- 1School of Basic Medical Sciences, Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, China
- 2Health Care Security and Pharmacoeconomic Evaluation Branch, China Medical Education Association, Beijing, China
- 3Department of Health Economics and Outcome Research, Hainan Zhongwei Institute of Health Economy Development, Haikou, Hainan, China
Background: The GEMSTONE-304 trial established the clinical benefits of sugemalimab plus chemotherapy in advanced esophageal squamous-cell carcinoma (ESCC). This study evaluates the cost-effectiveness of this regimen versus chemotherapy alone as the first-line treatment for advanced ESCC patients from the perspective of China’s health system.
Methods: We established a partitioned survival model based on GEMSTONE-304 trial data, we simulated lifetime outcomes through three health states: progression-free survival, progressive disease, and death. Key parameters included quality-adjusted life years (QALYs) and incremental cost-effectiveness ratio (ICER), analyzed with 5% discounting. Sensitivity analyses encompassed probabilistic, one-way, and scenario evaluations.
Results: The sugemalimab combination yielded 0.336 incremental QALYs at $ 44,182.03 additional cost (ICER = $ 131,544.70/QALY). PD-L1 subgroup ICERs exhibited dose-dependent efficacy: $ 187,421.63/QALY (Combined Positive Score (CPS) < 1), $ 175,689.56 (1 ≤ CPS < 10), and $ 130,349.21 (CPS ≥ 10). Scenario analysis demonstrated ICER reduction to $ 51,454.12/QALY under consideration of patient assistance program. None of the results demonstrated cost-effectiveness for this therapeutic regimen. Sensitivity analyses identified sugemalimab pricing as the dominant driver of ICER, while simultaneously validating the model’s internal and external validity. Price cap simulations determined that a minimum 91.20% price reduction is required to achieve cost-effectiveness.
Conclusion: Current pricing renders sugemalimab combination therapy economically unfavorable as first-line ESCC treatment in China. Strategic price adjustments could enhance cost-effectiveness potential.
1 Introduction
Esophageal cancer remains a major global health challenge (1, 2), ranking as the seventh most common malignancy in Chinese females and fifth in males, with approximately 224,000 new cases and 187,500 deaths annually (3). Histologically classified into esophageal adenocarcinoma (EAC) and esophageal squamous cell carcinoma (ESCC) (4, 5), the latter accounts for 80% of cases and presents particular therapeutic challenges (6). The absence of effective early markers and screening modalities often leads to late diagnosis (stages II or III), missing optimal treatment windows (7). Current first-line chemotherapy regimens (cisplatin/5-fluorouracil, paclitaxel/carboplatin (8)) demonstrate limited efficacy (9, 10), contributing to a dismal 5-year survival rate of 18% (11).
In recent years, immunotherapies targeting PD-1 (12–14) and PD-L1 (15–17) have significantly advanced ESCC treatment. Landmark trials including KEYNOTE-181 (pembrolizumab), ORIENT-15 (sintilimab), and CheckMate 648 (nivolumab) have demonstrated significant survival benefits in advanced ESCC, establishing immunotherapy as a first-line standard (18–20). Sugemalimab, a fully humanized PD-L1 monoclonal antibody (21),, binds to the PD-1 receptor and blocks the PD-1/PD-L1 interaction, thereby enhancing anti-tumor immune responses (22, 23). It has demonstrated good efficacy and safety in treating metastatic non-small-cell lung cancer (NSCLC) (24, 25) and relapsed or refractory extranodal natural killer/T-cell lymphoma (26). The GEMSTONE-304 trial first demonstrated that sugemalimab combined with chemotherapy significantly improves median overall survival (OS) (15.3 vs 11.5 months) and progression-free survival (PFS) (6.2 vs 5.4 months) in advanced ESCC (27). Notably, despite these clinical benefits, the high cost of sugemalimab necessitates a cost-effectiveness analysis to assess whether the incremental costs justify the survival benefits, crucial for optimizing healthcare resource utilization.
This study innovatively constructs a three-state partitioned survival model (PSM) (derived from GEMSTONE-304 data (28)) to evaluate long-term cost-effectiveness from the Chinese health system perspective. The analysis not only fills a critical gap in China’s immunotherapy economic evaluations but also provides pivotal evidence for dynamic China’s National Reimbursement Drug List (NRDL) adjustments and optimal allocation of cancer control resources, carrying significant policy implications for refining China’s precision medicine value assessment framework.
2 Materials and methods
2.1 Population
The study cohort was derived from the GEMSTONE-304 phase III trial (NCT04187352) involving 540 patients with unresectable locally advanced esophageal squamous cell carcinoma (27). Inclusion criteria comprised: 18–75 years old, ECOG PS 0-1, life expectancy ≥ 3 months, measurable lesions per RECIST 1.1, and adequate organ function. Exclusion criteria excluded non-ESCC histology, active central nervous system (CNS) metastases or carcinomatous meningitis, prior systemic therapy, PD-1/L1 inhibitor exposure, Human Immunodeficiency Virus/Acquired Immune Deficiency Syndrome tests, organ transplantation history, and concurrent malignancies (29). Patients were randomized to either sugemalimab plus chemotherapy (n=358) or chemotherapy alone (n=182). The intervention group received sugemalimab (1200 mg iv q3w) with cisplatin (80 mg/m2 iv d1) and 5-FU (800 mg/m2 iv d1–4 q3w), while the control group received identical chemotherapy regimens without sugemalimab. This therapeutic alignment ensured comparability between groups, with treatment continuation until disease progression or intolerable toxicity.
The model incorporated demographic parameters from the Chinese population, including an average body weight of 65 kg and body surface area of 1.72 m2 (29) All patients initiated first-line therapy until disease progression or intolerable toxicity, with subsequent second-line treatment continuing until death. As the GEMSTONE-304 trial did not specify second-line regimens, we adopted the Chinese Society of Clinical Oncology Guidelines for the Diagnosis and Treatment of Esophageal Cancer 2024 (CSCO 2024) guideline-recommended protocol using irinotecan hydrochloride injection (350 mg/m2 q3w) combined with tegafur/gimeracil/oteracil (S-1) capsules (60 mg bid) for both treatment arms to maintain model stability (30). This standardized approach mitigated potential bias from differential second-line therapies while reflecting current clinical practice patterns in China.
2.2 Model construction
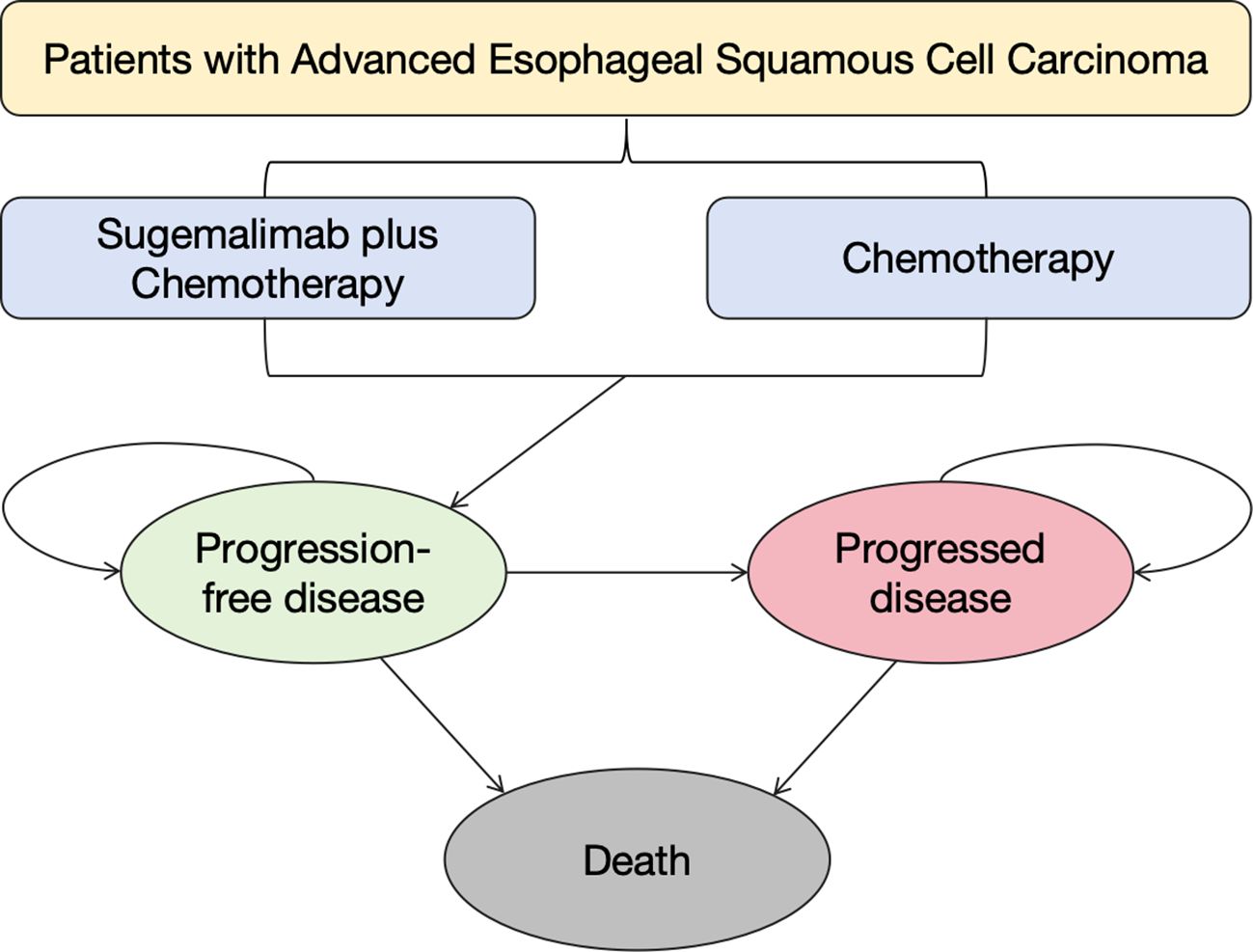
A PSM was developed to simulate disease progression in ESCC patients, incorporating three mutually exclusive health states: PFS, progressive disease (PD), and death (31). The model’s 3-week cycle length were aligned with the trial’s interval design, ensuring temporal consistency between intervention phases, and 10-year time horizon were calibrated to Chinese ESCC epidemiological data (5-year survival <18%) (11). All patients initiated in the PFS state, with transition probabilities between states derived from trial survival curves (Figure 1). The homogeneous baseline characteristics of trial participants (mean age 62.75 years, no baseline progression) enabled standardized state transition modeling. Cost and quality-adjusted life year (QALY) outcomes were discounted at 5% annually following the latest Chinese pharmacoeconomic guideline (32). This temporal framework captured both short-term treatment effects and long-term survival patterns, while the cycle length alignment with clinical trial intervals ensured therapeutic exposure accuracy.
2.3 Survival analysis
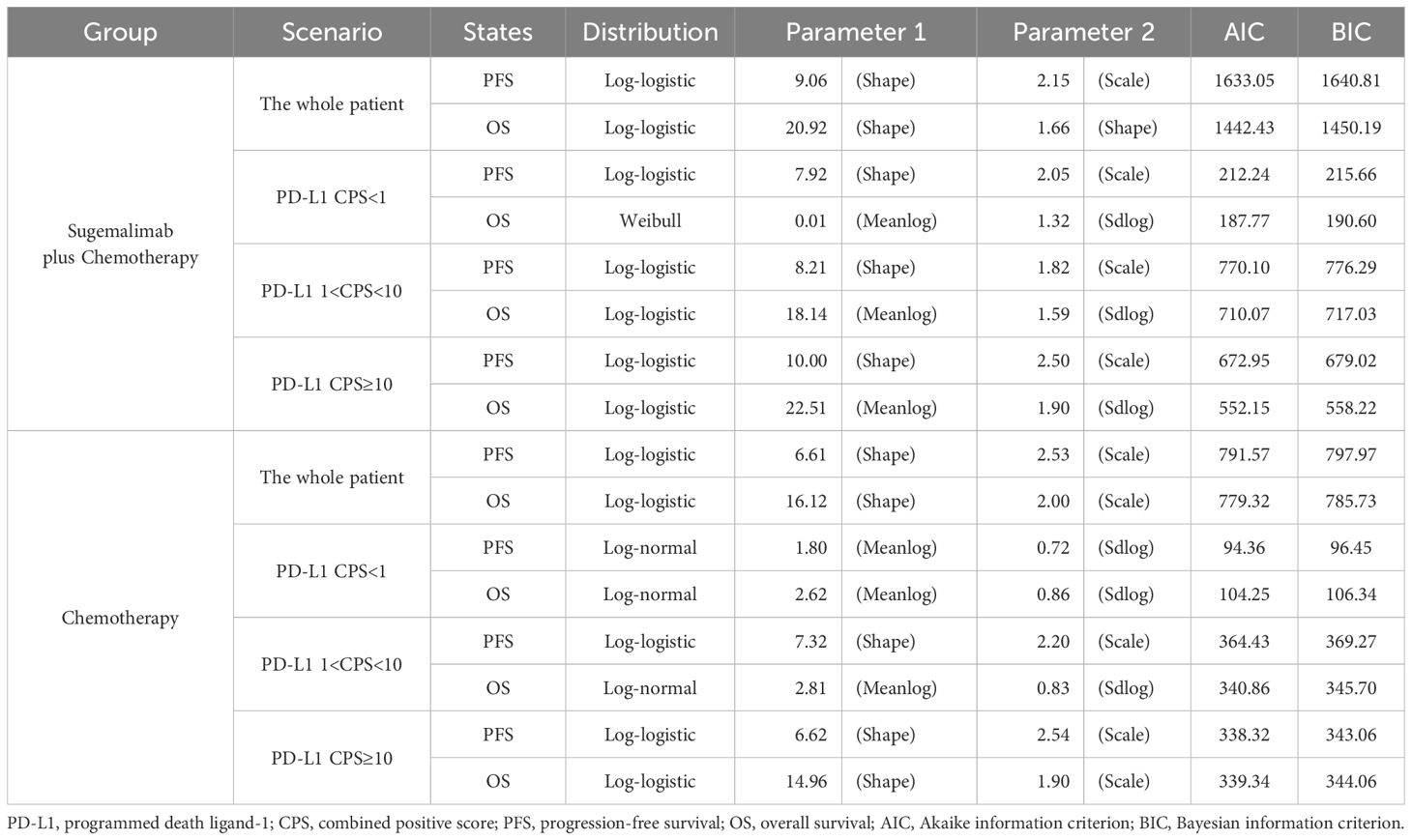
Survival data from the GEMSTONE-304 trial were extracted using GetData Graph Digitizer (33) and reconstructed in R software using the packages ‘survival’, ‘survHE’, and ‘survminer’. Six parametric distributions (Exponential, Weibull, Log-logistic, Log-normal, Gompertz and Generalized Gamma) were evaluated for PFS and OS curve fitting through maximum likelihood estimation (34). The optimal parametric models (Table 1) were selected based on statistical criteria (Akaike/Bayesian information criteria minimization) combined with visual examination of curve alignment, with detailed parameters documented in eTable 1. This methodology enabled extrapolation of trial survival outcomes to the 10-year model horizon, with cycle-specific health state occupancy proportions mathematically derived from the reconstructed survival curves (Figure 2).
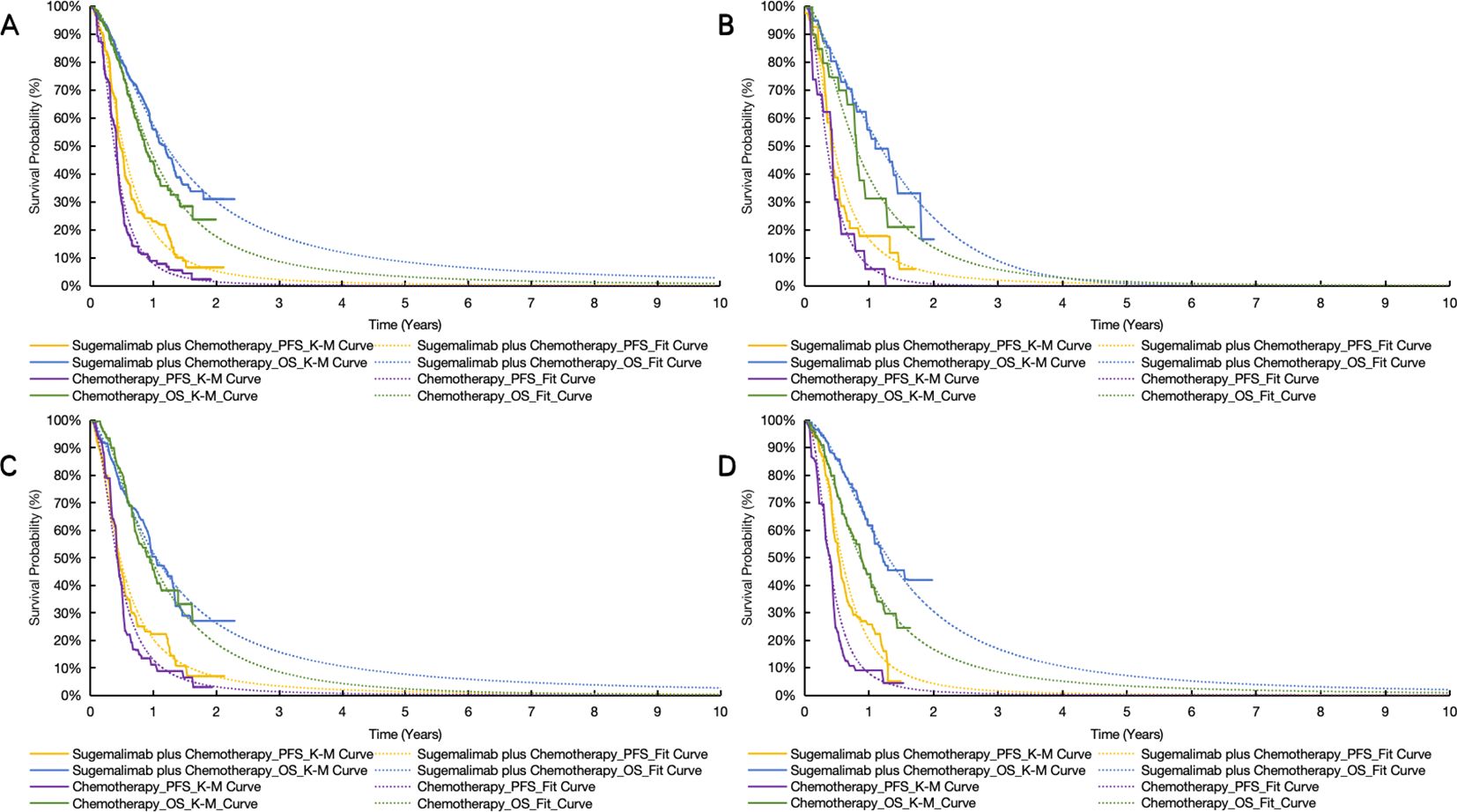
Figure 2. Original and reconstructed survival curves. (A) the whole patient. (B) patients with PD-L1 CPS < 1. (C) patients with PD-L1 1 < CPS < 10. (D) patients with PD-L1 CPS ≥ 10. OS indicates overall survival; PFS, progression-free survival; S + C, sugemalimab plus chemotherapy.
2.4 Cost and utility estimates
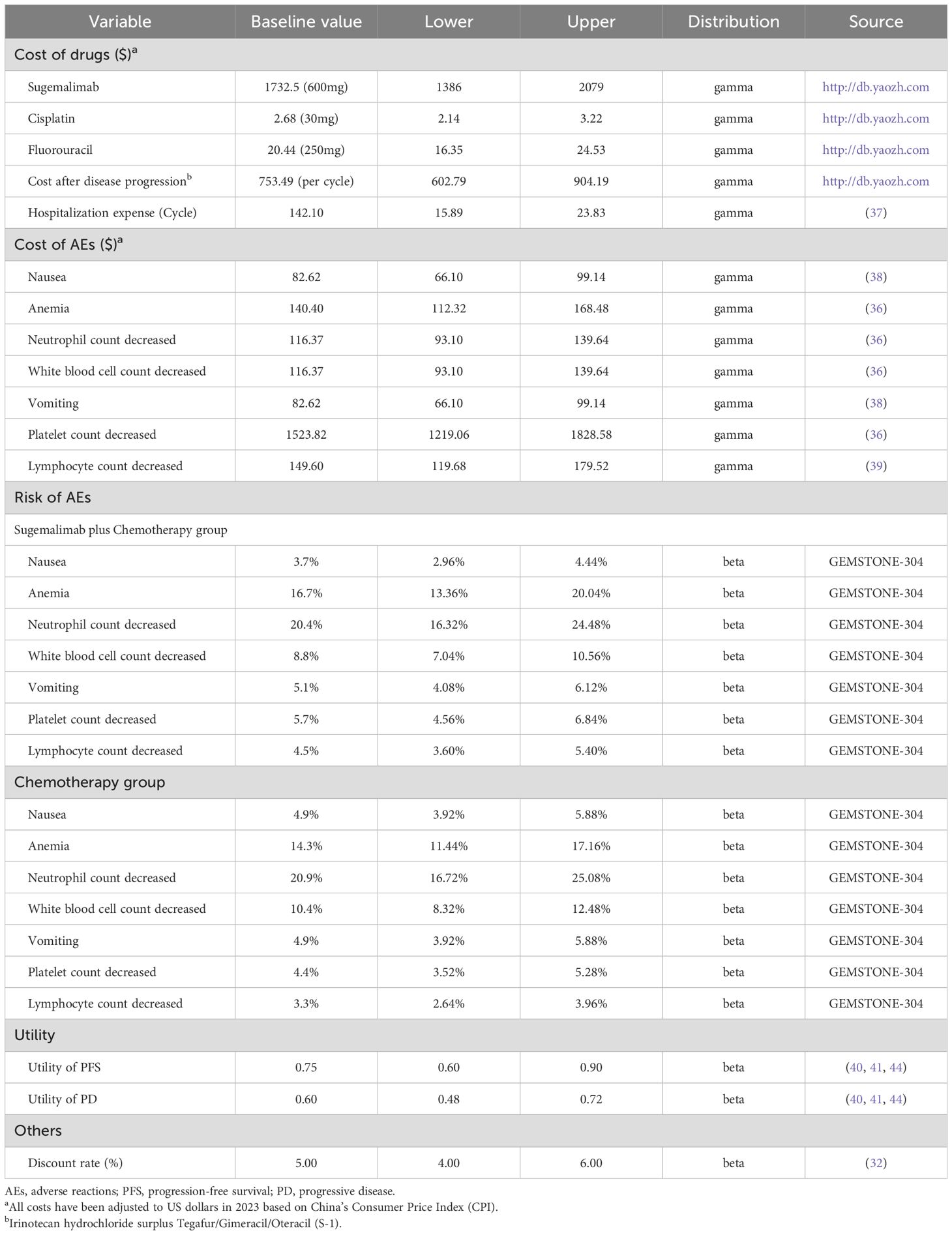
The cost-effectiveness analysis adopted the Chinese healthcare system perspective, incorporating direct medical costs encompassing drug acquisition, adverse event (AE) management, hospitalization, and progressive disease treatment. Drug costs for sugemalimab, cisplatin, 5-FU and camrelizumab were obtained from the Chinese health industry’s big data service platform (https://db.yaozh.com/), with AE-related expenses (grade ≥ 3 incidence ≥ 3%) and hospitalization fees derived from published literature (35–38). Specifically, severe hematologic toxicities (anemia, neutropenia, thrombocytopenia) and gastrointestinal events (nausea, vomiting) were included based on GEMSTONE-304 safety profiles (36–38). Health state utilities were derived from international ESCC studies due to lack of Chinese-specific data (39, 40). The willingness-to-pay (WTP) threshold was established at 1.94 times China’s 2024 per capita GDP ($ 23901.90/QALY), aligned with national guideline and community-based surveys (32, 41). All relevant inputs are presented in Table 2. According to the consumer price index (CPI) of the National Bureau of Statistics of China, all the cost parameters involved in this study are converted to 2023 (the latest consumer price index results released by China).
2.5 Sensitivity analysis
Sensitivity analyses were systematically conducted to validate model robustness and parameter influence on incremental cost-effectiveness ratios (ICERs). One-way sensitivity analysis (OWSA) examined individual parameter variations by ±20% when literature-based ranges were unavailable, while probabilistic sensitivity analysis (PSA) employed 1,000 Monte Carlo simulations with Gamma distributions for cost parameters and Beta distributions for utilities/AE risks (42). Key drivers of cost-effectiveness were visualized through tornado diagrams, with uncertainty characterized via incremental cost-effectiveness scatterplots and cost-effectiveness acceptability curves (CEACs).
To address limitations in Chinese-specific health utility data, sensitivity analyses incorporated alternative utility estimates from international ESCC studies (43–45), systematically evaluating cross-cultural variations in quality-of-life valuations.
2.6 Subgroup and scenario analyses
Subgroup analyses evaluated cost-effectiveness variations across PD-L1 expression subgroups (CPS < 1, 1 ≤ CPS < 10, CPS ≥ 10) using biomarker-stratified survival data (44). Scenario analysis modeled the economic impact of implementing the NSCLC patient assistance program (PAP) for ESCC treatment, assuming hypothetical approval. The PAP structure incorporates three donation phases: initial (2 purchased cycles + 2 free), secondary (2 + 25 cycles), and tertiary (1 + 3 cycles) support tiers. At the current NSCLC price ($1,732.5/600mg), the analysis assumed 100% PAP participation with progressive cost reductions. This dual approach assessed both biological heterogeneity through PD-L1 stratification and financial accessibility via pricing scenarios, maintaining consistent efficacy assumptions across analyses.
3 Results
3.1 Base–case analysis results
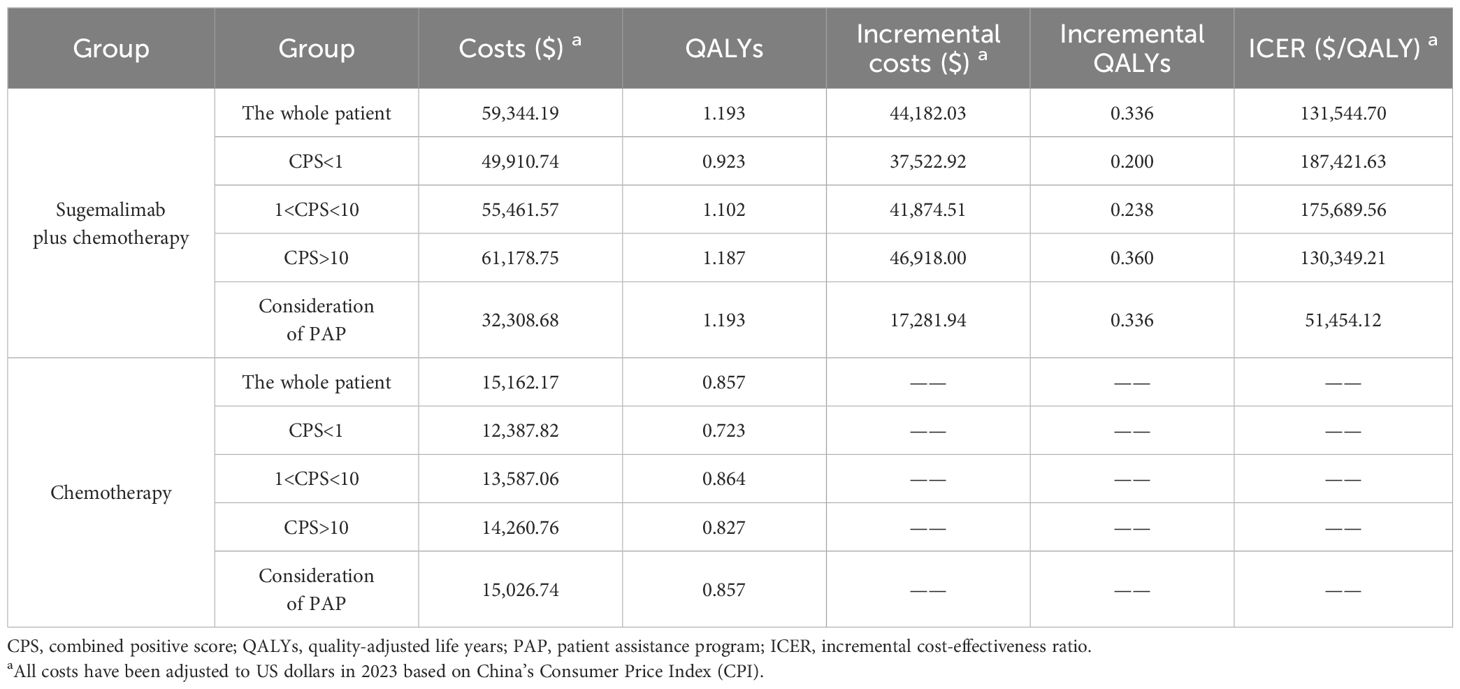
The base-case analysis demonstrated differential economic outcomes across treatment arms (Table 3). For the whole patient, sugemalimab combination therapy yielded an incremental 0.336 QALYs at an additional cost of $ 44,182.03 versus chemotherapy alone, resulting in an ICER of $ 131,544.70/QALY, exceeding the predefined WTP threshold. Subgroup analyses revealed biomarker-dependent cost-effectiveness gradients: PD-L1 CPS ≥10 subgroup showed optimal economic performance (ICER = $ 130,349.21/QALY), while CPS <1 subgroup exhibited limited value (ICER = $ 187,421.63/QALY). Scenario analysis incorporating patient assistance programs reduced the ICER to $ 51,454.12/QALY, yet remained above conventional affordability benchmarks. These findings underscore the necessity of strategic price adjustments to align sugemalimab’s cost with health system sustainability requirements.
3.2 Sensitivity analyses
3.2.1 One-way sensitivity analysis
The OWSA identified sugemalimab’s unit price as the principal ICER determinant across all analytical scenarios (Figure 3). For the whole patient, the package price of sugemalimab, utility of PD, and utility of PFS were the factors with the significant influence (Figure 3A). Subgroup analyses revealed consistent price sensitivity across difference PD-L1 expression strata (Figures 3B–D). Scenario analysis incorporating PAPs demonstrated amplified sensitivity to disease progression costs, highlighting financial vulnerability in late-line treatment phases (Figure 3E). These findings collectively establish drug pricing as the critical leverage point for value assessment in China’s reimbursement framework.
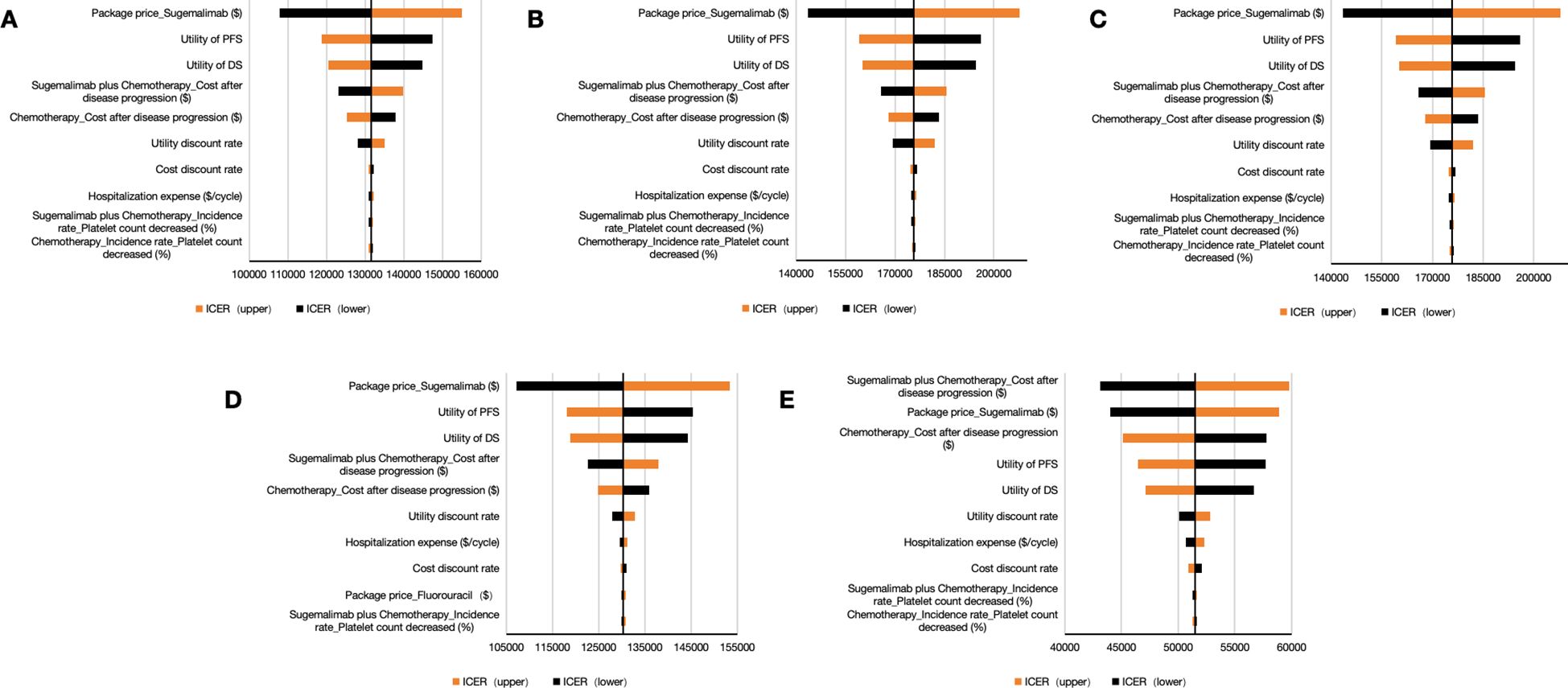
Figure 3. Tornado diagrams for one-way sensitivity analysis. (A) the whole patient. (B) patients with PD-L1 CPS < 1. (C) patients with PD-L1 1 < CPS < 10. (D) patients with PD-L1 CPS ≥ 10. (E) Consideration of PAP. PFS indicates progression-free survival; PD, progressive disease; S + C, sugemalimab plus chemotherapy; ICER, incremental cost-effectiveness ratio; PAP, patient assistance program.
3.2.2 Probabilistic sensitivity analysis
PSA demonstrated limited cost-effectiveness of sugemalimab combination therapy. The Monte Carlo simulations revealed all scenario outcomes in the northeast quadrant of the cost-effectiveness plane, exceeding the predefined WTP threshold (Figure 4). This pattern persisted across population subgroups, with the CEAC indicating zero probability of the intervention being cost-effective through 1,000 iterations (Figures 5A–E).
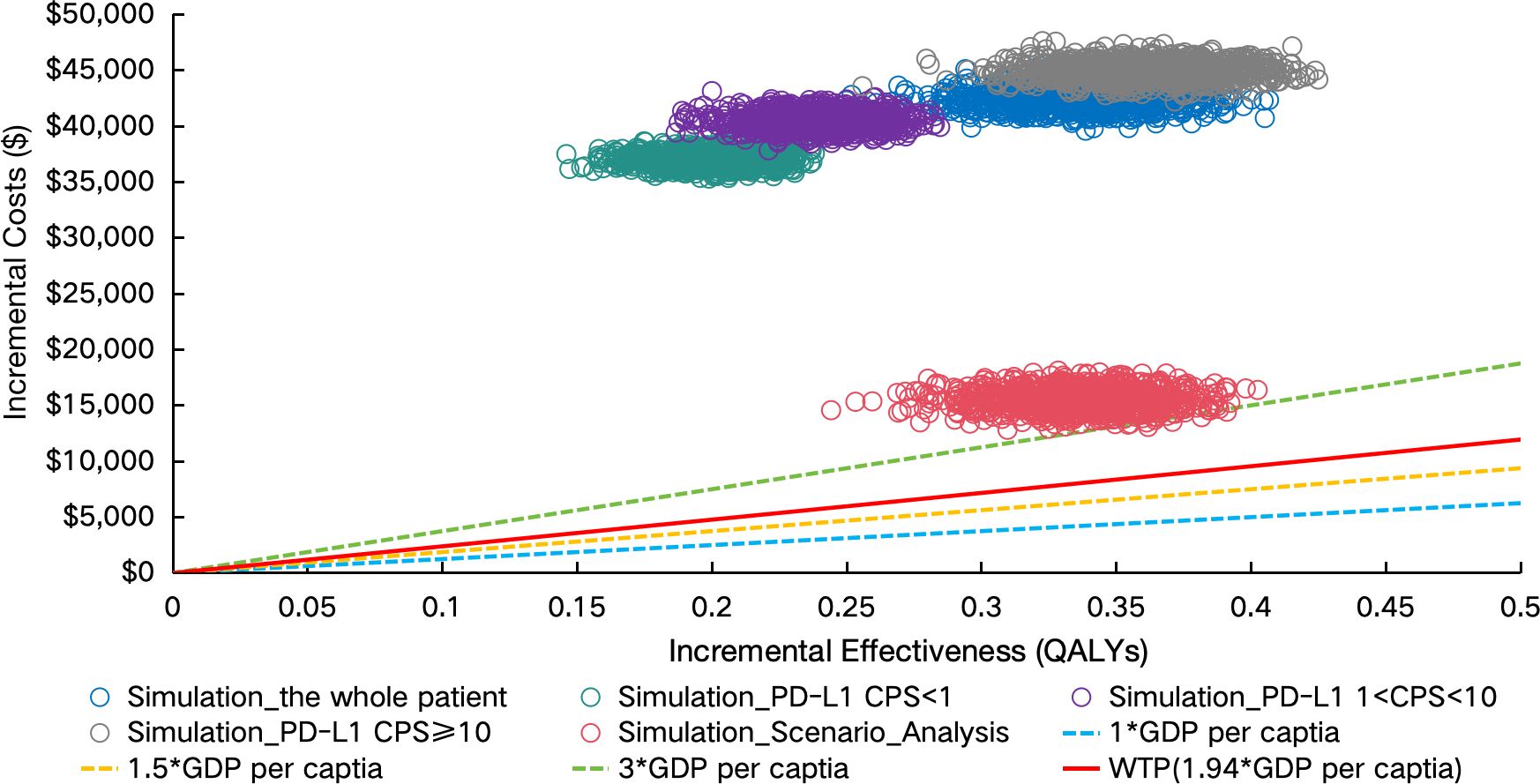
Figure 4. Incremental cost-effectiveness scatter plot. PD-L1 indicates programmed death ligand-1; CPS, combined positive score; GDP, gross domestic product; WTP, willingness to pay; QALYs, Quality-adjusted life years; PAP, patient assistance program.
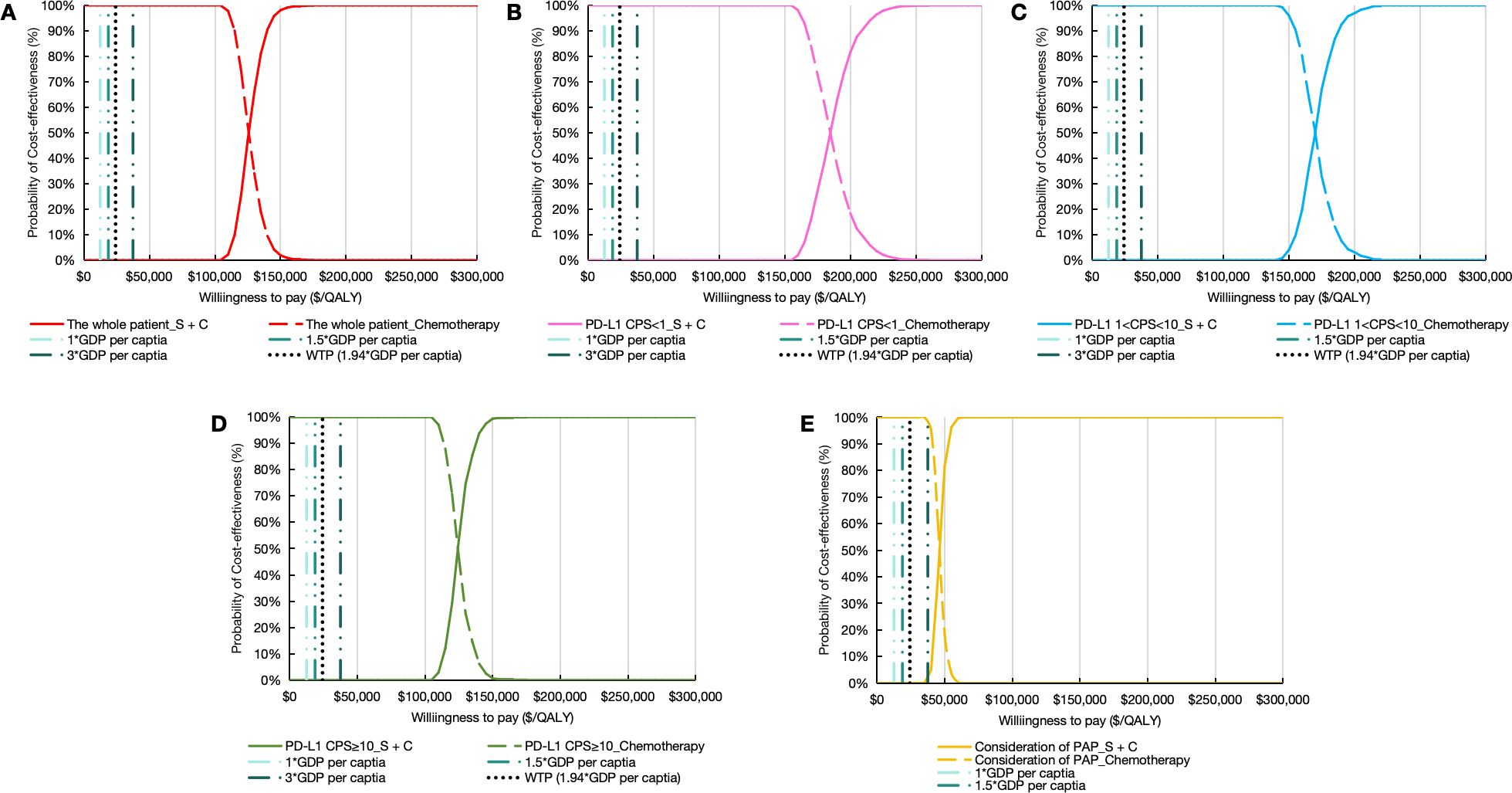
Figure 5. Cost-effectiveness acceptability curve. (A) the whole patient. (B) patients with PD-L1 CPS < 1. (C) patients with PD-L1 1 < CPS < 10. (D) patients with PD-L1 CPS ≥ 10. (E) Consideration of PAP. PD-L1 indicates programmed death ligand-1; CPS, combined positive score; S + C, sugemalimab plus chemotherapy; GDP, gross domestic product; WTP, willingness to pay; QALYs, quality-adjusted life years; PAP, patient assistance program.
3.2.3 ICERs based on different parameters of health utility value
Scenario analyses evaluating health utility parameters revealed consistent patterns in ICERs. All sensitivity scenarios maintained the rank order of ICER magnitudes regardless of the utility weights assigned to PFS and PD states. The intervention’s ICER values systematically exceeded the predefined WTP threshold across parameter combinations (eTable 2). Notably, variations in utility parameters failed to produce any scenario where combination therapy became economically favorable compared to chemotherapy alone.
3.3 Price cap simulations for sugemalimab
Given sugemalimab’s exclusion from China’s NRDL, price cap simulations were conducted to identify economically viable price points under China’s WTP framework for end-stage conditions (45).
Price cap simulations demonstrated relationships between drug cost reductions and cost-effectiveness probabilities. The intervention only achieved cost-effectiveness when sugemalimab package pricing was reduced by 91.24% from baseline ($ 1732.50 to $ 151.74) in the overall population. Patient subgroups stratified by PD-L1 expression levels required greater price concessions, with required reductions ranging from 91.11% to 94.38% across CPS categories. Complementary analysis incorporating PAP identified a separate viability threshold at $ 445.67 (74.28% reduction), though this remained substantially above conventional cost-effectiveness benchmarks (Figure 6).
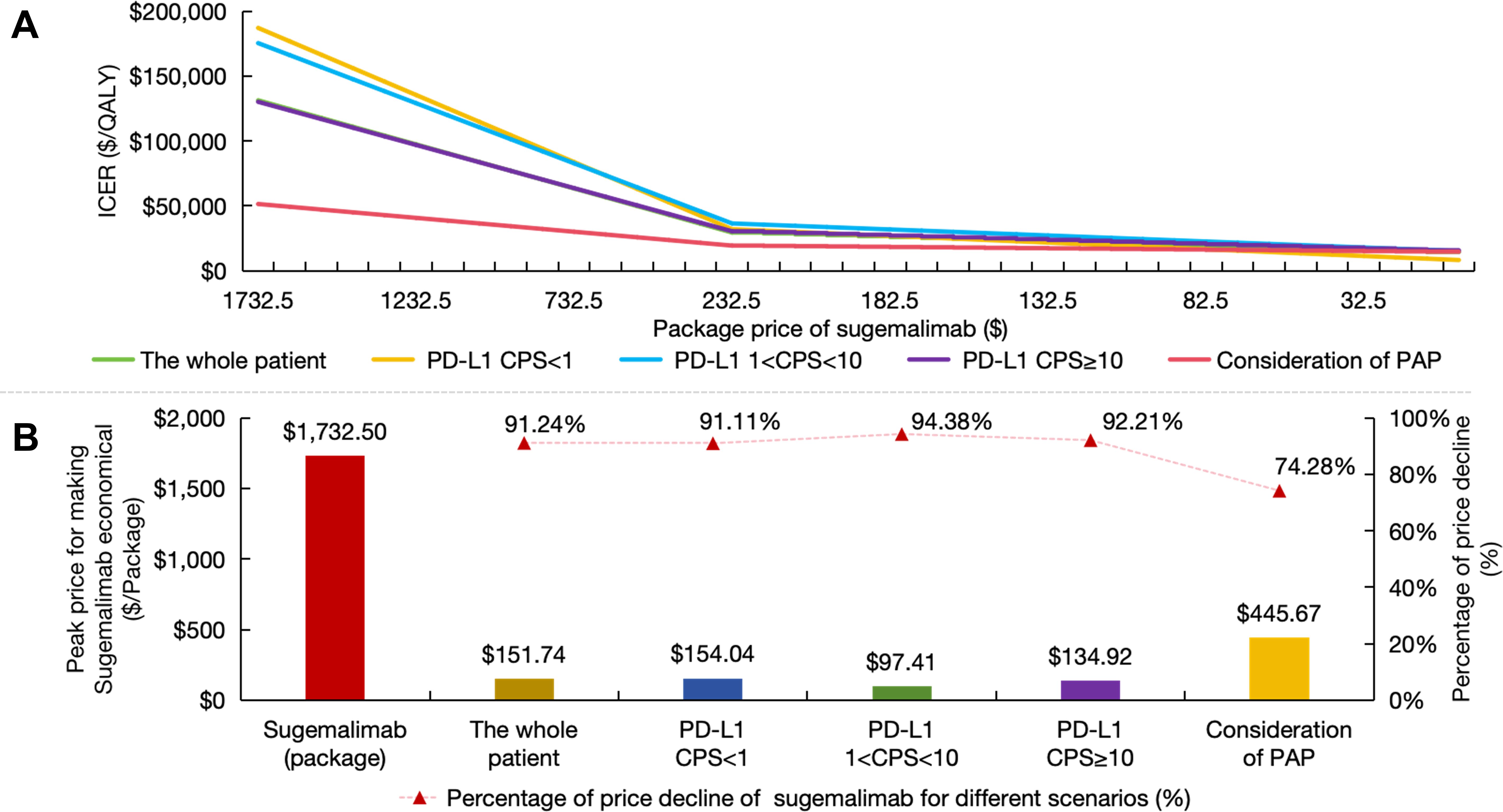
Figure 6. Price caps for sugemalimab. (A) The ICER variation curve with the decline in the package price of sugemalimab; (B) Price caps and reduction range that render sugemalimab economically viable under different scenarios. PD-L1 indicates programmed death ligand-1; CPS, combined positive score; PAP, patient assistance program; ICER, incremental cost-effectiveness ratio.
These findings collectively indicate that NRDL inclusion would necessitate unprecedented price restructuring for sugemalimab. Achieving the identified price thresholds could theoretically improve immunotherapy accessibility for ESCC patients, though the required magnitude of price adjustments (exceeding 90% in most scenarios) presents substantial implementation challenges for manufacturers and payers.
4 Discussion
The current therapeutic landscape for advanced ESCC in China remains dominated by conventional chemotherapy, radiotherapy, and surgical interventions, particularly for late-stage patients ineligible for curative resection (30). While these established modalities provide modest survival benefits, their clinical utility is constrained by suboptimal efficacy profiles and significant quality-of-life compromises. The recent introduction of immunotherapeutic agents like sugemalimab represents a paradigm shift in treatment approaches, though systemic barriers persist in translating clinical trial efficacy into real-world accessibility. Geographic disparities in healthcare resource distribution create pronounced inequities, while economic constraints further exacerbate this divide, as high out-of-pocket costs frequently lead to treatment discontinuation or suboptimal dosing regimens, particularly under China’s evolving universal healthcare coverage framework.
This study conducts a cost-effectiveness evaluation of sugemalimab-chemotherapy as first-line therapy for advanced ESCC using a PSM. The base-case analysis demonstrated an incremental gain of 0.336 QALYs at an additional cost of $ 44,182.03 compared to chemotherapy alone, with the regimen failing to meet the predefined WTP threshold. This result aligns with a prior pharmacoeconomic study (46), which concluded that the intervention lacks economic viability in the whole ESCC patients. Building upon this consensus, our analysis extends the evidence through three critical dimensions. First, CPS-stratified subgroup analyses systematically confirmed economic infeasibility across all PD-L1 expression levels. Second, PAP scenarios marginally decreased incremental costs, but failed to present cost-effectiveness advantages. Third, price cap simulations identified a 91.2% reduction requirement for sugemalimab’s current price to align with China’s pharmacoeconomic benchmarks.
The OWSA identified sugemalimab’s unit cost as the most predominant driver of ICER variability. In parallel, PSA incorporating cohort-wide, CPS-subgroup, and PAP scenarios demonstrated complete alignment with base-case outcomes. Monte Carlo simulations yielded zero ICER pairs within the cost-effective quadrant (ICER < the predefined WTP threshold), with corresponding CEACs also showing 0% probability of economic viability. These deterministic and stochastic analyses collectively indicate that no examined population subset or pricing modification achieves cost-effectiveness under the current pricing model. Sugemalimab has obtained regulatory approval in China for five oncologic indications, including squamous/non-squamous NSCLC and ESCC. Notably, published cost-effectiveness evaluations in NSCLC (47–53) all corroborate the systemic misalignment between its clinical benefits and current pricing across indications. This recurrent pharmacoeconomic paradox might arises from dual mechanisms: 1) The multi-indication development paradigm, while enabling R&D cost amortization through expanded therapeutic applications, paradoxically amplifies payer demands for volume-based price concessions during NRDL negotiations; 2) China’s value assessment framework continues to prioritize cost containment over therapeutic innovation for multi-indication biologics. Consequently, implementing an indication-stratified pricing framework—establishing disease-specific price thresholds coupled with volume-price agreements—may constitute the optimal strategic pathway to reconcile manufacturer returns with healthcare system sustainability.
Notably, several methodological assumptions inherent to our analytical framework may constrain the interpretative validity of the findings, introducing potential biases and uncertainties. Firstly, although the GEMSTONE-304 trial was conducted in China with domestic investigators, the absence of explicit regional recruitment documentation prevents confirmation of complete geographical representativeness between trial participants and broader ESCC populations (27). Secondly, the inherent selection bias of RCTs—enforcing restrictive eligibility criteria—may systematically overestimate therapeutic efficacy while underestimating safety risks prevalent in heterogeneous patient populations, as clinically significant comorbidities frequently excluded from RCT protocols routinely influence treatment feasibility and outcomes in real-world clinical settings. Thirdly, the exclusive focus on direct medical costs systematically underestimates societal economic burden by omitting direct non-medical costs and indirect costs (such as caregiving expenses, productivity losses, and ancillary medical supplies, etc) (32). Fourthly, the AE analysis framework exhibits dual constraints: it only incorporates grade ≥3 AEs with >3% incidence (nausea, cytopenias, etc.), excluding low-grade toxicities, while deriving management costs from literature sources rather than real-world claims data; and the treatment cost data of AEs in this study comes from the published relevant literature rather than real-world data, which cannot represent the true medical economic level in China (32). Fifthly, the binary treatment comparison (sugemalimab-chemo vs chemo alone) oversimplifies clinical decision-making. Only comparing the outcomes of two interventions may not provide sufficient guidance for clinicians faced with multiple treatment options in real-world clinical settings (54). Despite these constraints, the model’s adherence to trial-derived survival parameters maintains internal validity for the evaluated treatment paradigm. Through structural validation of the partitioned survival architecture, we optimized extrapolation robustness while constraining assumptions within evidence boundaries, thereby enabling contextually optimized cost-effectiveness inference that aligns with clinical decision-making frameworks.
5 Conclusions
This study reveals that sugemalimab-chemotherapy, while providing survival benefits, fails to demonstrate cost-effectiveness advantages in China’s healthcare context for advanced ESCC—consistent across overall and subgroup analyses. Patient assistance programs mitigate but cannot overcome the regimen’s economic barriers. Until further clinical evidence establishes superior safety-efficacy advantages of sugemalimab-chemotherapy, strategic price realignment remains the essential mechanism to balance therapeutic innovation with equitable accessibility, thereby aligning with China’s healthcare affordability priorities.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
QC: Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. QS: Conceptualization, Data curation, Methodology, Project administration, Software, Visualization, Writing – original draft, Writing – review & editing. BL: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported in part by grants from the from the National Natural Science Foundation of China (Grant number 82374314 (Baixue, Li)).
Acknowledgments
The authors thank all the local participants for their support and hard work in launching and implementing this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1459695/full#supplementary-material
References
1. Liu Q, Cui X, Yu X, Bian B-S-J, Qian F, Hu X-g, et al. Cripto-1 acts as a functional marker of cancer stem-like cells and predicts prognosis of the patients in esophageal squamous cell carcinoma. Mol Cancer. (2017) 16(1):81. doi: 10.1186/s12943-017-0650-7
2. Liu Y, Ge Q, Xu S, Li K, and Liu Y. Efficacy and safety of anlotinib plus programmed death-1 blockade versus anlotinib monotherapy as second or further-line treatment in advanced esophageal squamous cell carcinoma: A retrospective study. Front Oncol. (2022) 12:942678. doi: 10.3389/fonc.2022.942678
3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
4. Gong W, Xu J, Wang Y, Min Q, Chen X, Zhang W, et al. Nuclear genome-derived circular RNA circPUM1 localizes in mitochondria and regulates oxidative phosphorylation in esophageal squamous cell carcinoma. Signal Transduct Target Ther. (2022) 7(1):40. doi: 10.1038/s41392-021-00865-0
5. Zhao X, Huang L, Lu Y, Jiang W, Song Y, Qiu B, et al. PIWIL2 interacting with IKK to regulate autophagy and apoptosis in esophageal squamous cell carcinoma. Cell Death Differ. (2021) 28:1941–54. doi: 10.1038/s41418-020-00725-4
6. Xi Y, Lin Y, Guo W, Wang X, Zhao H, Miao C, et al. Multi-omic characterization of genome-wide abnormal DNA methylation reveals diagnostic and prognostic markers for esophageal squamous-cell carcinoma. Signal Transduct Target Ther. (2022) 7(1):53. doi: 10.1038/s41392-022-00873-8
7. Chen J, Duan Z, Liu Y, Fu R, and Zhu C. Ginsenoside Rh4 Suppresses Metastasis of Esophageal Cancer and Expression of c-Myc via Targeting the Wnt/β-Catenin Signaling Pathway. Nutrients. (2022) 14(15):3042. doi: 10.3390/nu14153042
8. Zhao F, Li Y, Dong Z, Zhang D, Guo P, Li Z, et al. Identification of A risk signature based on lactic acid metabolism-related lncRNAs in patients with esophageal squamous cell carcinoma. Front Cell Dev Biol. (2022) 10:845293. doi: 10.3389/fcell.2022.845293
9. Abnet CC, Arnold M, and Wei W-Q. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology. (2018) 154:360–73. doi: 10.1053/j.gastro.2017.08.023
10. Smyth EC, Lagergren J, Fitzgerald RC, Lordick F, Shah MA, Lagergren P, et al. Oesophageal cancer. Nat Rev Dis Primers. (2017) 3:17048. doi: 10.1038/nrdp.2017.48
11. Xi Y, Shen Y, Wu D, Zhang J, Lin C, Wang L, et al. CircBCAR3 accelerates esophageal cancer tumorigenesis and metastasis via sponging miR-27a-3p. Mol Cancer. (2022) 21(1):145. doi: 10.1186/s12943-022-01615-8
12. Khan Z, Hammer C, Carroll J, Di Nucci F, Acosta SL, Maiya V, et al. Genetic variation associated with thyroid autoimmunity shapes the systemic immune response to PD-1 checkpoint blockade. Nat Commun. (2021) 12(1):3355. doi: 10.1038/s41467-021-23661-4
13. Chen Y-X, Wang Z-X, Jin Y, Zhao Q, Liu Z-X, Zuo Z-X, et al. An immunogenic and oncogenic feature-based classification for chemotherapy plus PD-1 blockade in advanced esophageal squamous cell carcinoma. Cancer Cell. (2023) 41:919–932.e5. doi: 10.1016/j.ccell.2023.03.016
14. Sun J-M, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. (2021) 398:759–71. doi: 10.1016/s0140-6736(21)01234-4
15. Song Y, Zhang B, Xin D, Kou X, Tan Z, Zhang S, et al. First-line serplulimab or placebo plus chemotherapy in PD-L1-positive esophageal squamous cell carcinoma: a randomized, double-blind phase 3 trial. Nat Med. (2023) 29:473–82. doi: 10.1038/s41591-022-02179-2
16. Yan T, Cui H, Zhou Y, Yang B, Kong P, Zhang Y, et al. Multi-region sequencing unveils novel actionable targets and spatial heterogeneity in esophageal squamous cell carcinoma. Nat Commun. (2019) 10(1):1670. doi: 10.1038/s41467-019-09255-1
17. Yan X, Duan H, Ni Y, Zhou Y, Wang X, Qi H, et al. Tislelizumab combined with chemotherapy as neoadjuvant therapy for surgically resectable esophageal cancer: A prospective, single-arm, phase II study (TD-NICE). Int J Surge. (2022) 103:106680. doi: 10.1016/j.ijsu.2022.106680
18. Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. New Engl J Med. (2022) 386:449–62. doi: 10.1056/NEJMoa2111380
19. Kojima TA-O, Shah MA-O, Muro K, Francois E, Adenis A, Hsu CH, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. (2020) 38:4138–48. doi: 10.1200/JCO.20.01888
20. Lu Z, Wang J, Shu Y, Liu L, Kong L, Yang L, et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial. Bmj. (2022) 377:e068714. doi: 10.1136/bmj-2021-068714
21. Gong J, Cao J, Zhang Q, Xu N, Zhao Y, Xing B, et al. Safety, antitumor activity and biomarkers of sugemalimab in Chinese patients with advanced solid tumors or lymphomas: results from the first-in-human phase 1 trial. Cancer Immunol Immunother. (2022) 71:1897–908. doi: 10.1007/s00262-021-03102-3
22. Wei SC, Duffy CR, and Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. (2018) 8:1069–86. doi: 10.1158/2159-8290.Cd-18-0367
23. Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. (2018) 24:541–50. doi: 10.1038/s41591-018-0014-x
24. Zhou Q, Chen M, Jiang O, Pan Y, Hu D, Lin Q, et al. Sugemalimab versus placebo after concurrent or sequential chemoradiotherapy in patients with locally advanced, unresectable, stage III non-small-cell lung cancer in China (GEMSTONE-301): interim results of a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. (2022) 23:209–19. doi: 10.1016/s1470-2045(21)00630-6
25. Zhou C, Wang Z, Sun Y, Cao L, Ma Z, Wu R, et al. Sugemalimab versus placebo, in combination with platinum-based chemotherapy, as first-line treatment of metastatic non-small-cell lung cancer (GEMSTONE-302): interim and final analyses of a double-blind, randomised, phase 3 clinical trial. Lancet Oncol. (2022) 23:220–33. doi: 10.1016/s1470-2045(21)00650-1
26. Huang HA-O, Tao RA-O, Hao S, Yang Y, Cen HA-O, Zhou H, et al. Sugemalimab monotherapy for patients with relapsed or refractory extranodal natural killer/T-cell lymphoma (GEMSTONE-201): results from a single-arm, multicenter, phase II study. J Clin Oncol. (2023) 41:3032–41. doi: 10.1200/JCO.22.02367
27. Li J, Chen Z, Bai Y, Liu B, Li Q, Zhang J, et al. First-line sugemalimab with chemotherapy for advanced esophageal squamous cell carcinoma: a randomized phase 3 study. Nat Med. (2024) 30:740–8. doi: 10.1038/s41591-024-02797-y
28. Connock M, Auguste P, Dussart C, Guyotat J, and Armoiry X. Cost-effectiveness of tumor-treating fields added to maintenance temozolomide in patients with glioblastoma: an updated evaluation using a partitioned survival model. J Neuro-Oncol. (2019) 143:605–11. doi: 10.1007/s11060-019-03197-w
29. Xie Q, Wen F, Wei YQ, Deng HX, and Li Q. Cost analysis of adjuvant therapy with XELOX or FOLFOX4 for colon cancer. Colorectal Dis. (2013) 15:958–62. doi: 10.1111/codi.12216
30. Committee CSoCOG. Chinese Society of Clinical Oncology Guidelines for the Diagnosis and Treatment of Esophageal Cancer 2024. Beijing: People’s Medical Publishing House (2024).
31. Palmer S, Borget I, Friede T, Husereau D, Karnon J, Kearns B, et al. A guide to selecting flexible survival models to inform economic evaluations of cancer immunotherapies. Value Health. (2023) 26:185–92. doi: 10.1016/j.jval.2022.07.009
32. Liu G. China Guidelines for Pharmaceutical Economics Evaluation 2020. Beijing: China Market Press (2020).
33. Liu J, Zhou WY, Luo XJ, Chen YX, Wong CW, Liu ZX, et al. Long noncoding RNA Regulating ImMune Escape regulates mixed lineage leukaemia protein-1-H3K4me3-mediated immune escape in oesophageal squamous cell carcinoma. Clin Trans Med. (2023) 13(9):e1410. doi: 10.1002/ctm2.1410
34. Guyot P, Ades AE, Ouwens MJNM, Welton NJ, and Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. (2012) 12:9. doi: 10.1186/1471-2288-12-9
35. Shen J, Du Y, Shao R, and Jiang R. First-line sintilimab plus chemotherapy in locally advanced or metastatic esophageal squamous cell carcinoma: A cost-effectiveness analysis from China. Front Pharmacol. (2022) 13:967182. doi: 10.3389/fphar.2022.967182
36. Chen Q, Shao M, Zhang R, and Chen X. Cost-utility analysis of the first-line treatment of advanced, recurrent or metastatic esophageal squamous cell carcinoma with Cindilizumab combined with chemotherapy. China Pharmacy. (2023) 34:345–9. doi: 10.6039/j.issn.1001-0408.2023.03.17
37. Giuliani J and Bonetti A. Cost-effectiveness of newer regimens for the prophylaxis of chemotherapy-induced nausea and vomiting: review of the literature and real-world data. Curr Opin Oncol. (2020) 32:269–73. doi: 10.1097/cco.0000000000000634
38. Rui M, Fei Z, Wang Y, Zhang X, Ma A, Sun H, et al. Cost-effectiveness analysis of sintilimab + chemotherapy versus camrelizumab amrelizumabapy for the treatment of first-line locally advanced or metastatic nonsquamous NSCLC in China. J Med Econ. (2022) 25:618–29. doi: 10.1080/13696998.2022.2071066
39. Wilke H, Muro K, Van Cutsem E, Oh S-C, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. (2014) 15:1224–35. doi: 10.1016/s1470-2045(14)70420-6
40. Yang F, Fu Y, Kumar A, Chen M, Si L, and Rojanasarot S. Cost-effectiveness analysis of camrelizumab in the second-line treatment for advanced or metastatic esophageal squamous cell carcinoma in China. Ann Trans Med. (2021) 9:1226–6. doi: 10.21037/atm-21-1803
41. Xu L, Chen M, Angell B, Jiang Y, Howard K, Jan S, et al. Establishing cost-effectiveness threshold in China: a community survey of willingness to pay for a healthy life year. BMJ Global Health. (2024) 9(1):e013070. doi: 10.1136/bmjgh-2023-013070
42. Briggs AH, Weinstein MC, Fenwick EAL, Karnon J, Sculpher MJ, and Paltiel AD. Model parameter estimation and uncertainty analysis. Med Decision Making. (2012) 32:722–32. doi: 10.1177/0272989x12458348
43. De Mello RA, Li S, Peng L, Tan C, Zeng X, Wan X, et al. Cost-Effectiveness of ramucirumab plus paclitaxel as a second-line therapy for advanced gastric or gastro-oesophageal cancer in China. PloS One. (2020) 15(5):e0232240. doi: 10.1371/journal.pone.0232240
44. Liu S, Dou L, and Li S. Cost-effectiveness analysis of PD-1 inhibitors combined with chemotherapy as first-line therapy for advanced esophageal squamous-cell carcinoma in China. Front Pharmacol. (2023) 14:1055727. doi: 10.3389/fphar.2023.1055727
45. Zhang PF, Xie D, and Li QA-O. Cost-effectiveness analysis of nivolumab in the second-line treatment for advanced esophageal squamous cell carcinoma. Future Oncol. (2020) 16:1189–98. doi: 10.2217/fon-2019-0821
46. Cai H, Fang L, and Zheng Z. Sugemalimab combined with chemotherapy for the treatment of advanced esophageal squamous cell carcinoma: a cost-effectiveness analysis. Front Pharmacol. (2024) 15:1396761. doi: 10.3389/fphar.2024.1396761
47. Chen P, Li Y, Jing X, Chen J, Chen S, and Yang Q. Cost-effectiveness analysis of sugemalimab in combination with chemotherapy as first-line treatment in Chinese patients with metastatic NSCLC. Lung Cancer. (2022) 174:157–64. doi: 10.1016/j.lungcan.2022.11.008
48. Zheng Z, Zhu H, Fang L, and Cai H. Cost-effectiveness analysis of sugemalimab vs. chemotherapy as first-line treatment of metastatic nonsquamous non-small cell lung cancer. Front Pharmacol. (2022) 13:996914. doi: 10.3389/fphar.2022.996914
49. Li W and Wan L. Cost-effectiveness analysis of sugemalimab vs. placebo, in combination with chemotherapy, for treatment of first-line metastatic NSCLC in China. Front Public Health. (2022) 10:1015702. doi: 10.3389/fpubh.2022.1015702
50. Cheng M, Shao Y, Li L, Jiang M, and Song Z. Cost-effectiveness of immunotherapies for advanced squamous non-small cell lung cancer: a systematic review. BMC Cancer. (2024) 24(1):312. doi: 10.1186/s12885-024-12043-w
51. Cheng R, Zhou Z, and Liu Q. The cost-effectiveness of sugemalimab plus chemotherapy as first-line treatment for metastatic squamous and non-squamous NSCLC in China. Adv Ther. (2023) 40:4298–309. doi: 10.1007/s12325-023-02594-y
52. Wang H, Liao L, Xu Y, Long Y, Wang Y, and Zhou Y. Economic evaluation of first-line sugemalimab plus chemotherapy for metastatic non-small cell lung cancer in China. Front Oncol. (2022) 12:1081750. doi: 10.3389/fonc.2022.1081750
53. Püsküllüoğlu M, Li W, and Wan L. A trial-based cost-utility analysis of sugemalimab vs. placebo as consolidation therapy for unresectable stage III NSCLC in China. PloS One. (2023) 18(6):e0286595. doi: 10.1371/journal.pone.0286595
Keywords: cost-effectiveness, sugemalimab, esophageal squamous cell carcinoma, partitioned survival model, PD-L1
Citation: Chen Q, Sun Q and Li B (2025) Sugemalimab plus chemotherapy vs. chemotherapy for treatment of Chinese patients with esophageal squamous cell carcinoma: a cost effectiveness analysis to inform decision making. Front. Oncol. 15:1459695. doi: 10.3389/fonc.2025.1459695
Received: 04 July 2024; Accepted: 23 May 2025;
Published: 05 June 2025.
Edited by:
Mingzhou Guo, People’s Liberation Army General Hospital, ChinaReviewed by:
Salvatore Lavalle, San Raffaele Hospital (IRCCS), ItalyAhmed Amin, Kafrelsheikh University, Egypt
Copyright © 2025 Chen, Sun and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baixue Li, YmFpeHVlbGVlQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Qiuping Chen
Qiuping Chen Quan Sun
Quan Sun Baixue Li
Baixue Li