Abstract
We report a case of patient with Stage IIIa Hepatocellular Carcinoma Complicated by Early Gastric Cancer. Although Stage IIIa liver cancer can be treated with surgery, the overall prognosis of surgery is not ideal. Alternatively, conversion therapy is reported with different effectiveness towards stage IIIa liver cancer. Herein, this study shared the successful conversion of a patient with stage IIIa liver cancer and with early gastric cancer at Lanzhou University First Hospital, which is hoped to engage clinicians in evaluation and discussion.
1 Introduction
Primary liver cancer (PLC) accounts for 8.2% of all cancer deaths worldwide, and is the fourth most lethal malignancy (1, 2). There are 782,000 deaths and 841,000 new cases of PLC worldwide every year, with East Asia being the region with the highest prevalence of the disease (1). Hepatocellular carcinoma (HCC) constitutes for 85-90% of primary liver malignancies, and is the fourth most prevalent and the second most deadly cancer in China (3).
Barcelona clinic liver cancer (BCLC) classification has been widely validated. Due to variability of HCC prevalence, phenotype, and treatment response in China, specific guidelines for Chinese patients have been established (4). China liver cancer staging (CNLC) system was found in 2017, and has been used since then. In CNLC system, each stage of BCLC 0/A, B, and C is divided into two sub-stages, including stages Ia, Ib, IIa, IIb, IIIa, and IIIb (5).
For CNLC stage IIIa primary liver cancer, most patients have no indication for surgical resection (6), and for those with good liver function, the Chinese liver cancer staging system recommends TACE as the preferred treatment (5), in addition to systemic anti-tumor therapy (chemotherapy, targeted and immunotherapy) and radiotherapy (internal and external radiotherapy), which can be applied alone or in combination. The combination of TACE with targeted and immunotherapy treatment has significantly prolonged progression-free survival and overall survival (7–10). However, the outcomes remain still limited. In the present study, we report on a case, in which complicated by early gastric cancer. We hope this case will help clinicians to improve the treatment strategy for HCC.
2 Case report
The patient was a 76-year-old male who presented to Lanzhou University First Hospital on August 5, 2021, with upper abdominal bloating and mild pain persisting for over one month. He reported no other symptoms, and an abdominal ultrasound conducted at another local hospital indicated the presence of a liver mass. His medical history included chronic hepatitis B for over 30 years, hypertension, diabetes, and cataract surgery with intraocular lens implantation. Upon physical examination, the patient exhibited normal skin and mucosal color without jaundice, a flat abdomen devoid of abdominal wall varices, and no remarkable gastrointestinal contour or peristalsis. There was no tenderness upon abdominal palpation, and the liver was palpable below the rib margin. Murphy’s sign was negative, and bowel sounds were noted at a rate of four times per minute. Laboratory tests revealed the following values within normal limits: white blood cell count (WBC: 5.03 × 10^9/L), neutrophil ratio (NEUT%: 68.8%), hemoglobin (HGB: 124 g/L), and platelet count (PLT: 205 × 10^9/L). Tumor markers were elevated, including alpha-fetoprotein (AFP: 289 U/mL), carbohydrate antigen 19-9 (CA 19-9: 109 U/mL), carcinoembryonic antigen (CEA: 1.4 ng/mL), and ferritin (519 ng/mL). Biochemical analysis indicated that aspartate aminotransferase (AST: 67 U/L), alanine aminotransferase (ALT: 216 U/L), total bilirubin (TBIL: 16.4 µmol/L), direct bilirubin (DBIL: 4.8 µmol/L), alkaline phosphatase (ALP: 216 U/L), gamma-glutamyl transferase (GGT: 371 U/L), and glucose (GLU: 6.59 mmol/L) were outside normal ranges. Tests for hepatitis B (2+, 5+) and hepatitis B virus DNA (HBV DNA < 100 IU/mL) were negative. Brain natriuretic peptide (BNP: 143.4 pg/mL) was outside the normal range. The enhanced abdominal CT scan revealed a large mass-like abnormal enhancement in the right lobe of the liver, suggesting hepatocellular carcinoma (HCC) with portal vein thrombosis in the main trunk as well as the left and right branches, cirrhosis, and portal hypertension (including esophageal and gastric fundus varices). Magnetic resonance imaging (MRI) of the liver revealed a large abnormally enhanced mass in the right lobe, suggesting hepatocellular carcinoma (HCC) with intratumoral hemorrhage and portal vein thrombosis in the main trunk as well as the left and right branches; cirrhosis, splenomegaly, a small amount of ascites, liver disease, and gallbladder disease (Figure 1). The patient was diagnosed with HCC of the right lobe, post-hepatitis B cirrhosis, grade 3 hypertension (very high risk), and type 2 diabetes, classified as stage IIIa according to the CNLC staging system (Figure 2). An initial multidisciplinary team (MDT) discussion resulted in a treatment plan that included hepatic artery infusion chemotherapy (HAIC) treatment (FOLFOX), combined with 200 mg sintilimab and sorafenib 0.4 g bid targeted therapy. The patient was discharged on August 16th, 2021. After discharge, oral sorafenib 0.4g bid targeted therapy was prescribed. On the second admission, a second MDT discussion was held to adjust the protocol: HAIC treatment and percutaneous liver puncture for radioactive 125I particle implantation, combined with 200 mg sintilimab and sorafenib targeted therapy. After the fifth admission, there was no significant change in the portal vein tumor thrombus. The third MDT was conducted, and on March 29, 2022, “percutaneous hepatic puncture radioactive 125I particle implantation” radiation therapy was performed. Additionally, because the patient could not tolerate bisphosphonates, the treatment was changed to once-daily targeted therapy with 8 mg of lenvatinib. The patient underwent abdominal CT (plain + enhanced) at the sixth admission on June 21, 2022, compared with the previous scan on March 28, 2022 showed a lesion in the right lobe of the liver with little change, along with occlusion of the main portal vein and portal spongiosis, which remained little changed from the previous scan (Figure 2). After comprehensive consideration of the timing of surgical resection following successful conversion, the decision was made to perform surgical treatment. Subsequently, another preoperative evaluation was conducted, which included gastroscopy, revealing a 0-Ia+IIc type lesion can be seen near the anterior wall of the cardia, with a size of about 3 × 2 cm and chronic atrophic gastritis (Figure 3A). After the fourth MDT, the gastric tumor was initially resected via laparoscopy combined with endoscopy under general anesthesia on July 4, 2022 (endoscopic mucosal resection, partial gastrectomy, and D1 lymph node dissection). Due to the patient’s poor cardiopulmonary function during the operation, the laparoscopic surgery was interrupted and converted into an open procedure for right hepatectomy and cholecystectomy (Figure 3B). The postoperative gastric histopathology report indicated ectopic hyperplasia of adenoepithelial hyperdifferentiated epithelial endothelium and focal carcinomatous lesions (Figure 3C). Two trans arterial chemoembolization (TACE) procedures were performed on September 13, 2022, and April 3, 2023, as adjuvant treatment following hepatic resection, in accordance with the Guidelines for the Diagnosis and Treatment of Primary Hepatocellular Carcinoma (2022 edition). Postoperative abdominal CT and MRI were performed on December 18, 2023, which revealed the disappearance of the portal vein thrombus and widening of the hepatic fissure (Figure 4). Remarkably, the patient demonstrated a favorable prognosis, recovering well and remaining free of tumor recurrence or metastasis during over eight months of follow-up.
Figure 1
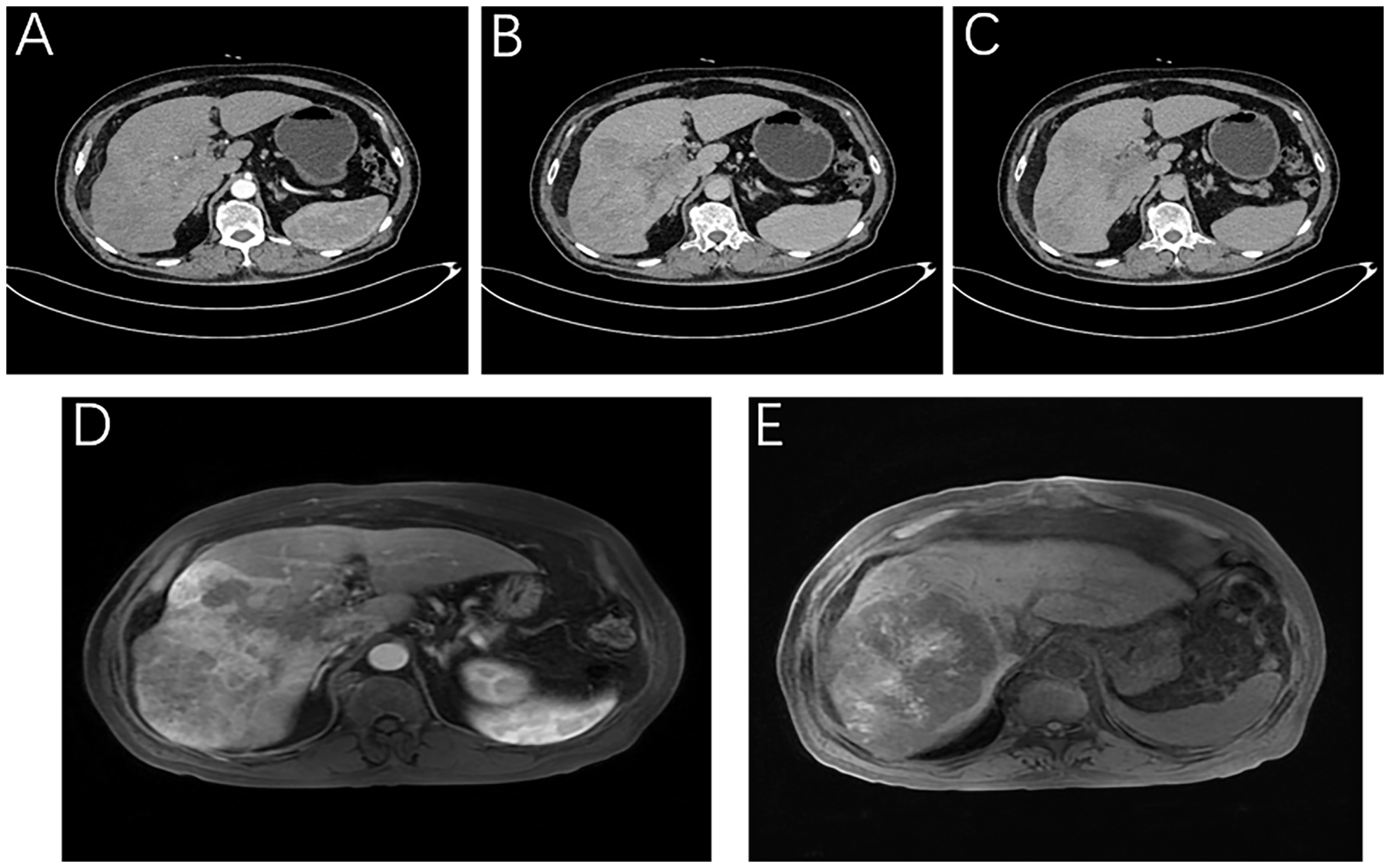
Imaging of the patient at the time of initial diagnosis. Liver-specific MRI: (A) Arterial phase, (B) Portal vein stage (blood vessel), (C) Grace; Enhanced abdominal CT: (D) Portal stage: portal vein thrombosis, (E) T1WI intratumor hemorrhage.
Figure 2
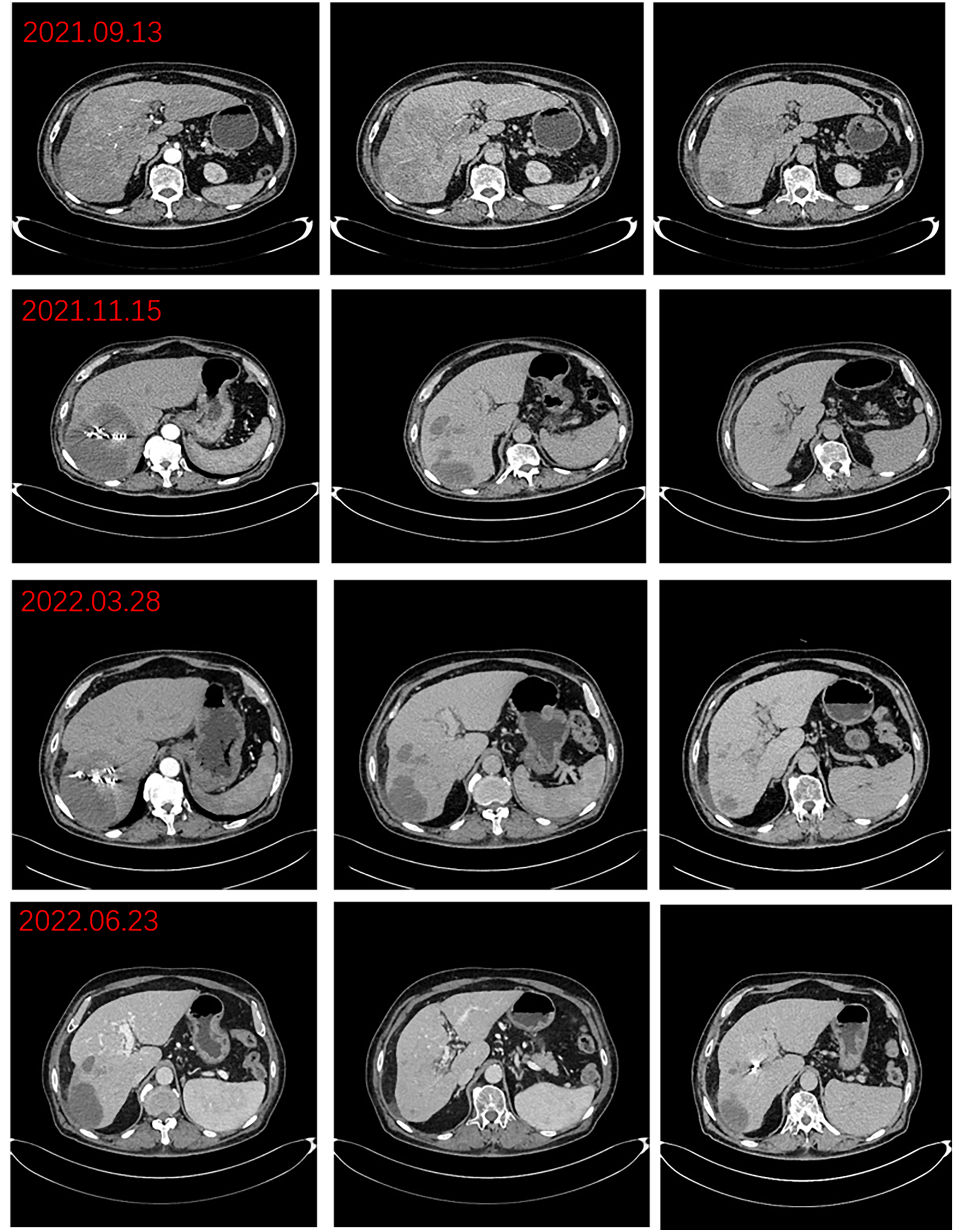
Preoperative imaging changes of hepatocellular carcinoma lesions.
Figure 3
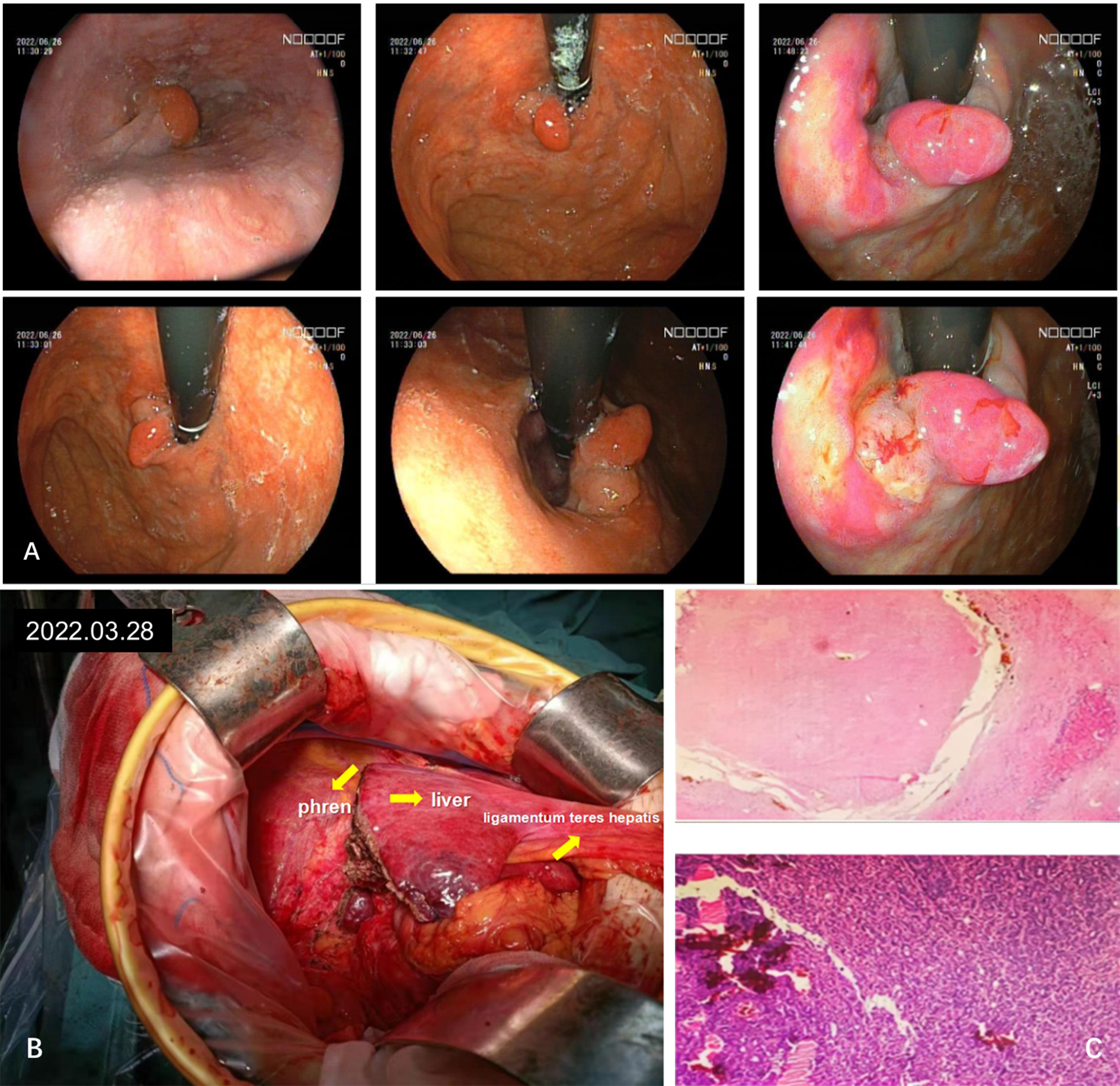
Diagnosis and intraoperative imaging of hepatocellular carcinoma combined with early gastric cancer. (A) A gastroscopic lesion of type 0-Ia+IIc is observed near the anterior wall of the cardia. (B) Intraoperative image. (C) Extensive sampling of liver tissue revealed all necrotic tissue, while gastric tissue exhibited chronic inflammation and focal low-grade intraepithelial tumors, as observed by hematoxylin-eosin (HE) staining.
Figure 4
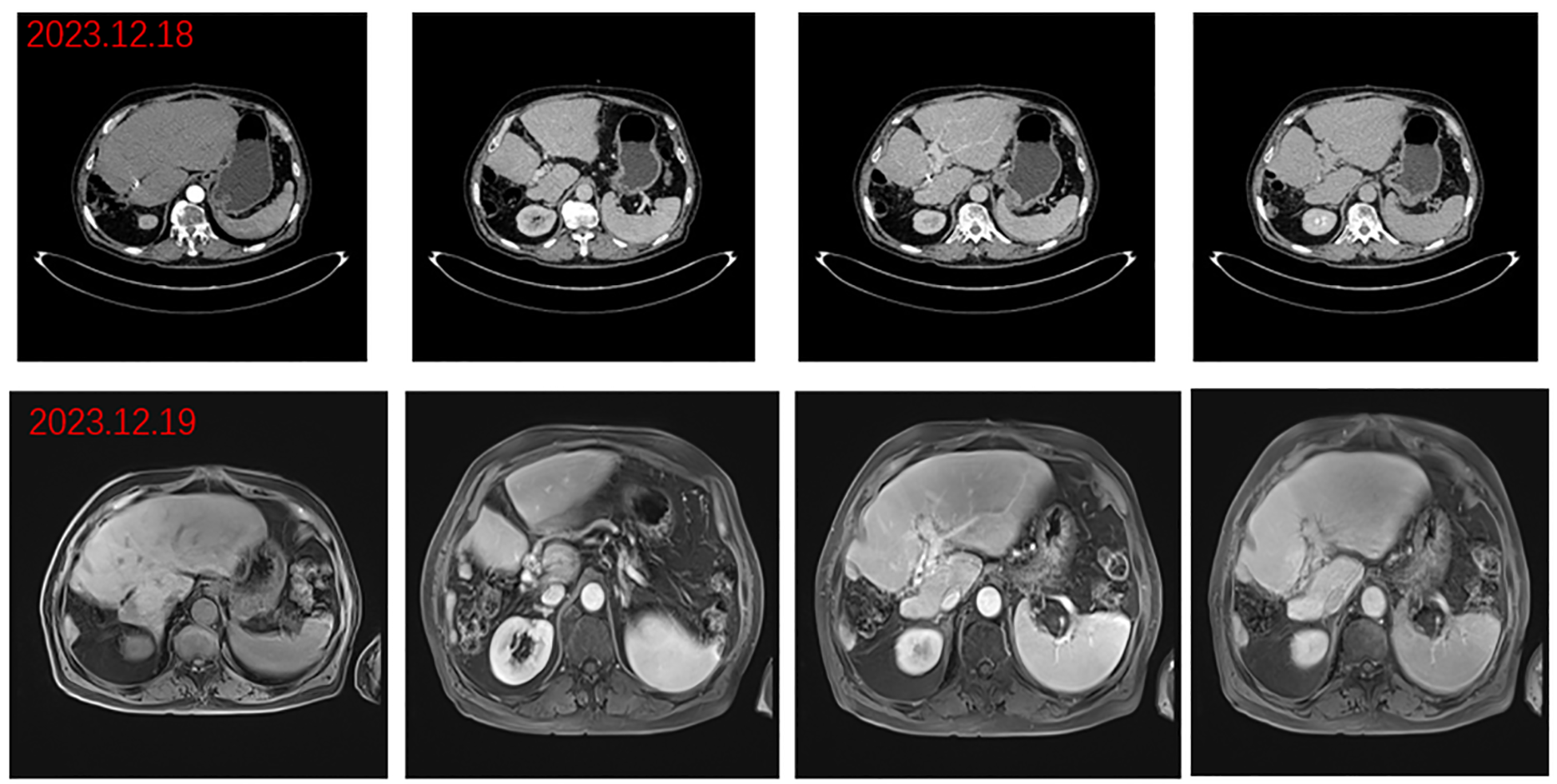
Postoperative images. Arterial, portal, and delayed phase CT: disappearance of portal cancer embolus and resection of right lobe lesion and portal phase, delayed phase abdominal MR.
3 Discussion
In China, liver cancer accounts for 75%–85% of malignant tumors in the digestive system (11); however, cholangiocarcinoma stands up to 10%–15% of malignant tumors in digestive system (12). Early stage of liver cancer can be treated with surgery, radiofrequency ablation, and other therapies, with a median survival time of over five years. For surgically resected patients, the five-year survival rate can reach 70%, and the surgical mortality rate is less than 5% (13, 14). Patients with advanced-stage liver cancer and poor prognosis which results from the fact that liver disease cannot undergo radical surgery directly (15). Therefore, conversion therapy for liver cancer is a clinically important issue.
Recently, the widespread application of targeted drugs (e.g., small molecule tyrosine kinase inhibitors (TKIs) and angiogenesis inhibitors) and immune checkpoint inhibitors (ICIs) has ushered in a new era of targeted immunotherapy for liver cancer treatment and conversion therapy. In recent clinical practice, combined targeted immunotherapy and local treatment have facilitated surgical resection for initially unresectable liver cancer patients with expert consensus (16).
According to the guideline of Primary Liver Cancer Diagnosis and Treatment Guidelines (2022 edition), multiple targeted drugs (e.g., donolutamide, lenvatinib, sorafenib, apatinib, bevacizumab, regorafenib, cabozantinib, and ramolutamide) and immunotherapeutic drugs (e.g., tislelizumab, camrelizumab, durvalumab + tremolutamide, and nivolumab + ipilimumab) are recommended for the first-line/second-line treatment of advanced liver cancer (17). Commonly used drug combinations for transformation therapy include Coley’s combination (TKI + ICI) (18–20), T+A (atezolizumab + bevacizumab) (21), and the double D combination (sintilimab + zolbetuximab) (22). In addition, local treatments, such as transcatheter arterial chemoembolization (TACE), hepatic artery infusion chemotherapy (HAIC), radiotherapy, and ablation, are often used in combination with targeted immunosuppressants for conversion therapy. The triple combination of TACE, ICI, and TKI has a higher success rate of surgical resection after HCC conversion therapy and a better prognosis than the combination of TACE and TKI (23, 24).
The timing of surgery should be based on tumor response. The main pathological response (MPR) refers to a significant reduction in the proportion of viable tumor cells to a clinically significant threshold. Pathological complete response refers to the absence of viable tumor cells in the resected specimen after complete evaluation of all sampled areas, including lymph nodes, cancer emboli, and distant metastases. Radiographic evaluation should use the modified RECIST (mRECIST) criteria instead of traditional RECIST v1.1 criteria for evaluating liver lesions’ response to treatment. In addition, the timing of surgery should also consider the safety of the operation, an important aspect to evaluate before transformation resection. This not only requires to assess the necessary safety checks for general liver resection surgery but also to investigate the potential effect of earlier conversion therapy on the liver. When stage IIIa liver cancer is combined with early gastric cancer (EGC), the LECS function can be selected. EGC refers to gastric cancer that invades no deeper than the submucosa layer, regardless of lymph node metastasis (T1 or any N stage). Deng et al. reported that for gastric body type IIa + IIb lesions diagnosed with high-grade intraepithelial neoplasia, ESD was performed (25). For gastric cancer staging, cT1b(SM)N0 is recommended for surgical resection with D1 or D1 + lymph node dissection. In 2012, Nunobe et al. proposed LECS for early gastric cancer. Generally, LECS is easier to perform, has a shorter operation time, and has fewer complications compared to ESD.
Recently, the concept and process of conversion therapy have become increasingly sophisticated (26). Hence, liver cancer patients will be able to receive alternative conversion therapy in the future. However, there remain many clinical issues that require further evaluation.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/s.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XS: Writing – original draft, Writing – review & editing. RC: Investigation, Resources, Writing – original draft. LW: Conceptualization, Project administration, Writing – original draft. FD: Conceptualization, Data curation, Writing – original draft. HN: Conceptualization, Data curation, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study is supported by 2022 Gansu Province Traditional Chinese Medicine Industry Innovation Consortium Project (22ZD6FA021-4); 2003 Gansu Province Science and Technology Plan Project (23YFFA0034).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Bray F Ferlay J Soerjomataram I Siegel RL Torre LA Jemal A . Global cancer statistics 2018: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2
Siegel RL Miller KD Fuchs HE Jemal A . Cancer statistics, 2022. CA: Cancer J Clin. (2022) 72:7–33. doi: 10.3322/caac.21708
3
Zhou M Wang H Zeng X Yin P Zhu J Chen W et al . Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: A systematic analysis for the global burden of disease study 2017. Lancet (London England). (2019) 394:1145–58. doi: 10.1016/s0140-6736(19)30427-1
4
Zhou J Sun HC Wang Z Cong WM Wang JH Zeng MS et al . Guidelines for diagnosis and treatment of primary liver cancer in China (2017 edition). Liver Cancer. (2018) 7:235–60. doi: 10.1159/000488035
5
Xie DY Ren ZG Zhou J Fan J Gao Q . 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. (2020) 9:452–63. doi: 10.21037/hbsn-20-480
6
Han K Kim JH . Transarterial chemoembolization in hepatocellular carcinoma treatment: barcelona clinic liver cancer staging system. World J Gastroenterol. (2015) 21:10327–35. doi: 10.3748/wjg.v21.i36.10327
7
Cao Y Ouyang T Xiong F Kan X Chen L Liang B et al . Efficacy of apatinib in patients with sorafenib-transarterial chemoembolization refractory hepatocellular carcinoma: A retrospective study. Hepatol Int. (2021) 15:1268–77. doi: 10.1007/s12072-021-10198-3
8
Ding X Sun W Li W Shen Y Guo X Teng Y et al . Transarterial chemoembolization plus lenvatinib versus transarterial chemoembolization plus sorafenib as first-line treatment for hepatocellular carcinoma with portal vein tumor thrombus: A prospective randomized study. Cancer. (2021) 127:3782–93. doi: 10.1002/cncr.33677
9
Fidelman N . A leap from tace to tace potentiated by immune response modulation and angiogenesis inhibition for patients with intermediate-stage hepatocellular carcinoma: leap-012 phase 3 randomized control trial protocol. Cardiovasc interventional Radiol. (2022) 45:413–4. doi: 10.1007/s00270-021-03010-0
10
Wang J Li X Wang F Shi D Zhang J . Anlotinib followed by transarterial chemoembolization and radiofrequency ablation is a safe and effective initial treatment for hepatocellular carcinoma patients with portal vein tumor thrombus: A retrospective case series study. J Cancer Res Ther. (2021) 17:619–24. doi: 10.4103/jcrt.JCRT_1253_20
11
Sung H Ferlay J Siegel RL Laversanne M Soerjomataram I Jemal A et al . Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
12
Vogel A Meyer T Sapisochin G Salem R Saborowski A . Hepatocellular carcinoma. Lancet (London England). (2022) 400:1345–62. doi: 10.1016/s0140-6736(22)01200-4
13
Yuan S Zhou L . Therapeutic strategies for downstaging hepatocellular carcinoma. J Clin Hepatol Digestion. (2020) 36. doi: 10.3969/j.issn.1001-5256.2020.02.002
14
Liver Cancer Conversion Therapy Collaboration Group of the Liver Cancer Professional Committee of the Chinese Anticancer Association . Chinese expert consensus on liver cancer conversion therapy (2021 edition). Chin J Digestive Surg. (2021) 20:600–16. doi: 10.3760/cma.j.cn115610-20210521-00223
15
Huang A Guo D Zhou J . Transformational therapy for liver cancer in the era of targeted and immune therapies. J Pract Oncol. (2023) 38:101–4. doi: 10.13267/j.cnki.syzlzz.2023.017
16
Sun HC Zhou J Wang Z Liu X Xie Q Jia W et al . Chinese expert consensus on conversion therapy for hepatocellular carcinoma (2021 edition). Hepatobiliary Surg Nutr. (2022) 11:227–52. doi: 10.21037/hbsn-21-328
17
Department of Medical Administration NHCotPsRoC . Diagnosis and treatment guideline for primary liver cancer (2022 edition). Chin J Hepatol. (2022) 30:367–88.
18
Zhu XD Huang C Shen YH Ji Y Ge NL Qu XD et al . Downstaging and resection of initially unresectable hepatocellular carcinoma with tyrosine kinase inhibitor and anti-pd-1 antibody combinations. Liver Cancer. (2021) 10:320–9. doi: 10.1159/000514313
19
Yi Y Sun BY Weng JL Zhou C Zhou CH Cai MH et al . Lenvatinib plus anti-pd-1 therapy represents a feasible conversion resection strategy for patients with initially unresectable hepatocellular carcinoma: A retrospective study. Front Oncol. (2022) 12:1046584. doi: 10.3389/fonc.2022.1046584
20
Finn RS Ikeda M Zhu AX Sung MW Baron AD Kudo M et al . Phase ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin oncology: Off J Am Soc Clin Oncol. (2020) 38:2960–70. doi: 10.1200/jco.20.00808
21
Finn RS Qin S Ikeda M Galle PR Ducreux M Kim TY et al . Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. New Engl J Med. (2020) 382:1894–905. doi: 10.1056/NEJMoa1915745
22
Ren Z Xu J Bai Y Xu A Cang S Du C et al . Sintilimab plus a bevacizumab biosimilar (Ibi305) versus sorafenib in unresectable hepatocellular carcinoma (Orient-32): A randomised, open-label, phase 2-3 study. Lancet Oncol. (2021) 22:977–90. doi: 10.1016/s1470-2045(21)00252-7
23
Qu WF Ding ZB Qu XD Tang Z Zhu GQ Fu XT et al . Conversion therapy for initially unresectable hepatocellular carcinoma using a combination of toripalimab, lenvatinib plus tace: real-world study. BJS Open. (2022) 6:zrac114. doi: 10.1093/bjsopen/zrac114
24
Chen S Wu Z Shi F Mai Q Wang L Wang F et al . Lenvatinib plus tace with or without pembrolizumab for the treatment of initially unresectable hepatocellular carcinoma harbouring pd-L1 expression: A retrospective study. J Cancer Res Clin Oncol. (2022) 148:2115–25. doi: 10.1007/s00432-021-03767-4
25
Deng R Chang Z Wu J Sun F Teng C . A case of early gastric cancer with primary liver cancer. Modern Digestion Interventional Diagnosis. (2020) 25:1134.
26
Duan C Duan J Wu L Yang F Liu X . Analysis of the efficacy of sequential surgery after interventional therapy combined with targeted immunotherapy for initially non-resectable liver cancer. Abdominal Surgery. 37(2):117–23. doi: 10.3969/j.issn.1003-5591.2024.02.008
Summary
Keywords
liver cancer, targeted therapy, radiotherapy, early gastric cancer, stage IIIA
Citation
Song X, Cai R, Wang L, Ding F and Ni H (2024) A case report of a patient with stage IIIa hepatocellular carcinoma complicated by early gastric cancer. Front. Oncol. 14:1440171. doi: 10.3389/fonc.2024.1440171
Received
12 July 2024
Accepted
04 November 2024
Published
25 November 2024
Volume
14 - 2024
Edited by
Pasquale Cianci, Azienda Sanitaria Localedella Provincia di Barletta Andri Trani (ASL BT), Italy
Reviewed by
Masaichi Ohira, Osaka City University, Japan
Vincenzo Lizzi, Azienda Ospedaliero-Universitaria Ospedali Riuniti di Foggia, Italy
Updates
Copyright
© 2024 Song, Cai, Wang, Ding and Ni.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaojing Song, songxiaojing4227@126.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.