- 1Neuro-Oncology Unit, Meyer Children’s Hospital IRCCS, Florence, Italy
- 2Pathology Unit, Meyer Children’s Hospital IRCCS, Florence, Italy
- 3Radiology Unit, Meyer Children’s Hospital IRCCS, Florence, Italy
- 4Neurosurgery Unit, Meyer Children’s Hospital IRCCS, Florence, Italy
- 5Pediatric Hematology/Oncology and HSCT Department, Meyer Children’s Hospital IRCCS, Florence, Italy
Introduction: In neuro-oncological pediatric patients under 3 years of age, chemotherapy intensified to high doses (high-dose chemotherapy, HDC) represents the cornerstone to avoid the potential toxicity of radiotherapy. Combination treatment with gemcitabine–oxaliplatin (GemOx) was administered for infant- type cerebral tumors as a bridge toward autologous hematopoietic transplantation to achieve clinical and neuroradiological permissiveness to HDC and to raise the possibility of second-look neurosurgery.
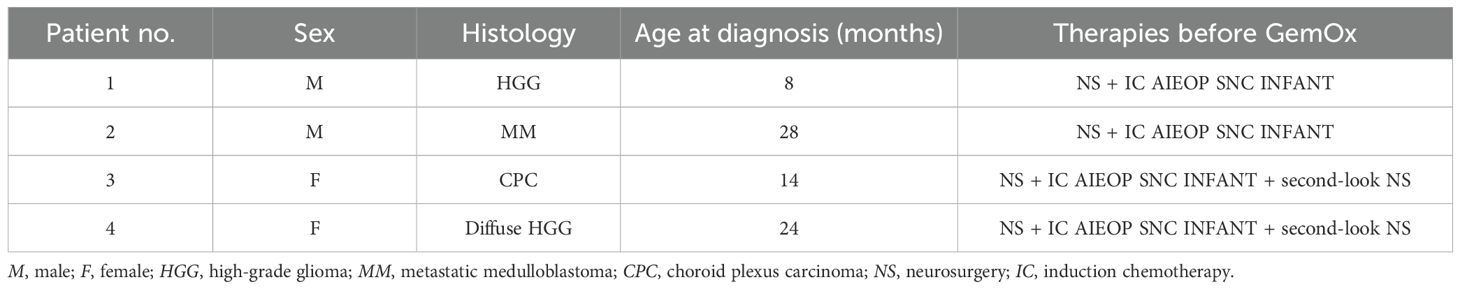
Methods: From May 2017 to May 2023, at Meyer Children’s Hospital IRCSS in Florence (Italy), four patients, with a median age of 19 months (with two high- grade gliomas, a metastatic medulloblastoma, and a choroid plexus carcinoma CNS WHO grade 3), were subjected to partial neurosurgical removal and induction therapy delivered according to the Italian program for malignant cerebral tumors under 3 years. To delay HDC, either for disease reassessment or for temporary unfitness, GemOx cycles were administered. Gemcitabine 1,000 mg/m2 and oxaliplatin 100 mg/m2 were given on day 1 every 21–28 days for one to six cycles.
Results: The treatment was well tolerated overall, except for severe platelet hematological toxicity in a patient, which required dose reduction to 75%. After GemOx, one patient was also subjected to further neurosurgery. Bridge therapy made it possible to submit patients to HDC in safety, in permissive clinical conditions, and after assessment of disease stability.
Conclusion: In infant-type cerebral tumors eligible for HDC, GemOx could be a possible strategy in the case of post-induction residual disease to exclude uncertain evolution or when waiting for clinical suitability for second surgery and intensified treatment. The therapy was overall safe and well tolerated. This approach resulted incisive in the therapeutic or palliative choice for extremely young patients with aggressive brain tumors.
1 Introduction
Gemcitabine (Gem) and oxaliplatin (Ox) are both active drugs for a large variety of solid tumors, with no overlapping toxicity (1). Gemcitabine (2′,2′-difluoro-2′-deoxycytidine) is a synthetic deoxycytidine that exhibits its cytotoxic effects primarily through the inhibition of DNA synthesis (2). It remains a cornerstone in locally advanced or metastatic pancreatic cancer (2). Oxaliplatin, a platinum-based chemotherapeutic drug, has been shown to play a definite role in the management of colorectal cancer, representing a new standard of treatment in the adjuvant setting and for metastatic diseases (3). Oxaliplatin is a diaminocyclohexane (DACH)-platinum compound that is active through the formation of DNA adducts, which are not recognized by mismatch repair complexes (4). The absence of nephrotoxicity, its lack of need for intravenous hydration, and its lower gastrointestinal and hematological toxicity rates are the main advantages of oxaliplatin over cisplatin (1). Numerous clinical studies have evaluated the combination of gemcitabine–oxaliplatin (GemOx) in adult solid tumors, including a phase III trial in pancreatic cancer (4) and a phase II study in advanced biliary tract carcinomas (5).
The pharmacokinetics of GemOx do not differ significantly in children compared with those in adults (4). In 2011, Geoerger et al. performed a multicenter, non-randomized phase II study to assess the objective response rates after four cycles of gemcitabine in combination with oxaliplatin in children and adolescents with relapsed or refractory solid tumors, inclusive of medulloblastoma and other central nervous system (CNS) tumors (4). The GemOx combination administered on a biweekly schedule showed an acceptable safety profile, but limited activity, in this pediatric cohort (4). A further improved tumor control was observed with prolonged treatment, particularly in a case of medulloblastoma and a papillary tumor of the pineal region (4).
Moreover, Bender et al., in a retrospective, single-center study, reported on a single-institution experience combining anti-CD20 therapy with GemOx for mature B-cell non-Hodgkin’s lymphoma (NHL) in children and adolescents who were unfit for intensive chemotherapy due to significant comorbidities (6). Indeed, for patients who cannot tolerate intensive regimens due to underlying comorbidities, the optimal treatment strategy remains unknown (6).
In children younger than 3 years with brain tumors, the main therapeutic approaches are surgery and chemotherapy to delay radiotherapy: indeed, the exposure of immature CNS to radiotherapy can induce early and severe cognitive deficits (7). In our institution, the preferred chemotherapy approach is delivered according to the Italian program for malignant CNS tumors under 3 years schedule (8). The induction phase consists of four courses and it includes the following drugs: methotrexate, vincristine, etoposide, cyclophosphamide, carboplatin. Peripheral blood stem cells are collected for rescue therapy after the second course. The intensification and consolidation phases include two high-dose chemotherapy (HDC) regimens (7). In 2020, our group evaluated the safety and the effectiveness of high-dose thiotepa and carboplatin/thiotepa followed by stem cell rescue in congenital brain tumors, which showed a high response rate (7).
However, to date, there are limited data on the treatment of infant patients who are clinically not suitable to receive intensified treatments, either temporarily due to reversible comorbidities or due to the need for further disease response assessment. Therefore, in our cohort of these patients, GemOx therapy was administered as a bridge toward autologous hematopoietic transplantation in the event of uncertain response to the induction therapy needing evolution re-evaluation, while waiting for clinical permissiveness for more intensive treatments, or when considering the possibility of a second-look neurosurgery before intensified regimens.
2 Patients and methods
This retrospective, single-center study was approved by the Ethical Committee and was performed in accordance with the principles of the Declaration of Helsinki. A total of four patients (two male and two female children; median age, 19 months; age range, 8–28 months) affected by infant-type brain tumors and who were treated at the Neuro Oncology Unit in Meyer Children’s Hospital IRCCS in Florence, Italy, were included. The included patients presented the following: a high-grade glioma (HGG) in the right insular–temporal site (patient 1), a metastatic medulloblastoma (patient 2), a right intraventricular occipital–parietal choroid plexus carcinoma (patient 3), and a diffuse HGG spreading from the left nuclear thalamus (patient 4). The presenting symptoms were related to the tumor location: gaze deviation to the left and left upper limb seizure in patient 1, gait instability in patient 2, vomiting and drowsiness in patient 3, and right-sided hyposthenia in patient 4. At diagnosis, all patients were subjected to partial neurosurgical removal and induction therapy according to the Italian program for malignant CNS tumors under 3 years schedule. Response was determined with magnetic resonance imaging (MRI) according to the RECIST criteria (9). At post-induction re-evaluation, in patient 1, progressive disease could not be excluded, while the other patients presented stable disease. Two patients (patients 3 and 4) received second-look surgery after induction to achieve better disease control. At the end of the induction treatment, all patients developed reversible comorbidities, such as reduced feeding and asthenia, which are typical of oncological patients, but are more pronounced in such infant pediatric age.
The patients, diagnoses, and treatments before GemOx are summarized in Table 1.
To delay HDC for uncertain response to the induction chemotherapy needing re-evaluation, to consider the possibility of a second-look neurosurgery before intensified regimens, and to allow infant patients to adequately recover from previous treatments in view of intensive therapy, given also the extremely young age, between May 2017 and May 2023, the included patients received GemOx cycles as a bridge therapy to HDC. This combination therapy was chosen based on previous promising data in adult advanced solid tumors (1) and in children with relapsed or refractory solid tumors, inclusive of medulloblastoma and other CNS tumors (4), as well as in children affected by mature B-cell NHL who are unfit for intensive chemotherapy (6).
Gemcitabine (as a 100-min infusion, 1,000 mg/m2 or 33.3 mg/kg in the case of weight <10 kg) and oxaliplatin (as a 2-h infusion, 100 mg/m2 or 3.3 mg/kg in the case of weight <10 kg) were administered on day 1 of each cycle, planned every 21–28 days, dependent on blood count recovery (absolute neutrophil count ≥1,000/μl and platelets ≥100,000/μl) and permissive clinical/performance status. Toxicities were graded according to the Common Terminology Criteria for Adverse Events (CTCAE), version 5.0.
3 Results
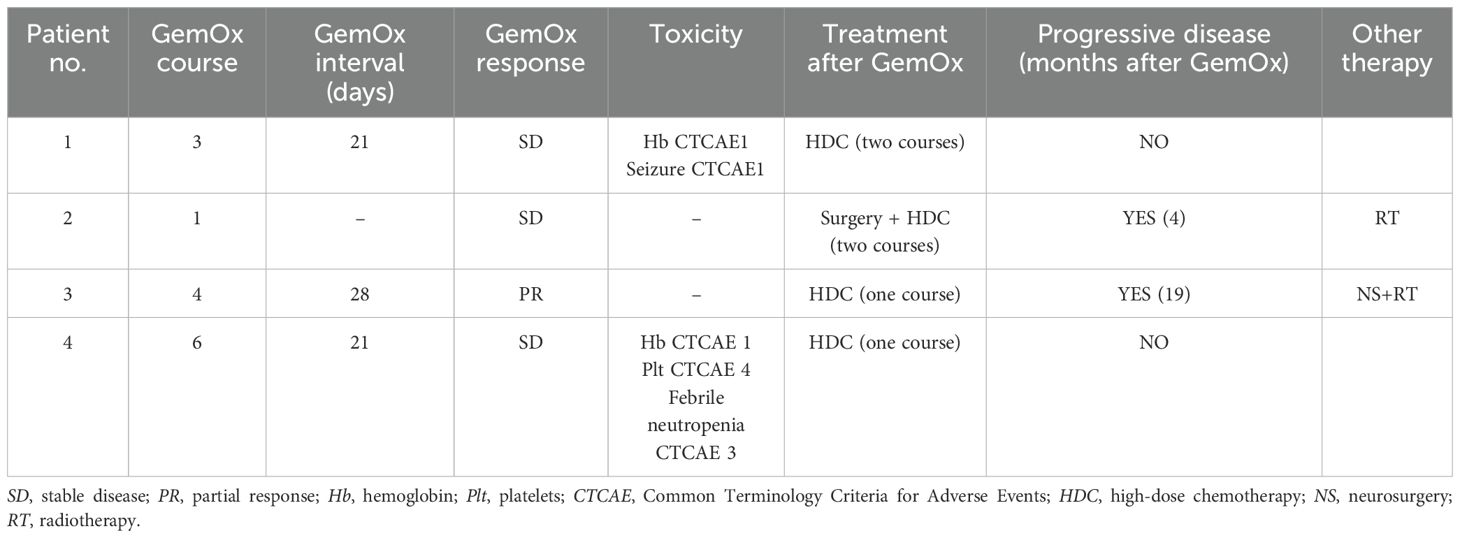
A total of 14 GemOx courses were administered. The median number of GemOx cycles per patient was 3.5 (range, one to six courses). The courses were given in a day hospital regimen. The total number of administered courses was established based on the purpose and the achievement of the desired clinical status and stable disease response.
At MRI disease assessment, maximum every four cycles, all patients showed optimal disease control (three stable disease and one partial response). After the GemOx cycles, all patients presented good clinical conditions, with good nutritional status, and were recovering from reversible comorbidities and the health decline intrinsically due to the induction chemotherapy. Considering the achieved optimal condition, one patient (patient 2) was also subjected to further surgery. All four patients were safely exposed to HDC (one to two courses) with subsequent autologous stem cell transplant, without complications (two HDC courses in patients 1 and 2 and one HDC course in patients 3 and 4). One or two courses of HDC were administered according to the clinical status, age of the patient, histology, and the attainment of pretreatment goals based on primary tumor extension.
At the end of HDC, three patients presented disease stability. However, the disease was progressive in one patient (patient 2); therefore, a radiotherapy course was delivered considering the achievement of permissive age. Patient 3 progressed 19 months after the end of GemOx, requiring further surgery (gross total resection, GTR) and radiotherapy.
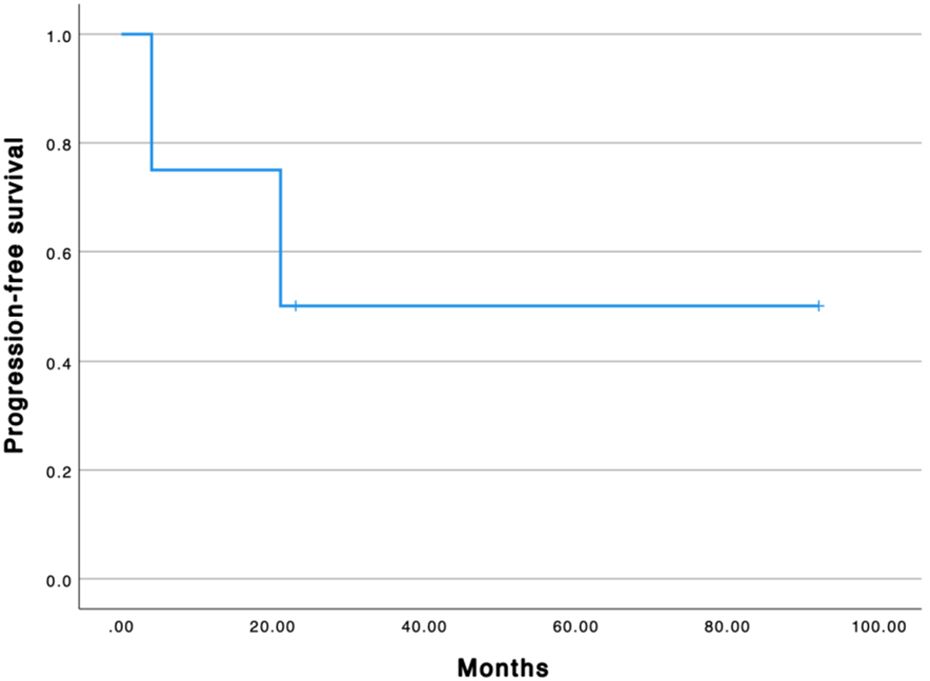
The median progression-free survival from the first GemOx is 22 months (Figure 1). All patients are alive. The median overall survival from the first GemOx is 65 months.
GemOx was well tolerated overall, with the most common toxicities being hematologic (Table 2). Only one patient had grade 4 thrombocytopenia during therapy, which required dose reduction to 75% of the total dose. The same patient presented febrile neutropenia CTCAE 3. No infusion reactions or other acute adverse events were reported. No granulocyte colony-stimulating factor was required among courses.
The GemOx response and toxicity and the post-GemOx treatments are reported in Table 2.
4 Discussion
In this infant malignant brain tumor cohort, the GemOx combination therapy represented a safe and encouraging strategy as a bridge therapy to HDC and autologous transplantation. The bridge time before intensive therapy was extremely useful for the achievement of clinical permissiveness after previous treatments and for better disease status and responsiveness evaluation. To date, there are no alternative strategies for infant patients with brain tumors temporarily not suitable to receive HDC.
The GemOx combination therapy was chosen based on previously reported promising data. It is known that the GemOx combination is active and well tolerated in advanced biliary tract adenocarcinoma (10), representing a standard first-line treatment (11). In this setting, further studies are emerging on its combination with immune checkpoint inhibitors (12) or with anti-programmed cell death ligand 1/vascular endothelial growth factor inhibitors (13).
GemOx has been studied as a first-line therapy for advanced pancreatic cancer (14), showing evidence of activity in a phase II trial (15). In recent years, in this context, it has been combined with encouraging treatments, such as high-intensity focused ultrasound (HIFU) ablation, showing clinical benefits (16).
GemOx has also been considered in other solid cancers. In 2006, Kakolyris et al. evaluated the activity and tolerance of this regimen in pretreated patients with advanced non-small cell lung cancer (NSCLC), showing relative activity and good tolerance (17). In a phase III randomized trial, GemOx was also evaluated against carboplatin–paclitaxel as a first-line therapy in this type of cancer (18). In 2009, Ray-Coquard et al. reported on the activity of GemOx in advanced ovarian carcinoma in early progression resistant to taxane–platinum treatment (19). In 2013, Vici et al. also examined the efficacy and safety of GemOx in recurrent, platinum-resistant ovarian cancer in a multicenter phase II clinical trial, showing encouraging activity and manageable toxicity (20). In 2007, Caruba et al. investigated the efficacy and safety of GemOx in patients with metastatic breast cancer heavily treated with anthracycline and taxane (21), while in 2017 Rizzo et al. reported GemOx as an active regimen in the treatment of luminal and human epidermal growth factor receptor 2-positive metastatic breast cancer (22). GemOx has also been proposed in urogenital tumors (23). A phase II study on GemOx was conducted in cisplatin-”unfit” patients with stage IV transitional cell bladder cancer (23). Pectasides et al. performed a phase II study evaluating GemOx in patients with cisplatin-refractory germ cell tumors, showing an encouraging 14% long-term disease-free status (24). GemOx was also assessed in recurrent nasopharyngeal carcinoma (25). In a phase II trial involving previously untreated patients with advanced hepatocellular carcinoma (HCC), the GemOx regimen appeared to be well tolerated and active (26). A large retrospective multicenter study on advanced HCC confirmed that GemOx is effective with manageable toxicity, permitting potentially curative treatment that was not initially feasible in a significant proportion of patients (27).
In view of the encouraging reported data on the adult population, GemOx has been evaluated in children with relapsed or refractory solid tumors, inclusive of medulloblastoma and other CNS tumors (4). In a biweekly schedule, an acceptable safety profile was reported, but with limited activity (4).
With regard to the hematological field, it has shown high efficacy with a low toxicity profile in elderly patients with relapsed and refractory diffuse large B-cell lymphoma (28), and it has been studied as an additional option in patients with recurrent/refractory primary CNS lymphomas (29). It was also evaluated in combination with rituximab as a viable treatment option for children with NHL who are unfit for intensive chemotherapy (6). Considering other GemOx add-on therapies, in The Lancet, the STARGLO trial by Abramson et al. introduced glofitamab (Glofit), a CD20 × CD3 bispecific antibody, combined with GemOx as a promising alternative for transplant-ineligible patients, meeting its primary endpoint (30). Otham et al. evaluated the safety and efficacy of atezolizumab plus rituximab and GemOx (R-GemOx+Atezo) in relapsed/refractory diffuse large B-cell lymphoma transformed from indolent B-cell lymphomas, demonstrating tolerance and promising preliminary efficacy (31). Moreover, in a multicenter, single-arm, phase 2 trial, Tian et al. assessed the safety and activity of another programmed cell death protein 1 inhibitor plus P-GemOx (pegaspargase, gemcitabine, and oxaliplatin) in the first-line setting for advanced extranodal natural killer/T-cell lymphoma, also showing safeness and activity in this case (32). For solid tumors, Dong et al. explored the performance of GemOx plus immunotherapy and next-generation sequencing (NGS)-guided targeted therapy for local advanced or metastatic biliary tract cancer, showing promising data (33). A different route of administration for GemOx has also been proposed: GemOx hepatic arterial infusion chemotherapy plus systemic chemotherapy in combination with an immune checkpoint inhibitor has been recently administered in patients with large unresectable intrahepatic cholangiocarcinoma (34). The combination of GemOx and immunotherapy was assessed as a first-line therapy for advanced intrahepatic cholangiocarcinoma, opening the landscape for a phase III, multicenter, double-blind, randomized study to validate the findings (35). A few studies have explored the combination of GemOx and bevacizumab. In 2023, Wang et al. investigated the efficacy and safety of atezolizumab, bevacizumab, and GemOx in advanced biliary tract cancer, exploring the potential biomarkers related to the response (13). In 2018, Bréchon et al. assessed and compared the efficacy of GemOx plus bevacizumab and GemOx alone in metastatic carcinoma of the biliary tract, concluding with an increase in progression-free survival and manageable toxicity with the combination therapy (36). Antiangiogenic therapy has been the most investigated strategy for glioblastoma in the last decade (37), and bevacizumab-containing regimens have shown promising activity in relapsed/refractory brain tumors (38). In our opinion, this could be an inspiring GemOx add-on avenue for research in the field of brain cancer.
There are different treatment schedules, and not one is universally established, the most common being gemcitabine over 30 or 100 min on days 1 and 8 and oxaliplatin as a 2-h infusion on day 1 or day 8 of a 21-day cycle or biweekly schedules with gemcitabine on day 1 and oxaliplatin on day 1 or day 2 of a 14-day cycle (4). In our cohort, as GemOx was used as a bridge capable of maintaining disease control rather than a salvage treatment, and taking the frail age of the patients into consideration, we decided to prolong the inter-cycle interval, administering GemOx on day 1 of a 21- to 28-day cycle.
Myelosuppression, asthenia, and nausea/vomiting have been reported as the major toxicities of gemcitabine (1). On the other hand, cumulative peripheral neurotoxicity is the main side effect of oxaliplatin (1). Demols et al. described that grade III/IV non-neurologic toxicities occurred in 36.3% of GemOx-treated adult patients, while grade I, II, and III neuropathy occurred in 51%, 9%, and 12% patients, respectively (14). Raspagliesi et al. reported thrombocytopenia as the dose-limiting toxicity in patients who received GemOx as a second-line treatment for refractory ovarian cancer (39). However, it must be considered that patients treated with GemOx are usually heavily pretreated with chemotherapy; therefore, the add-on toxicity might play a role. In our cohort, one patient experienced grade 4 thrombocytopenia during GemOx therapy, which required dose reduction. No neurotoxicity was reported during the follow-up.
There are a few therapeutic options other than intensive chemotherapy up to HDC that are possible in children under 3 years with cerebral tumors who should not be exposed to radiotherapy, being more vulnerable to hypothalamic–pituitary dysfunction (40) and to the risk of cognitive sequelae that impact long-term quality of life (41). To date, data on HDC unfitness have been limited.
Moreover, neoadjuvant chemotherapy may reduce the tumor vascularity and volume (42), facilitating maximal tumor resection (43): a GTR of pediatric brain tumors is one of the most important predictors of outcome (42). Second-look surgery appears to be feasible, safe, and effective with regard to the volumetric outcome parameters (44). Based on individual cases, GemOx bridge treatment may also permit a more accurate evaluation of the possibility of reintervention to safely achieve maximal tumor resection.
In conclusion, combination treatment with GemOx should be a viable option strategy in partially removed infant brain tumors in the case of temporary unsuitability for intensified treatments or while waiting for evolution verification, especially in radiotherapy delaying programs. In our cohort, the therapy was overall safe and well tolerated, except for the grade 4 hematological toxicity in only one patient. Although this is a small case series, based on our experience, the GemOx approach should result incisive in the therapeutic or palliative choice for extremely young patients with aggressive brain tumors.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Pediatric Ethics Committee of the Tuscany Region. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
BC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Writing – original draft. CF: Supervision, Validation, Visualization, Writing – review & editing. MT: Writing – review & editing. MN: Writing – review & editing, Resources. MG: Writing – review & editing. LGi: Writing – review & editing. BT: Writing – review & editing. CM: Writing – review & editing. AB: Writing – review & editing. LD’I: Writing – review & editing. FG: Writing – review & editing. MS: Writing – review & editing. VT: Writing – review & editing. LGe: Validation, Writing – review & editing. IS: Conceptualization, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported in part by funds from the ‘Current Research Annual Funding’ of the Italian Ministry of Health.
Acknowledgments
This study was conducted in accordance with the principles of the Declaration of Helsinki. The studies involving human participants were reviewed and approved by Pediatric Ethics Committee of the Tuscany Region. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Meriggi F, Zaniboni A. Gemox: A widely useful therapy against solid tumors-review and personal experience. J Chemother. (2010) 22:298–303. doi: 10.1179/joc.2010.22.5.298
2. Beutel AK, Halbrook CJ. Barriers and opportunities for gemcitabine in pancreatic cancer therapy. Am J Physiology-Cell Physiol. (2023) 324:C540–52. doi: 10.1152/ajpcell.00331.2022
3. Comella P. Role of oxaliplatin in the treatment of colorectal cancer. TCRM. (2009) 229:229–38. doi: 10.2147/TCRM.S3583
4. Geoerger B, Chisholm J, Le Deley MC, Gentet JC, Zwaan CM, Dias N, et al. Phase II study of gemcitabine combined with oxaliplatin in relapsed or refractory paediatric solid Malignancies: An innovative therapy for children with Cancer European Consortium Study. Eur J Cancer. (2011) 47:230–8. doi: 10.1016/j.ejca.2010.09.015
5. André T, Reyes-Vidal JM, Fartoux L, Ross P, Leslie M, Rosmorduc O, et al. Gemcitabine and oxaliplatin in advanced biliary tract carcinoma: a phase II study. Br J Cancer. (2008) 99:862–7. doi: 10.1038/sj.bjc.6604628
6. Bender JD, Rubinstein JD, Mizukawa B, Perentesis JP, Pommert L. Use of gemcitabine, oxaliplatin, and anti-CD20 therapy in children and adolescents with non-Hodgkin lymphoma unfit for intensive therapy. Pediatr Blood Cancer. (2023) 70:e30214. doi: 10.1002/pbc.30214
7. Guidi M, Giunti L, Buccoliero AM, Santi M, Spacca B, De Masi S, et al. Use of high-dose chemotherapy in front-line therapy of infants aged less than 12 months treated for aggressive brain tumors. Front Pediatr. (2020) 8:135. doi: 10.3389/fped.2020.00135
8. Gandola L, Massimino M, Cefalo G, Solero C, Spreafico F, Pecori E, et al. Hyperfractionated accelerated radiotherapy in the milan strategy for metastatic medulloblastoma. JCO. (2009) 27:566–71. doi: 10.1200/JCO.2008.18.4176
9. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. JNCI: J Natl Cancer Inst. (2000) 92:205–16. doi: 10.1093/jnci/92.3.205
10. André T, Tournigand C, Rosmorduc O, Provent S, Maindrault-Goebel F, Avenin D, et al. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol. (2004) 15:1339–43. doi: 10.1093/annonc/mdh351
11. Lagenfelt H, Blomstrand H, Elander NO. Real-world evidence on palliative gemcitabine and oxaliplatin (GemOx) combination chemotherapy in advanced biliary tract cancer. Cancers. (2021) 13:3507. doi: 10.3390/cancers13143507
12. Chen X, Wu X, Wu H, Gu Y, Shao Y, Shao Q, et al. Camrelizumab plus gemcitabine and oxaliplatin (GEMOX) in patients with advanced biliary tract cancer: a single-arm, open-label, phase II trial. J Immunother Cancer. (2020) 8:e001240. doi: 10.1136/jitc-2020-001240
13. Wang K, Liu ZH, Yu HM, Cheng YQ, Xiang YJ, Zhong JY, et al. Efficacy and safety of a triple combination of atezolizumab, bevacizumab plus GEMOX for advanced biliary tract cancer: a multicenter, single-arm, retrospective study. Therap Adv Gastroenterol. (2023) 16:175628482311606. doi: 10.1177/17562848231160630
14. Demols A, Peeters M, Polus M, Marechal R, Gay F, Monsaert E, et al. Gemcitabine and oxaliplatin (GEMOX) in gemcitabine refractory advanced pancreatic adenocarcinoma: a phase II study. Br J Cancer. (2006) 94:481–5. doi: 10.1038/sj.bjc.6602966
15. Alberts SR, Townley PM, Goldberg RM, Cha SS, Sargent DJ, Moore DF, et al. Gemcitabine and oxaliplatin for metastatic pancreatic adenocarcinoma: a North Central Cancer Treatment Groupphase II study. Ann Oncol. (2003) 14:580–5. doi: 10.1093/annonc/mdg170
16. Tao Sf, Gu Wh, Gu Jc, Zhu Ml, Wang Q, Zheng Lz. A retrospective case series of high-intensity focused ultrasound (HIFU) in combination with gemcitabine and oxaliplatin (Gemox) on treating elderly middle and advanced pancreatic cancer. OTT. (2019) 12:9735–45. doi: 10.2147/OTT.S220299
17. Kakolyris S, Ziras N, Vamvakas L, Varthalitis J, Papakotoulas P, Syrigos K, et al. Gemcitabine plus oxaliplatin combination (GEMOX regimen) in pretreated patients with advanced non-small cell lung cancer (NSCLC): A multicenter phase II study. Lung Cancer. (2006) 54:347–52. doi: 10.1016/j.lungcan.2006.09.001
18. Weissman CH, Reynolds CH, Neubauer MA, Pritchard S, Kobina S, Asmar L. A phase III randomized trial of gemcitabine–oxaliplatin versus carboplatin–paclitaxel as first-line therapy in patients with advanced non-small cell lung cancer. J Thorac Oncol. (2011) 6:358–64. doi: 10.1097/JTO.0b013e3181ffe8ef
19. for the GINECO group, Ray-Coquard I, Weber B, Cretin J, Haddad-Guichard Z, Lévy E, et al. Gemcitabine-oxaliplatin combination for ovarian cancer resistant to taxane-platinum treatment: a phase II study from the GINECO group. Br J Cancer. (2009) 100:601–7. doi: 10.1038/sj.bjc.6604878
20. Vici P, Sergi D, Pizzuti L, Mariani L, Arena MG, Barba M, et al. Gemcitabine-oxaliplatin (GEMOX) as salvage treatment in pretreated epithelial ovarian cancer patients. J Exp Clin Cancer Res. (2013) 32:49. doi: 10.1186/1756-9966-32-49
21. CAruba T, Cottu PH, Madelaine-Chambrin I, Espié M, Misset JL, Gross-Goupil M. Gemcitabine?Oxaliplatin combination in heavily pretreated metastatic breast cancer: A pilot study on 43 patients. Breast J. (2007) 13:165–71. doi: 10.1111/j.1524-4741.2007.00391.x
22. Rizzi A, Aroldi F, Bertocchi P, Prochilo T, Mutti S, Savelli G, et al. GEMOX: an active regimen for the treatment of luminal and human epidermal growth factor receptor 2-positive metastatic breast cancer. Chemotherapy. (2017) 62:30–3. doi: 10.1159/000445936
23. Eroglu Z, Fruehauf JP. A phase II study of gemcitabine and oxaliplatin in advanced transitional cell carcinoma of the bladder. Cancer Chemother Pharmacol. (2013) 72:263–7. doi: 10.1007/s00280-013-2178-x
24. Pectasides D, Pectasides M, Farmakis D, Aravantinos G, Nikolaou M, Koumpou M, et al. Gemcitabine and oxaliplatin (GEMOX) in patients withcisplatin-refractory germ cell tumors: a phase II study. Ann Oncol. (2004) 15:493–7. doi: 10.1093/annonc/mdh103
25. Ma BBY, Hui EP, Wong SCC, Tung SY, Yuen KK, King A, et al. Multicenter phase II study of gemcitabine and oxaliplatin in advanced nasopharyngeal carcinoma—correlation with excision repair cross-complementing-1 polymorphisms. Ann Oncol. (2009) 20:1854–9. doi: 10.1093/annonc/mdp065
26. Louafi S, Boige V, Ducreux M, Bonyhay L, Mansourbakht T, de Baere T, et al. Gemcitabine plus oxaliplatin (GEMOX) in patients with advanced hepatocellular carcinoma (HCC): Results of a phase II study. Cancer. (2007) 109:1384–90. doi: 10.1002/cncr.v109:7
27. Zaanan A, Williet N, Hebbar M, Dabakuyo TS, Fartoux L, Mansourbakht T, et al. Gemcitabine plus oxaliplatin in advanced hepatocellular carcinoma: A large multicenter AGEO study. J Hepatol. (2013) 58:81–8. doi: 10.1016/j.jhep.2012.09.006
28. Shen QD, Zhu HY, Wang L, Fan L, Liang JH, Cao L, et al. Gemcitabine-oxaliplatin plus rituximab (R-GemOx) as first-line treatment in elderly patients with diffuse large B-cell lymphoma: a single-arm, open-label, phase 2 trial. Lancet Haematol. (2018) 5:e261–9. doi: 10.1016/S2352-3026(18)30054-1
29. Collignon A, Houillier C, Ahle G, Chinot O, Choquet S, Schmitt A, et al. (R)-GEMOX chemotherapy for unfit patients with refractory or recurrent primary central nervous system lymphoma: a LOC study. Ann Hematol. (2019) 98:915–22. doi: 10.1007/s00277-018-3564-6
30. Sureda A, Pavlosky A. Glofit-GemOx: a new treatment paradigm in relapsed or refractory diffuse large B-cell lymphoma? Lancet. (2024) 404:1899–901. doi: 10.1016/S0140-6736(24)02138-X
31. Othman T, Frankel P, Allen P, Popplewell LL, Shouse G, Siddiqi T, et al. Atezolizumab combined with immunogenic salvage chemoimmunotherapy in patients with transformed diffuse large B-cell lymphoma. Haematologica. (2025) 110(1):142–52. doi: 10.3324/haematol.2024.285185.
32. Tian XP, Cai J, Xia Y, Zhang YC, Wang L, Liu PP, et al. First-line sintilimab with pegaspargase, gemcitabine, and oxaliplatin in advanced extranodal natural killer/T cell lymphoma (SPIRIT): a multicentre, single-arm, phase 2 trial. Lancet Haematol. (2024) 11:e336–44. doi: 10.1016/S2352-3026(24)00066-8
33. Dong X, Zhang Z, Zhang Q, Chen L, Cao G, Liu C, et al. Triple therapy in biliary tract cancers: GemOX plus immune checkpoint inhibitor in combination with lenvatinib or NGS-guided targeted therapy. J Cancer Res Clin Oncol. (2023) 149:1917–27. doi: 10.1007/s00432-022-04166-z
34. Ni Jy, Sun Hl, Guo Gf, Zhou X, Wei Jx, Xu Lf. Hepatic arterial infusion of GEMOX plus systemic gemcitabine chemotherapy combined with lenvatinib and PD-1 inhibitor in large unresectable intrahepatic cholangiocarcinoma. Int Immunopharmacol. (2024) 140:112872. doi: 10.1016/j.intimp.2024.112872
35. Shi GM, Huang XY, Wu D, Sun HC, Liang F, Ji Y, et al. Toripalimab combined with lenvatinib and GEMOX is a promising regimen as first-line treatment for advanced intrahepatic cholangiocarcinoma: a single-center, single-arm, phase 2 study. Sig Transduct Target Ther. (2023) 8:106. doi: 10.1038/s41392-023-01317-7
36. Bréchon M, Dior M, Dréanic J, Brieau B, Guillaumot MA, Brezault C, et al. Addition of an antiangiogenic therapy, bevacizumab, to gemcitabine plus oxaliplatin improves survival in advanced biliary tract cancers. Invest New Drugs. (2018) 36:156–62. doi: 10.1007/s10637-017-0492-6
37. Pellerino A, Bruno F, Soffietti R, Rudà R. Antiangiogenic therapy for Malignant brain tumors: does it still matter? Curr Oncol Rep. (2023) 25:777–85. doi: 10.3171/2014.11.PEDS14334
38. Schiavetti A, Varrasso G, Mollace MG, Dominici C, Ferrara E, Papoff P, et al. Bevacizumab-containing regimen in relapsed/progressed brain tumors: a single-institution experience. Childs Nerv Syst. (2019) 35:1007–12. doi: 10.1007/s00381-019-04117-z
39. Raspagliesi F, Zanaboni F, Vecchione F, Hanozet F, Scollo P, Ditto A, et al. Gemcitabine combined with oxaliplatin (GEMOX) as second-line chemotherapy in patients with advanced ovarian cancer refractory or resistant to platinum and taxane. Oncology. (2004) 67:376–81. doi: 10.1159/000082921
40. Lebbink CA, Ringers TP, Schouten-van-Meeteren AYN, van Iersel L, Clement SC, Boot AM, et al. Prevalence and risk factors of hypothalamic–pituitary dysfunction in infant and toddler childhood brain tumor survivors. Eur J Endocrinol. (2021) 185:597–606. doi: 10.1530/EJE-21-0137
41. Al Dahhan NZ, Cox E, Nieman BJ, Mabbott DJ. Cross-translational models of late-onset cognitive sequelae and their treatment in pediatric brain tumor survivors. Neuron. (2022) 110:2215–41. doi: 10.1016/j.neuron.2022.04.009
42. Van Poppel M, Klimo P, Dewire M, Sanford RA, Boop F, Broniscer A, et al. Resection of infantile brain tumors after neoadjuvant chemotherapy: the St. Jude experience. J Neurosurg Pediatr. (2011) 8:251–6. doi: 10.3171/2011.6.PEDS11158
43. Iwama J, Ogiwara H, Kiyotani C, Terashima K, Matsuoka K, Iwafuchi H, et al. Neoadjuvant chemotherapy for brain tumors in infants and young children. PED. (2015) 15:488–92. doi: 10.3171/2014.11.PEDS14334
Keywords: brain tumors, gemcitabine, oxaliplatin, high-dose chemotherapy, autologous stem cell transplantation, pediatric oncology
Citation: Castelli B, Fonte C, Tellini M, Di Nicola M, Guidi M, Giunti L, Tirinnanzi B, Marzano C, Buccoliero AM, D’Incerti L, Giordano F, Scagnet M, Tintori V, Genitori L and Sardi I (2025) Gemcitabine–oxaliplatin as a bridge therapy toward autologous hematopoietic stem cell transplantation in infant-type brain tumors. Front. Oncol. 15:1476411. doi: 10.3389/fonc.2025.1476411
Received: 05 August 2024; Accepted: 07 March 2025;
Published: 13 May 2025.
Edited by:
Anand Singh, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Sandeep Dembla, University of Texas MD Anderson Cancer Center, United StatesAjeena Ramanujan, University of Texas MD Anderson Cancer Center, United States
Copyright © 2025 Castelli, Fonte, Tellini, Di Nicola, Guidi, Giunti, Tirinnanzi, Marzano, Buccoliero, D’Incerti, Giordano, Scagnet, Tintori, Genitori and Sardi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Barbara Castelli, YmFyYmFyYS5jYXN0ZWxsaUBtZXllci5pdA==
 Barbara Castelli
Barbara Castelli Carla Fonte1
Carla Fonte1 Marco Tellini
Marco Tellini Marco Di Nicola
Marco Di Nicola Ludovico D’Incerti
Ludovico D’Incerti Flavio Giordano
Flavio Giordano Mirko Scagnet
Mirko Scagnet Lorenzo Genitori
Lorenzo Genitori Iacopo Sardi
Iacopo Sardi