- 1Department of Medical Oncology, IRCCS San Raffaele Scientific Institute, Milan, Italy
- 2School of Medicine and Surgery, Vita-Salute San Raffaele University, Milan, Italy
Background: Nausea and vomiting are common side effects of Trastuzumab Deruxtecan (T-DXd), but guidelines for optimal management were not initially available. This retrospective single-center study aimed at evaluating the efficacy of two antiemetic regimens in patients receiving T-DXd.
Methods: Data from metastatic breast cancer patients receiving T-DXd were collected. Two groups were defined: patients treated with 5-HT3 receptor antagonists (RA) ± dexamethasone (5-HT3-group) and patients treated with a fixed oral combination of netupitant (NK1RA) and palonosetron ± dexamethasone (NK1 group). Physicians preferentially offered the NK1 regimen to patients at higher risk of nausea and vomiting based on internal recommendations. Only nausea and vomiting during cycles 1 and 2 were considered. Comparisons of nausea and vomiting by the antiemetic prophylaxis group were assessed using chi-square.
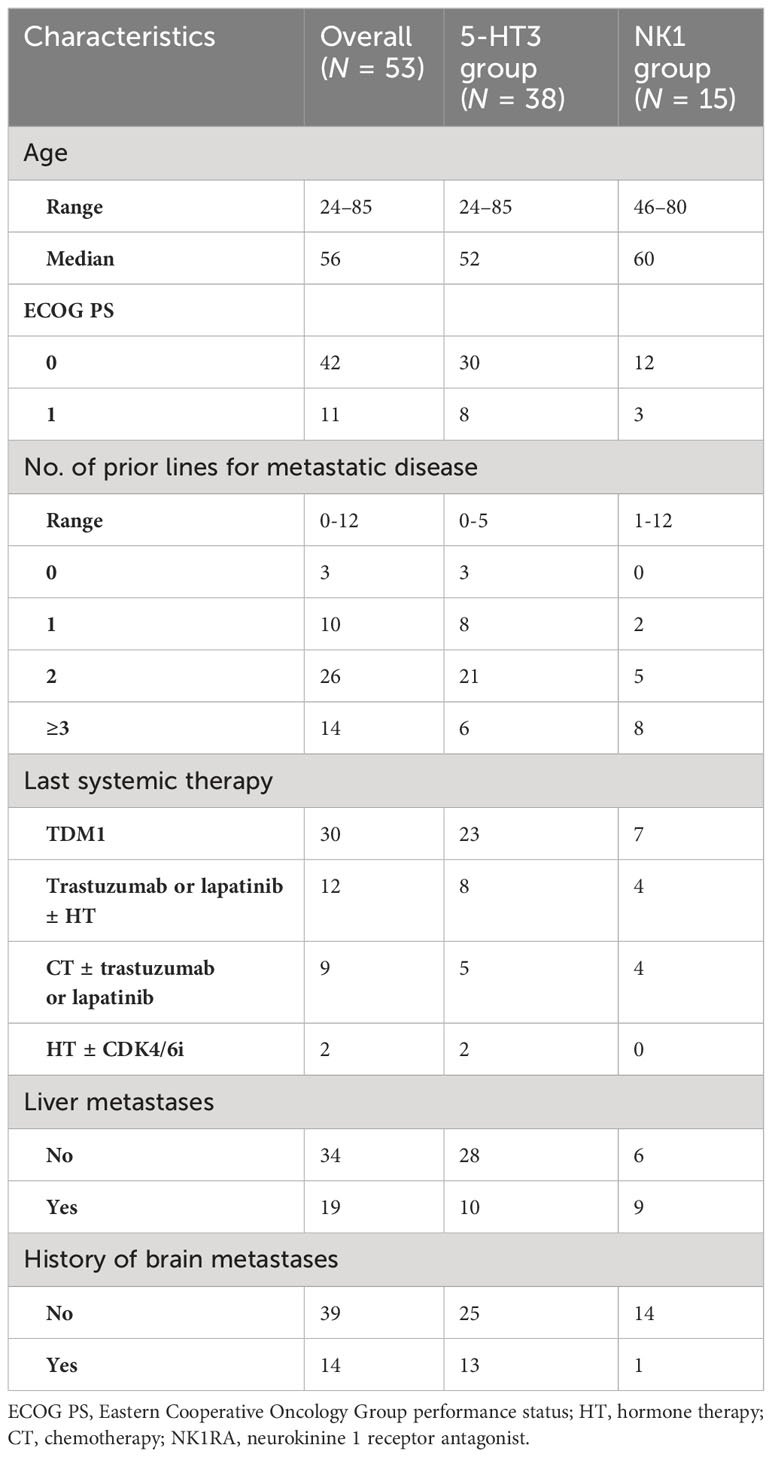
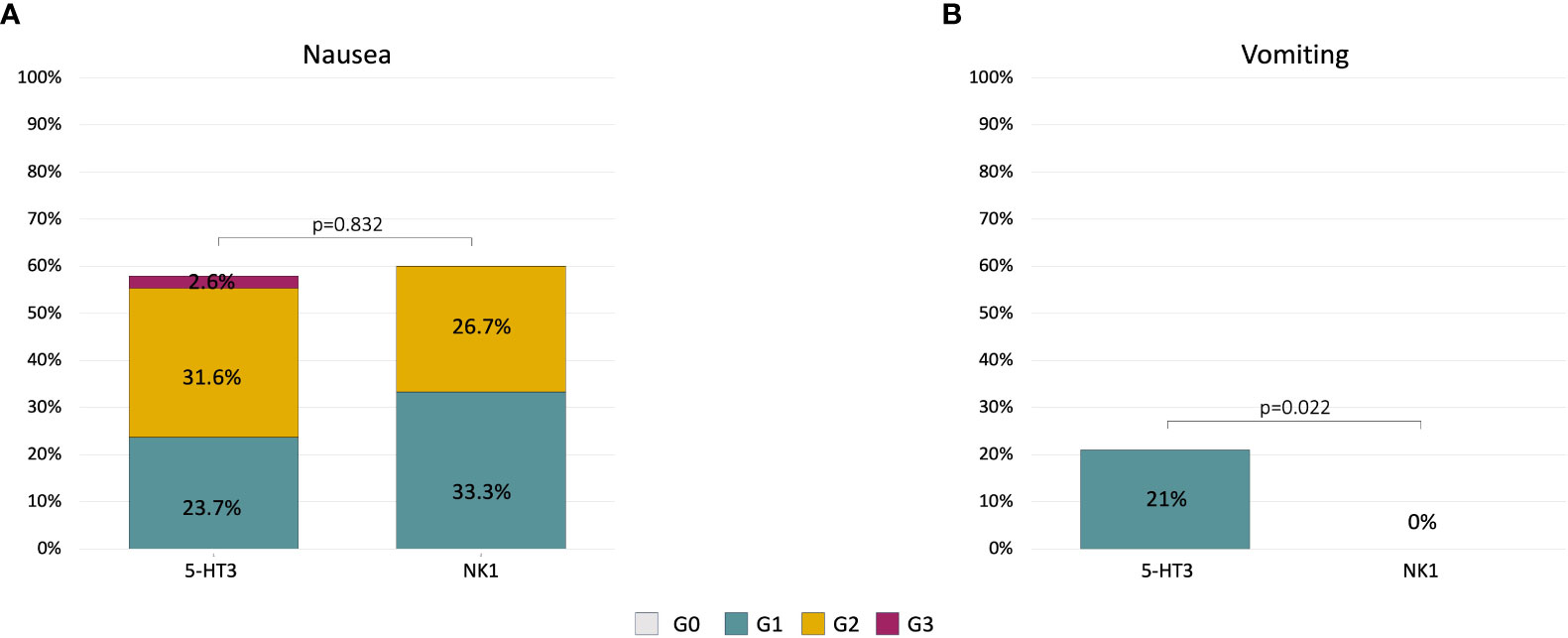
Results: A total of 53 patients were included in the analysis. At cycle 1, 72% and 28% of patients received the 5-HT3 and NK1 prophylaxis, respectively. Overall, 58% reported nausea, with no differences between groups (58% vs. 60%; p = 0.832), but with a trend for lower grade in the NK1 group (33.3% G1; 26.7% G2) compared to the 5-HT3 group (23.7% G1; 31.6% G2; 2.6% G3). Vomiting was reported by 21% and 0% of patients in the 5-HT3 and the NK1 group, respectively (p = 0.054). Among the 15 patients in the 5-HT3 group with nausea at cycle 1 who escalated to NK1 at cycle 2, nausea decreased from 100% to 53% (p = 0.022) and vomiting decreased from 47% to 13% (p = 0.046).
Conclusions: The NK1 regimen improved vomiting control at cycle 1 and, when introduced at cycle 2, significantly improved both nausea and vomiting. The biased NK1 selection for higher-risk patients may have dampened the differences between groups at cycle 1. These findings support enhanced control of T-DXd-related nausea and vomiting with NK1RA.
Introduction
Trastuzumab Deruxtecan (T-DXd) is a HER2-directed antibody–drug conjugate (ADC) composed of a humanized immunoglobulin G1 anti-HER2 monoclonal antibody and a topoisomerase I inhibitor cytotoxic payload, covalently linked by a tetrapeptide-based cleavable linker (1).
T-DXd has demonstrated clinically meaningful activity across a broad range of HER2-expressing solid tumors (2) and is currently approved as a standard of care treatment for HER2-expressing metastatic breast cancer, HER2-positive gastric or gastroesophageal junction adenocarcinoma, and HER2-mutant non-small cell lung cancer (3–8).
Despite its generally acceptable safety profile, T-DXd presents some non-negligible side effects that require appropriate clinical management. Specifically, it has been linked to a notable occurrence of nausea and vomiting, reaching 75% and 45%, respectively, in clinical trials (3–8).
These side effects may arise very early in the treatment course and persist for an extended period (9), inevitably exerting a negative impact on the quality of life for patients if not adequately managed (10).
At the time of its initial clinical development, the emetogenic potential of T-DXd was not well known, and early trial protocols did not include recommendations for any specific antiemetic prophylaxis. Furthermore, guidelines for the optimal management of T-DXd-related nausea and vomiting were not available until 2020, when the ASCO and NCCN guidelines categorized T-DXd as a moderate emetic risk anticancer agent (11, 12). Therefore, the early management of T-DXd-related nausea and vomiting was mostly empirical.
Between November and December 2020, three virtual focus groups of Italian oncologists were held to raise awareness of the importance of appropriate antiemetic prophylaxis for T-DXd and to provide oncologists with practical guidance for effective management (13). Consistent with the 2020 ASCO and NCCN guidelines, the panelists endorsed the antiemetic protocol defined at the San Raffaele Hospital, recommending a two-drug prophylaxis regimen with a 5-HT3 receptor antagonist (RA) plus dexamethasone for most patients, with the option to add an NK1 RA from the first cycle in the presence of individual risk factors, or at subsequent cycles in case of suboptimal control of nausea during cycle 1 (13).
Here, we present real-world data regarding the management of T-DXd-related nausea and vomiting in patients with metastatic breast cancer using this antiemetic protocol.
Materials and methods
Patients
Data from patients with metastatic breast cancer who had received T-DXd as of February 2023 were retrospectively collected from the healthcare services’ information system of the San Raffaele Hospital in Milan, Italy. The available information on the antiemetic prophylaxis used and the occurrence of nausea and vomiting at cycle 1 and subsequent cycles of T-DXd treatment were recorded. Only patients with reliable information on antiemetic prophylaxis type and severity of nausea and vomiting according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 were included in the analysis.
Procedures
Two groups of patients were defined: those treated with a 5-HT3 RA with or without dexamethasone (5-HT3 group) and those treated with a fixed oral combination of NK1RA (netupitant) and palonosetron with or without dexamethasone (NK1 group). Patients considered at higher risk of nausea and vomiting by physicians according to their individual risk factors were preferentially offered the NK1RA-containing regimen in line with internal recommendations (13). Only nausea and vomiting occurring at cycles 1 and 2 were considered for the analysis. Grading of nausea and vomiting was assessed by the treating physician and recorded in the medical chart.
Statistics
Descriptive statistics were used to summarize patient and tumor characteristics. Comparisons of nausea and vomiting by the antiemetic prophylaxis group were assessed using Pearson’s chi-squared test. All p-values were two-sided, and statistical significance was set at p ≤ 0.05. Only univariate analysis was performed.
Ethical issues
This retrospective study analyzed data that have been anonymized from previously collected patient information and did not involve any intervention or impact on patient care. All patients had provided informed consent before undergoing treatment with T-DXd. The study protocol (“TD-rNV”) was approved by the institutional Ethics Committee and followed local regulation and ethical guidelines.
Results
Between July 2018 and February 2023, 62 patients with HER2+ metastatic breast cancer were treated with T-DXd at our institution. After excluding patients without reliable information on antiemetic prophylaxis type and severity of nausea and vomiting, 53 patients were included in the analysis.
Baseline patient and disease characteristics are reported in Table 1. Median age was 56 years (range, 24–85 years); 75% of patients had received two or more previous lines of therapy for metastatic disease; 57% had received TDM1 as the latest systemic therapy. Thirty-six percent of patients had liver metastases and 26% had a history of brain metastases.
At cycle 1, 28% and 72% of patients received antiemetic prophylaxis with a three-drug (NK1 group) and a two-drug (5-HT3 group) regimen, respectively.
At cycle 1, 58% of patients reported nausea of any grade, with no significant differences between groups (58% in the 5-HT3 group and 60.0% in the NK1 group, p = 0.832) (Figure 1A). A numerical trend for lower-grade nausea in the NK1 group compared to the 5-HT3 group was observed. Nausea of grades 1, 2, and 3 was recorded in 24%, 32%, and 3%, respectively, in the 5-HT3 group, compared to 33%, 27%, and 0%, respectively, in the NK1 group (Figure 1A).
Overall, 15% of patients reported vomiting of any grade during cycle 1, 21% in the 5-HT3 group (all G1) and 0% in the NK1 group (p = 0.054) (Figure 1B).
Among the 22 patients in the 5-HT3 group with nausea at cycle 1, 15 patients immediately escalated to the NK1 regimen at cycle 2. Within this group, a statistically significant reduction of nausea and vomiting incidence was observed at cycle 2. Nausea decreased from 100% any grade (26% G1, 67% G2, and 7% G3) at cycle 1 to 53% any grade (13% G1, 40% G2) at cycle 2 (p = 0.022) (Figure 2A). Vomiting decreased from 47% (all G1) at cycle 1 to 13% (all G1) at cycle 2 (p = 0.046) (Figure 2B).
Figure 2 Alluvial plots describing the change of nausea (A) and vomiting (B) severity in patients (n = 15) who did not achieve an optimal control of symptoms at cycle 1 with 5-HT3 and escalated to the NK1 regimen at cycle 2.
No dose reductions or interruptions have been observed in either group during the first two cycles.
Discussion
Nausea and vomiting are among the most common toxicities associated with T-DXd. Since these side effects can significantly impair patient’s quality of life and adherence to treatment, their proper management is essential to ensure that patients derive the maximum benefit from T-DXd, while avoiding potential detrimental consequences.
Based on early clinical trial data, T-DXd was previously categorized as moderately emetogenic (11, 12). However, with the maturation of real-world experience suggesting an inadequate nausea control with the two-drug prophylaxis in a non-negligible proportion of patients (13, 14), in January 2023, the NCCN guidelines re-categorized T-DXd as a highly emetogenic agent, modifying the recommendation to endorse a three-drug antiemetic regimen for all patients (15). In January 2024, the ESMO guidelines have incorporated explicit recommendations for the management of T-DXd-related nausea and vomiting, categorizing it as a pharmaceutical agent at the high end of the moderate category (16). The recommendations advise the implementation of a three-drug regimen that includes an NK1RA, aligning with the established approach for Carboplatin with AUC ≥5 (16).
Recently, a randomized study comparing antiemetic prophylaxis with either a two- or three-drug regimen for patients with breast cancer scheduled to receive T-DXd was conducted (17). Patients were randomly assigned to receive granisetron and dexamethasone or granisetron, dexamethasone, and aprepitant. The primary endpoint was complete response rate, defined as no emesis or no rescue therapy during the overall phase (0–120 h after initiating T-DXd). Complete response rates were 36.8% and 70.0% with the two- and the three-drug regimen, respectively (odds ratio = 0.1334; p-value = 0.019), with a more pronounced difference between the two regimens in the delayed phase (24–120 h, 36.8% vs. 75%) and extended-delayed phase (24–168 h, 31.6% vs. 70%) (17).
Our real-world experience aligns with these data and further emphasizes that a two-drug antiemetic prophylaxis cannot be considered effective. The NK1RA-based regimen demonstrated a meaningful improvement in vomiting control at cycle 1 and, when introduced as rescue at cycle 2, significantly improved both nausea and vomiting. The biased preferential selection of the NK1 regimen for higher-risk patients may have dampened the differences between the two- and the three-drug regimen in nausea control at cycle 1 and therefore an improved control of nausea with the NK1 regimen in an unselected population cannot be ruled out.
Nevertheless, these data indicate that despite the use of a three-drug prophylaxis regimen including an NK1RA, some patients may continue to experience nausea and vomiting during T-DXd treatment. This could be at least partly attributed to the unique pharmacokinetic properties of T-DXd. Indeed, pharmacokinetic studies showed that free molecules of exatecan, the T-DXd payload, are released into the systemic circulation at levels that remain pharmacologically active for extended durations (18, 19), supporting the hypothesis that circulating free payloads could contribute not only to the efficacy but also to the toxicity profile of T-DXd. This phenomenon may lead to the activation of different pathways of nausea and vomiting, potentially necessitating antiemetic strategies that remain effective for an extended period.
Olanzapine is an atypical antipsychotic drug with broad-spectrum antiemetic properties, attributed to its action on multiple neurotransmitter receptors (20, 21). Several studies demonstrated that olanzapine-containing three- or four-drug antiemetic regimens are effective for preventing acute and delayed nausea and vomiting with both moderately or highly emetogenic anticancer regimens (22, 23). Moreover, some data suggest that, when combined with 5-HT3 RA and dexamethasone, olanzapine is more effective than an NK1 RA in preventing delayed nausea (24, 25). For this reason, there is an increasing interest in investigating the use of olanzapine to prevent and treat nausea and vomiting linked to T-DXd. The ongoing ERICA study (26) is a multicenter, randomized, double-blind, placebo-controlled phase II trial designed to assess the effectiveness of an olanzapine-based antiemetic regimen for the management of T-DXd-related nausea and vomiting in patients with HER2-positive metastatic breast cancer. Although the results of this study will certainly provide a contribution to the field, it is noteworthy that the study was initiated when T-DXd was still classified as moderately emetogenic. Therefore, some may raise concerns about the appropriateness of the control arm, represented by the two-drug regimen of 5HT3 RA and dexamethasone.
Despite the demonstrated cost-effectiveness of olanzapine (27), clinicians sometimes hesitate to prescribe it due to concerns related to its toxicity profile, which could, in turn, impact patients’ quality of life. In fact, common adverse events with olanzapine include fatigue, postural hypotension, anticholinergic side effects, and sedation (28). Increasing evidence suggests that a low dose of olanzapine (i.e., 5 mg PO daily) may be equally effective but better tolerated than the initial standard 10-mg dose (29, 30), and even lower doses (2.5 mg daily) may provide a comparable antiemetic effect with increased patient tolerability (31).
Low-dose olanzapine may represent an additional useful agent for the management of T-DXd-related nausea and vomiting, particularly in those cases with a delayed presentation of symptoms.
Strengths of our study include its substantial sample size, as to the best of our knowledge, it represents the largest real-world series investigating T-DXd-related nausea and vomiting to date. Furthermore, the monocentric nature of this study allows for high homogeneity in the selection of antiemetic prophylaxis, adding to the reliability of our findings. However, there are limitations to our analysis. Firstly, because of the retrospective nature of the study, it was not possible to control for the distribution of all variables that could have influenced the results, such as concomitant medications that might interfere with the onset of nausea and vomiting during treatment or individual patient risk factors for chemotherapy-induced nausea and vomiting, such as history of alcohol intake, morning sickness, motion sickness, anxiety, and history of vomiting during prior therapy. Secondly, some patients did not receive dexamethasone within the antiemetic prophylaxis because they initiated T-DXd treatment in 2018, a period during which concerns about potential interactions between dexamethasone and T-DXd existed. This may have partially dampened the overall efficacy of prophylaxis. Moreover, for this analysis, we did not consider data regarding symptomatology occurring beyond cycle 2, and therefore, we did not collect late events that might have led to treatment delays or dose reductions in subsequent cycles. Nevertheless, it is well known that T-DXd-related nausea occurs at the beginning of treatment, and the likelihood of these events occurring for the first time after cycles 2 or 3 is extremely low (9). Lastly, the monocentric nature of the study may also be considered a drawback and could potentially limit the generalizability of the observed results.
In conclusion, the effective management of T-DXd-related nausea and vomiting is critical to enhance patients’ quality of life and ensure optimal treatment adherence. While common, these symptoms can be effectively managed in the majority of cases with the use of adequate antiemetic prophylaxis protocols. Our findings support enhanced control of nausea and vomiting with the use of a three-drug antiemetic regimen including an NK1RA, in line with the most recent antiemetic guidelines for T-DXd.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by San Raffaele Hospital institutional review board. The studies were conducted in accordance with the local legislation and institutional requirements. This retrospective study analyzed data that have been anonymized from previously collected patient information and did not involve any intervention or impact on patient care. All patients had provided informed consent before undergoing treatment with T-DXd.
Author contributions
GN: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. MN: Writing – original draft, Writing – review & editing. LS: Writing – original draft, Writing – review & editing. GV: Writing – original draft, Writing – review & editing. AR: Writing – original draft, Writing – review & editing. SZ: Writing – original draft, Writing – review & editing. PZ: Writing – original draft, Writing – review & editing. MP: Writing – original draft, Writing – review & editing. CB: Writing – original draft, Writing – review & editing. MM: Writing – original draft, Writing – review & editing. DA: Writing – original draft, Writing – review & editing. GB: Conceptualization, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing. LL: Conceptualization, Data curation, Formal Analysis, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work has been supported in part by Fondazione AIRC per la Ricerca sul Cancro (IG2018 - ID21787, P.I. GB).
Conflict of interest
GV has served on the advisory boards for Gilead; has received honoraria for speakers’ bureaus from Novartis, Lilly; support for travel, accommodations, expenses from: Lilly and Pfizer. AR support for travel, accommodations, expenses from: Gilead, LeoPharma. SZ support for travel, accommodations, expenses from: Pfizer, D Sankyo. MP support for travel, accommodations, expenses from: Novartis, Gilead. GB has received consulting fee from Roche, AstraZeneca, Novartis, MSD, Sanofi, Daiichi Sankyo, and Exact Sciences; honoraria for speakers’ bureaus from Roche, Pfizer, Astra- Zeneca, Lilly, Novartis, Neopharm Israel, MSD, Chugai, Daiichi Sankyo, EISAI, and Exact Sciences; support for travel, accommodations, expenses from Roche, Pfizer, and AstraZeneca; is co-inventor of ‘European patent Application N. 12195182.6 and 12196177.5 titled “PDL-1 expression in anti-HER2 therapy” -Roche- Issued no compensation provided; has served on the advisory boards for Pfizer, Roche, Daiichi Sankyo, Lilly, MSD, Novartis, AstraZeneca, Genomic Health, EISAI, Gilead, Seagen. LL has served on the advisory boards for: Lilly, Exact Sciences, AstraZeneca, Italfarmaco, Daiichi Sankyo, Accord, Seagen; has received consulting fee from: Exact Sciences, Helsinn, EISAI, Daiichi Sankyo; honoraria for speakers’ bureaus from: Gilead, Exact Sciences, Helsinn, Lilly; support for travel, accommodations, expenses from: Lilly, Gilead, Accord.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nakada T, Sugihara K, Jikoh T, Abe Y, Agatsuma T. The latest research and development into the antibody-drug conjugate, [fam-] trastuzumab deruxtecan (DS-8201a), for HER2 cancer therapy. Chem Pharm Bull (Tokyo). (2019) 67:173–85. doi: 10.1248/cpb.c18-00744
2. Meric-Bernstam F, Makker V, Lee JY, Oaknin A, Oh DY, Banerjee S, et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: primary results from the DESTINY-panTumor02 phase II trial. J Clin Oncol. (2024) 42:47–58. doi: 10.1200/JCO.23.02005
3. Modi S, Saura C, Yamashita T, Yamashita T, Park YH, Kim SB, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. (2020) 382:610–21. doi: 10.1056/NEJMoa1914510
4. André F, Hee Park Y, Krop I, Kim SB, Takano T, Im SA, et al. Trastuzumab deruxtecan versus treatment of physician's choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): a randomised, open-label, multicentre, phase 3 trial. Lancet. (2023) 401:1773–85. doi: 10.1016/S0140-6736(23)00725-0
5. Modi S, Jacot W, Cameron DA, Yamashita T, Sohn J, Vidal M, et al. DESTINY-breast04 trial investigators. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. (2022) 387(1):9–20.
6. Shitara K, Bang YJ, Iwasa S, Iwasa S, Sugimoto N, Ryu MH, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. (2020) 382:2419–30. doi: 10.1056/NEJMoa2004413
7. Li BT, Smit EF, Goto Y, Nakagawa K, Udagawa H, Mazières J, et al. Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. N Engl J Med. (2022) 386:241–51. doi: 10.1056/NEJMoa2112431
8. Siena S, Di Bartolomeo M, Raghav K, Masuishi T, Loupakis F, Kawakami H, et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): a multicentre, open-label, phase 2 trial. Lancet Oncol. (2021) 22:779–89. doi: 10.1016/S1470-2045(21)00086-3
9. Hamilton EP, Petry V, Yeo W, Kim SB, Bianchini G, Yamashita T, et al. Trastuzumab deruxtecan (T-DXd) versus trastuzumab emtansine (T-DM1) in patients (pts) with HER2-positive (HER2+) unresectable and/or metastatic breast cancer (mBC): Safety follow-up of the randomized, phase 3 study DESTINY-Breast03. ASCO 2022 Annual Meeting.
10. Ueno N, Jacot W, Yamashita T, Sohn J, Tokunaga E, Prat A, et al. Patient-reported outcomes (PROs) from DESTINY-Breast04, a randomized phase III study of trastuzumab deruxtecan (T-DXd) vs treatment of physician's choice (TPC) in patients (pts) with HER2-low metastatic breast cancer (MBC). ESMO Congress (2022). doi: 10.1016/j.annonc.2022.07.256
11. Hesketh PJ, Kris MG, Basch E, Bohlke K, Barbour SY, Clark-Snow RA, et al. Antiemetics: ASCO guideline update. J Clin Oncol. (2020) 38:2782–97. doi: 10.1200/JCO.20.01296
12. NCCN guidelines antiemesis (2020). Available online at: https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf.
13. Bianchini G, Arpino G, Pappagallo G, Biganzoli L, Lonardi S, Puglisi F, et al. Emetogenicity of antibody-drug conjugates (ADCs) in solid tumors with a focus on trastuzumab deruxtecan: insights from an italian expert panel. Cancers (Basel). (2022) 14(4):1022. doi: 10.3390/cancers14041022
14. Stankowicz M, Mauro L, Harnden K, Pennisi A. Management of chemotherapy-induced nausea and vomiting with trastuzumab deruxtecan: A case series. Breast Care. (2021) 16:408–11. doi: 10.1159/000511049
15. NCCN guidelines antiemesis (2023). Available online at: https://www.nccn.org/guidelines/guidelines-detail?category=3&id=1415.
16. Herrstedt J, Clark-Snow R, Scotté F, Ruhlmann A, Molassiotis I. 2023 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting. ESMO Open. (2024) 102195:2059–7029. doi: 10.1016/j.esmoop.2023.102195
17. Iihara H, Shimokawa M, Futamura M, Bando H, Niwa Y, Mizuno Y, et al. Doublet or triplet antiemetic prophylaxis for nausea and vomiting induced by trastuzumab deruxtecan: an open-label, randomized, and multicenter exploratory phase 2 study. J Cancer. (2023) 14(14):2644–54. doi: 10.7150/jca.87169
18. Tarcsa E, Guffroy MR, Falahatpisheh H, Phipps C, Kalvass JC. Antibody-drug conjugates as targeted therapies: Are we there yet? A critical review of the current clinical landscape. Drug Discovery Today Technol. (2020) 37:13–22. doi: 10.1016/j.ddtec.2020.07.002
19. Doi T, Shitara K, Naito Y, Naito Y, Shimomura A, Fujiwara Y, et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2- targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose escalation study. Lancet Oncol. (2017) 18:1512–22. doi: 10.1016/S1470-2045(17)30604-6
20. Navari RM, Qin R, Ruddy KJ, Liu H, Powell SP, Bajaj M, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med. (2016) 375:134–42. doi: 10.1056/NEJMoa1515725
21. Hashimoto H, Abe M, Tokuyama O, Mizutani H, Uchitomi Y, Yamaguchi T, et al. Olanzapine 5 mg plus standard antiemetic therapy for the prevention of chemotherapy-induced nausea and vomiting (J-FORCE): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2020) 21:242–9. doi: 10.1016/S1470-2045(19)30678-3
22. Chow R, Chiu L, Navari R, Passik S, Chiu N, Popovic M, et al. Efficacy and safety of olanzapine for the prophylaxis of chemotherapy-induced nausea and vomiting (CINV) as reported in phase I and II studies: a systematic review. Support Care Cancer. (2016) 24:1001–8. doi: 10.1007/s00520-015-3000-6
23. Sutherland A, Naessens K, Plugge E, Ware L, Head K, Burton MJ, et al. Olanzapine for the prevention and treatment of cancer-related nausea and vomiting in adults. Cochrane Database Syst Rev. (2018) 9:CD012555. doi: 10.1002/14651858.CD012555.pub2
24. Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol. (2011) 9:188–95. doi: 10.1016/j.suponc.2011.05.002
25. Zhang Z, Zhang Y, Chen G, Hong S, Yang Y, Fang W, et al. Olanzapine-based triple regimens versus neurokinin-1 receptor antagonist-based triple regimens in preventing chemotherapy-induced nausea and vomiting associated with highly emetogenic chemotherapy: a network meta-analysis. Oncologist. (2018) 23:603–16. doi: 10.1634/theoncologist.2017-0378
26. Sakai H, Takiguchi D, Takano T, Ozaki Y, Ishiguro H, Nozawa K, et al. Multicentre, randomised, double-blind, placebo-controlled phase II study of prophylactic olanzapine for patients with metastatic breast cancer receiving T-DXd treatment: protocol for the ERICA study (WJOG14320B). BMJ Open. (2023) 13(4):e070304. doi: 10.1136/bmjopen-2022-070304
27. Chow R, Chiu L, Herrstedt J, Aapro M, Lock M, DeAngelis C, et al. Cost-efectiveness analysis of olanzapine-containing antiemetic therapy for the prophylaxis of chemotherapy-induced nausea and vomiting (CINV) in highly emetogenic chemotherapy (HEC) patients. Support Care Cancer. (2021) 29:4269–75. doi: 10.1007/s00520-020-05977-x
28. Chow R, Chiu L, Navari R, Passik S, Chiu C, Popovic M, et al. Efcacy and safety of olanzapine for the prophylaxis of chemotherapy-induced nausea and vomiting (CINV) as reported in phase I and II studies: a systematic review. Support Care Cancer. (2016) 24(2):1001–8. doi: 10.1007/s00520-015-3000-6
29. Chow R, Navari RM, Terry B, DeAngelis C, Prsic EH. Olanzapine 5 Mg vs 10 Mg for the prophylaxis of chemotherapy-induced nausea and vomiting: a network meta-analysis. Support Care Cancer. (2022) 30:1015–8. doi: 10.1007/s00520-021-06606-x
30. Yanai T, Iwasa S, Hashimoto H, Ohyanagi F, Takiguchi T, Takeda K, et al. A double-blind randomized phase II dose-finding study of olanzapine 10 Mg or 5 Mg for the prophylaxis of emesis induced by highly emetogenic cisplatin-based chemotherapy. Int J Clin Oncol. (2018) 23:382–8. doi: 10.1007/s10147-017-1200-4
31. Bajpai J, Kapu V, Gupta S, Rath S, Kumar S, Sekar A, et al. Low-dose versus standard-dose olanzapine with triple antiemetic therapy for prevention of highly emetogenic chemotherapy-induced nausea and vomiting in patients with solid tumours: a single-centre, open-label, non-inferiority, randomised, controlled, phase 3 trial. Lancet Oncol. (2024), 1470–2045. doi: 10.1016/S1470-2045(23)00628-9
Keywords: nausea, vomiting, antiemetic prophylaxis, Trastuzumab Deruxtecan, breast cancer
Citation: Notini G, Naldini MM, Sica L, Viale G, Rognone A, Zambelli S, Zucchinelli P, Piras M, Bosi C, Mariani M, Aldrighetti D, Bianchini G and Licata L (2024) Management of Trastuzumab Deruxtecan-related nausea and vomiting in real-world practice. Front. Oncol. 14:1374547. doi: 10.3389/fonc.2024.1374547
Received: 22 January 2024; Accepted: 23 February 2024;
Published: 11 March 2024.
Edited by:
Alessandra Fabi, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Carmen Criscitiello, University of Milan, ItalyYukinori Ozaki, Cancer Institute Hospital of Japanese Foundation for Cancer Research, Japan
Copyright © 2024 Notini, Naldini, Sica, Viale, Rognone, Zambelli, Zucchinelli, Piras, Bosi, Mariani, Aldrighetti, Bianchini and Licata. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luca Licata, bGljYXRhLmx1Y2FAaHNyLml0; Giampaolo Bianchini, YmlhbmNoaW5pLmdpYW1wYW9sb0Boc3IuaXQ=
†These authors have contributed equally to this work and share last authorship
‡ORCID: Giampaolo Bianchini, orcid.org/0000-0002-6790-6267
Luca Licata, orcid.org/0000-0002-0331-3585
 Giulia Notini
Giulia Notini Matteo Maria Naldini1,2
Matteo Maria Naldini1,2 Luca Licata
Luca Licata