- 1Department of Oncology, Guangzhou Fuda Cancer Hospital, Guangzhou, China
- 2Department of Surgery and Anesthesia, Guangzhou Fuda Cancer Hospital, Guangzhou, China
- 3Central Laboratory, Guangzhou Fuda Cancer Hospital, Guangzhou, China
Cryoablation has emerged as a promising local treatment technique for early breast cancer, garnering significant interest in recent years. This review delves into the fundamental principles of cryoablation, its clinical applications, efficacy assessments, and comparisons with traditional treatment modalities. We explore the potential advantages and challenges associated with incorporating cryoablation into the management of early breast cancer. Furthermore, we analyze current research developments and future directions in this field, aiming to provide valuable insights for clinical practice and enhance patient care in breast cancer management.
1 Introduction
Breast cancer remains one of the most prevalent malignancies globally, with a significant impact on women’s health. The rising incidence rates have prompted a critical reevaluation of treatment strategies, particularly for early-stage breast cancer, which presents unique challenges and opportunities for innovative therapies (1–4). Traditional treatment options such as surgery, radiation therapy, and chemotherapy have been the cornerstone of management. However, the focus is shifting towards minimizing treatment-related morbidity while maximizing patient quality of life. In this context, cryoablation has emerged as a promising minimally invasive alternative to conventional surgical approaches, offering potential benefits in terms of safety, efficacy, and patient satisfaction (5–7).
The treatment landscape for early-stage breast cancer has evolved, emphasizing the need for less aggressive interventions without compromising oncological outcomes. Surgical options, such as lumpectomy and mastectomy, while effective, often entail significant physical and emotional burdens on patients. Cryoablation has emerged as a promising minimally invasive technique for the treatment of various cancers, including early-stage breast cancer (8–14). This technique involves the application of extreme cold to destroy cancerous tissues, offering potential advantages over conventional treatments, such as reduced recovery times and fewer complications (15–17). Early clinical trials have demonstrated that cryoablation can achieve comparable local control rates to surgical excision, particularly in tumors less than 2 cm in size, with low recurrence rates reported (18, 19). Moreover, cryoablation can be performed in an outpatient setting under local anesthesia, thereby reducing recovery times and enhancing patient comfort (20).The rise of cryoablation in oncology is driven by its ability to precisely target tumor cells while sparing surrounding healthy tissues, thus minimizing collateral damage and enhancing patient quality of life (21–24).
Despite the promising results, the integration of cryoablation into clinical practice requires careful consideration of patient selection criteria and comprehensive management protocols. The current literature highlights a need for standardized guidelines to optimize patient outcomes and ensure that cryoablation is utilized effectively within the broader spectrum of breast cancer treatment (25, 26). This review aims to clarify cryoablation’s role in early-stage breast cancer management, highlighting benefits and limitations to aid clinicians and researchers in optimizing treatment protocols.
2 Cryoablation for early breast cancer
2.1 Principles and equipment of cryoablation technology
2.1.1 Basic principles of cryoablation
Cryoablation is a minimally invasive technique that employs extreme cold to destroy cancer cells (14, 27, 28). The fundamental principle involves the application of freezing temperatures to induce cellular injury and death (29, 30). This process is typically achieved using cryoprobes that deliver liquid nitrogen or argon gas to the target tissue, creating an “iceball” that encompasses the tumor (31). The rapid freezing and slow thawing cycles cause ice crystals to form within the cells, leading to cellular dehydration, membrane rupture, and ultimately, cell death through necrosis and apoptosis (32, 33). Additionally, cryoablation induces vascular damage, resulting in ischemia and further contributing to tumor destruction (33–35). The technique’s efficacy is enhanced by the immune response triggered by the release of tumor antigens during the cryoablation process, potentially aiding in the systemic eradication of cancer cells (16, 36–40).
The effectiveness of cryoablation is particularly notable in the treatment of early-stage breast cancer, where it has been demonstrated to achieve high rates of tumor ablation and low recurrence rates (41). Studies indicate that cryoablation can be performed safely in an outpatient setting, often under local anesthesia, which minimizes patient discomfort and recovery time compared to traditional surgical approaches (18, 25, 42). The technique is gaining traction as a viable alternative to surgical resection, especially for patients with small, low-risk tumors who may not tolerate more invasive procedures (43).
2.1.2 Mechanism of cryoablation
The mechanism of cryoablation involves several key processes that contribute to its effectiveness in tumor destruction. Initially, the application of extreme cold leads to the formation of ice crystals within the tumor cells, which disrupts cellular membranes and organelles, ultimately causing cell death through necrosis. This process is facilitated by the rapid cooling of the tissue, which can reach temperatures as low as -40°C, effectively inducing irreversible damage to the cellular structure (19, 44–46). Moreover, cryoablation has been found to induce an inflammatory response that can enhance the immune system’s ability to recognize and attack tumor cells (47–49). The necrotic tissue releases tumor-specific antigens into the systemic circulation, potentially activating both innate and adaptive immune responses (16, 40, 50). This immunological effect is particularly relevant in the context of combining cryoablation with immunotherapy, as it may enhance the overall therapeutic efficacy against the tumor (16, 51, 52). Additionally, cryoablation precisely targets tumors while protecting healthy tissue, minimizing damage and preserving adjacent structures, aided by imaging guidance like ultrasound or MRI for real-time monitoring (53) (Figure 1).
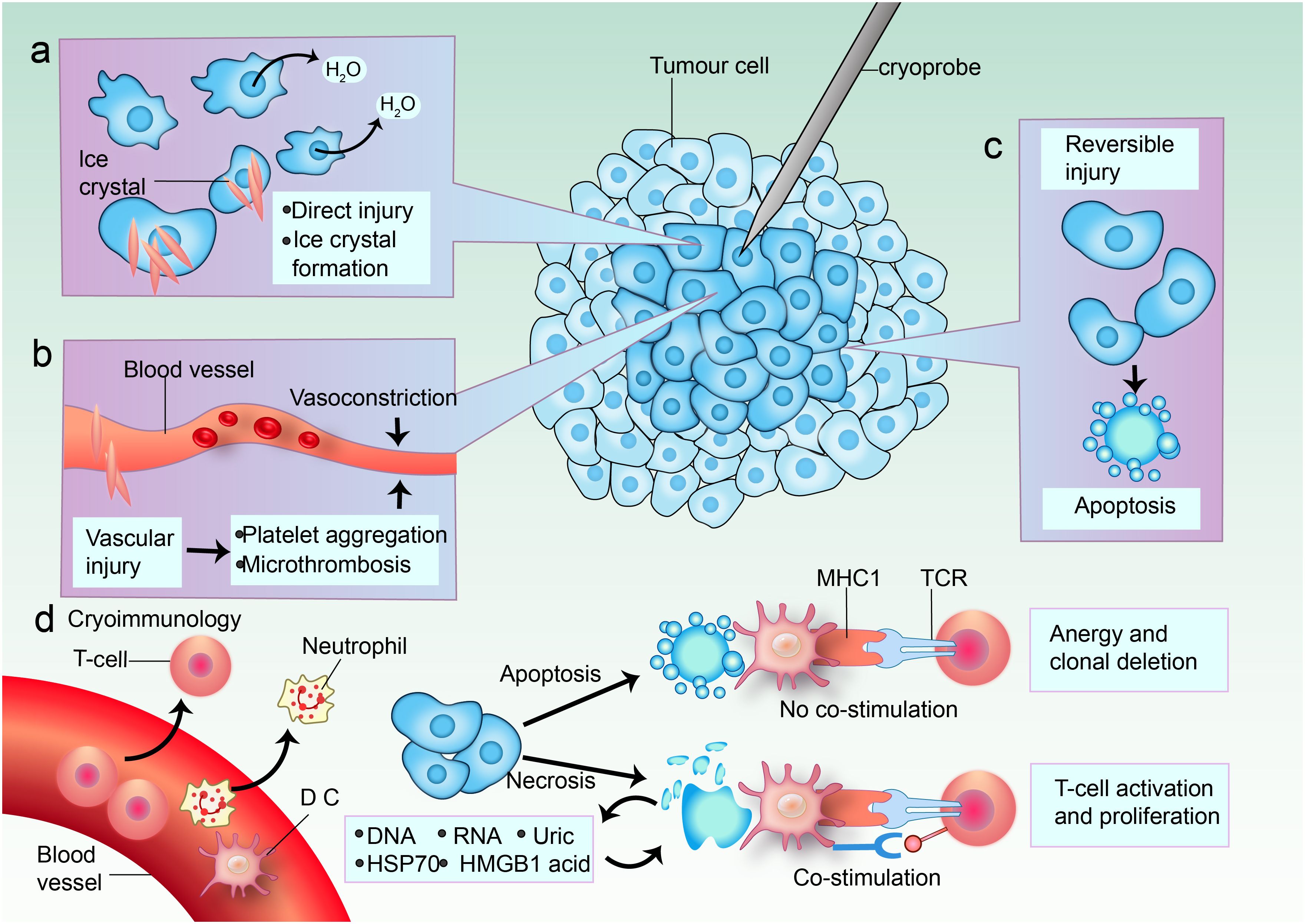
Figure 1. Mechanisms of cell death in cryoablation. (a) In the center of the cryoablative lesion lies a sharply delineated area of frozen necrosis where direct injury takes place. Here, the temperature rapidly drops below -40°C, causing ice to form from the extracellular space inward. This leads to a hypertonic extracellular environment and osmotic cell shrinkage due to fluid shifting out of the cell. The formation of ice crystals increases direct damage. (b) Cold-induced vascular injury causes harm to endothelial cells and cell junctions, resulting in platelet aggregation and microthrombosis. Vasoconstriction occurs in response to cooling temperatures. Freezing also leads to a hyperemic response and increased vascular permeability. The consequence is ischemia, causing further coagulative necrosis. (c) Apoptosis occurs in a peripheral zone of sublethal cold temperatures, and this is likely induced by reversible damage. (d) Blood vessels supply immune cell infiltrates. Both enhanced and reduced anti-tumor immunity can be triggered by cryoablation; immunomodulation might depend on the predominant mode of cell death. Some tumor cells undergo apoptosis. When antigen-presenting cells (APCs) such as dendritic cells (DCs) and macrophages phagocytose tumor cells after apoptosis without danger signals, the tumor antigens are presented on major histocompatibility complex (MHC) class I molecules without co-stimulation of T cells. The dying cells can even secrete immunosuppressive cytokines, such as interleukin-10 (IL-10) and transforming growth factor-β (TGFβ). This induces anergy and clonal deletion. Other tumor cells are necrotic, and they release their extracellular contents: DNA, RNA, heat shock protein 70 (HSP70), uric acid, and high mobility group protein B1 (HMGB1). Pro-inflammatory cytokines induce DCs to take up more antigens and express danger signals via co-stimulatory molecules that are necessary to prime nearby T cells. TCR, T cell receptor.
2.1.3 Cryoablation equipment and techniques
Cryoablation equipment is characterized by its precision, safety, and efficacy. These systems are designed to deliver controlled freezing cycles, ensuring complete ablation of the target tissue while minimizing damage to adjacent structures, including real-time imaging capabilities, such as ultrasound, CT, or MRI guidance, which allow for accurate placement of cryoprobes and monitoring of the ablation process (54). Additionally, these devices offer adjustable cryoprobe sizes and configurations, enabling customization based on tumor size and location (55). Safety mechanisms, such as temperature sensors and feedback systems, are integrated to prevent over-freezing and reduce the risk of complications (56).
2.2 Clinical application of cryoablation in early breast cancer
2.2.1 Definition and treatment principles of early breast cancer
Early breast cancer is typically defined as cancer that is confined to the breast or has only spread to nearby lymph nodes but not to distant parts of the body (57–59). The primary treatment principles for early breast cancer include local control of the tumor and prevention of systemic recurrence (3, 60–62). Local control is often achieved through surgery, which may be followed by radiation therapy, while systemic recurrence is managed with adjuvant therapies such as chemotherapy, hormone therapy, and targeted therapy (63). The goal is to eliminate cancer cells and reduce recurrence risk, improving survival and quality of life (64).
2.2.2 Indications for cryoablation in early breast cancer treatment
Cryoablation is indicated for early breast cancer in patients with small, localized tumors under 1.5 cm, especially low-grade, hormone receptor-positive ones (43, 65, 66). Patients unsuitable for surgery may benefit from this treatment, while contraindications include large tumors, multifocal disease, and significant comorbidities (25). Moreover, patients with certain types of breast cancer, such as triple-negative breast cancer, may not be ideal candidates for cryoablation due to the aggressive nature of the disease and the potential for higher recurrence rates. It is essential for healthcare providers to conduct a thorough assessment of each patient’s clinical profile before recommending cryoablation as a treatment option.
2.2.3 Clinical trials and research findings
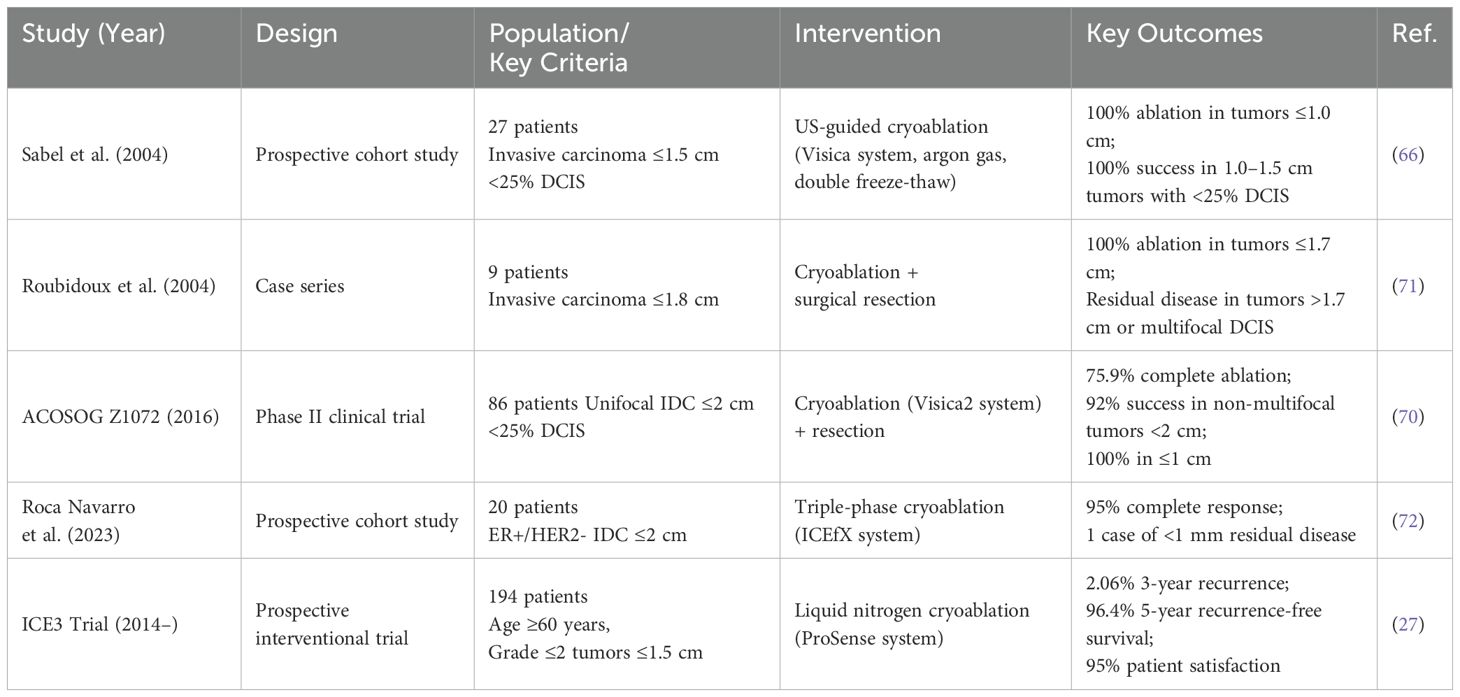
Cryoablation has emerged as a minimally invasive therapeutic modality for both benign and malignant breast pathologies (26, 67). For fibroadenoma management, this technique demonstrates sustained efficacy with longitudinal studies reporting >85% volumetric reduction and excellent post-procedural cosmesis (68, 69). The clinical application has expanded to invasive carcinomas, achieving histological ablation success rates of 76-100% in neoplasms ≤2 cm when utilizing modern cryogenic systems (27, 70) (Table 1).
Early pivotal work by Sabel et al. (66) utilized an argon gas-based cryoablation system (Visica) delivering probe temperatures of -160°C via a double freeze-thaw protocol. In 27 patients with invasive carcinomas, 100% ablation success was achieved for tumors ≤1.0 cm (Table 1). For lesions 1.0–1.5 cm, equivalent efficacy required <25% ductal carcinoma in situ (DCIS) on core biopsy. Technical refinements during the trial, including probe insulation optimization (2.7 mm diameter probe with distal 4 cm cooling), highlighted the critical interplay between device design and tumor size limitations. Concurrently, Roubidoux et al. (71) reported complete tumor eradication in 9 patients with invasive carcinomas ≤1.7 cm, while residual disease was observed in larger tumors (1.8 cm) or those with undetected multifocal DCIS, reinforcing the importance of preoperative imaging-pathology correlation (Table 1).
The ACOSOG Z1072 phase II trial (70) prospectively evaluated cryoablation in 86 patients with unifocal invasive ductal carcinoma (IDC) ≤2 cm and <25% DCIS. Employing the Visica2 system with pre- and post-procedural MRI, pathologic analysis revealed: 75.9% complete ablation across all cases, 92% success in non-multifocal tumors <2 cm, 100% efficacy in tumors ≤1.0 cm. These findings underscored multifocality as a key predictor of treatment failure.
Recent advancements by Roca Navarro et al. (72) integrated magnetic seed localization (Sentimag) with triple-phase cryoablation (ICEfX system, 14-G/17-G probes) under continuous ultrasound guidance (Table 1). In 20 patients with ER+/HER2- IDC <2 cm, histologic analysis post-excision (6–49 days) demonstrated 95% complete ablation (19/20 cases), with a single case showing <1 mm residual invasive disease. Critical Exclusion Criteria: Invasive lobular carcinoma remains contraindicated due to its multicentric growth pattern and poor correlation between imaging findings and pathologic extent (73).
The therapeutic paradigm for early-stage breast cancer is being redefined by three landmark clinical trials evaluating cryoablation as definitive treatment. The ICE3 trial (NCT02200705), initiated in 2014, enrolled 194 elderly patients (mean age 75 years) with ultrasound-visible unifocal IDC ≤1.5 cm (ER/PR+, HER2-, Nottingham grade ≤2) (27) (Table 1). Utilizing the ProSense liquid nitrogen system (-170°C), this protocol achieved 35–40 mm ice balls through stab-incision placement with dual freeze-thaw cycles. Three-year interim analysis revealed 2.06% ipsilateral recurrence (4/194), comparable to surgical outcomes, with 20.8% experiencing minor adverse events (ecchymosis, transient cryodermatitis) (27) (Table 1). Extended 5-year follow-up demonstrated 96.4% local control (7 recurrences) and universal satisfaction with cosmetic outcomes, despite three undetermined breast cancer-related mortalities (74).
These trials collectively suggest cryoablation achieves 96-99% 5-year local control in carefully selected, imaging-concordant IDC. However, critical knowledge gaps persist regarding optimal patient selection biomarkers, DCIS component thresholds, and long-term survival equivalence to surgical resection. The integration of genomic profiling (COOL-IT) and artificial intelligence-driven thermal monitoring represents the next frontier in ablative precision oncology.
2.3 Evaluation of the efficacy of cryoablation
2.3.1 Tumor control rate
The tumor control rate is a critical metric for evaluating the efficacy of cryoablation. It reflects the ability of the procedure to effectively manage tumor growth and prevent recurrence. Various studies have reported favorable tumor control rates across different types of cancers treated with cryoablation. For example, in patients with renal cell carcinoma, cryoablation has shown a local recurrence-free survival rate of approximately 88.1% at one year, with significant long-term control observed in many cases (75). In breast cancer, cryoablation has demonstrated a complete ablation rate of 90%, indicating its effectiveness in achieving tumor control without the need for extensive surgical procedures (18). Additionally, a meta-analysis comparing cryoablation with radiofrequency ablation for non-small cell lung cancer revealed that cryoablation had superior disease-free survival rates, further supporting its efficacy as a treatment option (76). These findings collectively highlight that cryoablation can achieve high tumor control rates, making it a valuable option in the oncological treatment landscape, particularly for patients with localized tumors.
2.3.2 Analysis of postoperative complications and safety
The safety profile of cryoablation is a significant aspect of its overall efficacy evaluation. While the technique is generally associated with fewer complications compared to traditional surgical approaches, it is essential to analyze the types and rates of postoperative complications that may arise. Studies indicate that cryoablation is associated with a lower incidence of severe complications, particularly in comparison to more invasive surgical procedures (77–79). Additionally, a study on cryoablation for breast lesions reported no major complications in patients, emphasizing the procedure’s safety (80). Minor complications like pain or swelling can occur, but the overall risk is low, making cryoablation an appealing option for patients wanting less invasive treatments. Furthermore, advanced imaging in cryoablation improves precision, reducing complications from misplacement or inadequate tumor treatment (53). Cryoablation’s safety profile, with low complications and good outcomes, confirms its viability in modern oncology.
2.3.3 Imaging techniques for post-cryoablation efficacy assessment
Ultrasound: Ultrasound has become the most commonly used method for assessing treatment efficacy after cryoablation due to its real-time capabilities, ease of operation, and lack of radiation exposure. Post-cryoablation ultrasound evaluates treatment efficacy by detecting changes in low echo areas and the absence of Doppler blood flow signals. This is particularly helpful for accurately locating tumor boundaries and assessing immediate treatment effects (18, 81, 82).
Magnetic Resonance Imaging (MRI): MRI provides clear images of breast tumors and surrounding structures, making it widely used for assessing the necrosis extent in the ablation area and the presence of residual tumor activity. However, the MRI-guided cryoablation process is time-consuming and requires expensive equipment, posing significant challenges for its widespread clinical adoption (83, 84).
Computed Tomography (CT): CT, with its high spatial resolution, clearly shows breast tumor lesions and surrounding structures, making it especially suitable for deeper or more complex lesions (23, 85). However, CT guidance involves radioactive radiation, which limits its conventional application.
2.4 Comparison of cryoablation with traditional treatment methods
Cryoablation is a minimally invasive treatment with advantages over surgery and radiation, offering a compelling option for tumors while reducing risks. The technique involves the application of extreme cold to destroy cancerous cells, resulting in less damage to surrounding healthy tissue, shorter recovery times, and improved cosmetic outcomes. Compared to surgical resection, which often requires significant recovery time and carries the risks of major surgery, cryoablation is associated with lower complication rates and can be performed on an outpatient basis (53, 86, 87). This suggests that cryoablation may be a suitable alternative for patients who are not candidates for surgery due to comorbidities or personal preferences.
2.4.1 Comparison with surgical resection
When comparing cryoablation to surgical resection, it is essential to consider both the efficacy and safety profiles of the two approaches. Surgical resection remains the gold standard for many solid tumors, particularly when complete removal of the tumor is feasible. However, the invasiveness of surgical procedures can lead to longer hospital stays, increased postoperative pain, and a higher risk of complications (88). In contrast, cryoablation is associated with shorter recovery times and less postoperative discomfort. Studies have reported that patients undergoing cryoablation experience significantly lower rates of treatment-emergent adverse events compared to those undergoing surgical resection (89, 90). Moreover, cryoablation can be repeated for tumor recurrence, offering flexibility, while preserving healthy tissue and organ function, making it appealing for localized tumors despite surgical resection being more definitive (91, 92). Ultimately, the choice between cryoablation and surgical resection should be individualized based on tumor characteristics, patient preferences, and overall health status.
2.4.2 Combined application with radiotherapy
The combination of cryoablation with radiotherapy represents a promising strategy for enhancing treatment efficacy in various malignancies. While cryoablation effectively targets and destroys tumor cells through extreme cold, radiotherapy complements this by delivering ionizing radiation to eliminate residual cancer cells and reduce the risk of local recurrence (22, 93, 94). This synergistic approach has shown potential in improving overall survival rates and disease-free survival (94). The integration of cryoablation with radiotherapy may also exploit the abscopal effect, where localized treatment leads to systemic immune responses that can target distant metastases (95). For example, in patients with metastatic dedifferentiated liposarcoma, cryoablation has been observed to induce immune-mediated regression of untreated metastases, suggesting that combining local therapies with immunotherapy could enhance therapeutic outcomes (96). Furthermore, using cryoablation as a bridging therapy prior to immunotherapy, such as CAR-T cell therapy, has shown promising results in reducing tumor burden and improving response rates (97). Overall, the combination of cryoablation with radiotherapy offers a multifaceted approach to cancer treatment, potentially maximizing therapeutic efficacy while minimizing treatment-related morbidity.
2.5 Future directions of cryoablation
Cryoablation, a minimally invasive technique that employs extreme cold to destroy abnormal tissue, has shown promising results in various clinical applications. As the field continues to evolve, the future of cryoablation appears to be shaped by significant technological advancements and innovations. Recent developments in imaging techniques, such as MRI and ultrasound, have enhanced the precision of cryoablation procedures, allowing for better targeting of lesions and improved patient outcomes (98). Moreover, the integration of robotic systems and automated devices is expected to streamline the procedure, reduce operator variability, and enhance safety during ablation. Innovations in cryoablation devices, including multi-needle systems that allow for simultaneous treatment of multiple sites, are also on the horizon (99). Furthermore, the incorporation of nanotechnology in cryoablation could improve the delivery of cryogenic agents and enhance the effectiveness of the treatment by modulating the immune response post-ablation (100, 101). Overall, the technological progress in cryoablation is likely to expand its applications across various malignancies and benign conditions, leading to more effective and personalized treatment options.
2.5.1 Technological advances and innovations
The cryoablation technology is advancing rapidly, with innovations improving efficacy and safety, particularly in imaging modalities like real-time MRI and ultrasound, which enhance tissue localization and reduce damage to healthy structures (53). Advancements in cryoablation devices, like temperature-sensitive materials and better cryogenic agents, will optimize freezing for more effective ablation (102). Furthermore, the integration of AI and machine learning in cryoablation systems promises to automate procedures and enhance decision-making in treatment (103, 104). These innovations improve cryoablation’s precision and safety while enabling new tumor treatments.
2.5.2 Clinical applications: prospects and challenges
The clinical application of cryoablation is expanding, with promising prospects in treating a variety of conditions (99). Cryoablation’s effectiveness as a minimally invasive alternative is likely to boost its clinical adoption. However, several challenges remain. One of the primary obstacles is the need for standardized protocols and guidelines to ensure consistent outcomes across different institutions and practitioners (105). Additionally, while cryoablation is associated with lower morbidity compared to surgical resection, concerns regarding the long-term efficacy and potential for recurrence still need to be addressed through ongoing research and clinical trials (103). Furthermore, the integration of cryoablation into multimodal treatment plans, particularly in combination with immunotherapy or targeted therapies, presents both opportunities and challenges that require careful consideration and investigation (16, 37, 105–107). Overall, while the future of cryoablation is promising, addressing these challenges will be crucial for its successful implementation in clinical settings.
3 Conclusion
Cryoablation for early-stage breast cancer shows promise but has limitations; it offers minimally invasive options to reduce morbidity while ensuring effective outcomes, necessitating comparison with traditional surgeries. Balancing diverse findings on cryoablation is challenging; some studies show good tumor control and cosmetic results, while others question long-term efficacy and recurrence. This highlights the need for multi-center trials to clarify cryoablation’s role in breast cancer care, especially regarding survival and quality of life. Future research should identify patient-specific factors influencing cryoablation success, such as genetic markers and tumor characteristics, to develop personalized treatment plans. Incorporating cryoablation into comprehensive strategies could improve early-stage breast cancer management. Integrating cryoablation into clinical practice offers a non-invasive alternative for patients not suited for surgery, promoting a multidisciplinary approach for balanced treatment options. In conclusion, cryoablation shows promise for early-stage breast cancer, but further research is needed to refine its role in personalized treatment and improve patient outcomes.
Author contributions
YX: Formal analysis, Writing – original draft, Writing – review & editing. HL: Writing – review & editing. TY: Project administration, Writing – review & editing. XZ: Project administration, Writing – review & editing. BL: Project administration, Writing – review & editing. YM: Formal analysis, Project administration, Writing – review & editing. LN: Funding acquisition, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by grants from the International Science Foundation of Guangzhou Fuda Cancer Hospital (Y2021-MS-02); Science and Technology Program of Guangzhou, No. 202201020023; Science and Technology Program of Guangzhou, No. 20220102009.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Burstein HJ, Curigliano G, Thürlimann B, Weber WP, Poortmans P, Regan MM, et al. Customizing local and systemic therapies for women with early breast cancer: the St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann Oncol. (2021) 32:1216–35. doi: 10.1016/j.annonc.2021.06.023
2. Purswani JM, Hardy-Abeloos C, Perez CA, Kwa MJ, Chadha M, and Gerber NK. Radiation in early-stage breast cancer: moving beyond an all or nothing approach. Curr Oncol. (2022) 30:184–95. doi: 10.3390/curroncol30010015
3. Thomas M, Kelly ED, Abraham J, and Kruse M. Invasive lobular breast cancer: A review of pathogenesis, diagnosis, management, and future directions of early stage disease. Semin Oncol. (2019) 46:121–32. doi: 10.1053/j.seminoncol.2019.03.002
4. Harbeck N and Gnant M. Breast cancer. Lancet. (2017) 389:1134–50. doi: 10.1016/S0140-6736(16)31891-8
5. Shimanuki Y, Hashimoto H, Kawazoe H, Uozumi R, Udagawa R, Watabe D, et al. Preventive effects of self-administered cryotherapy on paclitaxel-induced peripheral neuropathy in patients with early-stage breast cancer: a propensity score analysis. Pharmazie. (2021) 76:261–5. doi: 10.1691/ph.2021.1398
6. Balic M, Thomssen C, Gnant M, and Harbeck N. St. Gallen/Vienna 2023: optimization of treatment for patients with primary breast cancer - A brief summary of the consensus discussion. Breast Care (Basel). (2023) 18:213–22. doi: 10.1159/000530584
7. Nigdelis MP, Karamouzis MV, Kontos M, Alexandrou A, Goulis DG, and Lambrinoudaki I. Updates on the treatment of invasive breast cancer: Quo Vadimus? Maturitas. (2021) 145:64–72. doi: 10.1016/j.maturitas.2020.11.006
8. Palussière J, Cazayus M, Cousin S, Cabart M, Chomy F, Catena V, et al. Is there a role for percutaneous ablation for early stage lung cancer? What is the evidence? Curr Oncol Rep. (2021) 23:81. doi: 10.1007/s11912-021-01072-4
9. Luo J, Dong Z, Xie H, Zhang W, An L, Yu Z, et al. Efficacy and safety of percutaneous cryoablation for elderly patients with small hepatocellular carcinoma: A prospective multicenter study. Liver Int. (2022) 42:918–29. doi: 10.1111/liv.15169
10. Xue K, Liu X, Xu X, Hou S, Wang L, and Tian B. Perioperative outcomes and long-term survival of cryosurgery on unresectable pancreatic cancer: a systematic review and meta-analysis. Int J Surg. (2024) 110:4356–69. doi: 10.1097/JS9.0000000000001407
11. Alexander ES, Petre EN, Offin M, Zauderer M, Zhao K, Sotirchos V, et al. Safety and efficacy of percutaneous cryoablation for primary and metastatic pleural based tumors. Eur J Radiol. (2024) 175:111465. doi: 10.1016/j.ejrad.2024.111465
12. Bodard S, Marcelin C, Kastler A, Dimopoulos PM, Petre EN, Frandon J, et al. Safety and efficacy of cryoablation of Soft-Tissue tumors. Br J Radiol. (2024) 1–14. doi: 10.1093/bjr/tqae075
13. Barjolle I, Ah-Thiane L, Frampas E, Karam G, Rigaud J, and David A. Efficacy and safety of cryoablation for localized renal tumor as an alternative approach to partial nephrectomy. Front Oncol. (2023) 13:1235705. doi: 10.3389/fonc.2023.1235705
14. Vogl TJ, Bielfeldt J, Kübler U, and Adwan H. CT-guided percutaneous cryoablation of breast cancer: A single-center experience. Cancers (Basel). (2024) 16:2373. doi: 10.3390/cancers16132373
15. Duraes M, Garbay M, Ferrer C, Duflos C, and Rathat G. Effect of whole-body cryotherapy versus placebo cryotherapy on joint pain induced by aromatase inhibitors in women with early stage breast cancer: a randomised clinical trial. BMJ Open. (2023) 13:e071756. doi: 10.1136/bmjopen-2023-071756
16. Galati F, Marra A, Cicciarelli F, Pasculli M, Maroncelli R, Rizzo V, et al. Cryoablation for the treatment of breast cancer: immunological implications and future perspectives. Utopia or reality? Radiol Med. (2024) 129:222–8. doi: 10.1007/s11547-024-01769-z
17. Korpan NN. A history of cryosurgery: its development and future. J Am Coll Surg. (2007) 204:314–24. doi: 10.1016/j.jamcollsurg.2006.11.006
18. Galati F, Pasculli M, Maroncelli R, Rizzo V, Moffa G, Cerbelli B, et al. Ultrasound-guided cryoablation of early breast cancer: safety, technical efficacy, patients' satisfaction, and outcome prediction with MRI/CEM: a pilot case-control study. Eur Radiol Exp. (2024) 8:120. doi: 10.1186/s41747-024-00515-4
19. Huang ML, Tomkovich K, Lane DL, Katta R, Candelaria RP, and Santiago L. Breast cancer cryoablation fundamentals past and present: technique optimization and imaging pearls. Acad Radiol. (2023) 30:2383–95. doi: 10.1016/j.acra.2023.05.019
20. Korpan NN, Xu K, Schwarzinger P, Watanabe M, Breitenecker G, and Patrick LP. Cryo-assisted resection en bloc, and cryoablation in situ, of primary breast cancer coupled with intraoperative ultrasound-guided tracer injection: A preliminary clinical study. Technol Cancer Res Treat. (2018) 17:1533034617746294. doi: 10.1177/1533034617746294
21. Sun X, Xu S, Li Y, Lv X, Wei M, and He M. Efficacy and safety of PARP inhibitors in the treatment of BRCA-mutated breast cancer: an updated systematic review and meta-analysis of randomized controlled trials. Expert Rev Clin Pharmacol. (2023) 16:245–56. doi: 10.1080/17512433.2023.2188193
22. Deipolyi AR, Ward RC, Riaz A, Vogl TJ, Simmons RM, Pieper CC, et al. Locoregional therapies for primary and metastatic breast cancer: AJR expert panel narrative review. AJR Am J Roentgenol. (2024) 222:e2329454. doi: 10.2214/AJR.23.29454
23. Corines MJ, Sogani J, Hogan MP, Mango VL, and Bryce Y. The role of contrast-enhanced mammography after cryoablation of breast cancer. AJR Am J Roentgenol. (2024) 222:e2330250. doi: 10.2214/AJR.23.30250
24. Mokbel K, Kodresko A, Ghazal H, Mokbel R, Trembley J, and Jouhara H. The evolving role of cryosurgery in breast cancer management: A comprehensive review. Cancers (Basel). (2023) 15:4272. doi: 10.3390/cancers15174272
25. Pigg N and Ward RC. Cryoablation for the treatment of breast cancer: A review of the current landscape and future possibilities. Acad Radiol. (2023) 30:3086–100. doi: 10.1016/j.acra.2023.06.030
26. Holmes D and Iyengar G. Breast cancer cryoablation in the multidisciplinary setting: practical guidelines for patients and physicians. Life (Basel). (2023) 13:1756. doi: 10.3390/life13081756
27. Fine RE, Gilmore RC, Dietz JR, Boolbol SK, Berry MP, Han LK, et al. Cryoablation without excision for low-risk early-stage breast cancer: 3-year interim analysis of ipsilateral breast tumor recurrence in the ICE3 trial. Ann Surg Oncol. (2021) 28:5525–34. doi: 10.1245/s10434-021-10501-4
28. Lanza E, Palussiere J, Buy X, Grasso RF, Beomonte Zobel B, Poretti D, et al. Percutaneous image-guided cryoablation of breast cancer: A systematic review. J Vasc Interv Radiol. (2015) 26:1652–1657.e1651. doi: 10.1016/j.jvir.2015.07.020
29. Chen Z, Meng L, Zhang J, and Zhang X. Progress in the cryoablation and cryoimmunotherapy for tumor. Front Immunol. (2023) 14:1094009. doi: 10.3389/fimmu.2023.1094009
30. Kwak K, Yu B, Lewandowski RJ, and Kim D-H. Recent progress in cryoablation cancer therapy and nanoparticles mediated cryoablation. Theranostics. (2022) 12:2175–204. doi: 10.7150/thno.67530
31. Overduin CG, Bomers JGR, Jenniskens SFM, Hoes MF, Ten Haken B, de Lange F, et al. T1-weighted MR image contrast around a cryoablation iceball: a phantom study and initial comparison with in vivo findings. Med Phys. (2014) 41:112301. doi: 10.1118/1.4896824
32. Kwong A, Co M, and Fukuma E. Prospective clinical trial on expanding indications for cryosurgery for early breast cancers. Clin Breast Cancer. (2023) 23:363–8. doi: 10.1016/j.clbc.2023.01.007
33. Takada M and Toi M. Cryosurgery for primary breast cancers, its biological impact, and clinical outcomes. Int J Clin Oncol. (2019) 24:608–13. doi: 10.1007/s10147-019-01448-4
34. Ma J, Yu X, Lv J, Lin D, Lin J, Bai Y, et al. Cryotherapy mediates histopathological and microstructural changes during the treatment of skin and subcutaneous tumors in dogs. Cryobiology. (2021) 98:164–71. doi: 10.1016/j.cryobiol.2020.11.006
35. Beji H, Pilleul F, Picard R, Tredan O, Bouhamama A, Peix M, et al. Percutaneous cryoablation of breast tumours in patients with stable metastatic breast cancer: safety, feasibility and efficacy. Br J Radiol. (2018) 91:20170500. doi: 10.1259/bjr.20170500
36. Cebula H, Noel G, Garnon J, Todeschi J, Burckel H, de Mathelin M, et al. The Cryo-immunologic effect: A therapeutic advance in the treatment of glioblastomas? Neurochirurgie. (2020) 66:455–60. doi: 10.1016/j.neuchi.2020.06.135
37. McArthur HL, Diab A, Page DB, Yuan J, Solomon SB, Sacchini V, et al. A pilot study of preoperative single-dose ipilimumab and/or cryoablation in women with early-stage breast cancer with comprehensive immune profiling. Clin Cancer Res. (2016) 22:5729–37. doi: 10.1158/1078-0432.CCR-16-0190
38. Sardela de Miranda F, Castro M, Remmert N, Singh SP, Layeequr Rahman R, and Melkus MW. Leveraging cryoablation and checkpoint inhibitors for high-risk triple negative breast cancer. Front Immunol. (2023) 14:1258873. doi: 10.3389/fimmu.2023.1258873
39. Regen-Tuero HC, Ward RC, Sikov WM, and Littrup PJ. Cryoablation and immunotherapy for breast cancer: overview and rationale for combined therapy. Radiol Imaging Cancer. (2021) 3:e200134. doi: 10.1148/rycan.2021200134
40. Korpan NN, Goltsev AN, Dronov OI, and Bondarovych MO. Cryoimmunology: Opportunities and challenges in biomedical science and practice. Cryobiology 100. (2021) 100:1–11. doi: 10.1016/j.cryobiol.2021.02.005
41. Tan E, Chong J, Pua U, Tan EY, and Mok WY. Local recurrence and residual tumor rates following cryoablation for small early-stage breast cancers: systemic review and meta-analysis. Breast Cancer. (2025) 32:69–78. doi: 10.1007/s12282-024-01643-w
42. Yang M, Han B, and Ye P. Cryoablation for breast cancer: a narrative review of advances, clinical applications, and future challenges. Transl Cancer Res. (2025) 14:1467–78. doi: 10.21037/tcr-24-1415
43. Ciambella CC and Takabe K. Cryotherapy in the treatment of early-stage breast cancer. World J Oncol. (2024) 15:737–43. doi: 10.14740/wjon1909
44. Korpan NN, Hochwarter G, and Sellner F. Cryoscience and cryomedicine: new mechanisms of biological tissue injury following low temperature exposure. Exp study. Klin Khir. (2009) 7:80–5.
45. Erinjeri JP and Clark TW. Cryoablation: mechanism of action and devices. J Vasc Interv Radiol. (2010) 21:S187–191. doi: 10.1016/j.jvir.2009.12.403
46. Baust JG, Gage AA, Bjerklund Johansen TE, and Baust JM. Mechanisms of cryoablation: clinical consequences on Malignant tumors. Cryobiology. (2014) 68:1–11. doi: 10.1016/j.cryobiol.2013.11.001
47. Lett K, Zhang Y, and Nishimura N. Neurological and inflammatory effects of radio frequency and cryoablation in a rat sciatic nerve model of submucosal nerve ablation. Am J Rhinol Allergy. (2022) 36:628–37. doi: 10.1177/19458924221099377
48. Tan H, Jiang Y, Shen L, Nuerhashi G, Wen C, Gu L, et al. Cryoablation-induced neutrophil Ca2+ elevation and NET formation exacerbate immune escape in colorectal cancer liver metastasis. J Exp Clin Cancer Res. (2024) 43:319. doi: 10.1186/s13046-024-03244-z
49. Sardela de Miranda F, Martinez-Marin D, Babcock RL, Castro M, Boligala GP, Khan SY, et al. Cryoablation of primary breast cancer tumors induces a systemic abscopal effect altering TIME (Tumor Immune Microenvironment) in distant tumors. Front Immunol. (2024) 15:1498942. doi: 10.3389/fimmu.2024.1498942
50. Tian Y, Qi X, Jiang X, Shang L, Xu K, and Shao H. Cryoablation and immune synergistic effect for lung cancer: A review. Front Immunol. (2022) 13:950921. doi: 10.3389/fimmu.2022.950921
51. Liu Q, Zhang C, Chen X, and Han Z. Modern cancer therapy: cryoablation meets immune checkpoint blockade. Front Oncol. (2024) 14:1323070. doi: 10.3389/fonc.2024.1323070
52. Yu Z, Wang D, Qi Y, Liu J, Zhou T, Rao W, et al. Autologous-cancer-cryoablation-mediated nanovaccine augments systematic immunotherapy. Mater Horiz. (2023) 10:1661–77. doi: 10.1039/D3MH00092C
53. Roknsharifi S, Wattamwar K, Fishman MDC, Ward RC, Ford K, Faintuch S, et al. Image-guided microinvasive percutaneous treatment of breast lesions: where do we stand? Radiographics. (2021) 41:945–66. doi: 10.1148/rg.2021200156
54. Ashokkumar P, Siroraj P, Govindarajan Valanthan Veda G, and Vegesana Krishnakumar Raja BK. Cryosurgery of multiple haemangiomas of oral cavity. BMJ Case Rep 15. (2022) 15:e253654. doi: 10.1136/bcr-2022-253654
55. Ang J, Kim S, Watson KD, Pierezan F, Berg KJ, Yazdi Z, et al. Liquid nitrogen cryosurgery for cutaneous and ocular neoplasms in Koi (Cyprinus carpio) and goldfish (Carassius auratus): eight cases (2018-2019). J Zoo Wildl Med. (2021) 52:763–73. doi: 10.1638/2020-0102
56. Jin K, Qiu S, Zheng X, Li Y, Zhang S, Li J, et al. Cryotherapy shows no inferiority compared with radical Prostatectomy for low-risk and intermediate-risk localized Prostate Cancer: a real-world study from the SEER database. J Cancer. (2020) 11:5738–45. doi: 10.7150/jca.38323
57. Brett JO and Mayer EL. New developments in systemic management for high-risk early-stage hormone-receptor-positive, HER2-negative breast cancer. Curr Treat Options Oncol. (2023) 24:594–610. doi: 10.1007/s11864-023-01082-3
58. Whitman GJ and Destounis SV. Screening mammography with AI and supplemental breast US: current status. Radiology. (2024) 312:e241362. doi: 10.1148/radiol.241362
59. Katz SJ, Abrahamse P, Furgal A, Hodan R, Tocco RS, Ward KC, et al. Testing, and family communication into survivorship after diagnosis of breast cancer. J Clin Oncol. (2024) 42:3123–29. doi: 10.1200/JCO.24.00122
60. Casey DL, Gupta GP, and Ollila DW. The role of intraoperative radiation in early-stage breast cancer. Clin Breast Cancer. (2021) 21:103–11. doi: 10.1016/j.clbc.2020.12.007
61. Yarnold JR. Early stage breast cancer: treatment options and results. Br Med Bull. (1991) 47:372–87. doi: 10.1093/oxfordjournals.bmb.a072477
62. Loibl S, André F, Bachelot T, Barrios CH, Bergh J, Burstein HJ, et al. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. (2024) 35:159–82. doi: 10.1016/j.annonc.2023.11.016
63. Trapani D, Ginsburg O, Fadelu T, Lin NU, Hassett M, Ilbawi AM, et al. Global challenges and policy solutions in breast cancer control. Cancer Treat Rev. (2022) 104:102339. doi: 10.1016/j.ctrv.2022.102339
64. Zhang L, Wang Y, Meng W, Zhao W, and Tong Z. Cardiac safety analysis of anti-HER2-targeted therapy in early breast cancer. Sci Rep. (2022) 12:14312. doi: 10.1038/s41598-022-18342-1
65. Toi M, Kinoshita T, Benson JR, Jatoi I, Kataoka M, Han W, et al. Non-surgical ablation for breast cancer: an emerging therapeutic option. Lancet Oncol. (2024) 25:e114–25. doi: 10.1016/S1470-2045(23)00615-0
66. Sabel MS, Kaufman CS, Whitworth P, Chang H, Stocks LH, Simmons R, et al. Cryoablation of early-stage breast cancer: work-in-progress report of a multi-institutional trial. Ann Surg Oncol. (2004) 11:542–9. doi: 10.1245/ASO.2004.08.003
67. Oueidat K, Baird GL, Barclay-White B, Kozlowski K, Plaza MJ, Aoun H, et al. Cryoablation of primary breast cancer in patients ineligible for clinical trials: A multiinstitutional study. AJR Am J Roentgenol. (2024) 223:e2431392. doi: 10.2214/AJR.24.31392
68. Niu L, Wu B, and Xu K. Cryosurgery for breast fibroadenomas. Gland Surg. (2012) 1:128–31. doi: 10.1148/radiol.2341030931
69. Kaufman CS, Littrup PJ, Freman-Gibb LA, Francescatti D, Stocks LH, Smith JS, et al. Office-based cryoablation of breast fibroadenomas: 12-month followup. J Am Coll Surg. (2004) 198:914–23. doi: 10.1016/j.jamcollsurg.2004.02.014
70. Simmons RM, Ballman KV, Cox C, Carp N, Sabol J, Hwang RF, et al. A phase II trial exploring the success of cryoablation therapy in the treatment of invasive breast carcinoma: results from ACOSOG (Alliance) Z1072. Ann Surg Oncol. (2016) 23:2438–45. doi: 10.1245/s10434-016-5275-3
71. Roubidoux MA, Sabel MS, Bailey JE, Kleer CG, Klein KA, and Helvie MA. Small (< 2.0-cm) breast cancers: mammographic and US findings at US-guided cryoablation–initial experience. Radiology. (2004) 233:857–67. doi: 10.1148/radiol.2333031734
72. Roca Navarro MJ, Garrido Alonso D, Navarro Monforte Y, García Martínez F, Díaz de Bustamante Durbán T, Córdoba Chicote MV, et al. Efficacy of ultrasound-guided cryoablation in treating low-risk breast cancer. Radiologia (Engl Ed). (2023) 65:112–21. doi: 10.1016/j.rxeng.2023.03.002
73. Cazzato RL, Garnon J, Ramamurthy N, Koch G, Tsoumakidou G, Caudrelier J, et al. Percutaneous image-guided cryoablation: current applications and results in the oncologic field. Med Oncol. (2016) 33:140. doi: 10.1007/s12032-016-0848-3
74. Fine RE, Gilmore RC, Tomkovich KR, Dietz JR, Berry MP, Hernandez LE, et al. Cryoablation without excision for early-stage breast cancer: ICE3 trial 5-year follow-up on ipsilateral breast tumor recurrence. Ann Surg Oncol. (2024) 31:7273–83. doi: 10.1245/s10434-024-16181-0
75. Uka M, Iguchi T, Okawa N, Matsui Y, Tomita K, Umakoshi N, et al. Percutaneous cryoablation for clinical T3a renal cell carcinoma (< 7 cm) with segmental vein involvement or perinephric fat invasion based on preoperative evaluation of high-resolution multidetector computed tomography scan. Jpn J Radiol. (2022) 40:1201–9. doi: 10.1007/s11604-022-01297-8
76. Xu Z, Wang X, Ke H, and Lyu G. Cryoablation is superior to radiofrequency ablation for the treatment of non-small cell lung cancer: A meta-analysis. Cryobiology. (2023) 112:104560. doi: 10.1016/j.cryobiol.2023.104560
77. Khairy P, Chauvet P, Lehmann J, Lambert J, Macle L, Tanguay J-F, et al. Lower incidence of thrombus formation with cryoenergy versus radiofrequency catheter ablation. Circulation. (2003) 107:2045–50. doi: 10.1161/01.CIR.0000058706.82623.A1
78. Abrishami Kashani M, Murphy MC, Saenger JA, Wrobel MM, Tahir I, Mrah S, et al. Risk of persistent air leaks following percutaneous cryoablation and microwave ablation of peripheral lung tumors. Eur Radiol. (2023) 33:5740–51. doi: 10.1007/s00330-023-09499-y
79. Avitall B and Kalinski A. Cryotherapy of cardiac arrhythmia: From basic science to the bedside. Heart Rhythm. (2015) 12:2195–203. doi: 10.1016/j.hrthm.2015.05.034
80. Plaza MJ, Kumar AV, and Sanchez-Gonzalez MA. Safety and efficacy of ultrasound-guided cryoablation for benign breast fibroepithelial lesions. J Breast Imaging. (2019) 1:324–8. doi: 10.1093/jbi/wbz047
81. Ward RC, Lourenco AP, and Mainiero MB. Ultrasound-guided breast cancer cryoablation. AJR Am J Roentgenol. (2019) 213:716–22. doi: 10.2214/AJR.19.21329
82. Kawamoto H, Tsugawa K, Furuya Y, Sakamaki K, Kakimoto S, Kitajima M, et al. Percutaneous ultrasound-guided cryoablation for early-stage primary breast cancer: a follow-up study in Japan. Breast Cancer. (2024) 31:695–704. doi: 10.1007/s12282-024-01584-4
83. Machida Y, Shimauchi A, Igarashi T, and Fukuma E. MRI findings after cryoablation of primary breast cancer without surgical resection. Acad Radiol. (2019) 26:744–51. doi: 10.1016/j.acra.2018.07.012
84. Huang ML, Lane DL, and Santiago L. MRI-guided breast cryoablation with the patient in prone position. AJR Am J Roentgenol. (2024) 222:e2330754. doi: 10.2214/AJR.23.30754
85. Adachi T, Machida Y, Fukuma E, and Tateishi U. Fluorodeoxyglucose positron emission tomography/computed tomography findings after percutaneous cryoablation of early breast cancer. Cancer Imaging. (2020) 20:49. doi: 10.1186/s40644-020-00325-y
86. Khan SY, Snitman A, Habrawi Z, Crawford S, Melkus MW, and Layeequr Rahman R. The role of cryoablation in breast cancer beyond the oncologic control: COST and breast-Q patient-reported outcomes. Ann Surg Oncol. (2023) 30:1029–37. doi: 10.1245/s10434-022-12570-5
87. Pusceddu C, Paliogiannis P, Nigri G, and Fancellu A. Cryoablation in the management of breast cancer: evidence to date. Breast Cancer (Dove Med Press). (2019) 11:283–92. doi: 10.2147/BCTT.S197406
88. Miller DL, Hutchins J, Ferguson MA, Barhoush Y, Achter E, and Kuckelman JP. Intercostal nerve cryoablation during lobectomy for postsurgical pain: A safe and cost-effective intervention. Pain Ther. (2025) 14:317–28. doi: 10.1007/s40122-024-00694-3
89. Chen Y, Xu C, Mou Z, Hu Y, Yang C, Hu J, et al. Endoscopic cryoablation versus radical nephroureterectomy for upper tract urothelial carcinoma. Eur Urol Oncol. (2024) 7:1453–61. doi: 10.1016/j.euo.2024.04.012
90. Ko SE, Lee MW, Rhim H, Kang TW, Song KD, Cha DI, et al. Comparison of procedure-related complications between percutaneous cryoablation and radiofrequency ablation for treating periductal hepatocellular carcinoma. Int J Hyperthermia. (2020) 37:1354–61. doi: 10.1080/02656736.2020.1849824
91. Pal K, Awad A, Yevich S, Kuban JD, Tam AL, Huang SY, et al. Safety and efficacy of percutaneous cryoablation for recurrent or metastatic soft-tissue sarcoma in adult patients. AJR Am J Roentgenol. (2024) 223:e2431490. doi: 10.2214/AJR.24.31490
92. Sundelin MO, Lagerveld B, Ismail M, Keeley FX, and Nielsen TK. Repeated cryoablation as treatment modality after failure of primary renal cryoablation: A European registry for renal cryoablation multinational analysis. J Endourol. (2019) 33:909–13. doi: 10.1089/end.2019.0444
93. Liu Y-E, Zong J, Chen X-J, Zhang R, Ren X-C, Guo Z-J, et al. Cryoablation combined with radiotherapy for hepatic Malignancy: Five case reports. World J Gastrointest Oncol. (2020) 12:237–47. doi: 10.4251/wjgo.v12.i2.237
94. Shibamoto Y and Takano S. Non-surgical definitive treatment for operable breast cancer: current status and future prospects. Cancers (Basel). (2023) 15:1864. doi: 10.3390/cancers15061864
95. Ali Mohammad S, Hak A, Pogu SV, and Rengan AK. Radiotherapy, photodynamic therapy, and cryoablation-induced abscopal effect: Challenges and future prospects. Cancer Innov. (2023) 2:323–45. doi: 10.1002/cai2.v2.5
96. Wetterwald L, Papadopoulos S, Tsoumakidou G, Boughdad S, Ferraro D, Koulouris P, et al. Abscopal effect induced by cryoablation in a 55-year-old patient with metastatic dedifferentiated liposarcoma: a case report. Ann Transl Med. (2024) 12:94. doi: 10.21037/atm-23-1868
97. Zhang X, Wu J, Qiao L, Chen L, Chen C, Zhang H, et al. Case report: Cryoablation as a novel bridging strategy prior to CAR-T cell therapy for B cell Malignancies with bulky disease. Front Oncol. (2023) 13:1008828. doi: 10.3389/fonc.2023.1008828
98. Wimper Y, Fütterer JJ, and Bomers JGR. MR imaging in real time guiding of therapies in prostate cancer. Life (Basel). (2022) 12:302. doi: 10.3390/life12020302
99. Khandpur U, Haile B, and Makary MS. Early-stage renal cell carcinoma locoregional therapies: current approaches and future directions. Clin Med Insights Oncol. (2024) 18:11795549241285390. doi: 10.1177/11795549241285390
100. Liu J and Deng ZS. Nano-cryosurgery: advances and challenges. J Nanosci Nanotechnol. (2009) 9:4521–42. doi: 10.1166/jnn.2009.1264
101. Ou W, Stewart S, White A, Kwizera EA, Xu J, Fang Y, et al. In-situ cryo-immune engineering of tumor microenvironment with cold-responsive nanotechnology for cancer immunotherapy. Nat Commun. (2023) 14:392. doi: 10.1038/s41467-023-36045-7
102. Ofosu A, Ramai D, and Adler DG. Endoscopic ultrasound-guided ablation of pancreatic cystic neoplasms: ready for prime time? Ann Gastroenterol. (2019) 32:39–45. doi: 10.20524/aog.2018.0331
103. Gao X, Liu K, Zhao X, Lv X, Wu X, Ren C, et al. Global research trends in catheter ablation and surgical treatment of atrial fibrillation: A bibliometric analysis and science mapping. Front Surg. (2022) 9:1048454. doi: 10.3389/fsurg.2022.1048454
104. Moreira P, Tuncali K, Tempany C, and Tokuda J. AI-based isotherm prediction for focal cryoablation of prostate cancer. Acad Radiol. (2023) 30 Suppl 1:S14–s20. doi: 10.1016/j.acra.2023.04.016
105. Wei Z, Yu X, Huang M, Wen L, and Lu C. Nanoplatforms potentiated ablation-immune synergistic therapy through improving local control and suppressing recurrent metastasis. Pharmaceutics. (2023) 15:1456. doi: 10.3390/pharmaceutics15051456
106. Olagunju A, Forsman T, and Ward RC. An update on the use of cryoablation and immunotherapy for breast cancer. Front Immunol. (2022) 13:1026475. doi: 10.3389/fimmu.2022.1026475
Keywords: cryoablation, early breast cancer, local treatment, efficacy assessment, clinical applications
Citation: Xing Y, Li H, Yuan T, Zou X, Liang B, Ma Y and Niu L (2025) Cryoablation in the treatment of early breast cancer: a comprehensive analysis. Front. Oncol. 15:1469684. doi: 10.3389/fonc.2025.1469684
Received: 24 July 2024; Accepted: 29 April 2025;
Published: 20 May 2025.
Edited by:
Isabella Castellano, University of Turin, ItalyReviewed by:
Xiaodong Zheng, Chongqing University, ChinaNikolai Korpan, International Institute of Cryosurgery, Austria
Manuela Durando, A.O.U. Città della Salute e della Scienza di Torino, Italy
Copyright © 2025 Xing, Li, Yuan, Zou, Liang, Ma and Niu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lizhi Niu, bml1Ym9zaGlAZnVkYWhvc3BpdGFsLmNvbQ==; Yangyang Ma, bWF5YW5neWFuZzY4NkAxNjMuY29t
†ORCID: Yanli Xing, orcid.org/0009-0004-7318-3968
Hongmei Li, orcid.org/0000-0002-9620-6104
Ting Yuan, orcid.org/0009-0004-1633-3851
Xiaojiao Zou, orcid.org/0000-0002-6403-7553
Bing Liang, orcid.org/0009-0001-6862-5010
 Yanli Xing1†
Yanli Xing1† Yangyang Ma
Yangyang Ma Lizhi Niu
Lizhi Niu