- 1Oncology Service, University Hospital Geneva, Geneva, Switzerland
- 2Precision Oncology, University Hospital Geneva, Geneva, Switzerland
- 3Pathology Department, University Hospital Geneva, Geneva, Switzerland
Background: The neurofibromin 1 (NF1) protein regulates the downstream RAS/RAF/MEK/ERK pathway and functions as a tumor suppressor. Somatic pathogenic mutations in NF1 are found in approximately 4.7%–10% of NSCLC, with a higher frequency in lung adenocarcinomas, reaching up to 15% in certain cohorts. Trametinib, a MEK inhibitor, has demonstrated activity in tumors with NF1 alteration in preclinical models, and clinical activity in low-grade glioma and plexiform neurofibromas in neurofibromatosis type 1. Trametinib had only limited clinical efficacy in other tumor types with NF1 mutations in the NCI-Match trial. However, the sole NSCLC patient that was evaluable for response in the NCI-Match trial benefited from a deep partial response. More data for the activity of MEK inhibitors in NF1 altered NSCLC are needed.
Cases presentation: We report here a series of four NSCLC patients with NF1 pathogenic mutations treated with trametinib. All patients underwent extensive molecular testing with next-generation sequencing (custom 462-gene panel) and copy number variation analysis and were deemed to have potential NF1-loss-driven tumors after a case discussion in a multidisciplinary molecular tumor board. Two patients exhibited homozygous NF1 LOF alterations, whereas two patients had heterozygous loss-of-function alterations. All patients were treated with oral trametinib 2 mg once daily, after failure of standard therapies. Trametinib was administered for a maximum duration of 9 weeks. The best response observed was a stable disease in one patient. All patients died within 3 months of treatment initiation. No side effects warranted treatment cessation.
Conclusion: In this small case series, NSCLC patients with NF1 alterations did not derive clinical benefit from trametinib. While these data do not support trametinib as a treatment option for NF1-mutated NSCLC, larger studies are required to draw firm conclusions.
Introduction
The neurofibromin 1 (NF1) gene encodes for a small GTPase-activating protein that binds to the RAS family of proteins and functions as a tumor-suppressor gene. NF1 binds to KRAS, HRAS, and NRAS favoring their inactive GDP-bound state, thus governing cellular growth and differentiation (1). Germline NF1 mutation is associated with neurofibromatosis type 1, characterized by the development of neurofibromas. Individuals with this syndrome also face an increased risk of various cancers, including malignant peripheral nerve sheath tumor (MPNST), leukemia, glioma, and breast cancer (2). Acquired somatic mutations in the NF1 gene have been identified in a diverse array of malignancies that lack any connection with neurofibromatosis type 1 (3). Notably approximately 8% of non-small cell lung cancers (NSCLC) carry pathogenic mutations in NF1 (4), although most studies do not report the percentage of patients with complete inactivation of both alleles, which is probably lower.
The enhanced understanding of tumor biology and the identification of drivers in NSCLC have enabled the development of targeted therapies that have revolutionized the management of these patients and improved outcomes. Trametinib, a mitogen-activated protein kinase (MEK) inhibitor targeting MEK1 and MEK2, crucial components of the MAPK/ERK pathway, has emerged as a potential treatment of NF1 altered cancers (5, 6). It showed promising results in patients with unresectable plexiform neurofibromas and low-grade glioma in neurofibromatosis type 1 (5). However, the efficacy of trametinib in other tumors harboring NF1 mutations appears limited. In the NCI-MATCH trial, which included patients with NF1-, GNAQ-, or GNA11- mutant tumors, three patients with NF1-mutated NSCLC were initially enrolled, but only one was evaluable for response, showing a near-complete response. The other two patients were not evaluable due to rapid disease progression or early withdrawal (7). We are not aware of other studies or case reports detailing the response of NF1-altered non-small cell lung cancer to MEK inhibitors. It is therefore unclear whether a clinically meaningful benefit can be derived from MEK inhibitors in this setting.
We present a series of four patients with NF1 altered metastatic lung adenocarcinoma treated with trametinib in our institution, and a comprehensive review of the literature.
Case presentation
Molecular analysis
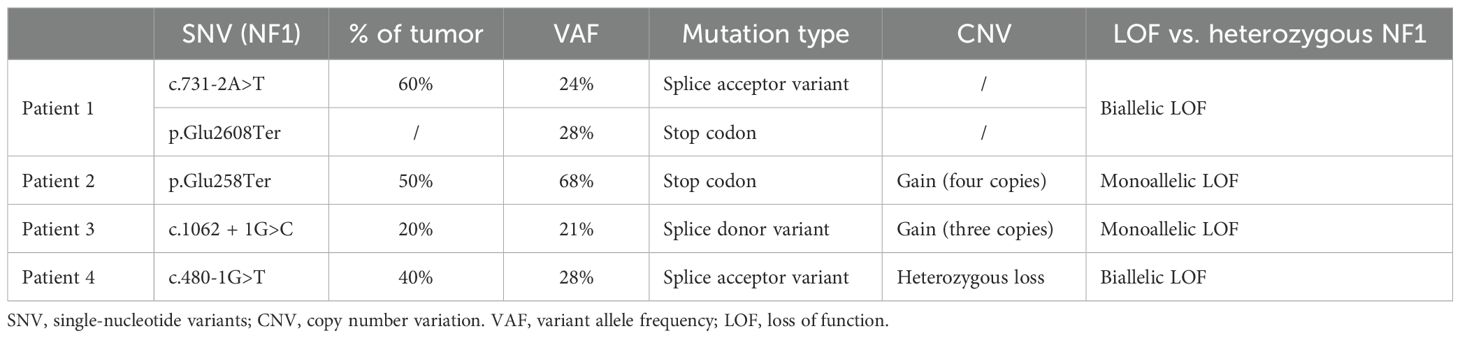
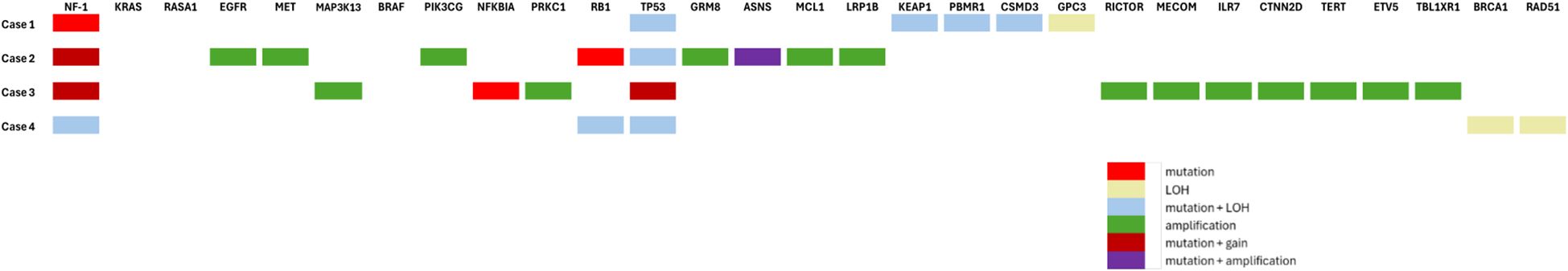
Within our institution, patients with NSCLC progressing after first-line systemic therapy are systematically discussed at the molecular tumor board (TB). The four patients described below underwent the same extensive molecular analysis through next-generation sequencing (custom 462 gene panel, SureSelectHS capture-based Agilent) and copy number variations analysis (Oncoscan Assay kit, cat. 902,695; Thermo Fisher Scientific) as previously described (8). These analyses are routinely performed before discussion at our institution TB. The size of the NGS panel is >1 Mb. The Cancer Gene Census, COSMIC (Catalogue of Somatic Mutations In Cancer) (9), CIViC (Clinical Interpretations of Variants in Cancer) (10), and OncoKb (11) were used for variant interpretation and classification. For NF1 splice variant mutations, pathogenicity was determined based on SpliceAI (12) and Pangolin (13) algorithms. The clinical characteristics and treatment outcomes are summarized in Figure 1. A description of the different genomic alterations found for each patient is provided in Table 1 and Figure 2.
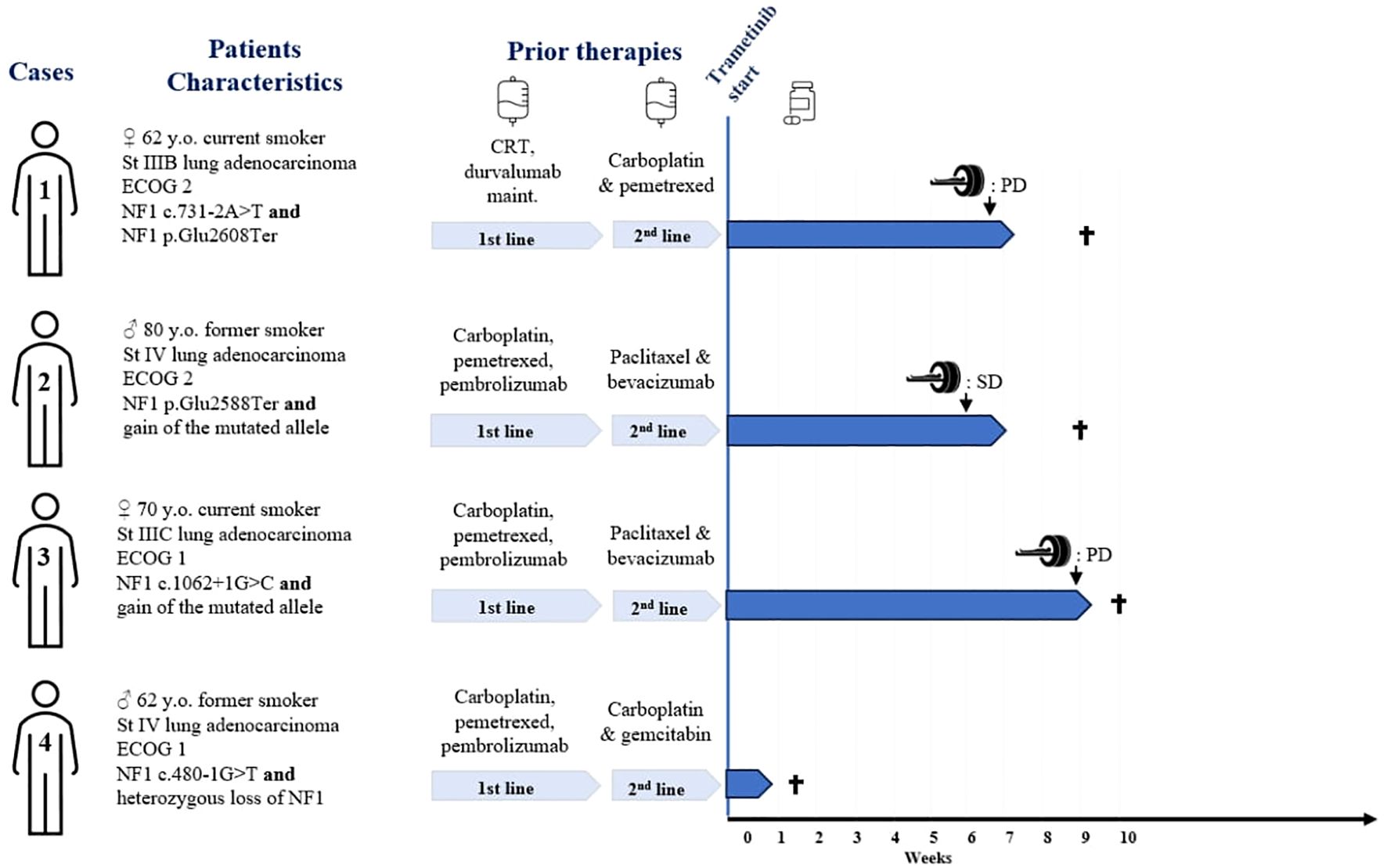
Figure 1. Patients' characteristics, and treatment outcomes. St, stage; y.o., years old; CRT, concurrent chemoradiotherapy; PD, progressive disease per RECIST criteria; SD, stable disease per RECIST criteria. † = death.
Trametinib was proposed for each case after discussion in the molecular tumor board based on preclinical rationale, suggesting that MEK inhibition can be effective in NF1-deficient tumors (14, 15), and by extrapolation from evidence in other NF1-altered cancers (6). The off-label use of trametinib and its therapeutic trial nature were clearly discussed with each patient individually. As this was an off-label use, approval for coverage was obtained from the health insurance on a case-by-case basis.
Case 1
The first case was a 62-year-old woman and active smoker, diagnosed with stage IIIB according to the 8th edition of the TNM, PD-L1 negative lung adenocarcinoma in September 2020. She underwent concomitant chemotherapy (carboplatin and paclitaxel) and radiotherapy, followed by maintenance durvalumab for 1 year. Two months after stopping immunotherapy, the patient presented with peritoneal metastatic relapse, biopsy-proven. First-line chemotherapy was started with carboplatin and pemetrexed allowing a stable disease, followed by pemetrexed maintenance, until a new metastatic progression in November 2022.
Two pathogenic NF1 mutations were identified (Table 1). Following TB proposition, the patient was started on trametinib in March 2023. At the onset of treatment, the patient had an ECOG of 2 and the Charlson score was 7 points. The treatment course was complicated by a grade 3 skin rash, requiring topical corticosteroid treatment and oral doxycycline. The first CT scan was performed at 7 weeks, showing progressive disease with a 70% increase in size of the lesions, per RECIST criteria (Figure 3). Treatment was discontinued, and the patient died rapidly.
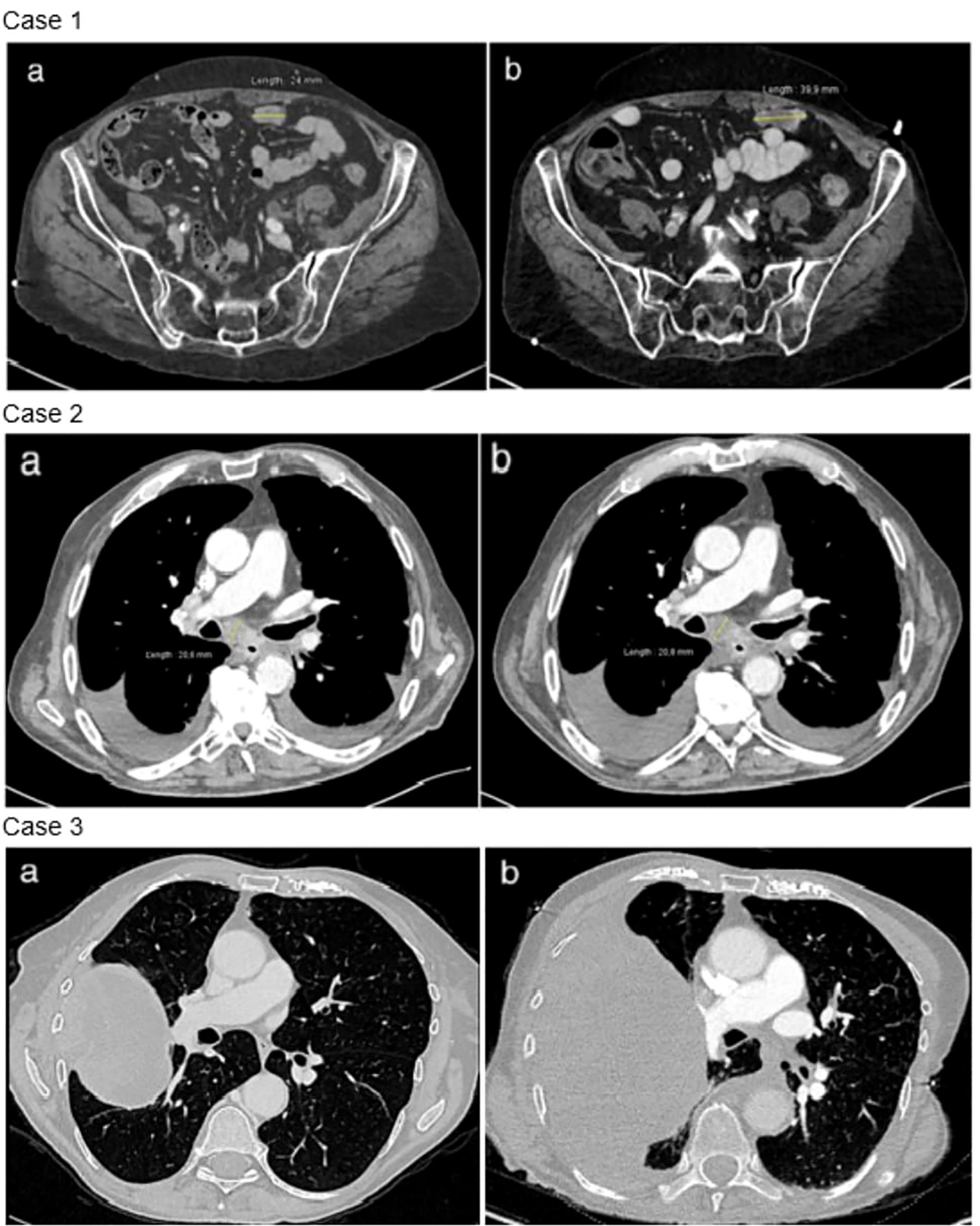
Figure 3. Radiological evolution under trametinib. Case 1: disease progression between baseline thoraco-abdominal computed tomography (a) and after 7 weeks of trametinib (b). Example of a peritoneal metastasis. Case 2: stable disease between baseline thoraco-abdominal computed tomography (a) and after 6 weeks of trametinib (b). Case 3: disease progression between baseline thoraco-abdominal computed tomography (a) and after 8 weeks of trametinib (b).
Case 2
The second case was an 80-year-old man, with a 65 pack-years smoking history. In January 2022, he was diagnosed with a metastatic adenocarcinoma PD-L1 negative. The initial treatment consisted in 4 cycles of carboplatin–pemetrexed–pembrolizumab administered. Due to her stable disease, maintenance with pemetrexed–pembrolizumab was started and then discontinued after one cycle due to grade 3 fatigue treatment-related reasons.
The patient was relatively well until November 2022, when he presented with pleural and pulmonary progression on his CT scan. Second-line treatment with paclitaxel and bevacizumab was started. The patient experienced poor clinical tolerance to chemotherapy with grade 3 fatigue, grade 4 thrombopenia, and grade 3 leucopenia. Further molecular analysis revealed a stop codon mutation in the NF1 gene, as well as an increased copy number (4 copies) of the NF1 gene. No alteration in RASA1 was found. Because of the allelic frequency, the mutated variant was likely located on the gained allele (Table 1). After discussion, and considering the poor patient tolerance to chemotherapy, a PS ECOG 2, and a Charlson score of 15 points, a treatment with trametinib was started in February 2023. The patient coped moderately with trametinib and complained of cough, and developed grade 2 creatinine elevation, grade 2 thrombocytopenia, and grade 1 anemia. All the side effects, except cough, were considered treatment related. None of these side effects required dose suspension or dose reduction.
After 6 weeks of trametinib, a CT scan showed stable disease per RECIST criteria (Figure 3). The clinical course was then complicated by an accidental fall and a hip fracture, followed by a heart failure with acute pulmonary edema. Trametinib treatment was immediately suspended, and the patient passed away 2 weeks later.
Case 3
The third case was a 70-year-old woman, diagnosed with unresectable stage IIIC pulmonary adenocarcinoma of the right upper lobe, with a PDL1 expression of 10%. The patient underwent first-line chemo-immunotherapy, consisting of four cycles of carboplatin, pemetrexed, and pembrolizumab. After an initial partial response, the patient experienced progressive disease during his maintenance with pemetrexed–pembrolizumab.
Second-line treatment with paclitaxel and bevacizumab was administered for 3 cycles until January 2022. Molecular analysis revealed a mutation in a splice donor consensus sequence of the NF1 gene with a gain of the mutated allele, based on the allele frequency and potentially resulting in partial NF1 inactivation (Table 1). No activating co-mutation of the RAS/MAPK pathway was identified, notably no RASA1 alteration. Subsequently, treatment with 2 mg of trametinib was initiated in February 2023. The patient had a ECOG 2 and a Charlson comorbidity index of 9 points. She experienced a grade 1 cutaneous rash and diarrhea. After 8 weeks of treatment, the CT scan showed thoracic and CNS progression (Figure 3). Trametinib was discontinued and, due to a rapid deterioration, the patient was started on best supportive care and died in April 2023.
Case 4
The fourth case was a 62-year-old-man diagnosed with metastatic lung adenocarcinoma, with a PDL1 expression of 10%. The patient received first-line treatment with carboplatin, pemetrexed, and pembrolizumab with a partial response. After progression 1 year later, the patient had second-line treatment with carboplatin and gemcitabine for 3 months before experiencing further metastatic progression. Molecular analysis revealed a pathogenic mutation in the NF1 gene with a loss of the other allele (Table 1). No co-mutation with RASA1 was identified. The patient had an ECOG 2 and a Charlson comorbidity index of 7 points. Trametinib was initiated, but the patient experienced a very rapid deterioration of his general condition, resulting in death 1 week later. Clinical worsening occurred as a result of disease progression and was not attributed to trametinib toxicity.
Discussion
NF1 mutations have been described in both lung adenocarcinomas and squamous cell carcinomas, at rates of 8.3% and 12%, respectively (4), with a lower prevalence in Asian populations (9, 10). NF1 is a large gene consisting of 60 exons (>280 kb, 60 exons), which can harbor a wide diversity of alterations—including nonsense, missense, frameshift, splice-site variants, indels, and large deletions—rendering comprehensive analysis technically challenging. This likely contributed to the relatively limited studies of NF1-mutant cancers compared with other oncogenic drivers. It was observed that most non-small cell lung cancer cases with NF1 mutation do not exhibit co-mutations (16, 17). In the large genomic cohort analyzed by Bowman et al., KRAS mutations were the most frequent co-alteration (16.5%), followed by EGFR (6.8%) and BRAF (3.9%) alterations in white populations. These KRAS mutations were predominantly located in positions G12 and G13 (4). Similar patterns of co-mutations and smoking association were also described by Tlemsani et al. (18) In a previous observational study, most patients with NF1 mutations were current or former smokers (88%). In the Asian population, a significantly higher number of co-mutations of NF1 with known oncogenic mutations are described, predominantly involving EGFR mutations, of which we know that their prevalence in this population is higher (3). Whether NF1 mutations carry a prognostic role in NSCLC remains unclear and appears to be context dependent.
Prognosis of patients with tumors carrying a NF1-inactivating mutation appear comparable with those with KRAS mutations in codon 12 or 13 and less favorable than patients with mutated EGFR (4). Interestingly, in the Chinese population, an NF1 mutation appears to be associated with a better prognosis in the case of the EGFR mutant/TP53 wild type compared with those without the NF1 mutation (19). In patients with mutated EGFR, the co-occurrence of a TP53 mutation and an alteration in a tumor-suppressor gene such as NF1 appears to have a worse prognosis (20). In a study by Liu et al., NF1 mutations were associated with improved survival in patients receiving immunotherapy, but with poorer outcomes under targeted therapies for patients with EGFR-mutated or ALK-rearranged NSCLC (21). Other mutations co-occurring with NF1 are worth noting like RASA1, a Ras-GTPase-activating protein (22), as RASA1/NF1 co-mutation appears to be mutually exclusive with other driver mutations and to be more sensitive to MEK inhibition in preclinical models (15).
Literature on trametinib treatment for mutated NF1 tumors is almost non-existent. The NCI-MATCH trial evaluated the efficacy of trametinib in patients with advanced neoplasia carrying an NF1 mutation within one of its sub-protocols (7). Patients eligible for participation had advanced solid tumor, lymphoma, or multiple myeloma that had progressed after at least one line of systemic treatment. Three patients diagnosed with lung cancer were initially enrolled in the study; however, only one patient was evaluable for response, demonstrating an almost complete response with a median progression-free survival of 6.4 months. Notably, no co-mutations were identified in this case, notably no KRAS alteration (4). While both NF1 loss and KRAS activating mutation lead to MAPK pathway activation and sensitivity to MEK inhibition in vitro, the use of MEK inhibitors has failed to demonstrate efficacy for patients with KRAS-mutated non-small cell lung cancer, likely due to the presence of several mechanisms of bypass of the MAPK pathway inhibition, such as increased AKT signaling in KRAS-altered tumors (23). In the National Lung Matrix trial, an umbrella study, the use of selumetinib, a MEK inhibitor, plus docetaxel in 14 patients with NF1 loss showed an objective response rate of 31% with a median PFS of 5.3 months. Eligible patients received previous anticancer treatment or refused standard-of-care first line. Unfortunately, the isolated efficacy of selumetinib is not known in this study. The results may have been driven by the use of docetaxel (24). The trial NCT03232892 aimed to investigate the efficacy of trametinib in patients diagnosed with non-small cell lung cancer and bearing NF1 mutations. Regrettably, the trial was prematurely terminated due to insufficient participant recruitment.
The four cases we described are therefore the largest cases series ever reported on the use of trametinib, a MEK inhibitor, in patients with metastatic lung adenocarcinomas harboring NF1 alterations. All patients had altered performance status (ECOG score 1-2) at treatment initiation and received trametinib as a third-line treatment. Although some patients experienced severe adverse effects such as grade 3 skin rash and hematologic toxicities, these were managed effectively without requiring treatment discontinuation or dose reduction. This suggests that trametinib can be tolerated in this heavily pretreated population, provided careful monitoring is performed, including regular clinical assessments, blood counts, and liver and kidney function tests, with dose adjustments or temporary interruptions considered for grade ≥2 adverse events. However, careful monitoring remains essential. The treatment was moderately tolerated, but no effects required treatment discontinuation. Two out of three patients progressed on treatment according to RECIST criteria quite quickly at the first CT scan. The third patient achieved stable disease but sadly died after an accidental fall quite at the beginning of his treatment. The fourth patient died after only one week of trametinib treatment and could not be formally evaluated. In summary, one of three patients may have derived some radiological benefit from trametinib, although no deep response was seen.
A few factors may have contributed to the lack of response to trametinib in our cases. First and most importantly, two out of the four patients presented only one pathogenic mutation along with a gain of the NF1 gene—probably the mutated allele, suggesting that the other wild-type allele might still be functional. In these cases, the monoallelic alteration of NF1 might not be sufficient to activate the MAPK pathway on its own, therefore contributing to treatment resistance. Second, no concurrent alterations of the MAPK/ERK pathways were found in any patient. While certain mutations appear to confer resistance to MEK inhibitors in this scenario (e.g., KRAS), other alterations, such as loss of RASA1, have been shown to greatly enhance the sensitivity to MEK inhibition in NF1-deficient cell lines, highlighting maybe the importance of considering not only the NF1 mutation but the entire genetic context. In patient 3, whether the MAP3K13 amplification could have promoted a bypass survival mechanism through upregulation of the JNK signaling axis remains uncertain (25). In this perspective, it may be worthwhile in future research to explore combination therapies that address compensatory pathways or resistance mechanisms that limit the effectiveness of MEK inhibition in this setting. Finally, all patients were treated in the third-line setting and had altered performance status and short expected survival.
Conclusions
We report four cases of metastatic lung adenocarcinoma with complete or partial NF1 inactivation treated with single-agent trametinib at the third line. None derived clinically significant benefit. Our observations question the use of trametinib in this population in particular in the presence of only one allele mutated, although the small number of patients, their heterogeneous molecular presentations, and the initiation of treatment at third-line therapy are important limitations. Comprehensive studies including more patients and a thorough analysis of all MAPK genes are necessary to advance our understanding of this subset of patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
FK: Writing – original draft. MB: Writing – original draft. CV: Methodology, Writing – original draft, Writing – review & editing. PT: Validation, Writing – review & editing. AA: Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank the patient and families for their agreement to the publication of this report.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wilson BN, John AM, Handler MZ, and Schwartz RA. Neurofibromatosis type 1: New developments in genetics and treatment. J Am Acad Dermatol. (2021) 84:1667–76. doi: 10.1016/j.jaad.2020.07.105
2. Rasmussen SA and Friedman JM. NF1 gene and neurofibromatosis 1. Am J Epidemiol. (2000) 151:33–40. doi: 10.1093/oxfordjournals.aje.a010118
3. Pan Y, Yuan C, Cheng C, Zhang Y, Ma Y, Zheng D, et al. Frequency and clinical significance of NF1 mutation in lung adenocarcinomas from East Asian patients. Int J Cancer. (2019) 144:290–6. doi: 10.1002/ijc.31871
4. Redig AJ, Capelletti M, Dahlberg SE, Sholl LM, Mach S, Fontes C, et al. Clinical and molecular characteristics of NF1-mutant lung cancer. Clin Cancer Res. (2016) 22:3148–56. doi: 10.1158/1078-0432.CCR-15-2377
5. Perreault S, Larouche V, Tabori U, Hawkin C, Lippé S, Ellezam B, et al. A phase 2 study of trametinib for patients with pediatric glioma or plexiform neurofibroma with refractory tumor and activation of the MAPK/ERK pathway: TRAM-01. BMC Cancer. (2019) 19:1250. doi: 10.1186/s12885-019-6442-2
6. Wang D, Ge L, Guo Z, Li Y, Zhu B, Wang W, et al. Efficacy and safety of trametinib in neurofibromatosis type 1-associated plexiform neurofibroma and low-grade glioma: A systematic review and meta-analysis. Pharmaceuticals. (2022) 15:956. doi: 10.3390/ph15080956
7. Wisinski KB, Flamand Y, Wilson MA, Luke JJ, Tawbi HA, Hong F, et al. Trametinib in patients with NF1-, GNAQ-, or GNA11-mutant tumors: results from the NCI-MATCH ECOG-ACRIN trial (EAY131) subprotocols S1 and S2. JCO Precis Oncol. (2023), e2200421. doi: 10.1200/PO.22.00421
8. Astaras C, De Vito C, Chaskar P, Bornand A, Khanfir K, Sciarra A, et al. The first comprehensive genomic characterization of rectal squamous cell carcinoma. J Gastroenterol. (2023) 58:125–34. doi: 10.1007/s00535-022-01937-w
9. Sondka Z, Bamford S, Cole CG, Ward SA, Dunham I, and Forbes SA. The COSMIC Cancer Gene Census: describing genetic dysfunction across all human cancers. Nat Rev Cancer. (2018) 18:696–705. doi: 10.1038/s41568-018-0060-1
10. Griffith M, Spies NC, Krysiak K, McMichael JF, Coffman AC, Danos AM, et al. CIViC is a community knowledgebase for expert crowdsourcing the clinical interpretation of variants in cancer. Nat Genet. (2017) 49:170–4. doi: 10.1038/ng.3774
11. Chakravarty D, Gao J, Phillips S, Kundra R, Zhang H, Wang J, et al. OncoKB: A precision oncology knowledge base. JCO Precis Oncol. (2017), 1–16. doi: 10.1200/PO.17.00011
12. Jaganathan K, Kyriazopoulou Panagiotopoulou S, McRae JF, Darbandi SF, Knowles D, Li YI, et al. Predicting splicing from primary sequence with deep learning. Cell. (2019) 176:535–548.e24. doi: 10.1016/j.cell.2018.12.015
13. Zeng T and Li YI. Predicting RNA splicing from DNA sequence using Pangolin. Genome Biol. (2022) 23:103. doi: 10.1186/s13059-022-02664-4
14. See WL, Tan I-L, Mukherjee J, Nicolaides T, and Pieper RO. Sensitivity of glioblastomas to clinically available MEK inhibitors is defined by neurofibromin 1 deficiency. Cancer Res. (2012) 72:3350–9. doi: 10.1158/0008-5472.CAN-12-0334
15. Hayashi T, Desmeules P, Smith RS, Drilon A, Somwar R, and Ladanyi M. RASA1 and NF1 are preferentially co-mutated and define A distinct genetic subset of smoking-associated non–small cell lung carcinomas sensitive to MEK inhibition. Clin Cancer Res. (2018) 24:1436–47. doi: 10.1158/1078-0432.CCR-17-2343
16. Bowman L, Tiu R, Smyth EN, Willard MD, Li L, Beyrer J, et al. Clinical characteristics, treatments, and concurrent mutations in non–small cell lung cancer patients with NF1 mutations. Clin Lung Cancer. (2021) 22:32–41.e1. doi: 10.1016/j.cllc.2020.09.011
17. Collisson EA, Campbell JD, Brooks AN, Berger AH, Lee W, Chmielecki J, et al. Comprehensive molecular profiling of lung adenocarcinoma. Nature. (2014) 511:543–50. doi: 10.1038/nature13385
18. Tlemsani C, Pécuchet N, Gruber A, Laurendeau I, Danel C, Riquet M, et al. NF1 mutations identify molecular and clinical subtypes of lung adenocarcinomas. Cancer Med. (2019) 8:4330–7. doi: 10.1002/cam4.2175
19. Tian H, Chen Z, Jie G-L, Wang Z, Yan H, Wu S, et al. Prognostic features and comprehensive genomic analysis of NF1 mutations in EGFR mutant lung cancer patients. Cancer Med. (2023) 12:396–406. doi: 10.1002/cam4.4925
20. Stockhammer P, Grant M, Wurtz A, Foggetti G, Expósito F, Gu J, et al. Co-occurring alterations in multiple tumor suppressor genes are associated with worse outcomes in patients with EGFR-mutant lung cancer. J Thorac Oncol. (2024) 19:240–51. doi: 10.1016/j.jtho.2023.10.001
21. Liu R, Rizzo S, Wang L, Chaudhary N, Maund S, Garmhausen MR, et al. Characterizing mutation-treatment effects using clinico-genomics data of 78,287 patients with 20 types of cancers. Nat Commun. (2024) 15:10884. doi: 10.1038/s41467-024-55251-5
22. Kitajima S and Barbie DA. RASA1/NF1-mutant lung cancer: racing to the clinic? Clin Cancer Res. (2018) 24:1243–5. doi: 10.1158/1078-0432.CCR-17-3597
23. Jänne PA, van den Heuvel MM, Barlesi F, Cobo M, Mazieres J, Crinò L, et al. Selumetinib plus docetaxel compared with docetaxel alone and progression-free survival in patients with KRAS-mutant advanced non–small cell lung cancer: the SELECT-1 randomized clinical trial. JAMA. (2017) 317:1844–53. doi: 10.1001/jama.2017.3438
24. Middleton G, Fletcher P, Popat S, Savage J, Summers Y, Greystoke A, et al. The National Lung Matrix Trial of personalized therapy in lung cancer. Nature. (2020) 583:807–12. doi: 10.1038/s41586-020-2481-8
Keywords: trametinib, NF1 mutation, non-small-cell lung cancer, targeted therapy, case report
Citation: Kim F, Borgeaud M, De Vito C, Tsantoulis P and Addeo A (2025) Case Report: Trametinib in the treatment of patients with metastatic lung adenocarcinoma harboring NF1 mutation: a case series and literature review. Front. Oncol. 15:1481284. doi: 10.3389/fonc.2025.1481284
Received: 15 August 2024; Accepted: 30 September 2025;
Published: 15 October 2025.
Edited by:
Christos Adamopoulos, National and Kapodistrian University of Athens, GreeceReviewed by:
Robert Kortum, Uniformed Services University of the Health Sciences, United StatesJean-Stephane Giraud, INSERM U1016 Institut Cochin, France
Copyright © 2025 Kim, Borgeaud, De Vito, Tsantoulis and Addeo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Floryane Kim, ZmxvcnlhbmUua2ltQGh1Zy5jaA==
†These authors have contributed equally to this work
 Floryane Kim
Floryane Kim Maxime Borgeaud
Maxime Borgeaud Claudio De Vito3
Claudio De Vito3 Alfredo Addeo
Alfredo Addeo