- 1Department of Shandong Provincial Key Laboratory of Precision Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, Shandong, China
- 2Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, Shandong, China
- 3Department of Shandong Provincial Key Laboratory of Precision Oncology, Shandong University Cancer Center, Jinan, Shandong, China
- 4Cancer Center, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
- 5Lung Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China
Background and purpose: Immunotherapy, with or without radiotherapy (iRT or ICIs-nonRT), is the standard treatment for non–small cell lung cancer (NSCLC). Nonetheless, the response to the treatment varies among patients. Given the established role of aspartate aminotransferase/alanine transaminase (AST/ALT) ratio in predicting cancer prognosis, we sought to identify whether the pre-treatment AST/ALT ratio has the potential to serve as a prognostic factor for NSCLC patients receiving ICIs-nonRT and iRT.
Materials and methods: We retrospectively analyzed NSCLC patients who received immunotherapy between April 2018 and March 2021. Patients were classified into iRT group and ICIs-nonRT group and further classified based on AST/ALT ratio cut-off values. The Kaplan-Meier (KM) method estimated the time-to-event endpoints (progression-free survival (PFS) and overall survival (OS)
Results: Of the cohort, 239 underwent ICIs-nonRT and 155 received iRT. Higher AST/ALT ratios correlated with worse outcomes in the ICIs-nonRT group but indicated better outcomes in those who received iRT. Multivariate analysis validated AST/ALT ratio as an independent prognostic factor. For AST/ALT ratios between 0.67-1.7, both ICIs-nonRT and iRT yielded similar treatment outcomes; with AST/ALT ratios greater than 1.7, iRT could be a more favorable treatment option (P=0.038). Conversely, for ratios less than 0.67, ICIs-nonRT could be a more favorable treatment option (P=0.073).
Conclusions: The pre-treatment AST/ALT ratio demonstrates potential as a prognostic marker for treatment outcomes in NSCLC patients receiving either ICIs-nonRT or iRT. This finding could help guide clinicians in selecting more effective treatment protocols, thereby enhancing patient prognosis.
1 Introduction
Lung cancer remains the leading cause of cancer-related fatalities (1), with non-small cell lung cancer (NSCLC) accounting for approximately 85% of lung cancer cases (2). Among these, advanced NSCLC presents an especially grim prognosis, exhibiting a 5-years survival rate ranging from 10% to 30% (3).
Over time, therapeutic advances such as immunotherapy and radiotherapy have shown promise in improving outcomes for NSCLC patients (4). Although immunotherapy offers a durable response and long-term survival for a fraction of patients, resistance to this treatment is unfortunately commonplace, with hyperprogression observed in some instances (5). As a potential solution, the combination of immunotherapy and radiotherapy (iRT) is being considered as a more promising way to treat NSCLC. This combined strategy aims to enhance positive immunoregulation while significantly attenuating negative immune resistance, thereby potentially providing superior survival prognosis (6, 7). However, resistance to both radiotherapy and immunotherapy remains a challenge, leading to poor response to iRT in some patients (8). To date, a definitive biomarker guiding clinicians to judiciously apply either iRT or immunotherapy without radiotherapy (ICIs-nonRT) to the appropriate patient population has not been identified.
The aspartate aminotransferase/alanine transaminase (AST/ALT) ratio of serum levels was first proposed by Fernando De Ritis in 1957 as an indicator of hepatocellular damage or death (9), has lately been identified as a significant prognostic factor for several types of cancers, including bladder cancer (10), testicular cancer (11), hepatocellular carcinomas (12), pancreatic cancer (13) and prostate cancer (14), but there is scarce data regarding the role of AST/ALT ratio as a prognostic factor in NSCLC. Additionally, increasing number of studies have shown that reprogramming of glutamine metabolism is a putative determinant of the anti-tumor immune response in the tumor microenvironment (TME) (15). AST and ALT play crucial roles in glutamine metabolism. Malignant tumors, in order to ensure sufficient energy, exhibit increased glutamine metabolism in addition to the “Warburg effect” to sustain nucleotide biosynthesis and the synthesis of non-essential amino acids in proliferating tumor cells (16–18). Tumor cells transport glutamine into cells through specific transporters, and then convert it into glutamate under the action of glutaminase (AST, ALT and Phosphoserine Aminotransferase 1), and further convert it into α-ketoglutarate (α-KG), which enters the Tricarboxylic Acid cycle (TCA) and participates in the onset, development and dissemination of tumors (19, 20). Similar to malignant cells, immune cell activation also requires the uptake of glutamine (21). Immune cells uptake glutamine at similar or higher rates than glucose (22), with glutamine partially oxidized to CO2 within immune cells and converted to glutamate, alanine, and aspartate. This unique transformation is vital for immune cell function (15, 23). The appropriate concentration of glutamine promotes the expression of lymphocyte surface markers such as CD71, CD25, and CD45RO, as well as the production of cytokines such as IL-6, gamma-interferon (IFN-γ), and TNF-α (24–27). Glutamine metabolism also plays a major role in the activation of lymphocytes and is necessary for the differentiation of B lymphocytes into plasma cells and lymphoblasts. At the same time, glutamine is also necessary for T and B lymphocytes, for their proliferation, protein and antibody synthesis, and IL-2 production (28). Glutamine metabolism also plays a key role in regulating macrophage activation, and the synthesis and secretion of pro-inflammatory cytokines, such as IL-1, TNF-α and IL-6. In addition, α-KG produced by glutamine metabolism promotes the differentiation of M2 macrophages (29, 30).
Therefore, given the impact of glutamine metabolism on tumor immune response and the significant role of AST and ALT in this process, our aim is to explore the relationship between the easily accessible hematological marker AST/ALT ratio and the prognosis of non-small cell lung cancer patients receiving ICIs-nonRT and iRT, to aid in more precise clinical treatment.
2 Materials and methods
2.1 Study population
This retrospective study was conducted at a single institution. According to the AJCC 8th TNM and systematic staging imaging, including computed tomography (CT), positron emission tomography (PET), PET/CT, and contrast-enhanced magnetic resonance imaging, we identified 491 stage III and IV NSCLC patients who received non-operative immunotherapy between April 2018 and March 2021. Patients were then stratified into two groups: an iRT group and an ICIs-nonRT group, based on the inclusion and exclusion of radiotherapy in combination with immunotherapy. Additionally, within each group, patients were further subdivided based on the optimal cut-off values of AST/ALT ratio. Hematological indicators of AST and ALT were recorded within 5 days before the initiation of the first immunotherapy. The study excluded patients who lacked complete hematological parameters prior to their first dose of ICIs-nonRT or iRT, and those who received immunotherapy at other institutions. The detailed exclusion criteria are shown in Figure 1.
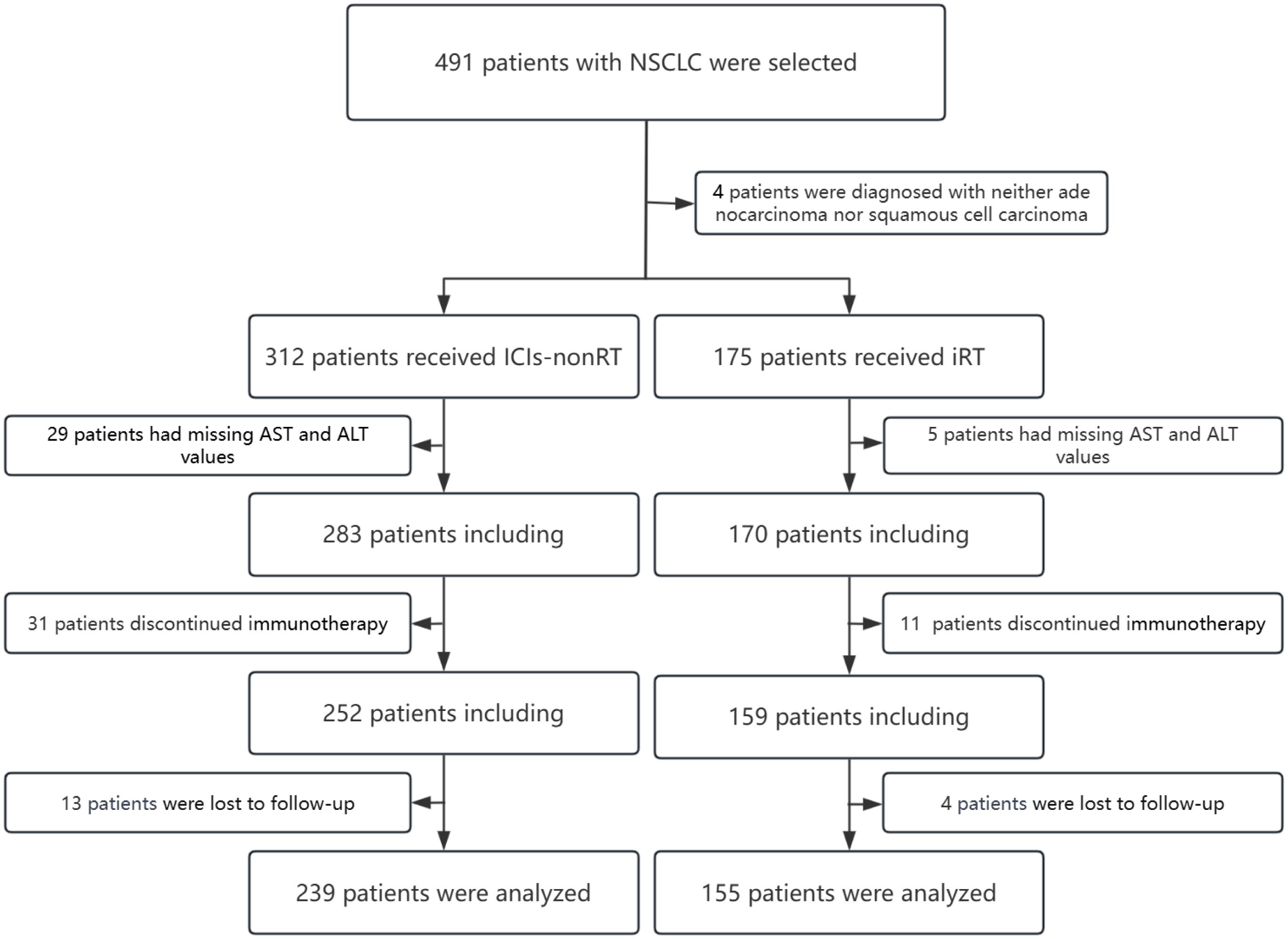
Figure 1. Flow chart of patient selection for this analysis. NSCLC, non-small cell lung cancer; ICIs-nonRT, immunotherapy without radiotherapy; iRT, immunotherapy combined with radiotherapy.
2.2 Treatment
In the ICIs-nonRT group, an overwhelming 98% of patients were treated with anti‐programmed cell death-1 (PD-1) immunotherapy, while a smaller fraction of only 2% received immunotherapy with an anti-programmed cell death ligand-1 (PD-L1) agent. Similarly, in the iRT group, a majority of 92% of patients were treated with anti‐PD-1 immunotherapy, with a marginally higher proportion of 8% received immunotherapy with an anti-PD-L1 agent. Regarding radiation therapy, within the iRT group, a predominant 96% of patients received intensity-modulated radiation therapy (IMRT), with the remainder divided between 1% receiving volumetric modulated arc therapy (VMAT) and 3% receiving three-dimensional conformal radiation therapy (3D-CRT). Detailed information on immunosuppressive agents and radiotherapy mode in each group was provided in Supplementary Notes 1, 2.
2.3 Statistical analysis
The primary endpoint of the study was overall survival (OS), while the secondary endpoint was progression-free survival (PFS). Both OS and PFS were defined from the first day of iRT or ICIs-nonRT until the event occurrence or the last follow-up. Continuous data are presented as median with 25th-75th percentiles or mean ± standard deviation and compared with the nonparametric Mann-Whitney U test or independent samples t test. The normality of distribution of continuous data was evaluated using the Kolmogorov-Smirnov test. Categorical variables are presented as count and proportions (%) and compared using the chi-squared test. The optimal cut-off values of continuous variables were calculated using X-tile software (http://www.tissuearray.org/rimmlab) The Kaplan-Meier (KM) method estimated the time-to-event endpoints (OS and PFS) and the log-rank test compared among subgroups. Univariate Cox proportional hazard model was used to evaluate each potential predictor, and P-values ≤0.10 were enrolled in multivariate analysis. A trichotomy KM curve was plotted to determine the precise range of AST/ALT levels, and the reliability of the range of the identified optimal biomarkers was verified by KM curves. All the P-values were two sided, and P ≤ 0.05 was considered to have a significant statistical difference.
GraphPad Prism 9 software was used to generate the KM curve. Univariate and multivariate Cox regressions were analyzed using the Xiantao academic analysis tool (www.xiantao.love). Descriptive statistics were performed using SPSS software (version 27.0; IBM Corp, Armonk, NY).
3 Results
3.1 Patient characteristics
A total of 394 eligible patients were ultimately enrolled in this study, including 239 in the ICIs-nonRT group and 155 in the iRT group. The baseline characteristics of each group are listed in Tables 1, 2. The median follow-up time for the ICIs-nonRT group and the iRT group was 29 months and 30 months, respectively (P=0.279). The number of PFS events was 164 in the ICIs-nonRT group and 114 in the iRT group. The number of OS events was 101 in the ICIs-nonRT group and 75 in the iRT group.
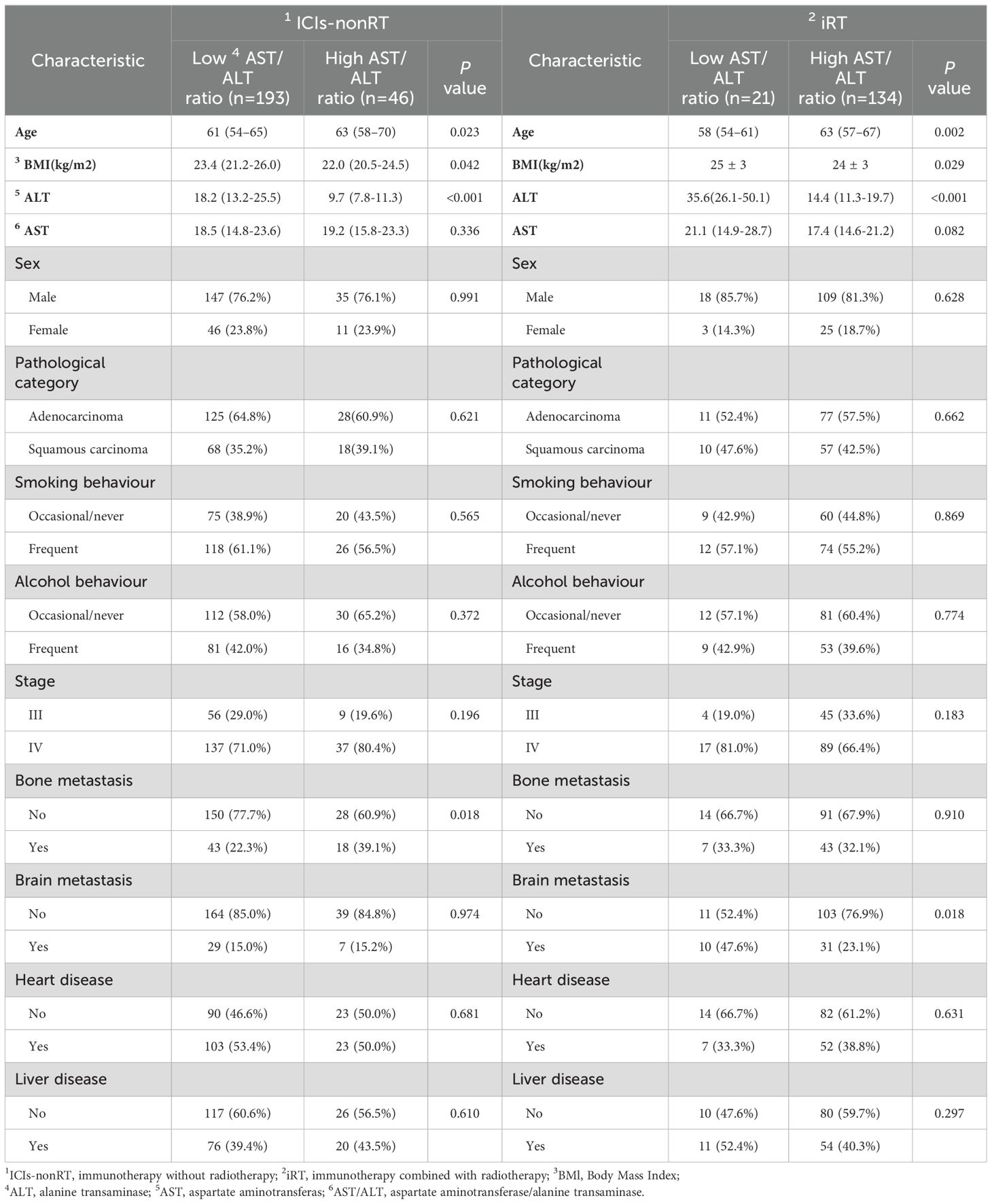
Table 2. Characteristics of all patients according to the level of pre-treatment AST/ALT ratio in ICIs-nonRT and iRT groups.
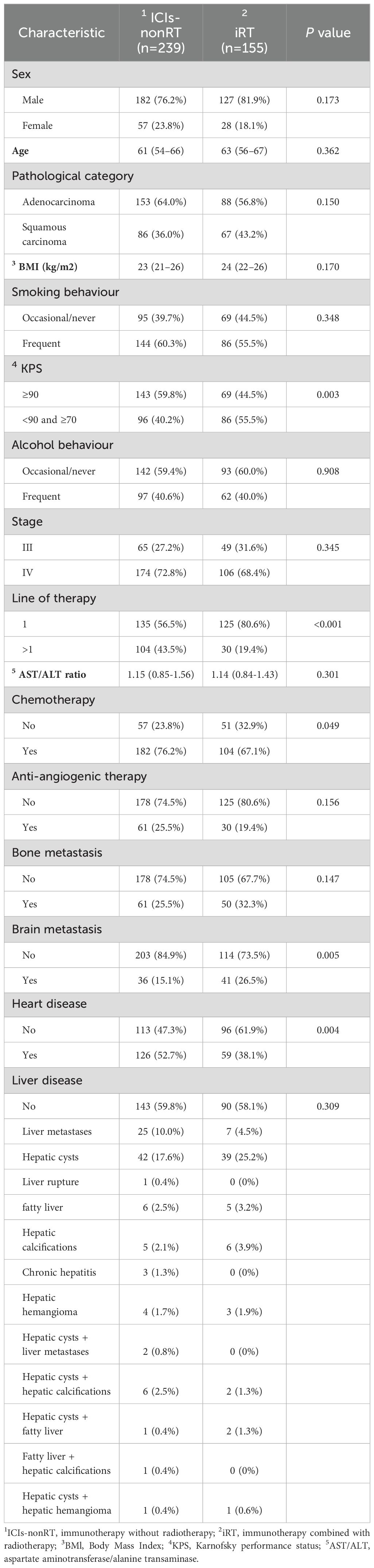
In the ICIs-nonRT group, patients were more likely to be treated with immunotherapy combined with chemotherapy, the majority of patients had a KPS≥90 (59.8%) and a higher prevalence of heart disease (including coronary artery disease, arrhythmia, pericardial disease, heart failure, valvular heart disease, and so on) was noted before the initiation of ICIs-nonRT treatment. In the iRT group, more patients received iRT as a first line of treatment, had a KPS<90 and ≥70(55.5%), and had brain metastases before iRT. By contrast, no significant difference was observed in the sex, age, pathological category, body mass index (BMI), smoking behavior, alcohol behavior, stage, AST/ALT ratio levels, anti-angiogenic therapy, bone metastasis and liver disease (including liver metastasis, hepatic cysts, fatty liver, chronic hepatitis, liver rupture, and so on) between ICIs-nonRT and iRT groups (Table 1).
In addition, our analysis identified that patients in both the ICIs-nonRT and iRT groups with high pre-treatment AST/ALT ratios (ICIs-nonRT group: AST/ALT ratio >1.7, n = 46; iRT group: AST/ALT ratio >0.67, n = 134) exhibited certain common characteristics: these patients were generally older and had lower BMI and ALT levels. Furthermore, in the ICIs-nonRT and iRT groups, it was observed that there were no significant differences between these patients with pre-treatment low and high AST/ALT ratios in the heart disease, liver disease, smoking habits, alcohol consumption, and AST levels, sex, pathological category, and tumor stage (Table 2).
3.2 Survival analysis according to AST/ALT ratio
Figure 2 depicts KM analyses for PFS and OS according to the AST/ALT ratio. Patients were stratified based on the optimal AST/ALT ratio cut-off values. For the ICIs-nonRT group, patients with higher AST/ALT ratios (PFS: >1.64; OS: >1.7) were associated with worse PFS (P=0.017) and OS (P=0.002) compared to those with a lower ratio (PFS: <1.64; OS: <1.7) (Figures 2A, C). In contrast, in the iRT group, patients with a higher AST/ALT ratio (PFS: >0.67; OS: >0.67) had a significantly improved PFS (P=0.010) and OS (P=0.012) compared to those with lower AST/ALT ratios (PFS: <0.67; OS: <0.67) (Figures 2B, D).
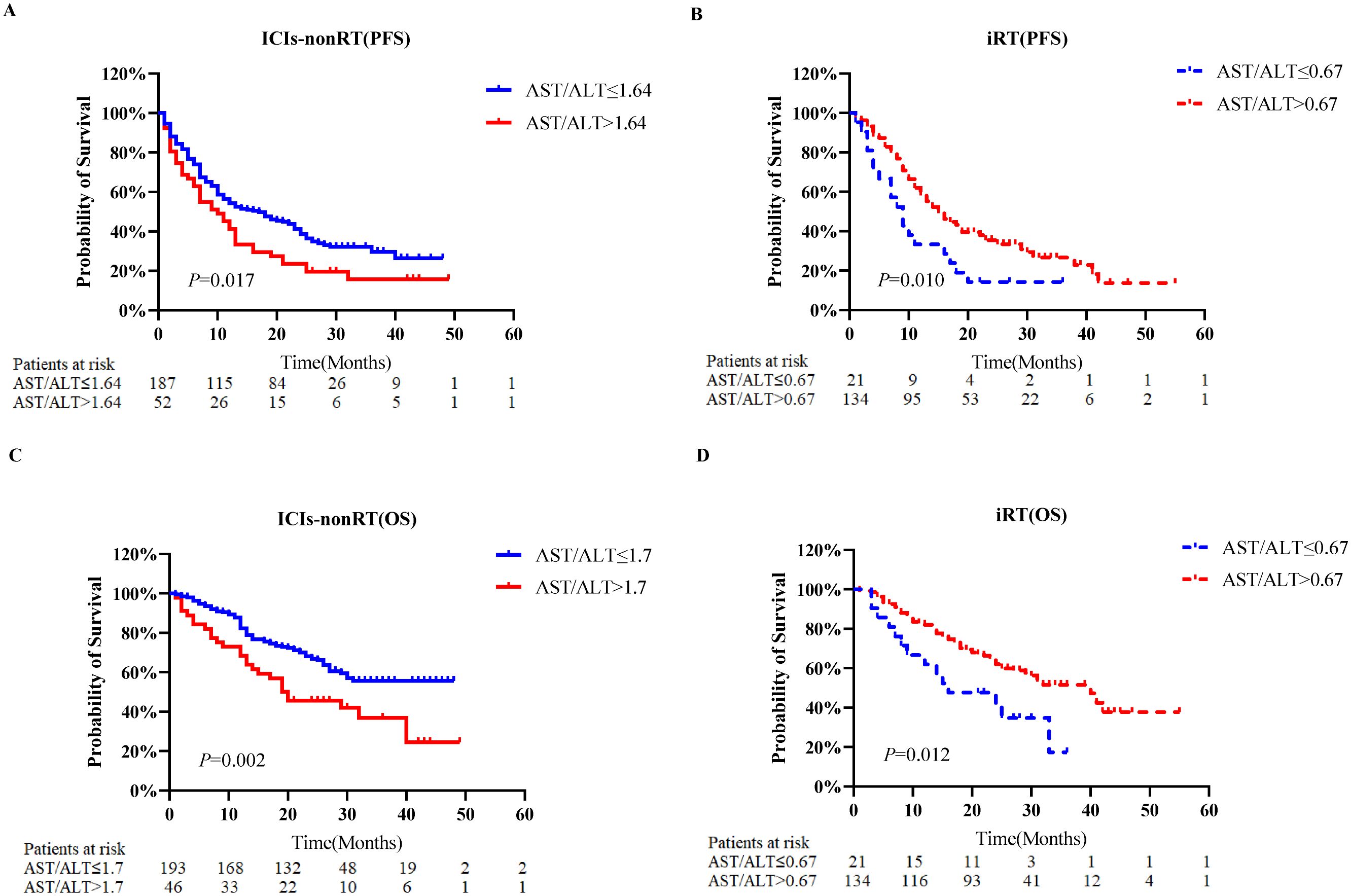
Figure 2. Kaplan-Meier probability plots of overall survival and progression-free survival according to the pre-treatment AST/ALT ratio category. (A) Progression-free survival of patients with non-small cell lung cancer who received ICIs-nonRT. (B) Progression-free survival of patients with non-small cell lung cancer who received iRT. (C) Overall survival of patients with non-small cell lung cancer who received ICIs-nonRT. (D) Overall survival of patients with non-small cell lung cancer who received iRT. PFS, progression-free survival; OS, overall survival; AST/ALT, aminotransferase/alanine transaminase; ICIs-nonRT, immunotherapy without radiotherapy; iRT, immunotherapy combined with radiotherapy.
3.3 Univariate and multivariate analysis of factors influencing survival
Independent predictors of PFS and OS for NSCLC patients were identified using the Cox proportional hazards regression models (Tables 3, 4).
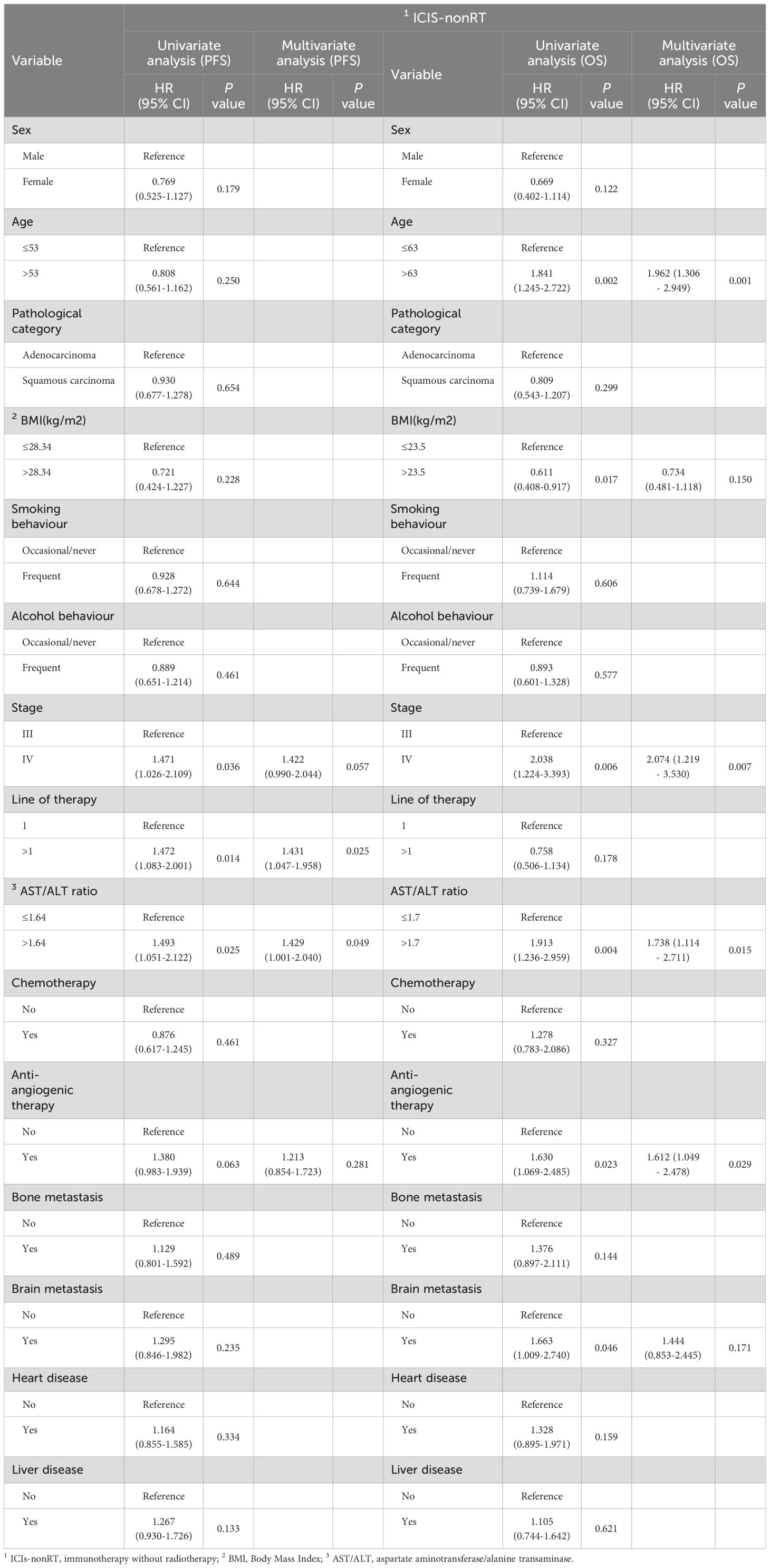
Table 3. Univariate and multivariate analyses of OS and PFS for different characteristics of NSCLC patients in the ICIs-nonRT group.
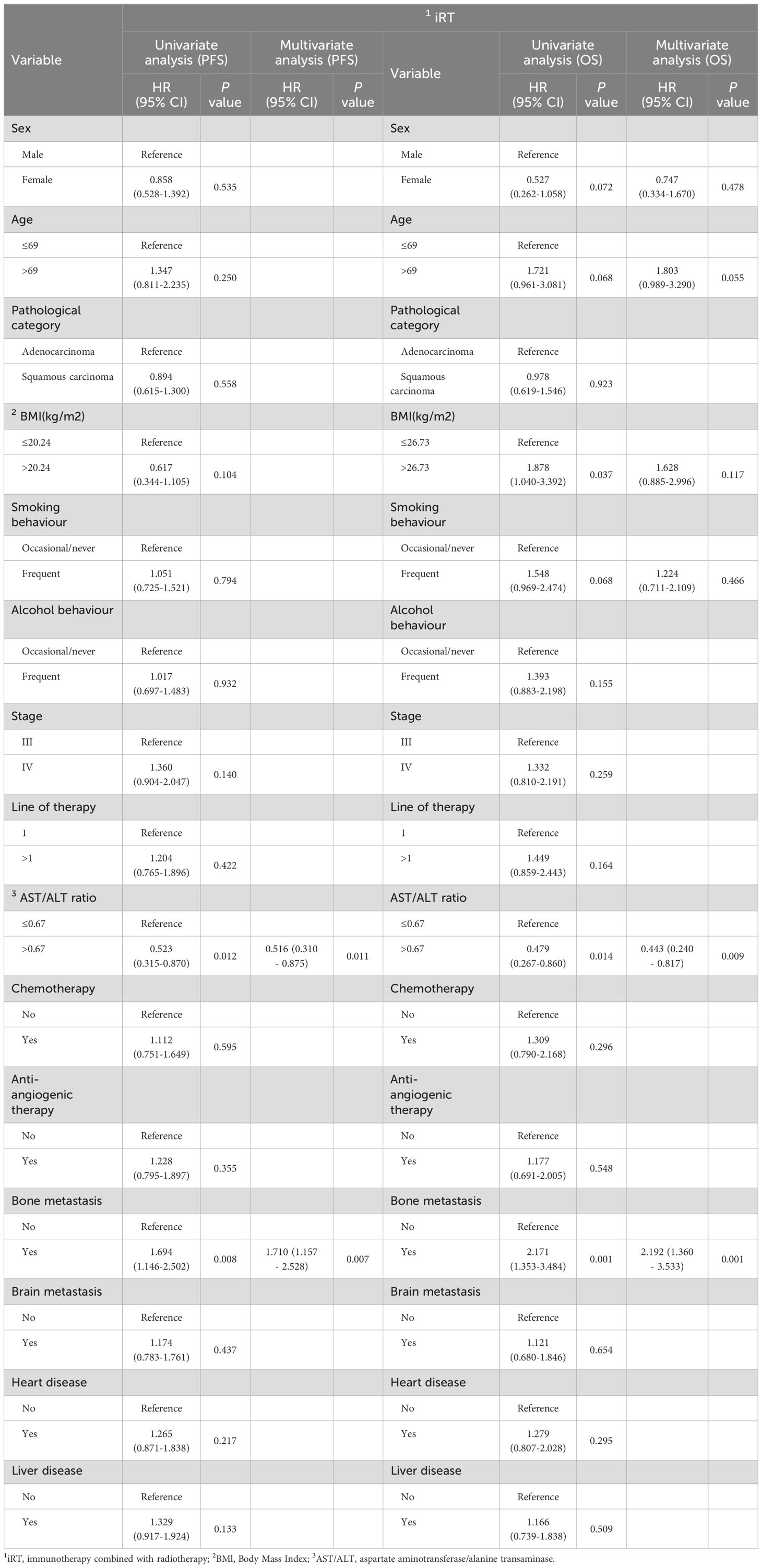
Table 4. Univariate and multivariate analyses of OS and PFS for different characteristics of NSCLC patients in the iRT group.
Univariate and multivariate Cox regression analyses for the ICIs-nonRT group are presented in Table 3. Univariate Cox analysis identified stage, line of therapy and the AST/ALT ratio as statistically significant PFS predictors, and age, BMI, stage, the AST/ALT ratio, anti-angiogenic therapy and brain metastasis as significant OS predictors. Multivariate Cox analysis established that the pre-treatment AST/ALT ratio remained an independent prognostic factor for PFS (HR=1.429, 95% CI 1.001-2.040, P=0.049) and OS (HR=1.738, 95% CI 1.114-2.711, P=0.015). Alongside the AST/ALT ratio, line of therapy remained a significant prognostic factor for PFS, and age, stage, and anti-angiogenic therapy remained significant prognostic factors for OS according to the multivariate Cox regression analysis.
Univariate and multivariate Cox regression analyses of the iRT group are also shown in Table 4. Univariate Cox analysis identified the AST/ALT ratio and bone metastasis as statistically significant PFS predictor, and BMI, the AST/ALT ratio and bone metastasis as statistically significant OS predictors. Subsequent multivariate regression analysis confirmed the pre-treatment AST/ALT ratio as an independent prognostic factor for PFS (HR=0.516, 95% CI 0.310-0.875, P=0.011) and OS (HR=0.443, 95% CI 0.240-0.817, P=0.009). Furthermore, in the multivariate Cox regression analysis, in addition to the pre-treatment AST/ALT ratio, bone metastasis remained a significant prognostic factor for PFS and OS predictors.
3.4 Determination of the AST/ALT ratio range
To enhance oncological treatment decisions, we attempted to determine the precise range of the AST/ALT ratio (Figures 3A, B). The trichotomy KM curve of the AST/ALT ratio revealed that in the ICIs-nonRT group, patients with an AST/ALT ratio >1.7 had the poorest OS (P=0.009), while no difference was observed between the subgroup of patients with an AST/ALT ratio ranging from 1.11 to 1.7 and <1.11 (Figure 3A). In contrast, in the iRT group, patients with an AST/ALT ratio <0.67 had the worst OS (P=0.010), and no difference was found between the subgroup of patients with an AST/ALT ratio ranging from 0.67 to 1.48 and >1.48 (Figure 3B). To better elucidate our findings (Figures 3A, B), a schematic was plotted in Figure 3C. This schematic reveals that patients with an AST/ALT ratio ranging from 0.67 to 1.7 experienced a similar prognosis under ICIs-nonRT and iRT for NSCLC. To further confirm the reliability of the range, we validated the above results using the KM curve (Figures 3D–F) and reached the same conclusion. The KM curve indicated that for patients with a pre-treatment AST/ALT ratio ranging from 0.67 to 1.7, survival curves of ICIs-nonRT and iRT closely overlapped, indicating that both treatment modalities offer equivalent therapeutic effects in patients with NSCLC (Figure 3D). For patients with a pre-treatment AST/ALT ratio >1.7, iRT was associated with a better prognosis (P=0.038) than ICIs-nonRT (Figure 3E). For patients with a pre-treatment AST/ALT ratio <0.67, despite the comparison between ICIs-nonRT and iRT (P=0.073) not reaching statistical significance, a clear trend could be discerned (Figure 3F).

Figure 3. Determine the range of AST/ALT ratio in predicting OS for NSCLC patients receiving ICIs-nonRT and iRT. Kaplan-Meier probability plots of overall survival according to tertiles of the pre-treatment AST/ALT ratio: ICIs-nonRT group (A) and iRT group (B); The schematic (C) summarizes the findings of Figures (A, B) Green indicates better patient survival. Red indicates poorer patient survival; Figures (D–F) validates the results of Figure (C) Kaplan-Meier probability plot of overall survival for patients receiving ICIs-nonRT and iRT: patients with pre-treatment AST/ALT ratios in the range of 0.67-1.7 (D), pre-treatment AST/ALT ratios >1.7 (E) and pre-treatment AST/ALT ratios <0.67 (F). OS, overall survival; AST/ALT, aminotransferase/alanine transaminase; ICIs-nonRT, immunotherapy without radiotherapy; iRT, immunotherapy combined with radiotherapy. *, P-value less than 0.05; **, P-value less than 0.01; ns, P-value is not significant.
In addition, this trend could also be observed when predicting PFS based on the AST/ALT ratio (Supplementary Figure 1). The KM curve suggested that for patients with an AST/ALT ratio ranging from 0.67 to 1.64, both iRT and ICIs-nonRT resulted in comparable prognosis for patients with NSCLC (Supplementary Figure 1D). However, for patients with an AST/ALT ratio >1.64 and <0.67, no statistically significant difference was found between ICIs-nonRT and iRT (Supplementary Figures 1E, F). This lack of significant difference might be attributed to mechanisms of immune checkpoint inhibitors, which combat tumors by modulating tumor growth kinetics rather than solely through direct cytotoxic effects (31), leading to meaningful improvements in OS with minimal or no enhancements in PFS.
4 Discussion
Although numerous studies link elevated levels of AST/ALT ratio with decreased disease control and survival across different tumor types (10–14), the AST/ALT ratio’s impact in NSCLC patients receiving immunotherapy remains undefined.
Our study elucidated that high AST/ALT ratio correlated with a worse prognosis in patients receiving ICIs-nonRT, yet conversely associated with a more favorable prognosis in those receiving iRT. Additionally, we leveraged stratified Kaplan-Meier curves based on the AST/ALT ratio to demonstrate that this ratio could aid clinicians in applying either ICIs-nonRT or iRT more effectively for specific patient populations.
Prior research delineated the relationship between the AST/ALT ratio and patient prognosis, revealing that an elevated pre-treatment AST/ALT ratio was linked to a poor prognosis (32). Our data echoed these findings, indicating that NSCLC patients with a high pre-treatment AST/ALT ratio (>1.7) undergoing ICIs-nonRT treatment tended to have a poorer prognosis. However, the precise mechanisms connecting a high AST/ALT ratio and poor prognosis remain speculative, though theories regarding glutamate metabolism may offer some explanations. Glutaminolysis, a metabolic process prevalent in all proliferating cells, especially in tumor cells (18), involves conversion of glutamine to glutamate, catalyzed by Glutaminase (GLS) (33). This transformation allows cancer cells to replenish the tricarboxylic acid cycle (TCA, Krebs cycle) with α-KG as carbon source. Glutamate can be further transformed to α-KG through three aminotransferase pathways, namely ALT, AST, and phosphoserine aminotransferase1 (PSAT1). Each pathway generates a unique amino acid byproduct in addition to α-KG, with ALT being crucial in α-KG production (34, 35). Previous studies suggested that aggressive cancer cells, known for their enhanced metabolic rate, demonstrate lower serum ALT levels compared to their less invasive counterparts, likely due to increased ALT consumption (36). Besides, theories suggest that glucose metabolism and anaerobic glycolysis, wherein AST plays a significant role, might also underpin these observations. Such metabolic adaptations, known as the Warburg effect, may result in an elevated AST/ALT ratio. Nevertheless, a comprehensive understanding of these mechanisms warrants further research.
Our study also identified a unique prospective relationship between the AST/ALT ratio and prognosis. Specifically, NSCLC patients with a pre-treatment AST/ALT ratio exceeding 1.7 undergoing iRT treatment demonstrated a better prognosis compared to those with a lower AST/ALT ratio. This novel observation has rarely been reported in previous studies, and the specific mechanism is not yet clear. Previous studies have shown that radiation may stimulate resident immune cells (37, 38) and promote the influx of circulating immune cells into the tumor microenvironment (39). Similar to malignant cells, T cell activation requires glutamine uptake, and glutamine blockade inhibits oxidative and glycolytic metabolism in cancer cells, leading to decreased hypoxia, acidosis, and nutrient depletion. In contrast, the response of effector T cells to glutamine antagonists is characterized by a significant upregulation of oxidative metabolism and the adoption of a long-lived, highly activated phenotype (21). Therefore, we speculate that the better prognosis of patients with a pre-treatment AST/ALT ratio exceeding 1.7 after receiving iRT may be due to their lower ALT levels, which reduce glutamine metabolism, promote T cell proliferation and activation, reverse the inhibitory immune microenvironment, and enhance the anti-tumor immune response. Further, glutamine deprivation in cancer cells might augment oxidative stress response and reactive oxygen species (ROS) generation, leading to DNA damage and enhanced radiosensitivity (40). However, for patients with a pre-treatment AST/ALT ratio below 0.67, iRT showed a poor prognosis compared to ICIs-nonRT. While the mechanisms underlying this shift remain ambiguous, it has been speculated that radiation-resistant cells characterized by low glycolysis, reduced mitochondrial respiration, decreased TCA cycle activity, elevated glutamine anabolism might contribute to a lower AST/ALT ratio (41). However, the exact mechanisms necessitate further exploration.
Another important finding from our study involves patients with an AST/ALT ratio between 0.67 and 1.7, where ICIs-nonRT and iRT demonstrated identical prognosis in NSCLC patients. However, the detailed mechanism is still unclear and requires further investigation.
Despite our study’s novel insights into tailoring treatment modalities based on the AST/ALT ratio in NSCLC patients, it bears several limitations. These encompass potential unknown confounders and selection bias associated with retrospective studies, unidentified causes of liver enzyme alterations, uncertainty regarding the full extent of underlying disease in our study cohort, and a single pre-treatment measurement of aminotransferases. Therefore, we cannot ensure that all abnormalities truly indicate disease states. Finally, future prospective studies and external validation are necessary to determine the optimal cut-off of the AST/ALT ratio.
5 Conclusion
In this study, we identified the pre-treatment AST/ALT ratio as a reliable prognostic factor for survival in NSCLC patients receiving ICIs-nonRT and iRT. Interestingly, our findings challenge the conventional view that high AST/ALT ratios correlated with poor prognosis. For NSCLC patients treated with ICIs-nonRT, high AST/ALT ratios signified a poorer prognosis. Contrarily, a completely different scenario was observed in patients receiving iRT, where high AST/ALT ratios were linked to a favorable prognosis. These findings indicate that the AST/ALT ratio could serve as a valuable tool in customizing treatment for NSCLC patients. When the serum AST/ALT ratio of patients ranges from 0.67 to 1.7, ICIs-nonRT and iRT treatment seem to yield comparable outcomes. However, when the serum AST/ALT ratio is greater than 1.7, iRT appeared to be a more advantageous treatment compared to ICIs-nonRT. Conversely, ICIs-nonRT showed a superior outcome for patients with an AST/ALT ratio below 0.67 compared to iRT.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Ethics statement
This study was approved by the Ethics Review Committee of Shandong Cancer Hospital and complied with the provisions of the Declaration of Helsinki. This study was a retrospective analysis and informed consent was not required. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YZ: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. JZ: Data curation, Formal analysis, Methodology, Validation, Writing – original draft, Writing – review & editing. SS: Data curation, Writing – review & editing. JM: Data curation, Writing – review & editing. FW: Supervision, Writing – review & editing. MW: Supervision, Writing – review & editing. JY: Supervision, Writing – review & editing. DC: Supervision, Writing – review & editing, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Science Foundation of Shandong (grant numbers ZR2020MH277, ZR2020LZL014, ZR2021YQ52, ZR2020LZL016, ZR201911040452); National Natural Science Foundation of China (grant number 82172676, 81627901, 81972863 and 82030082); and Bethune Charitable Foundation (grant number 2021434953); the Academic Promotion Program of Shandong First Medical University (grant number 2019ZL002); Research Unit of Radiation Oncology, Chinese Academy of Medical Sciences (grant number 2019RU071).
Acknowledgments
We extremely express our gratitude to our colleagues who assisted to improve our paper from the department of Shandong Provincial Key Laboratory of Precision Oncology.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1389804/full#supplementary-material
Glossary
NSCLC: Non-small cell lung cancer
AST/ALT: Aspartate aminotransferase/alanine transaminase
iRT: Immunotherapy combined with radiotherapy
ICIs-nonRT: Immunotherapy without radiotherapy
PFS: Progression-free survival
OS: Overall survival
CT: Computed tomography
PET: Positron emission tomograph
PD-1: Programmed cell death-1
PD-L1: Programmed cell death ligand-1
IMRT: Intensity-modulated radiation therapy
VMAT: Volumetric modulated arc therapy
3D-CRT: Three-dimensional conformal radiation therapy
BMI: Body mass index
KM: Kaplan-Meier
GLS: Glutaminas
TCA: Krebs cycle: Tricarboxylic acid cycle
α-KG: α-ketoglutarate
PSAT1: Phosphoserine aminotransferase 1
ROS: Reactive oxygen species
IFN-γ: γ-interferon
TME: Tumor microenvironment
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA A Cancer J Clin. (2022) 72:7–33. doi: 10.3322/caac.21708
2. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. (2008) 83:584–94. doi: 10.4065/83.5.584
3. Higgins KA, Puri S, Gray JE. Systemic and radiation therapy approaches for locally advanced non–small-cell lung cancer. JCO. (2022) 40:576–85. doi: 10.1200/JCO.21.01707
4. Spigel DR, Faivre-Finn C, Gray JE, Vicente D, Planchard D, Paz-Ares L, et al. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. JCO. (2022) 40:1301–11. doi: 10.1200/JCO.21.01308
5. Chen R, Manochakian R, James L, Azzouqa A-G, Shi H, Zhang Y, et al. Emerging therapeutic agents for advanced non-small cell lung cancer. J Hematol Oncol. (2020) 13:58. doi: 10.1186/s13045-020-00881-7
6. Chen Y, Gao M, Huang Z, Yu J, Meng X. SBRT combined with PD-1/PD-L1 inhibitors in NSCLC treatment: a focus on the mechanisms, advances, and future challenges. J Hematol Oncol. (2020) 13:105. doi: 10.1186/s13045-020-00940-z
7. Theelen WSME, Chen D, Verma V, Hobbs BP, Peulen HMU, Aerts JGJV, et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir Med. (2021) 9:467–75. doi: 10.1016/S2213-2600(20)30391-X
8. Theelen WSME, Peulen HMU, Lalezari F, van der Noort V, De Vries JF, Aerts JGJV, et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non–small cell lung cancer: results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol. (2019) 5:1276. doi: 10.1001/jamaoncol.2019.1478
9. De Ritis F, Coltorti M, Giusti G. An enzymic test for the diagnosis of viral hepatitis: The transaminase serum activities. Clin Chim Acta. (2006) 369:148–52. doi: 10.1016/j.cca.2006.05.001
10. Ha Y-S, Kim SW, Chun SY, Chung J-W, Choi SH, Lee JN, et al. Association between De Ritis ratio (aspartate aminotransferase/alanine aminotransferase) and oncological outcomes in bladder cancer patients after radical cystectomy. BMC Urol. (2019) 19:10. doi: 10.1186/s12894-019-0439-7
11. Gorgel SN, Akin Y, Koc EM, Kose O, Ozcan S, Yilmaz Y. Impact of increased aspartate aminotransferase to alanine aminotransferase (De Ritis) ratio in prognosis of testicular cancer. Investig Clin Urol. (2019) 60:169. doi: 10.4111/icu.2019.60.3.169
12. Liu C, Jia B, Zou B, Du H, Yan L, Yang J, et al. Neutrophil-to-lymphocyte and aspartate-to-alanine aminotransferase ratios predict hepatocellular carcinoma prognosis after transarterial embolization. Medicine. (2017) 96:e8512. doi: 10.1097/MD.0000000000008512
13. Riedl JM, Posch F, Prager G, Eisterer W, Oehler L, Sliwa T, et al. The AST/ALT (De Ritis) ratio predicts clinical outcome in patients with pancreatic cancer treated with first-line nab-paclitaxel and gemcitabine: post hoc analysis of an Austrian multicenter, noninterventional study. Ther Adv Med Oncol. (2020) 12:175883591990087. doi: 10.1177/1758835919900872
14. Wang H, Fang K, Zhang J, Jiang Y, Wang G, Zhang H, et al. The significance of De Ritis (aspartate transaminase/alanine transaminase) ratio in predicting pathological outcomes and prognosis in localized prostate cancer patients. Int Urol Nephrol. (2017) 49:1391–8. doi: 10.1007/s11255-017-1618-7
15. Ma G, Zhang Z, Li P, Zhang Z, Zeng M, Liang Z, et al. Reprogramming of glutamine metabolism and its impact on immune response in the tumor microenvironment. Cell Commun Signal. (2022) 20:114. doi: 10.1186/s12964-022-00909-0
16. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
17. Hsu PP, Sabatini DM. Cancer cell metabolism: warburg and beyond. Cell. (2008) 134:703–7. doi: 10.1016/j.cell.2008.08.021
18. Fernandez-de-Cossio-Diaz J, Vazquez A. Limits of aerobic metabolism in cancer cells. Sci Rep. (2017) 7:13488. doi: 10.1038/s41598-017-14071-y
19. Zhang J, Pavlova NN, Thompson CB. Cancer cell metabolism: the essential role of the nonessential amino acid, glutamine. EMBO J. (2017) 36:1302–15. doi: 10.15252/embj.201696151
20. Daye D, Wellen KE. Metabolic reprogramming in cancer: Unraveling the role of glutamine in tumorigenesis. Semin Cell Dev Biol. (2012) 23:362–9. doi: 10.1016/j.semcdb.2012.02.002
21. Leone RD, Zhao L, Englert JM, Sun I-M, Oh M-H, Sun I-H, et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science. (2019) 366:1013–21. doi: 10.1126/science.aav2588
22. Newsholme P. Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J Nutr. (2001) 131:2515S–22S. doi: 10.1093/jn/131.9.2515S
23. Curi R, Newsholme P, Marzuca-Nassr GN, Takahashi HK, Hirabara SM, Cruzat V, et al. Regulatory principles in metabolism–then and now. Biochem J. (2016) 473:1845–57. doi: 10.1042/BCJ20160103
24. Curi R, Lagranha CJ, Doi SQ, Sellitti DF, Procopio J, Pithon-Curi TC, et al. Molecular mechanisms of glutamine action. J Cell Physiol. (2005) 204:392–401. doi: 10.1002/jcp.20339
25. Curi R, Lagranha CJ, Doi SQ, Sellitti DF, Procopio J, Pithon-Curi TC. Glutamine-dependent changes in gene expression and protein activity. Cell Biochem Funct. (2005) 23:77–84. doi: 10.1002/cbf.1165
26. Roth E, Oehler R, Manhart N, Exner R, Wessner B, Strasser E, et al. Regulative potential of glutamine—relation to glutathione metabolism. Nutrition. (2002) 18:217–21. doi: 10.1016/S0899-9007(01)00797-3
27. Hiscock N, Petersen EW, Krzywkowski K, Boza J, Halkjaer-Kristensen J, Pedersen BK. Glutamine supplementation further enhances exercise-induced plasma IL-6. J Appl Physiol. (2003) 95:145–8. doi: 10.1152/japplphysiol.00471.2002
28. Crawford J, Cohen HJ. The essential role of L-glutamine in lymphocyte differentiation in vitro. J Cell Physiol. (1985) 124:275–82. doi: 10.1002/jcp.1041240216
29. Cruzat V, Macedo Rogero M, Noel Keane K, Curi R, Newsholme P. Glutamine: metabolism and immune function, supplementation and clinical translation. Nutrients. (2018) 10:1564. doi: 10.3390/nu10111564
30. Liu P-S, Wang H, Li X, Chao T, Teav T, Christen S, et al. α-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat Immunol. (2017) 18:985–94. doi: 10.1038/ni.3796
31. Merino M, Kasamon Y, Theoret M, Pazdur R, Kluetz P, Gormley N. Irreconcilable differences: the divorce between response rates, progression-free survival, and overall survival. JCO. (2023) 41:2706–12. doi: 10.1200/JCO.23.00225
32. Ghahari M, Salari A, Ghafoori Yazdi M, Nowroozi A, Fotovat A, Momeni SA, et al. Association between preoperative de ritis (AST/ALT) ratio and oncological outcomes following radical cystectomy in patients with urothelial bladder cancer. Clin Genitourinary Cancer. (2022) 20:e89–93. doi: 10.1016/j.clgc.2021.10.007
33. Cluntun AA, Lukey MJ, Cerione RA, Locasale JW. Glutamine metabolism in cancer: understanding the heterogeneity. Trends Cancer. (2017) 3:169–80. doi: 10.1016/j.trecan.2017.01.005
34. Smith B, Schafer XL, Ambeskovic A, Spencer CM, Land H, Munger J. Addiction to coupling of the warburg effect with glutamine catabolism in cancer cells. Cell Rep. (2016) 17:821–36. doi: 10.1016/j.celrep.2016.09.045
35. Hao Y, Samuels Y, Li Q, Krokowski D, Guan B-J, Wang C, et al. Oncogenic PIK3CA mutations reprogram glutamine metabolism in colorectal cancer. Nat Commun. (2016) 7:11971. doi: 10.1038/ncomms11971
36. Conde VR, Oliveira PF, Nunes AR, Rocha CS, Ramalhosa E, Pereira JA, et al. The progression from a lower to a higher invasive stage of bladder cancer is associated with severe alterations in glucose and pyruvate metabolism. Exp Cell Res. (2015) 335:91–8. doi: 10.1016/j.yexcr.2015.04.007
37. Osman AM, Sun Y, Burns TC, He L, Kee N, Oliva-Vilarnau N, et al. Radiation triggers a dynamic sequence of transient microglial alterations in juvenile brain. Cell Rep. (2020) 31:107699. doi: 10.1016/j.celrep.2020.107699
38. Lai Y-C, Hsieh C-Y, Lu K-Y, Sung C-H, Ho H-Y, Cheng M-L, et al. Monitoring early glycolytic flux alterations following radiotherapy in cancer and immune cells: hyperpolarized carbon-13 magnetic resonance imaging study. Metabolites. (2021) 11:518. doi: 10.3390/metabo11080518
39. Kang J-H, Woo JK, Jang Y-S, Oh SH. Radiation potentiates monocyte infiltration into tumors by ninjurin1 expression in endothelial cells. Cells. (2020) 9:1086. doi: 10.3390/cells9051086
40. Mukha A, Kahya U, Dubrovska A. Targeting glutamine metabolism and autophagy: the combination for prostate cancer radiosensitization. Autophagy. (2021) 17:3879–81. doi: 10.1080/15548627.2021.1962682
Keywords: non-small cell lung cancer, aspartate aminotransferase, alanine transaminase, AST/ALT ratio, immunotherapy without radiotherapy, immunotherapy combined with radiotherapy
Citation: Zhang Y, Zhang J, Shang S, Ma J, Wang F, Wu M, Yu J and Chen D (2024) The AST/ALT ratio predicts survival and improves oncological therapy decisions in patients with non-small cell lung cancer receiving immunotherapy with or without radiotherapy. Front. Oncol. 14:1389804. doi: 10.3389/fonc.2024.1389804
Received: 22 February 2024; Accepted: 31 July 2024;
Published: 26 August 2024.
Edited by:
Mayank Singh, All India Institute of Medical Sciences, IndiaReviewed by:
Mattia Falchetto Osti, Sapienza University of Rome, ItalyHaihua Yang, Wenzhou Medical University, China
Hidekazu Tanaka, Yamaguchi University Graduate School of Medicine, Japan
Copyright © 2024 Zhang, Zhang, Shang, Ma, Wang, Wu, Yu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinming Yu, c2R5dWppbm1pbmdAMTI2LmNvbQ==; Dawei Chen, ZGF2ZTA1MDVAeWVhaC5uZXQ=
†These authors have contributed equally to this work and share first authorship
 Yanyan Zhang1,2†
Yanyan Zhang1,2† Meng Wu
Meng Wu Jinming Yu
Jinming Yu Dawei Chen
Dawei Chen