- 1Department of Gastroenterology and Hepatology, The Fourth Hospital of Hebei Medical University, Shijiazhuang, China
- 2Department of Oncology, The Handan Central Hospital, Handan, China
- 3Department of Cardiovascular, The Fourth Hospital of Hebei Medical University, Shijiazhuang, China
- 4Department of Oncology, The Qinhuangdao First Hospital, Qinhuangdao, China
- 5Department of Rheumatology, The Fourth Hospital of Hebei Medical University, Shijiazhuang, China
Background: PD-1 inhibitors combined with chemotherapy have become the standard first-line treatment for advanced gastric cancer (GC), but their efficacy in young GC patients is unknown. This study aimed to evaluate the efficacy of immunotherapy in young GC patients and explore new treatment strategies for this population.
Methods: Clinicopathological data of young unresectable GC patients were collected from multiple centres. We defined young as ≤45 years. Statistical analyses were conducted with SPSS IBM for Windows version 24.0.
Results: In total, 225 young unresectable GC patients were registered. Their clinicodemographic characteristics included female predominance (60.9%), poor differentiation (86.7%), high family history of cancer (14.2%), low HER2 expression (12.2%), PD-L1 expression (43.0%) and mismatch repair (MMR) deficiency (1.0%), and a high proportion of peritoneal metastasis (49.3%). After screening, 134 patients were included for analysis: 63 received dual chemotherapy (mFOLFOX6, XELOX, SOX and two-drug containing paclitaxel), 32 PD-1 inhibitors plus dual chemotherapy (mFOLFOX6, XELOX, SOX and two-drug containing paclitaxel), and 39 triple regimens (two-drug chemotherapy combined with apatinib or trastuzumab, or triple chemotherapy based on platinum, fluorouracil and paclitaxel). The effectiveness analysis revealed no significant difference in the disease control rate (DCR) between the dual chemotherapy group and the PD-1 inhibitor plus dual chemotherapy group (P=0.787), but triple regimens led to the best DCR (71.4% vs. 68.8% vs. 94.9%, all P<0.05). Kaplan–Meier curves showed median progression-free survival (PFS) times of the three groups of 4.7, 4.7 and 9.2 months, respectively. The median overall survival (OS) was 13.9, 11.0 and 15.9 months, respectively. Multivariate analyses showed that triple regimens were an independent prognostic factor for PFS [hazard ratio (HR) 0.430, 95% confidence interval (CI) 0.263-0.700; P=0.001]. Detailed survival analysis demonstrated that patients receiving intraperitoneal infusion of paclitaxel followed by intravenous paclitaxel combined with S-1 and apatinib oral therapy had better PFS (P=0.014) and OS (P=0.013) than those receiving other regimens.
Conclusion: Young patients with GC have unique clinical characteristics and are not sensitive to immunotherapy. Triple regimens, especially intraperitoneal infusion of paclitaxel followed by intravenous paclitaxel combined with S-1 and apatinib oral therapy, deserve to be studied as first-line therapies.
Introduction
In 2022, gastric cancer (GC) became the fifth most common cancer and the fifth leading cause of cancer-related death worldwide (1). Although the incidence and mortality of GC have declined over several decades in most parts of the world, the disease burden of GC remains high in Asia. In China, GC is the fifth most frequently diagnosed cancer and the third leading cause of cancer-related death (2). GC typically occurs in middle-aged and elderly patients, especially those aged ≥65 years, but can occur in young patients, although it is largely ignored in the clinic. Recent findings indicate an increasing trend in GC incidence rates among young patients. At present, there is no clear age limit for young patients with GC. In some studies, young GC is defined as GC before age 40, while in others, the definition has included all patients diagnosed before age 45 (3–6). Whether the cut-off is 40 or 45 years, the incidence of young GC patients has increased annually (7, 8). The occurrence of GC in young patients is less affected by environmental factors and more strongly related to heredity (9, 10). Compared with elderly patients, young patients commonly have more aggressive tumours, more advanced disease at presentation, and worse prognoses (11). Meanwhile, due to the lack of obvious early symptoms and delayed diagnosis, young patients often present at an advanced stage when seeking medical care, resulting in the loss of opportunities for radical surgery (12). Therefore, actively exploring pathological molecular characteristics and advancing precision therapy for this condition are of critical significance.
In recent years, immune checkpoint inhibitors (ICIs) have achieved remarkable success across various cancer types, offering a promising advancement in the treatment of GC. Supported by global multicentre phase III clinical trials, programmed death 1 (PD-1) inhibitors combined with chemotherapy have been approved as the standard first-line therapeutic regimen for advanced GC (13–16). However, current treatment strategies for advanced GC remain undifferentiated by age, and no consensus clinical guidelines specifically address the management of young patients in international practice. A recent study demonstrated that immune responses differ with sex and age, with female and young patients having lower response rates to immunotherapy (17). In another multi-institutional retrospective study including 538 patients with advanced melanoma, the association between age and the response to anti-PD1 therapy was evaluated. The study showed that patients younger than 62 years were less likely to benefit from pembrolizumab treatment, with a disease control rate of 52% compared to 63% in older patients. The investigators also noted that the likelihood of response increased with age and that the odds ratios of progressing on pembrolizumab decreased 13% with every decade of patient age (18). Therefore, the efficacy of immunotherapy in young patients still needs to be clarified.
Our previous study demonstrated that young gastrointestinal patients (aged <45 years) receiving PD-1 inhibitor therapy exhibited significantly suboptimal treatment responses and inferior survival outcomes compared to elderly cohorts (19). In this study, we retrospectively analysed the clinical data of young GC patients aged ≤45 years, summarized their clinicopathological characteristics, and compared the efficacy of different first-line treatment schemes, especially PD-1 inhibitors combined with dual chemotherapy, to further verify the efficacy of immunotherapy in young patients and provide data supporting the realization of precision treatment for young GC patients.
Methods
Patient screening
Patients were selected for this multicentre retrospective cohort study through the Hebei Gastric Cancer Collaborative Network Database (http://hbss.suvalue.com/), which collected data from the Fourth Hospital of Hebei Medical University, a large cancer centre in Hebei Province, China, between January 2019 and January 2022. To expand the database and exclude single-centre errors, data from two other research centres (Handan Central Hospital and Qinhuangdao First Hospital) were also collected.
Inclusion criteria
The inclusion criteria were as follows: (i) age 45 years or younger at diagnosis; (ii) histological diagnosis of adenocarcinoma and a diagnosis of stage IV GC, with GC staging based on the 8th edition of the American Joint Committee on Cancer (AJCC) TNM classification. The exclusion criteria were as follows: (i) age>45 years; (ii) insufficient staging information or uncertainty of distant metastasis; (iii) the coexistence of other malignancies; or (iv) other pathological subtypes of gastric malignancies, such as gastric small cell carcinoma, neuroendocrine carcinoma and lymphoma.
Data acquisition
The following detailed information on the clinicopathologic characteristics of patients was collected from medical records and follow-up systems: age, sex, family history of tumours, tumour location, degree of tumour, human epidermal growth factor receptor 2 (HER2) status, programmed cell death-ligand 1 (PD-L1) expression, microsatellite status, performance status (PS) score, metastatic sites, number of metastatic lesions, history of childbearing (for female patients), operation type, history of palliative chemotherapy, and laboratory tests, including carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9. The study protocol was approved by the institutional review board of each participating hospital before initiation of the study (2020KS001). All procedures followed medical ethical standards and were carried out in strict accordance with the Declaration of Helsinki and its amendments and other applicable local laws and regulations. Due to the retrospective nature of this study, informed consent was waived by the ethics committee.
Treatment response assessment
All patients received enhanced thoracic-abdominal-pelvic computed tomography (CT) scans and/or endoscopy before starting the treatments. The CT image sets were retrieved and reassessed according to the 8th edition of the AJCC staging manual. Patients received palliative chemotherapy according to the recommendation of the guidelines and the patient’s preference. Treatment continued until disease progression, treatment-related adverse events, death or loss to follow-up. The Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1 was used to evaluate the efficacy of chemotherapy. The modified RECIST 1.1 for immune-based therapeutic (iRECIST) was used to evaluate the immunotherapy response. Complete response (CR) was defined as the complete disappearance of the target lesion after chemotherapy. Partial response (PR) was defined as a reduction in the total diameter of each target lesion by 30% or more. Progressive disease (PD) was defined as at least a 20% increase in the sum of the long diameters of all target lesions and an absolute increase of more than 5 mm or the appearance of new lesions. Stable disease (SD) was defined as no change in target lesions. The objective response rate (ORR) was defined as the percentage of patients with a CR or PR among all the treated patients. The disease control rate (DCR) was defined as the percentage of patients who achieved CR, PR and SD.
Follow-up
All patients were followed up via rehospitalization or re-examinations in the outpatient clinic or by telephone until mortality due to any reason or loss to follow-up. Progression-free survival (PFS) was calculated from the date of starting first-line treatment to the date of disease progression or death, whichever occurred first. Overall survival (OS) was calculated from the date of treatment initiation to the date of patient death or the patient’s last follow-up date.
Statistical analyses
The SPSS 24.0 software package (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 9 (GraphPad Software, San Diego, California) were used for statistical analyses. Qualitative variables and continuous variables are described as frequencies, percentages, means, standard deviations, and medians. Group comparisons of qualitative variables were performed using Pearson’s chi-squared test or two-sided Fisher’s exact test, while continuous variables were compared by means of Student’s t tests. Based on Cohen’s w effect size, we need a minimum sample size of 102 to obtain the statistical difference. The basic characteristics of the patients were evaluated by descriptive analysis. Kaplan–Meier curves were used for analyses of survival outcomes, and the log-rank test was carried out to test for statistical significance. Cox regression was used for multivariate analysis with the forward stepwise method. P<0.05 was considered statistically significant, and all reported P values are two-sided.
Results
Patient characteristics and the selection of first-line treatments
From January 2019 to January 2022, 225 unresectable GC patients aged ≤45 years were included according to the inclusion and exclusion criteria. The characteristics of the patients are summarized in Table 1. The median age was 37 years (range, 21-45), with 32 patients (32/225, 14.2%) younger than 30. There was a significant predominance of female patients (137/225, 60.9%), and 126 (126/137, 92.0%) of them had a history of childbirth. Notably, among all the female patients who had a history of childbirth, 52 (52/126, 41.3%) had a history of caesarean section, which suggests that caesarean section may be related to the occurrence of GC. There were 109 patients with a PS score of 0 (109/225, 48.4%) and 116 patients with a PS score ≥1 (116/225, 51.6%). In total, 14.2% (32/225, 14.2%) of the GC patients had a family history of tumours. The highest proportion of tumours were in the gastric body (136/225, 60.4%), followed by the gastric antrum (51/225, 22.7%) and gastric cardia (38/225, 16.9%). The vast majority of patients had poorly differentiated tumours (195/225, 86.7%). HER2 status was examined in 172 patients, with 21 (21/172, 12.2%) showing positive expression (immunohistochemistry, IHC 3+ or IHC 2+, fluorescence in situ hybridization, FISH amplification). Data on PD-L1 expression were available for 93 patients, 40 (40/93, 43.0%) showing positive expression (combined positive score, CPS≥1). The proportion of patients with CPS≥5 was 20.4% (19/93, 20.4%), and that with CPS≥10 was 8.6% (8/93, 8.6%). Among the 96 patients whose microsatellite status was evaluated, only 1 (1/96, 1.0%) had deficient mismatch repair, dMMR/microsatellite instability-high, and MSI-H. Among the 225 patients, 47 (47/225, 20.9%) patients received palliative surgery, including palliative gastric resection (partial or total) and ovarian resection. There were 49.3% (111/225, 49.3%) of patients with peritoneal metastases at diagnosis.
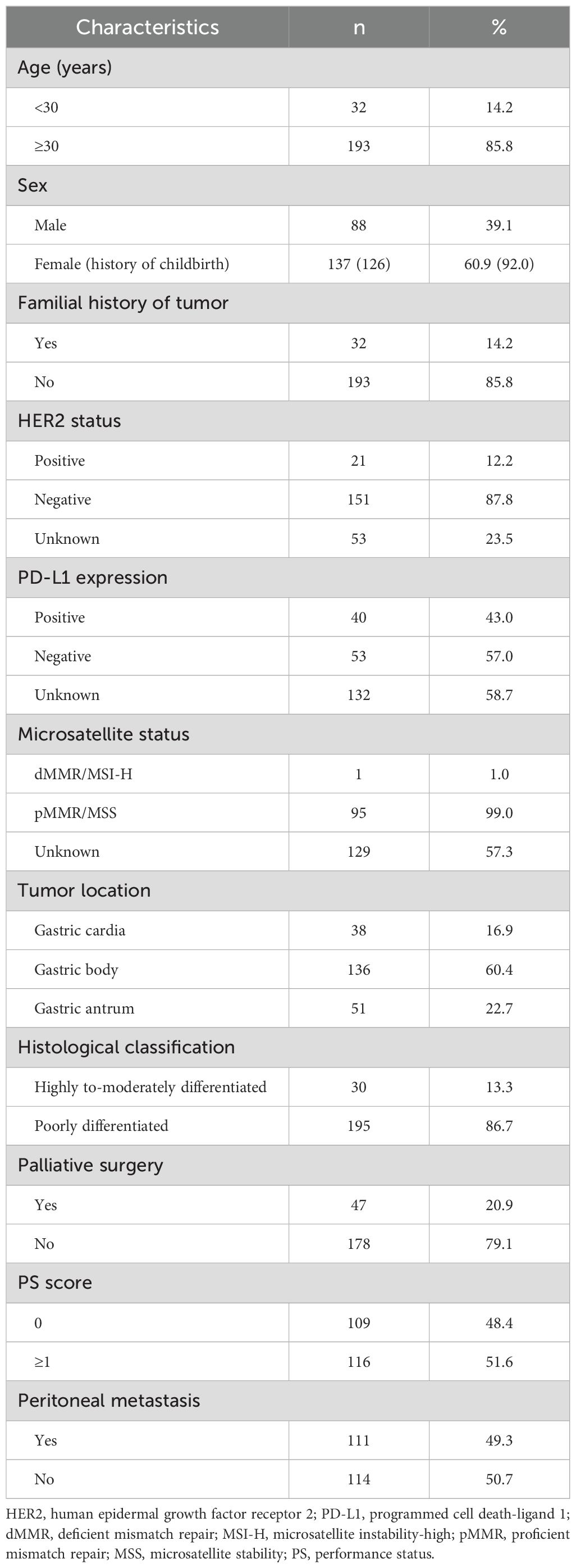
Table 1. Clinicopathological characteristics of young unresectable gastric cancer patients aged ≤45 years (n=225).
Among the 225 patients, 202 received first-line chemotherapy. Among them, 6 patients received monotherapy chemotherapy based on fluorouracil and paclitaxel; 107 received dual chemotherapy based on platinum, fluorouracil, and paclitaxel (mFOLFOX6, XELOX, SOX and two-drug containing paclitaxel); 41 received a PD-1 inhibitor (nivolumab, pembrolizumab, tislelizumab, camrelizumab, toripalimab, or sintilimab) plus dual chemotherapy (mFOLFOX6, XELOX, SOX and two-drug containing paclitaxel); and 48 received triple regimens including two-drug chemotherapy combined with apatinib or trastuzumab, intraperitoneal infusion of paclitaxel followed by intravenous paclitaxel combined with S-1 and apatinib oral therapy or triple chemotherapy based on platinum, fluorouracil and paclitaxel. The selection of first-line treatments for patients stratified by sex is shown in Figure 1.
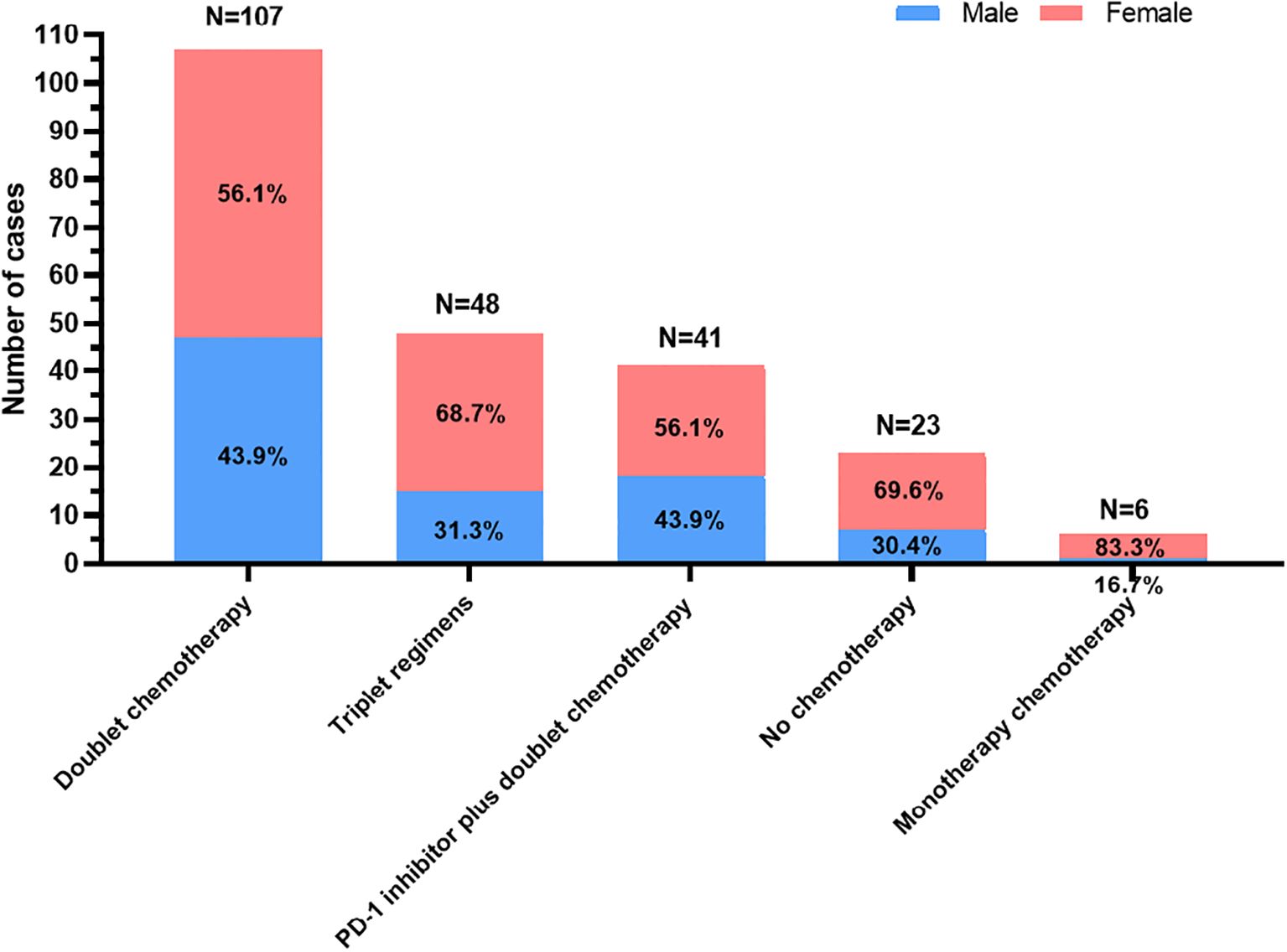
Figure 1. The selection of first-line treatments for young unresectable gastric cancer patients stratified by sex.
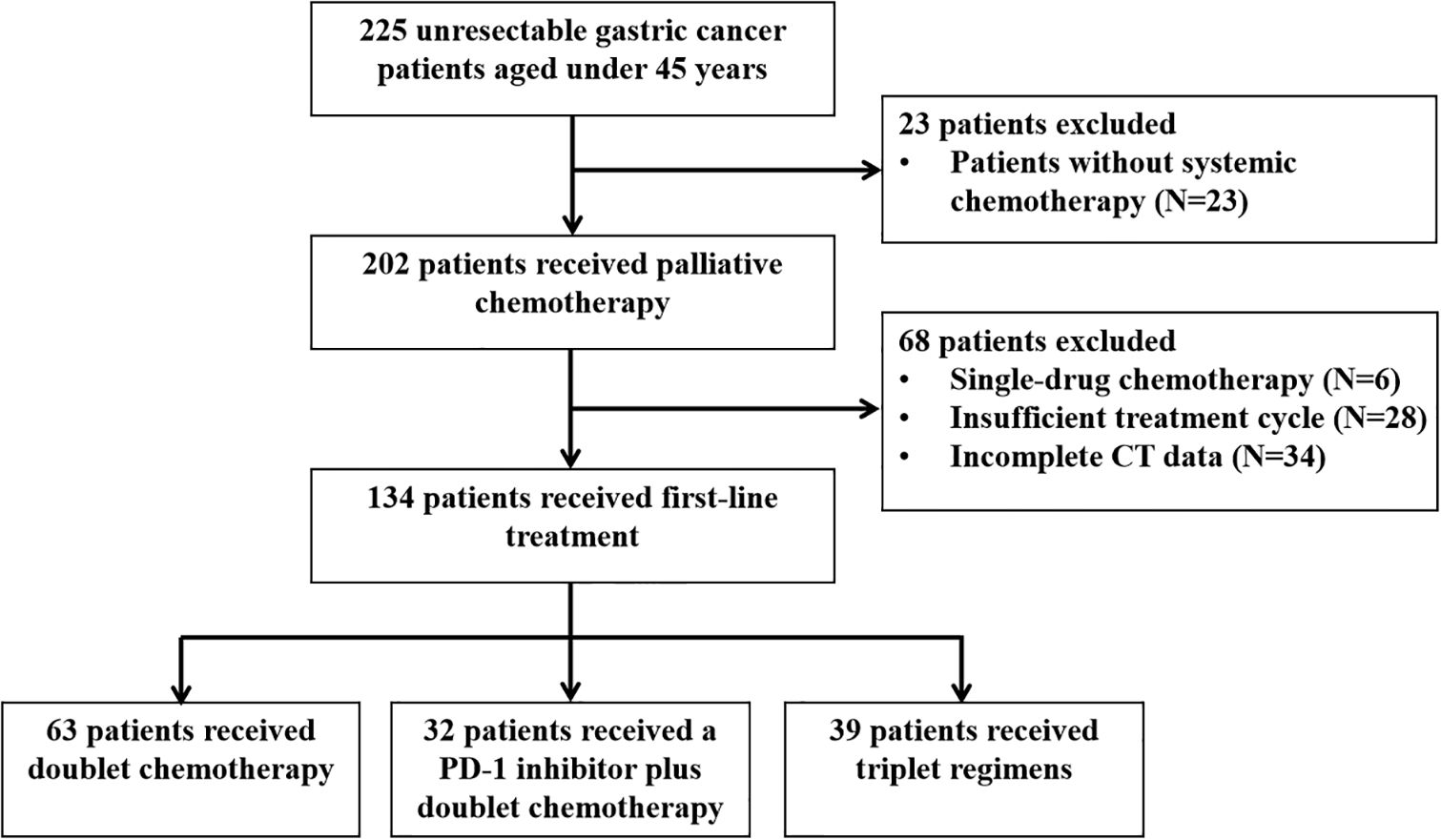
The efficacy of different first-line therapy regimens in young GC patients
After screening, 134 patients who received at least two cycles of chemotherapy as first-line therapy and had complete data were reviewed. The selection procedure of the study cohort is shown in Figure 2. Among them, 63 received dual chemotherapy, 32 received a PD-1 inhibitor plus dual chemotherapy, and 39 received triple regimens. As shown in Table 2, compared with the PD-1 inhibitor plus dual chemotherapy and triple regimens groups, the proportion of undetected PD-L1 expression was significantly higher in the dual chemotherapy group (P=0.011). Meanwhile, there were more patients in the triplet regimen group that did not receive later-line treatment than in the other two groups (P=0.018). Other baseline characteristics, such as age, sex, tumour location, histological classification, PS score, peritoneal metastasis, number of metastatic lesions, familial history of tumour, history of palliative surgery, and CEA and CA19-9 levels at the initiation of first-line therapy, were not significantly different among the three groups.
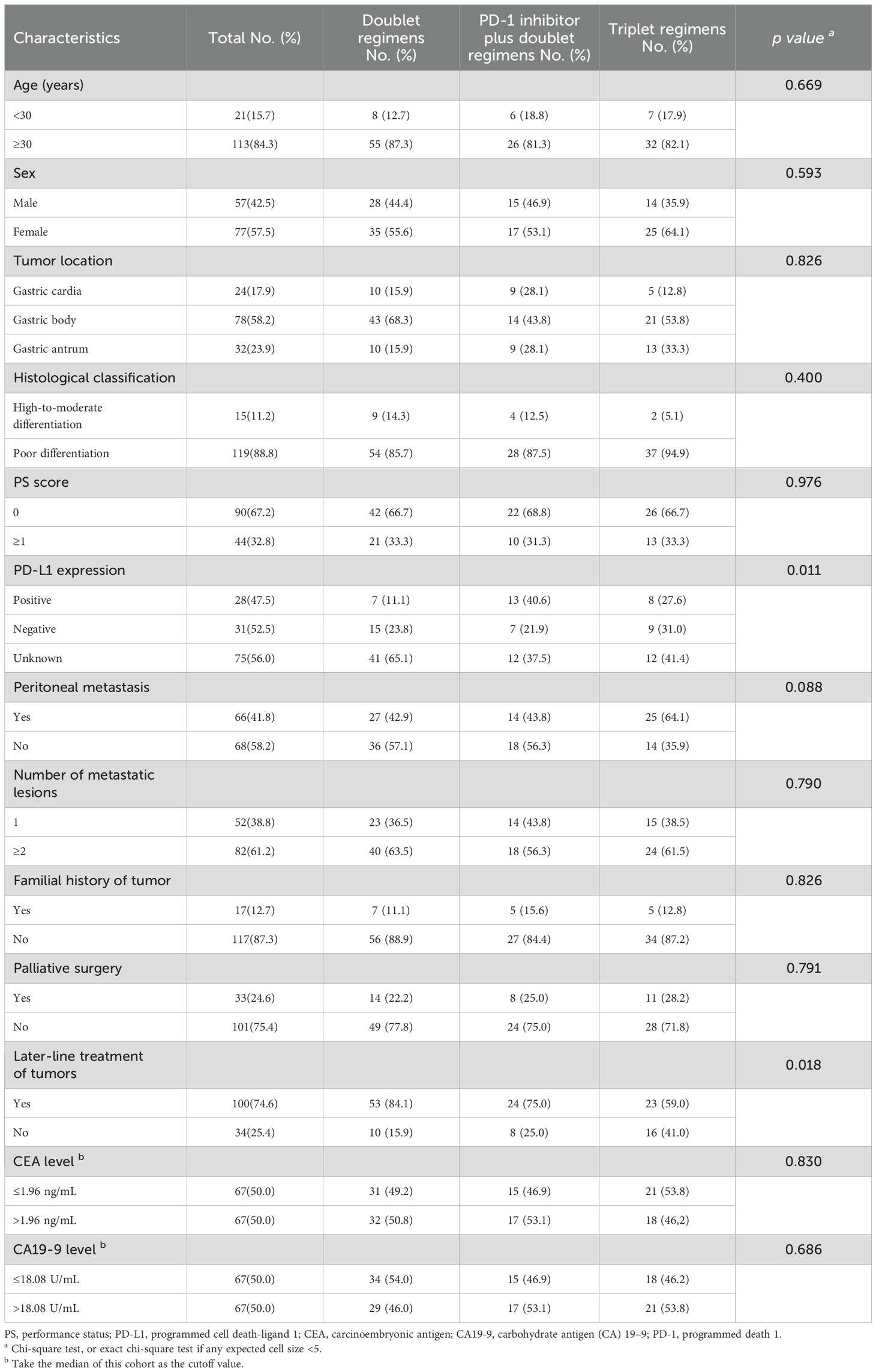
Table 2. The baseline characteristics of young unresectable gastric cancer patients in different treatment groups (n=134).
The efficacy of different first-line therapy regimens in young unresectable GC patients was evaluated according to RECIST 1.1 and iRECIST. As shown in Table 3, the ORR of the dual chemotherapy group was 15.9% (10/63), that of the PD-1 inhibitor plus dual chemotherapy group was 12.5% (4/32), and that of the triple regimen group was 23.1% (9/39). The triple regimen group had an advantage in ORR, although the difference was not significant in the current small sample of patients (all P>0.05). The DCRs of the three groups were 71.4% (45/63), 68.8% (22/32) and 94.9% (37/39), respectively. There was no significant difference between the dual chemotherapy group and the PD-1 inhibitor plus dual chemotherapy group (P=0.787); however, the DCR was significantly higher in the triple regimen group than in the other two groups (all P<0.05).
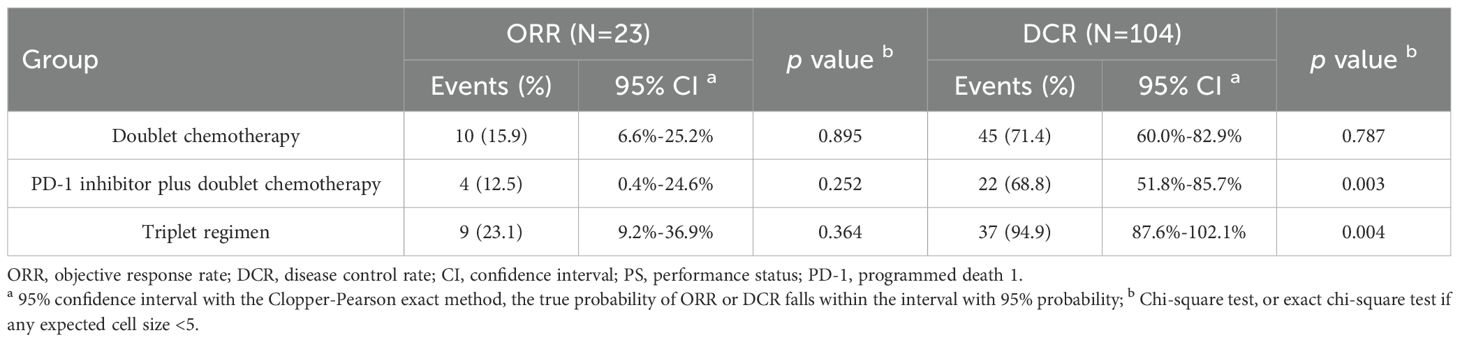
Table 3. The clinical response to different first-line therapy regimens in young unresectable gastric cancer patients.
Clinical outcome analysis in young unresectable GC patients
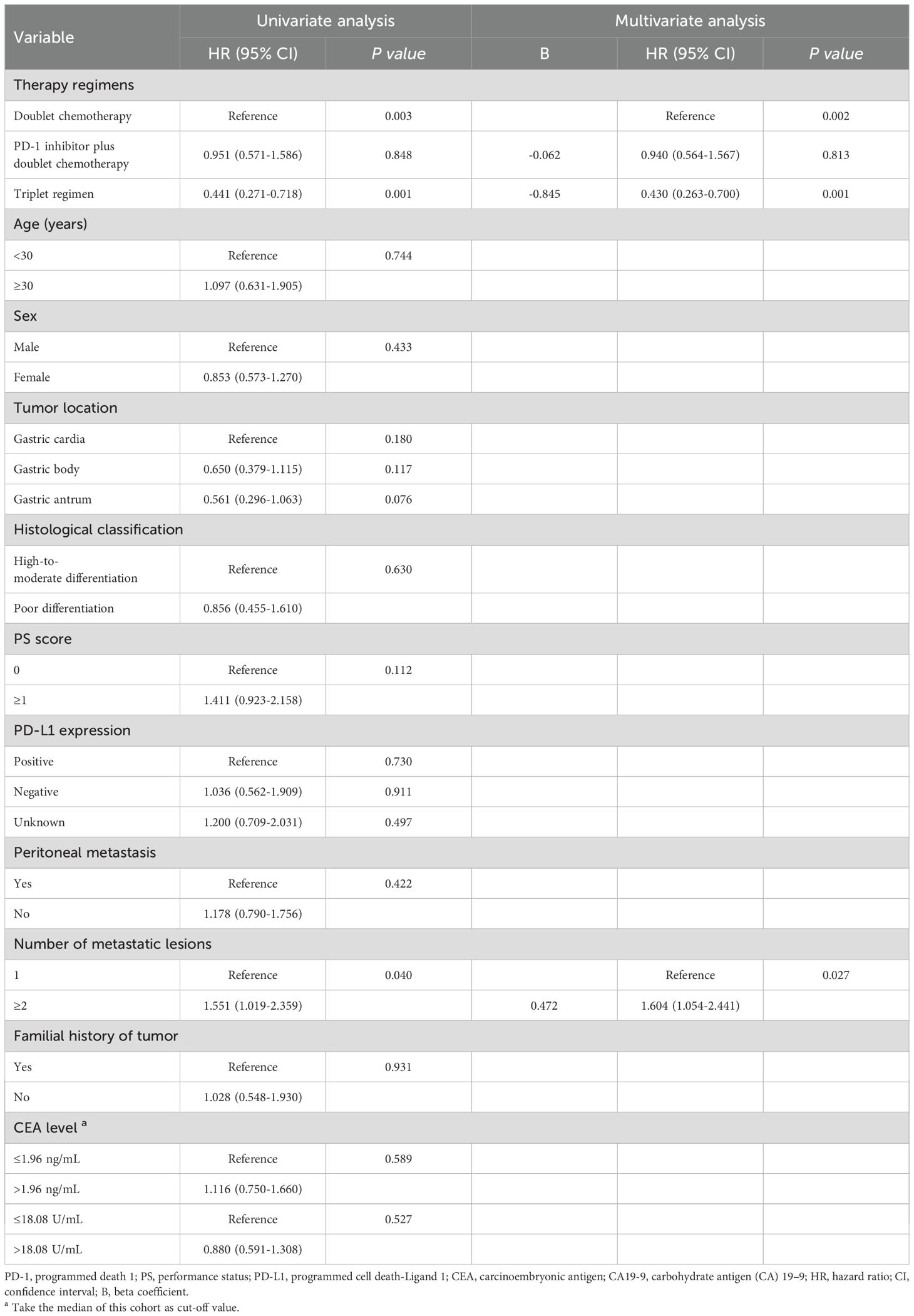
Univariate analysis was performed to find clinical features that might affect the PFS of young unresectable GC patients. The median PFS durations of the three groups were 4.7, 4.7 and 9.2 months, respectively. As shown in Table 4, there was no significant difference in PFS between the dual chemotherapy group and the PD-1 inhibitor plus dual chemotherapy group (P=0.848, Figure 3A), but the patients who received triple regimens had the best PFS (P=0.001). Patients with one metastatic lesion had a prolonged PFS compared with those with two or more metastatic lesions (P=0.040). In the Cox proportional hazards model, triplet regimens were independently associated with the best PFS [hazard ratio (HR) 0.430, 95% confidence interval (CI) 0.263-0.700, P=0.001]. In addition, the number of metastatic lesions (HR 1.604, 95% CI 1.054-2.441, P=0.027) was found to be independently associated with PFS (Table 4).
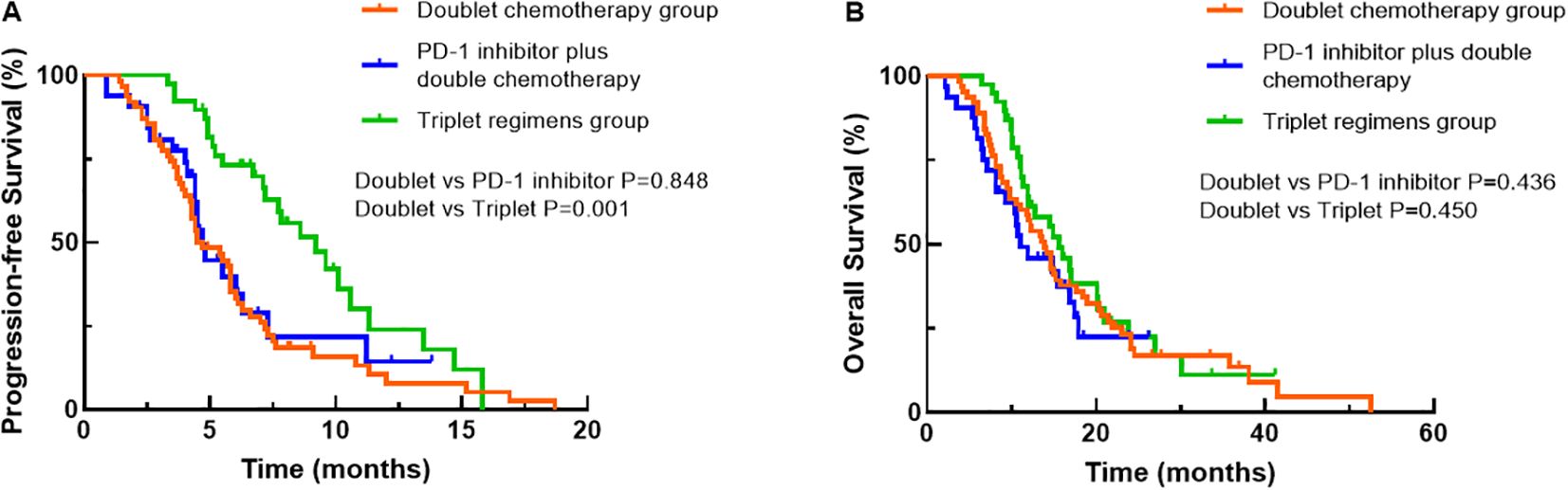
Figure 3. Comparisons of progression-free survival (A) and overall survival (B) among the treatment groups using Kaplan–Meier curves.
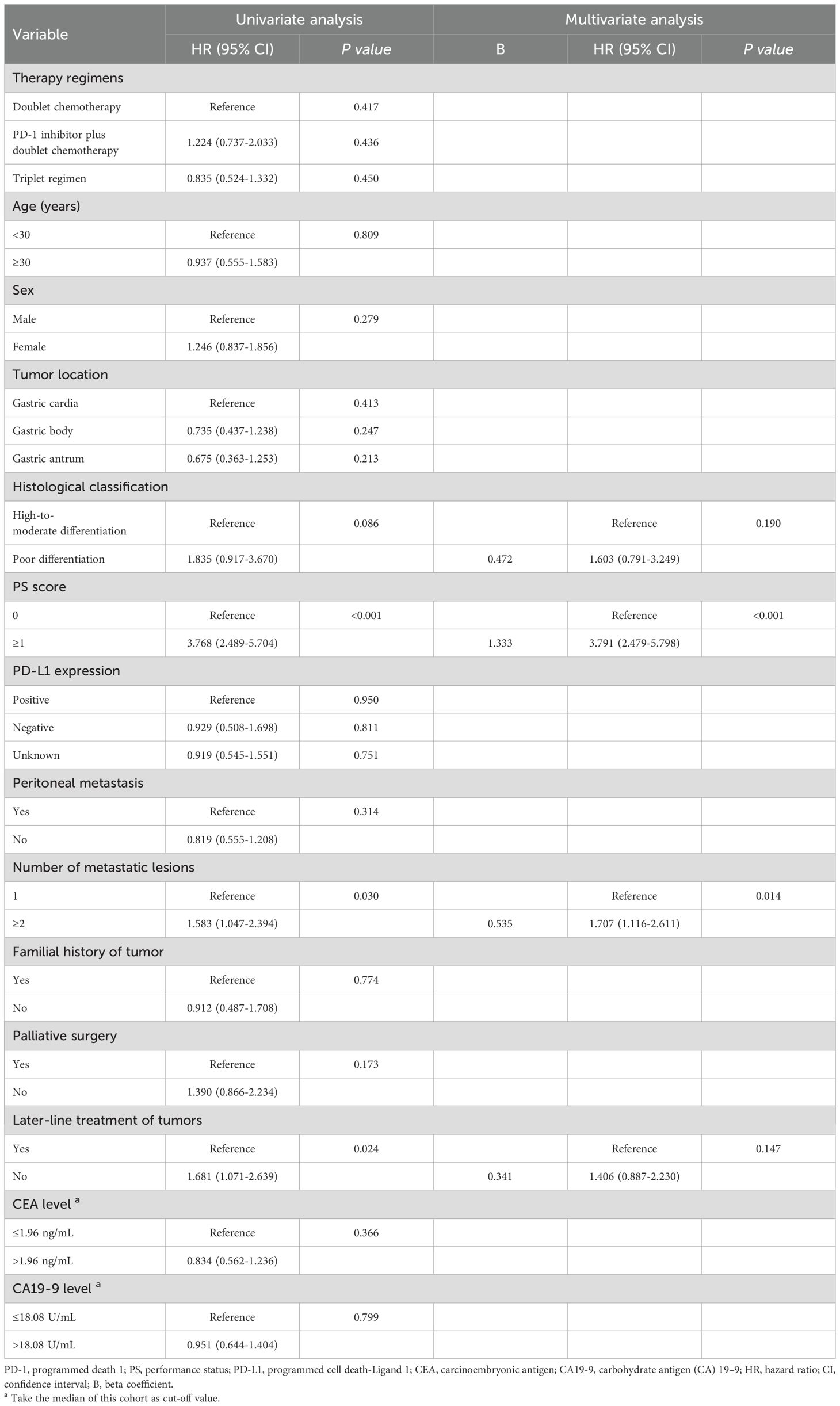
Regarding OS, at the data cut-off (September 30, 2022), 103 (76.9%) deaths occurred, 29 (21.6%) patients were alive, and 2 (1.5%) patients were lost to follow-up. The corresponding median OS durations of the three groups were 13.9 months, 11.0 months and 15.9 months, respectively. When OS was compared among the three groups, patients treated with triple regimens tended to have better survival than patients treated with dual chemotherapy and PD-1 inhibitors plus dual chemotherapy, although the difference did not reach significance (all P>0.05, Figure 3B). Nevertheless, PS score (P<0.001), number of metastatic lesions (P=0.030) and later-line treatment of tumours (P=0.024) were correlated with the OS of young GC patients by univariate analysis (Table 5). Based on the outcomes, we conducted further multivariate Cox analysis. The results indicated that PS score (HR 3.791, 95% CI 2.479-5.798, P<0.001) and the number of metastatic lesions (HR 1.707, 95% CI 1.116-2.611, P=0.014) were independent factors for the OS of young unresectable GC patients (Table 5).
Next, we performed a more detailed survival analysis based on different treatment regimens. As presented in Figure 4, PFS and OS did not differ among the different dual chemotherapy groups (mFOLFOX6, XELOX, SOX and two-drug containing paclitaxel) or the PD-1 inhibitor plus different dual chemotherapy groups (mFOLFOX6, XELOX, SOX and two-drug containing paclitaxel). The patients who received intraperitoneal infusion of paclitaxel followed by intravenous paclitaxel combined with S-1 and apatinib oral therapy had a significantly longer OS than patients treated with other triple regimens (P=0.014), but the PFS did not differ among the different triple regimens (P=0.205). When intraperitoneal infusion of paclitaxel followed by intravenous paclitaxel combined with S-1 and apatinib oral therapy was compared with the two-drug combination of paclitaxel plus PD-1 inhibitor or all two-drug combinations containing paclitaxel, the patients treated with intraperitoneal infusion of paclitaxel followed by intravenous paclitaxel combined with S-1 and apatinib oral therapy had better PFS (P=0.014) and OS (P=0.013).
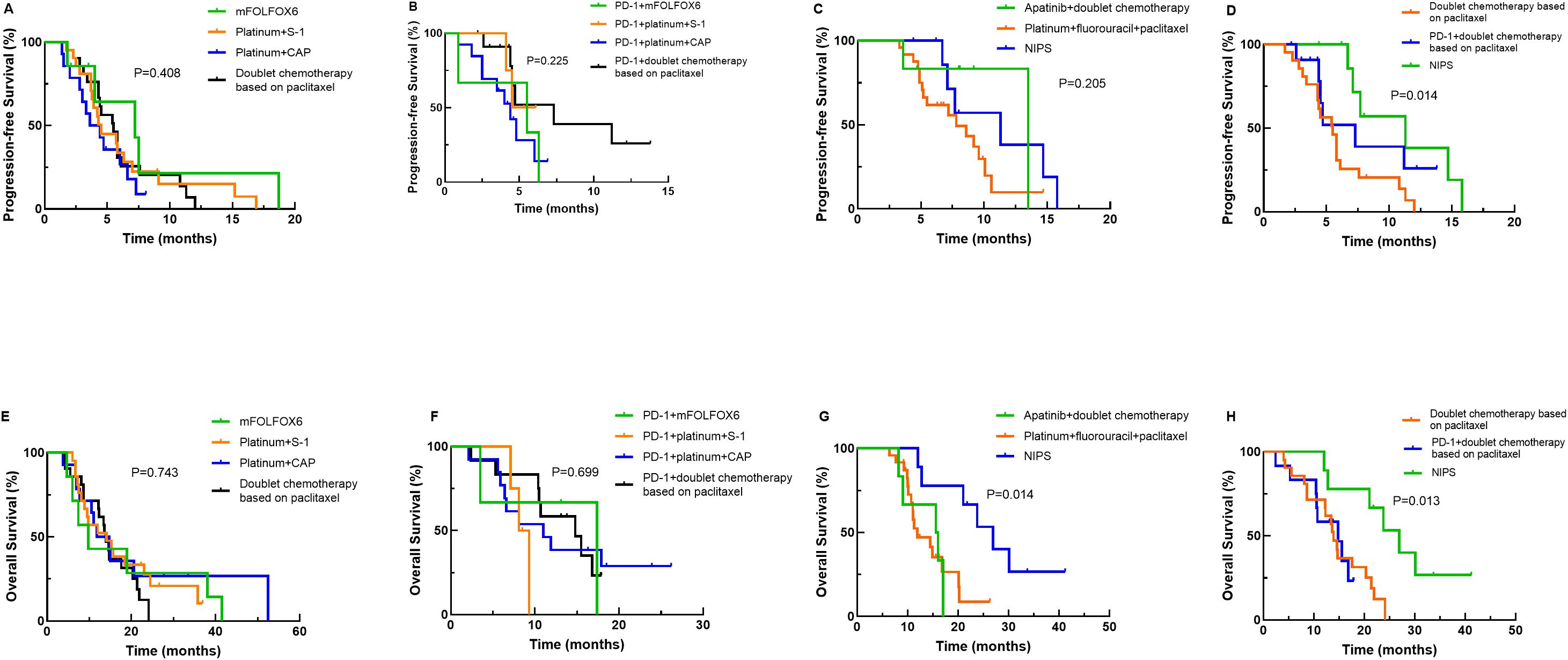
Figure 4. Kaplan–Meier curves for progression-free survival (PFS) and overall survival (OS) stratified by more detailed treatment regimens. (A) PFS curves for comparisons between different doublet chemotherapy regimens; (B) PFS curves for PD-1 inhibitor combined with different doublet chemotherapy regimens; (C) PFS curves for various triplet regimens; (D) PFS curves for the neoadjuvant intraperitoneal and systemic chemotherapy (NIPS) group, the paclitaxel-based doublet chemotherapy group, and the PD-1 inhibitor combined with paclitaxel-based doublet chemotherapy group; (E) OS curves for comparisons between different doublet chemotherapy regimens; (F) OS curves for PD-1 inhibitor combined with different doublet chemotherapy regimens; (G) OS curves for various triplet regimens; (H) OS curves for the NIPS group, the paclitaxel-based doublet chemotherapy group, and the PD-1 inhibitor combined with paclitaxel-based doublet chemotherapy group. Note: mFOLFOX6, oxaliplatin+calcium folinate+5-fluorouracil.
Discussion
The incidence of GC in young individuals has increased steadily in recent decades (7, 8). Previous research has revealed that the detailed clinicopathologic features and prognostic factors of young patients with GC are still debated. Therefore, we conducted the current study to investigate a young group of patient under the age of 45 years. In our analyses, female patients constituted a higher proportion of the entire group, with a male-to-female ratio of approximately 1:1.56. Previous reports have shown that this incidence of GC is higher among males and patients of advanced age, but among younger patients, the incidence is higher among females (20–22). Our study supports this finding of a higher proportion of female patients among young patients. The reason for the female predominance among young patients remains unclear. Several studies have suggested that sex hormones, especially oestrogen, may play an important role in the development of GC in young people (23). Zhong et al. showed that oestrogen-related receptors are highly expressed in GC tissues, where they can promote the migration and invasion of cancer cells (24). Harrison et al. found that oestradiol is trophic to GC cell lines in vitro and suggested that positivity for oestradiol receptor D5 expression is an independent negative prognostic factor for patients with GC (25). In addition, sex hormones and sex chromosome-related genes are also the main factors driving these differences in immunity (26, 27). The molecular mechanisms underlying sex-based differences in nonresponsiveness to ICIs, such as oestrogen-mediated recruitment of myeloid-derived suppressive cells (MDSCs) and Tregs to the TME, which are known to be involved in resistance to ICIs, have recently been characterized in mouse models (28). Interestingly, our study found that 41.3% of all female patients with a history of childbirth underwent caesarean section, while the rate of caesarean section in China is only 34.9% (29). The relatively high proportion of patients with GC who underwent caesarean section suggests that caesarean section may be related to the occurrence of GC. However, there are currently few studies on the relationship between the medical history of female patients and GC, and further research is necessary to explore this further. In summary, GC can occur in young female patients, especially those who undergo caesarean section, and symptoms suggestive of GC should be investigated aggressively.
Currently, the occurrence and progression of GC are multifactor and multistep processes that are the result of interactions between the environment and heredity. However, the incidence of GC among young patients is less affected by environmental factors and more related to heredity (9, 10). Familial clustering was found in 11.4% of GC patients and was more notable in young patients (30). In our study, 14.2% of GC patients had a family history of tumours, consistent with other reports (6, 31). The most common hereditary GC is hereditary diffuse gastric cancer (HDGC), which is an autosomal dominant genetic syndrome often caused by E-cadherin gene (CDH1) mutation. Decreased or dysfunctional e-cadherin can lead to decreased adhesion between cells, increasing tumour aggressiveness (32, 33). Due to the limited research scope, we did not sequence the exons of tumour tissues. Nonetheless, it is important to carry out regular endoscopic screening for young patients who have a family history of tumours or genetic mutations who are at risk of GC.
In addition, recent studies have shown that young GC patients have a different molecular expression profile than old patients, including a lower frequency of HER2 overexpression and mismatch repair (MMR) deficiency (34, 35). In our cohort, HER2 status was examined in 172 patients, with 12.2% showing positive expression. According to the HER-EAGLE study, only 9.2% HER2 positivity was detected in patients before age 55 (36). This difference from our study may be explained by the study era and geographic variations. Recent studies have shown that HER2 itself regulates the recruitment of tumour-infiltrating immune cells by inducing the expression of chemokine ligand 2 (CCL2) and PD-1 ligands, thereby enhancing the efficacy of immunotherapy (37). The KEYNOTE-811 study showed that pembrolizumab combined with trastuzumab and chemotherapy increased the ORR of patients with HER2-positive advanced GC to 74.4%, which was significantly higher than that of the control group. Based on this study, immunotherapy combined with anti-HER2 therapy plays an important role in the first-line treatment of advanced GC (16). Microsatellite status was detected in 96 patients, with only 1 of them having dMMR/MSI-H in our survey. The low incidence of MMR deficiency in young patients with GC is probably related to the high proportion of diffuse tumours, wherein MSI is less common, and to the fact that genomically stable tumours are usually diagnosed at an earlier age (38). Patients with MMR deficiency tend to generate more neoantigens and are more immunogenic, thus responding better to ICIs (39). At present, MSI status is the most accurate molecular marker for predicting the efficacy of immunotherapy. In addition, PD-L1 expression was evaluated in 93 patients in our study, 43.0% of whom exhibited positive PD-L1 expression. PD-L1 positivity was defined as a combined positive score (CPS) ≥1. Rha SY et al. included a large cohort of 574 GC patients and demonstrated that 67.4% of all patients had a CPS ≥1 (40). Another analysis showed a positive correlation between PD-L1 expression and the CPS and ICIs response in patients with GC (41). PD-L1 expression has rarely been reported in young GC patients, so these results may provide a reference for further exploration. In conclusion, young GC patients may not be good candidates for immunotherapy, so novel therapies involving different approaches are needed.
Recently, based on the phase III clinical trials, PD-1 inhibitors combined with dual chemotherapy have become the standard first-line treatment for advanced GC patients (13–16). However, therapeutic options for GC are not stratified by age worldwide, and young GC patients may not be good candidates for immunotherapy. In a recent study evaluating the relationship between age and immunotherapy in patients with lung adenocarcinoma, the tumour mutation burden (TMB) of patients in the older group (>50 years) was significantly higher than that of patients in the younger group (≤50 years), and the OS of patients receiving immunotherapy was longer in the older group (42). Specific genetic mutations also influence responses to ICIs. Integrating genomic profiles from TCGA and clinical immunotherapy data from MSKCC, Guan R et al. identified age-related mutational disparities: young patient cohorts exhibited higher frequencies of IDH1, BRAF, and ATRX mutations, whereas TP53, TTN, MUC16, and LRP1B mutations were more prevalent in older cohorts (43). Notably, BRAF mutations appeared to confer inferior responses to ICIs, while patients with LRP1B mutations exhibited significantly higher TMB and improved clinical outcomes compared to wild-type counterparts (44, 45). We also previously reported that PD-1 inhibitors therapy has lower efficacy against gastrointestinal cancer in younger patients (aged <45 years) (18). In this study, we focused on young unresectable GC patients, compared the efficacy of different first-line treatment regimens, and found that PD-1 inhibitors plus dual chemotherapy were not superior to dual chemotherapy alone. The low response rate to anti-PD-1 therapy in young GC patients is not only related to the molecular expression profile of young GC patients themselves but also may be due to the higher incidence of young GC in female patients. A recent study revealed that young and female patients accumulate driver mutations in their tumours that are less readily presented by their major histocompatibility complex (MHC) molecules (17), further suggesting that these effects are strong and complementary. Together, current knowledge provides a rationale for the paradigm that immune selection exerts its Toll differently concerning age and sex, with a strong immunoediting effect being observed in young and female patients. In addition, this study found that 49.3% of young GC patients had peritoneal metastasis at the first visit, which is a negative clinical indicator of immunotherapeutic efficacy. In a multicentre biomarker cohort study on the efficacy of nivolumab treatment for GC, the results showed that patients with nonperitoneal metastases had a higher response rate to immunotherapy than those with peritoneal metastases (46). Previous studies have pioneered the integration of machine learning and single-cell sequencing analysis to systematically investigate the mechanisms underlying immunotherapy efficacy in lung adenocarcinoma (47–49). Our future studies will employ scRNA-seq and spatial transcriptomics to map age-stratified immune landscapes. Coupled with deep learning models (50), this will identify novel targets such as exhausted CD8+T cell subsets or immunosuppressive CD8+ Treg populations, which may underlie the attenuated immunotherapy efficacy in young patients.
However, young patients are in relatively good physical condition, have few complications, have high tumour malignancy, experience rapid disease progression, and need and tolerate more aggressive treatment options. In the present study, we compared the efficacy and prognostic impact of different first-line treatment regimens in young unresectable GC patients. Triple regimens provided a significant DCR and PFS advantage compared with dual chemotherapy and PD-1 inhibitors plus dual chemotherapy. Although baseline imbalances in patient characteristics, temporal variations, and genetic heterogeneity may compromise the generalizability of the findings, this study provided compelling evidence supporting that these young GC patients may benefit from a stronger chemotherapy regimen. Next, we performed a more detailed survival analysis based on different treatment regimens and found that intraperitoneal infusion of paclitaxel followed by intravenous paclitaxel combined with S-1 and apatinib oral therapy had better PFS and OS than other regimens, which may be related to the higher proportion of patients with peritoneal metastasis in this study. For patients with peritoneal metastasis, due to the existence of a plasma-peritoneal barrier, it is difficult for chemotherapeutic drugs with large molecules to penetrate the barrier to act on metastases in the abdominal cavity, so systemic chemotherapy has little effect on patients with peritoneal metastasis. Intraperitoneal perfusion chemotherapy provides a new treatment option for patients with peritoneal metastasis. Meanwhile, young GC patients frequently present as a poorly differentiated or undifferentiated adenocarcinoma, characterized by heightened tumour cell proliferative activity and a larger proportion of cells residing in active phases of the cell cycle. This aggressive proliferative phenotype may render these tumours more sensitive to paclitaxel, a taxane-class chemotherapeutic agent that targets rapidly dividing cells by stabilizing microtubules and disrupting mitotic spindle dynamics, thereby inducing cell cycle arrest and apoptosis (6). PHOENIX series studies in Japan have confirmed that intraperitoneal infusion of paclitaxel followed by intravenous paclitaxel combined with S-1 oral therapy is a safe and feasible treatment method for GC with peritoneal metastasis (51). In addition, apatinib, as a highly selective VEGFR-2 inhibitor, exhibits fewer off-target effects compared to multi-targeted TKIs, enhancing its therapeutic index in GC (52). Therefore, intraperitoneal infusion of paclitaxel followed by intravenous oral paclitaxel combined with S-1 and apatinib may be recommended as a preferred option for young patients. However, higher quality trials, better patient selection, and multicentre randomized controlled trials are needed to support this conclusion. In summary, there is no doubt that young GC patients have fewer complications, better PS, and adaptability to aggressive treatment. Our results may support an argument that is worth verifying in future research that young GC patients should receive more aggressive treatment.
Our study has several limitations. First, this study was retrospective in nature. Second, the relatively small sample size limited the reliability of the conclusions. Incomplete data on molecular markers such as HER2, MMR status and PD-L1 expression, particularly the unexplored association between PD-L1 expression levels and the therapeutic efficacy of immunotherapy in young GC patients, may introduce bias into the study outcomes. Nevertheless, this study revealed critical clinical characteristics of young GC patients, including a female predominance, lower rates of HER-2 positivity, reduced prevalence of PD-L1 expression, diminished proportions of MSI-H/dMMR, and a higher incidence of peritoneal metastasis. Furthermore, our exploration provided real-world data for the precision first-line treatment of young patients. Prospective validation of age-stratified treatment protocols, particularly in immunotherapy trials, is urgently warranted to translate these findings into clinical practice.
Conclusions
This study revealed important clinicopathological features of young unresectable GC patients, which may lead to a poor response to ICIs treatment in this population. In terms of first-line treatment in young patients, PD-1 inhibitors plus dual chemotherapy were not superior to dual chemotherapy alone, but triple regimens, especially intraperitoneal infusion of paclitaxel followed by intravenous paclitaxel combined with S-1 and apatinib oral therapy, were superior to other regimens. To our knowledge, this is the first study to explore first-line treatment specific to the population of young GC patients. Prospective new studies are needed to provide more information on this subject.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The study protocol was reviewed and approved by the ethics committee of the Fourth Hospital of Hebei Medical University (2020KS001). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Due to the retrospective nature of this study, informed consent was waived by the same ethics committee.
Author contributions
YW: Data curation, Formal Analysis, Funding acquisition, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. YL: Investigation, Methodology, Writing – original draft, Writing – review & editing. ZL: Investigation, Methodology, Writing – original draft, Writing – review & editing. JL: Investigation, Methodology, Writing – original draft, Writing – review & editing. HX: Investigation, Methodology, Writing – original draft, Writing – review & editing. RZ: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. FZ: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. ZG: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by Medical Science Research Project of Hebei(Grant no. 20241557), and S&T Program of Hebei (Grant no. 21377759D).
Acknowledgments
The authors would like to thank the patients who consented to participate and release their personal data for the scientific purposes of this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Zhou Y, Song K, Chen Y, Zhang Y, Dai M, Wu D, et al. Burden of six major types of digestive system cancers globally and in China. Chin Med J (Engl). (2024) 137:1957–64. doi: 10.1097/CM9.0000000000003225
3. De B, Rhome R, Jairam V, Özbek U, Holcombe RF, Buckstein M, et al. Gastric adenocarcinoma in young adult patients: patterns of care and survival in the United States. Gastric Cancer. (2018) 21:889–99. doi: 10.1007/s10120-018-0826-x
4. Tekesin K, Emin Gunes M, Tural D, Akar E, Zirtiloglu A, Karaca M, et al. Clinicopathological characteristics, prognosis and survival outcome of gastric cancer in young patients: A large cohort retrospective study. J Buon. (2019) 24:672–8.
5. Rona KA, Schwameis K, Zehetner J, Samakar K, Green K, Samaan J, et al. Gastric cancer in the young: An advanced disease with poor prognostic features. J Surg Oncol. (2017) 115:371–5. doi: 10.1002/jso.24533
6. Huang Q, Zheng X, Jiao Y, Lei Y, Li X, Bi F, et al. A distinct clinicopathological feature and prognosis of young gastric cancer patients aged ≤ 45 years old. Front Oncol. (2021) 11:674224. doi: 10.3389/fonc.2021.674224
7. Wong MCS, Huang J, Chan PSF, Choi P, Lao XQ, Chan SM, et al. Global incidence and mortality of gastric cancer, 1980-2018. JAMA Netw Open. (2021) 4:e2118457. doi: 10.1001/jamanetworkopen.2021.18457
8. Wang Z, El-Serag HB, Thrift AP. Increasing incidence of advanced non-cardia gastric cancers among younger hispanics in the USA. Dig Dis Sci. (2021) 66:1669–72. doi: 10.1007/s10620-020-06397-x
9. Nishimura S, Yashiro M, Sera T, Yamamoto Y, Kushitani Y, Sugimoto A, et al. Feasibility of identifying patients at high risk of hereditary gastric cancer based on clinicopathological variables. Anticancer Res. (2019) 39:5057–64. doi: 10.21873/anticanres.13698
10. Machlowska J, Kapusta P, Baj J, Morsink FHM, Wołkow P, Maciejewski R, et al. High-throughput sequencing of gastric cancer patients: unravelling genetic predispositions towards an early-onset subtype. Cancers (Basel). (2020) 12:1981. doi: 10.3390/cancers12071981
11. Cheng L, Chen S, Wu W, Kuo ZC, Wei Z, Meng S, et al. Gastric cancer in young patients: a separate entity with aggressive features and poor prognosis. J Cancer Res Clin Oncol. (2020) 146:2937–47. doi: 10.1007/s00432-020-03268-w
12. Krishnamoorthi N, Charles L, Nisha Y, Dubashi B, Ganesan P, Kayal S, et al. Aggressive histology and extensive metastasis characteristic of very young gastric cancer (Less than 30 years): A retrospective clinical audit. South Asian J Cancer. (2023) 12:326–33. doi: 10.1055/s-0043-1761284
13. Janjigian YY, Ajani JA, Moehler M, Shen L, Garrido M, Gallardo C, et al. First-line nivolumab plus chemotherapy for advanced gastric, gastroesophageal junction, and esophageal adenocarcinoma: 3-year follow-up of the phase III checkMate 649 trial. J Clin Oncol. (2024) 42:2012–20. doi: 10.1200/JCO.23.01601
14. Rha SY, Oh DY, Yañez P, Bai Y, Ryu MH, Lee J, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. (2023) 24:1181–95. doi: 10.1016/S1470-2045(23)00515-6
15. Xu J, Jiang H, Pan Y, Gu K, Cang S, Han L, et al. Sintilimab plus chemotherapy for unresectable gastric or gastroesophageal junction cancer: the ORIENT- 16 randomized clinical trial. JAMA. (2023) 330:2064–74. doi: 10.1001/jama.2023.19918
16. Janjigian YY, Kawazoe A, Bai Y, Xu J, Lonardi S, Metges JP, et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE- 811 randomised placebo-controlled trial. Lancet. (2023) 402:2197–208. doi: 10.1016/S0140-6736(23)02033-0
17. Castro A, Pyke RM, Zhang X, Thompson WK, Day CP, Alexandrov LB, et al. Strength of immune selection in tumors varies with sex and age. Nat Commun. (2020) 11:4128. doi: 10.1038/s41467-020-17981-0
18. Kugel CH 3rd, Douglass SM, Webster MR, Kaur A, Liu Q, Yin X, et al. Age correlates with response to anti-PD1, reflecting age-related differences in intratumoral effector and regulatory T-cell populations. Clin Cancer Res. (2018) 24:5347–56. doi: 10.1158/1078-0432
19. Wang Y, Zhang S, Zhang F, Wang L, Wu C, Zhang X, et al. Young patients show poor efficacy for immune checkpoint inhibitor combined therapy in metastatic gastrointestinal cancers. Front Oncol. (2023) 13:1155019. doi: 10.3389/fonc.2023.1155019
20. Ławniczak M, Gawin A, Jaroszewicz-Heigelmann H, Rogoza-Mateja W, Białek A, Kulig J, et al. Analysis of clinicopathologic characteristics of gastric cancer in patients ≤40 and ≥40 years of age. Scand J Gastroenterol. (2020) 55:62–6. doi: 10.1080/00365521.2019.1699597
21. Park KB, Jun KH. Clinicopathological features and prognosis of gastric cancer in young patients. J Minim Invasive Surg. (2020) 23:161–2. doi: 10.7602/jmis.2020.23.4.161
22. Sandeep B, Huang X, Li Y, Mao L, Gao K, Xiao Z. Gastric carcinoma in young patients and its clinicopathological characteristics and prognosis. Gastroenterol Res Pract. (2020) 2020:7378215. doi: 10.1155/2020/7378215
23. Kim JH, Boo YJ, Park JM, Park SS, Kim SJ, Kim CS, et al. Incidence and long-term outcome of young patients with gastric carcinoma according to sex: does hormonal status affect prognosis? Arch Surg. (2008) 143:1062–7. doi: 10.1001/archsurg.143.11.1062
24. Zhong Y, He K, Shi L, Chen L, Zhou B, Ma R, et al. Down-regulation of estrogen-related receptor alpha (ERRα) inhibits gastric cancer cell migration and invasion in vitro and in vivo. Aging (Albany NY). (2021) 13:5845–57. doi: 10.18632/aging.202508
25. Harrison JD, Watson S, Morris DL. The effect of sex hormones and tamoxifen on the growth of human gastric and colorectal cancer cell lines. Cancer. (1989) 63:2148–51. doi: 10.1002/1097-0142(19890601)63:11<2148::aid-cncr2820631113>3.0.co;2-c
26. Schurz H, Salie M, Tromp G, Hoal EG, Kinnear CJ, Möller M. The X chromosome and sex-specific effects in infectious disease susceptibility. Hum Genomics. (2019) 13:2. doi: 10.1186/s40246-018-0185-z
27. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. (2016) 16:626–38. doi: 10.1038/nri.2016.90
28. Milette S, Hashimoto M, Perrino S, Qi S, Chen M, Ham B, et al. Sexual dimorphism and the role of estrogen in the immune microenvironment of liver metastases. Nat Commun. (2019) 10:5745. doi: 10.1038/s41467-019-13571-x
29. Li HT, Luo S, Trasande L, Hellerstein S, Kang C, Li JX, et al. Geographic variations and temporal trends in cesarean delivery rates in China, 2008-2014. JAMA. (2017) 317:69–76. doi: 10.1001/jama.2016.18663
30. Yaghoobi M, Rakhshani N, Sadr F, Bijarchi R, Joshaghani Y, Mohammadkhani A, et al. Hereditary risk factors for the development of gastric cancer in younger patients. BMC Gastroenterol. (2004) 4:28. doi: 10.1186/1471-230X-4-28
31. Pocurull A, Herrera-Pariente C, Carballal S, Llach J, Sánchez A, Carot L, et al. Clinical, molecular and genetic characteristics of early onset gastric cancer: analysis of a large multicenter study. Cancers (Basel). (2021) 13:3132. doi: 10.3390/cancers13133132
32. Gullo I, Devezas V, Baptista M, Garrido L, Castedo S, Morais R, et al. Phenotypic heterogeneity of hereditary diffuse gastric cancer: report of a family with early-onset disease. Gastrointest Endosc. (2018) 87:1566–75. doi: 10.1016/j.gie.2018.02.008
33. Setia N, Wang CX, Lager A, Maron S, Shroff S, Arndt N, et al. Morphologic and molecular analysis of early-onset gastric cancer. Cancer. (2021) 127:103–14. doi: 10.1002/cncr.33213
34. Mun DG, Bhin J, Kim S, Kim H, Jung JH, Jung Y, et al. Proteogenomic characterization of human early-onset gastric cancer. Cancer Cell. (2019) 35:111–24.e10. doi: 10.1016/j.ccell.2018.12.003
35. Moelans CB, Milne AN, Morsink FH, Offerhaus GJ, van Diest PJ. Low frequency of HER2 amplification and overexpression in early onset gastric cancer. Cell Oncol (Dordr). (2011) 34:89–95. doi: 10.1007/s13402-011-0021-0
36. Kim WH, Gomez-Izquierdo L, Vilardell F, Chu KM, Soucy G, Dos Santos LV, et al. HER2 status in gastric and gastroesophageal junction cancer: results of the large, multinational HER-EAGLE study. Appl Immunohistochem Mol Morphol. (2018) 26:239–45. doi: 10.1097/PAI.0000000000000423
37. Triulzi T, Forte L, Regondi V, Di Modica M, Ghirelli C, Carcangiu ML, et al. HER2 signaling regulates the tumor immune microenvironment and trastuzumab efficacy. Oncoimmunology. (2018) 8:e1512942. doi: 10.1080/2162402X.2018.1512942
38. Li J. Gastric cancer in young adults: A different clinical entity from carcinogenesis to prognosis. Gastroenterol Res Pract. (2020) 2020:9512707. doi: 10.1155/2020/9512707
39. Huang SC, Ng KF, Yeh TS, Cheng CT, Lin JS, Liu YJ, et al. Subtraction of Epstein-Barr virus and microsatellite instability genotypes from the Lauren histotypes: Combined molecular and histologic subtyping with clinicopathological and prognostic significance validated in a cohort of 1,248 cases. Int J Cancer. (2019) 145:3218–30. doi: 10.1002/ijc.32215
40. Rha SY, Ku GY, Kim HS, Chung HC, Amlashi FG, Maru DM, et al. PD-L1 expression and overall survival in Asian and western patients with gastric cancer. Future Oncol. (2022) 18:2623–34. doi: 10.2217/fon-2022-0103
41. Lei M, Siemers NO, Pandya D, Chang H, Sanchez T, Harbison C, et al. Analyses of PD-L1 and inflammatory gene expression association with efficacy of nivolumab ± Ipilimumab in gastric cancer/gastroesophageal junction cancer. Clin Cancer Res. (2021) 27:3926–35. doi: 10.1158/1078-0432
42. Li P, Che S, Qi Y, Luo N, Lin Q, Zhu X, et al. Age-dependent genomic characteristics and their impact on immunotherapy in lung adenocarcinoma. J Cancer Res Clin Oncol. (2023) 149:2997–3007. doi: 10.1007/s00432-022-04195-8
43. Guan R, Lyu Q, Lin A, Liang J, Ding W, Cao M, et al. Influence of different age cutoff points on the prediction of prognosis of cancer patients receiving ICIs and potential mechanistic exploration. Front Oncol. (2021) 11:670927. doi: 10.3389/fonc.2021.670927
44. Proietti I, Skroza N, Michelini S, Mambrin A, Balduzzi V, Bernardini N, et al. BRAF inhibitors: molecular targeting and immunomodulatory actions. Cancers (Basel). (2020) 12:1823. doi: 10.3390/cancers12071823
45. Chen H, Chong W, Wu Q, Yao Y, Mao M, Wang X. Association of LRP1B mutation with tumor mutation burden and outcomes in melanoma and non-small cell lung cancer patients treated with immune check-point blockades. Front Immunol. (2019) 10:1113. doi: 10.3389/fimmu.2019.01113
46. Hagi T, Kurokawa Y, Kawabata R, Omori T, Matsuyama J, Fujitani K, et al. Multicentre biomarker cohort study on the efficacy of nivolumab treatment for gastric cancer. Br J Cancer. (2020) 123:965–72. doi: 10.1038/s41416-020-0975-7
47. Zhang P, Feng J, Rui M, Xie J, Zhang L, Zhang Z. Integrating machine learning and single-cell analysis to uncover lung adenocarcinoma progression and prognostic biomarkers. J Cell Mol Med. (2024) 28:e18516. doi: 10.1111/jcmm.18516
48. Zhang P, Yang Z, Liu Z, Zhang G, Zhang L, Zhang Z, et al. Deciphering lung adenocarcinoma evolution: Integrative single-cell genomics identifies the prognostic lung progression associated signature. J Cell Mol Med. (2024) 28:e18408. doi: 10.1111/jcmm.18408
49. Zhang L, Cui Y, Mei J, Zhang Z, Zhang P. Exploring cellular diversity in lung adenocarcinoma epithelium: Advancing prognostic methods and immunotherapeutic strategies. Cell Prolif. (2024) 57:e13703. doi: 10.1111/cpr.13703
50. Zhang L, Cui Y, Zhou G, Zhang Z, Zhang P. Leveraging mitochondrial-programmed cell death dynamics to enhance prognostic accuracy and immunotherapy efficacy in lung adenocarcinoma. J Immunother Cancer. (2024) 12:e010008. doi: 10.1136/jitc-2024-010008
51. Ishigami H, Fujiwara Y, Fukushima R, Nashimoto A, Yabusaki H, Imano M, et al. Phase III trial comparing intraperitoneal and intravenous paclitaxel plus S-1 versus cisplatin plus S-1 in patients with gastric cancer with peritoneal metastasis: PHOENIX-GC trial. J Clin Oncol. (2018) 36:1922–9. doi: 10.1200/JCO.2018.77.8613
Keywords: young gastric cancer, clinicopathological features, PD-1 inhibitors, triple regimens, prognostic analysis
Citation: Wang Y, Li Y, Liu Z, Liu J, Xu H, Zhang R, Zhang F and Guo Z (2025) Immune checkpoint inhibitors plus chemotherapy in the first-line treatment of young unresectable gastric cancer patients: a multicentre real-world study. Front. Oncol. 15:1476402. doi: 10.3389/fonc.2025.1476402
Received: 05 August 2024; Accepted: 26 March 2025;
Published: 11 April 2025.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Xiaowei Zhang, Fudan University Shanghai Cancer Centre, ChinaPengpeng Zhang, Nanjing Medical University, China
Copyright © 2025 Wang, Li, Liu, Liu, Xu, Zhang, Zhang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhanjun Guo, empndW81ODg2QGFsaXl1bi5jb20=
 Yingnan Wang
Yingnan Wang Yan Li2
Yan Li2 Zheng Liu
Zheng Liu Fengbin Zhang
Fengbin Zhang Zhanjun Guo
Zhanjun Guo