- 1Department of Thoracic Surgery, Heping Hospital Affiliated to Changzhi Medical College, Changzhi, Shanxi, China
- 2Department of Rehabilitation, Heping Hospital Affiliated to Changzhi Medical College, Changzhi, Shanxi, China
Background: Newly identified as a radiological concept, interstitial lung abnormalities (ILA) is emerging as a prognostic factor for lung cancer. Yet, debates persist regarding the prognostic significance of ILA in lung cancer. Our inaugural meta-analysis aimed to investigate the correlation between ILA and lung cancer outcomes, offering additional insights for clinicians in predicting patient prognosis.
Methods: Articles meeting the criteria were found through PubMed, the Cochrane Library, EMBASE, and Web of Science by February 29, 2024. The outcomes evaluated were the survival rates such as overall survival (OS), disease-free survival (DFS), progression-free survival (PFS), and cancer-specific survival (CSS).
Results: A total of 12 articles with 4416 patients were included in this meta-analysis. The pooled results showed that lung cancer patients with interstitial lung abnormalities had an inferior OS (n=11; HR=2.22; 95% CI=1.68-2.95; P<0.001; I2 = 72.0%; Ph<0.001), PFS (n=3; HR=1.59; 95% CI=1.08-2.32; P=0.017; I2 = 0%; Ph=0.772), and CSS (n=2; HR=4.00; 95% CI=1.94-8.25; P<0.001; I2 = 0%; Ph=0.594) than those without, however, the ILA was not significantly associated with the DFS (n=2; HR=2.07; 95% CI=0.94-7.02; P=0.066; I2 = 90.4%; Ph=0.001). Moreover, lung cancer patients with ILA were significantly correlated with male (OR=2.43; 95% CI=1.48-3.98; P<0.001), smoking history (OR=2.11; 95% CI=1.37-3.25; P<0.001), advanced age (OR=2.50; 95% CI=1.56-4.03; P<0.001), squamous carcinoma (OR=0.42; 95% CI=0.24-0.71; P=0.01), and EGFR mutation (OR=0.50; 95% CI=0.32-0.78; P=0.002). The correlation between ILA and race, stage, ALK, however, was not significant.
Conclusion: ILA was a availability factors of prognosis in patients with lung cancers. These findings highlight the importance of early pulmonary fibrosis, namely ILA for prognosis in patients with lung cancer, and provide a partial rationale for future clinical work.
1 Introduction
Lung cancer still stands as one of the malignancies with the highest mortality and morbidity globally (1, 2). Despite advancements in treatment options for lung cancer, survival rates have increased from previous levels, yet the overall prognosis remains grim. As a result, there is a continuous effort to investigate the various factors influencing the prognosis of individuals diagnosed with lung cancer.
ILA were initially detected as a radiologic discovery based on incidental CT findings of abnormalities impacting over 5% of lung regions, including ground-glass or reticular abnormalities, lung distortion, traction bronchiectasis, honeycombing, and non-emphysematous cysts (3). ILA is considered to be an early stage of fibrotic lung disease (4), and there is a strong correlation between ILA and lung cancer (5). Numerous studies (5, 6) indicating an increased occurrence of lung cancer and heightened pulmonary complications following cancer therapy in ILA patients. Additionally, some studies (7–14) suggest that combined ILA significantly worsens the prognosis in lung cancer patients. Conversely, conflicting studies (15–18) have shown no clear correlation between ILA and survival outcome among lung cancer patients. These results are inconclusive, leading to a continued debate over the association of ILA with lung cancer prognosis. Thus, we conducted a comprehensive meta-analysis of existing literature regarding the prognosis of lung cancer patients with ILA.
2 Methods
2.1 Literature search strategies
The current meta-analysis accompanied the PRISMA statement (19). On February 29, 2024, PubMed, EMBASE, Web of science, and the Cochrane Library were retrieved for English language studies. The search term consists of a combination of the following medical subject heading (MeSH) and free text words: (lung carcinoma OR lung adenocarcinoma OR lung neoplasm OR lung cancer) AND (interstitial lung abnormality OR ILA OR subclinical ILD OR early ILD) AND (prognosis OR prognoses OR survival OR outcome) AND (computed tomography). Additionally, we manually retrieved the reference lists of the publications that qualified. Details of the literature search are presented in Supplementary Table 1.
2.2 Inclusion and exclusion criteria
After the initial search and removal of the duplicates, screening of the title and abstract was done by 2 independent investigators (TXL and GXT). Full texts were retrieved for studies that satisfied the inclusion criteria. Any disagreements were resolved through discussion. Bibliography sections of the included studies were further searched for additional relevant papers.
Inclusion Criteria
Utilizing the Population-Intervention-Control-Outcome-Study (PICOS) framework, the inclusion criteria for the current study were developed as follows: patient population consisting of individuals diagnosed with lung carcinoma through pathological or histological means; Exposure-intervention involving a computed tomography (CT) scan of the lungs identifying ILA, with a positive diagnosis by a radiologist; Control group comprised of individuals whose lung CT scans showed no signs of ILA or interstitial lung disease; Desired outcomes consisted of English-language studies examining the relationship between ILA and prognosis in lung cancer patients, including OS, DFS, PFS, and CSS, as well as hazard ratio (HR) and 95% confidence interval (CI) related to patient survival; And study design encompassing both retrospective and prospective English-language publications. Classification of patients into ILA group and non-ILA group for the intervention and control groups was based on the definition of ILA identified in chest CT scans.
Exclusion Criteria
Conference abstracts, case reports, or comments were excluded. Studies that compared only the clinicopathologic features and not the prognostic value of ILA were excluded.
2.3 Data extraction and quality assessment
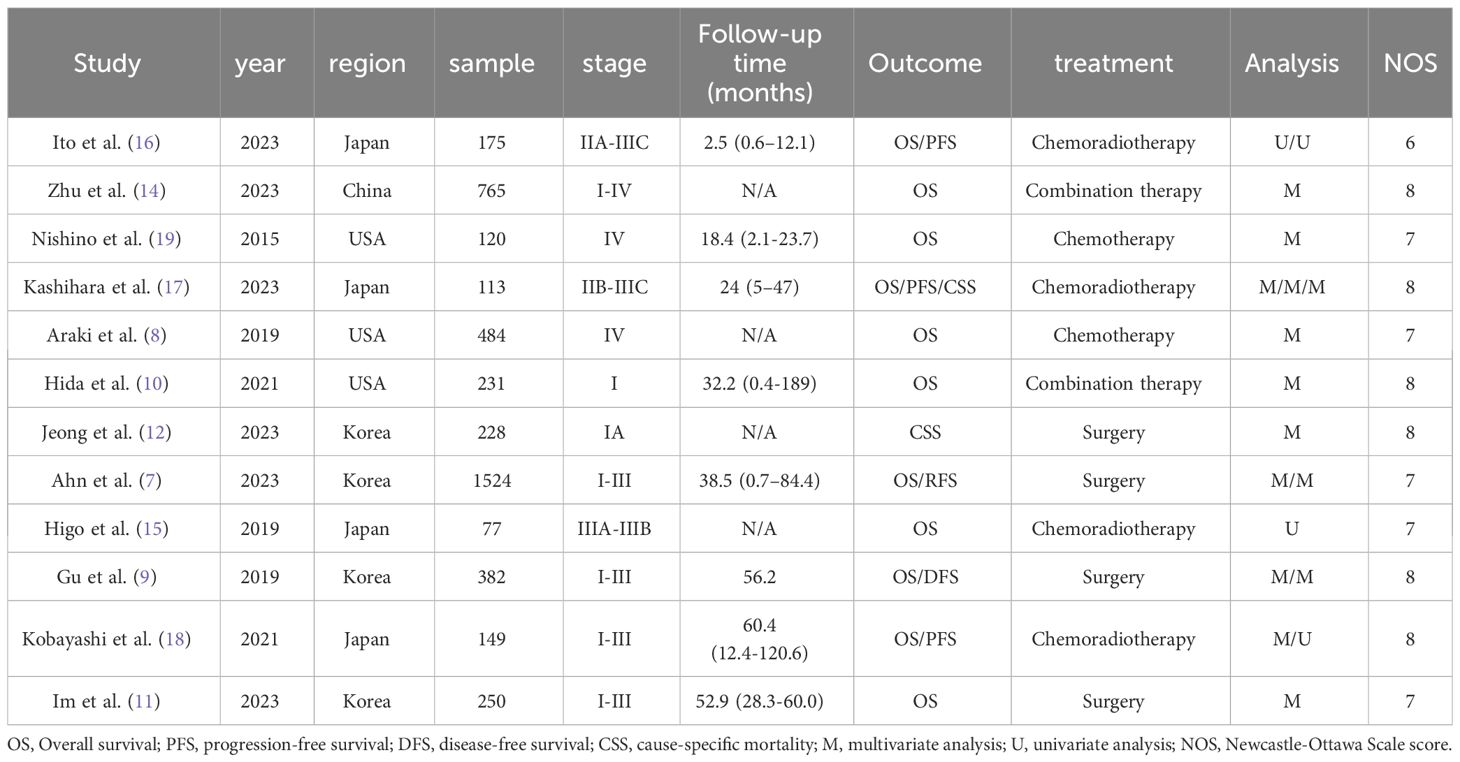
The main focus of data extraction was on the author, year of publication, region of study, design of the study, period of study, size of the sample, ILA incidence, types of cancer, treatment methods, duration of follow-up, and study outcomes. The quality of observational studies was assessed using the Newcastle-Ottawa Scale (NOS) score (20). Literature was considered high-quality if it scored above six. Two authors (GXT and TXL) independently double-checked all of the aforementioned steps, and any discrepancies were resolved by a third author (YSZ).
2.4 Statistical methods
Stata 16.0 was utilized for the statistical analysis. OS is determined as the period from the initiation of therapy until decease, irrespective of the reason. A fatality linked to lung cancer or a respiratory ailment is counted as a cause-specific decease, and the period from the initiation of therapy until a cause-specific decease is designated as CSS. Within this meta-analysis, DFS and RFS in the included studies were jointly denoted as DFS, which is characterized as the duration from surgery until the reappearance of the disease or decease from any cause (event). The combined HR and 95%CI were utilized to assess the connection between ILA and prognosis in lung cancer patients. Multivariate HR and 95% CI data were directly obtained from the study. In cases where multivariate HR was not provided, univariate HR data was extracted. An HR greater than 1 (with a 95%CI not overlapping 1) suggested that ILA was linked to a poorer prognosis in patients, rather than the opposite being true. In terms of examining the relationship between ILA and clinical and pathological characteristics of lung cancer patients, the combined odds ratio (OR) and 95%CI were employed. An OR greater than or less than 1 (with a 95%CI not overlapping 1) indicated a correlation between ILA and clinicopathological features in lung cancer patients, and not the contrary. The chisquared test was employed to calculate the statistical heterogeneity. A random effect model was applied when P<0.1 and I2>50% indicated high heterogeneity; otherwise, the fixed effect model was utilized. Egger’s tests were conducted to assess publication bias, and in cases of significant bias, the trim-and-fill method was applied to adjust the results. Sensitivity analysis was carried out to evaluate result stability, with each study being excluded independently.
3 Results
3.1 Characteristics of studies
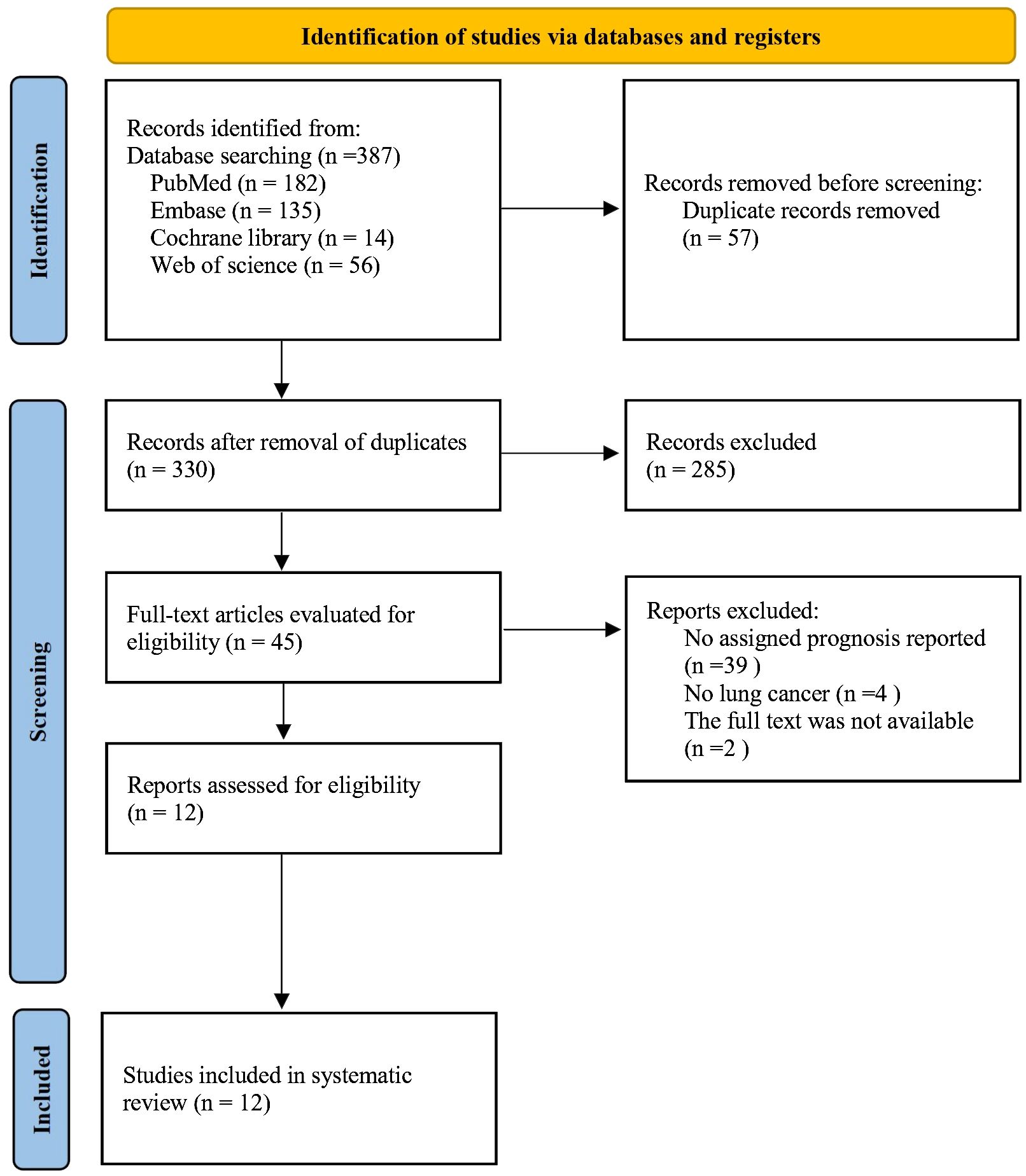
After the initial search, 57 duplicate studies were removed. Then there were 285 articles deleted after carefully reading the titles and abstracts. Later, the full texts of the remaining 45 articles were further assessed.
12 articles (7–18) involving 4416 patients were ultimately included. The PRISMA flow diagram is provided in Figure 1. The main characteristics of the studies included are shown in Table 1. All the 12 studies were retrospective. Four studies were from Korea and Japan, respectively, three studies were carried out in USA, the remaining one in China. Ten studies involved the treatment of NSCLC, one studies included small-cell lung cancer (SCLC), and one study had both NSCLC and SCLC arms. The sample sizes varied from 73 to 1,524. The incidence rate of ILA ranged from 3.93% to 37.87%, with an average incidence of 14.77%. The NOS scores for the 12 articles ranged from 6 to 8, which represented a low risk of bias (Table 1).
3.2 ILA and overall survival
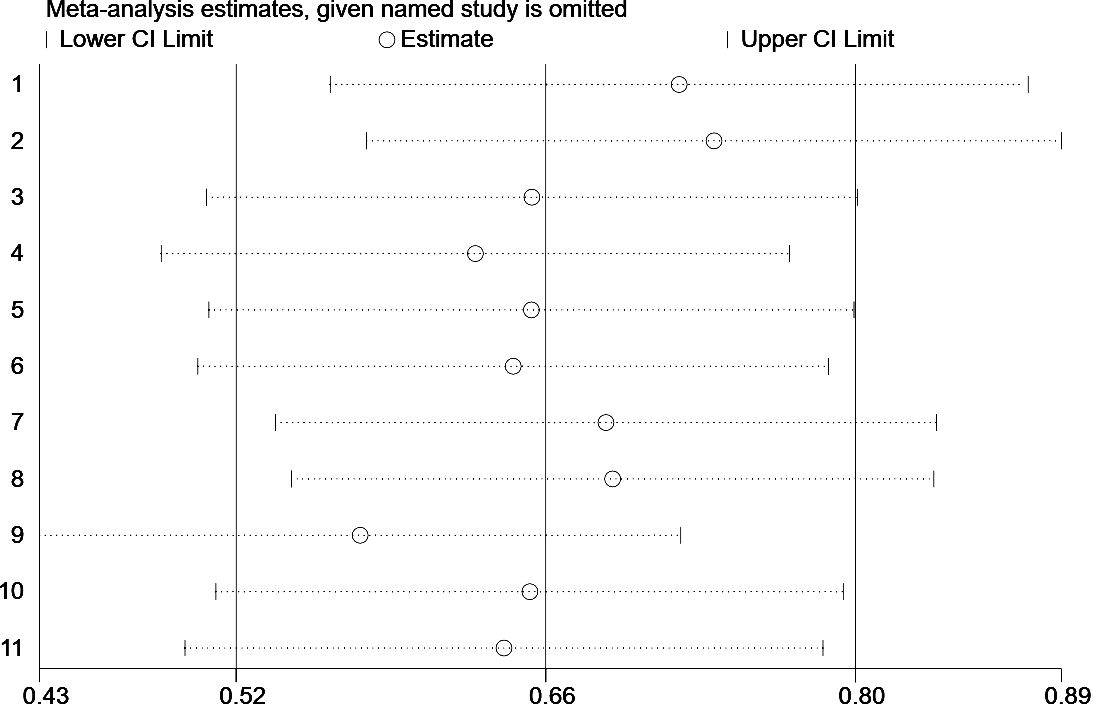
In total, 11 articles (7–11, 13–18) involving 4188 patients explored the association between ILA and OS in lung cancer patients. The pooled HR was (n=11; HR=2.22; 95% CI=1.68-2.95; P<0.001; I2 = 72.0%; Ph<0.001), implying that the lung cancer patients with ILA raised death risk by 122% compared to those without (Figure 2).
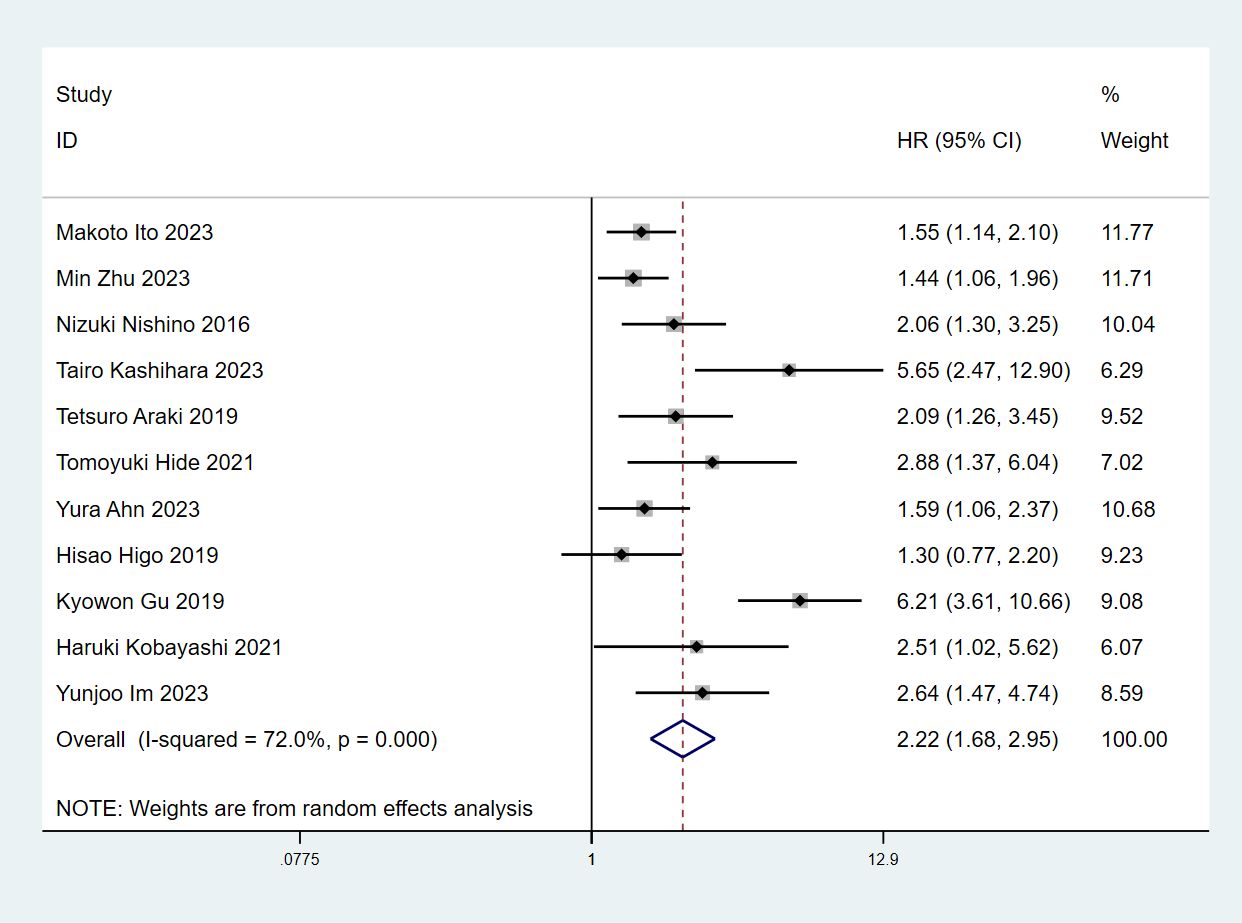
Figure 2 Forest plots of the association between ILA and the overall survival of patients with lung cancer.
Since there was significant heterogeneity, a random effects model was used (I2 = 73.9%, P<0.001). We then conducted subgroup analyses based on study region, sample size, cancer types, treatment and analysis. The results were consistent with the above findings (Table 2, Supplementary Figures S1–5).
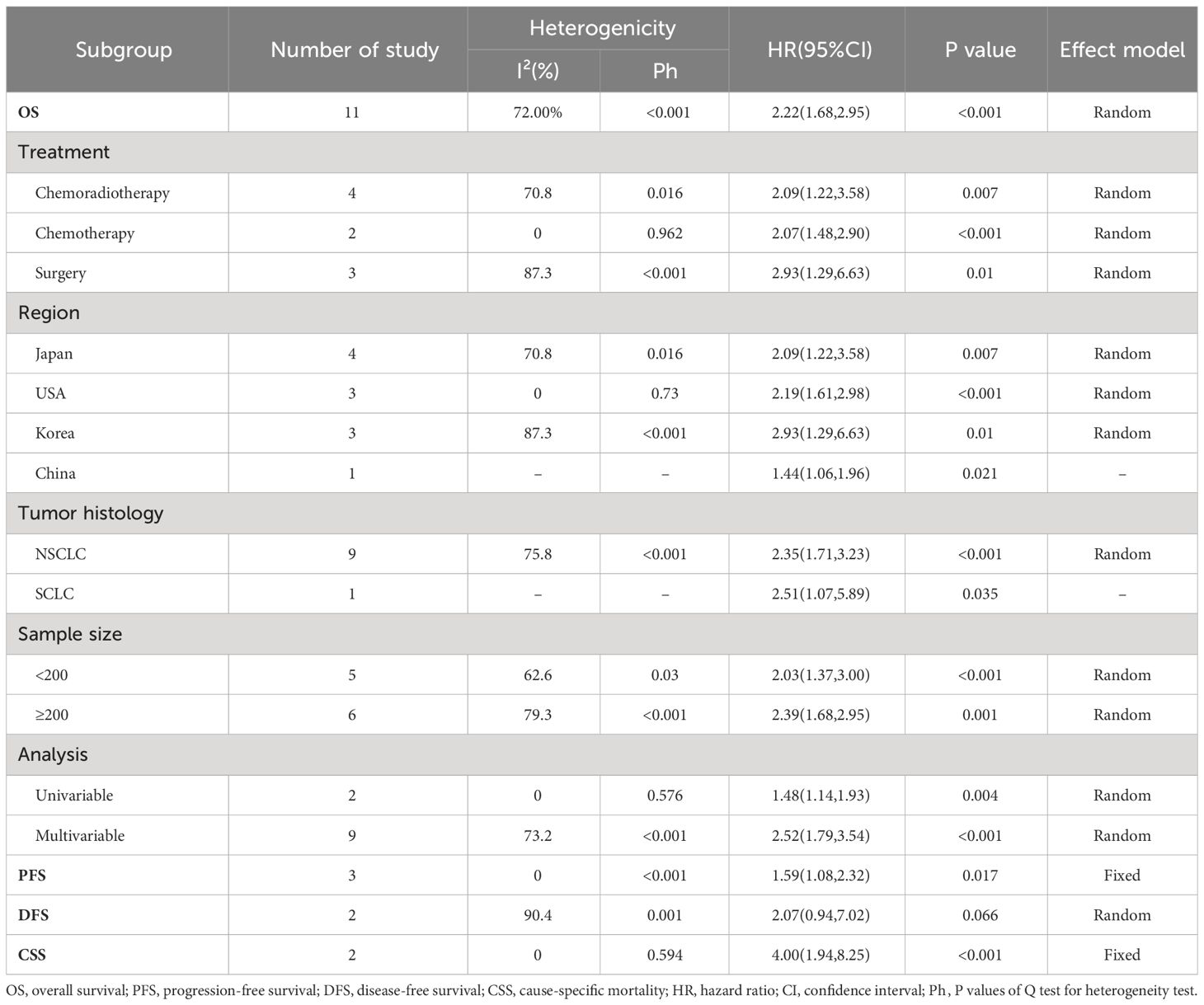
Table 2 Subgroup analysis of the association between ILA and overall survival in patients with lung cancer.
3.3 ILA and progression-free survival
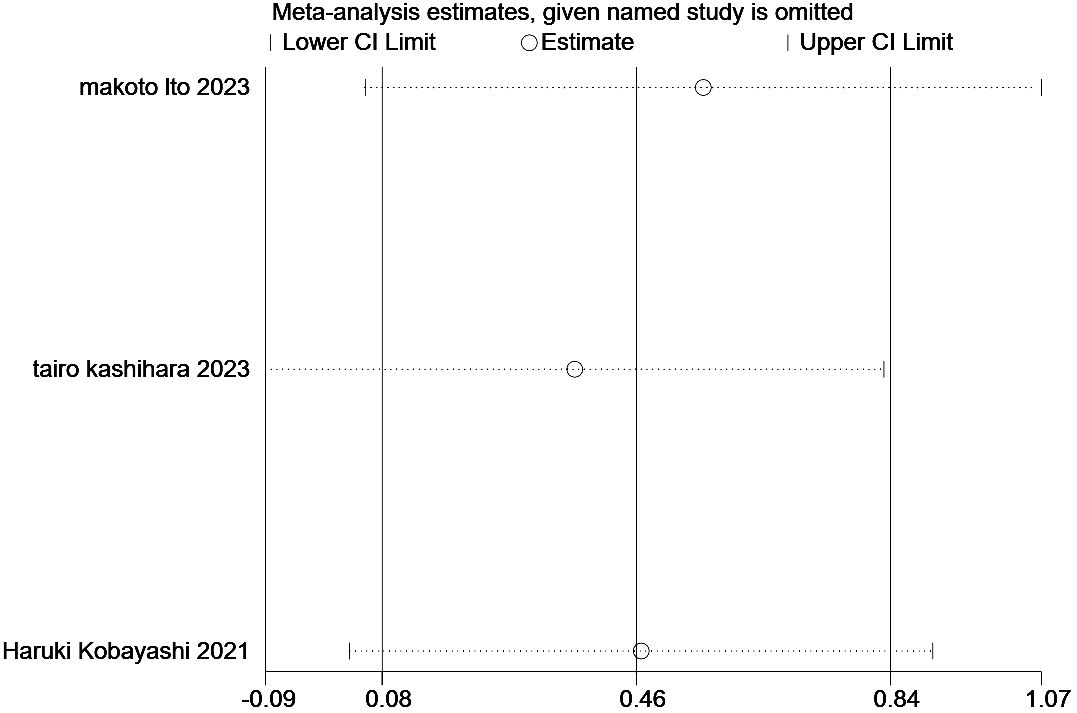
The relationship between ILA and PFS was examined using prognostic data from 3 studies (16–18) involving 355 participants.A combined analysis demonstrated that patients with ILA was significantly correlated with shortened PFS (HR=1.59; 95% CI=1.08-2.32; P=0.017) than those without, with no significant heterogeneity identified between studies (I2 = 0%; P=0.772) (Figure 3).
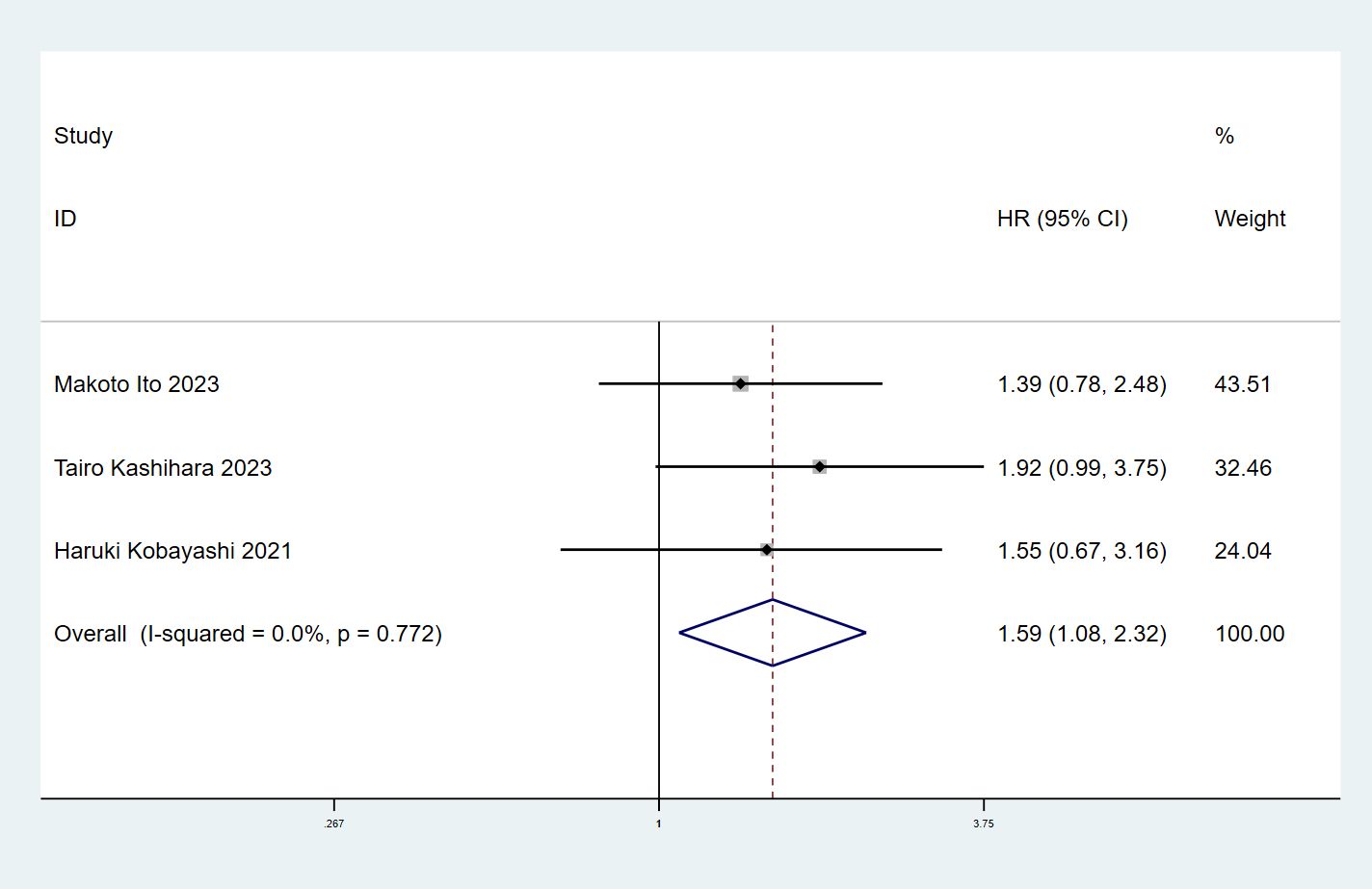
Figure 3 Forest plots of the association between ILA and the progression-free survival of patients with lung cancer.
3.4 ILA and disease-free survival
There were 2 studies (7, 9) with a total of 1906 patients investigating the correlation between ILA and DFS. Significant heterogeneity was observed in the included studies (I2 = 90.4%, P = 0.001), so a random effects model was used. Interestingly, the results indicated that the combined ILA patients had no significant association with DFS (HR: 2.07, 95% CI: 0.94–7.02, P = 0.066) (Figure 4).
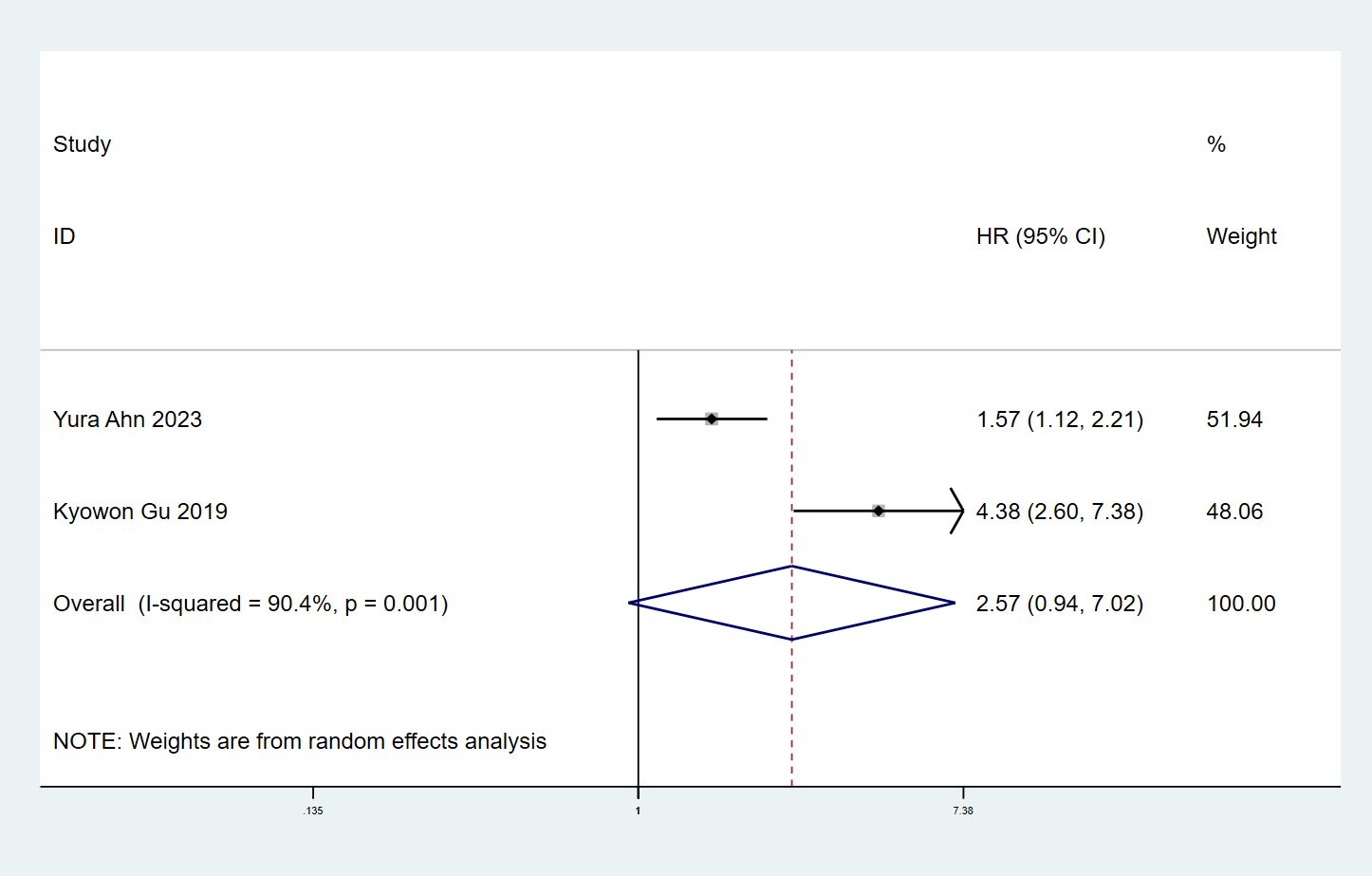
Figure 4 Forest plots of the association between ILA and the disease-free survival of patients with lung cancer.
3.5 ILA and cancer-specific survival
The association between ILA and CSS in cancer patients was explored in two articles (12, 17) with 341 individuals. We found significant correlation between ILA and CSS in lung cancer patients (HR=4.00, 95% CI: 1.94-8.25, P<0.001) using a fixed effect model (I2 = 0%, P= 0.594) (Figure 5).
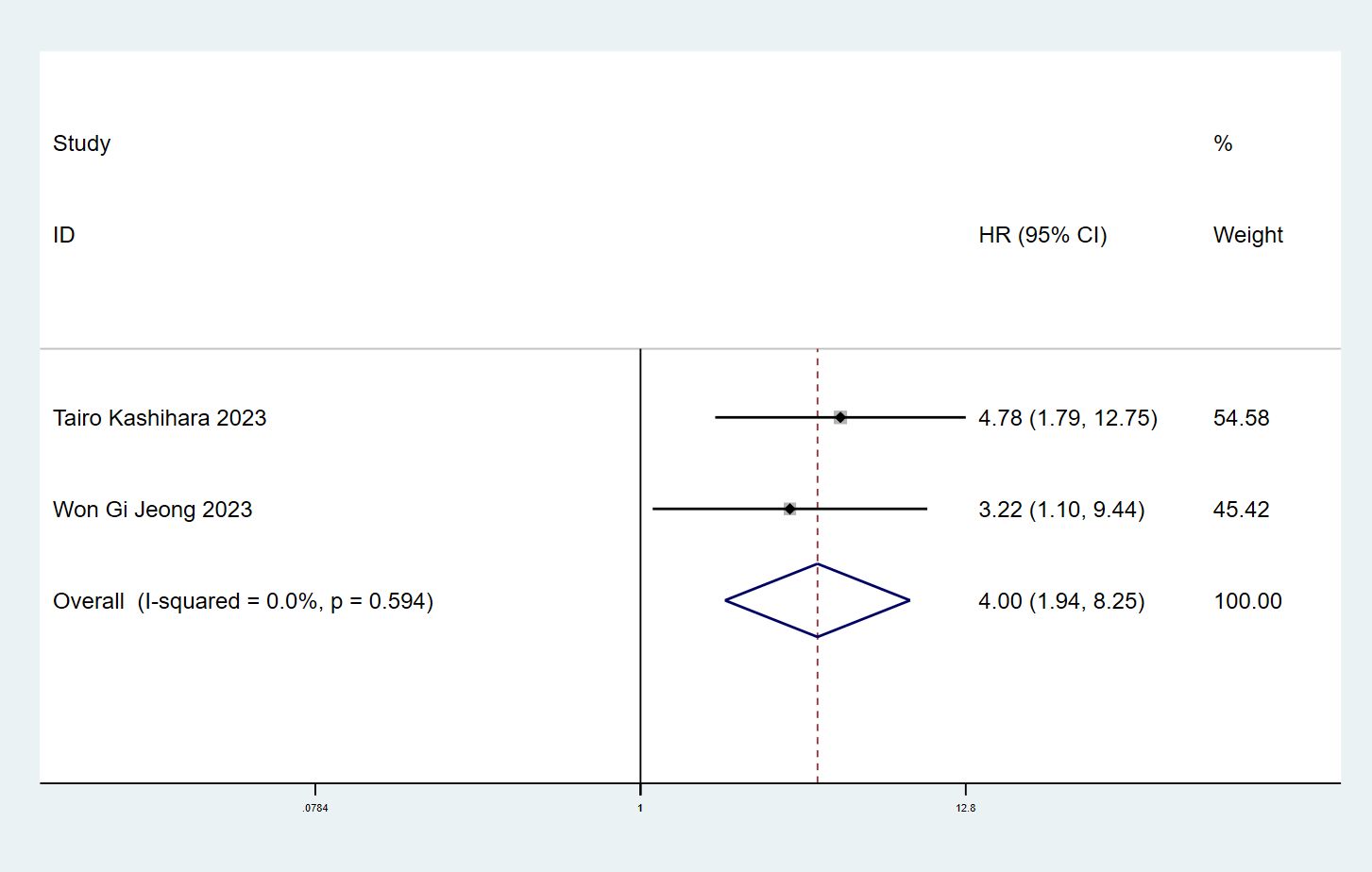
Figure 5 Forest plots of the association between ILA and the cause-specific mortality of patients with lung cancer.
3.6 Association of ILA with clinicopathological characteristics
Of the articles included for analysis in the present study, a total of 8 studies, involving 2330 patients with lung cancer, reported a relationship between the ILA and clinicopathological factors of lung cancer. As shown by the combined results in Table 3, patients with ILA were significantly associated with male (OR= 2.43; 95% CI= 1.48-3.98; P<0.001), smoking history (OR= 2.11; 95% CI=1.37-3.25; P<0.001), advanced age (OR=2.50; 95% CI= 1.56-4.03; P<0.001), squamous carcinoma (OR=0.42; 95% CI=0.24-0.71; P=0.01), and EGFR mutation (OR=0.50; 95% CI=0.32-0.78; P=0.002). There was no significant correlation, however, between the ILA patients and Race (OR=1.92; 95% CI= 0.51-7.18; P= 0.334), Stage (OR= 0.93; 95% CI= 0.65-1.34; P= 0.708), ALK (OR= 0.30; 95% CI= 0.11-1.38; P= 0.144) (Supplementary Figures S6–13).
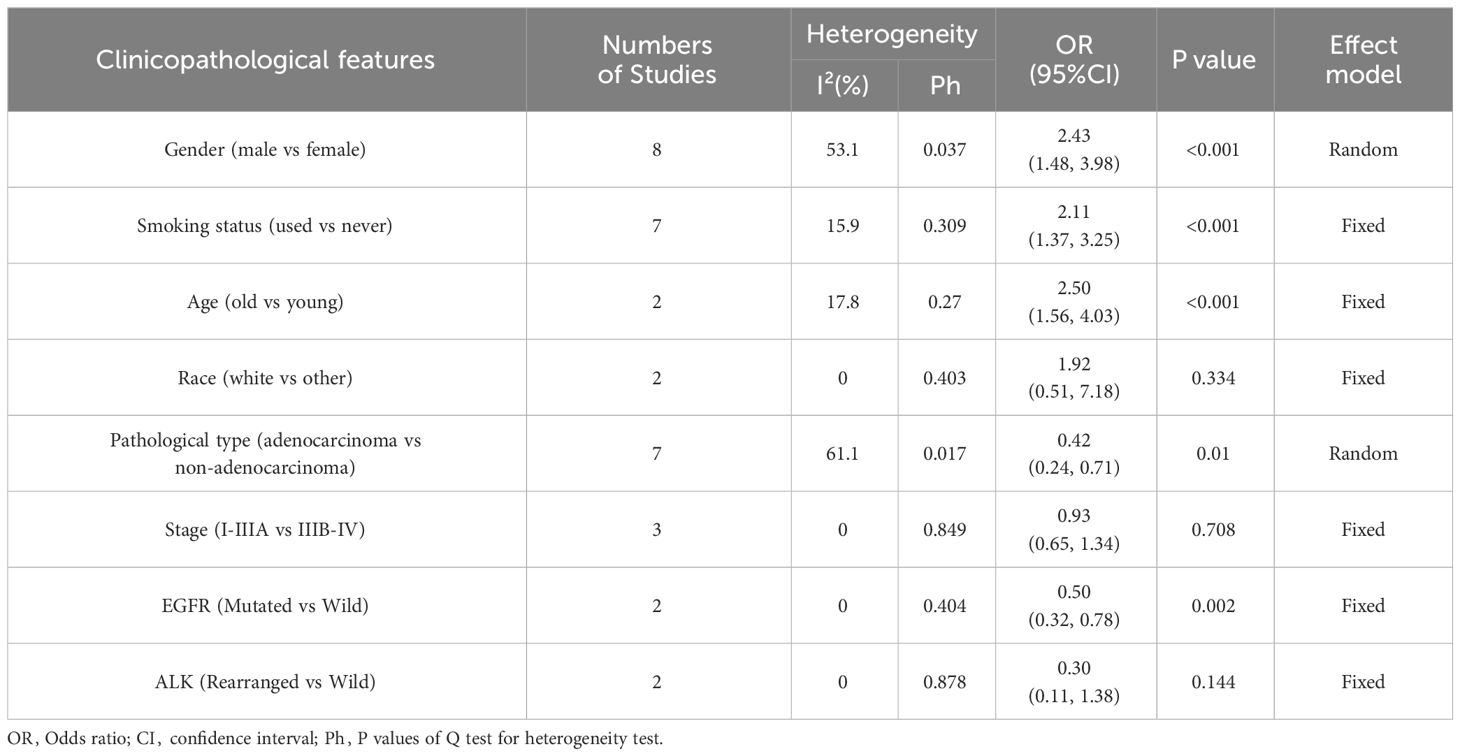
Table 3 The correlation between ILA and clinicopathological characteristics in patients with lung cancer.
3.7 Sensitivity analysis
In the present study, we performed a sensitivity analysis on the relationship between the ILA and OS (Figure 6) and PFS (Figure 7), through which we determined that the significance of ILA in predicting OS and PFS in patients with lung cancer did not change after eliminating any single article.
3.8 Publication bias
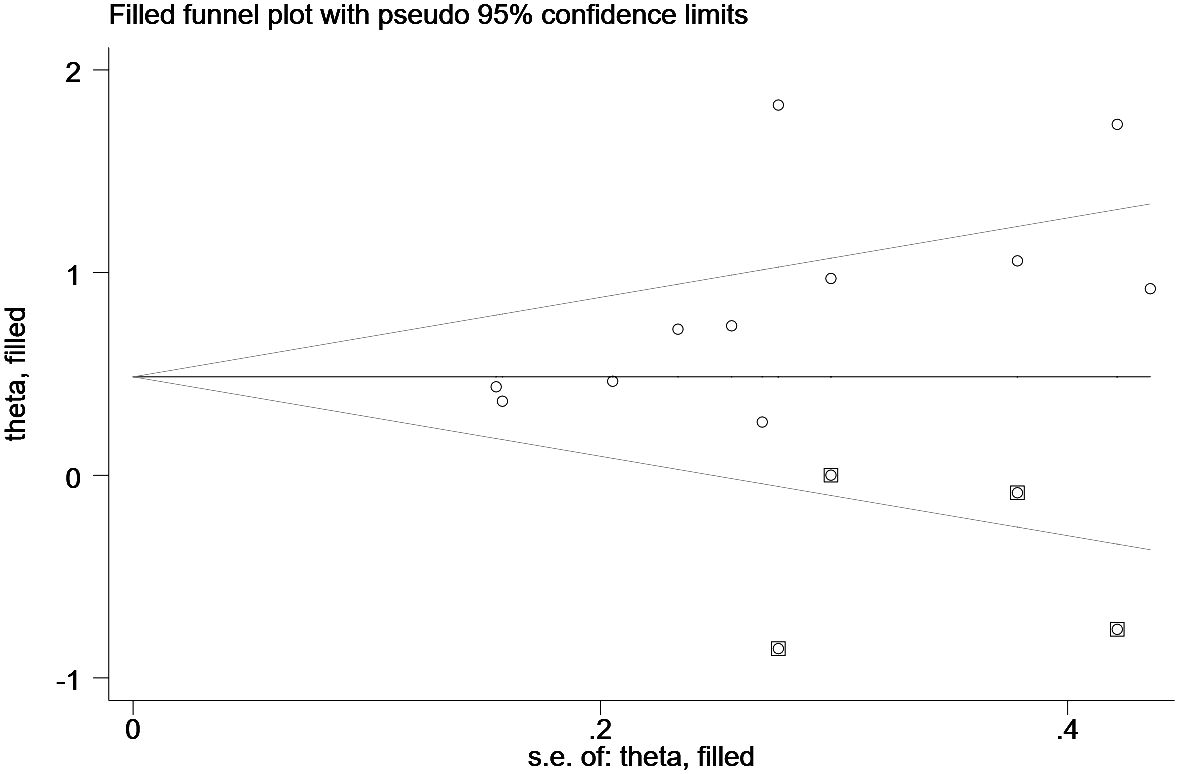
Funnel plot and Egger’s test evaluated the inclusion for publication bias. For the Meta-analysis of the relationship between ILA and OS, the meta-analysis was biased (P = 0.04) (Figure 8); Next, the trim and fill method was utilized to calculate the number of missing studies in OS. By factoring in the missing hypothesis studies, the combined HR of OS was recalculated but was not substantially different. As a result, the publication bias had little impact, and the outcome was stable (Figure 9).
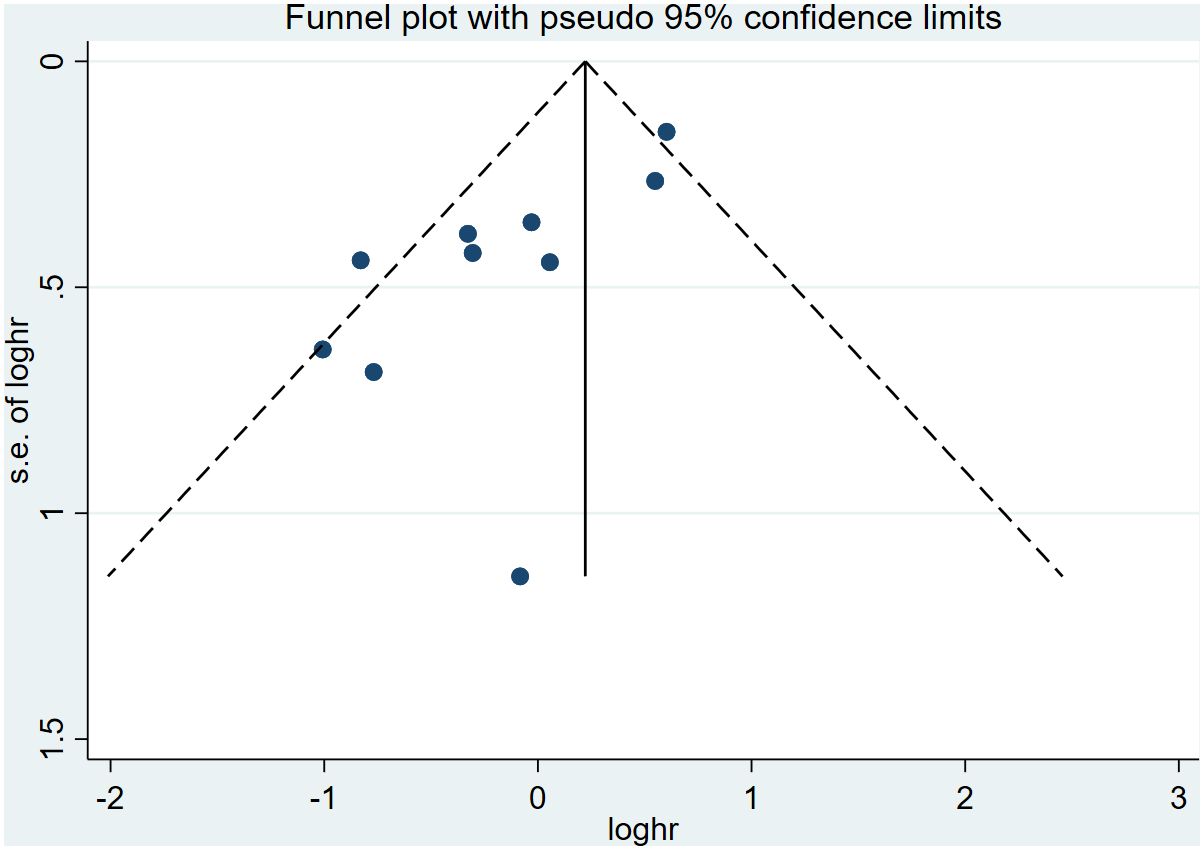
Figure 8 Funnel plots showing the publication bias of the included studies in the overall survival analysis.
4 Discussion
Washko et al. (21, 22) first introduced the concept of interstitial pulmonary abnormalities (ILA) to assess interstitial changes in the lungs using high-resolution computed tomography (HRCT) in 2010. Subsequent research has shown that ILA is linked to the prognosis of lung cancer. Grantorser et al. (6) conducted meta-analysis revealed that ILA is associated with a higher risk of lung cancer and increased mortality among ILA patients. Additionally, Tepatel et al. (23) conducted an extensive cohort study demonstrated that ILA is linked to the progression of interstitial lung disease (ILD) and postoperative complications in lung cancer patients. These findings suggest that ILA could be a valuable marker for predicting poor outcomes in lung cancer and may indicate an increased risk of the disease, serving as a prognostic indicator.
In this meta-analysis, we aimed to assess the prognostic significance of ILA in lung cancer. The study included 12 research articles with a total of 4248 cases. Our findings showed that individuals with ILA face a heightened risk of OS, PFS, and CSS along with poorer combined HRs. Our meta-analysis did not uncover the predictive significance of ILA for DFS due to the constraints resulting from a limited sample size, leading to decreased statistical efficiency. This issue may stem from inadequate statistical power, given that only 2 studies examined DFS in relation to ILA. Additional investigations are essential to explore the predictive value of ILA for DFS in lung cancer patients and to aid in risk assessment for disease management and survival. Subsequent large-scale prospective studies are necessary to confirm our conclusions. The correlation between ILA and OS was consistent across various factors such as treatment approaches, geographical regions, tumor types, sample sizes, and analytical methods. However, Higo et al. (15) reported no significant difference in 2-year OS between lung cancer patients with ILA and those without ILA, possibly due to the previous ambiguity in defining ILA. On the other hand, Kobayashi et al. (18) Ito et al. (16) and Kashihara et al. (17) discovered that ILA was not an independent prognostic factor for PFS in lung cancer patients undergoing chemoradiotherapy. Interestingly, combined results indicated that patients with ILA had a poorer PFS (HR =1.55, 95% CI: 0.67-3.16; P=0.258). Several potential explanations for these conflicting outcomes have been suggested, such as the inclusion of only lung cancer patients receiving chemoradiotherapy in these studies, thereby excluding some ILA patients in good health, as well as the limited sample size of the studies.
Moreover, ILA was found to be significantly associated with such clinicopathologic features as male, advanced age, smoking history, squamous cell carcinoma, and EGFR mutated, which suggests that male, advanced age, smoking history, squamous cell carcinoma, and EGFR mutated patients with lung cancer tend to suffer from ILA. The content of these risk factors consists of clinical information readily available in daily clinical practice. It will help develop treatment and prevention strategies for this hidden disease and could provide very useful information for clinicians to improve outcomes for lung cancer patients.
However, the mechanism between ILA and the prognosis of lung cancer has not been elucidated. Previous studies suggest that ILA may represent an early stage of pulmonary fibrosis (PF) in unknown interstitial lung disease (ILD) (4). Research has shown that fibrotic lung disease and lung cancer may share common pathobiological processes, such as genetic alterations (24), epigenetic similarities (including DNA methylation and altered mRNA expression profiles) (24), as well as abnormalities in intercellular communication, alterations in intracellular signaling pathways (25), increased programmed cell death-ligand 1 (PD-L1) signaling (26) and overexpression of transforming growth factor (TGF)-β molecules (27, 28). These factors create a conducive microenvironment for cancer growth and invasion, thus indicating that patients with ILA are at a higher risk for a poor prognosis.
However, it is important to acknowledge the limitations of this meta-analysis. Firstly, all the studies reviewed were retrospective in nature, which raises concerns about potential bias. Secondly, the studies we included were mainly from the United States and Asia, and there was a lack of studies on Caucasian and African populations. Thirdly, the limited number of eligible studies prevented a thorough analysis of potential sources of heterogeneity. Lastly, publication bias could not be completely ruled out, as studies with positive results may have a higher likelihood of being published.
In summary, ILA lung cancer patients are associated with poorer OS, RFS and CSS, so ILA can be used as a potential predictor of lung cancer prognosis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
XT: Conceptualization, Data curation, Resources, Software, Visualization, Writing – original draft, Writing – review & editing, Methodology. YS: Conceptualization, Data curation, Investigation, Validation, Writing – original draft, Writing – review & editing. XG: Data curation, Resources, Software, Writing – original draft, Methodology, Visualization. SY: Conceptualization, Data curation, Investigation, Validation, Writing – original draft. WZ: Formal analysis, Resources, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1397246/full#supplementary-material
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. (2021) 71:7–33. doi: 10.3322/caac.21654
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Hatabu H, Hunninghake GM, Richeldi L, Brown KK, Wells AU, Remy-Jardin M, et al. Interstitial lung abnormalities detected incidentally on CT: a Position Paper from the Fleischner Society. Lancet Respir Med. (2020) 8:726–37. doi: 10.1016/s2213-2600(20)30168-5
4. Abu Qubo A, Numan J, Snijder J, Padilla M, Austin JHM, Capaccione KM, et al. Idiopathic pulmonary fibrosis and lung cancer: future directions and challenges. Breathe (Sheff). (2022) 18:220147. doi: 10.1183/20734735.0147-2022
5. Whittaker Brown SA, Padilla M, Mhango G, Powell C, Salvatore M, Henschke C, et al. Interstitial lung abnormalities and lung cancer risk in the national lung screening trial. Chest. (2019) 156:1195–203. doi: 10.1016/j.chest.2019.06.041
6. Grant-Orser A, Min B, Elmrayed S, Podolanczuk AJ, Johannson KA. Prevalence, risk factors, and outcomes of adult interstitial lung abnormalities: A systematic review and meta-analysis. Am J Respir Crit Care Med. (2023) 208:695–708. doi: 10.1164/rccm.202302-0271OC
7. Ahn Y, Lee SM, Choi S, Lee JS, Choe J, Do KH, et al. Automated CT quantification of interstitial lung abnormality and interstitial lung disease according to the Fleischner Society in patients with resectable lung cancer: prognostic significance. Eur Radiol. (2023) 33:8251–62. doi: 10.1007/s00330-023-09783-x
8. Araki T, Dahlberg SE, Hida T, Lydon CA, Rabin MS, Hatabu H, et al. Interstitial lung abnormality in stage IV non-small cell lung cancer: A validation study for the association with poor clinical outcome. Eur J Radiol Open. (2019) 6:128–31. doi: 10.1016/j.ejro.2019.03.003
9. Gu K, Lee HY, Lee K, Choi JY, Woo SY, Sohn I, et al. Integrated evaluation of clinical, pathological and radiological prognostic factors in squamous cell carcinoma of the lung. PloS One. (2019) 14:e0223298. doi: 10.1371/journal.pone.0223298
10. Hida T, Hata A, Lu J, Valtchinov VI, Hino T, Nishino M, et al. Interstitial lung abnormalities in patients with stage I non-small cell lung cancer are associated with shorter overall survival: the Boston lung cancer study. Cancer Imaging. (2021) 21:14. doi: 10.1186/s40644-021-00383-w
11. Im Y, Chung MP, Lee KS, Han J, Chung MJ, Kim HK, et al. Impact of interstitial lung abnormalities on postoperative pulmonary complications and survival of lung cancer. Thorax. (2023) 78:183–90. doi: 10.1136/thoraxjnl-2021-218055
12. Jeong WG, Kim YH. Survival impact of fibrotic interstitial lung abnormalities in resected stage IA non-small cell lung cancer. Br J Radiol. (2023) 96:20220812. doi: 10.1259/bjr.20220812
13. Nishino M, Cardarella S, Dahlberg SE, Araki T, Lydon C, Jackman DM, et al. Interstitial lung abnormalities in treatment-naïve advanced non-small-cell lung cancer patients are associated with shorter survival. Eur J Radiol. (2015) 84:998–1004. doi: 10.1016/j.ejrad.2015.01.021
14. Zhu M, Yi J, Su Y, Zhang Y, Gao Y, Xu X, et al. Newly diagnosed non-small cell lung cancer with interstitial lung abnormality: Prevalence, characteristics, and prognosis. Thorac Cancer. (2023) 14:1874–82. doi: 10.1111/1759-7714.14935
15. Higo H, Kubo T, Makimoto S, Makimoto G, Ihara H, Masaoka Y, et al. Chemoradiotherapy for locally advanced lung cancer patients with interstitial lung abnormalities. Jpn J Clin Oncol. (2019) 49:458–64. doi: 10.1093/jjco/hyz016
16. Ito M, Katano T, Okada H, Sakuragi A, Minami Y, Abe S, et al. Subpleural fibrotic interstitial lung abnormalities are implicated in non-small cell lung cancer radiotherapy outcomes. Radiol Oncol. (2023) 57:229–38. doi: 10.2478/raon-2023-0018
17. Kashihara T, Nakayama Y, Okuma K, Takahashi A, Kaneda T, Katagiri M, et al. Impact of interstitial lung abnormality on survival after adjuvant durvalumab with chemoradiotherapy for locally advanced non-small cell lung cancer. Radiother Oncol. (2023) 180:109454. doi: 10.1016/j.radonc.2022.109454
18. Kobayashi H, Wakuda K, Naito T, Mamesaya N, Omori S, Ono A, et al. Chemoradiotherapy for limited-stage small-cell lung cancer and interstitial lung abnormalities. Radiat Oncol. (2021) 16:52. doi: 10.1186/s13014-021-01780-y
19. Page MJ, Mckenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. (2021) 372:n71. doi: 10.1136/bmj.n71
20. Cook DA, Reed DA. Appraising the quality of medical education research methods: the Medical Education Research Study Quality Instrument and the Newcastle-Ottawa Scale-Education. Acad Med. (2015) 90:1067–76. doi: 10.1097/acm.0000000000000786
21. Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, et al. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. (2011) 364:897–906. doi: 10.1056/NEJMoa1007285
22. Washko GR, Lynch DA, Matsuoka S, Ross JC, Umeoka S, Diaz A, et al. Identification of early interstitial lung disease in smokers from the COPDGene Study. Acad Radiol. (2010) 17:48–53. doi: 10.1016/j.acra.2009.07.016
23. Patel AS, Miller E, Regis SM, Hunninghake GM, Price LL, Gawlik M, et al. Interstitial lung abnormalities in a large clinical lung cancer screening cohort: association with mortality and ILD diagnosis. Respir Res. (2023) 24:49. doi: 10.1186/s12931-023-02359-9
24. Kinoshita T, Goto T. Molecular mechanisms of pulmonary fibrogenesis and its progression to lung cancer: A review. Int J Mol Sci. (2019) 20:1461. doi: 10.3390/ijms20061461
25. Stella GM, Gentile A, Balderacchi A, Meloni F, Milan M, Benvenuti S. Ockham’s razor for the MET-driven invasive growth linking idiopathic pulmonary fibrosis and cancer. J Transl Med. (2016) 14:256. doi: 10.1186/s12967-016-1008-4
26. Guo X, Sunil C, Adeyanju O, Parker A, Huang S, Ikebe M, et al. PD-L1 mediates lung fibroblast to myofibroblast transition through Smad3 and β-catenin signaling pathways. Sci Rep. (2022) 12:3053. doi: 10.1038/s41598-022-07044-3
27. Saito A, Horie M, Micke P, Nagase T. The role of TGF-β Signaling in lung cancer associated with idiopathic pulmonary fibrosis. Int J Mol Sci. (2018) 19:3611. doi: 10.3390/ijms19113611
Keywords: lung cancer, meta-analysis, interstitial lung abnormality, prognosis, evidence-based medicine
Citation: Tang XL, Sun YB, Guo XT, Yang SZ and Zhang WP (2024) Prognostic impact of interstitial lung abnormalities in lung cancer: a systematic review and meta-analysis. Front. Oncol. 14:1397246. doi: 10.3389/fonc.2024.1397246
Received: 07 March 2024; Accepted: 24 April 2024;
Published: 10 May 2024.
Edited by:
Emilio Bria, Università Cattolica del Sacro Cuore, ItalyReviewed by:
Stephen J. Kuperberg, Wake Forest University, United StatesGeorgii Vasiukov, Vanderbilt University, United States
Copyright © 2024 Tang, Sun, Guo, Yang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen-Ping Zhang, emhhbmd3ZW5waW5nNjhAMTI2LmNvbQ==
 Xian-Liang Tang
Xian-Liang Tang Yin-Bo Sun1
Yin-Bo Sun1