- 1College of First Clinical Medicine, Shandong University of Traditional Chinese Medicine, Jinan, China
- 2Department of Otorhinolaryngology, the Second Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 3Department of Thyroid and Breast Surgery, Weifang People’s Hospital, Weifang, China
- 4College of Traditional Chinese Medicine, Weifang Medical University, Weifang, China
- 5Department of Oncology, Weifang Traditional Chinese Hospital, Weifang, China
- 6Department of Cardiovascular, Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
Background: Patients with schizophrenia are at a higher risk of developing cancer. However, the causal relationship between schizophrenia and different tumor types remains unclear.
Methods: Using a two-sample, two-way Mendelian randomization method, we used publicly available genome-wide association analysis (GWAS) aggregate data to study the causal relationship between schizophrenia and different cancer risk factors. These tumors included lung adenocarcinoma, lung squamous cell carcinoma, small-cell lung cancer, gastric cancer, alcohol-related hepatocellular cancer, tumors involving the lungs, breast, thyroid gland, pancreas, prostate, ovaries and cervix, endometrium, colon and colorectum, and bladder. We used the inverse variance weighting (IVW) method to determine the causal relationship between schizophrenia and different tumor risk factors. In addition, we conducted a sensitivity test to evaluate the effectiveness of the causality.
Results: After adjusting for heterogeneity, evidence of a causal relationship between schizophrenia and lung cancer risk was observed (odds ratio [OR]=1.001, 95% confidence interval [CI], 1.000–1.001; P=0.0155). In the sensitivity analysis, the causal effect of schizophrenia on the risk of lung cancer was consistent in both direction and degree. However, no evidence of causality or reverse causality between schizophrenia and other tumors was found.
Conclusion: This study elucidated a causal relationship between the genetic predictors of schizophrenia and the risk of lung cancer, thereby providing a basis for the prevention, pathogenesis, and treatment of schizophrenia in patients with lung cancer.
1 Introduction
Schizophrenia is a serious mental illness that affects the thinking, behavior, and emotions of patients (1). Recent studies have demonstrated a complex relationship between schizophrenia and tumor incidence (2).
Symptoms of schizophrenia include hallucinations, delusions, and confusion (3, 4), whereas tumors are pathological changes caused by excessive cell growth. Although they seem unrelated, the relationship between them may be more complicated than anticipated. However, whether there is a causal relationship between schizophrenia and tumors remains controversial. Some observational studies have suggested a causal relationship between schizophrenia and tumor risk (5), whereas others have not (6). In addition, the incidence of tumors in patients with schizophrenia differs from that in the general population. Patients with schizophrenia may have a lower cancer incidence (7); however, this does not imply people with schizophrenia will not develop tumors but that the risk is relatively low. In contrast, a significant difference was observed in the overall incidence of cancer among people diagnosed with mental disorders, and tumors are often diagnosed at an advanced stage in patients with mental disorders (8). Patients with schizophrenia may be at increased risk of developing certain types of tumors following antipsychotic treatment (9), which could be attributed to lifestyle, drug therapy, and genetic factors.
Although the relationship between schizophrenia and tumors is not fully understood, this possible link is of considerable importance for the research and treatment of schizophrenia. Understanding this relationship may help us better understand the pathological mechanisms of schizophrenia and provide new implications for future treatment strategies. The relationship between schizophrenia and tumors is complex and delicate (2). Further research is needed to explore and understand this relationship to provide a scientific basis for the prevention and treatment of schizophrenia and tumors.
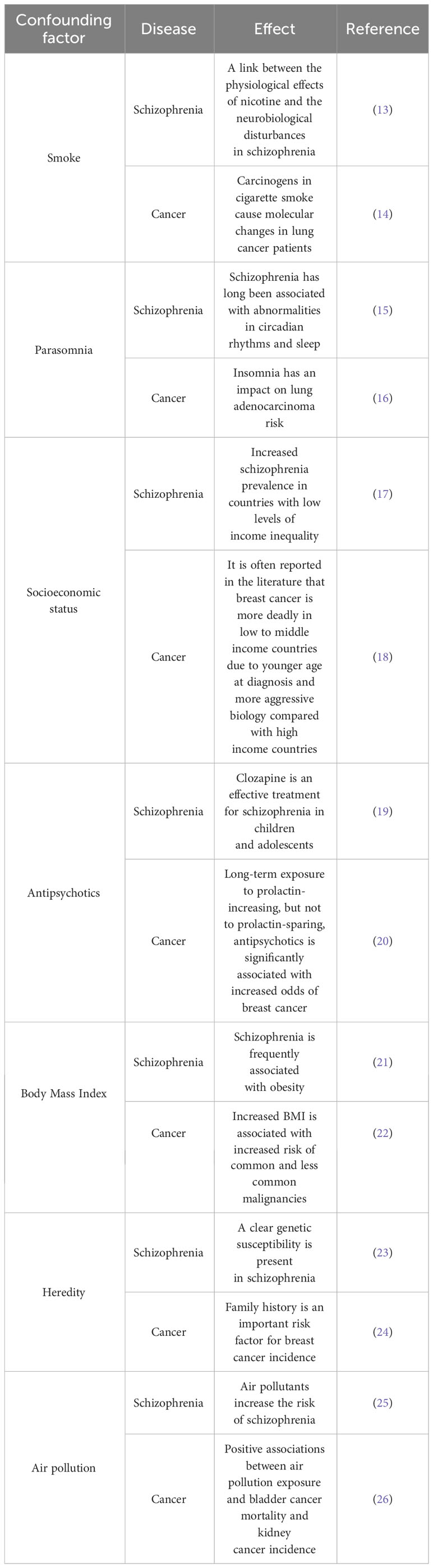
When observational studies explore the causal association between schizophrenia and cancer, the level of evidence for causal inference is often low due to confounding factors such as smoking, body mass index, socioeconomic status (SES), antipsychotic drugs, and genetics (10–12). Table 1 summarized the common confounding factors affecting schizophrenia and tumors. Among the diverse epidemiological research methods, randomized controlled trials are the strongest research design in clinical trials because of their randomness, control, and blindness. Randomized controlled trials are the “gold standard” to verify causality (27). However, because the research and implementation cost too much time, money and manpower; Strict inclusion criteria may lead to a decrease in the representativeness of the test population to the target population. The standard interventions used are not completely consistent with clinical practice; Limited sample size and short follow-up lead to inadequate detection of rare adverse events. These limitations pose challenges in extrapolating the findings of Randomized Controlled Trial (RCT) to practical clinical applications. In addition, for rare and life-threatening diseases that lack effective treatments, routine RCTS may be difficult to implement, require high time costs, or may raise ethical concerns. Therefore, randomized controlled trials face various challenges in clinical practice (28–30).
Mendelian randomization (MR) is a statistical method for evaluating etiological inferences in epidemiological studies. MR is based on genome-wide sequencing data and uses genetic variations with strong correlations to exposure factors as tool variables to assess causal relationships between exposure factors and clinical outcomes (31). During meiosis, alleles are randomly separated; therefore, MR can reduce deviations caused by confounding factors (32).
In this study, a large-scale genome-wide association analysis (GWAS) dataset was used to conduct a two-sample MR study to explore the causal relationship between schizophrenia and the risk of multiple cancers. These tumors included lung adenocarcinoma, lung squamous cell carcinoma, small-cell lung cancer, gastric cancer, alcohol-related hepatocellular cancer, tumors involving the lungs, breast, thyroid gland, pancreas, prostate, ovaries and cervix, endometrium, colon and colorectum, and bladder. This study will help reveal the causal relationship between schizophrenia and cancer, which may aid in prevention and treatment.
2 Materials and methods
2.1 Data sources
We used publicly available schizophrenia (52017 cases and 75889 controls, European origin) GWAS data (33) and selected a statistically significant Single Nucleotide Polymorphism (SNP) (p < 5 × 10-8) that was closely related to schizophrenia from the GWAS. We used publicly available oncology data from the MRC Integrative Epidemiology Unit (IEU, https://gwas.mrcieu.ac.uk//; accessed on September 17, 2023).
Because the data in this study were obtained from a public database, ethical approval from the participants was not required.
2.2 P value and F-statistics calculation
We used F-statistics to evaluate the statistical strength. The F-statistics are equal to ((N-k-1)/k) × (R2/(1-R2)), where N and k represent the sample size and number of SNP, respectively (34). An F-statistic < 10 indicates that the genetic variation used is a weak tool variable that may contribute to a bias. We determined the variance in phenotype explained by each instrument by R2: R2 = [2 × EAF × (1-EAF) × (β) 2]/[(2 × EAF × (1-EAF) × (β) 2) + (2 × EAF × (1-EAF) × N × se (β) 2)], where EAF is the effect allele frequency, β is the effect quantity, N is the sample size, and Se (β) is the standard error of the effect quantity. We used R2 and F-statistics to evaluate the statistical power of each tool variable. Supplementary Table S1 shows the number of MR statistical powers and valid tool variables for each pair.
2.3 Statistical analysis
The causal relationship between exposure (schizophrenia) and outcome (tumor) was determined using two-sample Mendelian randomization (35). Figure 1 illustrates the study process. The inverse variance weighting (IVW) method was used to estimate the causal relationship between exposure and outcome (36). This method ignores the existence of intercept terms and fits the reciprocal of the outcome variance as a weight; therefore, it can reliably estimate the impact of exposure on the outcome under the condition of heterogeneity between tool variables (37). The results are presented as odds ratios (OR) and 95% confidence intervals (CI). IVW is the most effective MR method; however, it assumes that all tool variables are valid. If the horizontal multiplicity is not zero, a bias exists. The weighted median method used most SNPs to determine causality (38). Therefore, we conducted a sensitivity analysis to ensure the accuracy of the causal effect results, including the leave-one-out sensitivity test, heterogeneity test, and pleiotropy test. The main purpose of the leave-one-out sensitivity test was to calculate the MR results of the remaining instrumental variable (IV) after eliminating it. If there is a substantial difference between the estimated MR and the summary results of other IVs after excluding a certain IV, the MR results are sensitive to the IV. The main purpose of the heterogeneity test was to examine the differences between the different IVs. Moreover, if there are considerable differences between different IVs, the heterogeneity of these IVs is large. The multiplicity test mainly tests whether there is horizontal multiplicity in multiple IV. The MR–Egger regression intercept is commonly used to express horizontal multiplicity if the intercept is not zero. In addition, the MR-PRESSO package is a commonly used R packet for testing horizontal multiplicity. MR analysis was performed using the R package “Two Sample MR” (version 0.5.7) in R (version 4.3.1).
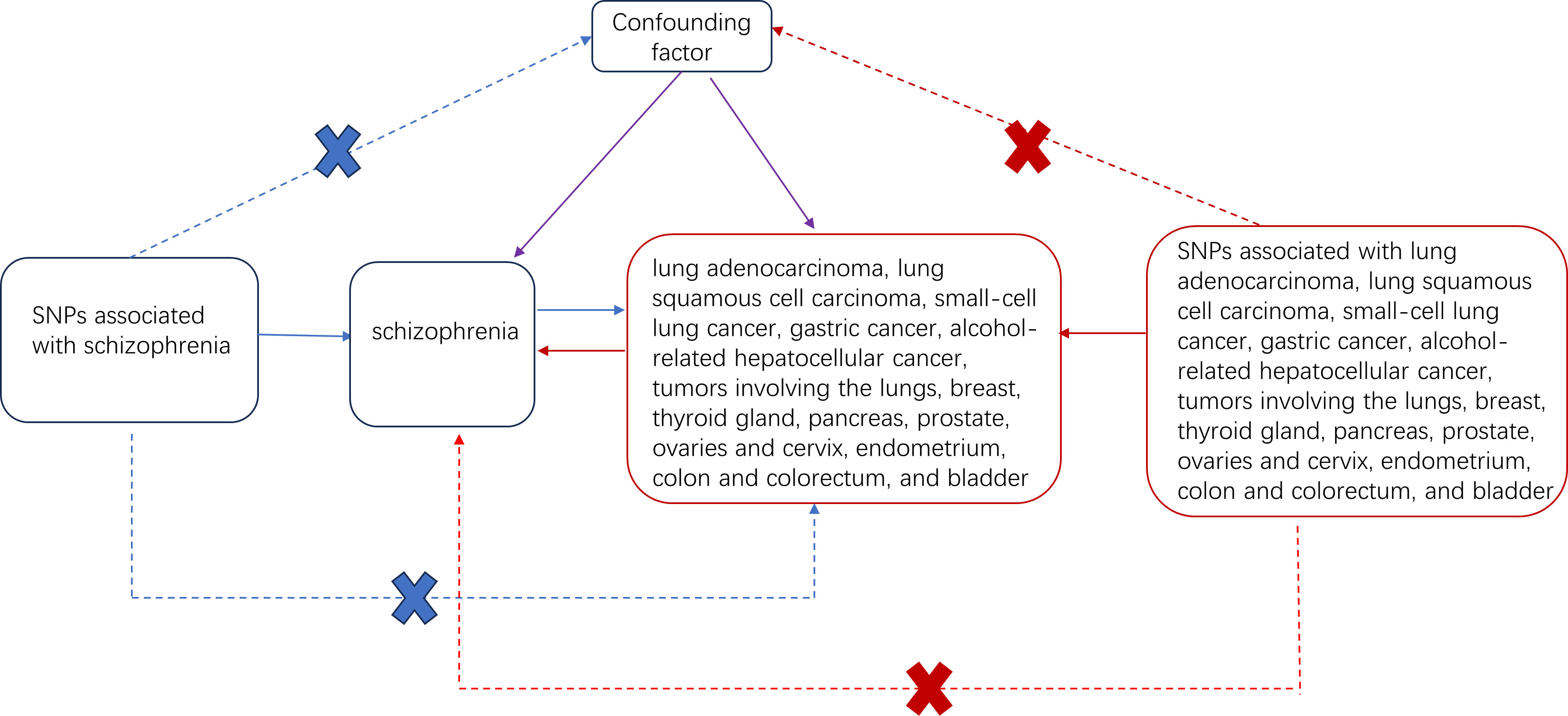
Figure 1 Two-sample Mendelian randomized study design for schizophrenia and different cancers. Solid blue lines indicate the association between tool variables (SNP) and exposure (schizophrenia) and between exposure and outcome (different types of tumors). The red solid line indicates the correlation of reverse causality. The dotted line with crossover indicates that this association conforms to two basic assumptions of Mendelian randomization: 1. Genetic variation (SNP) is independent of the confounding factor between exposure and outcome 2. Genetic variation affects the results only through exposure.
3 Results
3.1 Causality between schizophrenia and tumor
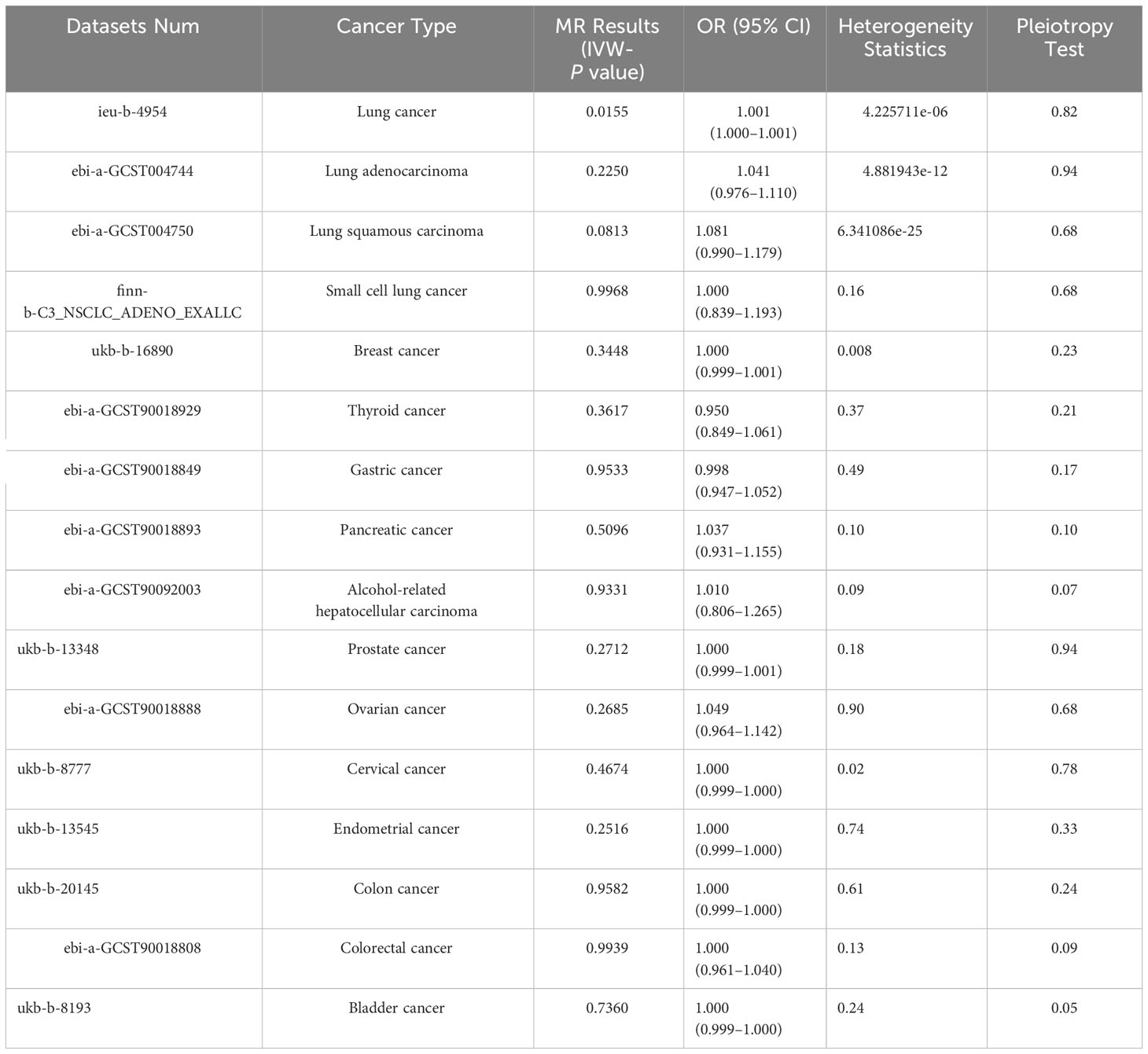
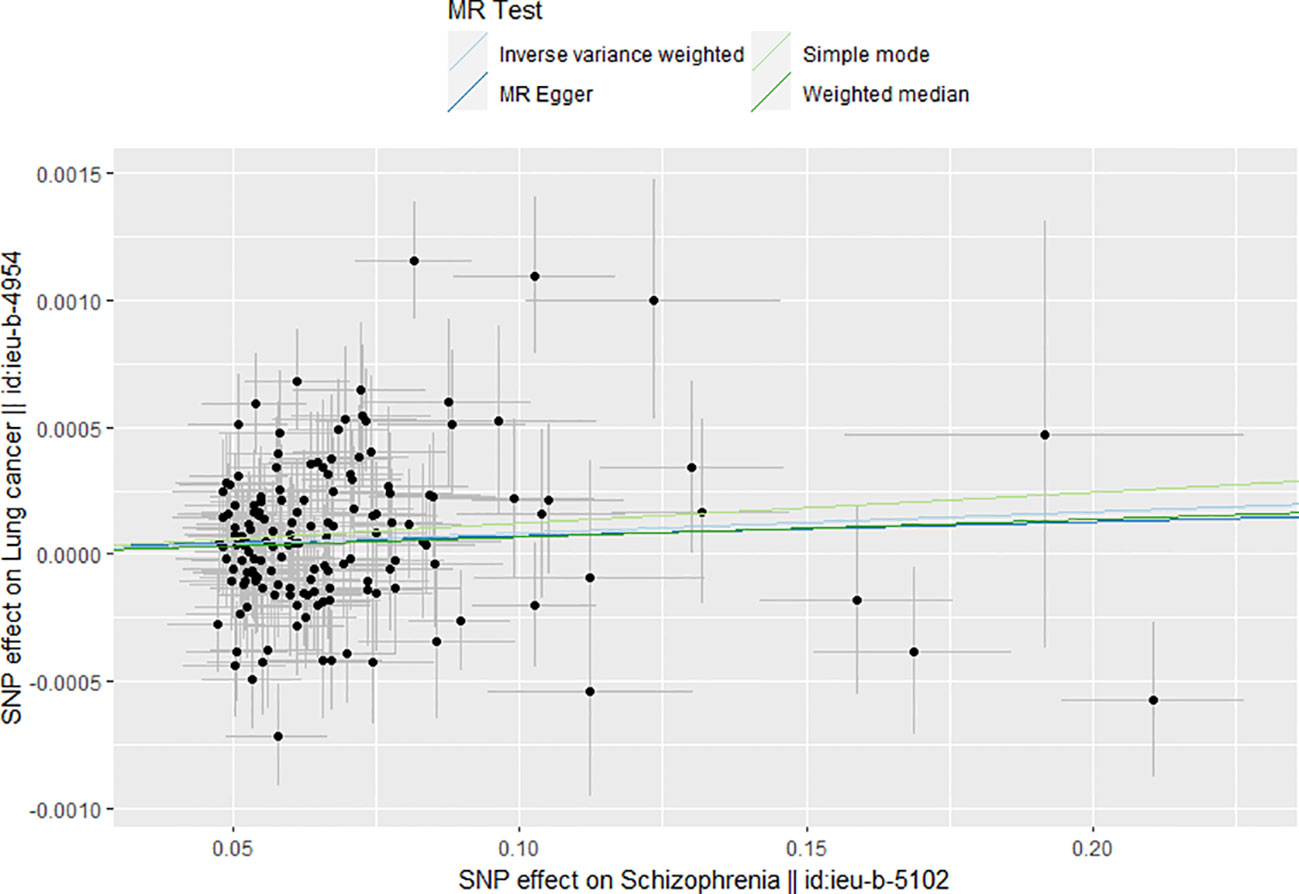
Table 2 shows the results of the MR analysis of different tumors in patients with schizophrenia, which shows that schizophrenia is associated with an increased risk of lung cancer (Figure 2). Lung cancer (OR=1.001, 95% CI: 1.000–1.001, P=0.0155), lung adenocarcinoma (OR=1.041, 95% CI: 0.976–1.110, P=0.2250), lung squamous cell carcinoma (OR=1.081, 95% CI: 0.990–1.179, P=0.0813), small cell lung cancer (OR=1.000, 95% CI: 0.839–1.193, P=0.9968), breast cancer (OR=1.000, 95% CI: 0.999–1.001, P=0.3448), thyroid cancer (OR=0.950, 95% CI:0.849–1.061, P=0.3617), gastric cancer (OR=0.998, 95% CI: 0.947–1.052, P=0.9533), pancreatic cancer (OR=1.037, 95% CI: 0.931–1.155, P=0.5096), alcohol-related hepatocellular carcinoma (OR=1.010, 95% CI: 0.806–1.265, P=0.9331), prostate cancer (OR=1.000, 95% CI: 0.999–1.001, P=0.2712), ovarian cancer (OR=1.049, 95% CI: 0.964–1.142). Cervical cancer (OR=1.000, 95% CI: 0.999–1.000, P=0.4674), endometrial cancer (OR=1.000, 95% CI: 0.999–1.000, P=0.2516), colon cancer (OR=1.000, 95% CI: 0.999–1.000, P=0.9582), colorectal cancer (OR=1.000, 95% CI: 0.961–1.040, P=0.9939), bladder cancer (OR=1.000, 95% CI: 0.999–1.000, P=0.7360). Table 2 shows that the heterogeneity tests of lung cancer, lung adenocarcinoma, lung squamous carcinoma and breast cancer are all much less than 0.05. This suggests that the results may be heterogeneous. We used the random-effect method to estimate the MR effect. From the results of the random-effect model, it is not difficult to see that there is a causal relationship between schizophrenia and lung cancer (pval < 0.05), and schizophrenia increases the risk of lung cancer (b > 0). This may suggest that there is a statistical causal relationship between schizophrenia and lung cancer (P= 0.0155). In the pleiotropy test, the P values of all outcomes are greater than 0.05. There is no statistical difference, so it can be considered that there is no horizontal pleiotropy. So the genetic variation in this study does not affect the outcome variables other than exposure. The leave-one-out sensitivity analysis in Figure 3 shows that all beta values are in the same direction, which means that the positive causal relationship between exposure and outcome still exists after any single SNP is removed.
3.2 Causal relationship between tumor and schizophrenia
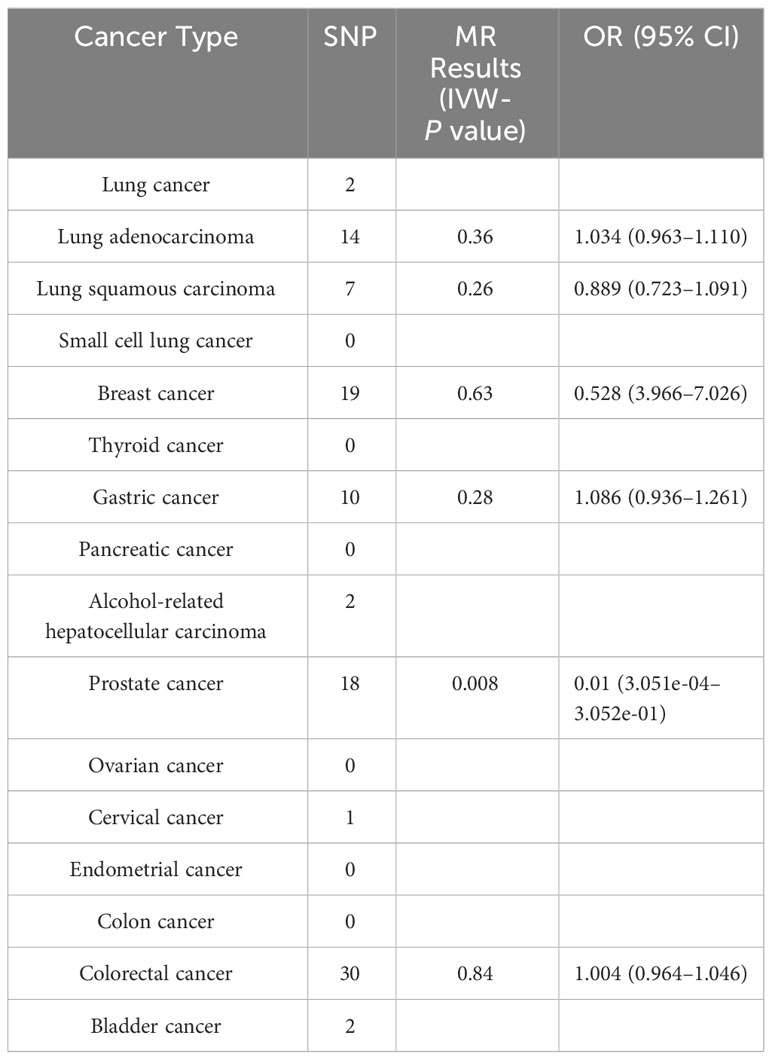
To analyze the causal effects of different tumors on the risk of schizophrenia, we first identified SNPs that are closely related to different tumors. Among the 16 tumor types, only 6 had a strong correlation SNP, 6 had no strong correlation SNP, and 4 had <5 SNP. Therefore, we could not conduct an MR analysis of these 10 tumors. The IVW-P value of prostate cancer was 0.008 in the reverse MR analysis; however, the beta value of the MR–Egger was not consistent with the direction of IVW in further MR analysis, and the result was invalid. Therefore, we found no effect of tumors on the risk of schizophrenia, indicating that almost all tumors had no significant effect on the risk of schizophrenia (P > 0.05; Table 3).
4 Discussion
To date, the possibility of a causal relationship between schizophrenia and tumor incidence remains controversial. Cohort studies by some scholars have shown that there is no significant difference in the incidence rates of colorectal cancer, breast cancer, lung cancer, and all common cancers between patients with schizophrenia and patients without schizophrenia (39, 40). However, some scholars have also found in cohort studies that, under the influence of different confounding factors, the incidence rates of schizophrenia and different cancers in different groups have completely different effects. For example, under the influence of the confounding factor of smoking, the incidence of lung cancer in male patients with schizophrenia is reduced (41), while the incidence of lung cancer and breast cancer in women is increased (42). This shows that confounding factors do have a significant impact between the two, and the way of impact is uncertain. The relationship between schizophrenia and cancer is controversial, possibly owing to research design and confounding factors such as smoking or diet, antipsychotics, and different cancer screening and treatment methods (11, 12). Moreover, cancer is a geriatric disease, and the life expectancy of patients with schizophrenia is shortened by 10–25 years (43). The advantage of MR is to control confounding factors, so that we can get more reliable and accurate results between exposure and outcome at the genetic level. This study examined the causal relationship between genetically predicted schizophrenia and the risk of various cancers, including lung adenocarcinoma, lung squamous cell carcinoma, small-cell lung cancer, gastric cancer, alcohol-related hepatocellular cancer, tumors involving the lungs, breast, thyroid gland, pancreas, prostate, ovaries and cervix, endometrium, colon and colorectum, and bladder. We found suggestive evidence (OR=1.001, 95% CI:1.000–1.001, P=0.0155) of genetic predictions of causality between schizophrenia and lung cancer; however, reverse causality could not be determined. This study demonstrated that schizophrenia is associated with an increased risk of lung cancer. A literature search revealed that the association between schizophrenia and lung cancer involves different mechanisms. Shaw et al. (44) showed that antipsychotic drugs fluspirilene, penfluridol, and pimozide induce apoptosis and inhibit metastasis through p53, STAT3, STAT5, protein phosphatase 2A, cholesterol homeostasis, integrin, autophagy, USP1, wnt/β-catenin signal transduction and DNA repair in vivo and in vitro. In addition, penfluridol and pimozide have synergistic effects with existing chemotherapeutic drugs such as cisplatin. Accumulating evidence suggests that antipsychotics are potential anticancer agents. Zuber et al. have shown that the relationship between schizophrenia and lung cancer may be related to common genetic risk factors; smoking is closely related to schizophrenia and lung cancer, and the variation in nicotinic acetylcholine receptors may lead to this overlap (43). In addition, the anti-schizophrenic drug penfluridol can inhibit the growth and metastasis of various G0/G1 stagnant lung cancer cells by increasing the expression of p21/p27 and reducing the expression of the cyclin-CDK complex. Penfluridol is an inexpensive drug approved by the Food and Drug Administration. This study demonstrated the potential of anti-schizophrenic drugs in the treatment of lung cancer.
This study had several advantages. First, we used two-sample MR analysis to minimize the deviation caused by confounding factors. Second, we performed an MR analysis of schizophrenia and 16 types of cancer; these 16 types of tumors are common and representative in the clinic. In addition, the database sources for each pair of exposures and results did not overlap and were of European origin, ensuring the basic requirements for the MR analysis of the two samples. Fourth, the exposed F-statistics were all greater than 10, indicating that there was no weak tool deviation. Fifth, to ensure the validity of the causal effect results, we conducted a sensitivity analysis, including the leave-one-out sensitivity, heterogeneity, and pleiotropy tests.
Despite these advantages, our study had certain limitations. First, we used only GWAS data on PGC schizophrenia; therefore, the results may be inadequate. In addition, because the two samples of the MR analysis required ethnic consistency, our study focused on the European population and did not cover the Asian and African populations, which may have led to incomplete results. In addition, population stratification may interfere with the causal relationship between schizophrenia and tumors (45). Although the population studied was European, the internal structure of the population and potential sex factors were not considered. Sex differences in the incidence of schizophrenia have been reported (46), and sex differences between schizophrenia and lung cancer have gained considerable attention. Furthermore, on reversing the MR analysis, we found that the number of strong correlations SNP was relatively small, resulting in invalid results that limited our ability to draw real causality conclusions. In addition, the OR value is 1.001, indicating that there is a potential correlation between schizophrenia and lung cancer. Schizophrenia is a risk factor for lung cancer, but the possible risk factors are not very obvious. It is necessary to further explore the relationship between schizophrenia and lung cancer in the future. This result also suggests that we need to pay more attention to the incidence of lung cancer in patients with schizophrenia in the future. Finally, our study aimed to determine whether there is a causal relationship between schizophrenia and lung cancer; however, the underlying mechanism has not yet been studied. Future investigations analyzing the possible mechanisms increasing the risk of lung cancer in patients with schizophrenia are warranted.
5 Conclusion
A two-sample MR analysis revealed a potential causal relationship between the genetic prediction of schizophrenia and lung cancer using schizophrenia as an exposure factor and 16 cancer types as outcomes. However, the causal relationship between lung cancer and schizophrenia has not been determined, and the causal effect and reverse causal relationship of schizophrenia on other types of cancer have not been determined. This demonstrates the importance of preventing schizophrenia in the prevention and treatment of lung cancer. Future large-scale prospective studies and more in-depth mechanism research are required to validate the study findings.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
XTZ: Writing – original draft, Writing – review & editing. QL: Writing – original draft, Writing – review & editing. SHL: Writing –review & editing. ZLS: Writing – review & editing. LQW: Writing – review & editing. CGS: Writing – review & editing. XNC: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (81973677;82174222) and Qingdao Science and Technology Public-interest Demonstration Project (23-2-8-smjk-1-nsh).
Acknowledgments
Thanks to the editors and reviewers for their hard work and important comments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1321445/full#supplementary-material
Abbreviations
CI, confidence interval; GWAS, genome-wide association analysis; IV, instrumental variable; IVW, inverse variance weighting; MR, Mendelian randomization; OR, odds ratio; RCT, Randomized Controlled Trial; SNP, Single Nucleotide Polymorphism.
References
1. Canessa N, Iozzino L, Andreose S, Castelletti L, Conte G, Dvorak A, et al. RISK aversion in Italian forensic and non-forensic patients with schizophrenia spectrum disorders. PloS One (2023) 18:e0289152. doi: 10.1371/journal.pone.0289152
2. Hamoud A, Bach K, Kakrecha O, Henkel N, Wu X, McCullumsmith R, et al. Adenosine, schizophrenia and cancer: does the purinergic system offer a pathway to treatment? Int J Mol Sci (2022) 23(19):11835. doi: 10.3390/ijms231911835
3. Kendler K. Phenomenology of schizophrenia and the representativeness of modern diagnostic criteria. JAMA Psychiatry (2016) 73:1082–92. doi: 10.1001/jamapsychiatry.2016.1976
4. Rapin L, Dohen M, Lœvenbruck H, Whitman J, Metzak P, Woodward T. Hyperintensity of functional networks involving voice-selective cortical regions during silent thought in schizophrenia. Psychiatry Res (2012) 202:110–7. doi: 10.1016/j.pscychresns.2011.12.014
5. Cui Y, Lu W, Shao T, Zhuo Z, Wang Y, Zhang W. Severe mental illness and the risk of breast cancer: A two-sample, two-step multivariable Mendelian randomization study. PloS One (2023) 18:e0291006. doi: 10.1371/journal.pone.0291006
6. Ge F, Huo Z, Liu Y, Du X, Wang R, Lin W, et al. Association between schizophrenia and prostate cancer risk: Results from a pool of cohort studies and Mendelian randomization analysis. Compr Psychiatry (2022) 115:152308. doi: 10.1016/j.comppsych.2022.152308
7. Grassi L, Riba M. Cancer and severe mental illness: Bi-directional problems and potential solutions. Psycho-oncology (2020) 29:1445–51. doi: 10.1002/pon.5534
8. Wootten J, Wiener J, Blanchette P, Anderson K. Cancer incidence and stage at diagnosis among people with psychotic disorders: Systematic review and meta-analysis. Cancer Epidemiol (2022) 80:102233. doi: 10.1016/j.canep.2022.102233
9. Nordentoft M, Plana-Ripoll O, Laursen T. Cancer and schizophrenia. Curr Opin Psychiatry (2021) 34:260–5. doi: 10.1097/YCO.0000000000000697
10. Hong X, Yin J, Wang B. [Probe variables: a tool for identification of unmeasured confounders in an observational study]. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi (2021) 42:735–9. doi: 10.3760/cma.j.cn112338-20200315-00355
11. Hippisley-Cox J, Vinogradova Y, Coupland C, Parker C. Risk of Malignancy in patients with schizophrenia or bipolar disorder: nested case-control study. Arch Gen Psychiatry (2007) 64:1368–76. doi: 10.1001/archpsyc.64.12.1368
12. Ji J, Sundquist K, Ning Y, Kendler K, Sundquist J, Chen X. Incidence of cancer in patients with schizophrenia and their first-degree relatives: a population-based study in Sweden. Schizophr Bull (2013) 39:527–36. doi: 10.1093/schbul/sbs065
13. Uneri O, Tural U, Cakin Memik N. [Smoking and schizophrenia: where is the biological connection?]. Turk psikiyatri dergisi = Turkish J Psychiatry (2006) 17:55–64.
14. Jassem E, Szymanowska A, Siemińska A, Jassem J. [Smoking and lung cancer]. Pneumonologia i alergologia polska (2009) 77:469–73.
15. Yates N. Schizophrenia: the role of sleep and circadian rhythms in regulating dopamine and psychosis. Rev Neurosci (2016) 27:669–87. doi: 10.1515/revneuro-2016-0030
16. Wang J, Tang H, Duan Y, Yang S, An J. Association between sleep traits and lung cancer: A mendelian randomization study. J Immunol Res (2021) 2021:1893882. doi: 10.1155/2021/1893882
17. Chow J, Chien T, Lin C, Chou W. Income inequality and schizophrenia: Increased schizophrenia prevalence in countries with low levels of income inequality. Asian J Psychiatry (2020) 48:101910. doi: 10.1016/j.ajp.2019.101910
18. Chamberlin M, Booth C, Brooks G, Manirakiza A, Rubagumya F, Vanderpuye V. More drugs versus more data: the tug of war on cancer in low- and middle-income countries. Hematology/oncology Clinics North America (2024) 38:229–38. doi: 10.1016/j.hoc.2023.06.010
19. Sarkar S, Grover S. Antipsychotics in children and adolescents with schizophrenia: a systematic review and meta-analysis. Indian J Pharmacol (2013) 45:439–46. doi: 10.4103/0253-7613.117720
20. Taipale H, Solmi M, Lähteenvuo M, Tanskanen A, Correll C, Tiihonen J. Antipsychotic use and risk of breast cancer in women with schizophrenia: a nationwide nested case-control study in Finland, The lancet. Psychiatry (2021) 8:883–91. doi: 10.1016/S2215-0366(21)00241-8
21. McWhinney S, Brosch K, Calhoun V, Crespo-Facorro B, Crossley N, Dannlowski U, et al. Obesity and brain structure in schizophrenia - ENIGMA study in 3021 individuals. Mol Psychiatry (2022) 27:3731–7. doi: 10.1038/s41380-022-01616-5
22. Renehan A, Tyson M, Egger M, Heller R, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet (London England) (2008) 371:569–78. doi: 10.1016/S0140-6736(08)60269-X
23. Khan Z, Martin-Montañez E, Muly E. Schizophrenia: causes and treatments. Curr Pharm design (2013) 19:6451–61. doi: 10.2174/1381612811319360006
24. Brewer H, Jones M, Schoemaker M, Ashworth A, Swerdlow A. Family history and risk of breast cancer: an analysis accounting for family structure. Breast Cancer Res Treat (2017) 165:193–200. doi: 10.1007/s10549-017-4325-2
25. Duan J, Cheng Q, Luo X, Bai L, Zhang H, Wang S, et al. Is the serious ambient air pollution associated with increased admissions for schizophrenia? Sci total Environ (2018) 644:14–9. doi: 10.1016/j.scitotenv.2018.06.218
26. Zare Sakhvidi M, Lequy E, Goldberg M, Jacquemin B. Air pollution exposure and bladder, kidney and urinary tract cancer risk: A systematic review. Environ pollut (Barking Essex 1987) (2020) 267:115328. doi: 10.1016/j.envpol.2020.115328
27. Skou S, Lind M, Hölmich P, Jensen H, Jensen C, Afzal M, et al. Study protocol for a randomised controlled trial of meniscal surgery compared with exercise and patient education for treatment of meniscal tears in young adults. BMJ Open (2017) 7:e017436. doi: 10.1136/bmjopen-2017-017436
28. Sholapurkar S. Re: RCT evidence should not drive everything we do clinically: Trials and errors: Role of RCTs and EBM will be enhanced by discussion about limitations, avoidance of pitfalls and judicious application to clinical practice. BJOG an Int J obstetrics gynaecology (2017) 124:1449–50. doi: 10.1111/1471-0528.14677
29. Huang D. Challenges in randomized controlled trials and emerging multiple sclerosis therapeutics. Neurosci Bull (2015) 31:745–54. doi: 10.1007/s12264-015-1560-6
30. Yang A, Singh N, Varshney U. Mobile health interventions and RCTs: structured taxonomy and research framework. J Med Syst (2022) 46:66. doi: 10.1007/s10916-022-01856-6
31. Holmes M, Ala-Korpela M, Smith G. Mendelian randomization in cardiometabolic disease: challenges in evaluating causality, Nature reviews. Cardiology (2017) 14:577–90. doi: 10.1038/nrcardio.2017.78
32. Zheng J, Baird D, Borges M, Bowden J, Hemani G, Haycock P, et al. Recent developments in mendelian randomization studies. Curr Epidemiol Rep (2017) 4:330–45. doi: 10.1007/s40471-017-0128-6
33. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature (2014) 511:421–7. doi: 10.1038/nature13595
34. Ren F, Shang Q, Zhao S, Yang C, Feng K, Liu J, et al. An exploration of the correlations between seven psychiatric disorders and the risks of breast cancer, breast benign tumors and breast inflammatory diseases: Mendelian randomization analyses. Front Psychiatry (2023) 14:1179562. doi: 10.3389/fpsyt.2023.1179562
35. Burgess S, Scott R, Timpson N, Davey Smith G, Thompson S. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol (2015) 30:543–52. doi: 10.1007/s10654-015-0011-z
36. Yang M, Wan X, Zheng H, Xu K, Xie J, Yu H, et al. No evidence of a genetic causal relationship between ankylosing spondylitis and gut microbiota: A two-sample Mendelian randomization study. Nutrients (2023) 15(4):1057. doi: 10.3390/nu15041057
37. Bowden J, F. Del Greco M, Minelli C, Smith GD, Sheehan N, Thompson J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med (2017) 36:1783–802. doi: 10.1002/sim.7221
38. Burgess S, Davey Smith G, Davies N, Dudbridge F, Gill D, Glymour M, et al. Guidelines for performing Mendelian randomization investigations: update for summer 2023. Wellcome Open Res (2019) 4:186. doi: 10.12688/wellcomeopenres.15555.1
39. Osborn D, Limburg H, Walters K, Petersen I, King M, Green J, et al. Relative incidence of common cancers in people with severe mental illness. Cohort study in the United Kingdom THIN primary care database. Schizophr Res (2013) 143:44–9. doi: 10.1016/j.schres.2012.11.009
40. Zhuo C, Zhuang H, Gao X, Triplett P. Lung cancer incidence in patients with schizophrenia: meta-analysis. Br J Psychiatry J Ment Sci (2019) 215:704–11. doi: 10.1192/bjp.2019.23
41. Dalton S, Mellemkjaer L, Thomassen L, Mortensen P, Johansen C. Risk for cancer in a cohort of patients hospitalized for schizophrenia in Denmark, 1969-1993. Schizophr Res (2005) 75:315–24. doi: 10.1016/j.schres.2004.11.009
42. Pettersson D, Gissler M, Hällgren J, Ösby U, Westman J, Bobo W. The overall and sex- and age-group specific incidence rates of cancer in people with schizophrenia: a population-based cohort study. Epidemiol Psychiatr Sci (2020) 29:e132. doi: 10.1017/S204579602000044X
43. Zuber V, Jönsson E, Frei O, Witoelar A, Thompson W, Schork A, et al. Identification of shared genetic variants between schizophrenia and lung cancer. Sci Rep (2018) 8:674. doi: 10.1038/s41598-017-16481-4
44. Shaw V, Srivastava S, Srivastava S. Repurposing antipsychotics of the diphenylbutylpiperidine class for cancer therapy. Semin Cancer Biol (2021) 68:75–83. doi: 10.1016/j.semcancer.2019.10.007
45. Sanderson E, Richardson T, Hemani G, Davey Smith G. The use of negative control outcomes in Mendelian randomization to detect potential population stratification. Int J Epidemiol (2021) 50:1350–61. doi: 10.1093/ije/dyaa288
Keywords: schizophrenia, cancer, mendelian randomization, causality, GWAS
Citation: Zhou X, Liu Q, Liu S, Wang L, Sun Z, Sun C and Cui X (2024) Genetic prediction of the causal relationship between schizophrenia and tumors: a Mendelian randomized study. Front. Oncol. 14:1321445. doi: 10.3389/fonc.2024.1321445
Received: 02 November 2023; Accepted: 26 January 2024;
Published: 16 February 2024.
Edited by:
Sigrid A. Langhans, Nemours Children’s Health, United StatesReviewed by:
Shahram Arsang-Jang, Medical College of Wisconsin, United StatesMassimo Tusconi, University of Cagliari, Italy
Semra Bulbuloglu, Istanbul Aydın University, Türkiye
Copyright © 2024 Zhou, Liu, Liu, Wang, Sun, Sun and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changgang Sun, enl4eXNjZ0B3Zm1jLmVkdS5jbg==; Xiangning Cui, Y3VpeGlhbmduaW5nQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Xintong Zhou1†
Xintong Zhou1† Qi Liu
Qi Liu Shihan Liu
Shihan Liu Zhongli Sun
Zhongli Sun Changgang Sun
Changgang Sun Xiangning Cui
Xiangning Cui