- 1Department of Gynecology, The Seventh Affiliated Hospital of Sun Yat-Sen University, Shenzhen, China
- 2Guangdong Provincial Key Laboratory of Digestive Cancer Research, Department of Scientific Research Center, The Seventh Affiliated Hospital of Sun Yat-sen University, Shenzhen, China
Background: The prognostic significance of Treg and Th17 cells, as well as their ratio (Th17/Treg), in cervical cancer remains a topic of debate. Our study aimed to clarify their association with patient survival and clinical outcomes in cervical cancer through a comprehensive meta-analysis.
Materials and methods: We conducted a comprehensive search in PubMed, Embase, and Web of Science to identify eligible studies. Studies related to cervical cancer and involving Treg cells or Th17 cells were included. For prognostic analysis, we collected Hazard Ratio values of patient survival. For studies focusing on clinical characteristics, we selected mean and standard deviation values for further analysis. This study was registered at PROSPERO (ID:CRD42024546507).
Results: Out of the 2949 records initially retrieved, we ultimately included 21 studies in our analysis. High levels of Treg cells were found to be correlated with shorter survival in patients with cervical cancer. Subgroup analysis revealed that the prognostic effect of Treg cells on cervical cancer was not influenced by their source or definition. However, analyses of different survival measures indicated that only Overall Survival showed a correlation with Treg cell levels. Additionally, Treg cells were associated with clinical staging. High-grade Th17 cells were associated with lymphatic metastases and advanced clinical stage. The Th17/Treg ratio was found to be elevated in both cervical intraepithelial neoplasia and cervical cancer patients compared to controls.
Discussion: Despite limitations such as heterogeneity among selected studies and inadequate subgroup analyses, our study contributes to a deeper understanding of the significance of Treg cells in the onset and progression of cervical cancer. It also provides valuable insights for future research in immunotherapy.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42024546507.
Introduction
Cervical cancer (CC) is the fourth most common cancer in the women and an important public health problem affecting middle-aged women, especially in under-developed sectors (1). Although various approaches, such as refraining from smoking, utilizing barrier contraception, and receiving vaccinations, can diminish the occurrence of cervical lesion (2), persistent human papillomavirus (HPV) infection is still considered the leading cause of CC (3). The progression from persistent HPV infection to cervical warts, benign cervical dysplasia, cervical intraepithelial neoplasia (CIN), and eventually invasive CC is a long-term process (4). The host’s immune system plays an important role in this process. Immune escape of CC cells is associated with downregulation of the immune system locally and evasion of detection, including changes in Treg cells, major histocompatibility complex (MHC), immune checkpoint molecules, etc. (5). Put succinctly, the current standard for treating precancerous conditions and early-stage cervical cancer predominantly involves surgical intervention. In contrast, high-grade or metastatic cervical cancer are predominantly addressed through hysterectomy combined with radiochemotherapy (6). Concurrently, immunotherapy is emerging as a significant therapeutic alternative, with an increasing array of clinical trials bolstering its potential use in clinical settings. In the realm of immunotherapy, the therapeutic efficacy is closely related to the function of the host’s immune system, especially the state of immune cells. Here, we would like to focus on the two type of immune cells, Treg and Th17, to unfold our subsequent discussion.
Treg cells are the Foxp3-expressing CD4+ T cells whose function include preventing autoimmunity and maintaining immune homeostasis. Mechanically, the HPV16 E7 protein can cause excessive proliferation of squamous epithelium of the cervix, modulate epithelial dendritic cells, suppress T helper type 1 immune responses, and simultaneously promote T cell differentiation into Tregs. The upregulation of Treg cell infiltration is considered an important obstacle to induce robust anti-tumor immunity, for suppressing effector T cells through their surface co-stimulatory signals (such as CD80/CD86-CD28 and ICOSLG-ICOS) (7–9). Several studies have suggested that a high frequency of tumor-infiltrating effector Treg cells is significantly associated with poor prognosis in a variety of solid tumors (10), including CC (11). Targeting Treg cells in tumor microenvironment (TME) reverses the immunosuppressive environment and allows immune cells to recognize CC cells, a strategy considered a viable immunotherapy for CC (5).
Th17 cells are recognized to have both anti-tumor and pro-tumor effects. On the one hand, Th17 cells may indirectly mediate their antitumor activity by promoting the recruitment of other effector immune cells (12), such as NK cells and tumor-infiltrating IFN-γ effector T cells (13). On the other hand, Th17 cells can be recruited to the tumor area by stromal cells or tumor cells through the secretion of chemokines such as IL-12 and IL-23 (14, 15). Subsequently, Th17 can cause resistance to radiotherapy and chemotherapy, recurrence, or distant metastasis of cervical cancer through the IL-17-AKT signaling pathway (16). Given its duality, the role of Th17 cells in the development of CC needs to be further explored.
In addition, both Treg and Th17 cells are considered to be malleable, adapting to different signal cues in a changing environment (17). Treg cells are able to reacquire characteristics of Th17 cells under influence of cytokines like IL-1β, IL-6 (17, 18). Th17 cells also have the ability to transdifferentiate into Treg cells (19). Studies on Treg/Th17 ratio in the peripheral blood of lung cancer have found that a higher ratio is correlated with a lower survival rate (20). Imbalances in the Th17/Treg ratio remain elusive in CC (21).
In 2023, the European Society of Medical Oncology (ESMO) reported on two clinical trials that could potentially change clinical practice in the treatment of cervical cancer. Among them, the CALLA study(NCT03830866) failed to demonstrate the efficacy of durvalumab in combination with chemoradiotherapy for locally advanced cervical cancer (22). Additionally, the KEYNOTE-A18 (NCT 04221945) trial focused on the efficacy of chemoradiation therapy combined with either placebo or pembrolizumab, with continued use of placebo or pembrolizumab for maintenance therapy post-treatment (23). The 24-month progression-free survival rates for the experimental and control groups were 67.8% and 57.5%, respectively, with a statistically significant difference (24). T cells are an important medium for the action of immune checkpoint inhibitors, and the inconsistent results of the two immunosuppressive agent-related clinical trials also suggest the importance of conducting in-depth research into the heterogeneity of T cells themselves or the intercellular communication that mediates the differences in the efficacy of immunotherapy. Therefore, in this meta-analysis, we further explored the prognostic significance of Treg and Th17 cells, as well as the imbalance of Th17/Treg ratio in CC patients.
Materials and methods
This meta-analysis was performed as the Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) guidelines (Supplementary Table 1) and was registered at PROSPERO (ID:CRD42024546507).
Search strategy and information sources
A comprehensive literature search was performed in PubMed, Embase, and Web of science from inception to December 26, 2023, without restrictions in publication type or language. The search query was as follows: (“Th17 Cells” OR “T-Lymphocytes, Regulatory”) AND (“Uterine Cervical Neoplasms” OR “Uterine Cervical Dysplasia). The search terms actually used consist of Medical Subject Heading (MeSH) terms and their synonyms, more specific terms are presented in Supplementary Data Sheet 1. Two review authors screened the titles and abstracts of the retrieved articles independently. Then the full texts of the selected literatures were further reviewed for eligibility, and differences of opinion between reviewers are resolved by consensus.
Study selection and quality assessment
The inclusion criteria were: 1, original research; 2, studies evaluating human subjects; 3, full text available; 4, studies with titles or abstracts, including CC (CIN) and Treg cells (Th17 cell); 5, studies using flow cytometry or immunohistochemistry to assess the accessible proportion of circulating or tumor-infiltrating Treg cells (Th17 cell) in CC (CIN) patients; 6, studies that reported the Correlation of Treg cell(Th17 cell) with prognosis and clinical features; 7, for prognostic study selected the studies that presented hazard ratio (HR) for survival and its 95% confidence interval (95% CI) or Kaplan-Meier Graphs which can extract data. The exclusion criteria were: 1, Studies that are not published in English as their first language;2, Reviews, clinical case study, Meta-Analysis, meeting abstracts and letter; 3, secondary literature or duplicate published data; 4, Special types of CC, such as adenocarcinoma; 5, insufficient data can be extracted for later evaluation.
Different quality assessment methods were selected for different types of studies. For cohort studies on prognosis, the Newcastle-Ottawa Scale (NOS) (25) was selected for quality assessment. For cross-sectional studies on clinical features, quality assessment tool revised by Agency for Healthcare Research and Quality (AHRQ) (26) was used.
Data extraction
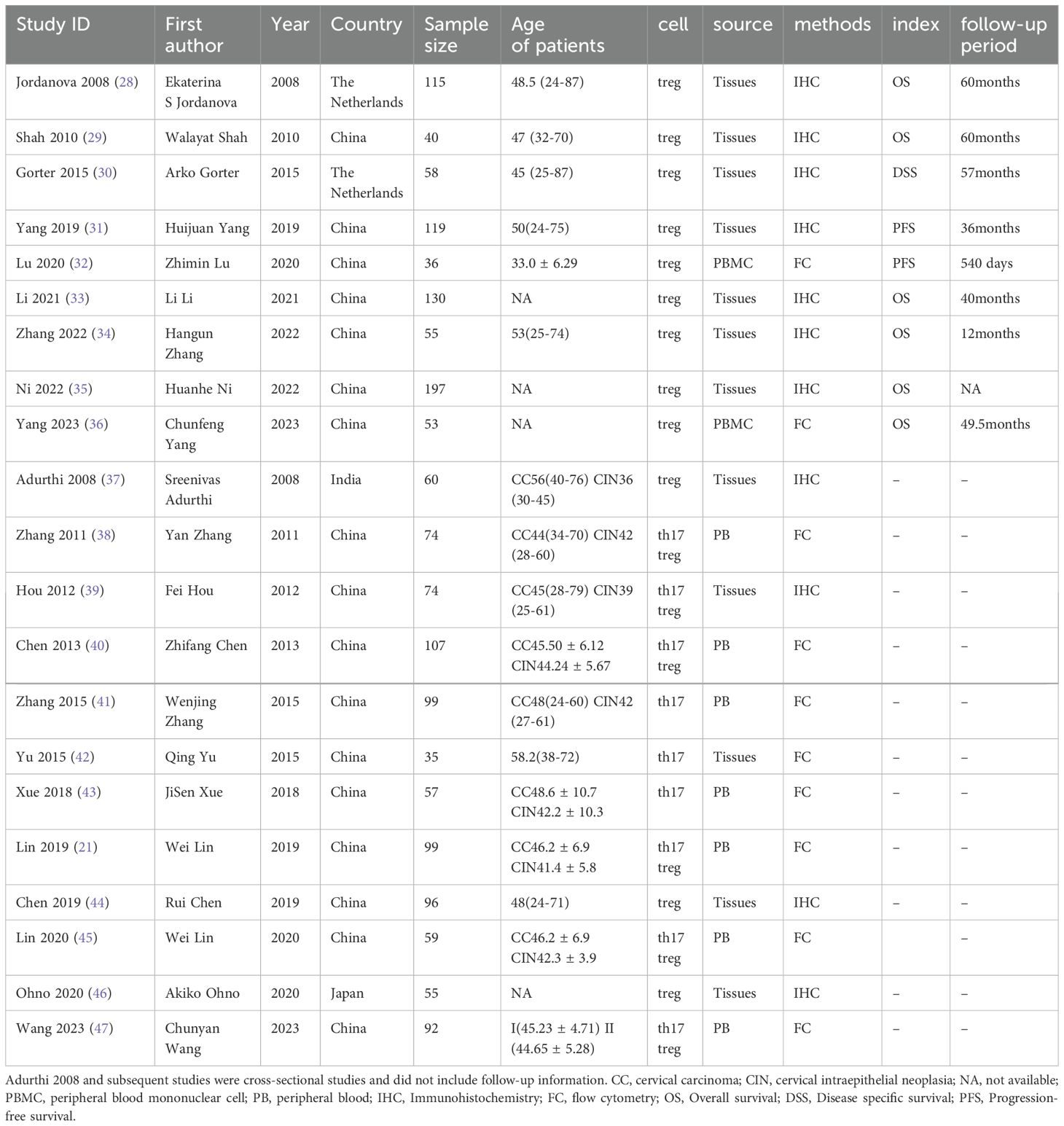
Two reviewers independently extracted basic characters and detailed information required for the research from the included studies. Disagreements were discussed and judged with the participation of the third reviewer. The table of basic Characteristics includes: first author, year, country, sample size, age of patients, cell, source of cells, method, index and follow-up period. Information about prognosis, including HR for survival and its 95% CI were also collected. For studies that did not provide HR directly, data were extracted from Kaplan–Meier (KM) plot using engauge digitizer (software downloaded from https://markummitchell.github.io/engauge-digitizer/), and obtain HR and 95% CI through the method recommend by Tierney etal (27). And preferentially extract HR from multivariate analysis, otherwise univariate analysis is chosen. Mean and standard deviation (SD) were extracted from the included studies to study the clinical characteristics of CC.
Assessment of risk of bias and sensitivity analysis
We used funnel plot, begg test and egger test to measure publication bias. Sensitivity analysis was performed using the one-by-one elimination method, which eliminated the included studies one by one, combined the other studies, and observed the changes in the pooled effect size before and after the exclusion. Publication bias and sensitivity analyses were performed using Stata/SE 12.0 for Window [32-bit].
Data synthesis
Meta-analysis was performed using Review Manager (RevMan) [Computer program]. Version 5.4. The Cochrane Collaboration, 2020. To study the relationship between the prognosis of CC patients and Treg cell (Th17 cell), the high Treg cell level was used as the experimental group and the low Treg cell level was used as the control group, and the HR and 95% CI of each study were merged. A two-tailed P-value <0.05 was considered statistically significant. If I2> 50% or p<0.10, the degree of heterogeneity was regarded as significant, and random-effects models were selected for analysis. Otherwise, data were merged using the fixed-effects model. Subgroup analyses were performed based on the source of the Treg cell (peripheral blood (PB) or tissue), the different prognostic index selected [Overall Survival (OS), Progression-Free Survival (PFS) or Disease Specific Survival (DSS)], and the different definitions of the Treg cell (foxp3 or other). For the prognostic studies of Th17 cell, no subgroup analyses were performed due to the small number of studies.
To investigate the relationship between Treg cell, Th17 cell, Th17/Treg cell and the clinical features of patients with CC, lymphatic metastasis, clinical stage, CIN, or CC were selected as study indicators. And it is carried out separately according to the cell (tumor-infiltrating or circulating). Mean and standard deviation (SD) of each study were merged to measure the differences of immune cells in patient with different clinical features.
Results
Study characteristics
A flowchart illustrating the study selection process is shown in Figure 1. Initially, 2949 records were identified through our search. After removing 1008 duplicate records, 1914 records remained. 1804 articles were excluded after reviewing the titles and abstracts, and 66 reports were selected for retrieval. We reviewed the full text of the remaining studies. There were 21 studies (21, 28–47) included in the review, 9 of which provided prognostic information. Main characteristics of the included studies were listed in Table 1. The included studies comprised 9 cohort studies and 12 cross-sectional studies, with a total of 2023 samples. Of these, 12 studies provided data on Treg cells, 3 studies provided data on Th17 cells, and the remaining 6 studies provided data on both Treg and Th17 cells. Twelve provided information on cells in tumor tissue and the remaining 9 studies provided information on cells in PBMC or PB. Eleven studies used immunofluorescence assay and 10 used flow cytometry.
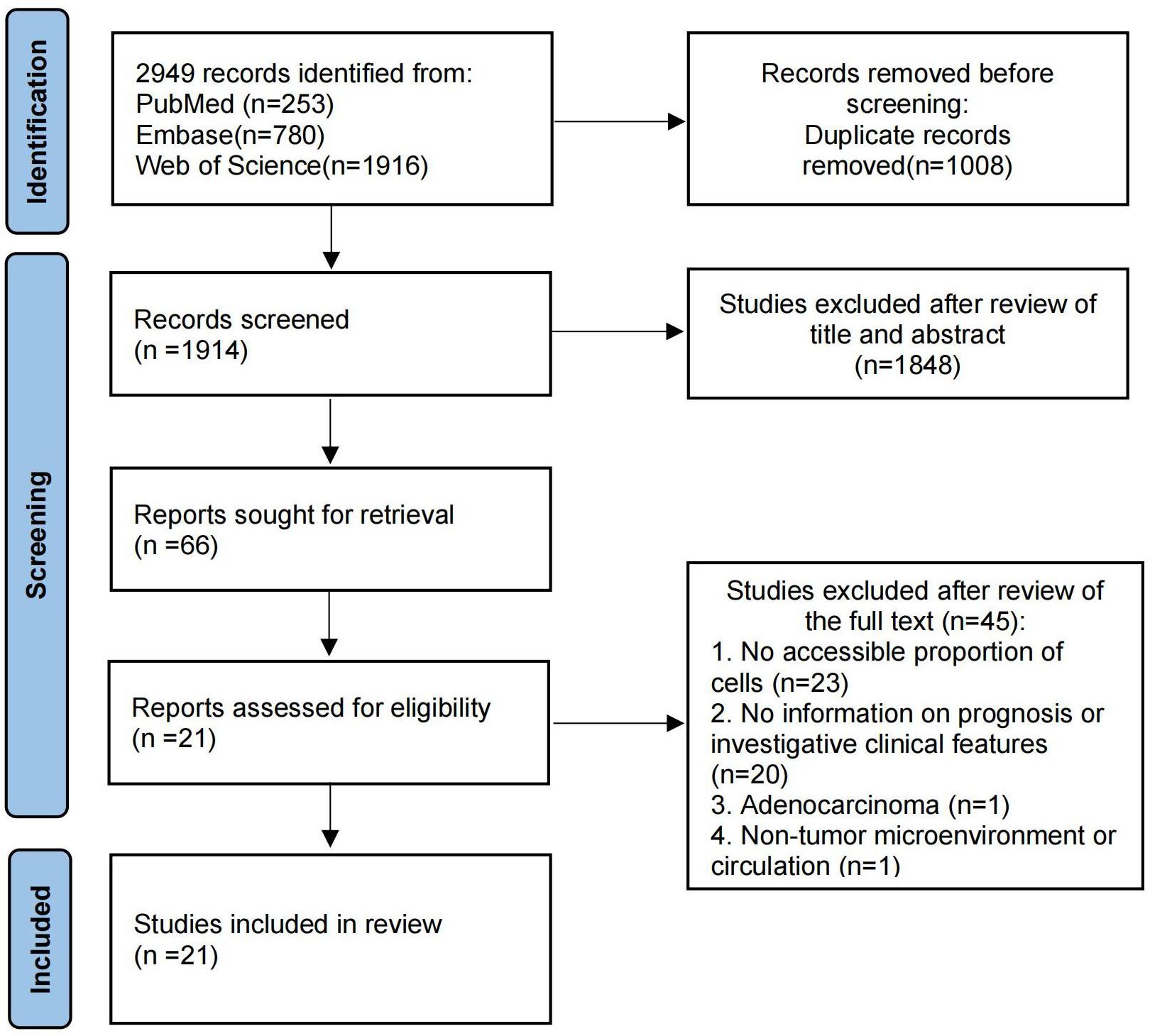
Figure 1. The flow chart of the article search and inclusion process following the PRISMA guidelines.
We utilized NOS and AHRQ tools for quality assessment. For the cohort studies, NOS scores showed: 1 article scored 8 points, 4 articles scored 7 points, and the remaining 4 articles scored 6 points. For cross-sectional studies, AHRQ scores showed: 1 article scored 8 points, 5 articles scored 7 points, and the remaining 6 articles scored 6 points. Although there is currently no uniform standard for the rating of NOS and AHRQ tools, we can still consider the quality of articles to be above medium.9 cohort studies used NOS for quality assessment, with an average score of 6.67. The remaining studies employed a quality assessment tool revised by AHRQ, with an average score of 6.58. Details of the quality assessment were provided in Supplementary Table 2.
The prognostic value of Treg and Th17 cells
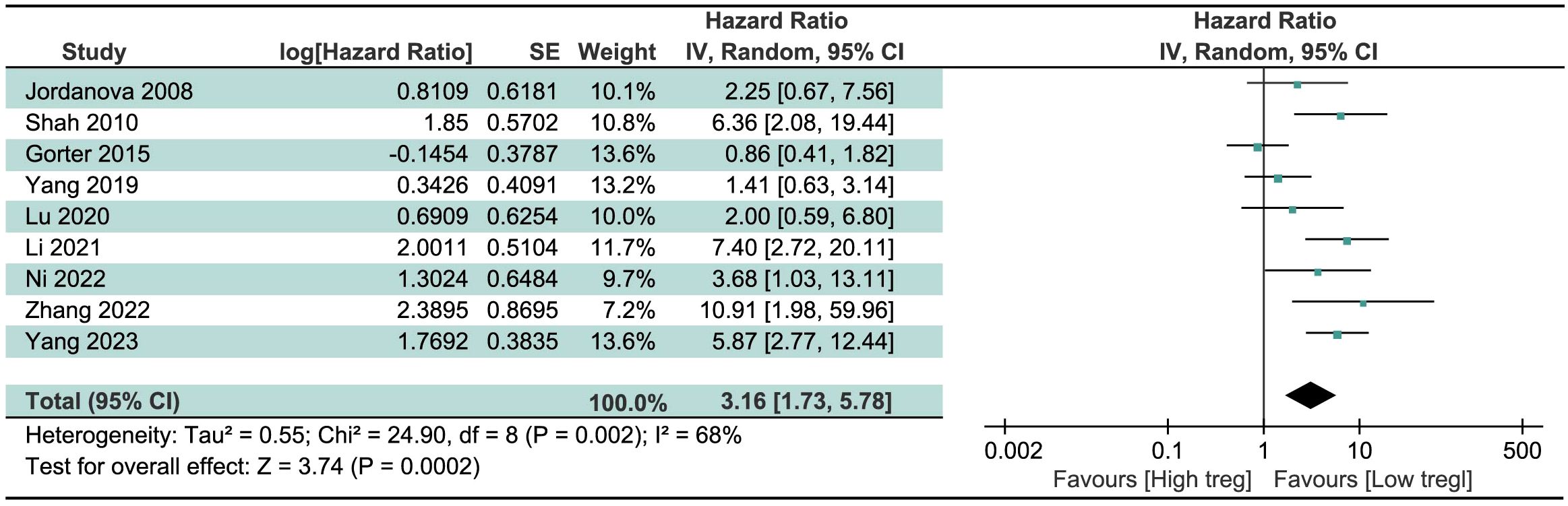
We pooled HR and 95% CI of 9 studies about the significance of Treg cells on the survival of CC patients, regardless of the source of Treg cells and the survival index chosen. Result in Figure 2 revealed that high levels of Treg cells were associated with a poor prognosis, indicative of shortened survival, in CC patients. The merged HR was 3.16 (95% CI=1.73 to 5.78, P=0.0002). Due to significant heterogeneity among studies (I2 = 68%, P=0.002), the random-effects model was chosen.
Similarly, we tried to pool HR and 95% CI studies on the prognostic value of Th17 cells in CC patients. However, we only found two relevant studies that were suitable for inclusion. One study suggested that high levels of Th17 cells were associated with a poor prognosis (48), while the other indicated the opposite effect, that high levels of Th17 cell with an improved prognosis (49). Therefore, it is not yet possible to draw conclusions about the prognostic effect of Th17 cells on CC patients, and more relevant studies are required for further investigation.
Subgroup analysis
Subgroup analyses were performed based on different sources of Treg cells, different survival index selected, and different definitions of Treg cells, the results were shown in Figure 3. Through subgroup analysis of different sources of Treg cells, we found that high levels of Treg cells in PB or tumor tissue were associated with poor prognosis in CC patients, with no significant difference observed between the two subgroups. This meant that Treg cells, both in the circulation and in the tumor microenvironment, indicate poor prognosis in cervical cancer. Analysis of different survival index found that high levels of Treg cells were associated with shortened OS, not PFS and DSS, and there were statistically significant differences between different subgroups. This indicated that the effect of Treg cells on cervical cancer is more reflected in overall survival, and may be less related to the recurrence of cervical cancer. High levels of Treg cells with different definitions were associated with poor prognosis, and there was no statistically significant difference between different definitions. This further indicated that Foxp3 is a highly representative surface molecule of Treg cells.

Figure 3. Forest plot presenting subgroup analyses for Treg. (A) Subgroup analyses according to different sources of Treg. (B) Subgroup analyses according to different survival index. (C) Subgroup analyses according to different definitions of Treg.
Publication bias and sensitivity analysis
The funnel plot (Figure 4A) showed that there was acceptable symmetry and heterogeneity. The begg test (Figure 4B) and egger test (Figure 4C) were generally consistent with the funnel plot, showing p value of 0.754 and 0.263, respectively, which were not statistically significant and did not have publication bias.
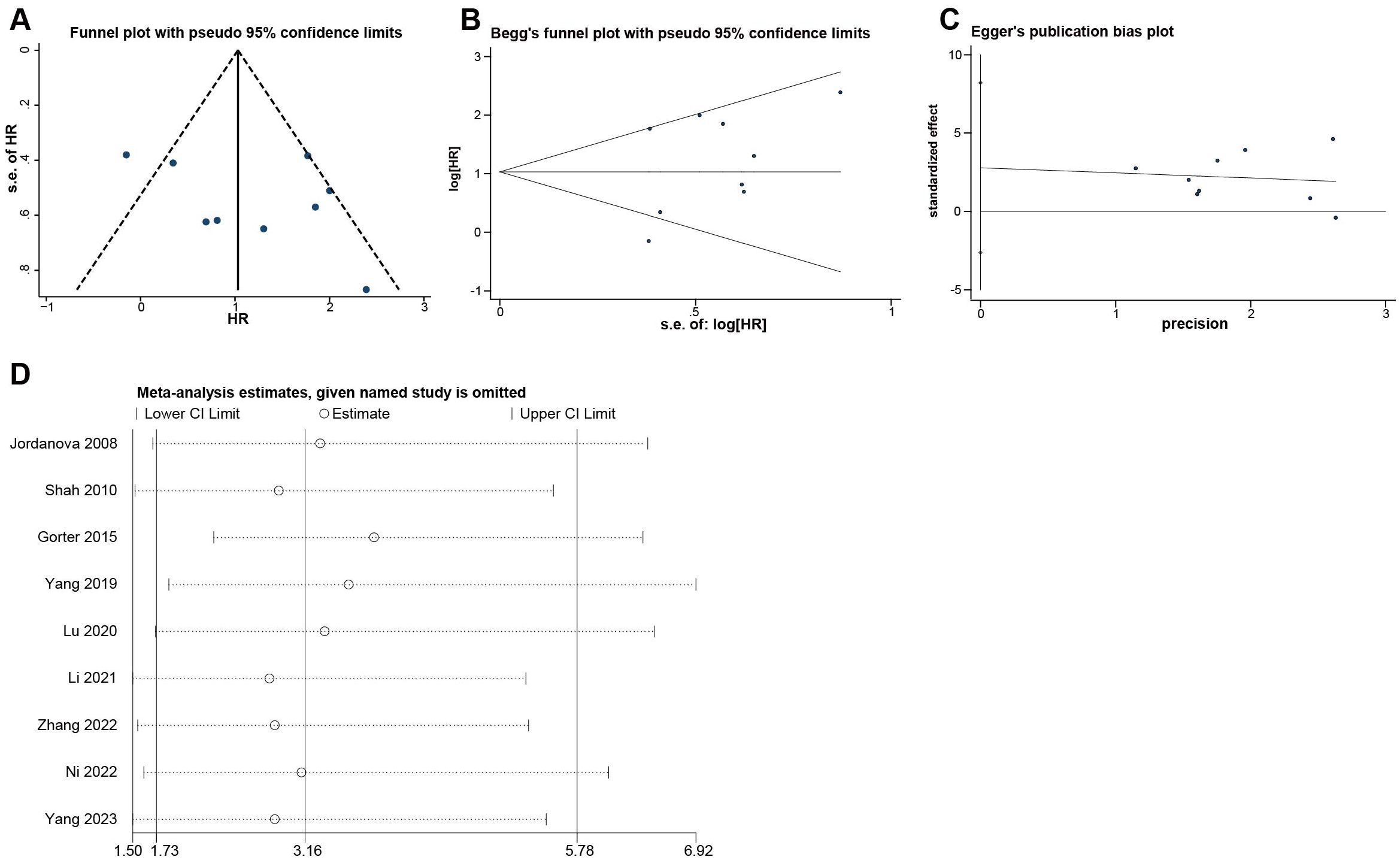
Figure 4. Evaluation of potential publication bias and Sensitivity analysis of the included researches on Tregs. (A) Funnel plot. (B) Begg’s funnel plot. (C) Egger’s publication bias plot. (D) Sensitivity analysis.
Sensitivity analysis (Figure 4D) was performed using the one-by-one elimination method. No matter which study is excluded, the 95% CI of the combined effect size was above 1.50, which proved that our study results were robust.
The value of Treg and Th17 cells, and Th17/Treg ratio in clinical features of CC patients
To further explore the relationship between Treg cells, Th17 cells, and Th17/Treg ratio and the prognosis of CC patients, we investigated their effect on the clinical features of CC patients. We mainly studied lymphoid metastasis, clinical stage, CIN and CC differences, and conducted analysis separately according to the cell source. The results showed that the Treg cells in PB in patients with FIGO stage II CC was higher than that in patients with stage I cancer (P<0.00001, Figure 5A). Patients with lymphoid metastases and higher FIGO stage have more Th17 cells in PB (P<0.0001, Figure 5B; P=0.02, Figure 5C). CC patients had higher levels of Treg cells in tumor tissue than CIN patients (P=0.01, Figure 5D). Although no significant difference of Th17/Treg ratio in PB was found between CIN and CC patients (P=0.36 Figure 5G), Th17/Treg ratio was elevated in both CIN and CC patients compared with controls (P<0.00001, Figure 5E; P=0.005, Figure 5F). Higher levels of Th17/Treg ratio have been found to be associated with lymphatic metastases (38) and higher FIGO stage (47), but the number of studies is too small to perform meta-analysis. No statistically significant differences were found in the remaining analyses (Supplementary Figure 1).
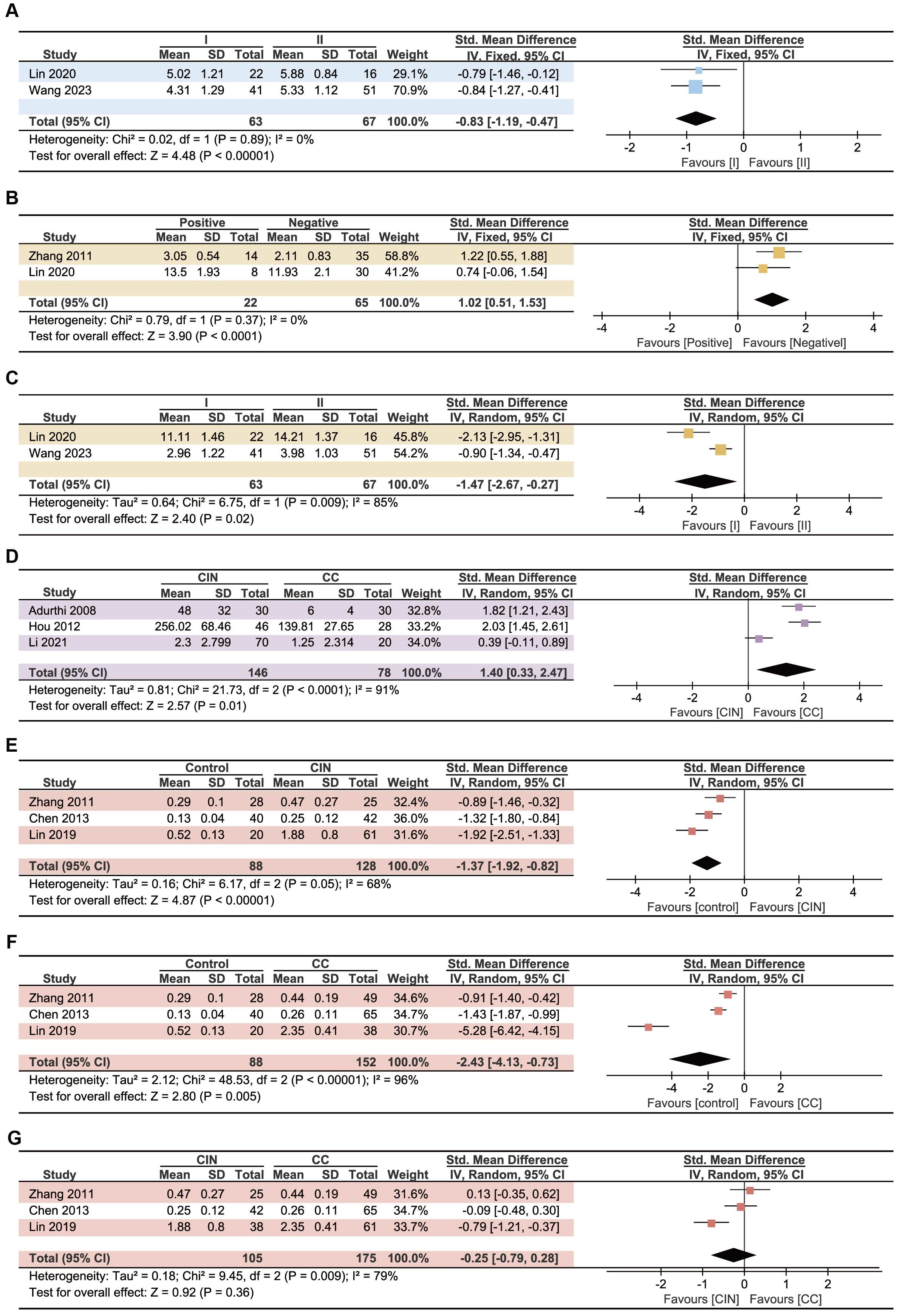
Figure 5. Forest plots assessing the association between Treg, Th17 and CC patients’ clinical features. (A) Relationship between Treg cells in PB and clinical stage. (B) Relationship between Th17 cells in PB and lymphoid metastases. (C) Relationship between Th17 cells in PB and clinical stage. (D) Differences of Treg cells in tumor tissue between CIN and CC patients. (E) Differences of Th17/Treg ratio between control and CIN patients. (F) Differences of Th17/Treg ratio between control and CC patients. (G) Differences of Th17/Treg ratio between CIN and CC patients.
Discussion
The prognostic value of Treg cells in cancer remains controversial, with high levels of Treg cells infiltration thought to be associated with a poor prognosis in most solid tumors, while opposite outcomes have been observed in head and neck cancers and colorectal cancers (50, 51). In CC, current research suggests that high levels of Treg cell are associated with a poor prognosis (52). However, the specific indicators of its impact, such as OS or DFS are still inconclusive. And most studies tend to focus on tumor-infiltrating Treg cells, with less attention paid to the impact of Treg cells in PB. We further determined the prognostic significance of Treg cell in CC patients through meta-analysis, and found that Treg cells in both tumor tissue and PB was negatively correlated with survival through subgroup analysis, and Treg cells has more of an impact on OS and is not statistically significant for metrics such as DFS. Foxp3, as a specific transcription factor for Treg cells, plays an important role in the development and functionality of Treg cells (53). Although Foxp3 was found to be expressed not only in Treg cells but also in some non-lymphocytes, including normal cells as well as cancer cells, we did not find a significant difference in the prognostic effect of Foxp3-defined Treg cell from other definitions (54).
The effect of Th17 cells on tumors is thought to be dual, but unfortunately the relationship between Th17 cells and survival in CC patients could not be meta-analyzed in our study due to the insufficient number of studies that could be included. However, in studies of the clinical characteristics of CC patients, we found that in CC patients with lymphatic metastases and higher FIGO stage, a higher density of Th17 cells was exhibited in PB. At the same time, we did not find a statistically significant association between Th17 cells infiltrating tumor tissue and the clinical stage of CC patients. It has been found that late plasticity makes mature Th17 cells have the characteristics of Th1, thereby exerting anti-tumor effects (55). Whether the difference in the results of our study of Th17 cells in PB and tumor tissues is related to the effect of TME on Th17 cells, which leads to a plasticity change in Th17 cells, is a question worth further exploring. In addition, Punt et al. found that IL-17 in tumor stroma is mainly expressed by neutrophils, mast cells, and innate lymphocytes, while Th17 cells are a secondary group of IL-17. Their study suggests that IL-17, which is predominantly expressed by neutrophils, primarily promotes tumor growth and is associated with poor prognosis in early disease (49). Whether this will have an impact on our findings needs to be further explored.
Although we did not find a difference in Th17/Treg ratio between CIN and CC in our study, it remains a mystery whether this ratio will be further imbalanced with tumor progression. Further exploration of the changes of Th17 and Treg cells in the process of tumor progression will not only help to understand the role they play in tumorigenesis and development, but also guide the immunotherapy of CC to a certain extent.
Although our research provides some useful information, there are still some limitations should be addressed. Firstly, we included studies that were highly heterogeneous and were described using a random-effects model. Possible sources of heterogeneity include differences in survival index selected, differences methods to detect cells and so on. High heterogeneity may affect the accuracy of the study results. Secondly, we were unable to obtain data on individual patients in the studies, and we extracted data from the Kaplan–Meier (KM) plot for calculation if HR values were not provided in the prognostic study, which may cause some error. Thirdly, for prognostic studies we only included studies that provided HR or extractable HR, and in studies of clinical features we only focused on SMD, so there may have been a lot of studies on prognosis and clinical features that were excluded. Fourthly, the prognostic role of Treg cell may vary due to clinical stage, molecular subtype, etc. (50), but limited by the number of studies we included and insufficient information, no relevant subgroup analyses were conducted. Inadequate subgroup analyses may compromise the full understanding of the relationship between Tregs and prognosis of cervical cancer and prevent the discovery of sources of heterogeneity. Fifthly, we also excluded the study of other types of cervical cancer (like adenocarcinoma), which also makes the study limited. Finally, although the funnel plot, Begg test, and Egger test indicate no significant publication bias, there is still the possibility of error due to the small number of studies and large heterogeneity. Sensitivity analysis and exclusion of low-quality studies were used to deal with publication bias.
Our study demonstrated that Treg cells have a negative impact on prognosis in CC, primarily reflected by a reduction in OS. Additionally, elevated levels of Treg cells in peripheral blood have been linked to more advanced clinical stages of the disease. Although we were unable to draw firm conclusions about the prognostic value of Th17 cells for CC, we found that patients with higher clinical stages and lymphatic metastases had higher levels of Th17 cell in their peripheral blood. In addition, we demonstrated that Th17/Treg ratio was higher than controls in both CIN and CC. But how Treg/Th17 specifically affect CC tumor cells still require more experiments, including in vivo and in vitro experiments, to transform this finding into clinical trials. In the future, co-cultured experiments of Treg/Th17 and cervical cancer cell are needed to be conduct. Based on robust in vitro experimental evidence and an understanding of the mechanisms of interaction between the two types of cells, animal experiments to elaborate on the deep mechanisms of their relationship with patient prognosis in vivo are indispensable. Therefore, our research findings do indeed have significant limitations when translated into clinical applications, which also motivates us to further refine our studies.
In the future, we hope to promote this study by conducting cohort study in multiple centers to expand the sample size, verifying the conclusion of this study in real world at the same time. Subsequently, starting from ample clinical evidence, we can guide the experimental design and practice of cellular and animal-based basic experiments. This will further prove or elucidate the role of Th17/Treg in mediating the efficacy of PD-1/PD-L1 at the cellular/animal level. At the same time, in both in vivo and in vitro experiments, we will continuously improve the methods for measuring the Treg, Th17 and their ratio, and ultimately use the existing clinical cohorts to conduct specific and effective tests. In a word, further study of Treg and Th17 cell, and Th17/Treg ratio on tumors is conducive to a better understanding of tumor development and can also better guide tumor immunotherapy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
JWZ: Conceptualization, Data curation, Formal analysis, Visualization, Writing – original draft. JJZ: Conceptualization, Data curation, Formal analysis, Visualization, Writing – review & editing. ZTG: Data curation, Validation, Writing – original draft. XML: Data curation, Validation, Writing – original draft. TL: Writing – review & editing. ML: Writing – review & editing. CLZ: Conceptualization, Funding acquisition, Supervision, Writing – original draft. LZ: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by National Natural Science Foundation of China (Grant No. 82002899, 32100564, 82172883), the Science and Technology Planning Project of Guangdong Province (No. 2021B1212040006), Guangdong Basic and Applied Basic Research Foundation (Grant No. 2023A1515012662, 2022A1515220110, 2022A1515012444), Open Funds of State Key Laboratory of Oncology in South China (Grant No. HN2023-01), the Research Start-up Fund of the Seventh Affiliated Hospital, Sun Yat-sen University (Grant No.ZSQYRSSFAR0001), Shenzhen Medical Research Fund, Shenzhen, China (Grant No. A2303011) the Shenzhen Science and technology Program(Grant No. JCYJ20220530144607017) and Shenzhen Key Laboratory of Chinese Medicine Active substance screening and Translational Research (Grant No. ZDSYS20220606100801003).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1442103/full#supplementary-material
References
1. Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob Health. (2019) 8:e191–203. doi: 10.1016/S2214-109X(19)30482-6
2. Golia D’Augè T, Cuccu I, Etrusco A, D’Amato A, Laganà AS, D’Oria O, et al. State of the art on HPV-related cervical lesions. Ital J Gynaecol Obstet. (2024) 36:135. doi: 10.36129/jog.2024.161
3. Rahangdale L, Mungo C, O’Connor S, Chibwesha CJ, Brewer NT. Human papillomavirus vaccination and cervical cancer risk. BMJ. (2022) 379:e070115. doi: 10.1136/bmj-2022-070115
4. Revathidevi S, Murugan AK, Nakaoka H, Inoue I, Munirajan AK. APOBEC: A molecular driver in cervical cancer pathogenesis. Cancer Lett. (2021) 496:104–16. doi: 10.1016/j.canlet.2020.10.004
5. Ferrall L, Lin KY, Roden RBS, Hung C-F, Wu T-C. Cervical cancer immunotherapy: Facts and hopes. Clin Cancer Res. (2021) 27:4953–73. doi: 10.1158/1078-0432.CCR-20-2833
6. Bogani G, Sopracordevole F, Ciavattini A, Ghelardi A, Vizza E, Vercellini P, et al. HPV-related lesions after hysterectomy for high-grade cervical intraepithelial neoplasia and early-stage cervical cancer: A focus on the potential role of vaccination. Tumori J. (2023) 110(2):139–45. doi: 10.1177/03008916231208344
7. Qu X, Wang Y, Jiang Q, Ren T, Guo C, Hua K, et al. Interactions of Indoleamine 2,3-dioxygenase-expressing LAMP3+ dendritic cells with CD4+ regulatory T cells and CD8+ exhausted T cells: synergistically remodeling of the immunosuppressive microenvironment in cervical cancer and therapeutic implications. Cancer Commun. (2023) 43:1207–28. doi: 10.1002/cac2.12486
8. Savage PA, Klawon DEJ, Miller CH. Regulatory T cell development. Annu Rev Immunol. (2020) 38:421–53. doi: 10.1146/annurev-immunol-100219-020937
9. Wu M-Y, Kuo T-Y, Ho H-N. Tumor-infiltrating lymphocytes contain a higher proportion of FOXP3(+) T lymphocytes in cervical cancer. J Formos Med Assoc. (2011) 110:580–6. doi: 10.1016/j.jfma.2011.07.005
10. Sakaguchi S, Mikami N, Wing JB, Tanaka A, Ichiyama K, Ohkura N. Regulatory T cells and human disease. Annu Rev Immunol. (2020) 38:541–66. doi: 10.1146/annurev-immunol-042718-041717
11. Tang Y, Zhang AXJ, Chen G, Wu Y, Gu W. Prognostic and therapeutic TILs of cervical cancer—current advances and future perspectives. Mol Ther Oncolytics. (2021) 22:410–30. doi: 10.1016/j.omto.2021.07.006
12. Zou W, Restifo NP. TH17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. (2010) 10:248–56. doi: 10.1038/nri2742
13. Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. (2009) 114(6):1141–9. doi: 10.1182/blood-2009-03-208249
14. Walch-Rückheim B, Mavrova R, Henning M, Vicinus B, Kim Y-J, Bohle RM, et al. Stromal Fibroblasts Induce CCL20 through IL6/C/EBPβ to Support the Recruitment of Th17 Cells during Cervical Cancer Progression. Cancer Res. (2015) 75:5248–59. doi: 10.1158/0008-5472.CAN-15-0732
15. Walch-Rückheim B, Ströder R, Theobald L, Pahne-Zeppenfeld J, Hegde S, Kim Y-J, et al. Cervical cancer-instructed stromal fibroblasts enhance IL23 expression in dendritic cells to support expansion of th17 cells. Cancer Res. (2019) 79:1573–86. doi: 10.1158/0008-5472.CAN-18-1913
16. Theobald L, Stroeder R, Melchior P, Iordache II, Tänzer T, Port M, et al. Chemoradiotherapy-induced increase in Th17 cell frequency in cervical cancer patients is associated with therapy resistance and early relapse. Mol Oncol. (2021) 15:3559–77. doi: 10.1002/1878-0261.13095
17. Knochelmann HM, Dwyer CJ, Bailey SR, Amaya SM, Elston DM, Mazza-McCrann JM, et al. When worlds collide: Th17 and treg cells in cancer and autoimmunity. Cell Mol Immunol. (2018) 15:458–69. doi: 10.1038/s41423-018-0004-4
18. Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. (2008) 29:44–56. doi: 10.1016/j.immuni.2008.05.007
19. Downs-Canner S, Berkey S, Delgoffe GM, Edwards RP, Curiel T, Odunsi K, et al. Suppressive IL-17A+Foxp3+ and ex-Th17 IL-17AnegFoxp3+ treg cells are a source of tumour-associated treg cells. Nat Commun. (2017) 8:14649. doi: 10.1038/ncomms14649
20. Marshall EA, Ng KW, Kung SHY, Conway EM, Martinez VD, Halvorsen EC, et al. Emerging roles of T helper 17 and regulatory T cells in lung cancer progression and metastasis. Mol Cancer. (2016) 15:67. doi: 10.1186/s12943-016-0551-1
21. Lin W, Niu Z, Zhang H, Kong Y, Wang Z, Yang X, et al. Imbalance of th1/th2 and th17/treg during the development of uterine cervical cancer. Int J Clin Exp Pathol. (2019) 12:3604–12.
22. Mayadev J, Nunes AT, Li M, Marcovitz M, Lanasa MC, Monk BJ. CALLA: Efficacy and safety of concurrent and adjuvant durvalumab with chemoradiotherapy versus chemoradiotherapy alone in women with locally advanced cervical cancer: a phase III, randomized, double-blind, multicenter study. Int J Gynecol Cancer. (2020) 30:1065–70. doi: 10.1136/ijgc-2019-001135
23. Lorusso D, Xiang Y, Hasegawa K, Scambia G, Leiva M, Ramos-Elias P, et al. Pembrolizumab or placebo with chemoradiotherapy followed by pembrolizumab or placebo for newly diagnosed, high-risk, locally advanced cervical cancer (ENGOT-cx11/GOG-3047/KEYNOTE-A18): a randomised, double-blind, phase 3 clinical trial. Lancet. (2024) 403:1341–50. doi: 10.1016/S0140-6736(24)00317-9
24. García E, Ayoub N, Tewari KS. Recent breakthroughs in the management of locally advanced and recurrent/metastatic cervical cancer. J Gynecol Oncol. (2024) 35:e30. doi: 10.3802/jgo.2024.35.e30
25. Ottawa Hospital Research Institute. Available online at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed March 31, 2024).
26. Rostom A, Dubé C, Cranney A, Saloojee N, Sy R, Garritty C, et al. Appendix D. Quality assessment forms, in: Celiac Disease (2004). Agency for Healthcare Research and Quality (US. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK35156/ (Accessed March 31, 2024).
27. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
28. Jordanova ES, Gorter A, Ayachi O, Prins F, Durrant LG, Kenter GG, et al. Human leukocyte antigen class I, MHC class I chain-related molecule a, and CD8+/regulatory T-cell ratio: Which variable determines survival of cervical cancer patients? Clin Cancer Res. (2008) 14:2028–35. doi: 10.1158/1078-0432.CCR-07-4554
29. Shah W, Yan X, Jing L, Zhou Y, Chen H, Wang Y. A reversed CD4/CD8 ratio of tumor-infiltrating lymphocytes and a high percentage of CD4(+)FOXP3(+) regulatory T cells are significantly associated with clinical outcome in squamous cell carcinoma of the cervix. Cell Mol Immunol. (2011) 8:59–66. doi: 10.1038/cmi.2010.56
30. Gorter A, Prins F, van Diepen M, Punt S, van der Burg SH. The tumor area occupied by tbet+ cells in deeply invading cervical cancer predicts clinical outcome. J Transl Med. (2015) 13:295. doi: 10.1186/s12967-015-0664-0
31. Yang H, Ye S, Goswami S, Li T, Wu J, Cao C, et al. Highly immunosuppressive HLADRhi regulatory T cells are associated with unfavorable outcomes in cervical squamous cell carcinoma. Int J Cancer. (2020) 146:1993–2006. doi: 10.1002/ijc.32782
32. Lu Z, Zhu M, Marley JL, Bi K, Wang K, Zhai M, et al. The combined action of monocytic myeloid-derived suppressor cells and mucosal-associated invariant T cells promotes the progression of cervical cancer. Int J Cancer. (2021) 148:1499–507. doi: 10.1002/ijc.33411
33. Li L, Xu X-T, Wang L-L, Qin S-B, Zhou J-Y. Expression and clinicopathological significance of foxp3 and VISTA in cervical cancer. Am J Transl Res. (2021) 13:10428–38.
34. Zhang H, Tan S, Fang C, Zhang Q, Cao X, Liu Y. PD-1/PD-l1 correlates with the efficacy of the treatment of concurrent chemoradiotherapy in cervical cancer. Front Oncol. (2022) 12:858164. doi: 10.3389/fonc.2022.858164
35. Ni H, Zhang H, Li L, Huang H, Guo H, Zhang L, et al. T cell-intrinsic STING signaling promotes regulatory T cell induction and immunosuppression by upregulating FOXP3 transcription in cervical cancer. J Immunother Cancer. (2022) 10:e005151. doi: 10.1136/jitc-2022-005151
36. Yang C, Zhang Y, Wang R, Cheng B, Wu Y, Fu X. IL-10+CD19+ regulatory B cells induce CD4+foxp3+regulatory T cells in serum of cervical cancer patients. Autoimmunity. (2024) 57:2290909. doi: 10.1080/08916934.2023.2290909
37. Adurthi S, Krishna S, Mukherjee G, Bafna UD, Devi U, Jayshree RS. Regulatory T cells in a spectrum of HPV-induced cervical lesions: Cervicitis, cervical intraepithelial neoplasia and squamous cell carcinoma. Am J Reprod Immunol. (2008) 60:55–65. doi: 10.1111/j.1600-0897.2008.00590.x
38. Zhang Y, Ma D, Zhang Y, Tian Y, Wang X, Qiao Y, et al. The imbalance of th17/treg in patients with uterine cervical cancer. Clin Chim Acta. (2011) 412:894–900. doi: 10.1016/j.cca.2011.01.015
39. Hou F, Li Z, Ma D, Zhang W, Zhang Y, Zhang T, et al. Distribution of th17 cells and foxp3-expressing T cells in tumor-infiltrating lymphocytes in patients with uterine cervical cancer. Clin Chim Acta. (2012) 413:1848–54. doi: 10.1016/j.cca.2012.07.012
40. Chen Z, Ding J, Pang N, Du R, Meng W, Zhu Y, et al. The th17/treg balance and the expression of related cytokines in uygur cervical cancer patients. Diagn Pathol. (2013) 8:61. doi: 10.1186/1746-1596-8-61
41. Zhang W, Tian X, Mumtahana F, Jiao J, Zhang T, Croce KD, et al. The existence of th22, pure th17 and th1 cells in CIN and cervical cancer along with their frequency variation in different stages of cervical cancer. BMC Cancer. (2015) 15:717. doi: 10.1186/s12885-015-1767-y
42. Yu Q, Lou X, He Y. Preferential recruitment of th17 cells to cervical cancer via CCR6-CCL20 pathway. PloS One. (2015) 10:e0120855. doi: 10.1371/journal.pone.0120855
43. Xue J, Wang Y, Chen C, Zhu X, Zhu H, Hu Y. Effects of th17 cells and IL-17 in the progression of cervical carcinogenesis with high-risk human papillomavirus infection. Cancer Med. (2018) 7:297–306. doi: 10.1002/cam4.1279
44. Chen R, Gong Y, Zou D, Wang L, Yuan L, Zhou Q. Correlation between subsets of tumor-infiltrating immune cells and risk stratification in patients with cervical cancer. PeerJ. (2019) 7:e7804. doi: 10.7717/peerj.7804
45. Lin W, Zhang H-L, Niu Z-Y, Wang Z, Kong Y, Yang X-S, et al. The disease stage-associated imbalance of th1/th2 and th17/treg in uterine cervical cancer patients and their recovery with the reduction of tumor burden. BMC Womens Health. (2020) 20:126. doi: 10.1186/s12905-020-00972-0
46. Ohno A, Iwata T, Katoh Y, Taniguchi S, Tanaka K, Nishio H, et al. Tumor-infiltrating lymphocytes predict survival outcomes in patients with cervical cancer treated with concurrent chemoradiotherapy. Gynecol Oncol. (2020) 159:329–34. doi: 10.1016/j.ygyno.2020.07.106
47. Wang C, Fan L, Hu J. Comparison of lymphocyte subsets, inflammatory factors and postoperative infection in cervical cancer at stage I and stage II. J Biol Regulators Homeostatic Agents. (2023) 37:3523–8. doi: 10.23812/j.biol.regul.homeost.agents.20233707.348
48. Yu Q, Lou X-M, He Y. Prediction of local recurrence in cervical cancer by a cox model comprised of lymph node status, lymph-vascular space invasion, and intratumoral th17 cell-infiltration. Med Oncol. (2014) 31:795. doi: 10.1007/s12032-013-0795-1
49. Punt S, Fleuren GJ, Kritikou E, Lubberts E, Trimbos JB, Jordanova ES, et al. Angels and demons: Th17 cells represent a beneficial response, while neutrophil IL-17 is associated with poor prognosis in squamous cervical cancer. OncoImmunology. (2015) 4:e984539. doi: 10.4161/2162402X.2014.984539
50. Shang B, Liu Y, Jiang S, Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: A systematic review and meta-analysis. Sci Rep. (2015) 5:15179. doi: 10.1038/srep15179
51. Cho JH, Lim YC. Prognostic impact of regulatory T cell in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Oral Oncol. (2021) 112:105084. doi: 10.1016/j.oraloncology.2020.105084
52. Hu J, Wu X, Huang P, Teng F, Wang Y, Xue F. The proportion and prognostic significance of T-regulatory cells in patients with gynecological cancers: A systematic review and meta-analysis. J Cancer. (2020) 11:3340–8. doi: 10.7150/jca.42472
53. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. (2008) 133:775–87. doi: 10.1016/j.cell.2008.05.009
54. Martin F, Ladoire S, Mignot G, Apetoh L, Ghiringhelli F. Human FOXP3 and cancer. Oncogene. (2010) 29:4121–9. doi: 10.1038/onc.2010.174
Keywords: uterine cervical neoplasms, uterine cervical dysplasia, regulatory T-lymphocytes, Th17 cells, prognosis
Citation: Zhang J, Zhan J, Guan Z, Lin X, Li T, Li M, Zhang C and Zhong L (2024) The prognostic value of Th17/Treg cell in cervical cancer: a systematic review and meta-analysis. Front. Oncol. 14:1442103. doi: 10.3389/fonc.2024.1442103
Received: 01 June 2024; Accepted: 26 August 2024;
Published: 11 September 2024.
Edited by:
Andrea Giannini, Umberto 1 Hospital, ItalyReviewed by:
Ilaria Cuccu, Sapienza University of Rome, ItalyMarcia Antoniazi Michelin, Universidade Federal do Triângulo Mineiro, Brazil
Ma Cailing, Memorial Sloan Kettering Cancer Center, United States
Copyright © 2024 Zhang, Zhan, Guan, Lin, Li, Li, Zhang and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Zhong, emhvbmdsaUBzeXN1c2guY29t; Changlin Zhang, emhhbmdjaGFuZ2xpbkBzeXN1c2guY29t
†These authors have contributed equally to this work and share first authorship
 Jingwei Zhang
Jingwei Zhang Jijie Zhan
Jijie Zhan Ziting Guan
Ziting Guan Xinmei Lin
Xinmei Lin Tian Li
Tian Li Miao Li
Miao Li Changlin Zhang
Changlin Zhang Li Zhong
Li Zhong