- 1Department of Breast Surgery, The Affiliated Hospital of Guizhou Medical University, Guiyang, Guizhou, China
- 2Department of Thoracic Surgery, The Affiliated Hospital of Guizhou Medical University, Guiyang, Guizhou, China
- 3Department of Breast Oncoplastic Surgery, Hunan Cancer Hospital, Changsha, China
Synovial sarcoma (SS) is a rare and aggressive malignancy that primarily affects young people and usually occurs in the extremities. Breast involvement is uncommon, and males with breast SS are even rarer. We present a rare case of a 17-year-old boy with a progressively growing mass found in his right breast, which was confirmed as SS through pathological diagnosis following surgery.
1 Introduction
Synovial sarcoma (SS) is a rare mesenchymal malignancy primarily affecting adolescents and young adults, commonly arising at the extremities (1, 2). Breast cancer in males is a rare condition, accounting for approximately 1% of all breast cancers and typically being estrogen receptor-positive (3). Breast SS in males is extremely rare. Currently, no such cases have been reported in the literature. This report describes the first known case of breast SS in a male.
2 Case presentation
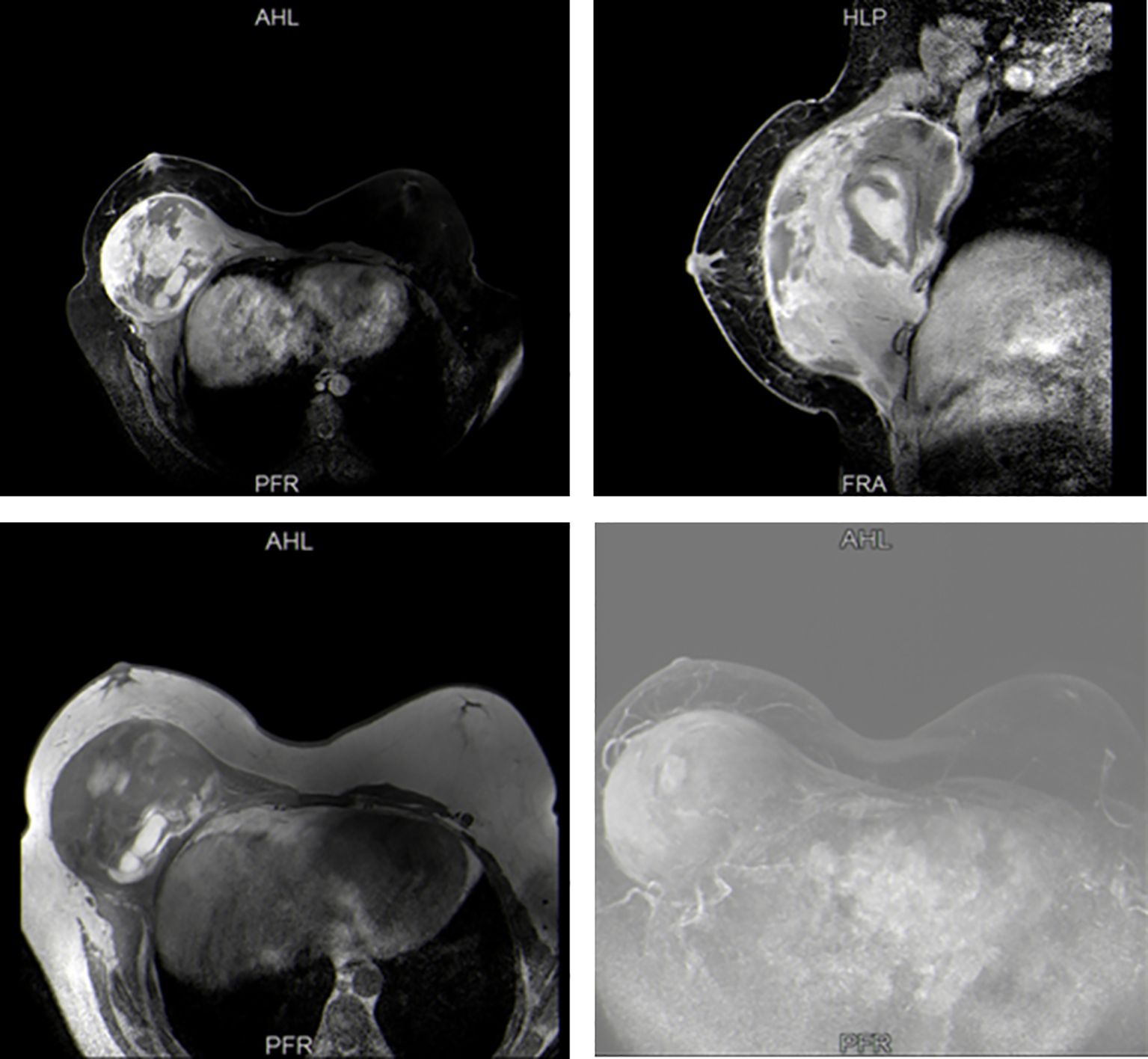
A 17-year-old boy presented to our hospital with a 10-month history of a rapidly enlarging mass in right breast. The patient had no other obvious symptoms except for mild tenderness in the mass over the past 6 months. He did not seek medical attention promptly due to a lack of family history of breast cancer. On physical examination, a hard lump measuring 30 × 30 cm, with poor mobility and an indistinct boundary, occupying almost his entire right breast (Figure 1). Moreover, this boy had an enlarged left breast. He had no palpable lymphadenopathy, in the axilla, supraclavicular or cervical regions. Sex hormones tests showed increased levels of prolactin and progesterone. Breast ultrasound indicated gynecomastia in the left breast and a solid cystic mass in the right breast adjacent to the chest wall. Magnetic resonance imaging (MRI) confirmed a space-occupying lesion in the right breast and chest, accompanied by hemorrhage and necrosis (Figure 2). An ultrasound-guided biopsy puncture of the right breast was performed. Immunohistochemical staining results were as follows: Vim (+), Desmin (-), SMA (+), Calponin (+), Caldesmon (-), CK (+), EMA (+), CK7 (+), CK19 (+), SS18-SSX (+), TLE1 (+), CK5/6 (-), CKL (-), CKH (-), P63 (-), S100 (+),sox10 (-), CD57 (+), GFAP (-), ER (+), PR (-), CD34 (-), STAT6 (-), BCL-2 (+), CD99 (+), and Ki-67 (+). Furthermore, fluorescence in situ hybridization (FISH) confirmed SS18 gene translocation. Based on these findings, the patient was diagnosed with SS in the right breast and gynecomastia in the left breast.
3 Treatment
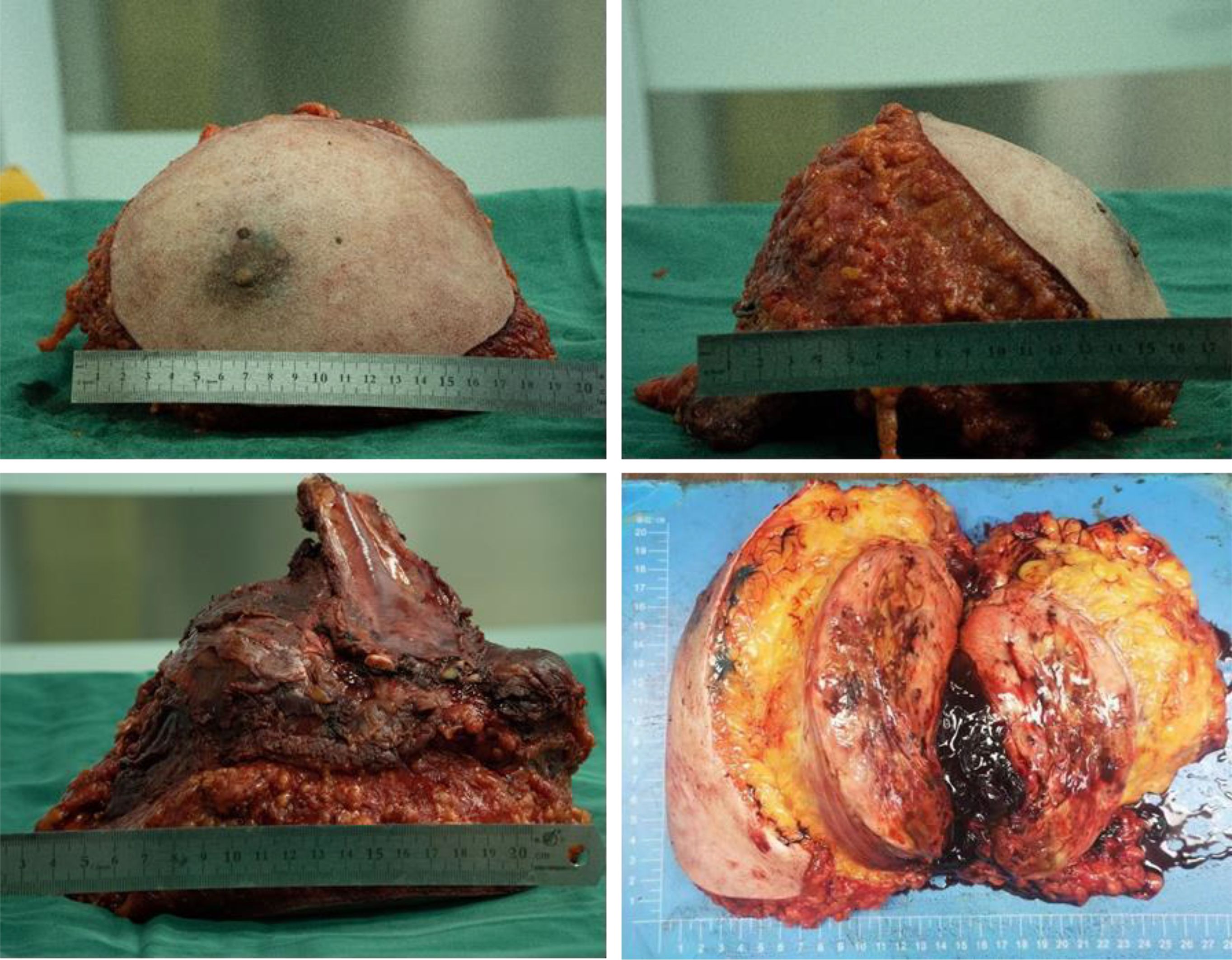
The patient underwent subcutaneous mastectomy of the left breast to address gynecomastia. For the right breast, an extended radical mastectomy was performed on right breast after intraoperative freezing pathology confirmed the absence of sentinel lymph node metastasis (0/2) in the right axilla (Figure 3). The target lesion involved the proximal portions of the 4th and 5th ribs, and therefore, the 4th and 5th ribs and parietal pleura between them were removed. The subcutaneous tissues at 4, 3, 1, 12, 6, 10, 9, 7 o ‘clock of the right breast tumor resection, as well as the tissues at the lower, upper, and internal and external margins of the ribs, were sent for frozen pathological examination to ensure that the surgical margins were negative. The Matrix RIB fixation system was used for thoracoplasty (Figures 4A-C). The right pedicled rectus abdominis flap was used for chest wall reconstruction (Figures 4D, E). The inferior epigastric vessels of the flap were anastomosed to the lateral thoracic vessels to ensure an adequate blood supply. Postoperative pathological immunohistochemistry confirmed that the malignant tumor originated from the breast, and the final diagnosis was right emulsified carcinoma (carcinosarcoma). The main tumor was biphasic synovial sarcoma. As shown in Figure 5, immunohistochemical results of Radical resection of right breast carcinoma: (Pathological ID: 23003336): SS18-SSX (+), STAT6(-), S100 (-), SMA (-), TLE1 (+), Desmin (-), CK5/6 (focal +), CK20 (-), CK (focal +), CK8/18 (-), CKH(-), CKL (-), ER (focal +), Mammaglobin (focal +), GATA3(-), GCDFP-15(-), EMA (focal +), Ki-67(+, 70%), P63(Individual cell+), and Vim (+). No lymph node metastasis was observed in the nine sentinel lymph nodes of the right axilla (0/9). The survival status of the flaps was monitored by assessing surface color and blood supply every 2 hours within 48 hours and every 4 hours on the third day. Postoperative recovery was uneventful with good flap vitality (Figure 6).
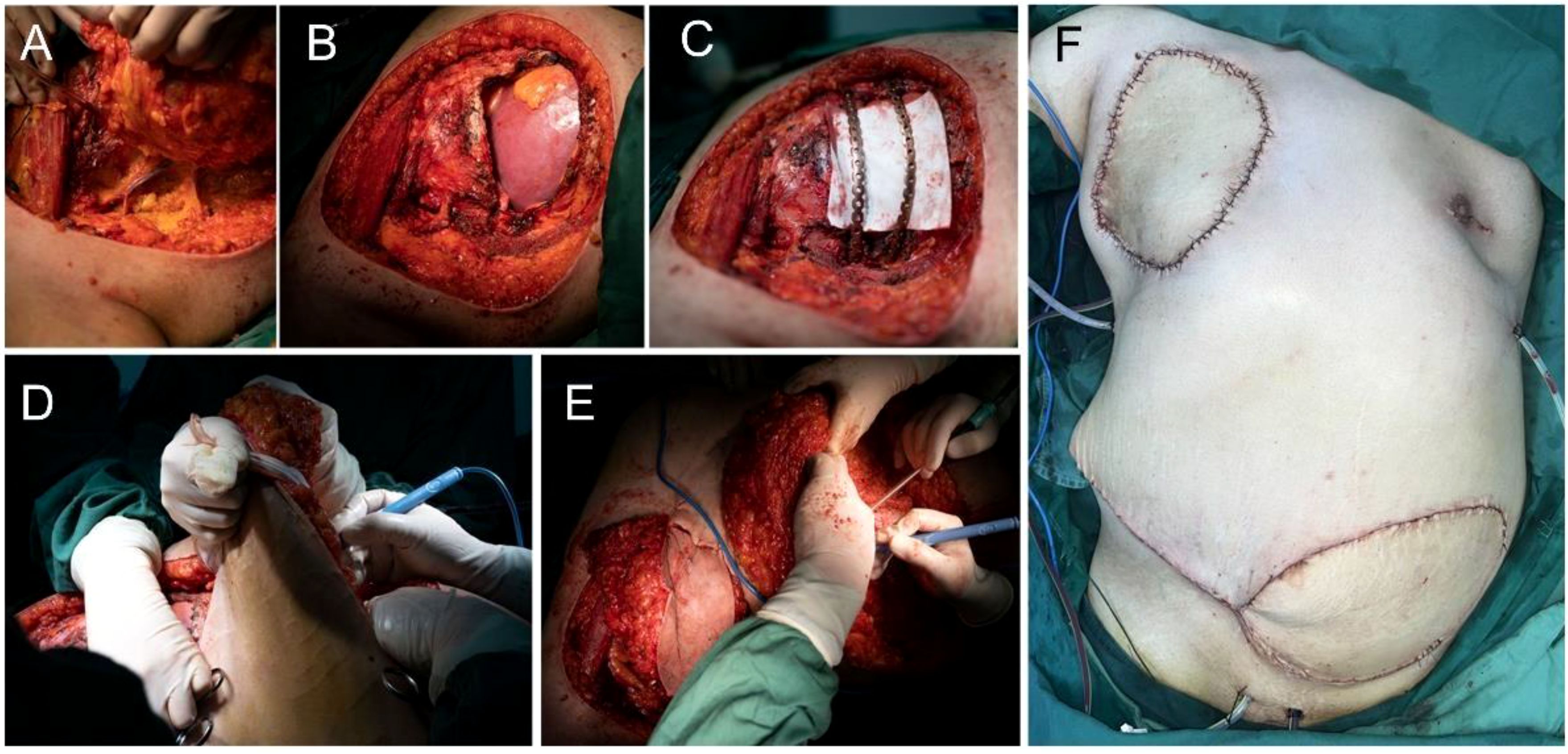
Figure 4. Surgical procedure. (A-C) Extended radical mastectomy and chest wall reconstruction. (D, E) The right pedicled rectus abdominis flap (F).
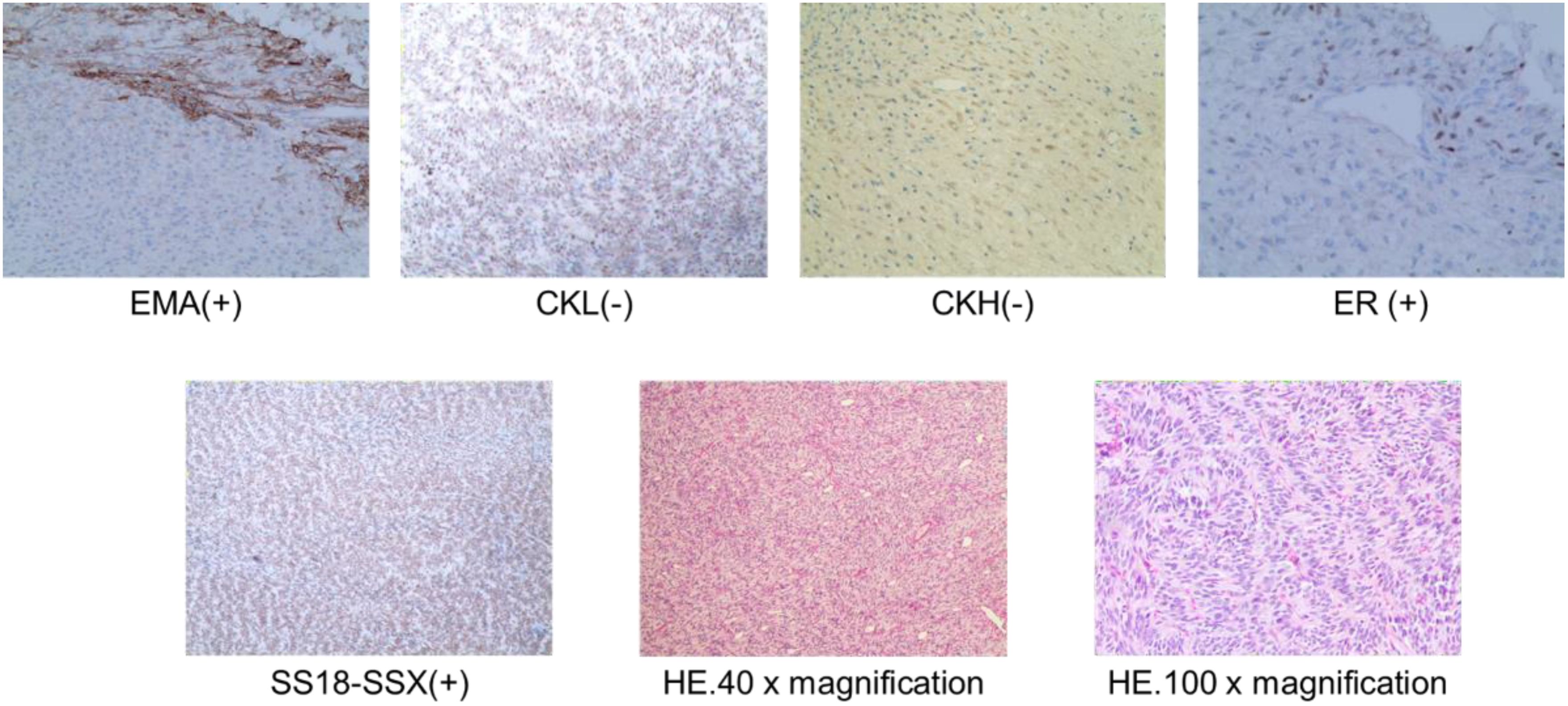
Figure 5. Postoperative pathological HE staining and immunohistochemistry of right breast carcinoma (Pathological ID: 23003336).
4 Discussion
Synovial sarcoma is a highly aggressive soft-tissue malignancy primarily affecting adolescents and young adults, often found in the lower extremities (1, 2). Recent literature describes a case of a 44-year-old woman with SS in the thigh that recurred and metastasized to the breast and lungs following surgery (4). Primary breast SS is exceedingly rare, particularly in adolescent males (5). Male breast cancer accounts for only 1% of all breast cancer cases. To the best of the authors’ knowledge, only three cases of female breast SS have been reported (6–8), and we report the first documented case of breast SS in males.
SYT-SSX is a reliable indicator for the pathological diagnosis of SS (9, 10). In this case, SS in this young boy was confirmed through preoperative biopsy and postoperative pathology, which identified SS18 (18q11) (SYT) and SS18 gene translocation (11, 12). Surgery remains the best treatment option for SS. The role of chemotherapy in the treatment of SS remains debatable; it is generally used for salvage therapy in advanced stages of the disease (13). Although synovial sarcomas are equally insensitive to radiotherapy, experts consider a combination of radiotherapy and surgery for high-risk patients (e.g., grade 3 tumors, deep tumors, or tumors larger than 5 cm). Although the surgical margin of the patient reported in this case was negative, the patient had a large tumor, and postoperative radiotherapy is still strongly recommended to reduce the risk of recurrence. As the patient was lost to follow-up, whether if they received radiotherapy was subsequently administered could not be determined.
Previous research has demonstrated that tumor size (P<0.005) and age (P=0.024) are significantly negatively associated with cancer-specific survival (CSS) in SS, regardless of tumor location and site (14). The lesions in the right breast mass in this case were large and extended into the 4th and 5th ribs. Restoring both functionality and aesthetics was critical. Along with thoracic reconstruction, a pedicled transverse rectus abdominis myocutaneous (TRAM) flap was used to fill the cavity created by the removal of the pectoralis major and minor muscles. The TRAM flap is a common choice for breast construction (15). Importantly, in this case, the inferior epigastric vessels of the flap were supercharged by anastomosing them to the lateral thoracic vessels when signs of venous congestion appeared. This strategy has not been previously reported in cases of SS. This is the first reported case of breast and chest wall reconstruction for SS. When the patient returned to the hospital 12 days after surgery, the incision healed well and the appearance was satisfactory (Figure 6). The patient was lost to follow-up due to electing to continue subsequent treatment at another institution.
Synovial sarcoma is a highly aggressive cancer with a poor prognosis. The 5-year overall survival for SS is approximately 60.5%, and half of the patients will develop metastatic recurrence, with a 5-year survival rate of 14.4% (16, 17). Therefore, there is an urgent need to develop more effective strategies for SS. The disease is driven by the pathognomonic t (X;18) chromosomal translocation and subsequent formation of the SS18:SSX fusion oncogenes (18). A recent phase 1 clinical trial (NCT03132922) has highlighted the efficacy of T-cell receptor therapy in treating SS (19). Given the role of SS18-SSX in epigenetic regulation and the effect of BET inhibitors on cell cycle regulators such as MYC, p21, CDK4, and CDK6, BET inhibitors targeting the intrinsic apoptosis pathway regulated by SS18-SSX represent a promising potential therapeutic option (20). In addition, inhibiting CDK9 in sarcomas has been shown to downregulate the expression of some oncogenes and reduce the proliferation and growth of various sarcoma cells. Targeting CDK9 in cancer has yielded promising results in both preclinical and clinical studies (21). We look forward to further advancements in effective treatments for SS that could improve prognosis and prolong survival time.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Affiliated Hospital of Guizhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by- product of routine care or industry. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
WY: Writing – original draft. DH: Writing – original draft. FY: Data curation, Writing – original draft. LM: Resources, Writing – original draft. ZJ: Methodology, Writing – original draft. SD: Methodology, Supervision, Writing – original draft. SL: Methodology, Supervision, Writing – review & editing. LK: Writing – review & editing, Conceptualization, Methodology, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by grants from the National Natural Science Foundation of China (82060480) and Doctoral research start-up Fund of Guizhou Medical University (Gyfybsky-2021-42, Gyfybsky-2023-19).
Acknowledgments
We acknowledge Zhang Mai from the Second People’s Hospital of Guiyang for his contribution to photo production.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cheng Y, Shen Z, Gao Y, Chen F, Xu H, Mo Q, et al. Phase transition and remodeling complex assembly are important for SS18-SSX oncogenic activity in synovial sarcomas. Nat Commun. (2022) 13:2724. doi: 10.1038/s41467-022-30447-9
2. Sultan I, Rodriguez-Galindo C, Saab R, Yasir S, Casanova M, and Ferrari A. Comparing children and adults with synovial sarcoma in the Surveillance, Epidemiology, and End Results program, 1983 to 2005: an analysis of 1268 patients. Cancer. (2009) 115:3537–47. doi: 10.1002/cncr.24424
3. Rolfes M, Borde J, Möllenhoff K, Kayali M, Ernst C, Gehrig A, et al. Prevalence of cancer predisposition germline variants in male breast cancer patients: results of the german consortium for hereditary breast and ovarian cancer. Cancers (Basel). (2022) 14:3292. doi: 10.3390/cancers14133292
4. Khanal P, Baral B, Pande P, Neupane S, and Joshi R. Recurrent synovial sarcoma with breast and pulmonary nodule: A case report. JNMA J Nepal Med Assoc. (2024) 62:52–4. doi: 10.31729/jnma.8408
5. Kerouanton A, Jimenez I, Cellier C, Laurence V, Helfre S, Pannier S, et al. Synovial sarcoma in children and adolescents. J Pediatr Hematol Oncol. (2014) 36:257–62. doi: 10.1097/MPH.0000000000000154
6. Tormo V and Andreu FJ. Primary breast synovial sarcoma: a rare primary breast neoplasm. Clin Transl Oncol. (2009) 11:854–5. doi: 10.1007/s12094-009-0456-1
7. Do Q, Katiyar V, Breaux A, and Singh V. Primary monophasic breast synovial sarcoma in a female patient. BMJ Case Rep. (2021) 14:e242313. doi: 10.1136/bcr-2021-242313
8. Doyle VJ, Bateman AC, and Theaker JM. An unusual breast mass: primary synovial sarcoma. BMJ Case Rep. (2013) 2013:bcr2013010468. doi: 10.1136/bcr-2013-010468
9. Xie Y, Törnkvist M, Aalto Y, Nilsson G, Girnita L, Nagy B, et al. Editorial Expression of Concern: Gene expression profile by blocking the SYT-SSX fusion gene in synovial sarcoma cells. Identification of XRCC4 as a putative SYT-SSX target gene. Oncogene. (2023) 42:783. doi: 10.1038/s41388-022-02552-y
10. Qi Y, Dong SS, He YL, Liu ZH, Huang YL, Wang N, et al. SYT-SSX1 enhances the invasiveness and maintains stem-like cell properties in synovial sarcoma via induction of TGF-β1/Smad signaling. BMC Cancer. (2022) 22:166. doi: 10.1186/s12885-022-09229-5
11. Zheng L, Wang XI, Chen S, Moosvi AM, Wan DQ, and Zhang S. Two cases of biphasic synovial sarcoma with expression of PAX8 and ER: A diagnostic pitfall. Int J Gynecol Pathol. (2023) 42:234–40. doi: 10.1097/PGP.0000000000000892
12. Jiang H, Ma G, Nie Z, Zhu J, Yan Q, Chen H, et al. A case of a 22-year-old man with primary synovial sarcoma of the parapharyngeal space with an AR somatic mutation: A case report and review of the literature. SAGE Open Med Case Rep. (2022) 10:2050313X211068646. doi: 10.1177/2050313
13. Vining CC, Sinnamon AJ, Ecker BL, Kelz RR, Fraker DL, Roses RE, et al. Adjuvant chemotherapy in resectable synovial sarcoma. J Surg Oncol. (2017) 116:550–8. doi: 10.1002/jso.24688
14. Smolle MA, Parry M, Jeys L, Abudu S, and Grimer R. Synovial sarcoma: Do children do better? Eur J Surg Oncol. (2019) 45:254–60. doi: 10.1016/j.ejso.2018.07.0066
15. Yi KH, Lee HJ, Lee JH, Seo KK, and Kim HJ. Application of botulinum neurotoxin injections in TRAM flap for breast reconstruction: intramuscular neural arborization of the rectus abdominis muscle. Toxins (Basel). (2021) 13:269. doi: 10.3390/toxins1304026
16. Desar IME, Fleuren EDG, and van der Graaf WTA. Systemic treatment for adults with synovial sarcoma. Curr Treat Options Oncol. (2018) 19:13. doi: 10.1007/s11864-173018-0525-117
17. Yuan J, Li X, and Yu S. Molecular targeted therapy for advanced or metastatic soft tissue sarcoma. Cancer Control. (2021) 28:10732748211038424. doi: 10.1177/10732748211038424
18. Kerrison WGJ, Ning J, Krasny L, Arthur A, Guljar N, Elms ML, et al. Characterisation of a novel cell line (ICR-SS-1) established from a patient-derived xenograft of synovial sarcoma. Cells. (2022) 11:2418. doi: 10.3390/cells11152418
19. D’Angelo SP, Araujo DM, Abdul Razak AR, Agulnik M, Attia S, Blay JY, et al. Afamitresgene autoleucel for advanced synovial sarcoma and myxoid round cell liposarcoma (SPEARHEAD-1): an international, open-label, phase 2 trial. Lancet. (2024) 403:1460–71. doi: 10.1016/S0140-6736(24)00319-2
20. Kotani Y, Imura Y, Nakai S, Chijimatsu R, Takami H, Inoue A, et al. Therapeutic potential of bromodomain and extra-terminal domain inhibitors for synovial sarcoma cells. Cancers (Basel). (2024) 16:1125. doi: 10.3390/cancers16061125
Keywords: breast synovial sarcoma, chest wall reconstruction, breast reconstruction, vascular anastomosis, case report
Citation: Yue W, Hua D, Yumei F, Mengyi L, Jian Z, Dajiang S, Shu L and Ke L (2025) Case Report: A rare case of male breast synovial sarcoma. Front. Oncol. 15:1469910. doi: 10.3389/fonc.2025.1469910
Received: 24 July 2024; Accepted: 30 April 2025;
Published: 26 May 2025.
Edited by:
Alessandro De Vita, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), ItalyReviewed by:
Indranil Chakrabarti, All India Institute of Medical Sciences, Kalyani (AIIMS Kalyani), IndiaJoseph M Escandón, Wyckoff Heights Medical Center, United States
Copyright © 2025 Yue, Hua, Yumei, Mengyi, Jian, Dajiang, Shu and Ke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhang Jian, MTM5ODUxMTQxMjBAMTYzLmNvbQ==; Song Dajiang, c2RqNDFAMTYzLmNvbQ==; Liu Shu, ZHJsaXVzaHVAMTYzLmNvbQ==; Luo Ke, NTQxNzQ0MjVAcXEuY29t
†These authors have contributed equally to this work and share first authorship
 Wang Yue
Wang Yue Ding Hua1†
Ding Hua1† Song Dajiang
Song Dajiang Liu Shu
Liu Shu