the analysis, the heterogeneity of the Aidi injection subgroup was due to the relatively high proportion of males in the population included in Ma JJ (2017) (89, 121). Additionally, Ma JJ (2017) and (89) did not mention the pathological type of the included population. The heterogeneity of the Kanglaite injection subgroup was attributed to the fact that (118) only included patients in stage IIIb-IV and that (158) used antiemetic treatment before chemotherapy. The heterogeneity of the Shenqifuzheng injection subgroup arose from (82), in which all patients received antiemetics before chemotherapy. Moreover (111), did not mention the pathological type of the included population. The heterogeneity of the Lanxiangxi injection subgroup arose from the pathological type of the included population in (157), which was only adenocarcinoma. After excluding the literature with large heterogeneity, the combined statistical values were as follows: Kanglaite injection subgroup, RR=0.50, 95% CI: 0.39–0.65, P<0.001; I2=0%); Shenqifuzheng injection subgroup, RR=0.67, 95% CI: 0.56–0.81, P<0.001; I2=0%; and Lanxiangxi injection subgroup, RR=1.04, 95% CI: 0.94–1.15, P=0.50; I2=0%. In this study, the conclusions were not significantly different from the original results, and the literature heterogeneity within the subgroups had no significant effect on the study results. For the Aidi injection subgroup, the literature with large heterogeneity was excluded, and the random effects model was used to combine the results. The statistical value differed from the original result (RR=0.95, 95% CI: 0.88–1.03, P=0.23; I2=12%), and the literature heterogeneity had a certain impact on the meta-analysis. After excluding the heterogeneity, it was found that the Aidi injection exhibited no obvious advantage in terms of the incidence of nausea and vomiting between the treatment group and the control group. Because less than two articles were retrieved for the Javanica oil emulsion and Shenfu injection subgroups, only descriptive analysis was carried out without merging. Overall, the Shenfu injection subgroup positively affected the incidence of nausea and vomiting; however, the Javanica oil emulsion injection subgroup had no obvious advantage. Forest plot of nausea and vomiting is provided in the Supplementary Figure 5.
3.4.8 Diarrhea
A total of 17 RCTs involving 10 CHIs reported outcomes for diarrhea. No statistical heterogeneity was found in the combined analysis (I2=0%, P=0.85); hence, the fixed effect model was used. The results showed that the incidence of diarrhea in the treatment group was significantly lower than that in the control group (RR=0.59, 95% CI: 0.48–0.73, P<0.001). Subgroup analysis according to the type of CHIs showed that the incidence of diarrhea was significantly lower in the Compound Kushen injection subgroup (RR=0.56, 95% CI: 0.40–0.77, P=0.001; I2=0%). Because less than two articles were retrieved for the Javanica oil emulsion and Xiaoaiping injection subgroups, only descriptive analysis was carried out without merging. Overall, the two had no apparent advantage in the incidence of diarrhea. Forest plot of diarrhea is provided in the Supplementary Figure 6.
3.4.9 Constipation
A total of 6 RCTs covering 4 CHIs reported constipation outcomes. Little heterogeneity was observed in the combined analysis (I2=13%, P=0.33). The results showed that the incidence of constipation in the treatment group was significantly lower than that in the control group (RR=0.68, 95% CI: 0.54–0.86, P=0.001). The results of subgroup analysis according to CHIs type showed that the incidence of constipation was significantly reduced in the Compound Kushen injection subgroup (RR=0.66, 95% CI: 0.49–0.89, P=0.01; I2=49%). Forest plot of constipation is provided in the Supplementary Figure 7.
3.4.10 Publication bias
Figure 7 shows that the funnel plot based on ORR, DCR, QoL, leukopenia, anemia, thrombocytopenia, nausea and vomiting, diarrhea, and constipation is asymmetric, indicating a certain publication bias.
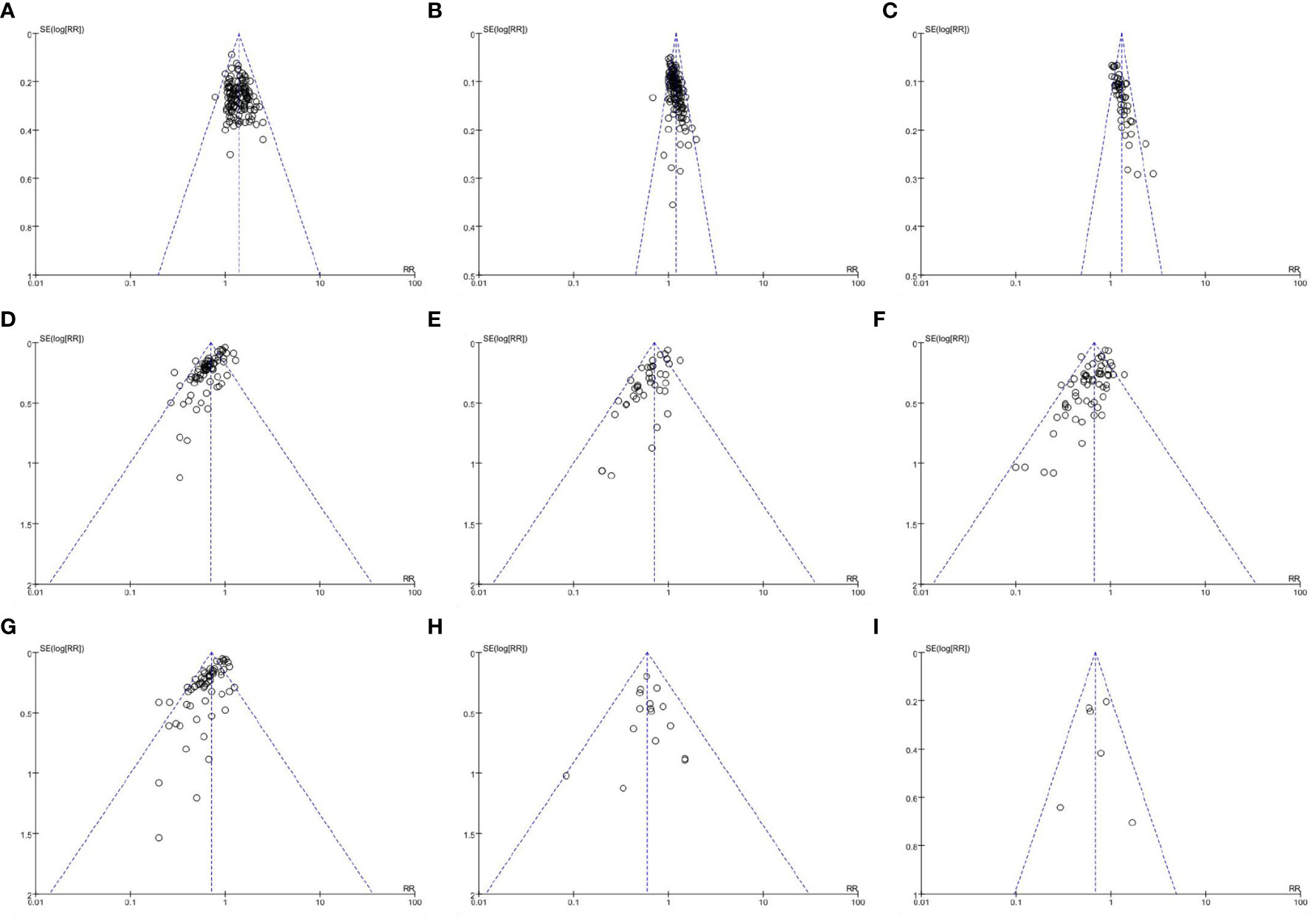
Figure 7 Funnel plots of outcomes. (A) objective response rate; (B) disease control rate; (C) quality of life; (D) leukopenia; (E) anemia; (F) thrombocytopenia; (G) nausea and vomiting; (H) diarrhea; (I) constipation.
3.4.11 Additional analyses
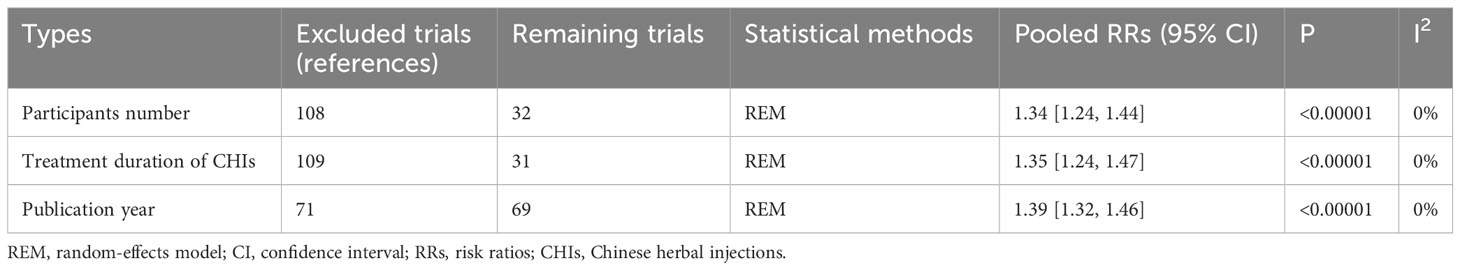
In terms of ORR, the pooled results of RCTs showed that the ORR of the treatment group was significantly higher than that of the control group (RR=1.35, 95% CI: 1.30–1.41, P<0.001). Sensitivity analysis showed that when only 32 RCTs with ≥50 participants (RR=1.34, 95% CI: 1.24–1.44, P<0.001), 31 RCTs with treatment duration ≥4 cycles (RR=1.35, 95% CI: 1.24–1.47, P<0.001), or 69 RCTs published within the last 5 years (RR=1.39, 95% CI: 1.32–1.46, P<0.001) were included, ORR only changed slightly from the previous results.
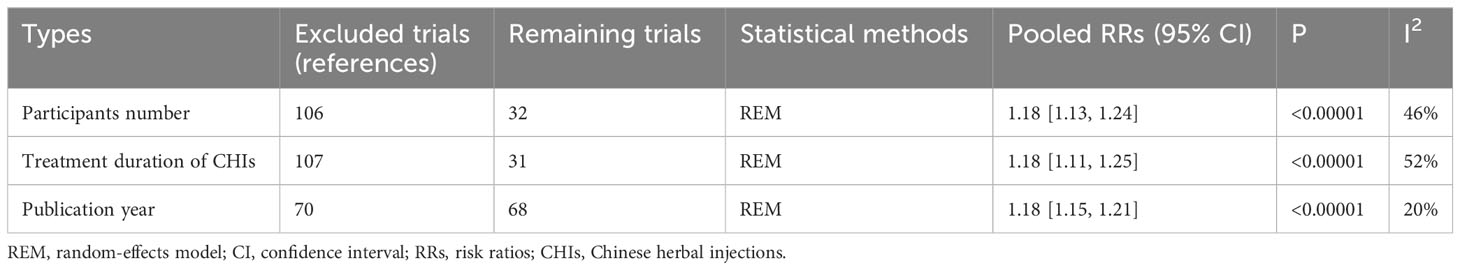
The pooled results of RCTs showed that the DCR of the treatment group was significantly higher than that of the control group (RR=1.15, 95% CI: 1.13–1.18, P<0.001). Sensitivity analysis showed that when only 32 RCTs with ≥50 participants (RR=1.18, 95% CI: 1.13–1.24, P<0.00001), 31 RCTs with treatment duration ≥4 cycles (RR=1.18, 95% CI: 1.11–1.25, P<0.001), or 68 RCTs published in the last 5 years (RR=1.18, 95% CI: 1.15–1.21, P<0.001) were included, DCR only changed slightly from the previous results. These analyses showed that the pooled results for the primary outcome measures were robust both before and after the removal of relevant trials (Tables 4, 5).
The required sample size of the TSA can estimate the meta-analysis and reduce the possibility of a false positive result. This study selected bilateral inspection method, setting I error probability for α at 0.05, statistical test force at 80%, merge relative risk loss at 20%, the incidence of positive events ORR control group at 35.83%, and the positive event rate in the DCR control group at 69.71%. TSA analysis of ORR and DCR showed that the cumulative Z value had passed the traditional threshold value and TSA value, and the cumulative information had reached the expected value. Therefore, TSA analysis confirmed that there was definite evidence that CHIs combined with PBC in the treatment of NSCLC could improve ORR and DCR (Figure 8).
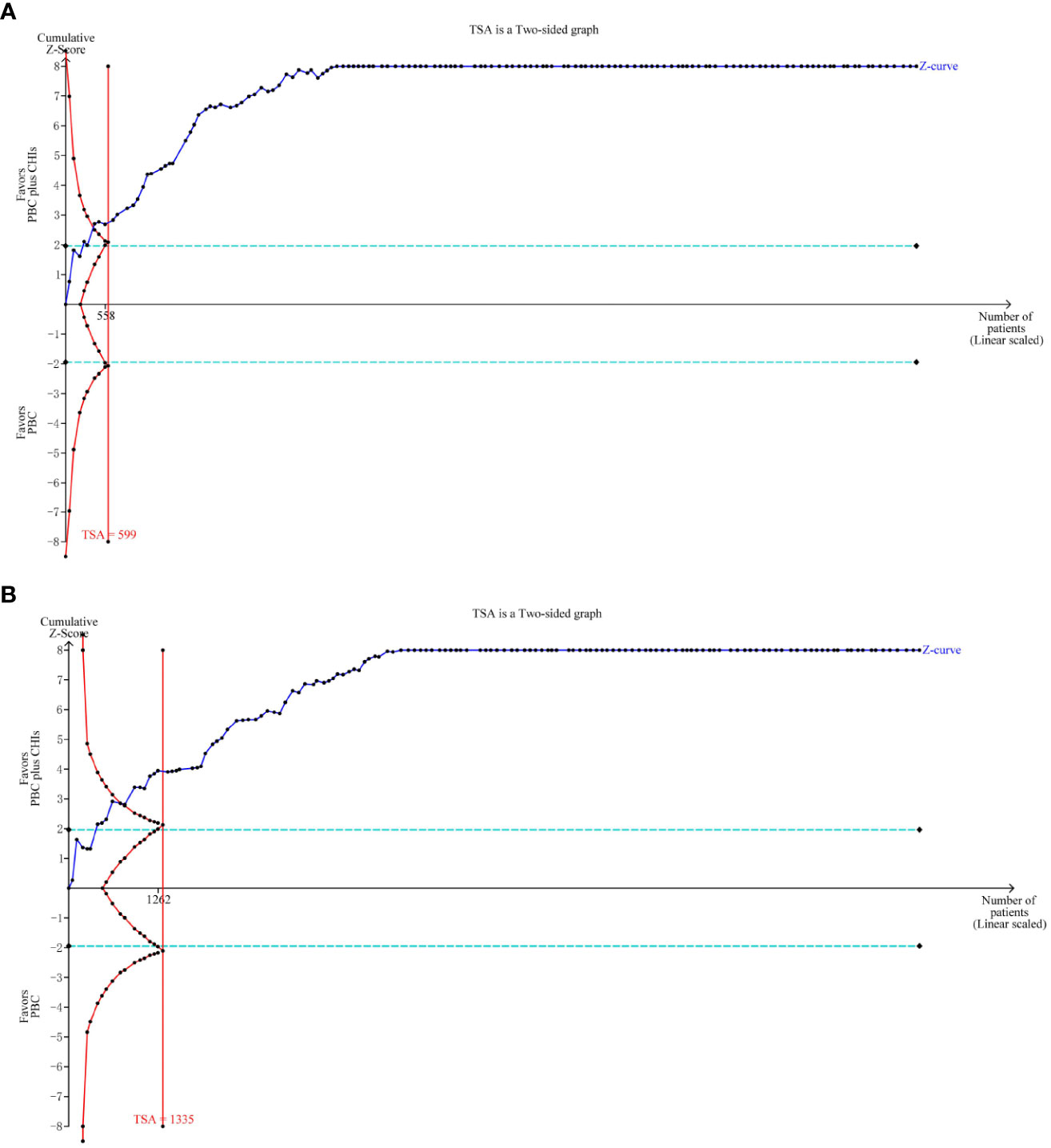
Figure 8 Trial sequential analysis (TSA) on outcomes. TSA analysis of ORR and DCR showed that the cumulative Z value had passed the traditional threshold value and TSA value, and the cumulative information had reached the expected value. (A) objective response rate; (B) disease control rate.
4 Discussion
This study differs from the previous meta-analyses of CHIs and has certain advantages. First, there exists a wide variety of CHIs, and the efficacy of multiple CHIs when combined with PBC remains insufficiently compared. Therefore, we selected a variety of CHIs commonly used in clinical practice to conduct a systematic review of effectiveness and safety to provide a reference for the selection of CHIs in the treatment of NSCLC. Second, this study included high-quality literature and conducted detailed subgroup analysis and sensitivity tests according to different PBC chemotherapy regimens, yielding robust results.
Based on published RCTs, we conducted a meta-analysis of the efficacy and safety of CHIs when combined with PBC in the treatment of advanced NSCLC. Overall, compared with PBC, CHIs when combined with PBC exhibited a significant improvement in ORR, DCR, and QoL. And the incidence of leukopenia, anemia, thrombocytopenia, nausea and vomiting, diarrhea, constipation, and other adverse reactions was significantly reduced. Among them, the heterogeneity of ORR, DCR, QoL, diarrhea, and constipation was low, and the reliability of the results was high. Although the heterogeneity of leukopenia, anemia, thrombocytopenia, and nausea and vomiting indexes was high after the combined analysis, the sensitivity analysis showed that heterogeneity did not significantly affect the results and also had certain reliability. Sensitivity analysis showed that the results of the primary outcome index were also stable. In terms of methodological assessment, the overall quality of the included studies was moderate, and the GRADE assessment showed that the quality of evidence was low, which may be related to study design, publication bias, and heterogeneity.
PBC, as the first-line treatment for non-positive gene-driven advanced NSCLC, has been widely used in clinical practice and has a significant effect. However, the toxic side effects of PBC can interrupt the treatment process and reduce patients’ QoL Therefore, reducing adverse reactions while pursuing efficacy has become one of the urgent challenges in the treatment of NSCLC. TCM, which has been inherited for thousands of years, has accumulated rich experience in tumor treatment, which can be used as one of the ideas to solve the problem. Compound Kushen injection is an herbal extract of Sophora flavescens and Smilax glabra. Pharmacological studies have found that it can improve the immune level of patients, inhibit the proliferation of tumor cells, and play a role in anti-cancer therapy (164). Aidi injection is composed of extracts from cantharis, ginseng, Astragalus membranaceus, Acanthopanax senticosusm, and other drugs, with the main active ingredients being cantharidin, ginsenoside, and astragaloside. Many studies have shown that Aidi injection has anti-tumor activity, immunomodulatory effect, and adverse event reduction effect (12). The main components of Huachansu injection include toad venom ligands, alkaloids, and peptides. Pharmacological studies have shown that Huachansu injection has multiple anti-tumor effects, such as inducing tumor cell apoptosis, inhibiting tumor cell proliferation, invasion, and metastasis, and reversing drug resistance (165, 166). Shenmai, Shenfu, and Kangai injections all contain ginseng. Ginsenoside, the main component of ginseng, can induce lung cancer cell apoptosis and inhibit lung cancer cell proliferation (167, 168). However, the main components of Shenfu injection are ginsenoside compounds, which also have similar effects (169). In addition to ginseng, Shenmai injection also includes Ophiopogon japonicus, which has been shown to enhance cisplatin-induced apoptosis by regulating mitofusin-2 (Mfn2)-dependent mitochondrial dynamics in lung adenocarcinoma cells (170). Kangai injection can inhibit the proliferation of gastric cancer cells through IL-6/STAT3 pathway (171). Shenqifuzheng injection, which is composed of Codonopsis pilosula and Astragalus membranaceus extracts, has the effect of supplementing Qi deficiency and strengthening vital energy. Similar to the Shenmai injection, it has a synergistic effect on chemotherapy through cell cycle arrest and initiation of mitochondrial apoptosis, which is involved in the up-regulation of Mfn2 expression (172). The Kanglaite injection is another TCM injection extracted from coix seeds. Studies have found that its anti-tumor effect is related to inducing cancer cell apoptosis and improving immune function (173). The main component of Xiaoaiping injection is marsdenia tenacissima. Modern pharmacological studies have confirmed that the clearance vine can induce apoptosis and inhibit autophagy in NSCLC cells to play an anti-tumor role (174).
From the perspective of clinical practice, our results also show that CHIs based on Chinese herbal extracts can improve the efficacy of PBC in NSCLC and reduce the incidence of adverse reactions. Although the results showed that CHIs when combined with PBC were more effective and safer than PBC alone in the treatment of advanced NSCLC, it was unclear which CHIs played a role in this process; therefore, we conducted a subgroup analysis according to the type of CHIs.
Regarding the primary outcome indicators, ORR and DCR, 9 CHIs including Aidi injection, Compound Kushen injection, Huachansu injection, Kanglaite injection, Kangai injection, Lanxiangxi injection, Shenmai injection, Shenqifuzheng injection, and Xiaoaiping injection could improve ORR and DCR. Additionally, the heterogeneity of the combined analysis of 7 CHIs such as Aidi injection, Compound Kushen injection, Kanglaite injection, Kangai injection, Shenmai injection, Shenqifuzheng injection, and Xiaoaiping injection was low, and the reliability of the results was high. The heterogeneity of Huachansu injection in DCR and Lanxiangxi injection in ORR and DCR was high; however, because heterogeneity did not affect the results, the combined results of the two injections also have certain reliability. It is worth noting that Shenfu injection exhibited particularly unique effects, playing a positive role in DCR improvement; however, its effect on ORR improvement was not significant. The two CHIs of Javanica oil emulsion injection and Lentinan injection had no significant effect on improving ORR and DCR, and the heterogeneity of Javanica oil emulsion injection was low, yielding highly reliable results. However, Shenfu injection and Lentinan injection were not combined owing to the small number of studies, and the reliability of the results was hence relatively insufficient.
In terms of QoL improvement, 9 CHIs, Aidi injection, Compound Kushen injection, Huachansu injection, Javanica oil emulsion injection, Kanglaite injection, Kangai injection, Shenmai injection, Shenqifuzheng injection, and Xiaoaiping injection could improve QoL. The heterogeneity of the above injections was low, and the reliability of the results was high. Lanxiangxi injection had no obvious advantage; however, due to the small number of studies, The studies on Lanxiangxi injection was not combined. Hence, the results need to be further evaluated for reliability.
Regarding safety improvement, Aidi injection, Compound Kushen injection, Shenmai injection, Shenqifuzheng injection, Xiaoaiping injection and other five types of CHIs played a positive role in bone marrow suppression. Compound Kushen injection, Shenqifuzheng injection, Xiaoaiping injection, and the other three CHIs exhibited low heterogeneity and high reliability after combination. In contrast, Aidi injection and Shenmai injection had high heterogeneity in terms of the incidence of leukopenia and thrombocytopenia after combination. However, the heterogeneity did not affect the results; hence, the effects of the combined injection also have certain reliability. At the same time, studies have shown that Lanxiangxi injection has no apparent advantage in the prevention and treatment of bone marrow suppression, and the heterogeneity of leukopenia and thrombocytopenia after the combination is high. However, the heterogeneity does not affect the results. Therefore, the combined effects of this injection also have some reliability. Among other injections, Kangai injection and Shenfu injection played a positive role in leukopenia and thrombocytopenia; however, owing to the small number of literature, the reliability of the results needs to be further verified. Kangai injection showed no apparent advantage in alleviating the incidence of anemia. At the same time, Huachansu injection played a positive role in Leukopenia. However, these interpretations excluded the results after the combination of studies with higher heterogeneity, which needs to be further confirmed. Javanica oil emulsion injection did not show obvious advantages. It is worth noting that Kanglaite injection did not offer apparent advantages in alleviating anemia and thrombocytopenia incidence, and the heterogeneity in Anemia was high. However, the combined results were also reliable, because they did not affect the results. It showed an advantage in alleviating the incidence of leukopenia, but it needs to be further confirmed because it is the result of excluding the pooled studies with high heterogeneity. Compound Kushen injection had a positive effect on the digestive tract reaction. In terms of other injections such as Kanglaite injection, Kangai injection, Shenfu injection, and Shenmai injection, six types of CHIs such as Shenqifuzheng injection played a positive role in alleviating the incidence of nausea and vomiting. Among them, Kangai injection and Shenqifuzheng injection had high heterogeneity after being combined. However, the heterogeneity did not affect the results; hence, the combined results of these two injections also have certain reliability. However, the Shenfu injection was not combined due to the small number of studies, and the reliability of the results needs to be further verified. Javanica oil emulsion injection did not show any advantage in alleviating the incidence of nausea, vomiting, and diarrhea. However, owing to the poor availability of studies, the reliability of the results still needs to be further verified. Xiaoaiping injection showed an advantage in nausea and vomiting but not in diarrhea. Aidi injection and Lanxiangxi injection did not show obvious advantages in nausea and vomiting. However, Aidi injection was the result of the combination of studies excluding high heterogeneity; therefore, it needs to be further confirmed. The heterogeneity of Lanxiangxi injection was higher, but the heterogeneity did not affect the results.
In summary, the Compound Kushen injection has shown positive effects in improving the efficacy and QoL and reducing side effects, which can be recommended for clinical application. Aidi injection, Kanglaite injection, Kangai injection, Shenmai injection, Shenqifuzheng injection, and CHIs such as Xiaoaiping injection positively improve the efficacy and QoL. However, they exhibit insufficient effects in reducing toxic and side effects and hence can be used for secondary recommendations. Finally, Huachansu injection, Lanxiangxi injection, Javanica oil emulsion injection, Lentinan injection, Shenfu injection and other CHIs only showed advantages in some indicators, indicating that CHIs may not be recommended for the treatment of advanced NSCLC when the evidence is insufficient, and the research on these CHIs should be strengthened in the future for more reliable evidence-based medical application.
This study also has some limitations. First, all the included studies were conducted in China, which may have caused regional bias. Second, the number of studies on some CHIs is small, and hence, the efficacy may be exaggerated; therefore, the results obtained need to be further verified. Third, the study’s results showed asymmetric funnel plots and possible publication bias. Fourth, most included studies did not mention allocation concealment and blinding. Although our study included the RCTs with the highest level of evidence, methodologic shortcomings may exaggerate the clinical effect and further affect the credibility of the results. Fifth, the study period of the included studies was short, and the long-term endpoints such as OS and PFS were not reported; therefore, the level of evidence is insufficient when applied to the long-term treatment of tumors. In general, the number of studies on some CHIs is limited, and the overall quality of research needs further improvement. In the future, high-quality RCTs with long-term endpoint results are needed to further verify the efficacy and safety of CHI in combination with PBC in advanced NSCLC treatment.
5 Conclusion
From the available evidence, CHIs combined with PBC in the treatment of advanced NSCLC can improve ORR, DCR and QoL and alleviate the occurrence of adverse reactions such as leukopenia, anemia, thrombocytopenia, nausea and vomiting, diarrhea, and constipation. Among the studied CHIs, Compound Kushen injection has advantages in efficacy and safety and can be recommended for clinical application. However, considering the limitations of the existing evidence, more high-quality RCTs are needed to further verify the application of CHIs. Moreover, clinicians are advised to be cautious when applying the results of this study.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
KC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Visualization, Writing – original draft, Writing – review & editing. SH: Conceptualization, Data curation, Formal analysis, Software, Visualization, Writing – original draft, Writing – review & editing. DW: Conceptualization, Data curation, Formal analysis, Software, Visualization, Writing – original draft, Writing – review & editing. CQ: Data curation, Writing – original draft. ZW: Data curation, Writing – original draft. JW: Data curation, Writing – original draft. WH: Conceptualization, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the National Natural Science Foundation of China (No. 82074239), National Administration Traditional Chinese Medicine Evidence based capacity building project of TCM (No. 60101) and China Academy of Chinese Medical Sciences Innovation Fund (No. CI2021A01801).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1307836/full#supplementary-material
Supplementary Figure 1 | Risk of bias summary. Methodological quality evaluation of the included literature.
Supplementary Figure 2 | Forest plot of leukopenia in PBC versus PBC plus CHIs. The meta-analysis results, stratified by the type of CHIs, showed differences in leukopenia between PBC and PBC plus CHIs. CHIs, Chinese herbal injections; PBC, platinum-based chemotherapy.
Supplementary Figure 3 | Forest plot of anemia in PBC versus PBC plus CHIs. The meta-analysis results, stratified by the type of CHIs, showed differences in anemia between PBC and PBC plus CHIs. CHIs, Chinese herbal injections; PBC, platinum-based chemotherapy.
Supplementary Figure 4 | Forest plot of thrombocytopenia in PBC versus PBC plus CHIs. The meta-analysis results, stratified by the type of CHIs, showed differences in thrombocytopenia between PBC and PBC plus CHIs. CHIs, Chinese herbal injections; PBC, platinum-based chemotherapy.
Supplementary Figure 5 | Forest plot of nausea and vomiting in PBC versus PBC plus CHIs. The meta-analysis results, stratified by the type of CHIs, showed differences in nausea and vomiting between PBC and PBC plus CHIs. CHIs, Chinese herbal injections; PBC, platinum-based chemotherapy.
Supplementary Figure 6 | Forest plot of diarrhea in PBC versus PBC plus CHIs. The meta-analysis results, stratified by the type of CHIs, showed differences in diarrhea between PBC and PBC plus CHIs. CHIs, Chinese herbal injections; PBC, platinum-based chemotherapy.
Supplementary Figure 7 | Forest plot of constipation in PBC versus PBC plus CHIs. The meta-analysis results, stratified by the type of CHIs, showed differences in constipation between PBC and PBC plus CHIs. CHIs, Chinese herbal injections; PBC, platinum-based chemotherapy.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc (2008) 83(5):584–94. doi: 10.4065/83.5.584
3. Schuette WH, Groschel A, Sebastian M, Andreas S, Muller T, Schneller F, et al. A randomized phase II study of pemetrexed in combination with cisplatin or carboplatin as first-line therapy for patients with locally advanced or metastatic non-small-cell lung cancer. Clin Lung Cancer (2013) 14(3):215–23. doi: 10.1016/j.cllc.2012.10.001
4. Ferry D, Billingham L, Jarrett H, Dunlop D, Woll PJ, Nicolson M, et al. Carboplatin versus two doses of cisplatin in combination with gemcitabine in the treatment of advanced non-small-cell lung cancer: Results from a British Thoracic Oncology Group randomised phase III trial. Eur J Cancer (2017) 83:302–12. doi: 10.1016/j.ejca.2017.05.037
5. Griesinger F, Korol EE, Kayaniyil S, Varol N, Ebner T, Goring SM. Efficacy and safety of first-line carboplatin-versus cisplatin-based chemotherapy for non-small cell lung cancer: A meta-analysis. Lung Cancer (2019) 135:196–204. doi: 10.1016/j.lungcan.2019.07.010
6. Chu Q, Vincent M, Logan D, Mackay JA, Evans WK. Taxanes as first-line therapy for advanced non-small cell lung cancer: a systematic review and practice guideline. Lung Cancer (2005) 50(3):355–74. doi: 10.1016/j.lungcan.2005.06.010
7. Tsiouda T, Sardeli C, Porpodis K, Pilikidou M, Apostolidis G, Kyrka K, et al. Sex differences and adverse effects between chemotherapy and immunotherapy for non-small cell lung cancer. J Cancer (2020) 11(11):3407–15. doi: 10.7150/jca.40196
8. Chen Y, Zhu J, Zhang W. Antitumor effect of traditional Chinese herbal medicines against lung cancer. Anticancer Drugs (2014) 25(9):983–91. doi: 10.1097/CAD.0000000000000127
9. Chen S, Flower A, Ritchie A, Liu J, Molassiotis A, Yu H, et al. Oral Chinese herbal medicine (CHM) as an adjuvant treatment during chemotherapy for non-small cell lung cancer: A systematic review. Lung Cancer (2010) 68(2):137–45. doi: 10.1016/j.lungcan.2009.11.008
10. Li J, Li HZ, Zhu GH, Gao RK, Zhang Y, Hou W, et al. Efficacy and safety of Kanglaite injection combined with first-line platinum-based chemotherapy in patients with advanced NSCLC: a systematic review and meta-analysis of 32 RCTs. Ann Palliat Med (2020) 9(4):1518–35. doi: 10.21037/apm-20-616
11. Chen H, Yao X, Li T, Lam CW, Zhang R, Zhang H, et al. Compound Kushen injection combined with platinum-based chemotherapy for stage III/IV non-small cell lung cancer: A meta-analysis of 37 RCTs following the PRISMA guidelines. J Cancer (2020) 11(7):1883–98. doi: 10.7150/jca.40267
12. Wang J, Li G, Yu L, Mo T, Wu Q, Zhou Z. Aidi injection plus platinum-based chemotherapy for stage IIIB/IV non-small cell lung cancer: A meta-analysis of 42 RCTs following the PRISMA guidelines. J Ethnopharmacol (2018) 221:137–50. doi: 10.1016/j.jep.2018.04.013
13. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev (2015) 4:1. doi: 10.1186/2046-4053-4-1
14. Lin H. Clinical application manual of Chinese patent tumor drugs. Beijing: People's Medical Publishing House (2011).
15. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer (1981) 47(1):207–14. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6
16. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst (2000) 92(3):205–16. doi: 10.1093/jnci/92.3.205
17. Yano T, Hamatake M, Tokunaga S, Okamoto T, Yamazaki K, Miura T, et al. A prospective observational study of postoperative adjuvant chemotherapy for non-small cell lung cancer in elderly patients (>/= 75 years). Int J Clin Oncol (2022) 27(5):882–8. doi: 10.1007/s10147-022-02143-7
18. Wang S, Wang X, Zhou T, Hu S, Tian P, Li Z, et al. Effectiveness and safety of Chinese herbal injections combined with fluoropyrimidine and oxaliplatin-based chemotherapy for advanced colorectal cancer: a systematic review and meta-analysis of 63 randomized controlled trials. J Cancer (2021) 12(23):7237–54. doi: 10.7150/jca.60895
19. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.3. Chichester, UK: Cochrane (2022). Available at: www.training.cochrane.org/handbook.
20. Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ (2004) 328(7454):1490. doi: 10.1136/bmj.328.7454.1490
21. Wang D, Liu J, Jiang M. Shenqi Fuzheng Injection adjuvant chemotherapy on effectiveness and immune function of advanced gastric cancer: A Meta-analysis. Drug Eval Res (2021) 44(10):2225–33. doi: 10.7501/j.issn.1674-6376.2021.10.025
22. Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol (2008) 61(10):991–6. doi: 10.1016/j.jclinepi.2007.11.010
23. Ai L, Kou L, Yin H, Tang H, Chen H. Efficacy of xiaoaiping injection combined with docetaxel and carboplatin in the treatment of advanced lung adenocarcinoma. Modern Med J China (2015) 17(11):39–41. doi: 10.3969/j.issn.1672-9463.2015.11.012
24. Bao Z, He Y. Effects of Shenqi Fuzheng Injection Combined with chemotherapy on peripheral blood T lymphocyte subsets and tumor markers in elderly patients with advanced non-small cell lung cancer. Chin J Gerontol (2019) 39(10):2359–61. doi: 10.3969/j.issn.1005-9202.2019.10.019
25. Bian F, Wang Y, Liu Y, Wang X. Effect of cinobufagin injection combined with GP regimen on advanced non-small cell lung cancer. Shandong Med J (2015) 55(38):75–6. doi: 10.3969/j.issn.1002-266X.2015.38.031
26. Bian L, Liu C, Zhong W, Chen Y, Liu P. Effect of Shenmai injection combined with chemotherapy on advanced non-small cell lung cancer. Shandong Med J (2005) 34):37–8. doi: 10.3969/j.issn.1002-266X.2005.34.018
27. Cao J, Zhou J, Yang D, Chu J. Effect of cinobufagin injection combined with chemotherapy in the first-line treatment of advanced non-small cell lung cancer. J Int Oncol (2016) 43(10):741–3. doi: 10.3760/cma.j.issn.1673-422X.2016.10.005
28. Cao Y, Li P, Tan K, Chen R. Clinical observation of Shenmai injection in the prevention and treatment of chemotherapy adverse reactions in advanced non-small cell lung cancer. Chin J Integr Med (2006) 06):550–2. doi: 10.3321/j.issn:1003-5370.2006.06.018
29. Cao Y. Clinical study of Brucea javanica oil emulsion injection combined with chemotherapy in the treatment of advanced non-small cell lung cancer. Acta Chin Med Pharmacol (2013) 41(01):44–6. doi: 10.3969/j.issn.1002-2392.2013.01.016
30. Chen C. A randomized parallel controlled study of Kanglaite injection combined with GP regimen in the treatment of advanced non-small cell lung cancer. J Pract Traditional Chin Internal Med (2018) 32(03):46–8. doi: 10.13729/j.issn.1671-7813.z20180074
31. Chen L. Effect of Kangai Injection on immune function and quality of life of patients with advanced non-small cell lung cancer during chemotherapy. Guiding J Traditional Chin Med Pharm (2014) 20(05):29–31. doi: 10.13862/j.cnki.cn43-1446/r.2014.05.010
32. Chen L, Chai F, Fu S, Zhu S, Wang Y. Effect of Shenmai injection combined with paclitaxel and carboplatin in the treatment of non-small cell lung cancer. Med Forum (2021) 25(28):4017–9. doi: 10.19435/j.1672-1721.2021.28.008
33. Chen W, Wei T. Clinical efficacy of Kanglaite injection combined with gemcitabine cisplatin in the treatment of advanced non-small cell lung cancer and its effect on immune function of patients. Chin J Clin Oncol Rehabil (2016) 23(07):814–7. doi: 10.13455/j.cnki.cjcor.2016.07.13
34. Chen W, Lei L, Cai S, Chen L, Dang H. Clinical efficacy of Kanglaite injection combined with chemotherapy in the treatment of advanced non-small cell lung cancer. Prog Mod BioMed (2015) 15(20):3869–71. doi: 10.13241/j.cnki.pmb.2015.20.017
35. Chen X, Liang W. The curative effect of Kangai injection in the adjuvant treatment of advanced lung cancer and its effect on related cytokines. J China Prescription Drug (2021) 19(07):99–100. doi: 10.3969/j.issn.1671-945X.2021.07.047
36. Chen Y. Effect of GP regimen combined with Kanglaite in the treatment of advanced non-small cell lung cancer. Med J Chin People's Health (2018) 30(08):73–5. doi: 10.3969/j.issn.1672-0369.2018.08.036
37. Chen Y, Li Q, Zhang C, Li S. Effects of xiaoaiping injection combined with TP Chemotherapy on immune status, adverse reactions and quality of life in patients with advanced NSCLC. J Int Oncol (2019) 01):17–21. doi: 10.3760/cma.j.issn.1673-422X.2019.01.004
38. Chen Y, Sun Y, Ou W, Qin A. Clinical observation of Shenqi Fuzheng Injection combined with chemotherapy in the treatment of non-small cell lung cancer. Chin J Clin Rational Drug Use (2018) 11(19):86–7. doi: 10.15887/j.cnki.13-1389/r.2018.19.046
39. Chen Y, Zhuang W, Liu Y. Efficacy of elemene combined with DC regimen in the treatment of advanced non-small cell lung cancer. Shandong Med J (2008) 30):54–5. doi: 10.3969/j.issn.1002-266X.2008.30.024
40. Chen Z, Lu H. Treatment of non-small cell lung cancer with integrated traditional Chinese and Western Medicine. Chin J Exp Tradit Med Formulae (2013) 19(11):322–4. doi: 10.11653/syfj2013110322
41. Cheng Z, Xi F. A randomized parallel controlled study of Shenqi Fuzheng Injection Combined with chemotherapy in the treatment of advanced non-small cell lung cancer (Qi deficiency). J Pract Traditional Chin Internal Med (2017) 31(12):43–5. doi: 10.13729/j.issn.1671-7813.2017.12.15
42. Chi H, Weng J, Cheng S. Effect of cinobufagin combined with docetaxel carboplatin chemotherapy on patients with advanced non-small cell lung cancer. Chin Rem Clin (2019) 19(22):3919–21. doi: 10.11655/zgywylc2019.22.039
43. Cui Y, Wang W. Clinical observation of Aidi injection combined with NP regimen in the treatment of advanced non-small cell lung cancer. Chin J Cancer Prev Treat (2005) 06):456–8. doi: 10.3969/j.issn.1673-5269.2005.06.016
44. Dai Z, Dong S. Observe the effect of compound Sophora flavescens injection + chemotherapy in the treatment of elderly non-small cell lung cancer. Med Diet Health (2020) 18(04):105–6.
45. Ding C, Yang L. Shenqi Fuzheng Injection combined with chemotherapy in the treatment of 35 cases of advanced non-small cell lung cancer. Shanxi J Tradit Chin Med (2012) 33(01):30–2. doi: 10.3969/j.issn.1000-7369.2012.01.016
46. Ding P, Huang H, Lin K. Observation on efficacy of Shenqifuzheng Injection combined with chemotherapy in treatment of elderly patients with non small cell lung cancer. Eval Anal Drug-Use Hospitals China (2016) 16(04):461–3. doi: 10.14009/j.issn.1672-2124.2016.04.012
47. Gao L. Clinical study of Yanshu compound Kushen Injection in treatment of lung cancer. Liaoning J Tradit Chin Med (2019) 46(12):2583–5. doi: 10.13192/j.issn.1000-1719.2019.12.034
48. Gao Y. Efficacy and safety of DP chemotherapy regimen combined with Kangai injection in the treatment of advanced non-small cell lung cancer. Guide China Med (2019) 17(28):157–8. doi: 10.15912/j.cnki.gocm.2019.28.127
49. Gao Z, Xu X. Clinical effect of Kangai injection combined with chemotherapy on patients with advanced non-small cell lung cancer and its influence on quality of life. Chin J Clin Rational Drug Use (2021) 14(29):86–8. doi: 10.15887/j.cnki.13-1389/r.2021.29.034
50. Geng K, Dong J, Su H, Dai C, Chen J, Yue X. Efficacy and safety of Aidi injection combined with gemcitabine and cisplatin in the treatment of advanced non-small cell lung cancer. Henan Med Res (2020) 29(12):2231–2. doi: 10.3969/j.issn.1004-437X.2020.12.059
51. Guang X. Comprehensive evaluation of Kangai Injection combined with chemotherapy in the treatment of advanced NSCLC. J North Pharm (2015) 12(10):26–7.
52. Gu N, Li Z. Clinical observation of Xiaoaiping Injection combined with chemotherapy in treatment with advanced non-small cell lung cancer. China Health Standard Manage (2016) 7(19):120–1. doi: 10.3969/j.issn.1674-9316.2016.19.081
53. Guo W, Zhang G, Li J. Effect of Addie injection combined with PC chemotherapy on bone marrow suppression and quality of life in patients with advanced non-small cell lung cancer. J Med Forum (2017) 38(11):46–8. doi: 10.ssss/j.issn.1672-3422.2017.11.015
54. Gu Y, Pang L. Data from: Docetaxel+cisplatin scheme of joint compound sophora injection curativeeffect analysis for the treatment of advanced non-small cell lung cancer. Beijing, China: CNKI (2016). Available at: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=ZYZZ201604001614&DbName=CPFD2016.
55. Han R, Wang A, Yang G, Yang Y, Liu B, Cao X, et al. Effect of aidi injection on chemotherapy and immune function of advanced non small cell lung cancer. Chin J Gerontol (2015) 35(17):4878–9. doi: 10.3969/j.issn.1005-9202.2015.17.067
56. He L, Wu X, Yu X, Chen H. Clinical effect and quality of life of patients with advanced lung cancer treated with Kanglaite injection. Modern Pract Med (2017) 29(03):329–30. doi: 10.3969/j.issn.1671-0800.2017.03.023
57. He W. Effect of Shenqi Fuzheng injection assisted with GP regimen on advanced non-small cell lung cancer. Pract Clin J Integrated Traditional Chin Western Med (2021) 21(11):44–5. doi: 10.13638/j.issn.1671-4040.2021.11.022
58. He Z, Liu Y, Wu Y. Effective and safety of Addie Injection and docetaxel and carboplatin chemotherapy for non-small cell lung cancer. Chin Arch Tradit Chin Med (2016) 34(06):1459–61. doi: 10.13193/j.issn.1673-7717.2016.06.051
59. Huang W, Zheng J, Gao B. Clinical efficacy of Aidi injection combined with GP regimen in the treatment of advanced non-small cell lung cancer and its influence on the immune function of patients. J Med Theory Pract (2017) 30(07):995–7. doi: 10.19381/j.issn.1001-7585.2017.07.029
60. Hu Q, Qi H. Clinical efficacy and serum SIL-2R、CEA、VEGF level changes of Addie Injection combined with docetaxel and carboplatin for NSCLC. Pract J Cancer (2015) 30(10):1469–72. doi: 10.3969/j.issn.1001-5930.2015.10.013
61. Hu X, Liu X, Chen X. Clinical effect of Xiaoaiping Injection combined with gemcitabine and carboplatin in treatment of IIIB and IV non-small cell lung cancer. Drug Eval Res (2017) 40(02):266–9. doi: 10.7501/j.issn.1674-6376.2017.02.026
62. Hu Y, Cui Q, Ma D, Geng L. Effect of Xiaoaiping injection on T lymphocyte subsets in patients with non-small cell lung cancer. Chin J Clin (2019) 47(05):570–3. doi: 10.3969/j.issn.2095-8552.2019.05.022
63. Jia J. Effect of Kanglaite injection on immune function and adverse reactions in advanced NSCLC patients with chemotherapy. J Community Med (2018) 16(05):11–3.
64. Jiang H, Zheng K, Tong L. Effect of Kangai injection combined with gemcitabine+cisplatin regimen on the efficacy and quality of life of patients with advanced non-small cell lung cancer. Chin J Primary Med Pharm (2018) 25(11):1447–51. doi: 10.3760/cma.j.issn.1008-6706.2018.11.023
65. Ji S, Ma D, Cui H, Chen H. Clinical observation of cinobufotalin injection combined with docetaxel and secaplatin in the treatment of advanced non-small cell lung cancer. Chongqing Med (2017) 46(34):4831–3. doi: 10.3969/j.issn.1671-8348.2017.34.026
66. Liang J, Cai H, Chen Z. Effects of kanglaite injection adjuvant NP chemotherapy on lung cancer. J Gannan Med Univ (2018) 38(03):232–5. doi: 10.3969/j.issn.1001-5779.2018
67. Lian Z, Lu Y, Hou E, Wang X. Combination of GP regimen and Kanglaite in the treatment of advanced non-small cell lung cancer. Chin J Lung Cancer (2006) 01):74–7.
68. Li H, Shang G, Dong L, Chen Y, Li Y, Wang W, et al. Shenmai Injection combined with systemic chemotherapy treatment of advanced non-small cell lung cancer and effect on TNF-α in serum. Chin Arch Tradit Chin Med (2012) 30(11):2442–5. doi: 10.13193/j.archtcm.2012.11.76.lihj.080
69. Li H, Huang J, Lei Y. Effect of Shenqi Fuzheng Injection on the efficacy and adverse reaction of TP chemotherapy scheme in the treatment of non small cell lung cancer. Chin J Clin Rational Drug Use (2012) 5(09):77–8. doi: 10.15887/j.cnki.13-1389/r.2012.09.113
70. Li H. Clinical effect of Kanglaite Injection combined with gemcitabine and cisplatin on advanced non-small-cell lung cancer and the impact on immune function. Pract J Cardiac Cereb Pneumal Vasc Dis (2017) 25(05):121–3. doi: 10.3969/j.issn.1008-5971.2017.05.033
71. Li J, Han L, Yuan B, Dai X. Clinical observation on Kangai Injection combined with chemotherapy in the treatment of advanced non small cell lung cancer. J China Traditional Chin Med Inf (2011) 3(10):53–5.
72. Lin X. Clinical observation of Aidi Injection combined with paclitaxel and cisplatin regimen in the treatment of advanced non-small cell lung cancer. Med Innovation China (2018) 15(03):62–5. doi: 10.3969/j.issn.1674-4985.2018.03.016
73. Li Q, Chen B. Clinical effects of Xiaoaiping Injection combined with GP chemotherapy regimen in treating patients with advanced non - small cell lung cancer. Chin Arch Tradit Chin Med (2016) 34(04):785–7. doi: 10.13193/j.issn.1673-7717.2016.04.004
74. Li S, Ma N, Wang G, Zhang H, Li H. 30 cases of advanced non small cell lung cancer treated with Shenmai Injection combined with chemotherapy. Shandong J Traditional Chin Med (2005) 09):532–3. doi: 10.16295/j.cnki.0257-358x.2005.09.009
75. Li S, Sun C, Sun Q, Chen S, Zhang Y. Clinical study of compound Matrine Injection combined with chemotherapy in the treatment of non-small cell lung cancer. Liaoning J Tradit Chin Med (2017) 44(03):556–7. doi: 10.13192/j.issn.1000-1719.2017.03.038
76. Liu H, Nie Q, Lu Y, Liu X. Clinical efficacy of Aidi injection combined with GP regimen on advanced non-small cell lung cancer and its effect on serum VEGF expression and immune function. Jiangxi J Traditional Chin Med (2019) 50(03):42–4.
77. Liu J, Wen F, Li B. Clinical effect of comprehensive treatment of Elemene combined with Gemcitabine and Cisplatin in the treatment of advanced non -small cell lung cancer. China Med Her (2015) 12(35):80–2.
78. Liu J, An Y, Yang W, Zhao K. Clinical study on Xiaoaiping Injection combined with GP regimen in the treatment of advanced lung squamous cell carcinom. Med Innovation China (2016) 13(13):97–101. doi: 10.3969/j.issn.1674-4985.2016.13.027
79. Liu Q, Fan R, Zhou L. Therapeutic effect of Aidi injection combined with TP regimen on non-small cell lung cancer. Contemp Med (2020) 26(07):139–41. doi: 10.3969/j.issn.1009-4393.2020.07.055
80. Liu S, Li X, Liu J, Xu Y. Effects of brucea javanica oil emulsion injection on the cisplatin chemotherapy regimen in the treatment of non-small cell lung cancer and its toxic and side. Clin Res Pract (2019) 4(29):56–8. doi: 10.19347/j.cnki.2096-1413.201929024
81. Liu Y, Ding X, Liu M. Guanqi fuzheng injection combined with GP chemotherapy to treat non-small cell lung cancer. Shenzhen J Integrated Traditional Chin Western Med (2021) 31(19):48–50. doi: 10.16458/j.cnki.1007-0893.2021.19.019
82. Luo S, Huang Y, Shan H, Yang Y, Mo C, Wu X. Clinical observation on the treatment of advanced non-small cell lung cancer with Shenqi Fuzheng injection combined with paclitaxel and cisplatin. Chin Clin Oncol (2007) 05):381–2. doi: 10.3969/j.issn.1009-0460.2007.05.019
83. Lu X, Yang Z, Deng C. Influence of Shenfu injection with chemotherapy on advanced non small cell lung cancer immmune function. Acta Chin Med (2010) 25(06):1049–51.
84. Lv J. Clinical observation of Shenqi Fuzheng Injection as an adjuvant chemotherapy in the treatment of advanced non small cell Lung cancer. China Med Her (2008) 5(36):73–4. doi: 10.3969/j.issn.1673-7210.2008.36.045
85. Ma J, Zhan Y, Huang C. Effect of Aidi injection combined with chemotherapy on non-small cell lung cancer. Guangxi Med J (2010) 32(08):932–3.
86. Ma J. Efficacy of lentinan combined with NP regimen in treatment of patients with advanced nonsmall-cell lung cancer and its effect on immunologic function. Chin J Biochem Pharm (2014) 34(08):154–6.
87. Jun-jie MA, Hui-ping L. Efficacy of Aidi Injection on overexpression of P-Glycoprotein induced by vinorelbine and cisplatin regimen in patients with non-small cell lung cancer. Chin J Integr Med (2017) 23(07):504–9.
88. Ma M. Effect of Aidi injection combined with GP chemotherapy on the immune function and coagulation function of patients with advanced NSCLC of qi yin deficiency type. Asia-Pacific Traditional Med (2017) 13(21):108–10. doi: 10.11954/ytctyy.201721046
89. Mao G, Li S, Ni G, Liu M, Feng Y. Clinical observation on Aidi Injection combined with Pemetrexed and Platinum drugs in the first-Line treatment of non-small cell lung carcinoma(Lung Adenocarcinoma). Eval Anal Drug-Use Hospitals China (2021) 21(10):1215–8. doi: 10.14009/j.issn.1672-2124.2021.10.016
90. Ma Y, Wang W. Clinical efficacy and safety of Aidi Injection combined with GC regimen for treatment of advanced non-small cell lung cancer. J Guangzhou Univ Tradit Chin Med (2020) 37(11):2098–102. doi: 10.13359/j.cnki.gzxbtcm.2020.11.009
91. Mei C, Wang K, Lei N, Mei Z. Clinical observation of xiaoaiping injection combined with TP regimen in the treatment of advanced non-small cell lung cancer. China Pharm (2015) 26(11):1531–3. doi: 10.6039/j.issn.1001-0408.2015.11.31
92. Miao C, Yu Q, Liang H. Clinical observation of cinobufagin injection combined with chemotherapy in the treatment of advanced non-small cell lung cancer. Chin J Integr Med (2007) 07):657–8. doi: 10.3321/j.issn:1003-5370.2007.07.027
93. Mo Y. Addie Injection combined with Docetaxel and Cisplatin chemotherapy on treatment of non small cell lung cancer. J Henan Univ Sci Technol (Medical Science) (2015) 33(04):277–8. doi: 10.15926/j.cnki.issn1672-688x.2015.04.013
94. Mu Q, Li Y. Effect of Kanglaite injection combined with DC chemotherapy regimen on serum T cell subsets and quality of life in patients with advanced non-small cell lung cancer. J Med Forum (2018) 39(10):120–1.
95. Niu H, Wu C, Jiang J, Xu B, Zhao J, Zhou W, et al. Clinical observation of Brucea javanica oil emulsion injection combined with DP regimen in the treatment of advanced non-small cell lung cancer. Chin J Postgraduates Med (2011) 22):13–6. doi: 10.3760/cma.j.issn.1673-4904.2011.22.005
96. Peng S. Effect of Aidi injection combined with chemotherapy in the treatment of advanced non-small cell lung cancer. Healthmust-Readmagazine (2020) 36):46.
97. Ren Z, Ren X, Zhang Y, Han Y, Zhang W. Observation on the multi index curative effect of compound sophora flavescens injection combined with chemotherapy in the treatment of advanced non-small cell lung cancer. Chin J Surg Oncol (2017) 9(06):370–2. doi: 10.3969/j.issn.1674-4136.2017.06.009
98. Sang X. Addition of Compound Kushen Injection to Vinorelbine Plus Cisplatin for patients with nonsmall cell lung cancer: clinical observation. Eval Anal Drug-Use Hospitals China (2012) 12(07):645–8. doi: 10.14009/j.issn.1672-2124.2012.07.014
99. Shen J, Qin H, Liu J, Huang C. Data from: Clinical observation on the combination of Shenmai injection and NP regimen in the treatment of 30 cases of advanced non-small cell lung cancer. Shanxi, China: Wangfang Data (2012). Available at: http://www.wanfangdata.com.cn/details/detail.do?_type=conference&id=7731976.
100. Shen L, Qiu W, Lan C, Yu X, Du L. Curative observation of Xiaoaiping Injection combined with TP regimen in the treatment of patients with advanced non-small cell lung cancer. Chin Foreign Med Res (2015) 13(32):8–9. doi: 10.14033/j.cnki.cfmr.2015.32.004
101. Shen R, Zhang Y, Yu P, Liu J. Effect of Aidi injection combined with gemcitabine and cisplatin in the treatment of advanced lung cancer. Modern Med Health Res Electronic J (2021) 5(08):29–31.
102. Shi Z, Chen F, Luo J. Observation of Shenmai injection combined chemotherapy protocols on no small-cell lung cancer in advanced stage. Hebei J Traditional Chin Med (2008) 30(11):1200–2. doi: 10.3969/j.issn.1002-2619.2008.11.050
103. Song F, Song F, Li T, Li J, Wu X, Kang N. Elemene combined with NP chemotherapy in the treatment of advanced non-small-cell lung carcinoma. Med Recapitulate (2015) 21(09):1710–2. doi: 10.3969/j.issn.1006-2084.2015.09.068
104. Su J. Effect of PC chemotherapy regimen combined with Kanglaite injection in the treatment of advanced lung adenocarcinoma. Henan Med Res (2018) 27(06):1017–8. doi: 10.3969/j.issn.1004.437X.2018.06.025
105. Sun S, Zhu X, Huang J, Xu T, Lin Y. Effect of Kanglaite injection combined with chemotherapy for improvement of quality of life in advanced stage NSCLC patients. J Pract Oncol (2012) 27(05):506–10. doi: 10.13267/j.cnki.syzlzz.2012.05.025
106. Tang X. Clinical observation of aidi injection combined with docetaxel and cisplatin in the treatment of 36 patients with advanced non-small cell lung cancer. Cancer Res Clin (2012) 05):343–5. doi: 10.3760/cma.j.issn.1006-9801.2012.05.022
107. Tang X, Liu J, Zhao H. Efficacy of Shenmai Injection combined with gemcitalbine and cisplatin in the treatment of lung adenocarcinoma and its effect on the changes of tumor CT net added value. China Pharm (2016) 27(09):1191–4. doi: 10.6039/j.issn.1001-0408.2016.09.13
108. Tan H, Tan C, Feng X, Pan B. Efficacy of GP chemotherapy combined with Aidi Injection on elderly advanced non-small cell lung cancer and its effects on immune function and quality of life of patients. Pract J Cancer (2020) 35(08):1327–30. doi: 10.3969/j.issn.1001-5930.2020.08.028
109. Tian H, Yu S, Wang B, Liang G, Huang X. Effect of Fructus Bruceae Oil Emulsion on cellular immune function and quality of life in patients with non small cell lung cancer. Chin J Integr Med (2007) 02):157–9. doi: 10.3321/j.issn:1003-5370.2007.02.016
110. Wang C, Wang C. Clinical observation of Kanglaite injection combined with docetaxel cisplatin regimen in the treatment of advanced non-small cell lung cancer. Chin Rem Clin (2018) 18(06):989–91. doi: 10.11655/zgywylc2018.06.062
111. Wang H. Evaluation of Shenqi Fuzheng Injection assisted by GP regimen chemotherapy in the treatment of stage IIIB∼IV non small cell lung cancer. Clin Med (Lond) (2021) 41(11):123–5. doi: 10.19528/j.issn.1003-3548.2021.011.047
112. Wang J, Li G, Gong S. Clinical study of GP regimen combined with Brucea javanica oil emulsion in first-line treatment of advanced non-small cell lung cancer. Chin J Postgraduates Med (2012) 24):11–3. doi: 10.3760/cma.j.issn.1673-4904.2012.24.005
113. Wang J. Effect of Compound Sophora flavescens Injection combined with Gemcitabine and Cisplatin in the treatment of non small cell lung cancer. Pract Clin J Integrated Traditional Chin Western Med (2020) 20(13):94–6. doi: 10.13638/j.issn.1671-4040.2020.13.047
114. Wang L, Men X, Gao S. Clinical observation on the effect of Brucea Javanica Oil Emulsion combined with chemotherapy in the treatment for middle-advanced nonsmall-cell lung cancer. Med Recapitulate (2015) 21(05):879–81. doi: 10.3969/j.issn.1006-2084.2015.05.042
115. Wang T, Dou C. Clinical observation on Shenqi Fuzheng injection combined with chemotherapy for 41 cases of non-small cell lung cancer. J Tradit Chin Med (2014) 55(09):775–7. doi: 10.13288/j.11-2166/r.2014.09.015
116. Wang Y. Clinical study of Compound Kushen Injection combined with chemotherapy on advanced non-small cell lung cancer. Acta Chin Med (2015) 30(12):1710–1. doi: 10.16368/j.issn.1674-8999.2015.12.591
117. Wang Y, Bai H, Li Q, Li L, Jing X, Jiang H, et al. A clinical study of Shenfu injection combined with GP regimen in the treatment of vitalenergy deficiency type patients with advanced non-small cell lung cancer. J Mod Oncol (2016) 24(13):2081–5. doi: 10.3969/j.issn.1672-4992.2016.13.020
118. Wang Y, Ye L, Liu K. Effects of Kanglaite Injection combined with chemotherapy in treatment of non-small-cell lung cancer. J Hubei Univ Med (2013) 32(05):387–90. doi: 10.7543/j.issn.1006-9674.2013.05.006
119. Wang Y, Yu S, Jin C. Clinical observation on 44 cases of non-small cell lung cancer treated with integrated traditional Chinese and western medicine. Chin J Modern Drug Appl (2013) 7(19):152. doi: 10.14164/j.cnki.cn11-5581/r.2013.19.022
120. Wang Z, Guo W, Gong Y, Wang D. Effect of Kangai injection combined with PC chemotherapy regimen on patients with advanced non-small cell lung cancer. China Clin Pract Med (2018) 02):4–5. doi: 10.3760/cma.j.issn.1673-8799.2018.02.002
121. Wen H. Observation on the therapeutic effect of integrated traditional Chinese and western medicine on elderly patients with advanced non-small cell lung cancer. Mod J Integr Tradit Chin West Med (2014) 23(21):2325–6. doi: 10.3969/j.issn.1008-8849.2014.21.018
122. Wu L, Jiang B, Yang J, Li H. 30 cases of advanced non small cell lung cancer treated with Shenqi Fuzheng Injection combined with chemotherapy. Chin J Integr Med (2004) 06):567–8. doi: 10.3321/j.issn:1003-5370.2004.06.031
123. Wu Z. Adjuvant effect of Shenqi Fuzheng Injection on advanced NSCLC chemotherapy patients. Smart Healthcare (2019) 5(12):103–4. doi: 10.19335/j.cnki.2096-1219.2019.12.051
124. Xiao X. Clinical study of Kanglaite Injection combined with PC chemotherapy in the treatment of stage III B-IV non small cell lung cancer. J Shanxi Health Vocat Coll (2020) 30(03):62–4.
125. Xing J, Wang L, Lv H, Zhou Q, Qi X. Application of Aidi combined with NP regimen in the treatment of elderly non-small cell lung cancer. Chin J Primary Med Pharm (2014) 21(02):281. doi: 10.3760/cma.j.issn.1008-6706.2014.02.056
126. Xiong X, Sun D. Synergistic and toxigenic effects of Aidi Injection on chemotherapy in advanced non-small cell lung cancer effects on serum VEGF and T lymphocyte subsets. Chin Arch Tradit Chin Med (2021) 39(11):217–20. doi: 10.13193/j.issn.1673-7717.2021.11.051
127. Xu D. Therapeutic effect of Aidi Injection combined with chemotherapy on advanced non-small cell lung cancer. Med Inf (2021) 34(22):127–9. doi: 10.3969/j.issn.1006-1959.2021.22.039
128. Xu J, Zheng R, Wang Y. The clinical efficacy and safety of elemene injection combined with GP chemotherapy in the treatment of advanced non-small cell lung cancer. Pract J Cancer (2018) 33(11):1878–81. doi: 10.3969/j.issn.1001-5930.2018.11.041
129. Xu L, Wu D. Clinical effects of Shenmai Injection combined with PC chemotherapy in the treatment of lung adenocarcinoma patients. Med Equip (2021) 34(21):127–9. doi: 10.3969/j.issn.1002-2376.2021.21.059
130. Xu Y, Li Y. Effect of Compound Sophora flavescens Injection combined with chemotherapy on regulatory T and B lymphocytes in peripheral blood of patients with advanced NSCLC. J Clin Res (2017) 34(11):2192–4. doi: 10.3969/j.issn.1671-7171.2017.11.039
131. Xu Z. Effect of Aidi injection combined with GP chemotherapy regimen on patients with advanced non-small cell lung cancer. Henan Med Res (2020) 29(27):5133–5. doi: 10.3969/j.issn.1004-437X.2020.27.060
132. Yang B. Analysis of curative effect of integrated traditional Chinese and western medicine on advanced non-small cell lung cancer. Asia-Pacific Traditional Med (2014) 10(15):83–4.
133. Yang G, Wang X, Lin H, Li P, Chen X, Yang Y, et al. Data from: Clinical observation of Kanglaite combined with chemotherapy in the treatment of advanced non-small cell lung cancer. Beijing, China: Wangfang Data (2010). Available at: http://www.wanfangdata.com.cn/details/detail.do?_type=conference&id=7372425.
134. Yan Q, Xing G, Pan Q. Effect of GP regimen combined with Kanglaite on advanced non-small cell lung cancer. Pract Clin J Integrated Traditional Chin Western Med (2018) 18(09):112–3. doi: 10.13638/j.issn.1671-4040.2018.09.056
135. Yao D, Cai Y, Chen Y. Clinical observation of Shenqi Fuzheng injection combined with GP regimen in the treatment of advanced non-small cell lung cancer. Chin J Clin Res (2013) 26(12):1378–9.
136. Ye C, Zhao F, Xing S, Wang J. Clinical observation of Kanglaite Injection combined with Gemcitabine Cisplatin regimen for advanced non-small cell lung cancer. Clin Res (2019) 27(07):24–6.
137. Ye H, Qin B, Su M, Wang Z, Wang Z. Clinical effect of Brucea javanica oil emulsion combined with chemotherapy on advanced non-small cell lung cancer. Guiding J Traditional Chin Med Pharm (2015) 21(07):35–8. doi: 10.13862/j.cnki.cn43-1446/r.2015.07.012
138. Ye Z. Clinical analysis of Kangai injection plus chemotherapy in the treatment of advanced non-small cell lung cancer. Chin Baby (2018) 7):91. doi: 10.3969/j.issn.1671-2242.2018.07.084
139. Yu F, Li K. Clinical observation of Shenqi Fuzheng Injection as an adjuvant chemotherapy in the treatment of non small cell lung cancer. Chin J Integr Med (2007) 02):166–7. doi: 10.3321/j.issn:1003-5370.2007.02.020
140. Yu F, Jiang D, Zhao Y, Liu C, Yang G. Control effect of DP chemotherapy regimen combined with Kangai injection on advanced non-small cell lung cancer. Chin J Public Health Eng (2018) 17(02):278–80.
141. Zhang H, Zhang X. Clinical observation of Aidi injection combined chemotherapy for advanced nonsmall-cell lung cancer. J Hunan Univ Chin Med (2009) 29(04):65–6. doi: 10.3969/j.issn.1674-070X.2009.04.022
142. Zhang H, Wei W, Yan L, Liu H. Clinical observation of Compound Matrine Injection combined with TP chemotherapy in the treatment of non small cell lung cancer. Heilongjiang Med J (2019) 43(01):65–7. doi: 10.3969/j.issn.1004-5775.2019.01.27
143. Zhang J, Shi X, Meng W, Ma F, Wang Z, Zhao J. Efficacy of treatment of Aidi injection combination pemetrexed and cisplatin for advanced lung adenocarcinoma. Chin J Clin Oncol Rehabil (2015) 22(07):799–801. doi: 10.13455/j.cnki.cjcor.2015.07.10
144. Zhang J, Yang J, Yuan X. Clinical Observation on the Treatment of Advanced Non small Cell Lung Cancer with Compound Sophora flavescens Injection. New J Tradit Chin Med (2015) 47(01):190–2. doi: 10.13457/j.cnki.jncm.2015.01.091
145. Zhang J, Yang L. Clinical observation of combined Kangai injection with TP in the treatment of advanced non-small cell lung cancer. J Mod Oncol (2010) 18(06):1132–4. doi: 10.3969/j.issn.1672-4992.2010.06.31
146. Zhang K, Feng B, Yang J. Effect analysis of Kanglaite Injection combined with chemotherapy for patients with advanced nonsmall cell lung cancer. Chin J Modern Drug Appl (2021) 15(08):107–9. doi: 10.14164/j.cnki.cn11-5581/r.2021.08.040
147. Zhang M. Clinical efficacy and safety of Kanglaite injection in patients with advanced non-small cell lung cancer. J Med Theory Pract (2019) 32(01):54–6. doi: 10.19381/j.issn.1001-7585.2019.01.025
148. Zhang M, He W, Zhang H, Liu X. Efficacy of adjuvant treatment with sophora flavescens injection for advanced non-small cell lung cancer. Chin J Clin Oncol Rehabil (2019) 26(03):327–9. doi: 10.13455/j.cnki.cjcor.2019.03.20
149. Zhang S, Wang Y, Kang W. Kang Ai Injection combined with platinum - containing chemotherapy in the treatment of advanced non-small cell lung cancer in older patients. Int J Geriatrics (2021) 42(05):284–6. doi: 10.3969/j.issn.1674-7593.2021.05.008
150. Zhang Z, Zhang S, Huo X. Effect of Compound Sophora flavescens injection combined with pemetrexed+cisplatin regimen on quality of life, immune function and tumor markers in patients with advanced non-small cell lung cancer. China Clin Pract Med (2019) 02):21–2. doi: 10.3760/cma.j.issn.1673-8799.2019.02.006
151. Zhao J, Li J. Application effect of Aidi injection combined with GP scheme in advanced non-small cell lung cancer. Clin Res Pract (2019) 4(25):117–9. doi: 10.19347/j.cnki.2096-1413.201925049
152. Zhao K, Yang J, Wang J, Li Y, Wang H, Zhang A. Influence of Compound Matrine Injection combined with GP regimen on therapeutic efficacy and the quality of life in patients with advanced non-small cell lung cancer. Pract Prev Med (2012) 19(12):1853–4. doi: 10.3969/j.issn.1006-3110.2012.12.036
153. Zhao L, Cui Q, Cui L, Jiao F. Evaluation of shenmai injection combined with chemotherapy in the treatment of advanced non-small cell lung cancer. Pract Clin J Integrated Traditional Chin Western Med (2018) 18(05):97–8. doi: 10.13638/j.issn.1671-4040.2018.05.049
154. Zhao Q. Clinical observation of Shenqi Fuzheng Injection in the treatment of advanced non small cell carcinoma. Guide China Med (2019) 17(15):194. doi: 10.15912/j.cnki.gocm.2019.15.146
155. Zhao W, Chen D, Chen J. Observation of short-term therapeutic and adverse effect of lentinan and gemcitabine plus cisplatin as the first-line treatment for advanced non-small cell lung cancer. Chin J Clin Pharmacol Ther (2013) 18(01):71–7.
156. Zhao Z, Ma Y. Effect of Shenqi Fuzheng Injection on the adverse reactions of chemotherapy in patients with advanced non-small cell lung cancer. Med J Wuhan Univ (2014) 35(05):744–6. doi: 10.14188/j.1671-8852.2014.05.062
157. Zhou Y, Zhang Y, Sun L. Efficacy and safety of elemene injection combined with PC chemotherapy in the treatment of advanced non-small cell lung cancer. Mod J Integr Tradit Chin West Med (2020) 29(06):615–8. doi: 10.3969/j.issn.1008-8849.2020.06.011
158. Zhu J, You Y. Improvement effect of Kanglaite Injection combined with GP program on the immunity of patients with non-small-cell lung cancer at advanced stage. Henan Traditional Chin Med (2016) 36(11):1943–5. doi: 10.16367/j.issn.1003-5028.2016.11.0781
159. Zhu J. Effect of Aidi injection and docetaxel combined with carboplatin chemotherapy on non-small cell lung cancer. J Bethune Med Sci (2017) 15(01):38–40. doi: 10.16485/j.issn.2095-7858.2017.01.016
160. Zhu L, Guo J. Clinical study of Yanshu injection on treating advanced non-small cell lung cancer. Chin J Clin Oncol Rehabil (2011) 18(04):380–5. doi: 10.13455/j.cnki.cjcor.2011.04.032
161. Zhu Q, Chen Y. Clinical observation of Taxotere plus Cisplatin chemotherapy in combination with Aidi Injection in the treatment of non-small cell advanced lung cancer. Chin J Pharmacoepidemiol (2006) 03):137–9. doi: 10.3969/j.issn.1005-0698.2006.03.004
162. Zhu S, Wang H, Xu Y, Chen J, Zhang B. Clinical study of Kangai Injection combined with PP regimenin the treatment of advanced lung adenocarcinoma patients with spleen-lung Qi deficiency. Her Med (2020) 39(04):572–6. doi: 10.3870/j.issn.1004-0781.2020.04.026
163. Ni M, Wu Z, Wang H, Zhou W, Wu C, Stalin A, et al. A multidimensional bayesian network meta-Analysis of Chinese herbal injections for treating non-small cell lung cancer with gemcitabine and cisplatin. Front Pharmacol (2021) 12:739673. doi: 10.3389/fphar.2021.739673
164. Wang D, Xu Y, Huang T, Peng W, Zhu D, Zhou X, et al. Clinical efficacy and safety of NSCLC ancillary treatment with compound Kushen injection through immunocompetence regulation: A systematic review and meta-analysis. Phytomedicine (2022) 104:154315. doi: 10.1016/j.phymed.2022.154315
165. Wang DL, Qi FH, Tang W, Wang FS. Chemical constituents and bioactivities of the skin of Bufo bufo gargarizans Cantor. Chem Biodivers (2011) 8(4):559–67. doi: 10.1002/cbdv.201000283
166. Zhan X, Wu H, Wu H, Wang R, Luo C, Gao B, et al. Metabolites from bufo gargarizans (Cantor 1842): A review of traditional uses, pharmacological activity, toxicity and quality control. J Ethnopharmacol (2020) 246:112178. doi: 10.1016/j.jep.2019.112178
167. Ge G, Yan Y, Cai H. Ginsenoside rh2 inhibited proliferation by inducing ROS mediated ER stress dependent apoptosis in lung cancer cells. Biol Pharm Bull (2017) 40(12):2117–24. doi: 10.1248/bpb.b17-00463
168. Zhao M, Chen Q, Xu W, Wang H, Che Y, Wu M, et al. Total ginsenosides extract induce autophagic cell death in NSCLC cells through activation of endoplasmic reticulum stress. J Ethnopharmacol (2019) 243:112093. doi: 10.1016/j.jep.2019.112093
169. Zheng Q, Wang X, Liu J, Peng C, Xiong L, Zhu Y, et al. Identification of chemical constituents in Shenfu injection and study on anti-inflammatory activities of its polyacetylene compounds. China Pharm (2022) 33(16):1931–6. doi: 10.6039/j.issn.1001-0408.2022.16.03
170. Chen Y, Sun Y, Zhao Q, Liu C, Wang C. Shenmai injection enhances cisplatin-induced apoptosis through regulation of Mfn2-dependent mitochondrial dynamics in lung adenocarcinoma A549/DDP cells. Cancer Drug Resist (2021) 4(4):1047–60. doi: 10.20517/cdr.2021.94
171. Zheng CL, Hou KZ, Wang AQ, Fang WX, Yu ST, Liang JE, et al. Kang-ai injection inhibits gastric cancer cells proliferation through IL-6/STAT3 pathway. Chin J Integr Med (2022) 28(6):524–30. doi: 10.1007/s11655-020-3265-6
172. Xiong Y, Zhao Q, Gu L, Liu C, Wang C. Shenqi Fuzheng injection reverses cisplatin resistance through mitofusin-2-mediated cell cycle arrest and apoptosis in A549/DDP cells. Evid Based Complement Alternat Med (2018) 2018:8258246. doi: 10.1155/2018/8258246
173. Wen J, Yang T, Wang J, Ma X, Tong Y, Zhao Y. Kanglaite Injection combined with chemotherapy versus chemotherapy alone for the improvement of clinical efficacy and immune function in patients with advanced non-small-cell Lung cancer: a systematic review and meta-analysis. Evid Based Complement Alternat Med (2020) 2020:8586596. doi: 10.1155/2020/8586596
Keywords: advanced non-small cell lung cancer, Chinese herbal injection, meta-analysis, platinum-based chemotherapy, randomized controlled trial, systematic review
Citation: Cao K, Hu S, Wang D, Qiao C, Wang Z, Wang J and Hou W (2024) Clinical efficacy and safety of Chinese herbal injections in combination with platinum-based chemotherapy for advanced non-small cell lung cancer: a systematic review and meta-analysis of 140 randomized controlled trials. Front. Oncol. 14:1307836. doi: 10.3389/fonc.2024.1307836
Received: 05 October 2023; Accepted: 16 January 2024;
Published: 02 February 2024.
Edited by:
Prem Prakash Kushwaha, Case Western Reserve University, United StatesReviewed by:
Ankit Kushwaha, Stanford University, United StatesAshutosh Prince, Cleveland State University, United States
Copyright © 2024 Cao, Hu, Wang, Qiao, Wang, Wang and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Hou, aG91d2VpMTk2NEAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Kangdi Cao
Kangdi Cao Shuaihang Hu1†
Shuaihang Hu1† Dandan Wang
Dandan Wang Chenxi Qiao
Chenxi Qiao Wei Hou
Wei Hou