- The First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
Background: Breast cancer has emerged as the predominant malignant neoplasm globally, with potential implications for patient prognosis based on blood lipid profiles. This study aims to systematically review and meta-analyze the influence of lipid levels on the prognostic outcomes of individuals with breast cancer.
Methods: A thorough search was performed across multiple academic databases, including Embase, Cochrane, PubMed, Web of Science, CNKI, and Wanfang Database, up to March 2024. A meta-analysis was conducted to assess the impact of total cholesterol (TC), triglycerides (TG), low-density lipoprotein-cholesterol (LDL-C), and high-density lipoprotein-cholesterol (HDL-C) on the prognosis of Breast Cancer. The primary outcome measure was hazard ratios (HR) for overall survival (OS) and/or disease-free survival (DFS).
Results: Eight studies meeting inclusion criteria from a total of 13,292 were included in the meta-analysis. The systematic review and meta-analysis demonstrate an association between lower HDL-C levels and poorer survival outcomes. However, the statistical analysis did not find significant associations between HDL-C, TG, and LDL-C levels and the prognosis of breast cancer patients.
Conclusion: While our analysis reveals a link between reduced HDL-C levels and unfavorable survival outcomes, the statistical evidence does not support significant connections between HDL-C, TG, and LDL-C concentrations and the prognostic landscape for breast cancer patients. Further research is warranted to explore these relationships more comprehensively.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO, identifier CRD42021297118.
1 Introduction
Based on the most recent data published by the International Agency for Research on Cancer, the global incidence of new cases of breast cancer in 2022 has reached 2.3 million, making it the second most prevalent form of cancer worldwide. Furthermore, breast cancer has now surpassed gastric cancer to become the fourth leading cause of cancer-related mortality globally (2024). In China, the incidence of female breast cancer reached 357,200 cases in 2022, positioning it as the fifth most prevalent cancer (1) and supplanting colon and rectal cancer as the fifth leading cause of cancer mortality among women (2). Factors such as patient lifestyle, adjuvant therapy for breast cancer, and the significant reduction in estrogen levels due to menopause can contribute to the development of dyslipidemia in individuals with breast cancer (3). Nature has reported that obese individuals are at a heightened risk for developing breast cancer (4, 5), with a 25% increase in mortality among breast cancer patients who are obese (6). Additionally, a meta-analysis has indicated that obesity is linked to poorer disease-free survival (DFS) and overall survival (OS) across all subtypes of breast cancer (7). The prevalence of obesity among breast cancer patients is as high as 70% (8), and obese individuals typically exhibit elevated blood lipid levels (9). Following chemotherapy and endocrine therapy, blood lipid levels tend to remain abnormal in this population (10, 11). Hence, blood lipids play a role in influencing the prognosis of breast cancer, yet there is a scarcity of systematic reviews examining the relationship between lipids and breast cancer prognosis.
The findings of a meta-analysis examining the relationship between metabolic syndrome and breast cancer prognosis revealed conflicting results regarding the impact of dyslipidemia on breast cancer outcomes (12). Given the lack of a clear correlation, further research is warranted to explore the potential role of lipids in breast cancer prognosis. Importantly, identifying factors that influence the participation and adherence to breast cancer screening programs, such as the finding by Esra Bayrakceken et al. (13) that high blood lipid level affected the time women get mammography screening, can provide valuable insights into improving early detection and treatment outcomes. Similarly, understanding the relationship between lipid profiles and breast cancer prognosis may offer critical information for developing targeted interventions to enhance patient outcomes and reduce mortality rates. Thus, this study aims to fill this gap in the literature by systematically reviewing the available evidence on the association between blood lipid levels and breast cancer prognosis.
2 Methods
2.1 Protocol and registration
This systematic review has been conducted following the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (14) and has been registered in the PROSPERO International Register of Systematic Reviews under the registration number CRD42021297118.
2.2 Search strategy
A systematic search of electronic databases, including Embase, Cochrane, PubMed, Web of Science, CNKI, and Wanfang Database, was conducted by trained librarians to identify studies on the relationship between “breast cancer” and lipid profiles (HDL-C, LDL-C, Total cholesterol, Triglycerides) from inception to March 2024. References from included studies and review articles will be examined to locate additional relevant studies. The search strategy may be updated before final data analysis to include any newly published studies. The full search strategy is provided in online Supplementary Box 1.
2.3 Inclusion/exclusion criteria
Inclusion criteria encompassed clinical trials and cohort studies without type restrictions, involving participants over 18 years old diagnosed with primary breast cancer. Outcome indicators included hazard ratios for overall survival and/or disease-free survival in breast cancer patients with varying lipid levels. Exclusion criteria comprised non-clinical research such as cell studies and animal experiments, as well as redundant publications and literature reviews. Additionally, patients with other malignancies were excluded from clinical trials.
2.4 Data management and analysis
The results obtained from the electronic database search were imported into EndNote X9, a software utilized for managing academic literature, where duplicate titles were eliminated. Titles and abstracts were reviewed by two authors, with any discrepancies resolved through discussion. Articles deemed potentially eligible were subjected to full-text screening. A minimum of two reviewers independently assessed the full-text articles, and data extraction was conducted by two authors using standardized forms. Study authors were contacted for supplementary information as needed.
The meta-analysis examined the association between lipid levels and prognosis in breast cancer patients. Heterogeneity among the included studies was assessed using the I2 statistic, which quantifies the proportion of total variance attributable to differences between studies rather than random error. The analyses were performed using Review Manager 5.
2.5 Assessment of study quality
Two authors conducted a risk of bias assessment utilizing the Newcastle-Ottawa scale (15). This assessment was applied to both observational and interventional studies due to the authors’ post hoc analyses in clinical trials, where populations were randomized to different treatments. In these trials, levels of HDL-C, LDL-C, TC, and TG were found to be unrelated to the randomization process.
3 Results
3.1 Study selection
Figure 1 presents a summary of the publications reviewed. A literature search of electronic databases yielded 13,292 results, with secondary searches of the reference lists of retained full-text articles yielding no additional articles. After removing duplicates, a total of 6,164 studies were included, with 542 focusing on HDL-C, 1,268 on LDL-C, 1,798 on TG, and 2,559 on TC (some studies focused on multiple lipid indicators simultaneously). The majority of studies were excluded based on titles and abstracts. Following the application of pre-screening eligibility criteria, 27 full-text articles were evaluated. Three full texts were unavailable for retrieval. Sixteen articles were excluded with justification, while eight articles met the inclusion criteria.
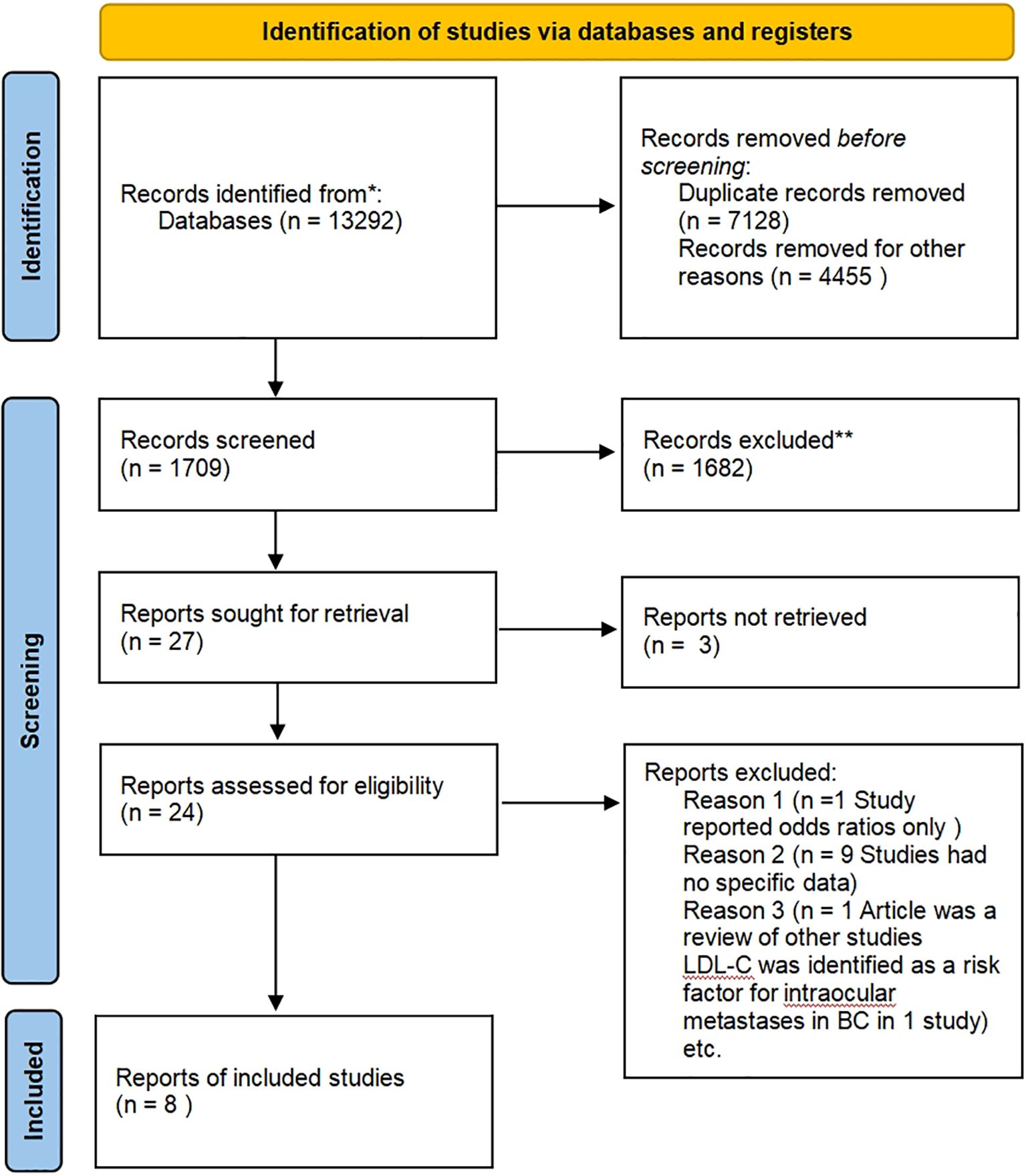
Figure 1. PRISMA diagram outlining the search strategy and selection of studies included in this review.
3.2 Study characteristics
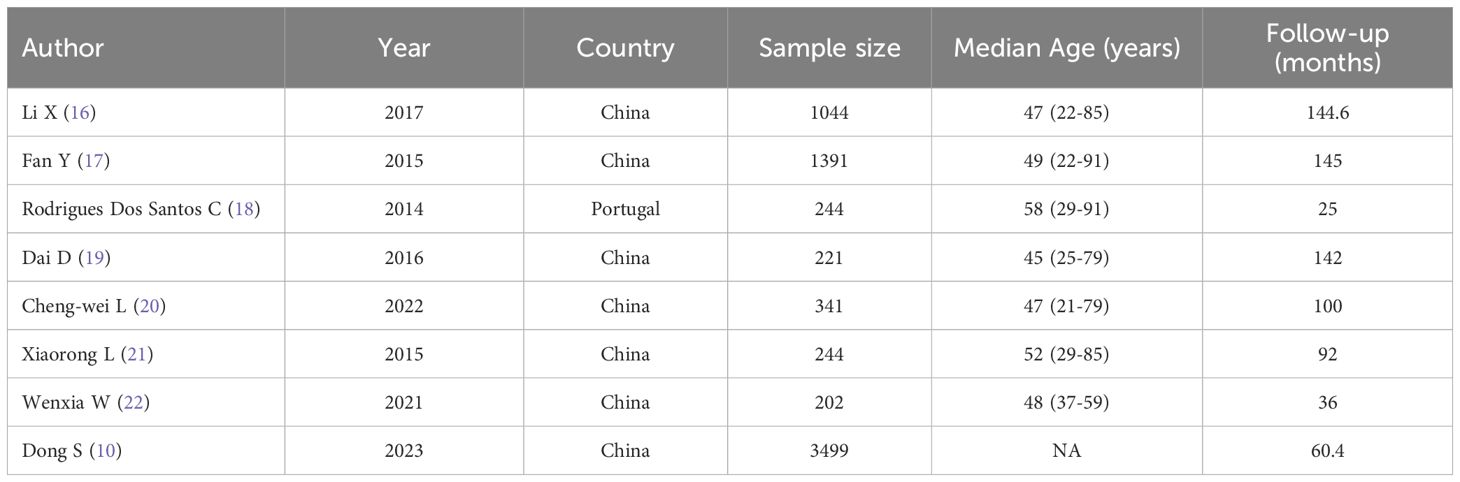
The attributes of the studies incorporated are delineated in Table 1, encompassing a total of 7186 participants. All eight studies were cohort studies focusing on female breast cancer patients, with sample sizes ranging from 202 to 3499. The predominant geographical location of the studies was China.
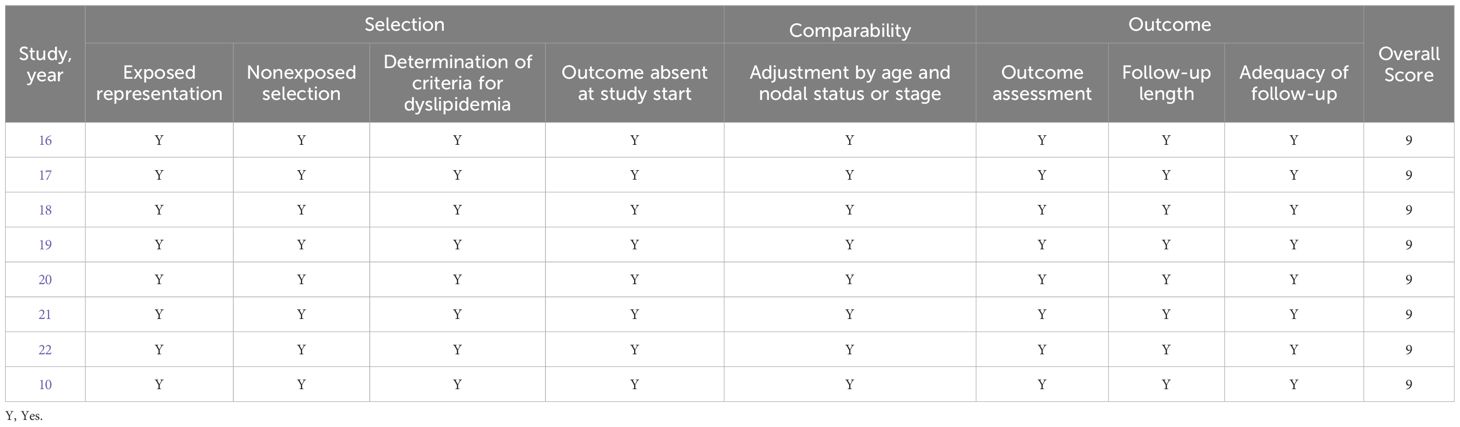
3.3 Quality appraisal results
Given that the studies incorporated in the analysis are all cohort studies, the Newcastle-Ottawa Quality Assessment Scale for cohort studies was employed for the purpose of evaluation. The quality assessment outcomes of the included studies are presented in Table 2.
3.4 Key findings of the included studies
Five studies were conducted to examine the impact of HDL-C levels on breast cancer prognosis. One retrospective study by Li X et al. (16) demonstrated a significant association between lower HDL-C levels and poorer OS in multivariate analysis (HR=0.528; 95%CI: 0.302-0.923; P=0.025), although no statistical significance was found for DFS. Another retrospective study by Fan Y et al. (17) found that low HDL-C levels were independently associated with poor prognosis for recurrence-free survival (RFS) (HR=3.266, 95%CI:2.087-5.112, P<0.0001) and OS (HR=3.071, 95%CI:1.732-5.445, P<0.0001) in patients with triple-negative breast cancer (TNBC). Decreased levels of HDL-C have been associated with poorer outcomes in terms of RFS and OS specifically in patients with TNBC, as opposed to non-TNBC patients. LI C et al. (20) demonstrated that elevated HDL-C levels were correlated with improved DFS in TNBC patients in a COX multivariate analysis (HR=3.916, 95% CI:1.355-11.313, P=0.012). Additionally, a study by Wu W et al. (22) revealed that a baseline HDL-C level below 1.14 mmol/L was associated with increased risk of recurrence (HR=2.907, 95% CI:1.024-8.255, P=0.045) and mortality (HR=8.718, 95% CI: 1.148-66.198, P=0.036) after adjusting for factors such as menopausal status, BMI, blood pressure, and blood glucose levels. A study by Dong S et al. (10) found that HDL was related to more favorable clinical outcomes (HR=0.70, 95% CI: 0.56–0.89, P<0.01). No significant relationship with OS was observed.
Five studies were included in the quality-effect meta-analysis (Figure 2), revealing an association between decreased levels of HDL-C and diminished disease-free survival (HR=1.05, 95% CI:0.87-1.28, P=0.60) and overall survival (HR=1.10, 95% CI:0.83-1.47, P=0.49) in breast cancer patients. In summary, lower HDL-C levels are correlated with unfavorable prognostic outcomes in breast cancer patients, characterized by reduced survival duration, heightened likelihood of disease recurrence, metastasis, and mortality, but there was no statistical significance.
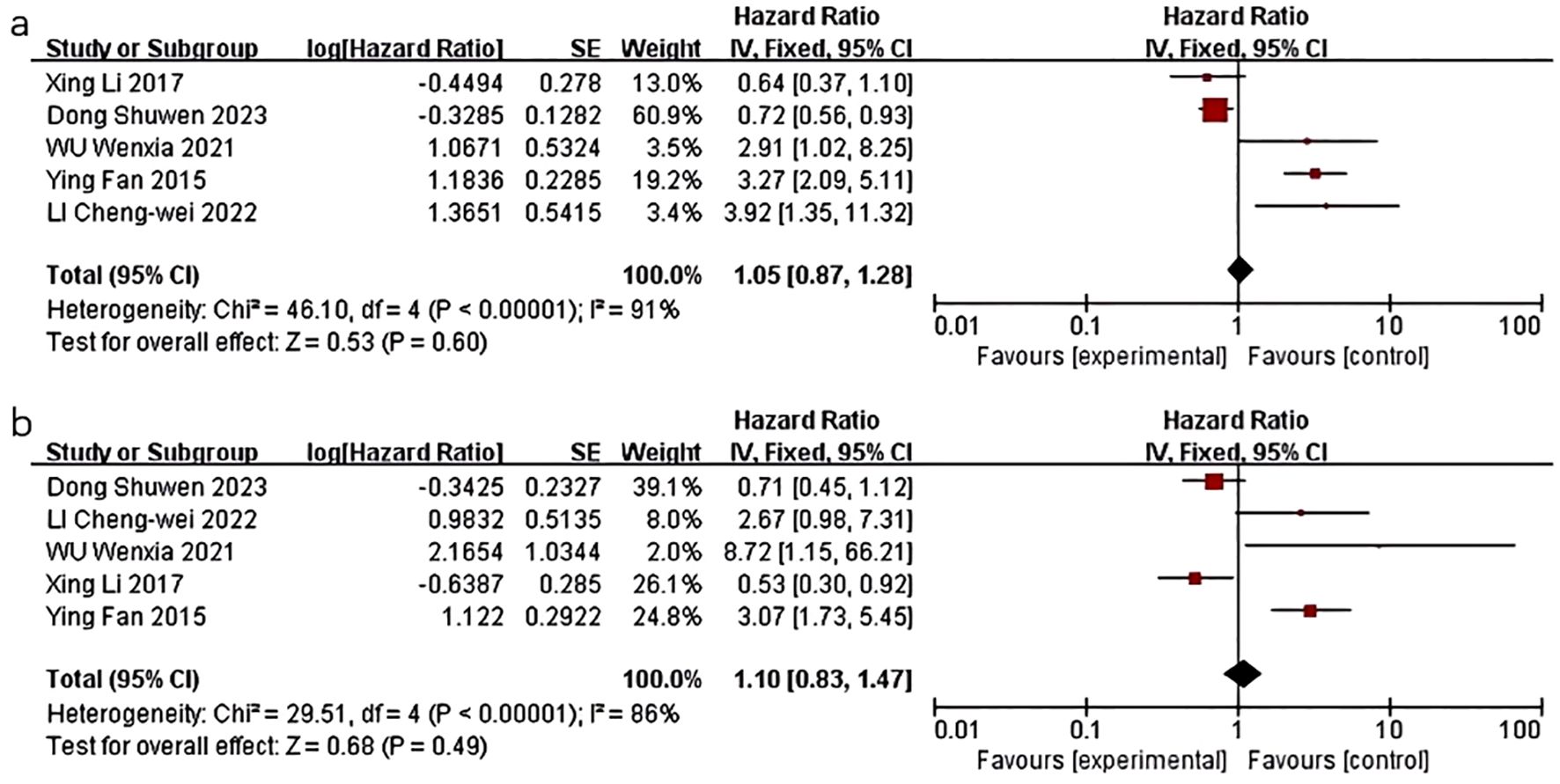
Figure 2. Association of lower HDL-C levels with DFS and OS of breast cancer patients. (a) Association of lower HDL-C levels with DFS of breast cancer patients; (b) Association of lower HDL-C levels with OS of breast cancer patients.
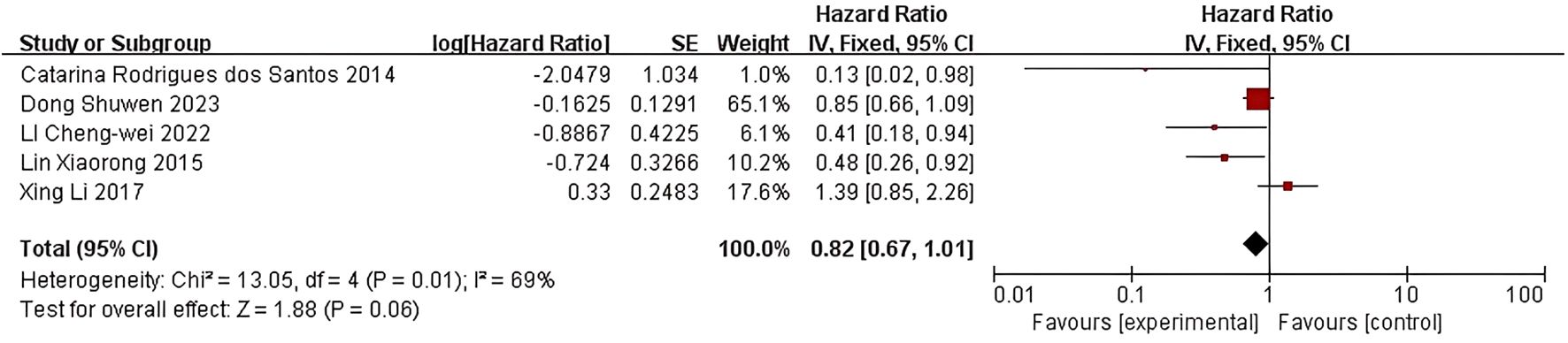
Five studies examined the correlation between LDL-C levels and breast cancer prognosis. Rodrigues dos Santos et al. (18) discovered that LDL-C levels exceeding 117 mg/dL were linked to decreased disease-free survival (HR=0.129; 95% CI:0.017-0.978; P=0.048). LI C et al. (20) also observed that lower LDL-C levels were associated with improved disease-free survival (HR=0.412, 95% CI: 0.180-0.946, P=0.037). In the study conducted by Lin Xiaorong et al. (21), the Cox regression model demonstrated a significantly poorer DFS in the group with LDL-C levels exceeding 3.08 mmol/L compared to those with LDL-C levels at or below 3.08 mmol/L. This finding suggests that LDL-C levels serve as independent prognostic factors for DFS (HR=0.4848; 95% CI: 0.2556-0.9194; P=0.0026). Conversely, in the research conducted by Li, X et al. (16)and Dong S et al. (10), no statistically significant association was observed between LDL-C levels and either DFS or OS.
Five studies were included in the quality-effect meta-analysis (Figure 3), which indicated no significant association between lower LDL-C levels and disease-free survival (HR=0.82, 95% CI:0.67-1.01, P=0.06) in breast cancer patients.
In the context of TG levels and the prognosis of breast cancer, Li et al. (16) discovered that decreased TG levels were significantly linked to a poorer DFS outcome (HR=0.569; 95%CI: 0.370-0.873; P=0.010). Conversely, LI C et al. (20) found that low TG levels were associated with a beneficial DFS outcome (HR=0.408, 95% CI: 0.175-0.951, P=0.038). Similarly, Danian Dai et al. (19) observed that in univariate analysis, shorter DFS was correlated with higher TG levels (HR=1.811; 95%CI:1.174-2.793; P=0.007), as well as shorter OS (HR=1.710, 95%CI:1.099-2.661, P=0.017). However, the results of the multivariate survival analysis model indicated that TG levels were not statistically significant in relation to DFS and OS outcomes. Dong S et al. (10) found that patients with sustained high levels of TG had poorer RFS (HR=1.90, 95% CI:1.16–3.11), but no statistically significant association was observed between TG levels and OS.
The quality-effect meta-analysis incorporated four studies and indicated that there was no statistically significant correlation between triglyceride levels and disease-free survival (HR=1.02, 95%CI 0.82-1.27, P=0.86) or overall survival (HR=0.88, 95%CI:0.66-1.18, P=0.40) (Figure 4).
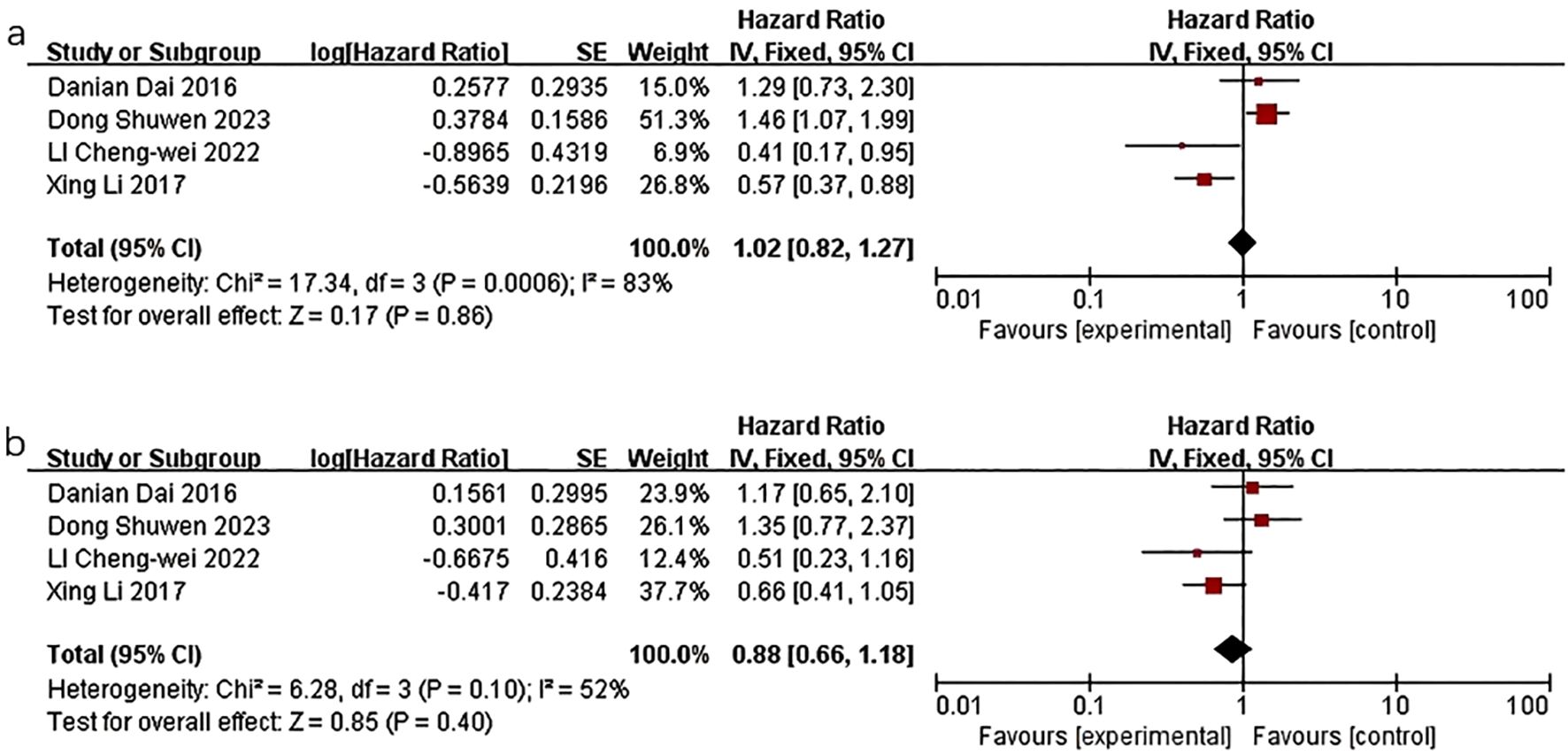
Figure 4. Association of lower TG levels with DFS and OS of breast cancer patients. (a) Association of lower TG levels with DFS of breast cancer patients. (b) Association of lower TG levels with OS of breast cancer patients.
The study conducted by Lin Xiaorong et al. (21) utilized a Cox regression model to demonstrate a lower DFS rate in the group with TC levels greater than or equal to 5.2 mmol/L compared to those with TC levels less than 5.2 mmol/L. This finding indicates that TC is an independent prognostic factor for DFS, with a hazard ratio of 0.4747 (95%CI: 0.2732-0.8245; P=0.008). However, study conducted by Dong S et al. (10) observed that no significant association was observed between TC levels and prognosis.
4 Discussion
This systematic review and meta-analysis demonstrate an association between lower HDL-C levels and poorer survival outcomes. However, the statistical analysis did not find significant associations between HDL-C, TG, and LDL-C levels and the prognosis of breast cancer patients.
This study undertook a systematic review of clinical literature examining the impact of lipid levels on breast cancer prognosis. The review revealed various shortcomings in the existing studies, such as inconsistent criteria for evaluating dyslipidemia and variations in the demographics of the study populations. These variations stem from differences in factors such as race, age, breast cancer subtype, geographic location, and dietary patterns, potentially resulting in disparate prognostic outcomes across studies.
In the United States, the prevalence of obesity is 41.9% (23), with individuals of African descent exhibiting the highest susceptibility and those of Asian descent displaying the lowest susceptibility (24). Conversely, in China, the prevalence of overweight/obesity stands at 48.9%, with a greater representation of males (25). It is widely recognized that obesity serves as a risk factor for the initiation and advancement of breast cancer, and dyslipidemia, a prevalent complication of obesity (25), and it must not be overlooked for its potential impact on breast cancer. The variations in blood lipid levels among breast cancer patients of diverse racial and geographical backgrounds necessitate further investigation into their respective impacts on the prognosis of the disease.
Moreover, research indicates that there are varying correlations between lipid levels and distinct subtypes of breast cancer. Specifically, studies have demonstrated that estrogen receptor (ER) and progesterone receptor (PR) positive breast cancer exhibit a negative association with HDL-C and LDL-C levels, with HDL-C levels being independently linked to ER and PR positive breast cancer in postmenopausal women (26). Furthermore, abnormal levels of TC, LDL-C, HDL-C, and apolipoprotein A1 (ApoA1) are closely associated with different molecular subclasses of breast cancer and are correlated with the expression of the Ki-67 protein. Notably, abnormalities in TC, LDL-C, HDL-C, and ApoA1 are more prevalent in the TNBC and human epidermal growth factor receptor 2 (HER2)-positive breast cancer subtypes compared to the luminal subtype (27). The assessment of lipid levels in individuals diagnosed with primary invasive breast cancer enables the identification of potential diagnostic indicators and therapeutic targets for the disease, offering novel insights that may enhance prognostic accuracy and inform tailored treatment strategies for distinct molecular subtypes of breast cancer.
Dyslipidemia is a significant risk factor for cardiovascular disease (28), with elevated LDL-C levels being particularly linked to an increased risk of atherosclerotic cardiovascular disease resulting from the accumulation of plaque within arterial walls (29). There exists compelling evidence supporting the notion that the trajectory of atherosclerotic cardiovascular disease can be altered by reducing LDL-C levels (30), thereby mitigating the risk of its occurrence. In the context of breast cancer, a notable association has been established between HDL-C levels and the risk of developing breast cancer (31), with studies demonstrating a significant inverse relationship between HDL-C levels and triple-negative breast cancer (17). While elevated HDL-C levels are not a necessary factor in the onset of TNBC, they hold prognostic significance for tumor recurrence and cancer-specific mortality in TNBC patients. Consequently, monitoring and standardizing HDL levels in TNBC subtypes may be of particular importance. Further investigation into the association between blood lipids and breast cancer prognosis is warranted to enhance the targeted prevention and treatment of breast cancer recurrence or metastasis.
The rising prevalence of breast cancer on a global scale has positioned it as the second most prevalent form of cancer (32). Factors such as patient lifestyle, adjuvant therapy for breast cancer, and the significant reduction in estrogen levels due to menopause can all contribute to the development of dyslipidemia in affected individuals (Center and Society, 2022). Therefore, it is imperative to investigate the potential relationship between blood lipid levels and the prognosis of breast cancer. This systematic review and meta-analysis provides a comprehensive analysis of the existing clinical literature, highlighting the intricate relationship between blood lipid levels and breast cancer prognosis. It offers valuable insights that could enhance prognostic accuracy. However, the study is limited by several factors. Firstly, there is inconsistency in evaluating dyslipidemia across different studies, which can affect the comparability of results. Secondly, the study population demographics vary significantly, with most included studies focusing on Chinese individuals, which limits the generalizability of the findings to other racial and ethnic groups. Furthermore, the study did not perform subgroup analysis based on molecular subtypes, which could have provided more detailed insights into the relationship between blood lipids and breast cancer prognosis. Other factors such as age, breast cancer subtype, geographic location, and dietary patterns also vary across studies, potentially leading to disparate prognostic outcomes. Lastly, while the study identifies associations between blood lipid levels and breast cancer prognosis, further research, particularly through multicenter, large-sample cohort studies, is needed to more definitively establish these relationships and their clinical implications.
5 Conclusion
While our analysis reveals a link between reduced HDL-C levels and unfavorable survival outcomes, the statistical evidence does not support significant connections between HDL-C, TG, and LDL-C concentrations and the prognostic landscape for breast cancer patients. Further research is warranted to explore these relationships more comprehensively.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
JS: Conceptualization, Data curation, Investigation, Visualization, Writing – original draft. YJ: Conceptualization, Data curation, Investigation, Writing – original draft. QY: Formal analysis, Supervision, Writing – review & editing. HW: Data curation, Formal analysis, Writing – review & editing. XG: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by The National Natural Science Foundation of China (No.82374447, No.82074438).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1496468/full#supplementary-material
Abbreviations
TC, Total cholesterol; TG, Triglycerides; LDL-C, Low-density lipoprotein-cholesterol; HDL-C, High-density lipoprotein-cholesterol; HR, Hazard ratios; OS, Overall survival; DFS, Disease-free survival; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RFS, Recurrence-free survival; TNBC, Triple-negative breast cancer; ER, Estrogen receptor; PR, Progesterone receptor; ApoA1, Apolipoprotein A1; HER2, Human epidermal growth factor receptor 2.
References
1. Zheng RS, Chen R, Han BF, Wang SM, Li L, Sun KX, et al. Cancer incidence and mortality in Chin. Zhonghua Zhong Liu Za Zhi. (2024) 46:221–31. doi: 10.3760/cma.j.cn112152-20240119-00035
2. Qi J, Li M, Wang L, Hu Y, Liu W, Long Z, et al. National and subnational trends in cancer burden in China 2005-20: an analysis of national mortality surveillance data. Lancet Public Health. (2023) 8:e943–55. doi: 10.1016/s2468-2667(23)00211-6
3. Center B.C.E.C.O.N.C.Q.C and Society H.M.P.C.O.B.B.C.P.A.C. Comprehensive management guideline for breast cancer follow-up and healthcare,(2022 edition). Chin J Oncol. (2022) 44:1–28. doi: 10.3760/cma.j.cn112152-20211029-00798
4. Lagarde CB, Kavalakatt J, Benz MC, Hawes ML, Arbogast CA, Cullen NM, et al. Obesity-associated epigenetic alterations and the obesity-breast cancer axis. Oncogene. (2024) 43:763–75. doi: 10.1038/s41388-024-02954-0
5. Nguyen HL, Geukens T, Maetens M, Aparicio S, Bassez A, Borg A, et al. Obesity-associated changes in molecular biology of primary breast cancer. Nat Commun. (2023) 14:4418. doi: 10.1038/s41467-023-39996-z
6. Bonet C, Crous-Bou M, Tsilidis KK, Gunter MJ, Kaaks R, Schulze MB, et al. The association between body fatness and mortality among breast cancer survivors: results from a prospective cohort study. Eur J Epidemiol. (2023) 38:545–57. doi: 10.1007/s10654-023-00979-5
7. Lohmann AE, Soldera SV, Pimentel I, Ribnikar D, Ennis M, Amir E, et al. Association of obesity with breast cancer outcome in relation to cancer subtypes: A meta-analysis. J Natl Cancer Inst. (2021) 113:1465–75. doi: 10.1093/jnci/djab023
8. Sparano JA, Wang M, Zhao F, Stearns V, Martino S, Ligibel JA, et al. Obesity at diagnosis is associated with inferior outcomes in hormone receptor-positive operable breast cancer. Cancer. (2012) 118:5937–46. doi: 10.1002/cncr.27527
9. Okekunle AP, Yie GE, Song S, Kim Z, Youn HJ, Cho J, et al. Association of lipid profile with obesity among breast cancer survivors: a cross-sectional study. Lipids Health Dis. (2022) 21:66. doi: 10.1186/s12944-022-01674-2
10. Dong S, Yu J, Chen X, and Shen K. Association of serum lipid levels and clinical outcomes in early breast cancer patients. Ther Adv Med Oncol. (2023) 15:17588359231177004. doi: 10.1177/17588359231177004
11. Ma Y, Lv M, Yuan P, Chen X, and Liu Z. Dyslipidemia is associated with a poor prognosis of breast cancer in patients receiving neoadjuvant chemotherapy. BMC Cancer. (2023) 23:208. doi: 10.1186/s12885-023-10683-y
12. Dong S, Wang Z, Shen K, and Chen X. Metabolic syndrome and breast cancer: prevalence, treatment response, and prognosis. Front Oncol. (2021) 11:629666. doi: 10.3389/fonc.2021.629666
13. Bayrakçeken E, Yaralı S, and Alkan ÖChecktae. Identify risk factors affecting participation of Turkish women in mammography screening for breast cancer prevention. Breast Cancer Res Treat. (2024) 205:487–95. doi: 10.1007/s10549-024-07296-9
14. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. Bmj. (2021) 372:n160. doi: 10.1136/bmj.n160
15. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
16. Li X, Tang H, Wang J, Xie X, Liu P, Kong Y, et al. The effect of preoperative serum triglycerides and high-density lipoprotein-cholesterol levels on the prognosis of breast cancer. Breast. (2017) 32:1–6. doi: 10.1016/j.breast.2016.11.024
17. Fan Y, Ding X, Wang J, Ma F, Yuan P, Li Q, et al. Decreased serum HDL at initial diagnosis correlates with worse outcomes for triple-negative breast cancer but not non-TNBCs. Int J Biol Markers. (2015) 30:e200–7. doi: 10.5301/jbm.5000143
18. Rodrigues Dos Santos C, Fonseca I, Dias S, and Mendes de Almeida JC. Plasma level of LDL-cholesterol at diagnosis is a predictor factor of breast tumor progression. BMC Cancer. (2014) 14:132. doi: 10.1186/1471-2407-14-132
19. Dai D, Chen B, Wang B, Tang H, Li X, Zhao Z, et al. Pretreatment TG/HDL-C ratio is superior to triacylglycerol level as an independent prognostic factor for the survival of triple negative breast cancer patients. J Cancer. (2016) 7:1747–54. doi: 10.7150/jca.15776
20. Cheng-wei L, Meng-xiang Q, Tie-cheng L, and Gong-sheng J. Effect of preoperative total cholesterol and high-density lipoprotein-cholesterol levels on the prognosis of breast cancer. Chin J Gen Pract. (2022) 20:796–800. doi: 10.16766/j.cnki.issn.1674-4152.002457
21. Xiaorong L, Di H, Jingqi C, and Zhiyong W. The serum levels of total cholesterol and LDL-cholesterol in prognostic effect for breast tumol. Lingnan Modern Clinics Surg. (2015) 15:17–21. doi: 10.1007/s10549-005-6654-9
22. Wenxia W and Meng Z. Study on the effect of baseline blood lipid level on the proanosis of patients with non-triple-negative breast cancer. Chin Gen Pract. (2021) 24:3316–22. doi: 10.12114/j.issn.1007-9572.2021.01.009
23. Hu FB. Obesity in the USA: diet and lifestyle key to prevention. Lancet Diabetes Endocrinol. (2023) 11:642–3. doi: 10.1016/s2213-8587(23)00194-8
24. Nielsen J, Narayan KV, and Cunningham SA. Incidence of obesity across adulthood in the United States 2001-2017-a national prospective analysis. Am J Clin Nutr. (2023) 117:141–8. doi: 10.1016/j.ajcnut.2022.10.012
25. Chen K, Shen Z, Gu W, Lyu Z, Qi X, Mu Y, et al. Prevalence of obesity and associated complications in China: A cross-sectional, real-world study in 15.8 million adults. Diabetes Obes Metab. (2023) 25:3390–9. doi: 10.1111/dom.15238
26. Wang X, Wang Y, Wang M, Chen X, Cui W, and Chen X. The association between serum lipid levels and histological type of breast cancer. Eur J Med Res. (2022) 27:154. doi: 10.1186/s40001-022-00784-y
27. Sun XB, Liu WW, Wang B, Yang ZP, Tang HZ, Lu S, et al. Correlations between serum lipid and Ki-67 levels in different breast cancer molecular subcategories. Oncol Lett. (2023) 25:53. doi: 10.3892/ol.2022.13639
28. Thongtang N, Sukmawan R, Llanes EJB, and Lee ZV. Dyslipidemia management for primary prevention of cardiovascular events: Best in-clinic practices. Prev Med Rep. (2022) 27:101819. doi: 10.1016/j.pmedr.2022.101819
29. Gebreegziabiher G, Belachew T, Mehari K, and Tamiru D. Prevalence of dyslipidemia and associated risk factors among adult residents of Mekelle City, Northern Ethiopia. PloS One. (2021) 16:e0243103. doi: 10.1371/journal.pone.0243103
30. Tokgozoglu L, Orringer C, Ginsberg HN, and Catapano AL. The year in cardiovascular medicine 2021: dyslipidaemia. Eur Heart J. (2022) 43:807–17. doi: 10.1093/eurheartj/ehab875
31. Katzke VA, Sookthai D, Johnson T, Kühn T, and Kaaks R. Blood lipids and lipoproteins in relation to incidence and mortality risks for CVD and cancer in the prospective EPIC-Heidelberg cohort. BMC Med. (2017) 15:218. doi: 10.1186/s12916-017-0976-4
Keywords: blood lipid levels, breast cancer, prognosis, disease-free survival, overall survival
Citation: Song J, Jin Y, Yu Q, Wu H and Gao X (2025) Impact of blood lipid levels on breast cancer prognosis: a systematic review and meta-analysis. Front. Oncol. 15:1496468. doi: 10.3389/fonc.2025.1496468
Received: 14 September 2024; Accepted: 17 June 2025;
Published: 01 July 2025.
Edited by:
Duygu Aydemir, Koç University, TürkiyeReviewed by:
Maria Antonietta Castiglione Morelli, University of Basilicata, ItalyÖmer Alkan, Atatürk University, Türkiye
Copyright © 2025 Song, Jin, Yu, Wu and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiufei Gao, Z2FveGl1ZmVpQHpjbXUuZWR1LmNu
†ORCID: Jiaqing Song, orcid.org/0000-0002-8281-407X
Ying Jin, orcid.org/0009-0003-8208-1149
Qinghong Yu, orcid.org/0000-0003-0710-8383
Hongting Wu, orcid.org/0009-0006-3730-4560
Xiufei Gao, orcid.org/0000-0002-2560-7920
 Jiaqing Song
Jiaqing Song Ying Jin†
Ying Jin† Hongting Wu
Hongting Wu Xiufei Gao
Xiufei Gao