- 1Four Department of Oncology, Jilin Cancer Hospital, Changchun, Jilin, China
- 2Department of Integrated TCM & Western Medicine, Jilin Cancer Hospital, Changchun, Jilin, China
- 3Department of Radiology, Jilin Cancer Hospital, Changchun, Jilin, China
Background: Inflammatory myofibroblastic tumor of the urinary bladder (IMTUB) is a rare tumor with low postoperative recurrence and metastasis. Due to the lack of clinical evidence, the optimal treatment paradigm for patients with IMTUB has not yet been established.
Case presentation: We reported a case of a 55-year-old man who was diagnosed with bladder malignancy after transurethral resection of a bladder tumor, and then tumor metastasis was treated by traditional Chinese medicine. Following further disease progression, he was admitted to our hospital, where the diagnosis was revised to IMTUB with multiple metastases and TPM4–anaplastic lymphoma kinase (ALK) fusion by computed tomography (CT) scan, pathological diagnosis, immunohistochemistry, and genetic testing. The patient subsequently received 225 mg ensartinib once daily. Symptoms improved and achieved partial response (PR) with acceptable toxicities.
Conclusion: Ensartinib may provide a new therapeutic direction with promising efficacy and an acceptable safety profile for IMTUB with ALK fusion. Further clinical investigation is needed to identify its efficacy and safety.
1 Introduction
Inflammatory myofibroblastic tumor (IMT) is a distinctive moderate malignant mesenchymal tumor with a global incidence of 0.04%–0.7% (1). In addition, IMT affects a variety of organs but is highly rare in the urinary bladder, where anaplastic lymphoma kinase (ALK) fusion is present in approximately 50% of cases (2, 3). Surgical resection is the standard treatment strategy of IMT of the urinary bladder (IMTUB), and the prognosis is relatively good (4). Only a few patients have postoperative recurrence and metastasis, and their treatment options are relatively limited (5, 6).
Ensartinib is a novel, potent, and highly selective next-generation ALK inhibitor that exhibits a broader inhibitory profile and demonstrates potent antitumor activity with favorable safety (7, 8). Furthermore, it has been shown to effectively inhibit ALK fusions in certain cancers (8, 9). Currently, there are no reports on treating IMTUB with ensartinib. Here, we reported the treatment results of ensartinib in a patient with multiple metastases and TPM4-ALK fusion of primary IMTUB, symptomatic improvement, and rapid decline in tumor burden with partial response (PR). Our patient was reported to provide new insight and evidence for the diagnosis and treatment of IMTUB.
2 Case presentation
A 55-year-old man initially presented with painless gross hematuria and received transurethral resection of a bladder tumor, subsequently considered as bladder malignancy by pathological examination (the specific record was unknown) at a Japanese hospital. Then, positron emission tomography (PET)–computed tomography (CT) revealed bladder tumor metastasis in our hospital and received Chinese medicine treatment (the specific prescription was unknown) at a local Chinese medicine clinic. However, a CT performed at Jilin People’s Hospital suggested further deterioration.
Upon admission to our department, the patient underwent a thorough evaluation, which included imaging, histopathological analysis, immunohistochemical studies, and next-generation sequencing (NGS). CT combined with magnetic resonance imaging (MRI) revealed space-occupying lesions of the bladder, considered to be a malignant tumor and recurrent, involving the left ureter, left seminal vesicle gland, and the anterior wall of the rectum (Figure 1A); pelvic lymph nodes were slightly larger than normal, and peritoneal thickening with multiple nodules led to the consideration of metastasis (Figure 1A); tiny nodules were present in the left upper lobe of the lung. Cystoscopy combined with histopathological examination revealed inflammatory cell infiltration of the urothelial mucosa and proliferating spindle cells present in the stroma, which was confirmed as a spindle cell tumor (Figure 2A). Immunohistochemistry (IHC) showed strong positive ALK (+) and Vimentin (+) staining, along with WT-1 (+), Ki-67 (approximately 20%+), CK (−), EMA (−), P40 (−), SMA (−), CD34 (+), Bcl-2 (focal weak +), S-100 (−), CD45 (−), PHH3 (+), CDla (−), and Desmin (−) (Figure 2B). NGS revealed three somatic cell variants, in which the representation mutation was TPM4-ALK Exon8:Exon20 fusion, with an abundance of 12.5%. Based on these findings, the patient was diagnosed with IMTUB, pelvic lymph node metastasis, peritoneal metastasis, and pulmonary nodules.
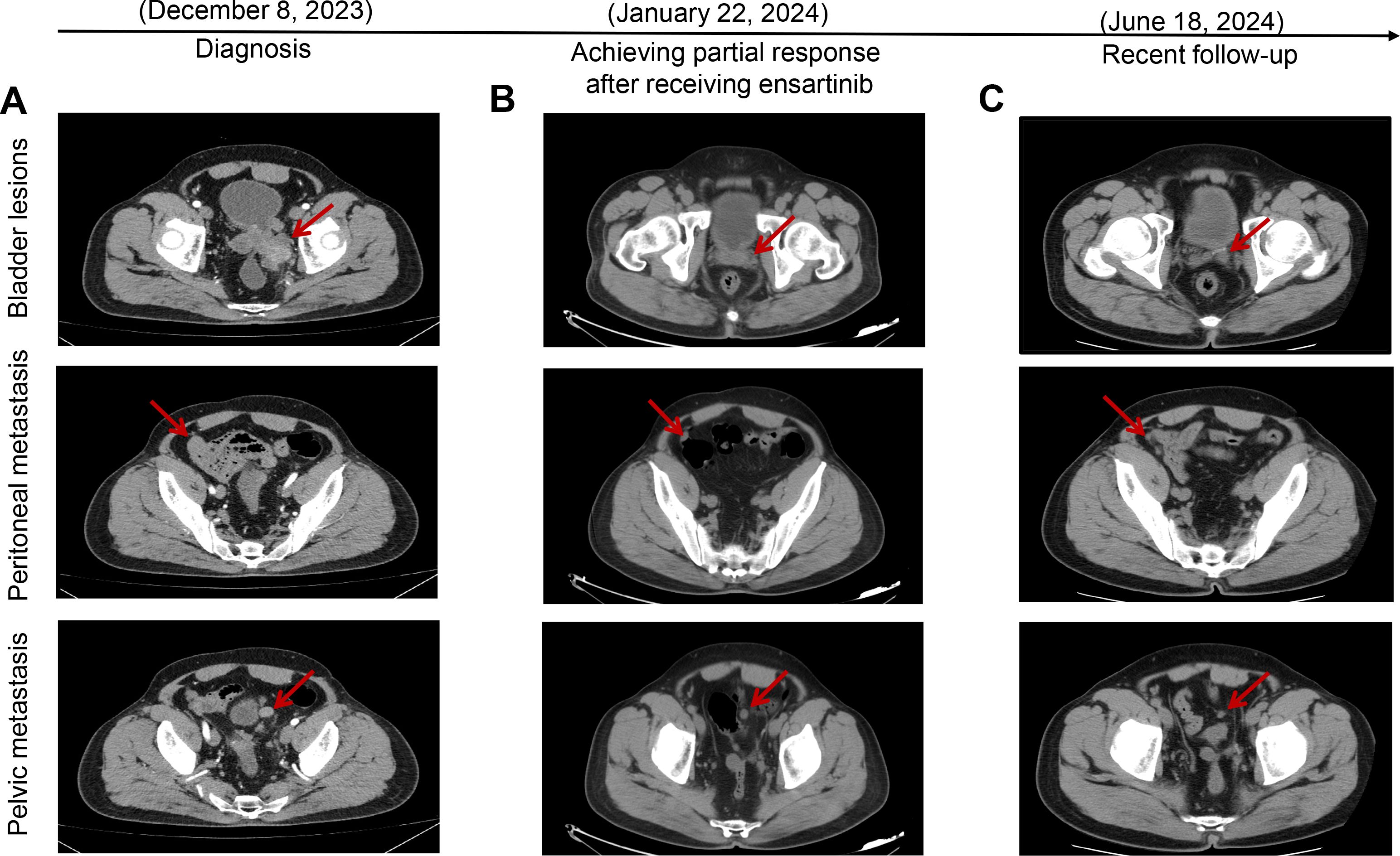
Figure 1. Target lesions of the patient with IMTUB by CT scans. (A) CT scans indicated space-occupying lesions of the bladder, with peritoneal and pelvic metastasis. (B) CT scans revealed tumors had decreased in size, achieving partial response after receiving ensartinib treatment. (C) Recent follow-up CT scans showed that the patient maintained partial response. IMTUB, inflammatory myofibroblastic tumor of the urinary bladder; CT, computed tomography.
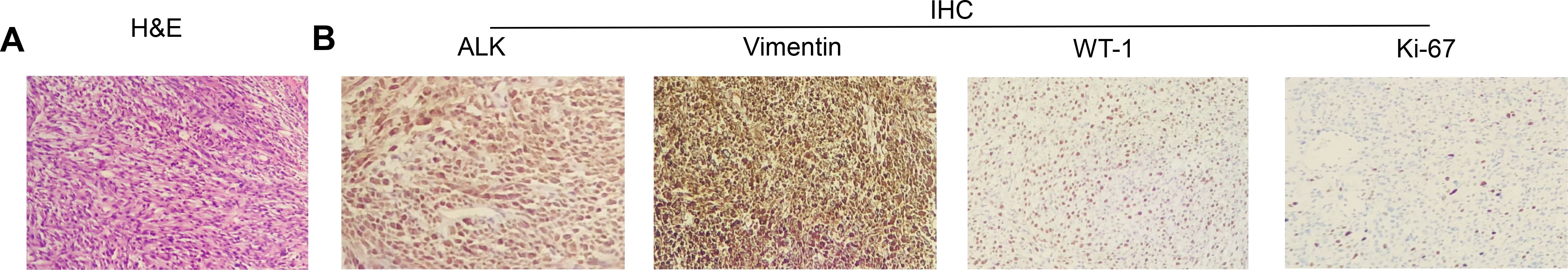
Figure 2. Morphologic features and IHC staining of the IMTUB samples. (A) Representative pictures of H&E staining (×100). (B) IHC analysis of ALK, Vimentin, WT-1, and Ki-67 expression levels (×100). H&E, hematoxylin and eosin; IHC, immunohistochemistry; IMTUB, inflammatory myofibroblastic tumor of the urinary bladder.
Given the presence of IMTUB with TPM4-ALK fusion and the treatment history, off-label treatment with ensartinib was considered, based on its mechanism of action as well as previous case reports demonstrating success with its use. The patient received oral ensartinib 225 mg once daily initiated on December 20, 2023. However, after approximately 1 month of treatment, the patient experienced swelling and a rash on the face, which were assessed by the investigator to be related to ensartinib and resolved soon with 40 mg/day furosemide tablets and 80 mg/day spironolactone tablets. Tumor evaluations were performed via CT, which revealed that both the bladder tumor and the metastatic sites had decreased in size compared to previous examinations on January 22, 2024 (Figure 1). According to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1, the better response evaluation was evaluated as PR. The recent follow-up CT showed that the patient maintained PR for nearly 5 months (Figure 1C). The targeted therapy of ensartinib was continued, and regular outpatient follow-ups continued.
3 Discussion
IMTUB, a neoplasm of intermediate biologic potential with a local tumor recurrence rate of only 4% after surgery and just a few cases of metastasis to other organs, has been reported (6, 10). It presented challenges in treatment due to the rarity and complexity of its recurrence and metastasis, and conventional cytotoxic chemotherapy regimens seem to be ineffective (11, 12). In addition, the ALK fusion genes have been identified as tumor driver genes and potential therapeutic targets, and targeted therapy with ALK inhibitors, such as lorlatinib and alectinib, exhibited favorable antitumor activity in IMTUB with ALK fusion (13, 14). However, to our knowledge, this is the first case report describing the postoperative recurrence of IMTUB with multiple metastases and TPM4-ALK fusion treated with ensartinib.
The majority of IMTUB patients are young and female, with no obvious specificity, and the most common clinical manifestations are hematuria, frequency-dysuria syndrome, and bladder outlet obstruction (15). IMTUB radiographic examination usually indicates a space-occupying lesion in the bladder, which can be easily misdiagnosed as bladder malignancy due to tumor bleeding or surrounding blood clot accumulation. The definite diagnosis needs to rely on pathological and immunohistochemical results (16). Additionally, IMTUB is mostly a benign process, but a few cases have local invasion, recurrent, or malignant transformation (17, 18). Here, we described an extremely unusual case of recurrent IMTUB with multiple metastases, where the possible mechanism of recurrence was TPM N-terminal coiled-coil domains fused to the ALK C-terminal kinase domain, leading to abnormal proliferation and malignant transformation (19). In this context, ALK inhibition appears to be a necessary condition for preventing further deterioration.
The ALK inhibitor ensartinib is a promising treatment for ALK-positive non-small-cell lung cancer (NSCLC) and showed superior efficacy to crizotinib in systemic (20). However, unlike in NSCLC, evidence on the efficacy of ensartinib in IMT is limited. A 66-year-old man with IMT bone metastasis accompanied by GCC2-ALK fusion and another man with pulmonary IMT underwent postoperative disease progression with STRN-ALK fusion both obtained PR following treatment with ensartinib (21, 22). The TPM4-ALK fusion retains the complete kinase domain of ALK and has been identified in both pulmonary IMT and peritoneal IMT cases; however, treatment with entrectinib and crizotinib did not yield the expected therapeutic outcomes (23, 24). In addition, other novel ALK inhibitors, such as ceritinib, brigatinib, and alectinib, while proven to cause tumor shrinkage, inevitably lead to drug resistance or severe adverse reactions (14, 25). In this case, combined with previous treatment history and current condition, the patient adopted ensartinib therapy. After administration of the medication, the patient’s condition significantly improved, and he achieved PR, suggesting that the TPM4-ALK fusion gene may be a potential target for ensartinib in treating IMTUB. However, the specific efficacy of this treatment would need to be confirmed through large-scale clinical trials. Furthermore, regarding the adverse reactions, this patient experienced swelling and a rash on the face following the administration of ensartinib. Indeed, the rash and other skin toxic effects have been noted as the most frequently observed toxicities associated with ensartinib, as reported in various studies (20, 26). However, the mechanism behind ensartinib-induced skin toxicity remains unclear. Nevertheless, it has been reported that the concentration of ensartinib in the skin was 9.0× higher than in the plasma, potentially explaining the high frequency of skin-related side effects observed with its use (27). Importantly, the unique disease characteristics and genetic variations in each patient can greatly influence their response to the medication, thus necessitating the development of targeted treatment plans tailored to individual patients, potentially leading to greater clinical benefits for them.
There are some limitations in this case report. First, this case report involved experience with a single patient; it does not provide sufficient evidence to prove the efficacy of ensartinib in treating this population. Second, the inherent design of case reports may bias our findings and limit their generalizability. Finally, due to the limited research on ensartinib for the treatment of IMTUB, we lack comparative data to position our findings against other studies, so the findings of this case should be interpreted with caution.
4 Conclusion
In conclusion, the finding from this case suggests that TPM4-ALK may be used as a new target for IMTUB TKI treatment with ensartinib, which may provide a new therapeutic direction with promising efficacy and an acceptable safety profile. However, further clinical studies are needed to verify its benefits comprehensively.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Jilin Cancer Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HR: Data curation, Resources, Writing – original draft. QC: Data curation, Resources, Writing – review & editing. XC: Data curation, Resources, Writing – review & editing. DS: Data curation, Resources, Writing – review & editing. ZZ: Data curation, Resources, Writing – review & editing. FC: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Panagiotopoulos N, Patrini D, Gvinianidze L, Woo WL, Borg E, Lawrence D. Inflammatory myofibroblastic tumour of the lung: a reactive lesion or a true neoplasm? J Thorac Dis. (2015) 7:908–11. doi: 10.3978/j.issn.2072-1439.2015.04.60
2. Dergamoun H, El Gdaouni A, Taoufik A, Ziouziou I. Inflammatory myofibroblastic tumor of the urinary bladder: a case report. Pan Afr Med J. (2023) 44:119. doi: 10.11604/pamj.2023.44.119.38156
3. Lovly CM, Gupta A, Lipson D, Otto G, Brennan T, Chung CT, et al. Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov. (2014) 4:889–95. doi: 10.1158/2159-8290.CD-14-0377
4. Kovach SJ, Fischer AC, Katzman PJ, Salloum RM, Ettinghausen SE, Madeb R, et al. Inflammatory myofibroblastic tumors. J Surg Oncol. (2006) 94:385–91. doi: 10.1002/jso.20516
5. Gass J, Beaghler M, Kwon M. Inflammatory myofibroblastic tumor of the urinary bladder: a case report. J Endourology Case Rep. (2019) 5:31–3. doi: 10.1089/cren.2018.0096
6. Teoh JYC, Chan N-H, Cheung H-Y, Hou SSM, Ng C-F. Inflammatory myofibroblastic tumors of the urinary bladder: A systematic review. Urology. (2014) 84:503–8. doi: 10.1016/j.urology.2014.05.039
7. Ma Y, Pan H, Liu Y, Zhang Y, Hong S, Huang J, et al. Ensartinib in advanced ALK-positive non-small cell lung cancer: a multicenter, open-label, two-staged, phase 1 trial. J Thoracic Dis. (2022) 14:4751. doi: 10.21037/jtd-22-1606
8. Yang Y, Zhou J, Zhou J, Feng J, Zhuang W, Chen J, et al. Efficacy, safety, and biomarker analysis of ensartinib in crizotinib-resistant, ALK-positive non-small-cell lung cancer: a multicentre, phase 2 trial. Lancet Respir Med. (2020) 8:45–53. doi: 10.1016/s2213-2600(19)30252-8
9. Childress MA, Himmelberg SM, Chen H, Deng W, Davies MA, Lovly CM. ALK fusion partners impact response to ALK inhibition: differential effects on sensitivity, cellular phenotypes, and biochemical properties. Mol Cancer Res. (2018) 16:1724–36. doi: 10.1158/1541-7786.Mcr-18-0171
10. Libby EK, Ellis LT, Weinstein S, Hammer RD, Murray KS. Metastatic inflammatory myofibroblastic tumor of the bladder. Urol Case Rep. (2019) 23:10–2. doi: 10.1016/j.eucr.2018.11.007
11. Nakano K. Inflammatory myofibroblastic tumors: recent progress and future of targeted therapy. Japanese J Clin Oncol. (2023) 53:885–92. doi: 10.1093/jjco/hyad074
12. Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. (2007) 31:509–20. doi: 10.1097/01.pas.0000213393.57322.c7
13. Fujiki T, Sakai Y, Ikawa Y, Takenaka M, Noguchi K, Kuroda R, et al. Pediatric inflammatory myofibroblastic tumor of the bladder with ALK–FN1 fusion successfully treated by alectinib. Pediatr Blood Cancer. (2023) 70:e30172. doi: 10.1002/pbc.30172
14. Reinhart S, Trachsel Y, Fritz C, Wagner U, Bode-Lesniewska B, John H, et al. Inflammatory myofibroblastic tumor of the bladder with FN1-ALK gene fusion: different response to ALK inhibition. Urology. (2020) 146:32–5. doi: 10.1016/j.urology.2020.09.026
15. Song D, Jiao W, Gao Z, Liu N, Zhang S, Zong Y, et al. Inflammatory myofibroblastic tumor of urinary bladder with severe hematuria: A Case report and literature review. Med (Baltimore). (2019) 98:e13987. doi: 10.1097/md.0000000000013987
16. Chen C, Huang M, He H, Wu S, Liu M, He J, et al. Inflammatory myofibroblastic tumor of the urinary bladder: an 11-year retrospective study from a single center. Front Med. (2022) 9. doi: 10.3389/fmed.2022.831952
17. Kim HW, Choi YH, Kang SM, Ku JY, Ahn JH, Kim JM, et al. Malignant inflammatory myofibroblastic tumor of the bladder with rapid progression. Korean J Urol. (2012) 53:657–61. doi: 10.4111/kju.2012.53.9.657
18. Dishop MK, Warner BW, Dehner LP, Kriss VM, Greenwood MF, Geil JD, et al. Successful treatment of inflammatory myofibroblastic tumor with Malignant transformation by surgical resection and chemotherapy. J Pediatr Hematol Oncol. (2003) 25:153–8. doi: 10.1097/00043426-200302000-00014
19. Lawrence B, Perez-Atayde A, Hibbard MK, Rubin BP, Dal Cin P, Pinkus JL, et al. TPM3-ALK and TPM4-ALK oncogenes in inflammatory myofibroblastic tumors. Am J Pathol. (2000) 157:377–84. doi: 10.1016/S0002-9440(10)64550-6
20. Horn L, Wang Z, Wu G, Poddubskaya E, Mok T, Reck M, et al. Ensartinib vs crizotinib for patients with anaplastic lymphoma kinase–positive non–small cell lung cancer: A randomized clinical trial. JAMA Oncol. (2021) 7:1617–25. doi: 10.1001/jamaoncol.2021.3523
21. Li X, Zheng J, Li X, Chen Y, Liu K, Li F, et al. Case Report: Ensartinib for gastric epithelioid inflammatory myofibrosarcoma with STRN-ALK fusion. Front Oncol. (2023) 13:1252221. doi: 10.3389/fonc.2023.1252221
22. Tosefsky K, Rebchuk AD, Martin KC, Chen DW, Yip S, Makarenko S. Preoperative corticosteroids reduce diagnostic accuracy of stereotactic biopsies in primary central nervous system lymphoma: A systematic review and meta-analysis. Neurosurgery. (2022) 10:1227. doi: 10.1227/neu.0000000000002944
23. Wong HH, Bentley H, Bulusu VR, Anyaegbu G, Watkins J, Horan G, et al. Lorlatinib for the treatment of inflammatory myofibroblastic tumour with TPM4-ALK fusion following failure of entrectinib. Anti-Cancer Drugs. (2020) 31:1106–10. doi: 10.1097/CAD.0000000000000994
24. Tsakiri K, Kotoula V, Lakis S, Müller J, Fostira F, Bobos M, et al. Crizotinib failure in a TPM4-ALK–rearranged inflammatory myofibroblastic tumor with an emerging ALK kinase domain mutation. JCO Precis Oncol. (2017) 1:1–7. doi: 10.1200/PO.17.00015
25. Kyi C, Friedman CF, Mueller JJ, Benayed R, Ladanyi M, Arcila M, et al. Uterine mesenchymal tumors harboring ALK fusions and response to ALK-targeted therapy. Gynecol Oncol Rep. (2021) 37:100852. doi: 10.1016/j.gore.2021.100852
26. Zhou F, Yang Y, Zhang L, Cheng Y, Han B, Lu Y, et al. Expert consensus of management of adverse drug reactions with anaplastic lymphoma kinase tyrosine kinase inhibitors. ESMO Open. (2023) 8:101560. doi: 10.1016/j.esmoop.2023.101560
Keywords: ensartinib, inflammatory myofibroblastic tumor, urinary bladder, multiple metastases, TPM4-ALK
Citation: Ren H, Cheng Q, Chen X, Sui D, Zhang Z and Chen F (2025) Case Report: Clinical response of ensartinib for inflammatory myofibroblastic tumor of the urinary bladder with multiple metastases and TPM4-ALK fusion. Front. Oncol. 15:1481602. doi: 10.3389/fonc.2025.1481602
Received: 16 August 2024; Accepted: 03 April 2025;
Published: 02 May 2025.
Edited by:
Brigida Anna Maiorano, IRCCS Casa Sollievo della Sofferenza Hospital, ItalyCopyright © 2025 Ren, Cheng, Chen, Sui, Zhang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei Chen, MjYyMDg0MzdAcXEuY29t
 Hongtao Ren1
Hongtao Ren1 Fei Chen
Fei Chen