- 1Department of Breast Medical Oncology, Liaoning Cancer Hospital and Institute, Shenyang, China
- 2Department of Breast Medical Oncology, Zhejiang Cancer Hospital, Hangzhou, China
- 3Breast Internal Medicine Department, Hunan Cancer Hospital, Changsha, China
- 4Department of Breast Medical Oncology, Shengjing Hospital of China Medical University, Shenyang, China
- 5Department of Breast Disease, The Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital, Zhengzhou, China
- 6Department of Oncology, The First Hospital of Jilin University, Changchun, China
- 7Department of Breast Oncology, Anshan Cancer Hospital, Anshan, China
Background: Until now there has been no comprehensive data from extensive samples to evaluate the therapeutic potential of inetetamab in metastatic breast cancer (MBC) patients who had a history of trastuzumab treatment. Some previous studies had either small sample sizes from single-center, or partial enrolled patients without prior exposure to trastuzumab. This study aimed to provide a deep analysis of inetetamab-based therapy in this specific population.
Methods: A multicenter retrospective study collected clinicopathological data from a total of 500 patients between Jul 2020 and Oct 2023. Progression-free survival (PFS) was estimated as the primary endpoint. Secondary endpoints included objective response rate (ORR), disease control rate (DCR) and adverse events (AEs). The association of risk factors with the effect of inetetamab treatment on PFS was evaluated using Cox proportional hazards regression analysis.
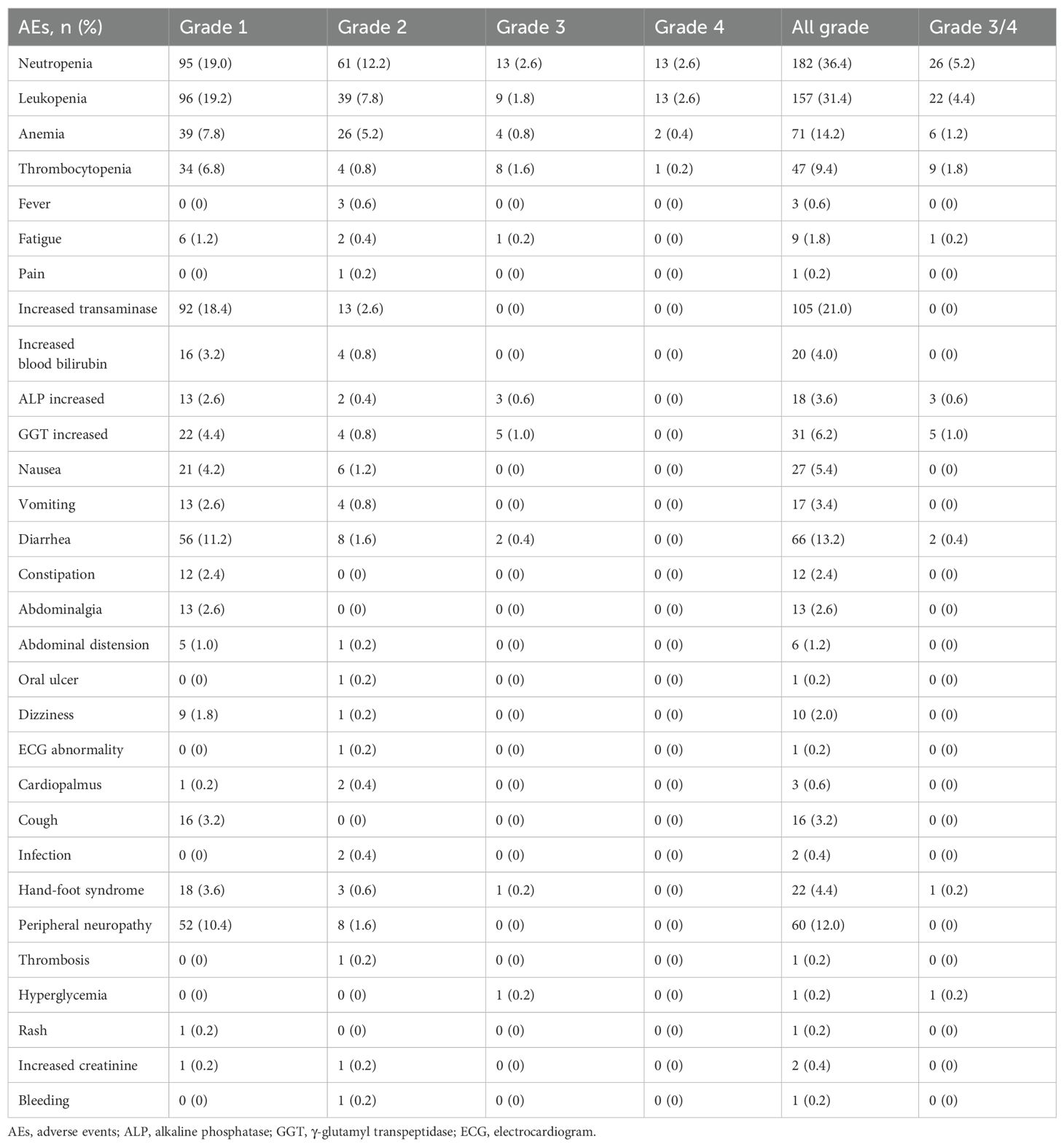
Results: In the overall cohort, we observed a median PFS of 8.0 months, an ORR of 28.6% and a DCR of 89.2% (median treatment line: third line). Meanwhile, patients who received a combination treatment regime of inetetamab + tyrosine kinase inhibitors (TKIs) + chemotherapy achieved the most prolonged PFS of 9.0 months and the higher ORR of 29.3%. Ki67 index, distant lymph nodes metastasis and treatment strategy were independent predictors of PFS. The most common all-grade AEs were neutropenia (182/500, 36.4%) and leukopenia (157/500, 31.4%).
Conclusions: Inetetamab was promising in HER2-positive MBC patients who had prior exposure to trastuzumab, offering a new option for patients with a prior failure of trastuzumab.
1 Introduction
According to the latest statistics from the World Health Organization (WHO), global new breast cancer cases surpassed 2.3 million in 2022, representing 11.7% of all cancer cases worldwide and claiming approximately 670,000 lives. It remains the most commonly diagnosed cancer among women globally (1). Data from the National Cancer Center of China indicate that in 2022, China recorded approximately 357,000 new breast cancer cases and about 75,000 deaths, posing a serious threat to women’s health in China (2). HER2 gene amplification or overexpression is found in 20%-30% of breast cancer cases (3). The overexpression of HER2 is closely correlated with the incidence, progression and outcome of breast cancer. Strong invasion, inadequate histological differentiation, and a high rate of metastasis and recurrence characterize HER2-positive breast cancer (4).
The recommended first-line therapy for HER2-positive metastatic breast cancer (MBC) involves trastuzumab in Chinese Society of Clinical Oncology (CSCO) breast cancer guideline. tyrosine kinase inhibitors (TKIs) or antibody-drug conjugate (ADC) have emerged as the second-line treatment strategy for HER2-positive MBC patients who have failed trastuzumab treatment in China (5). Despite the outstanding performance of ADCs, their high costs remain a significant burden for most Chinese MBC patients, particularly in economically underdeveloped regions, forcing many patients to forgo treatment due to financial constraints (6). Unfortunately, most Chinese patients who have failed both trastuzumab and TKIs could only have limited benefits from attempting trastuzumab-based regimens again (7).
Inetetamab is the first developed novel anti-HER2 monoclonal antibody in China, offering a more economical and effective therapy option (8, 9). A Phase III clinical trial known as HOPES study reported that inetetamab + vinorelbine could significantly prolong the progression-free survival (PFS) compared to chemotherapy alone (39.6 weeks vs. 14.0 weeks, p < 0.0001) (10). Subsequently, inetetamab has been approved by the China National Medical Products Administration (NMPA) for the treatment of HER2-positive MBC in June 2020. Meanwhile, the first-line subgroup analysis in this HOPES study showed that inetetamab exhibited a median PFS of 11.1 months for the first-line treatment in HER2-positive MBC patients, which was comparable to that of trastuzumab. Fortunately, the incidence of adverse events (AEs) did not increase significantly (11).
However, there had been a lack of comprehensive data from large sample sizes to evaluate inetetamab-based therapy in HER2-positive MBC patients who were previously treated with trastuzumab. The sample sizes in the previous studies were relatively small (12, 13). Not all enrolled patients had previously received trastuzumab treatment (12). And the study of Yu et al. was single-center (13). These factors resulted in several important limitations. Based on the current situation, this study with a larger-scale patients aimed to analyze the efficacy and safety of inetetamab-based therapy in HER2-positive MBC patients who had previously received trastuzumab treatment, exploring a more economical and effective therapeutic option.
2 Methods
2.1 Study design
A multi-center retrospective study, led by Liaoning Cancer Hospital & Institute in China from Jul 2020 to Oct 2023, was registered at https://www.ClinicalTrials.gov (NCT06305702). The informed consent was waived to be written as it solely utilized pre-existing data and records collected during the period of investigation.
2.2 Patients
The inclusion criteria were as follows: (I) an age of at least 18 years or older; (II) pathologically diagnosed HER2-positive MBC; (III) immunohistochemistry (IHC) 3+ or IHC 2+ with fluorescence in situ hybridization (FISH) positive; (IV) at least one measurable lesion as defined in the Response Criteria Evaluation in Solid Tumors version 1.1 (RECIST v1.1) (14); (V) prior trastuzumab therapy (≥1 cycle administered at any disease stage, either early or metastatic); (VI) receiving inetetamab-based therapy in the MBC stage; (VII) retaining traceable medical history records.
The exclusion criteria included: (I) pregnant or lactating women; (II) patients previously diagnosed with other malignant tumors; (III) patients with severe and uncontrolled infectious diseases, severe underlying medical conditions, or psychiatric illnesses; (IV) ineligible for this study by the researchers’ assessment.
2.3 Trial endpoints
The primary end point of the trial was PFS which was defined as the duration from randomization until first evidence of tumor progression or death from any cause. Secondary endpoints were objective response rate (ORR) which was defined as the proportion of patients that achieved a complete response (CR) or partial response (PR), and disease control rate (DCR) which was calculated as the percentage of patients whose therapeutic intervention had led to a CR, PR or stable disease (SD). RECIST v1.1 was used to assess the tumor treatment response in all the enrolled patients with measurable lesions (14). Other secondary endpoints encompassed AEs, which was estimated according to CTCAE version 5.0.
2.4 Procedures
Basic information, laboratory and imaging examination results, treatment history, tumor characteristics, metastatic sites, hormone receptor/HER2 status, etc. were retrospectively collected from the electronic medical record system in the involved hospitals. Treatment details, clinical efficacy assessments, safety evaluations, and other pertinent data from subsequent follow-up visits were also comprehensively gathered.
2.5 Statistical analysis
The Kaplan-Meier method was utilized to estimate the survival curves, and the Cox proportional hazards model was used to analyze hazard ratio (HR) with corresponding 95% confidence intervals (CIs). Relevant variables whose p values were < 0.2 in the univariate analysis were included into the multivariate regression model to determine risk factors for PFS. The relatively liberal threshold is recommended in the literature to avoid excluding potentially important predictors (12, 15). For the safety analysis, the numbers and percentages of patients with AEs of each grade were calculated. All statistical analyses were conducted using SAS version 9.4 and R software version 4.3.2.
3 Results
3.1 Patient characteristics
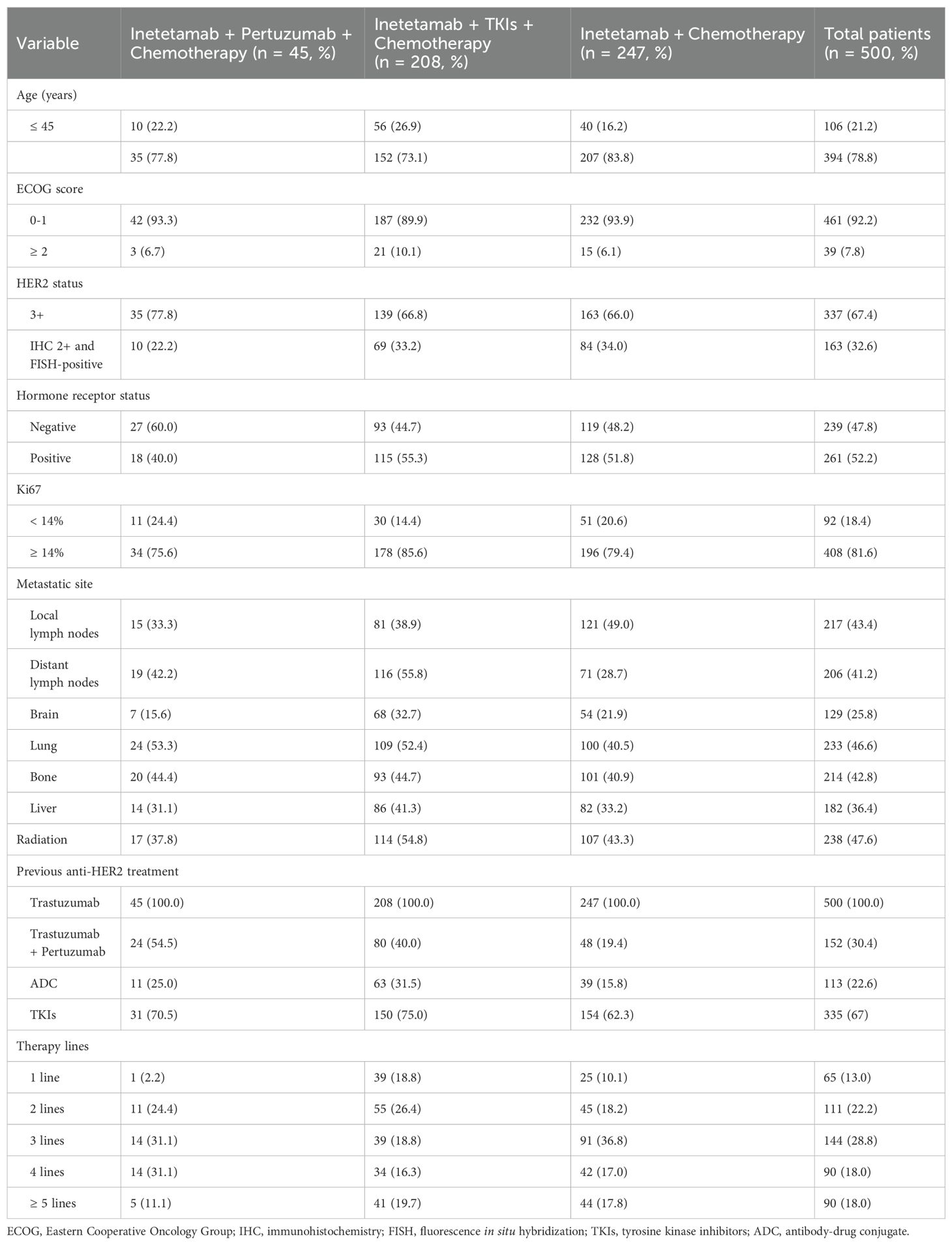
A total of 500 patients was enrolled in this study from Jul 2020 to Oct 2023. Table 1 presented patient characteristics. The overall population had a median age of 54 years (range 26 to 85), with 394 patients (78.8%) being older than 45 years. Additionally, 461 patients (92.2%) had an Eastern Cooperative Oncology Group (ECOG) score of 0-1, and 337 patients (67.4%) were defined as IHC 3+. Lung was the most common observed metastatic site (233 patients, 46.6%), followed by local lymph nodes (217 patients, 43.4%), bone (214 patients, 42.8%), distant lymph nodes (206 patients, 41.2%), liver (182 patients, 36.4%) and brain (129 patients, 25.8%). In the previous treatment, all 500 enrolled patients had received trastuzumab (100%) and other anti-HER2 regimens including 152 patients (30.4%) with trastuzumab + pertuzumab, 113 patients (22.6%) with HER2-ADC, and 335 patients (67%) with TKIs.
3.2 Patient treatment
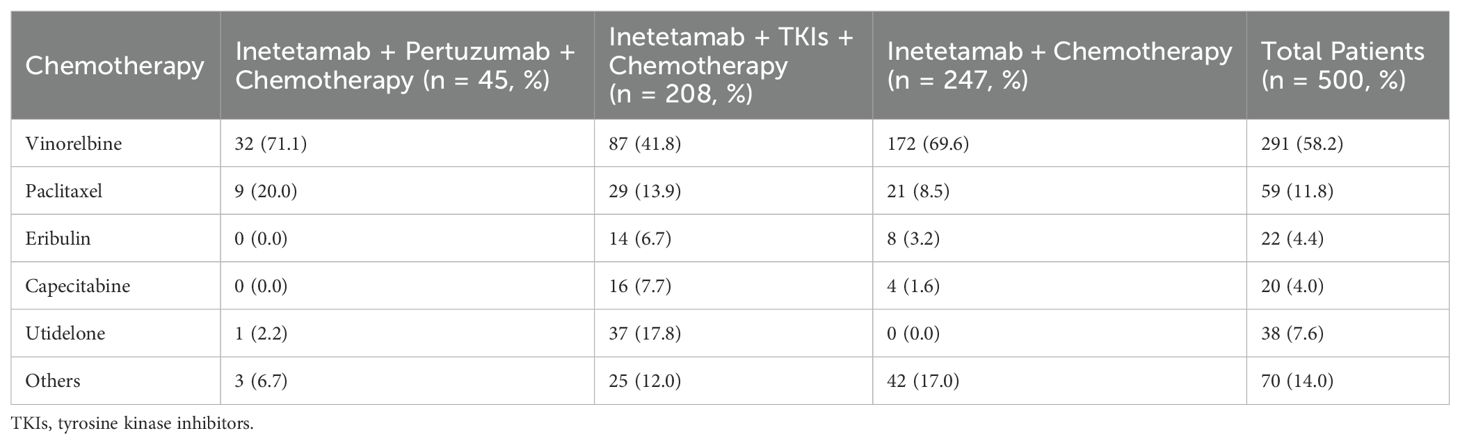
Most patients were given the chemotherapeutic agents plus anti-HER2 drugs (Table 2), including inetetamab + chemotherapy (247/500, 49.4%), inetetamab + TKIs + chemotherapy (208/500, 41.6%) and inetetamab + pertuzumab + chemotherapy (45/500, 9.0%). Meanwhile, most patients received vinorelbine (chemotherapy drug) (291/500, 58.2%), followed by paclitaxel (59/500, 11.8%), utidelone (38/500, 7.6%), eribulin (22/500, 4.4%), capecitabine (20/500, 4.0%), and others (70/500, 14.0%).
3.3 Efficacy in overall population
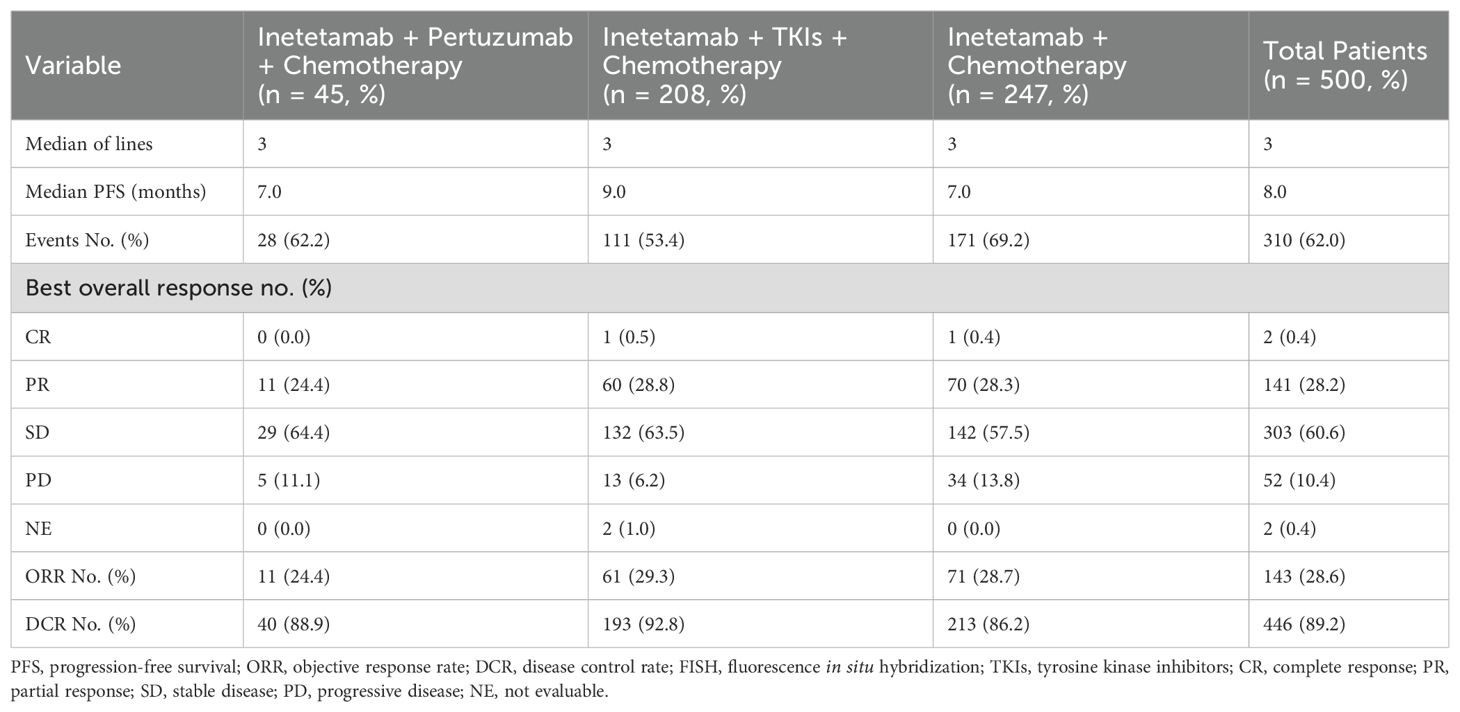
The median PFS was 8.0 months in the overall cohort (Table 3, Figure 1A). Of the 500 included patients, there were 2 patients (0.4%) with CR, 141 patients (28.2%) with PR, 303 patients (60.6%) with SD, and 52 patients (10.4%) with progressive disease (PD). Overall, the ORR and DCR were calculated to be 28.6% and 89.2%, respectively.
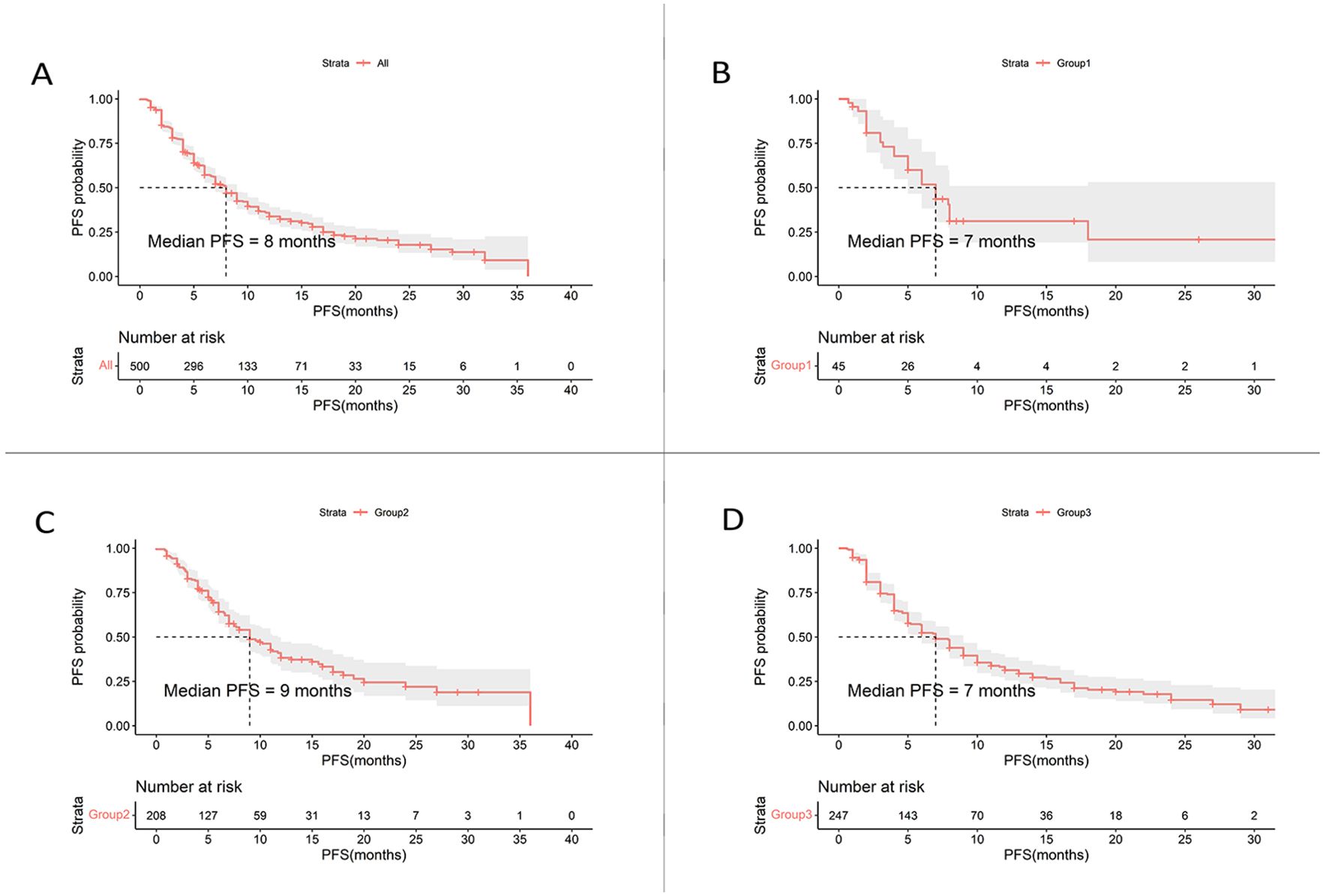
Figure 1. Kaplan-Meier curves of PFS for HER2-positive MBC patients. (A) Kaplan-Meier estimates for the entire cohort (n = 500). (B) Kaplan-Meier estimates for patients treated with inetetamab + pertuzumab + chemotherapy (n = 45). (C) Kaplan-Meier estimates for patients treated with inetetamab + TKIs + chemotherapy (n = 208). (D) Kaplan-Meier estimates for patients treated with inetetamab + chemotherapy (n = 247). PFS, progression-free survival; TKIs, tyrosine kinase inhibitors.
Patients who received inetetamab + TKIs + chemotherapy treatment achieved the most prolonged PFS of 9.0 months, which surpassed those of other regimens (Figures 1B–D) including inetetamab + pertuzumab + chemotherapy (7.0 months) as well as inetetamab + chemotherapy (7.0 months). The ORRs for the various treatment regimens were listed as follows: inetetamab + TKIs + chemotherapy (29.3%), inetetamab + chemotherapy (28.7%), and inetetamab + pertuzumab + chemotherapy (24.4%). Additionally, the combination treatment of inetetamab + TKIs + chemotherapy demonstrated an outstanding DCR of 92.8%, followed by inetetamab + pertuzumab + chemotherapy (88.9%) as well as inetetamab + chemotherapy (86.2%).
3.4 Subgroup analysis of PFS based on inetetamab + TKIs + chemotherapy
As mentioned above, patients who received inetetamab + TKIs + chemotherapy treatment achieved a longer PFS of 9.0 months. To explore determinants of this clinical benefit, we conducted both univariate and multivariate analyses across common baseline variables (Table 4).
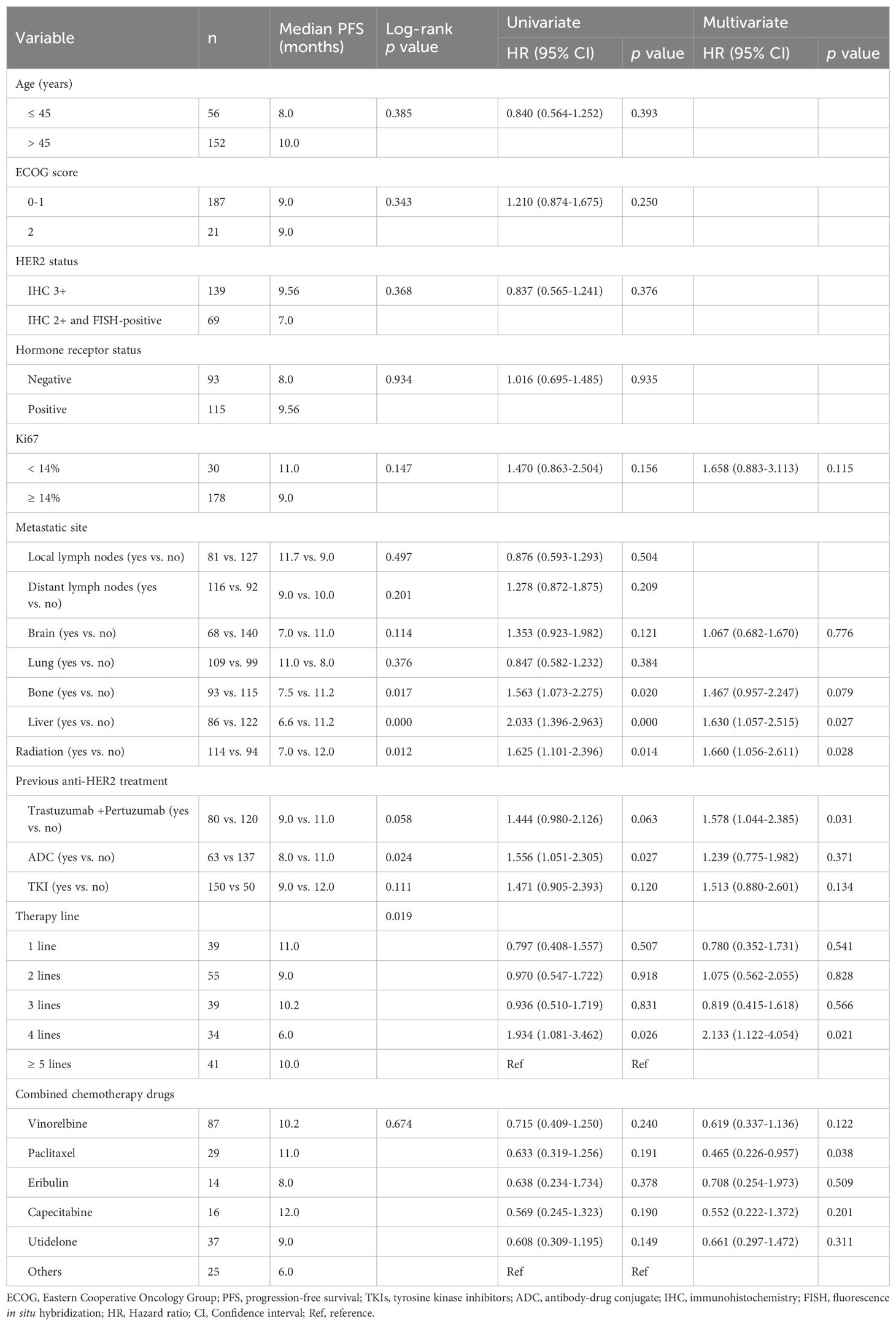
Univariate screening identified bone metastasis (7.5 vs. 11.2 months, p = 0.017), liver metastasis (6.6 vs. 11.2 months, p < 0.001), prior ADC exposure (8.0 vs. 11.0 months, p = 0.024), radiotherapy history (7.0 vs. 12.0 months, p = 0.012) and therapy line (p = 0.019) as factors significantly associated with PFS (Figure 2). Variables with p < 0.20 were entered into a multivariate Cox model, whitch additionally included Ki-67 index, brain metastasis, prior trastuzumab + pertuzumab, prior TKI use, and the specific cytotoxic partner.
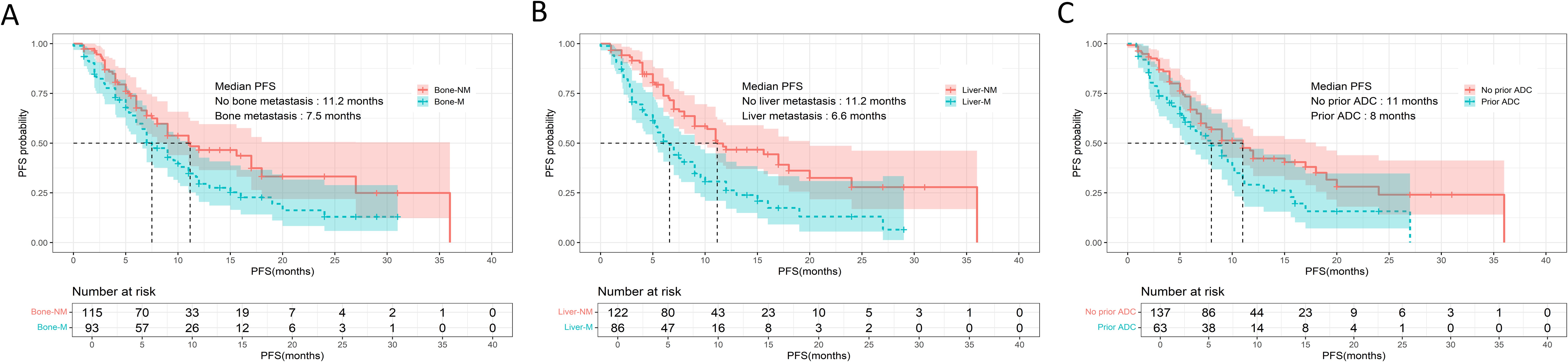
Figure 2. Kaplan-Meier curves of PFS for patients treated with inetetamab + TKIs + chemotherapy. (A) PFS analysis of patients with (n = 93) and without (n = 115) bone metastasis. (B) PFS analysis of patients with (n = 86) and without (n = 122) liver metastasis. (C) PFS analysis of patients with (n = 63) and without (n = 137) prior ADC treatment. PFS, progression-free survival; TKIs, tyrosine kinase inhibitors.
After adjustment, liver metastasis [HR 1.630, 95% CI 1.057-2.515; p = 0.027], history of radiotherapy [HR 1.660, 95% CI 1.056-2.611; p = 0.028], fourth-line vs. ≥ fifth-line therapy [HR 2.133, 95% CI 1.122-4.054; p = 0.021], and prior trastuzumab + pertuzumab treatment [HR 1.578, 95% CI 1.044 -2.385; p = 0.031] remained independently predictive of shorter PFS. Conversely, paclitaxel as the chemotherapy backbone was independently associated with prolonged PFS [HR 0.465, 95% CI 0.226-0.957, p = 0.038].
3.5 Cox regression analysis of PFS
The univariate analysis (Table 5) indicated that Ki67 index, distant lymph nodes metastasis, previous TKIs treatment and treatment strategy were significantly associated with the PFS (all p values < 0.05). Meanwhile, relevant variables that exhibited p-values less than 0.2 in the univariate analysis were exported into the multivariate regression model, including age, HER2 expression, Ki67 index, distant lymph nodes metastasis, brain metastasis, bone metastasis, liver metastasis, a history of radiation therapy, previous anti-HER2 treatment (TKIs or ADC), and treatment strategy (inetetamab + TKIs + chemotherapy). The Cox multivariate regression results revealed that the following factors were independently predictive of PFS: Ki67 index [HR 1.361, 95% CI 1.004-1.846; p = 0.047], distant lymph nodes metastasis [HR 1.437, 95% CI 1.126-1.835; p = 0.004], and treatment strategy (inetetamab + TKIs + chemotherapy) [HR 0.607, 95% CI 0.469-0.787; p < 0.000].
3.6 Safety
The most common all-grade AEs (Table 6) in all the enrolled patients were neutropenia (182/500, 36.4%) and leukopenia (157/500, 31.4%), followed by increased transaminase (105/500, 21.0%), anemia (71/500, 14.2%), and diarrhea (66/500, 13.2%). Among them, the most common grades 3/4 AEs included neutropenia (26/500, 5.2%), leukopenia (22/500, 4.4%), thrombocytopenia (9/500, 1.8%), anemia (6/500, 1.2%), and GGT increased (5/500, 1.0%). No treatment-related deaths occurred. No specific cardiotoxicity was observed with inetetamab, including decreased left ventricular ejection fraction (LVEF) and congestive heart failure (CHF).
4 Discussion
In recent years, the proportion of patients who received trastuzumab in the neoadjuvant stage has reached up to 80% and will continue to rise in the future (5). This trend suggests that HER2-positive MBC patients treated with trastuzumab will also gradually increase. Currently, this was the first multi-center real-world study to directly investigate inetetamab-based therapy in HER2-positive MBC patients, all of whom had prior exposure to trastuzumab. Although inetetamab-based treatment had been evaluated in the previous studies, their sample sizes were relatively small (n ≤ 141) (12, 13). And not all enrolled patients had previously received trastuzumab (12). The study led by Yu et al. was single-center (13). These factors limited the previous studies to fully understand the efficacy and safety of inetetamab-based therapy in patients who had received trastuzumab. Therefore, we carried out this multi-center retrospective real-world study with larger-scale patients (n = 500). Specifically, all the enrolled patients had prior exposure to trastuzumab therapy.
Our study demonstrated that inetetamab-based treatment exhibited significant clinical efficacy for HER2-positive MBC patients, especially in prolonging PFS that was comparable or superior to current mainstream anti-HER2 regimens. All enrolled patients had prior trastuzumab treatment, with the majority receiving TKIs and/or ADCs. Despite the heavily pretreated patients (median treatment line: third line), the inetetamab combination achieved a median PFS of 8.0 months. We conducted an indirect comparative analysis with key HER2-targeted therapies. The NCCN-recommended third-line standard regimen of trastuzumab + tucatinib + capecitabine demonstrated a median PFS of 7.8 months in the HER2CLIMB trial (16, 17), whereas inetetamab-based therapy reached 8.0 months, suggesting a potential advantage in patients with extensive prior trastuzumab exposure.
Importantly, benefit was consistent across multiple treatment lines (18). As first-line treatment, the regimen achieved a median PFS of 11.0 months. Although lower than the 18.7 months with pertuzumab + trastuzumab + docetaxel (CLEOPATRA trial), this result is remarkable given all patients with prior trastuzumab exposure. In second-line therapy, median PFS was 9.0 months, surpassing the 6.9 months reported for T-DM1 (EMILIA trial). Third-line use yielded 7.0 months, comparable to the tucatinib regimen. Fourth-line administration achieved 7.7 months, exceeding the 6.9 months in the control group (trastuzumab/lapatinib + capecitabine, DESTINY-Breast02 trial). Even in fifth-line or beyond, median PFS was maintained at 6.0 months. These data demonstrate sustained antitumor activity and position inetetamab as a viable option for HER2-positive MBC after trastuzumab exposure.
Multivariate analysis of the overall study population confirmed that the treatment strategy of inetetamab + TKIs + chemotherapy was an independent protective factor for PFS (HR 0.607, 95% CI 0.469-0.787; p < 0.001). It revealed that the combination regimen achieved a median PFS of 9 months, an ORR of 29.3%, and a DCR of 92.8%, aligning with the 8.2 months reported by Liu et al. (12). Further multivariate Cox analysis within the inetetamab + TKIs + chemotherapy subgroup indicated that patients with absence of liver metastasis, no prior trastuzumab + pertuzumab therapy, no radiotherapy history, and those receiving paclitaxel-based chemotherapy derived significantly greater PFS benefits. The favorable outcome observed in the ≥ 5-line subgroup potentially reflects the regimen’s particular efficacy in highly selected late-line patients.
Although the median PFS was numerically longer in the overall population receiving capecitabine (12 months), univariate analysis indicated that no single chemotherapeutic agent had a statistically significant impact on PFS (all p > 0.20). Sensitivity analyses further suggested that heterogeneity in chemotherapy regimens could still confound inter-group comparisons (Supplementary Material, Supplementary Tables S1, S2). Therefore, multivariate models require simultaneous adjustment for key prognostic variables to accurately assess treatment differences. In the multivariate analysis of the inetetamab + TKIs + chemotherapy subgroup, capecitabine did not demonstrate an independent benefit (p = 0.201), whereas paclitaxel was independently associated with significantly prolonged PFS (p = 0.038).
Mechanistically, inetetamab acts extracellularly by blocking HER2 ligand binding, mediating antibody-dependent cellular cytotoxicity (ADCC), and promoting receptor internalization and degradation. TKI acts intracellularly by irreversibly inhibiting HER2/EGFR kinase activity, thereby blocking downstream PI3K/AKT and MAPK signaling pathways. Paclitaxel stabilizes microtubules, arrests mitosis, and induces mitochondrial apoptosis. Furthermore, its ability to upregulate HER2 expression enhances the targeted binding efficiency of inetetamab, creating a therapeutic positive feedback loop. Thie synergistic action is expected to further enhance anti-tumor efficacy (19, 20). The PHILA phase III study demonstrated that adding pyrotinib to trastuzumab + docetaxel significantly extended the median PFS from 10 to 24 months (21). Therefore, the inetetamab + TKIs + paclitaxel regimen warrants further validation in prospective studies.
Additionally, analysis of the overall population identified Ki-67 index and distant lymph node metastasis as independent predictors of PFS. Elevated Ki67 index, an indicative of rapid tumor proliferation and high malignancy, contributed to increased treatment challenges and the risk of drug resistance, thereby exhibiting a negative correlation with PFS. Moreover, distant lymph node metastasis means the dissemination of breast cancer, necessitating complex treatment strategies and often predicting a poor prognosis. Thus, monitoring Ki67 index and accurately assessing the status of lymph node metastasis are of paramount importance for tailoring treatment plans and better patient outcomes. Brain, lung and liver were the most common metastatic sites in HER2-positive breast cancer patients (22). But in contrast to several previous studies (13, 23–26), our study found that these metastatic sites were not significant predictors of PFS. This discrepancy could be attributed to differences in patient characteristics, study design or sample size. Only in the subgroup analysis was liver metastasis confirmed as an independent risk factor for shorter PFS (p = 0.027), while bone metastasis and prior ADC exposure lost significance in the multivariable model (p > 0.05). This indicates that the univariate PFS differences observed for the latter two variables are driven mainly by confounding effects of tumor burden and treatment history rather than by independent biological interactions.
The study identified a manageable spectrum of treatment-emergent adverse events, most of which were not attributed to inetetamab. Importantly, no cardiotoxicity was observed including LVEF decline or CHF, yielding a cardiac safety advantage over trastuzumab + anthracyclines (11.3% events: 8.7% LVEF decline, 2.35% CHF and over T-DXd in DESTINY-Breast03 (2.3% asymptomatic LVEF decline) (18). Classic HER2-targeted toxicities were also markedly reduced. Diarrhea and rash were predominantly low-grade; hepatic enzyme elevations were mild; and no cases of interstitial lung disease were observed, in contrast to the approximately 15.4% incidence reported with T-DXd (18). Ocular toxicities and severe hypersensitivity reactions were not observed, and hematologic events were limited to grade 1–2 neutropenia and leukopenia, without necessitating treatment discontinuation. Overall, inetetamab demonstrated a favorable safety profile in patients with HER2-positive MBC, supporting its potential as a well-tolerated and effective therapeutic option in this population.
Economic considerations further support the adoption of inetetamab-based regimens. Trastuzumab (Herceptin®) has undergone multiple price reductions since its launch in China over 20 years ago. Following its inclusion in the 2017 National Reimbursement Drug List (NRDL), the price for 440 mg/vial formulation plummeted by 78% (from RMB 24,500 to RMB 5,500). Nonetheless, it remains a heavy financial burden for most Chinese patients. Similarly, T-DXd (Enhertu®) is currently priced at RMB 3,480 for 100 mg/vial formulation. In contrast, the domestic anti-HER2 monoclonal antibody inetetamab was approved in June 2020 and entered the NRDL in 2021, offering 150 mg/vial (RMB 1,368) and 50 mg/vial (RMB 590). These flexible strengths enable precise weight-based dosing, eliminate wastage, and substantially ease both financial and clinical burdens.
There are some limitations in our study. As a multicenter retrospective analysis, it is susceptible to potential information bias and incomplete medical records, which restricted the number of covariates incorporated into the Cox model. Additionally, due to feasibility and time constraints, OS was not systematically collected during the design phase, with PFS serving as the sole primary efficacy endpoint. To minimize bias, we implemented stringent eligibility criteria and harmonized data-capture protocols across all participating centers. Subsequent manual verification and systematic data-cleaning eliminated erroneous or inconsistent records, ensuring enhanced data accuracy and consistency.
In conclusion, this large-scale, multi-center real-world study of 500 HER2-positive MBC patients demonstrated that inetetamab-based regimens deliver consistent PFS benefits with a favorable safety profile. Prospective randomized trials comparing inetetamab + TKIs + paclitaxel against alternative regimens, enhanced by biomarker-guided patient stratification, are now imperative for advancing personalized care paradigms.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
This study was approved by the Ethics Committee of Liaoning Cancer Hospital & Institute (20220692). The protocol was registered at http://www.ClinicalTrials.gov (NCT06305702). The informed consent was waived to be written in this retrospective study as it solely utilized pre-existing data and records collected during the period of investigation.
Author contributions
YaD: Formal Analysis, Investigation, Project administration, Resources, Validation, Writing – original draft. TS: Investigation, Methodology, Project administration, Resources, Validation, Writing – review & editing, Conceptualization. ZC: Investigation, Resources, Validation, Writing – review & editing. QO: Investigation, Resources, Validation, Writing – review & editing. KL: Investigation, Resources, Validation, Writing – review & editing. MY: Investigation, Resources, Validation, Writing – review & editing. ZLv: Investigation, Resources, Validation, Writing – review & editing. ZLi: Investigation, Resources, Validation, Writing – review & editing. LM: Investigation, Resources, Validation, Writing – review & editing. XuL: Investigation, Resources, Validation, Writing – review & editing. YuD: Investigation, Resources, Validation, Writing – review & editing. MJ: Investigation, Resources, Validation, Writing – review & editing. YW: Investigation, Resources, Validation, Writing – review & editing. XG: Investigation, Resources, Validation, Writing – review & editing. JX: Investigation, Resources, Validation, Writing – review & editing. XiL: Investigation, Resources, Validation, Writing – review & editing. CJ: Investigation, Resources, Validation, Writing – review & editing. YE: Investigation, Resources, Validation, Writing – review & editing. LJ: Investigation, Resources, Validation, Writing – review & editing. HC: Investigation, Resources, Validation, Writing – review & editing. YfJ: Investigation, Resources, Validation, Writing – review & editing. JW: Investigation, Resources, Validation, Writing – review & editing. HL: Investigation, Resources, Validation, Writing – review & editing. LZ: Investigation, Resources, Validation, Writing – review & editing. YjJ: Investigation, Resources, Validation, Writing – review & editing. ZG: Investigation, Resources, Validation, Writing – review & editing. FD: Investigation, Resources, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
The authors express their sincere gratitude to the patients, clinicians, pathologists, and statisticians who contributed to this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1496371/full#supplementary-material
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21763
2. Zheng R, Chen R, Han B, Wang S, Li L, Sun K, et al. Cancer incidence and mortality in China, 2022. Chin J Oncol. (2024) 46:221–31. doi: 10.3760/cma.j.cn112152-20231009-00123
3. Cao M and Chen W. Interpretation on the global cancer statistics of GLOBOCAN 2020. Chin J Front Med Sci. (2021) 13:63–9. doi: 10.12037/YXQY.2024.06-01
4. Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. (2000) 406:747–52. doi: 10.1038/35021093
5. Sun C, Huang X, Li W, and Yin Y. Update and interpretation of CSCO guidelines in 2023: HER2-positive advanced breast cancer. J Prac Oncol. (2023) 38:505–8. doi: 10.13267/j.cnki.syzlzz.2023.079
6. Zhu Y, Liu K, Wang M, Wang K, and Zhu H. Trastuzumab deruxtecan versus trastuzumab emtansine for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: A cost-effectiveness analysis. Breast. (2022) 66:191–8. doi: 10.1016/j.breast.2022.10.010
7. Lv H, Yan M, Sun T, Ling X, Liu X, Yang J, et al. Anti-HER2 antibody inetetamab plus camrelizumab and utidelone for pretreated HER2-positive metastatic breast cancer: Final results from the phase 2 ICU trial. J Clin Oncol. (2023) 41:1042. doi: 10.1200/JCO.2023.41.16_suppl.1042
8. Zhang X, Chen J, Weng Z, Li Q, Zhao L, Yu N, et al. A new anti-HER2 antibody that enhances the anti-tumor efficacy of trastuzumab and pertuzumab with a distinct mechanism of action. Mol Immunol. (2020) 119:48–58. doi: 10.1016/j.molimm.2020.01.009
9. Wang X, Liu P, Lv F, and Tan Q. Evaluation of critical quality attributes of an anti-HER2 humanized monoclonal antibody drug. Chin Pharm J. (2015) 50:1054–61. doi: 10.11669/cpj.2015.12.014
10. Bian L, Xu B, Di L, Wang T, Wang X, Jiao S, et al. Phase III randomized controlled, multicenter, prospective study of recombinant anti-HER2 humanized monoclonal antibody (Cipterbin) combined with vinorelbine in patients with HER2 positive metastatic breast cancer: the HOPES Study. Natl Med J China. (2020) 100:2351–7. doi: 10.3760/cma.j.cn112137-20200116-00105
11. Wang T, Zhang P, Di L, Wang X, Yang J, Tong Z, et al. Efficacy and safety of inetetamab in combination with chemotherapy as first-line treatment of HER2-positive metastatic breast cancer: a subgroup analysis in the HOPES study. Transl Breast Cancer Res. (2022) 3:1–10. doi: 10.21037/tbcr-21-42
12. Liu B, Xie N, Tian C, Feng R, Hu Z, Li J, et al. Exploring the clinical outcomes and safety profile of inetetamab treatment in metastatic breast cancer patients: A multicenter assessment of a Chinese-origin recombinant Anti-HER2 monoclonal antibody. Breast. (2023) 72:103597. doi: 10.1016/j.breast.2023.103597
13. Liu X, Zhang P, Li C, Song X, Liu Z, Shao W, et al. Efficacy and safety of inetetamab-containing regimens in patients with HER2-positive metastatic breast cancer: a real-world retrospective study in China. Front Oncol. (2023) 13:1136380. doi: 10.3389/fonc.2023.1136380
14. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
15. Sun GW, Shook TL, and Kay GL. Inappropriate use of bivariable analysis to screen risk factors for use in multivariable analysis. J Clin Epidemiol. (1996) 49:907–16. doi: 10.1016/0895-4356(96)00025-x
16. Gradishar WJ, Moran MS, Abraham J, Abramson V, Aft R, Agnese D, et al. NCCN guidelines® Insights: breast cancer, version 4. 2023. J Natl Compr Canc Netw. (2023) 21:594–608. doi: 10.6004/jnccn.2023.0031
17. Curigliano G, Mueller V, Borges V, Hamilton E, Hurvitz S, Loi S, et al. Tucatinib versus placebo added to trastuzumab and capecitabine for patients with pretreated HER2+ metastatic breast cancer with and without brain metastases (HER2CLIMB): final overall survival analysis. Ann Oncol. (2022) 33:321–9. doi: 10.1016/j.annonc.2021.12.005
18. Mandó P, Waisberg F, Pasquinelli R, Rivero S, Ostinelli A, and Perazzo F. HER2-directed therapy in advanced breast cancer: benefits and risks. Onco Targets Ther. (2023) 19:115–32. doi: 10.2147/OTT.S335934
19. Liu S, Lan B, Li L, Qian H, and Ma F. Unraveling the synergistic mechanisms of pyrotinib in combination with trastuzumab: Insights from the PHILA trial. J Clin Oncol. (2024) 42:e13015. doi: 10.1200/JCO.2024.42.16_suppl.e13015
20. Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. (2004) 6:117–27. doi: 10.1016/j.ccr.2004.06.022
21. Zhou Y, Wang H, Yang J, Wang F, Dong D, Zhao X, et al. Comparison of the prognostic effect of pyrotinib plus trastuzumab and chemotherapy different lines therapy in HER2-positive advanced breast cancer. J Chemother. (2025) 37:135–45. doi: 10.1080/1120009X.2024.2335714
22. Battisti NML, Tong D, Ring A, and Smith I. Long-term outcome with targeted therapy in advanced/metastatic HER2-positive breast cancer: the Royal Marsden experience. Breast Cancer Res Treat. (2019) 178:401–8. doi: 10.1007/s10549-019-05406-6
23. Waks AG and Winer EP. Breast cancer treatment: A review. JAMA. (2019) 321:288–300. doi: 10.1001/jama.2018.19323
24. Deluche E, Antoine A, Bachelot T, Lardy-Cleaud A, Dieras V, Brain E, et al. Contemporary outcomes of metastatic breast cancer among 22,000 women from the multicentre ESME cohort 2008-2016. Eur J Cancer. (2020) 129:60–70. doi: 10.1016/j.ejca.2020.01.016
25. Swain SM, Miles D, Kim SB, Im YH, Im SA, Semiglazov V, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. (2020) 21:519–30. doi: 10.1016/S1470-2045(19)30863-0
26. Xu B, Li W, Zhang Q, Li Q, Wang X, Li H, et al. Pertuzumab, trastuzumab, and docetaxel for Chinese patients with previously untreated HER2-positive locally recurrent or metastatic breast cancer (PUFFIN): a phase III, randomized, double-blind, placebo-controlled study. Breast Cancer Res Treat. (2020) 182:689–97. doi: 10.1007/s10549-020-05728-w
Keywords: breast cancer, inetetamab, HER2, monoclonal antibody, real-word data
Citation: Duan Y, Sun T, Chen Z, Ouyang Q, Li K, Yan M, Lv Z, Li Z, Man L, Luo X, Dong Y, Jing M, Wang Y, Guo X, Xu J, Li X, Jiang C, E Y, Jiang L, Cao H, Jia Y, Wu J, Li H, Zhang L, Jiang Y, Gao Z and Dong F (2025) Real-world efficacy and safety of inetetamab-based therapy in HER2-positive metastatic breast cancer patients with prior exposure to trastuzumab. Front. Oncol. 15:1496371. doi: 10.3389/fonc.2025.1496371
Received: 14 September 2024; Accepted: 28 July 2025;
Published: 02 September 2025.
Edited by:
Cristian Scatena, University of Pisa, ItalyReviewed by:
Hasanain Odhar, Al-Zahrawi University College, IraqElias Antonio Gracia Medina, Institute of Oncology and Radiobiology, Cuba
Copyright © 2025 Duan, Sun, Chen, Ouyang, Li, Yan, Lv, Li, Man, Luo, Dong, Jing, Wang, Guo, Xu, Li, Jiang, E, Jiang, Cao, Jia, Wu, Li, Zhang, Jiang, Gao and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Sun, amlhbm9uZ0AxMjYuY29t; Zhanhong Chen, Y3pyZWRAc2luYS5jb20=
 Yangyang Duan1
Yangyang Duan1 Tao Sun
Tao Sun Quchang Ouyang
Quchang Ouyang Min Yan
Min Yan Zheng Lv
Zheng Lv Junnan Xu
Junnan Xu Ying E
Ying E Yufeng Jia
Yufeng Jia Jie Wu
Jie Wu Huan Li
Huan Li Zhichao Gao
Zhichao Gao