- 1Department of Pulmonary and Critical Care Medicine, The First Hospital of China Medical University, Shenyang, China
- 2China Medical University, Shenyang, China
- 3Department of Laboratory Medicine, The First Hospital of China Medical University, Shenyang, China
Background: The clinical benefits of postoperative chemotherapy for non-small cell lung cancer (NSCLC) have plateaued, thus highlighting the need for novel strategies. This meta-analysis evaluated the efficacy and safety of adjuvant immunotherapy in patients with completely resected NSCLC and wild-type epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK).
Methods: PubMed, Web of Science, Embase, and the Cochrane Library were searched up to February 12, 2025, for studies assessing adjuvant immunotherapy in NSCLC. Primary endpoints included disease-free survival (DFS), overall survival (OS), correlation between subgroup characteristics and efficacy, and safety outcomes, including treatment-related adverse events (TRAEs), severe adverse events (SAEs), and treatment discontinuation.
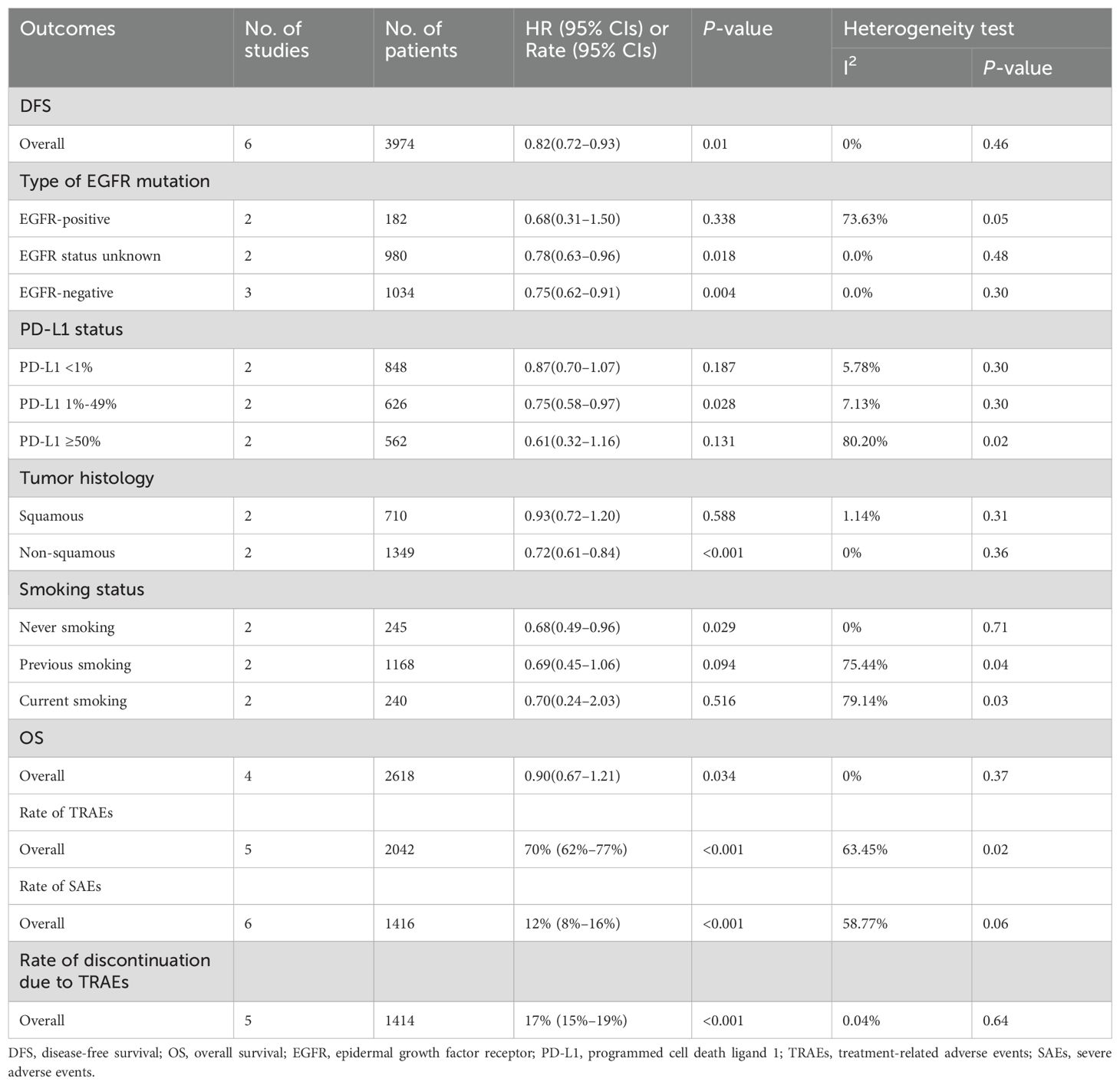
Results: Twelve articles involving 4048 patients were included. Adjuvant immunotherapy significantly improved DFS in patients with resected stage IB–III NSCLC than supportive care or placebo (hazard ratio [HR]: 0.82, 95% confidence interval [CI]: 0.72–0.93, p = 0.01; I2 = 0%, p = 0.46). However, the OS benefit was not significant (HR: 0.9, 95% CI: 0.67–1.21, p = 0.34). DFS benefit was observed in EGFR-negative (HR: 0.75, 95% CI: 0.62–0.91, I2 = 0%), EGFR status unknown (HR: 0.78, 95% CI: 0.63–0.96, I2 = 0%), programmed cell death ligand 1 (PD-L1) 1–49% (HR: 0.75, 95% CI: 0.58–0.97, I2 = 7.13%), non-squamous cell carcinoma (HR: 0.72, 95% CI: 0.61–0.84, I2 = 0%), and never-smoking (HR: 0.68, 95% CI: 0.49–0.96, I2 = 0%) subgroups. The pooled incidences of TRAEs, SAEs, and discontinuation of treatment due to toxicity were 70% (95% CI: 62%–77%), 12% (95% CI: 8%–16%), and 17% (95% CI: 15–19%), respectively.
Conclusions: Adjuvant immunotherapy improved DFS in patients with completely resected NSCLC, particularly those who were EGFR-negative, had PD-L1 levels of 1–49%, had non-squamous cell carcinoma, or never smoked.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO, identifier CRD42024547752.
1 Introduction
Lung cancer is a leading cause of cancer-related deaths worldwide (1). The 5-year survival rate of patients with non-small cell lung cancer (NSCLC) remains poor (2). Complete surgical excision is the primary approach to achieving long-term survival. However, postoperative recurrence occurs in 28–68.2% of patients with completely resected NSCLC (3–5), and only 37.1% of them receive standard adjuvant chemotherapy (6). However, the 5-year overall survival (OS) rate for those receiving postoperative chemotherapy remains between 44.5% and 69% (7–10). Therefore, novel individualized adjuvant treatments are needed to decrease postoperative recurrence and improve survival in patients with radically resected NSCLC.
Wu et al. (11) found that compared to placebo, osimertinib significantly improved the disease-free survival (DFS) of patients with epidermal growth factor receptor (EGFR) mutation-positive, completely resected NSCLC (hazard ratio [HR]: 0.20, p < 0.001). In addition, the ALINA trial results demonstrated that alectinib therapy significantly improved DFS in patients with completely resected NSCLC harboring anaplastic lymphoma kinase (ALK) rearrangement compared to standard adjuvant chemotherapy (12). Therefore, the National Comprehensive Cancer Network guidelines recommend osimertinib and alectinib use in patients with EGFR and ALK mutations, respectively. Real-world data suggest that the efficacy of postoperative EGFR-tyrosine kinase inhibitors (TKIs) alone is equivalent to that of adjuvant chemotherapy followed by EGFR-TKIs (13).
However, the efficacy of immune checkpoint inhibitors (ICIs) administered to patients with NSCLC after surgical resection is inconsistent. Felip et al. (14) reported that atezolizumab could significantly prolong DFS in patients with resected stage II–IIIA NSCLC after standard adjuvant chemotherapy, with the most significant benefit observed in those with programmed cell death ligand 1 (PD-L1) ≥ 50%. Conversely, a real-world study by Yang et al. (15, 16) indicated that adjuvant immunotherapy was not statistically significant. Besse et al. (17) showed that adjuvant pembrolizumab significantly improved DFS in patients with stage II–IIIA NSCLC but not in those with PD-L1 ≥ 50%. Another study reported that 50% of patients undergoing adjuvant immunotherapy experienced grade 3 or higher adverse events (AEs) (18).
Given these conflicting findings, it remains unclear whether the variability in adjuvant immunotherapy efficacy is associated with PD-L1 positivity and expression levels. Furthermore, histological subtypes may influence treatment response. Smoking is a well-established risk factor for lung cancer; however, its impact on adjuvant immunotherapy efficacy remains unexplored. To address these uncertainties, we conducted a meta-analysis summarizing the available evidence up to February 12, 2025.
2 Materials and methods
2.1 Literature search
This study was conducted in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) reporting checklist (19). The study was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42024547752) before data extraction. Two investigators (Hong Huang and Hongyu Jin) independently searched PubMed, Web of Science, the Cochrane Library, and Embase until February 12, 2025. The search terms included (“surgery” or “lobectomy” or “resection”), (“adjuvant immunotherapy” or “adjuvant immune checkpoint inhibitor”) and (“non-small cell lung cancer” or “NSCLC” or “carcinoma, non-small cell lung”). The reference lists of the retrieved publications were reviewed to identify additional eligible articles. Recent abstracts and data from international conferences, including the American Association for Cancer Research, the American Society of Clinical Oncology, and the European Society for Medical Oncology, were reviewed. The search was limited to publications in English.
2.2 Selection criteria
Articles meeting the following criteria were included: (i) studies on patients with completely resected stage I–III NSCLC; (ii) use of immune checkpoint inhibitors used after surgery; and (iii) reported outcomes such as DFS, OS, incidence of treatment-related adverse events (TRAEs), severe adverse events (SAEs), and discontinuation of adjuvant immunotherapy due to TRAEs. If multiple articles were derived from the same trial, the one reporting the most recent data or the largest sample size was selected for inclusion. Meta-analyses, reviews, and case reports were excluded. Two reviewers independently screened and assessed the studies, and any disagreements were resolved through discussion.
2.3 Outcome measures and data collection
The primary outcomes were DFS and OS. Secondary outcomes included the incidence of TRAEs, SAEs, and treatment discontinuation due to toxicity. Hong Huang and Pengchen Bao independently reviewed all articles. Data on population characteristics, study design, adjuvant treatment regimens, endpoints, and other study details were extracted using templated forms. Delei Kong was consulted to resolve disagreements. We contacted the corresponding authors for missing data when needed.
2.4 Quality assessment
Huang and Jin independently performed a quality assessment. Discrepancies were discussed with Delei Kong, who made the final decision. Non-randomized studies were assessed using the ROBINS-I tool (20). The Cochrane risk-of-bias tool was applied to randomized controlled trials (RCTs).
2.5 Statistical analysis
Stata version 16.0 was used to analyze the data. The risk of bias figure was created using RevMan 5.3.5. The pooled HR for DFS and OS were calculated. Non-comparative binary data of AEs were pooled as N (%) with a 95% confidence interval (CI). Random-effects models (Restricted Maximum Likelihood) and Knap–Hartung adjustments were used (21). The chi-square test (χ2) and inconsistency test (I2) were applied to evaluate the statistical heterogeneity between the articles. P < 0.1, or I2 > 50% indicated significant heterogeneity. A sensitivity analysis was performed by omitting one study at a time to explore the sources of heterogeneity and assess the reliability of the results. A fixed-effects model was used for additional sensitivity analysis when there was no heterogeneity (I2 = 0%). Publication bias was assessed using forest plots and Egger’s tests. The explanation for this inconsistency follows the prescription in the Cochrane Handbook. Statistical significance was set at P < 0.05. significant.
3 Results
3.1 Literature selection
Supplementary Figure S1 presents the details of the literature screening process. The search yielded 2187 articles. After removing 667 duplicates, 1459 studies were excluded based on titles and abstracts. Sixty-one full-text studies and conference abstracts were carefully reviewed for eligibility. Eleven articles were excluded as they did not include immunotherapy as a postoperative adjuvant regimen. Twenty-nine studies were excluded because they did not report the relevant outcomes. Three conference papers and one article were excluded because their data had already been published in Felip 2021 (15), O’Brien 2022 (22), and Shin 2024 (23). Five studies (18, 24–27) were excluded because they included patients with incomplete resection.
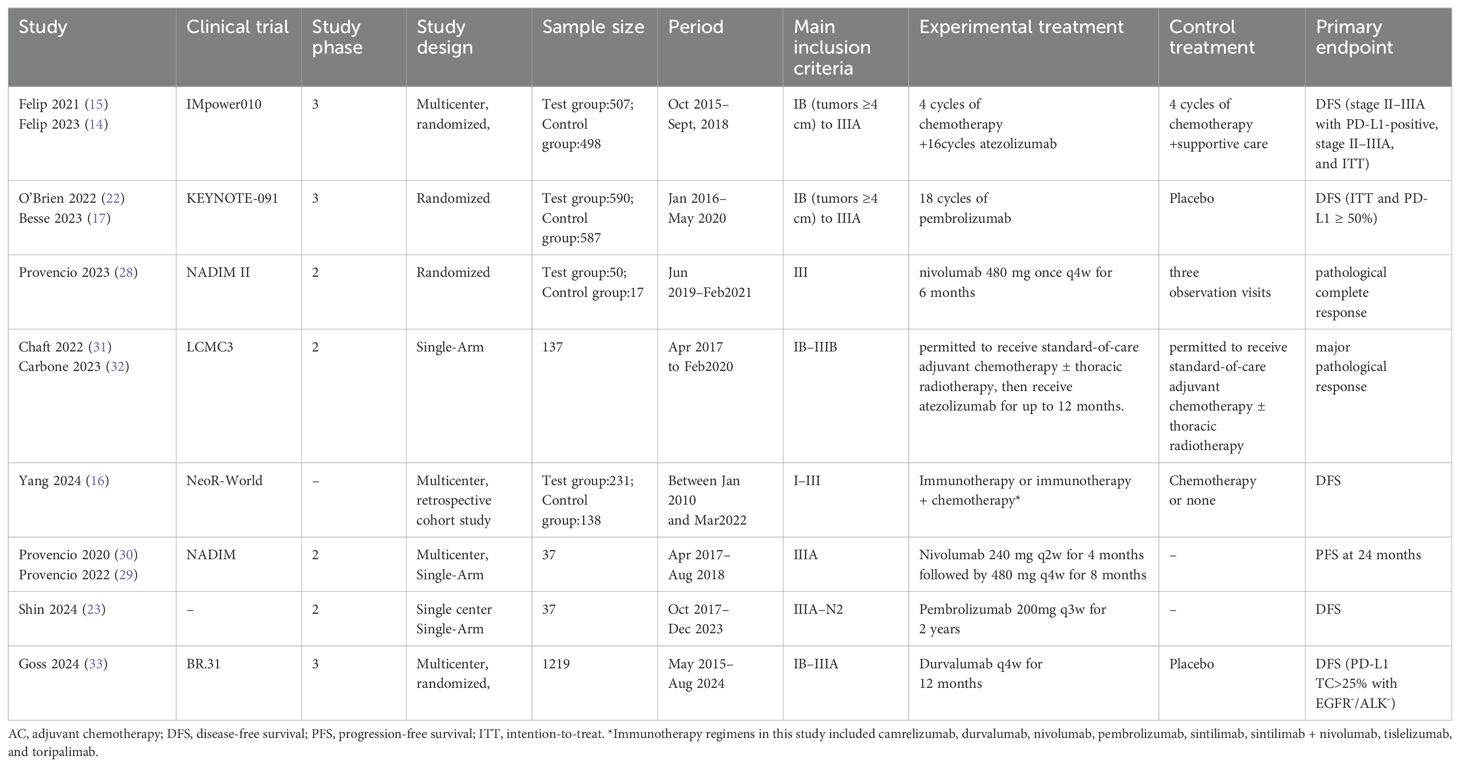
Finally, 12 articles (14–17, 22, 23, 28–33) from 8 clinical trials, covering 4048 patients, were included in the meta-analysis. The characteristics of the included clinical trials are summarized in Table 1. Patients with EGFR-positive tumors were excluded from studies by Provencio et al. (29, 30) (28), Chaft et al. (31), and Carbone et al. (32). However, in the Felip 2021 (15) and O’Brien 2022 (22) studies, 11.6% and 6.2%, of patients were EGFR-positive, respectively. In these two studies, 52.4% and 36.9 of the patients were EGFR mutation-negative, whereas the mutation status of the remaining patients was unknown. The immunotherapy regimens included atezolizumab, pembrolizumab, nivolumab, camrelizumab, durvalumab, sintilimab, tislelizumab, and toripalimab. A summary of the risk of bias in RCTs and non-RCTs is provided in Supplementary Figure S2 and Supplementary Table S2.
3.2 Clinical efficacy of adjuvant immunotherapy
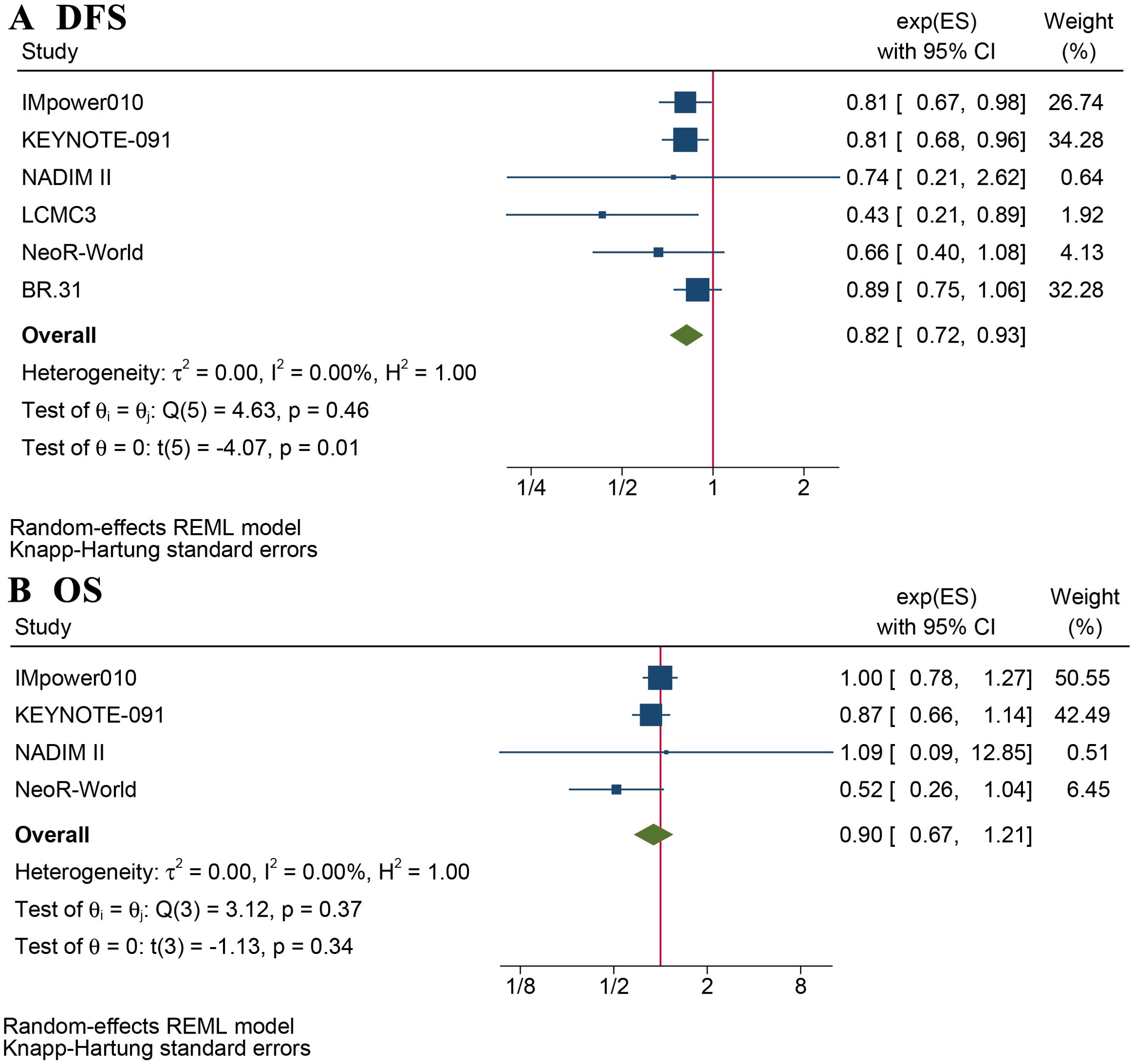
Eight articles reported DFS and/or OS (Supplementary Table S3). The mean follow-up ranged from 21.6 –71 months. The DFS benefit of adjuvant immunotherapy treatment in completely resected stage IB–III NSCLC was statistically significant (HR: 0.82, 95% CI: 0.72–0.93, p = 0.01), without statistical heterogeneity (I2 = 0%, p = 0.5), as shown in Figure 1A. The OS benefit was not statistically significant (HR 0.90, 95% CI: 0.67–1.21, p = 0.34), as shown in Figure 1B.
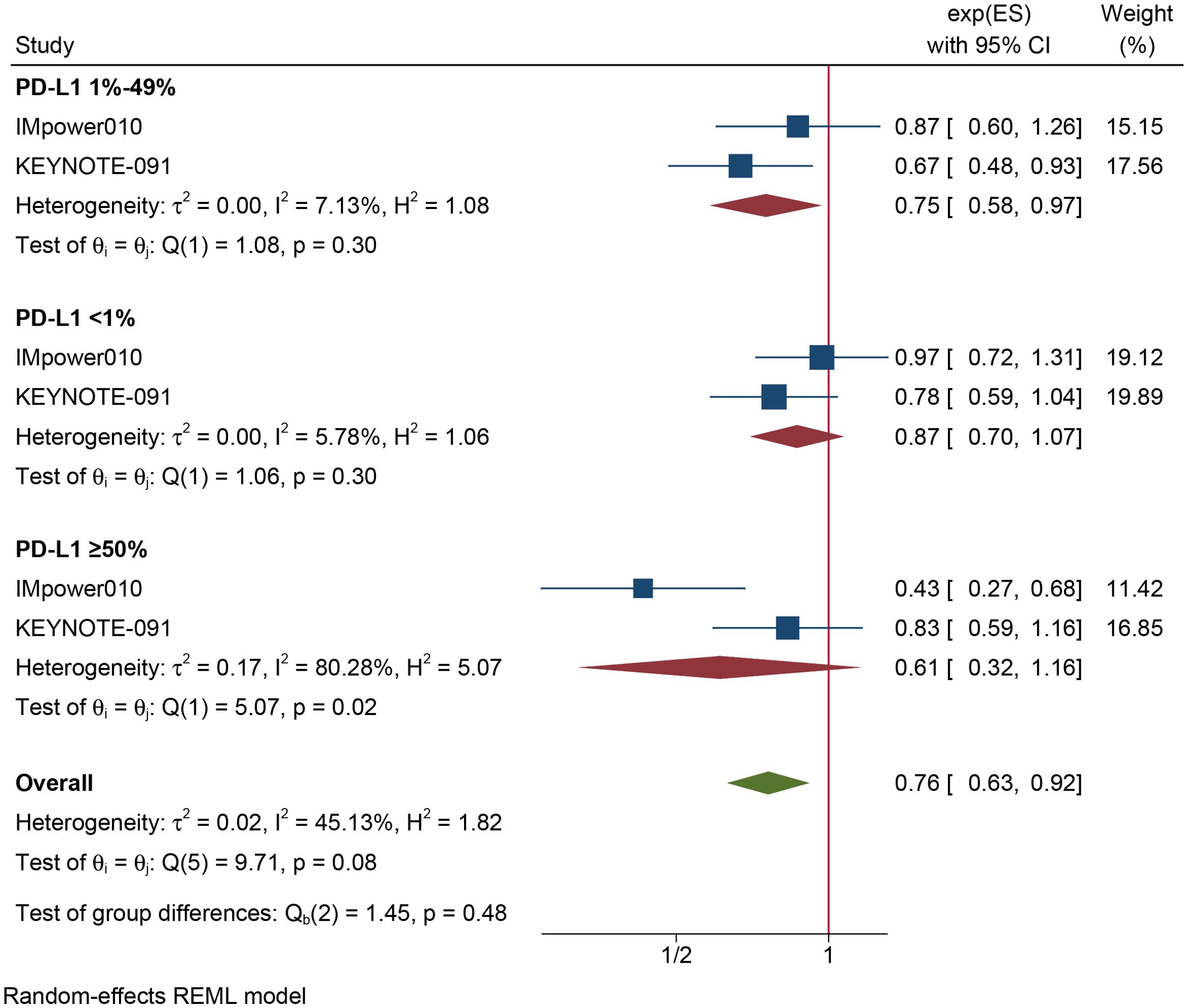
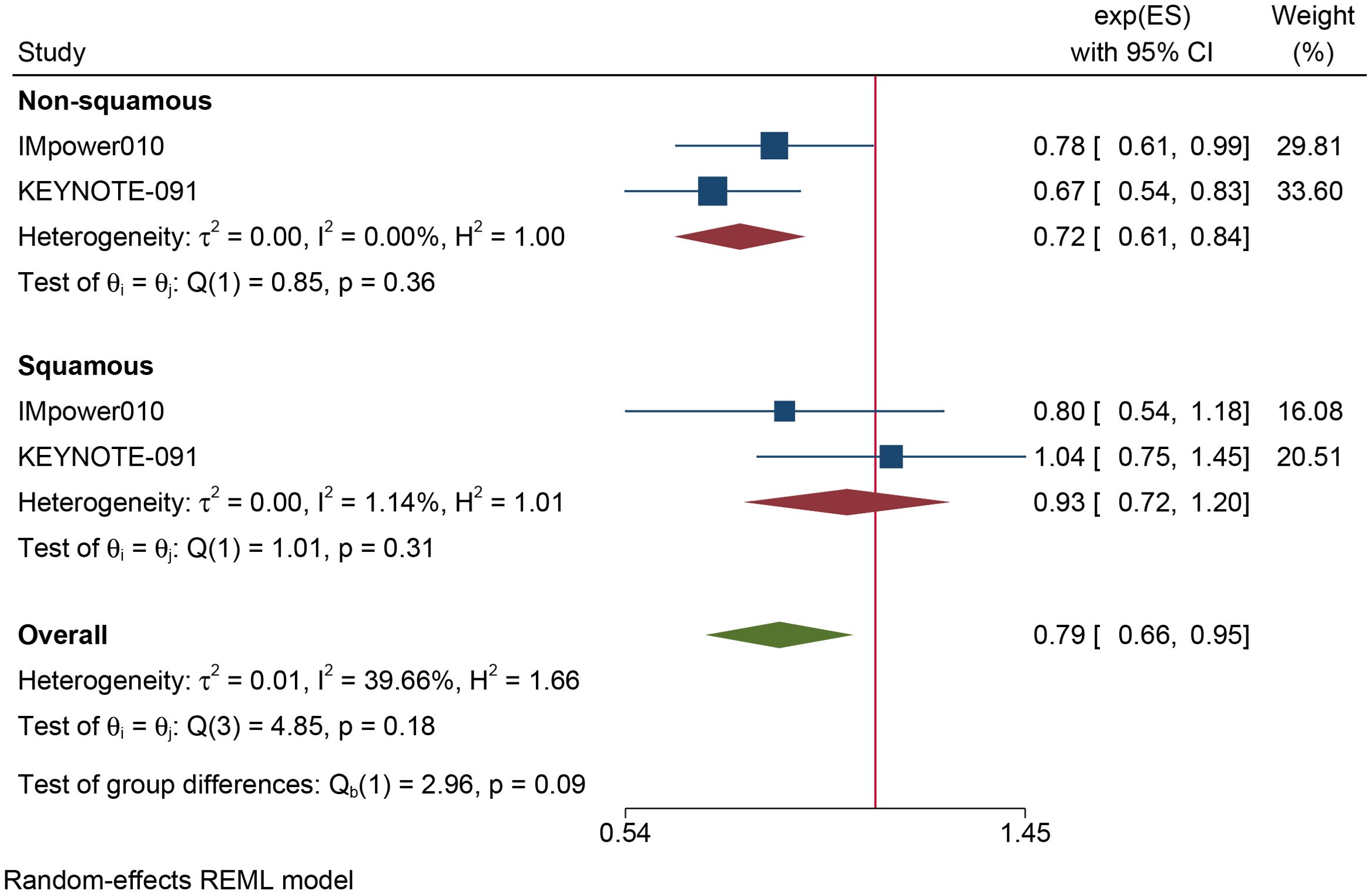
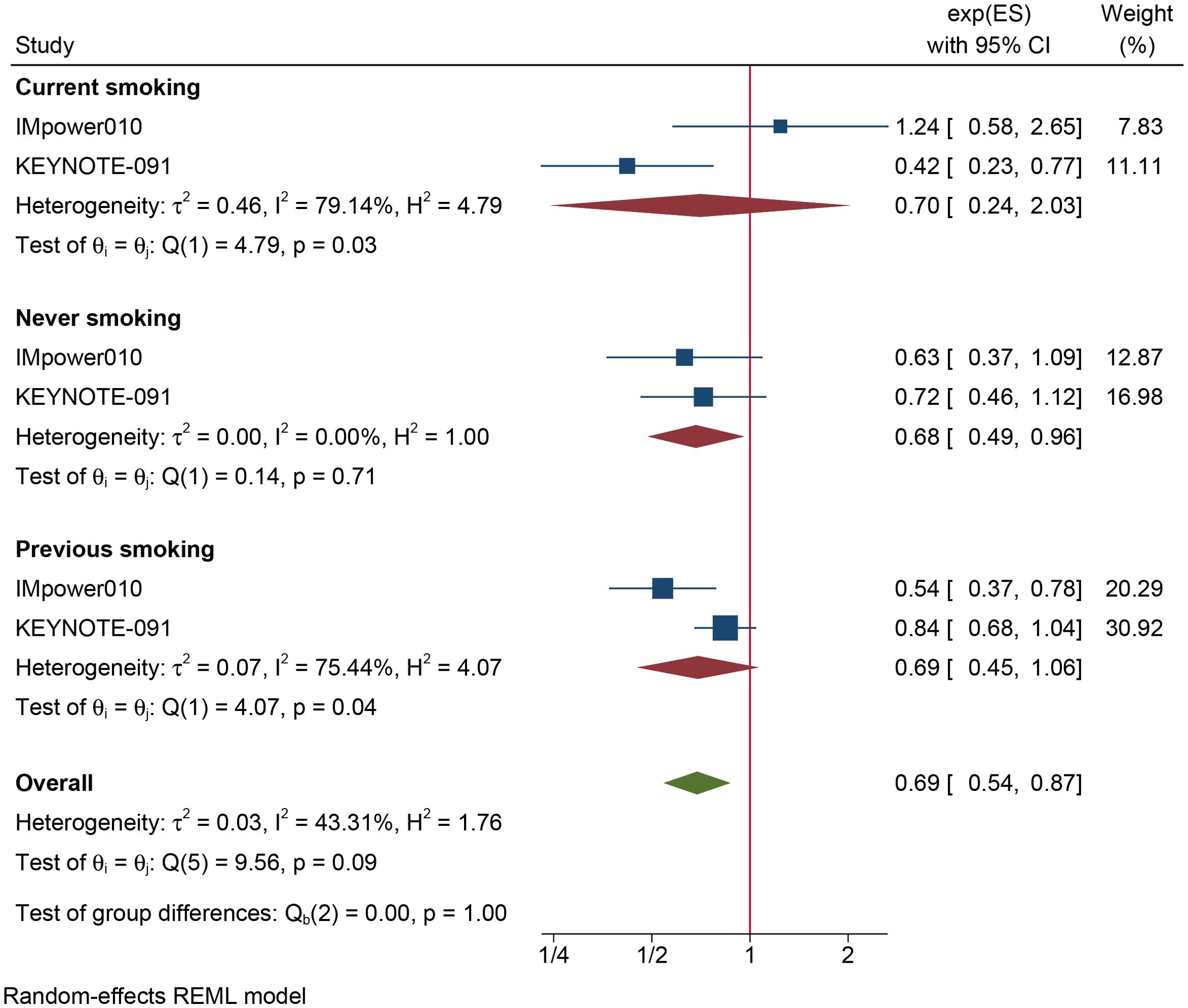
The results of the subgroup analysis are as follows: (1) DFS was significantly improved by adjuvant immunotherapy in patients who were EGFR-negative (HR: 0.75, 95% CI: 0.62–0.91, I2 = 0%) and in those with unknown EGFR status (HR 0.78, 95% CI: 0.63–0.96, I2 = 0%) but not in those who were EGFR-positive (HR: 0.68, 95% CI: 0.31–1.5, I2 = 73.6%) (see Figure 2). (2) As shown in Figure 3, DFS benefit was statistically significant in patients with PD-L1 expression of 1–49% (HR: 0.75, 95% CI: 0.58–0.97, I2 = 7.13%) but not in those who were PD-L1-negative (HR: 0.87, 95% CI: 0.70–1.07, I2 = 5.78%) or those with PD-L1 ≥50% (HR: 0.61, 95% CI: 0. 32–1.16, I2 = 80.28%). (3) Similarly, DFS benefit was significant in patients with non-squamous cell carcinoma (HR: 0.72, 95% CI: 0.61–0.84, I2 = 0%) but not in those with squamous cell carcinoma (HR: 0.93, 95% CI: 0.72–1.20, I2 = 1.14%) (see Figure 4). (4) Notably, adjuvant immunotherapy demonstrated a statistically significant DFS benefit in never-smoking patients (HR: 0.68, 95% CI: 0.49–0.96, I2 = 0%). However, neither previous nor current smokers experienced a significant DFS benefit from immunotherapy (Figure 5). The aggregated findings are detailed in Table 2.
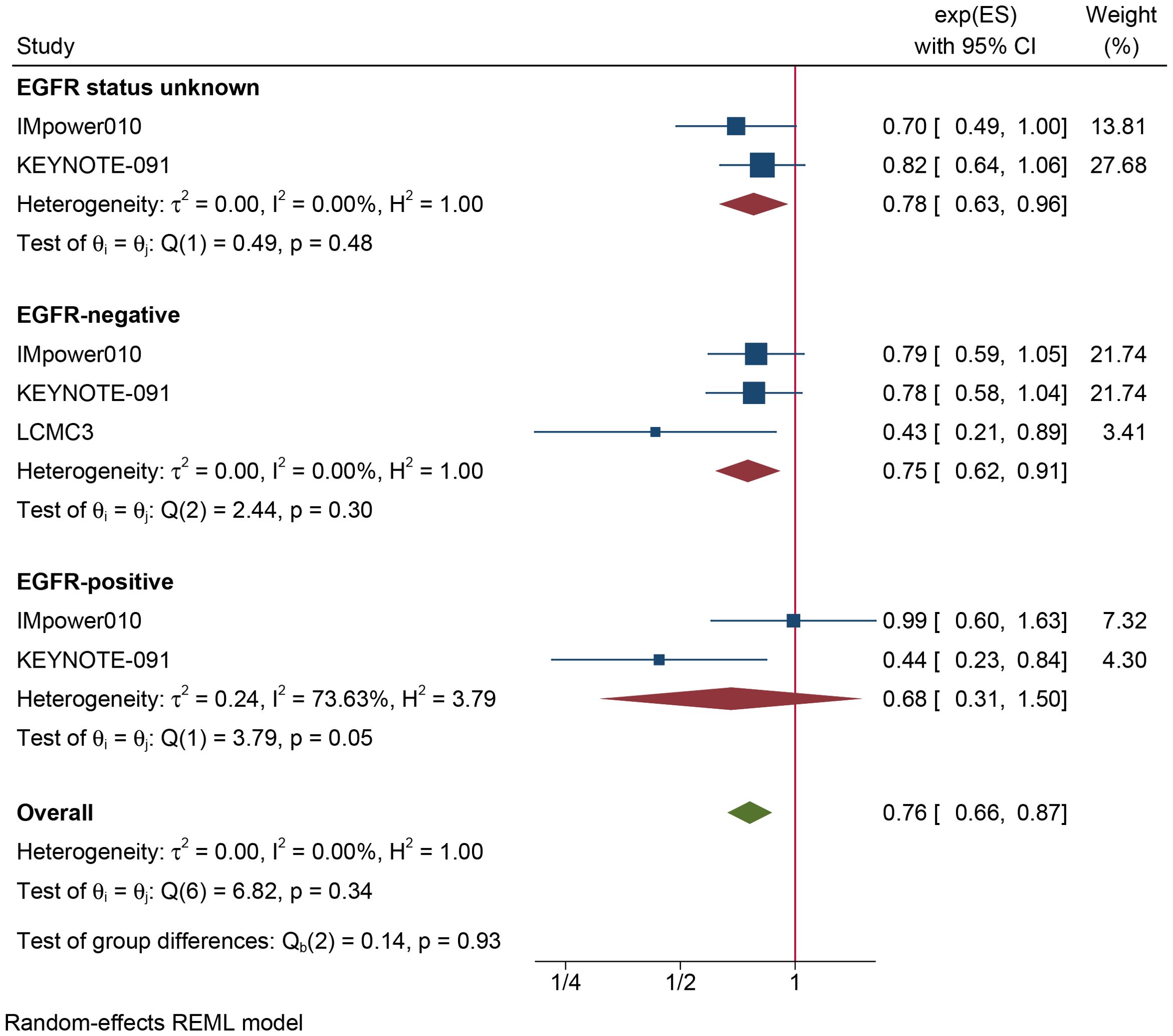
Figure 2. Forest plot of disease-free survival of subgroup analysis based on the type of EGFR mutation.
3.3 Safety of adjuvant immunotherapy
Supplementary Table S3 summarizes the rates of TRAEs and SAEs reported in these studies. The pooled incidence of TRAEs was 70% (95% CI: 62%–77%) (Figure 6A). The estimated incidence of SAEs, defined as grade 3–5 TRAEs, was 12% (95% CI: 8%–16%) (Figure 6B). Figure 6C shows that the incidence of adjuvant immunotherapy discontinuation due to TRAEs was 17% (95% CI: 15%–19%). A summary of the pooled results is provided in Table 2.
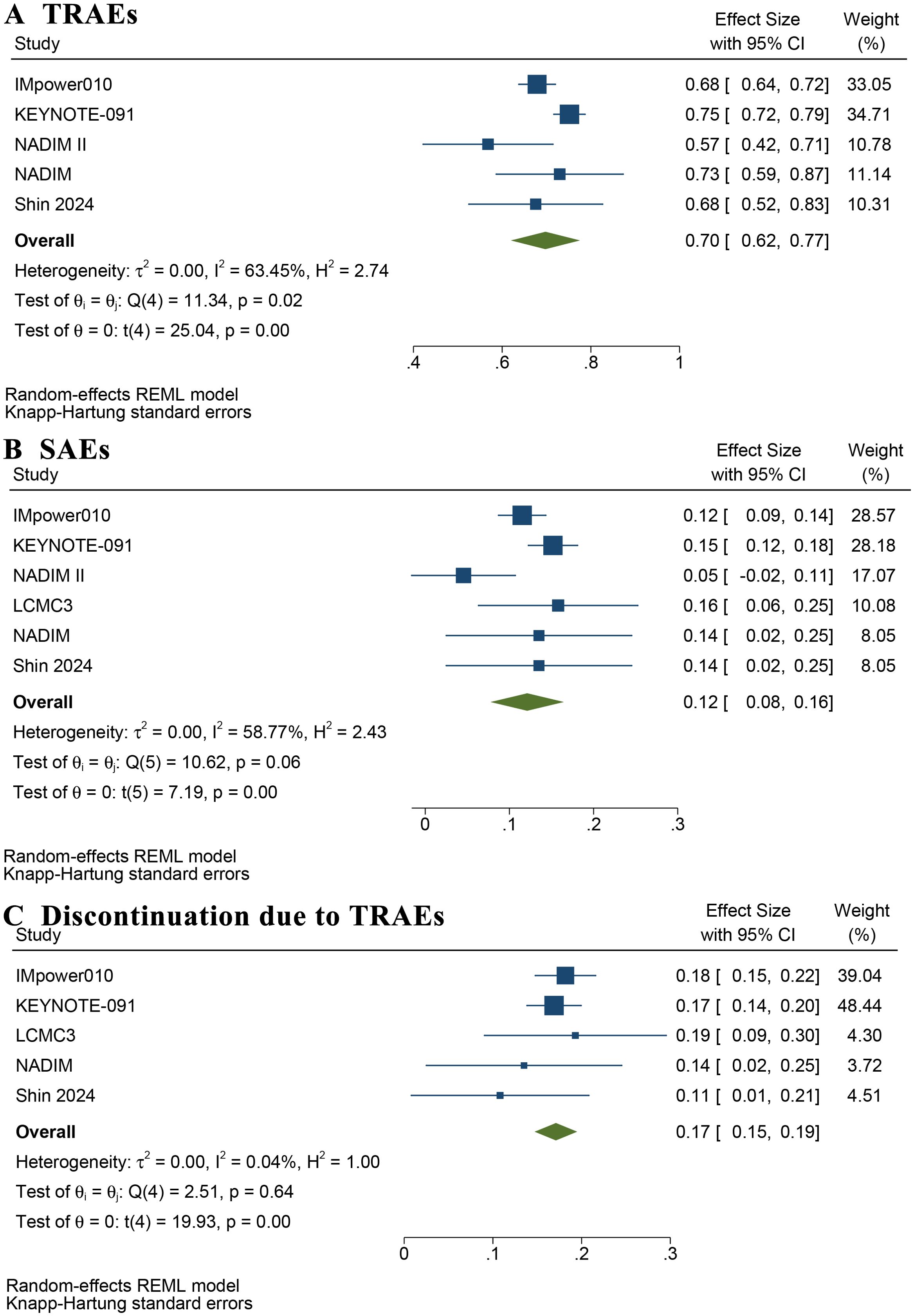
Figure 6. Forest plot of rate of treatment-related adverse events (A), severe adverse events (B), and discontinuation of adjuvant immunotherapy due to treatment-related adverse events (C).
Supplementary Figures S3 and S4 summarize the profiles of immune-mediated and severe immune-mediated AEs, respectively. Immune-mediated AEs with an incidence >5% included hypothyroidism (19.2%), rash (18.4%), pneumonitis (10.2%), and hyperthyroidism (8.7%). The most prevalent severe immune-mediated AEs included severe skin reactions (1.9%), hepatitis (1.2%), pneumonitis (1.1%), and rashes (0.9%).
3.4 Sensitivity analysis and publication bias
Leave-one-out sensitivity analyses indicated that no single study significantly affected the pooled DFS and OS results (see Supplementary Figure S5). The pooled HRs for DFS (0.82, 95% CI: 0.74–0.90) and OS (0.90, 95% CI: 0.76–1.08) from the fixed-effects model closely matched those from the random-effects method (see Supplementary Figure S6). These results confirm the robustness of our findings. Furthermore, the heterogeneity in TRAE incidence significantly decreased after excluding the KEYNOTE-091 trial (22) (Supplementary Figure S7). Similarly, removing the KEYNOTE-091 (22) or NADIM II (28) trials appreciably reduced the heterogeneity in SAE rates (Supplementary Figure S8). The funnel plots (Supplementary Figure S9), and Egger’s test (Supplementary Table S4) showed no significant publication bias.
4 Discussion
ICIs have become a critical treatment pillar for advanced-stage NSCLC without oncogenic drivers. Their efficacy as neo/adjuvant or perioperative immunotherapy in resectable NSCLC has been confirmed in previous meta-analysis results see Supplementary Table S3). However, no study has specifically summarized the evidence on adjuvant immunotherapy in patients with completely resected IB–III NSCLC.
Nuccio et al. (34) conducted a meta-analysis by examining clinicopathological factors influencing ICI benefits in early-stage lung cancer and comparing neoadjuvant and perioperative strategies. Their analysis, which included two RCTs on adjuvant immunotherapy, found that it prolonged DFS. Expanding upon this with updated data and additional studies, we found that adjuvant ICI therapy significantly prolonged DFS—particularly in EGFR-negative, PD-L1 (1–49%), non-squamous carcinoma, or never-smokers—it did not improve OS in completely resected stage IB–III NSCLC. These findings have clinical implications for selecting adjuvant therapy in this patient population.
It is extensively accepted that upregulated PD-L1 expression in tumor cells plays an essential role in tumor immune evasion. Specifically, PD-L1 binding to PD-1 inhibits T cell proliferation and survival through the PI3K/Akt pathway and disrupts T cell differentiation by blocking the synthesis and secretion (35, 36). Additionally, PD1/PD-L1 signaling inhibits T cell function by attenuating CD28 costimulatory signaling (37).
Consequently, the PD-1/PD-L1 blockade therapy, which directly restores suppressed host antitumor immune responses, has significantly improved the clinical prognosis of many patients with advanced and resectable NSCLC (38–40). Our study further confirms the DFS benefits of adjuvant ICI therapy in patients who have undergone complete resection. The non-significant OS benefit may occur because the median OS in the intention-to-treat (ITT) population has yet to be reached in two large RCT studies. Another important consideration is that none of the included studies identified OS as the primary endpoint, which may have resulted in insufficient power to detect statistically significant differences in OS (41).
PD-L1 expression in tumors is often used to select suitable candidates for immunotherapy. It is generally believed that the higher the expression of PD-L1, the better the therapeutic effect of immunotherapy. Indeed, the study in 2021 by Felip et al. (15) found that DFS has significantly improved in the PD-L1 ≥50% population but not in the PD-L1 of 1–49% and PD-L1<1% populations, based on data from 882 stage II to IIIA patients followed 32.2 months (IQR 27.4–38.3). However, the other RCT in 2022 (22), which included 1177 stage IB to IIIA patients, followed at 35.6 months (IQR 27.1–45.5), indicating the significant DFS benefit in the PD-L1 of 1–49% patients, but not in the PD-L1 ≥50% and PD-L1 <1% populations. The investigators considered that random factors contributed to the better-than-expected DFS performance in the control group of patients with PD-L1 ≥50%, and further extended follow-up periods were needed to identify the survival advantage of the experimental group. Nevertheless, the final DFS analysis, based on the data from 51.7 months (range 32.7–84.2) follow-up, indicated the same result (17). Our pooled results further showed that patients with PD-L1 of 1–49% benefited from postoperative immunotherapy, whereas those with PD-L1 expression ≥50% did not. These findings suggest we should be cautious when applying postoperative immunotherapy in patients with PD-L1 expression ≥ 50%. More RCT trials are required to support and verify these results.
Our findings indicate that DFS benefits were observed only in the never-smoker population. Given that smoking increases the tumor mutation burden (TMB) in tumor cells (42), smokers tend to benefit from immunotherapy than never-smokers. However, the effect of smoking on immunotherapy efficacy in advanced NSCLC has been inconsistent across clinical strategies. In first-line treatment, smokers benefit more from immunotherapy alone than never-smokers (43). Conversely, when first-line chemoimmunotherapy is used, both smoking and nonsmoking subgroups experience similar clinical benefits (44).
The efficacy of different immunotherapeutic agents in NSCLC also varies by smoking status. A meta-analysis (45) demonstrated that both smokers and never-smokers with NSCLC gained significant OS benefits from pembrolizumab plus chemotherapy, with a reduced risk of death of 32–46% in smokers and 70–84% in never-smokers, respectively. However, only smokers benefited from atezolizumab combined with chemotherapy. These differences may stem from variations in the pathological molecular characteristics and immune microenvironments of the tumors. Filetti et al. (46) conducted a retrospective analysis of 142 patients with PD-L1 expression >50% who received first-line pembrolizumab monotherapy. Their results showed that among patients with high TMB, never-smokers exhibited a higher overall response rate (100% vs. 73%) and longer median OS (27.95 months vs. 17.65 months) than smokers. Further analysis suggested that this difference was caused by the enrichment of DNA damage response signaling pathways in the tumor tissues of never-smokers.
The superior DFS benefits observed in never-smokers in our meta-analysis may be attributed to similar tumor molecular characteristics. We plan to further verify these findings in future clinical studies. Additionally, given the limited number of studies included in the subgroup analysis and the high heterogeneity in both the previous and current smoking subgroups, the conclusion that DFS benefits were absent in these two populations should be interpreted cautiously.
Our subgroup analysis indicated that adjuvant immunotherapy significantly improved DFS in patients with resected EGFR-negative NSCLC and non-squamous cell carcinoma. Consistent with our findings, previous studies indicate that patients with advanced NSCLC harboring EGFR mutations derive limited benefit from immunotherapy (47–49). Most clinical studies have shown that tumors with EGFR mutations exhibit lower PD-L1 expression than those with wild-type EGFR (50–52). Conversely, wild-type EGFR tumors are characterized by higher TMB (53), stronger T-cell clonality (54), and significant infiltration of CD8+ T cells (53), all of which contribute to enhanced immunotherapy efficacy. Future studies are needed to validate the efficacy across different histological types and molecular subgroups of NSCLC.
This meta-analysis had several limitations. First, the number of trials enrolled in this study was relatively small, and the inclusion of some single-arm trials and retrospective cohort studies may have influenced the overall level of evidence. However, our findings were based on data from 4048 patients, and the quality of the included studies was acceptable, with no significant bias. Additionally, the OS and safety outcomes were consistent with those of previous studies. Second, the OS data were derived from an interim analysis, as the follow-up endpoints of large RCTs had not yet been reached. A longer follow-up period is needed to determine whether a significant improvement in the OS of the ITT population exists. Third, among the included studies, patients in the NADIM II, NADIM, and Shin 2024 trials only received adjuvant immunotherapy alone. In the NeoR-World, KEYNOTE-091, and IMpower010 trials, 84.8% to 100% of participants additionally underwent 1-4 cycles of adjuvant chemotherapy. In the BR.31 trial, patients were permitted to receive adjuvant chemotherapy, while in the LCMC3 trial, adjuvant chemotherapy with or without radiotherapy was allowed, but data on combination therapy were unclear. We intended to perform a subgroup analysis to evaluate the confounding effects of chemotherapy combination therapy. However, insufficient data prevented this execution.
Overall, our findings suggest that adjuvant immunotherapy improves DFS in patients with resected NSCLC, particularly in those who are EGFR-negative, have PD-L1 expression of 1–49%, have non-squamous cell carcinoma, and have never smoked. This treatment regimen demonstrates a favorable safety and tolerability profile. These results highlight adjuvant immunotherapy as a promising therapeutic option for IB–III NSCLC following complete resection. Further clinical trials are warranted to clarify its role across different NSCLC stages.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
HH: Conceptualization, Data curation, Investigation, Writing – original draft, Writing – review & editing. PB: Data curation, Resources, Software, Writing – review & editing. HJ: Project administration, Software, Writing – review & editing. WL: Investigation, Writing – review & editing. HS: Conceptualization, Software, Writing – review & editing. ZQ: Formal analysis, Supervision, Writing – review & editing. YP: Methodology, Resources, Writing – review & editing. XS: Funding acquisition, Methodology, Writing – review & editing. DK: Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the National Natural Science Foundation of China—Youth Science Fund (82002219).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1493221/full#supplementary-material
Abbreviations
NSCLC, non-small cell lung cancer; EGFR, epidermal growth factor receptor; TRAEs, treatment-related adverse events; SAEs, severe adverse events; DFS, disease-free survival; HR, hazard ratio; CI, confidence interval; ICIs, immune checkpoint inhibitors; PD-L1, programmed death-ligand 1; OS, overall survival; PD-1, programmed death 1; ALK, anaplastic lymphoma kinase; ITT, intention-to-treat.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Chansky K, Detterbeck FC, Nicholson AG, Rusch VW, Vallières E, Groome P, et al. The IASLC lung cancer staging project: external validation of the revision of the TNM stage groupings in the eighth edition of the TNM classification of lung cancer. J Thorac Oncol. (2017) 12:1109–21. doi: 10.1016/j.jtho.2017.04.011
3. Sekihara K, Hishida T, Yoshida J, Oki T, Omori T, Katsumata S, et al. Long-term survival outcome after postoperative recurrence of non-small-cell lung cancer: who is 'cured' from postoperative recurrence? Eur J Cardiothorac Surg. (2017) 52:522–8. doi: 10.1093/ejcts/ezx127
4. Higashiyama M, Miyazaki R, Yamamoto H, Anayama T, Kikuchi S, Hirohashi K, et al. Preoperative AminoIndex Cancer Screening (AICS) abnormalities predict postoperative recurrence in patients undergoing curative resection for non-small cell lung cancer. BMC Cancer. (2020) 20:1100. doi: 10.1186/s12885-020-07575-w
5. Martin J, Ginsberg RJ, Venkatraman ES, Bains MS, Downey RJ, Korst RJ, et al. Long-term results of combined-modality therapy in resectable non-small-cell lung cancer. J Clin Oncol. (2002) 20:1989–95. doi: 10.1200/JCO.2002.08.092
6. Ni J, Guo T, Li Y, Yang X, Li Y, Zou L, et al. Patterns and risks of postoperative recurrence in completely resected EGFR-mutant non-small cell lung cancer: prognostic significance of routine immunohistochemical markers. Transl Lung Cancer Res. (2019) 8:967–78. doi: 10.21037/tlcr.2019.12.02
7. Douillard J-Y, Rosell R, De Lena M, Carpagnano F, Ramlau R, Gonzáles-Larriba JL, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. (2006) 7:719–27. doi: 10.1016/S1470-2045(06)70804-X
8. Winton T, Livingston R, Johnson D, Rigas J, Johnston M, Butts C, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. (2005) 352:2589–97. doi: 10.1056/NEJMoa043623
9. Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon J-P, Vansteenkiste J. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. (2004) 350:351–60. doi: 10.1056/NEJMoa031644
10. Pignon J-P, Tribodet H, Scagliotti GV, Douillard J-Y, Shepherd FA, Stephens RJ, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. (2008) 26:3552–9. doi: 10.1200/JCO.2007.13.9030
11. Wu Y-L, Tsuboi M, He J, John T, Grohe C, Majem M, et al. Osimertinib in resected -mutated non-small-cell lung cancer. N Engl J Med. (2020) 383:1711–23. doi: 10.1056/NEJMoa2027071
12. Wu YL, Dziadziuszko R, Ahn JS, Barlesi F, Nishio M, Lee DH, et al. Alectinib in resected ALK-positive non-small-cell lung cancer. N Engl J Med. (2024) 390:1265–76. doi: 10.1056/NEJMoa2310532
13. Liu J-F, Sun X-S, Yin J-H, Xu X-E. Adjuvant EGFR-TKI therapy in resected EGFR-mutation positive non-small cell lung cancer: A real-world study. Front In Oncol. (2023) 13:1132854. doi: 10.3389/fonc.2023.1132854
14. Felip E, Altorki N, Zhou C, Vallières E, Martínez-Martí A, Rittmeyer A, et al. Overall survival with adjuvant atezolizumab after chemotherapy in resected stage II-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase III trial. Ann Oncol. (2023) 34:907–19. doi: 10.1016/j.annonc.2023.07.001
15. Felip E, Altorki N, Zhou C, Csőszi T, Vynnychenko I, Goloborodko O, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. (2021) 398:1344–57. doi: 10.1016/S0140-6736(21)02098-5
16. Yang Z, Wang S, Yang H, Jiang Y, Zhu L, Zheng B, et al. Treatment patterns and clinical outcomes of patients with resectable non-small cell lung cancer receiving neoadjuvant immunochemotherapy: A large-scale, multicenter, real-world study (NeoR-World). J Thorac Cardiovasc Surg. (2024) 168:1245–58.e17. doi: 10.1016/j.jtcvs.2024.02.006
17. Besse B, Havel L, Peters S, Marreaud SI, Jha N, Oselin K, et al. 120MO Adjuvant pembrolizumab versus placebo for early-stage NSCLC after resection and optional chemotherapy: Updated results from PEARLS/KEYNOTE-091. Immuno-Oncology Technology. (2023) 20:100592. doi: 10.1016/j.iotech.2023.100592
18. Rothschild SI, Zippelius A, Eboulet EI, Savic Prince S, Betticher D, Bettini A, et al. SAKK 16/14: durvalumab in addition to neoadjuvant chemotherapy in patients with stage IIIA(N2) non-small-cell lung cancer-A multicenter single-arm phase II trial. J Clin Oncol. (2021) 39:2872–80. doi: 10.1200/JCO.21.00276
19. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
20. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. (2016) 355:i4919. doi: 10.1136/bmj.i4919
21. Langan D, Higgins JPT, Jackson D, Bowden J, Veroniki AA, Kontopantelis E, et al. A comparison of heterogeneity variance estimators in simulated random-effects meta-analyses. Res Synth Methods. (2019) 10:83–98. doi: 10.1002/jrsm.v10.1
22. O'Brien M, Paz-Ares L, Marreaud S, Dafni U, Oselin K, Havel L, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. (2022) 23:1274–86. doi: 10.1016/S1470-2045(22)00518-6
23. Shin J, Park S, Kim KH, Shin EC, Jung HA, Cho JH, et al. Adjuvant pembrolizumab in patients with stage IIIA/N2 non-small cell lung cancer completely resected after neoadjuvant concurrent chemoradiation: A prospective, open-label, single-arm, phase 2 trial. Cancer Res Treat. (2024) 56:1084–95. doi: 10.4143/crt.2024.084
24. Cascone T, Awad MM, Spicer JD, He J, Lu S, Sepesi B, et al. Perioperative nivolumab in resectable lung cancer. N Engl J Med. (2024) 390:1756–69. doi: 10.1056/NEJMoa2311926
25. Zhao ZR, Yan WP, Yu XY, Zhang JB, Fang YF, Ma K, et al. Adjuvant immunotherapy does not improve survival in non-small cell lung cancer with major/complete pathologic response after induction immunotherapy. J Thorac Cardiovasc Surg. (2024) S0022-5223(24)01109-7. doi: 10.1016/j.jtcvs.2024.11.028
26. Cerqueira ER, Batista PM, Almeida MF, Rego MAC, Ribeiro-Pereira ACP, Alencar F, et al. The journey of stage III and IV non-small cell lung cancer patients in the Brazilian private healthcare system: a retrospective study. Front Oncol. (2023) 13:1257003. doi: 10.3389/fonc.2023.1257003
27. Dong Y, Xu L, Si H, Wu J, Chen T, Wen J, et al. P2.07C.02 prognostic impact of adjuvant immunotherapy in patients with non-small cell lung cancer following neoadjuvant immunotherapy. J Thorac Oncol. (2024) 19:S241. doi: 10.1016/j.jtho.2024.09
28. Provencio M, Nadal E, González-Larriba JL, Martínez-Martí A, Bernabé R, Bosch-Barrera J, et al. Perioperative nivolumab and chemotherapy in stage III non-small-cell lung cancer. N Engl J Med. (2023) 389:504–13. doi: 10.1056/NEJMoa2215530
29. Provencio M, Serna-Blasco R, Nadal E, Insa A, García-Campelo MR, Casal Rubio J, et al. Overall Survival and Biomarker Analysis of Neoadjuvant Nivolumab Plus Chemotherapy in Operable Stage IIIA Non-Small-Cell Lung Cancer (NADIM phase II trial). J Clin Oncol. (2022) 40:2924–33. doi: 10.1200/JCO.21.02660
30. Provencio M, Nadal E, Insa A, García-Campelo MR, Casal-Rubio J, Dómine M, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. (2020) 21:1413–22. doi: 10.1016/S1470-2045(20)30453-8
31. Chaft JE, Oezkan F, Kris MG, Bunn PA, Wistuba II, Kwiatkowski DJ, et al. Neoadjuvant atezolizumab for resectable non-small cell lung cancer: an open-label, single-arm phase II trial. Nat Med. (2022) 28:2155–61. doi: 10.1038/s41591-022-01962-5
32. Carbone D, Waqar SN, Chaft J, Kris MG, Johnson BE, Lee JM, et al. 145MO Updated survival, efficacy and safety of adjuvant (adj) atezolizumab (atezo) after neoadjuvant (neoadj) atezo in the phase II LCMC3 study. J Thorac Oncol. (2023) 18:S90–S1. doi: 10.1016/S1556-0864(23)00339-8
33. Goss G, Darling GE, Westeel V, Nakagawa K, Massuti Sureda B, Perrone F, et al. LBA48 CCTG BR.31: A global, double-blind placebo-controlled, randomized phase III study of adjuvant durvalumab in completely resected non-small cell lung cancer (NSCLC). Ann Oncol. (2024) 35:S1238. doi: 10.1016/j.annonc.2024.08.2289
34. Nuccio A, Viscardi G, Salomone F, Servetto A, Venanzi FM, Riva ST, et al. Systematic review and meta-analysis of immune checkpoint inhibitors as single agent or in combination with chemotherapy in early-stage non-small cell lung cancer: Impact of clinicopathological factors and indirect comparison between treatment strategies. Eur J Cancer. (2023) 195:113404. doi: 10.1016/j.ejca.2023.113404
35. Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. (2010) 236:219–42. doi: 10.1111/j.1600-065X.2010.00923.x
36. Patsoukis N, Brown J, Petkova V, Liu F, Li L, Boussiotis VA. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. (2012) 5:ra46. doi: 10.1126/scisignal.2002796
37. Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science. (2017) 355:1428–33. doi: 10.1126/science.aaf1292
38. Rodríguez-Abreu D, Powell SF, Hochmair MJ, Gadgeel S, Esteban E, Felip E, et al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: protocol-specified final analysis from KEYNOTE-189. Ann Oncol. (2021) 32:881–95. doi: 10.1016/j.annonc.2021.04.008
39. Paz-Ares L, Vicente D, Tafreshi A, Robinson A, Soto Parra H, Mazières J, et al. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol. (2020) 15:1657–69. doi: 10.1016/j.jtho.2020.06.015
40. Zhang W, Liang Z, Zhao Y, Li Y, Chen T, Li W, et al. Efficacy and safety of neoadjuvant immunotherapy plus chemotherapy followed by adjuvant immunotherapy in resectable non-small cell lung cancer: a meta-analysis of phase 3 clinical trials. Front In Immunol. (2024) 15:1359302. doi: 10.3389/fimmu.2024.1359302
41. Merino M, Kasamon Y, Theoret M, Pazdur R, Kluetz P, Gormley N. Irreconcilable differences: the divorce between response rates, progression-free survival, and overall survival. J Clin Oncol. (2023) 41:2706–12. doi: 10.1200/JCO.23.00225
42. Wang X, Ricciuti B, Nguyen T, Li X, Rabin MS, Awad MM, et al. Association between smoking history and tumor mutation burden in advanced non-small cell lung cancer. Cancer Res. (2021) 81:2566–73. doi: 10.1158/0008-5472.CAN-20-3991
43. El-Osta H, Jafri S. Predictors for clinical benefit of immune checkpoint inhibitors in advanced non-small-cell lung cancer: a meta-analysis. Immunotherapy. (2019) 11:189–99. doi: 10.2217/imt-2018-0086
44. El-Osta HE, Mott FE, Burt BM, Wang DY, Sabichi AL. Predictors of benefits from frontline chemoimmunotherapy in stage IV non-small-cell lung cancer: a meta-analysis. Oncoimmunology. (2019) 8:e1665974. doi: 10.1080/2162402X.2019.1665974
45. Sheng L, Gao J, Xu Q, Zhang X, Huang M, Dai X, et al. Selection of optimal first-line immuno-related therapy based on specific pathological characteristics for patients with advanced driver-gene wild-type non-small cell lung cancer: a systematic review and network meta-analysis. Ther Adv Med Oncol. (2021) 13:17588359211018537. doi: 10.1177/17588359211018537
46. Filetti M, Occhipinti M, Cirillo A, Scirocchi F, Ugolini A, Giusti R, et al. Exploring genomic biomarkers for pembrolizumab response: A real-world approach and patient similarity network analysis reveal DNA response and repair gene mutations as a signature. Cancers (Basel). (2024) 16:3955. doi: 10.3390/cancers16233955
47. Lee CK, Man J, Lord S, Links M, Gebski V, Mok T, et al. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer-A meta-analysis. J Thorac Oncol. (2017) 12:403–7. doi: 10.1016/j.jtho.2016.10.007
48. Lee CK, Man J, Lord S, Cooper W, Links M, Gebski V, et al. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non-small cell lung carcinoma: A systematic review and meta-analysis. JAMA Oncol. (2018) 4:210–6. doi: 10.1001/jamaoncol.2017.4427
49. Rizvi NA, Hellmann MD, Brahmer JR, Juergens RA, Borghaei H, Gettinger S, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. (2016) 34:2969–79. doi: 10.1200/JCO.2016.66.9861
50. Dietel M, Savelov N, Salanova R, Micke P, Bigras G, Hida T, et al. Real-world prevalence of programmed death ligand 1 expression in locally advanced or metastatic non-small-cell lung cancer: The global, multicenter EXPRESS study. Lung Cancer. (2019) 134:174–9. doi: 10.1016/j.lungcan.2019.06.012
51. Gainor JF, Shaw AT, Sequist LV, Fu X, Azzoli CG, Piotrowska Z, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: A retrospective analysis. Clin Cancer Res. (2016) 22:4585–93. doi: 10.1158/1078-0432.CCR-15-3101
52. Schoenfeld AJ, Rizvi H, Bandlamudi C, Sauter JL, Travis WD, Rekhtman N, et al. Clinical and molecular correlates of PD-L1 expression in patients with lung adenocarcinomas. Ann Oncol. (2020) 31:599–608. doi: 10.1016/j.annonc.2020.01.065
53. Dong Z-Y, Zhang J-T, Liu S-Y, Su J, Zhang C, Xie Z, et al. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. Oncoimmunology. (2017) 6:e1356145. doi: 10.1080/2162402X.2017.1356145
Keywords: adjuvant immunotherapy, immune checkpoint inhibitors, ICIs, non-small cell lung cancer, NSCLC
Citation: Huang H, Bao P, Jin H, Li W, Shen H, Qin Z, Pan Y, Su X and Kong D (2025) Adjuvant immunotherapy improves survival in completely resected stage IB–III NSCLC: a systematic review and meta-analysis. Front. Oncol. 15:1493221. doi: 10.3389/fonc.2025.1493221
Received: 08 September 2024; Accepted: 20 March 2025;
Published: 08 April 2025.
Edited by:
Mohamed Rahouma, NewYork-Presbyterian, United StatesReviewed by:
Joseph Gergi Kattan, Hôtel-Dieu de France, LebanonAlberto Pavan, Azienda ULSS 3 Serenissima, Italy
Copyright © 2025 Huang, Bao, Jin, Li, Shen, Qin, Pan, Su and Kong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Delei Kong, a29uZ2RlbGVpQDEyNi5jb20=
 Hong Huang
Hong Huang Pengchen Bao2
Pengchen Bao2 Hongyu Jin
Hongyu Jin Wenyang Li
Wenyang Li Hui Shen
Hui Shen Ying Pan
Ying Pan Delei Kong
Delei Kong