- 1Department of Oncology, Tianjin Union Medical Center, The First Affiliated Hospital of Nankai University, Tianjin, China
- 2Tianjin Cancer Institute of Integrative Traditional Chinese and Western Medicine, Tianjin, China
- 3The Institute of Translational Medicine, Tianjin Union Medical Center, The First Affiliated Hospital of Nankai University, Tianjin, China
- 4Nankai University School of Medicine, Tianjin, China
A 58-year-old female patient with bilateral breast cancer developed unexpected hematochezia at a frequency of approximately 10 episodes per day following adjuvant chemotherapy, with the emergency endoscopy reporting superficial ulcers throughout the entire colon, suggesting a diagnosis of ulcerative colitis (UC). Given the absence of preexisting autoimmune history or detectable autoantibodies, we supposed that the onset of UC was closely related to the chemotherapy. The complex bidirectional relationship between autoimmune rheumatic diseases and cancer continues to be elucidated. Variations in autoimmune disease type, duration, and specific clinical/laboratory features may modulate cancer risk, either increasing or decreasing susceptibility to certain malignancies. These associations could potentially inform type-specific cancer screening strategies. Furthermore, the widespread use of immune checkpoint inhibitors across multiple tumor types, along with their associated inflammatory syndromes, has significant implications for the development and management of autoimmune rheumatic diseases. Herein, we report this case, which could be one of the few bilateral breast cancer cases to be reported with ulcerative colitis, and conducted a literature review of the bidirectional association of breast cancer and autoimmune diseases.
Introduction
Ulcerative colitis (UC), one of the two primary forms of inflammatory bowel disease (IBD), is characterized by mucosal inflammation extending continuously from the rectum to the proximal colon (1). While the exact etiology of IBD remains incompletely understood, current evidence suggests a multifactorial pathogenesis involving molecular and cellular mechanisms, microbial factors, microbiome interactions, genetic predisposition, and immune dysregulation (2). Similar to other chronic inflammatory conditions, UC is associated with an elevated risk of malignancy. Notably, colitis-associated cancer (CAC)—colorectal cancer being the most prevalent—demonstrates risk stratification based on factors including age at diagnosis, disease duration, and disease severity (3). Given the elevated cancer risk associated with autoimmune diseases (AIDs), there has been growing attention on the long-term management of patients with pre-existing AIDs (4). Recent studies have yielded divergent findings regarding the cancer risks of different AIDs, with some conditions, such as rheumatoid arthritis (RA), potentially conferring a reduced risk rather than an increased one (5). Using breast cancer as an example, recent research has revealed that the impact of autoimmune disorders on breast cancer development extends far beyond the initially recognized association with thyroid disease, particularly the involvement of both anti-thyroid peroxidase (anti-TPO) and anti-thyroglobulin (anti-TG) antibodies (6, 7). Furthermore, mounting evidence confirms a bidirectional interplay between AIDs and cancer, wherein certain malignancies may significantly alter susceptibility to subsequent autoimmune conditions. This relationship is exemplified by a nationwide population-based cohort study (Chen et al.), which revealed that breast cancer patients showed markedly reduced incidence rates of systemic lupus erythematosus (SLE), RA, and Sjögren’s syndrome (SS) compared to matched controls (8, 9).
Case presentation
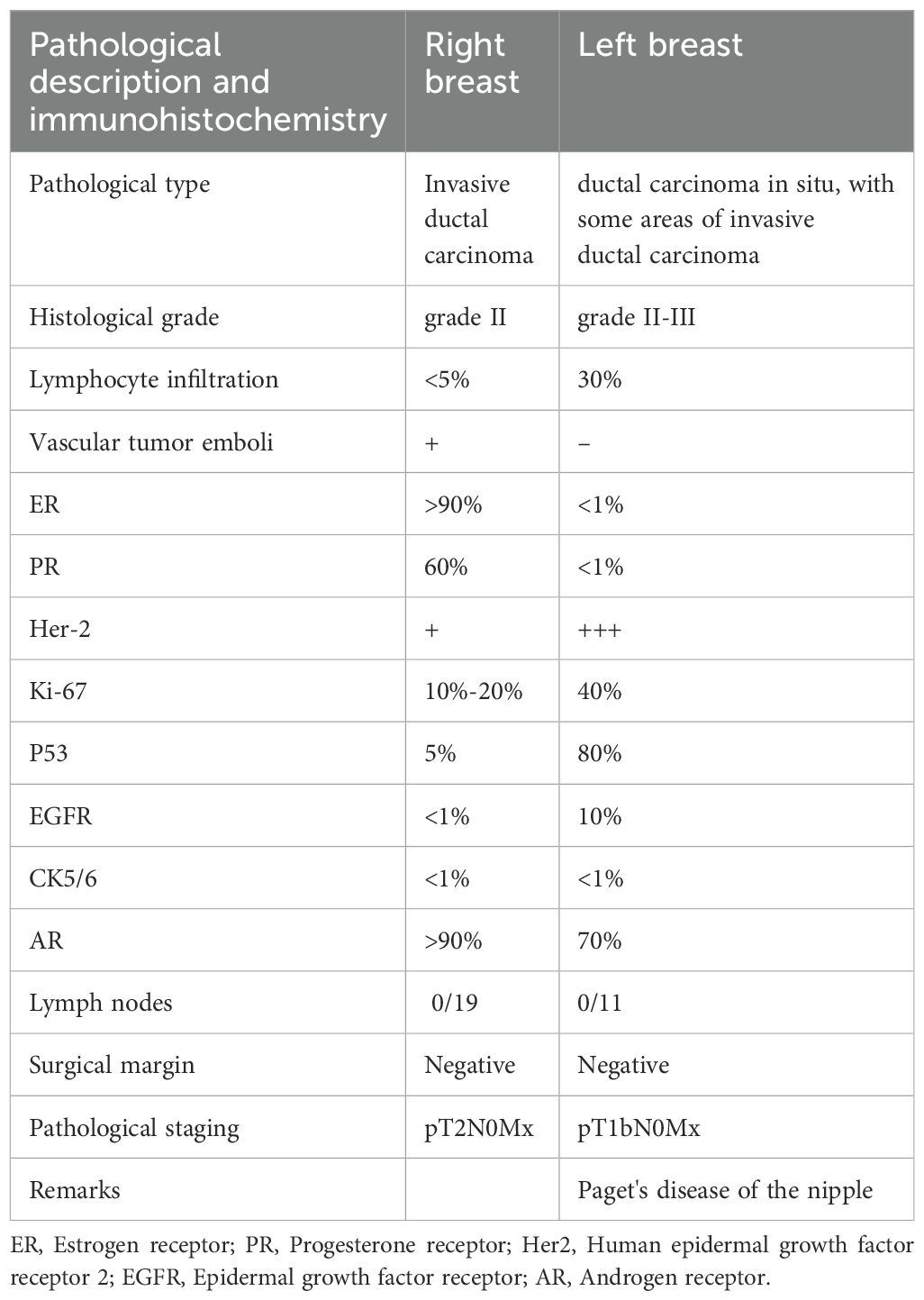
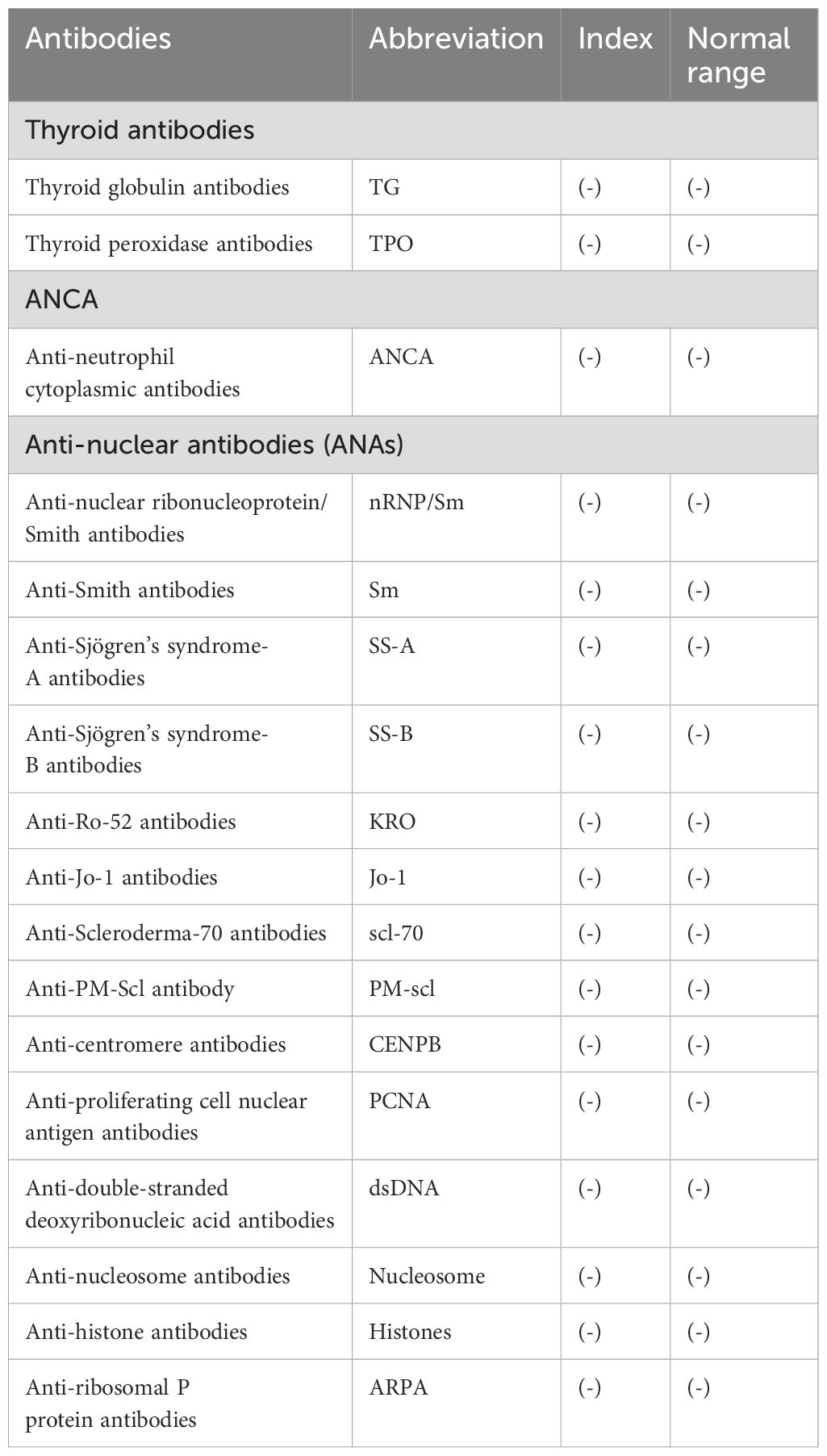
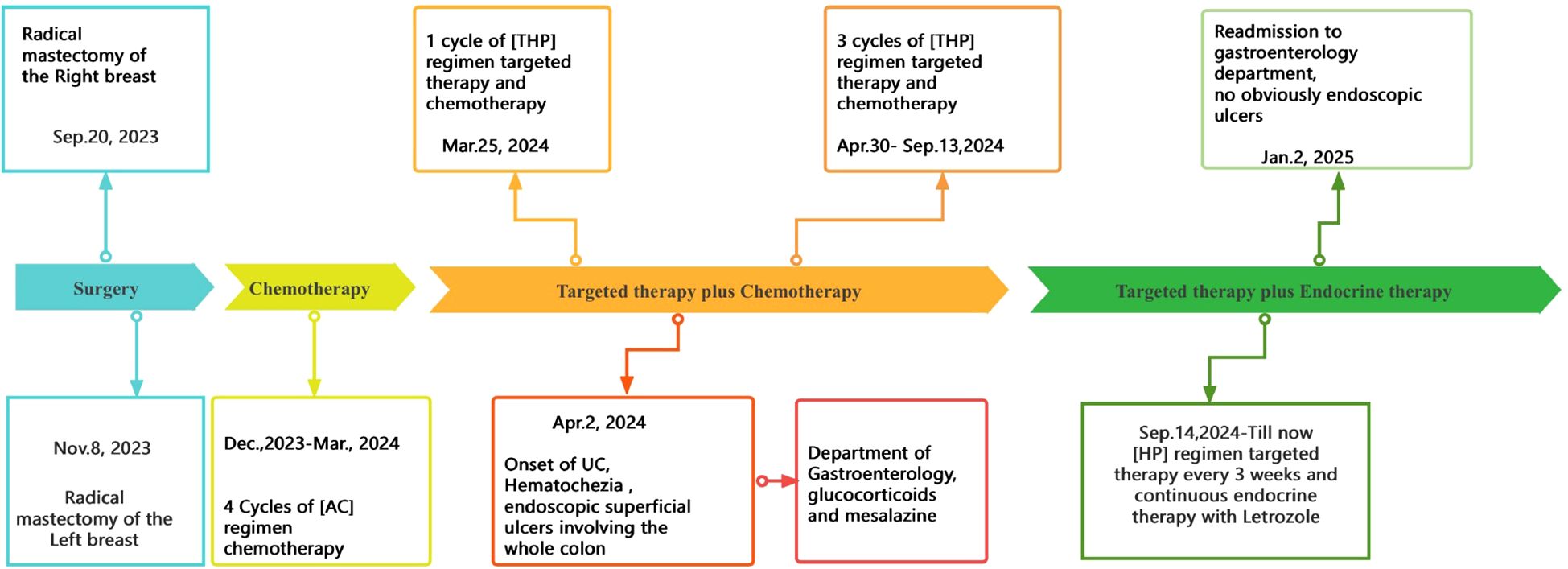
A 58-year-old female patient underwent sequential radical mastectomies, first on the right breast (20 September 2023) and then followed by the left breast (8 November 2023). Histopathological analysis revealed the following: Right breast: invasive ductal carcinoma (Estrogen receptor (ER) >90%, Progesterone receptor (PR) 60%, Human epidermal growth factor receptor 2 (Her2) 1+, Ki-67 10-20%), pathological stage pT2N0Mx; Left breast: predominantly ductal carcinoma in situ with focal invasive ductal carcinoma (ER <1%, PR <1%, HER2 3+, Ki-67 40%), pathological stage pT1bN0Mx (Table 1). The patient initiated the AC-THP adjuvant chemotherapy regimen, completing four cycles (cyclophosphamide 0.8g d1 + doxorubicin liposome 50mg d1, Q3W). During the fifth cycle (trastuzumab 560mg d0 + pertuzumab 840mg d0 + albumin-bound paclitaxel 400mg d1), she developed acute hematochezia (approximately 10 episodes per day). Emergency endoscopy demonstrated diffuse superficial colonic ulcers (Figure 1), though a biopsy was refused by the patient due to bleeding concerns. Given the result of negative stool cultures and C. difficile toxin assays, the absence of serum autoimmune antibodies (Table 2), and the clinical improvement following glucocorticoid and mesalazine therapy, combined with the endoscopic findings, the patient was diagnosed with UC. Follow-up endoscopy (January 2, 2025) showed complete ulcer resolution (Figure 2). The complete treatment timeline summarizing the main events is shown in Figure 3.
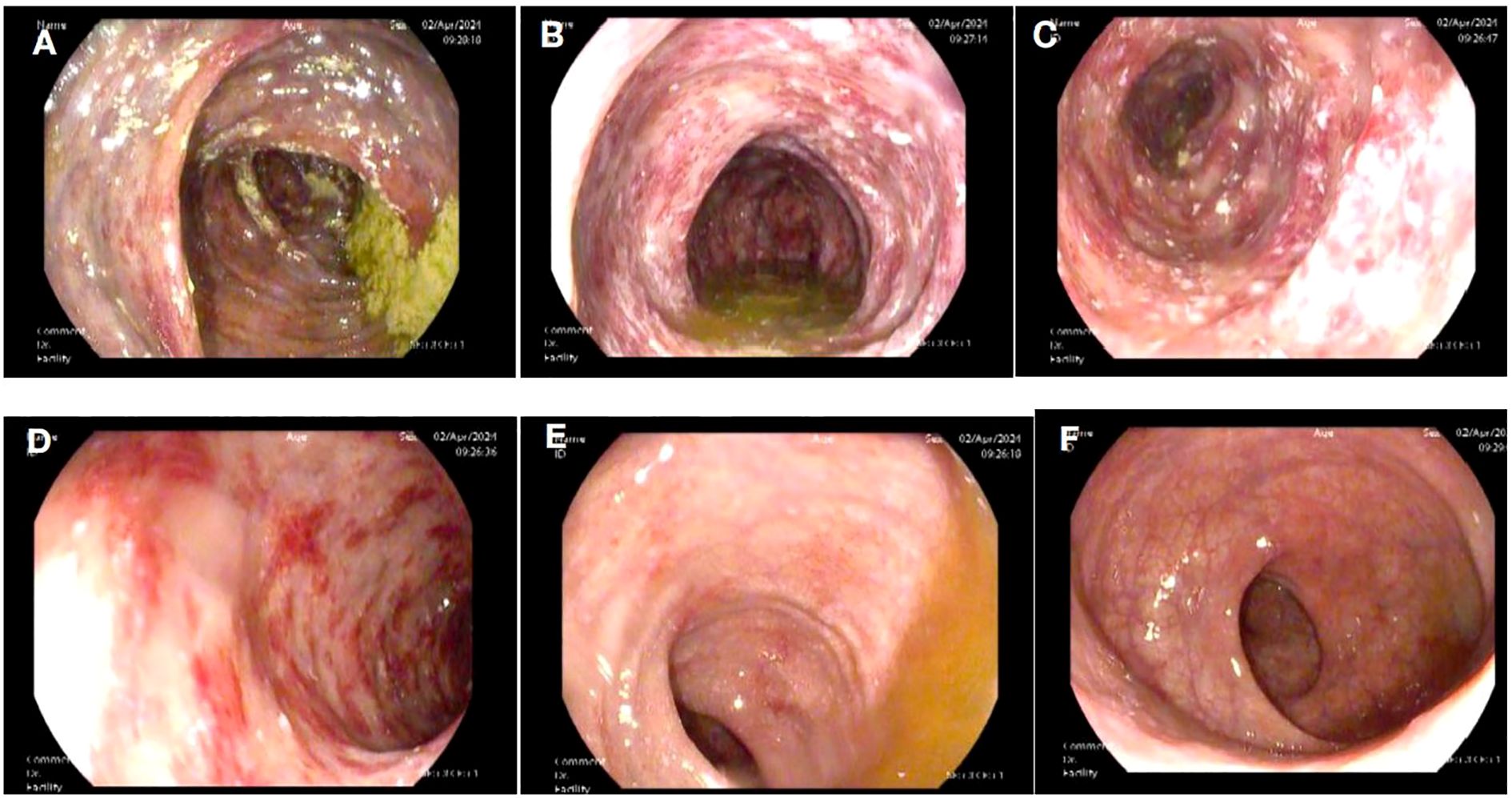
Figure 1. Superficial ulcers involving the whole colon. Superficial ulcers in the ascending colon (A), transverse colon (B), descending colon (C), and sigmoid colon (D). No obvious ulcers in the rectosigmoid junction (E) and rectum (F).
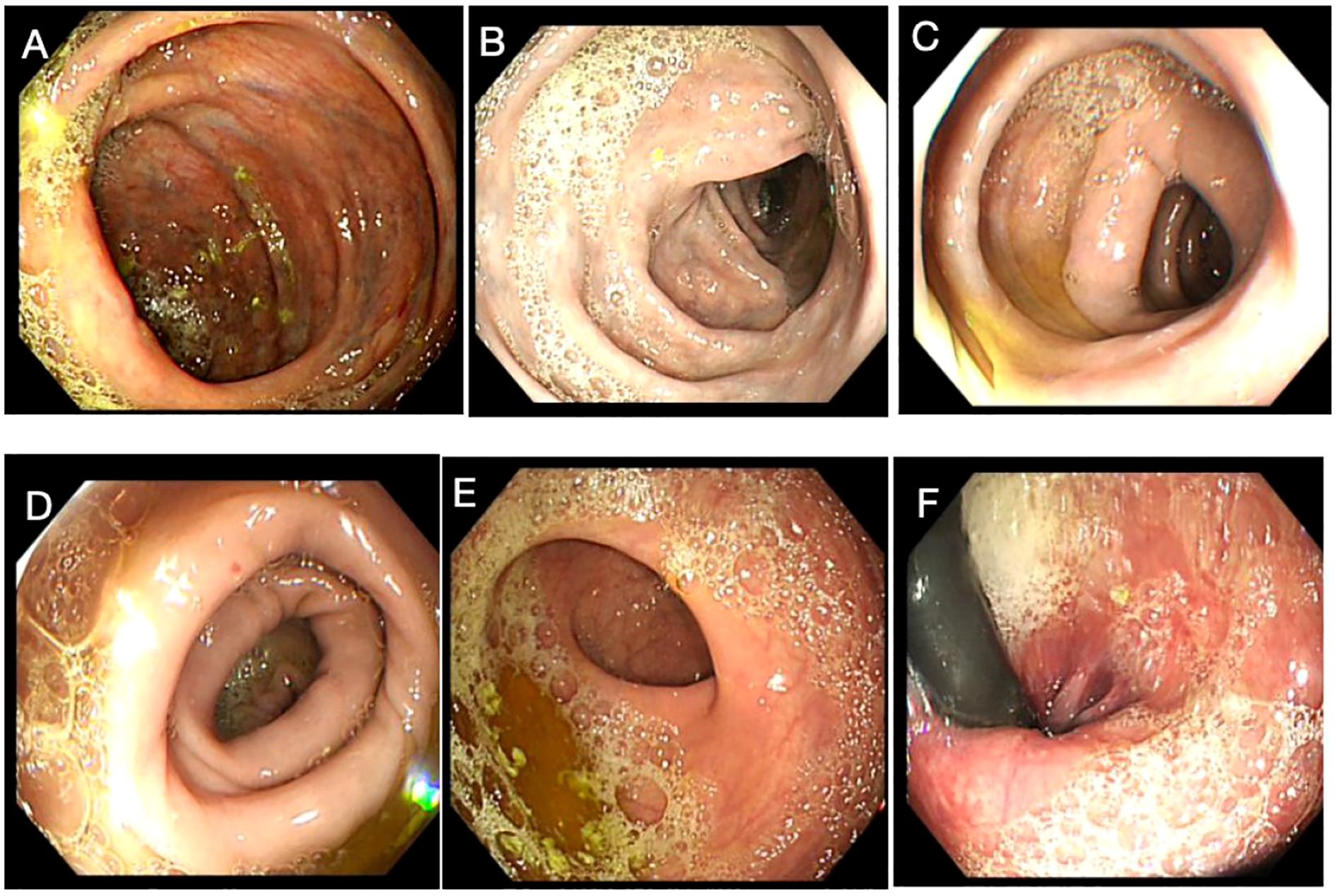
Figure 2. Endoscopic findings upon remission of the colitis. No obvious ulcers were detected in the ascending colon (A), transverse colon (B), descending colon (C), sigmoid colon (D), rectosigmoid junction (E), and rectum (F).
Discussion
Bilateral breast cancer represents a distinct and relatively uncommon form of malignant breast neoplasia, characterized by independent primary tumors originating from the ductal and/or lobular epithelium in both breasts, either synchronously or metachronously. Since Kilgore’s initial description in 1921 (10), reported cases have progressively increased, with synchronous bilateral breast cancer (SBBC) constituting ≤5% of all breast malignancies (11). UC, an idiopathic chronic inflammatory disorder of the colonic mucosa (with potential pancolonic involvement), typically manifests with relapsing-remitting hematochezia (12). We herein present a rare case of synchronous bilateral breast cancer complicated by UC. The mechanisms underlying CAC involve multiple molecular pathways that may include genetic mutations, such as TP53 gene alterations; inflammatory signaling, such as TLR4-MyD88-dependent NF-κB activation, which perpetuates a pro-inflammatory microenvironment; the prostaglandin pathway, such as COX-2-mediated PGE2 production, promoting angiogenesis and immune modulation via PI3K signaling; metabolic reprogramming, such as PI3K/AKT activation by cytokines that facilitates GLUT4 translocation, enhancing TH2 and TH17 lymphocyte metabolism; genetic regulation, such as modifications in key regulatory genes (IL23R and rs10889677 [c.*309C>A]) of the PI3K/AKT signaling pathway. These molecular alterations interact with immune components (macrophages, neutrophils, and T-regulatory cells) and cellular processes (autophagy), ultimately driving CAC development (13).
Similarities in the pathologies of autoimmune diseases and cancers can be traced back at least 30 years. Autoimmune diseases result from the breakdown of peripheral immune tolerance, specifically, the disruption of the delicate balance between immune tolerance and immune activation. This equilibrium is normally maintained by specialized cell subsets, such as regulatory T cells (Tregs) and B cells (Bregs), macrophages, fibroblasts, and myeloid-derived suppressor cells (MDSCs) (14). When this balance is disturbed, it leads to pathological immune overactivation. While the recruitment of tolerogenic immune cell subsets and evasion of immune surveillance are recognized as the hallmarks of cancer, often termed “cancer tolerance”, malignant cells achieve this through multiple mechanisms. These include the expression of immune checkpoint proteins, impaired antigen presentation, epithelial-to-mesenchymal transition (EMT), and dysregulated RNA editing. A very important part of this complex network is the microbiome, as both the microbiome and its metabolites can exert pro-tumor function, and a gut microbiota imbalance can cause gastrointestinal cancers or IBD (15). Inflammatory cytokines and growth factors mediate cell proliferation, and proteinases, especially the collagenase, matrix metalloproteinase-1 (MMP-1), contribute to disease progression by remodeling the extracellular matrix and modulating the microenvironment (16). Chronic inflammation caused by autoimmune diseases or the anti-rheumatic therapies and oncogenic virus infections, such as human papillomavirus, hepatitis B and C viruses, and Epstein–Barr virus, which are difficult to treat, may contribute to the development and progression of cancer (8). Autoimmuity-induced chronic inflammation and tissue damage may produce inflammatory mediators, such as TNF-α, interleukin (IL)-6, tumor growth factor (TGF)-β, and IL-10, leading to the failure of Treg-mediated immune tolerance and stimulating the development of malignant tumors through various mechanisms such as DNA damage, inactivation of tumor-suppressor genes, triggering of angiogenesis, and enhancing invasiveness (17).
Conversely, anti-tumor immune responses may cross-react with self-tissues, leading to the development of autoimmunity (6). Previous studies indicate that patients with pre-existing autoimmune diseases often exhibit shortened survival, primarily attributable to the direct or indirect immune-related adverse events (irAEs) induced by anti-tumor therapies such as chemotherapy, radiation therapy, and, notably, the accumulated application of immune checkpoint inhibitor in the management of advanced tumors (18), leading to the flare-up or onset of autoimmune diseases (8, 19, 20). Numerous chemotherapeutic agents have been implicated in the development of autoimmune-like manifestations, especially bleomycin, gemcitabine, carboplatin, and paclitaxel have been associated with scleroderma-like syndromes and Raynaud’s phenomenon (21). Aromatase inhibitors (AIs), a mainstay of hormone receptor-positive breast cancer treatment, frequently induce arthralgias (22). Radiotherapy targeting the head and neck regions can induce xerostomia that clinically resembles primary Sjögren’s syndrome. Emerging evidence suggests that approximately 50% of breast cancer patients may develop radiation-induced cutaneous fibrosis at treatment sites, though current studies remain limited by small cohort sizes and methodological constraints (23). In patients with SLE, low levels of transforming growth factor-beta 1 (TGF-β1) correlate with increased disease severity. Therefore, radiotherapy-induced TGF-β release may have therapeutic potential in SLE by compensating for this deficiency (24). ICIs induce uncontrolled T-cell activation, resulting in systemic inflammatory syndromes that may involve multiple organ systems. These irAEs can manifest as autoimmune-like pathologies affecting the skin, endocrine, gastrointestinal, cardiovascular, pulmonary, and neurological systems (25, 26). The patient in this case report received multiple chemotherapy, targeted, and endocrine therapies. While treatment-induced colitis has not been previously reported with these specific regimens, the potential for immune-related adverse events, including autoimmune-like manifestations such as colitis, cannot be ruled out.
Autoimmune thyroid diseases
The association between breast cancer and thyroid autoimmunity has attracted sustained research interest owing to the shared biological characteristics of thyroid and mammary gland tissues. A previous meta-analysis (27) demonstrated an elevated breast cancer risk among patients with autoimmune thyroiditis, particularly in those with anti-thyroid peroxidase (anti-TPO) and anti-thyroglobulin (anti-TG) antibodies. This association may stem from shared iodine-concentrating capacity via the sodium/iodide symporter (NIS) in both epithelial tissues or the abundance of TSH receptor-rich adipose tissue in breast parenchyma (28). While NIS protein expression is too limited to serve as a primary autoantigen, current evidence highlights the following two key antigenic links: thyroid peroxidase and its mammary homolog lactoperoxidase (both expressed in breast tissue) (29) and thyroid hormone receptor α 2 (TRα2), which modulates breast cancer signaling pathways. Notably, TRα2 overexpression correlates with improved survival outcomes in patients with breast cancer and is particularly upregulated in BRCA1-associated breast cancers, serving as a positive prognostic marker for both 5-year and overall survival. Additional mechanistic insights include the presence of estrogen receptors in abnormal thyroid tissue, potentially explaining thyroid dysfunction in breast cancer patients and shared endocrine stimuli affecting both organs (30, 31). These findings suggest diagnostic and therapeutic opportunities through targeting thyroid-breast antigenic connections (6).
Rheumatoid arthritis
RA has been associated with an increased risk of hematological and solid malignancies for many years, even more common during treatment with disease-modifying anti-rheumatic drugs (DMARDs) and biotherapy (32). However, in recent years, multiple studies have demonstrated that RA does not uniformly elevate cancer risk, but rather exhibits tumor-specific and sex-dependent variations in malignancy incidence. Patients with RA have an increased risk of non-Hodgkin lymphoma, but a lower risk of gastric cancer (33). Men have a reduced risk of rectal and renal malignancies, while women have a reduced risk of gastric and rectal cancers, and a reduced risk of hepatic malignancies is found in both men and women (34). Regarding hematological tumors, the risk of lymphoma was significantly higher in RA patients of both sexes, but the incidence of leukemia was significantly lower in women (35).
Epidemiological studies demonstrate a bidirectional reduction in disease risk between RA and breast cancer. Women with RA show decreased breast cancer incidence, while breast cancer survivors exhibit similarly reduced RA risk (5, 36). Notably, adjuvant antihormonal therapies (tamoxifen or aromatase inhibitors) do not increase RA risk compared to other breast cancer treatments, a finding replicated in Chinese populations (7). The MMP-1 system reveals important pathophysiological connections between these conditions. Initially characterized for its collagenolytic activity, MMP-1 now demonstrates pleiotropic functions through interactions with Chemokine receptor CXCR-4 and protease-activated receptor-1 (PAR-1). These interactions establish critical signaling networks. The MMP-1/CXCR4 axis modulates activated fibroblast behavior in both RA and cancer. The MMP-1/PAR-1 autocrine/paracrine loop amplifies extracellular matrix remodeling and cellular responses. Emerging therapies targeting MMP-1 and associated G protein-coupled receptors highlight shared pathogenic mechanisms between autoimmunity and oncogenesis (10). While more specific than previous agents, these therapies underscore the fundamental biological parallels between RA and certain malignancies.
Systemic lupus erythematosus
The association between SLE and breast cancer remains controversial. While early studies suggested a protective effect of SLE against breast cancer, more recent investigations have yielded conflicting results. A Brazilian cohort study (n=100) reported elevated breast and cervical cancer incidence in patients with SLE vs. the general population (37). Large international studies demonstrated reduced breast cancer risk in patients with SLE (38, 39). Age-adjusted analyses show comparable or slightly increased breast cancer risk in patients with SLE vs. controls (40). A multicenter international study (n=16,409) found no SLE-specific factors explaining cancer risk variations (41). Longitudinal data indicate that age is the primary breast cancer risk factor in patients with SLE (41). A Mendelian randomization analysis revealed population-specific effects, with a significant risk reduction in East Asian cohorts (OR 0.85, 95% CI 0.78-0.93) but no association in European populations (42). A five-gene SLE prognostic signature (RACGAP1, HMMR, TTK, TOP2A, and KIF15) effectively stratified breast cancer survival risk (43). These contradictory findings highlight the need for more large prospective cohort studies, population-specific risk assessments, and mechanistic investigations into SLE-related immunomodulatory effects.
Other autoimmune diseases
A study based on a Spanish population showed an increased overall cancer risk in patients with systemic sclerosis (SSc) compared with the general population. A high risk in lung, breast cancer, and hematological malignancies was also reported, with the presence of anticentromere antibodies an independent favorable factor for decreasing cancer risk (44). A similar outcome was reported in a large-scale cohort study conducted in China (4). For primary Sjögren’s syndrome (pSS), there could be geographical differences in its association with breast cancer risk; patients with pSS in European countries exhibited a lower risk of breast cancer, different from Asia and Argentina (44). Dedousis et al. conducted a survival analysis that found that there was a higher prevalence of rheumatoid arthritis, Crohn’s disease, ulcerative colitis, and systemic lupus erythematosus in patients with breast cancer compared to age-matched cohorts in the general population. The presence of an autoimmune diagnosis was associated with a lower overall survival (OS) in stages I–III breast cancer and improved OS in patients with stage IV disease, suggesting that anti-tumor immunity plays an important role in advanced breast cancer and could potentially be exploited to improve the effectiveness of immunotherapy (45). High breast cancer risk was also found in type 1 diabetes mellitus (T1DM) and psoriasis (Pso) (5, 46), whereas breast cancer patients with multiple sclerosis suffered a modest progression during breast cancer treatment (47). It is generally accepted that during the 3 to 5 years before and after the diagnosis, patients with inflammatory myopathies have the highest risk of developing certain types of cancers, with adenocarcinoma being the most common histological type, among which the breasts could be a susceptible site (48). In autoimmune retinopathy, anti-TULP1 AAbs disrupt protein translocation to the outer segments and are involved in photoreceptor degeneration in a manner similar to the degeneration induced by mutations in the TULP1 gene. The detection of anti-TULP1 AAbs in patients with breast cancer suggests that anti-TULP1 AAbs have the potential to be a biomarker for cancer-associated autoimmune retinopathy (49).
Conclusion
Breast cancer and autoimmune diseases represent two prevalent conditions that threaten women’s health, with growing evidence suggesting their complex bidirectional relationship. This case highlights the co-occurrence of breast cancer and UC, offering several important insights. While colorectal cancer remains the predominant malignancy associated with UC, this case suggests the potential oncogenic effects of UC beyond the colon. The patient’s diverse therapeutic regimens (chemotherapy, targeted agents, and endocrine therapy) may have contributed to UC development, consistent with established evidence linking anticancer treatments and autoimmune-like manifestations. However, there is also a limitation as the exact causal relationship between breast cancer and UC in this case remains undetermined. This also calls for clinical vigilance regarding autoimmune manifestations in cancer patients and the requirement for large-cohort studies to elucidate the disease-disease interactions and treatment-related autoimmune effects to provide guidelines for oncologists, immunologists, and gastroenterologists to optimize care for patients with overlapping cancer and autoimmune diseases.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XQ: Writing – original draft. MZ: Writing – original draft. JZ: Writing – original draft. HW: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The work was funded by the National Natural Science Foundation of China(Grant No. 82070206); Tianjin Key Medical Discipline (Specialty) Construction Project (Grant No. TJYXZDXK-053B); Tianjin Health Research Project (Grant No. TJWJ2023MS013).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
UC, Ulcerative colitis; IBD, Inflammatory bowel disease; CAC, Colitis-associated cancer; AIDs, Autoimmune diseases; AI, Aromatase inhibitors; TPO, Thyroid peroxidase; TG, Thyroglobulin; SBBC, Synchronous bilateral breast cancer; ICIs, Immune checkpoint inhibitors; MMP-1, Matrix metalloproteinase-1; TNF-α, Tumor necrosis factor-α; TGF-β, Tumor growth factor -β; NIS, Sodium/iodine sympathetic apparatus; TRα2, Thyroid hormone receptor α2; BRCA1, Breast cancer 1; RA, Rheumatoid arthritis; DMARDs, Disease-modifying antirheumatic drugs; CXCR-4, Chemokine receptor -4; PAR-1, Protease-activated receptor-1; SDF-1, Stromal cell-derived factor-1; SLE, Systemic lupus erythematosus; SSc, Systemic sclerosis; pSS, Primary Sjögren’s syndrome; T1DM, Type-1 diabetes mellitus; PsO, Psoriasis; MS, Multiple sclerosis.
References
1. Kobayashi T, Siegmund B, Le Berre C, Wei SC, Ferrante M, Shen B, et al. Ulcerative colitis. Nat Rev Dis Primers. (2020) 6:74. doi: 10.1038/s41572-020-0205-x
2. Guan Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J Immunol Res. (2019), 7247238. doi: 10.1155/2019/7247238
3. Olén O, Erichsen R, Sachs MC, Pedersen L, Halfvarson J, Askling J, et al. Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet. (2020) 395:123–31. doi: 10.1016/S0140-6736(19)32545-0
4. Zhou Z, Liu H, Yang Y, Zhou J, Zhao L, Chen H, et al. The five major autoimmune diseases increase the risk of cancer: epidemiological data from a large-scale cohort study in China. Cancer Commun (Lond). (2022) 42:435–46. doi: 10.1002/cac2.12283
5. Fischer S, Meisinger C, and Freuer D. Autoimmune diseases and female-specific cancer risk: A systematic review and meta-analysis. J Autoimmun. (2024) 144:103187. doi: 10.1016/j.jaut.2024.103187
6. Muller I and Barrett-Lee PJ. The antigenic link between thyroid autoimmunity and breast cancer. Semin Cancer Biol. (2020) 64:122–34. doi: 10.1016/j.semcancer.2019.05.013
7. Rahman S, Archana A, Jan AT, Dutta D, Shankar A, Kim J, et al. Molecular insights into the relationship between autoimmune thyroid diseases and breast cancer: A critical perspective on autoimmunity and ER stress. Front Immunol. (2019) 10:344. doi: 10.3389/fimmu.2019.00344
8. Masetti R, Tiri A, Tignanelli A, Turrini E, Argentiero A, Pession A, et al. Autoimmunity and cancer. Autoimmun Rev. (2021) 20:102882. doi: 10.1016/j.autrev.2021.102882
9. Chen H-H, Lin C-H, Chen D-Y, Chao W-C, Chen Y-H, Hung W-T, et al. Risk of major autoimmune diseases in female breast cancer patients: A nationwide, population-based cohort study. PloS One. (2019) 14:e0222860. doi: 10.1371/journal.pone.0222860
10. Kilgore AR. The incidence of cancer in the second breast. J Am Med Assoc. (1921) 77:454–7. doi: 10.1001/jama.1921.02630320038011
11. Verkooijen HM, Chatelain V, Fioretta G, Vlastos G, Rapiti E, Sappino AP, et al. Survival after bilateral breast cancer: results from a population-based study. Breast Cancer Res Treat. (2007) 105:347–57. doi: 10.1007/s10549-006-9455-x
12. Ordás I, Eckmann L, Talamini M, Baumgart DC, and Sandborn WJ. Ulcerative colitis. Lancet. (2012) 380:1606–19. doi: 10.1016/S0140-6736(12)60150-0
13. Yamamoto-Furusho JK and Gutierrez-Herrera FD. Molecular mechanisms and clinical aspects ofColitis-associated cancer in ulcerative colitis. Cells. (2025) 14:162. doi: 10.3390/cells14030162
14. Qi X, Jiang H, Liu P, Xie N, Fu R, Wang H, et al. Increased myeloidderived suppressor cells in patients with myelodysplastic syndromes suppress CD8+ T lymphocyte function through the STAT3-ARG1 pathway. Leukemia Lymphoma. (2020) 62:218–23. doi: 10.1080/10428194.2020.1817431
15. Sakowska J, Arcimowicz Ł, Jankowiak M, Papak I, Markiewicz A, Dziubek K, et al. Autoimmunity and cancer—Two sides of the same coin. Front Immunol. (2022) 13:793234. doi: 10.3389/fimmu.2022.793234
16. Eck SM, Blackburn JS, Schmucker AC, Burrage PS, and Brinckerhoff CE. Matrix metalloproteinase and G protein coupled receptors: co-conspirators in the pathogenesis of autoimmune disease and cancer. J Autoimmun. (2009) 33:214–21. doi: 10.1016/j.jaut.2009.09.011
17. Balkwill F and Mantovani A. Inflammation and cancer: back to Virchow? Lancet. (2001) 357:539–45. doi: 10.1016/S0140-6736(00)04046-0
18. Fiorentino V, Tralongo P, Larocca LM, Pizzimenti C, Martini M, and Pierconti F. First-line ICIs in renal cell carcinoma. Hum Vaccin Immunother. (2023) 19:2225386. doi: 10.1080/21645515.2023.2225386
19. Valencia JC, Egbukichi N, and Erwin-Cohen RA. Autoimmunity and cancer, the paradox comorbidities challenging therapy in the context of preexisting autoimmunity. J Interferon Cytokine Res. (2019) 39:72–84. doi: 10.1089/jir.2018.0060
20. Tison A, Garaud S, Chiche L, Cornec D, and Kostine M. Immune-checkpoint inhibitor use in patients with cancer and pre-existing autoimmune diseases. Nat Rev Rheumatol. (2022) 18:641–56. doi: 10.1038/s41584-022-00841-0
21. Cappelli LC and Shah AA. The relationships between cancer and autoimmune rheumatic diseases. Best Pract Res Clin Rheumatol. (2020) 34:101472. doi: 10.1016/j.berh.2019.101472
22. Gibson L, Lawrence D, Dawson C, and Bliss J. Aromatase inhibitors for treatment of advanced breast cancer in postmenopausal women. Cochrane Database Systematic Rev. (2009) 4:Cd003370. doi: 10.1002/14651858.CD003370.pub3
23. Shah DJ, Hirpara R, Poelman CL, Woods A, Hummers LK, Wigley FM, et al. Impact of radiation therapy on scleroderma and cancer outcomes in scleroderma patients with breast cancer. Arthritis Care Res (Hoboken). (2018) 70:1517–24. doi: 10.1002/acr.23505
24. Becker-Merok A, Eilertsen GØ, and Nossent JC. Levels of transforming growth factor-b are low in systemic lupus erythematosus patients with active disease. J Rheumatol. (2010) 37:2039–45. doi: 10.3899/jrheum.100180
25. Postow MA, Sidlow R, and Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. New Engl J Med. (2018) 378:158–68. doi: 10.1056/NEJMra1703481
26. Khoja L, Day D, Chen TW, Siu LL, and Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol: Off J Eur Soc Med Oncol. (2017) 28:2377–85. doi: 10.1093/annonc/mdx286
27. Hardefeldt PJ, Eslick GD, and Edirimanne S. Benign thyroid disease is associated with breast cancer: a meta-analysis. Breast Cancer Res Treat. (2012) 133:1169–77. doi: 10.1007/s10549-012-2019-3
28. Grani G, Dicorato P, Dainelli M, Coletta I, Calvanese A, Del Sordo M, et al. Thyroid diseases in women with breast cancer. Clin Ter. (2012) 163:e401–4.
29. Muller I, Pinchera A, Fiore E, Belardi V, Rosellini V, Giustarini E, et al. High prevalence of breast cancer in patients with benign thyroid diseases. J Endocrinol Investig. (2011) 34:349–52. doi: 10.1007/BF03347458
30. Michalaki V, Kondi-Pafiti A, Gennatas S, Antoniou A, Primetis H, and Gennatas C. Breast cancer in association with thyroid disorders. J Balk Union Oncol. (2009) 14:425–8.
31. Kapdi JJ and Wolfe JN. Breast cancer relationship to thyroid supplements for hypothyroidism. JAMA. (1976) 236:1124–7. doi: 10.1001/jama.1976.03270110022020
32. Ehrenfeld M. Cancer and autoimmunity. In: Anaya JM, Cervera R, Levy RA, Rojas-Villarraga A, and Shoenfeld Y, editors. Autoimmunity: From Bench to Bedside, Bogota (Colombia): El Rosario University Press (2013). p. 683–9.
33. Chang SH, Park JK, Lee YJ, Yang JA, Lee EY, Song YW, et al. Comparison of cancer incidence among patients with rheumatic disease: a retrospective cohort study. Arthritis Res Ther. (2014) 16:428. doi: 10.1186/s13075-014-0428-x
34. Hashimoto A, Chiba N, Tsuno H, Komiya A, Furukawa H, Matsui T, et al. Incidence of Malignancy and the risk of lymphoma in Japanese patients with rheumatoid arthritis compared to the general population. J Rheumatol. (2015) 42:564–71. doi: 10.3899/jrheum.140533
35. Lin YC, Chou HW, Tsai WC, Yen JH, Chang SJ, and Y.C. Lin. The age-risk relationship of hematologic Malignancies in patients with rheumatoid arthritis: a nationwide retrospective cohort study. Clin Rheumatol. (2015) 34:1195–202. doi: 10.1007/s10067-015-2972-4
36. Wadström H, Pettersson A, Smedby KE, and Askling J. Risk of breast cancer before and after rheumatoid arthritis, and the impact of hormonal factors. Ann Rheum Dis. (2020) 79:581–6. doi: 10.1136/annrheumdis-2019-216756
37. Skare TL and da Rocha BV. Breast and cervical cancer in patients with systemic lupus erythematosus. Rev Bras Ginecol Obstet. (2014) 36:367–71. doi: 10.1590/SO100-720320140005052
38. Bernatsky S, Ramsey-Goldman R, Labrecque J, Joseph L, Boivin JF, Petri M, et al. Cancer risk in systemic lupus: an updated international multi-centre cohort study. J Autoimmun. (2013) 42:130–5. doi: 10.1016/j.jaut.2012.12.009
39. Goobie GC, Bernatsky S, Ramsey-Goldman R, and Clarke AE. Malignancies in systemic lupus erythematosus: a 2015 update. Curr Opin Rheumatol. (2015) 27:454–60. doi: 10.1097/BOR.0000000000000202
40. Khaliq W, Qayyum R, Clough J, Vaidya D, Wolff AC, and D.M. Becker. Comparison of breast cancer risk in women with and without systemic lupus erythematosus in a Medicare population. Breast Cancer Res Treat. (2015) 151:465–74. doi: 10.1007/s10549-015-3412-5
41. Bernatsky S, Ramsey-Goldman R, Petri M, Urowitz MB, Gladman DD, Fortin PF, et al. Breast cancer in systemic lupus. Lupus. (2016) 26:311–5. doi: 10.1177/0961203316664595
42. Li W, Wang R, and Wang W. Exploring the causality and pathogenesis of systemic lupus erythematosus in breast cancer based on Mendelian randomization and transcriptome data analyses. Front Immunol. (2023) 13:1029884. doi: 10.3389/fimmu.2022.1029884
43. Carbonell C, Marcos M, Guillén-Del-Castillo A, Rubio-Rivas M, Argibay A, Marín-Ballvé A, et al. Standardized incidence ratios and risk factors for cancer in patients with systemic sclerosis: Data from the Spanish Scleroderma Registry (RESCLE). Autoimmun Rev. (2022) 21:103167. doi: 10.1016/j.autrev.2022.103167
44. Deng J, Liu M, Xiao R, Wang J, Liao X, Ye Z, et al. Risk, incidence, and mortality of breast cancer in primary sjögren’s syndrome: A systematic review and meta-analysis. Front Immunol. (2022) 13:904682. doi: 10.3389/fimmu.2022.904682
45. Dedousis D, Zhang AL, Vassiliou AN, Cao S, Yammani D, Kyasaram RK, et al. Survival in elderly patients with breast cancer with and without autoimmune disease. Cancer Med. (2023) 12:13086–99. doi: 10.1002/cam4.5989
46. Xiong F, Wang J, Nierenberg JL, Van Blarigan EL, Kenfield SA, Chan JM, et al. Diabetes mellitus and risk of breast cancer: a large-scale, prospective, population-based study. Br J Cancer. (2023) 129:648–55. doi: 10.1038/s41416-023-02345-4
47. Nylander AN, Singh J, Poole S, Anderson A, Marrie RA, Rugo H, et al. Clinical course of multiple sclerosis and patient experiences during breast cancer treatment. Mult Scler. (2023) 29:967–78. doi: 10.1177/13524585231175975
48. Oldroyd A, Sergeant JC, New P, McHugh NJ, Betteridge Z, Lamb JA, et al. The temporal relationship between cancer and adult onset anti-transcriptional intermediary factor 1 antibody-positive dermatomyositis. Rheumatol (Oxford England). (2019) 58:650–5. doi: 10.1093/rheumatology/key357
Keywords: ulcerative colitis, bilateral breast cancer, adjuvant chemotherapy, target therapy, autoimmune disease
Citation: Qi X, Zhang M, Zhu J and Wang H (2025) Case report of concurrent ulcerative colitis and bilateral breast cancer: a literature review of the bidirectional association between autoimmune diseases and breast cancer. Front. Oncol. 15:1495731. doi: 10.3389/fonc.2025.1495731
Received: 13 September 2024; Accepted: 19 May 2025;
Published: 11 June 2025.
Edited by:
Kosuke Kawaguchi, Kyoto University, JapanReviewed by:
Marcos Edgar Herkenhoff, Santa Catarina State University, BrazilVincenzo Fiorentino, University of Messina, Italy
Copyright © 2025 Qi, Zhang, Zhu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huaqing Wang, aHVhcWluZ3dAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Xiao Qi
Xiao Qi Miao Zhang1,2,3†
Miao Zhang1,2,3†