- 1Department of Otolaryngology and Head and Neck Surgery, Keio University School of Medicine, Shinjuku-ku, Tokyo, Japan
- 2Division of Otolaryngology and Head and Neck Surgery, Saiseikai Utsunomiya Hospital, Utsunomiya-Shi, Tochigi, Japan
Objectives: Evaluation of sarcopenia accompanied by systemic inflammation status is a more beneficial prognostic marker than sarcopenia alone in various cancers. However, few studies have focused on this combination in patients with head and neck squamous cell cancer (HNSCC). In this study, we investigated how the combination of sarcopenia and systemic inflammation could affect survival in patients with HNSCC. Moreover, we explored which systemic inflammation markers could be better prognostic indicators when accompanied by sarcopenia.
Materials and methods: We retrospectively reviewed the medical records of patients with HNSCC treated between 2012 and 2016. Sarcopenia was defined by the skeletal muscle area measured on a computed tomography image slice at the level of the third cervical vertebra. The neutrophil/lymphocyte, platelet/lymphocyte, and lymphocyte/monocyte ratios (NLR, PLR, and LMR, respectively) were used as systemic inflammation markers that were combined with sarcopenia to evaluate prognosis.
Results: A total of 100 patients were enrolled, and 71 patients were considered sarcopenia. Patients with sarcopenia had significantly lower LMR and higher NLR and PLR. They also showed worse overall survival (OS) and progression-free survival (PFS). The comparative assessment of multiple combination patterns of sarcopenia and systemic inflammation indices proved that sarcopenia plus LMR considered as most reliable indicator for prognosis in HNSCC patients. Sarcopenia plus low LMR was a significantly poor prognostic factor both for OS and PFS with greater HR values than sarcopenia alone.
Conclusions: The combination of sarcopenia and LMR was considered the most sensitive prognostic factor in patients with HNSCC, suggesting it might be beneficial for identifying poor outcome risks.
Introduction
Numerous studies have described that malnutrition and systemic inflammation closely correlate to poor outcomes in patients with malignant tumors (1, 2). Sarcopenia, a loss of skeletal muscle mass (SMM), muscle strength, or loss of physical function, is among malnutrition status indicators associated with poor outcomes such as physical disability, poor life quality (3), and worse prognosis in patients with several cancers. In particular, patients with head and neck squamous cell cancer (HNSCC) are at risk for sarcopenia as the tumor site might cause dysphagia and difficulties in swallowing, and a recent study described that sarcopenia increased chemotherapeutic toxicity and is an independent risk factor for poor overall survival (OS) in patients with HNSCC (4).
Meanwhile, systemic inflammation also plays an important role in cancer patients. The neutrophil/lymphocyte, platelet/lymphocyte, and lymphocyte/monocyte ratio (NLR, PLR, and LMR, respectively) are widely used as systemic inflammation markers. Previous studies have demonstrated that higher NLR and PLR as well as lower LMR levels are associated with poor outcomes in patients with cancer (5–8).
Sarcopenia closely correlates to systemic inflammation, which causes muscle degeneration, leading to sarcopenia in patients with cancer (9, 10). Considering these aspects, recent studies described that sarcopenia with systemic inflammation is central to determining survival in various cancers. However, few studies have examined how sarcopenia accompanied by systemic inflammation could affect the prognosis of patients with HNSCC. Therefore, in this study, we investigated whether the combined evaluation of sarcopenia and systemic inflammation could serve as a reliable prognostic marker in patients with HNSCC who underwent curative therapy by comparing several combination patterns including sarcopenia with NLR, PLR, and LMR.
Materials and methods
Study design and patients
In this retrospective study, we included a total of 100 patients with HNSCC who had received initial treatment for primary HNSCC such as cancer of the oropharynx, hypopharynx, and larynx between February 2012 and March 2016. Exclusion criteria involved patients with missing data and undergoing palliative therapy only. All clinical data were collected using electronic medical records. This study was approved by the appropriate institutional research ethics committee (reference numbers: 2019-29) and was conducted according to the tenets of the Declaration of Helsinki. The requirement for informed consent was waived owing to the retrospective nature of the analysis.
Treatment protocol
Patients were treated with surgery, radiotherapy (RT) alone, and concurrent chemoradiotherapy (CCRT) considering various factors such as age, stage of disease, risk factors, performance status, and comorbidities. The listed treatments were initiated according to the guidelines of the National Comprehensive Cancer Network. Briefly, T1 and T2 cases prefer RT alone (total of 60–66 Gy) or transoral surgery, while T3 and T4 cases were administered CCRT (Cisplatin; 80 mg/m2, infused on days 1, 22, and 43, RT; a total of 66 Gy) or surgery (total laryngectomy or pharyngolaryngectomy) based on several factors of the patients. In advanced cases, surgery was preferred.
Measurement of skeletal muscle cross-sectional area and sarcopenia definition
In all 100 cases, cervical computed tomography (CT) imaging was obtained before the treatment. According to a previously described method by Swarz et al. (11), SMM was determined in each patient. Briefly, a single axial CT slide at level C3, displaying the entire vertebral arc, was selected first, when the C3 vertebra was scrolled from a caudal to a cephalic direction. The paravertebral muscle and both sternocleidomastoid muscle segments were highlighted in red and traced manually using the ImageJ software (Figure 1). We calculated the sum of the delineated areas of both the paravertebral and sternocleidomastoid muscles at the level of C3 vertebrae, defined as the cross-sectional muscle area (CSA) at level C3. Next, we estimated CSA at level L3 using the prediction method previously described by Swartz et al. (11). The estimated CSA at level L3 was normalized for the height by dividing it by the squared height, defined as the lumber skeletal muscle index (LSMI, cm2/m2).
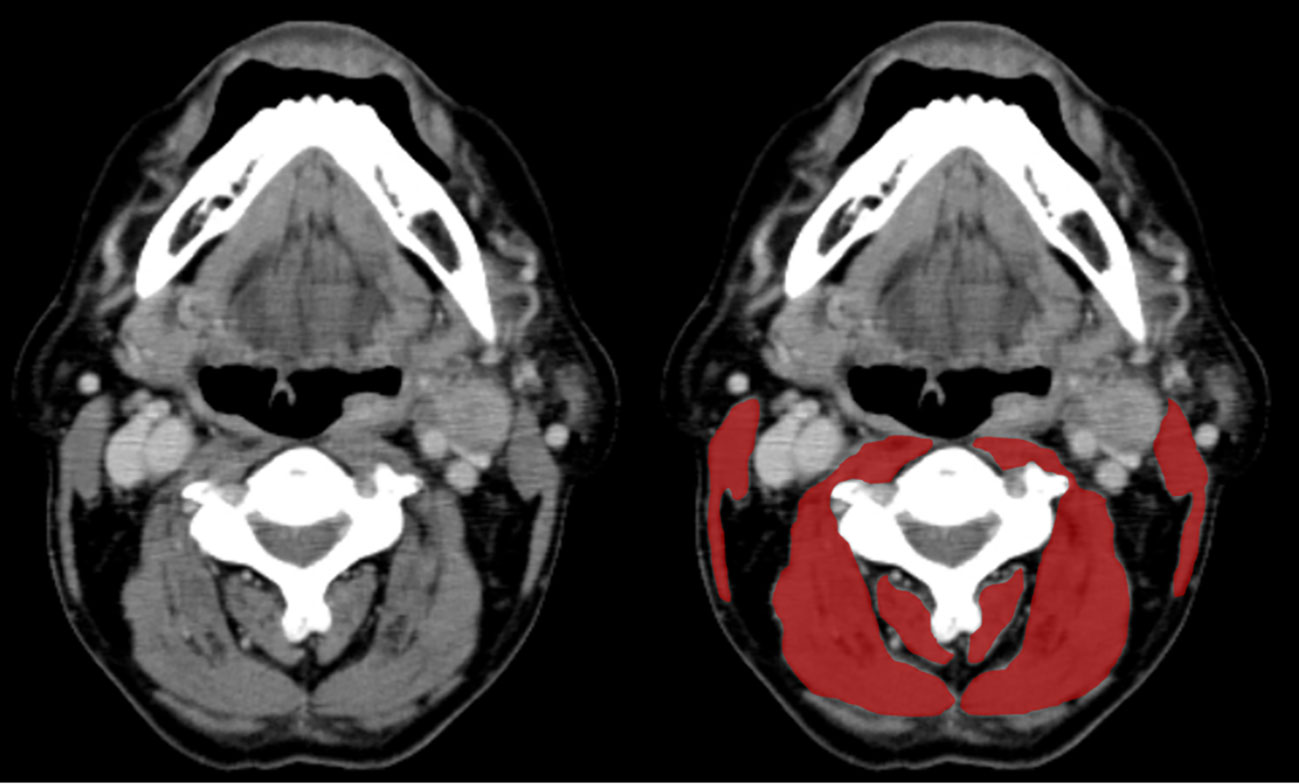
Figure 1 Paravertebral and sternocleidomastoid muscle area measurement at the level of the C3 vertebra. In the left axial CT slide, the muscle tissue is unsegmented. The right CT slide shows both the paravertebral and sternocleidomastoid segmented muscles in red.
Sarcopenia is characterized by an LSMI below 43.2cm2/m2 (12) according to the international consensus. In our study, although only low LSMI was used for sarcopenia definition, albumin and BMI were also collected as indicators reflecting nutritional status. Based on institutional criteria, the cut-off values of albumin and BMI were set at 3.5 and 18.5, respectively.
Systemic inflammation markers
The blood cell counts of the patients were measured within one week of treatment administration. NLR, PLR, and LMR were calculated by dividing the neutrophil count by the lymphocyte count, the platelet count by the lymphocyte count, and the lymphocyte count by the monocyte count, respectively. Low values of LMR and high values of NLR and PLR suggest high inflammatory status. We developed Receiver Operating Characteristic (ROC) curves for the NLR, PLR, and LMR using OS as the primary endpoint. NLR ≥ 2.180 was defined as high with an area under the curve (AUC), sensitivity, and specificity of 0.62, 60.0%, and 62.0%, respectively. A high PLR was defined as ≥ 112.8 with an AUC, sensitivity, and specificity of 0.59, 42.0%, and 84.0%, respectively. A lower LMR was defined as ≤ 4.118 with an AUC, sensitivity, and specificity of 0.62, 68.0%, and 58.0%, respectively.
Statistical analysis
Continuous variables are shown as the median (or mean) and range, while we presented categorical variables as frequencies. For comparisons between groups, we analyzed continuous data using the Mann–Whitney U test, while we performed categorical data analysis using Fisher’s exact test or the Chi-square test. We defined OS as the time from diagnosis until the last follow-up or death from any cause. We defined progression-free survival (PFS) as the time from diagnosis until the detection of the first detection of disease progression, the last follow-up, or death from any cause. We also compared how potential risk factors (age, sex, primary site, T or N classification, treatment type, anemia, BMI, sarcopenia, NLR, PLR, or LMR) could affect OS and PFS using the log-rank test and analyzed by generating Kaplan–Meier survival curves. We used Cox hazard regression analysis to perform multivariable analysis on the variables with P-values of p < 0.05 in the univariate analysis and clinically important OS and PFS predictors. HRs and their corresponding 95% confidence intervals are presented. We compared the combined prognostic factor of sarcopenia and systemic inflammation markers (NLR, PLR, and LMR) according to the ROC curve and also calculated the AUC. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, the referred interface is a modified version of R commander designed to add statistical functions that are frequently used in biostatistics. P < 0.05 was considered statistically significant.
Results
Patient characteristics
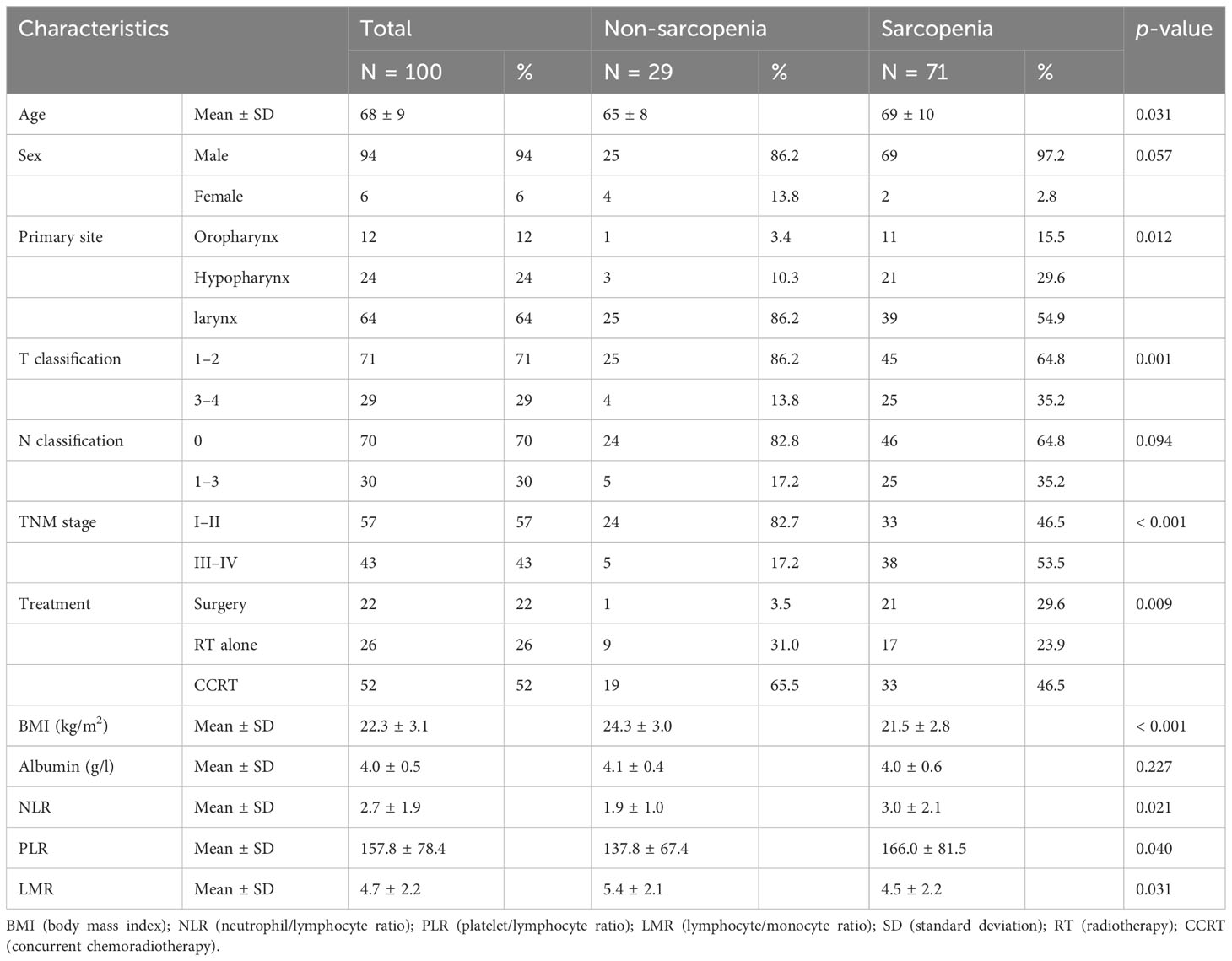
In a total of 100 patients, 94 were men and the median age at diagnosis was 69 years (range, 39-92 years). Of these, 12, 24, and 64 patients suffered from oropharyngeal, hypopharyngeal, and laryngeal cancer, respectively. The patients were divided into non-sarcopenia and sarcopenia groups, according to the LSMI cut-off described in the Material and Methods section. Table 1 presents the patient characteristics in the non-sarcopenia (n = 29, 29%) and sarcopenia (n = 71, 71%) groups. Patients in the sarcopenia group were older, at a more advanced T- and TNM stage, and displayed lower BMI compared to those in the non-sarcopenia group while values of albumin did not show significant differences. Concerning the inflammatory markers, NLR and PLR were significantly higher in the sarcopenia than in the non-sarcopenia group (p = 0.021 and 0.031, respectively) and LMR was significantly lower in the sarcopenia than in the non-sarcopenia group (p = 0.040).
Survival and prognostic factor analysis
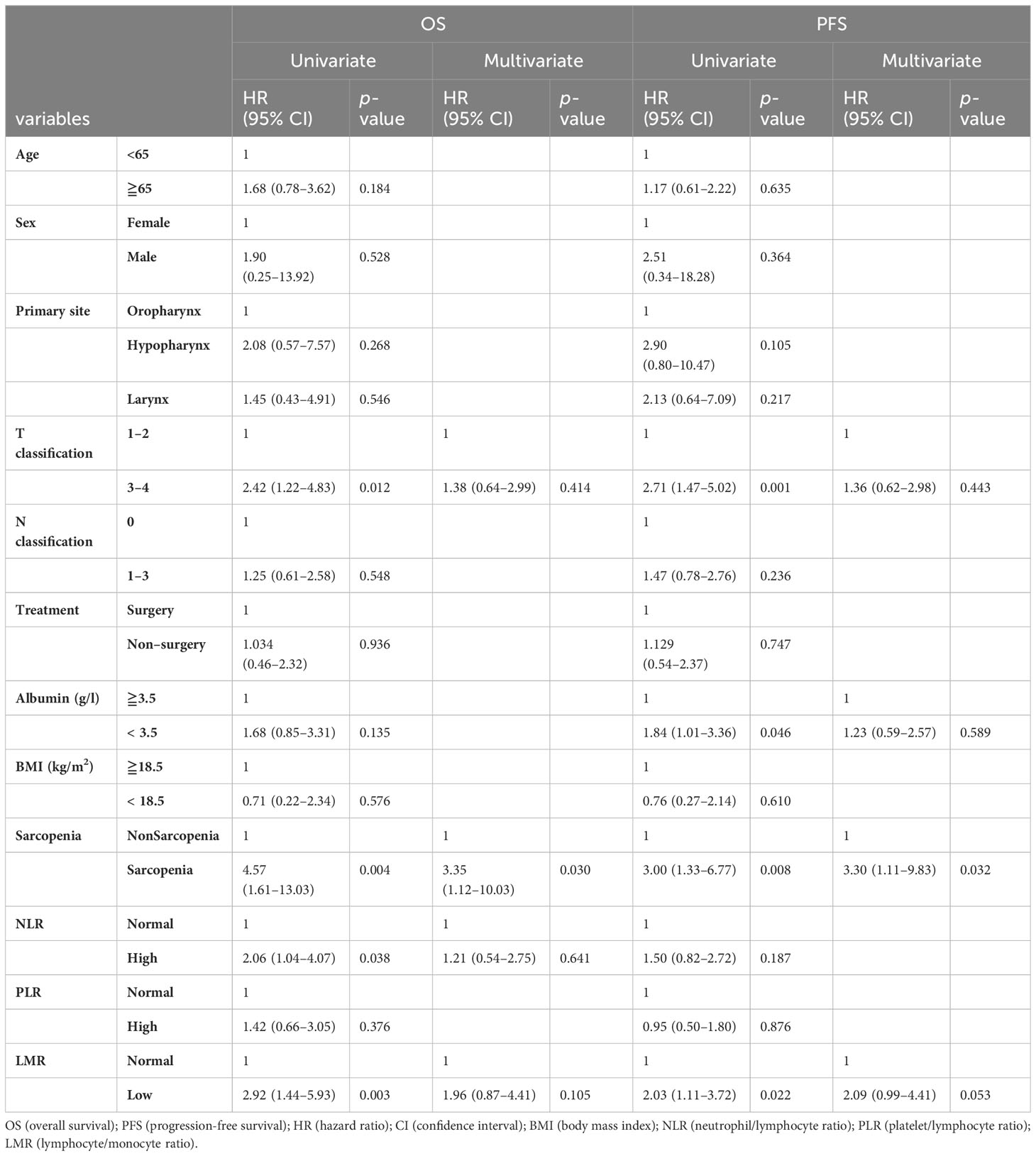
Twenty-eight patients died over a median follow-up duration of 78 months (range, 1–138 months). The 3-year OS and PFS rates among all 100 patients were 79 and 77%, respectively. Our univariate analysis revealed that NLR was associated with OS, but not with PFS (Table 2). Moreover, the univariate analysis showed that T classification, sarcopenia, and LMR were associated both with OS and PFS. Multivariate analysis using factors that showed significant differences in univariate analysis revealed that only sarcopenia was a significant predictor of both OS and PFS. According to the Kaplan–Meier analysis, the patients with sarcopenia had poorer OS (log-rank test: p = 0.002; Figure 2A) and PFS (log-rank test: p = 0.005; Figure 2B) than those with non-sarcopenia.
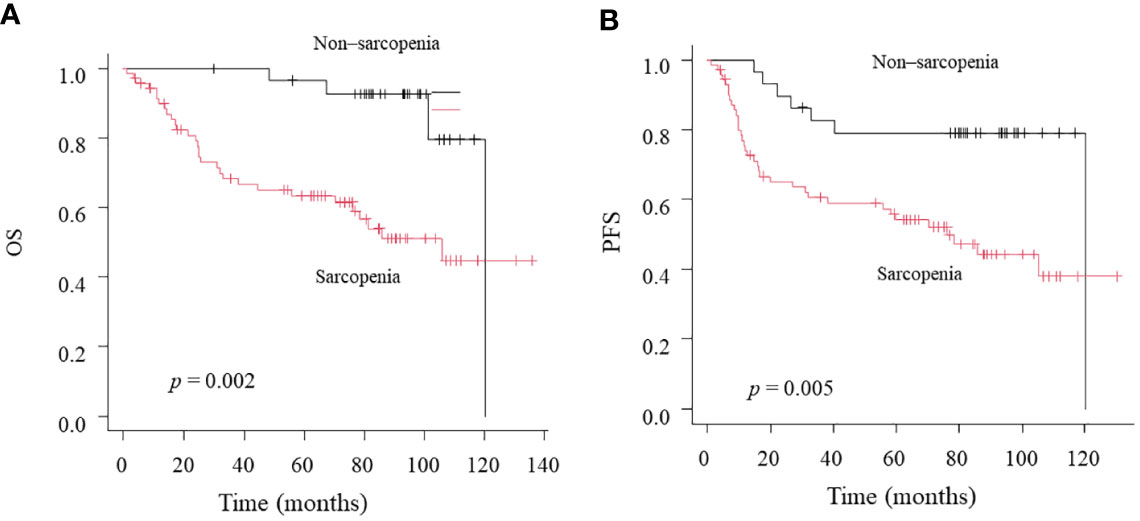
Figure 2 Kaplan-Meier curves comparing OS (A) and PFS (B) between the Non-sarcopenia and Sarcopenia.
ROC analysis of sarcopenia plus NLR, PLR, and LMR
Neither systemic inflammation index alone was a significant predictor in multivariate analysis, but we also evaluated whether their utility could be improved by combining them with sarcopenia. We created ROC curves and compared the AUC values to assess the discrimination ability of each prognostic score. The AUC values of the sarcopenia alone, sarcopenia plus NLR, sarcopenia plus PLR, and sarcopenia plus LMR for OS were 0.678 (95%CI = 0.570–0.786), 0.756 (95%CI = 0.662–0.850), 0.727 (95%CI = 0.624–0.829), and 0.752 (95%CI = 0.655–0.848), respectively. Although the combination of sarcopenia and NLR, PLR or LMR showed significantly greater AUC values than sarcopenia alone, there were no significant differences between sarcopenia plus NLR, sarcopenia plus PLR and sarcopenia plus LMR.
The effects of sarcopenia and systemic inflammation indices on survival
To compare the utility of three different combination patterns of sarcopenia and systemic inflammatory indices, we stratified patients into sarcopenia plus high inflammatory status and sarcopenia plus low inflammatory status using NLR, PLR, and LMR. Patients with sarcopenia plus high NLR had a worse OS than patients with sarcopenia plus low NLR (5-year OS; 66.7% vs. 39.4%, log-rank test: p = 0.043; Figure 3A). Similarly, patients with sarcopenia plus low LMR had significantly worse OS than those with sarcopenia plus high LMR (5-year OS; 67.6% vs. 37.8%, log-rank test: p = 0.012; Figure 3C). However, the combined index of sarcopenia and PLR did not show significant differences between two groups (5-year OS; 80.0% vs. 55.7%, log-rank test: p = 0.377; Figure 3B). On the other hand, regarding PFS, the combination of sarcopenia and LMR is the only indicator that showed significant differences between two groups (5-year PFS; 61.8% vs 32.4%, log-rank test: p = 0.033; Figure 4C), while sarcopenia plus NLR (5-year PFS; 60. 6% vs. 34.2%, log-rank test: p = 0.070; Figure 4A) and sarcopenia plus PLR (5-year PFS; 60.0% vs. 52.9%, log-rank test: p = 0.876; Figure 4B) showed no statistical differences, suggesting that the combination of sarcopenia and LMR is the most reliable prognostic index. We thus used this parameter in the subsequent subgroup analysis. The group of patients with sarcopenia plus low LMR had a higher percentage of oro-hypopharyngeal cancer (51.4% vs. 38.2%) and Stage III-IV cancers (62.2% vs. 44.1%) compared to those with sarcopenia plus high LMR. As for the treatment, the group of patients with sarcopenia plus low LMR tended to perform surgery compared to those with sarcopenia plus high LMR (37.8% vs. 20.6%), but there were no significant differences concerning treatment choice.
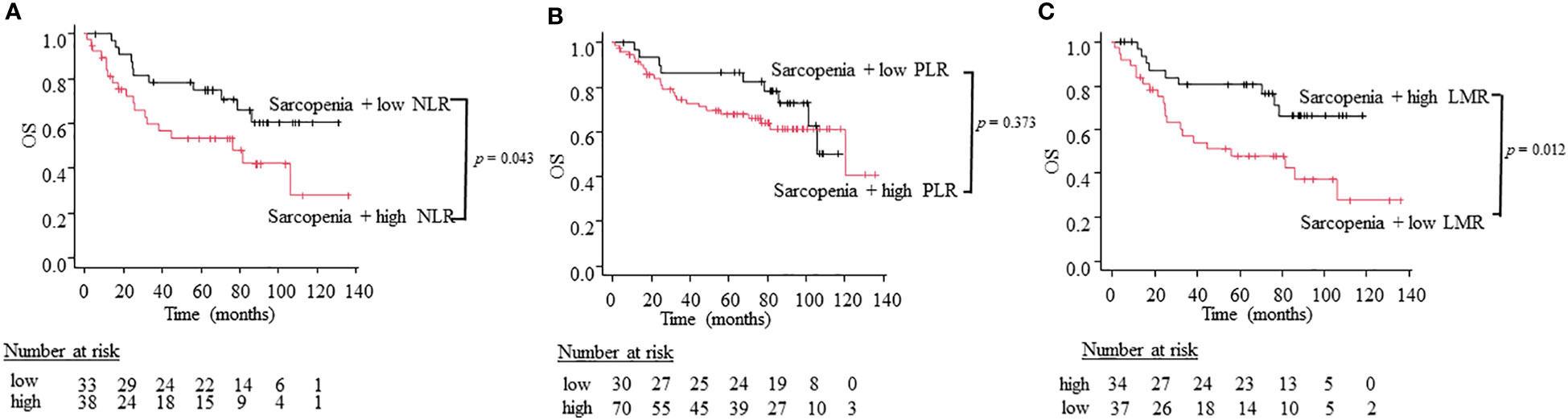
Figure 3 Kaplan-Meier curves comparing OS between the sarcopenia plus high inflammation status and low inflammation status defined by NLR (A), PLR (B), and LMR (C).
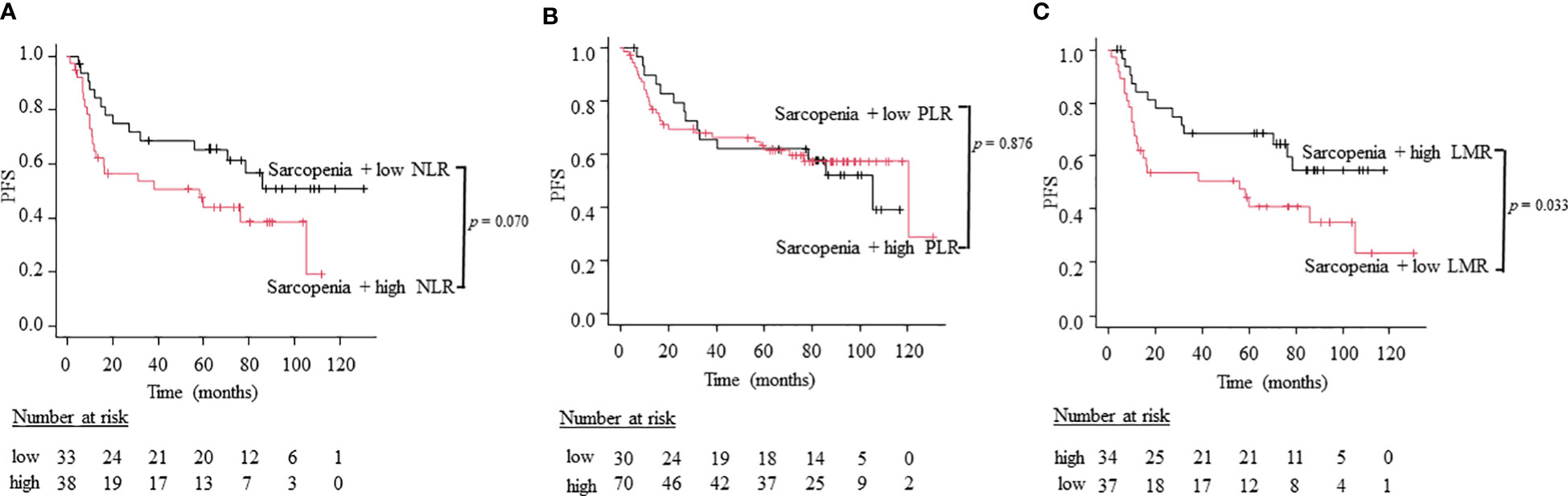
Figure 4 Kaplan-Meier curves comparing PFS between the sarcopenia plus high inflammation status and low inflammation status defined by NLR (A), PLR (B), and LMR (C).
We then performed a multivariate analysis including sarcopenia plus LMR status, T classification, and albumin which showed significant differences in univariate analysis. The results revealed that sarcopenia plus low LMR was a significantly poor prognostic factor both for OS and PFS with greater HR values than sarcopenia alone (Table 3).
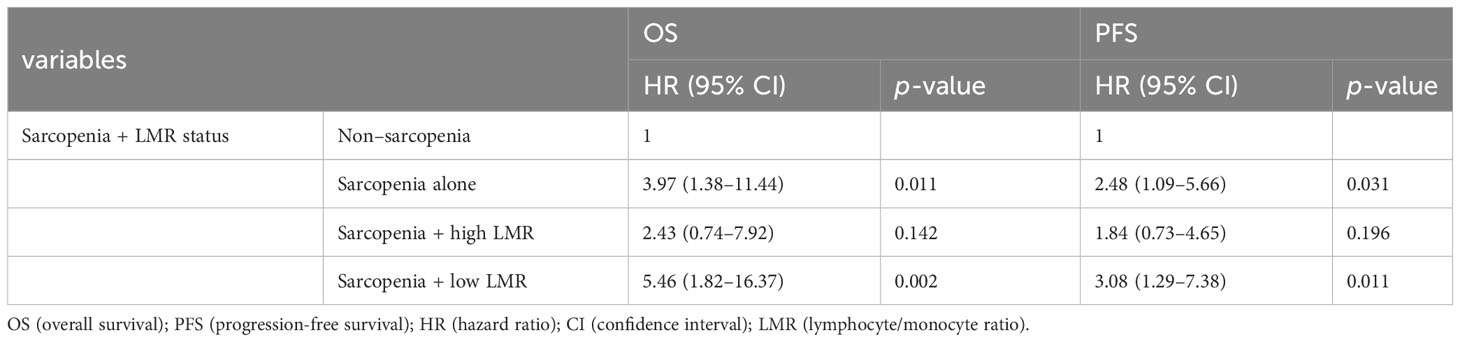
Table 3 Prognostic factor for OS and PFS in the multivariate analysis using the combined index of sarcopenia and LMR.
Discussion
Sarcopenia is reportedly highly associated with poor treatment outcomes in various cancer types, including HNSCC (4, 13, 14). Several studies demonstrated that sarcopenia correlates to increased surgical complications, such as delayed surgical wound healing (15), increased incidence of pharyngocutaneous fistula (15), surgical site infection (16), and postoperative delirium (17). Other studies reported that sarcopenia is associated with chemoradiation-induced toxicities in patients with HNSCC, as follows: mucositis, dysphagia, and dose-limiting chemotherapeutic toxicities (13). These side effects prevent the completion of full chemotherapeutic or radiation treatment cycles and lead to poor treatment outcomes. Moreover, several studies described that sarcopenia is an independent poor prognostic factor for OS and PFS in patients with HNSCC (14, 18). In our study, HNSCC patients with sarcopenia displayed poorer OS and PFS than those without sarcopenia. In addition, sarcopenia accompanied by systemic inflammation was closely associated with poor OS and PFS, and it was considered a more sensitive indicator than sarcopenia alone.
Systemic inflammation is intimately involved in tumor development, invasion, and metastasis (19). Moreover, several inflammatory markers, including NLR, PLR, and LMR, reportedly correlated with clinical outcomes in patients with HNSCC. Neutrophils release various inflammatory mediators that affect tumor angiogenesis, reduce T lymphocyte function, and promote tumor cell growth and metastasis (20–22). Lymphocytes also release several factors, that inhibit antitumor immunity and promote tumor growth and metastasis (23, 24), leading to an altered tumor microenvironment. Moreover, increased lymphocyte infiltration in the tumor microenvironment was reportedly associated with a better response to cytotoxic treatment and prognosis in patients with cancer (25). Platelets and monocytes promote tumor progression (26, 27). Platelets are activated in tumor cells and release several cytokines, thereby promoting tumor proliferation, metastatic potential, and angiogenesis (26). Monocytes infiltrate into the tumor cells, promote tumor progression and invasion, and suppress immune cell function (27). Several studies reported that higher NLR and PLR as well as lower LMR were independent poor prognostic factors in patients with HNSCC (6–8, 28, 29). However, we could not identify both NLR alone and LMR alone as an independent prognostic factor in our study. Multiple studies have described that systemic inflammation closely correlates to sarcopenia. Systemic inflammation could promote muscle catabolism through pro-inflammatory cytokines such as interleukin-6, tumor necrosis factor-alpha, and transforming growth factor-beta (30, 31). Furthermore, muscle breakdown might further exacerbate the existing systemic inflammation (32), resulting in a detrimental inflammation-myopia cycle (32). Higher NLR and PLR as well as lower LMR reportedly correlated with a higher sarcopenia incidence (7, 8, 33, 34). However, only a few studies have explored the relationship between systemic inflammation markers (NLR, PLR, and LMR) and sarcopenia in patients with HNSCC. In our study, we demonstrated that higher NLR and PLR as well as lower LMR were significantly associated with sarcopenia.
NLR, PLR, and LMR are reportedly poor prognostic factors in patients with HNSCC, although all are single prognostic factors. Recently, the combination of sarcopenia and systemic inflammation markers reportedly improved prognosis accuracy. Sarcopenia accompanied by systemic inflammation affects the prognosis in patients with various cancers. However, only a few studies have evaluated the efficacy of combining sarcopenia and inflammation on the prognosis of patients with HNSCC. Yamahara et al. (34) described that sarcopenia accompanied by high PLR was the most significant independent risk factor for OS and DFS. Cho et al. (35) reported that sarcopenia accompanied by high NLR was the most significant risk factor of poor OS and PFS, reflecting a very aggressive status in patients with HNSCC. Moreover, several studies described that sarcopenia plus lower LMR was an independent poor prognostic factor in various cancers (6–8). However, all these studies have examined single combination patterns of sarcopenia and systemic inflammatory markers (NLR, PLR, and LMR). In our study, we evaluated multiple combination patterns using comparative assessment and revealed that sarcopenia plus low LMR is a more perceptive indicator of poor prognosis than sarcopenia alone. As no studies have compared the efficacy of different combinations of indicators, our findings provide novel scientific contributions to cancer treatment. For high-risk patients detected by the combined index of sarcopenia and LMR, it may be useful to consider supportive therapy such as nutritional intervention with close monitoring. Immunonutrition is emerging as a promoting intervention that can attenuate sarcopenia-related inflammation to improve outcomes (36). They contain unique ingredients, such as arginine, omega-3 fatty acids, and dietary nucleotides that modulate prostaglandin E2 production, decrease IL-6 production, and promote T-cell differentiation (37). The previous paper reported that the use of immunonutrition for five days before surgery was associated with a significant reduction in the incidence of wound abscesses and orocutaneous or pharyngocutaneous fistulas compared to the control group (38), which may affect prognosis by allowing transition to appropriate adjuvant postoperative therapy.
Our study has some limitations. First, inevitable bias might be present in a single-center retrospective study related to sample size. Second, the CSA estimation method at L3 based on the CSA at C3 is uncertain. Jung et al. (39) reported another predictive model for estimating the CSA at L3 different from the approach of Swartz et al. (11). Moreover, they demonstrated that CSA at C3 alone displayed high predictability for estimating OS after definitive treatment for patients with advanced-stage HNSCC (39), leading to making the conversion from CSA at C3 to L3 unnecessary. Third, in previous studies, no consensus has been reached on the cut-off for sarcopenia, making result comparison difficult. Further studies with increased sample sizes would be required to support our findings.
Conclusion
In this study, we described that sarcopenia accompanied by low LMR significantly correlated with poor OS and PFS in patients with HNSCC undergoing curative therapy. The combination of these two measures might be beneficial for identifying patients with HNSCC at risk of poor outcomes.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies were approved by Research ethics committee of Saiseikai Utsunomiya Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The requirement for informed consent was waived owing to the retrospective nature of the analysis.
Author contributions
KK: Conceptualization, Writing – review & editing, Data curation, Formal Analysis, Writing – original draft. TK: Conceptualization, Writing – review & editing, Investigation, Methodology, Project administration, Supervision, Validation. YS: Conceptualization, Investigation, Methodology, Project administration, Supervision, Validation, Writing – review & editing. MU: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing. HS: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing. YF: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing. SS: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing. HO: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
SMM, skeletal muscle mass; OS, overall survival; PFS, progression-free survival; HNSCC, head and neck squamous cell cancer; NLR, neutrophil/lymphocyte ratio; PLR, platelet/lymphocyte ratio; LMR, lymphocyte/monocyte ratio; RT, radiotherapy; CCRT, concurrent chemotherapy; CT, computed tomography; LSMI, lumber skeletal muscle index; ROC, receiver operating characteristic; AUC, area under the curve; BMI, body mass index; HR, hazard ratio.
References
1. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. (2014) 15:e493–503. doi: 10.1016/S1470-2045(14)70263-3
2. Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur J Cancer. (2016) 57:58–67. doi: 10.1016/j.ejca.2015.12.030
3. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. (2010) 39:412–23. doi: 10.1093/ageing/afq034
4. van Rijn-Dekker MI, van den Bosch L, van den Hoek JGM, Bijl HP, van Aken ESM, van der Hoorn A, et al. Impact of sarcopenia on survival and late toxicity in head and neck cancer patients treated with radiotherapy. Radiother Oncol. (2020) 147:103–10. doi: 10.1016/j.radonc.2020.03.014
5. Zhang Y, Jiang C, Li J, Sun J, Qu X. Prognostic significance of preoperative neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in patients with gallbladder carcinoma. Clin Transl Oncol. (2015) 17:810–8. doi: 10.1007/s12094-015-1310-2
6. Song HN, Kim JY, Kim JM, Kang KM, Choi HS, Jeong JH, et al. Sarcopenia using pectoralis muscle area and lymphocyte-to-monocyte ratio (LMR) are independent prognostic factors in patients for nonmetastatic breast cancer. Med (Baltimore). (2022) 101:e32229. doi: 10.1097/MD.0000000000032229
7. Liang H, Peng H, Chen L. Prognostic value of sarcopenia and systemic inflammation markers in patients undergoing definitive radiotherapy for esophageal cancer. Cancer Manag Res. (2021) 13:181–92. doi: 10.2147/CMAR.S288522
8. Lin JX, Lin JP, Xie JW, Wang JB, Lu J, Chen QY, et al. Prognostic value and association of sarcopenia and systemic inflammation for patients with gastric cancer following radical gastrectomy. Oncologist. (2019) 24:e1091–e101. doi: 10.1634/theoncologist.2018-0651
9. Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS Jr. NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science. (2000) 289:2363–6. doi: 10.1126/science.289.5488.2363
10. Baracos VE. Regulation of skeletal-muscle-protein turnover in cancer-associated cachexia. Nutrition. (2000) 16:1015–8. doi: 10.1016/S0899-9007(00)00407-X
11. Swartz JE, Pothen AJ, Wegner I, Smid EJ, Swart KM, de Bree R, et al. Feasibility of using head and neck CT imaging to assess skeletal muscle mass in head and neck cancer patients. Oral Oncol. (2016) 62:28–33. doi: 10.1016/j.oraloncology.2016.09.006
12. Wendrich AW, Swartz JE, Bril SI, Wegner I, de Graeff A, Smid EJ, et al. Low skeletal muscle mass is a predictive factor for chemotherapy dose-limiting toxicity in patients with locally advanced head and neck cancer. Oral Oncol. (2017) 71:26–33. doi: 10.1016/j.oraloncology.2017.05.012
13. Nagpal P, Pruthi DS, Pandey M, Yadav A, Singh H. Impact of sarcopenia in locally advanced head and neck cancer treated with chemoradiation: An Indian tertiary care hospital experience. Oral Oncol. (2021) 121:105483. doi: 10.1016/j.oraloncology.2021.105483
14. Surov A, Wienke A. Low skeletal muscle mass predicts relevant clinical outcomes in head and neck squamous cell carcinoma. A Meta Analysis Ther Adv Med Oncol. (2021) 13:17588359211008844. doi: 10.1177/17588359211008844
15. Bril SI, Pezier TF, Tijink BM, Janssen LM, Braunius WW, de Bree R. Preoperative low skeletal muscle mass as a risk factor for pharyngocutaneous fistula and decreased overall survival in patients undergoing total laryngectomy. Head Neck. (2019) 41:1745–55. doi: 10.1002/hed.25638
16. Makiguchi T, Yamaguchi T, Nakamura H, Suzuki K, Harimoto N, Shirabe K, et al. Impact of skeletal muscle mass volume on surgical site infection in free flap reconstruction for oral cancer. Microsurgery. (2019) 39:598–604. doi: 10.1002/micr.30494
17. Makiguchi T, Yamaguchi T, Nakamura H, Ogawa M, Harimoto N, Shirabe K, et al. Impact of skeletal muscle mass on postoperative delirium in patients undergoing free flap repair after oral cancer resection. J Plast Surg Handb Surg. (2020) 54:161–6. doi: 10.1080/2000656X.2020.1724545
18. Li S, Wang T, Tong G, Li X, You D, Cong M. Prognostic impact of sarcopenia on clinical outcomes in Malignancies treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Front Oncol. (2021) 11:726257. doi: 10.3389/fonc.2021.726257
19. Coussens LM, Werb Z. Inflammation and cancer. Nature. (2002) 420:860–7. doi: 10.1038/nature01322
20. Tazzyman S, Lewis CE, Murdoch C. Neutrophils: key mediators of tumour angiogenesis. Int J Exp Pathol. (2009) 90:222–31. doi: 10.1111/j.1365-2613.2009.00641.x
21. Sabbione F, Gabelloni ML, Ernst G, Gori MS, Salamone G, Oleastro M, et al. Neutrophils suppress γδ T-cell function. Eur J Immunol. (2014) 44:819–30. doi: 10.1002/eji.201343664
22. Fridlender ZG, Albelda SM, Granot Z. Promoting metastasis: neutrophils and T cells join forces. Cell Res. (2015) 25:765–6. doi: 10.1038/cr.2015.62
23. Ruffell B, DeNardo DG, Affara NI, Coussens LM. Lymphocytes in cancer development: polarization towards pro-tumor immunity. Cytokine Growth Factor Rev. (2010) 21:3–10. doi: 10.1016/j.cytogfr.2009.11.002
24. Smith HA, Kang Y. The metastasis-promoting roles of tumor-associated immune cells. J Mol Med (Berl). (2013) 91:411–29. doi: 10.1007/s00109-013-1021-5
25. Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. (2013) 31:860–7. doi: 10.1200/JCO.2011.41.0902
26. Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. (2011) 11:123–34. doi: 10.1038/nrc3004
27. Izumi Y, Kanayama M, Shen Z, Kai M, Kawamura S, Akiyama M, et al. An antibody-drug conjugate that selectively targets human monocyte progenitors for anti-cancer therapy. Front Immunol. (2021) 12:618081. doi: 10.3389/fimmu.2021.618081
28. Mascarella MA, Mannard E, Silva SD, Zeitouni A. Neutrophil-to-lymphocyte ratio in head and neck cancer prognosis: A systematic review and meta-analysis. Head Neck. (2018) 40:1091–100. doi: 10.1002/hed.25075
29. Du J, Liu J, Zhang X, Chen X, Yu R, Gu D, et al. Pre-treatment neutrophil-to-lymphocyte ratio predicts survival in patients with laryngeal cancer. Oncol Lett. (2018) 15:1664–72. doi: 10.3892/ol.2017.7501
30. Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. (2012) 16:153–66. doi: 10.1016/j.cmet.2012.06.011
31. Feliciano EMC, Kroenke CH, Meyerhardt JA, Prado CM, Bradshaw PT, Kwan ML, et al. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS study. JAMA Oncol. (2017) 3:e172319. doi: 10.1001/jamaoncol.2017.2319
32. Kalinkovich A, Livshits G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev. (2017) 35:200–21. doi: 10.1016/j.arr.2016.09.008
33. Liaw FY, Huang CF, Chen WL, Wu LW, Peng TC, Chang YW, et al. Higher platelet-to-lymphocyte ratio increased the risk of sarcopenia in the community-dwelling older adults. Sci Rep. (2017) 7:16609. doi: 10.1038/s41598-017-16924-y
34. Yamahara K, Mizukoshi A, Lee K, Ikegami S. Sarcopenia with inflammation as a predictor of survival in patients with head and neck cancer. Auris Nasus Larynx. (2021) 48:1013–22. doi: 10.1016/j.anl.2021.03.021
35. Cho Y, Kim JW, Keum KC, Lee CG, Jeung HC, Lee IJ. Prognostic significance of sarcopenia with inflammation in patients with head and neck cancer who underwent definitive chemoradiotherapy. Front Oncol. (2018) 8:457. doi: 10.3389/fonc.2018.00457
36. Chen J, Dennis SK, Abouyared M. Sarcopenia and microvascular free flap reconstruction. Curr Opin Otolaryngol Head Neck Surg. (2021) 29:419–23. doi: 10.1097/MOO.0000000000000756
37. Aida T, Furukawa K, Suzuki D, Shimizu H, Yoshidome H, Ohtsuka M, et al. Preoperative immunonutrition decreases postoperative complications by modulating prostaglandin E2 production and T-cell differentiation in patients undergoing pancreatoduodenectomy. Surgery. (2014) 155:124–33. doi: 10.1016/j.surg.2013.05.040
38. Aeberhard C, Mayer C, Meyer S, Mueller SA, Schuetz P, Stanga Z, et al. Effect of preoperative immunonutrition on postoperative short-term outcomes of patients with head and neck squamous cell carcinoma. Head Neck. (2018) 40:1057–67. doi: 10.1002/hed.25072
Keywords: sarcopenia, nutrition, systemic inflammation, overall survival, progression-free survival, prognostic marker, head and neck cancer
Citation: Kasahara K, Kono T, Sato Y, Ueno M, So H, Fuse Y, Shinden S and Ozawa H (2024) Sarcopenia accompanied by systemic inflammation can predict clinical outcomes in patients with head and neck cancer undergoing curative therapy. Front. Oncol. 14:1378762. doi: 10.3389/fonc.2024.1378762
Received: 30 January 2024; Accepted: 26 February 2024;
Published: 14 March 2024.
Edited by:
Nobuhiko Oridate, Yokohama City University, JapanCopyright © 2024 Kasahara, Kono, Sato, Ueno, So, Fuse, Shinden and Ozawa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takeyuki Kono, dGFrZS5rMTIyN0BnbWFpbC5jb20=
 Ken Kasahara1,2
Ken Kasahara1,2 Takeyuki Kono
Takeyuki Kono Hiroyuki Ozawa
Hiroyuki Ozawa