- 1Department of Thoracic Surgery, The Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, China
- 2Department of Oncology, Shangrao People’s Hospital, Shangrao, China
- 3Pharmacy Intravenous Admixture Services, The Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, China
Background: Combining PD-1/PD-L1 inhibitors with chemotherapy (PIC) is a standard first-line treatment for advanced non-small cell lung cancer (NSCLC). The addition of bevacizumab to this regimen (PD-1/PD-L1 inhibitors+bevacizumab+chemotherapy [PIBC]) remains controversial regarding its potential to enhance antitumor efficacy in clinical practice. This meta-analysis aims to compare the antitumor effectiveness and safety profiles of PIBC with PIC.
Methods: We systematically searched six databases to identify eligible RCTs. The primary outcomes were overall survival (OS) and progression-free survival (PFS), while the secondary outcomes included treatment responses and adverse events (AEs).
Results: Three RCTs (IMpower150, jRCT2080224500, and ORIENT-31) comprising a total of 1529 patients were analyzed. The PIBC regimen significantly improved PFS (hazard ratio [HR]: 0.76 [0.66, 0.87], P < 0.0001), objective response rate (risk ratio [RR]: 1.36 [1.22, 1.51], P < 0.00001), and disease control rate (RR: 1.06 [1.00, 1.12], P = 0.04). The PFS rates were also higher in the PIBC group at 6 and 18 months. Both groups showed similar results in terms of OS, 3–36 month OS rates, and total AEs. However, the PIBC group exhibited a higher incidence of grade 3–5 AEs, serious AEs, grade 3–5 treatment-related AEs (TRAEs) and serious TRAEs. The most frequent grade 3–5 AEs in the PIBC group included anorexia (36.40%), decreased neutrophil count (16.25%), neutropenia (13.50%), reduced white blood cell count (12.12%), and febrile neutropenia (9.42%).
Conclusions: PIBC appears to be better than PIC for advanced NSCLC offering improved PFS and response rates (ORR and DCR). However, its higher incidence of AEs requires cautious attention.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42024559146, identifier CRD42024559146.
Introduction
In recent decades, non-small cell lung cancer (NSCLC) has remained one of the leading cause of incidence and mortality (1). The treatment landscape for advanced NSCLC has significantly evolved in recent years. Chemotherapy was previously the standard treatment for advanced NSCLC, but its limited efficacy often resulted in suboptimal patient outcomes (2). The advent of PD-1/PD-L1 inhibitors has provided new hope, offering improved survival for these patients (3). However, the efficacy of immunotherapy alone has varies across different patient populations (4).
The combination of antiangiogenic agents, such as bevacizumab, with PD-1/PD-L1 inhibitors and chemotherapy (PIBC) shows promise as a treatment strategy for advanced NSCLC. This regimen aims to enhance antitumor efficacy by targeting multiple pathways involved in tumor growth and progression (5). Despite its potential benefits, the clinical advantage of adding bevacizumab to PD-1/PD-L1 inhibitors and chemotherapy remains controversial (6–8). Recent randomized controlled trials (RCTs), including IMpower150, jRCT2080224500, and ORIENT-31, have generated substantial data comparing PIBC with PD-1/PD-L1 inhibitors plus chemotherapy (PIC) (9–11). While these studies provide valuable insights, they also underscore the need for a comprehensive analysis to determine the true clinical value of these regimens.
This meta-analysis systematically reviews and synthesizes data from these RCTs to assess the efficacy and safety of PIBC versus PIC, providing a robust foundation for optimizing treatment protocols in advanced NSCLC.
Materials and methods
Search strategy
The search strategy employed keywords such as “PD-1/PD-L1 (See Supplementary Table S1 for details)”, “Bevacizumab”, “Lung cancer”, and “Randomized”. A comprehensive search was conducted across six databases (PubMed, ScienceDirect, the Cochrane Library, Scopus, EMBASE, and Web of Science) from their inception to March 12, 2025 (Supplementary Table S1). Additionally, the reference lists of included studies were examined to identify further eligible RCTs.
Selection criteria
Inclusion criteria (PICOS):
1. Participants (P): advanced NSCLC.
2. Intervention (I) and control (C): directly comparing PIBC (B includes bevacizumab and its biosimilars) and PIC.
3. Outcomes (O): survival, survival rate, responses, and adverse events (AEs).
4. Study design (S): phase 3 RCTs.
Exclusion criteria: animal experiments, reviews, meta-analyses, case reports and conference articles.
Data extraction
Data were extracted by two investigators: study characteristics (geographic region, phase, etc.), patient demographics (ECOG PS, TNM Stage, etc.), survival outcomes (overall survival [OS] and progression-free survival [PFS]), survival rates (OS rate [OSR] and PFS rate [PFSR]), responses (objective response rate [ORR], disease control rate [DCR], etc.), and AEs (total, grade 3-5, etc.). Missing data was obtained by contacting the corresponding authors of the included studies. Discrepancies were resolved through re-evaluation.
Outcome assessments
OS and PFS were subgroup analyzed based on age, sex, race, ECOG PS, smoking status, pathological type, stage, brain metastases, liver metastases, PD-L1 combined positive score (CPS), PD-1/PD-L1 inhibitors type, and epidermal growth factor receptor (EGFR)-mutant.
Quality assessment
We assessed the quality of RCTs using the Cochrane Risk Assessment Tool and the Jadad scale, which assigns up to 5 points based on randomization, blinding, and participant inclusion. A score of 3 or higher indicates high-quality studies (12, 13). The overall certainty of the evidence was evaluated using the GRADE approach, which considers risk of bias, indirectness, imprecision, and publication bias. This framework categorizes certainty into four levels: very low, low, moderate, and high (14).
Statistical analysis
The combined data were analyzed using Review Manager 5.3 and STATA 12.0. Survival variables were assessed with the hazard ratio (HR), while dichotomous variables were evaluated with the risk ratio (RR). The OSR was examined at 6–36 months, and the PFSR at 6–24 months. The I² statistic and χ² test were utilized to evaluate heterogeneity. A fixed-effects model was applied when I² was less than 50% or P was greater than 0.1, indicating no notable heterogeneity; otherwise, a random-effects model (This model accounts for both within-study and between-study variability, making it suitable for analyzing data from studies that may have different underlying effect sizes due to variations in study populations, interventions, or other factors.) was used. Statistical significance was defined as P < 0.05. Publication bias was assessed through funnel plot visual inspection. Sensitivity analyses were conducted for primary outcomes (OS, PFS, and ORR) and outcomes with significant heterogeneity. This study adhered to PRISMA guidelines (Supplementary Table S2) and was registered in PROSPERO (ID: CRD42024559146).
Results
Search results
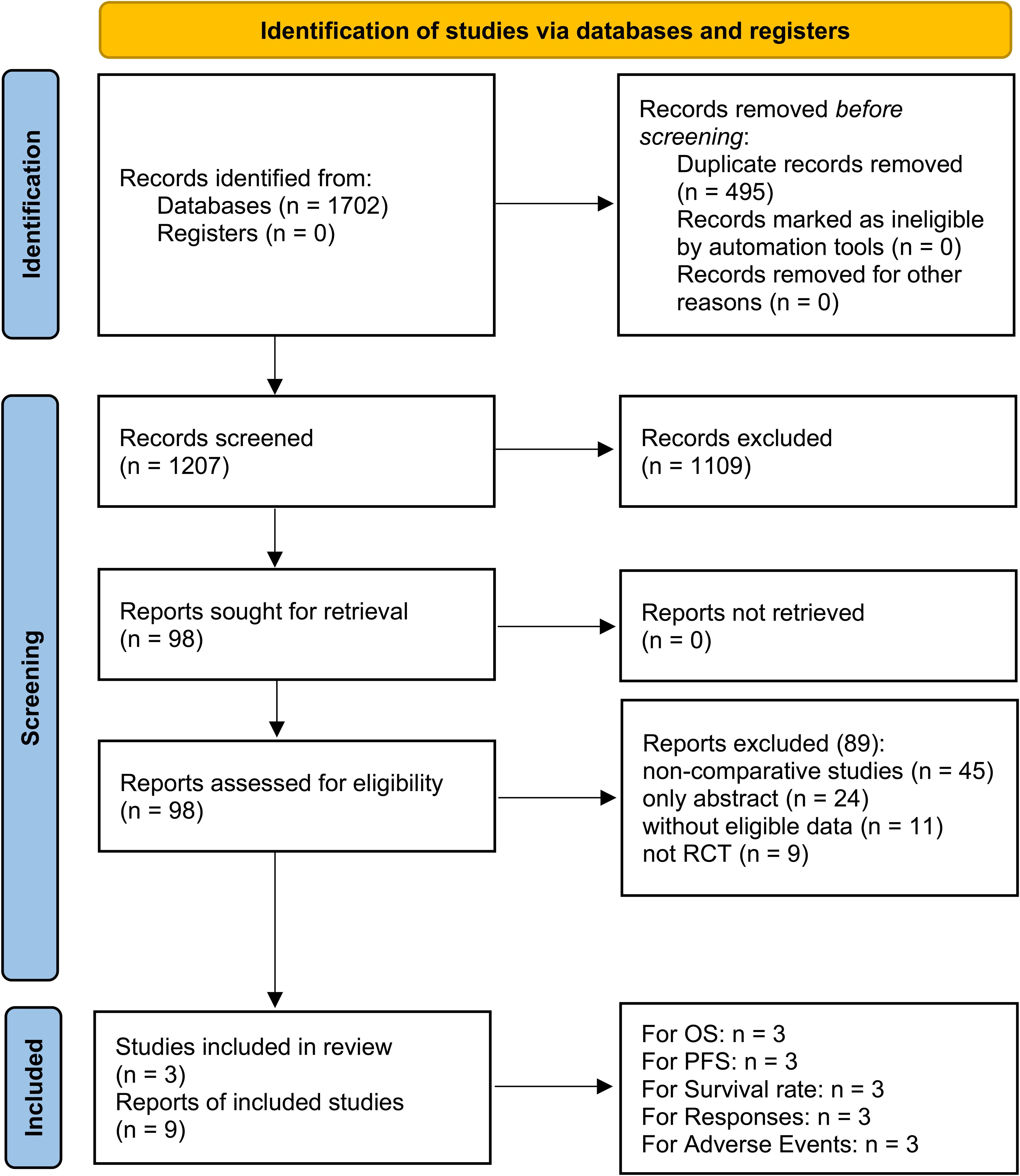
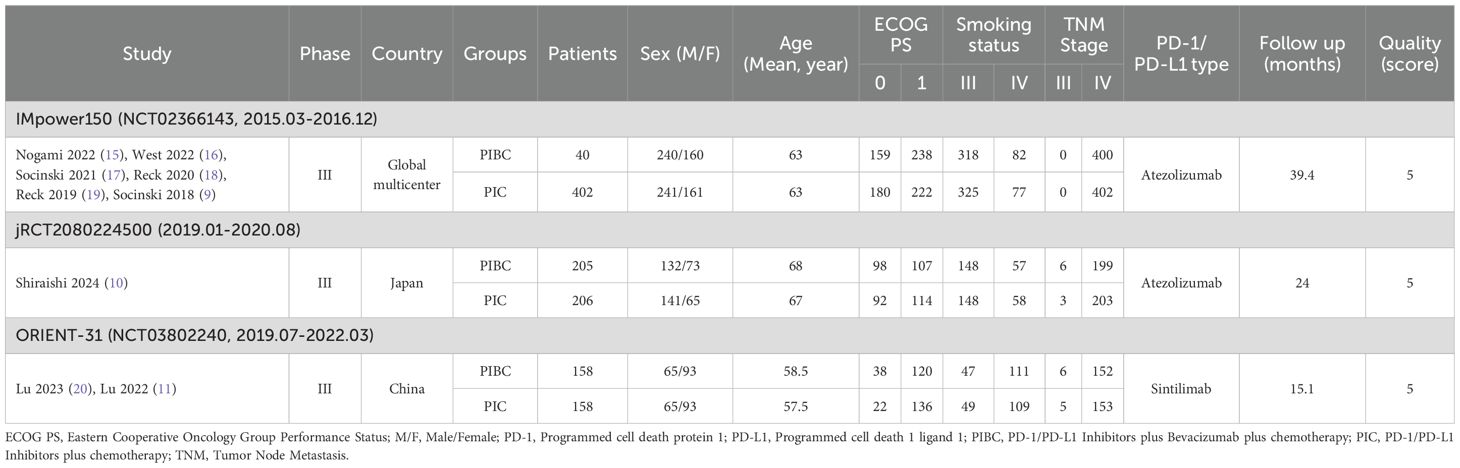
Nine studies derived from three RCTs (IMpower150, jRCT2080224500, and ORIENT-31) were included, comprising 763/766 patients in the PIBC/PIC groups (Figure 1) (9–11, 15–20). IMpower150 is a global multicenter study, whereas jRCT2080224500 and ORIENT-31 were conducted in Asia. All three studies were classified as high quality (Supplementary Figure S1, Supplementary Table S3). According to the GRADE approach, the certainty of evidence ranged from moderate to high (Supplementary Table S4). Table 1 provides a summary of the baseline information of the included RCTs.
Survival
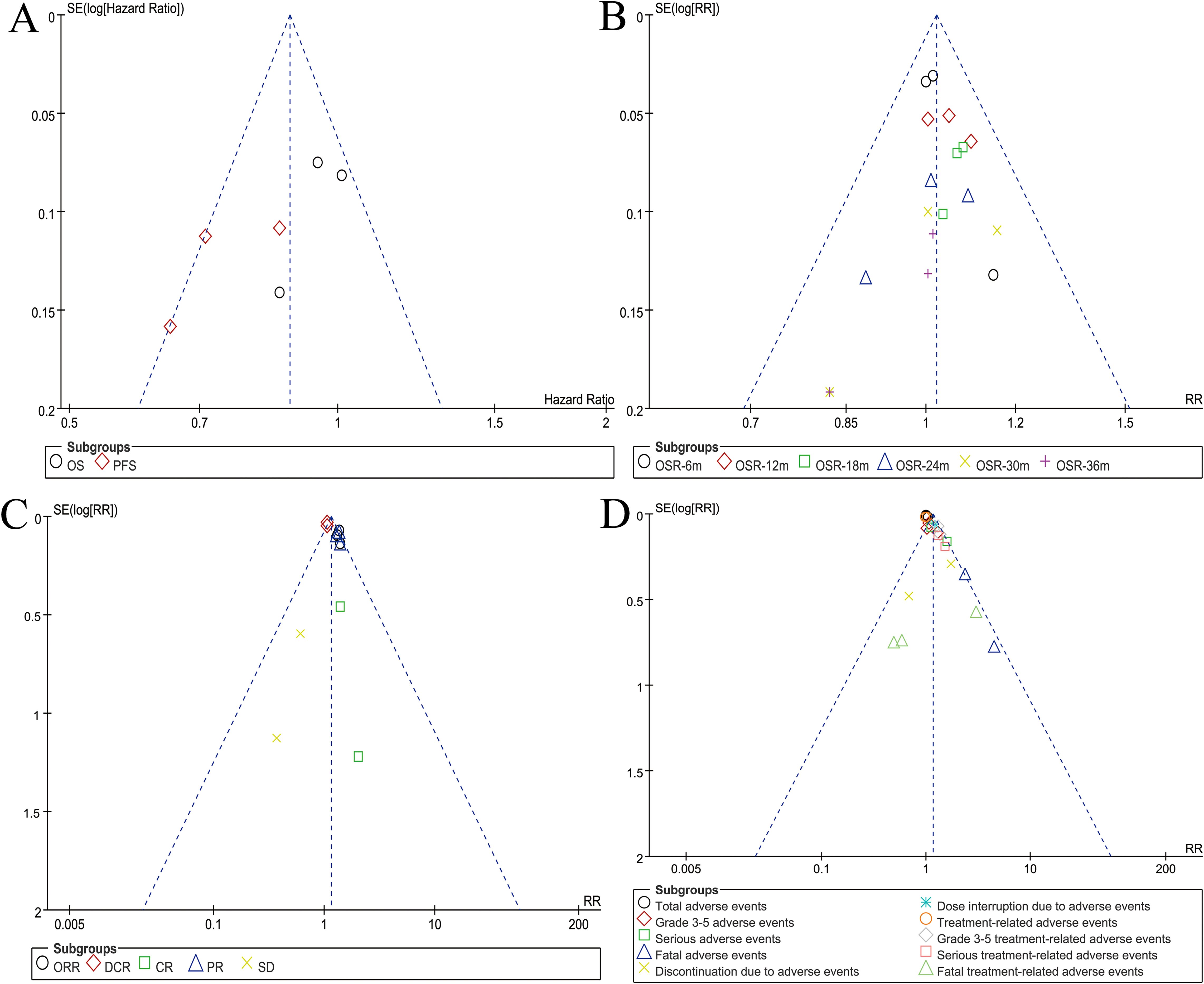
OS was comparable between the two groups (HR: 0.96 [0.87, 1.06], P = 0.43) (Figure 2). The OSR at 6–36 months showed no significant difference between two groups (Supplementary Figure S2). Detailed comparisons of OSR and its temporal changes over time are presented in Figures 3A, C. Older age may be a favorable factor for the PIBC group (Supplementary Table S5).
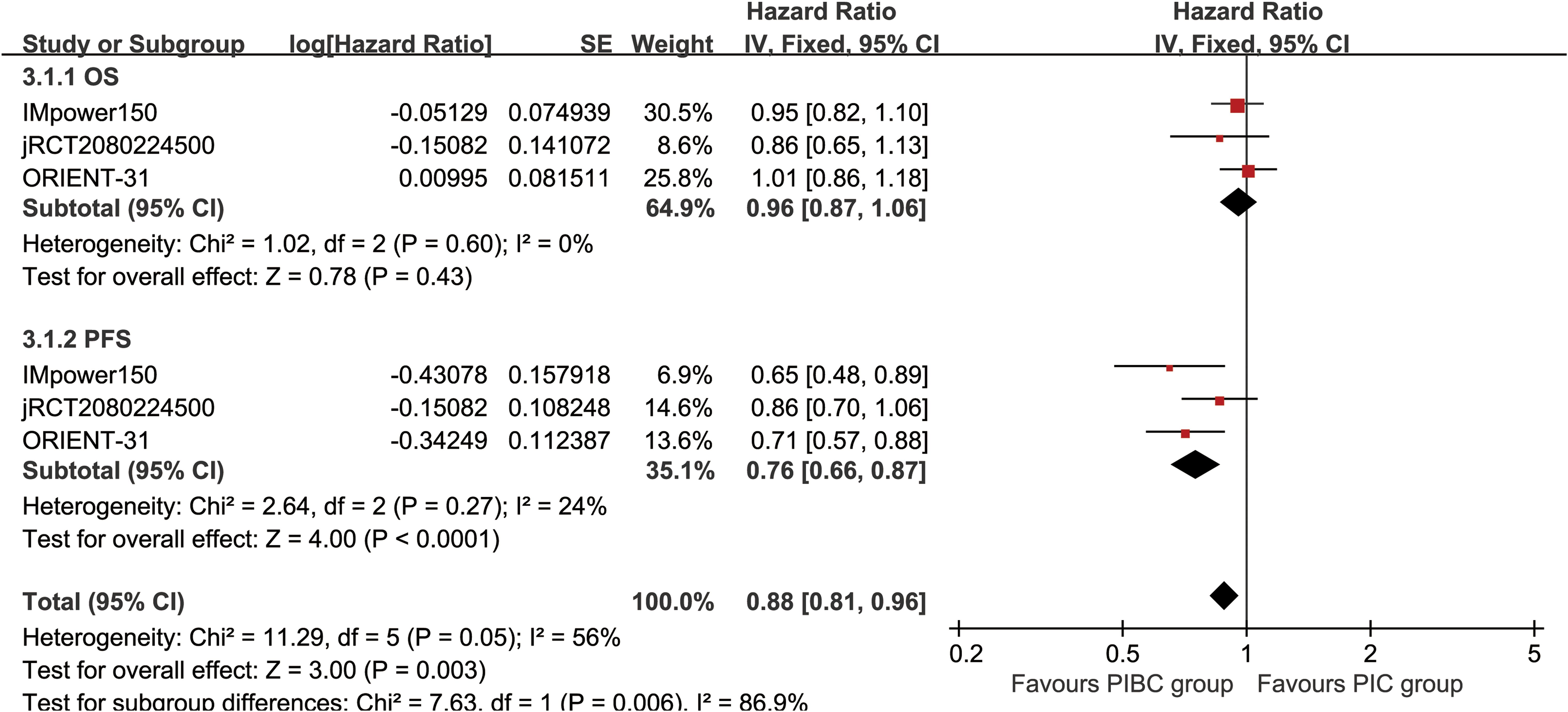
Figure 2. Forest plots of overall survival and progression-free survival associated with PIBC versus PIC.
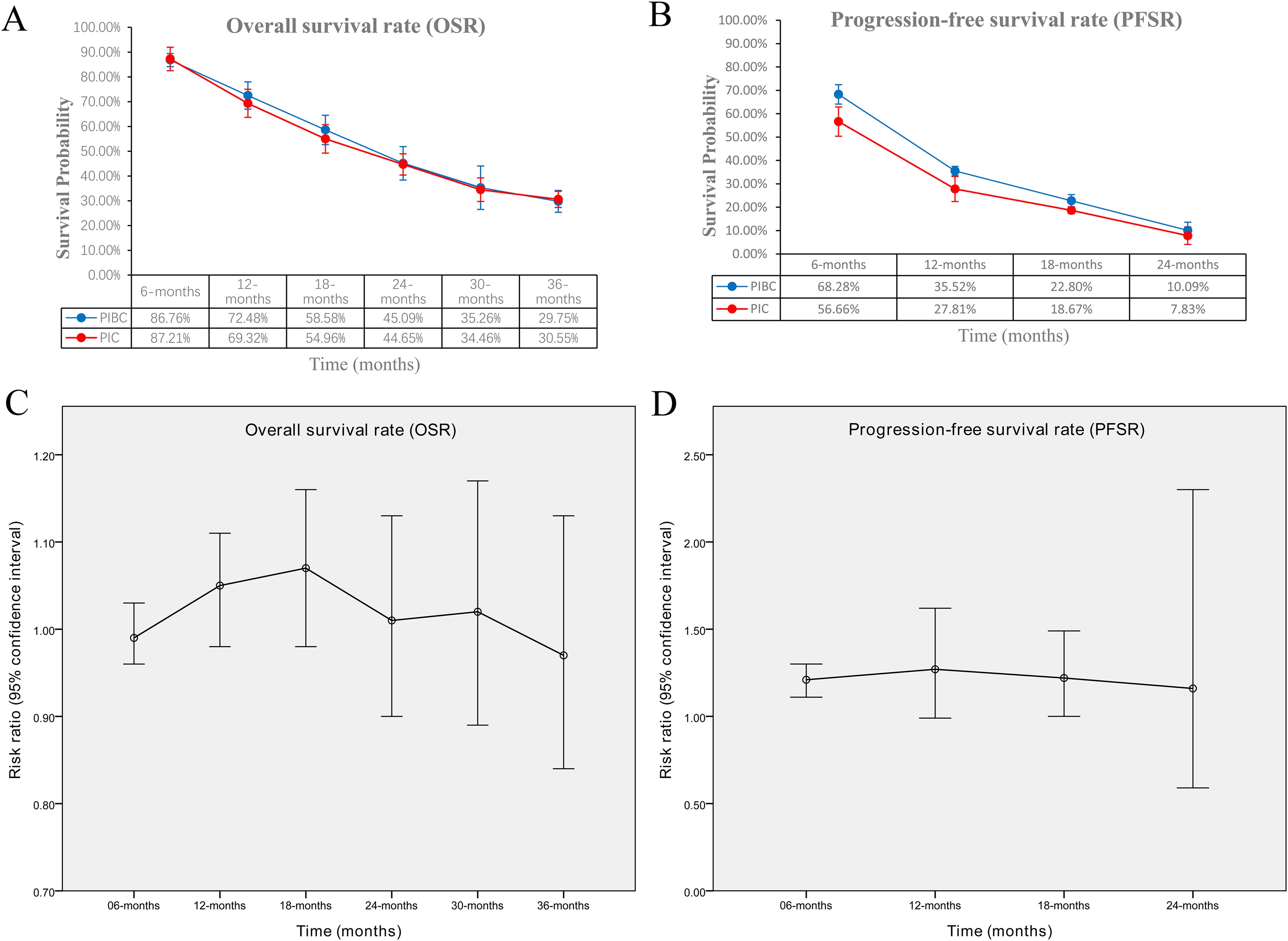
Figure 3. Comparisons of OSR and PFSR. (A) OSR at 3–36 months between the two groups; (B) PFSR at 3–24 months between the two groups; (C) trend of risk ratios in OSR; (D) trend of risk ratios in PFSR.
PFS was significantly better in the PIBC group (HR: 0.76 [0.66, 0.87], P < 0.0001) (Figure 2). The PFSRs were notably higher in the PIBC group at both 6 months (HR: 1.21 [1.11, 1.30], P < 0.00001) and 18 months (HR: 1.22 [1.00, 1.49], P = 0.05). (Supplementary Figure S3). Detailed comparisons of PFSR and its changes over time are presented in Figures 3B, D. Sex- female, smoking status-never, PD-L1 CPS <1%, and driver gene alterations-positive may be favorable factors for the PIC group (Supplementary Table S5).
Responses
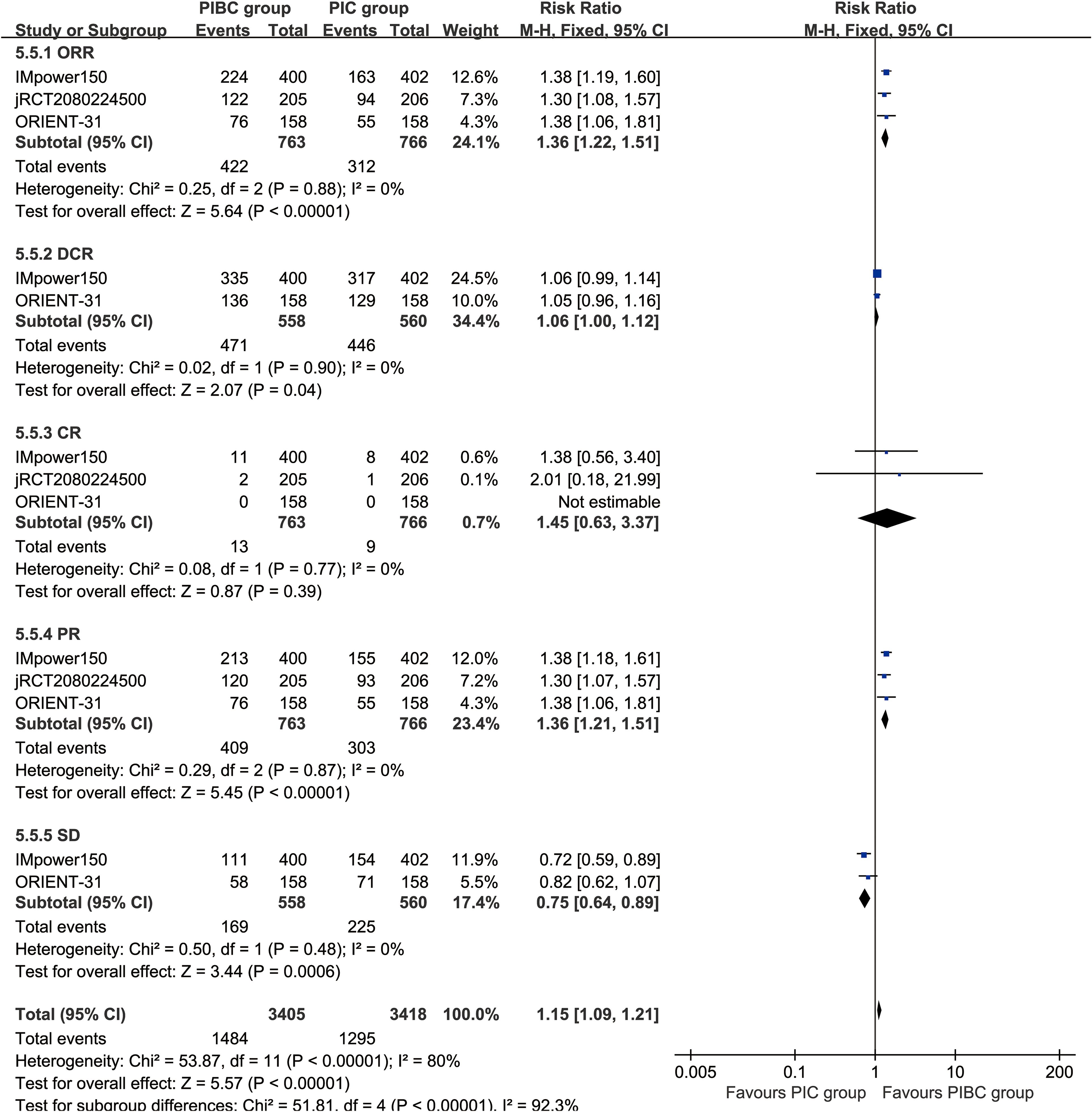
The ORR (RR: 1.36 [1.22, 1.51], P < 0.00001), DCR (RR: 1.06 [1.00, 1.12], P = 0.04), and partial response [PR] (RR: 1.36 [1.21, 1.51], P < 0.00001) were significantly higher in the PIBC group. Although the CR (RR: 1.45 [0.63, 3.37], P = 0.39) favored the PIBC group, the difference was not statistically significant. Conversely, the rate of stable disease [SD] (RR: 0.75 [0.64, 0.89], P = 0.0006) was higher in the PIC group (Figure 4).
Safety
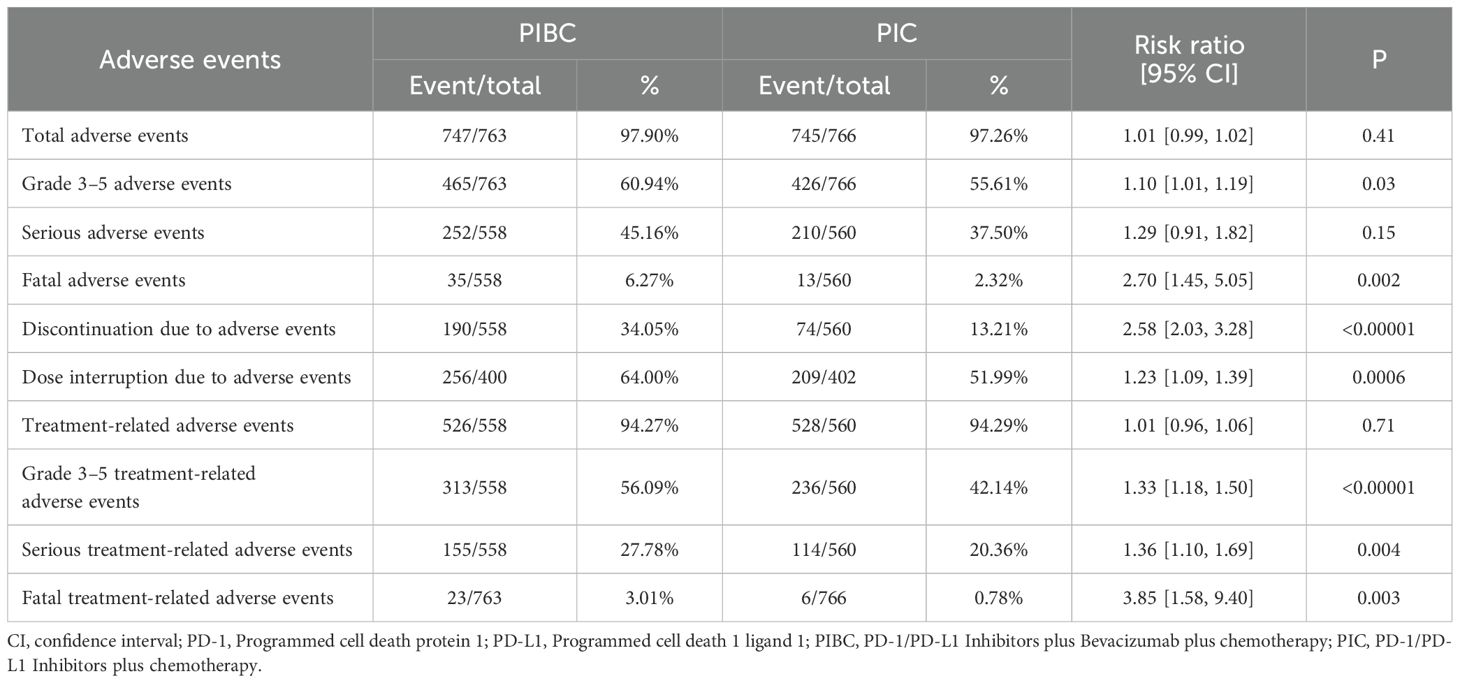
In summary, the rates of grade 3–5 AEs (RR: 1.10 [1.01, 1.19], P = 0.03), fatal AEs (RR: 2.70 [1.45, 5.05], P = 0.002), discontinuations due to AEs (RR: 2.58 [2.03, 3.28], P < 0.00001), dose interruptions due to AEs (RR: 1.23 [1.09, 1.39], P = 0.0006), grade 3–5 treatment-related AEs (TRAEs) (RR: 1.33 [1.18, 1.50], P < 0.00001), serious TRAEs (RR: 1.36 [1.10, 1.69], P = 0.004), and fatal TRAEs (RR: 3.85 [1.58, 9.40], P = 0.003) were significantly higher in the PIBC group. Total AEs, serious AEs, and total TRAEs showed no significant difference between two groups (Table 2, Supplementary Figure S4).
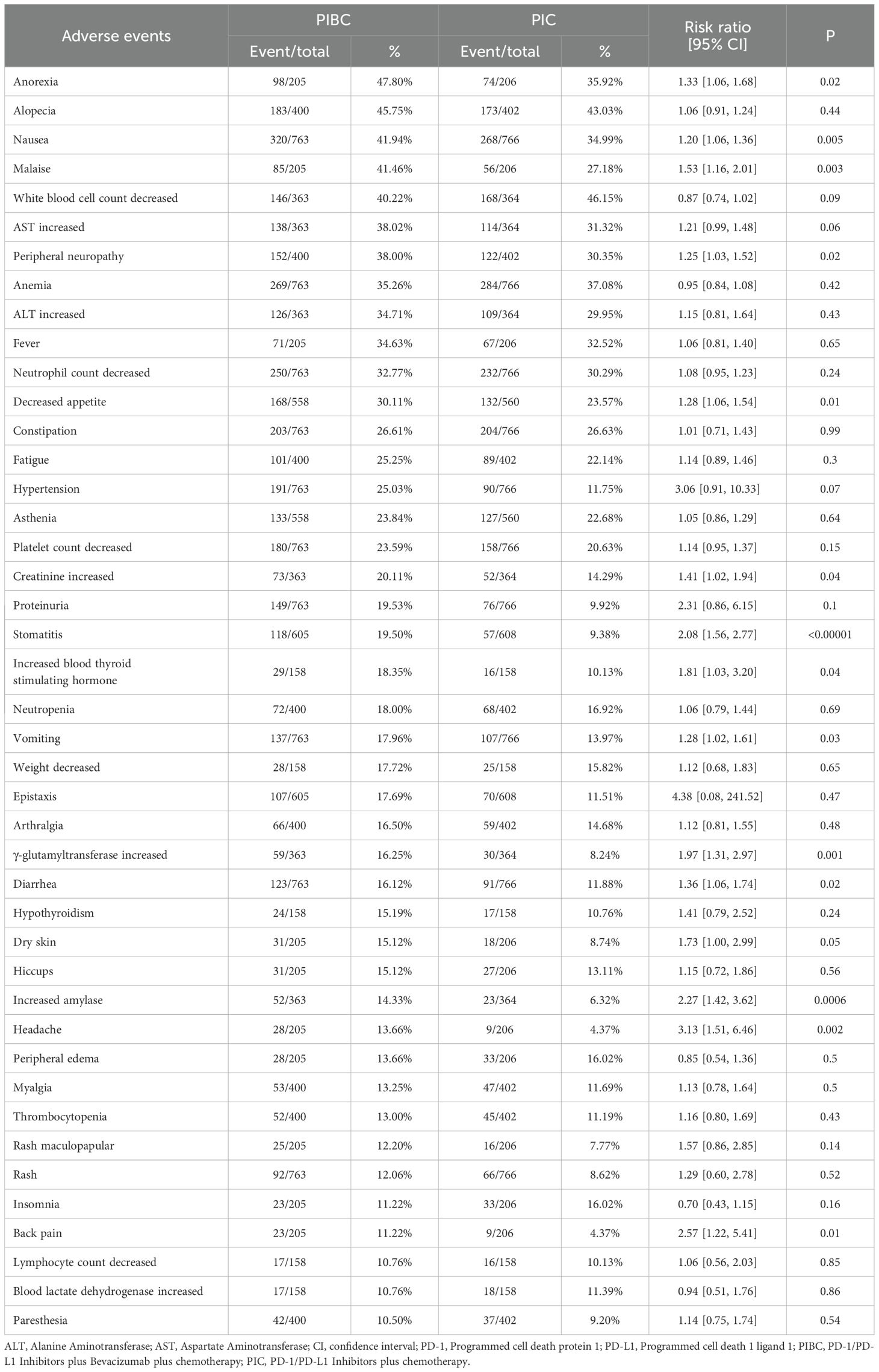
Regarding any grade AEs, the PIBC group exhibited higher rates of anorexia, nausea, malaise, peripheral neuropathy, decreased appetite, elevated creatinine, stomatitis, increased blood thyroid stimulating hormone, vomiting, elevated γ-glutamyltransferase, diarrhea, dry skin, elevated amylase, headache, back pain, and febrile neutropenia (Table 3, Supplementary Table S5).
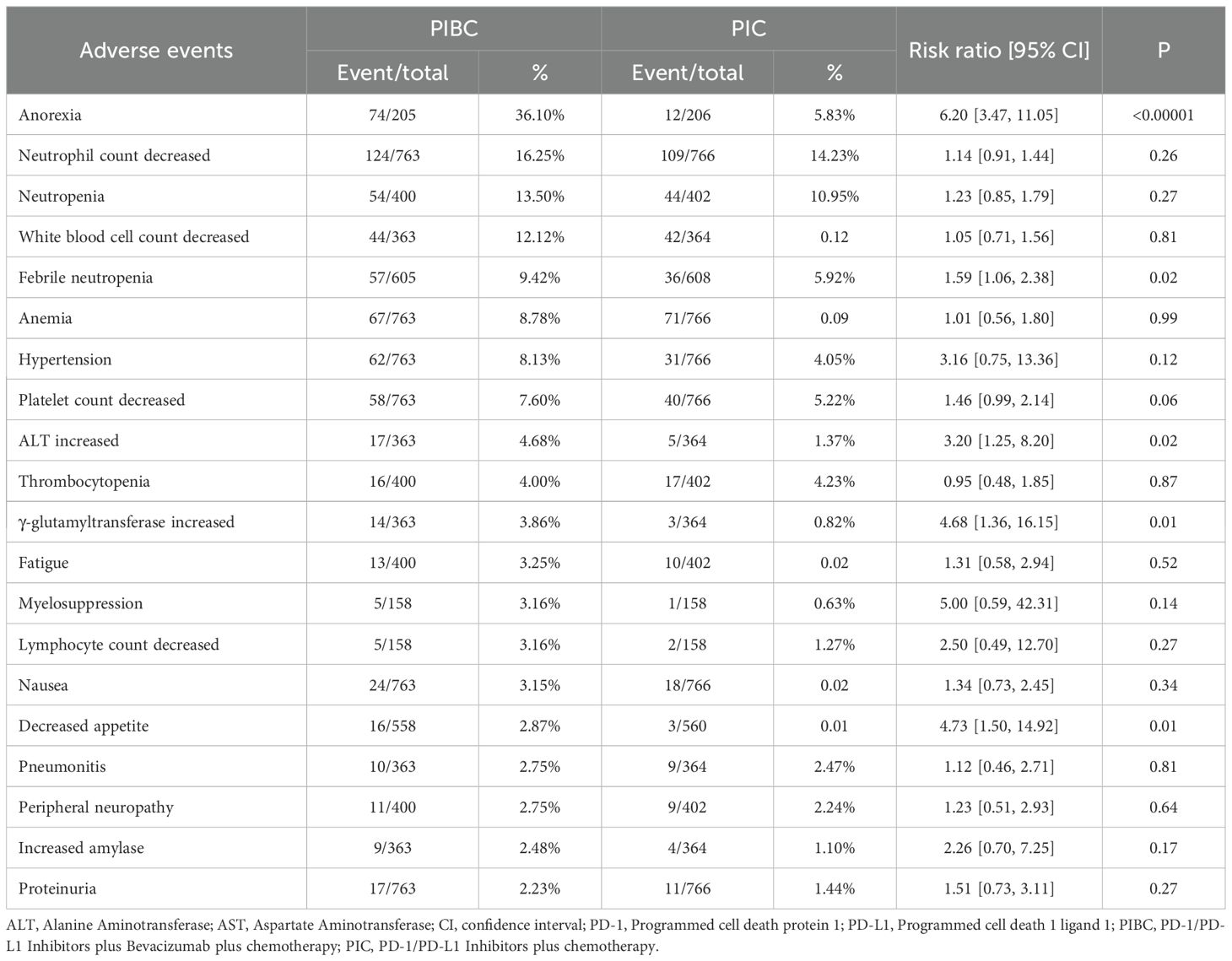
For grade 3–5 AEs, the PIBC group had more instances of grade 3–5 anorexia, febrile neutropenia, elevated ALT, increased γ-glutamyltransferase, and decreased appetite (Table 4, Supplementary Table S6).
Sensitivity analysis
Sensitivity analyses for PFSR-12m, rash, and anemia were conducted, demonstrated that omitting any individual study did not alter the results’ reliability (Supplementary Figure S5). Similarly, for the main outcomes (OS, PFS, and ORR), omitting any individual study also did not alter the results’ reliability (Supplementary Figure S6).
Publication bias
Symmetry in funnel plots for survival, OSR, responses, and safety summary suggested an acceptable level of publication bias (Figure 5).
Discussion
PIC is a common first-line treatment for NSCLC without driver gene mutations or for NSCLC with driver gene mutations that have developed resistance to targeted therapy (9–11). However, the addition of bevacizumab (PIBC) to the regimen remains controversial regarding its potential to enhance antitumor efficacy in clinical practice. The ATTLAS study demonstrated that adding atezolizumab and bevacizumab to chemotherapy significantly improved progression-free survival and objective response rates in EGFR- or ALK-mutated NSCLC patients who had progressed after tyrosine kinase inhibitor therapy (21). Similarly, the IMpower151 trial compared atezolizumab plus bevacizumab and chemotherapy with bevacizumab and chemotherapy alone in the first-line treatment of metastatic nonsquamous NSCLC, further supporting the potential of multi-agent immunotherapy regimens (22). However, both the ATTLAS and IMpower151 studies were excluded because they did not include a PIC arm, which is essential for directly addressing our research question regarding the additive value of bevacizumab. Ultimately, our meta-analysis directly compared the efficacy and safety of PIBC versus PIC using data from three phase 3 RCTs (IMpower150, jRCT2080224500, and ORIENT-31) (9–11). The results indicated that the PIBC regimen significantly improved PFS, ORR, and DCR. Additionally, the PFS rates at 6 and 18 months were higher in the PIBC group. Both groups were similar in terms of OS, OS rates at 3–36 months, and total AEs. However, the PIBC group exhibited higher rates of grade 3–5 TRAEs, serious TRAEs, and fatal TRAEs.
The primary advantage of the PIBC regimen lies in its superior PFS, a finding consistent with IMpower150 and ORIENT-31 (9, 11). The ORR and DCR were also notably higher in the PIBC group. These findings suggest a more robust tumor response and stabilization, consistent with the enhanced anti-angiogenic and immune-modulatory effects of the combination therapy (23, 24). The addition of bevacizumab appears to potentiate PD-1/PD-L1 inhibitors by normalizing tumor vasculature, improving immune cell infiltration, and enhancing chemotherapy delivery (25, 26). Despite these improvements in PFS and ORR, the OS benefit was less pronounced. These findings are further supported by a recent retrospective cohort study by Yang et al. (2024), which evaluated the efficacy and safety of PIBC regimen in 65 patients with driver gene-negative advanced-stage lung adenocarcinoma (27). Their results showed a significantly improved median PFS in the PIBC group compared to the bevacizumab plus chemotherapy (BC) group, while OS showed a non-significant trend in favor of PBC (20.6 vs. 15.9 months; P = 0.115). Multivariate Cox regression confirmed the PIBC regimen as an independent factor for prolonged PFS. However, no statistically significant OS benefit was observed, consistent with our meta-analysis findings. This discrepancy between PFS and OS may be explained by several factors. Firstly, subsequent lines of therapy post-progression may influence OS outcomes, as patients who progress on one therapy often receive additional treatments that can affect survival (28). Secondly, the development of resistance mechanisms, such as upregulation of alternative growth pathways or immune evasion strategies, may mitigate the long-term benefits of the initial treatment (29, 30). Furthermore, the aggressive nature of advanced NSCLC, particularly in patients with high tumor burden or poor performance status, may limit the potential for OS improvement despite initial PFS gains (31). The heterogeneity of patient populations across different studies also contributes to the varied survival outcomes. Differences in PD-L1 expression, and other molecular characteristics can influence response to therapy and long-term survival (32, 33). Patients with high PD-L1 expression may experience enhanced benefits from PIBC therapy due to more effective immune modulation and improved response rates. Conversely, patients with lower PD-L1 expression may not achieve the same level of efficacy, potentially requiring alternative therapeutic approaches (34, 35). Moreover, tumor stage at diagnosis can influence outcomes, with more advanced stages potentially showing variable responses due to differences in tumor burden and microenvironment (36). The addition of bevacizumab may enhance the effectiveness of PD-1/PD-L1 inhibitors by normalizing tumor vasculature, thereby improving drug delivery and immune cell infiltration in the tumor microenvironment (37). However, this combination may also increase the risk of severe adverse events, particularly in patients with pre-existing inflammatory conditions or compromised organ function (38). The dual impact of immune modulation and vascular normalization highlights the need for careful patient selection to maximize benefits while minimizing risks (39). Integrating molecular profiling and biomarker-driven strategies into clinical practice could further refine patient selection and optimize treatment outcomes.
While PIBC offers promising efficacy, it raises significant safety concerns. The PIBC also raises significant safety concerns, particularly regarding severe TRAEs. Grade 3–5 AEs were notably more prevalent in the PIBC group, including anorexia, febrile neutropenia, ALT increased, hypertension, proteinuria, and hemorrhage, in line with bevacizumab’s established safety profile (40). The heightened risk of hypertension is particularly concerning, necessitating careful monitoring and management to prevent cardiovascular complications. Proteinuria and hemorrhage also warrant close surveillance through regular renal function tests and bleeding assessments (41, 42). Managing these AEs requires a multidisciplinary approach, including routine monitoring, early symptom detection, and timely intervention (43, 44). Prophylactic measures, such as the use of granulocyte colony-stimulating factors (G-CSFs) to manage neutropenia, are essential to minimize treatment disruptions and maintain dose intensity (45, 46). The potential for severe TRAEs underscores the need for comprehensive patient education and the implementation of rapid-response protocols to manage complications effectively (47). Patient selection is crucial when considering PIBC. Factors such as performance status, prior treatment history, and comorbid conditions should be evaluated to balance the benefits of extended PFS against the risks of severe TRAEs. Personalized treatment plans, informed by biomarkers like PD-L1 expression, can optimize therapeutic outcomes (48, 49). Additionally, further clinical trials are needed to refine the safety profile of PIBC and develop strategies to mitigate AEs. For instance, prophylactic antihypertensive medications or bevacizumab dose modifications may help manage TRAEs more effectively (50, 51).
Our findings offer important insights into the comparative efficacy and safety of PIBC versus PIC; however, several limitations should be acknowledged, and potential solutions should be considered to improve future research in this field. First, restricting the analysis to English-language articles introduces language bias. Future meta-analyses should consider incorporating studies in multiple languages, potentially with professional translation support, to reduce potential selection bias. Second, the inclusion of only three RCTs limits the generalizability of our findings. Expanding the analysis by incorporating ongoing or recently completed RCTs could provide a more comprehensive and up-to-date evaluation of PIBC versus PIC. Third, the lack of individual patient data (IPD) precluded an IPD meta-analysis, which could have enhanced clinical relevance. Fourth, our study only included trials evaluating Atezolizumab and Sintilimab, so the results may not be representative of other PD-1/PD-L1 inhibitors. Further meta-analyses incorporating data from trials evaluating other PD-1/PD-L1 inhibitors, such as pembrolizumab, nivolumab, and durvalumab, are necessary to validate our findings. Fifth, the predominance of Asian patients in the included studies may limit the applicability of the results to other populations. Future studies should aim to incorporate data from more diverse geographic regions to enhance the external validity of the findings. Sixth, none of the included RCTs reported quality of life outcomes, which are essential for interpreting the real-world impact of treatments, especially when differences in toxicity profiles are observed. This represents an important gap in the current evidence and should be a key focus in future clinical research on PIBC regimens.
Conclusion
PIBC appears to be superior to PIC for advanced NSCLC offering improved PFS and higher response rates (ORR and DCR). However, OS and OSR at 6 to 36 months were comparable between the two groups. The increased risk of severe AEs necessitates cautious use and proactive management. Further research, including IPD meta-analyses, large multi-regional trials, and biomarker-driven studies, is needed to refine patient selection, identify predictive biomarkers, and develop strategies to mitigate adverse effects.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
CS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. YQ: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. HF: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. YH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by National Natural Science Foundation of China (NSFC, Grant number: 81560345). The funding had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Acknowledgments
The authors thank professor Wenxiong Zhang, MD (Department of Thoracic Surgery, The second affiliated hospital of Nanchang University) for his data collection and statistical advice.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1496611/full#supplementary-material
Supplementary Figure 1 | Cochrane risk assessment.
Supplementary Figure 2 | Forest plots of OSR at 6–36 months associated with PIBC versus PIC.
Supplementary Figure 3 | Forest plots of PFSR at 6–24 months associated with PIBC versus PIC.
Supplementary Figure 4 | Forest plots of safety summary associated with PIBC versus PIC.
Supplementary Figure 5 | Sensitivity analysis of PFSR-12m (A), rash (B), and anemia (C).
Supplementary Figure 6 | Sensitivity analysis of OS (A), PFS (B), and ORR (C).
Abbreviations
AE, Adverse Event; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; CI, confidence interval; CPS, Combined positive score; CR, Complete response; DCR, Disease control rate; ECOG PS, Eastern Cooperative Oncology Group Performance Status; EGFR, Epidermal growth factor receptor; GRADE, Grading of Recommendations, Assessment, Development, and Evaluation; HR, Hazard ratio; M/F, Male/Female; ORR, Objective response rate; OS, Overall survival; OSR, Overall survival rate; PD-1, Programmed cell death protein 1; PD-L1, Programmed cell death 1 ligand 1; PFS, Progression-free survival; PFSR, Progression-free survival rate; PIBC, PD-1/PD-L1 inhibitors plus bevacizumab plus chemotherapy; PIC, PD-1/PD-L1 inhibitors plus chemotherapy; PICOS, Participants, Intervention, Control, Outcomes, Study design; PR, Partial response; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, Randomized controlled trial; RR, Risk ratio; SD, Stable disease; TMB, Tumor mutational burden; TNM, Tumor Node Metastasis; TRAEs, Treatment-related adverse events.
References
1. Siegel RL, Giaquinto AN, and Jemal A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
2. Non-Small Cell Lung Cancer Collaborative Group. Chemotherapy and supportive care versus supportive care alone for advanced non-small cell lung cancer. Cochrane Database Syst Rev. (2010) 2010:CD007309. doi: 10.1002/14651858.CD007309.pub2
3. Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomized controlled trial. Lancet. (2020) 387:1540–50. doi: 10.1016/S0140-6736(15)01281-7
4. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Updated analysis of KEYNOTE-024: Pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. (2021) 39:2339–49. doi: 10.1200/JCO.18.00149
5. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. New Engl J Med. (2020) 378:2288–301. doi: 10.1056/NEJMoa1716948
6. Hopkins AM, Kichenadasse G, McKinnon RA, Abuhelwa AY, Logan JM, Badaoui S, et al. Efficacy of first-line atezolizumab combination therapy in patients with non-small cell lung cancer receiving proton pump inhibitors: post hoc analysis of IMpower150. Br J Cancer. (2022) 126:42–7. doi: 10.1038/s41416-021-01606-4
7. de Castro G Jr, Kudaba I, Wu YL, Lopes G, Kowalski DM, Turna HZ, et al. Five-year outcomes with pembrolizumab versus chemotherapy as first-line therapy in patients with non-small-cell lung cancer and programmed death ligand-1 tumor proportion score ≥ 1% in the KEYNOTE-042 study. J Clin Oncol. (2023) 41:1986–91. doi: 10.1200/JCO.21.02885
8. Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med. (2021) 9:305–14. doi: 10.1016/S2213-2600(20)30365-9
9. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. (2018) 378:2288–301. doi: 10.1056/NEJMoa1716948
10. Shiraishi Y, Kishimoto J, Sugawara S, Mizutani H, Daga H, Azuma K, et al. Atezolizumab and platinum plus pemetrexed with or without bevacizumab for metastatic nonsquamous non-small cell lung cancer: A phase 3 randomized clinical trial. JAMA Oncol. (2024) 10:315–24. doi: 10.1001/jamaoncol.2023.5258
11. Lu S, Wu L, Jian H, Chen Y, Wang Q, Fang J, et al. Sintilimab plus bevacizumab biosimilar IBI305 and chemotherapy for patients with EGFR-mutated non-squamous non-small-cell lung cancer who progressed on EGFR tyrosine-kinase inhibitor therapy (ORIENT-31): first interim results from a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. (2022) 23:1167–79. doi: 10.1016/S1470-2045(22)00382-5
12. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. (1996) 17:1–12. doi: 10.1016/0197-2456(95)00134-4
13. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
14. Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, and Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. (2011) 64:380–2. doi: 10.1016/j.jclinepi.2010.09.011
15. West HJ, McCleland M, Cappuzzo F, Reck M, Mok TS, Jotte RM, et al. Clinical efficacy of atezolizumab plus bevacizumab and chemotherapy in KRAS-mutated non-small cell lung cancer with STK11, KEAP1, or TP53 comutations: subgroup results from the phase III IMpower150 trial. J Immunother Cancer. (2022) 10:e003027. doi: 10.1136/jitc-2021-003027
16. Nogami N, Barlesi F, Socinski MA, Reck M, Thomas CA, Cappuzzo F, et al. IMpower150 final exploratory analyses for atezolizumab plus bevacizumab and chemotherapy in key NSCLC patient subgroups with EGFR mutations or metastases in the liver or brain. J Thorac Oncol. (2022) 17:309–23. doi: 10.1016/j.jtho.2021.09.014
17. Socinski MA, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, et al. IMpower150 final overall survival analyses for atezolizumab plus bevacizumab and chemotherapy in first-line metastatic nonsquamous NSCLC. J Thorac Oncol. (2021) 16:1909–24. doi: 10.1016/j.jtho.2021.07.009
18. Reck M, Wehler T, Orlandi F, Nogami N, Barone C, Moro-Sibilot D, et al. Safety and patient-reported outcomes of atezolizumab plus chemotherapy with or without bevacizumab versus bevacizumab plus chemotherapy in non-small-cell lung cancer. J Clin Oncol. (2020) 38:2530–42. doi: 10.1200/JCO.19.03158
19. Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. (2019) 7:387–401. doi: 10.1016/S2213-2600(19)30084-0
20. Lu S, Wu L, Jian H, Cheng Y, Wang Q, Fang J, et al. Sintilimab plus chemotherapy for patients with EGFR-mutated non-squamous non-small-cell lung cancer with disease progression after EGFR tyrosine-kinase inhibitor therapy (ORIENT-31): second interim analysis from a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Respir Med. (2023) 11:624–36. doi: 10.1016/S2213-2600(23)00135-2
21. Park S, Kim TM, Han JY, Lee GW, Shim BY, Lee YG, et al. Randomized study of atezolizumab plus bevacizumab and chemotherapy in patients with EGFR- or ALK-mutated non-small-cell lung cancer (ATTLAS, KCSG-LU19-04). J Clin Oncol. (2024) 42:1241–51. doi: 10.1200/JCO.23.01891
22. Zhou C, Dong X, Chen G, Wang Z, Wu X, Yao Y, et al. OA09.06 IMpower151: phase III study of atezolizumab + Bevacizumab + Chemotherapy in 1L metastatic nonsquamous NSCLC. J Thorac Oncol. (2023) 18:S64–5. doi: 10.1016/j.jtho.2023.09.059
23. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. (2016) 375:1823–33. doi: 10.1056/NEJMoa1606774
24. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. (2017) 389:255–65. doi: 10.1016/S0140-6736(16)32517-X
25. Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. (2017) 376:629–40. doi: 10.1056/NEJMoa1612674
26. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready N, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. (2015) 373:1627–39. doi: 10.1056/NEJMoa1507643
27. Yang X, Li X, Huang K, and Zhuang X. Evaluation of the efficacy of PD-1/PD-L1 inhibitor plus bevacizumab and chemotherapy for the treatment of patients with driver gene-negative advanced-stage lung adenocarcinoma: A retrospective cohort study. Oncol Lett. (2024) 29:53. doi: 10.3892/ol.2024.14799
28. Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. (2016) 387:1540–50. doi: 10.1016/S0140-6736(15)01281-7
29. Garassino MC, Gadgeel S, Speranza G, Felip E, Esteban E, Dómine M, et al. Pembrolizumab plus pemetrexed and platinum in nonsquamous non-small-cell lung cancer: 5-year outcomes from the phase 3 KEYNOTE-189 study. J Clin Oncol. (2023) 41:1992–8. doi: 10.1200/JCO.22.01989
30. Halmos B, Burke T, Kalyvas C, Vandormael K, Frederickson A, and Piperdi B. Pembrolizumab+chemotherapy versus atezolizumab+chemotherapy+/-bevacizumab for the first-line treatment of non-squamous NSCLC: A matching-adjusted indirect comparison. Lung Cancer. (2021) 155:175–82. doi: 10.1016/j.lungcan.2021.03.020
31. Satouchi M, Nosaki K, Takahashi T, Nakagawa K, Aoe K, Kurata T, et al. First-line pembrolizumab vs chemotherapy in metastatic non-small-cell lung cancer: KEYNOTE-024 Japan subset. Cancer Sci. (2021) 112:5000–10. doi: 10.1111/cas.v112.12
32. Hegde PS and Chen DS. Top 10 challenges in cancer immunotherapy. Immunity. (2020) 52:17–35. doi: 10.1016/j.immuni.2019.12.011
33. Postow MA, Sidlow R, and Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. (2018) 378:158–68. doi: 10.1056/NEJMra1703481
34. Chen R, Zinzani PL, Lee HJ, Armand P, Johnson NA, Brice P, et al. Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow-up of KEYNOTE-087. Blood. (2019) 134:1144–53. doi: 10.1182/blood.2019000324
35. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. (2018) 36:1714–68. doi: 10.1200/JCO.2017.77.6385
36. Di Federico A, Alden SL, Smithy JW, Ricciuti B, Alessi JV, Wang X, et al. Intrapatient variation in PD-L1 expression and tumor mutational burden and the impact on outcomes to immune checkpoint inhibitor therapy in patients with non-small-cell lung cancer. Ann Oncol. (2024) 35(10):902–13. doi: 10.1016/j.annonc.2024.06.014
37. Xue L, Gao X, Zhang H, Tang J, Wang Q, Li F, et al. Antiangiogenic antibody BD0801 combined with immune checkpoint inhibitors achieves synergistic antitumor activity and affects the tumor microenvironment. BMC Cancer. (2021) 21:1134. doi: 10.1186/s12885-021-08859-5
38. Ge Y, Zhan Y, He J, Li J, Wang J, Wei X, et al. PD-(L)1 inhibitors plus bevacizumab and chemotherapy as first-line therapy in PD-L1-negative metastatic lung adenocarcinoma: a real-world data. J Cancer Res Clin Oncol. (2024) 150:135. doi: 10.1007/s00432-024-05637-1
39. Xia R, Li Y, Yang L, and Huang M. Immunotherapy or antiangiogenic therapy plus chemotherapy as first-line treatment of patients with PD-L1(-) advanced non-squamous non-small cell lung cancer in a Chinese cohort. Cancer Med. (2023) 12:14282–92. doi: 10.1002/cam4.v12.13
40. Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. (2017) 376:2415–26. doi: 10.1056/NEJMoa1613493
41. Garon EB, Cho BC, Luft A, Alatorre-Alexander J, Geater SL, Kim SW, et al. Patient-reported outcomes with durvalumab, with or without tremelimumab, plus chemotherapy as first-line treatment for metastatic non-small-cell lung cancer (POSEIDON). Lung Cancer. (2023) 186:107422. doi: 10.1016/j.lungcan.2023.107422
42. Borghaei H, O’Byrne KJ, Paz-Ares L, Ciuleanu TE, Yu X, Pluzanski A, et al. Nivolumab plus chemotherapy in first-line metastatic non-small-cell lung cancer: results of the phase III CheckMate 227 Part 2 trial. ESMO Open. (2023) 8:102065. doi: 10.1016/j.esmoop.2023.102065
43. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. (2017) 377:1919–29. doi: 10.1056/NEJMoa1709937
44. Lou Y, Xu J, Zhang Y, Lu J, Chu T, Zhang X, et al. Chemotherapy plus EGFR-TKI as first-line treatment provides better survival for advanced EGFR-positive lung adenocarcinoma patients: updated data and exploratory in vitro study. Target Oncol. (2020) 15:175–84. doi: 10.1007/s11523-020-00708-y
45. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. (2018) 378:113–25. doi: 10.1056/NEJMoa1713137
46. Gettinger S, Horn L, Jackman D, Hellmann M, Antonia S, Spigel D, et al. Five-year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer: results from the CA209–003 study. J Clin Oncol. (2018) 36:1675–84. doi: 10.1200/JCO.2017.77.0412
47. Yu H, Chen P, Xia L, Fu S, Chen C, Zhang X, et al. PD-1/PD-L1 inhibitor plus chemotherapy versus bevacizumab plus chemotherapy in first-line treatment for non-squamous non-small-cell lung cancer. J Immunother Cancer. (2021) 9:e003431. doi: 10.1136/jitc-2021-003431
48. Ferrara R, Imbimbo M, Malouf R, Paget-Bailly S, Calais F, Marchal C, et al. Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer. Cochrane Database Syst Rev. (2021) 4:CD013257. doi: 10.1002/14651858.CD013257.pub3
49. Hui W, Song R, Tao H, Gao Z, Zhu M, Zhang M, et al. Cost-effectiveness of first-line immunotherapy combinations with or without chemotherapy for advanced non-small cell lung cancer: a modelling approach. BMC Cancer. (2023) 23:442. doi: 10.1186/s12885-023-10938-8
50. Xu Z, Zhang H, Ma G, Meng W, Du J, Wu X, et al. Real−world evidence of advanced non−small cell lung carcinoma treated with an immune checkpoint inhibitor plus chemotherapy. Oncol Lett. (2024) 28:405. doi: 10.3892/ol.2024.14538
Keywords: PD-1/PD-L1 inhibitors, bevacizumab, chemotherapy, non-small cell lung cancer, meta-analysis
Citation: Song C, Qiu Y, Fan H and Han Y (2025) PD-1/PD-L1 inhibitors plus bevacizumab plus chemotherapy versus PD-1/PD-L1 inhibitors plus chemotherapy for advanced non-small cell lung cancer: a phase 3 RCT based meta-analysis. Front. Oncol. 15:1496611. doi: 10.3389/fonc.2025.1496611
Received: 14 September 2024; Accepted: 29 April 2025;
Published: 21 May 2025.
Edited by:
Paul Takam Kamga, Université de Versailles Saint-Quentin-en-Yvelines, FranceReviewed by:
Alessandro Gonfiotti, University of Florence, ItalyQingbin Cui, University of Toledo College of Medicine and Life Sciences, United States
Guanming Jiang, Tenth Affiliated Hospital, Southern Medical University (Dongguan People’s Hospital), China
Copyright © 2025 Song, Qiu, Fan and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongqing Han, aGFueW9uZ3FpbmcyNThAMTI2LmNvbQ==
 Chao Song1
Chao Song1 Yongqing Han
Yongqing Han