- Department of Nuclear Medicine, Shengli Clinical Medical College of Fujian Medical University, Fujian Provincial Hospital, Fuzhou University Affiliated Provincial Hospital, Fuzhou, China
Objectives: The prognostic value of thyroglobulin antibody (TgAb) and its trends during follow-up periods may guide the treatment plans in patients with differentiated thyroid cancer (DTC) following surgery, however, there is still a lack of sufficient data. This study aims to evaluate the impact of change trends in TgAb levels on the prognosis of patients with DTC and to explore its potential application in clinical practice.
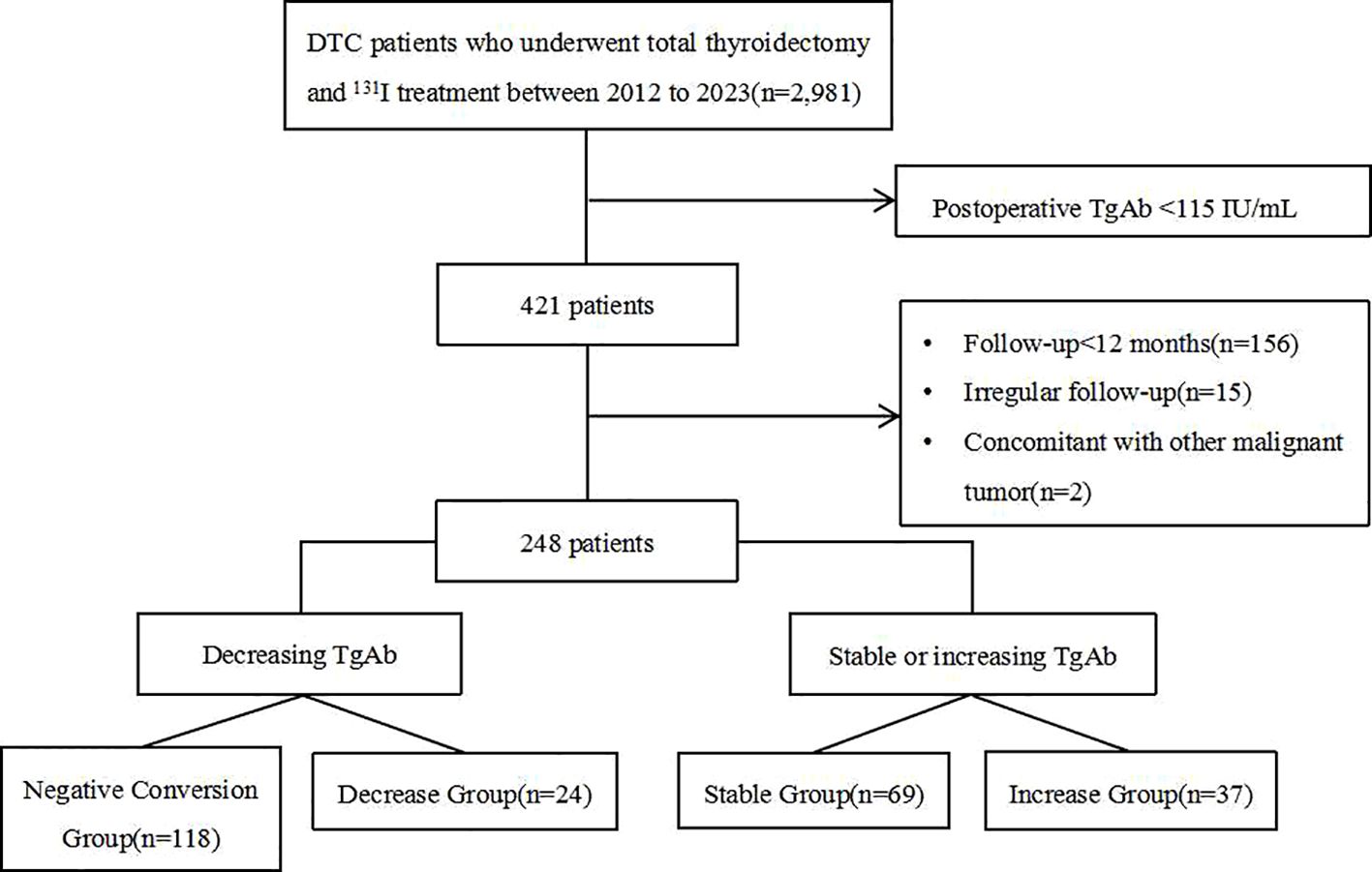
Materials and methods: A retrospective analysis was conducted on the medical records of 2,981 DTC patients who underwent surgery followed by 131I treatment. Among these, 248 patients with positive TgAb before treatment and had a follow-up period at least 12 months were included. Patients were categorized into four subgroups based on changes in TgAb levels: the Negative Conversion Group (TgAb shifted from positive to sustained negativity), the Decrease Group (TgAb decreased by more than 50% but remained positive), the Stable Group (TgAb fluctuated by ≤ 50% throughout follow-up), and the Increase Group (TgAb increased by 50% or more). Clinical and histopathological data among the four groups, as well as disease persistence/recurrence status after 131I treatment, were compared.
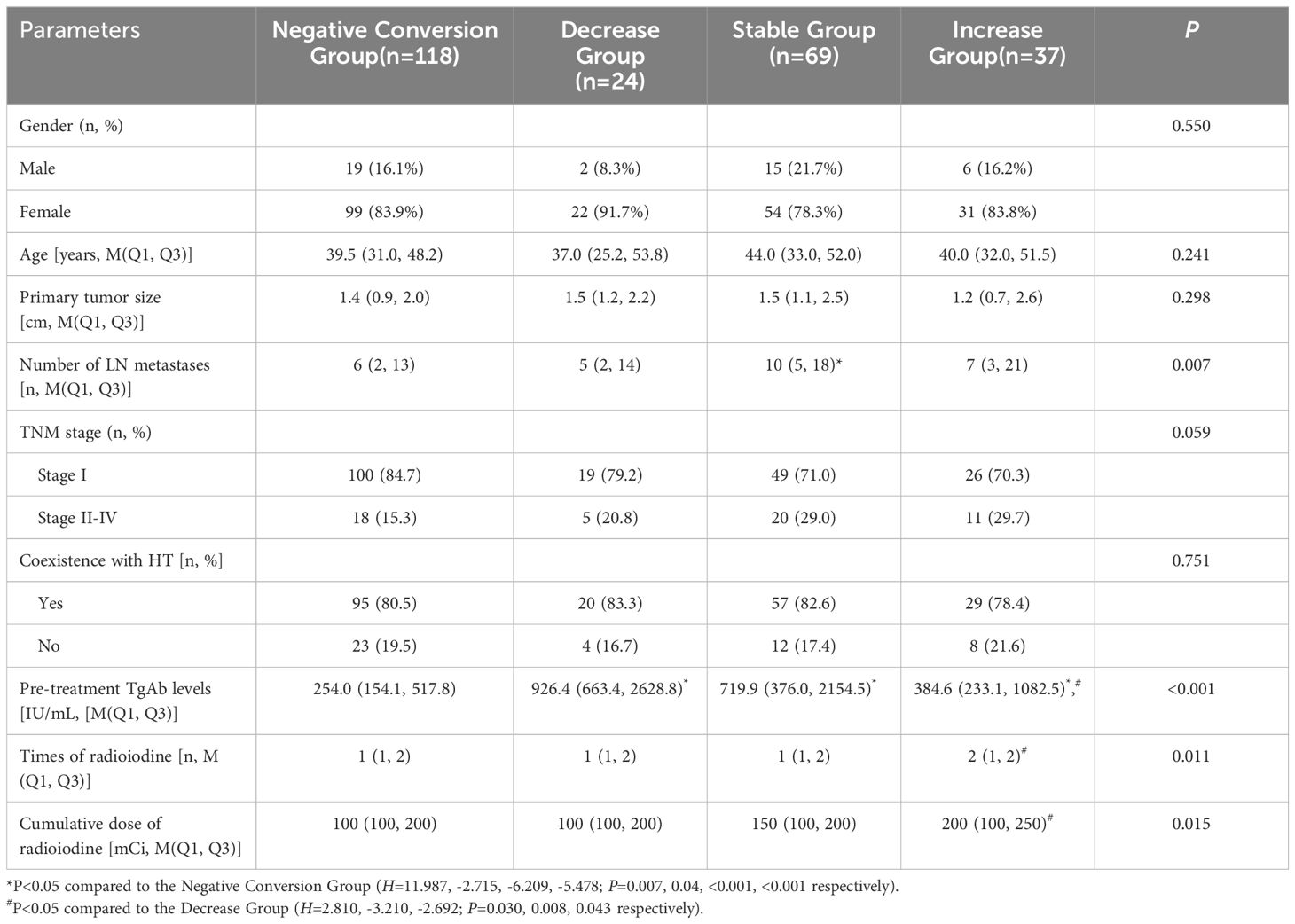
Results: Pre-treatment TgAb levels in the Negative Conversion Group were significantly lower than those in the other three groups (P<0.001). Compared to the Negative Conversion Group, the Stable Group had more postoperative lymph node metastases (P<0.05). Although pre-treatment TgAb levels in the Increase Group were lower than those in the Decrease Group, the Increase Group required significantly more treatments and a higher total dose of 131I (P<0.05). Analysis of the relationship between TgAb trends and treatment outcomes revealed 34 cases of recurrent/persistent DTC. The Negative Conversion Group had significantly better outcomes than the Stable Group and Increase Group (P<0.001, P=0.007), while the Decrease Group showed better outcomes than the Stable Group (P=0.045).
Conclusions: Negative conversion or a decrease in TgAb levels was associated with a favorable prognosis, whereas stable or increased TgAb levels indicated a higher risk of persistent/recurrent DTC. For patients with positive TgAb serum levels, monitoring the TgAb trend changes during follow-up should be a clinical priority, with timely adjustments to individualized treatment plans.
1 Introduction
Patients with differentiated thyroid carcinoma (DTC) generally have a favorable prognosis, with a 10-year survival rate exceeding 90%. However, recurrence occurs in 5% to 20% of patients, and due to its high incidence, DTC is the leading cause of mortality among endocrine tumors (1). Thyroglobulin (Tg), a thyroid-specific protein, is recognized as a specific tumor marker for the postoperative diagnosis and monitoring of persistent/recurrent disease in DTC (2). TgAb may arise as a response to the release of Tg produced by thyroid cancer cells or thyroid tissue (3). In principle, following total thyroidectomy and 131I treatment, TgAb levels in DTC patients should gradually decline in TgAb levels to negativity due to the absence of Tg antigen stimulation from cleared thyroid follicular cells. Clinically, about 20% to 30% of DTC patients test positive for TgAb (4, 5), which interferes with the accuracy of Tg detection and thereby limits its utility as a tumor marker during follow-up. Consequently, managing follow-up and selecting of treatment options for postoperative TgAb-positive DTC patients remain challenging.
Some researchers have suggested that serum TgAb could serve as a surrogate tumor marker for disease recurrence during long-term follow-up in patients undergoing combined 131I treatment after DTC surgery (6, 7). However, this hypothesis remains contentious, with no consensus on follow-up protocols or the prognostic value of TgAb positivity in DTC patients (8). Furthermore, the relatively low prevalence of TgAb positivity and the prolonged time required for seroconversion to negativity pose challenges for research. Many studies (6, 9) are limited by small sample sizes and short follow-up periods.
This study aims to evaluate the prognostic significance of TgAb change trends in postoperative TgAb-positive DTC patients after 131I treatment. By retrospectively analyzing a large dataset spanning over a decade and comparing clinical, histopathological, and treatment outcomes across different TgAb trajectories, this study seeks to enhance the clinical applicability of TgAb as an alternative tumor marker.
2 Methods
2.1 Study population
A retrospective analysis was performed on 2,981 DTC patients who underwent 131I treatment between January 2012 and September 2023, patients with positive TgAb before 131I treatment were included in the study if they met all of the following criteria. The inclusion criteria were 1) patients underwent total thyroidectomy with a confirmed pathological diagnosis and complete postoperative clinical data, 2) the first 131I ablation treatment was performed in our department, 3) serum TgAb levels were positive (≥115 IU/mL) before the initial 131I treatment, with subsequent measurements conducted using standardized equipment and assay methods, 4) regular follow-up for at least 12 months after 131I treatment, with complete relevant serological and imaging examinations, and 5) no history of medications that could interfere with TgAb levels. The exclusion criteria were 1) postoperative pathology revealed other thyroid cancer types or DTC combined with other thyroid cancers, 2) presence of other malignant tumors or severe hepatic or renal insufficiency, as well as cardiovascular system diseases, or 3) lack of standardized treatment and regular follow-up in our hospital led to incomplete relevant information. This study adhered to the principles of the Declaration of Helsinki, and all patients provided written informed consent.
2.2 131I treatment and follow-up
131I treatment protocols were implemented in accordance with the Guidelines for 131I Treatment of Differentiated Thyroid Carcinoma. Patients discontinued levothyroxine and followed a low-iodine diet for 3-4 weeks before 131I therapy. Pre-treatment evaluations included measurements of Tg, TgAb, and TSH levels, along with complete blood counts and liver and kidney function tests. Imaging studies, such as neck ultrasound, chest CT, and thyroid static imaging, were routinely performed. For suspected distant metastasis, additional diagnostics, including Dx-WBS with 131I, 18F-FDG PET/CT, or local CT/MRI, were conducted. Post-treatment whole-body scans (Rx-WBS) were performed 4-7 days after 131I therapy. The first outpatient follow-up occurred two months post-treatment, during which levothyroxine doses were adjusted based on test results. Subsequent follow-ups were scheduled every 3-6 months and included thyroid function tests and/or neck ultrasound. Additional imaging studies were performed as needed to monitor distant metastases. In cases of suboptimal treatment outcomes, repeat 131I treatment was administered at 6-12 month intervals as necessary.
2.3 Laboratory measurement
Serum levels of TSH, Tg, and TgAb were measured using electrochemiluminescence immunoassay (ECLIA) on the cobas 6000 analyzer (Roche Diagnostics, Germany). The measurement range for TgAb was 10-4000 IU/mL. Due to the heterogeneity of autoantibodies, which could lead to non-linear sparse phenomena, samples exceeding the upper limit of detection were not diluted. For this study, TgAb levels ≥4000 IU/mL were recorded as 4000 IU/mL.
2.4 Imaging examination
Rx-WBS was performed 4-7 days following oral administration of the therapeutic dose of 131I, while Dx-WBS was conducted 48 hours after ingestion of the diagnostic dose of 131I. A Discovery 670 dual-head SPECT/CT imaging system (GE Healthcare) was used, equipped with high-energy parallel hole collimators. Data acquisition was performed at a scanning speed of 9.2-10 cm per minute. Routine neck fusion tomography was conducted, with additional fusion imaging of suspicious areas when necessary.
2.5 Assessment of persistent/recurrent DTC post-131I treatment
The presence of persistent/recurrent DTC after 131I treatment was determined by integrating imaging results, including 131I-WBS, neck ultrasound, and chest CT, with cytological or histological examinations where applicable. According to the Consensus on the Diagnosis and Treatment of Recurrent Metastatic DTC by the Thyroid Cancer Expert Committee of the Chinese Society of Clinical Oncology, persistent/recurrent DTC is defined as the detection of new lesions or tumor remnants during follow-up after treatments such as surgery, thyroid clearance, and/or TSH suppression (10). Recurrence or residual tumors could occur in the thyroid bed or extrathyroidal sites via lymphatic, hematogenous, or implantation routes.
2.6 Observational indicators
The observational indicators included gender, age, primary tumor size, number of lymph node metastases, TNM stage, coexistence with Hashimoto’s thyroiditis (HT), pre-131I treatment TgAb levels, times of radioiodine, cumulative dose of radioiodine, and tumor persistence/recurrence status. Tumor TNM stage was conducted following the 8th edition of the AJCC Cancer Staging Manual by the American Joint Committee on Cancer and the International Union Against Cancer (11).
2.7 Grouping
Patients were categorized based on change trends in TgAb levels at the final follow-up, using definitions from existing literatures (6, 12): Negative Conversion Group: TgAb shifted from positive to sustained negativity(negativity defined as <115 IU/mL). Decrease Group: TgAb levels decreased by more than 50% but remained positive. Stable Group: TgAb levels fluctuated by ≤50% (increase or decrease) throughout the follow-up period. Increase Group: TgAb levels increased by more than 50%.
2.8 Statistical methods
Data were compiled into an Excel database and analyzed using SPSS 26.0 software. Quantitative data with a normal distribution were expressed as the Mean ± SD, and intergroup comparisons were performed using one-way ANOVA. Quantitative data that did not conform to a normal distribution were reported as medians (Q1, Q3), with intergroup comparisons made using the Kruskal-Wallis H test. Categorical data were expressed as percentages (%), and intergroup comparisons were made using the Pearson chi-square test or Fisher’s exact test. Kaplan-Meier survival analysis was employed to evaluate the relationship between the groups and disease persistence/recurrence. And the predictive value of relevant clinical factors for disease persistence/recurrence was analyzed using the Receiver Operating Characteristic (ROC) curve. Statistical significance was defined as P<0.05.
3 Results
3.1 Group distribution
A total of 248 eligible DTC patients with positive TgAb were included in this study (Figure 1). The cohort comprised 42 males and 206 females with an average age of 41.7 ± 13.0 years. Among them, there were 242 cases of papillary thyroid carcinoma, 5 cases of follicular thyroid carcinoma, and 1 case of mixed thyroid carcinoma. The distribution across the groups was as follows: the Negative Conversion Group (118 cases, 47.6%), Decrease Group (24 cases, 9.7%), Stable Group (69 cases, 27.8%), and Increase Group (37 cases, 14.9%).
3.2 Comparison of clinical and histopathological data among different groups
As shown in Table 1, the pre-treatment TgAb levels differed significantly between the Negative Conversion Group and the other three groups (P<0.05). A statistically significant difference was observed in the number of lymph node metastases when comparing the Stable Group with the Negative Conversion Group (P<0.05). Significant differences were also found in pre-treatment TgAb levels, times of radioiodine, and cumulative dose of radioiodine between the Increase Group and Decrease Group (P<0.05). No statistically significant differences were observed in gender, age, primary tumor size, TNM stage, or coexistence with HT among the four groups (all P>0.05).
3.3 Analysis of the relationship between TgAb change trends and persistent/recurrent DTC
Following 131I treatment, 34 patients had persistent/recurrent disease at the final follow-up. Breakdown by group: Negative Conversion Group, 6 cases; Decrease Group, 2 cases;Stable Group, 16 cases; Increase Group, 10 cases. Seven patients were confirmed to have radioiodine refractory-differentiated thyroid cancer (RAIR-DTC), including 1 case in the Negative Conversion Group, 4 cases in the Stable Group (Figure 2), and 2 cases in the Increase Group. Kaplan-Meier survival analysis curves (Figure 3) demonstrated statistically significant differences in disease persistence/recurrence among the groups. Specifically, the Negative Conversion Group exhibited a significantly lower rate of disease persistence/recurrence compared to both the Stable Group (X²=19.830, P<0.001) and Increase Group(X²=7.373, P=0.007). Additionally, the Stable Group had a higher rate than the Decrease Group (X²=4.014, P=0.045).
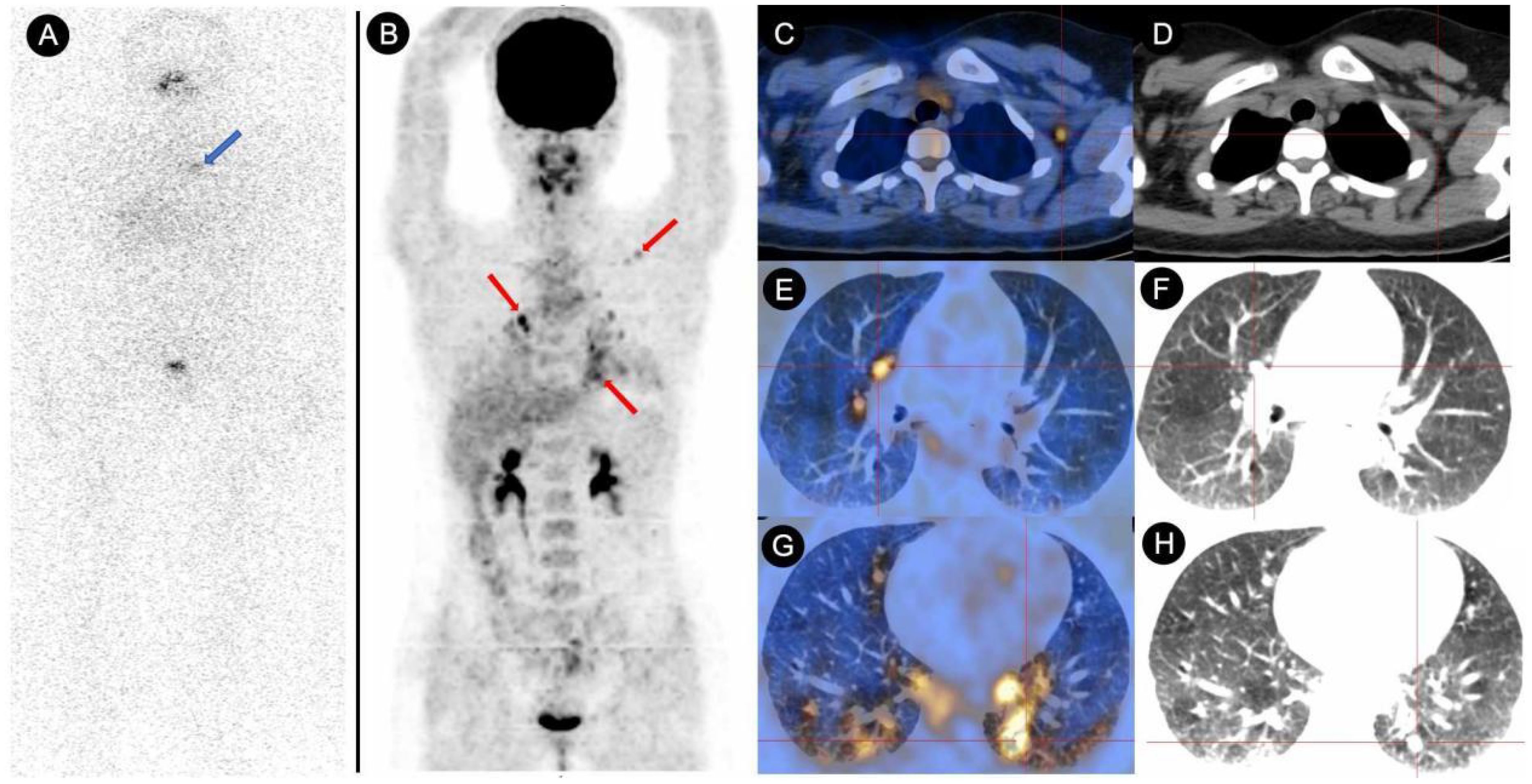
Figure 2. Comparative analysis of 131I-WBS and 18F-FDG PET/CT in a DTC patient with persistently elevated TgAb (>4000 IU/mL) over a 48-month follow-up after 131I treatment. (A) The 131I-WBS revealed no uptake of 131I in any lesions throughout the body (the area indicated by the blue arrow showed abnormal accumulation of 131I, which was confirmed by SPECT/CT to be physiological uptake by the thymus). (B–H) The whole-body MIP (Maximum Intensity Projection) image from the 18F-FDG PET/CT scan, as well as the fused PET/CT and CT images of metastatic lesions, indicating multiple lymph node metastases and lung metastases (red arrow and cross).
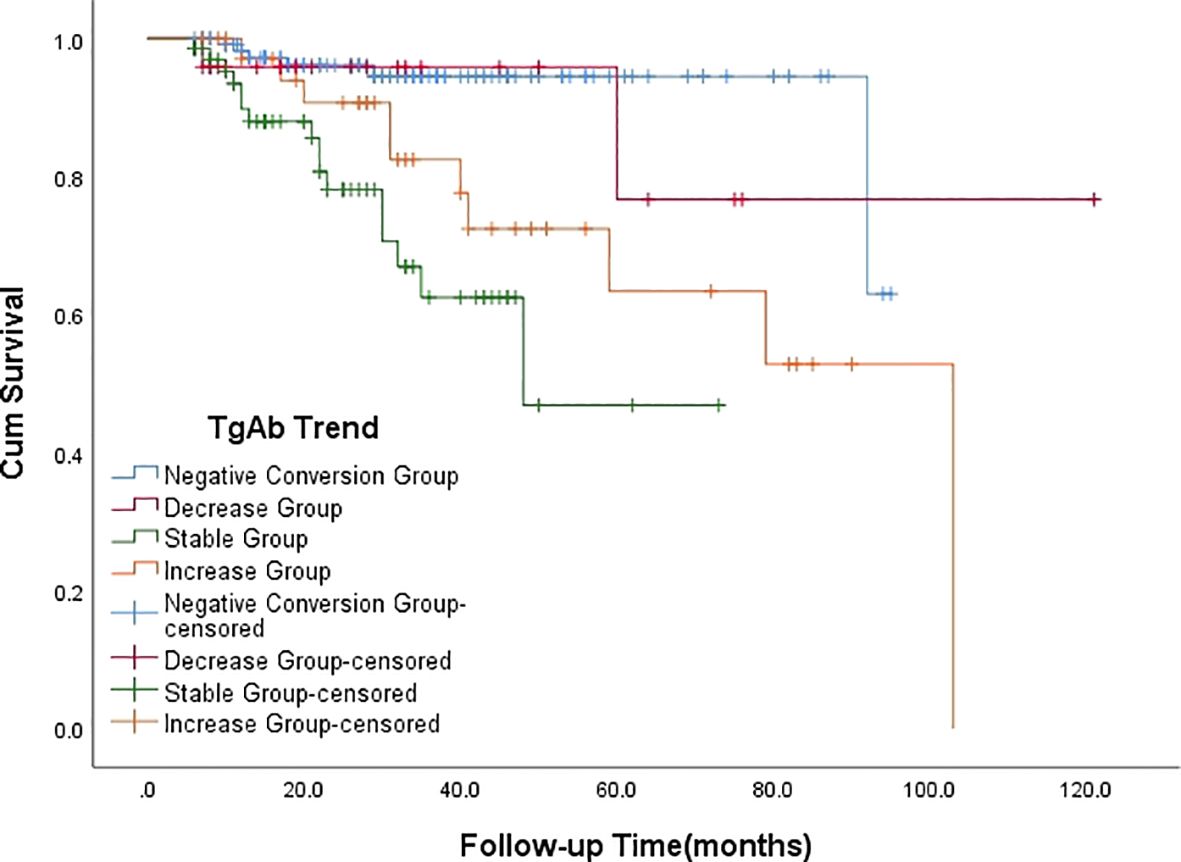
Figure 3. Kaplan-Meier survival analysis curve depicting the relationship between the outcome of TgAb and the persistence/recurrence of disease.
3.4 Analysis of the relationship between relevant initial clinical factors and persistent/recurrent DTC
No statistically significant differences were observed in gender, primary tumor size, number of lymph node metastases, or coexistence with HT between patients with persistent/recurrent DTC and those without (all P>0.05). However, patients with persistent/recurrent DTC were significantly older, had higher level of pre-treatment TgAb levels, and were at a higher TNM stage compared to those without (all P<0.05) (Table 2). The age threshold predictive of persistent/recurrent DTC was 48.5 years, with an area under the ROC curve (AUC) of 0.630 (95% CI: 0.522-0.739; P=0.015), yielding a sensitivity and specificity of 52.9% and 74.8%, respectively. Regarding pre-treatment TgAb levels, the optimal cutoff for predicting persistent/recurrent DTC was 659.75 IU/mL, achieving an AUC of 0.749 (95% CI: 0.659-0.839; P<0.001), with sensitivity and specificity for this prediction were 76.5% and 70.6%, respectively (Figure 4).
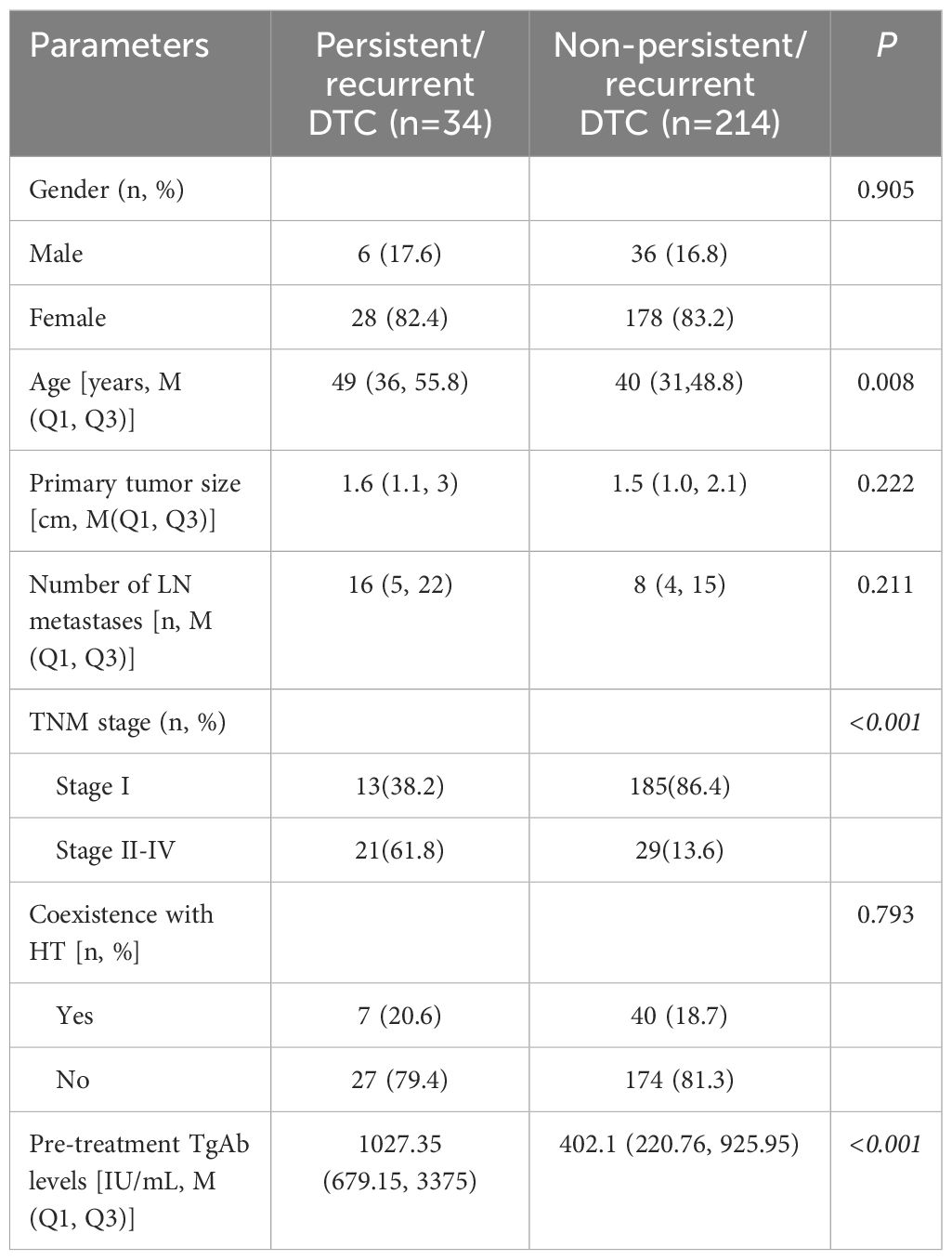
Table 2. Comparison of clinical and histopathological data before the first 131I treatment mong patients with different treatment outcomes.
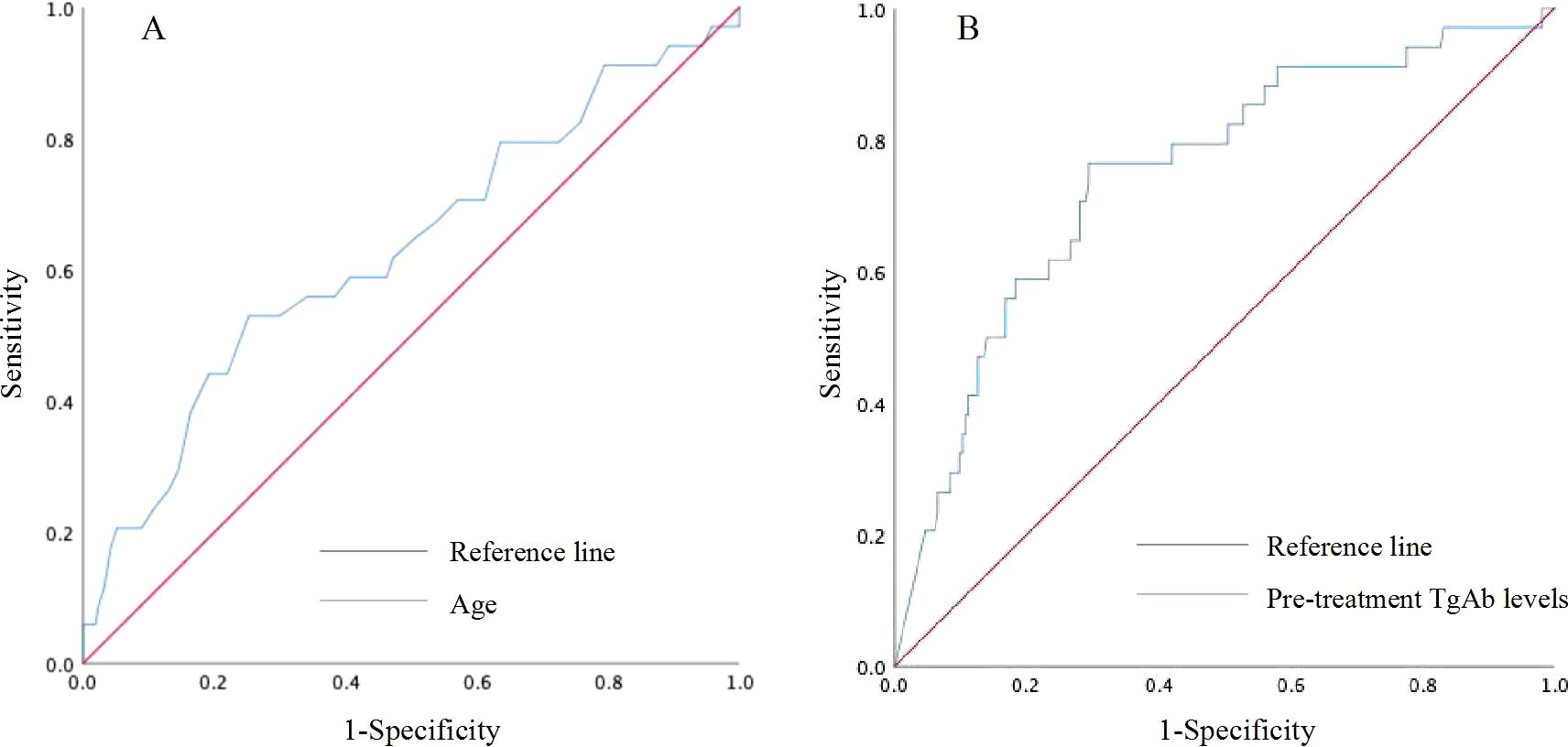
Figure 4. Receiver operating characteristic (ROC) curve for predicting persistent/recurrent DTC based on age (A) and pre-treatment TgAb levels (B).
4 Discussion
By analyzing the trends of TgAb levels during follow-up after 131I treatment in DTC patients, this study has revealed a significant correlation between these changes and the risk of disease persistence/recurrence. These findings not only provides a new perspective for the prognostic assessment of DTC patients but also lays the foundation for the development of individualized treatment strategies. Specifically, the dynamic changes in TgAb levels can offer clinical physicians more precise indicators for disease monitoring, enabling the early identification of patients at high risk for disease progression. This, in turn, allows for timely adjustments to treatment plans, with the aim of achieving better therapeutic outcomes.
The secretion of Tg antigen by residual thyroid tissue or DTC lesions is a necessary condition for the continuous production of TgAb. TgAb levels can reflect the characteristics of the primary DTC lesion to some extent, and high levels of TgAb may indicate a longer tumor size, multiple lesions, and a higher probability of capsular invasion and extrathyroidal extension (13–15). Even though some scholars suggest that the presence of TgAb lacks predictive value for the persistent/recurrent DTC, suggesting that biochemical prediction of recurrence or persistence is not always possible (9).The majority of opinions still indicate that in patients treated with 131I, pre-treatment TgAb levels can be used to estimate disease risk (16), moreover, sequential changes in TgAb levels after treatment are effective predictors of disease prognosis and contribute to clinical decision-making (1, 6, 17). A research by Ernaga-Lorea A et al. (13) indicates that a 1% higher decrease in TgAb levels leads to a 1.6% decrease in the risk of persistence/recurrence during the follow-up. Our study demonstrates that approximately 57.3% of TgAb-positive patients experienced a gradual decrease in TgAb levels after 131I treatment, with the majority achieving a negative conversion over an extended follow-up period. The incidence of disease persistence/recurrence was lower and the prognosis was better in the Negative Conversion Group compared to both the Stable Group and the Increase Group, as well as in the Decrease Group compared to the Stable Group, aligning with the results of most studies (18, 19). For these patients, serial assessments of highly sensitive Tg and TgAb may suffice for monitoring.
After total thyroidectomy and 131I treatment, an increasing TgAb level over time is associated with a higher risk of structural disease and may predict tumor recurrence and metastasis (12, 19, 20). This study revealed that patients in the Increase Group showing worse prognostic outcomes and requiring more frequent and higher doses of radioiodine compared to the Decrease Group. Therefore, in patients with rising TgAb levels, heightened surveillance is necessary, incorporating a combination of serological and imaging diagnostic procedures.
The greatest controversy has been the significance of a stable TgAb level over time for disease prognosis in recent years. A study by de Meer et al. (6) found no correlation between persistent TgAb and higher recurrence or mortality rates. However, our study shows that approximately 23.2% of patients in the Stable Group experienced persistent/recurrent DTC, significantly higher than the Decrease Group. The study by Sun D et al. (20) also indicates that patients with long-term stable TgAb levels appear to have a higher risk of developing structural disease compared to those whose TgAb levels significantly decrease. This discrepancy may be related to differences in sample size and the comprehensiveness of imaging examinations between studies. For example, our study found that a considerable proportion of patients with persistent/recurrent disease in the Stable Group had dedifferentiated iodine-refractory DTC, making the lesions more elusive and prone to being overlooked. Clinically, there is a tendency to focus more on patients with rising TgAb levels and conduct further examinations, while those with stable levels may not receive the same attention. This could lead to delays in diagnosis and disease progression in these patients.
This study also revealed that patients in the Stable Group had more postoperative lymph node metastases than those in Negative Conversion Group. The number of lymph node metastases reflects tumor invasiveness and metastatic potential, and an increase in this number is generally associated with disease progression and poorer prognosis (21, 22), approximately 15% of thyroid cancer patients with regional lymph node metastases experience regional invasion, distant metastasis, and therapeutic resistance (23). The mechanism by which TgAb contributes to lymph node metastasis of DTC is not yet fully understood. The underlying reason for higher levels of TgAb in DTC patients with lymph node metastasis may be that advanced tumors can elicit a stronger immune response within the thyroid or lymph nodes, leading to enhanced expression of Tg or an increased capacity to induce the production of TgAb (24). Therefore, patients exhibiting consistently high and stable serum TgAb levels should be managed in a manner similarly to those with elevated TgAb levels. It is advisable to consider additional structural, functional, or hybrid imaging examinations during their follow-up care (25). Especially when there are suspect lesions but the 131I-WBS is negative, further complementary examinations such as 18F-FDG PET/CT and histopathology should be considered.
Our study demonstrate that TgAb levels before initial 131I treatment is an important potential predictor for prognosis of DTC patients, the pre-treatment TgAb levels in the Negative Conversion Group is significantly lower than the other groups. This finding aligns with previous research, which suggests that lower TgAb levels are associated with better treatment responses and outcomes (26). However, changes of TgAb levels may lag behind the progression of the disease and can be influenced by a multitude of factors, including the patient’s immune system, the immunogenicity of Tg, and the development of the tumor. We observed that the Decrease Group had the highest pre-treatment TgAb levels, yet did not exhibit adverse prognostic outcomes. For those patients, one possible explanation is that a relatively high level of TgAb may require a longer time to achieve negative conversion. Therefore, for patients who enter the follow-up period after treatment, the trend of TgAb changes is more informative for monitoring prognosis than its absolute value, a perspective also proposed by Bueno et al. (27).
The main strengths of our study include the strict follow-up for a median duration of nearly three years, standardized diagnosis and treatment processes at a large tertiary hospital, and the homogeneity of the DTC patient samples from the same geographical area in southeast China over more than a decade. While the data provided are not novel, they contribute to the scientific understanding of a clinically significant topic. However, this study also has several limitations. Firstly, the single-center, retrospective design may introduce selection bias. Secondly, the long time span, variations in technical standards, incomplete clinical parameters such as genetic testing and immunological analysis, and varying degrees of detail in postoperative pathological descriptions have led to a considerable amount of data being excluded from the study.
In conclusion, when TgAb is positive before the initial 131I treatment, the change trends of TgAb during follow-up should be a critical focus in clinical practice. Particularly for patients over 48.5 years old, with pre-treatment TgAb levels ≥ 659.75 IU/mL or with a higher TNM stage that associated with an increased risk of persistent/recurrent DTC. Negative conversion or a decrease in TgAb levels signifies a good prognosis, while stable or increased TgAb levels indicate a higher risk of tumor persistence or recurrence.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
All patients followed the principles of the Declaration of Helsinki, and the study protocol was approved by the Ethics Committee of Shengli Clinical Medical College of Fujian Medical University, Fujian Provincial Hospital, Fuzhou University Affiliated Provincial Hospital (K2023-09-002). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because this is a retrospective study.
Author contributions
HG: Data curation, Methodology, Visualization, Writing – original draft, Writing – review & editing. WC: Resources, Supervision, Writing – review & editing. ZL: Data curation, Methodology, Writing – review & editing. YL: Data curation, Methodology, Writing – original draft. SC: Data curation, Methodology, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was funded by Wu Jie Ping Medical Foundation Clinical Research Special Grant Program (Grant no. 320.6750.2023-03-41).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA. (2017) 317:1338–48. doi: 10.1001/jama.2017.2719
2. Nuclear Medicine Association of Chinese Medical Association. 131I guidelines of the treatment of differentiated thyroid cancinoma (2021 edition). Chin J Nucl Med Mol Imaging. (2021) 41:218–41. doi: 10.3760/cma.j.cn321828-20201113-00412
3. Sanjari M, Ordooei M, Amirkhosravi L, Naghibzadeh-Tahami A, Nazemi S. The effect of positive thyroglobulin antibodies on the prognosis and treatment response in patients with papillary thyroid carcinoma. Heliyon. (2024) 10:e26092. doi: 10.1016/j.heliyon.2024.e26092
4. Dekker BL, van der Horst-Schrivers ANA, Sluiter WJ, Brouwers AH, Lentjes EGWM, Heijboer AC, et al. Clinical applicability of low levels of thyroglobulin autoantibodies as cutoff point for thyroglobulin autoantibody positivity. Thyroid. (2019) 29:71–8. doi: 10.1089/thy.2018.0195
5. Lee ZJO, Eslick GD, Edirimanne S. Investigating antithyroglobulin antibody as a prognostic marker for differentiated thyroid cancer: A meta-analysis and systematic review. Thyroid. (2020) 30:1601–12. doi: 10.1089/thy.2019.0368
6. De Meer SGA, Vorselaars WMCM, Kist JW, Stokkel MPM, de Keizer B, Valk GD, et al. Follow-up of patients with thyroglobulin-antibodies: rising Tg-Ab trend is a risk factor for recurrence of differentiated thyroid cancer. Endocr Res. (2017) 42:302–10. doi: 10.1080/07435800.2017.1319858
7. Chai H, Zhu ZJ, Chen ZQ, Yu YL. Diagnostic value of Tg and TgAb for metastasis following ablation in patients with differentiated thyroid carcinoma coexistent with Hashimoto thyroiditis. Endocr Res. (2016) 41:218–22. doi: 10.3109/07435800.2015.1010210
8. Morbelli S, Ferrarazzo G, Pomposelli E, Pupo F, Pesce G, Calamia I, et al. Relationship between circulating anti-thyroglobulin antibodies (TgAb) and tumor metabolism in patients with differentiated thyroid cancer (DTC): prognostic implications. J Endocrinol Invest. (2017) 40:417–24. doi: 10.1007/s40618-016-0578-6
9. Turanli S, Mersin HH. Serum antithyroglobulin antibody levels are not a good predictive factor on detection of disease activity in patients with papillary thyroid carcinoma. J Cancer Res Ther. (2020) 16:624–29. doi: 10.4103/jcrt.JCRT_340_17
10. Lin YS, Zhang B, Liang ZY, Niu LJ, He XY, Chen LB, et al. Consensus on the diagnosis and treatment of recurrent metastatic differentiated thyroid cancer. China Oncol. (2015) 25:481–96.
11. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. (2017) 67:93–9. doi: 10.3322/caac.21388
12. Reverter JL, Rosas-Allende I, Puig-Jove C, Zafon C, Megia A, Castells I, et al. Prognostic significance of thyroglobulin antibodies in differentiated thyroid cancer. J Thyroid Res. (2020) 2020:8312628. doi: 10.1155/2020/8312628
13. Ernaga-Lorea A, Hernández-Morhain MC, Anda-Apiñániz E, Pineda-Arribas JJ, Migueliz-Bermejo I, Eguílaz-Esparza N, et al. Prognostic value of change in anti-thyroglobulin antibodies after thyroidectomy in patients with papillary thyroid carcinoma. Clin Transl Oncol. (2018) 20:740–44. doi: 10.1007/s12094-017-1782-3
14. Li C, Yu W, Fan J, Li G, Tao X, Feng Y, et al. Thyroid functional parameters and correlative autoantibodies as prognostic factors for differentiated thyroid cancers. Oncotarget. (2016) 7:49930–38. doi: 10.18632/oncotarget.10236
15. Qin J, Yu Z, Guan H, Shi L, Liu Y, Zhao N, et al. High thyroglobulin antibody levels increase the risk of differentiated thyroid carcinoma. Dis Markers. (2015) 2015:648670. doi: 10.1155/2015/648670
16. Rosario PW, Côrtes MCS, Franco Mourão G. Follow-up of patients with thyroid cancer and antithyroglobulin antibodies: a review for clinicians. Endocr Relat Cancer. (2021) 28:111–19. doi: 10.1530/ERC-21-0012.[CC-16
17. Liu QH, Yin MT, Li GX. Antithyroglobulin antibody variation during follow-up has a good prognostic value for preoperative antithyroglobulin antibody-positive differentiated thyroid cancer patients: a retrospective study in southwest China. Front Endocrinol (Lausanne). (2021) 12:774275. doi: 10.3389/fendo.2021.774275
18. Côrtes MCS, Rosario PW, Oliveira LFF, Calsolari MR. Clinical impact of detectable antithyroglobulin antibodies below the reference limit (borderline) in patients with papillary thyroid carcinoma with undetectable serum thyroglobulin and normal neck ultrasonography after ablation: a prospective study. Thyroid. (2018) 28:229–35. doi: 10.1089/thy.2017.0350
19. Jia X, Pang P, Wang L, Zhao L, Jiang L, Song Y, et al. Clinical analysis of preoperative anti-thyroglobulin antibody in papillary thyroid cancer between 2011 and 2015 in Beijing, China: a retrospective study. Front Endocrinol. (2020) 11:452. doi: 10.3389/fendo.2020.00452
20. Sun D, Zheng X, He X, Huang C, Jia Q, Tan J, et al. Prognostic value and dynamics of antithyroglobulin antibodies for differentiated thyroid carcinoma. biomark Med. (2020) 14:1683–92. doi: 10.2217/bmm-2019-0432
21. Sun YG, Chen F, Sun QL, Tian JY, He XC. The number of metastatic lymph nodes optimizes staging in patients aged 55 years or older with papillary thyroid cancer. Front Endocrinol (Lausanne). (2022) 13:1026737. doi: 10.3389/fendo.2022.1026737
22. Lee J, Song Y, Soh EY. Prognostic significance of the number of metastatic lymphnodes to stratify the risk of recurrence. World J Surg. (2014) 38:858–62. doi: 10.1007/s00268-013-2345-6
23. Zhi J, Wu Y, Hu L, Zhao J, Liu H, Ruan X, et al. Assessment of the prognostic value and N1b changes of the eighth TNM/AJCC staging system for differentiated thyroid carcinoma. Int J Clin Oncol. (2020) 25:59–66. doi: 10.1007/s10147-019-01522-x
24. Wassner AJ, Della Vecchia M, Jarolim P, Feldman HA, Huang SA. Prevalence and significance of thyroglobulin antibodies in pediatric thyroid cancer. J Clin Endocrinol Metab. (2017) 102:3146–53. doi: 10.1210/jc.2017-00286
25. Giovanella L, D’Aurizio F, Algeciras-Schimnich A, Görges R, Petranovic Ovcaricek P, Tuttle RM, et al. Thyroglobulin and thyroglobulin antibody: an updated clinical and laboratory expert consensus. Eur J Endocrinol. (2023) 189:11–27. doi: 10.1093/ejendo/lvad109
26. Ora M, Nazar AH, Mishra P, Barai S, Arya A, Pradhan PK, et al. Clinical outcome of patients with differentiated thyroid cancer and raised antithyroglobulin antibody levels: a retrospective study. Thyroid Res. (2021) 14:8. doi: 10.1186/s13044-021-00099-w
Keywords: differentiated thyroid carcinoma, 131I, thyroglobulin antibody, prognosis, persistent/recurrent DTC
Citation: Ge H, Chen W, Lin Z, Li Y and Chen S (2025) Analysis of the prognostic value of thyroglobulin antibody change trends during follow-up after 131I treatment in patients with differentiated thyroid carcinoma. Front. Oncol. 15:1496594. doi: 10.3389/fonc.2025.1496594
Received: 14 September 2024; Accepted: 17 January 2025;
Published: 04 February 2025.
Edited by:
Thomas Joseph Fahey Iii, Cornell University, United StatesReviewed by:
Xiaotian Xia, Huazhong University of Science and Technology, ChinaYoulutuziayi Rixiati, Fudan University, China
Copyright © 2025 Ge, Chen, Lin, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenxin Chen, d2VueGluY2h6dEBhbGl5dW4uY29t
 Hua Ge
Hua Ge Wenxin Chen
Wenxin Chen Zhiyi Lin
Zhiyi Lin