- 1Department of Gastrointestinal Surgery The Second Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 2Department of Gastrointestinal Surgery, Shandong Cancer Hospital and Institute, Shandong First Medical University & Shandong Academy of Medical Science, Jinan, Shandong, China
Objective: To explore the feasibility, safety, and potential association between hyperthermic intraperitoneal chemotherapy (HIPEC) and peritoneal recurrence in patients with locally advanced gastric cancer following D2 radical surgery.
Materials and methods: A retrospective analysis was conducted on 156 patients with locally advanced gastric cancer treated with D2 surgery at two centers between 2014 and 2023. Clinical outcomes and adverse events were assessed.
Results: Baseline characteristics were comparable between the HIPEC group (n = 70) and the surgery-only group (n = 86). The 2-year peritoneal recurrence rate was lower in the HIPEC group (18.6% vs. 34.9%, P=0.0206). Several factors—including high Charlson Comorbidity Index, advanced T stage, vascular invasion, intraoperative blood loss, and absence of HIPEC—were associated with higher recurrence risk. No significant differences were observed in perioperative complications between the groups or among different HIPEC frequencies (all P > 0.05).
Conclusion: In this real-world dual-center study, HIPEC following D2 surgery was found to be feasible and safe, and was associated with a reduced risk of peritoneal recurrence in patients with locally advanced gastric cancer. These observational findings warrant further validation in randomized controlled trials.
Introduction
Gastric cancer remains a major global health burden, ranking as the fifth most common cancer and the fourth leading cause of cancer-related deaths worldwide (1). Current treatment options for gastric cancer include surgery, chemotherapy, targeted therapy, radiation therapy, and immunotherapy (2–5). However, in recent years, these treatments have shown limited clinical progress, with a median survival of only about 8 months for gastric cancer (6). Currently, the first-line treatment for locally advanced gastric cancer includes D2 radical surgery combined with systemic chemotherapy, according to major guidelines, both postoperative adjuvant chemotherapy and perioperative systemic therapy are recommended, depending on clinical stage and patient condition (7). It has been reported that perioperative systemic chemotherapy for locally advanced gastric cancer patients undergoing D2 radical surgery can significantly prolong patients’ survival (8).
Peritoneal metastasis is a major form of locally advanced gastric cancer, and for potentially curable gastric cancer patients, the peritoneum is one of the most common sites of recurrence (9). Peritoneal metastasis can lead to refractory ascites, progressive intestinal obstruction, and severe abdominal pain, significantly affecting patients’ overall survival and quality of life. Currently, there are no effective treatments to prevent the recurrence of peritoneal metastasis. Even for locally advanced gastric cancer patients undergoing D2 radical surgery combined with postoperative systemic chemotherapy, the postoperative peritoneal metastasis rate and 5-year mortality remain high (6, 10). Currently, cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) has become the main treatment for ovarian cancer, appendiceal mucinous neoplasms, pseudomyxoma peritonei of colonic origin (PMP), mesothelioma, and peritoneal metastasis (11–15). Prospective local-regional treatment using cytoreductive surgery and hyperthermic intraperitoneal chemotherapy has multiple advantages, including the significant removal of tumor burden through surgical resection and localized hyperthermic chemotherapy aiding in eradicating microscopic metastases and invisible free tumor cells (16).
As early as the 1990s, scholars initiated clinical studies to explore the potential effectiveness and safety of prophylactic hyperthermic intraperitoneal chemotherapy (HIPEC) following surgery for gastric cancer (17, 18). However, the early evidence was limited by suboptimal study designs and immature technical platforms. In recent years, with improvements in HIPEC-related theory, equipment, and implementation, there has been renewed interest in its clinical value. To further investigate the feasibility, safety, and potential association between postoperative prophylactic HIPEC and peritoneal recurrence, we conducted a dual-center retrospective study based on real-world data from patients with locally advanced gastric cancer treated at the Second Hospital of Shandong University and Shandong Cancer Hospital.
Materials and methods
Study population and grouping
Using a descriptive case series research method, clinical and pathological data of locally advanced gastric cancer patients who underwent D2 radical surgery (with or without HIPEC) at the Second Hospital of Shandong University and Shandong Cancer Hospital, affiliated with the First Medical University of Shandong Province, from January 2014 to February 2023 were retrospectively collected.
The inclusion criteria were as follows:(1) Age between 18 and 80 years;(2) Postoperative pathological confirmation of locally advanced gastric cancer;(3) No evidence of distant metastases detected on preoperative imaging;(4) Underwent D2 radical resection with R0 margins achieved;(5) Patients in the HIPEC group received at least one session of HIPEC;(6) No prior history of chemotherapy, radiotherapy, or immunotherapy;(7) No contraindications to chemotherapy based on laboratory tests, electrocardiogram (ECG), or comorbidities.
The exclusion criteria were as follows:(1) Incomplete clinicopathological data during the perioperative period or loss to follow-up;(2) Intraoperative identification of tumor metastasis;(3) Presence of other concurrent malignancies;(4) History of other malignant diseases;(5) Positive surgical resection margins;(6) Patients who did not receive adjuvant systemic chemotherapy, including those who failed to receive a standard regimen;(7) Postoperative survival time of less than two months.
All clinical decisions, including the use of HIPEC and adjuvant chemotherapy, were made by a multidisciplinary team (MDT) based on individual patient condition, tumor characteristics, and shared decision-making factors. This reflects real-world clinical practice, where therapeutic choices are often tailored rather than protocol-driven. However, the absence of explicitly standardized selection criteria may affect reproducibility and generalizability of the findings.
This study included a total of 156 patients, of which 70 patients were in the HIPEC group, receiving preventive HIPEC combined with postoperative adjuvant chemotherapy after D2 radical surgery. The remaining 86 patients were in the control group and received only postoperative adjuvant chemotherapy without preventive HIPEC. All patients received adjuvant chemotherapy with oxaliplatin and capecitabine (SOX regimen) within 6 weeks after surgery. Clinical and pathological data of all patients were collected.
Specific SOX Treatment Regimen for Gastric Cancer: Day 1 (D1): Oxaliplatin (130 mg/m²): Administered intravenously. D1–D14: S-1 (oral administration): Taken twice daily (once in the morning and once in the evening). Dose adjustment based on body surface area (BSA): BSA <1.25 m²: 40 mg per dose; 1.25–1.50 m²: 50 mg per dose; ≥1.50 m²: 60 mg per dose. D15–D21: Discontinue S-1. Treatment Cycle: Each chemotherapy cycle lasts 21 days, and patients receive a total of 6 cycles of the SOX regimen.
Tumor staging was determined by preoperative imaging, intraoperative findings, or pathological reports. The conduct of this study adhered to the ethical requirements outlined in the Helsinki Declaration, and it received approval from the hospital’s ethics committee with approval number: KYLL2024340, SDTHEC2024003170.
Treatment method
The HIPEC procedure was conducted as follows: Patients underwent exploratory laparotomy, laparoscopy, or robot-assisted radical gastrectomy under general anesthesia. During the surgery, four drainage tubes were placed in the abdominal cavity: two on both sides of the pelvis, one in the splenic fossa, and one on the hepatic diaphragmatic surface, each with multiple side holes. These tubes were respectively fixed to the right lower abdomen, left lower abdomen, right upper abdomen, and left upper abdomen. The tubes in the splenic fossa and on the hepatic diaphragmatic surface were used for fluid inflow, while those in the pelvic cavity were used for fluid outflow. The first HIPEC treatment was performed immediately after surgery, followed by subsequent abdominal closed HIPEC from postoperative day 2 to postoperative day 7. The second HIPEC treatment was administered 48 hours after surgery, depending on the patient’s postoperative recovery status. The third treatment was contingent upon the patient’s tolerance and willingness to receive therapy, with each treatment spaced 48 hours apart. Fentanyl and bupivacaine were used to relieve pain during HIPEC. HIPEC was performed in a regular ward. Isotonic dialysis fluid (4000 ml) was pumped into the abdominal cavity using an automatic pump at a perfusion rate of 600 ml/min and a temperature of 42-43 °C (monitored and controlled by the HIPEC machine). Perfusion time was 60 minutes to ensure uniform distribution of the perfusate, followed by fluid removal after perfusion. Postoperative hydration included intravenous fluid maintenance, and urine output was monitored according to standard treatment protocols. After the completion of HIPEC, the drainage tubes were retained for 2–3 days to assist in draining residual fluids from the abdominal cavity. Antitumor agents for HIPEC were selected based on the Expert Consensus on Clinical Application of Intraperitoneal Hyperthermic Chemotherapy in China (2019 edition). The key parameters of the HIPEC procedure were as follows: 1. Chemotherapeutic agents: Drugs such as cisplatin, paclitaxel, and oxaliplatin were used, with dosages adjusted according to body surface area and standard systemic chemotherapy reference guidelines. 2. Temperature: The perfusate was maintained at (43 ± 0.1)°C. 3. Duration: Each infusion lasted 60 to 90 minutes, with 60 minutes being the most common. 4. Number of cycles: For patients undergoing multiple C-HIPEC sessions, treatments were administered at 24-hour intervals, with a total of 1 to 3 sessions.
Evaluation criteria
(1) Evaluation of patients’ preoperative medical history using the Charlson Comorbidity Index (CCI) (19);(2) Evaluation of gastric cancer patients using the American Joint Committee on Cancer (AJCC) TNM staging system, including assessment of tumor infiltration depth, lymph node metastasis, and presence of distant metastasis;(3) Evaluation criteria for adverse events. Adverse reactions refer to any adverse or unexpected signs (including abnormal laboratory findings), symptoms, or diseases temporally associated with medical treatment or procedures, regardless of whether they are considered related to medical treatment or management. Adverse event recording: Two associate chief physicians recorded adverse events of grade 2 or above occurring during the patient’s treatment observation period based on the Common Terminology Criteria for Adverse Events (CTCAE 5.0) published by the U.S. Department of Health and Human Services. These events include hypoalbuminemia, bone marrow suppression, incision-related complications, intra-abdominal infection, pulmonary infection, gastric emptying disorder, anemia, postoperative bleeding, anastomotic leakage, intestinal obstruction, abdominal distension, and liver function impairment. Finally, a senior chief physician reviewed the above adverse events and made a safety assessment of the patients.
Data collection
Data collection was conducted by retrieving patient information from the medical record retrieval system, laboratory information system, and pathology system of the Second Hospital of Shandong University and Shandong Cancer Hospital, affiliated with the First Medical University of Shandong Province. Data were collected and compiled using Excel spreadsheets. The collected indicators mainly included the following aspects: (1) General patient information, including age, gender, body mass index (BMI), medical history, and ASA classification; (2) Preoperative laboratory indicators, including albumin, hemoglobin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT); (3) Postoperative pathological indicators, including tumor maximum diameter, tumor location, tumor differentiation, presence of neural invasion, presence of vascular invasion, lymph node metastasis status, tumor infiltration depth, and Her2 status; (4) Perioperative adverse events; (5) Relationship between the number of HIPEC treatments and perioperative adverse events.
Outcome evaluation
postoperative follow-up data for each patient were collected and compiled through outpatient reviews or telephone follow-ups. According to the research objectives of this study, the follow-up endpoint was determined to be March 1, 2024. Patients were followed up every 3 months for the first 2 years postoperatively, and then every 6 months for the next 3 years postoperatively. After 5 years postoperatively, patients were followed up annually.
Abdominal thin-layer enhanced CT (abdominal + pelvic) was determined to be the preferred imaging modality for detecting peritoneal metastasis in gastric cancer. Typical manifestations of gastric cancer peritoneal metastasis include uneven thickening of the peritoneum, high enhancement or nodules, nodular thickening of the mesentery, as well as significant accumulation of fluid in the abdominal and pelvic cavities, along with indirect signs such as dilation of the bile ducts, ureters, and intestines.
Statistical analysis
Statistical analysis was conducted using IBM SPSS Statistics v25.0 software. For normally distributed continuous data, the mean ± standard deviation (±s) was used, and intergroup comparisons were performed using the t-test. Skewed distributed continuous data were represented as median (P25, P75) or median (interquartile range, IQR), and intergroup comparisons were conducted using the Mann-Whitney U test. Categorical data were presented as frequencies (percentages), and intergroup comparisons were performed using the chi-square test or Fisher’s exact test. Kaplan-Meier curves were employed to represent survival data, and Cox regression analysis was used to evaluate the impact of various clinical factors on postoperative peritoneal metastasis recurrence. Logistic regression models were utilized for univariate and multivariate analysis of adverse events in patients with locally advanced gastric cancer. Factors with P<0.20 in univariate analysis were included in multivariate analysis, and factors with P<0.05 in multivariate analysis were considered independent influencing factors. A two-tailed P value <0.05 was considered statistically significant.
Results
Patient characteristics
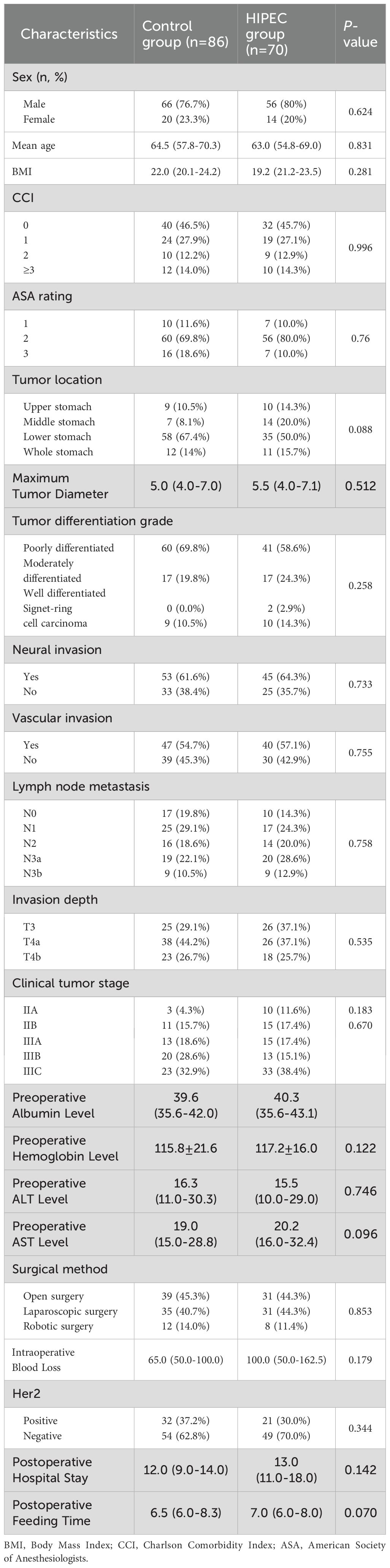
A total of 156 patients met our inclusion and exclusion criteria. Among these patients, 70 were in the HIPEC group, and 86 were in the control group. The two groups were well balanced in terms of age, gender, ASA score, body mass index, Charlson comorbidity index, tumor location, tumor maximum diameter, tumor differentiation, pathological T stage, neural invasion, clinical tumor stage, vascular invasion, intraoperative blood loss, Her2 gene status, postoperative hospital stay, time to oral intake postoperatively, preoperative albumin level, preoperative hemoglobin level, preoperative aspartate aminotransferase level, and preoperative alanine aminotransferase level (Table 1).
Peritoneal recurrence analysis
Through outpatient reviews, telephone inquiries, and other methods, follow-up was conducted for discharged patients. Among the 156 patients, the follow-up duration exceeded 1 year (12 months) for all. In the HIPEC group, the shortest time to peritoneal recurrence after surgery was 2.4 months, with a median time to peritoneal recurrence of 65.3 months (range: 56.6–74.1 months). In the control group, the shortest time to peritoneal recurrence after surgery was 2.1 months, with a median time to peritoneal recurrence of 51.0 months (range: 26.9–75.1 months). The rates of peritoneal recurrence at 1 year and 2 years were 12.9% (9/70) and 18.6% (12/70) in the HIPEC group, respectively, and 20.9% (18/86) and 34.9% (30/86) in the control group, respectively. The difference in postoperative peritoneal recurrence between the two groups was statistically significant (log-rank, P=0.0206) (Figure 1).
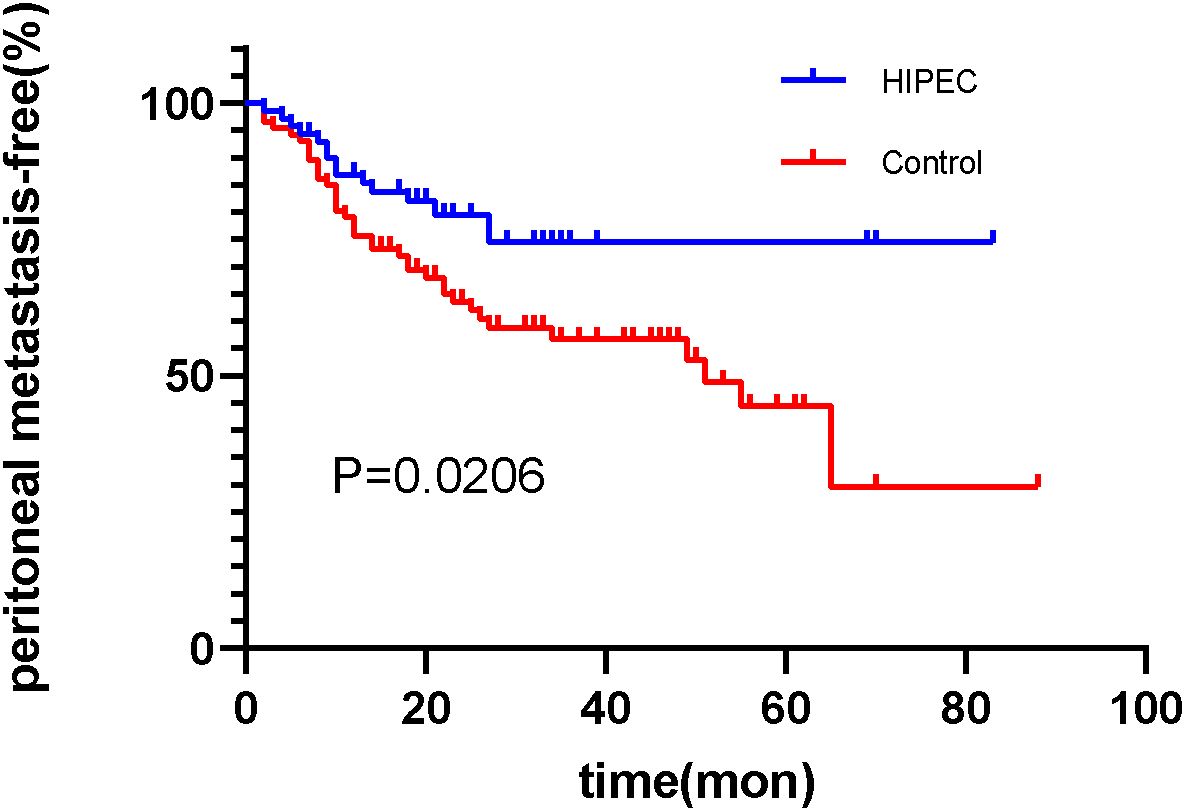
Figure 1. The survival analysis function of peritoneal recurrence after surgery in the HIPEC group and the control group. (Log-rank test, P=0.0206).
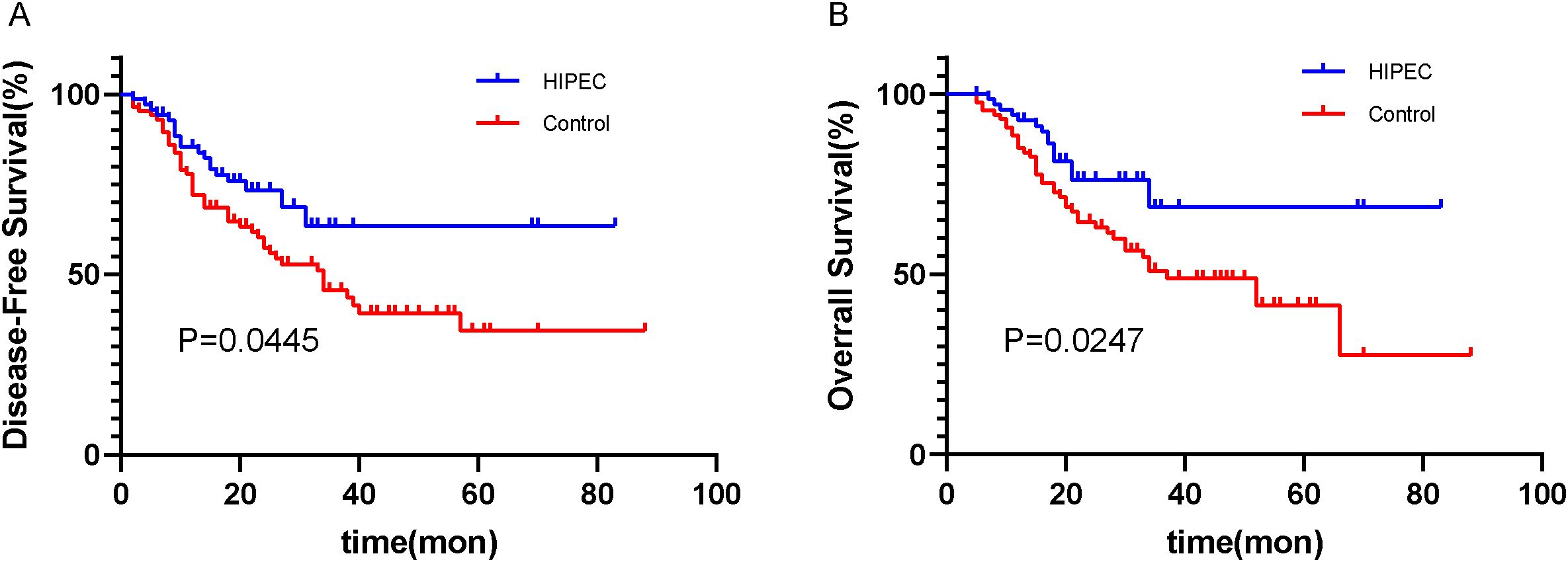
The median follow-up time for data analysis was 20.0 months (range: 12.0–33.0 months). All patients received adjuvant chemotherapy (oxaliplatin + capecitabine for 6 cycles) after surgery. Among the two groups, the disease-free survival (DFS) rate in the HIPEC group was 72.9% (51/70), compared to 46.5% (40/86) in the control group (Figure 2A). Furthermore, the overall survival (OS) rate was 78.6% (55/70) in the HIPEC group and 52.3% (45/86) in the control group (Figure 2B). The differences in both disease-free survival (DFS) and overall survival (OS) between the two groups were statistically significant, with P=0.0445 for DFS and P=0.0247 for OS, respectively (Figure 2).
We conducted univariate and multivariate Cox regression analyses to determine the factors influencing postoperative peritoneal recurrence. The results showed that a higher Charlson comorbidity index score, higher T stage with greater tumor infiltration, positive vascular invasion, absence of hyperthermic intraperitoneal chemotherapy (HIPEC) treatment, and greater intraoperative blood loss were independent risk factors for peritoneal recurrence after D2 radical resection in patients with locally advanced gastric cancer (Table 2).
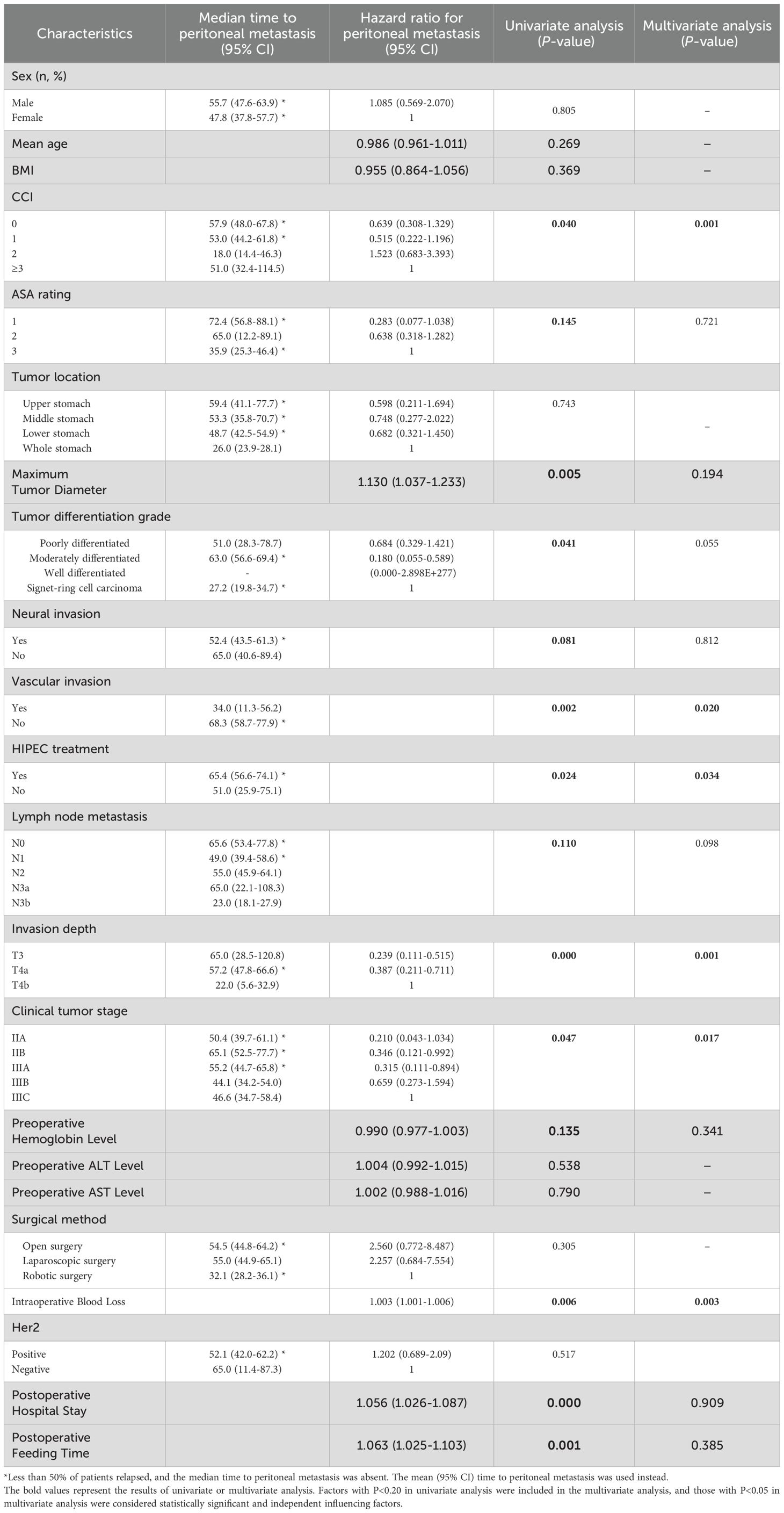
Table 2. Univariate and multivariate Cox analyses of postoperative recurrence in patients with locally advanced gastric cancer in the HIPEC group and the pure surgery group.
Complications
Adverse events occurring during the perioperative period were evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE) published by the U.S. Department of Health and Human Services. Only events of grade 2 or higher were recorded.
In the HIPEC group, 23 patients (32.9%) experienced a total of 37 adverse events, with the most common being postoperative hypoalbuminemia (12/70, 17.1%), pulmonary infections (6/70, 8.6%), and postoperative bleeding (3/70, 4.3%). Of these, the majority were grade 2 in severity; however, there were 5 grade 3 events, including severe hypoalbuminemia (n=2), postoperative bleeding requiring intervention (n=2), and myelosuppression (n=1). Some complications, such as mild peritoneal irritation or transient myelosuppression, were considered potentially related to the HIPEC procedure.
In the control group, 44 patients (51.2%) experienced 58 adverse events, with the most frequent being postoperative hypoalbuminemia (15/86, 17.4%), anemia (11/86, 12.8%), and pulmonary infections (8/86, 9.3%). The control group had 6 grade 3 adverse events, including severe anemia (n=2), intra-abdominal infections requiring intravenous antibiotics (n=2), and anastomotic leakage requiring intervention (n=2).
All adverse events in both groups were managed conservatively, and symptoms were relieved in most cases. Statistical analysis revealed no significant differences in the incidence of individual adverse events between the HIPEC and control groups (all P >0.05). A detailed breakdown of the types, frequency, and distribution of adverse events is presented in Table 3.
Moreover, we conducted a subgroup analysis based on the number of HIPEC sessions received by patients in the HIPEC group to evaluate whether treatment frequency was associated with a difference in perioperative adverse events. Patients were stratified into subgroups receiving 1, 2, or 3 HIPEC treatments.
The results showed no statistically significant differences in the incidence of any specific adverse events among the three subgroups (all P > 0.05). Most adverse events occurred in patients who received only a single HIPEC treatment, likely reflecting the fact that the majority of patients in our cohort underwent one session. No grade 3 adverse events were observed in patients receiving more than one HIPEC treatment. These findings suggest that increasing the frequency of HIPEC does not appear to increase the risk of perioperative complications in patients with locally advanced gastric cancer. No adverse events were found to be uniquely or significantly associated with the HIPEC procedure. While myelosuppression and postoperative bleeding were observed slightly more frequently in the HIPEC group, the differences were not statistically significant. Detailed data are presented in Table 4.
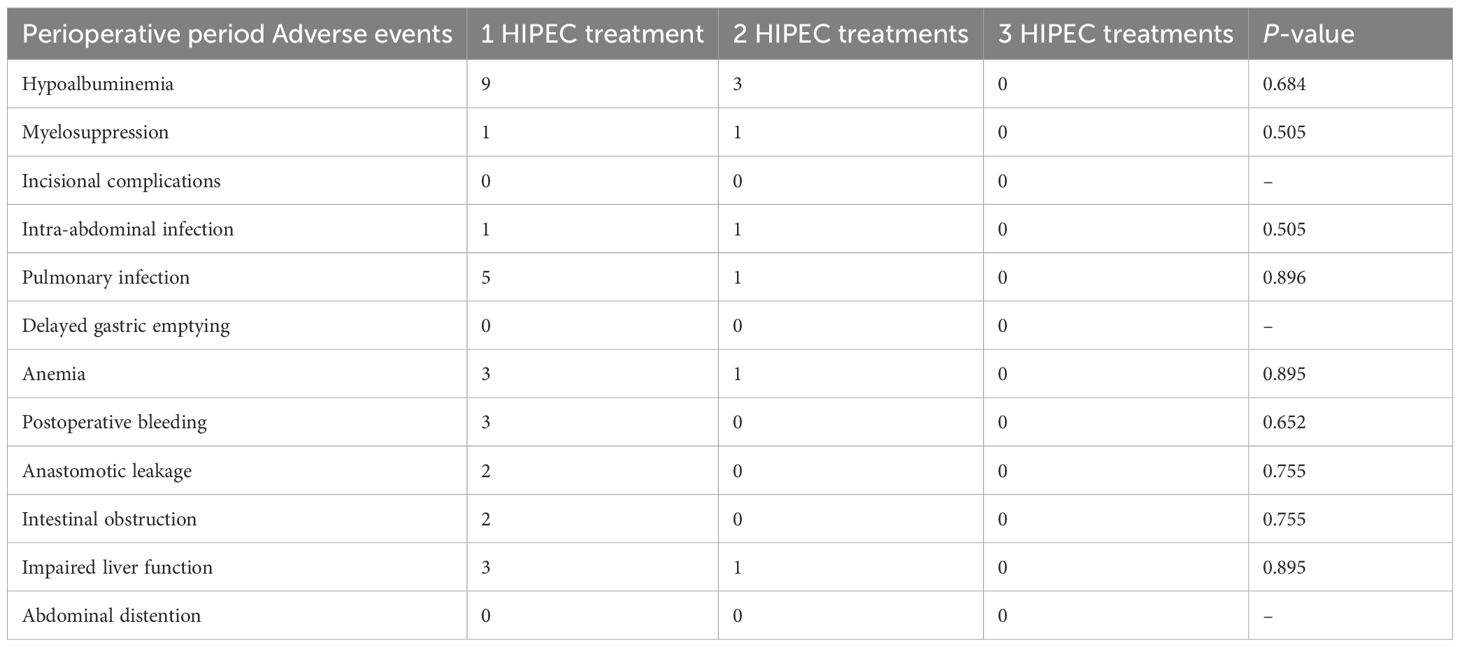
Table 4. Incidence of perioperative adverse events by number of HIPEC treatments in patients with locally advanced gastric cancer.
Discussion
Gastric cancer is one of the most common malignant tumors in East Asia, especially in China, where its incidence has been steadily increasing (20). Currently, radical surgical resection is the only curative treatment strategy. Multiple retrospective and prospective studies have shown that radical resection surgery can significantly improve the prognosis of patients (21, 22). Peritoneal recurrence is the most common form of recurrence after radical surgery for gastric cancer, which can lead to intestinal obstruction and malignant ascites (23). This significantly affects the quality of life for patients and shortens overall survival, making it one of the main causes of death among patients (9). However, peritoneal metastasis after radical gastric cancer surgery is almost unavoidable (24, 25). Some studies have indicated that during surgery, the extent of tumor cell spread into the abdominal cavity is higher than during initial exploration, suggesting that surgery can directly promote iatrogenic dissemination of tumor cells, increasing the likelihood of peritoneal metastasis (26). In addition to the increased risk of peritoneal spread due to serosal infiltration of gastric tumors, surgical procedures can also allow cancer cells to infiltrate the abdominal cavity from surgical margins, blood vessels, or lymphatic vessels, ultimately leading to peritoneal metastasis. For patients with advanced gastric cancer with peritoneal metastasis, clinicians employ multimodal treatment strategies to extend survival. A study by Runcong Nie and colleagues shows that conversion surgery can prolong survival in patients with advanced peritoneal metastatic gastric cancer, offering potential survival benefits (27). They also found that palliative gastrectomy combined with first-line chemotherapy can extend survival in patients without multiple distant metastases (28). To address the risk of postoperative peritoneal metastasis in patients with locally advanced gastric cancer, hyperthermic intraperitoneal chemotherapy (HIPEC) has been increasingly explored in recent years. Several randomized controlled trials have reported its potential benefits and safety in this setting (29–33). However, real-world evaluations of the feasibility and safety of combining D2 radical surgery with HIPEC remain limited, particularly in non-randomized, practice-based contexts. Further investigation is needed to assess its clinical applicability and generate hypotheses for future prospective studies.
This is a two-center, retrospective study. The purpose of this study is to explore the feasibility and safety of using hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with locally advanced gastric cancer following D2 radical surgery, and to investigate its potential association with peritoneal recurrence. The results indicate that patients in the HIPEC group had lower peritoneal recurrence rates compared to the surgery-alone group, suggesting that HIPEC may be associated with a reduced risk of peritoneal recurrence and improved prognosis in patients with locally advanced gastric cancer. Independent risk factors for peritoneal recurrence include high Charlson comorbidity index, advanced T stage, vascular invasion, significant intraoperative blood loss, and the absence of postoperative HIPEC treatment. Additionally, the incidence of perioperative adverse events was similar between the two groups, indicating that HIPEC does not increase the risk of such events after D2 radical surgery. These findings support the combination of HIPEC with D2 gastrectomy and highlight the importance of personalized selection of HIPEC candidates for risk stratification.
Previous studies, including those by Yarema et al. and M. Yu Reutovich, have shown that HIPEC significantly reduces peritoneal recurrence and improves disease-free survival compared to surgery alone (30, 34). Our findings align with these studies, showing a lower peritoneal recurrence rate (18.6% vs. 34.9%, P=0.0206) in the HIPEC group. The absence of HIPEC was associated with a higher recurrence risk; however, these associations should be interpreted cautiously due to the non-randomized nature of treatment allocation. These results suggest that combining HIPEC with D2 radical surgery may offer clinical benefits in improving patient prognosis for those with locally advanced gastric cancer. In addition to identifying HIPEC treatment as an independent prognostic factor, this study also explored other variables associated with peritoneal recurrence. Consistent with previous findings that tumor infiltration depth, lymph node metastasis, and neural invasion contribute to recurrence risk (35–37). Our analysis further highlighted the prognostic significance of a high Charlson comorbidity index, positive vascular invasion, advanced T stage, higher clinical stage, and substantial intraoperative blood loss.
HIPEC treatment, as an invasive procedure, has drawn attention due to the adverse events associated with this therapeutic approach. Reported adverse events during the perioperative period of HIPEC treatment include leukopenia, peritoneal infection, anastomotic leakage, severe bleeding, acute kidney injury, and intestinal perforation (38–40). These adverse events may also have a detrimental effect on long-term survival outcomes (41). However, with the passage of time, as HIPEC techniques continue to develop and improve, the incidence and mortality rates of related adverse events have gradually decreased (42, 43). Controversy still exists regarding whether there is a difference in the occurrence of complications between patients receiving HIPEC treatment after radical surgery compared to those undergoing radical surgery alone. Felix Merboth et al. reported a retrospective analysis study involving 58 patients, where there was no difference in postoperative complications between the HIPEC group and the radical surgery alone group (both 25%) (44). Jing Zhang et al. reported a retrospective analysis study involving 78 patients with T4 stage gastric cancer, dividing them into single HIPEC group (40 cases) and multiple HIPEC treatment group (38 cases). Both groups experienced mild renal dysfunction, mild hepatic dysfunction, low platelet count, and low white blood cell levels, but the difference in adverse events between the two groups was not statistically significant (P > 0.05) (45). Lili Li et al. employed a novel HIPEC protocol using ultrasound-guided puncture needles to establish a closed-loop HIPEC circulation under local anesthesia. Compared to the traditional method, using smaller diameter needles without the need for catheter insertion made the HIPEC treatment process more tolerable. The study results showed no significant difference in adverse events between the HIPEC treatment group and the control group. Mild procedure-related side effects observed mainly included self-resolving puncture site pain and mild peritonitis (29). In this study, there was no difference in the occurrence of perioperative adverse events between the HIPEC group and the radical surgery alone group (P > 0.05), and there was no statistically significant difference in the occurrence of adverse events between different frequencies of HIPEC treatment groups (P > 0.05). Overall, adjunctive HIPEC therapy appears to be effective and safe.
Despite advances in HIPEC, several key challenges persist. First, the indications for HIPEC in gastric cancer and the criteria for selecting patients who will benefit remain unclear. Although the Peritoneal Cancer Index (PCI) is widely used and validated as an independent prognostic factor for peritoneal metastasis in gastric cancer, its clinical utility is debated (46, 47). For example, up to 40% of patients with negative preoperative imaging have peritoneal metastases detected during diagnostic laparoscopy (48). Moreover, even if laparoscopy reveals no macroscopic disease, up to 13% of patients have positive peritoneal cytology and later develop peritoneal recurrence (49). Which clinical or molecular subgroups benefit most from HIPEC is also unknown, partly because clinicians often lack data on key biomarkers (e.g., PD-L1 expression, tumor mutational burden, microsatellite stability, CLDN18.2 status). Notably, high PD-L1 expression correlates with improved survival in gastric cancer, particularly in patients with peritoneal metastases. In contrast, CLDN18.2 positivity and HER2 overexpression are linked to poorer outcomes (50–53). Moreover, the distribution, metabolism, and mechanism of action of chemotherapeutic agents during HIPEC within the peritoneum and tumor tissue remain unclear. In vitro studies have shown that miR−218 enhances the sensitivity of gastric cancer cells to cisplatin, and HIPEC has been reported to significantly upregulate miR−218 levels (54). Additionally, hyperthermia markedly increases the cytotoxicity of 5−fluorouracil (5−FU) in gastric cancer cells, induces PARP and caspase−3 cleavage to promote apoptosis, and significantly upregulates PD−L1 expression on the tumor cell surface (55). Furthermore, HIPEC protocols lack standardization. Published reports reveal significant variability in drug selection, dosing, perfusion temperature, and duration across centers, hindering outcome comparisons (30, 56–59). Therefore, multicenter randomized controlled trials are needed to systematically evaluate these parameters and establish optimized, standardized protocols. Finally, patients receiving HIPEC after D2 gastrectomy remain at risk for complications such as leukopenia, peritoneal infection, severe hemorrhage, renal dysfunction, and intestinal perforation. The relationship between hyperthermic perfusion and postoperative complications remains controversial (45, 60–62). Optimizing perfusion temperature and duration, co-administering protective agents, and refining perioperative management guidelines may reduce these complication rates (63–65). Over the next few years, the HIPEC field is poised for major advances in multiple areas.
First, multicenter randomized trials will systematically compare key variables—drug selection, dosage, perfusion temperature, and duration—to establish standardized global protocols. Second, integrating molecular and imaging biomarkers (e.g., genomic alterations, protein expression, and preoperative imaging) will enable precise patient selection and risk stratification for HIPEC. Third, new heat−stable chemotherapeutics, nanocarriers, and liposomal systems will improve intraperitoneal drug delivery and tumor penetration while minimizing toxicity to healthy tissues. Fourth, combining HIPEC with immunotherapies—such as checkpoint inhibitors, CAR−T cells, or cancer vaccines—may enhance tumor immunogenicity, overcome “cold” tumor resistance, and increase therapeutic efficacy. Finally, large-scale clinical studies will build integrated clinical and genomic HIPEC databases. Machine learning models can then predict treatment outcomes and complication risks, supporting personalized HIPEC strategies.
This study has several limitations. First, its retrospective design and relatively short follow-up durations may affect the accuracy of recurrence assessment. Second, patients who did not receive standard adjuvant chemotherapy were excluded, which may introduce allocation bias. Third, the treatment allocation was non-randomized and influenced by real-world factors such as economic constraints, which may lead to selection bias and limit the ability to draw causal conclusions. Therefore, the observed associations between HIPEC and clinical outcomes should be interpreted with caution, and not as definitive evidence of efficacy. Lastly, although this was a dual-center study, the overall sample size was relatively limited, which may affect the generalizability of the findings. Future large-scale, multicenter randomized controlled trials are needed to validate these findings and support causal interpretation.
Conclusion
The study results suggest that adjuvant HIPEC following D2 radical surgery may be associated with a reduced risk of peritoneal recurrence in patients with locally advanced gastric cancer. In our cohort, combining D2 radical surgery with HIPEC—and performing multiple HIPEC sessions—was not associated with an increased risk of perioperative adverse events. However, some patients did not undergo HIPEC due to economic constraints, resulting in non-random treatment allocation and introducing potential selection bias. This form of bias cannot be addressed through statistical adjustment and may affect the interpretation of causality. Therefore, the findings should be interpreted as observational associations rather than definitive treatment effects. Standardized protocols for patient selection and technical parameters (perfusate type, duration, flow rates, temperature) are needed, and future studies using propensity score–based methods and large multicenter randomized controlled trials are essential to further evaluate the efficacy and safety of HIPEC.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Scientific Research, The Second Hospital of Shandong University; Ethics Committee of the Affiliated Cancer Hospital of Shandong First Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
TZ: Data curation, Methodology, Software, Writing – original draft, Writing – review & editing. HY: Investigation, Software, Writing – review & editing. LW: Data curation, Writing – review & editing. SZ: Data curation, Writing – review & editing. CZ: Data curation, Writing – review & editing. JC: Supervision, Writing – review & editing. DW: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank The Department of Gastrointestinal Surgery, The Second Hospital, Cheeloo College of Medicine/The Shandong Cancer Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wong MCS, Huang J, Chan PSF, Choi P, Lao XQ, Chan SM, et al. Global incidence and mortality of gastric cancer, 1980 - 2018. JAMA network Open. (2021) 4:e2118457. doi: 10.1001/jamanetworkopen.2021.18457
2. Sahin TK, Ayasun R, Rizzo A, and Guven DC. Prognostic value of neutrophil-to-eosinophil ratio (Ner) in cancer: A systematic review and meta-analysis. Cancers. (2024) 16(21):3689. doi: 10.3390/cancers16213689
3. Sahin TK, Rizzo A, Aksoy S, and Guven DC. Prognostic significance of the royal marsden hospital (Rmh) score in patients with cancer: A systematic review and meta-analysis. Cancers. (2024) 16(10):1835. doi: 10.3390/cancers16101835
4. Guven DC, Erul E, Kaygusuz Y, Akagunduz B, Kilickap S, De Luca R, et al. Immune checkpoint inhibitor-related hearing loss: A systematic review and analysis of individual patient data. Supportive Care cancer: Off J Multinational Assoc Supportive Care Cancer. (2023) 31:624. doi: 10.1007/s00520-023-08083-w
5. Vitale E, Rizzo A, Maistrello L, Guven DC, Massafra R, Mollica V, et al. Sex differences in adverse events among cancer patients receiving immune checkpoint inhibitors: the mouseion-07 systematic review and meta-analysis. Sci Rep. (2024) 14:28309. doi: 10.1038/s41598-024-71746-z
6. Li K, Zhang A, Li X, Zhang H, and Zhao L. Advances in clinical immunotherapy for gastric cancer. Biochim Biophys Acta Rev Cancer. (2021) 1876:188615. doi: 10.1016/j.bbcan.2021.188615
7. Ajani JA, D’Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, et al. Gastric cancer, version 2.2022, nccn clinical practice guidelines in oncology. J Natl Compr Cancer Network: JNCCN. (2022) 20:167–92. doi: 10.6004/jnccn.2022.0008
8. Katai H, Ishikawa T, Akazawa K, Isobe Y, Miyashiro I, Oda I, et al. Five-year survival analysis of surgically resected gastric cancer cases in Japan: A retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese gastric cancer association (2001 - 2007). Gastric cancer: Off J Int Gastric Cancer Assoc Japanese Gastric Cancer Assoc. (2018) 21:144–54. doi: 10.1007/s10120-017-0716-7
9. Ikoma N, Chen HC, Wang X, Blum M, Estrella JS, Fournier K, et al. Patterns of initial recurrence in gastric adenocarcinoma in the era of preoperative therapy. Ann Surg Oncol. (2017) 24:2679–87. doi: 10.1245/s10434-017-5838-y
10. Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S - 1, an oral fluoropyrimidine. New Engl J Med. (2007) 357:1810–20. doi: 10.1056/NEJMoa072252
11. van Driel WJ, Koole SN, Sikorska K, Schagen van Leeuwen JH, Schreuder HWR, Hermans RHM, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. New Engl J Med. (2018) 378:230–40. doi: 10.1056/NEJMoa1708618
12. Sugarbaker PH, Graves T, DeBruijn EA, Cunliffe WJ, Mullins RE, Hull WE, et al. Early postoperative intraperitoneal chemotherapy as an adjuvant therapy to surgery for peritoneal carcinomatosis from gastrointestinal cancer: pharmacological studies. Cancer Res. (1990) 50:5790–4.
13. Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin oncology: Off J Am Soc Clin Oncol. (2012) 30:2449–56. doi: 10.1200/jco.2011.39.7166
14. Feldman AL, Libutti SK, Pingpank JF, Bartlett DL, Beresnev TH, Mavroukakis SM, et al. Analysis of factors associated with outcome in patients with Malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy. J Clin oncology: Off J Am Soc Clin Oncol. (2003) 21:4560–7. doi: 10.1200/jco.2003.04.150
15. Elias D, Goéré D, Dumont F, Honoré C, Dartigues P, Stoclin A, et al. Role of hyperthermic intraoperative peritoneal chemotherapy in the management of peritoneal metastases. Eur J Cancer (Oxford England: 1990). (2014) 50:332–40. doi: 10.1016/j.ejca.2013.09.024
16. Li Y, Zhou YF, Liang H, Wang HQ, Hao JH, Zhu ZG, et al. Chinese expert consensus on cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal Malignancies. World J Gastroenterol. (2016) 22:6906–16. doi: 10.3748/wjg.v22.i30.6906
17. Fujimoto S, Takahashi M, Mutou T, Kobayashi K, and Toyosawa T. Successful intraperitoneal hyperthermic chemoperfusion for the prevention of postoperative peritoneal recurrence in patients with advanced gastric carcinoma. Cancer. (1999) 85:529–34. doi: 10.1002/(SICI)1097-0142(19990201)85:3<529::AID-CNCR3>3.0.CO;2-9
18. Kunisaki C, Shimada H, Nomura M, Akiyama H, Takahashi M, and Matsuda G. Lack of efficacy of prophylactic continuous hyperthermic peritoneal perfusion on subsequent peritoneal recurrence and survival in patients with advanced gastric cancer. Surgery. (2002) 131:521–8. doi: 10.1067/msy.2002.123769
19. Bannay A, Chaignot C, Blotière PO, Basson M, Weill A, Ricordeau P, et al. The best use of the charlson comorbidity index with electronic health care database to predict mortality. Med Care. (2016) 54:188–94. doi: 10.1097/mlr.0000000000000471
20. Morgan RJ Jr., Synold TW, Xi B, Lim D, Shibata S, Margolin K, et al. Phase I trial of intraperitoneal gemcitabine in the treatment of advanced Malignancies primarily confined to the peritoneal cavity. Clin Cancer research: an Off J Am Assoc Cancer Res. (2007) 13:1232–7. doi: 10.1158/1078-0432.Ccr-06-1735
21. Speyer JL, Sugarbaker PH, Collins JM, Dedrick RL, Klecker RW Jr., and Myers CE. Portal levels and hepatic clearance of 5-fluorouracil after intraperitoneal administration in humans. Cancer Res. (1981) 41:1916–22.
22. Bonnot PE, Piessen G, Kepenekian V, Decullier E, Pocard M, Meunier B, et al. Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastases (Cyto-chip study): A propensity score analysis. J Clin oncology: Off J Am Soc Clin Oncol. (2019) 37:2028–40. doi: 10.1200/jco.18.01688
23. Sugarbaker PH. Peritoneal metastases from gastrointestinal cancer. Curr Oncol Rep. (2018) 20:62. doi: 10.1007/s11912-018-0703-0
24. Coccolini F, Cotte E, Glehen O, Lotti M, Poiasina E, Catena F, et al. Intraperitoneal chemotherapy in advanced gastric cancer. Meta-Analysis Randomized Trials. Eur J Surg oncology: J Eur Soc Surg Oncol Br Assoc Surg Oncol. (2014) 40:12–26. doi: 10.1016/j.ejso.2013.10.019
25. Lee JH, Son SY, Lee CM, Ahn SH, Park DJ, and Kim HH. Factors predicting peritoneal recurrence in advanced gastric cancer: implication for adjuvant intraperitoneal chemotherapy. Gastric cancer: Off J Int Gastric Cancer Assoc Japanese Gastric Cancer Assoc. (2014) 17:529–36. doi: 10.1007/s10120-013-0306-2
26. Yu XF, Ren ZG, Xue YW, Song HT, Wei YZ, and Li CM. D2 lymphadenectomy can disseminate tumor cells into peritoneal cavity in patients with advanced gastric cancer. Neoplasma. (2013) 60:174–81. doi: 10.4149/neo_2013_023
27. Nie R, Yuan S, Li Y, Chen S, Li S, Yang L, et al. Risk-stratification model to select conversion surgery for advanced gastric cancer patients. Chin J Cancer Res = Chung-kuo yen cheng yen chiu. (2019) 31:178–87. doi: 10.21147/j.issn.1000-9604.2019.01.13
28. Nie RC, Chen S, Yuan SQ, Chen XJ, Chen YM, Zhu BY, et al. Significant role of palliative gastrectomy in selective gastric cancer patients with peritoneal dissemination: A propensity score matching analysis. Ann Surg Oncol. (2016) 23:3956–63. doi: 10.1245/s10434-016-5223-2
29. Liu L, Sun L, Zhang N, Liao CG, Su H, Min J, et al. A novel method of bedside hyperthermic intraperitoneal chemotherapy as adjuvant therapy for stage-iii gastric cancer. Int J hyperthermia: Off J Eur Soc Hyperthermic Oncology North Am Hyperthermia Group. (2022) 39:239–45. doi: 10.1080/02656736.2022.2028018
30. Yarema R, Mielko J, Fetsych T, Ohorchak M, Skorzewska M, Rawicz-Pruszyński K, et al. Hyperthermic intraperitoneal chemotherapy (Hipec) in combined treatment of locally advanced and intraperitonealy disseminated gastric cancer: A retrospective cooperative central-eastern european study. Cancer Med. (2019) 8:2877–85. doi: 10.1002/cam4.2204
31. Yu P, Huang X, Huang L, Dai G, Xu Q, Fang J, et al. Hyperthermic intraperitoneal chemotherapy (Hipec) plus systemic chemotherapy versus systemic chemotherapy alone in locally advanced gastric cancer after D2 radical resection: A randomized-controlled study. J Cancer Res Clin Oncol. (2023) 149:11491–8. doi: 10.1007/s00432-023-05019-z
32. Rau B, Lang H, Koenigsrainer A, Gockel I, Rau HG, Seeliger H, et al. Effect of hyperthermic intraperitoneal chemotherapy on cytoreductive surgery in gastric cancer with synchronous peritoneal metastases: the phase iii gastripec-I trial. J Clin oncology: Off J Am Soc Clin Oncol. (2024) 42:146–56. doi: 10.1200/jco.22.02867
33. Patel M, Arora A, Mukherjee D, and Mukherjee S. Effect of hyperthermic intraperitoneal chemotherapy on survival and recurrence rates in advanced gastric cancer: A systematic review and meta-analysis. Int J Surg (London England). (2023) 109:2435–50. doi: 10.1097/js9.0000000000000457
34. Reutovich MY, Krasko OV, and Sukonko OG. Hyperthermic intraperitoneal chemotherapy in serosa-invasive gastric cancer patients. Eur J Surg oncology: J Eur Soc Surg Oncol Br Assoc Surg Oncol. (2019) 45:2405–11. doi: 10.1016/j.ejso.2019.07.030
35. Loggie BW, Fleming RA, McQuellon RP, Russell GB, and Geisinger KR. Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for disseminated peritoneal cancer of gastrointestinal origin. Am surgeon. (2000) 66:561–8. doi: 10.1177/000313480006600607
36. Yonemura Y, de Aretxabala X, Fujimura T, Fushida S, Katayama K, Bandou E, et al. Intraoperative chemohyperthermic peritoneal perfusion as an adjuvant to gastric cancer: final results of a randomized controlled study. Hepato-gastroenterology. (2001) 48:1776–82.
37. Graziosi L, Mencarelli A, Renga B, Santorelli C, Cantarella F, Bugiantella W, et al. Gene expression changes induced by hipec in a murine model of gastric cancer. In Vivo (Athens Greece). (2012) 26:39–45.
38. Sugarbaker PH, Mora JT, Carmignani P, Stuart OA, and Yoo D. Update on chemotherapeutic agents utilized for perioperative intraperitoneal chemotherapy. oncologist. (2005) 10:112–22. doi: 10.1634/theoncologist.10-2-112
39. Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, et al. Chemotherapy for advanced gastric cancer. Cochrane Database systematic Rev. (2010) (3):Cd004064. doi: 10.1002/14651858.CD004064.pub3
40. Cohen MS, Al-Kasspooles MF, Williamson SK, Henry D, Broward M, and Roby KF. Combination intraperitoneal chemotherapy is superior to mitomycin C or oxaliplatin for colorectal carcinomatosis in vivo. Ann Surg Oncol. (2010) 17:296–303. doi: 10.1245/s10434-009-0669-0
41. Lubrano J, Bachelier P, Paye F, Le Treut YP, Chiche L, Sa-Cunha A, et al. Severe postoperative complications decrease overall and disease free survival in pancreatic ductal adenocarcinoma after pancreaticoduodenectomy. Eur J Surg oncology: J Eur Soc Surg Oncol Br Assoc Surg Oncol. (2018) 44:1078–82. doi: 10.1016/j.ejso.2018.03.024
42. Wu Y, Zheng X, Sun C, Wang S, Ding S, Wu M, et al. Hyperthermic intraperitoneal chemotherapy for patients with gastric cancer based on laboratory tests is safe: A single chinese center analysis. BMC Surg. (2022) 22:342. doi: 10.1186/s12893-022-01795-6
43. Manzanedo I, Pereira F, Rihuete Caro C, Pérez-Viejo E, Serrano Á, Gutiérrez Calvo A, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (Hipec) for gastric cancer with peritoneal carcinomatosis: multicenter study of spanish group of peritoneal oncologic surgery (Gecop). Ann Surg Oncol. (2019) 26:2615–21. doi: 10.1245/s10434-019-07450-4
44. Merboth F, Garcia S, V Renesse J, Distler M, Welsch T, Weitz J, et al. Comparative analysis of postoperative complications after cytoreductive surgery and hipec in gastric cancer. Oncol Res Treat. (2022) 45:45–53. doi: 10.1159/000520330
45. Zhang J, Sun Y, Bai X, Wang P, Tian L, Tian Y, et al. Single versus multiple hyperthermic intraperitoneal chemotherapy applications for T4 gastric cancer patients: efficacy and safety profiles. Front Oncol. (2023) 13:1109633. doi: 10.3389/fonc.2023.1109633
46. Yang XJ, Huang CQ, Suo T, Mei LJ, Yang GL, Cheng FL, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase iii randomized clinical trial. Ann Surg Oncol. (2011) 18:1575–81. doi: 10.1245/s10434-011-1631-5
47. Magge D, Zenati M, Mavanur A, Winer J, Ramalingam L, Jones H, et al. Aggressive locoregional surgical therapy for gastric peritoneal carcinomatosis. Ann Surg Oncol. (2014) 21:1448–55. doi: 10.1245/s10434-013-3327-5
48. Wu X, Li Z, Li Z, Jia Y, Shan F, Ji X, et al. Hyperthermic intraperitoneal chemotherapy plus simultaneous versus staged cytoreductive surgery for gastric cancer with occult peritoneal metastasis. J Surg Oncol. (2015) 111:840–7. doi: 10.1002/jso.23889
49. Thiels CA, Ikoma N, Fournier K, Das P, Blum M, Estrella JS, et al. Repeat staging laparoscopy for gastric cancer after preoperative therapy. J Surg Oncol. (2018) 118:61–7. doi: 10.1002/jso.25094
50. Chen XJ, Wei CZ, Lin J, Zhang RP, Chen GM, Li YF, et al. Prognostic significance of pd-L1 expression in gastric cancer patients with peritoneal metastasis. Biomedicines. (2023) 11(7):2003. doi: 10.3390/biomedicines11072003
51. Qi C, Chong X, Zhou T, Ma M, Gong J, Zhang M, et al. Clinicopathological significance and immunotherapeutic outcome of claudin 18.2 expression in advanced gastric cancer: A retrospective study. Chin J Cancer Res = Chung-kuo yen cheng yen chiu. (2024) 36:78–89. doi: 10.21147/j.issn.1000-9604.2024.01.08
52. Malla RR, Nellipudi HR, Srilatha M, and Nagaraju GP. Her-2 positive gastric cancer: current targeted treatments. Int J Biol macromolecules. (2024) 274:133247. doi: 10.1016/j.ijbiomac.2024.133247
53. Rizzo A, Mollica V, Marchetti A, Nuvola G, Rosellini M, Tassinari E, et al. Adjuvant pd-1 and pd-L1 inhibitors and relapse-free survival in cancer patients: the mouseion-04 study. Cancers. (2022) 14(17):4142. doi: 10.3390/cancers14174142
54. Zhang XL, Shi HJ, Wang JP, Tang HS, Wu YB, Fang ZY, et al. Microrna-218 is upregulated in gastric cancer after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy and increases chemosensitivity to cisplatin. World J Gastroenterol. (2014) 20:11347–55. doi: 10.3748/wjg.v20.i32.11347
55. Su BC, Chen GY, Yang CM, Chuang WT, Lin MC, Hsu PL, et al. Impacts of hyperthermic chemotherapeutic agent on cytotoxicity, chemoresistance-related proteins and pd-L1 expression in human gastric cancer cells. Int J hyperthermia: Off J Eur Soc Hyperthermic Oncology North Am Hyperthermia Group. (2024) 41:2310017. doi: 10.1080/02656736.2024.2310017
56. Koemans WJ, van der Kaaij RT, Wassenaar ECE, Boerma D, Boot H, Sikorska K, et al. Tumor characteristics and clinical outcome of peritoneal metastasis of gastric origin treated with a hyperthermic intraperitoneal chemotherapy procedure in the periscope I trial. J Surg Oncol. (2021) 123:904–10. doi: 10.1002/jso.26366
57. Zhu L, Xu Z, Wu Y, Liu P, Qian J, Yu S, et al. Prophylactic chemotherapeutic hyperthermic intraperitoneal perfusion reduces peritoneal metastasis in gastric cancer: A retrospective clinical study. BMC Cancer. (2020) 20:827. doi: 10.1186/s12885-020-07339-6
58. Saladino E, Fleres F, Mazzeo C, Pruiti V, Scollica M, Rossitto M, et al. The role of prophylactic hyperthermic intraperitoneal chemotherapy in the management of serosal involved gastric cancer. Anticancer Res. (2014) 34:2019–22.
59. Fan B, Bu Z, Zhang J, Zong X, Ji X, Fu T, et al. Phase ii trial of prophylactic hyperthermic intraperitoneal chemotherapy in patients with locally advanced gastric cancer after curative surgery. BMC Cancer. (2021) 21:216. doi: 10.1186/s12885-021-07925-2
60. Jafari MD, Halabi WJ, Stamos MJ, Nguyen VQ, Carmichael JC, Mills SD, et al. Surgical outcomes of hyperthermic intraperitoneal chemotherapy: analysis of the american college of surgeons national surgical quality improvement program. JAMA Surg. (2014) 149:170–5. doi: 10.1001/jamasurg.2013.3640
61. Mi DH, Li Z, Yang KH, Cao N, Lethaby A, Tian JH, et al. Surgery combined with intraoperative hyperthermic intraperitoneal chemotherapy (Ihic) for gastric cancer: A systematic review and meta-analysis of randomised controlled trials. Int J hyperthermia: Off J Eur Soc Hyperthermic Oncology North Am Hyperthermia Group. (2013) 29:156–67. doi: 10.3109/02656736.2013.768359
62. Lam JY, McConnell YJ, Rivard JD, Temple WJ, and Mack LA. Hyperthermic intraperitoneal chemotherapy + Early postoperative intraperitoneal chemotherapy versus hyperthermic intraperitoneal chemotherapy alone: assessment of survival outcomes for colorectal and high-grade appendiceal peritoneal carcinomatosis. Am J Surg. (2015) 210:424–30. doi: 10.1016/j.amjsurg.2015.03.008
63. Hübner M, Kusamura S, Villeneuve L, Al-Niaimi A, Alyami M, Balonov K, et al. Guidelines for Perioperative Care in Cytoreductive Surgery (Crs) with or without Hyperthermic Intraperitoneal Chemotherapy (Hipec): Enhanced Recovery after Surgery (Eras®) Society Recommendations - Part Ii: Postoperative Management and Special Considerations. Eur J Surg oncology: J Eur Soc Surg Oncol Br Assoc Surg Oncol. (2020) 46:2311–23. doi: 10.1016/j.ejso.2020.08.006
64. Liesenfeld LF, Wagner B, Hillebrecht HC, Brune M, Eckert C, Klose J, et al. Hipec-induced acute kidney injury: A retrospective clinical study and preclinical model. Ann Surg Oncol. (2022) 29:139–51. doi: 10.1245/s10434-021-10376-5
Keywords: intraperitoneal hyperthermic chemotherapy, gastric cancer, advanced stage, survival analysis, peritoneal metastasis recurrence
Citation: Zhang T, Yu H, Wang L, Zhao S, Zhao C, Chai J and Wang D (2025) Evaluation of the feasibility, safety, and preliminary effectiveness of hyperthermic intraperitoneal chemotherapy following radical surgery for locally advanced gastric cancer. Front. Oncol. 15:1498388. doi: 10.3389/fonc.2025.1498388
Received: 18 September 2024; Accepted: 20 August 2025;
Published: 09 September 2025.
Edited by:
Birendra Kumar Sah, Shanghai Jiao Tong University, ChinaReviewed by:
Nikolaos Benetatos, University of Patras, GreeceRun-Cong Nie, Sun Yat-Sen University Cancer Center (SYSUCC), China
Mahmoud Elshenawy, University of Menoufia, Egypt
Copyright © 2025 Zhang, Yu, Wang, Zhao, Zhao, Chai and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Chai, amNoYWlAc2RmbXUuZWR1LmNvbQ==; Dehai Wang, d2FuZ2RlaGFpMjAyNEAxMjYuY29t
†These authors have contributed equally to this work
 Tianze Zhang1†
Tianze Zhang1† Lang Wang
Lang Wang Shijun Zhao
Shijun Zhao Cheng Zhao
Cheng Zhao Jie Chai
Jie Chai Dehai Wang
Dehai Wang