- 1Department of Pharmacy, Shaukat Khanum Memorial Cancer Hospital and Research Centre, Lahore, Pakistan
- 2Punjab University College of Pharmacy, University of The Punjab, Lahore, Pakistan
- 3Department of Pathology, Shaukat Khanum Memorial Cancer Hospital and Research Centre, Lahore, Pakistan
- 4Basic Sciences Research, Shaukat Khanum Memorial Cancer Hospital and Research Centre, Lahore, Pakistan
- 5Department of Cancer Registry and Clinical Data Management, Shaukat Khanum Memorial Cancer Hospital and Research Centre, Lahore, Pakistan
- 6Department of Radiology, Shaukat Khanum Memorial Cancer Hospital and Research Centre, Lahore, Pakistan
- 7Department of Surgical Oncology, Shaukat Khanum Memorial Cancer Hospital and Research Centre, Lahore, Pakistan
Background: Tongue squamous cell carcinoma (TSCC) is a significant global health issue with high incidence and mortality rates. Current treatments involve surgery, radiotherapy, and chemotherapy; however, prognosis remains poor. Recent research highlights the crucial role of the tumor microenvironment, especially immune cells and checkpoints like PD-L1 and IDO, in TSCC progression.
Aim: This study aims to investigate the expression of IDO and PD-L1 in TSCC patients before and after chemotherapy and their association with patients’ clinicopathological characteristics.
Materials and Methods: This study involved 106 TSCC patients from Shaukat Khanum Memorial Cancer Hospital and Research Centre (SKMCH&RC) in Pakistan, with biopsies obtained from 2012 to 2022. Immunohistochemical analysis was performed on formalin-fixed, paraffin-embedded (FFPE) tumor samples to evaluate IDO and PD-L1 expression before and after chemotherapy. Data on patient demographics, tumor characteristics, and treatment were collected, and follow-up continued until January 2024.
Results: The cohort had a mean age of 48.9 years, with a predominance of male patients. Prior to chemotherapy, 83% of patients were IDO-negative, and 75.5% were PD-L1-negative. Post-chemotherapy, IDO expression increased to 24.5% of patients (n = 26), with 84.6% exhibiting low expression and 15.4% showing high expression. While PD-L1 expression was increased to 29.2% (n = 31), with 90.3% of the positive cases showing low expression and 9.7% high expression. IDO expression was notably higher in multifocal tumors and correlated with increased comorbidities post-chemotherapy. Despite changes in marker expression, there was no significant difference in survival rates associated with IDO or PD-L1 expression.
Conclusion: Chemotherapy appears to upregulate IDO and PD-L1 expressions in TSCC, highlighting the potential for integrating immunotherapy into treatment regimens. Further studies are needed to explore the dynamics of these biomarkers over time and their impact on patient outcomes, emphasizing the need for comprehensive therapeutic strategies.
Introduction
Oral cancer is one of the most prevalent malignancies of the head and neck region, posing significant challenges to patient well-being and healthcare systems (1). The recent data from the Globocan 2022 report reveals the extensive impact of oral cancer, with a global incidence reaching 389,846 cases and a mortality toll of 188,438 (2). Specifically in Pakistan, oral cancer holds the position of the second most prevalent tumor, contributing to a substantial burden with 15,915 reported cases and 10,181 associated deaths (3). Among head and neck cancer cases, oral cancer holds the leading position with a continuing rise in its incidence (1). Oral cancer is characterized by its highly malignant nature, marked by elevated rates of local recurrence and cervical lymph node metastasis (4, 5). The reported 5-year survival rate for oral cancer is approximately 50% (6). Currently, the preferred treatment for tongue cancer involves a combination of surgery, postoperative radiotherapy, and chemotherapy (7). However, the prognosis for oral cancer remains grim due to short-term recurrence and inadequate therapeutic efficacy, significantly impacting the quality of life for affected patients (8).
Increasing evidence highlights the pivotal role played by the interaction between tongue cancer cells and the surrounding microenvironment in the development and prognosis of tongue cancer (9). Predominantly, TSCC stands out for its marked infiltration of immune cells, characterizing it as an immunogenic tumor (10). Among the prevalent stromal components in tongue cancer, the infiltrated immune cells emerge as key contributors to the carcinogenic process (10).
The concept of cancer immunoediting is widely accepted, and tumor immune escape is considered an emerging hallmark of cancer (10). Cancer immunotherapy utilizing immune-checkpoint inhibitors has emerged as a highly effective therapeutic modality for various cancers, including tongue cancer (11–13). In several malignancies, programmed death ligand-1 (PD-L1) checkpoint blockades have shown substantial clinical efficacy (14–16). Recent evidence has pointed out that the expression of PD-1 and PD-L1 were considerably associated with local recurrence in patients with TSCC (13). Overexpression of PD-L1 on tumor cells inhibits the activation of T cells, leading to the progression of tumors (17–20). PD-L1 overexpression in TSCC correlates with advanced stage and shorter disease-free survival (21). Several ongoing clinical trials have demonstrated efficacy against recurrent/metastatic head and neck squamous cell carcinoma (HNSCC), leading to clinical approval in multiple countries following successful phase III trials (22). Thus, the focus on immune checkpoints represents a new area of investigation for novel cancer therapies (23–25). Another such prospective target in this realm is indoleamine 2,3-dioxygenase (IDO), a checkpoint protein influencing an immunosuppressive tumor microenvironment (26). IDO, a heme-containing enzyme, metabolizes L-tryptophan into kynurenine (27). The localized depletion of tryptophan hinders T-cell cytotoxicity, thereby inhibiting T-cell immune responses through the induction of regulatory T-cell differentiation (28, 29). IDO expression has been reported in many cancers, including breast, colorectal, ovarian, gastric, and oral cancer (28, 30–34). It is also associated with cancer poor prognosis in oral squamous cell carcinoma patients (33). The significance of IDO expression is underscored by its ability to predict tumor responsiveness to anti-IDO immunotherapy. Similarly, high PD-L1 expression is associated with an unfavorable prognosis in diverse tumors. Thus, evaluating the levels of IDO and PD-L1 expressions may offer insights into identifying patients who could potentially benefit from anti-IDO/anti-PD-L1 therapy or combination therapies. In this study’s context, we performed an immunohistochemical analysis to investigate the expressions of IDO and PD-L1 in individuals with TSCC.
Materials and methods
Patients and data
We conducted a retrospective cross-sectional analysis involving tongue cancer patients who were registered at Shaukat Khanum Memorial Cancer Hospital and Research Centre (SKMCH&RC) in Pakistan. The study cohort comprised (106) patients diagnosed with tongue cancer between 2012 and 2022. Biopsy samples were collected from all patients before chemotherapy at the time of their initial diagnosis. Following neoadjuvant chemotherapy, all patients underwent surgical resection, during which post-treatment tissue samples were obtained for comparative expression analysis. Following surgery, all patients received radiotherapy, with some also receiving concurrent chemoradiotherapy (CRT); however, they developed metastases at a later stage. Since the primary objective was to evaluate the effect of chemotherapy, biomarker expression was analyzed only before and after chemotherapy. The impact of radiotherapy on expression levels was not assessed. These patients also underwent surgery following chemotherapy, but they developed metastases at a later stage.
Moreover, all patients received cisplatin plus gemcitabine (GC) as neoadjuvant chemotherapy. The use of GC was based on prior experience at SKMCH&RC, Lahore, Pakistan. A previous study from our institution demonstrated that GC was well tolerated, had low toxicity, and showed significant antitumor activity in locally advanced head and neck cancer (HANC) (35). Building on these positive results and ongoing clinical success, GC has been adopted as the institutional neoadjuvant regimen for selected cases of advanced HANC.
Biopsies stored in formalin-fixed, paraffin-embedded (FFPE) blocks were obtained from the pathology department at SKMCH&RC. Extensive patient data, including demographics, pathological and radiological characteristics, as well as treatment specifics, were retrieved from SKMCH&RC’s electronic medical records system. Patient follow-up for survival analysis was extended until Jan 2024. The study received approval from the institutional review board (IRB) of SKMCH&RC (EX-05-12-22-02), with the IRB granting a waiver of informed consent due to the minimal risk posed to patients’ rights, safety, and well-being, given that the data and FFPE samples originated from archived records.
PD-L1 and IDO Expression analysis by immunohistochemistry
Two sections of FFPE tumor specimens of the same patients were cut at a thickness of 4 µm. IDO staining was performed using an anti-Indoleamine 2, 3-dioxygenase antibody (Cat # 86630S IDO (D5J4E™) Rabbit mAb); heat-mediated epitope retrieval with a Tris-EDTA buffer was performed. The immunoreactivity was detected by using the Dako EnVision kit (K8002). Normal human reactive lymph nodes served as a positive control. PD-L1 immunoreactivity was assessed by an immunohistochemical assay for FFPE tumor specimens. Slides were stained using an autostainer Link 48 (Dako Denmark) as per the manufacturer’s protocol. Slides were deparaffinized and antigen was retrieved simultaneously with the target retrieval low pH solution (#GV8005 Dako). PD-L1 antibody (22C3) and an automated staining procedure developed by DAKO. PD-L1 labeling was visualized using the Envision Flex detection kit DAKO (K8002). Normal human tonsils served as a positive control. Slides were visualized by an optical microscope (Provis AX-70, Olympus, Melville, NY).
Scoring
Pathologists assessed all the results. They performed a blind histopathologic evaluation. The discrepancies between the pathologists were examined mutually to reach a consensus and the mean score of both was considered a decisive score. The total IDO immunostaining scores were calculated as described earlier (36). The intensity was scored for IDO as negative (0), weak (1), moderate (2), or strong (3). The percentage of positive tumor cells was classified into four categories: diffuse (3+, 50–75%), focal (2+, 25–50%), sporadic (1+, 5–25%), and negative (0, 0%). The immunohistochemical expression of PD-L1 was calculated as described earlier (37). PD-L1 staining intensity was assessed as strong (3), moderate (2), weak (1), or negative (0). The percentage of tumor cells with positive staining was categorized according to the following formula: PD-L1 expression score (H score) (range, 0–9)=0×% of non-stained tumor cells +1×% of weakly stained tumor cells +2×% of moderately stained tumor cells +3×% of strongly stained tumor cells.
Currently CPS scoring is the standard practice in PD-L1 interpretation of Oral SCCs (38–40). The CPS scoring system was used to ensure uniformity and comparability with scoring system used for IDO IHC. The CPS score was used as a cut off for positive results and then as an additional step, the intensity of stain and overall percentage of positive cells were also considered (38, 41–44). This approach was followed to harmonize our IDO results, as both intensity and percentage evaluation for IDO and PD-L1 were applied.
Statistical analysis
Statistical analysis was performed by using SPSS software (version 20.0; SPSS, Chicago, IL, USA). Frequency and percentage were used for categorical variables while the median and range (min-max) were used for continuous variables. Bivariate analysis was done using chi-square or Fisher exact test (where necessary). For continuous explanatory variables such as age, the independent t-test was performed. In addition, McNemar’s test was performed to bifurcate the categorical pre and post data. Survival curves were generated using the Kaplan–Meier tool to estimate the probability of survival over time. Statistical significance was defined as a two-tailed P-value of 0.05.
Results
Patient demographics
The study included 106 participants with a mean age of 48.9 years (SD = 11.9) and a median age of 50.5 years, ranging from 20 to 78 years. Among the participants, 67.0% were male (n = 71) and 33.0% were female (n = 35). The demographic data reveals a predominantly middle-aged male cohort with a mean BMI (26.1 ± 4.5) in the overweight range. Most participants had no family history of disease or history of substance use. Most participants did not have comorbidities, but among those who did, hypertension was the most common condition (52.4%). Among 106 patients, 9.4% of tumors were poorly differentiated, 59.4% were moderately differentiated, and 31.1% were well differentiated as shown in Table 1. The majority of tumors in this cohort were moderately differentiated and primarily unifocal with uninvolved margins. The tumors exhibited a wide range in size and depth of invasion, with a notable proportion at advanced T stages. Despite extensive lymph node extraction, positive nodes were relatively few on average, although extra nodal extension, perineural invasion were significant concerns.
Immunohistochemical staining
The study assessed the expression of IDO and PD-L1 in 106 patients before and after chemotherapy. Prior to chemotherapy, IDO was negative in 83% of patients (n = 88) (Figure 1C) and positive in 17% (n = 18) (Figure 1A). Of the positive cases, 81.3% had low expression, while 16.7% had high expression. Post-chemotherapy, IDO expression increased to 24.5% of patients (n = 26), with 84.6% exhibiting low expression and 15.4% showing high expression (Figure 1D). Among those who were initially IDO positive, 38.9% (n = 7) became IDO negative (Figure 1B), and 61.1% (n = 11) remained positive. For PD-L1, prior to chemotherapy, 75.5% of patients (n = 80) were negative (Figure 2C) and 24.5% (n = 26) were positive (Figure 2A). Among the positive cases, 88.5% had low expression and 11.5% had high expression. After chemotherapy, the PD-L1 expression increased to 29.2% (n = 31), with 90.3% of the positive cases showing low expression and 9.7% high expression (Figure 2D). Our dataset shows that IDO and PD-L1 expressions increased after chemotherapy, suggesting that chemotherapy may upregulate these markers. The negative expression of both markers remain predominant (Figures 3D, E) though there is a slight increase in high expression cases post-chemotherapy (Figures 3A–C, F).
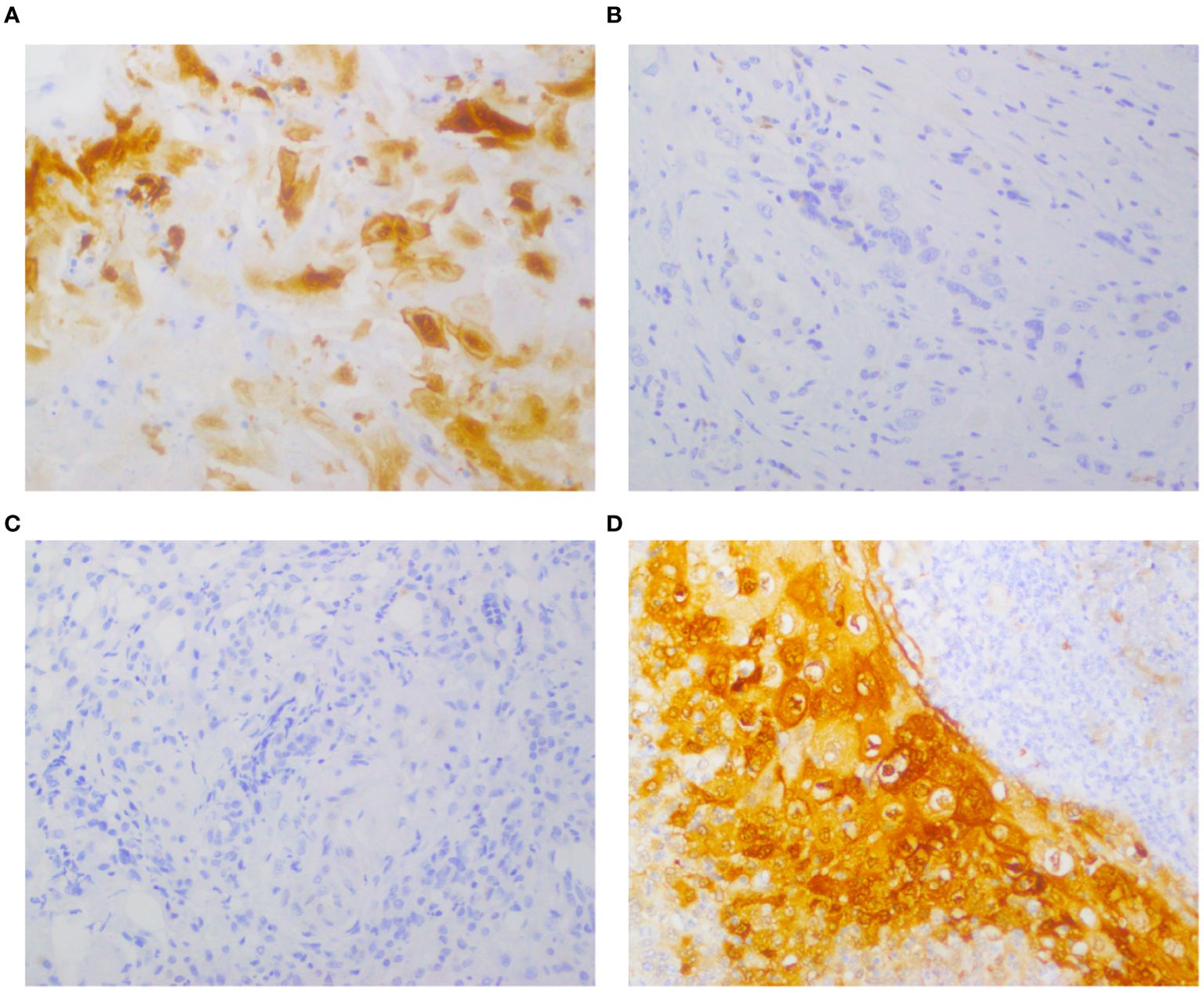
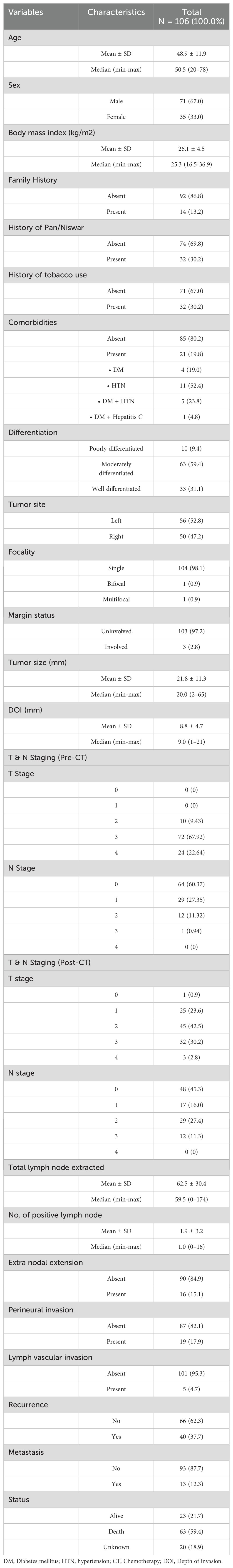
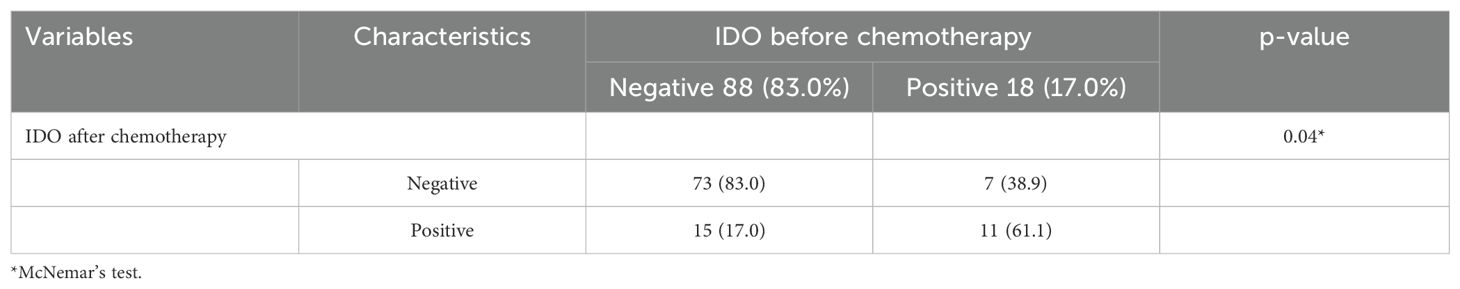
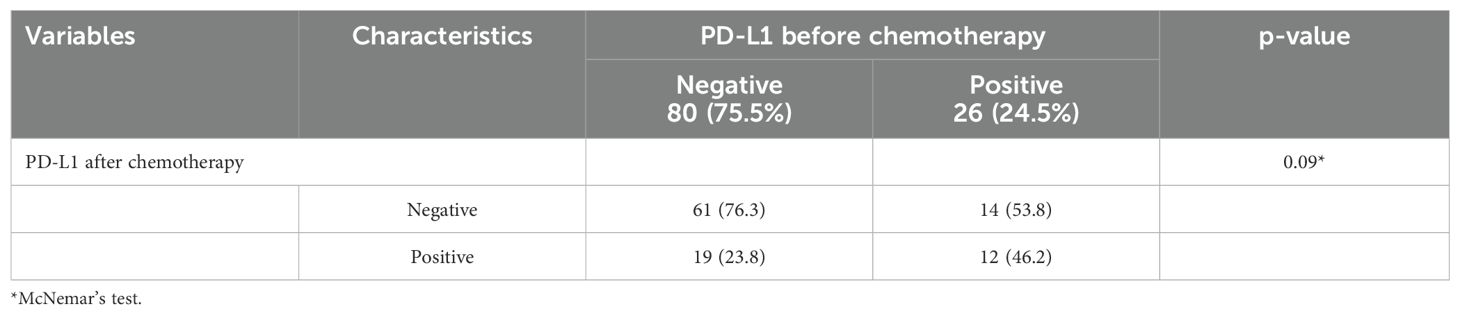
Figure 1. Expression of IDO before and after chemotherapy in the same patient, detected by immunohistochemical staining. Representative images of immunohistochemical staining for IDO in TSCC cases. (A, B) Positive IDO expression in tumor cells pre-chemotherapy; negative IDO expression post-chemotherapy. (C, D) Negative IDO expression pre-chemotherapy; positive IDO expression post-chemotherapy.
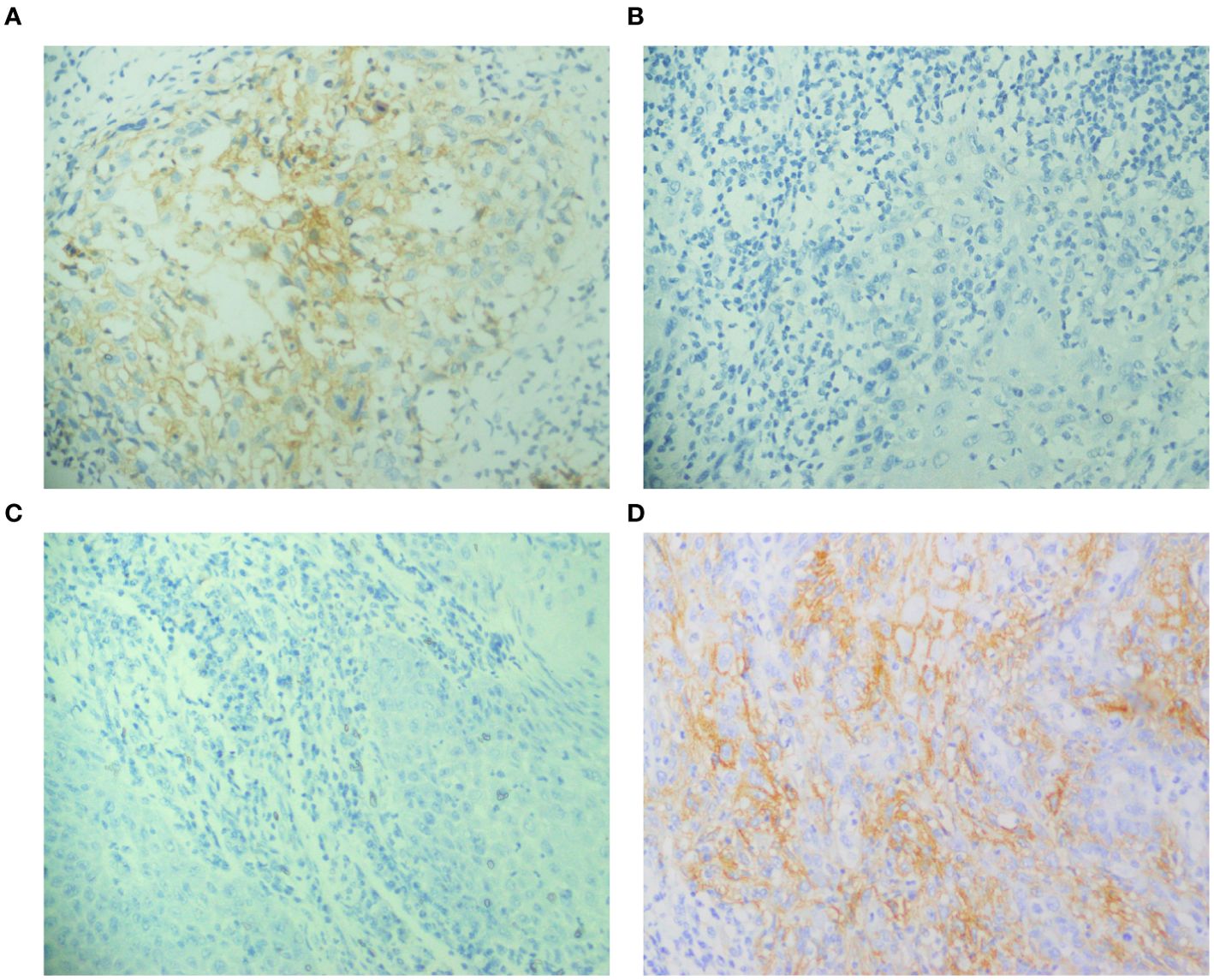
Figure 2. Expression of PD-L1 before and after chemotherapy in the same patient, detected by immunohistochemical staining. (A, B) Positive PD-L1 expression pre-chemotherapy; negative PD-L1 expression post-chemotherapy, (C, D) Negative PD-L1 expression pre-chemotherapy; positive PD-L1 expression post-chemotherapy.
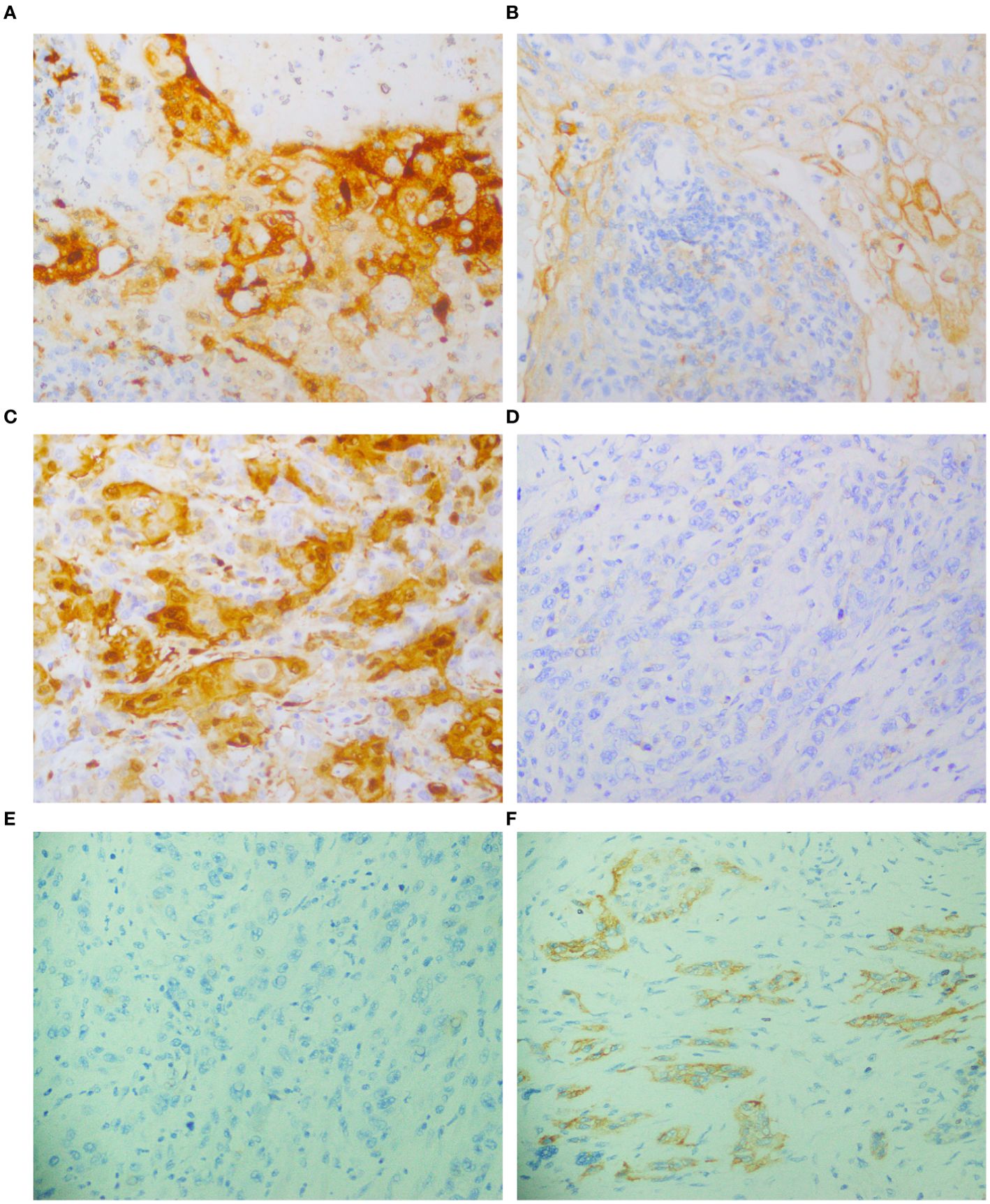
Figure 3. Expression of IDO and PD-L1 in the same patient, detected by immunohistochemical staining. (A, B) IDO positive expression; PD-L1 positive expression. (C, D) IDO positive expression; PD-L1 negative expression, (E, F) IDO negative expression; PD-L1 positive expression. All images were captured at 40X magnification.
Clinicopathological associations with IDO expression prior to chemotherapy
The study analyzed 106 patients to investigate the relationship between demographic and clinicopathological characteristics and IDO status before chemotherapy. A significant association was found between IDO positivity and PD-L1 positivity (p = 0.001), with 50% of IDO-positive patients also being PD-L1 positive. This indicates an immunosuppressive microenvironment in this subset of patients. No significant associations were observed between IDO status and factors such as age, sex, or tumor size. Additionally, we found that all multifocal tumors had positive IDO expression (p = 0.02), as shown in Table 2.
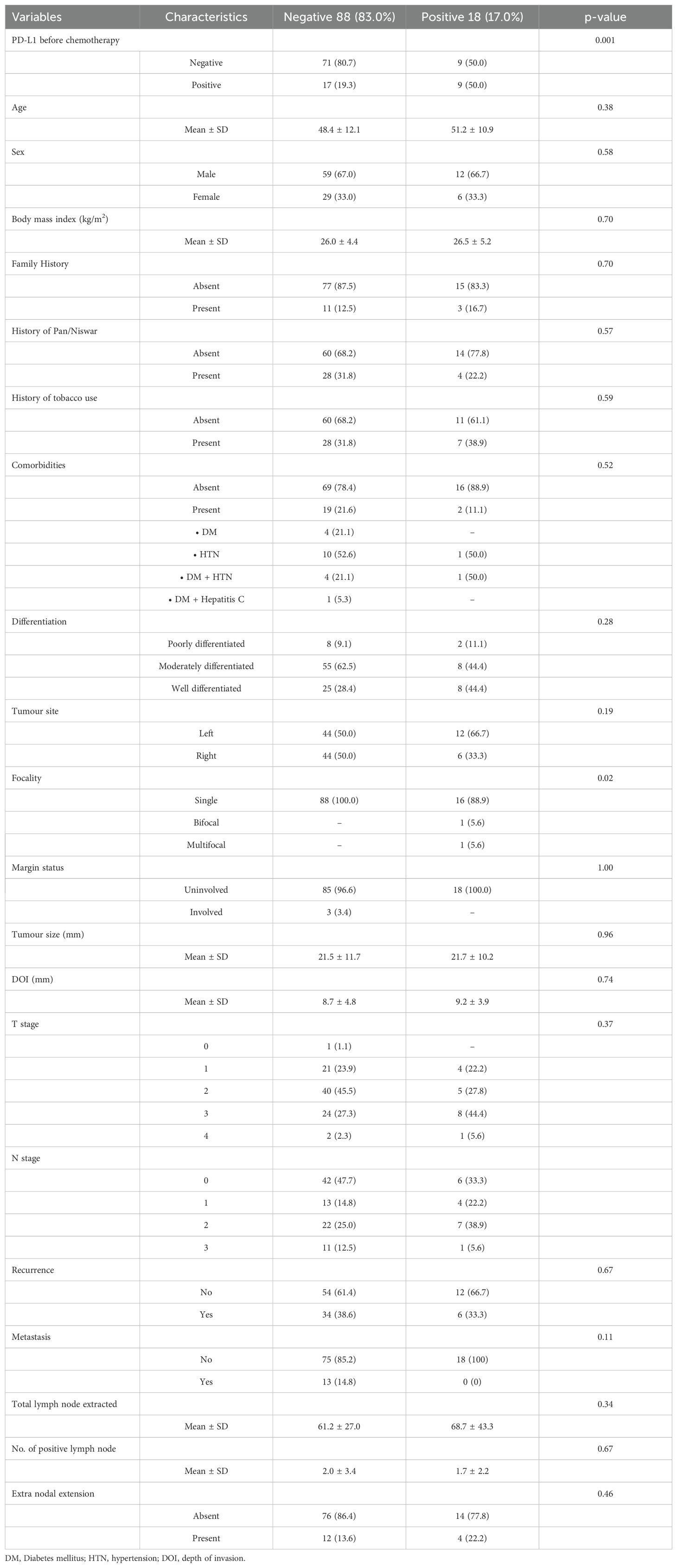
Table 2. Demographic and clinicopathological characteristics versus IDO (before chemotherapy) negative and positive.
Clinicopathological associations with IDO expression post chemotherapy
Post-chemotherapy, we observed a continued significant association between IDO positivity and PD-L1 status (p = 0.001). Additionally, IDO-positive patients had more comorbidities (p = 0.03). No significant differences were found for age, sex, BMI, family history, tobacco use, histology, tumor site, margin status, tumor size, depth of invasion, T stage, N stage, positive lymph nodes, and extranodal extension, as shown in Table 3.
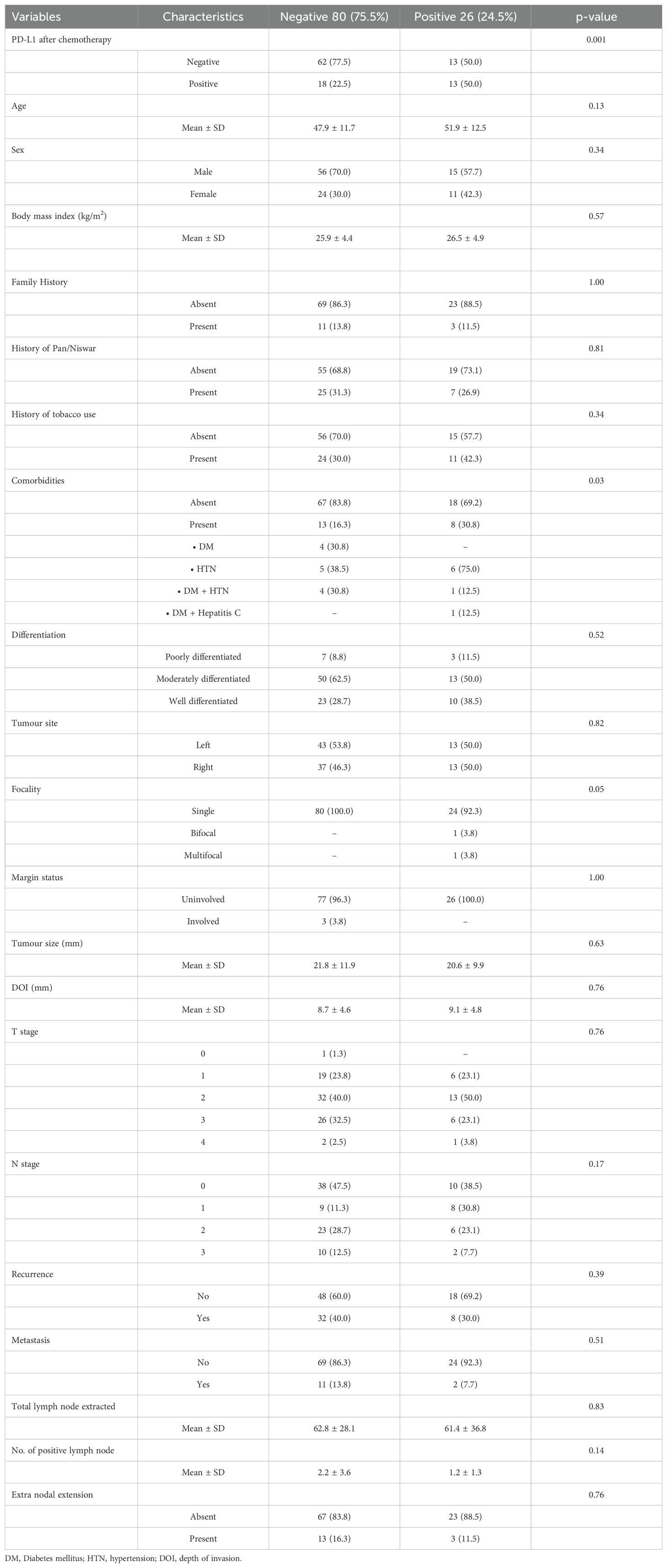
Table 3. Demographic and clinicopathological characteristics versus IDO (after chemotherapy) negative and positive.
Impact of chemotherapy on IDO and PD-L1 expression
In our dataset, 18 patients were IDO positive prior to chemotherapy. After chemotherapy, 7 of these patients became IDO negative. Conversely, among the 88 patients who were initially IDO negative, 15 became IDO positive, resulting in a total of 26 IDO-positive patients’ post-chemotherapy. This change in IDO expression was statistically significant (p = 0.04) according to McNemar’s test (Table 4). Chemotherapy significantly impacted IDO expression, with a notable proportion of initially IDO-positive patients maintaining their positive status post-treatment and some initially IDO-negative patients becoming positive.
Similarly, in our dataset, 26 patients were PD-L1 positive prior to chemotherapy (Table S1 in Supplementary Material). After treatment, 14 of these patients became PD-L1 negative (Figure 2B). Conversely, among the 80 patients who were initially PD-L1 negative, 19 became positive, resulting in a total of 31 PD-L1-positive patients’ post-chemotherapy (Table S2 in Supplementary Material). The change in PD-L1 expression approached statistical significance (p = 0.09) according to McNemar’s test (Table 5). Chemotherapy demonstrated a trend towards altering PD-L1 expression, with some patients switching their PD-L1 status post-treatment. Although the change did not reach statistical significance, the trend may suggest that chemotherapy may influence PD-L1 expression, potentially impacting the tumor’s immune evasion capabilities.
Survival analysis
In our study, 23 patients (21.7%) were alive at the time of last follow-up, while 63 patients (59.4%) had died while 20 (18.9) were unknown. The overall five-year survival rate was 49%, with a median survival time of 53 months, as depicted in Figure 4. We found no significant differences in survival associated with IDO and PD-L1 expression. Pre-chemotherapy, IDO-positive patients had a 68% five-year survival rate compared to 42% for IDO-negative patients (p = 0.18). Post-chemotherapy, these rates were 55% for IDO-positive and 43% for IDO-negative (p = 0.43). For PD-L1 expression, pre-chemotherapy survival was 51% for positive versus 44% for negative (p = 0.86), and post-chemotherapy, it was 45% for positive versus 47% for negative (p = 0.64).
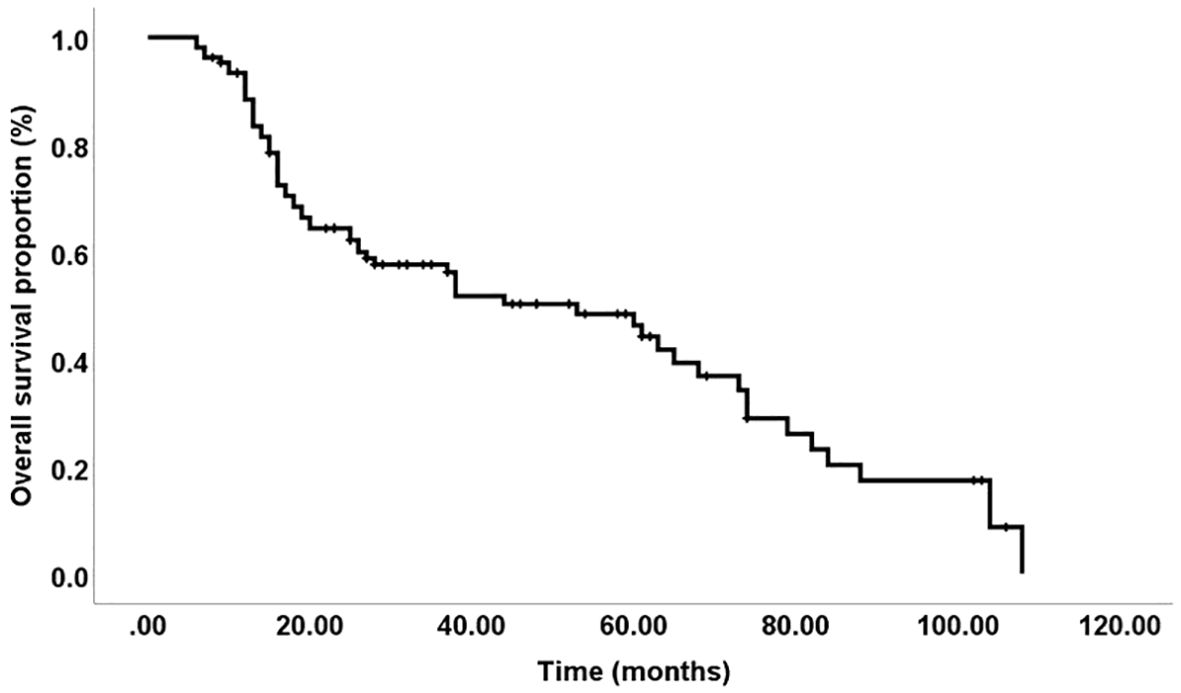
Figure 4. Overall survival: The five-year disease-free survival rate was 49%, with a median duration of 53 months.
Discussion
TSCC is one of the most prevalent malignancies of the head and neck region, constituting a significant global health concern with a survival rate of about 50% (45–47). Traditionally managed with surgery and radiotherapy or chemoradiotherapy, recent research has focused on immunotherapy to improve outcomes (47). The response rate to PD-1 and PD-L1 antibody treatment remains limited, with only about 20% of patients showing favorable outcomes in most solid tumors (48). PD-L1 is a transmembrane glycoprotein structurally characterized by extracellular IgV- and IgC-like domains (49). Under physiological conditions, PD-L1 is expressed by various immune cells, including macrophages, activated T and B lymphocytes, dendritic cells, and certain epithelial cells, particularly in response to pro-inflammatory signals (50). Within the tumor microenvironment, PD-L1 is frequently overexpressed on malignant cells as an adaptive mechanism to suppress cytotoxic T-cell activity and facilitate immune escape (51). This upregulation is often observed in immune-infiltrated tumors characterized by a high density of CD8+ T cells, Th1 cytokines, interferons, and distinct immunomodulatory gene signatures (52). Multiple oncogenic and inflammatory pathways regulate PD-L1 expression in digestive system cancers including the EGFR/ERK signaling axis in esophageal squamous cell carcinoma (53), the JAK/STAT pathway in gastric cancer (54), and the ERK/MAPK pathway in hepatocellular carcinoma (55). In addition, the IFN-γ–driven activation of the JAK/STAT pathway has been shown to mediate PD-L1 induction in myeloid leukemia as well as pancreatic and gastric cancers (56, 57).
Similarly, another potential focus is on IDO, a checkpoint protein implicated in creating an immunosuppressive environment within tumors (58). IDO is a cytosolic heme-containing enzyme that catalyzes the first and rate-limiting step in the metabolism of tryptophan (Trp) to kynurenine (Kyn) (59). It initiates the oxidative cleavage of the pyrrole ring of L-tryptophan to produce N-formylkynurenine, which is subsequently converted into L-kynurenine by kynurenine formamidase (60). Kynurenine and its downstream metabolites contribute to immunosuppression by inhibiting T-cell responses, enabling tumor cells to evade immune surveillance (61). L-kynurenine is further metabolized to anthranilic acid via kynureninase, then to 3-hydroxyanthranilic acid through kynurenine-3-monooxygenase, ultimately generating 2-amino-muconic acid, picolinic acid, and quinolinic acid (62). These downstream metabolites reinforce immune evasion by promoting regulatory T-cell differentiation and inhibiting effector T-cell activity, thereby fostering an immunosuppressive tumor microenvironment (63).
IDO plays a critical role in the pathogenesis of various conditions, including chronic inflammatory diseases, infections, and a wide range of malignancies (33, 64, 65). Its aberrant overexpression has been reported in several tumor types, including oral, colorectal, hepatocellular, ovarian cancers, and melanomas (33, 65). High IDO expression within the tumor microenvironment contributes to immune evasion, facilitates tumor progression and metastasis, and is strongly associated with poor clinical outcomes and decreased overall survival in cancer patients (66–68). Consistently, preclinical studies have demonstrated that IDO overexpression is linked to poor prognosis across multiple cancer types (69). Elevated IDO levels have been observed across various cancers such as breast, colorectal, ovarian, gastric cancer, and TSCC (30–34, 65), correlating with poorer prognosis in TSCC patients (70). This highlights the necessity for enhanced combined immunotherapeutic strategies and predictive biomarkers to effectively identify patients who could derive optimal benefit from these treatments.
In this study, we evaluated the expression of IDO and PD-L1 in 106 patients with tongue cancer both before and after chemotherapy. Our analysis indicates that chemotherapy significantly influences the expression of IDO and PD-L1. Prior to chemotherapy, the majority of patients were negative for IDO (83%) and PD-L1 (75.5%). Post-chemotherapy, there was an increase in the expression of both markers, with IDO positivity rising to 24.5% and PD-L1 positivity to 29.2%. This suggests that chemotherapy may upregulate these immunosuppressive markers, potentially impacting the tumor microenvironment and immune evasion mechanisms. The increase in IDO and PD-L1 expression post-chemotherapy could imply a chemoresistance mechanism, where tumors adapt to therapeutic pressures by enhancing immunosuppressive pathways.
Regarding the effect of chemotherapy on PD-L1 expression, several studies have documented changes in PD-L1 expression following traditional cancer treatments like chemotherapy, radiotherapy, or their combination. In a study conducted by Park BJ et al, chemoradiation therapy has shown to significantly alter PD-L1 expression in locoregional recurrent squamous cell carcinomas of the head and neck (71). This change in PD-L1 score post-treatment suggests a potential shift in the tumor’s immune microenvironment, which may influence the effectiveness of subsequent immunotherapeutic approaches (71). The findings highlight the importance of re-assessing PD-L1 status in recurrent tumors, as initial evaluations conducted prior to treatment may not accurately represent the post-therapy immune profile. A systematic review by Van den Ende et al. identified 48 studies examining PD-L1 expression changes post-treatment. Statistical analysis indicated that 30 of these studies reported increased PD-L1 expression in post-treatment specimens or treated versus untreated samples (72). However, Karpathiou et al. evaluated the stability of PD-L1 expression in 106 HNSCC tissue specimens over storage periods of 20–48 months (73). They reported a significant decline in PD-L1 tumor proportional score, immune cell expression, and combined positive score over time, indicating loss of antigenicity in archived FFPE samples (73). This degradation may lead to underestimation of PD-L1 expression, potentially influencing treatment decisions and study results depending on the interval between the first and second biopsy. Timely evaluation on freshly processed tissue is therefore recommended for accurate immunotherapy eligibility assessment.
Despite an extensive literature search, we found no studies addressing IDO status before and after chemotherapy specifically in oral squamous cell carcinoma. However, our findings align with other research indicating elevated IDO expression post-chemotherapy (74). IDO-inducing signals may be inherently present in the tumor’s inflammatory microenvironment and could be further triggered by dying cells and tumor antigens released during chemotherapy. Nevertheless, the precise extent of IDO production following chemotherapy remains largely unexplored (74).
Our study found a significant association between IDO and PD-L1 positivity both before and after chemotherapy, reinforcing the notion of an immunosuppressive microenvironment in IDO-positive patients. Multifocal tumors were also significantly associated with IDO positivity prior to chemotherapy, suggesting a potential link between tumor multiplicity and immunosuppressive marker expression. Post-chemotherapy, IDO-positive patients had more comorbidities, highlighting the complex interplay between patient health status and tumor biology.
The overall five-year survival rate for patients in this study was 49%, with a median survival time of 53 months. Despite investigating IDO and PD-L1 expressions, our analysis did not reveal significant differences in survival outcomes related to these markers. This finding suggests that, while IDO and PD-L1 are involved in the immunosuppressive environment of TSCC, their expression levels may not directly impact overall survival within this cohort. Further research could explore additional factors influencing survival and assess whether combining IDO and PD-L1 with other biomarkers might provide a more comprehensive understanding of patient prognosis.
To the best of our knowledge, no study has yet examined the expression of both biomarkers before and after chemotherapy. This highlights the strength of our research, providing valuable insights into the immunosuppressive dynamics within oral squamous cell carcinoma before and after chemotherapy. Future research should focus on longitudinal studies to monitor these markers over time. Additionally, further investigation into the molecular pathways driving these expression changes could uncover novel therapeutic targets.
The current study has some limitations. The retrospective design and reliance on existing patient records may introduce selection bias and limit data quality control. The cross-sectional analysis only captures data at two points, missing dynamic changes. A larger cohort would enhance statistical power and overall impact of the study.
Conclusion
The current study demonstrates that chemotherapy increases IDO and PD-L1 expression in TSCC, suggesting that incorporating immunotherapy could benefit patients undergoing chemotherapy. Future research should emphasize longitudinal studies and the combinatorial analysis of multiple biomarkers to gain a clearer understanding of immune marker expression dynamics in TSCC.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The current study involving human participants was approved by the Institutional Review Board (IRB) of SKMCH&RC under approval number EX-05-12-22-02. The IRB granted the waiver for informed consent for this study, which is in accordance with the Declaration of Helsinki. The FFPE samples were obtained from pathology archives and patient data was retrieved from medical records. All accessed data complied with relevant data protection and privacy regulations and posed minimal risk to participants.
Author contributions
MeF: Conceptualization, Data curation, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing. SB: Formal analysis, Investigation, Methodology, Resources, Writing – original draft. SR: Conceptualization, Project administration, Supervision, Writing – review & editing. AA: Conceptualization, Investigation, Project administration, Supervision, Writing – review & editing. MH: Data curation, Methodology, Software, Writing – original draft. MA: Data curation, Formal analysis, Software, Writing – original draft. AS: Data curation, Methodology, Resources, Writing – original draft. MT: Investigation, Methodology, Resources, Writing – original draft. US: Formal analysis, Methodology, Validation, Writing – review & editing. AL: Investigation, Project administration, Validation, Writing – review & editing. MuF: Investigation, Resources, Validation, Writing – review & editing. AF: Conceptualization, Formal analysis, Investigation, Project administration, Writing – original draft, Writing – review & editing. KA: Conceptualization, Investigation, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1495722/full#supplementary-material
References
1. Xu H, Gao Z, Liu H, An L, Yang T, Zhang B, et al. Associations of lifestyle factors with oral cancer risk: An umbrella review. J Stomatol Oral Maxillofac Surg. (2025) 126(3S):102234. doi: 10.1016/j.jormas.2025.102234
2. Ferlay J, Ervik M, Lam F, Laversanne M, Colombet M, Mery L, et al. Global Cancer Observatory: Cancer Today (version 1.1). Lyon, France: International Agency for Research on Cancer (2020). Available at: https://gco.iarc.who.int/today.
3. Ferlay J, Ervik M, Lam F, Laversanne M, Colombet M, Mery L, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer (20242024). Available at: https://gco.iarc.who.int/media/globocan/factsheets/populations/586-pakistan-factsheet.pdf.
4. Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. (2009) 45(4-5):309–16. doi: 10.1016/j.oraloncology.2008.06.002
5. Hao SP and Tsang NM. The role of supraomohyoid neck dissection in patients of oral cavity carcinoma. Oral Oncol. (2002) 38(3):309–12.
6. Shimojukkoku Y, Tomishima A, Ishida T, Kajiya Y, Oku Y, Kawaguchi K, et al. MCTP2 is a novel biomarker promoting tumor progression and nodal metastasis in oral squamous cell carcinoma. Sci Rep. (2025) 15(1):18456. doi: 10.1038/s41598-025-02094-9
7. St John MA, Abemayor E, and Wong DT. Recent new approaches to the treatment of head and neck cancer. Anti-cancer Drugs. (2006) 17(4):365–75.
8. Calabrese L, Bruschini R, Giugliano G, Ostuni A, Maffini F, Massaro MA, et al. Compartmental tongue surgery: long term oncologic results in the treatment of tongue cancer. Oral Oncol. (2011) 47(3):174–9.
9. Wahab A, Bello IO, Alabi RO, Mascitti M, Troiano G, Mauramo M, et al. Web-based prognostic tools for oral tongue cancer: An analysis of online predictors. Oral Diseases. (2024) 30(8):4867–77.
10. Chen SH, Hsiao SY, Chang KY, and Chang JY. New Insights Into Oral Squamous Cell Carcinoma: From Clinical Aspects to Molecular Tumorigenesis. Int J Mol Sci. (2021) 22(5):2252. doi: 10.3390/ijms22052252
11. Tao W, Li-Juan Z, Kan L, Jing-Yuan L, Xiang-Qi L, and Yu-Jie L. The Microenvironment of Tongue Cancer. Adv Exp Med Biol. (2020) 1296:49–78. doi: 10.1007/978-3-030-59038-3_4
12. Nishino M, Hatabu H, and Hodi FS. Imaging of Cancer Immunotherapy: Current Approaches and Future Directions. Radiology. (2019) 290(1):9–22. doi: 10.1148/radiol.2018181349
13. Kujan O, van Schaijik B, and Farah CS. Immune Checkpoint Inhibitors in Oral Cavity Squamous Cell Carcinoma and Oral Potentially Malignant Disorders: A Systematic Review. Cancers (Basel). (2020) 12(7):1937. doi: 10.3390/cancers12071937
14. Naruse T, Yanamoto S, Okuyama K, Ohmori K, Tsuchihashi H, Furukawa K, et al. Immunohistochemical Study of PD-1/PD-L1 Axis Expression in Oral Tongue Squamous Cell Carcinomas: Effect of Neoadjuvant Chemotherapy on Local Recurrence. Pathol Oncol Res. (2020) 26(2):735–42. doi: 10.1007/s12253-019-00606-3
15. Meng X, Huang Z, Teng F, Xing L, and Yu J. Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy. Cancer Treat Rev. (2015) 41:868–76. doi: 10.1016/j.ctrv.2015.11.001
16. Katsuya Y, Fujita Y, Horinouchi H, Ohe Y, Watanabe SI, and Tsuta K. Immunohistochemical status of PD-L1 in thymoma and thymic carcinoma. Lung Cancer. (2015) 88:154–9. doi: 10.1016/j.lungcan.2015.03.003
17. Jiang X, Wang J, Deng X, Xiong F, Ge J, Xiang B, et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer. (2019) 18:1–7.
18. Wang X, Teng F, Kong L, and Yu J. PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther. (2016) 9:5023–39. doi: 10.2147/OTT.S105862
19. Diskin B, Adam S, Cassini MF, Sanchez G, Liria M, Aykut B, et al. PD-L1 engagement on T cells promotes self-tolerance and suppression of neighboring macrophages and effector T cells in cancer. Nat Immunol. (2020) 21(4):442–54. doi: 10.1038/s41590-020-0620-x
20. Blank C, Gajewski TF, and Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother. (2005) 54(4):307–14. doi: 10.1007/s00262-004-0593-x
21. Patel SP and Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapyPD-L1 IHC as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. (2015) 14(5):847–56. doi: 10.1158/1535-7163.MCT-14-0983
22. Akisada N, Nishimoto K, Takao S, Gion Y, Marunaka H, Tachibana T, et al. PD-L1 expression in tongue squamous cell carcinoma. Med Mol Morphol. (2021) 54:52–9.
23. Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. (2016) 375:1856–67.
24. Sharma P and Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. (2015) 161:205–14. doi: 10.1016/j.cell.2015.03.030
25. Archilla-Ortega A, Domuro C, Martin-Liberal J, and Muñoz P. Blockade of novel immune checkpoints and new therapeutic combinations to boost antitumor immunity. J Exp Clin Cancer Res. (2022) 41:62. doi: 10.1186/s13046-022-02264-x
26. Shiravand Y, Khodadadi F, Kashani SMA, Hosseini-Fard SR, Hosseini S, Sadeghirad H, et al. Immune checkpoint inhibitors in cancer therapy. Curr Oncol. (2022) 29:3044–60. doi: 10.3390/curroncol29050247
27. Liu M, Wang X, Wang L, Ma X, Gong Z, Zhang S, et al. Targeting the IDO1 pathway in cancer: from bench to bedside. J Hematol Oncol. (2018) 11(1):100. doi: 10.1186/s13045-018-0644-y
28. Abd El-Fattah EE. IDO/kynurenine pathway in cancer: possible therapeutic approaches. J Transl Med. (2022) 20(1):347. doi: 10.1186/s12967-022-03554-w
29. Lee GK, Park HJ, Macleod M, Chandler P, Munn DH, and Mellor AL. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology. (2002) 107(4):452–60. doi: 10.1046/j.1365-2567.2002.01526.x
30. Yu J, Du W, Yan F, Wang Y, Li H, Cao S, et al. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol. (2013) 190(7):3783–97. doi: 10.4049/jimmunol.1201449
31. Brandacher G, Perathoner A, Ladurner R, Schneeberger S, Obrist P, Winkler C, et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res. (2006) 12(4):1144–51. doi: 10.1158/1078-0432.CCR-05-1966
32. Okamoto A, Nikaido T, Ochiai K, Takakura S, Saito M, Aoki Y, et al. Indoleamine 2,3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin Cancer Res. (2005) 11(16):6030–9. doi: 10.1158/1078-0432.CCR-04-2671
33. Laimer K, Troester B, Kloss F, Schafer G, Obrist P, Perathoner A, et al. Expression and prognostic impact of indoleamine 2,3-dioxygenase in oral squamous cell carcinomas. Oral Oncol. (2011) 47(5):352–7. doi: 10.1016/j.oraloncology.2011.03.007
34. Nishi M, Yoshikawa K, Higashijima J, Tokunaga T, and Kashihara H. Takasu Cet al. The Impact of Indoleamine 2,3-dioxygenase (IDO) Expression on Stage III Gastric Cancer. Anticancer Res. (2018) 38(6):3387–92. doi: 10.21873/anticanres.12605
35. Jamshed A, Hussain R, Ahmed S, Rehman K, Shehzad K, Muhammad B, et al. Cisplatin plus gemcitabine (GC) as induction chemotherapy in locally advanced head and neck cancer (HANC). J Clin Oncol. (2007) 25(18_suppl):6073.
36. Pan K, Wang H, Chen MS, Zhang HK, Weng DS, Zhou J, et al. Expression and prognosis role of indoleamine 2,3-dioxygenase in hepatocellular carcinoma. J Cancer Res Clin Oncol. (2008) 134(11):1247–53. doi: 10.1007/s00432-008-0395-1
37. Igarashi T, Teramoto K, Ishida M, Hanaoka J, and Daigo Y. Scoring of PD-L1 expression intensity on pulmonary adenocarcinomas and the correlations with clinicopathological factors. ESMO Open. (2016) 1(4):e000083. doi: 10.1136/esmoopen-2016-000083
38. Nocini R, Vianini M, Girolami I, Calabrese L, Scarpa A, Martini M, et al. PD-L1 in oral squamous cell carcinoma: A key biomarker from the laboratory to the bedside. Clin Exp Dent Res. (2022) 8(3):690–8. doi: 10.1002/cre2.590
39. Peña-Cardelles JF, Pozo-Kreilinger JJ, Roncador G, Esteban-Hernández J, Moro-Rodríguez JE, Sastre-Perona A, et al. Prognosis Value of Immunoregulatory Molecules in Oral Cancer Microenvironment: An Immunohistochemical Study. Biomedicines. (2022) 10(3):710. doi: 10.3390/biomedicines10030710
40. Cramer JD, Burtness B, and Ferris RL. Immunotherapy for head and neck cancer: Recent advances and future directions. Oral Oncol. (2019) 99:104460.
41. Chen X, Yao J, Zhang MY, Li R, Liu X, and Qu YQ. IDO1 Promotes the Progression of NSCLC by Regulating the Polarization of M2 Macrophages. Int J Gen Med. (2023) 16:1713–33. doi: 10.2147/IJGM.S398908
42. Noh BJ, Choi GM, Jang HJ, Ma CH, Oh HS, Kim M, et al. Prognostic implications of immune classification using IDO1 expression in extrahepatic bile duct carcinoma. Oncol Lett. (2022) 24:373.
43. Soliman H, Rawal B, Fulp J, Lee JH, Lopez A, Bui MM, et al. Analysis of indoleamine 2-3 dioxygenase (IDO1) expression in breast cancer tissue by immunohistochemistry. Cancer Immunol Immunother. (2013) 62:829–37. doi: 10.1007/s00262-013-1393-y
44. Chen SW, Li SH, Shi DB, Jiang WM, Song M, Yang AK, et al. Expression of PD-1/PD-L1 in head and neck squamous cell carcinoma and its clinical significance. Int J Biol Markers. (2019) 34(4):398–405.
45. Gormley M, Creaney G, Schache A, Ingarfield K, and Conway DI. Reviewing the epidemiology of head and neck cancer: definitions, trends and risk factors. Br Dent J. (2022) 233(9):780–6. doi: 10.1038/s41415-022-5166-x
46. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. (2021) 71(3):209–49. doi: 10.3322/caac.21660
47. Almangush A, Leivo I, and Mäkitie AA. Biomarkers for Immunotherapy of Oral Squamous Cell Carcinoma: Current Status and Challenges. Front Oncol. (2021) 11:616629. doi: 10.3389/fonc.2021.616629
48. Choi J, Lee HJ, Yoon S, Ryu HM, Lee E, Jo Y, et al. Blockade of CCL2 expression overcomes intrinsic PD-1/PD-L1 inhibitor-resistance in transglutaminase 2-induced PD-L1 positive triple negative breast cancer. Am J Cancer Res. (2020) 10:2878–94.
49. Sanmamed MF and Chen L. Inducible expression of B7-H1 (PD-L1) and its selective role in tumor site immune modulation. Cancer J. (2014) 20:256–61. doi: 10.1097/PPO.0000000000000061
50. Sharpe AH, Wherry EJ, Ahmed R, and Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. (2007) 8:239–45. doi: 10.1038/ni1443
51. Ohaegbulam KC, Assal A, Lazar-Molnar E, Yao Y, and Zang X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med. (2015) 21:24–33. doi: 10.1016/j.molmed.2014.10.009
52. Ji M, Liu Y, Li Q, Li XD, Zhao WQ, Zhang H, et al. PD-1/PD-L1 pathway in non-small-cell lung cancer and its relation with EGFR mutation. J Transl Med. (2015) 13:5. doi: 10.1186/s12967-014-0373-0
53. Ng HY, Li J, Tao L, Lam AK, Chan KW, Ko JMY, et al. Chemotherapeutic Treatments Increase PD-L1 Expression in Esophageal Squamous Cell Carcinoma through EGFR/ERK Activation. Transl Oncol. (2018) 11(6):1323–33. doi: 10.1016/j.tranon.2018.08.005
54. Mimura K, Teh JL, Okayama H, Shiraishi K, Kua LF, Koh V, et al. PD-L1 expression is mainly regulated by interferon gamma associated with JAK-STAT pathway in gastric cancer. Cancer Sci. (2018) 109(1):43–53. doi: 10.1111/cas.13424
55. Qin X, Liu C, Zhou Y, and Wang G. Cisplatin induces programmed death-1-ligand 1(PD-L1) over-expression in hepatoma H22 cells via Erk /MAPK signaling pathway. Cell Mol Biol (Noisy-le-grand). (2010) 56 Suppl:OL1366–72.
56. Bellucci R, Martin A, Bommarito D, Wang K, Hansen SH, Freeman GJ, et al. Interferon-γ-induced activation of JAK1 and JAK2 suppresses tumor cell susceptibility to NK cells through upregulation of PD-L1 expression. Oncoimmunology. (2015) 4:e1008824. doi: 10.1080/2162402X.2015.1008824
57. Imai D, Yoshizumi T, Okano S, Itoh S, Ikegami T, Harada N, et al. IFN-γ Promotes Epithelial-Mesenchymal Transition and the Expression of PD-L1 in Pancreatic Cancer. J Surg Res. (2019) 240:115–23. doi: 10.1016/j.jss.2019.02.038
58. Song X, Si Q, Qi R, Liu W, Li M, Guo M, et al. Indoleamine 2,3-Dioxygenase 1: A Promising Therapeutic Target in Malignant Tumor. Front Immunol. (2021) 12:800630. doi: 10.3389/fimmu.2021.800630
59. Meireson A, Devos M, and Brochez L. IDO Expression in Cancer: Different Compartment, Different Functionality? Front Immunol. (2020) :531491. doi: 10.3389/fimmu.2020.531491
60. Tang K, Wu YH, Song Y, and Yu B. Indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors in clinical trials for cancer immunotherapy. J Hematol Oncol. (2021) 14(1):68. doi: 10.1186/s13045-021-01080-8
61. Tomek P, Palmer BD, Flanagan JU, Sun C, Raven EL, and Ching LM. Discovery and evaluation of inhibitors to the immunosuppressive enzyme indoleamine 2,3-dioxygenase 1 (IDO1): Probing the active site-inhibitor interactions. Eur J Med Chem. (2017) 126:983–96. doi: 10.1016/j.ejmech.2016.12.029
62. Nguyen NT, Nakahama T, Le DH, Van Son L, Chu HH, and Kishimoto T. Aryl hydrocarbon receptor and kynurenine: recent advances in autoimmune disease research. Front Immunol. (2014) 5:551. doi: 10.3389/fimmu.2014.00551
63. Boasso A, Herbeuval JP, Hardy AW, Anderson SA, Dolan MJ, Fuchs D, et al. HIV inhibits CD4+ T-cell proliferation by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood. (2007) 109(8):3351–9. doi: 10.1182/blood-2006-07-034785
64. Mehraj V and Routy JP. Tryptophan Catabolism in Chronic Viral Infections: Handling Uninvited Guests. Int J Tryptophan Res. (2015) 8:41–8. doi: 10.4137/IJTR.S26862
65. Munn DH and Mellor AL. IDO in the Tumor Microenvironment: Inflammation, Counter-Regulation, and Tolerance. Trends Immunol. (2016) 37(3):193–207. doi: 10.1016/j.it.2016.01.002
66. Platten M, von Knebel Doeberitz N, Oezen I, Wick W, and Ochs K. Cancer Immunotherapy by Targeting IDO1/TDO and Their Downstream Effectors. Front Immunol. (2015) 5:673. doi: 10.3389/fimmu.2014.00673
67. Jiang T, Sun Y, Yin Z, Feng S, Sun L, and Li Z. Research progress of indoleamine 2,3-dioxygenase inhibitors. Future Med Chem. (2015) 7(2):185–201. doi: 10.4155/fmc.14.151
68. Liu X, Newton RC, Friedman SM, and Scherle PA. Indoleamine 2,3-dioxygenase, an emerging target for anti-cancer therapy. Curr Cancer Drug Targets. (2009) 9(8):938–52. doi: 10.2174/156800909790192374
69. Ball HJ, Sanchez-Perez A, Weiser S, Austin CJ, Astelbauer F, Miu J, et al. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. (2007) 396(1):203–13. doi: 10.1016/j.gene.2007.04.010. Erratum in: Gene. 2010 Oct 1;465(1-2):66.
70. Seppälä M, Halme E, Tiilikainen L, Luukkainen A, Laranne J, Rautiainen M, et al. The expression and prognostic relevance of indoleamine 2, 3-dioxygenase in tongue squamous cell carcinoma. Acta Oto-Laryngologica. (2016) 136(7):729–35.
71. Park BJ, Mattox AK, Clayburgh D, Patel M, Bell RB, Yueh B, et al. Chemoradiation therapy alters the PD-L1 score in locoregional recurrent squamous cell carcinomas of the head and neck. Oral Oncol. (2022) 135:106183. doi: 10.1016/j.oraloncology.2022.106183
72. Hassanian H, Asadzadeh Z, Baghbanzadeh A, Derakhshani A, Dufour A, Rostami Khosroshahi N, et al. The expression pattern of Immune checkpoints after chemo/radiotherapy in the tumor microenvironment. Front Immunol. (2022) 13:938063.
73. Karpathiou G, Vincent M, Dumollard JM, Mobarki M, and Péoc'h M. PD-L1 expression in head and neck cancer tissue specimens decreases with time. Pathol Res Pract. (2022) 237:154042. doi: 10.1016/j.prp.2022.154042
Keywords: tongue squamous cell carcinoma, IDO, PD-L1, chemotherapy, immunohistochemistry, biomarkers, immunotherapy
Citation: Fatima M, Bashir S, Raza SA, Alamgeer, Hassan M, Abu Bakar M, Sheikh AZ, Tahseen M, Sheikh UN, Loya A, Faisal M, Farooq A and Asghar K (2025) Expression of immune checkpoints (IDO and PD-L1) in oral tongue cancer patients: a 10-year retrospective cohort study in Pakistan. Front. Oncol. 15:1495722. doi: 10.3389/fonc.2025.1495722
Received: 13 September 2024; Accepted: 18 June 2025;
Published: 12 August 2025.
Edited by:
Raffaele Addeo, ASL Napoli 2 Nord Oncologia, ItalyReviewed by:
Mojgan Alaeddini, Tehran University of Medical Sciences, IranMaria Fiammetta Romano, University of Naples Federico II, Italy
Mario Pirozzi, University of Campania Luigi Vanvitelli, Italy
Copyright © 2025 Fatima, Bashir, Raza, Alamgeer, Hassan, Abu Bakar, Sheikh, Tahseen, Sheikh, Loya, Faisal, Farooq and Asghar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kashif Asghar, ZHJrYXNoaWZhc2doYXJAZ21haWwuY29t; Alamgeer, YWxhbWdlZXIucGhhcm1hY3lAcHUuZWR1LnBr
 Merium Fatima
Merium Fatima Shaarif Bashir3
Shaarif Bashir3 Syed Atif Raza
Syed Atif Raza Alamgeer
Alamgeer Muhammad Hassan
Muhammad Hassan Muhammad Abu Bakar
Muhammad Abu Bakar Ali Zafar Sheikh
Ali Zafar Sheikh Umer Nisar Sheikh
Umer Nisar Sheikh Asif Loya
Asif Loya Muhammad Faisal
Muhammad Faisal Asim Farooq
Asim Farooq Kashif Asghar
Kashif Asghar