- 1Department of Liver Disease and Digestive Interventional Radiology, National Clinical Research Center for Digestive Diseases and Xijing Hospital of Digestive Diseases, Air Force Medical University, Xi’an, Shaanxi, China
- 2Department of Liver Diseases and Interventional Radiology, Digestive Diseases Hospital, Xi’an International Medical Center Hospital, Northwestern University, Xi’an, Shaanxi, China
The progression pattern of tumors has an impact on the survival of patients with advanced hepatocellular carcinoma (HCC) and has been applied in the design of clinical trials for multiple second-line drugs. Previous research results have been contradictory, and the clinical impact of different progression patterns and their role in survival are still in question.
Purpose: The study aims to analyze the impact of different progression patterns and tumor burden size on survival of HCC patients, as well as their interactions, through a retrospective cohort study.
Patients and methods: The study involved 538 patients who had undergone treatment with sorafenib and had shown radiographic progression. The progression pattern was analyzed using Cox regression by including an interaction term between progression pattern and tumor burden, which was then visualized through a graphical analysis. Tumor burden was categorized into low, medium, and high subgroups based on the six-and-twelve criteria, allowing for an exploration of the effect of progression pattern on survival in different tumor burden situations.
Results: Compared to patients with only intrahepatic progression (NIH/IHG) with an overall survival (OS) of 14.1/19.9 months and post-progression survival (PPS) of 8.1/13.1 months respectively, patients with extrahepatic lesions (NEH/EHG) had worse overall and postprogressive survival (OS: 9.3/9.2 months, PPS: 4.9/5.1 months). The hazard ratio for extrahepatic progression (NEH/EHG) compared to intrahepatic progression (NIH/IHG) at low, medium, and high tumor burden were [HR 2.729, 95%CI 1.189-6.263], [HR 1.755, 95%CI 1.269-2.427], and [HR 1.117, 95%CI 0.832-1.499], respectively.
Conclusion: The study concluded that the interaction between the tumor progression patterns and tumor burden significantly affects the prognosis of HCC patients. As the tumor burden increases, the sensitivity of the patient’s risk of death to the progression pattern decreases. These findings are valuable in personalized treatment and trial design.
1 Introduction
Tumor progression is generally considered a discouraging event and is seen as a reflection of treatment failure that requires shifting to another treatment approach (1–3). However, in fact, progression may have different patterns, and the progression pattern is an important factor that affects the subsequent survival of liver cancer patients, distinguishing them according to the location of lesion progression (4–9). The 2020 trial design and endpoints in hepatocellular carcinoma: AASLD (American Association for the Study of Liver Diseases) consensus conference mentioned that the trial design of second-line treatment for advanced hepatocellular carcinoma should take into account the different progression patterns after first-line treatment and this has been applied to the clinical trial design of multiple second-line drugs (4, 7–10). Stratifying patients based on their progression patterns provides a validated predictor of treatment outcomes and has become a relevant parameter for informing patients, designing and analyzing clinical trials. In clinical trials, reasonable survival assumptions are key to determining the potential impact of new drugs on expected lifespan.
However, past related research has yielded conflicting results (Table 1) (11–17). In 2013, Maria Reig et al. for the first time explored the relationship between survival and progression patterns in patients who progressed after receiving sorafenib treatment and had good liver function and performance scores, and found that new extrahepatic lesions (NEH) were a poor prognostic factor, providing evidence that different types of progression should be considered when stratifying patients and emphasized the need for further analysis and clarification of the prognostic significance of different progression types (11). In 2015, Massimo Colombo et al. found that although NEH was an independent prognostic factor, the post-progression survival of patients with extrahepatic growth (EHG) was similar to that of NEH patients, with respective time periods of 3.2 and 3.1 months (12). In the sub-analysis of the SORAMIC trial in 2020, NEH was not a poor prognostic factor (with respective median survival times of 14.8 and 14.9 months compared to overall survival), and only lung metastasis was a poor prognostic factor (7.6 months) (15). In SIRT treatment, Bruno Sangro et al. found that the NEH or NIH progression patterns represented a poor prognosis (16). Of course, more research points to NEH as an independent prognostic factor.
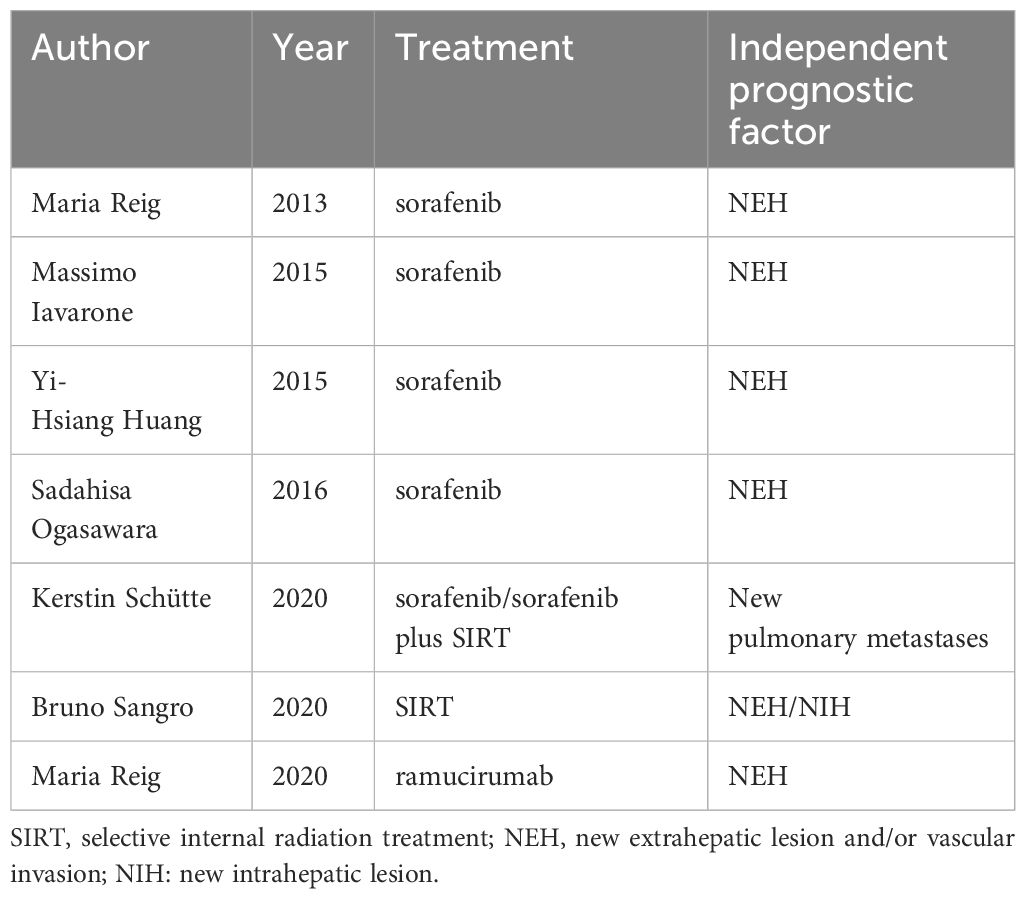
Table 1 Previous studies on the prognostic significance of progression patterns in patients with advanced hepatocellular carcinoma.
Advanced hepatocellular carcinoma is characterized by significant heterogeneity, with tumor burden, metastases, liver function reserve, and overall health status all significantly impacting patient survival time and quality of life (18). Moreover, studies have shown that even in patients with liver cancer accompanied by extrahepatic metastases, more than 80% of deaths are attributed to intrahepatic tumor progression, with liver failure resulting from late-stage progression being the main cause of death (19). Previous studies have also found that as tumor burden increases, the correlation between imaging response and survival rate after Transarterial chemoembolization (TACE) treatment weakens, possibly due to a balance between the positive impact of imaging response and the negative impact on liver function in patients with high tumor burden (20). Therefore, in the progression patterns of advanced liver cancer, can intrahepatic tumor burden affect the prognosis of progression patterns, with the negative impact on liver function resulting from the rapid deterioration in patients with high tumor burden being consistent with the negative impact of extrahepatic lesion progression?
Therefore, we propose the following hypothesis: In cases of low tumor burden, the appearance of extrahepatic lesion progression (NEH/EHG) signifies poor prognosis, but with increasing tumor burden, the sensitivity of extrahepatic lesion progression prognosis gradually decreases.
2 Patients and methods
2.1 Study population
This study retrospectively included 1048 patients with HCC who received sorafenib at our Center from January 2010 to October 2019, including patients with advanced HCC or those who were resistant to TACE therapy. Diagnosis was made by imaging or histological assessment according to American Association for the Study of Liver Diseases (AASLD) or European Association for the Study of Liver Diseases (EASL) guidelines. Exclusion criteria included: (1) accompanied by other malignant tumors; (2) Received any local treatment (ablation or TACE, etc.) within 4 weeks prior to the initiation of sorafenib; (3) Child-Pugh grade C patients; (4) Patients with ECOG physical status score over 2 points; (5) Patients who lacked progressive imaging until the last follow-up.
Patients received an initial dose of sorafenib of 400mg BID and the dose was adjusted in the event of intolerable adverse reactions. In the event of intolerable toxicity, the dose of sorafenib is reduced accordingly, or even temporary or permanent discontinuation of sorafenib therapy, but patients are usually encouraged to continue sorafenib therapy when adverse reactions can be tolerated.
2.2 Data collection
Commonly variables collected for the analysis were baseline demographic patient characteristics, radiological images and serum parameters.
Multiphase computed tomography (CT) or dynamic enhanced magnetic resonance imaging (MRI) imaging was performed before treatment initiation and every 8 weeks after treatment, and tumor response (complete response, partial response, disease stabilization, tumor progression) was evaluated according to modified response evaluation criteria in solid tumors (mRECIST) (21).
The progress time of imaging evaluation of patients was recorded, and the type of progress was registered: IHG: ≥20% increase in the size of intrahepatic lesions compared with baseline (intrahepatic growth); NIH: new intrahepatic lesion; EHG: ≥20% increase in the size of extrahepatic lesions compared with baseline (extrahepatic growth); NEH: new extrahepatic lesion and/or vascular invasion (11).
Tumor burden was categorized into low, medium, and high subgroups based on the six-and-twelve criteria (The sum of tumor numbers and maximum diameters was delimited by truncation values of six and twelve) (22).
The primary outcome points were overall survival and post-progression survival. Follow-up was conducted by a professional clinical follow-up team every 8 weeks until death or the last follow-up date or contact was lost. On October 9, 2021, a final follow-up was conducted and a final survival assessment was made for all patients. The procedures followed in this study conformed to the ethical guidelines of the Helsinki Declaration of 1975 and were approved by the Ethics Committee of Xijing Hospital (Xi’an, China). According to institutional guidelines, all patients signed a written informed consent for treatment and to provide their clinical data in subsequent research before receiving sorafenib therapy.
2.3 Statistical analysis
Quantitative variables are presented as means with standard deviation (SD) or medians with interquartile range median with interquartile range, and were compared by Student’s t test or Mann-Whitney U test. Categorical variables were presented as absolute and relative frequencies and compared by Chi-square test or Fisher’s exact test. The interaction multiplicative terms of progression pattern and tumor load were included in COX regression, and the interaction was analyzed by drawing viewable views. Survival curves were plotted using the Kaplan-Meier (KM) method. For all analyses, a corresponding p value less than 0.05 was considered statistically significant. All calculations were performed with SPSS v22 (SPSS Inc., Chicago, IL) and R version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
3 Results
3.1 Baseline characteristics
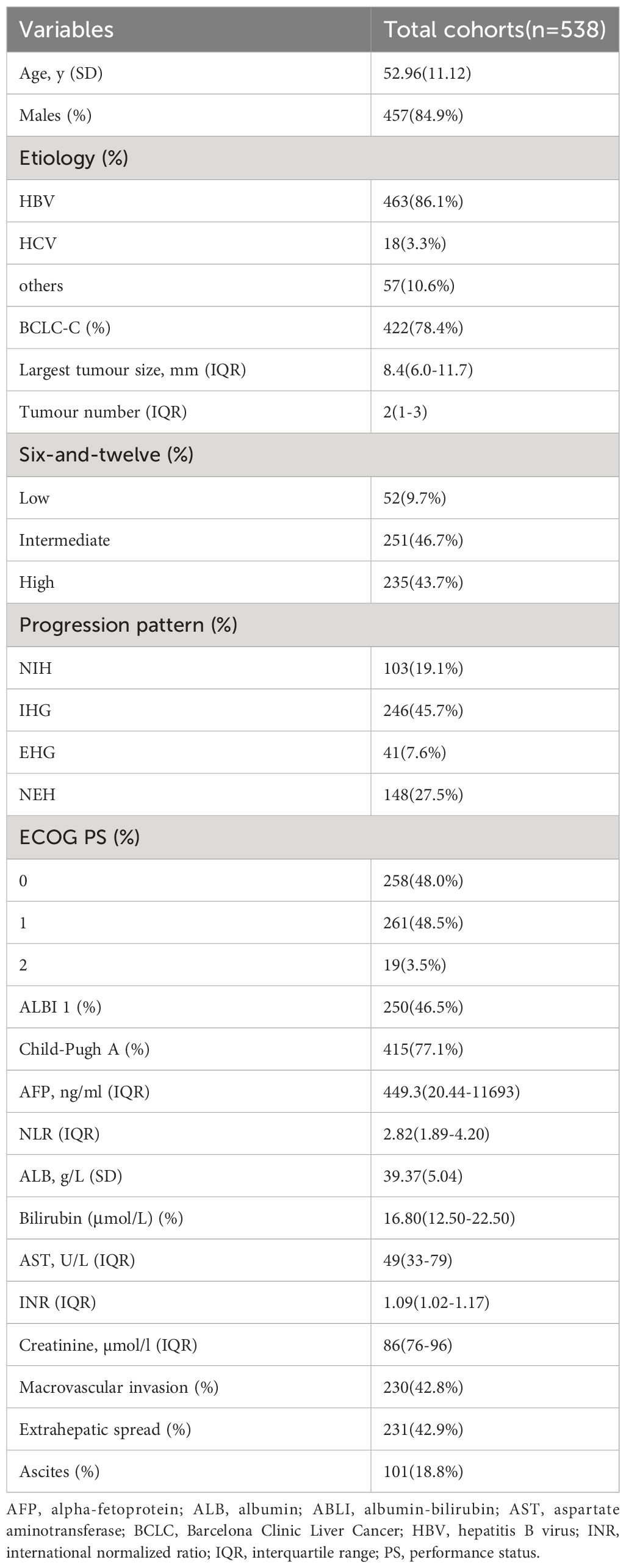
A total of 538 patients with hepatocellular carcinoma that progressed after receiving sorafenib were included in this study (Figure 1). Chronic hepatitis B virus infection was the main cause in 463 patients (86.1%). The patients were mainly with medium-high tumor burden: 52 patients (9.7%) in low-load group, 251 patients (46.7%) in medium-load group, and 235 patients (43.7%) in high-load group. In the mode of progression, there were 246 cases (45.7%) of intrahepatic lesions, 148 cases (27.5%) of new extrahepatic lesions, 103 cases (19.1%) of new intrahepatic lesions, and 41 cases (7.6%) of extrahepatic lesions. The baseline data of all patients are shown in Table 2.
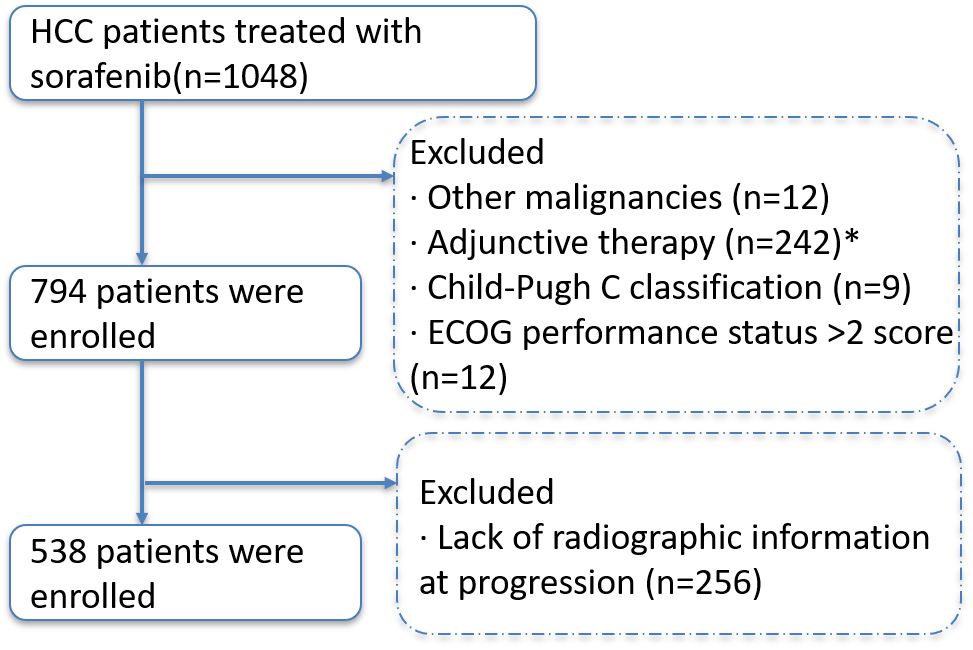
Figure 1 Selection flow diagram. Comment: * Received any local treatment (ablation or TACE, etc.) within 4 weeks prior to the initiation of sorafenib.
3.2 Survival analysis
The median follow-up was 11.8 months (IQR 6.2–24.7 months). In the general population, the median survival of patients with different progression modes (NEH, EHG, NIH, IHG) was 9.3 (95%CI 8.1-11.6) months, 9.2 (95%CI 6.2-11.8) months, 14.1 (95%CI 11.3-16.6) months, and 19.6 (95%CI 15.8-24.8) months, respectively (p<0.001); The post-progression survival was 4.9 (95%CI 3.8-6.4) months, 5.1 (95%CI 4.2-7.5) months, 8.1 (95%CI 7.0-10.1) months, 13.1 (95%CI 9.5-16.5) months, respectively (p<0.001) (Figure 2).
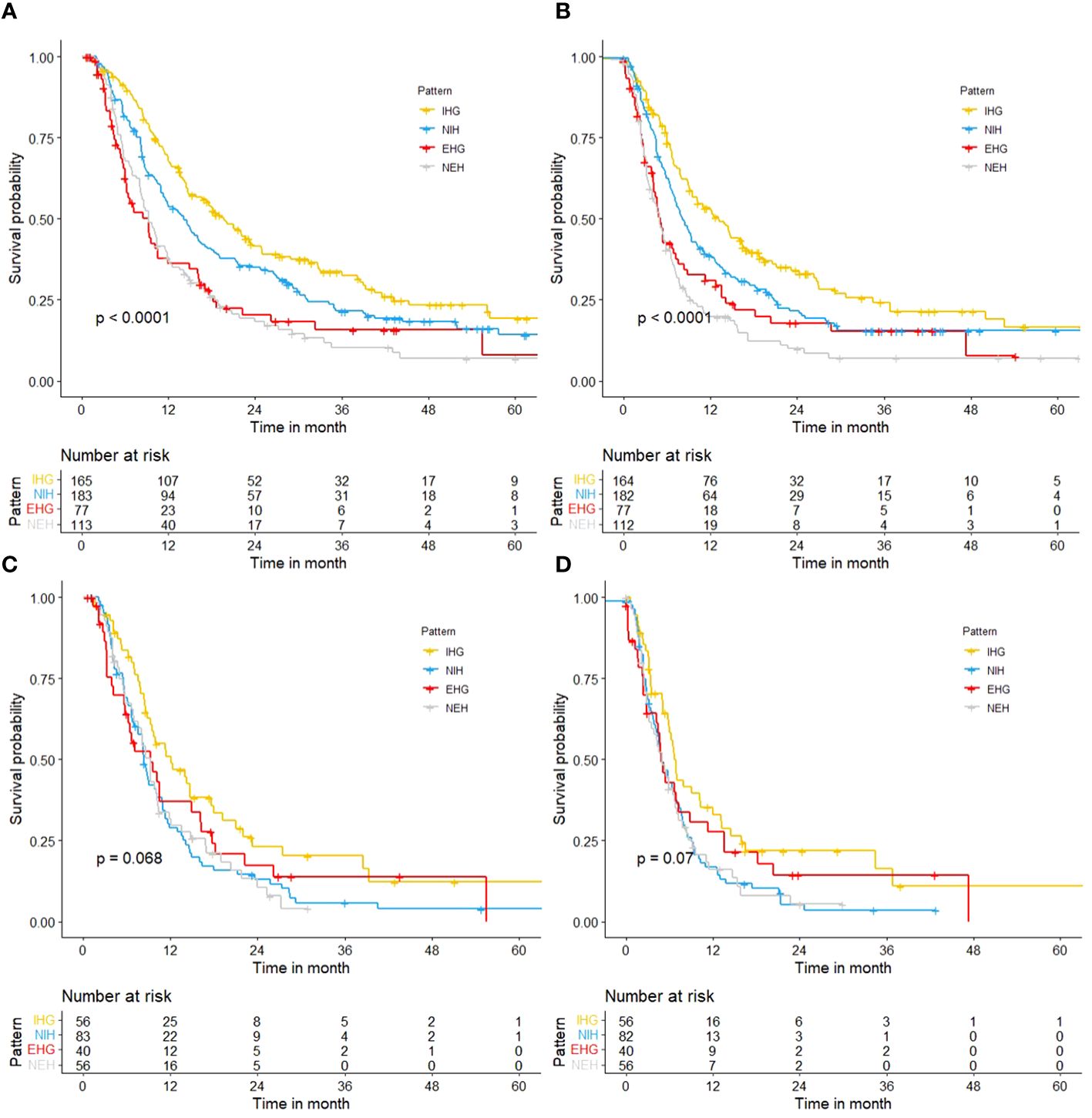
Figure 2 Kaplan-Meier curves. (A) OS of different progression patterns in total cohort; (B) PPS of different progression patterns in total cohort; (C) OS of different progression patterns in patients with a high tumor burden; (D) PPS of different progression patterns in patients with a high tumor burden.
But in people with a high tumor burden, the median survival of patients with different progression modes (NEH, EHG, NIH, IHG) was 9.2 (95%CI 7.0-10.4) months, 9.3 (95%CI 6.2-16.3) months, 8.3 (95%CI 7.5-10.8) months, and 12.1 (95%CI 9.3-17.9) months, respectively (p=0.068); The post-progression survival was 4.9 (95%CI 3.2-7.0) months, 4.8 (95%CI 4.2-11.3) months, 5.0 (95%CI 4.1-6.9) months, 6.6 (95%CI 5.9-12.0) months (p=0.07). and it was difficult to distinguish survival based on the mode of progression. The effect of progression patterns on patient survival was no longer statistically significant.
3.3 Interaction analysis
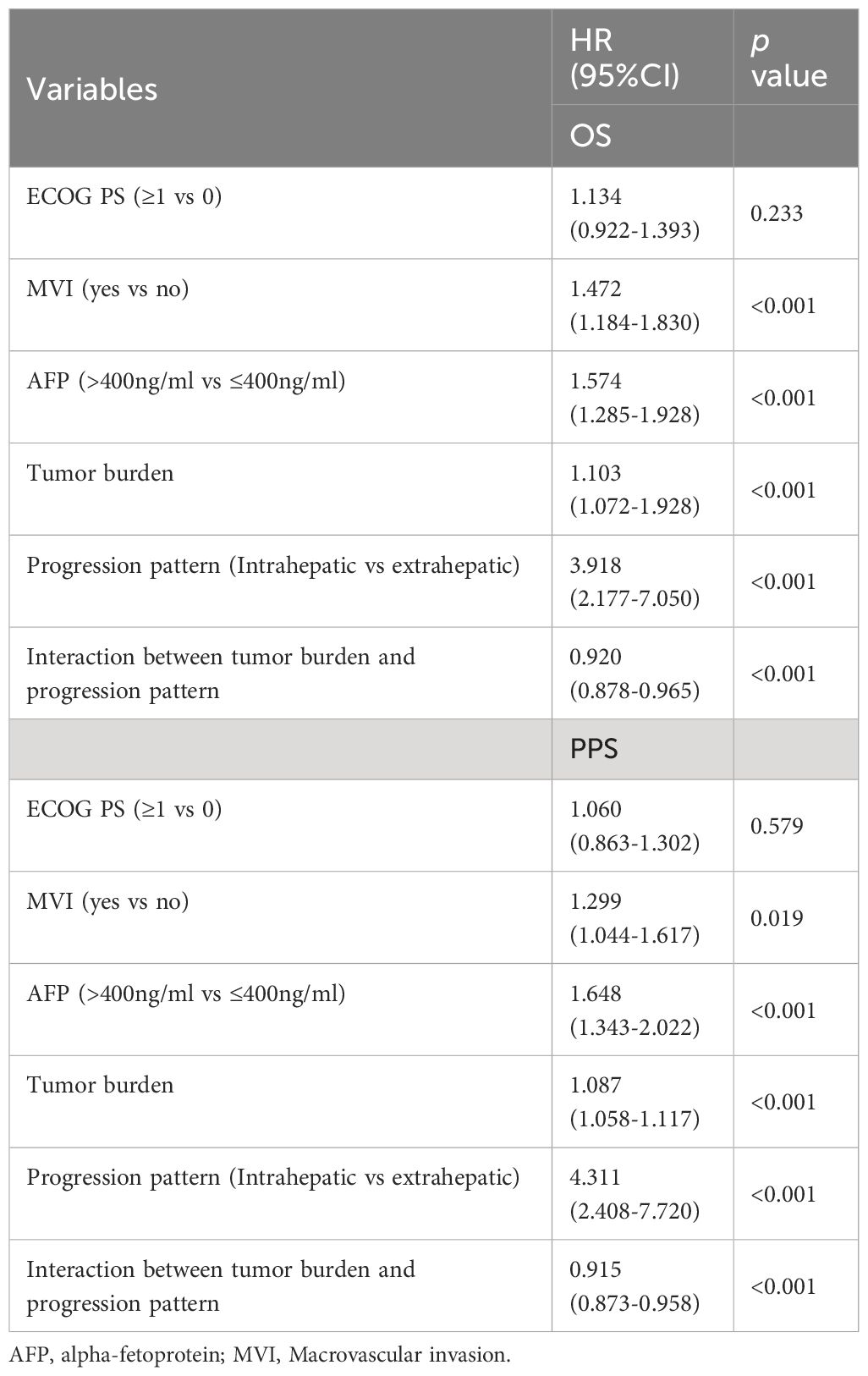
The multivariate Cox regression analysis included factors related to the guidelines recommended grouping criteria for clinical trials, in addition to tumor load and the multiplicative interaction terms of tumor load and pattern of progression (progression limited to intrahepatic/extrahepatic progression). The results showed that MVI, AFP, tumor burden, progression pattern and the multiplicative interaction terms of tumor load and mode of progression were statistically significant for the prognosis of patients (p< 0.001) (Table 3). A restricted cube plot (Figure 3) shows that the risk of death in patients with liver-limited progression (NIH/IHG) increases with the enlarging in tumor load until the risk is close to that in patients with extrahepatic progression at high tumor load.
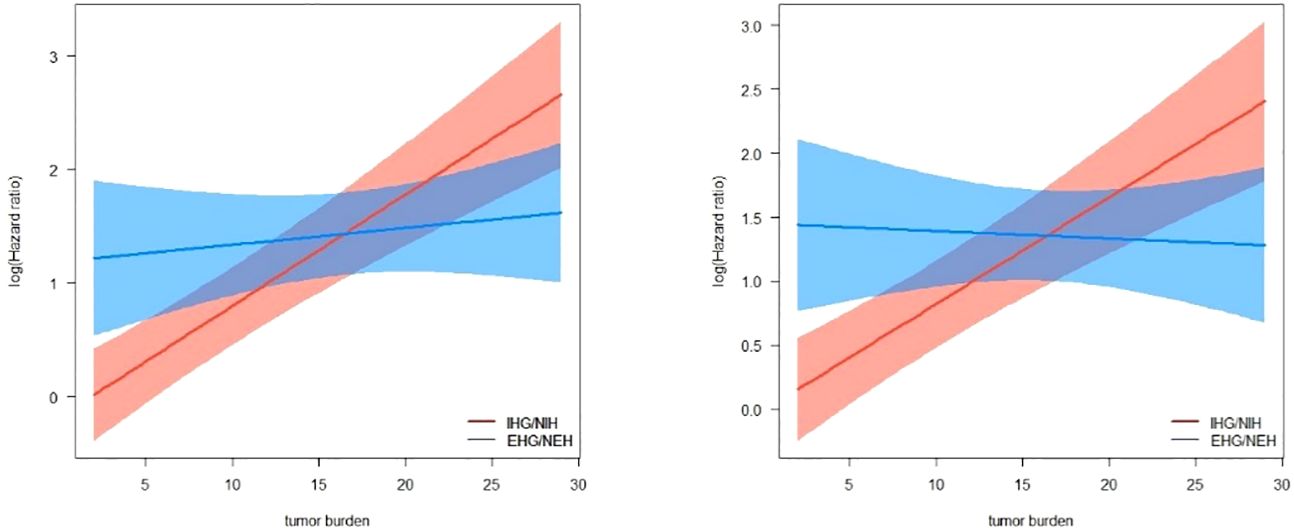
Figure 3 Interactive visualization of the interaction between tumor burden and progression patterns with OS/PPS as the endpoint.
3.4 Relationship between progression patterns and mortality risk under different tumor burden
Multivariate COX regression analysis was performed according to the clinical trial grouping criteria recommended by the guidelines. The pattern of progression was found to be independent prognostic factor p<0.001 for both overall median survival and post-progression survival (Table 4). Patients with extrahepatic progression or new extrahepatic progression had a 1.549 times higher risk of death (95%CI 1.256-1.909).
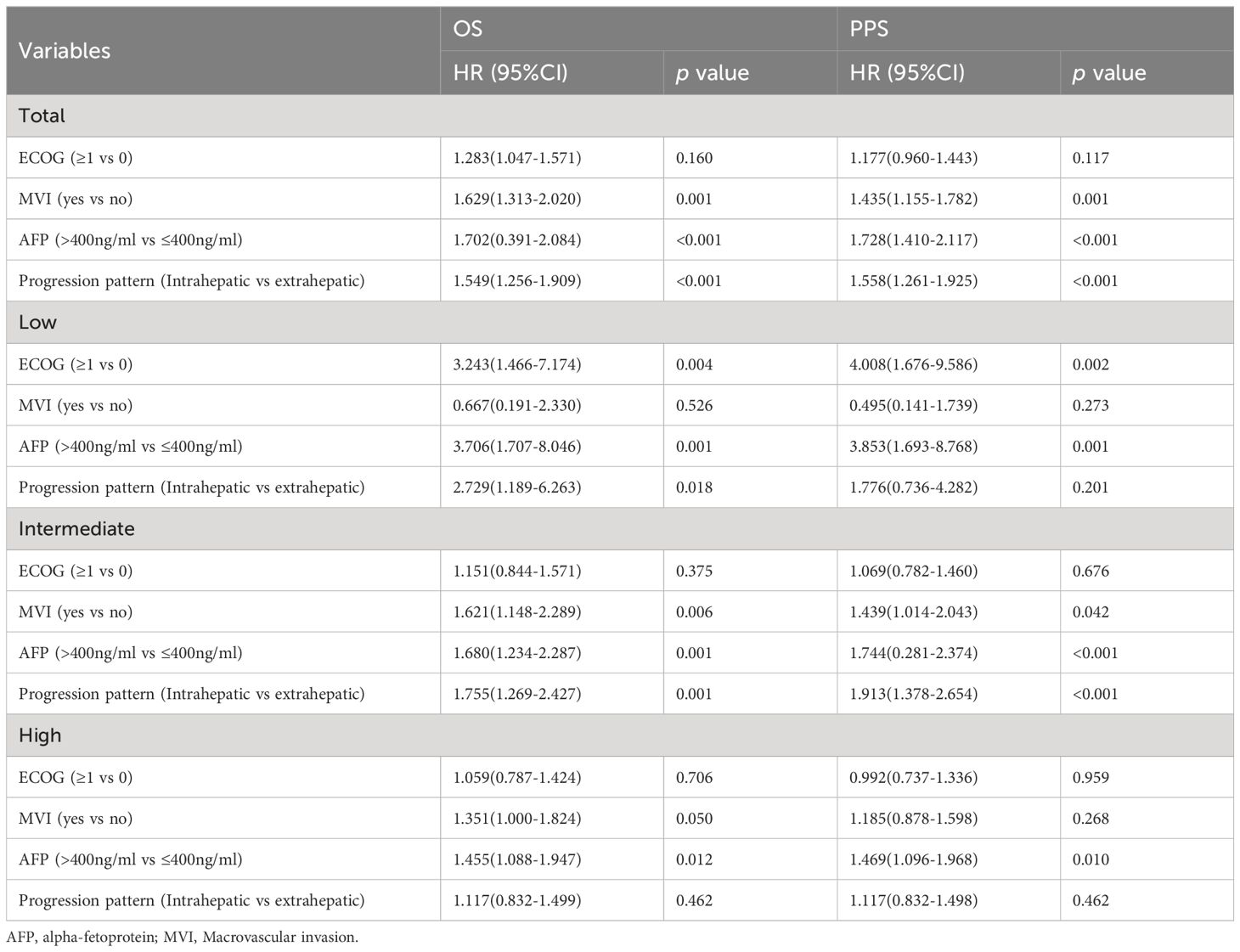
However, when subgroups were differentiated according to tumor load, it was found that the sensitivity of patients’ risk of death to the pattern of progression decreased with increasing tumor load. The hazard ratio for extrahepatic progression (NEH/EHG) compared to intrahepatic progression (NIH/IHG) at low, medium, and high tumor burden were [HR 2.729, 95%CI 1.189-6.263], [HR 1.755, 95%CI 1.269-2.427], and [HR 1.117, 95%CI 0.832-1.499], respectively.
4 Discussion
Based on a retrospective analysis of 538 patients with hepatocellular carcinoma who progressed after treatment with sorafenib, we found that there was a significant interaction between tumor burden and progression pattern on the outcome of hepatocellular carcinoma. At the same time, the changes of the relationship between death risk and progression pattern of patients under different tumor burden were further analyzed using tumor burden as stratification condition. Our study partly explains why previous studies have reached different conclusions about the progression pattern of advanced liver cancer tumors, in which tumor burden was not included in the analysis (11–17).
According to the tumor burden model established previously by our research group (six-and-twelve) (22), we divided the tumor burden into three subgroups of low, medium and high, and found that as the tumor burden increases, the sensitivity of the patient’s risk of death to the progression pattern decreases: In patients with low and moderate tumor loads, patients with extrahepatic progression (NEH/EHG) had a significantly worse prognosis than those with intrahepatic progression (NIH/IHG). In patients with high tumor burden, the progression pattern no longer significantly stratified patient survival. Meanwhile, in patients whose progression was limited to the liver (NIH/IHG), median survival of different subgroups of at-risk individuals was significantly differentiated based on tumor burden; However, it is difficult to distinguish different risk groups based on tumor burden when there is extrahepatic lesion progression. This may be because the negative effects of rapid deterioration of liver function in patients with high tumor burden after progression are similar to the negative effects of extra-hepatic lesion progression.
With the development of first-line therapy such as molecular targeted therapy and immunotherapy, the therapeutic strategy of second-line therapy is also about to change dramatically (23, 24). Appropriate stratification factors should be considered in the trial design of second-line therapy for advanced liver cancer, otherwise patient characteristics may influence clinical trial results in patients with treatment failure (4). Our study suggests that the interaction between progression patterns and tumor burden in trial design should be fully considered in trial design and personalized treatment.
In our study, high tumor burden and medium tumor burden together accounted for more than 90% of the total population, which is consistent with the popular situation of our country, and the influence of tumor burden on our patients is more important (25). In the general population, overall survival and post-progression of new and extra-hepatic lesions were poor (OS: 9.3, 9.2; PPS: 4.9 months, 5.1 months). For patients with high tumor burden, regardless of intrahepatic progression or extrahepatic progression, the survival time after progression was only about half a year (4.9 months, 4.8 months, 5.0 months, 6.6 months). Therefore, for patients with higher tumor burden and better liver function reserve, active treatment with higher objective remission rate and disease control rate is recommended, because the objective remission rate of sorafenib is low, once the tumor progression is not controlled, it is difficult to bring better survival benefits to patients. At the same time, our study found that AFP had significant prognostic significance in different subgroups, and its prognostic value and significance should be further explored.
Advanced liver cancer has considerable heterogeneity, and tumor burden, metastasis, liver function reserve, and systemic condition all have considerable influence on the survival time and quality of life of patients (18, 26). However, the degree of influence of these factors varies. In 2018, Giannini et al. used clinical characteristics to classify heterogeneous BCLC stage C patients. In a retrospective analysis of 835 patients with stage C BCLC, median overall survival was significantly different based on criteria leading to advanced tumor stage (ECOG score 1-2, macrovascular infiltration, or extrahepatic spread) (18). At the same time, these factors will also influence each other and interact with each other, instead of simply adding or subtracting. However, the current research mainly divides patients into different groups according to a certain factor. In the follow-up studies of prognostic factors, faced with complex individuals, we should study more the interaction between multiple factors, rather than just explore the independent prognostic effect of a single factor.
Intrahepatic tumor burden, macrovascular invasion and extrahepatic metastasis, as well as severe liver function impairment, are key factors for poor prognosis, and these factors are often reflected in multiple clinical prediction models (27–32). However, tumor burden is rarely considered in the trial design of second-line therapy, possibly because its role is often overwritten by adverse prognostic factors such as liver function and extrahepatic metastasis (7–10, 33, 34). Patients with high tumor burden are more likely to suffer from rapid deterioration of liver function or even liver failure, resulting in death, after the progression of intrahepatic tumor, and this risk should not be ignored (19). The liver function and tumor burden can be in a certain interaction between need further exploration in the future. On the one hand, normal liver tissue is affected in patients with large tumor burden, and people with poor liver function should be more than patients with small tumor burden. On the other hand, as the patient’s liver function declines, the sensitivity of tumor burden to survival prognosis may also gradually decrease.
The study also has some limitations. First, the risk of selection bias is inevitable in observational studies. Secondly, the cause of Chinese patients is mainly hepatitis B, and the tumor burden is also relatively high, which has some differences with other areas of tumors, and needs to be further verified in multi-center and other areas. At the same time, we only considered the interaction between tumor burden and progressive mode, and did not consider the interaction between tumor burden and progressive mode and liver function. This part of work needs to be further explored in the future.
5 Conclusion
In conclusion, interaction between the tumor progression patterns and tumor burden significantly affects the prognosis of HCC patients. As the tumor burden increases, the sensitivity of the patient’s risk of death to the progression pattern decreases. Therefore, the interaction between progression mode and tumor burden should be fully considered in trial design and personalized treatment.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, Guohong Han, upon reasonable request. Requests to access these datasets should be directed to Jun Sun, NDM4ODMxNDQzQHFxLmNvbQ==.
Ethics statement
The studies involving humans were approved by Ethics Committee of Xijing Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
JS: Writing – review & editing, Writing – original draft, Visualization, Software, Methodology, Investigation, Data curation, Conceptualization. DX: Writing – review & editing, Validation, Supervision, Software, Project administration, Methodology, Investigation, Conceptualization. WB: Writing – review & editing, Supervision, Resources, Project administration, Methodology, Investigation, Formal Analysis, Data curation, Conceptualization. XL: Writing – review & editing, Resources, Investigation, Data curation. EW: Writing – review & editing, Methodology, Investigation, Data curation, Conceptualization. ZY: Writing – review & editing, Resources, Project administration, Methodology, Investigation, Data curation, Conceptualization. GH: Writing – review & editing, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal Analysis, Data curation, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Easl clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. (2018) 69:182–236. doi: 10.1016/j.jhep.2018.03.019
2. Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. (2022) 400:1345–62. doi: 10.1016/S0140-6736(22)01200-4
3. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the american association for the study of liver diseases. Hepatology. (2018) 68:723–50. doi: 10.1002/hep.29913
4. Llovet JM, Villanueva A, Marrero JA, Schwartz M, Meyer T, Galle PR, et al. Trial design and endpoints in hepatocellular carcinoma: aasld consensus conference. Hepatology. (2021) 73 Suppl 1:158–91. doi: 10.1002/hep.31327
5. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. (2021) 7:6. doi: 10.1038/s41572-020-00240-3
6. Villanueva A. Hepatocellular carcinoma. N Engl J Med. (2019) 380:1450–62. doi: 10.1056/NEJMra1713263
7. Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (reach-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2019) 20:282–96. doi: 10.1016/S1470-2045(18)30937-9
8. Chau I, Peck-Radosavljevic M, Borg C, Malfertheiner P, Seitz JF, Park JO, et al. Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib: patient-focused outcome results from the randomised phase iii reach study. Eur J Cancer. (2017) 81:17–25. doi: 10.1016/j.ejca.2017.05.001
9. Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (resorce): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2017) 389:56–66. doi: 10.1016/S0140-6736(16)32453-9
10. Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. (2018) 379:54–63. doi: 10.1056/NEJMoa1717002
11. Reig M, Rimola J, Torres F, Darnell A, Rodriguez-Lope C, Forner A, et al. Postprogression survival of patients with advanced hepatocellular carcinoma: rationale for second-line trial design. Hepatology. (2013) 58:2023–31. doi: 10.1002/hep.26586
12. Iavarone M, Cabibbo G, Biolato M, Della Corte C, Maida M, Barbara M, et al. Predictors of survival in patients with advanced hepatocellular carcinoma who permanently discontinued sorafenib. Hepatology. (2015) 62:784–91. doi: 10.1002/hep.27729
13. Lee I, Chen Y, Chao Y, Huo TI, Li CP, Su CW, et al. Determinants of survival after sorafenib failure in patients with bclc-c hepatocellular carcinoma in real-world practice. Med (Baltimore). (2015) 94:e688. doi: 10.1097/MD.0000000000000688
14. Ogasawara S, Chiba T, Ooka Y, Suzuki E, Kanogawa N, Saito T, et al. Post-progression survival in patients with advanced hepatocellular carcinoma resistant to sorafenib. Invest New Drugs. (2016) 34:255–60. doi: 10.1007/s10637-016-0323-1
15. Schütte K, Schinner R, Fabritius MP, Möller M, Kuhl C, Iezzi R, et al. Impact of extrahepatic metastases on overall survival in patients with advanced liver dominant hepatocellular carcinoma: a subanalysis of the soramic trial. Liver Cancer. (2020) 9:771–86. doi: 10.1159/000510798
16. de la Torre-Aláez M, Jordán-Iborra C, Casadei-Gardini A, Bilbao JI, Rodriguez-Fraile M, Sancho L, et al. The pattern of progression defines post-progression survival in patients with hepatocellular carcinoma treated with sirt. Cardiovasc Intervent Radiol. (2020) 43:1165–72. doi: 10.1007/s00270-020-02444-2
17. Reig M, Galle PR, Kudo M, Finn R, Llovet JM, Metti AL, et al. Pattern of progression in advanced hepatocellular carcinoma treated with ramucirumab. Liver Int. (2021) 41:598–607. doi: 10.1111/liv.14731
18. Giannini EG, Bucci L, Garuti F, Brunacci M, Lenzi B, Valente M, et al. Patients with advanced hepatocellular carcinoma need a personalized management: a lesson from clinical practice. Hepatology. (2018) 67:1784–96. doi: 10.1002/hep.29668
19. Uchino K, Tateishi R, Shiina S, Kanda M, Masuzaki R, Kondo Y, et al. Hepatocellular carcinoma with extrahepatic metastasis. Cancer. (2011) 117:4475–83. doi: 10.1002/cncr.25960
20. Wang Z, Wang E, Bai W, Xia D, Ding R, Li J, et al. Exploratory analysis to identify candidates benefitting from combination therapy of transarterial chemoembolization and sorafenib for first-line treatment of unresectable hepatocellular carcinoma: a multicenter retrospective observational study. Liver Cancer. (2020) 9:308–25. doi: 10.1159/000505692
21. Lencioni R, Llovet J. Modified recist (mrecist) assessment for hepatocellular carcinoma. Semin Liver Dis. (2010) 30:52–60. doi: 10.1055/s-0030-1247132
22. Wang Q, Xia D, Bai W, Wang E, Sun J, Huang M, et al. Development of a prognostic score for recommended tace candidates with hepatocellular carcinoma: a multicentre observational study. J Hepatol. (2019) 70:893–903. doi: 10.1016/j.jhep.2019.01.013
23. Gordan JD, Kennedy EB, Abou-Alfa GK, Beg MS, Brower ST, Gade TP, et al. Systemic therapy for advanced hepatocellular carcinoma: asco guideline. J Clin Oncol. (2020) 38:4317–45. doi: 10.1200/JCO.20.02672
24. Cabibbo G, Reig M, Celsa C, Torres F, Battaglia S, Enea M, et al. First-line immune checkpoint inhibitor-based sequential therapies for advanced hepatocellular carcinoma: rationale for future trials. Liver Cancer. (2022) 11:75–84. doi: 10.1159/000520278
25. Wang ZX, Li J, Wang EX, Xia DD, Bai W, Wang QH, et al. Validation of the six-and-twelve criteria among patients with hepatocellular carcinoma and performance score 1 receiving transarterial chemoembolization. World J Gastroenterol. (2020) 26:1805–19. doi: 10.3748/wjg.v26.i15.1805
26. Yoo JJ, Chung GE, Lee JH, Nam JY, Chang Y, Lee JM, et al. Sub-classification of advanced-stage hepatocellular carcinoma: a cohort study including 612 patients treated with sorafenib. Cancer Res Treat. (2018) 50:366–73. doi: 10.4143/crt.2017.126
27. Labeur TA, Berhane S, Edeline J, Blanc JF, Bettinger D, Meyer T, et al. Improved survival prediction and comparison of prognostic models for patients with hepatocellular carcinoma treated with sorafenib. Liver Int. (2019) 40:215–28. doi: 10.1111/liv.14270
28. Berhane S, Fox R, Garcia-Finana M, Cucchetti A, Johnson P. Using prognostic and predictive clinical features to make personalised survival prediction in advanced hepatocellular carcinoma patients undergoing sorafenib treatment. Br J Cancer. (2019) 121:117–24. doi: 10.1038/s41416-019-0488-4
29. Edeline J, Blanc JF, Campillo-Gimenez B, Ma YT, King J, Faluyi O, et al. Prognostic scores for sorafenib-treated hepatocellular carcinoma patients: a new application for the hepatoma arterial embolisation prognostic score. Eur J Cancer. (2017) 86:135–42. doi: 10.1016/j.ejca.2017.08.036
30. Choi GH, Han S, Shim JH, Ryu MH, Ryoo BY, Kang YK, et al. Prognostic scoring models for patients undergoing sorafenib treatment for advanced stage hepatocellular carcinoma in real-life practice. Am J Clin Oncol. (2017) 40:167–74. doi: 10.1097/COC.0000000000000132
31. Adhoute X, Penaranda G, Raoul JL, Blanc JF, Edeline J, Conroy G, et al. Prognosis of advanced hepatocellular carcinoma: a new stratification of barcelona clinic liver cancer stage c: results from a french multicenter study. Eur J Gastroenterol Hepatol. (2016) 28:433–40. doi: 10.1097/MEG.0000000000000558
32. Takeda H, Nishikawa H, Osaki Y, Tsuchiya K, Joko K, Ogawa C, et al. Clinical features associated with radiological response to sorafenib in unresectable hepatocellular carcinoma: a large multicenter study in Japan. Liver Int. (2015) 35:1581–9. doi: 10.1111/liv.12591
33. Qin S, Li Q, Gu S, Chen X, Lin L, Wang Z, et al. Apatinib as second-line or later therapy in patients with advanced hepatocellular carcinoma (ahelp): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol Hepatol. (2021) 6:559–68. doi: 10.1016/S2468-1253(21)00109-6
Keywords: HCC, sorafenib, progression pattern, tumor burden, interaction
Citation: Sun J, Xia D, Bai W, Li X, Wang E, Yin Z and Han G (2024) Tumor burden affects the progression pattern on the prognosis in patients treated with sorafenib. Front. Oncol. 14:1405178. doi: 10.3389/fonc.2024.1405178
Received: 22 March 2024; Accepted: 05 April 2024;
Published: 23 April 2024.
Edited by:
Fabricio Ferreira Coelho, University of São Paulo, BrazilReviewed by:
Bin-Yan Zhong, The First Affiliated Hospital of Soochow University, ChinaFrancisco Tustumi, University of São Paulo, Brazil
Copyright © 2024 Sun, Xia, Bai, Li, Wang, Yin and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guohong Han, aGFuZ2hAZm1tdS5lZHUuY24=
 Jun Sun
Jun Sun Dongdong Xia1,2
Dongdong Xia1,2 Guohong Han
Guohong Han