- 1Department of Neurosurgery, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, China
- 2People’s Hospital of Guangdong Province Ganzhou Hospital, Ganzhou Municipal Hospital, Ganzhou, China
Background: Cancer-related fatigue (CRF) is one of the most prevalent symptoms which drastically affect patient health and quality of life. This study aimed to construct and validate a nomogram to accurately predict the occurrence of cancer-related fatigue in patients with glioma.
Methods: This cross-sectional study included 470 glioma patients from two hospitals (training cohort: n=284; validation cohort: n=186). All patients were categorized into two groups based on their Numerical Rating Scale scores of cancer-related fatigue: a no or mild fatigue group (scores 0-3) and a moderate to severe fatigue group (scores 4-10). LASSO model and multivariable logistic regression analyses were used to determine the significant risk factors contributing to the occurrence of cancer-related fatigue in glioma patients. A nomogram was constructed and its predictive accuracy and conformity was validated by ROC curves, calibration curves and decision curve analysis.
Results: Combining LASSO algorithm and multivariable logistic regression analyses, the cancer stage (p=0.014), and the scores of Perceived Social Support Scale (PSSS) (p<0.001), physical functioning (PF) (p<0.001), bodily pain (BP) (p=0.031), general health (GH) (p<0.001), and mental health (MH) (p=0.009) were the independent risk factors for cancer-related fatigue of glioma patients. A clinically quantitative predictive model nomogram was developed based on these extracted risk factors. The concordance-index of nomogram was 0.964 (0.935-0.993). The AUC values of nomogram were 0.916 (CI: 0.879-0.953) in the training cohort and 0.885 (CI: 0.829-0.941) in the validation cohort. The calibration curves of this nomogram exhibited a notable concordance with the ideal diagonal line. The decision curve analyses illuminated that this nomogram achieved high clinical net benefit.
Conclusion: The nomogram, incorporating the cancer stage of glioma, perceived social support, and quality of life of patients, demonstrated good accuracy and clinical practicality. It can serve as a valuable prediction and evaluation tool for anticipating the occurrence of cancer-related fatigue in patients with glioma.
Introduction
Glioma is the most prevalent primary intracranial tumor, representing 81% of malignant brain tumors (1). The incidence of gliomas is estimated to be around 5 to 10 cases per 100,000 people per year. Although relatively rare, gliomas can cause significant mortality and morbidity (2). The five-year survival rate for high-grade glioma is typically less than 10-15% (3). Glioma management is inherently complex. In most low-grade gliomas, the primary treatment involves maximal safe resection, which forms the foundation of therapeutic strategy (4). For patients with adult-type diffuse gliomas, the current standard of care typically involves a multimodal approach combining radiotherapy with chemotherapy, with some cases achieving remarkable long-term survival exceeding 10–20 years (5). However, avoiding surgical trauma-related harm and preventing radiotherapy- and chemotherapy-induced complications remain significant challenges (6). Additionally, the diagnostic process, treatment course, and long-term surveillance impose significant psychological distress, including clinically significant anxiety, depression, and fear of recurrence.
Cancer-related fatigue (CRF) is a debilitating and multidimensional symptom complex characterized by persistent physical exhaustion, emotional weariness, and cognitive impairment that disproportionately affects cancer patients (7, 8). Unlike general fatigue, cancer-related fatigue is more severe, persistent, overwhelming, and not easily alleviated by rest or sleep (9). People with cancer-related fatigue often describe their feelings of extreme tiredness, weakness, and weariness (10). In the majority of studies, 30% to 60% of patients report experiencing moderate to severe cancer-related fatigue during treatment, occasionally leading to the discontinuation of treatment (11). Research on long-term cancer survivors demonstrates that 25-33% of cancer survivors continue to experience chronic fatigue for up to 10 years following diagnosis (12). Cancer-related fatigue can hinder one’s ability to maintain work, social interactions, and daily routines. For some people with cancer, cancer-related fatigue may be more distressing than side effects like pain, nausea, or vomiting. It causes disruption in all aspects of life and might be a risk factor of reduced survival (13). Although the prevalence and course of fatigue in other cancer populations have been well-documented (14–16), its characteristics in glioma patients remain unknown. There is an urgent need for the development of prediction models. The proposed nomogram could offer a clinically practical tool for individualized risk prediction of cancer-related fatigue in glioma patients by visually integrating multiple prognostic factors. This modeling approach will forecast the occurrence of cancer-related fatigue in patients with glioma.
Materials and methods
Study participants
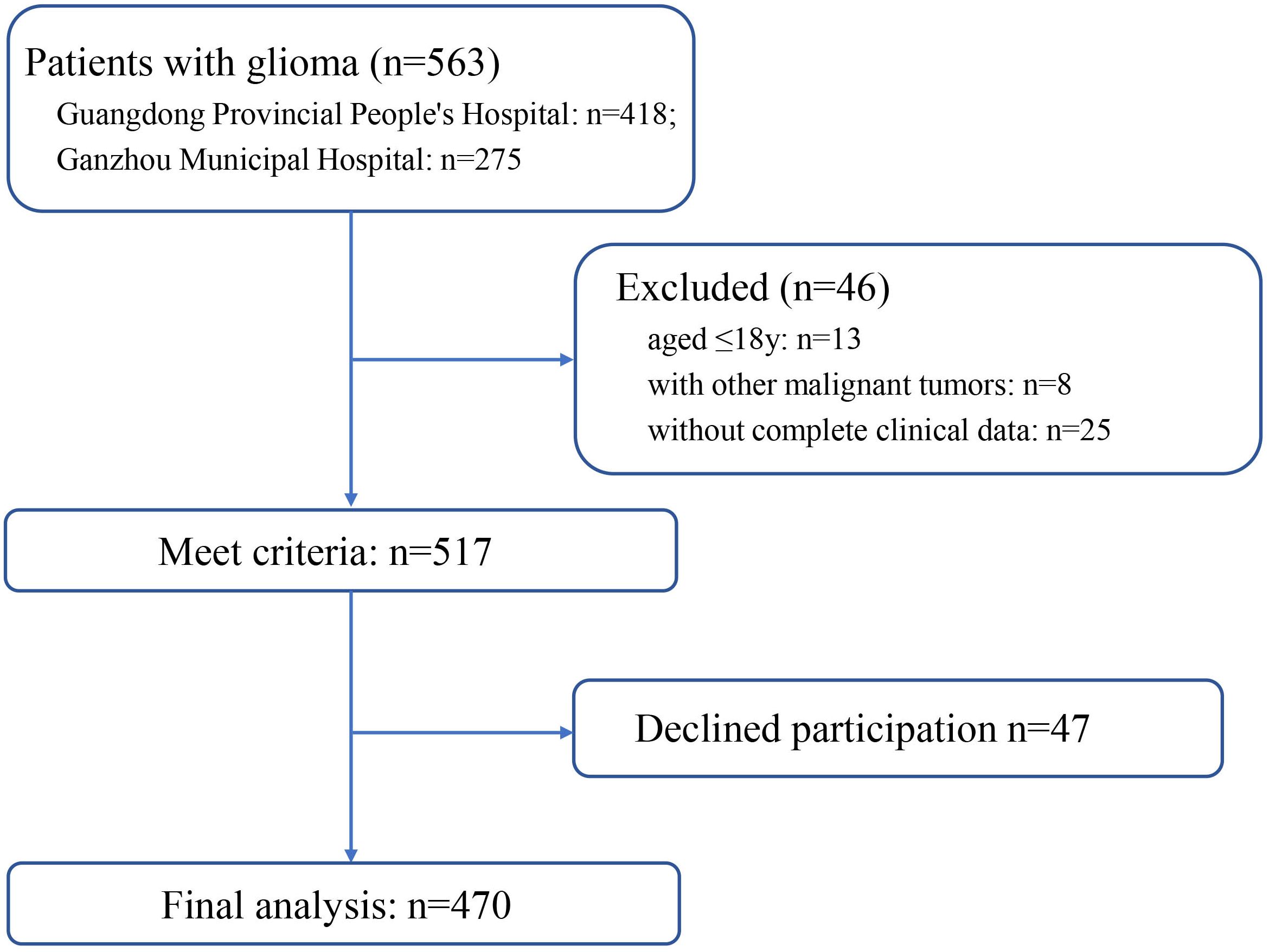
This cross-sectional study was conducted at the Guangdong Provincial People’s Hospital and People’s Hospital of Guangdong Province Ganzhou Hospital (Ganzhou Municipal Hospital), both of which are tertiary referral hospitals. Ethical approval for the use of clinical data for research purposes was granted by the Ethics Committee of Guangdong Provincial People’s Hospital. Between November 2022 and August 2024, we totally enrolled 470 patients from Guangdong Provincial People’s Hospital and People’s Hospital of Guangdong Province Ganzhou Hospital (Ganzhou Municipal Hospital). The flow diagram of patient screening process is shown in Figure 1. The study objective and data confidentiality were communicated to the participants before participating in the study. According to the declaration of Helsinki, written informed consents were acquired.
The inclusion criteria for the patients were as follows (1): age ≥ 18 years, (2) glioma confirmed through CT, MRI, and pathological examination, (3) diagnosed with glioma for the first time, (4) complete clinical data available, (5) patients with clear consciousness and literacy, capable of participating in scale assessment, (6) patients who voluntarily signed the informed consent form. Exclusion criteria were as follows: (1) psychiatric disorders, impaired mental status, or communication difficulties, (2) patients in pregnancy or lactation period, (3) concurrent diagnosis of other malignant tumors, (4) patients with acute infections or immune system disorders, (5) patients with an estimated postoperative survival time of less than 3 months, (6) history of brain inflammation or neurological deficits, (7) received psychotherapy or intervention within one year.
Measures
The basic sociodemographic data of participant was collected using a self-prepared sociodemographic questionnaire in Chinese. The information including age, gender, body mass index (BMI), occupation, marital status, educational level, and personal monthly income. Clinical data were retrieved from the hospital information and management system, including cancer stage, chemotherapy, self-care ability, insomnia, neuroticism, pain, depression, anxiety, and physical exercise. All the information was collected one month after the patient received treatment.
In this study, the Numerical Rating Scale (NRS) was used to measure the levels of cancer-related fatigue and the intensity of pain experienced by patients with glioma (17). The NRS typically consists of a horizontal line with numbers ranging from 0 to 10. The endpoints of the scale represented the levels of cancer-related fatigue and pain, with 0 indicating no fatigue or no pain and 10 indicating the worst possible fatigue or the worst possible pain.
In order to assess sleep quality and disturbances of glioma patients over a one-month time interval, the Pittsburgh Sleep Quality Index (PSQI) was used in this study (18). Each component score of the PSQI ranges from 0 to 3, with 3 indicating the greatest dysfunction or disturbance. The seven component scores are then summed to obtain a global PSQI score, which ranges from 0 to 21. Higher scores indicate poorer sleep quality.
The Perceived Social Support Scale (PSSS) was used to measure the patients’ perceptions of the support they received from their social network (19, 20). It includes items prompting individuals to rate their agreement with statements on social support. Patients rated their responses on a Likert scale of 1 to 7 or 1 to 5, where higher scores indicating stronger perceived social support.
Health-related quality-of-life was measured by the 36-item Short Form Health Survey (SF-36) (21, 22). This is a patient-reported questionnaire that covers eight health domains: physical functioning (PF), role physical (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role emotional (RE), and mental health (MH). Different options for each item have different score weightings. The final score ranges from 0 (worst general health status) to 100 (best health status) for each domain, and higher scores indicate better quality of life.
Risk factors extraction and analysis
In this study, all patients were categorized into two groups based on their NRS scores of cancer-related fatigue: a no or mild fatigue group (scores 0-3) and a moderate to severe fatigue group (scores 4-10). We have included 26 potential risk factors contributing to cancer related fatigue in glioma patients. There were 10 demographic and clinical factors, including age, BMI, occupation, marital status, education level, personal monthly income, cancer stage, chemotherapy, self-care ability, and physical exercise. There were 16 mental and psychological factors, including sleep quality, neuroticism, intensity of pain, depression, anxiety, perceived social support, quality-of-life, and so on.
Prior to risk factors selection, 26 potential risk factors were normalized using a z-score approach to eliminate index dimension differences of the data. In the training cohort, risk factor selection and model development were carried out. The least absolute shrinkage and selection operator (LASSO) regression with 10-fold cross-validation was employed to identify the risk factors with non-zero coefficients. Subsequently, a multivariable logistic regression analysis was performed on the risk factors identified by the LASSO model to determine significant risk factors for nomogram development.
Nomogram construction and evaluation
A nomogram was constructed with the significant risk factors extracted by LASSO model and multivariable logistic regression analyses. The receiver operating characteristic (ROC) curve and area under the curve (AUC) illustrated the predictive accuracy and conformity of this nomogram in the training cohort and validation cohort. The C-index and calibration plots visually revealed the predictive accuracy of nomogram. Decision curve analysis (DCA) was used to evaluate the clinical net benefit of nomogram.
Statistical analysis
All statistical analyses were performed by SPSS software version 25.0 (IBM Corporation; United States) and R software (version 3.4.1, Boston, MA, USA). The ages, BMI, and the scores of NRS, PSQI, PSSS and SF-36 were continuous variables approximately normally distributed, which were described using means and standard deviation (SD). The descriptive statistics for categorical variables were used frequencies and percentages. The differences between the training and validation cohorts in general characteristics were analyzed by Student’s t test or Pearson’s χ2 test. The multivariable logistic regression analyses were conducted to determine the hazard ratios (HR) and 95% confidence intervals (CI) for events. The variables with p<0.05 in multivariate logistic regression analyses were considered as the significant risk factors leading to cancer-related fatigue in glioma patients. We considered p<0.05 to be the statistically significance.
Results
General characteristics
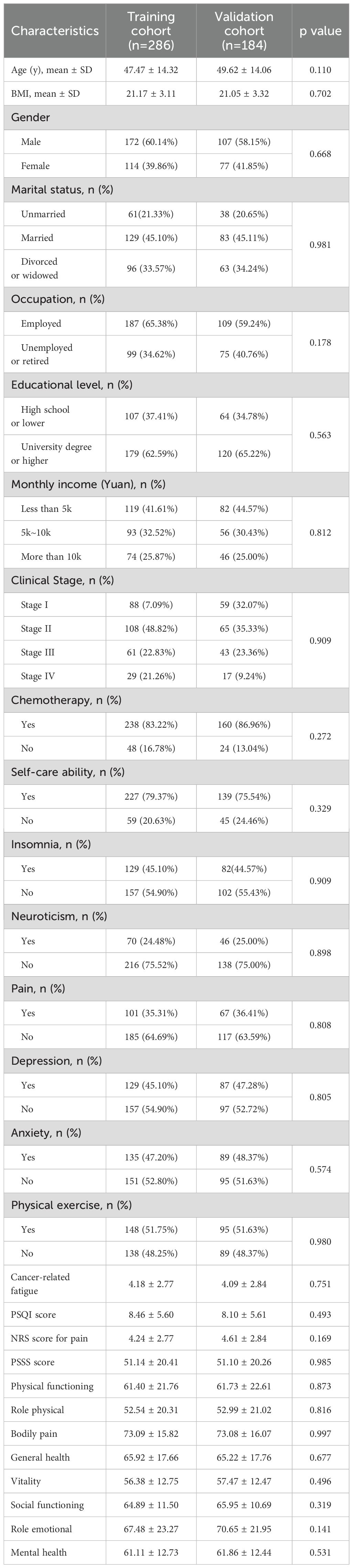
A total of 470 glioma patients were included in this study, with 284 patients as the training cohort and 186 patients as the validation cohort. The general characteristics of two cohorts were summarized in Table 1. No statistical difference was observed between two cohorts, indicating the grouping was randomized and reasonable. The Numerical Rating Scale (NRS) score of cancer-related fatigue was 4.18 ± 2.77 for training cohort and 4.09 ± 2.84 for validation cohort. In the training cohort, 129 glioma patients experienced no or mild cancer-related fatigue, whereas 155 patients reported moderate or severe cancer-related fatigue. In the validation set, 104 patients were divided into the no or mild fatigue group, and 82 patients were divided into the moderate or severe fatigue group.
Risk factors extraction
After LASSO algorithm, 12 risk factors were retained based on non-zero coefficients for the prediction of cancer-related fatigue (Figure 2). These factors included occupation, cancer stage, sleep quality, anxiety, the PSSS score, and the seven health domains of the SF-36, excluding role physical.
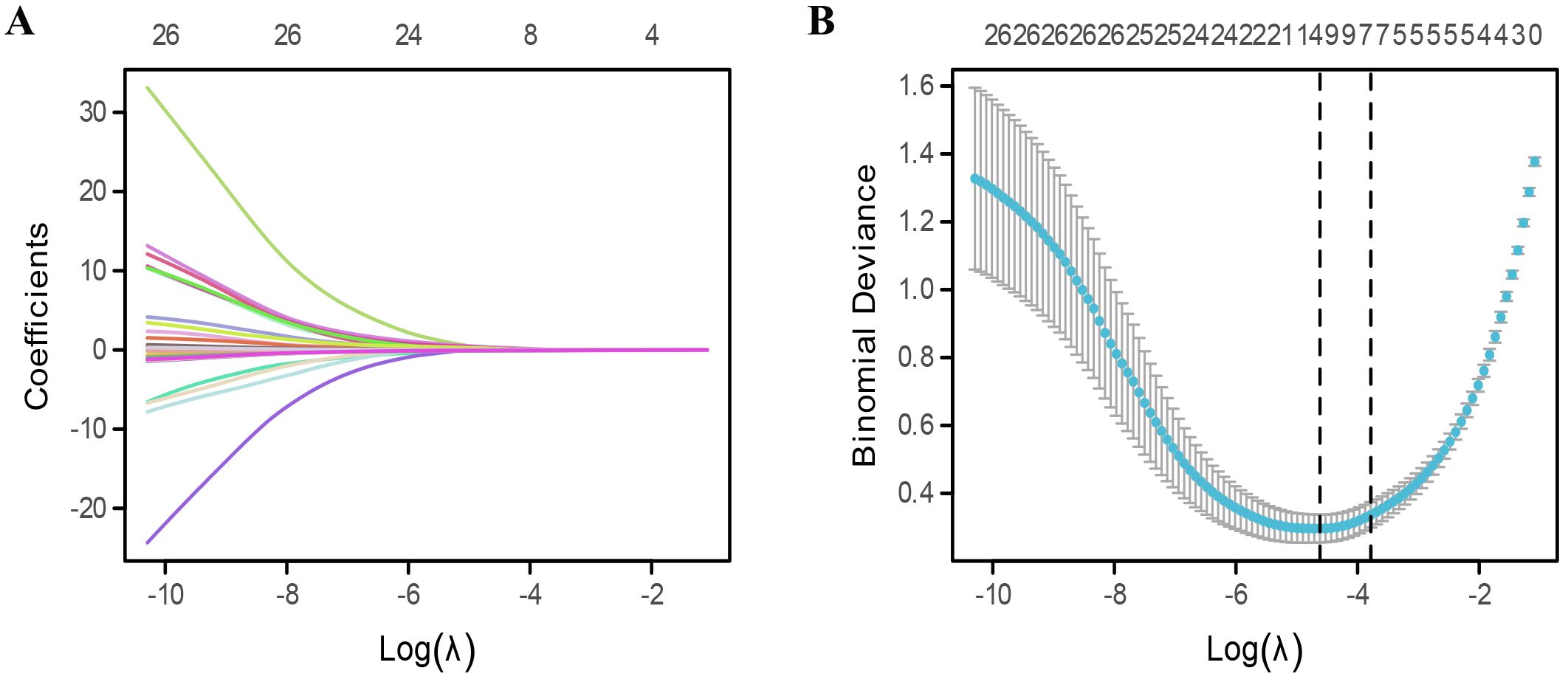
Figure 2. Extraction of risk factors for cancer-related fatigue. (A, B) Risk features extraction by using least absolute shrinkage and selection operator (LASSO) algorithm.
To determine the independent risk factors leading to cancer-related fatigue of glioma patients, multivariable logistic regression analyses were performed in the training cohort. As shown in Table 2, the cancer stage (p=0.014), and the scores of PSSS (p<0.001), physical functioning (PF) (p<0.001), bodily pain (BP) (p=0.031), general health (GH) (p<0.001), and mental health (MH) (p=0.009) were the independent risk factors for cancer-related fatigue of glioma patients.
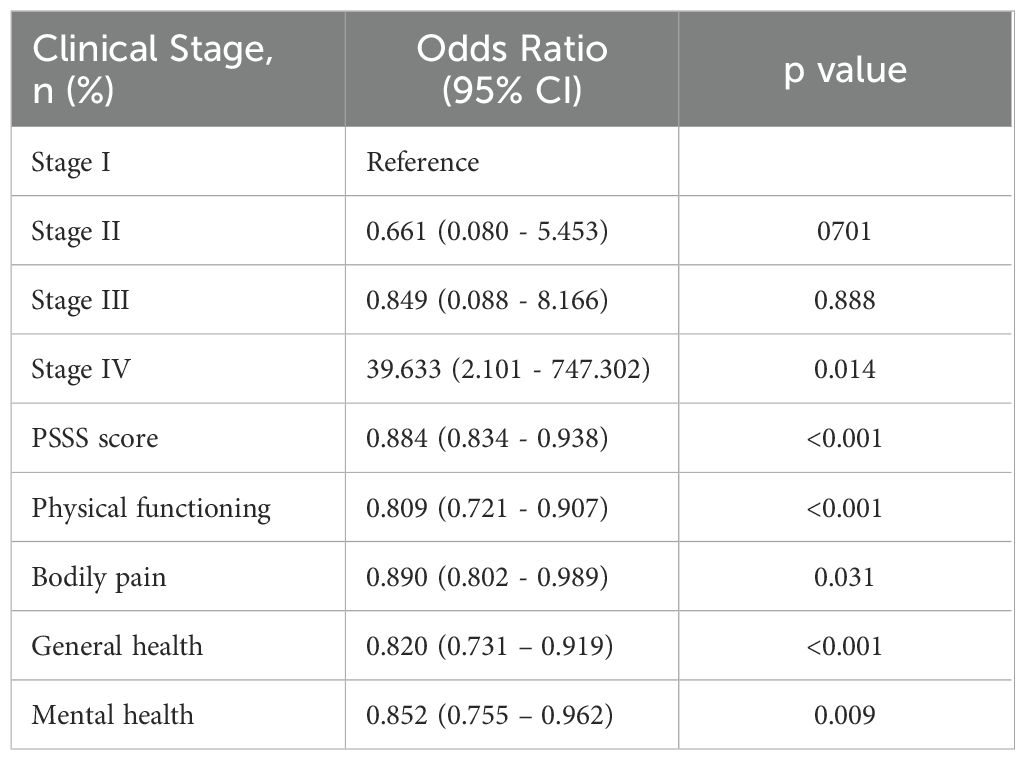
Table 2. Multiple logistic regression analysis of risk factors of cancer-related fatigue in thyroid cancers patients.
Construction and validation of nomogram
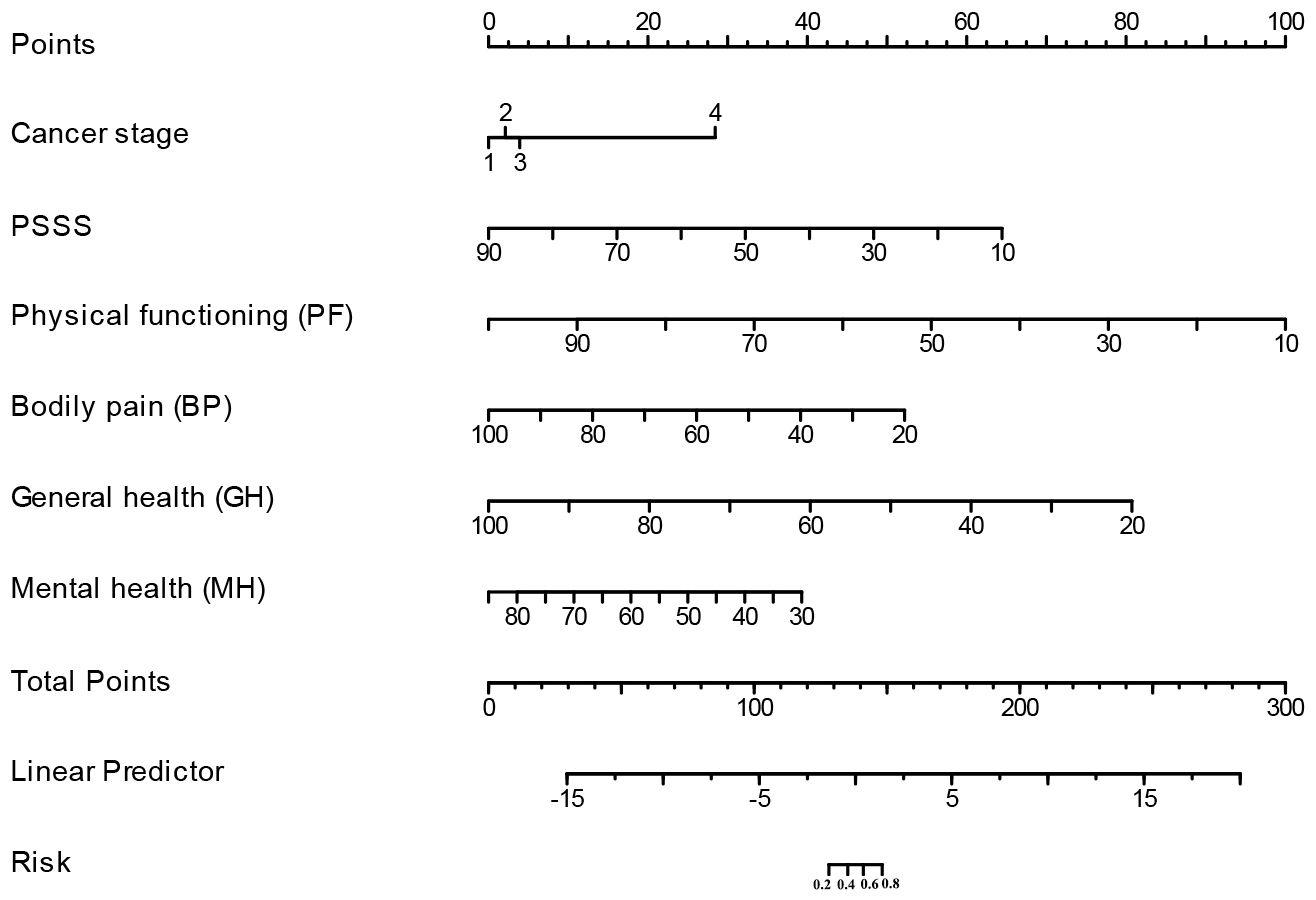
A clinically quantitative predictive model nomogram was developed based on the independent risk factors extracted above (Figure 3). The nomogram demonstrated excellent forecasting ability, with the concordance-index was 0.964 (0.935-0.993). For each glioma patient, higher total points indicated as a greater risk of developing cancer-related fatigue. We further examined the predictive ability of this nomogram on both the training and validation cohorts. The AUC values of nomogram were 0.916 (CI: 0.879-0.953) in the training cohort (Figure 4A) and 0.885 (CI: 0.829-0.941) in the validation cohort (Figure 4B). The calibration curves of this nomogram exhibited a notable concordance with the ideal diagonal line (Figures 4C, D). Moreover, the decision curve analyses (DCA) illuminated that the optimal utility of this nomogram which achieved the high clinical net benefit across the entire range of reasonable threshold probabilities in both training and validation cohorts (Figures 4E, F).

Figure 4. Calibration and discrimination of the nomogram. (A) ROC curve for training cohort. (B) ROC curve for validation cohort. (C) Calibration curve for training cohort. (D) Calibration curve for validation cohort. (E) Decision curve analysis for training cohort. (F) Decision curve analysis for validation cohort.
Discussion
Through LASSO and multivariable logistic regression analyses, six risk factors were independently associated with cancer-related fatigue in glioma patients. To be specific, the risk factors were the cancer stage of glioma, perceived social support, and quality-of-life of patients. The nomogram model incorporating these risk factors showed favorable discrimination in predicting the likelihood of cancer-related fatigue in glioma patients. This predictive tool provides a dependable quantitative evaluation for individual-level clinical prognostication.
Our study found that glioma patients in more advanced stages of cancer experienced more aggressive cancer-related fatigue. As cancer progresses to later stages, the tumor burden increases, leading to more extensive physical changes and metabolic alterations (23). Patients may encounter systemic symptoms such as pain, weight loss, and anemia, alongside undergoing more aggressive treatments (24). The cumulative effect of treatments, combined with the disease itself, can lead to emotional distress that compounds feelings of exhaustion (25). In addition, the glioma patients with later stages may struggle to maintain proper nutrition due to factors such as nausea, loss of appetite, or gastrointestinal issues. These nutritional deficiencies may contribute to decreased energy levels and heightened fatigue in glioma patients. Mustian KM et al. analyzed 113 studies involving a sample of 11,525 cancer patients and also found that cancer stage (nonmetastatic, metastatic, or mixed) was an independent variable influencing the treatment effectiveness of cancer-related fatigue (26). Consequently, it is essential to provide enhanced attention and psychological support to patients with advanced glioma (27). This approach not only addresses their emotional and mental well-being but also plays a significant role in mitigating cancer-related fatigue.
Our study revealed that the score of Perceived Social Support Scale was an independent risk factor for cancer-related fatigue in glioma patients. The patient with a strong perception of social support was less likely to experience cancer-related fatigue. This is in line with the conclusion drawn by Zhihui Gu et al. in their study of patients with cervical cancers (6). The perception of social support refers to an individual’s belief or feeling about the availability and adequacy of support from their social network, which includes family, friends, colleagues, and community members. Studies have suggested that cancer patients who perceive a consistent availability of social support were more likely to report higher levels of resilience and lower levels of distress, regardless of the type of cancer they have (28). This may be attributed to the social support derived from role relationships, which helps to stabilize and foster positive self-esteem and confidence, thereby enhancing patients’ ability to cope with stress and reducing cancer-related fatigue (29). Therefore, seeking available social support or providing support to patients with glioma may facilitate reducing the incidence of fatigue.
Furthermore, we found that the quality of life plays a crucial role in the occurrence of cancer-related fatigue in patients with glioma. Patients with a higher quality of life typically enjoy better physical and mental health, which can help alleviate their perception and experience of fatigue (30). They are more motivated to adhere to treatment regimens and possess positive emotional states that enable them to cope effectively with the stressors associated with cancer and its treatment, potentially reducing feelings of fatigue (31). A recent multi-center randomized controlled trial found that the patient with high quality of life often maintain an active lifestyle and engage in regular physical activity, which can enhance energy levels and resilience against fatigue (32). A Systematic Review included 20 articles also indicated that supervised moderate-hard resistance training with or without moderate-vigorous aerobic exercise and dietary intervention may improve quality of life and alleviate cancer-related fatigue in cancer patients (33).
Nevertheless, there were several limitations in the present study. Firstly, the small sample size may have restricted the ability to identify significant associations between risk factors and the occurrence of cancer-related fatigue. Consequently, larger prospective cohort studies are needed to explore this topic further. Secondly, it is necessary to conduct additional prospective validation studies from multiple centers to enhance the reliability of our findings. Thirdly, this study is limited by the lack of a formal pre-study sample size calculation, which may reduce confidence in the statistical power. Last but not least, the follow-up period in this study was limited to just one month. A Longer follow-up is required to assess more distant outcomes.
Conclusion
In conclusion, this study identified glioma cancer stage, perceived social support, and patient quality of life as independent risk factors for cancer-related fatigue. We developed and validated a nomogram that can noninvasively predict cancer-related fatigue in glioma patients. It would enable earlier detection and intervention for cancer-related fatigue, helping healthcare providers identify at-risk patients sooner. By facilitating timely interventions, it can improve the quality of life for glioma patients, ultimately enhancing their well-being and treatment outcomes.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
QW: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Resources, Software, Supervision, Writing – original draft, Writing – review & editing. CS: Data curation, Formal Analysis, Investigation, Resources, Software, Writing – original draft. MX: Investigation, Project administration, Resources, Software, Writing – review & editing. LO: Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by Guangdong Provincial Medical Science and Technology Research Fund Project, Grant number B2023024.
Acknowledgments
The authors would like to acknowledge the assistance of DeepSeek Chat (DeepSeek-V3) in improving the language and grammar of this manuscript. The AI model was accessed via https://www.deepseek.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chen R, Smith-Cohn M, Cohen AL, and Colman H. Glioma subclassifications and their clinical significance. Neurotherapeutics: J Am Soc Exp NeuroTherapeutics. (2017) 14:284–97. doi: 10.1007/s13311-017-0519-x
2. Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, et al. The epidemiology of glioma in adults: a "state of the science" review. Neuro-oncology. (2014) 16:896–913. doi: 10.1093/neuonc/nou087
3. Weller M, Wick W, Aldape K, Brada M, Berger M, Pfister SM, et al. Glioma. Nat Rev Dis primers. (2015) 1:15017. doi: 10.1038/nrdp.2015.17
4. Iorgulescu JB, Sun C, Neff C, and Cioffi G. Molecular biomarker-defined brain tumors: Epidemiology, validity, and completeness in the United States. Neuro-oncology (2022) 24:1989–2000. doi: 10.1093/neuonc/noac113
5. van den Bent MJ, Geurts M, French PJ, Smits M, Capper D, Bromberg JEC, et al. Primary brain tumours in adults. Lancet (London England). (2023) 402:1564–79. doi: 10.1016/s0140-6736(23)01054-1
6. Gu Z, Yang C, Zhang K, and Wu H. Development and validation of a nomogram for predicting sever cancer-related fatigue in patients with cervical cancer. BMC cancer. (2024) 24:492. doi: 10.1186/s12885-024-12258-x
7. Berger AM, Mooney K, Alvarez-Perez A, Breitbart WS, Carpenter KM, Cella D, et al. Cancer-related fatigue, version 2.2015. J Natl Compr Cancer Network: JNCCN. (2015) 13:1012–39. doi: 10.6004/jnccn.2015.0122
8. National Comprehensive Cancer Network. Cancer-related fatigue. Clin Practice Guidelines Onco. (2003) 1:308–31. doi: 10.6004/jnccn.2003.0029
9. David A, Hausner D, and Frenkel M. Cancer-related fatigue-is there a role for complementary and integrative medicine? Current Oncol. Rep. (2021) 23:145. doi: 10.1007/s11912-021-01135-6
10. Fabi A, Bhargava R, Fatigoni S, Guglielmo M, Horneber M, Roila F, et al. Cancer-related fatigue: ESMO Clinical Practice Guidelines for diagnosis and treatment. Ann oncology: Off J Eur Soc Med Oncol. (2020) 31:713–23. doi: 10.1016/j.annonc.2020.02.016
11. Ma Y, He B, Jiang M, Yang Y, Wang C, Huang C, et al. Prevalence and risk factors of cancer-related fatigue: A systematic review and meta-analysis. Int J Nurs Stud. (2020) 111:103707. doi: 10.1016/j.ijnurstu.2020.103707
12. Bower JE, Ganz PA, Desmond KA, Bernaards C, Rowland JH, Meyerowitz BE, et al. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer. (2006) 106:751–8. doi: 10.1002/cncr.21671
13. Bower JE. Cancer-related fatigue–mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. (2014) 11:597–609. doi: 10.1038/nrclinonc.2014.127
14. Cramp F and Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database systematic Rev. (2012) 11:Cd006145. doi: 10.1002/14651858.CD006145.pub3
15. Fox RS, Ancoli-Israel S, Roesch SC, Merz EL, Mills SD, Wells KJ, et al. Sleep disturbance and cancer-related fatigue symptom cluster in breast cancer patients undergoing chemotherapy. Supportive Care cancer: Off J Multinational Assoc Supportive Care Cancer. (2020) 28:845–55. doi: 10.1007/s00520-019-04834-w
16. Kang YE, Yoon JH, Park NH, Ahn YC, Lee EJ, and Son CG. Prevalence of cancer-related fatigue based on severity: a systematic review and meta-analysis. Sci Rep. (2023) 13:12815. doi: 10.1038/s41598-023-39046-0
17. Luo Q, Liu C, Zhou Y, Zou X, Song L, Wang Z, et al. Chinese cross-cultural adaptation and validation of the Well-being Numerical Rating Scales. Front Psychiatry. (2023) 14:1208001. doi: 10.3389/fpsyt.2023.1208001
18. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, and Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
19. Zimet GD, Powell SS, Farley GK, Werkman S, and Berkoff KA. Psychometric characteristics of the multidimensional scale of perceived social support. J Pers Assess. (1990) 55:610–7. doi: 10.1080/00223891.1990.9674095
20. Yang X, Xue M, Pauen S, and He H. Psychometric properties of the chinese version of multidimensional scale of perceived social support. Psychol Res Behavior Management (2024) 17:2233–41. doi: 10.2147/prbm.s463245
21. Jenkinson C, Coulter A, and Wright L. Short form 36 (SF36) health survey questionnaire: normative data for adults of working age. BMJ (Clinical Res ed). (1993) 306:1437–40. doi: 10.1136/bmj.306.6890.1437
22. Zhang Y, Qu B, Lun SS, Guo Y, and Liu J. The 36-item short form health survey: reliability and validity in Chinese medical students. Int J Med Sci. (2012) 9:521–6. doi: 10.7150/ijms.4503
23. Pace A, Dirven L, Koekkoek JAF, Golla H, Fleming J, Rudà R, et al. European Association for Neuro-Oncology (EANO) guidelines for palliative care in adults with glioma. Lancet Oncol. (2017) 18:e330–40. doi: 10.1016/s1470-2045(17)30345-5
24. Crooms RC, Johnson MO, Leeper H, Mehta A, McWhirter M, and Sharma A. Easing the journey-an updated review of palliative care for the patient with high-grade glioma. Current Onco Rep. (2022) 24:501–15. doi: 10.1007/s11912-022-01210-6
25. Ghiaseddin AP, Shin D, Melnick K, and Tran DD. Tumor treating fields in the management of patients with Malignant gliomas. Curr Treat options Oncol. (2020) 21:76. doi: 10.1007/s11864-020-00773-5
26. Mustian KM, Alfano CM, Heckler C, Kleckner AS, Kleckner IR, Leach CR, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: A meta-analysis. JAMA Oncol. (2017) 3:961–8. doi: 10.1001/jamaoncol.2016.6914
27. Koekkoek JAF, van der Meer PB, Pace A, and Hertler C. Palliative care and end-of-life care in adults with Malignant brain tumors. Neuro-oncology (2023) 25:447–56. doi: 10.1093/neuonc/noac216
28. Seiler A and Jenewein J. Resilience in cancer patients. Front Psychiatry. (2019) 10:208. doi: 10.3389/fpsyt.2019.00208
29. Bedaso A, Adams J, Peng W, and Sibbritt D. The relationship between social support and mental health problems during pregnancy: a systematic review and meta-analysis. Reprod Health. (2021) 18:162. doi: 10.1186/s12978-021-01209-5
30. Hare CJ, Crangle C, McGarragle K, Ferguson SE, and Hart TL. Change in cancer-related fatigue over time predicts health-related quality of life in ovarian cancer patients. Gynecologic Oncol. (2022) 166:487–93. doi: 10.1016/j.ygyno.2022.07.001
31. Liu L, He X, and Feng L. Exercise on quality of life and cancer-related fatigue for lymphoma survivors: a systematic review and meta-analysis. Supportive Care cancer: Off J Multinational Assoc Supportive Care Cancer. (2019) 27:4069–82. doi: 10.1007/s00520-019-04983-y
32. Dhillon HM, van der Ploeg HP, Bell ML, Boyer M, Clarke S, and Vardy J. The impact of physical activity on fatigue and quality of life in lung cancer patients: a randomised controlled trial protocol. BMC cancer. (2012) 12:572. doi: 10.1186/1471-2407-12-572
Keywords: nomogram, cancer-related fatigue, glioma, perceived social support, quality of life
Citation: Wu Q, Su C, Xing M and Ouyang L (2025) Development and validation of a nomogram for predicting cancer-related fatigue in patients with glioma: a multicenter study. Front. Oncol. 15:1497151. doi: 10.3389/fonc.2025.1497151
Received: 16 September 2024; Accepted: 26 May 2025;
Published: 16 June 2025.
Edited by:
Yi Xie, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Zhigang Chen, Anhui Medical University, ChinaMade Satya Nugraha Gautama, Ganesha University of Education, Indonesia
Copyright © 2025 Wu, Su, Xing and Ouyang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liangmei Ouyang, b3V5YW5nbGlhbmdtZWlAb3V0bG9vay5jb20=
 Qiuxia Wu
Qiuxia Wu Cuiqun Su1
Cuiqun Su1