- 1Department of Internal Medicine and Medical Specialties (Di.M.I.), School of Medicine, University of Genova, Genova, Italy
- 2Department of Medical Oncology, U.O. Clinica di Oncologia Medica, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ospedale Policlinico San Martino, Genova, Italy
- 3Translational and Clinical Research Institute, Centre for Cancer, Newcastle University, Newcastle upon Tyne, United Kingdom
- 4Mildred Scheel Cancer Career Centre HaTriCS4, University Cancer Centre Hamburg, University Medical Centre Eppendorf, Hamburg, Germany
- 5Department of Oncology, Hematology and Bone Marrow Transplantation with Division of Pneumology, University Medical Centre Eppendorf, Hamburg, Germany
- 6Candiolo Cancer Institute Fondazione del Piemonte per l’Oncologia-Istituto di Ricovero e Cura a Carattere Scientifico (FPO-IRCCS), Candiolo, Italy
- 7Medical Oncology Unit 1, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ospedale Policlinico San Martino, Genova, Italy
- 8Medical Oncology Unit, Ospedale San Paolo, Savona, Italy
- 9Department of Internal Medicine and Medical Specialties (Di.M.I.), University of Genova, Genova, Italy
- 10Endocrinology Unit, Department of Internal Medicine, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ospedale Policlinico San Martino, Genova, Italy
- 11Oncology Department, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Policlinico San Matteo, Pavia, Italy
- 12Endocrinology Unit, Department of Internal Medicine and Medical Specialties (Di.M.I.), Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ospedale Policlinico San Martino, University of Genova, Genova, Italy
Background: Human chorionic gonadotropin (hCG)–induced hyperthyroidism is a rare paraneoplastic syndrome observed in non-seminomatous testicular germ cell tumors, due to a cross-reaction between the β-subunit of hCG with the thyroid-stimulating hormone receptor. The precise prevalence of this paraneoplastic phenomenon is unclear as, in the majority of cases, hyperthyroidism remains subclinical.
Case presentation: Here, we present two cases of advanced metastatic non-seminomatous testicular germ cell tumors where patients exhibited signs and symptoms of thyrotoxicosis at primary diagnosis due to excessive serum β-hCG elevation, with complete remission of symptomatology after the start of oncological treatments and no signs of relapse at the time of publication of this report. Additionally, we provide a comprehensive review of the existing literature concerning this uncommon occurrence.
Conclusion: Despite being a rare event, the presence of hyperthyroidism or thyrotoxicosis without clear etiology in a young man should lead to consider less frequent causes such as testicular tumors. Even if patients typically have mild symptoms that resolve after chemotherapy, in rare cases, it can be a life-threatening condition that requires prompt recognition and specific intervention.
Introduction
The term “thyrotoxicosis” refers to a clinical condition that results from elevated circulating thyroid hormone action within the body. The main causes include Graves’ disease, toxic adenoma, toxic multinodular goitre and thyroiditis, as well as hyperthyroidism induced by iodinated contrast media or amiodarone. Rarely, thyrotoxicosis may also be related to a paraneoplastic syndrome (1–3).
Human chorionic gonadotropin (hCG)–induced hyperthyroidism represents a rare paraneoplastic syndrome in non-seminomatous testicular germ cell tumors (NSTGCTs), in which the excessive secretion of the β-subunit of hCG (β-hCG) results in hyperthyroidism through a cross-reaction with the thyroid-stimulating hormone (TSH) receptor (4). Excessive β-hCG levels can be present in NSTGCTs with a predominant choriocarcinoma component (5). The exact prevalence of this paraneoplastic phenomenon is unknown: in most cases, hyperthyroidism remains subclinical, and, thus, only a limited number of cases with clinically symptomatic hyperthyroidism have been reported in the literature.
Here, we present two cases of advanced NSTGCT with signs and symptoms of thyrotoxicosis at primary diagnosis due to excessive serum β-hCG elevations and a review of the available literature on this rare phenomenon.
Case presentation I
A previously healthy 38-year-old man presented to the Emergency Department of the “San Martino” University Hospital of Genoa with classic symptoms of thyrotoxicosis: tachycardia with palpitations, insomnia, restlessness, tremors in the limbs, loss of appetite, and weight loss of about 5 kg.
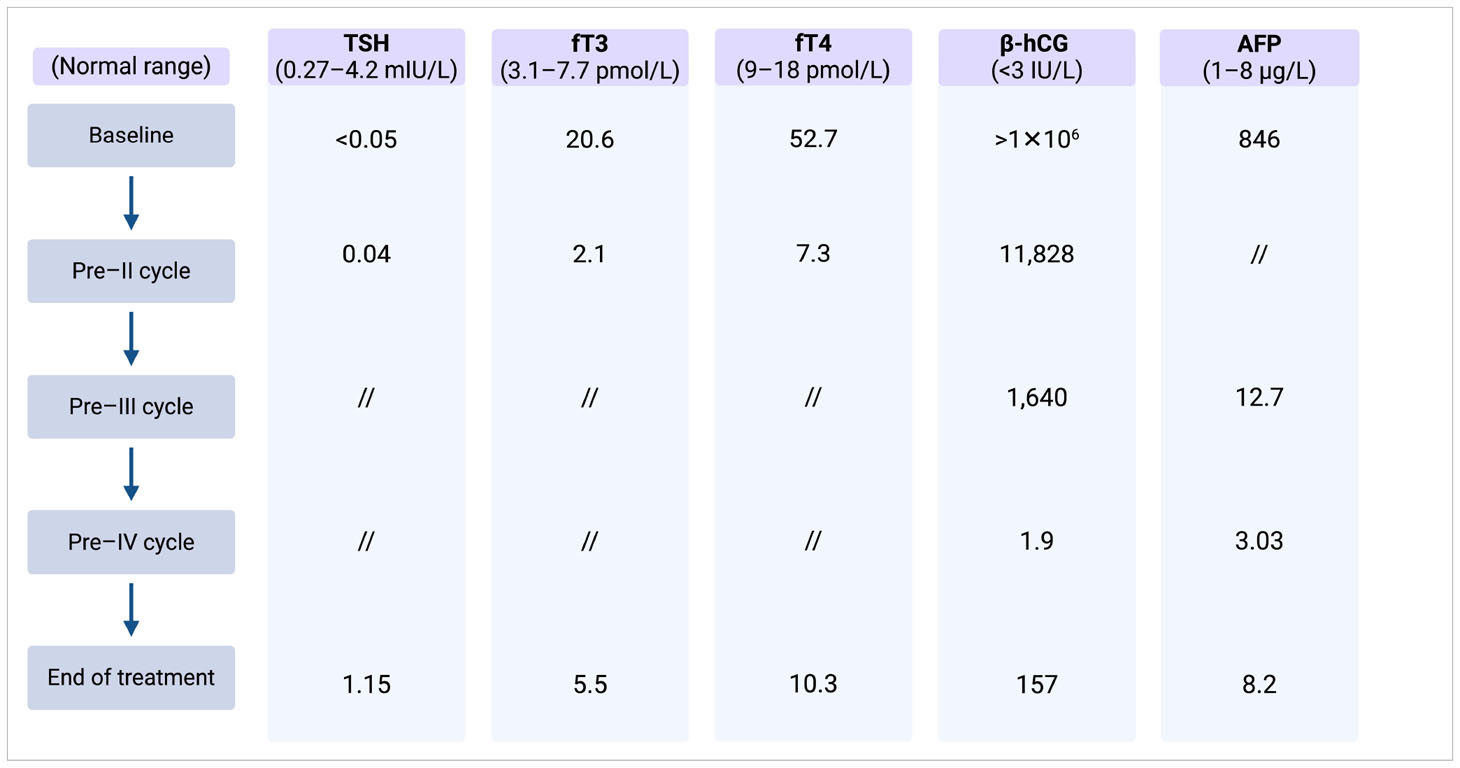
His thyroid function was tested, and the results validated the diagnosis of thyrotoxicosis [TSH suppressed and free T4 (fT4), 52.7 pmol/L; upper limit of normal (ULN), 18 pmol/L]. The remaining blood tests were normal, except for a mild anemia [hemoglobin (Hgb), 124 g/L; normal range, 140–180 g/L] and a slight elevation of Gamma-glutamyl transferase (GGT, 83 IU/L; ULN, 49 IU/L).
Clinically, a thyroid gland of increased volume was detected on cervical palpation but had no exophthalmos and no cervical lymphadenopathy.
A thyroid ultrasound (US) showed an enlarged and heterogeneous thyroid gland with hypervascular flow on Doppler US.
Further diagnostic work-up for hyperthyroidism including autoimmune antibodies as well as iodiuria yielded negative results.
Treatment with methimazole of 5 mg six times a day and propranolol of 20 mg twice a day was initiated, and the patients was discharged with close endocrinological follow-up.
However, shortly after, the patient was hospitalized because of worsening of the clinical condition with dyspnea and intense asthenia.
At admission, he was tachycardic, tachypneic, and fatigued. The vital signs were unremarkable, including an oxygen saturation of 99% on room air. Abnormal findings on physical examination included fine crepitations above both lungs and a significantly enlarged left testicle.
Initial laboratory investigation revealed microcytic anemia (Hgb, 91 g/L), excessively high serum β-hCG of >1 × 106 IU/L (ULN < 3 IU/L), α-fetoprotein (AFP) of 846 ng/mL (ULN < 13.6 ng/mL), and an elevated lactate dehydrogenase (LDH) of 981 U/L (normal range, 135–250 IU/L).
Despite the ongoing thyrostatic therapy, thyroid function tests revealed elevated serum fT3 and fT4 levels, along with a suppressed TSH level. Laboratory test results and markers trend are summarized in Table 1.
A testicular US revealed a heterogeneous left intratesticular mass of 9 cm × 10 cm × 11 cm in size, suspicious of a testicular primary tumor. A computed tomography (CT) scan of the head, chest, and abdomen showed diffuse metastases in the lungs, liver, bone, and retroperitoneal lymph nodes.
Clinical diagnosis of metastatic NSTGCT was made on the basis of the findings of a solid testicular mass with multiple metastases and very high levels of serum β-hCG, and the thyrotoxicosis was attributed to be a paraneoplastic phenomenon.
The patient underwent immediate left radical inguinal orchiectomy, which confirmed the diagnosis of an embryonal carcinoma of the testis, pT2 cN3 cM1b S3, Union for International Cancer Control (UICC) clinical stage IIIC (6).
He was classified as having a poor prognosis in view of his high level of β-hCG and metastatic sites, based on the International Germ Cell Cancer Collaborative Group (IGCCCG) criteria, and was started on standard conventional chemotherapy with cisplatin 20 mg/m² and etoposide 100 mg/m2 on days 1–5 and bleomycin of 30 mg on days 2, 9, and 16 Platinol, Etoposide and Bleomycin (PEB) for four treatment cycles (7).
After completing his first chemotherapy cycle, his serum β-hCG decreased significantly, and the thyroid function improved to near normalization (Table 1) with complete resolution of his symptoms. Methimazole was decreased from 30 mg to 10 mg daily and was discontinued at the start of the second cycle of chemotherapy.
After three cycles of treatment, his β-hCG plummeted to 1.9 IU/L but started to rise again subsequently in keeping with acquired cisplatin resistance.
At the end of the four cycles of therapy, a re-evaluation CT scan was performed with evidence of a marker-positive partial remission of all metastatic sites.
After 3 months, β-hCG increased to 310 IU/L, whereas the thyroid function remained normal. Consequently, the patient underwent salvage chemotherapy with one cycle of induction with paclitaxel of 250 mg/m2 on day 1 and ifosfamide of 3,000 mg/m2 on days 2–3 (TI) for stem cell mobilization and subsequent high-dose carboplatin Area Under the Curve (AUC) of 7 and etoposide of 400 mg/m2 on days 1–3 for three treatment cycles with autologous bone marrow stem cell support.
At the end of the high-dose chemotherapy, the patient’s β-hCG level decreased to 8 IU/L, and the CT scan still showed residual lesions in all metastatic sites.
After multidisciplinary discussion, it was decided to proceed with debulking surgery of the residual hepatic and retroperitoneal lesions with no viable germ cell tumor cells according to histopathology. Two irresectable liver lesions were additionally treated with radiofrequency ablation.
The patient was also started on maintenance oral therapy with VP100, which was suspended because of poor tolerance. Maintenance oral etoposide after salvage therapy is not a standard practice in Italy but retrospective data has hinted at its potential benefits (8), and the results of an ongoing randomized clinical trial are pending and will provide valuable insights (9).
At the last re-evaluation, β-hCG was stable at 8 IU/L, whereas the CT scan showed stable pulmonary micronodules and signs of hepatic metastasectomy and lymphadenectomy. To date, three years after completing the salvage treatment, the patient remains relapse-free and euthyroid, with ongoing regular intensive follow-up assessments.
Case presentation II
A previously healthy 18-year-old man presented at the Emergency Medicine Department of the University Medical Centre Hamburg-Eppendorf with quickly worsening shortness of breath, fatigue, polyuria, polydipsia, and unintended weight loss. Vital signs were abnormal with tachycardia and an oxygen saturation of 88% on room air.
Physical examination revealed reduced breathing sounds over both lungs and a slightly enlarged left testicle.
A chest X-ray showed multiple bi-pulmonary nodules mainly in the inferior lobes and the right middle lobe. A subsequent CT of thorax, abdomen, and pelvis confirmed the widespread pulmonary metastatic lesions and also showed a large centrally necrotic left-sided retroperitoneal lymph node of 5.3 cm × 4.6 cm adjacent to the pancreatic tail (Figure 1).
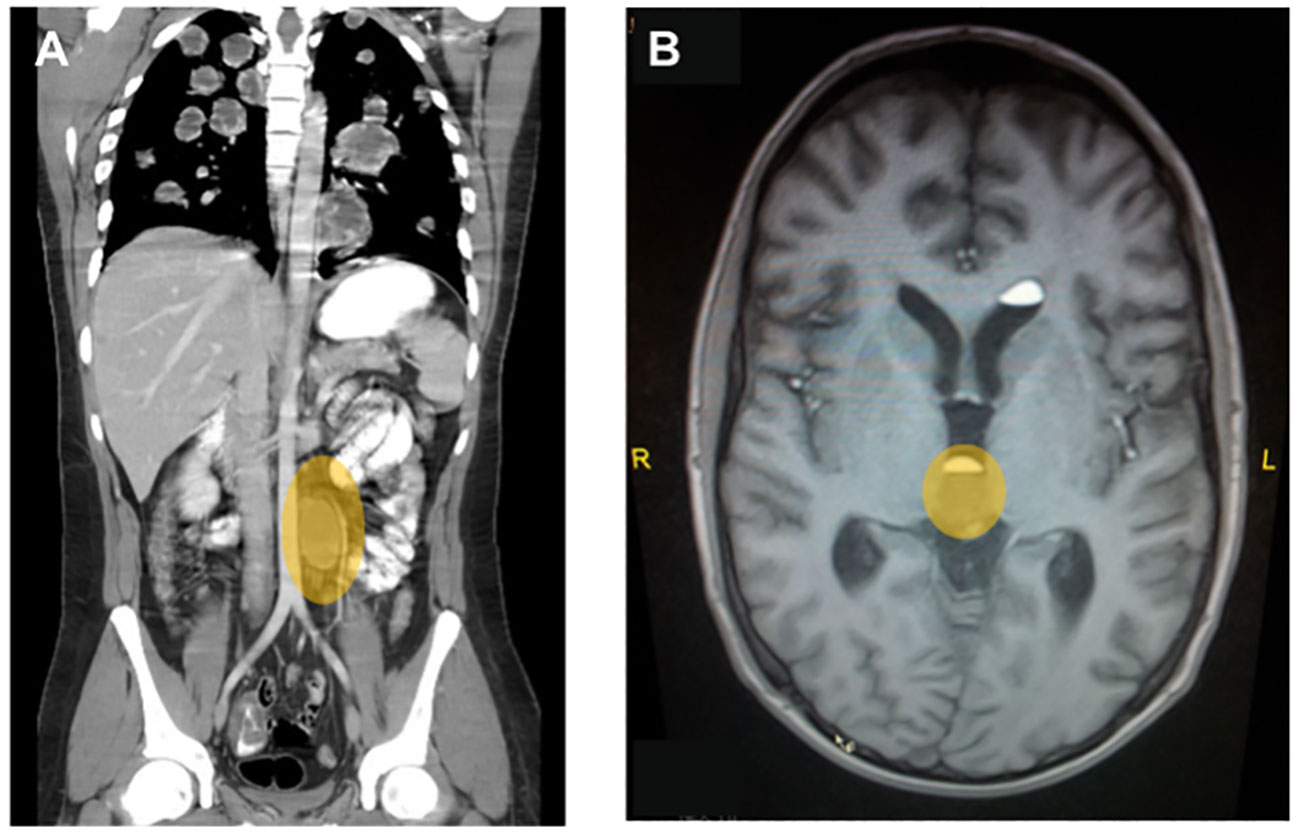
Figure 1 Representative diagnostic images at primary diagnosis for case 2. (A) Contrast-enhanced computed tomography of chest abdomen and pelvis showing multiple bilateral, centrally necrotic lung metastases and a singular retroperitoneal para-aortic lymph node metastasis. (B) T2-weighted image of a whole-brain MRI showing a central metastasis close to the pituitary gland, which lead to a clinically significant diabetes mellitus with polyuria and polydipsia.
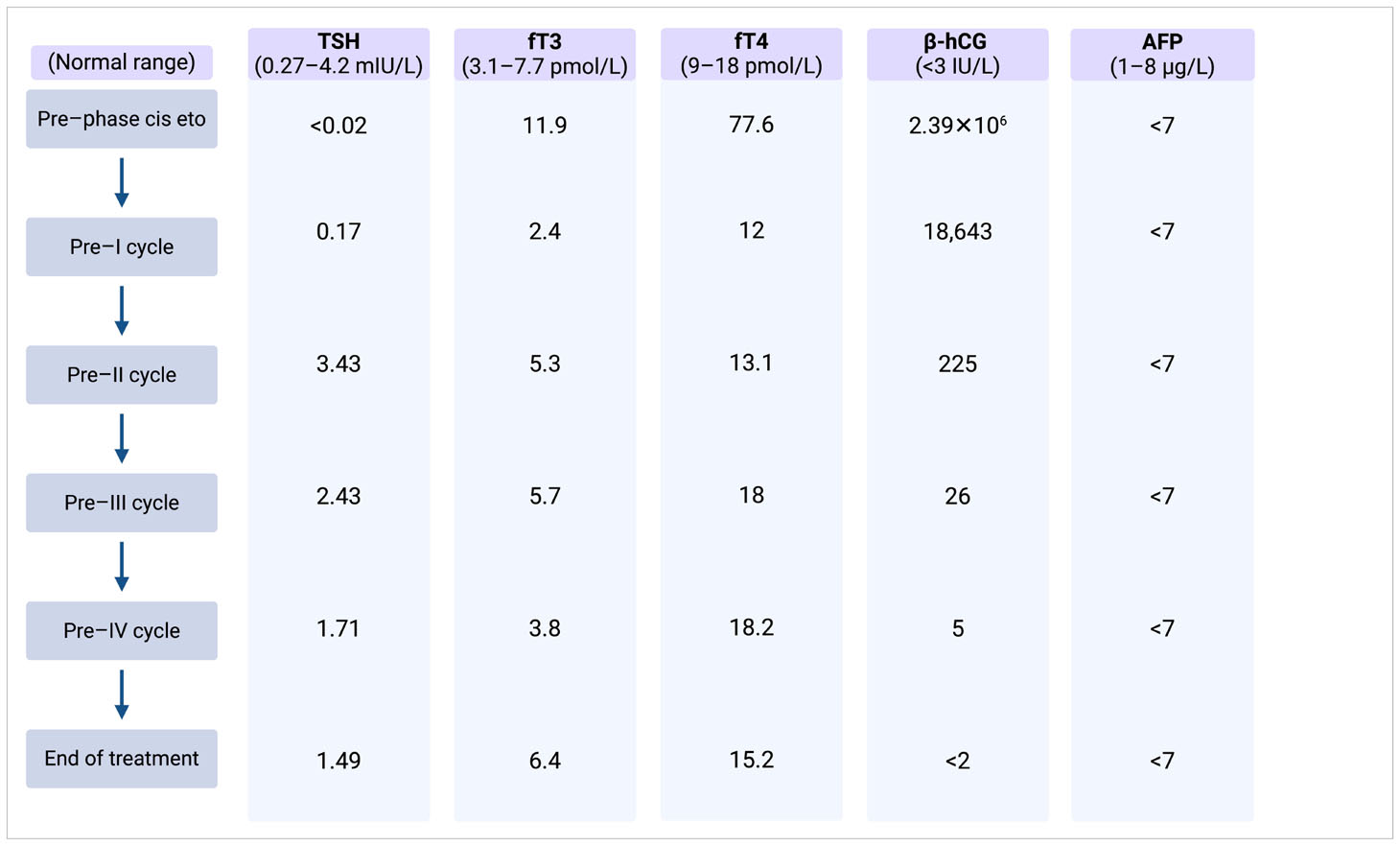
Thyroid function assessment prior to CT scanning revealed a suppressed TSH and a significantly elevated serum level of free T4 in line with thyrotoxicosis. Alongside, germ cell tumor serum tumor markers were found to be elevated with a massively elevated β-hCG of 2.39 × 106 IU/L (ULN < 3 IU/L), mildly elevated AFP at 21.3 µg/L (ULN, 8 µg/L), and an LDH of 835 IU/L (ULN, 250 IU/L). Laboratory test results and markers trend are summarized in Table 2.
An additional testicular US showed a large, hypovascularized lesion of 1.5 cm × 1.3 cm in the left testicle suspicious for a germ cell tumor.
Based on the clinical and laboratory findings, this patient was diagnosed with a metastatic non-seminoma, TNM stage cTx N3 M1b S3, clinical stage IIIC with poor prognosis according to the IGCCCG risk criteria (6, 7).
In light of the excessive β-hCG elevation and the extensive pulmonary metastases despite limited lymphonodal spread, it was assumed to be a predominant choriocarcinoma. Consequently, the patient underwent an additional magnetic resonance imaging (MRI) of the brain, which revealed metastases in the left hemisphere with perifocal edema, in the right splenium and at the base of the pituitary gland (Figure 1), the latter causing an additionally diagnosed diabetes insipidus as an explanation for his polydipsia and polyuria of about 10 L/day.
Because of the imminent respiratory failure and thyrotoxicosis, the patient was transferred to the intensive care unit and immediately commenced cytotoxic chemotherapy without primary orchiectomy.
Given the high risk of treatment-induced tumor lysis syndrome, the patient initially received one cycle of cytoreductive chemotherapy with cisplatin of 20 mg/m2 and etoposide of 75 mg/m2 on days 1–3, which lead to a major pulmonary bleeding and a pneumothorax, which required invasive ventilation due to global respiratory failure in terms of an acute respiratory distress syndrome showing the full clinical picture of a choriocarcinoma syndrome.
Following the diagnosis, in addition, carbimazole and propranolol were administered to manage the thyrotoxicosis, and nasal vasopressin was prescribed for the diabetes insipidus.
Upon clinical stabilization and extubation, first-line systemic therapy with cisplatin of 20 mg/m2, etoposide of 75 mg/m2, and ifosfamide of 1.2 g/m2 on days 1–5 etoposide (VePesid), ifosfamide and cisplatin (Platinol) (VIP) was given followed by successful autologous stem cell harvest of 3 × 6.3 × 106 CD34+ cells.
The patient was transferred to the Oncology Department and treated with three sequential cycles of high-dose VIP (HD-VIP; cisplatin of 20 mg/m2, etoposide of 300 mg/m2, and ifosfamide of 2.4 g/m2 on days 1–5) with autologous stem cell support on day 7.
The β-hCG values dropped continuously during the chemotherapy and reached normal range after the third cycle of HD-VIP achieving a marker-negative partial remission with residual masses in the retroperitoneum and lungs and equivocal changes on whole-brain MRI. The thyroid function parameters normalized quickly along the dropping β-hCG, but the patient still depended on desmopressin substitution to control the diabetes insipidus.
Six weeks after the completion of chemotherapy with continued normalized serum tumor markers, the patient underwent retroperitoneal residual mass resection and left-sided orchiectomy showing necrosis only in the resected specimens. Complete resection of the pulmonary masses was deemed unfeasible and thus omitted, whereas additional whole-brain radiotherapy (10 × 3 Gy fractions, cumulative dose 30 Gy) was applied to control the multifocal brain metastases.
After a follow-up of so far 10 years, the patient never relapsed and shows an ongoing marker-negative partial remission with small pulmonary nodules suspected as residual fibrotic residues.
Long-term sequelae of the intensified treatment include fatigue and an ongoing diabetes insipidus sufficiently controlled with transnasal desmopressin substitution.
Discussion
Testicular germ cell tumors (TGCTs) are a relatively rare type of cancer, accounting for about 1% of all cancers in men. However, TGCTs are the most common solid tumor in men aged between 20 and 34 years (10).
Expression of hCG physiologically originates from syncytiotrophoblastic cells of the placenta during pregnancy. Because non-seminomatous germ cell tumors originate from totipotential embryonic germ cell capable of generating all embryological differentiation lineages, including somatic (teratoma) and extra-embryonic (choriocarcinoma and yolk sac tumor), syncytiotrophoblastic cells can also be found in NSTGCTs and less prominently also in seminomas (11). All patients with choriocarcinomas and 40% to 60% of patients with embryonal cell carcinomas display elevated serum levels of β-hCG, whereas approximately 10%–20% of patients with pure seminoma have elevated serum β-hCG levels, which rarely exceed 500 IU/L (5). In non-seminomas, β-hCG serum levels independently predict patient survival and represent a risk factor in the IGCCCG risk classification for metastatic NSTGCTs (12). Moreover, β-hCG serum levels of >50,000 IU/L together with widespread lung metastases are risk factors for the development of choriocarcinoma syndrome, a rare complication with signs of pulmonary hemorrhage and respiratory distress syndrome (13). Paraneoplastic hCG production has also been described in liver, pancreatic, gastric, breast, kidney, and bladder cancers (5).
Interestingly, high β-hCG serum levels are linked to hyperthyroidism. This is due to a cross-reaction of β-hCG with the TSH receptor, which is based on the structural similarity between the two glycoprotein hormones (hCG and TSH) and their receptors. This not only elucidates the pathophysiology of gestational thyrotoxicosis but also clarifies the underlying mechanism of paraneoplastic hyperthyroidism in men with TGCTs exhibiting elevated serum β-hCG levels, as well as in women with gestational trophoblastic disease (1, 14, 15). Cross-activation of the TSH receptor by β-hCG occurs only at markedly high serum levels, given that β-hCG acts as a relatively weak agonist of the TSH receptor (1).
Oosting et al. evaluated prospectively the thyroid function of 144 patients treated with chemotherapy for disseminated NSTGCTs. They identified five patients with hyperthyroidism. All five patients had β-hCG levels >50,000 IU/L corresponding to 50% of patients with such high β-hCG levels, whereas none of the other 134 patients with low or intermediate hCG levels (β-hCG <5,000 IU/L or >5,000 but <50,000 IU/L) had hyperthyroidism (4). Furthermore, the likelihood of a patient developing thyrotoxicosis is influenced not just by the quantity but also by the quality of hCG, as structural changes in hCG can result in enhanced thyrotropic activity. Specifically, variants of hCG produced paraneoplastically in patients with cancer often display excessive sialylation, and the extent of this sialylation correlates with the thyrotropic activity of the hCG (16). Additionally, other molecular variants that enhance the thyrotropic potency of hCG include truncated molecules that lack the carboxyl-terminal (C-terminal) tail. Indeed, the role of C-terminal peptide seems to be to prevent hyperthyroidism during the first trimester of pregnancy, when a large amount of hCG is produced by the placenta, and this also explains why the very high hCG levels in pregnancy are not generally associated with thyrotoxicosis (14).
Primary treatment of paraneoplastic hyperthyroidism is to appropriately treat the hCG-secreting tumor. Meanwhile, hyperthyroidism should be treated to control the negative systemic consequences of thyrotoxicosis. The pharmacological treatment can include anti-thyroid drugs, inorganic iodide, corticosteroids, β-blockers, and antipyretic agents depending on clinical presentation (1). Treatment should be given according to local policy with the help of endocrinology specialists. Both presented patients received therapy with anti-thyroid drugs and β-blockers to control the excessive thyroid function. The first patient was treated with methimazole of 30 mg/day and propranolol of 40 mg/day, and the other patient received carbimazol of 15 mg/day and propranolol of 40 mg/day. In patients with significantly symptomatic thyrotoxicosis, the consideration of β-blockers is advised, as β-adrenergic blocking agents can alleviate symptoms by inhibiting the effects of adrenaline on the body. Additionally, at higher doses, propranolol is known to suppress the conversion of thyroxine (T4) to the more active triiodothyronine (T3), offering a therapeutic advantage in the regulation of thyroid hormone levels in cases of thyrotoxicosis (1).
For patients with metastatic TGCT and a poor prognosis (based on the criteria of the international classification IGCCCG), surgery alone is insufficient, but cisplatin-based chemotherapy is effective to control the disease and thereby decrease β-hCG levels, which, in turn, reduces thyrotoxic symptoms, as reported for both cases (17). Certainly, thyrotoxicosis encompasses an additional thread and may contribute to a critical condition, as described for case II, but curative chemotherapy must not be postponed but started immediately along initiation of thyrostatic treatment.
As the prevalence of clinically symptomatic hyperthyroidism in men with β-hCG–secreting TGCTs is low, there are only a few cases described in literature (18, 19).
In 2000, Goodarzi and Van Herle published a review of the literature since 1964 describing 17 cases of male TGCT patients with signs and symptoms of thyrotoxicosis due to excessively elevated β-hCG levels. For two patients, survival information was not available; seven patients were alive at the time of the case report; and among the eight patients that had died, one had succumbed to thyroid storm, whereas the others died as a consequence of tumor progression (19).
We performed a literature search using the electronic databases PubMed, including case reports on patients with testicular neoplasms and hyperthyroidism or thyrotoxicosis published since 2000 and excluding non-English articles. We used the following search terms: “(hyperthyroidism) AND (germ-cell tumor),” “(hyperthyroidism) AND (germ-cell cancer),” and “(hyperthyroidism) AND “(human chorionic gonadotropin)”.
In Table 3, the 14 cases reported in literature that we found are summarized (18–31). Patients’ age at diagnosis, when reported, ranged from 18 to 58 years. Most patients (9/17) began with symptoms related to hyperthyroidism, whereas, in other patients, the neoplastic disease presented with different symptoms (e.g., testicular pain, abdominal pain, dysuria, or black stools) despite the presence of hyperthyroidism on laboratory tests. Only 10 authors reported the tumor histological subtype: in particular, we found eight cases of choriocarcinoma, two of which were part of a mixed tumor (yolk sac tumor and embryonal carcinoma or yolk sac tumor and teratoma); the remaining two patients had, respectively, an embryonal carcinoma and a mixed germ cell tumor (seminoma, embryonal cancer, and teratoma). All patients had very high levels of β-HCG, varying from 1,063 IU/L to 1,118,053 IU/L, and the majority was at a metastatic stage. At the time of publication of the case reports, four patients were alive, nine patients were dead (seven patients died shortly after diagnosis and hospitalization and two after a few months due to disease progression), and one patient was lost to follow-up.
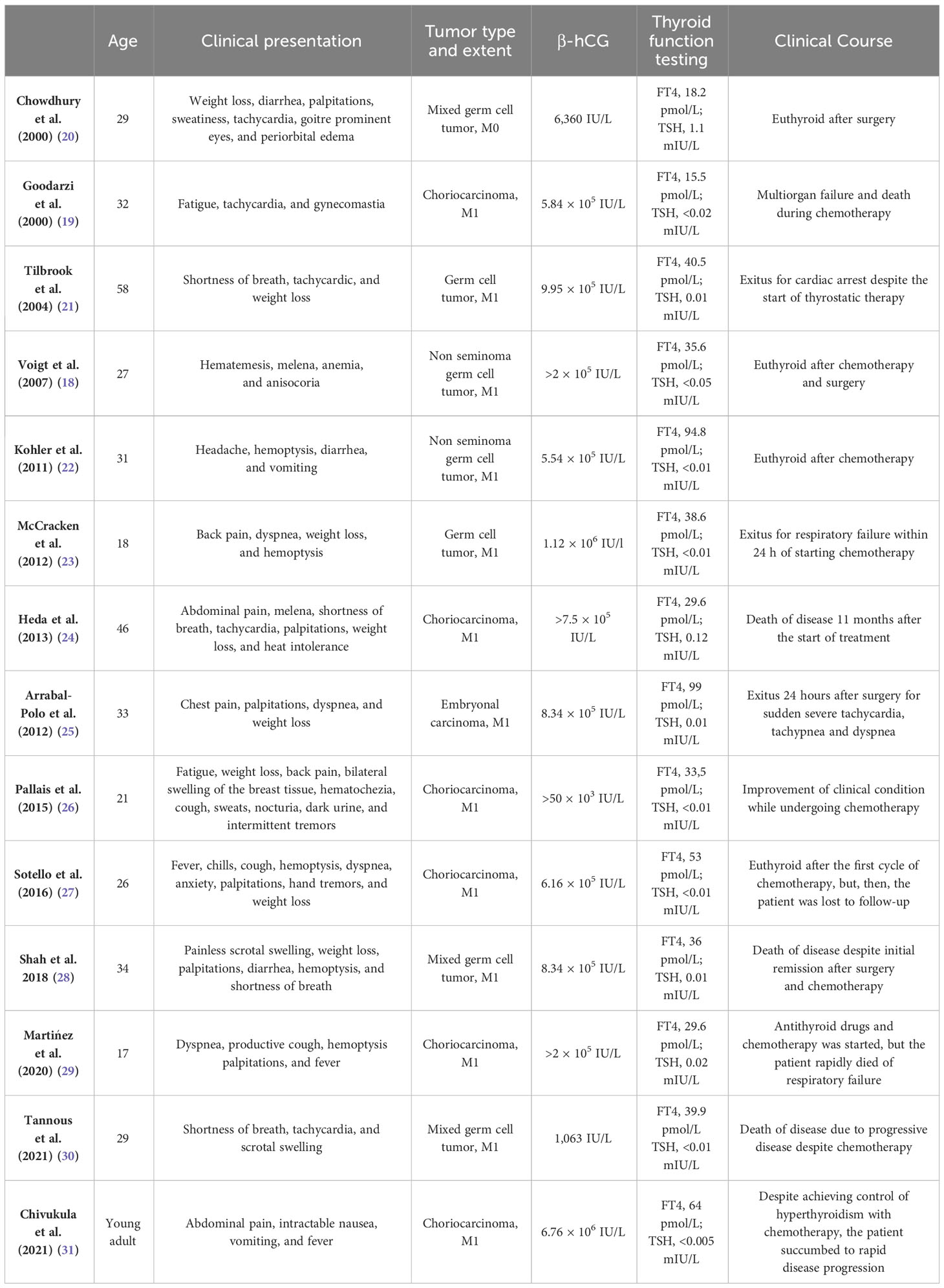
Table 3 Cases of hyperthyroidism and testicular germ cell tumors published in the literature since 2000.
Conclusion
Despite being a rare event, the presence of hyperthyroidism or thyrotoxicosis without clear etiology in a young man should lead to consider less frequent causes such as TGCTs.
Even if patients typically have mild symptoms that resolve after chemotherapy, in rare cases, a clinically significant thyrotoxicosis may arise, requiring immediate thyrostatic treatment along life-saving chemotherapy.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
DFa: Writing – original draft, Writing – review & editing. CO: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing. CS: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing. PR: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing. FCa: Writing – review & editing. MC: Writing – review & editing. SR: Writing – review & editing. FG: Writing – review & editing. GR: Supervision, Writing – review & editing. DFe: Supervision, Writing – review & editing. GF: Methodology, Supervision, Writing – review & editing. FCo: Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
SR received honoraria as a speaker at scientific events and travel accommodation from BMS, Amgen, GSK, Janssen, Astellas, and MSD; GF serves on the advisory boards of Astellas, Janssen, Pfizer, Bayer, MSD, and Merck and received travel accommodation from Astellas, Janssen, and Bayer.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, et al. 2016 American thyroid association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. (2016) 26:1343–421. doi: 10.1089/thy.2016.0229
2. Manso J, Piva I, Censi S, Clausi C, Bardi M, Schiavon B, et al. Safety and efficacy of prophylactic treatment for hyperthyroidism induced by iodinated contrast media in a high-risk population. Front Endocrinol (Lausanne). (2023) 14:1154251. doi: 10.3389/fendo.2023.1154251
3. Cappellani D, Marconcini G, Manetti L, Bartalena L, Bogazzi F. Real-life data on the effect of medical therapy for amiodarone-induced thyrotoxicosis on CV events and hospitalizations. J Clin Endocrinol Metab. (2023) 108:1298–307. doi: 10.1210/clinem/dgac756
4. Oosting SF, De Haas EC, Links TP, De Bruin D, Sluiter WJ, De Jong IJ, et al. Prevalence of paraneoplastic hyperthyroidism in patients with metastatic non-seminomatous germ-cell tumors. Ann Oncol. (2010) 21:104–8. doi: 10.1093/annonc/mdp265
5. Ehrlich Y, Beck SDW, Foster RS, Bihrle R, Einhorn LH. Serum tumor markers in testicular cancer. Urol Oncol: Semin Original Investigat. (2013) 31:17–23. doi: 10.1016/j.urolonc.2010.04.007
6. Brierley J, Gospodarowicz MK, Wittekind C eds. TNM classification of Malignant tumours. Eighth edition. Chichester, West Sussex, UK Hoboken, NJ: John Wiley & Sons, Inc (2017). p. 253.
7. Gillessen S, Sauvé N, Collette L, Daugaard G, De Wit R, Albany C, et al. Predicting outcomes in men with metastatic nonseminomatous germ cell tumors (NSGCT): results from the IGCCCG update consortium. JCO. (2021) 39:1563–74. doi: 10.1200/JCO.20.03296
8. Taza F, Abonour R, Zaid MA, Althouse SK, Anouti B, Akel R, et al. Maintenance oral etoposide after high-dose chemotherapy (HDCT) for patients with relapsed metastatic germ-cell tumors (mGCT). Clin Genitourinary Cancer. (2023) 21:213–20. doi: 10.1016/j.clgc.2023.01.004
9. Ashkar R, Adra N, Abonour R, Abu Zaid MI, Snow CI, Hanna NH, et al. Randomized phase 2 trial of maintenance oral etoposide or observation following high-dose chemotherapy for relapsed metastatic germ cell tumor. JCO. (2022) 40:TPS429–9. doi: 10.1200/JCO.2022.40.6_suppl.TPS429
10. Gaddam SJ, Chesnut GT. Testicle cancer. In: StatPearls. StatPearls Publishing, Treasure Island (FL (2023). Available at: http://www.ncbi.nlm.nih.gov/books/NBK563159/.
11. Oosterhuis JW, Looijenga LHJ. Testicular germ-cell tumours in a broader perspective. Nat Rev Cancer. (2005) 5:210–22. doi: 10.1038/nrc1568
12. International Germ Cell Cancer Collaborative Group. International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. JCO. (1997) 15:594–603. doi: 10.1200/JCO.1997.15.2.594
14. Yoshimura M, Hershman JM. Thyrotropic action of human chorionic gonadotropin. Thyroid. (1995) 5:425–34. doi: 10.1089/thy.1995.5.425
15. Steigbigel NH, Oppenheim JJ, Fishman LM, Carbone PP. Metastatic embryonal carcinoma of the testis associated with elevated plasma TSH-like activity and hyperthyroidism. N Engl J Med. (1964) 271:345–9. doi: 10.1056/NEJM196408132710704
16. Mann K, Schneider N, Hoermann R. Thyrotropic activity of acidic isoelectric variants of human chorionic gonadotropin from trophoblastic tumors. Endocrinology. (1986) 118:1558–66. doi: 10.1210/endo-118-4-1558
17. Honecker F, Aparicio J, Berney D, Beyer J, Bokemeyer C, Cathomas R, et al. ESMO Consensus Conference on testicular germ cell cancer: diagnosis, treatment and follow-up. Ann Oncol. (2018) 29:1658–86. doi: 10.1093/annonc/mdy217
18. Voigt W, Maher G, Wolf HH, Schmoll HJ. Human chorionic gonadotropin-induced hyperthyroidism in germ cell cancer – a case presentation and review of the literature. Oncol Res Treat. (2007) 30:330–4. doi: 10.1159/000101524
19. Goodarzi MO, Van Herle AJ. Thyrotoxicosis in a male patient associated with excess human chorionic gonadotropin production by germ cell tumor. Thyroid. (2000) 10:611–9. doi: 10.1089/thy.2000.10.611
20. Chowdhury TA, Tanchel BM, Jaganathan RS, Dodson PM. A toxic testicle. Lancet. (2000) 355:2046. doi: 10.1016/S0140-6736(00)02353-9
21. Tilbrook LK, Slater J, Blainey AD. Testicular germ cell tumour presenting as thyrotoxicosis. Ann Clin Biochem. (2004) 41:248–9. doi: 10.1258/000456304323019668
22. Kohler S, Tschopp O, Jacky E, Schmid C. Paraneoplastic hyperthyroidism. Case Rep. (2011) 2011:bcr0420114163–bcr0420114163. doi: 10.1136/bcr.04.2011.4163
23. McCracken EJ, Johnston PC, Lindsay JR, Mulholland C, McAleer JJA, Black RN. Testicular choriocarcinoma: an unusual case of paraneoplastic thyrotoxicosis. QJM. (2012) 105:675–7. doi: 10.1093/qjmed/hcr084
24. Heda P, Cushing G. Testicular choriocarcinoma presenting as hyperthyroidism. Am J Med. (2013) 126:e1–2. doi: 10.1016/j.amjmed.2013.07.017
25. Arrabal-Polo MA, Jimenez-Pacheco A, Arrabal-Martin M, Moreno-Jimenez J, Gutierrez-Tejero F, Galisteo-Moya R, et al. Hyperthyroidism as a clinical manifestation of a embryonal carcinoma of the testis. Acta Clin Belg. (2012) 67:214–6.
26. Pallais JC, McInnis M, Saylor PJ, Wu RI. Case 38-2015: A 21-year-old man with fatigue and weight loss. N Engl J Med. (2015) 373:2358–69. doi: 10.1056/NEJMcpc1506821
27. Sotello D, Rivas AM, Test VJ, Lado-Abeal J. Choriocarcinoma presenting with thyrotoxicosis. Proc (Bayl Univ Med Cent). (2016) 29:42–3. doi: 10.1080/08998280.2016.11929353
28. Shah D, Khalid N. Symptomatic hyperthyroidism in metastatic testicular mixed germ cell tumour. BMJ Case Rep. (2018) 2018:bcr2018224877,bcr-2018–224877. doi: 10.1136/bcr-2018-224877
29. Martínez JC, Ovalle-Zavala EA. Thyroid storm associated with testicular choriocarcinoma. Eur J Case Rep Internal Med. (2020) 7(10):69.
30. Tannous T, Miskovsky J, Keating M. Testicular germ cell tumors: paraneoplastic syndromes and the role of beta-human chorionic gonadotropin. Cureus. (2021) 13(4):e14286. doi: 10.7759/cureus.14286
Keywords: non-seminomatous testicular germ cell tumors, thyrotoxicosis, hyperthyroidism, human chorionic gonadotropin, TSH receptor
Citation: Favero D, Oing C, Seidel C, Rescigno P, Catalano F, Cremante M, Rebuzzi SE, Gatto F, Rosti G, Ferone D, Fornarini G and Cocchiara F (2024) Hyperthyroidism in non-seminomatous testicular germ cell tumors: two case reports and literature review. Front. Oncol. 14:1338438. doi: 10.3389/fonc.2024.1338438
Received: 14 November 2023; Accepted: 26 February 2024;
Published: 27 March 2024.
Edited by:
Michal Mego, Campus Bio-Medico University, ItalyReviewed by:
Jacopo Manso, University of Padua, ItalyAndrew Protheroe, Oxford University Hospitals NHS Trust, United Kingdom
Copyright © 2024 Favero, Oing, Seidel, Rescigno, Catalano, Cremante, Rebuzzi, Gatto, Rosti, Ferone, Fornarini and Cocchiara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giuseppe Fornarini, Z2l1c2VwcGUuZm9ybmFyaW5pQGhzYW5tYXJ0aW5vLml0
 Diletta Favero
Diletta Favero Christoph Oing3,4
Christoph Oing3,4 Christoph Seidel
Christoph Seidel Fabio Catalano
Fabio Catalano Sara Elena Rebuzzi
Sara Elena Rebuzzi Federico Gatto
Federico Gatto Giovanni Rosti
Giovanni Rosti Giuseppe Fornarini
Giuseppe Fornarini