- 1Department of Hematology, The 940th Hospital of Joint Logistics Support Force of Chinese People’s Liberation Army, Lanzhou, China
- 2Department of Pathology, The 940th Hospital of Joint Logistics Support Force of Chinese People’s Liberation Army, Lanzhou, China
- 3Department of Diagnostic Radiology, The 940th Hospital of Joint Logistics Support Force of Chinese People’s Liberation Army, Lanzhou, China
Background: Hemangiopericytoma (HPC) is a rare secondary tumor after allogeneic hematopoietic stem cell transplantation (allo-HSCT), which has not been reported in the literature. Herein, we reported a case of HPC after allo-HSCT.
Case description: We reported a case of a middle-aged female patient with primary plasma cell leukemia who presented lumbosacral pain and right lower limb pain and numbness on1684 days post-transplant. She underwent an MRI of the lumbar spine, which showed abnormal signal intensity in the spinal canal at the second through fifth lumbar spine vertebral levels. The patient was diagnosed with HPC based on a pathological biopsy of the diseased tissue in the spinal canal. Radiotherapy was administered to the lesion in the second through fifth lumbar vertebrae. The patient experienced less numbness and pain.
Conclusion: According to the literature, this is the first reported case of post-transplant HPC. Therefore, attention should be paid to secondary tumors after transplantation, especially rare tumors.
Introduction
Secondary tumors after allogeneic hematopoietic stem cell transplantation (allo-HSCT) have become an important factor affecting the long-term survival of patients (1). Patients after allo-HSCT have a 1.7-2.1 times higher risk of developing non-hematological second malignancies than the general population (2, 3). 5-10% of late deaths after allo-HSCT are caused by secondary tumors. Secondary tumors after allo-HSCT include cancers of the esophagus, thyroid and skin (2, 4, 5). However, to our knowledge, hemangiopericytoma (HPC) after hematopoietic stem cell transplantation (allo-HSCT) has not been reported. Herein, we report a first case of HPC after allo-HSCT, detailing the clinical and pathologic findings, treatment and follow-up. Additionally, the literature related to HPC was reviewed.
Case description
Clinical findings
A 48-year-old woman was diagnosed with primary plasma cell leukemia by bone marrow morphology and immunotyping (Figure 1). Her karyotype is 46, XX. The patient underwent bortezomib, doxorubicin liposomes, dexamethasone (PAD) chemotherapy regimen and achieved complete remission. The specific drugs and dosages of PAD regimen are bortezomib 2mg on d1, d4, d8, d11, doxorubicin liposomes 40mg on d1, dexamethasone 40mg on d1-4, d8-11. She received four 21-d cycles of PAD chemotherapy regimen. The patient went into complete remission as evidenced by a bone marrow biopsy. Then, she underwent HLA-matched sibling transplantation from her brother. The conditioning regimen consisted of fludarabine 50mg/d (-5d, -4d, -3d, -2d) and melphalan 150mg/d (-4d, -3d). She received 5.87×108 mononuclear cells/kg and 2.34×106 CD34+ cells/kg. The patient received a combination of cyclosporine, mycophenolate mofetil, and short-course MTX to prevent graft versus host disease (GVHD) after allo-HSCT. The patient underwent granulocyte implantation on day 14 and platelet implantation on day 16 after allo-HSCT. She underwent bone marrow cell morphology on day 28 after allo-HSCT and the result showed a complete remission. The patient’s chimerism was complete donor type. Since then, the patient’s condition has been stable at regular check-ups. She received lenalidomide maintenance therapy until one year after allo-HSCT. The patient developed oral chronic GVHD after 13 months of transplantation, which resolved after treatment with prednisone. Since then, the patient has led a normal life.
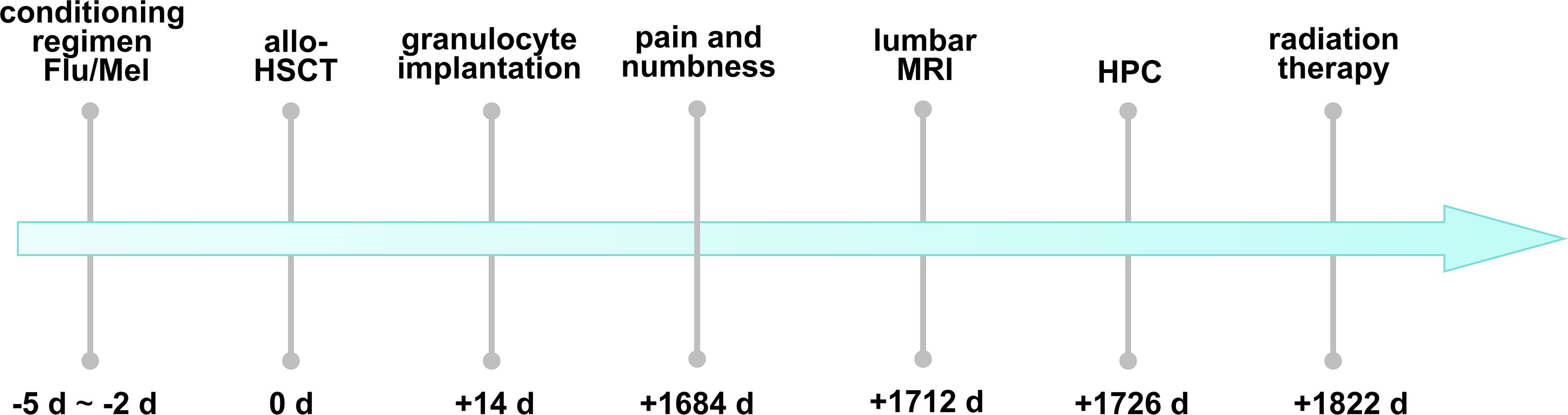
Figure 1. The timeline of the patient’s discomfort after allo-HSCT and the diagnosis and treatment of HPC. Flu, fludarabine; Mel, melphalan; allo-HSCT, allogeneic hematopoietic stem cell transplantation; MRI, magnetic resonance imaging; HPC, hemangiopericytoma.
On 1684 d after allo-HSCT, the patient presented pain in the lumbosacral region and numbness in the right lower limb without any obvious cause, and the muscle strength in her limbs was normal. The patient’s pain could not be alleviated after rest. Physical examination on admission revealed spinous process and paravertebral tenderness in the second through fifth lumbar vertebrae. The patient had skin numbness and decreased sensation below the middle and lower third of the right thigh. Therefore, she underwent lumbar MRI on 1712 days after allo-HSCT. The result suggested that there was an abnormal signal intensity in the patient’s spinal canal at the level of the second through fifth lumbar vertebrae, and space-occupying lesions were considered (Figure 2). She underwent lumbar intraspinal lesion resection under general anesthesia on 1720 days after allo-HSCT and the lesion was found to be located within the dura.

Figure 2. Magnetic resonance imaging (MRI) of the patient’s thoracic and lumbar vertebrae on the admission. Lumbar spine magnetic resonance imaging revealed a space-occupying lesion at the level of the second through fifth lumbar vertebrae. T1-weighted fluid-attenuated inversion recovery MRI (A) showed low signal intensity and inhomogeneously low signal intensity on T2-weighted MRI (B). The lesion (indicated by yellow lines) showed a remarkable enhancement on contrast-enhanced T1-weighted MRI (C).
Pathological findings
The pathological results of the resected tissue in the spinal canal of the patient showed that the tumor cells were densely distributed, with short fusiform cells, round nuclei and inconsistent sizes (Figure 3A). The mitotic figures of the tumor cells were >2/10 high-magnification fields and the tumor tissues surrounded the blood vessels. The results of immunohistochemical staining of the patient were as follows (Figures 3B–F): tumor cells were positive for vimentin, CD56, Fli-1 (partially weak), ERG (weak), p53 (small amount of weak), Ki67 (30%) while they were negative for CKp, Syn, S-100, SOX-10, CD99, MelanA, Calretinin, alpha-inhibin, GFAP, CD20, CD3, STAT-6, Desmin, SATB-2, LCA, CD34, O1ig-2. The pathological diagnosis of the patient was HPC, WHO grade 3 on 1822 days after allo-HSCT.
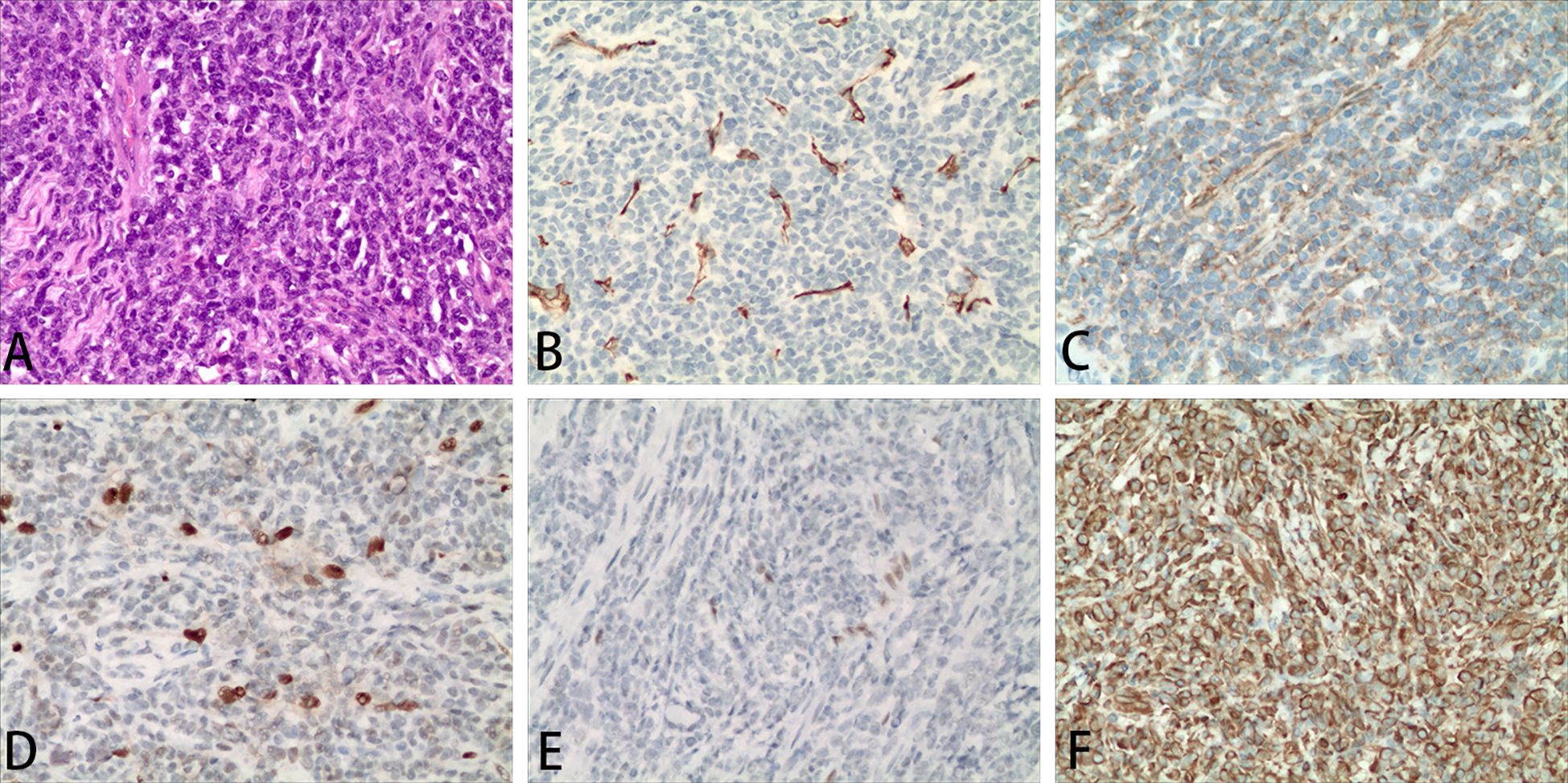
Figure 3. Representative images of the diseased tissue in the lumbar spinal canal of the patient. Hematoxylin and eosin staining of the diseased tissue (A). Representative immunohistochemical staining for CD34 (B), CD56 (C), ERG (D), Fli-1 (E), vimentin (F). Magnification 200×.
Treatment and follow-up
On 1822 days post-transplant, the patient received radiation therapy to the lesions of the lumbar spinal canal at the level of the second through fifth lumbar vertebrae. Gross tumor volume (GTV) is the location and range of lesions visible on CT. The planning target volume (PTV) was GTV with a 5mm expansion. The PTV prescription dose is 4500cGy/25f. The radiation oncologist reviewed the target volumes and treatment plans. Following radiation therapy, the patient experienced relief from pain and numbness in the lumbosacral region and right lower limb.
Discussion
It has been reported that the latency period of secondary tumors after allo-HSCT is 3-5 years (6). The 10-year cumulative incidence of secondary tumors after allo-HSCT ranged from 1.0% to 27% (5, 7–11). Several retrospective studies have concluded that the time from transplantation to the occurrence of secondary tumors is 6.7-11.7 years. Secondary tumors are found in patients aged 23.4-61 years after transplantation (5, 10, 11). A large clinical study from Europe enrolled 220,617 HSCT patients over 14 years and found that 1.8% patients developed secondary tumors with a median age at diagnosis of 59.1 years (12).
In our center, 320 patients have received allo-HSCT in the past 10 years and 182 patients have survived for more than 5 years. At present, 1.65% patients after allo-HSCT developed a secondary tumor, with a time range of 4.3 to 11.3 years and a median age of 48.2 years. The time of occurrence of secondary tumors in patients after allo-HSCT in our center was consistent with reports (10, 13). Thyroid cancer is the most common and earliest occurrence after allo-HSCT. Therefore, ultrasounds are recommended to screen for thyroid cancer every year (5, 14).
We report a patient diagnosed with intraspinal anaplastic HPC following allo-HSCT, which is the first reported case. HPC, first described in 1928, is a malignant tumor derived from perivascular cells and usually occurs in bone and soft tissue (15). According to the WHO pathological classification, HPC is WHO grade 2 and anaplastic HPC is WHO grade 3 (16). HPC often occurs in the intracranial space (17, 18) and rarely in the spinal canal (19). Due to the rarity of central nervous system (CNS) HPC and the paucity of randomized clinical trials, there are limited data on this disease and no authoritative guidelines for its management.
Ghose A et al. (18) analyzed 523 patients diagnosed with HPC and found that the average age at diagnosis was about 44 years old. The risk of local recurrence of HPC is high, and HPC may have extroneural metastasis. The most common sites of neurometastasis are lung, bone, liver, intraperitoneal, subcutaneous tissue, breast, pleura, and thyroid. Clinical manifestations of HPC vary according to tumor size and location and are prone to local recurrence after surgical resection (20).
Intraspinal HPC usually presents with sensory and motor abnormalities caused by spinal cord compression or nerve root irritation. Postoperative pathological diagnosis is the gold standard for HPC diagnosis. HPC appears as a typical antler-like vascular pattern, and the tumor cells are obviously deformed, being round, spindle-shaped, or irregular (21). The immunohistochemical manifestations of HPC were positive for CD99, Bcl-2, vimentin, and CD34 (21, 22). In contrast, S100, CD31 and EMA were not expressed (21, 23–25). It has been reported that intraspinal HPC has a high misdiagnosis rate due to lack of specificity in clinical symptoms and imaging examination, and needs to be differentiated from meningioma and schwannoma. According to the location of the tumor, intraspinal HPC can be divided into subdural type, extradural type and extradural type. Surgical resection of intraspinal HPC is the first choice, and vascular embolization is feasible when total resection is difficult.
According to literature, surgical treatment of the lesion site of HPC, especially complete resection (19) and adjuvant radiotherapy (26, 27), has a clear curative effect as first-line treatment (18). Spinal HPC should be removed as a whole to reduce surgical blood loss and possibly increase disease-free survival. Despite surgical removal of these apparently benign lesions, the high recurrence rate requires close follow-up (28). Intraspinal HPC is prone to early recurrence and metastasis (29), and studies have shown that adjuvant irradiation after surgery can reduce the risk of recurrence and metastasis (26, 30). CNS HPC has a poor response to chemotherapy (17), and some studies suggest that HPC outside the CNS may respond well to chemotherapeutic drugs including doxorubicin. In this case, the patient underwent complete surgical resection of the tumor tissue followed by radiotherapy, and the patient’s symptoms of pain and numbness were significantly reduced. This suggests that surgical resection combined with radiotherapy was effective for this patient (31). Notably, Wang K et al. (17) analyzed 1243 patients with HPC and multivariate analysis showed that surgical resection was clinically effective, while the clinical usefulness of adjuvant chemotherapy or radiotherapy is limited. Research shows that antiangiogenic agents, immunotherapy and RNA-targeting technologies are also beneficial for HPC (32), but further clinical studies are needed.
Several studies have confirmed that the prognosis of secondary tumors following allo-HSCT depends mainly on the type of tumor. Studies have reported that the 5-year overall survival rate of secondary pancreatic cancer, lung cancer, hepatobiliary cancer, esophageal cancer, brain cancer and gastric cancer after secondary tumor diagnosis is poor, and the median survival time is between 0.6 and 1 year. The major risk factors for death after secondary tumor diagnosis were age at transplantation, donor type, physical condition, and GVHD (12, 33).
Secondary tumors after allo-HSCT are more complex. Studies have suggested that anti-thymocyte globulin, chronic GVHD, total body irradiation, chemotherapeutic agents such as fludarabine, genetic susceptibility, and viral infection are risk factors for increased secondary tumors after transplantation (5, 34, 35). Skin and oropharyngeal squamous cell carcinoma are associated with chronic GVHD (12). Total body irradiation is mainly associated with an increased risk of non-squamous cell carcinoma (36). Secondary tumors are significantly correlated with radiation dose, and radiation is an important risk factor for breast cancer, thyroid cancer, CNS tumors, bone cancer and melanoma (5, 12). There were 18 cases (18/146) of secondary solid tumors in the total body irradiation based regimen group, much higher than 2 cases (2/280) in the non-irradiation based regimen group (5). Therefore, it has been suggested that in the long-term follow-up of allo-HSCT patients after irradiation, attention should be paid to secondary tumors in young patients (5, 37). There is literature describing potential mechanisms. The use of multiple cytotoxic drugs in the treatment of hematologic diseases, lethal doses of radiotherapy and chemotherapy pretreatment in patients undergoing transplantation, use of immunosuppressants after allo-HSCT, infection, and inflammatory damage caused by GVHD are all risk factors for secondary tumors (38–40).
In conclusion, the use of high-dose cytotoxic drugs, radiotherapy, immunosuppressant and GVHD during allo-HSCT are all related to the development of secondary tumors (12). According to Jacie Standards and international guidelines, screening for secondary tumors after transplantation includes abdominal ultrasound, thyroid ultrasound, and specialized examinations such as otolaryngology, dermatology, gynecology, mammary surgery, and stomatology (8, 35, 41).
Data availability statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YS: Writing – original draft, Writing – review & editing. GL: Conceptualization, Data curation, Writing – review & editing. HZ: Writing – review & editing. YW: Methodology, Writing – review & editing. YaH: Data curation, Methodology, Writing – review & editing. ST: Data curation, Methodology, Writing – review & editing. SL: Data curation, Methodology, Writing – review & editing. YiW: Data curation, Methodology, Writing – review & editing. HB: Data curation, Methodology, Writing – review & editing. RX: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Youth Science and Technology Fund of Gansu (23JRRA321), Innovation Base and Talent Program of Gansu (21JR7RA015), Science and Technology Development Plan Project of Lanzhou (2023-ZD-176), Key Research and Development Program of Gansu Province (22YF7FA106) and Hematology Medical Research Center of 940th Hospital of Joint Logistic Support Force (2021yxky078) and High-level Talents Project of 940th Hospital of Joint Logistics Support Force (2024-G3-11). Health industry research project of Gansu Province (GSWSHL2022-39).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nelson AS, Ashton LJ, Vajdic CM, Le Marsney RE, Daniels B, Nivison-Smith I, et al. Second cancers and late mortality in Australian children treated by allogeneic HSCT for haematological Malignancy. Leukemia. (2015) 29:441–7. doi: 10.1038/leu.2014.203
2. Park SS, Park SH, Han S. Risk of secondary nonhematologic Malignancies after allogeneic stem cell transplantation: A nationwide case-control cohort study. Int J Cancer. (2022) 151:1024–32. doi: 10.1002/ijc.34115
3. Yokota A, Ozawa S, Masanori T, Akiyama H, Ohshima K, Kanda Y, et al. Secondary solid tumors after allogeneic hematopoietic SCT in Japan. Bone Marrow Transplant. (2012) 47:95–100. doi: 10.1038/bmt.2011.23
4. Vasudevan NR, Yeung J, Pierre A, Wong R, Darling G, Kim J, et al. Outcomes of patients with esophageal cancer after allogeneic hematopoietic stem cell transplantation. J Gastrointest Oncol. (2022) 13:2705–12. doi: 10.21037/jgo-22-700
5. Keslova P, Formankova R, Riha P, Sramkova L, Snajderova M, Malinova B, et al. Total body irradiation is a crucial risk factor for developing secondary carcinomas after allogeneic hematopoietic stem cell transplantation in childhood. Neoplasma. (2020) 67:1164–9. doi: 10.4149/neo_2020_200214N131
6. Ringden O, Brazauskas R, Wang Z, Ahmed I, Atsuta Y, Buchbinder D, et al. Second solid cancers after allogeneic hematopoietic cell transplantation using reduced-intensity conditioning. Biol Blood Marrow Transplant. (2014) 20:1777–84. doi: 10.1016/j.bbmt.2014.07.009
7. Isabella G, Katharina A, Matthias E, Oliver K, Daniel W. Secondary solid Malignancies and precancerous lesions after allogeneic hematopoietic stem cell transplantation using non-total body irradiation-based conditioning in acute myeloid leukemia. J Cancer Res Clin Oncol. (2024) 150:152. doi: 10.1007/s00432-024-05679-5
8. Lupo-Stanghellini MT, Piemontese S, Assanelli A, Serpenti F, Mastaglio S, Clerici D, et al. Second solid cancers after hematopoietic stem cell transplantation: active surveillance during long-term follow-up. Hemasphere. (2021) 5:e654. doi: 10.1097/HS9.0000000000000654
9. Del GA, Rousseau A, Capes A, Michonneau D, Robin M, de Fontbrune FS, et al. Life expectancy and burden of late complications after reduced intensity conditioning allogeneic transplantation. Bone Marrow Transplant. (2022) 57:1365–72. doi: 10.1038/s41409-022-01715-5
10. Michelis FV, Kotchetkov R, Grunwald RM, Azeem A, Atenafu EG, Lipton JH, et al. Long-term incidence of secondary Malignancies after allogeneic hematopoietic cell transplantation: A single-center experience. Biol Blood Marrow Transplant. (2017) 23:945–51. doi: 10.1016/j.bbmt.2017.02.015
11. Kasai S, Itonaga H, Niino D, Miyoshi H, Kato T, Imanishi D, et al. Programmed death 1 ligand (PD-L1) in solid cancers after allogeneic hematopoietic stem cell transplantation: a retrospective analysis by the Nagasaki Transplant Group. Int J Hematol. (2020) 112:524–34. doi: 10.1007/s12185-020-02926-6
12. Tichelli A, Beohou E, Labopin M, Socie G, Rovo A, Badoglio M, et al. Evaluation of second solid cancers after hematopoietic stem cell transplantation in european patients. JAMA Oncol. (2019) 5:229–35. doi: 10.1001/jamaoncol.2018.4934
13. Nomura K, Iizuka T, Kaji D, Asano-Mori Y, Ochiai Y, Suzuki Y, et al. Secondary esophageal squamous cell carcinoma after hematopoietic stem cell transplantation. J Cancer Res Clin Oncol. (2021) 147:2137–44. doi: 10.1007/s00432-020-03500-7
14. Vivanco M, Dalle JH, Alberti C, Lescoeur B, Yakouben K, Carel JC, et al. Malignant and benign thyroid nodules after total body irradiation preceding hematopoietic cell transplantation during childhood. Eur J Endocrinol. (2012) 167:225–33. doi: 10.1530/EJE-12-0073
15. Castiglione M, Nardo L, Ottaviani F. Hemangiopericytoma arising from the cartilage of the external auditory canal. Head Neck. (2016) 38:E108–10. doi: 10.1002/hed.24328
16. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. (2007) 114:97–109. doi: 10.1007/s00401-007-0243-4
17. Wang K, Mei F, Wu S, Tan Z. Hemangiopericytoma: incidence, treatment, and prognosis analysis based on SEER database. BioMed Res Int. (2020) 2020:2468320. doi: 10.1155/2020/2468320
18. Ghose A, Guha G, Kundu R, Tew J, Chaudhary R. CNS hemangiopericytoma: A systematic review of 523 patients. Am J Clin Oncol. (2017) 40:223–7. doi: 10.1097/COC.0000000000000146
19. Das A, Singh PK, Suri V, Sable MN, Sharma BS. Spinal hemangiopericytoma: an institutional experience and review of literature. Eur Spine J. (2015) 24 Suppl 4:S606–13. doi: 10.1007/s00586-015-3789-1
20. Kumar R, Vaid VK, Kumar V, Kalra SK. Hemangiopericytoma of thoracic spine: a rare bony tumor. Childs Nerv Syst. (2007) 23:1215–9. doi: 10.1007/s00381-007-0372-z
21. Diensthuber M, Gotz F, Langer F, Lenarz T, Lenarz M. Extra- and intracranial dumbbell-shaped hemangiopericytoma. Eur Arch Otorhinolaryngol. (2008) 265:481–4. doi: 10.1007/s00405-007-0466-y
22. Peng Z, Wang Y, Wang Y, Li Q, Fang Y, Fan R, et al. Hemangiopericytoma/solitary fibrous tumor of the cranial base: a case series and literature review. BMC Surg. (2022) 22:289. doi: 10.1186/s12893-022-01718-5
23. Vilendecic M, Grahovac G, Lambasa S, Jelec V, Topic I. Unrecognized hemangiopericytoma of posterior cervical region with intracranial extension. J Craniomaxillofac Surg. (2012) 40:e51–3. doi: 10.1016/j.jcms.2011.01.019
24. Tan I, Soo MY, Ng T. Haemangiopericytoma of the trigeminal nerve. Australas Radiol. (2001) 45:350–3. doi: 10.1046/j.1440-1673.2001.00935.x
25. Patel AR, Flores BC, Ban VS, Hatanpaa KJ, Mickey BE, Barnett SL. Intracranial hemangiopericytomas: recurrence, metastasis, and radiotherapy. J Neurol Surg B Skull Base. (2017) 78:324–30. doi: 10.1055/s-0037-1599073
26. Combs SE, Thilmann C, Debus J, Schulz-Ertner D. Precision radiotherapy for hemangiopericytomas of the central nervous system. Cancer-Am Cancer Soc. (2005) 104:2457–65. doi: 10.1002/cncr.21448
27. Golub D, McBriar JD, Donaldson H, Wong T, Unadkat P, White TG, et al. Postoperative stereotactic radiosurgery for intracranial solitary fibrous tumors: systematic review and pooled quantitative analysis. J Neurooncol. (2023) 165:229–39. doi: 10.1007/s11060-023-04499-w
28. Betchen S, Schwartz A, Black C, Post K. Intradural hemangiopericytoma of the lumbar spine: case report. Neurosurgery. (2002) 50:654–7. doi: 10.1097/00006123-200203000-00045
29. Spitz FR, Bouvet M, Pisters PW, Pollock RE, Feig BW. Hemangiopericytoma: a 20-year single-institution experience. Ann Surg Oncol. (1998) 5:350–5. doi: 10.1007/BF02303499
30. Tobias S, Jahshan S, Grober Y, Soustiel JF. Skull base hemangiopericytomas. Acta Neurol Belg. (2022) 122:1537–45. doi: 10.1007/s13760-021-01812-0
31. Okubo T, Nagoshi N, Tsuji O, Tachibana A, Kono H, Suzuki S, et al. Imaging characteristics and surgical outcomes in patients with intraspinal solitary fibrous tumor/hemangiopericytoma: A retrospective cohort study. Global Spine J. (2023) 13:276–83. doi: 10.1177/2192568221994799
32. Ren C, D’Amato G, Hornicek FJ, Tao H, Duan Z. Advances in the molecular biology of the solitary fibrous tumor and potential impact on clinical applications. Cancer Metastasis Rev. (2024). doi: 10.1007/s10555-024-10204-8
33. Gunduz M, Ozen M, Sahin U, Toprak SK, Civriz BS, Kurt YM, et al. Subsequent Malignancies after allogeneic hematopoietic stem cell transplantation. Clin Transplant. (2017) 31. doi: 10.1111/ctr.12987
34. Bhatia S, Ramsay NK, Steinbuch M, Dusenbery KE, Shapiro RS, Weisdorf DJ, et al. Malignant neoplasms following bone marrow transplantation. Blood. (1996) 87:3633–9. doi: 10.1182/blood.V87.9.3633.bloodjournal8793633
35. Inamoto Y, Shah NN, Savani BN, Shaw BE, Abraham AA, Ahmed IA, et al. Secondary solid cancer screening following hematopoietic cell transplantation. Bone Marrow Transplant. (2015) 50:1013–23. doi: 10.1038/bmt.2015.63
36. Majhail NS, Brazauskas R, Rizzo JD, Sobecks RM, Wang Z, Horowitz MM, et al. Secondary solid cancers after allogeneic hematopoietic cell transplantation using busulfan-cyclophosphamide conditioning. Blood. (2011) 117:316–22. doi: 10.1182/blood-2010-07-294629
37. Danylesko I, Shimoni A. Second Malignancies after hematopoietic stem cell transplantation. Curr Treat Options Oncol. (2018) 19:9. doi: 10.1007/s11864-018-0528-y
38. Curtis RE, Metayer C, Rizzo JD, Socie G, Sobocinski KA, Flowers ME, et al. Impact of chronic GVHD therapy on the development of squamous-cell cancers after hematopoietic stem-cell transplantation: an international case-control study. Blood. (2005) 105:3802–11. doi: 10.1182/blood-2004-09-3411
39. Tanaka Y, Kurosawa S, Tajima K, Tanaka T, Ito R, Inoue Y, et al. Increased incidence of oral and gastrointestinal secondary cancer after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. (2017) 52:789–91. doi: 10.1038/bmt.2017.4
40. Akiyama M, Yamaoka M, Ohyama W, Yokoi K, Ashizuka S, Aizawa D, et al. Genetic profile and microsatellite instability in a case of secondary esophageal squamous cell carcinoma 12 years after allogeneic hematopoietic stem cell transplantation for aplastic anemia. J Pediatr Hematol Oncol. (2020) 42:302–6. doi: 10.1097/MPH.0000000000001355
Keywords: primary plasma cell leukemia, allogeneic hematopoietic cell transplantation, secondary tumors, hemangiopericytoma, case report
Citation: Shi Y, Liang G, Zhang H, Wang Y, Han Y, Tong S, Liang S, Wang Y, Bai H and Xi R (2024) Hemangiopericytoma following allogeneic hematopoietic stem cell transplantation in a patient with primary plasma cell leukemia: the first case report and literature review. Front. Oncol. 14:1467237. doi: 10.3389/fonc.2024.1467237
Received: 19 July 2024; Accepted: 21 October 2024;
Published: 14 November 2024.
Edited by:
Yan-Lai Tang, First Affiliated Hospital of Sun Yat-sen University, ChinaReviewed by:
Lindsey Sloan, University of Minnesota, United StatesHailong Yuan, First Affiliated Hospital of Xinjiang Medical University, China
Copyright © 2024 Shi, Liang, Zhang, Wang, Han, Tong, Liang, Wang, Bai and Xi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui Xi, MTM5MTk5MzE2MDlAMTM5LmNvbQ==
†These authors have contributed equally to the work
 Yajun Shi
Yajun Shi Guohao Liang1†
Guohao Liang1† Rui Xi
Rui Xi