- 1Department of Oncology, Akershus University Hospital (AHUS), Lorenskog, Norway
- 2Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway
- 3Department of Pathology, Oslo University Hospital, Oslo, Norway
- 4Department of Clinical Molecular Biology (EpiGen), Akershus University Hospital (AHUS), Lorenskog, Norway
Objective: In this study, we investigated pivotal molecular markers in human high-grade breast ductal carcinoma in situ (DCIS). Expression status of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth receptor 2 (HER2) was measured among various subtypes (Luminal (Lum) A, LumB HER2-, LumB HER2+, HER2-enriched and triple-negative).
Methods: In total, 357 DCIS cases were classified into respective subtypes, according to the 2013 St. Gallen guidelines. Each subtype was categorized into three subcategories: “Pure” (those without an invasive component), “W/invasive” (those with an invasive component), and “All” (the entire group of the given subtype). ER and PR expression were registered as intervals. Equivocal HER2 immunohistochemistry (IHC) cases (2+) were further investigated using dual-color in situ hybridization.
Results: The majority of patients (71%) were over the age of 50. We discovered no significant differences in the proportion of age between the “Pure” and “W/invasive” groups. There was no significant difference in ER/PR expression between “Pure” luminal subtypes of DCIS and “W/invasive” cases. We compared the HER2 IHC scores of “0”, “1+”, and “2+” among LumA and LumB HER2 subtypes and identified no statistically significant differences between “Pure” and “W/invasive” (p = 0.603). ER and PR expression ≥ 50% cutoff value was present in > 90% of all LumA cases. The incidences of cases with ER expression at cutoff values of < 10% and ≥ 50% in LumA were significantly different compared to other luminal subtypes (p < 0.0001). The proportion of cases with PR expression < 20% showed significant differences in the various luminal subtypes. In luminal B subtypes, low PR expression (< 20%) was significantly associated with both strong HER2 expression (3+) and the presence of an invasive component (p = 0.0001 and p = 0.0365, respectively).
Conclusions: ER and PR expression at ≥ 50% cutoff values were found in more than 90% of LumA cases. Samples with ER < 10% and ≥ 50% in LumA were significantly different compared to other luminal subtypes (p < 0.0001). Low PR expression in high-grade DCIS was strongly associated with HER2 overexpression (3+) and an invasive component (p = 0.0001 and p = 0.0365, respectively).
Introduction
Breast ductal carcinoma in situ (DCIS), a precancerous lesion that is considered to be a precursor of invasive breast carcinoma (IBC), is a frequent finding during modern breast cancer diagnostics after mammography screening was implemented (1). In industrialized countries, the incidence of DCIS is approximately 20%–25% of all malignant lesions detected in national screening programs (1, 2). Only a proportion of these lesions eventually transform into IBC. If left untreated, it is predicted that 8%–17.6% of DCIS cases may progress to IBC after 10 years, and in some studies, this percentage has been as high as 20%–30% (3). Many of these lesions are small when detected and guidelines for in situ lesions recommend either surgery alone or surgery followed by radiation as the usual treatment options. Treatment of DCIS is still exclusively determined by the extent and histological grade of the lesion (4). Our overall aim is to identify distinct subtypes of DCIS lesions that often progress to IBC, or not, to pave the way toward precision medicine for patients diagnosed with DCIS. We hypothesize that selected patients at high risk for the development of invasive cancers may require intensified, tailored, and targeted treatment, possibly including immunotherapy (e.g., anti-PD-1/PD-L1 (5, 6)) and anti-human epidermal growth factor receptor 2 (HER2) therapy, as offered to patients diagnosed with IBC (7–10). We believe that changing the current guidelines may not only have beneficial impacts on patients, avoiding under- and overtreatment, but also the health system will be able to better allocate funding to patients diagnosed with high-grade DCIS. To date, the utility of immunohistochemistry (IHC) markers has not been established in DCIS diagnostics, in contrast to IBC, where the hormone receptors (HRs) for estrogen (ER) and progesterone (PR), HER2, and Ki67 proliferation index are all deciding on a complex treatment algorithm. DCIS of the breast is a heterogeneous entity with nuclear atypia varying from mild to pronounced with various distinct growth patterns. We regularly confirm the presence of IHC-stained DCIS as part of the routine diagnosis of IBC but do not take much notice of it since these findings currently have no impact on therapy. Few studies have evaluated these markers in DCIS according to their molecular subtypes (11–13). In a previous study of high-grade breast DCIS, we reported the distribution of molecular subtypes. LumA and luminal B HER2-negative (LumB HER2−) cases together comprised 50.4%, luminal B HER2-positive (LumB HER2+) comprised 22.1%, HER2-enriched comprised 21.8%, and triple-negative (TPN) subtype comprised 5.6% of cases. We also identified the HER2-enriched subtype as a high-risk entity because it was significantly correlated with the presence of an invasive component (14, 15). This study aimed to investigate the pivotal and well-established molecular breast cancer markers, ER, PR, and HER2, in the respective subtypes of human high-grade DCIS, aiming to identify those DCIS lesions that most probably will progress to IBC.
Materials and methods
Our study material consisted of formalin-fixed and paraffin-embedded (FFPE) histopathological specimens from the consecutive patient cohort stored in the diagnostic archive at Akershus University Hospital, Norway. Collected between 1996 and 2018, these samples represented 494 female patients diagnosed with DCIS of the breast. Experienced breast pathologists actively graded the histopathological specimens using the Van Nuys classification system (15, 16). To our knowledge, this is the largest DCIS biobank that has been approved for cancer research purposes in Europe. We chose to focus on and investigate grade 3 (high-grade) DCIS cases because these lesions are thought to have the highest risk of recurrence and progression to IBC (16–19). A total of 357 high-grade DCIS cases were submitted to IHC analysis, stained for ER, PR, HER2, and Ki67, and subjected to further studies. These were classified into their respective subtypes in accordance with the 2013 St. Gallen International Consensus Conference Guidelines, currently established for molecular subtyping of IBC lesions (15). Briefly, according to this classification (20), the LumA subtype was defined when ER was positive (≥ 1%) and/or PR was ≥ 20%, HER2−, and Ki67 index was < 20%. LumB HER2− was defined as ER that was positive, HER2−, and Ki67 index expression was ≥ 20%, or when ER was ≥ 1%, Ki67 was ≥ 20% or PR expression was < 20%, and HER2 was negative. LumB HER2+ was defined when ER and/or PR were positive and HER2+ and Ki67 were at any value. HER2-enriched was defined as ER and PR negativity, HER2 positivity, and any Ki67 value. TPN was defined when ER, PR, and HER2 were negative and Ki67 was at any value. The general definitions of the molecular subtypes in IBC, according to IHC surrogate markers, are provided in Supplementary Table S1 (21–23). All procedures have been described in detail in our previous study (15). Each subtype was sorted into three subcategories: “Pure” (n = 306) meaning those without an invasive component; “W/invasive” (n = 51) meaning those with an invasive component; and “All” (n = 357) meaning the entire group of the given subtype. We decided to split the patients based on age (younger than 50 and older than 50), taking into account other studies that looked at the incidence and mortality rate of DCIS (2, 24).
Immunohistochemistry and dual-color silver-enhanced in situ hybridization
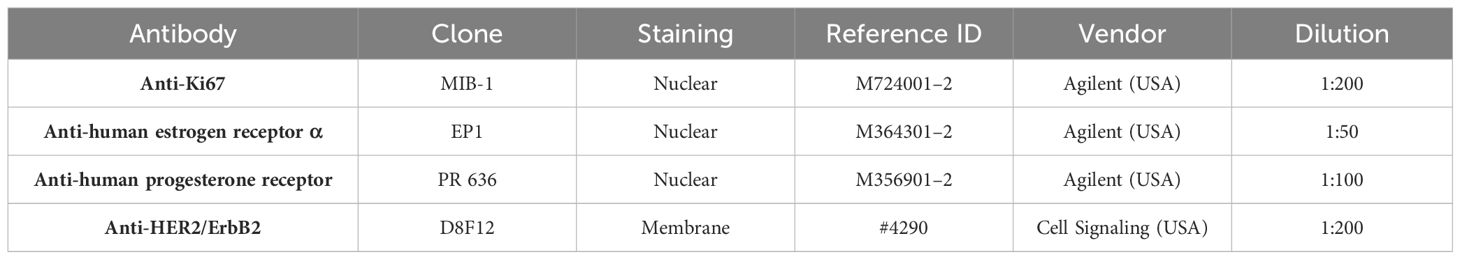
IHC staining for ER, PR, HER2, and Ki67 was performed using a Dako Autostainer (Agilent). Antigen retrieval was achieved in a PT-Link station by immersion in EnVision™ FLEX Target Retrieval Solution at a high pH (K8004, Agilent) and heating at 97°C for 20 min. Endogenous peroxidase activity was quenched by incubating the slides in the EnVision™ FLEX peroxidase blocking reagent (K8000, Agilent) for 5 min. For HER2 IHC, nonspecific staining was inhibited by an animal-free blocking solution 1× (No. 15019) for 30 min. Primary antibodies Ki67 (1:200), ER (1:50), and PR (1:100) were diluted in EnVision™ FLEX Antibody Diluent (K8006, Agilent); antibody HER2 (1:200) was diluted in SignalStain® Antibody Diluent (No. 8112, Cell Signaling), and slides were incubated with primary antibodies for 20–60 min at room temperature. For ER and PR IHC, rabbit (K800921–2, Agilent) and mouse (K800221–2, Agilent) linkers were added for 15 min for signal amplification after incubation with the primary antibody. This was followed by incubation with the ready‐to‐use secondary buffered solution (k8002, EnVision FLEX/HRP, Agilent) for 20 min. The sections were reacted with 3.30‐diamino‐benzidine tetrahydrochloride (DAB) solution for 10 min and counterstained with hematoxylin (link) (k8008, Agilent) for 5 min. In each run, a positive tissue control with invasive mammary carcinoma was included. Details of the antibody clones, staining, and dilutions are described in Table 1. The ER and PR IHC positivity was defined as ≥ 1% positive tumor (DCIS) cells in accordance with the updated guidelines of the American Society of Clinical Oncology (ASCO) and College of American Pathologists (CAP) (25) developed for IBC. We chose to divide the ER into the following intervals: < 1%, 1%–10%, > 10%–50%, and > 50%–100%, and PR in < 1%, 1%–20%, > 20%–50%, and > 50%–100%, based on St. Gallen 2013 and numerous other studies that examined the prognostic and predictive values in different HRs cutoff points (20, 26, 27). Ki67% IHC was estimated, by counting 200 DCIS cells in two separate hotspot foci, and the ratios were calculated and recorded as continuous values, rather than categorical values. Ki67 cutoff threshold was set at 20%, in accordance with the 2013 St. Gallen Recommendations (28) established for IBC. Three breast pathologists interpreted the IHC analysis in ER, PR, HER2, and Ki67. HER2 IHC was scored based on ASCO/CAP guidelines, as in routine diagnostics for IBC (29, 30). Briefly, HER2 was scored “0” when IHC staining was absent or membrane staining was weak and pale in ≤ 10% of DCIS cells. HER2 was considered “1+” when partial and incomplete membrane staining also showed a faint intensity within > 10% of the DCIS cells, and HER2 that was scored as “3+” showed strong and complete positive membrane staining in > 10% of DCIS cells. HER2 was identified as “2+” when the membrane was stained faint to moderately complete in > 10% of DCIS cells or strongly and completely in ≤ 10% of DCIS cells, which was considered equivocal and was subjected to further dual-color silver-enhanced in situ hybridization (dc-SISH) analysis performed on a Ventana BenchMark (Roche Diagnostics, Switzerland) machine using the fully automated Ultra-IHC/ISH Staining Module (31) with CC2 as a buffer. The dc-SISH results were interpreted in accordance with the ASCO/CAP comprehensive guidelines and algorithms established for IBC (32). We did not observe any changes in the quality or intensity of ER, PR, Ki67, HER2 IHC, or HER2 SISH, regardless of when the sample was taken.
Statistical analysis
GraphPad Prism version 9.4 was used for the statistical calculations. We applied different tests to calculate statistical significance, ensuring they were conducted only when appropriate. Pearson’s Chi-square (χ²) or Fisher’s exact tests were used to calculate p-values when comparing two proportions, using the contingency table. Statistical significance was set a priori at p < 0.05.
Results
Hormone receptor status
The majority of patients (71%) were older than 50 years (Supplementary Figures S1A, B; Table 2). There were no significant differences in the proportion of age in the “Pure” (n = 306) versus “W/invasive” (n = 51) groups. Of “All” cases, 98% of the LumA subtype showed an ER ≥ 50%. PR expression ≥ 50% was found in 91% of cases in this subtype. In general, the expression of PR was slightly lower than that of ER in the LumA subtype. Details of ER and PR expression in luminal subtypes (LumA, LumB HER2−, and LumB HER2+) according to 2013 St. Gallen consensus meeting guidelines are shown in Figures 1A-F; Supplementary Table S2. The incidence of ER-positive cases at a cutoff of < 10% in the LumA subtype was significantly lower than that in the LumB HER2− and LumB HER2+ subtypes (p < 0.0001, chi-square) (Figure 2A). In contrast, there was a statistically significant increase in ER expression at a cutoff of ≥ 50% in the LumA compared to the latter subtypes (p < 0.0001, chi-square) (Figure 2B). The proportion of PR-positive cases at a cutoff of < 20% showed significant differences in luminal subtypes between the LumA, LumB HER2−, and LumB HER2+ subtypes (3%, 47%, and 44%, respectively) (p < 0.0001, Chi-square) (Figure 2C). There was also a significantly higher proportion of patients with a PR ≥ 50% among the LumA subtype (p < 0.0001, Chi-square) (Figure 2D). We demonstrated that low PR expression (< 20%) was significantly associated with a concurrent invasive component (p = 0.0365, Fisher’s exact test) (Figure 3A). We observed no significant differences in ER expression (< 1%, 1%–10%, > 10%–50%, > 50%–100%) and PR expression (< 1%, 1%–20%, > 20%–50%, > 50%–100%) between luminal subtypes in “Pure” cases of high-grade DCIS and those “W/invasive” components.
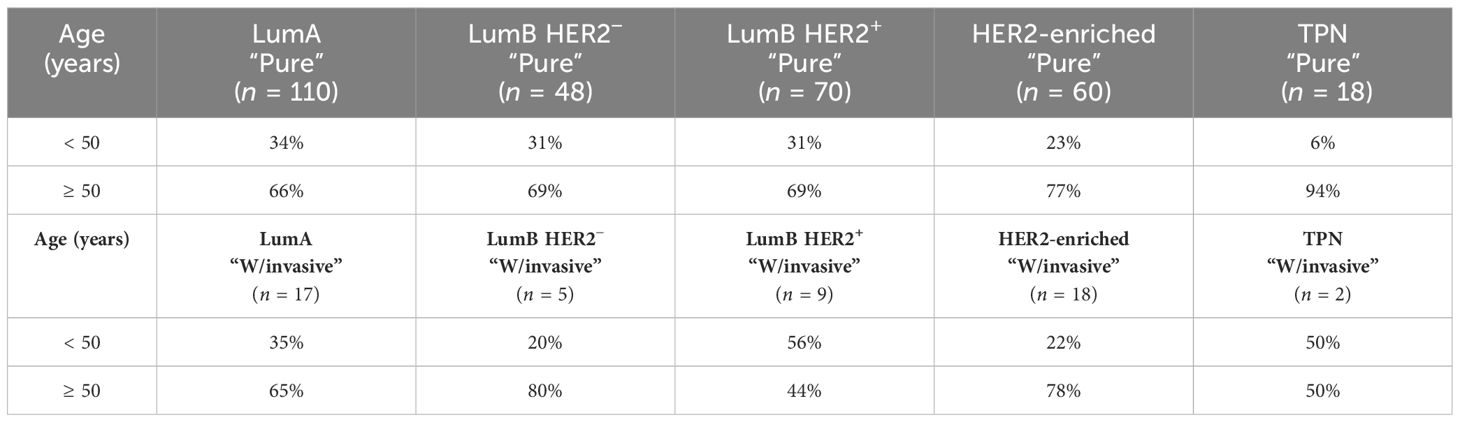
Table 2 Distribution of age ≶ 50 years is demonstrated among all subtypes that are further subcategorized in “Pure” and “W/invasive”, respectively.
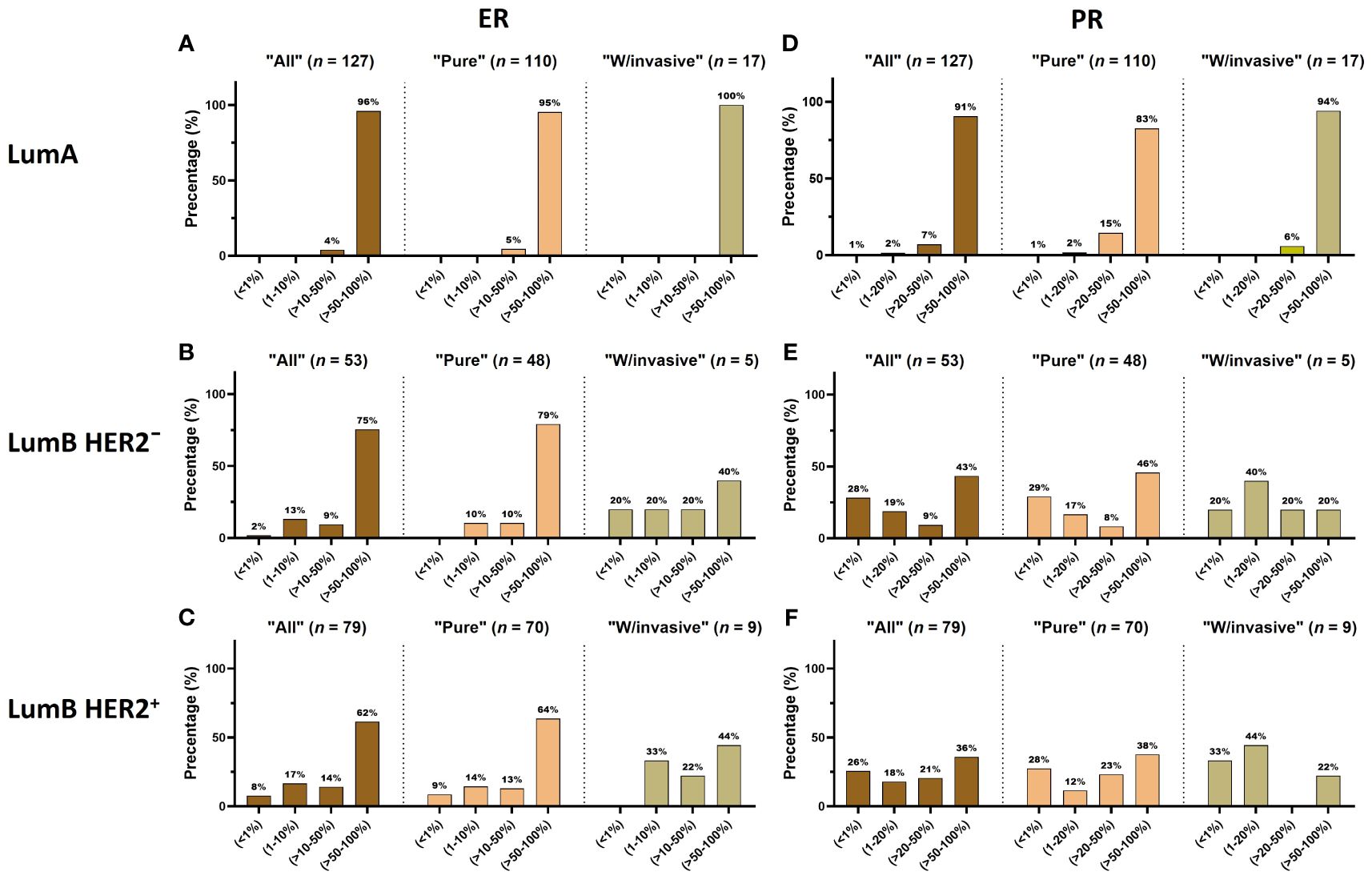
Figure 1 (A–F) The distribution of ER and PR expression is displayed in each luminal subtype (LumA, LumB HER2− and LumB HER2+). Each subtype is further subcategorized into “All”, “Pure”, and “W/invasive”, respectively.
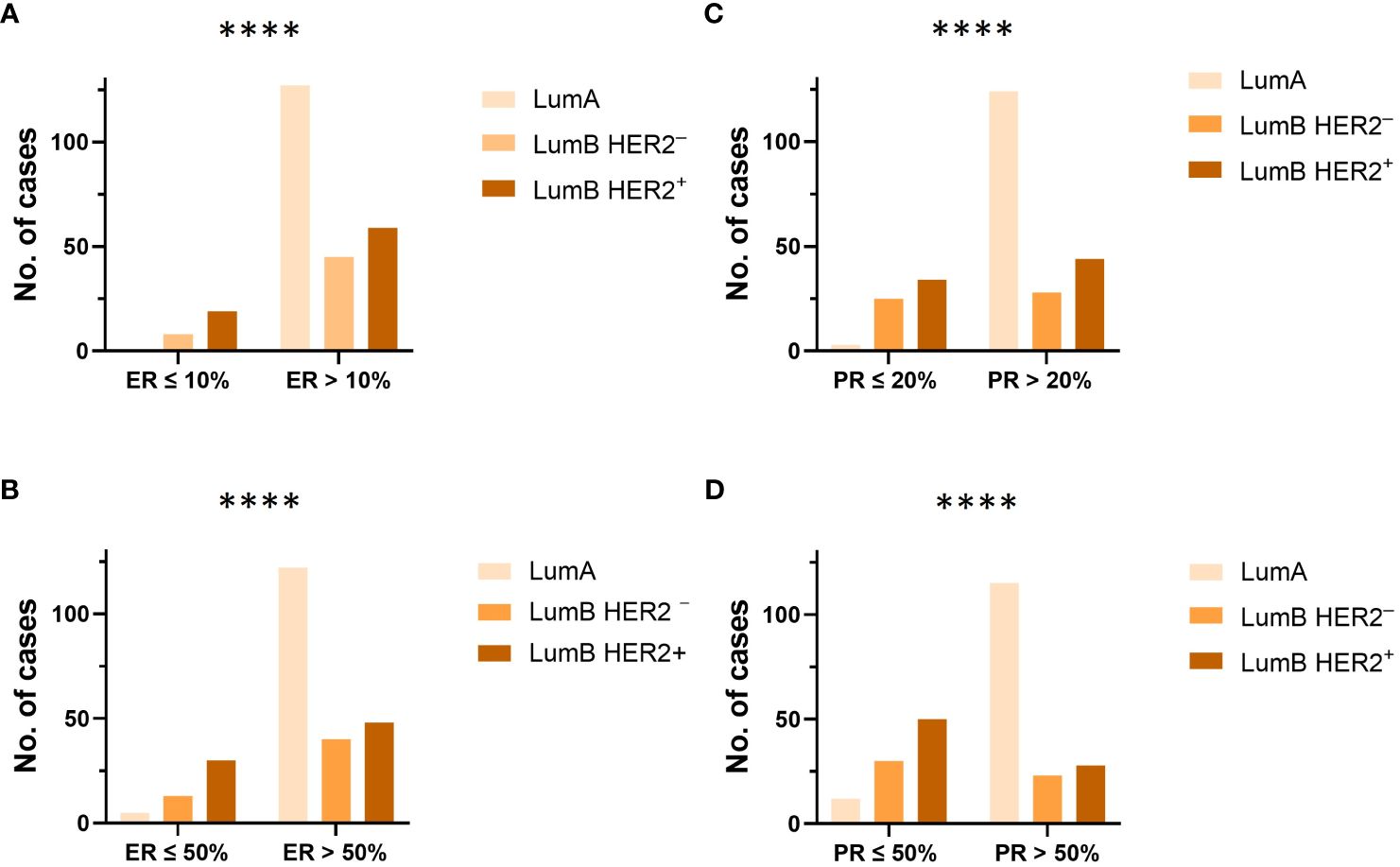
Figure 2 (A, B) The proportion and distribution of ER at cutoff values of 10% and 50%, were compared between each luminal subtype (LumA, LumB HER2− and LumB HER2+), respectively. ****p-value < 0.0001, Chi-square test. (C, D) The proportion and distribution of PR at cutoff values of 20% and 50%, were compared between each luminal subtype (LumA, LumB HER2−, and LumB HER2+), respectively. ****p-value < 0.0001, Chi-square test.
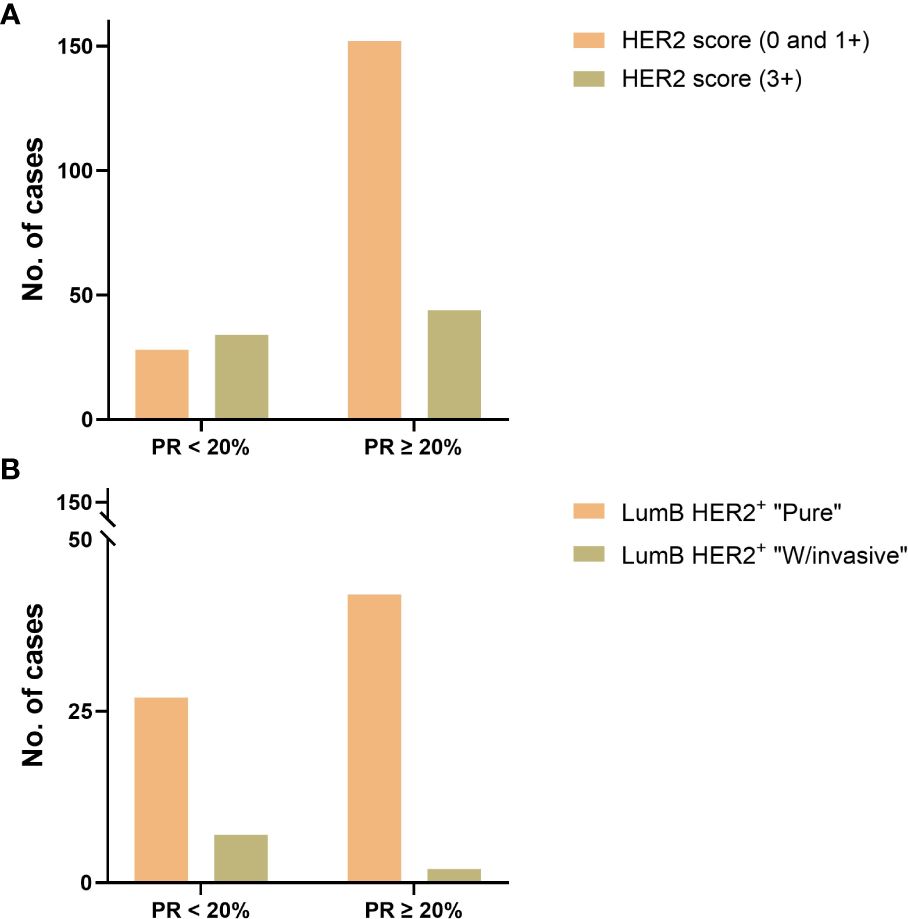
Figure 3 (A) PR expression status with a cutoff value of 20% was compared to HER2 IHC scores (“0”, “1+”, and “3+”) in luminal B subtypes and showed a significant difference (p-value = 0.0001, Fisher’s exact test) in favor of those with HER2 IHC score 3+. (B) PR expression status with a cutoff value of 20% in the LumB HER2+ subtype was compared in the “Pure” vs. “W/invasive” subcategories and showed a significant difference (p-value = 0.0365, Fisher’s exact test) in favor of the “W/invasive” component.
HER2 status
The distribution of HER2 IHC scores showed the proportions for score 0 as 41%; score 1+ as 13%; score 2+ as 4%; and score 3+ as 42%. Of the LumA cases, 96% were HER2−, with an IHC score of 0 or 1+ (Table 3). We compared the HER2 IHC 0, 1+, and 2+ scores among LumA and LumB HER2− subtypes and did not find statistically significant differences when the “Pure” and “W/invasive” were compared (p = 0.603, Chi-square). We found a significant association between HER2 overexpression (score 3+) and low PR expression (< 20%) in the luminal B subtypes (p = 0.0001, Fisher’s exact test) (Figure 3B). A total of 16 cases were HER2 IHC equivocal (2+ score) and subjected to HER2 dc-SISH analysis. Five cases belonged to LumA, one to LumB HER2−, seven to LumB HER2+, and one to HER2-enriched, whereas two samples belonged to the TPN subtype. Nonamplified cases had a mean of 3.1 HER2 gene signals and 2.3 chromosome 17 (CEP17) signals. Amplification by dc-SISH was observed in 50% of cases. The LumB HER2+ subtype accounted for 88% of all dc-SISH amplification cases. Figures 4A-F display dc-SISH staining in selected cases, from nonamplification to high amplification (clusters). Details of the dc-SISH findings, HER2 gene, and centromeric probe for CEP17 count and ratios are summarized in Table 4.
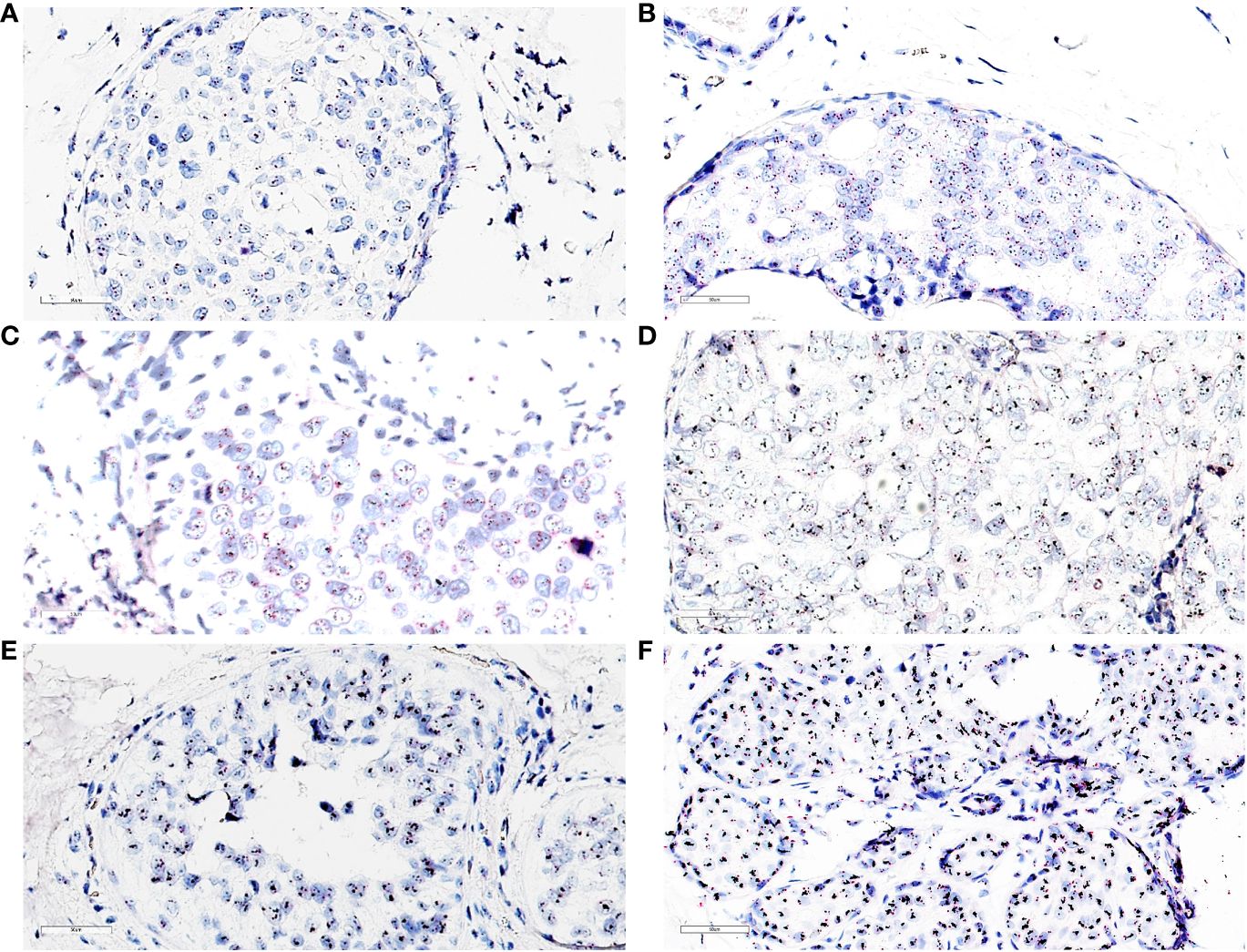
Figure 4 (A) dc-SISH of HER2 (black signals) and CEP17 (red signals) displaying nonamplification (disomy). HER2/CEP17 was equal to 1.25 (2.75/2.2); ×40 magnification. (B) dc-SISH of HER2 (black signals) and CEP17 (red signals) displaying a heterogenous nonamplification with two to four HER2 and CEP17 signals. HER2/CEP17 was equal to 1.16 (3.43/2.95); ×40 magnification. (C) dc-SISH of HER2 (black signals) and CEP17 (red signals) displaying tetrasomy nonamplification. HER2/CEP17 was equal to 1.16 (4.65/4.0); ×40 magnification. (D) dc-SISH of HER2 (black signals) and CEP17 (red signals) displaying amplification. HER2/CEP17 was equal to 4.67 (7.7/1.65); ×40 magnification. (E) dc-SISH of HER2 (black signals) and CEP17 (red signals) displaying HER2/CEP17 high amplification (cluster); ×40 magnification. (F) dc-SISH of HER2 (black signals) and CEP17 (red signals) displaying HER2/CEP17 high amplification (cluster) with retrograde lobular cancerization (cancerization of lobules) growth pattern; ×40 magnification.
Discussion
ER regulates PR expression in human breast tissue; thus, the latter hormone receptor is a clinical prognostic marker of ER action (33, 34). Therefore, low PR expression may indicate low or fading ER expression. Overall, our results demonstrated considerable heterogeneity in high-grade DCIS. High ER and PR expression were significantly more prevalent in the LumA subtype than in the LumB HER2− and LumB HER2+ subtypes. In contrast, low ER and PR expression were more common in the latter two subtypes (p < 0.0001, Chi-square). Our results are consistent with those made in IBC (23). Furthermore, we found that low PR expression was significantly associated with HER2 overexpression (IHC score 3+) in both luminal B subtypes and in those with an invasive component (p = 0.0001 and p = 0.0365, respectively; Fisher’s exact test). These findings indicate that low PR expression in DCIS is an independent marker for progression to IBC and upregulation of the HER2 gene, with poor outcomes in patients diagnosed with IBC (35–39). Konecny et al. (40) identified an inverse relationship between HR levels and HER2 gene amplification in a large number of human breast cancer tissues. Huang HJ et al. investigated the relationship between age, HRs, and HER2 status in female patients with invasive breast cancer (41). They found that the relationship between HRs and HER2 expression varied with patient age, with a negative correlation primarily observed in patients aged > 45 years. This is an intriguing finding given that the majority (71%) of patients in our study material were over the age of 50 years (Supplementary Figures S1A, B). Shah et al. (42) reported a significant difference between age and ER/PR expression, although they did not identify any significant variation between age and HER2 overexpression. In our earlier study, we identified that HER2 overexpression in cases classified as the HER2-enriched subtype was concurrent with early morphological invasive growth in high-grade DCIS (15). HER2 gene amplification in IBC is an independent poor prognostic factor (43, 44). The prognostic and predictive roles of PR in IBC have been reported by others in some previous studies (35, 36, 45–47). However, while drugs that target ER are common therapeutic tools for the treatment of patients diagnosed with IBC (48), an effective drug targeting PR has not yet been approved for the treatment of these patients (35). Further studies are necessary to investigate whether, in diagnosing IBC and DCIS, PR can possibly be a reliable and reproducible prognostic and predictive marker in selected patients. Our results showed that HER2 expression varied greatly, with no significant differences between the LumA and LumB HER2− subtypes (Table 3). The LumB HER2+ subtype had the highest number of equivocal (IHC 2+ score) lesions (7 of 16) and harbored an IHC 3+ score in 72 of 149 (48%) cases. The HER2-enriched subtype was uniform and had a strong IHC 3+ score in all except one case, whereas the LumB HER2+ subtype was quite heterogeneous, showing cluster amplification as well as polysomy and aneusomy with varying HER2 ratios, consistent with a lower grade of HER2 gene amplification. Our findings show that LumB subtypes are a heterogeneous family that displays a biological continuum of alterations in growth pattern, HR, and HER2 expression. Bediaga et al. (49) demonstrated that, in IBC, the LumB HER2 subtypes (HER2+ and HER2−) had both low and high DNA methylation categories, resulting in different epigenetic and clinical features. The high DNA methylation subgroup corresponded to the LumB HER2+ subtype, whereas the low DNA methylation group clustered with the LumA subtype. Our results also support this finding since only the present cutoff value for the Ki67 proliferation index determines whether a DCIS sample that is HR-positive and HER2−, is categorized as LumA or LumB HER2−. In the context of IBC, these cutoff values have been subject to discussions and changes in numerous studies (28, 50, 51). We investigated ER and PR expression in DCIS with various cutoff values and did not demonstrate any statistically significant differences between cases classified as “Pure” with those classified as “W/invasive”.
Strengths and limitations of the study
To our knowledge, this study material is the largest DCIS biobank that has been approved for cancer research purposes in Norway. It is a large collection of tissue material that represents a 22-year period and was obtained during the initial diagnosis. All samples were assessed and rated by qualified mammary pathologists. IHC analyses were performed in a single pathology department in accordance with national standards and recommendations.
Limitations of the study
Pathologists and clinicians are aware of the challenges associated with the Ki67 IHC assessment, which can lead to differences in the results of analyses within and between departments of pathology.
Conclusions
The majority of patients (71%) in our material study were over 50 years old. IHC analysis of ER and PR revealed a spectrum from low to high expression within the DCIS LumA and LumB subtypes, with the former displaying significantly higher expression than the latter (p < 0.0001). According to our results, the LumB subtypes are a diverse group that exhibits a biological continuum of changes in HR, growth pattern, and HER2 amplification. In this study, we demonstrated that low PR in high-grade DCIS was linked to concurrent HER2 overexpression as well as the coexistence of an invasive component in the luminal B subtypes (p = 0.0001 and p = 0.0365, respectively). PR and HER2 may have the potential to be incorporated into diagnostic tools as robust and reproducible prognostic and predictive markers for distinguishing high-risk in situ lesions that progress to IBC. The results of this study may contribute to the identification of high-risk patients with DCIS potentially in need of systemic adjuvant therapy. This question has to be addressed in clinical trials.
Data availability statement
The datasets for this article are not publicly available due to concerns regarding participant/patient anonymity. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Regional Committee for Medical and Health Research Ethics (REK). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because all living patients received an information letter in which the purpose of the project was described. The text of the information letter was approved by the Regional Committee for Medical and Health Research Ethics (REK) (case Nos. 29299 and 307976). A prepaid return envelope was enclosed in the letters patients received, in addition to a sheet to sign and return if they objected. If they agreed, they would not need to undertake any action. We received reservations from ten patients; consequently, their cases were excluded from further examinations.
Author contributions
HS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. LF: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing. DP: Formal analysis, Validation, Writing – review & editing. YL: Visualization, Writing – review & editing. SA: Formal analysis, Methodology, Writing – review & editing. JG: Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing. TS: Conceptualization, Data curation, Formal analysis, Investigation, Project administration, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Akershus University Hospital in Norway generously provided open-access funding. We extend our sincere appreciation to the National Network for Breast Cancer Research in Norway, Akershus University Hospital, Division of Medicine, and the University of Oslo, Faculty of Medicine (JG), for their valuable research support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1347166/full#supplementary-material
Abbreviations
DCIS, ductal carcinoma in situ; BC, invasive breast carcinoma; IHC, immunohistochemistry; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; Ki67, proliferation index; HR, hormone receptor; dc-SISH, dual-color silver-enhanced in situ hybridization; FFPE, formalin-fixed paraffin-embedded.
References
1. Ponti A, Lynge E, James T, Májek O, von Euler-Chelpin M, Anttila A, et al. International variation in management of screen-detected ductal carcinoma in situ of the breast. Eur J Cancer (Oxford England: 1990). (2014) 50:2695–704. doi: 10.1016/j.ejca.2014.07.019
2. Elin Wølner Bjørnson ÅSH, Hofvind S. Breast Screen Norway: 25years of organized screening. Oslo: Cancer Registry of Norway (2022). Available at: https://www.kreftregisteret.no/globalassets/mammografiprogrammet/rapporter-og-publikasjoner/2022–25-arsrapport_webversjon.pdf.
3. Ryser MD, Weaver DL, Zhao F, Worni M, Grimm LJ, Gulati R, et al. Cancer outcomes in DCIS patients without locoregional treatment. J Natl Cancer Instit. (2019) 111:952–60. doi: 10.1093/jnci/djy220
4. Parker S. Clinical guidelines for the management of breast cancer (2019). Available online at: https://www.england.nhs.uk/mids-east/wp-content/uploads/sites/7/2018/02/guidelines-for-the-management-of-breast-cancer-v1.pdf.
5. Ubago JM, Blanco LZ, Shen T, Siziopikou KP. The PD-1/PD-L1 axis in HER2+ Ductal carcinoma in situ (DCIS) of the breast. Am J Clin pathol. (2019) 152:169–76. doi: 10.1093/ajcp/aqz020
6. Wang X, Liu Y. PD-L1 expression in tumor infiltrated lymphocytes predicts survival in triple-negative breast cancer. Pathol Res practice. (2020) 216:152802. doi: 10.1016/j.prp.2019.152802
7. Mercogliano MF, Bruni S, Mauro FL, Schillaci R. Emerging targeted therapies for HER2-positive breast cancer. Cancers (Basel). (2023) 15(7):1987. doi: 10.3390/cancers15071987
8. Montagna E, Colleoni M. Hormonal treatment combined with targeted therapies in endocrine-responsive and HER2-positive metastatic breast cancer. Ther Adv Med Oncol. (2019) 11:1758835919894105. doi: 10.1177/1758835919894105
9. Hua X, Bi XW, Zhao JL, Shi YX, Lin Y, Wu ZY, et al. Trastuzumab plus endocrine therapy or chemotherapy as first-line treatment for patients with hormone receptor-positive and HER2-positive metastatic breast cancer (SYSUCC-002). Clin Cancer Res. (2022) 28:637–45. doi: 10.1158/1078-0432.CCR-21-3435
10. Iqbal N, Iqbal N. Human epidermal growth factor receptor 2 (HER2) in cancers: overexpression and therapeutic implications. Mol Biol Int. (2014) 2014:852748. doi: 10.1155/2014/852748
11. Davis JE, Nemesure B, Mehmood S, Nayi V, Burke S, Brzostek SR, et al. Her2 and ki67 biomarkers predict recurrence of ductal carcinoma in situ. Appl Immunohistochem Mol Morphol. (2016) 24:20–5. doi: 10.1097/PAI.0000000000000223
12. Fulawka L, Blaszczyk J, Tabakov M, Halon A. Assessment of Ki-67 proliferation index with deep learning in DCIS (ductal carcinoma in situ). Sci Rep. (2022) 12:3166. doi: 10.1038/s41598-022-06555-3
13. Petrone I, Resende, Rodrigues F, Valverde Fernandes P, Abdelhay E. Immunohistochemical biomarkers in ductal carcinoma in situ. Open J Pathol. (2020) 10:129–46. doi: 10.4236/ojpathology.2020.104013
14. Schandiz H, Park D, Kaiser YL, Lyngra M, Talleraas IS, Geisler J, et al. Abstract A015: A novel diagnostics approach to Ductal Carcinoma In Situ (DCIS) with potential impact on the therapeutic algorithms. Cancer Prev Res. (2022) 15(12_Supplement_1):A015. doi: 10.1158/1940–6215.DCIS22-A015
15. Schandiz H, Park D, Kaiser YL, Lyngra M, Talleraas IS, Geisler J, et al. Subtypes of high-grade breast ductal carcinoma in situ (DCIS): incidence and potential clinical impact. Breast Cancer Res Treat. (2023) 201:329–38. doi: 10.1007/s10549-023-07016-9
16. Silverstein MJ, Poller DN, Waisman JR, Colburn WJ, Barth A, Gierson ED, et al. Prognostic classification of breast ductal carcinoma-in-situ. Lancet. (1995) 345(8958):1154–7. doi: 10.1016/S0140-6736(95)90982-6
17. Maxwell AJ, Clements K, Hilton B, Dodwell DJ, Evans A, Kearins O, et al. Risk factors for the development of invasive cancer in unresected ductal carcinoma in situ. Eur J Surg Oncol. (2018) 44:429–35. doi: 10.1016/j.ejso.2017.12.007
18. Jones JL. Overdiagnosis and overtreatment of breast cancer: Progression of ductal carcinoma in situ: the pathological perspective. Breast Cancer Res. (2006) 8:204. doi: 10.1186/bcr1397
19. Hoffman AW, Ibarra-Drendall C, Espina V, Liotta L, Seewaldt V. Ductal carcinoma in situ: challenges, opportunities, and uncharted waters. Am Soc Clin Oncol Educ book. 2012:40–4. doi: 10.14694/EdBook_AM.2012.32.228
20. Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. (2013) 24:2206–23. doi: 10.1093/annonc/mdt303
21. Maisonneuve P, Disalvatore D, Rotmensz N, Curigliano G, Colleoni M, Dellapasqua S, et al. Proposed new clinicopathological surrogate definitions of luminal A and luminal B (HER2-negative) intrinsic breast cancer subtypes. Breast Cancer res: BCR. (2014) 16:R65. doi: 10.1186/bcr3679
22. Sali AP, Sharma N, Verma A, Beke A, Shet T, Patil A, et al. Identification of luminal subtypes of breast carcinoma using surrogate immunohistochemical markers and ascertaining their prognostic relevance. Clin Breast Cancer. (2020) 20:382–9. doi: 10.1016/j.clbc.2020.03.012
23. Orrantia-Borunda E A-NP, Acuña-Aguilar LE, Gómez-Valles FO, Ramírez-Valdespino CA. Subtypes of Breast Cancer. In: Breast Cancer. Exon Publications, Brisbane, Australia (2022).
24. Roca-Barceló A, Viñas G, Pla H, Carbó A, Comas R, Izquierdo Á, et al. Mortality of women with ductal carcinoma in situ of the breast: a population-based study from the Girona province, Spain (1994–2013). Clin Trans Oncol. (2019) 21:891–9. doi: 10.1007/s12094-018-1994-1
25. Allison KH, Hammond MEH, Dowsett M, McKernin SE, Carey LA, Fitzgibbons PL, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. (2020) 38:1346–66. doi: 10.1200/JCO.19.02309
26. Yu KD, Cai YW, Wu SY, Shui RH, Shao ZM. Estrogen receptor-low breast cancer: Biology chaos and treatment paradox. Cancer Commun (Lond). (2021) 41:968–80. doi: 10.1002/cac2.12191
27. Viale G, Regan MM, Maiorano E, Mastropasqua MG, Dell'Orto P, Rasmussen BB, et al. Prognostic and predictive value of centrally reviewed expression of estrogen and progesterone receptors in a randomized trial comparing letrozole and tamoxifen adjuvant therapy for postmenopausal early breast cancer: BIG 1–98. J Clin Oncol. (2007) 25:3846–52. doi: 10.1200/JCO.2007.11.9453
28. Harbeck N, Thomssen C, Gnant M. St. Gallen 2013: brief preliminary summary of the consensus discussion. Breast Care (Basel). (2013) 8:102–9. doi: 10.1159/000351193
29. Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human epidermal growth factor receptor 2 testing in breast cancer: american society of clinical oncology/college of american pathologists clinical practice guideline focused update. Arch Pathol Lab Med. (2018) 142:1364–82. doi: 10.5858/arpa.2018-0902-SA
30. Wolff AC, Somerfield MR, Dowsett M, Hammond MEH, Hayes DF, McShane LM, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology-college of American pathologists guideline update. J Clin Oncol. (2023) 41(22):3867–3872. doi: 10.1200/JCO.22.02864
31. VENTANA HER2 Dual ISH DNA Probe Cocktail: Roche (2020). Available online at: https://diagnostics.roche.com/content/dam/diagnostics/us/en/LandingPages/her2-dual-ish/VENTANA-HER2-Dual-ISH-Method-Sheet.pdf.
32. HER2 Breast Cancer Testing Guideline Update Algorithms: American Society of Clinical Oncology (ASCO) & The College of American Pathologists (CAP) (2023). Available online at: https://documents.cap.org/documents/her2_breast_update_algorithms_2023.pdf.
33. Hicks DG, Lester SC. Hormone Receptors (ER/PR). In: Hicks DG, Lester SC, editors. Diagnostic Pathology: Breast, 2nd ed. Elsevier, Philadelphia (2016) 430–9.
34. Diep CH, Ahrendt H, Lange CA. Progesterone induces progesterone receptor gene (PGR) expression via rapid activation of protein kinase pathways required for cooperative estrogen receptor alpha (ER) and progesterone receptor (PR) genomic action at ER/PR target genes. Steroids. (2016) 114:48–58. doi: 10.1016/j.steroids.2016.09.004
35. Ono M, Tsuda H, Yoshida M, Shimizu C, Kinoshita T, Tamura K. Prognostic significance of progesterone receptor expression in estrogen-receptor positive, HER2-negative, node-negative invasive breast cancer with a low ki-67 labeling index. Clin Breast Cancer. (2017) 17:41–7. doi: 10.1016/j.clbc.2016.06.012
36. Purdie CA, Quinlan P, Jordan LB, Ashfield A, Ogston S, Dewar JA, et al. Progesterone receptor expression is an independent prognostic variable in early breast cancer: a population-based study. Br J Cancer. (2014) 110:565–72. doi: 10.1038/bjc.2013.756
37. Paakkola NM, Karakatsanis A, Mauri D, Foukakis T, Valachis A. The prognostic and predictive impact of low estrogen receptor expression in early breast cancer: a systematic review and meta-analysis. ESMO Open. (2021) 6:100289. doi: 10.1016/j.esmoop.2021.100289
38. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. (1987) 235:177–82. doi: 10.1126/science.3798106
39. Ross JS, Fletcher JA. The HER-2/neu oncogene in breast cancer: prognostic factor, predictive factor, and target for therapy. Stem Cells. (1998) 16:413–28. doi: 10.1002/stem.160413
40. Konecny G, Pauletti G, Pegram M, Untch M, Dandekar S, Aguilar Z, et al. Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. JNCI: J Natl Cancer Instit. (2003) 95:142–53. doi: 10.1093/jnci/95.2.142
41. Huang HJ, Neven P, Drijkoningen M, Paridaens R, Wildiers H, Van Limbergen E, et al. Hormone receptors do not predict the HER2/neu status in all age groups of women with an operable breast cancer. Ann Oncol. (2005) 16:1755–61. doi: 10.1093/annonc/mdi364
42. Shah A, Haider G, Abro N, Bhutto S, Baqai TI, Akhtar S, et al. Correlation between age and hormone receptor status in women with breast cancer. Cureus. (2022) 14:e21652. doi: 10.7759/cureus.21652
43. Wang J, Xu B. Targeted therapeutic options and future perspectives for HER2-positive breast cancer. Signal Transduct Target Ther. (2019) 4:34. doi: 10.1038/s41392-019-0069-2
44. Luo C, Zhong X, Wang Z, Wang Y, Wang Y, He P, et al. Prognostic nomogram for patients with non-metastatic HER2 positive breast cancer in a prospective cohort. Int J Biol Mark. (2019) 34:41–6. doi: 10.1177/1724600818824786
45. Cao SS, Lu CT. Recent perspectives of breast cancer prognosis and predictive factors. Oncol Lett. (2016) 12:3674–8. doi: 10.3892/ol.2016.5149
46. Nicolini A, Ferrari P, Duffy MJ. Prognostic and predictive biomarkers in breast cancer: Past, present and future. Semin Cancer Biol. (2018) 52:56–73. doi: 10.1016/j.semcancer.2017.08.010
47. Badowska-Kozakiewicz AM, Patera J, Sobol M, Przybylski J. The role of oestrogen and progesterone receptors in breast cancer - immunohistochemical evaluation of oestrogen and progesterone receptor expression in invasive breast cancer in women. Contemp Oncol (Pozn). (2015) 19:220–5. doi: 10.5114/wo.2015.51826
48. Patel R, Klein P, Tiersten A, Sparano JA. An emerging generation of endocrine therapies in breast cancer: a clinical perspective. NPJ Breast Cancer. (2023) 9:20. doi: 10.1038/s41523-023-00523-4
49. Bediaga NG, Beristain E, Calvo B, Viguri MA, Gutierrez-Corres B, Rezola R, et al. Luminal B breast cancer subtype displays a dicotomic epigenetic pattern. SpringerPlus. (2016) 5:623. doi: 10.1186/s40064-016-2235-0
50. Focke CM, van Diest PJ, Decker T. St Gallen 2015 subtyping of luminal breast cancers: impact of different Ki67-based proliferation assessment methods. Breast Cancer Res Treat. (2016) 159:257–63. doi: 10.1007/s10549-016-3950-5
Keywords: ductal carcinoma in situ, invasive breast carcinoma, molecular subtypes, precision medicine, immunohistochemistry, hormone receptors, HER2, in situ hybridization
Citation: Schandiz H, Farkas L, Park D, Liu Y, Andersen SN, Geisler J and Sauer T (2024) Low progesterone receptor levels in high-grade DCIS correlate with HER2 upregulation and the presence of invasive components. Front. Oncol. 14:1347166. doi: 10.3389/fonc.2024.1347166
Received: 30 November 2023; Accepted: 04 June 2024;
Published: 26 June 2024.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Ossama Tawfik, Saint Luke’s Health System, United StatesAlisson Clemenceau, Yale University, United States
Copyright © 2024 Schandiz, Farkas, Park, Liu, Andersen, Geisler and Sauer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hossein Schandiz, aHNjaGFuZGl6QGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share last authorship
 Hossein Schandiz
Hossein Schandiz Lorant Farkas2,3
Lorant Farkas2,3 Torill Sauer
Torill Sauer