- 1Stem Cell and Cancer Biology Laboratory, School of Medical Science, University of Hyderabad, Telangana, India
- 2Thakkar Consultancy Services, Janjgir, Chhattisgarh, India
- 3Environmental Biotechnology and Genomics Division (SEP-EB), Council of Scientific and Industrial Research-National Environmental Engineering Research Institute (CSIR-NEERI), Nagpur, Maharashtra, India
- 4L V Prasad Eye Institute, Hyderabad, Telangana, India
A cancer stem cell (CSC) is an immortal cell that is capable of self-renewal, continuous proliferation, differentiation into various cancer cell lineages, metastatic dissemination, tumorigenesis, maintaining tumor heterogeneity, and resistance to conventional treatments. Targeted therapies have made huge advances in the past few years, but resistance is still a major roadblock to their success, in addition to their life-threatening side effects. Progressive treatments are now available, including immunotherapies, CRISPR-Cas 9, sonodynamic therapy, chemodynamic therapy, antibody–drug nanoconjugates, cell-based therapies, gene therapy, and ferroptosis-based therapy, which have replaced surgery, chemotherapy, and radiotherapy for cancer treatment. The challenge is to develop targeted treatment strategies that are effective in eradicating CSCs, as they are resistant to anticancer drugs, causing treatment failure, relapse, and recurrence of cancer. An overview of the fundamental characteristics of CSCs, drug resistance, tumor recurrence, and signaling pathways as well as biomarkers associated with their metastatic potential of CSC is elucidated in this review. The regulatory frameworks for manufacturing and conducting clinical trials on cancer therapy are explicated. Furthermore, we summarize a variety of promising nanocarriers (NCs) that have been used directly and/or synergistic therapies coupled with the therapeutic drug of choice for the detection, targeting, and imaging of CSCs to surmount therapeutic resistance and stemness-related signaling pathways and eradicate CSCs, hence alleviating the limitation of conventional therapies. Nanoparticle-mediated ablation therapies (NMATs) are also being argued as a method for burning or freezing cancer cells without undergoing open surgery. Additionally, we discuss the recent clinical trials testing exosomes, CRISPR/Cas9, and nanodrugs, which have already received approval for several new technologies, while others are still in the early stages of testing. The objective of this review is to elucidate the advantages of nanocarriers in conquering cancer drug resistance and to discuss the most recent developments in this field.
1 Introduction
The American Cancer Society estimates that cancer is the second leading cause of death in the United States, and the number of cancer cases is projected to increase to 2,041,910 in 2025, with cancer deaths expected to reach 618,120 (1). CSCs are vicious and can be a significant contributing factor to cancer treatment failure (2). Cancer stem cells (CSCs) are a small subset of cancer cells with regeneration capabilities and excessive tumorigenic potentials, involved crucially in tumor growth, progression, invasion, and metastasis (2, 3). Tumors are heterogeneous and contain both differentiated tumors and undifferentiated cancer stem cells (4). Numerous conventional modalities including surgery, chemotherapy, and radiotherapy are available for treating a wide range of malignancies (5, 6). Studies have revealed that CSCs have inherent drug resistance to conventional modalities as well as developmental plasticity (7, 8), allowing CSCs to differentiate into mature progeny (9). Moreover, differentiated cancer cells can undergo a stem cell-like transformation (10). Conventional treatments primarily target the tumor but often fail to eliminate drug-resistant CSCs due to the overexpression of anti-apoptotic proteins, ATP binding cassette (ABC) transporters, enhanced DNA damage response, elevated DNA repair, increased survival signaling, epithelial–mesenchymal transition (EMT) induction, epigenetic mechanism, hypoxia and low reactive oxygen species (ROS) level (11, 12), increased quiescence, increased autophagy, detoxifying enzymes (ALDH1), and signaling pathways (Wnt/β-catenin, Notch, hedgehog, Hippo, and PI3K/Akt, JAK/STAT), which led to drug resistance and tumor recurrence (12–15). The tumor tissue has an extracellular pH of 6.8 (acidic), favoring metalloproteinases, activating several signaling pathways, and serving as a blockade for many anticancer drugs that accentuate the malignancy and aggressiveness of cancer cells (16–18). Despite the availability of many treatment options, resistance to treatments still occurs, causing the cancer to recur, a phenomenon explained by CSC, imposing an innovative outlook for cancer treatment (11, 14).
The nanocarriers (NCs) used in cancer treatments are usually in the range of 20–200 nm, allowing them to circulate more quickly and absorb more readily into cells (19, 20). By virtue of their enhanced permeability and retention (EPR) effect, these NCs passively extravasate leaky tumor vessels and accumulate in tumors (21), allowing medications to be delivered to cancer cells and avoiding contact with healthy cells (21, 22). NCs are on the horizon as a novel breakthrough in targeted therapy. They provide altered therapeutic possibilities over conventional approaches and are impeccably able to modulate drug delivery and accumulate at target sites specifically to treat tumor-targeting CSCs (23–26). Biomedical researchers have increasingly embraced nanotechnology over the past decade and focused on the nanomaterial-loaded drug delivery (NDD) strategy targeting CSCs based on their markers (27), hence perceived by means of cell imaging, immunotherapy, multimodal synergistic therapies, siRNA delivery, and targeted cancer therapy (28–30). NDD via endocytosis bypasses the efflux pump, resulting in intracellular accumulation in CSCs (31, 32). The co-delivery of anticancer drugs, multiple drug resistance modulators, and CSC-targeting ligands using NDD could boost the specificity of CSC to surmount drug resistance (33–35). In addition to providing a comprehensive understanding of CSCs, our goal is to present a summary of recent cancer nanotherapies, both basic and applied, as well as new treatments that are currently being researched and hoped to overcome conventional treatment limitations. As innovative anticancer strategies, different approaches to diagnosis and therapy will be discussed, highlighting their current status in the clinical context. In this review, we introduce the treatment modalities, involving drug-loaded inorganic NCs, antibody–drug conjugates polymer-based NCs, self-assembling protein NCs, exosomes, and MXene, which have been reported to interact with tumor-associated stem cells, as well as with CSC-related signaling pathways, and are being used as diagnostic and therapeutic agents. The review describes the advances in technologies to reduce CSCs, including photothermal therapy (PTT) and nanoparticle-mediated ablation therapies (NMATs), and bioengineered exosomes’ role in antitumor therapies in order to encounter the prevailing complications of therapy resistance. Understanding the merits and limitations of these treatments offers new perspectives for clinical practice and groundbreaking research.
2 Cancer stem cells
2.1 Characteristics
A number of studies suggest that CSCs not only are responsible for tumor growth, maintenance, and resistance to chemotherapy and radiotherapy but also contribute to cancer recurrence after treatment since they can regenerate the tumor (10, 14, 36). However, non-CSCs are more differentiated and less likely to cause tumor growth or recurrence (37). CSCs may be resistant to therapy by activating survival pathways, remaining in quiescent states, increasing drug efflux, impairing apoptosis, and repairing DNA damage more efficiently (11, 12). A significant feature of CSCs is their capability to modify the surrounding stroma by secreting proteins and molecular components such as extracellular matrix (ECM) proteins, which helps in maintaining the CSCs in a dormant state to regulate their fate, plasticity, and resistance against conventional therapies (37–39). Their self-renewal capacity can lead to uncontrolled differentiation with transformed cellular and molecular phenotypes, resulting in the formation of heterogeneous primary and metastatic tumor cells that are resistant to treatment and contribute to tumor recurrence (38, 40). The major characteristics of CSCs include (2, 41–45) the following:
● self-renewal and differentiation properties,
● presence of specific surface markers for identification,
● ability to generate after transplantation,
● resistance to chemotherapy and radiotherapy,
● initiation of a new tumor through pre-existing CSCs,
● altered expression of transcription factors, receptors, and signaling pathways,
● ability to divide symmetrically into two CSCs or one CSC and one daughter cell,
● ability to thrive in hypoxic microenvironments,
● plasticity, which is the ability to adapt to new environments following phenotypic transition, and
● increased mobility, migratory, and invasive properties.
2.2 Chemoresistance of drug and self-renewal ability
The Darwinian notion of survival of the fittest applies to cancer cells attaining drug-resistant traits at molecular levels for survival (12, 39, 46, 47). Numerous in vitro and in vivo examinations have shown that conventional therapies induce CSCs, which later contribute to tumor relapse and therapy resistance (48). CSCs depend on multiple pathways for chemoresistance and self-renewal (10, 49). Thus, targeting these pathways can guide us to a strategic mechanism to overcome resistance. Notch1 signaling plays a key role in enhancing trastuzumab resistance in breast cancer cell lines BT474, SK-BR3, and MCF-7 cells; its inhibition, either genetic or pharmacological, enhances the sensitivity of these cells to the drug, i.e., making them more responsive to the drug’s effect (12). The Notch activity was boosted in both bulk and breast cancer stem-like cells in ER+ and HER2+ breast cancer cell lines upon treatment with tamoxifen or trastuzumab drugs, respectively (50). Knocking down Notch triggers significant growth arrest in these cells, leading to loss of stem-like characteristics such as self-renewal, tumor recurrence, resistance to drugs, and EMT (50). Significant molecular alteration was observed in breast cancer upon treatment of γ-secretase inhibitors, i.e., tamoxifen or letrozole, i.e., an aromatase inhibitor (reversible non-steroidal imidazole-based inhibitor) (12). As a first-line treatment for glioblastoma multiforme (GBM), humanized monoclonal anti-VEGF antibodies (bevacizumab) were effective in reducing tumor formation (12). The clinical benefit, however, lasted for a short time due to the development of resistant lineages and the dominance of VEGF-VEGFR2-Neuropilin-1 autocrine signaling over time, resulting in tumor relapses (51, 52). Glioblastoma CSCs (CD133+/Prominin-1) induced by radiotherapy can increase resistance by activating DNA checkpoints and repair pathways. Therefore, co-treatment with checkpoint inhibitors (Chk1 and Chk2) and radiotherapy increased the radiosensitization of glioblastoma CSCs (53). CSCs often confiscate pluripotent or oncofetal drivers, as they share critical features of embryonic stem cells for the expression of transcriptional factors such as SALL4, NANOG, KLF4, MYC, OCT4, and FOXM1 and signaling pathways such as Hedgehog, Notch, Hippo, Wnt/β-catenin, and TGF-β (54, 55). Lin28B (RNA-binding proteins that affect stem cell maintenance, metabolism, and oncogenesis) has been identified as an oncofetal circulator CSC marker and a crucial therapeutic target for hepatocellular carcinoma recurrence (56, 57). These oncofetal stem cell markers are not expressed by normal stem cells, so they serve as prime targets for therapy (12). Multiple cellular processes such as increased DNA damage and repair, entering into a dormant state, quick drug efflux, and anti-apoptotic protein overexpression, are mechanisms that lead to drug resistance (58, 59). Therefore, the removal of CSCs has become a prime target among the scientific fraternity. Nanocarriers have been proven a promising tool to deliver chemotherapeutic drugs at high dosages and release them to their target to control the CSCs, leading to overcoming the resistance and recurrence of CSCs (58).
2.3 CSC markers and challenges encountered in biomarker identification
Different cancers have distinct molecular and genetic profiles, which influence the markers expressed by CSCs (60). CSC markers mimic those of normal stem cells, resulting in difficulties in differentiated and targeted CSCs. CSC markers express differently in diverse microenvironments including inflammation, hypoxia, and cell–cell interaction, prompting the CSC features and marker expression (61). CSCs are also capable of sustaining genetic and epigenetic changes, which can alter their marker properties, depending on the mutational tumor sites and their evolutionary pathway (62, 63). In order to ensure a successful therapy, somatic stem cells (SSCs) should not experience any side effects; if we understand how CSCs and SSCs differ in their origin, self-renewal mechanism, and signaling pathways, we will be able to target CSC populations more effectively, protecting healthy cells and minimizing side effects.
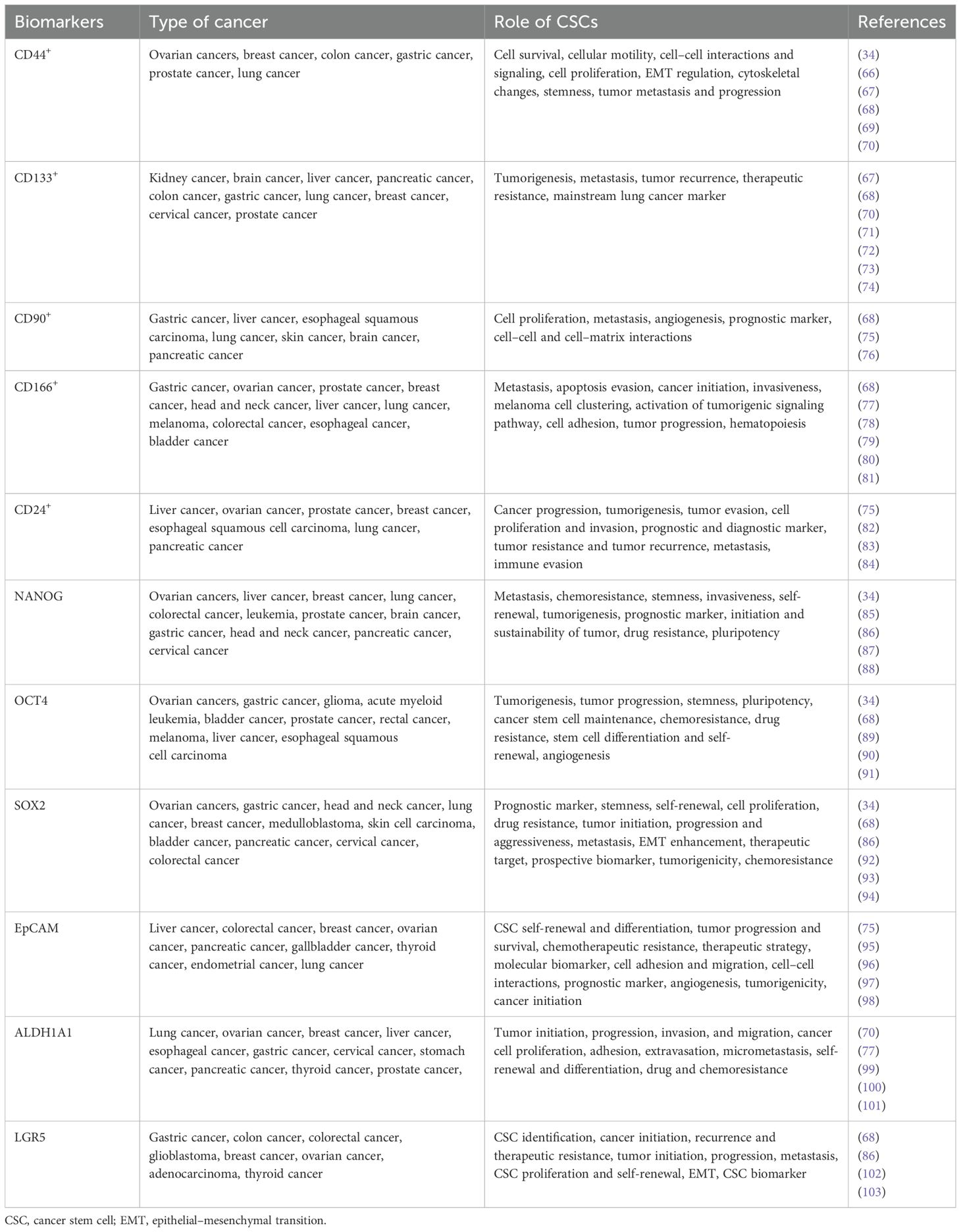
CSC targeting strategies have proved to be difficult due to phenotypic plasticity in tumors, which allows non-CSCs to acquire CSC traits, complicating CSC targeting strategies (39, 64). This necessitates the use of specific cell surface markers detected on CSCs for better results (38). Different markers can be expressed by CSCs depending on the tissue from which they originate (4, 11). Some of the markers associated with CSCs are cell surface markers, signaling pathways, transcription factors, and drug transporters, as well as genes, proteins, enzymes, and miRNA, which are responsible for self-renewal, immune evasion, metastasis, and treatment resistance (65) (Table 1, Figure 1A). The CSC-specific surface markers include CD24, CD26, CD44, CD133, CD166, aldehyde dehydrogenase (ALDH), and Ep‐CAM (also called CD326 or epithelial‐specific antigen/ESA) (61, 111, 112). The markers like CD24, CD34, CD44, CD133, CD166, and ALDH1 were used for the identification of CSCs in solid bulk tumors (61, 112). Common stem cell markers include CSC-specific markers such as CD34, CD44, CD123, CD133, c-kit, ABCG2, and ALDH, which have been reported in a wide range of malignancies (113, 114). CSCs are also intrinsically regulated by stemness-related transcription factors, such as OCT-4, SOX2, KLF4, c-MYC, STAT3, and NANOG, as well as epigenetics and epi-transcriptomics, which are important for stemness maintenance and plasticity (112). Figure 1A presents markers specific to cancer stem cells in different types of cancer (13, 104–110), and these markers are primarily useful for targeting CSCs for therapeutic purposes.
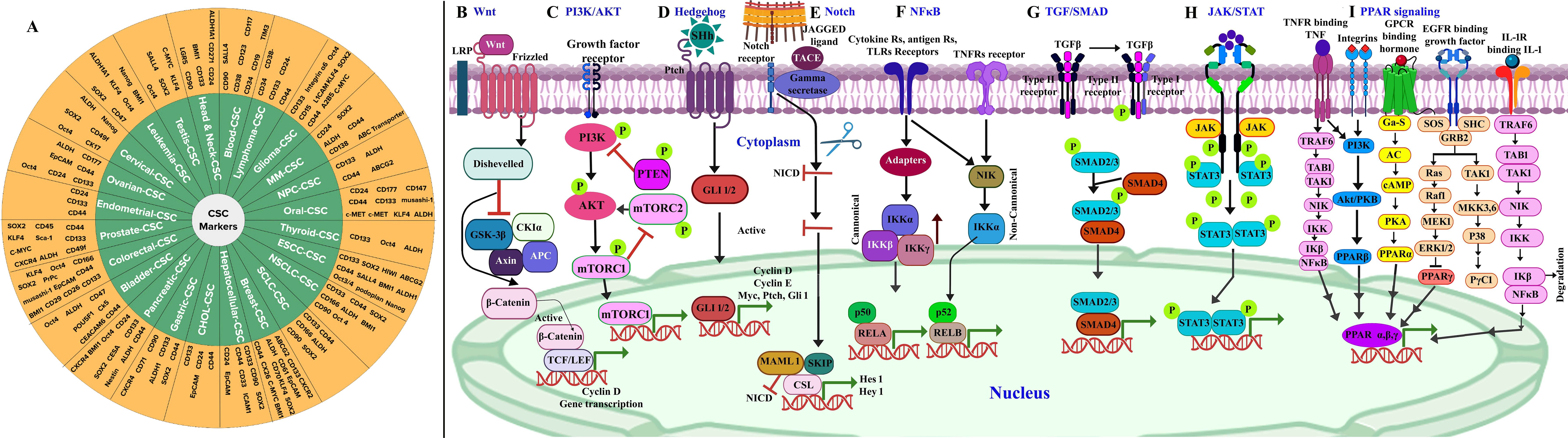
Figure 1. (A) Cancer stem cell markers in different types of cancer (13, 104–110). A schematic illustration of signaling pathways involved in cancer stem cells. (B) Wnt/β-catenin: targets include Wnt/Frizzled complexes, β-catenin/TCF, and CK1α. (C) PI3K/AKT: targets include PI3K complex, AKT1/2/3, and mTORC1/2. (D) Hedgehog: targets include SHh-Ptch interaction, SMO, and GLI. (E) Notch: targets include Notch and γ-secretase. (F) NF-κB: targets include IKKα/β/γ and NF-κB-inducing kinase (NIK). (G) TGF/SMAD: targets include TGF-β1/β2/β3, TβRI/II, and Smad3/4/5. (H) JAK/STAT: targets include JAK1/2/3 and STAT1/2/3/4/5. (I) PPAR: targets include PPARα/γ/δ signaling pathways. Adapted and modified using BioRender for illustrative purposes with permission from Chu et al. (2) (Copyright 2024).
2.4 CSC signaling pathways and FDA-approved drugs as inhibitors
The recurrence of CSCs is due to their resistance to existing conventional therapies, along with their high potential for metastasis and invasiveness (115). CSC signaling pathways are aberrantly activated in cancer, which govern self-renewal, cell proliferation, invasion, metastasis, and angiogenesis (44). The CSC transformation from a normal cell is due to accretions of genetic alterations, tumor suppressor genes, epigenetic modification [including (epi)methylation, demethylation, mutations, and rearrangements in the stem/progenitor pool (niche) and differentiated cells], and tumor microenvironment stimulation through extracellular signals (61, 112) A new challenge in cancer treatment is selecting the signaling networks that facilitate self-renewal, proliferation, and differentiation in CSCs that regulate tumorigenesis process. Most common CSCs associated with oncogenic cascades comprise Wnt/β-catenin (Figure 1B), phosphoinositide 3 kinase (PI3K)/AKT/mTOR (Figure 1C), hedgehog (Figure 1D), Notch (Figure 1E), NF-κB (Figure 1F), TGF-β/SMAD (Figure 1G), JAK/STAT (Figure 1H), and peroxisome proliferator-activated receptors (PPARs) (Figure 1I) (61, 116, 117). The effectiveness of small molecule inhibitors in cancer treatment is still challenged by minimal and short response values/duration, systemic toxicity, CSC biomarkers, and drug resistance (118). Currently, the Food and Drug Administration (FDA) has approved approximately 88 small molecule inhibitors for the treatment of cancer after clinical trials (118). The inhibitors that underwent clinical trials and target major pathways are implicated in the CSC pathway (Figure 2).
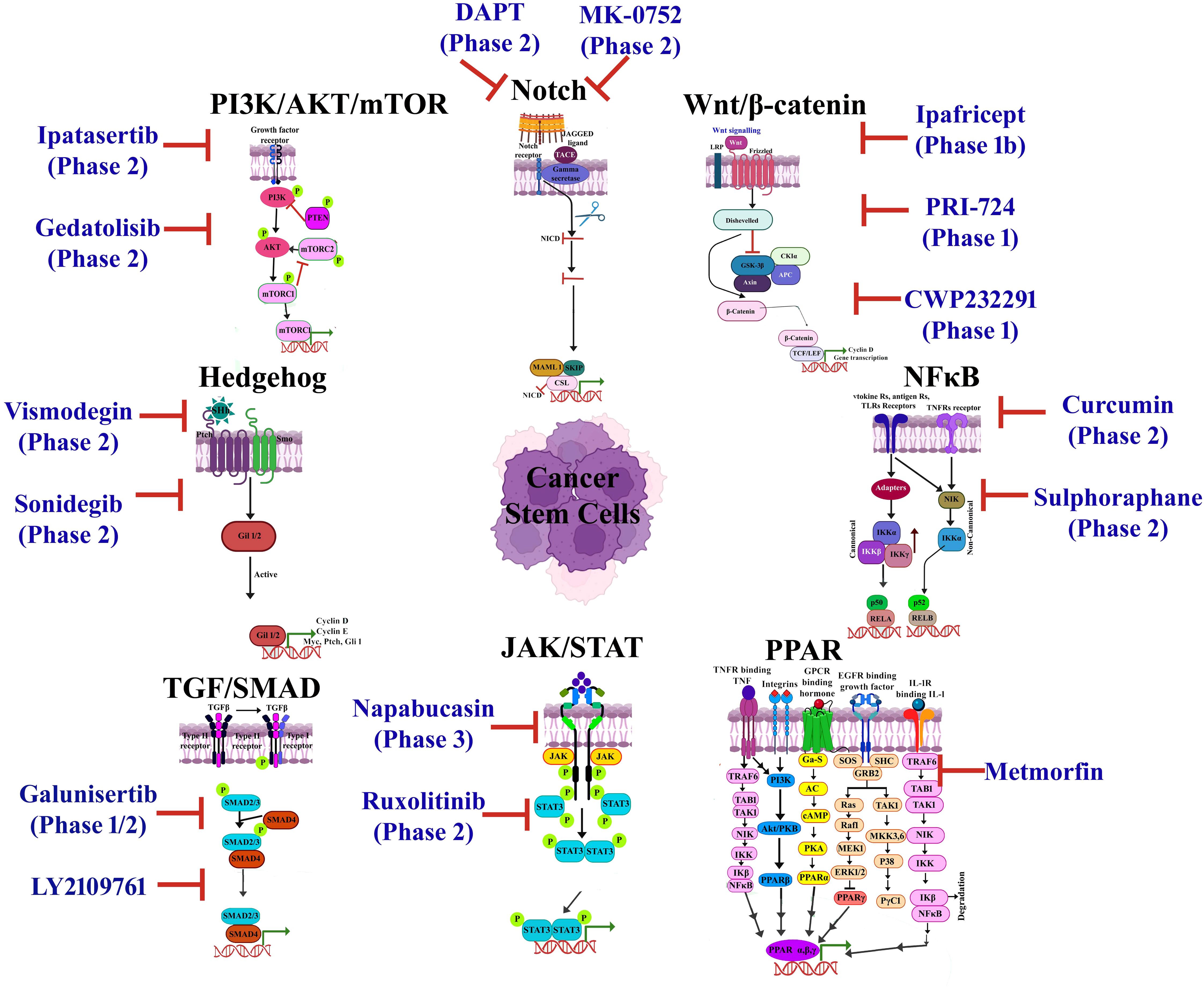
Figure 2. An overview of a few cancer stem cell (CSC)-targeted therapeutic agents that have been approved or are undergoing clinical trials. Adapted and modified with permission from PS Kharkar (Copyright 2020, American Chemical Society) (45, 119–123).
2.4.1 Wnt/β-catenin
The Wnt/β-catenin signaling cascade plays a key role in CSC biology, leading to self-renewal, uncontrolled cell proliferation, and differentiation (124). The dysregulation in Wnt/β-catenin signaling has been documented in a wide range of malignant cancers such as leukemia, colon, epidermal, breast, and cutaneous carcinoma (125, 126). Numerous methodologies have been upgraded for targeting Wnt/β-catenin cascade involving small molecules (ICG-001, PRI-724, E7386), which inhibit the interaction between TCF/LEF1 and β-catenin, thereby interrupting self-renewal property of CSC (124). Recent investigations used monoclonal antibodies (mAbs) against Wnt ligands and their subsequent receptors as a target for CSC-based therapy (127). The Wnt/B catenin signaling pathway targets a wide range of small molecules, including Wnt974, Wnt-C59, ONC201, Niclosamide, XAV939, Chelerythrine, FH535, IWR-1, IC-2, JIB-04, DTX and SFN, PP, OXT-328, AD, and Ts (Figure 2) (45, 128–135). Specifically, these compounds inhibit CSC progression/population, suppress self-renewal ability, attenuate CSC-mediated chemoresistance, and deregulate CSC markers and genes, resulting in drug resistance and compounding its sensitivity (45, 136–138).
2.4.2 Hh signaling
Hh signaling contributes significantly to various stages of cell development; mutation at any stage of the sonic hedgehog (Shh) pathway can lead to the advancement of numerous cancers such as melanoma, rhabdomyosarcoma, medulloblastoma, and basal cell carcinoma, as well as breast, pancreas, lung, liver, and prostate cancers (58, 139). Aberrant Hh signaling promotes CSC self-renewal and resistance to treatment and its hyperactivation (mutations/deregulation), which leads to tumorigenesis (41). Studies have shown that inhibiting the aberrantly active Hh pathway in non-small-cell lung cancer (NSCLC) using a Hh antagonist led to a significant reduction in cell viability and malignancy (140). CSC progression can be inhibited by small molecules including glasdegib, sonidegib, vismodegib, ciclesonide, cyclopamine, and GANT61 by suppressing Hh signaling (45, 64, 141–145) (Figure 2). These small molecules inhibit CSC marker expression, self-renewal and mammosphere formation, and CSC proliferation and survival (45, 64, 141–145).
2.4.3 Notch signaling
Notch signaling regulates cell-to-cell communication right from embryogenesis, cellular proliferation, differentiation, and even in apoptosis (146), also crucial for neural stem cell survival, immune regulation, colorectal epithelial maturation, breast development, and normal hematopoiesis (41). The Delta-like ligand 4 (Dll4) is one of the Notch signaling ligands that contribute to malignancy progression (147). There have been numerous reports of mutations of the Notch gene, including those of Dll4, which have been implicated in the growth of different types of gynecological tumors (148). Inhibitors of Notch act on N1ICD, γ-secretase, Hes-1, Hey-1, and Notch ligands to treat cancer and prevent recurrence (45, 149). Several pharmaceutical drugs, including MK-0752, PF-03084014, RO4929097, DAPT, and Quinomycin A (Figure 2) reduce the mammosphere formation, strike tumor regeneration, impede CSC growth, induce CSC differentiation, and decrease drug resistance in reverse, increasing drug sensitivity (45, 149–152).
2.4.4 TGF-β/SMAD
The tumor cells secrete interleukin-33 cytokine, which causes myeloid cell differentiation into macrophage and consequently stimulates TGF-β signals to reach cancer stem cells, resulting in the progression of malignant tumors and drug resistance (153). TGF-β serves as a significant target commonly for multiple malignant tumors (breast, lung, liver, colon, among others) and found to be involved in the initial developmental stage and maintenance of CSCs (154, 155). Few inhibitors that address the unmet clinical necessities in cancer immunotherapies targeting TGF-β/SMAD signaling pathway are galunisertib (LY2157299) (156), vactosertib (TEW-7197) (157), LY2109761 (158), LY3200882 (159), MDV6058 (PF-0695229), GFH018 (160), YL-13027 (157), AGMB-129 (ORG-129) (161), SH3051, Trabedersen (AP 12009) (157), fresolimumab (GC1008) (162), AVID200 (163), ABBV-151 (164), SRK-181 (165), and bintrafusp alfa (M7824) (166). The inhibitor shown in Figure 2 blocks the TGF-β/SMAD pathway (164, 167–172).
2.4.5 PPAR
The PPAR pathway activation (comprising PPARα, PPARδ, and PPARγ subtypes) involves the binding of G-protein-coupled receptors to its respective ligand, which leads to the induction of translocation of nuclear receptor protein PPAR responsible for gene expression (173–176). The PPARs are involved in cell proliferation modulation, apoptosis, cell survival (stimulatory or inhibitory effects on cancer progression), EMT process regulation, and stem cell-like properties of CSCs (116). PPARβ/δ also regulates tumor angiogenesis in vivo and in vitro in CSCs by the promotion of proangiogenic factors such as VEGF and interleukin-8 (IL-8) (177). PPARα and PPARβ/δ regulated CSCs for metabolic reprogramming in GBM, lung cancer, and mouse mammary gland carcinoma, suggesting its association with CSC metabolism (178). A clinical trial is being conducted with efatutazone and metformin, which target the PPAR signaling pathway (Figure 2) and have antiproliferative, anticancer stem cell activity, maintenance of chemosensitivity, apoptosis, and reverse chemotherapy resistance and reduce migration and metastasis of cancer cells (179, 180).
2.4.6 JAK/STAT
JAK/STAT signaling plays a crucial role in the development of multiple cancers and is directly associated with growth, metastasis, and progression whereas indirectly linked to the immune surveillance modulation and is activated during the recruitment and activation of JAK by the cytokine receptors (181, 182). The receptor tyrosine is then phosphorylated by JAK followed by the recruitment of STAT proteins (182, 183). The phosphorylation of STAT results in the translocation of its dimers to the cell nucleus for DNA binding to initiate the transcription of target genes (184). The JAK protein consists of JAK1–3 and Tyk2, whereas the STAT family comprises STAT5a, STAT5b, STAT1-4, and STAT6. In the case of high-grade gliomas, JAK1/2-STAT3 along with a hypoxia-induced pathway utilizing hypoxia-inducible factor 1α (HIF-1α) TF has been reported for enhancing the self-renewal capability of glioma stem-like cells (178, 181, 185). The antitumor molecules Pacritinib, fedratinib, tofacitinib, baricitinib, abrocitinib, filgotinib, oclacitinib, peficitinib, upadacitinib, deucravacitinib, and delgocitinib (186–193) have garnered interest as potential candidates for modulating the JAK/STAT pathway (Figure 2) and were found to be effective in reducing cell proliferation and viability, promoting apoptosis and obstructing invasion (189, 194).
2.4.7 PI3K/Akt
PI3K/Akt is an intracellular phosphatidylinositol kinase, while the mTOR pathway comprises a regulatory subunit p85 along with a catalytic subunit p110 having serine/threonine (Ser/Thr) kinase and phosphatidylinositol kinase (195, 196). The three isoforms of Akt (Akt1-3) are directly activated by PI3K (197). The mTOR complex is a downstream target gene with two multiprotein complexes (mTORC1 and mTORC2) (198, 199). mTORC2 phosphorylates the Ser473 residue of Akt (200), resulting in Akt activation (178). This pathway can be activated by various mechanisms including insulin-like growth factor (IGF)/IGFR, ErbB, and fibroblast growth factor (FGF)/FGFR signaling (116, 201). The PI3K/AKT/mTOR pathway is crucial for the growth of cancer cells, involved in the cell cycle, proliferation, quiescence, migration and invasion of CSCs, and therapeutic resistance (178, 202, 203). Clinical analysis of multiple small molecules dysregulating PI3K/AKT/mTOR pathway, including buparlisib (BKM120), pictilisib (GDC-0941), idelalisib, alpelisib (BYL719), serabelisib, taselisib (GDC-0032), gedatolisib (PF05212384), voxtalisib (SAR245409/XL765), MK2206, capivasertib (AZD5363), perifosine, uprosertib (GSK-2141795), aspirin, rapamycin, everolimus, temsirolimus, metformin, onatasertib (CC223), sapanisertib, and vistusertib (AZD2014) (204–207) (Figure 2) are found to reduce tumor progression and improve chemotherapy treatment efficacy (46, 47, 208–219).
2.4.8 NF-κβ
NF-κβ is a rapid inducible TF along with five different proteins, namely, RelB, NF-κβ1, NF-κβ2, p65, and c-Rel (220). The major physiological function of NF-κβ is p50-p65 dimer (221). The activity of NF-κβ complex is regulated by canonical and non-canonical signaling pathways (220, 222). Cytokines involved in tumor-promoting inflammation including TNF-α, IL-1, IL-6, COX2, iNOS, and MCP1, and factors like Cyclin E, Cyclin D, and proto-oncogen c-Myc are accountable for the activation of the NF-κβ pathway resulting in the cancer cell proliferation (160, 178, 223, 224). The NF-κβ pathway is involved in the stimulation, EMT, invasiveness, angiogenesis, apoptosis prevention, and metastasis of CSCs (160, 184). The NF-κB signaling cascade is reported to be targeted using ferulic acid, vanillic acid, curcumin, resveratrol, nobiletin, trilobatin, apigenin, cirsiliol, scutellarein, acacetin, chalcone 2, luteolin, anthocyanidin, ginsenoside Rg-3, chlorogenic acid, quercetin, dehydroxymethylepoxyquinomicin (DHMEQ), nepalolide A, and parthenolide (Figure 2), ensuring interruption in tumor growth and proliferation, considering low toxicity to healthy cells (224–239).
3 CRISPR/Cas9 technology for cancer therapy
CRISPR/Cas9 (Clustered regularly interspaced short palindromic repeat)/CRISPR-associated protein 9) is a revolutionary genome-editing tool that can be used to regulate endogenous gene expression by both gene insertion and knockout relying on the Cas9 protein and the guide RNA (gRNA), making it a very powerful and versatile tool (240, 241). The suppression of oncogenes or upregulation of tumor suppressor genes can improve targeted therapy by confronting drug resistance and improving immunotherapy with CRISPR/dCas9 (242). This tool has been used to treat a wide variety of cancers and has demonstrated prominent outcomes (243–246). Researchers using CRISPR/Cas9 technology have recognized novel genes for cancer treatment such as suppression of NAD kinase (NADK activates pentose phosphate pathway involved in cancer survival) or ketohexokinase (KHK suppression leads to elevated fructose metabolites intricate in liver cancer progression) and inhibit tumor growth (245). Chen and co-authors discovered that alisertib is more effective when HASPIN (histone H3-associated protein kinase) is inhibited through CRISPR/Cas9 in breast cancer (247). Protein-l-isoaspartate (d-aspartate) O-methyltransferase (PCMT1) promotes ovarian carcinogenesis through FAK-Src activation (248). Accordingly, many tumor-associated genes (oncogenes, drug-resistant genes, tumor suppressor genes, immune evasion genes, and metabolic reprogramming genes) are targeted through CRISPR-Cas9, for instance, KRAS, p53, EGFR, PTEN, Nestin, BRAF, HASPIN FGFR, FAK, BRCA gene, PIK3CA, VEGFR, HER2, LDHA, NADK, ALK, NOTCH1, PD-L1, ABCB1, TERT, and LGALS2 (190, 242, 245, 247–254). In 2016, Sichuan University’s West Society China Hospital recruited its first patient to test the effectiveness of CRISPR/Cas9 in cancer therapeutics (255). The use of CRISPR-edited T cells in a phase I clinical trial in patients with non-small-cell lung cancer has been demonstrated to be safe in human subjects with advanced non-small-cell lung cancer (256). The most significant barrier to clinical CRISPR/Cas9 applications is the lack of efficient and safe delivery systems (242). The delivery system must overcome many physical barriers, in addition to high encapsulation and biocompatibility, to deliver CRISPR/Cas9 components to the target, thereby attaining precise and effective treatment (242). There has been increasing attention to the application of non-viral vectors that rely on nanotechnology for anticancer cargo delivery (257). In addition to polymers, lipids, porous silicon, and mesoporous silica, have been used to treat different cancers because of their low immunogenicity, high biocompatibility, and ideal cargo delivery capabilities (242). Zhen et al. (258) injected nude mice with long-circulating pH-sensitive cationic liposomes targeted to splicing HPV16 E6/E7 cervical cancer cells, causing them to undergo apoptosis by inactivating them, thus inhibiting tumor growth without causing significant toxicity (258). In another study, multistage delivery nanoparticle (MDNP)/dCas9-miR-524 was administered to mice bearing MDA-MB-231 and LN-229 tumors, resulting in the significant upregulation of miR-524 expression (259). This upregulated expression then interferes with multiple signaling pathways associated with tumor proliferation, causing significant tumor growth retardation. MDNP was used to deliver CRISPR/Cas9, providing optimal efficiency in communicating with tumor tissues even in the face of multiple physiological barriers (259). Liu and colleagues discovered a nanoCRISPR system based on semiconductor polymers (SPs) that enables near-infrared (NIR) photoactivatable gene editing to advance the delivery proficiency of CRISPR and to improve cancer treatment effectiveness (259). This nanoCRISPR system can deliver sgRNA and generate heat using the photothermal effect when the NIR laser is irradiated (259). A localized heat event causes the dissociation of single-stranded DNA from single-stranded RNA to trigger sgRNA release, allowing precision cancer therapy using CRISPR (260).
3.1 Clinical trials of the CRISPR/Cas9 system for cancer therapy
We focused on published and ongoing clinical trials involving the CRISPR/Cas9 system’s capability of treating cancer. Phase I trials involving TALEN and CRISPR/Cas9 targeting HPV16 and HPV18 E6/E7 identifiers are underway to evaluate the safety and efficacy of the treatment for patients with HPV (+) CIN (261). In parallel, NCT04976218 specifies a phase I trial to evaluate CAR-EGFR-TGFR-KO T cells engineered through CRISPR/Cas9 to target TGF-β receptor II in previously treated EGFR-positive tumor cells (262). The CRISPR/Cas9 technology was used to knock out CD5 in CT125A cells (NCT04767308), a novel CAR T-cell therapy currently being tested in patients with relapsed/refractory CD5+ hematopoietic malignancies (263). The knockout of the PD-1 and TCCR genes using CRISPR/Cas9 was evaluated for safety, feasibility, in vivo persistence, and antitumor response in multiple solid tumor patients with mesothelin-positive cells (264). NCT03747965 is also associated with the CRISPR-engineered PD-1 gene knockout mesothelin-targeting CAR T-cell therapy for the treatment of neoplastic mesothelin-positive tumors in colorectal cancer (265). A trial evaluating the safety of PD-1 knockout T cells in patients with advanced esophageal cancer was completed and registered with identifier NCT03081715 (266). An alternative study identified as NCT05066165 aims to evaluate the activity and safety of NTLA-5001 in patients with acute myeloid leukemia following first-line or later treatment (267). NCT04035434 aims to investigate the safety and effectiveness of allogenic CRISPR/Cas9-engineered CTX110 T cells in patients with relapsed or refractory B-cell malignancies (268). C70-directed allogeneic CRISPR/Cas9-engineered CAR T-cell (CTX130) therapy in relapsed or refractory T-cell malignancies is being evaluated in another phase I study (NCT04502446) (269). Using premade allogeneic T cells from healthy donors (NCT05037669), a phase I study aims to evaluate the feasibility and safety of administering premanufactured allogeneic T cells that express CD19-targeting CAR knockouts targeting HLA class I, HLA class II molecules, and endogenous TCRs via CRISPR gene editing of beta-2 microglobulin, CIITA, and the T-cell receptor alpha chain (270). Phase I of the CTX120 study (NCT04244656) is evaluating the efficacy and safety of anti-BCMA-engineered T cells in patients with relapsed or refractory multiple myeloma (271). An open-label phase I study called COBALT-RCC (NCT04438083) will assess the efficacy, safety, and pharmacokinetics of CRISPR/Cas9-engineered T cells (CTX130) in patients with advanced, relapsed, or refractory renal cell carcinoma (272). NCT03166878 is a phase I/II study evaluating the efficacy and safety of UCART019 gene-edited allogeneic CD19-targeting CAR T cells in patients with relapsed or refractory CD19+ leukemia and lymphoma (273). Allogeneic gene-edited dual-specificity CD22, CD20, or CD19 CAR T cells are undergoing a phase I/II trial (NCT03398967) for treating patients with relapsed or refractory leukemia or lymphoma (274). Allogeneic TT52CAR19 T cells (NCT04557436), modified by CRISPR, are being studied in an open-label trial to treat relapsed or refractory CD19+ B-cell acute lymphoblastic leukemia in children (275). Trials utilizing CRISPR/Cas9-mediated CCR5 deletion of hematopoietic stem cells in HIV-1 and acute lymphoblastic leukemia patients have been partially successful (NCT03164137), which emphasizes a need for more efficient disruption of CCR5 in lymphocytes (276).
4 Regulatory landscape for nanodrug
Nanopharmaceutical development from the manufacturing to scale-up provisions may benefit from the 5R concept, which involves “right target/efficacy”, “right tissue/exposure”, “right patients”, and “right safety”, as proposed (277). Conventional drugs modified into nanoscales for targeted delivery can also be modified in terms of their pharmacokinetics, biodistribution, and toxicokinetic properties; as a result, they foster concerns over quality, safety, and efficacy (278). A number of regulatory authorities worldwide have developed guidelines/frameworks for nanopharmaceuticals in an attempt to ensure transparent, consistent, and predictable regulatory pathways considering safety as well as toxicity (279). Regulatory agencies in their respective jurisdictions include the US FDA, European Medicines Agency (EMA), and Central Drugs Standard Control Organisation, India (CDSCO) (278). The agencies have established guidelines for clinical trials, dossier submissions, and pharmacovigilance as a means of protecting public health (280). Participation by the US FDA is envisioned to establish a science-based approach to the regulation of nanomaterial-based products, build regulatory science knowledge, and facilitate the practice of nanomaterials in regulatory agencies (281). A number of nanodrugs have been developed and approved by the US FDA in collaboration with the National Nanotechnology Initiative (NNI) and Nanotechnology Characterization Laboratory (NCL), which may advance effectiveness and safety measures (281, 282). The EMA is also working to develop regulatory guidelines for the evaluation of nanomedicine products with the European Technology Platform on Nanomedicine (ETPN) and the European Nanomedicine Characterisation Laboratory (EU-NCL) (283). Nanotechnology products are regulated by the FDA and EMA as part of the Innovation Task Force (ITF), an international, multidisciplinary group that includes precise, regulatory, and legal expertise (283). India’s national regulatory authority oversees drug approvals and post-marketing surveillance through the CDSCO, an agency under the Ministry of Health & Family Welfare (284). Nanodrugs approved by the FDA for cancer treatment have different targets including protein synthesis, DNA damage, immunostimulation, microtubule, and hormone inhibition (284). The approved drugs include lipid-based nanoformulation metallic nanoparticles, polymer–drug conjugate drug-targeted antibodies, recombinant viruses, and herbal nanoparticles (285). The FDA- or EMA-approved drugs are DaunoXome®, Marqibo®, Doxil®, Aurimmune®, AuNPs®, Eligard®, SMANCS, Kadcyla®, Ontak ®, Gendicine®, Abraxane®, and nanoformulated curcumin (278, 284, 286). An overview of some FDA-approved nanodrugs is depicted in Table 2.
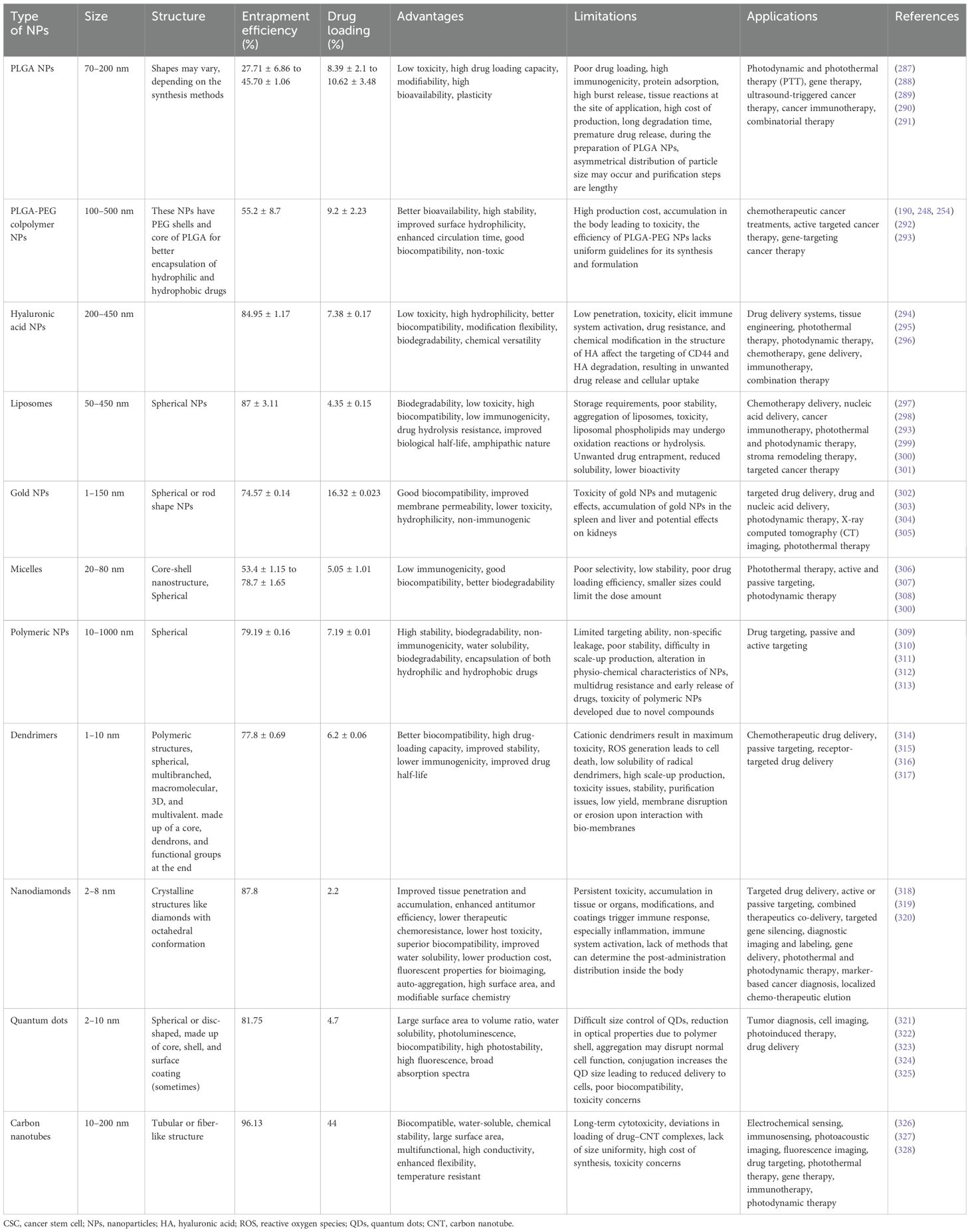
Table 2. An overview of different types of nanocarriers targeting CSC-specific markers/pathways and the characteristics of their shapes, sizes, and loading abilities, along with applications, advantages, and limitations of nanotherapeutic strategies.
5 Nanocarrier-mediated drug delivery to CSCs
Nanocarrier used to treat CSCs specifically offers great possibilities. CSC targeting nanocomposite is premeditated based on the notion of ligand–receptor interaction, as tumor tissues express numerous biomarkers distinctively from normal tissues (329–331). The common surface markers between CSCs and normal stem cells protect the latter from the damaging effects of chemotherapeutic agents, despite their similarity in surface markers (10). In addition to enhancing drug accumulation in CSCs, it also protects normal stem cells from therapy-based side effects (332). Compounding the drugs has another advantage in eliminating CSCs due to retrogressive drug resistance, constrained self-renewal, and promotion of differentiation (333, 334). Nanocarriers are colloidal systems with particle sizes below 1,000 nm (335) and were customized in the range of 10 and 200 nm (mainly for drug delivery) (336) to allow the NCs entry into blood vessels within the tumor (123). NCs are modulated with ligands using peptides, antibodies, small molecules, immunotherapeutics, and chemotherapeutics as well as natural polysaccharides that target receptors of CSCs displaying specific binding efficiency and subjected to both preclinical and clinical studies (337) (Table 3). A schematic illustration of the ability of ligand-modified NC to target cancer cells is shown in Figure 3. A wide range of nano-vehicles mostly enter the cytoplasm over the nucleus where anticancer drugs are highly effective (375). A drug molecule internalizing in the cytosol is unlikely to interact with a subcellular target; therefore, nanoparticle design and optimization are essential to allow cellular/nuclear targeting (376). As a result of leaky tumor vasculatures and poor lymphatic drainage, nanoparticles are more likely to accumulate in tumors than in normal tissues (377). A passive targeting strategy relies on the EPR effect, while active targeting includes targeting tumor cells via ligand-modified nanocarriers that interact with specific receptors (378). Numerous efficient ligands including folic acid, aptamers, hyaluronic acid, biotin, transferrin, peptides, antigens, antibodies, siRNA, small molecules, and FDA-approved drugs (378) have been widely discovered for selective cancer cells and CSCs, reducing localized toxicity, modulating tumor microenvironment, and overcoming blood–brain barrier and drug resistance (Figure 3). Kim et al. designed a dual-molecule liposome loaded with doxorubicin and DNA aptamers for the differentiation and targeting of breast tumor cell spheroids and CSCs; one aptamer targets the surface marker mucin 1 antigen (MUC1; a transmembrane glycoprotein) on breast tumor cells, while the other targets the CSC marker glycoprotein CD44 antigen (379). Cho examined CBP4, a small peptide that exhibits an affinity for CD133, a biomarker of glioblastoma cancer stem cells (380) conjugated with gold nanoparticles demonstrating fluorescent signals, was used in glioblastoma imaging and diagnosis. Another study found that topoisomerase inhibitor SN-38 conjugated nanoparticles packed with anti-CD133 antibodies bound efficiently to overexpressing CD133 cells (CD133Ab-NP-SN-38) in HCT116 colon cancer cells (381), showing cytotoxicity and inhibiting colony formation when compared with non-targeted nanoparticles (NP-SN-38) (381). An in vivo study in HCT116 xenograft nude mice (Figures 4A–D) demonstrated that CD133Ab-NP-SN-38 inhibited tumor growth and delayed tumor recurrence (381). Researchers have developed a polymeric chitosan-coated nanoparticle encapsulated with doxorubicin capable of binding specifically to CD44 receptors, thereby eliminating CD44+ cancer stem-like cells and reducing tumor size and cytotoxicity without causing systemic toxicity (382).
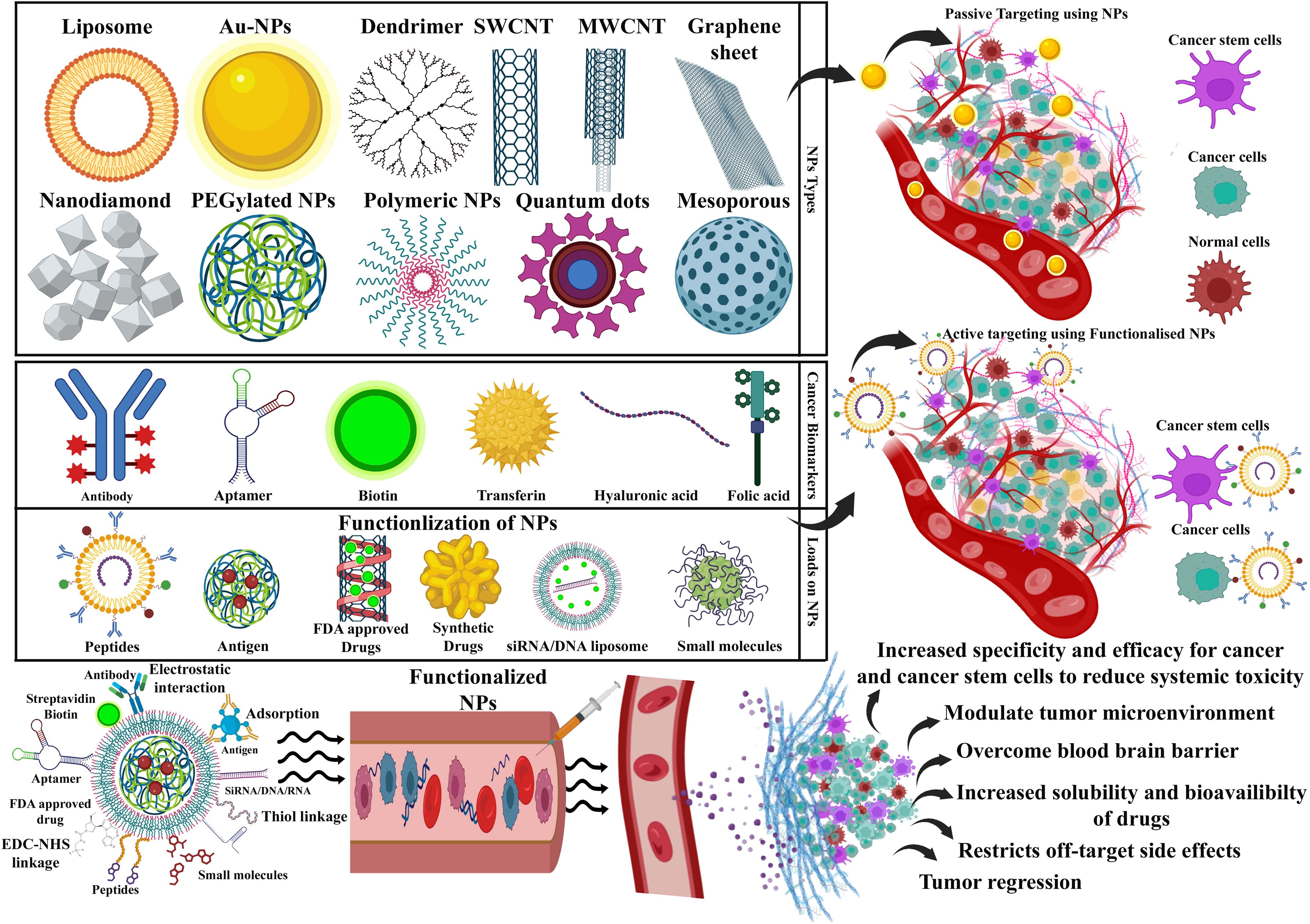
Figure 3. A schematic diagram showing that an array of nanocarriers modified with targeting ligands can, however, specifically attach to tumor cells’ receptors, permitting localized drug delivery or endocytosis. An illustration of active and passive targeting in antitumor nano-delivery systems. Passive targeting is achieved by delivering nanocarriers into tumor tissues via leaky tumor blood vessels, where they accumulate due to enhanced permeability and retention (EPR) effects. Illustration showing the ability of targeted cancer cells to absorb nanocarriers and their accumulation in tumors that exhibit tumor suppression. Image created with BioRender.com.
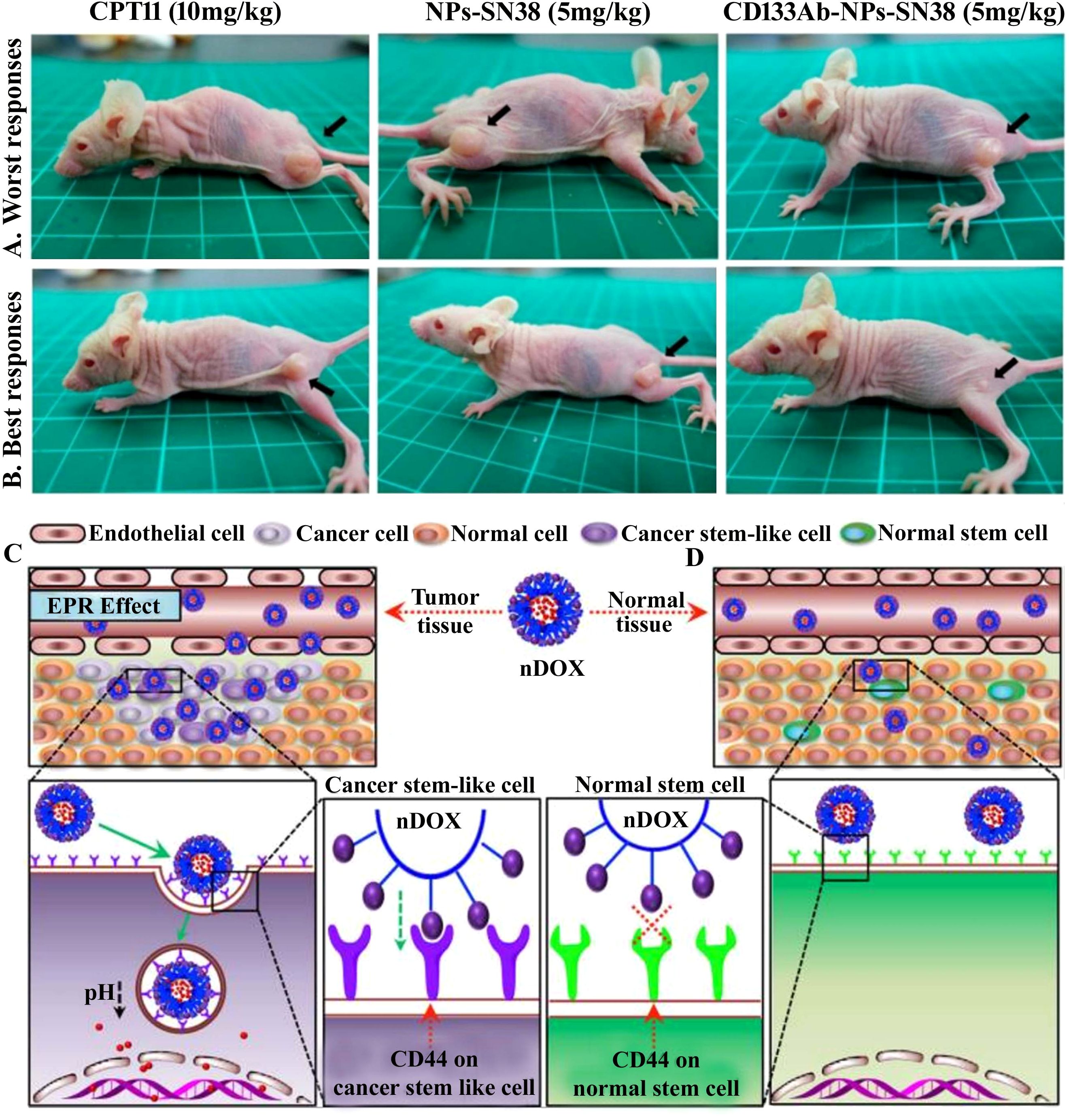
Figure 4. (A, B) CD133-positive (CD133+) cell was targeted with topoisomerase inhibitor SN-38-loaded nanoparticles conjugated with anti-CD133 antibody to resolve chemotherapy failure. HCT116 overexpress CD133 glycoprotein, which was efficiently bound by anti-CD133 antibody-conjugated SN-38-loaded nanoparticles (CD133Ab-NP-SN-38) demonstrated by the in vivo study. The tumor size depiction in mice treated with CPT11 (irinotecan, DNA topoisomerase I inhibitor) as control group, SN-38 nanoparticles (NPs), and CD133Ab-SN38 NPs in HCT116 xenograft model. This CD133Ab-NP-SN-38 combination thwarted tumor growth and hindered recurrence in xenograft model. Reprinted with permission from Ning et al. (381) (Copyright 2016, American Chemical Society). (C) CD44-overexpressing breast cancer stem cells (CSCs) were eradicated via chitosan-modified poly(ethylene glycol) (PEG)–poly(propylene glycol) (PPG)–PEG micelle crosslinking loaded with doxorubicin (DOX) in comparison with the free DOX application. DOX-loaded micelles facilitated increased DOX cytotoxicity on cancer stem cell (CSC)-expressing MCF7 breast tumor mouse model exhibiting CD44+ overexpression by six times compared to (D) normal tissue. Enhanced permeability and retention (EPR) effect of conjugated nanoparticles caused them to accumulate in tumor tissues more than they did in normal tissues. In addition, no noticeable systemic side effects were observed. Reused for illustrative purposes with permission from Rao et al. (382) (© 2015, American Chemical Society).
5.1 PLGA NCs
Poly(lactic-co-glycolic acid) (PLGA) is widely employed for the preparation of drug-loaded NCs due to its biodegradable properties and several applications in biomedical compounds (155). PLGA NCs are employed as a paclitaxel carrier in the case of ovarian cancer stem cells (378, 383). PEGylated poly(lactic-co-glycolic acid) carriers containing salinomycin (SAL-NP) and CD133 aptamers (Ap-SAL-NP) efficiently stopped the progression of CD133+ osteosarcoma cancer stem cells (384). Jin et al. demonstrated that GE11 peptides conjugated with PLGA NCs can deliver the conjugated anticancer agent, curcumin, to cells expressing EGFR receptor (EGFR) in vitro and in vivo (385). When these curcumin-loaded NCs were applied to breast cancer cells and tumor-bearing mice, the signaling of phosphoinositide 3-kinase was reduced, cancer cell viability was diminished, drug clearance from the bloodstream was attenuated, and tumor growth was reduced (385). After being delivered in the form of GE11-Cur-NPs, Cur rapidly accumulates within MCF-7 cells, suggesting active receptor-mediated endocytosis as well as passive uptake through the cell membrane (385). Pancreatic CSCs are inhibited by anthothecol-encapsulated PLGA NCs (Antho-NCs) through the inhibition of the sonic hedgehog pathway (386). Antho-NCs established show a therapeutic role demonstrated by reduced cell motility, migration, and invasion by upregulating E-cadherin and obstructing N-cadherin and Zeb1 (386). The antagonistic effect of Antho-NCs on pluripotency-maintaining factors and stem cell markers indicates that they are blocking CSC generation, disrupting Gli binding to DNA, and inhibiting Gli transcription (386). The dual inhibition of AKT and mTOR by nimbolide-loaded PLGA nanocarriers induces mesenchymal-to-epithelial transition in pancreatic cancer stem cells (387).
5.2 PLGA-PEG copolymer NCs
PLGA-PEG has been employed for the simultaneous delivery of various chemotherapeutic drugs for colorectal cancer therapy and lung cancer treatment (254). Dhar et al. (388) created prostate-specific membrane antigen (PSMA) targeting NCs using Pt(IV)-encapsulated PLGA–poly(ethylene glycol) (PEG)-functionalized controlled-release polymers for targeting cisplatin delivery to prostate CSCs (388). FA-modified NCs encapsulating CDDP and paclitaxel (PTX) exhibited superior targeting and antitumor efficacy against M109 cells (389). Cisplatin-encapsulating maleimide-polyethylene glycol-poly(d,l-lactic-co-glycolide) (mal-PEG-PLGA) in synergy with a CD44 monoclonal antibody produced via electrospray technique was effective at inhibiting ovarian cancer cell proliferation compared with cisplatin in free form and PLGA without CD44-conjugated NPs (390). Core-shell NCs fabricated using double emulsification of an amphiphilic copolymer, methoxy poly(ethylene glycol)-poly(lactide-co-glycolide) (mPEG-PLGA), were employed for simultaneous delivery with hydrophilic doxorubicin (DOX) and hydrophobic paclitaxel (TAX) (391). Despite the same concentrations of DOX and TAX, NCs suppressed tumor cell growth more efficiently than both on their own in A549, B16, and HepG2 cells (391). The authors suggested that DOX intercalates DNA, thereby interfering with transcription, which interrupts tubulin synthesis. The treatment also degrades microtubules, subsequently reducing microtubule content in tumor cells (391).
5.3 PLA-PEG NCs
Polylactic acid (PLA) is a biodegradable polymer as declared by the FDA, and it is found to be completely excreted through metabolism. Fabricated docetaxel (DTX) PLA NCs targeting lung cancer stem-like cells (CSLCs), on administration, indicated observable inhibition in tumor growth and anti-metastatic efficacy (392). Studies have shown that encapsulating salinomycin (SAL) in PLA NCs improved its pharmacokinetics and biodistribution profile, demonstrating efficacy against chemo-resistant cancer cells and CSCs (393). Also, when administered to Ehrlich ascites carcinoma (EAC) tumor-bearing mice, SAL: DOX co-loaded NCs caused significant tumor regression and complete inhibition of cancer recurrence (393, 394). Ahmadi-Nouraldinvand and colleagues designed PLA-PEG-based NCs, namely, PLA-chitosan-PEG-folic acid (COPA), PLA-chitosan-PEG-glucose (COPB), COPA and COPB (COPAB), and chitosan-PLA-PEG-FA/Glu/VEGF/siRNA/PTX (NCsAB/siRNA/paclitaxel for efficient siRNA and paclitaxel drug delivery to MCF-7 cells (395). The author opined that the release of siRNA and paclitaxel nanocarrier was favorable due to the acidic environment of tumor tissues (395). Curcumin and bortezomib, both slightly water-soluble anticancer drugs, were loaded as a complex (curc-BTZ) into methoxy-poly(ethylene glycol)-block-polylactic acid (mPEG-b-PLA) diblock copolymers, which demonstrated induced cytotoxicity in HeLa, MCF-7, and MDA-MB 231 cells (396).
5.4 Hyaluronic acid NCs
HA is an anionic, non-sulfated glycosaminoglycan that exhibits biocompatibility, biodegradability, and non-immunogenic properties, making it an excellent candidate for conjugating different drugs in cancer treatment (397). HA-functionalized NCs co-delivering camptothecin (CPT) and curcumin (CUR) (HA-CPT/CUR-NCs) exhibit synergistic anticancer effects, making HA-CPT/CUR-NCs a promising approach for colon cancer-targeted therapy (398). Inhibitory effects of naproxen nanoparticles coated with hyaluronic acid (HA) are demonstrated in breast cancer stem cells through modifications in the GSK-3β-related COX-independent pathway, providing a controlled release of naproxen, leading to apoptosis (399). An effective binding of HA (HA-eNCs) to CD44-enriched B16F10 cells was observed when all-trans-retinoic acid (ATRA)-encapsulated cationic albumin functionalized with HA (HA-eNCs) was applied to CSCs overexpressing CD44, triggering targeted delivery of drugs to eradicate CSCs (400).
5.5 Liposomes
Liposomes are characterized by self-accumulated vesicles consisting of a bilayer of lipids that completely encircles an internal aqueous phase (401). Liposomes can be a drug carrier to both hydrophilic and hydrophobic molecules, which is its major advantage (402). Using anti-CD44 antibodies, Wang et al. delivered liposomal NCs loaded with Dox and triple fusion (TF) genes consisting of the herpes simplex virus truncated thymidine kinase (HSV-ttk), renilla luciferase (Rluc), and red fluorescent protein (RFP) (403). As a result, non-invasive molecular imaging techniques were developed for monitoring and evaluating targeting efficacy and gene therapy in hepatocellular carcinoma (HCC) cells (403). CD133+ glioma stem cells undergo selective apoptosis and differentiate into non-stem-cell lineages following administration of dual-modified cationic liposomes (DP-CLP) with survivin siRNA and paclitaxel (404).
5.6 Gold nanocarriers
Gold nanocarriers (AuNCs) are known to lack the ability to induce adverse and acute toxicity; due to their unique optical properties, remarkable biocompatibility, easy turning of physicochemical properties, and surface chemistry (405, 406), they have been considered as a potential contrast agent in in vivo imaging (407). AuNCs conjugated with the antimetabolite 5-fluorouracil (5-FU) and CD133 antibody could enhance specific targeting by AuNPs and therefore reduce non-specific binding, thus reducing the possibility of systemic side effects in colorectal cancer CSCs (408). Gold nanoparticles were modified by modifying their surfaces with 6-mercapto-1-hexanol so that protoporphyrin IX and folic acid could be conjugated simultaneously for improved internalization through photochemical processes (409). The results showed that when compared to conventional photodynamic therapy, selective phototoxicity was increased in cancer cells (409). A combination of 5-aminolevulinic acid (5-ALA)-bound AuNCs and photodynamic therapy (PDT) decreased the invasion of cutaneous squamous cell carcinoma cells and the migration potential of the cells (410).
5.7 Micelles
Self-assembling nanomicelles (10–100 nm) are colloidal dispersions with a hydrophobic core and a hydrophilic shell (411). Boosted tumor suppression and apoptosis in vivo were observed in H460 human lung cancer cells and CSCs with the application of phenformin-loaded micelles (Phen M) along with gemcitabine-loaded micelles (Gem M) (412). The development of poly(styrene-b-ethylene oxide) (PS-b-PEO) and poly(lactic-co-glycolic) acid (PLGA) by double emulsions loaded with covalently bound temozolomide (TMZ) and/or RG7388 (idasanutlin) to CD133 aptamer, resulting in the possibility of targeting glioblastoma CSCs in combination with simultaneous diagnostic imaging, has been demonstrated (413). Ghosh and Biswas developed Pluronic P105 micelles loaded with doxorubicin and PTX loaded with dextran stearate were used to target melanoma folate-positive B16F10 cells and breast cancer cells (414).
5.8 Polymeric NCs
Polymeric NCs (PNCs) are hydrophilic cores that are surrounded by a polymeric substance with a size range of 1–1,000– nm used by Sun et al. to target gastrointestinal CSCs (415). NanoCurcTM, a polymer-encapsulated curcumin nanoparticle formulation, significantly enhanced brain CSC treatment by augmenting curcumin’s bioavailability and encouraging apoptosis, cell cycle arrest, growth reductions, and clonogenicity in brain CSCs with a reduction in CD133+ population of brain tumors (58). A study demonstrated efficient delivery of salinomycin to the EGFR−overexpressing osteosarcoma CSCs and cancer cells, which led to a reduced CSC population on osteosarcoma cells and CSCs by EGFR aptamer-bound, salinomycin-loaded polymer-lipid hybrid nanocarriers (EGFR-SNCs) (416).
5.9 Dendrimers
A dendrimer is defined as a three-dimensional macromolecule with multiple polymeric branching having the capability of structural modifications (417). Dendrimers are studied for their application in drug and gene delivery including poly(propylene imine) (PPI), poly−l−lysine (PLL), polyamidoamine (PAMAM), polyglycerol, poly(etherhydroxylamine) (PEHAM), and poly(ester amine) (PEA) (418). A temozolomide-loaded polyamide-amine dendrimer in a PAMAM delivery system was developed to explore its potential in targeting melanoma cells in vitro (419). Li et al. targeted CD44+ gastric cancer cells with hyaluronic acid-modified polyamidoamine dendrimer G5-entrapped gold NCs bound to the METase gene, resulting in repressed tumor growth of gastric cells (420). The study of Kesharwani et al. (421) utilized a CD44-targeted G4 PAMAM dendrimer combined with HA, followed by 3,4-difluorobenzylidene curcumin (CDF) for targeting MiaPaCa-2 and AsPC-1 cells. HA-PAMAM-CDF increased the cytotoxicity and antitumor activity in MiaPaCa-2 cells compared to AsPC-1 cells (421).
5.10 Quantum dots
Quantum dots (QDs) are semiconductor nanocarriers with excellent fluorescence properties and have been shown to possess significant imaging, sensing, and therapeutic advantages for cancer treatment in its earliest stages (422). QDs conjugated to anti-HER2 were used for immunolabeled breast and lung cancer cells and showed superior performance in a panel of lung cancer cells with differential HER2 expression, suggesting that they may be a useful tool for the identification of cancer biomarkers at an early stage (423). A study of QDs using EGFR mutation-specific antibodies showed superior effectiveness and sensitivity to traditional mainstays in determining patients’ disease status and therapeutic decisions (424). Researchers have demonstrated the performance of QD-based miRNA nanosensors for detecting point mutations in mir-1962a2, which is abnormally expressed in NSCLC patients’ lung tissues (425). To study the cytotoxicity pathway in hepatocellular carcinoma HepG2 cells, Nguyen et al. (426) synthesized a cadmium telluride quantum dot (CdTe-QD) method, which exhibited apoptosis in HepG2 cells following improved caspase-3 activity, poly ADP-ribose polymerase (PARP) cleavage, and phosphatidylserine externalization. Moreover, augmented activity of Fas levels and caspase-8 markers for extrinsic apoptosis pathway were also observed due to CdTe-QDs (426).
5.11 Nanodiamonds
Nanodiamonds (NDs) possess properties like biocompatibility and efficient drug delivery capability, making them a crucial nanoparticle-based vehicle (427). Nanodiamonds upon cracking form very-small-sized semi-octahedral carbon structures with crystallographic surfaces and sharp edges (428). Their surfaces can be used with small molecules, imaging agents, therapeutic biomolecules, genetic material, and targeting ligands, i.e., by a wide range of biological and chemical agents (429, 430). Chemoresistance was overwhelmed in hepatic cancer cell lines when an epirubicin-nanodiamond complex (EPND) was prepared, exhibiting enhanced efficiency compared with the original epirubicin (431). The dissociation of epirubicin from ND can be trigged by intracellularly charged protein molecules (431). It was found that micropinocytosis is crucial for the uptake of EPND, while inhibitors of clathrin-mediated endocytosis may weaken the uptake of EPND (431).
5.12 Carbon nanotubes
Carbon nanotubes consist of crystalline graphene; express exceptional properties like solubility in water, membrane penetration, discrimination of tumor retention, high drug loading capacity, less toxicity, and Raman properties; and are important for nanotechnology and clinical research (432–438). Research has shown that carbon nanotube-mediated thermal treatment can ablate both bulk breast tumors and breast cancer stem cells, impacting tumor growth, proliferation, and recurrence (439). A multimodal single-walled carbon nanotube (SWCNT) functionalized with CD44 antibodies established selective anti-CD44 targeting, providing effective therapy against breast CSCs (440). Distearoylphosphatidylethanolamine–hyaluronic acid (DSPE-HA) nanotubes were synthesized with a single coupling point in order to yield SWCNTs (DSPE-HA SWCNTs) with high dispersion and biocompatibility for targeting CD44-overexpressing cells (441). They developed novel drug delivery systems for epirubicin (EPI) using DSPE-HA SWCNTs as carriers, i.e., EPI-SWCNTsDSPE-HA (441). A549/Taxol cells and tumor spheroids were treated with EPI-SWCNT-DSPE-HA complexes for efficacy testing. It was found that EPI-SWCNT-DSPE-HA significantly increased intracellular EPI accumulation via CD44 receptor-mediated endocytosis in multidrug-resistant cancer cells (441).
6 Extracellular vesicles
6.1 Cellular exosomes: sources and structures
Extracellular vesicles (EVs) are lipid bilayer NCs found in the cytoplasm with diameters ranging from 30 to 2,000 nm, comprising sugars, nucleic acids, proteins, and lipid biomolecules (382). The smallest EVs ascended from multi-vesicular endosomes. Stem cells, cancer cells, immune cells, nerve cells, and other cell types secrete exosomes, which are found in saliva, amniotic fluid, tears, breast milk, platelets, plasma, red blood cells (RBCs), cerebrospinal fluid, bronchial fluid, synovial fluid, intestinal epithelium, nerve, urine, semen, lymph, bile, and stomach acid, are illustrated in Figure 5A (443–445). The plasma membranes of EVs begin budding inward, forming early endosomes that later develop into late endosomes after maturation and multivesicular bodies (MVBs) with intraluminal vesicles (ILVs) (446). Plasma membranes and MVBs fuse to release exosomes; microvesicles are delivered by direct budding of the plasma membrane externally (447). EVs have plenty of cargos, such as proteins, nucleic acids, metabolites, and lipids (448). EVs are uptaken by recipient cells via various processes including endocytosis, ligand–receptor interaction, and direct fusion as shown in Figure 5B (442).
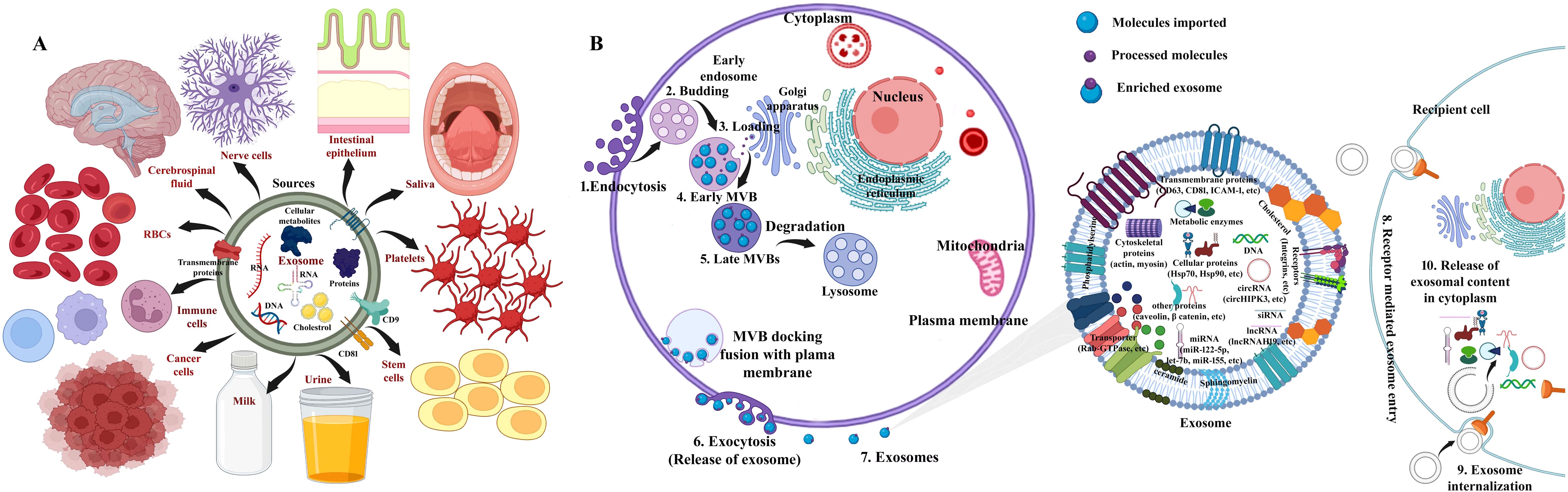
Figure 5. (A) Schematic depiction for sources of exosome: stem cells, cancer cells, immune cells, nerve cells, saliva, platelets, red blood cells (RBCs), breast milk, cerebrospinal fluid, intestinal epithelium, and urine, among others. (B) The generalized structure of exosomes and overview describing the biogenesis of exosomal stem cells and cargos and consequent intake of exosomes by recipient cells. (1) Fusion with target recipient cell, (2) endocytosis, and (3) interaction with specific receptors on cell surface and internalization. Source: Vakil et al. (442). Image was modified and recreated using BioRender.com.
6.2 Exosomes in antitumor therapy
In recent years, naturally secreted exosome vesicles have attracted significant attention as drug delivery vehicles due to their similarities with liposomes (449). A nanometric exosome is easily transported between cells; a lipid bilayer membrane protects bioactive molecules from degradation in the extracellular environment (449–451). Several advantages of exosomes have been demonstrated, including their ability to combat CSCs, lower immunostimulatory, extensive circulation time, and eminent loading efficacy, making them ideal as nanocarriers for drug loading and/or delivery (452, 453). Cheng and co-workers isolated exosomes from healthy hepatoma cells and transfected them using lentivirus expressing p120ctn; as a result, hepatocellular carcinoma cells formed fewer colonies, decreased proliferation, and inhibited migration (454). Furthermore, the exosomes with p120ctn expression reduced the tumor growth in in vivo hepatocellular carcinoma xenograft mice (454). It was also observed that exosome p120ctn did not impact PI3A/Akt or MEK/ERK pathways; however, STAT3 phosphorylation was vividly decreased in hepatocellular carcinoma cells, suggesting that the exosome p120ctn disables STAT3 to impede the hepatocellular carcinoma cell proliferation, metastasis, and expansion of the respective CSCs (454). Hu et al. (360, 455) reported that the exosomes secreted by stromal fibroblasts promote the reversion of phenotype and attainment of CSC characteristics in differentiated colorectal cancer cells by triggering Wnt signaling (360, 455). The in vitro and in vivo experiments suggested that inhibition of Wnt release using the porcupine inhibitor LGK974 curtailed the drug resistance in differentiated colorectal cells and possibly impacted CSC stemness (360, 455). Interestingly, a recent study determined that the migration and invasion of M2 macrophage-modulated colorectal cancer cells are controlled by M2 macrophage-derived exosomes, expressing higher levels of miR-21-5p and miR-155-5p, which are crucial to exosome-mediated colorectal cancer cell migration and invasion (456). Lin et al. (457) introduced that exosomal miR-21-5p derived from bladder cancer cells reversed phosphatase and tensin homolog instigation of the PI3K/AKT pathway in macrophages; in contrast, it induced STAT3 expression to promote the M2-polarized differentiation of tumor-associated macrophages (457). The secreted exosomal miR-21a-5p from the M2 macrophage induced the differentiation and proliferation of pancreatic cancer stem cells by targeting KLF3 for attenuating the stemness of pancreatic cancer (458). Moreover, downregulation of miR-21a 5p in M2 macrophage-induced EVs reduced the expression of Nanog/Oct4 and reduced sphere formation, colony formation, migration, invasion, and anti-apoptosis potency of pancreatic CSCs both in vitro and in vivo (458). The authors focused on miR-21-5p mediated KLF3 downregulation and targeted the differentiation ability of pancreatic stem cells (458). Unique miRNA has been found in prostate cancer exosomes resulting from cancerous stem cells and non-cancerous stem cells (459). Moreover, future cancer cell spread environments are prepared using CSC-derived exosomes (460).
The ability of NCs to induce autophagy has been reported for silver nanomaterials and carbon- and silicon-based nanomaterials (461–463). Pfeffer (464) reported the release of exosomes in certain cell types regulated by Rab27A (464) and Rab27B GTPases and their cognate effector proteins. As a next step, Chen et al. (465) investigated the impacts on parental cells subsequently preventing the exosomal release factor by impeding Rab27a-dependent exosome secretion (465). Downregulation of self-created exosome secretion (Rab27a) from metastatic hepatocellular carcinoma (MHCC97H) inhibited the migration, chemotaxis, and invasion of intrahepatic and lung metastasis via the MAPK/ERK signaling pathway, thereby targeting EMT (465). One of the most notable advantages of exosome-mediated doxorubicin delivery is its dramatic reduction in cardiotoxicity, which is commonly associated with doxorubicin in clinical applications (466). Yong et al. developed biocompatible tumor cell exosome-sheathed PSiNPs (E-PSiNPs) that can be exocytosed by tumor cells for targeted cancer chemotherapy (467). DOX was conjugated to luminescent porous silicon nanoparticles (PSiNPs, 150 nm) (DOX@PSiNPs) and incubated with H22 hepatocellular carcinoma tumor resulting in engulfment of exosome-sheathed (DOX@E-PSiNPs) (467). The DOX@E-PSiNPs enable them to enrich in vivo in both tumor cells and CSCs, resulting in DOX uptake by CSCs with eventual eradication of the CSCs (467) and facilitating the effectiveness in destroying subcutaneous, orthotopic, and metastatic cancer. The schematic illustration of nanocarrier design and its application in eradicating CSCs is presented in Figure 6A. Further, intravenous injections of free DOX, DOX@PSiNPs, and DOX@E-PSiNPs were used to determine whether DOX@E-PSiNPs penetrate deeply into tumors in xenograft mice of H22 hepatocellular carcinoma. The confocal microscopy images showed widespread distribution of DOX@E-PSiNPs in complete tumor sections after 24 h (Figure 6B), while DOX@PSiNPs and free DOX were mostly accumulated around the blood vessels as evidenced by FITC-CD31-labeled endothelial cells. A white line delineates the gap between the DOX distribution in blood vessels and the tumor parenchyma (467).

Figure 6. (A) The exosome-encapsulated porous silicon nanoparticles (E-PSiNPs) and DOX@E-PSiNP preparation as antitumor drug carriers are shown in schematic diagram. After incubation, DOX@E-PSiNPs are endocytosed into cancer cells, localized to multivesicular bodies (MVBs), and form autophagosomes. Exocytosis of DOX@E-PSiNPs occurs upon fusion of MVBs with cell membranes. A systemic injection of DOX@E-PSiNPs in tumor-bearing mice resulted in strong anticancer activity displaying accumulation in both cancer cells and cancer stem cells (CSCs) and penetrating deeply into tumor tissues. (B) Florescent images showing the localization of both DOX and CD31-labeled tumor blood vessels in tumors isolated from H22 tumor-bearing mice at 24 h after intravenous infusion of DOX alone, DOX@PSiNPs, and functional exosome-encapsulated DOX-PSiNPs (DOX@E-PSiNPs) at DOX dosage of 0.5 mg/kg; scale bar, 200 µm. A wide distribution of DOX@E-PSiNPs was evident after treatment; conversely, DOX and DOX@PSiNPs accumulated mostly around blood vessels as evidenced by stronger co-localization with FITC-CD31-labeled endothelial cells. A white line outlines the gap between the DOX distribution in blood vessels and the tumor parenchyma. Reused for illustrative purposes with permission from Yong et al. (467) (Copyright 2019, source: Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapyNature Communications).
6.3 Clinical trials of exosomes in cancer therapy
The European Union, Australia, and the United States have regulatory frameworks for manufacturing and conducting clinical trials, but there may be a need for guidelines dedicated to EV-based therapeutics (468). Exosomes have shown promising results in vitro and in animal models, indicating that they can be used to target CSCs; some clinical trials have already achieved significant results (469). Ascite (Aex)-derived exosomes together with granulocyte–macrophage colony-stimulating factor (GM-CSF) have been tested in a phase I clinical trial for the immunotherapy of colorectal cancer (470–472). This combinational immunotherapy shows the induction of beneficial tumor-specific antitumor cytotoxic T lymphocyte response, but not in the case of Aex alone, indicating the feasibility and better tolerance capability of patients with colorectal cancer (473). An intradermal and subcutaneous immunization of stage III/IV melanoma patients with autologous dendritic cell exosomes pulsed with melanoma-associated antigens family (MAGE 3) peptides was shown in a phase I trial (474). Exosome administration in these patients has proven to be safe and feasible, despite neither CD4+ nor CD8+ T cells specific to MAGE3 being detected in peripheral blood (474, 475). In a phase I study conducted by Morse et al. (476), patients with advanced non-small-cell lung cancer showed improved immune response and tumor progression after receiving dexosome (DC-derived exosomes loaded with the MAGE tumor antigens) immunotherapy. Pulsed dendritic cell EVs activated cytotoxic T cells against a growing tumor in immune-competent mice (477–479). According to Viaud et al. (480), dendritic cell-derived exosomes promote natural killer cell activation and result in anti-metastatic effects, which may be related to NKG2D ligands and IL-15Ralpha. Clinical regressions observed in the first phase I trial using peptide-pulsed Dex (dendritic cell-derived exosomes) were attributed to reduced T-cell response (481, 482). Phase II trials showed that IFN-γ-DC-derived exosomes were capable of boosting antitumor immunity in advanced non-small-cell lung cancer patients following phase I and preclinical trials (475). EVs generated by pulsed DCs, rather than those made by MHC class I and II peptides, induced the activation of B cells and promoted tumors (483, 484). When tumor EVs are combined with appropriate immune-stimulating adjuvants, their immune-inhibitory effect can be suppressed, enabling them to stimulate antitumor responses in advanced ovarian cancer (485–488). Further, antitumor vaccines have been developed using plasmid DNA and recombinant viruses that contain antigens fused to phosphatidylserine-binding domains of milk fat globule epidermal growth factor-factor VIII proteins (MFGE8, also known as lactadherin) (489, 490). This protein facilitates the binding of fusion proteins to EVs, making it a potential antitumor vaccine. An ongoing phase I clinical trial tests whether plant exosomes can deliver curcumin to colon tumors (491).
7 Nanoparticle-mediated ablation therapies
Challenges still exist in designing and assessing nanoformulation-mediated therapies that focus on CSCs. A prospect to address the constraint in CSC eradication is ablation therapy by means of heat or freezing kills the cancer cells, which causes necrosis and targets CSCs to undergo a cell death pathway (492–494), but was found to be limited due to their possible mutation to non-tumor tissues (495). Li et al. attempted NMATs to deliver uniform heat/freezing (concentrated to projected lesions) exposure to the solid tumor, protecting surrounding healthy tissues (496). An advanced NMAT cancer treatment, for instance, photothermal therapy, has been established and has become advanced against CSCs. PTT involves the killing of CSCs using high temperatures through NIR (493). NMATs are capable of penetrating deeper into regional tumor tissues to destroy CSCs (497). Nanocarriers like gold nanocarriers, carbon nanocarriers, MXenes, and iron oxide magnetic nanocarriers can produce high temperatures to convert absorbed energy into localized heat in tumors (439; 498–502).
7.1 Gold nanocarrier coupled with PTT
PTT has been demonstrated using nanospheres, nanocages, nanoshells, nanorods, and nanostars that exhibit surface plasmon resonance (SPR) in the NIR region, hence producing heat (503, 504). The findings of Atkinson et al. (505) on local hyperthermia delivered by Au nanoshells eliminated radio-resistant breast cancer stem cells, resulting in a reduction of tumor size and preventing the increased percentage of ALDH+ (505). Rastinehad et al. (506) tested gold nanoshells with PTT on prostate cancer and observed tumor reduction in 94% of patients without side effects (506). Tian et al. (507) fabricated the hollow gold nanospheres with CD271 monoclonal antibody to target osteosarcoma CSCs through PTT, causing cytotoxicity of osteosarcoma CSCs, resulting in apoptosis and DNA double-strand breaks (507). An investigation found that the synergistic combination of PTT and gold nanocages through the recognition of the sigma-2 ligand SV119 has the ability to eradicate breast CSCs (498). Using gold nanostars loaded with retinoic acid (RA) and dendritic polyglycerol (GNS-dPG) with multiple attachment sites of HA is effective in targeting CSCs (508). Liang and colleagues demonstrated that CSCs could be eradicated by means of a gold nanostar-based approach coupled with PTT and when modified with CD44v6 monoclonal antibodies are effective against gastric CSCs (499). The gold nanostar (GNS)-based PEGylated along with CD44v6 monoclonal antibody-conjugated nanoprobes (GNS-PEG-CD44v6, a test group) showed tremendous stability and biocompatibility (499). The investigators tested the synthesized GNS-PEG-CD44v6 (taken as the test group) to selectively eliminate gastric cancer stem cells (GCSCs), for which the CD44+-expressing spheroid colonies were incubated with the test group and GNS-PEG (taken as the control group), along with untreated GCSCs for comparison. Laser irradiation (1.5 W/cm2) was then applied to all groups for 5 min. The test group showed deteriorated colonies, in contrast to the control and untreated groups, under laser irradiation as represented in Figure 7A (499). In vivo photoacoustic (PA) imaging of a gastric tumor was carried out using a NIR laser (720 nm) with moderate energy to identify neovascularization and have a high PA contrast effect on the tumor. GNS-PEG-CD44v6 was tested and found to induce a steady upsurge in signal within 4 h. There was a strong signal fortification close to the stomach in subcutaneous tumors, indicating a gradual accumulation of GNS-PEG-CD44v6 and identifying the vascular system. PA images attained before and after injection (0, 2, 4, and 24 h) with GNS-PEG-CD44, GNS-PEG-CD44v6 (the first and third rows depict a subtumor, while the second and fourth display orthotopic tumor), and GNS-PEG (the fifth row denotes a subtumor) are presented in Figure 7B. The GNS enhanced the vessel signals and made them accumulate in the perivascular spaces and diffuse into the adjacent tumor tissues after which the signal was found to reduce nearly at 24 h. Correspondingly, GNS-PEG-CD44 doses resulted in parallel agglomeration, but the effects were less pronounced than GNS-PEG-CD44v6 (Figure 7B). Control PA signals did not show any robust enhancement, and in intravascular signals, only a slight increment was observed in 2–4 h (Figure 7B). Furthermore, GNS-PEG-CD44v6 was tested for its ability to selectively target GCSCs expressing CD44, with high efficiency of photothermal conversion and photothermal ablation (499). GNSs provided an attractive candidate for photothermal agents due to their significant heating capabilities (509, 510), thus overriding CSCs’ resistance to photodynamic therapy and general photothermal treatment. The investigators tested the potential of using GNS-PEG-CD44v6 as a smart imaging probe to detect GCSCs in gastric cancer (GC) using infrared microscopic imaging 499). After GNS-PEG-CD44v6 exposure, subcutaneous tumors from GC xenograft mice were irradiated with NIR lasers. Figures 7C–F show that the temperature of the treated tumor site significantly increased within 3 min after laser irradiation, and the color changed from blue to red as demonstrated by infrared imaging. Despite the absence of GNS-PEG-CD44v6 injection or laser irradiation, the infrared imaging signal did not change color, showing no apparent temperature variation when the temperature was increased (Figure 7E). In the xenograft mice, the tumor volume was unchanged (Figure 7G), while necrotic areas were observed in treated tumor tissues (Figure 7H), which may perhaps be due to the sharp structural features of nanostars, making it a more efficient photothermal transducer (511). An analysis of tumor growth curves from four groups after treatment with GNS-PEG-CD44, GNS-PEG, and PBS, respectively, as well as control groups without treatment. As a result of the GNS-PEG-CD44v6 treatment, the tumor volumes of the treated group showed a significant statistical difference and reduced after two weeks of therapy (Figure 7I). While GNS-PEG, based on passive targeting therapy, had negligible effect on tumor growth, the untreated groups and PBS groups plus NIR laser did not exhibit any significant therapeutic effect. Figure 6H illustrates the survival time of mice treated with GNS-PEG-CD44v6 was significantly longer than in control mice treated with GNS-PEG, PBS, or untreated (Figure 7J). This results from the fact that nanoprobes targeting GCSCs can extend tumor-bearing mice's survival time. A new study used aptamers conjugated with gold nanorods to specifically target prostate cancer stem cells in combination with NIR (512). Peng and Wang tailored gold nanorods with anti-CD133 monoclonal antibodies to selectively target and destroy CD133+ cells in glioblastoma cell lines in response to the laser beam (513).
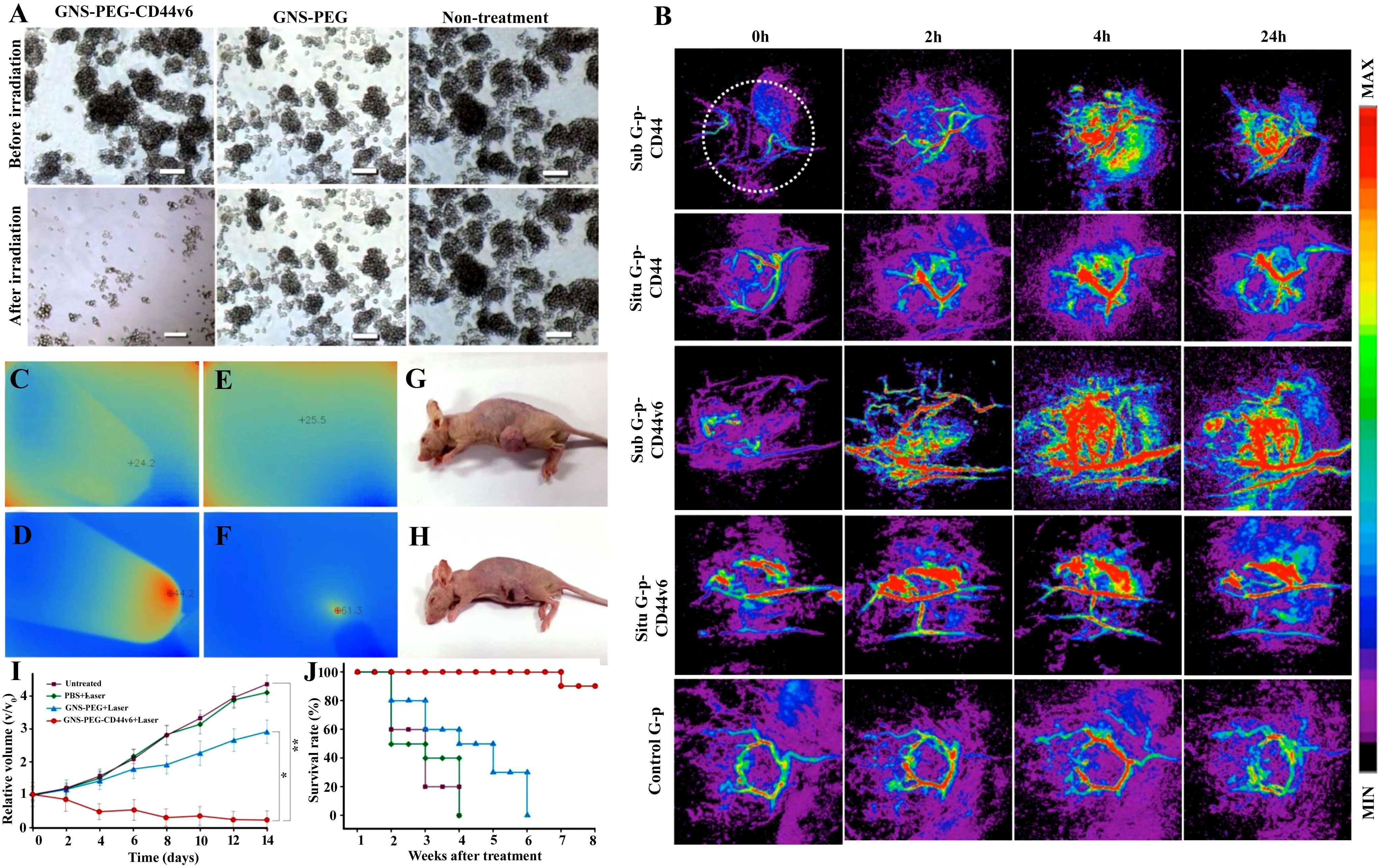
Figure 7. (A) A comparison of microscopy descriptions of gastric cancer stem cell (GCSC) spheroid colonies treated with GNS-PEG-CD44v6 and GNS-PEG irradiated for 24 h with near-infrared (NIR) laser (790 nm, 1.5 W/cm2, 5 min) showing damaged spheroid colonies in NIR irradiated GNS-PEG-CD44v6. (B) Photoacoustic (PA) images attained before and after injection (0, 2, 4, and 24 h) with GNS-PEG-CD44, GNS-PEG-CD44v6 (the first and third row depict a subtumor, while the second and fourth display orthotopic tumor taken as test group), and GNS-PEG (the fifth row denotes a subtumor taken as control). Observation of (C) deionized water and (D) GNS in a tube exposed to NIR radiation (790 nm, 0.3 W/cm2, 3 min) using infrared microscopic imaging. On NIR laser irradiation, subcutaneous GC tumors are shown (E) without and (F) with injections of GNS-PEG-CD44v6. An injection of GNS-PEG-CD44v6 was administered to a nude mouse of GC subcutaneous xenograft (G) with and (H) without laser exposure (790 nm, 0.3 W/cm2, 3 min). (I) Growth curves of GC tumors exposed to NIR laser treatment (790 nm, 0.8 W/cm2, 5 min), which were additionally treated with GNS-PEG-CD44v6, GNS-PEG, and PBS, along with the untreated control group. (J) Treatment-induced survivability rate (%) was assessed for GC tumor-bearing mice after 8 weeks in comparison to controls. Reused for illustrative purposes with permission from Liang et al. (499) (Copyright 2015, Ivyspring International Publisher, source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4493535/).
7.2 Carbon-based NCs with PTT
Carbon-based NCs with robust NIR absorption, thermal conductivity, ease of fabrication, and superior biocompatibility are a great choice for CSC-targeted therapy (514). Wang and colleagues found that carbon nanotubes (CNTs) coupled with CD133 monoclonal antibodies after NIR light exposure potentially reduced self-renewal and tumorigenesis of cancer stem cells in glioblastoma (515). The combination of organoselenium-modified CNTs with PTT successfully destroyed CSCs producing reactive oxygen species, resulting in apoptosis (516). A study reported that PTT with Multiwalled Carbon Nanotube (MWCNTs) eliminated both differentiated cells of tumor and tumor regression of breast cancer stem cells in vivo by necrotizing and destroying the CSCs (439). The transient receptor potential vanilloid family type 2 (TRPV2)-PEGylated carbon nanohorn (PCNH) was found to reduce cancer stemness in the presence of NIR irradiation (517) by activating Ca2+ influx, hence altering intracellular Ca2+ overload, which has been shown to cause apoptosis with TRPV2 overexpression. The human colorectal (HT-29) tumor growth reduction with the laser-driven TRPV2–PCNH was also experimented on nude mice (517), and the results are shown in Figures 8A–M. TRPV2–PCNH suppresses cancer stemness when exposed to NIR irradiation, contributing to intracellular Ca2+ overload that induces apoptosis (517). As shown in Figure 5, the researchers subcutaneously administered tumor xenograft mice with HT-29 colorectal cells or their TRPV2-overexpressing derivatives into their flanks in order to evaluate the effects of anticancer phototherapy. The xenograft mice were divided into groups: PBS as blank control, PBS+laser as laser control, PCNH as non-targeted nanoparticle control, PCNH+laser as non-targeted phototherapy control, TRPV2–PCNH as targeted nanoparticle control, and TRPV2–PCNH+laser as targeted phototherapy groups. Mice of both cell lines (HT-29 or transfected TRPV2 HT-29 cells) were intraperitoneally injected with 5 mg/kg doses of nanocomplexes every other day. Following 24 h of treatment, the mice were subjected to 5-min laser exposure (1 W, ~50 mW mm−2) to the right side of the tumor on days 2, 6, 9, 13, and 16 (Figure 8A). Thermographic infrared imaging of body surface temperatures was performed during laser irradiation (Figure 8B). HT-29–TRPV2 tumors from the group treated with TRPV2–PCNH+laser were the only ones to attain temperature levels above 52°C (activation threshold for TRPV2) (Figure 8C). Laser-irradiated PCNH or TRPV2–PCNH NPs reduced the rate of HT-29 (Figure 8D) and HT-29-TRPV2 (Figure 8E) tumor growth in mice compared with those receiving PBS. TRRV2–PCNH+laser suppressed HT-29–TRPV2 tumors more than any other treatment group, indicating that TRRV2–PCNH targets TRPV2-overexpressing cells selectively. A similar effect was observed in the TRRV2–PCNH+laser group in comparison with non-targeted phototherapy and blank control. HT-29–TRPV2 tumors exposed to laser irradiation were significantly smaller than tumors on the opposite flanks of the same mice without laser irradiation, whereas no effects of laser irradiation on HT-29 xenografts were observed (Figure 8F). The ability of laser-driven TRPV2–PCNH nanoparticles to regulate cancer stemness was evaluated via immunohistochemistry staining of Ki-67 and CD133, which are proliferation and stem cell markers, respectively. Laser irradiation resulted in significantly lower expression of both Ki-67 (Figure 8G) and CD133 (Figure 8H) markers in HT-29-TRPV2 tumor tissues. RT-qPCR investigation showed a decrease in mRNA levels of stemness-associated markers as a result of the laser irradiation effect on mouse tumor tissues tested with PCNH or TRPV2–PCNH NPs (Figure 8I). The resection of HT-29–TRPV2 tumors after treatment, following digestion and transplantation into nude mice (Figure 8J), resulted in aggressive tumor formation (100%) in non-irradiated tumors in contrast to less tumor growth (20%) in irradiated tumors (Figure 8K). The phototherapeutic efficacy of TRPV2–PCNH may improve drug resistance and inhibit cancer stemness. The U2OS osteosarcoma cells overexpressing TRPV2 when receiving TRPV2–PCNH+laser resulted in downregulated β-catenin (associated with carcinogenesis) expression in contrast to the non-laser-treated TRPV2–PCNH and control group (Figure 8L). To describe the mechanism, the authors examined laser-induced effects on protein kinase (PKCα), which in combination with Ca2+ phosphorylate β-catenin ultimately led to its reduced expression (518). The expression of protein kinase (PKCα) was upregulated in TRPV2-transfected MCF7 (breast cancer) cells following phototherapy revealed through Western blotting expression (Figure 8M). The non-phosphorylated and total β-catenin expressions in TRPV2-transfected MCF7 cells were reduced, whereas both protein expression levels were unchanged in MCF7 control (without TRPV2 transfection) (Figure 8M). Yu et al. (517) validated that Ca2+ influx induced by TRPV2–PCNH+laser stimulates PKCα, leading to downregulated Wnt/β-catenin signaling and related genes.
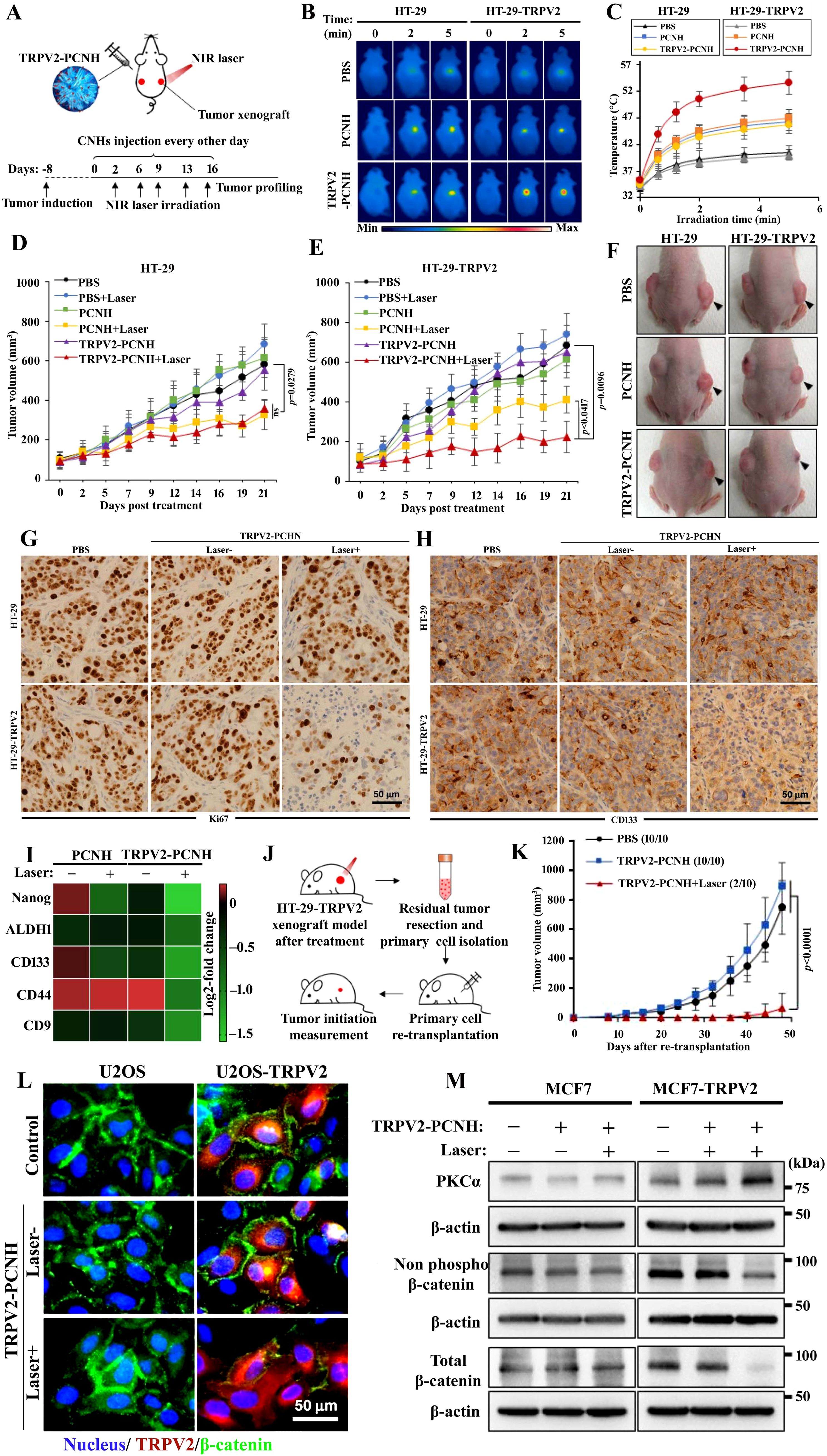
Figure 8. (A) A tumor xenograft model was established in mice on day 8 with the inoculation of HT-29 control cells and transfected TRPV2. The treatments PBS, PCHN, and TRPV2–PCNH were administered intraperitoneally (i.p.) on day 0 and administered every other day until day 16. Near-infrared (NIR) laser of 1,064 nm (1 W to 50 mW mm−2) was applied for 5 min to the right side of tumor on days 2, 6, 9, 13, and 16. (B) A thermographic infrared camera was used to monitor the surface temperature of the body during laser irradiation. (C) A laser-induced increase in temperature was observed in mice with HT-29 or HT-29–TRPV2 tumors after nanocomplex injection and measured at 24 (h) Tumor volume measured in (D) HT-29 and (E) HT-29-TRPV2 in different treatment groups with/without NIR laser exposure. A significant reduction in tumor volumes was observed in nude mice bearing HT-29–TRPV2 following TRPV2–PCNH+laser treatment. (F) Tumor-bearing nude mice with HT-29 and HT-29–TRPV2 photographed on day 16 (black arrows indicating irradiated tumors). Xenograft models overexpressing TRPV2 and TRPV2–PCNH+laser inhibit tumor reinitiation. Immunohistochemical analysis showed that expressions of (G) Ki-67 and (H) CD133 expressions were documented to reduce with TRPV2–PCNH+laser in primary tumor sections of xenografts overexpressing TRPV2. (I) The RT-qPCR analysis of tumors with TRPV2 overexpression from mice treated with TRPV2–PCNH and laser irradiation exhibited declines in mRNA of stemness-associated markers (Nanog, ALDH1, CD133, CD44, and CD9). (J) Methodology followed that of Yu et al. (517) for tumorigenesis experiments involving resection, isolation, re-implantation, and tumor initiation investigation. (K) The cells isolated from xenograft tumors showed reduction in tumor proliferation following TRPV2–PCNH+laser treatment in comparison with PBS and TRPV2–PCNH tested groups. (L) TRPV2–PCNH+laser irradiation in osteosarcoma cell line (U2OS) demonstrated reduced β-catenin expression as demonstrated by immunostaining images (Hoechst specifies nuclei, mCherry specifies TRPV2, and Alexa488 specifies β-catenin). (M) Western blotting illustrated downregulated non-phosphorylated and total β-catenin expression and upregulated PKCα expression in MCF7–TRPV2 cells with TRPV2–PCNH+laser treatment. β-Actin was used as a control for normalization. Reused for illustrative purposes with permission from Yu et al. (517) (Copyright 2020, Nature Communications, source: Photothermogenetic inhibition of cancer stemness by near-infrared-light-activatable nanocomplexes - PubMed (nih.gov)).
7.3 MXene with PTT
MXenes are 2D layered transition metal carbides with nitrides or carbonitrides and display robust absorption of NIR beam, causing hyperpyrexia in order to ablate tumors efficiently (519, 520). In situ growth of CdS on an ultrathin Nb2C nanosheet (MXene) produces M/CdS, which is then modified with CSCs targeting HA to form the nano-lymphatic (M/CdS-HA) material (502). HA-mediated tumor targeting and NIR-II (1,064 nm) laser irradiation targets the “nano-lymphatic” toward the tumor region, which reduces the tumor interstitial pressure (TISP) via PTT (Figure 9A). The tumor interstitial fluid pressure (TIFP) is decreased as a result of the temperature variation, prompting CdS to decompose the tumor interstitial fluid through pyroelectric catalysis. As a result of the reduction in TISP and TIFP, the “nano-lymphatic” penetrates deeper into tumors; at the same time, pyroelectric catalysis generates ROS in deep tumor stem cells, leading to apoptosis and necrosis due to oxidative damage. Figure 9B illustrates the pyroelectric effect and the relationship between temperature variation and pyroelectric current, whereby the motion of atoms in the pyroelectric material is influenced by temperature variation, leading to the change in polarization for a pyroelectric field integrated into it. This PTT and pyroelectric catalysis of M/CdS was visually illustrated via infrared thermal imaging (ITI), which demonstrated the exceptional result under laser irradiation at 3 min (Figure 9C) (502). The PTT effect, pyroelectric current, and potential response under 1,064-nm NIR-II irradiation of CdS, MXene, and MXene/CdS were evaluated and compared (Figure 9D). MXene and MXene/CdS displayed a substantial increase in temperature (ΔT = 45°C and 52°C) in 10 min compared to CdS (Figure 9D). Unlike MXene and CdS, M/CdS could produce significant amounts of O2 with 5 min of 1,064-nm laser irradiation, which showed water splitting by means of pyroelectric catalysis (Figure 9E) (502). Multicellular spheroids (MCSs) were formed with HeLa cells grown into a culture dish with an ultralow attachment surface. MCSs were previously treated with genistein (Gen), an endocytosis inhibitor, to interrupt the passage of M/CdS across the cell monolayer to study the relationship between diffusion and transcytosis (Figure 9F). The structural changes of MCSs with laser irradiation from day 0 to day 6 of MXene/CdS-HA/RB were first demonstrated by fluorescent imaging, as shown in Figure 9G. Blue fluorescence indicates the nucleus, while red fluorescence indicates the center of the MCSs, suggesting that MXene/CdS-HA was incorporated into MCSs on day 0 after laser irradiation. Upon laser irradiation, MCSs collapsed progressively on days 2, 4, and 6, indicating deep damage. The pyroelectric catalysis mechanism is presented in Figure 9H. MXene plasmon resonance and excitation of CdS were introduced in response to the NIR-II laser due to its large energy gap. Pyroelectric catalysis-based water splitting produced reactive oxygen species from the developed negative and positive oxygen species (Figure 9H). Since CdS and MXene have different Fermi levels (Ef) and work functions, the negative charges of CdS are transferred to MXene to equilibrate their Ef. Schottky barriers are formed when the energy band of CdS (n-type semiconductor) is bent upward during equilibrium (521). The light was converted into hyperpyrexia by MXene in combination with laser irradiation. The temperature variation due to the pyroelectric effect directed the negative charge of CdS to move along the Schottky junction toward MXene, prohibiting the backflow of negative charges (496). MXene could also be used as a cocatalyst to improve the catalytic efficiency of CdS. MXene could be used as a cocatalyst to enhance the catalytic efficiency of CdS. The excited negative charge was found to react with O2 and generated superoxide (•O2−) and hydroxyl (•OH) radicals, and the positive charge reacted with H2O to produce O2 and H+ as shown in Figure 9H (502). Lactic acid (LA) presented enhanced catalytic activity of laser-induced M/CdS-HA and antitumor efficacy (Figure 9I). As a result of LA overexpression in the tumor microenvironment, pyroelectric catalysis is prevented from interacting with positive charges, thus increasing ROS production. MXene/CdS-HA treatment of tumor blood vessels with/without 1,064-nm NIR-II laser exposure at various time intervals (0, 15, and 30 min) was visualized using photoacoustic illustration in Figure 9J. After 24 injections of M/CdS-HA, the blood perfusion remained very low without laser irradiation. The blood perfusion was enhanced with increasing time of laser exposure as a result of a decrease in TIP (502). Enhanced drug delivery from the blood to the tumor could be achieved by reducing the pressure difference between blood and tumor interstitial fluid, providing an effective force for the delivery of drugs from blood into bulk tumors. A white arrow indicates bleeding spots, which resulted from enhanced blood flow and hyperpyrexia-induced damage; at the same time, the enhanced blood circulation mediated by M/CdS-HA+L could increase the intratumoral oxygen (O2) content, improving hydrodynamic therapy (Figure 9K). The saline group was shown to exhibit hypoxia on irradiation with no significant change. M/CdS-HA+L exhibited an increment in O2 content and contributed to the increased blood perfusion (Figure 9K), which ultimately carried the nanomedicine to the tumor site. By regulating TIP, nanomedicine could effectively penetrate deeper into tumors, and the ROS resulting from the pyroelectric catalysis could further damage deep tumor stem cells (517).
Figure 9. MXene-mediated photothermal ablation of cancer stem cells (CSCs). (A) An overview of the synthesis and use of “nano-lymphatic” (M/CdS/HA) for increased tumor penetration, photothermal (NIR-II), and hydrodynamic therapies with the decrease in tumor interstitial pressure (TIP) via pyroelectric catalysis. (B) Pyroelectric effect with temperature variation depicted schematically. (C) The infrared thermal image (ITI) demonstrated the excellent photothermal effect of M/CdS under laser irradiation at 3 min and could confirm further usage for both PTT and pyroelectric catalysis (here, 0 min was considered as control). (D) Photothermal consequence of CdS, MXene, and MXene/CdS. (E) The curves of O2 generation due to CdS, MXene, and M/CdS under laser irradiation (1,064 nm, 1.0 W/cm2) in comparison to control. (F) The illustration of multicellular spheroid (MCS) formation and the penetration mechanism showing increased diffusion with rise in temperature (T↑), reduction in TIP, and transcytosis in MXene+laser, MXene/CdS, MXene/CdS+laser, and MXene/CdS+laser+genistein (Gen) treatments. (G) Confocal fluorescence imaging of MCSs on exposure with MXene/CdS-HA/RB with NIR-II laser irradiation (1,064 nm) from day 0 to 6 (scale bar = 100 μm). (H) Representation of pyroelectric catalysis for water splitting and induced reactive oxygen species (ROS) production in case of treatment. (I) Enhanced tumor penetration with improved blood perfusion due to TIP reduction and induced ROS generation due to lactic acid (LA) in the tumor. (J) MXene/CdS-HA treatment of tumor blood vessels with/without 1,064-nm NIR-II laser exposure at various time intervals (0, 15, and 30 min) was visualized using photoacoustic illustration. (K) Concentration of oxygen in tumor blood vessels treated with saline as a control and MXene/CdS-HA (with/without 2 min of 1,064-nm irradiation exposure) at various time intervals of 0, 15, and 30 min. Reused for illustrative purposes with permission from He et al. (502) (Copyright 2021, American Chemical Society, source: Pyroelectric Catalysis-Based “Nano-Lymphatic” Reduces Tumor Interstitial Pressure for Enhanced Penetration and Hydrodynamic TherapyACS Nano).
8 Conclusion
Current cancer treatment failures are thought to be rooted in CSCs, which are vastly resistant to conventional therapies, leading to recurrence and metastasis. A significant amount of investigation has promoted the practice of NCs for cancer therapy without targeting CSCs. Our review describes various functionalized NCs, EVs, and PTT mediated for improving CSC ablation. A major challenge in clinical translation research for specifically targeting CSCs with modified NCs and its outcome depends on factors involving specificity and protection. Developing clinical applications of modified NCs against CSCs requires multidisciplinary collaboration, as well as continuous basic and applied research aimed at understanding their properties. A state-of-the-art nanotechnology approach will also be required to develop more effective strategies for eradicating CSCs. It is very likely that the NCs targeting CSCs will attain efficacious clinical translation in the upcoming days, allowing patients to benefit from unique treatments.
Author contributions
DK: Conceptualization, Validation, Writing – original draft, Writing – review & editing. LT: Writing – original draft, Writing – review & editing. CA: Writing – original draft. SS: Writing – original draft. GV: Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. We would like to extend our sincere gratitude to the Institutions of Eminence (IoE)-RC4-21–006 of the University of Hyderabad, India, which provided financial support during our tenure.
Acknowledgments
Corrections of suggested grammatical errors during revision is accomplished using Wordtune.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
ABC transporters: ATP binding cassette transporters
ABCG2: ATP-binding cassette super-family G member 2
ALA: 5-aminolevulinic acid
ALDH: aldehyde dehydrogenase
ALDH1A1: aldehyde dehydrogenase 1 family, member A1
ALL: lymphoblastic leukemia
AML: acute myeloid leukemia
ANA: antinuclear antibodies
Antho-NPs: anthothecol-encapsulated PLGA nanoparticles
ATRA: all-trans-retinoic acid
AuNPs: gold nanoparticles
BTZ: bortezomib
CAP: cold atmospheric plasma
CAR T cells: chimeric antigen receptor T cells
Cas9: CRISPR-associated protein 9
CD: cluster of differentiation
CDDP: cisplatin (cis-diamminedichloroplatinum)
CDF: 3,4-difluorobenzylidene curcumin
CdTe-QDs: cadmium telluride quantum dots
CHO/CHOL: cholesterol
CNTs: carbon nanotubes
COPA: PLA-chitosan-PEG-folic acid
COPAB: COPA and COPB
COPB: PLA-chitosan-PEG-glucose
CPT: camptothecin
CRISPR: Clustered Regularly Interspaced Short Palindromic Repeats
CSCs: cancer stem cells
CTAB: cetyltrimethylammonium bromide
CXCR4: C-X-C chemokine receptor type 4
DAPT: N-[N-(3,5-difluorophenacetyl)-{{sc}}l{{/sc}}-alanyl]-S-phenylglycine t-butyl ester
Dex: dexamethasone
DLin-MC3-DMA: (6Z,9Z,28Z,31Z)-heptatriaconta-6,9,28,31-tetraen-19-yl-4-(dimethylamino)butanoate
DMPC: dimyristoyl phosphatidylcholine
DNA: deoxyribonucleic acid
DOPC: dioleoyl phosphatidylcholine
DOPE: dioleoyl phosphatidylethanolamine
DOTAP: dioleoyl trimethylammonium-propane
DOX: doxorubicin
DP-CLPs: dual-modified cationic liposomes
DPPC: dipalmitoyl phosphatidylcholine
DPPG: dipalmitoyl phosphatidylglycerol
DSPC: distearoyl phosphatidylcholine
DSPG: distearoyl phosphatidylglycerol
DTX: docetaxel
DX: dexamethasone (Dex)-associated liposomes
EAC: Ehrlich ascites carcinoma
ECM: extracellular matrix
EGFR: epidermal growth factor receptor
EMA: European Medicines Agency
EMT: epithelial–mesenchymal transition
EPC: ethyl phosphatidylcholine
EpCAM: epithelial cellular adhesion molecule
EPG: ethyl phosphatidylglycerol
EPR: enhanced permeability and retention
ESA: epithelial‐specific antigen
ESCC: esophageal squamous cell carcinoma
EVs: extracellular vesicles
FA: folic acid
FDA: Food and Drug Administration
FIH: first in human
GBM: glioblastoma multiforme
Gem: gemcitabine
Gen: genistein
GQDs: graphene quantum dots
HA: hyaluronic acid
HCC: hepatocellular carcinoma
HER2: human epidermal growth factor receptor 2
Hh: Hedgehog
HIV: human immunodeficiency virus
HNSCC: head and neck squamous cell carcinoma
HPV: human papillomavirus
HSP: heat shock proteins
HSPC: fully hydrogenated phosphatidylcholine
HT: human colorectal
IHC: immunohistochemistry
IL-2: interleukin-2
ILVs: intraluminal vesicles
IU: investigational use
KLF4: Krüppel-like factor 4
LA: lactic acid
LF: liposomal formulation
LGR5: leucine-rich repeat-containing G protein-coupled receptor 5
LiCl: lithium chloride
M/CdS: MXene-cadmium sulfide
mAbs: monoclonal antibodies
MAGE A3: melanoma antigen family A3
MCS: multicellular spheroid
MDR: multidrug resistance
MEK: mitogen-activated extracellular signal-regulated kinase
MH: 6-mercapto-1-hexanol
MHCC97H: metastatic hepatocellular carcinoma
miRNA-34: microRNA-34
MM: multiple melanoma
MMPs: matrix metalloproteinases
mPEG-b-PLA: methoxy-poly(ethylene glycol)-block-polylactic acid
MPEG-DSPE: N-(carbonyl-methoxy(polyethylene glycol)-2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine sodium salt
MSPC: monostearoyl phosphatidylcholine
MTD: maximum tolerable dose
MTX: methotrexate
MUC1: mucin 1
MVBs: multivesicular bodies
MWCNTs: Multiwalled carbon nanotubes
NAP: naproxen
NDD: nanomaterial-loaded drug delivery
NDs: nanodiamonds
NIR: near-infrared
NK cells: natural killer cells
NMATs: nanoparticle-mediated ablation therapies
NPC: nasopharyngeal
NPs: nanoparticles
NSCLC: non-small-cell lung cancer
OCT4: octamer-binding transcription factor 4
PAMAM: polyamidoamine
PARP: poly ADP-ribose polymerase
PBS: phosphate-buffered saline
PC: phosphatidylcholine
PCNH: PEGylated carbon nanohorn
PD-1: programmed cell death protein-1
PDT: photodynamic therapy
PEA: poly(ester amine)
PEG: poly(ethylene glycol)
PEG2000-C-DMG: α-(30-{[1,2-di(myristyloxy)propanoxy]carbonylamino}propyl)-ω-methoxy polyoxyethylene
PEG2000-DSPE: polyethylene glycol 2000-distearoyl phosphatidylethanolamine
PEHAM: polyglycerol, poly(etherhydroxylamine)
Phen: phenformin
PLA: polylactic acid
PLGA: poly(lactic-co-glycolic acid)
PNPs: polymeric nanoparticles
PEG: poly(ethylene glycol)-b-poly({{sc}}d{{/sc}},{{sc}}l{{/sc}}-lactide)
PPI: poly(propylene imine)
PpIX: protoporphyrin IX
PS-b-PEO: poly(styrene-b-ethylene oxide)
PSiNPs: porous silicon nanoparticles
PSMA: prostate-specific membrane antigen
PTT: photothermal therapy
PTX: paclitaxel
QD: quantum dots
RA: retinoic acid
RBCs: red blood cells
RCC: renal cell carcinoma
RNA: ribonucleic acid
ROS: reactive oxygen species
SA: streptavidin
SAL: salinomycin
SALL4: Sal-like protein 4
SAL-NP: salinomycin-loaded PEGylated poly(lactic-co-glycolic acid) nanoparticles
SCLC: small-cell lung cancer
siRNA: small interfering RNA
SMO: smoothened inhibitors
SOX2: (sex-determining region Y)-box 2
SPR: surface plasmon resonance
SWCNT: single-walled carbon nanotube
TCR: T-cell receptor
TGF-β: transforming growth factor beta
TIP: tumor interstitial pressure
TMZ: temozolomide
TNBC: triple-negative breast cancer
TNRPV2: transient receptor potential vanilloid family type 2
References
1. Siegel RL, Kratzer TB, Giaquinto AN, Sung H, and Jemal A. Cancer statistics 2025. CA Cancer J Clin. (2025) 75:10–45. doi: 10.3322/caac.21871
2. Chu X, Tian W, Ning J, Xiao G, Zhou Y, Wang Z, et al. Cancer stem cells: advances in knowledge and implications for cancer therapy. Signal Transduct Target Ther. (2024) 9:170. doi: 10.1038/s41392-024-01851-y
3. Dakal TC, Bhushan R, Xu C, Gadi BR, Cameotra SS, Yadav V, et al. Intricate relationship between cancer stemness, metastasis, and drug resistance. MedComm (Beijing). (2024) 5:e710. doi: 10.1002/mco2.710
4. Kapoor-Narula U and Lenka N. Cancer stem cells and tumor heterogeneity: Deciphering the role in tumor progression and metastasis. Cytokine. (2022) 157:155968. doi: 10.1016/j.cyto.2022.155968
5. Abbas Z and Rehman S. An overview of cancer treatment modalities. In: Neoplasm. London, United Kingdom: InTech (2018), 254. doi: 10.5772/intechopen.76558
6. Mathan SV, Rajput M, and Singh RP. Chemotherapy and radiation therapy for cancer. In: Understanding cancer. Academic press, Cambridge, Massachusetts, USA: Elsevier (2022). p. 217–36. doi: 10.1016/B978-0-323-99883-3.00003-2
7. Tao JJ, Visvanathan K, and Wolff AC. Long term side effects of adjuvant chemotherapy in patients with early breast cancer. Breast. (2015) 24:S149–53. doi: 10.1016/j.breast.2015.07.035
8. Oun R, Moussa YE, and Wheate NJ. The side effects of platinum-based chemotherapy drugs: a review for chemists. Dalton Trans. (2018) 47:6645–53. doi: 10.1039/C8DT00838H
9. Siqueira JM, Heguedusch D, Rodini CO, Nunes FD, and Rodrigues MFSD. Mechanisms involved in cancer stem cell resistance in head and neck squamous cell carcinoma. Cancer Drug Resist. (2023) 6:116–37. doi: 10.20517/cdr.2022.107
10. Phi LTH, Sari IN, Yang Y-G, Lee S-H, Jun N, Kim KS, et al. Cancer stem cells (CSCs) in drug resistance and their therapeutic implications in cancer treatment. Stem Cells Int. (2018) 2018:1–16. doi: 10.1155/2018/5416923
11. Dzobo K, Senthebane DA, Thomford NE, Rowe A, Dandara C, and Parker MI. Not everyone fits the mold: intratumor and intertumor heterogeneity and innovative cancer drug design and development. OMICS. (2018) 22:17–34. doi: 10.1089/omi.2017.0174
12. Khan SU, Fatima K, Aisha S, and Malik F. Unveiling the mechanisms and challenges of cancer drug resistance. Cell Commun Signal. (2024) 22:109. doi: 10.1186/s12964-023-01302-1
13. Mai Y, Su J, Yang C, Xia C, and Fu L. The strategies to cure cancer patients by eradicating cancer stem-like cells. Mol Cancer. (2023) 22:171. doi: 10.1186/s12943-023-01867-y
14. Mengistu BA, Tsegaw T, Demessie Y, Getnet K, Bitew AB, Kinde MZ, et al. Comprehensive review of drug resistance in mammalian cancer stem cells: implications for cancer therapy. Cancer Cell Int. (2024) 24:406. doi: 10.1186/s12935-024-03558-0
15. Shang T, Jia Z, Li J, Cao H, Xu H, Cong L, et al. Unraveling the triad of hypoxia, cancer cell stemness, and drug resistance. J Hematol Oncol. (2025) 18:32. doi: 10.1186/s13045-025-01684-4
16. Audero MM, Prevarskaya N, and Fiorio Pla A. Ca2+ Signalling and hypoxia/acidic tumour microenvironment interplay in tumour progression. Int J Mol Sci. (2022) 23:7377. doi: 10.3390/ijms23137377
17. Goswami KK, Banerjee S, Bose A, and Baral R. Lactic acid in alternative polarization and function of macrophages in tumor microenvironment. Hum Immunol. (2022) 83:409–17. doi: 10.1016/j.humimm.2022.02.007
18. Guo Y, Wang M, Zou Y, Jin L, Zhao Z, Liu Q, et al. Mechanisms of chemotherapeutic resistance and the application of targeted nanoparticles for enhanced chemotherapy in colorectal cancer. J Nanobiotechnol. (2022) 20:371. doi: 10.1186/s12951-022-01586-4
19. Sun T, Zhang YS, Pang B, Hyun DC, Yang M, and Xia Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew Chem Int Ed. (2014) 53:12320–64. doi: 10.1002/anie.201403036
20. Bravo M, Fortuni B, Mulvaney P, Hofkens J, Uji-i H, Rocha S, et al. Nanoparticle-mediated thermal Cancer therapies: Strategies to improve clinical translatability. J Control Release. (2024) 372:751–77. doi: 10.1016/j.jconrel.2024.06.055
21. Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, et al. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. (2016) 1:16014. doi: 10.1038/natrevmats.2016.14
22. Al-Thani AN, Jan AG, Abbas M, Geetha M, and Sadasivuni KK. Nanoparticles in cancer theragnostic and drug delivery: A comprehensive review. Life Sci. (2024) 352:122899. doi: 10.1016/j.lfs.2024.122899
23. Garbayo E, Pascual-Gil S, Rodríguez-Nogales C, Saludas L, Estella-Hermoso de Mendoza A, and Blanco-Prieto MJ. Nanomedicine and drug delivery systems in cancer and regenerative medicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. (2020) 12:e1637. doi: 10.1002/wnan.1637
24. Dhiman R, Bazad N, Mukherjee R, Himanshu G, Leal E, Ahmad S, et al. Enhanced drug delivery with nanocarriers: a comprehensive review of recent advances in breast cancer detection and treatment. Discov Nano. (2024) 19:143. doi: 10.1186/s11671-024-04086-6
25. Yan S, Na J, Liu X, and Wu P. Different targeting ligands-mediated drug delivery systems for tumor therapy. Pharmaceutics. (2024) 16:248. doi: 10.3390/pharmaceutics16020248
26. Spada A and Gerber-Lemaire S. Surface functionalization of nanocarriers with anti-EGFR ligands for cancer active targeting. Nanomaterials. (2025) 15:158. doi: 10.3390/nano15030158
27. Lyakhovich A and Lleonart ME. Bypassing mechanisms of mitochondria-mediated cancer stem cells resistance to chemo- and radiotherapy. Oxid Med Cell Longev. (2016) 2016:1716341. doi: 10.1155/2016/1716341
28. Chen W and Yang L. Targeted Delivery with Imaging Assessment of siRNA Expressing Nanocassettes into Cancer. Methods Mol Biol. (2016) 2016:49–59. doi: 10.1007/978-1-4939-3148-4_4
29. Duan H, Liu Y, Gao Z, and Huang W. Recent advances in drug delivery systems for targeting cancer stem cells. Acta Pharm Sin B. (2021) 11:55–70. doi: 10.1016/j.apsb.2020.09.016
30. dos Santos SN and Witney TH. Molecular imaging of cancer stem cells and their role in therapy resistance. J Nucl Med. (2025) 66:14–9. doi: 10.2967/jnumed.124.267657
31. Zhou M, Li L, Li L, Lin X, Wang F, Li Q, et al. Overcoming chemotherapy resistance via simultaneous drug-efflux circumvention and mitochondrial targeting. Acta Pharm Sin B. (2019) 9:615–25. doi: 10.1016/j.apsb.2018.11.005
32. Ulldemolins A, Seras-Franzoso J, Andrade F, Rafael D, Abasolo I, Gener P, et al. Perspectives of nano-carrier drug delivery systems to overcome cancer drug resistance in the clinics. Cancer Drug Resist. (2021) 4:44–68. doi: 10.20517/cdr.2020.59
33. Wei X, Song M, Li W, Huang J, Yang G, and Wang Y. Multifunctional nanoplatforms co-delivering combinatorial dual-drug for eliminating cancer multidrug resistance. Theranostics. (2021) 11:6334–54. doi: 10.7150/thno.59342
34. Wang C, Li F, Zhang T, Yu M, and Sun Y. Recent advances in anti-multidrug resistance for nano-drug delivery system. Drug Deliv. (2022) 29:1684–97. doi: 10.1080/10717544.2022.2079771
35. Sun X, Zhao P, Lin J, Chen K, and Shen J. Recent advances in access to overcome cancer drug resistance by nanocarrier drug delivery system. Cancer Drug Resist. (2023) 6:390–415. doi: 10.20517/cdr.2023.16
37. Xu J, Zhang H, Nie Z, He W, Zhao Y, Huang Z, et al. Cancer stem-like cells stay in a plastic state ready for tumor evolution. Neoplasia. (2025) 61:101134. doi: 10.1016/j.neo.2025.101134
38. Pérez-González A, Bévant K, and Blanpain C. Cancer cell plasticity during tumor progression, metastasis and response to therapy. Nat Cancer. (2023) 4:1063–82. doi: 10.1038/s43018-023-00595-y
39. Shi Z-D, Pang K, Wu Z-X, Dong Y, Hao L, Qin J-X, et al. Tumor cell plasticity in targeted therapy-induced resistance: mechanisms and new strategies. Signal Transduct Target Ther. (2023) 8:113. doi: 10.1038/s41392-023-01383-x
40. Ayob AZ and Ramasamy TS. Cancer stem cells as key drivers of tumour progression. J BioMed Sci. (2018) 25:20. doi: 10.1186/s12929-018-0426-4
41. Yadav AK and Desai NS. Cancer stem cells: acquisition, characteristics, therapeutic implications, targeting strategies and future prospects. Stem Cell Rev Rep. (2019) 15:331–55. doi: 10.1007/s12015-019-09887-2
42. Frąszczak K and Barczyński B. Characteristics of cancer stem cells and their potential role in endometrial cancer. Cancers (Basel). (2024) 16:1–20. doi: 10.3390/cancers16061083
43. Lim JR, Mouawad J, Gorton OK, Bubb WA, and Kwan AH. Cancer stem cell characteristics and their potential as therapeutic targets. Med Oncol. (2021) 38:1–12. doi: 10.1007/s12032-021-01524-8
44. Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang J, et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. (2020) 5:8. doi: 10.1038/s41392-020-0110-5
45. Yang Y, Li X, Wang T, Guo Q, Xi T, and Zheng L. Emerging agents that target signaling pathways in cancer stem cells. J Hematol Oncol. (2020) 13:60. doi: 10.1186/s13045-020-00901-6
46. Jin H, Wang L, and Bernards R. Rational combinations of targeted cancer therapies: background, advances and challenges. Nat Rev Drug Discov. (2023) 22:213–34. doi: 10.1038/s41573-022-00615-z
47. Jin N, Xia Y, and Gao Q. Combined PARP inhibitors and small molecular inhibitors in solid tumor treatment (Review). Int J Oncol. (2023) 62:28. doi: 10.3892/ijo.2023.5476
48. Liu L, Yang L, Yan W, Zhai J, Pizzo DP, Chu P, et al. Chemotherapy induces breast cancer stemness in association with dysregulated monocytosis. Clin Cancer Res. (2018) 24:2370–82. doi: 10.1158/1078-0432.CCR-17-2545
49. Rezayatmand H, Razmkhah M, and Razeghian-Jahromi I. Drug resistance in cancer therapy: the Pandora’s Box of cancer stem cells. Stem Cell Res Ther. (2022) 13:181. doi: 10.1186/s13287-022-02856-6
50. BeLow M and Osipo C. Notch signaling in breast cancer: A role in drug resistance. Cells. (2020) 9:2204. doi: 10.3390/cells9102204
51. Angom RS, Mondal SK, Wang F, Madamsetty VS, Wang E, Dutta SK, et al. Ablation of neuropilin-1 improves the therapeutic response in conventional drug-resistant glioblastoma multiforme. Oncogene. (2020) 39:7114–26. doi: 10.1038/s41388-020-01462-1
52. Douyère M, Chastagner P, and Boura C. Neuropilin-1: A key protein to consider in the progression of pediatric brain tumors. Front Oncol. (2021) 11:665634. doi: 10.3389/fonc.2021.665634
53. Alhaddad L, Osipov AN, and Leonov S. The molecular and cellular strategies of glioblastoma and non-small-cell lung cancer cells conferring radioresistance. Int J Mol Sci. (2022) 23:13577. doi: 10.3390/ijms232113577
54. Yan Q, Fang X, Li C, Lan P, and Guan X. Oncofetal proteins and cancer stem cells. Essays Biochem. (2022) 66:423–33. doi: 10.1042/EBC20220025
55. Zhang Z and Zhang Y. Transcriptional regulation of cancer stem cell: regulatory factors elucidation and cancer treatment strategies. J Exp Clin Cancer Res. (2024) 43:99. doi: 10.1186/s13046-024-03021-y
56. Nguyen LH, Robinton DA, Seligson MT, Wu L, Li L, Rakheja D, et al. Lin28b is sufficient to drive liver cancer and necessary for its maintenance in murine models. Cancer Cell. (2014) 26:248–61. doi: 10.1016/j.ccr.2014.06.018
57. Tian N, Shangguan W, Zhou Z, Yao Y, Fan C, and Cai L. Lin28b is involved in curcumin-reversed paclitaxel chemoresistance and associated with poor prognosis in hepatocellular carcinoma. J Cancer. (2019) 10:6074–87. doi: 10.7150/jca.33421
58. Lu B, Huang X, Mo J, and Zhao W. Drug delivery using nanoparticles for cancer stem-like cell targeting. Front Pharmacol. (2016) 7:84. doi: 10.3389/fphar.2016.00084
59. Tiek D and Cheng S-Y. DNA damage and metabolic mechanisms of cancer drug resistance. Cancer Drug Resist. (2022) 5:368–79. doi: 10.20517/cdr.2021.148
61. Najafi M, Farhood B, and Mortezaee K. Cancer stem cells (CSCs) in cancer progression and therapy. J Cell Physiol. (2019) 234:8381–95. doi: 10.1002/jcp.27740
62. Jin X, Jin X, and Kim H. Cancer stem cells and differentiation therapy. Tumor Biol. (2017) 39:101042831772993. doi: 10.1177/1010428317729933
63. Testa U, Petrucci E, Pasquini L, Castelli G, and Pelosi E. Ovarian cancers: genetic abnormalities, tumor heterogeneity and progression, clonal evolution and cancer stem cells. Medicines. (2018) 5:16. doi: 10.3390/medicines5010016
64. Cazet AS, Hui MN, Elsworth BL, Wu SZ, Roden D, Chan C-L, et al. Targeting stromal remodeling and cancer stem cell plasticity overcomes chemoresistance in triple negative breast cancer. Nat Commun. (2018) 9:2897. doi: 10.1038/s41467-018-05220-6
65. Naz F, Shi M, Sajid S, Yang Z, and Yu C. Cancer stem cells: a major culprit of intra-tumor heterogeneity. Am J Cancer Res. (2021) 11:5782–811.
66. Resendiz-Hernández M, García-Hernández AP, Silva-Cázares MB, Coronado-Uribe R, Hernández-de la Cruz ON, Arriaga-Pizano LA, et al. MicroRNA-204 regulates angiogenesis and vasculogenic mimicry in CD44+/CD24– breast cancer stem-like cells. Noncoding RNA. (2024) 10:14. doi: 10.3390/ncrna10010014
67. Peng L, Xiong Y, Wang R, Xiang L, Zhou H, and Fu Z. The critical role of peroxiredoxin-2 in colon cancer stem cells. Aging. (2021) 13:11170–87. doi: 10.18632/aging.202784
68. Chen Z, Gao Y, Zhang P, Liu Y, Wei B, Chen L, et al. Identification of gastric cancer stem cells with CD44 and Lgr5 double labelling and their initial roles on gastric cancer Malignancy and chemotherapy resistance. Cell Biol Toxicol. (2025) 41:-14. doi: 10.1007/s10565-024-09960-8
69. Wang X, Cai J, Zhao L, Zhang D, Xu G, Hu J, et al. NUMB suppression by miR-9-5P enhances CD44+ prostate cancer stem cell growth and metastasis. Sci Rep. (2021) 11:1–11. doi: 10.1038/s41598-021-90700-x
70. Pustovalova M, Blokhina T, Alhaddad L, Chigasova A, Chuprov-Netochin R, Veviorskiy A, et al. CD44+ and CD133+ Non-small cell lung cancer cells exhibit DNA damage response pathways and dormant polyploid giant cancer cell enrichment relating to their p53 status. Int J Mol Sci. (2022) 23:4922. doi: 10.3390/ijms23094922
71. Liu F and Qian Y. The role of CD133 in hepatocellular carcinoma. Cancer Biol Ther. (2021) 22:291–300. doi: 10.1080/15384047.2021.1916381
72. Zheng W, Peng W, Qian F, Zhang M, Duan B, Fan Z, et al. Vitamin D suppresses CD133+/CD44 + cancer stem cell stemness by inhibiting NF-κB signaling and reducing NLRP3 expression in triple-negative breast cancer. Cancer Chemother Pharmacol. (2024) 94:67–78. doi: 10.1007/s00280-024-04660-w
73. He L, Qian H, Seyiti A, Yang C, Shi N, Chen C, et al. CD133+/ABCC5+ cervical cancer cells exhibit cancer stem cell properties. Heliyon. (2024) 10:e37066. doi: 10.1016/j.heliyon.2024.e37066
74. Kim B, Sohn HM, Hyun H, and Lim W. Effect of HDAC9 inhibition on epithelial-mesenchymal transition in CD133+ prostate cancer cell lines. J Chemother. (2022) 34:45–54. doi: 10.1080/1120009X.2021.1963615
75. Asakura N, Nakamura N, Muroi A, Nojima Y, Yamashita T, Kaneko S, et al. Expression of cancer stem cell markers epcam and cd90 is correlated with anti- and pro-oncogenic epha2 signaling in hepatocellular carcinoma. Int J Mol Sci. (2021) 22:1–13. doi: 10.3390/ijms22168652
76. Kumar A, Bhanja A, Bhattacharyya J, and Jaganathan BG. Multiple roles of CD90 in cancer. Tumor Biol. (2016) 37:11611–22. doi: 10.1007/s13277-016-5112-0
77. Kim DK, Ham MH, Lee SY, Shin MJ, Kim YE, Song P, et al. CD166 promotes the cancer stem-like properties of primary epithelial ovarian cancer cells. BMB Rep. (2020) 53:622–7. doi: 10.5483/BMBRep.2020.53.12.102
78. Xu L, Mohammad KS, Wu H, Crean C, Poteat B, Cheng Y, et al. Cell adhesion molecule CD166 drives Malignant progression and osteolytic disease in multiple myeloma. Cancer Res. (2016) 76:6901–10. doi: 10.1158/0008-5472.CAN-16-0517
79. Levin TG, Powell AE, Davies PS, Silk AD, Dismuke AD, Anderson EC, et al. Characterization of the intestinal cancer stem cell marker CD166 in the human and mouse gastrointestinal tract. Gastroenterology. (2010) 139:2072–82. doi: 10.1053/j.gastro.2010.08.053
80. Ferragut F, Vachetta VS, Troncoso MF, Rabinovich GA, and Elola MT. ALCAM/CD166: A pleiotropic mediator of cell adhesion, stemness and cancer progression. Cytokine Growth Factor Rev. (2021) 61:27–37. doi: 10.1016/J.CYTOGFR.2021.07.001
81. El-Ashmawy NE, Salem ML, Abd El-Fattah EE, and Khedr EG. Targeting CD166+ lung cancer stem cells: Molecular study using murine dendritic cell vaccine. Toxicol Appl Pharmacol. (2021) 429:115699. doi: 10.1016/J.TAAP.2021.115699
82. Yang Y, Zhu G, Yang L, and Yang Y. Targeting CD24 as a novel immunotherapy for solid cancers. Cell Commun Signal. (2023) 21:1–14. doi: 10.1186/s12964-023-01315-w
83. Hong P, Xu T, Xu J, Chen W, Hu H, Chen J, et al. CD24 promotes metastasis and chemoresistance by directly targeting Arf6-ERK pathway in esophageal squamous cell carcinoma. Cancer Lett. (2024) 594:216994. doi: 10.1016/J.CANLET.2024.216994
84. Gu Y, Zhou G, Tang X, Shen F, Ding J, and Hua K. The biological roles of CD24 in ovarian cancer: old story, but new tales. Front Immunol. (2023) 14:1183285. doi: 10.3389/fimmu.2023.1183285
85. Vasefifar P, Motafakkerazad R, Maleki LA, Najafi S, Ghrobaninezhad F, Najafzadeh B, et al. Nanog, as a key cancer stem cell marker in tumor progression. Gene. (2022) 827:146448. doi: 10.1016/J.GENE.2022.146448
86. Saito M. Novel roles of nanog in cancer cells and their extracellular vesicles. Cells. (2022) 11:3881. doi: 10.3390/cells11233881
87. Rodrigo JP, Villaronga MÁ, Menéndez ST, Hermida-Prado F, Quer M, Vilaseca I, et al. A novel role for nanog as an early cancer risk marker in patients with laryngeal precancerous lesions. Sci Rep. (2017) 7:1–7. doi: 10.1038/s41598-017-11709-9
88. Alemohammad H, Asadzadeh Z, Motafakker azad R, Hemmat N, Najafzadeh B, Vasefifar P, et al. Signaling pathways and microRNAs, the orchestrators of NANOG activity during cancer induction. Life Sci. (2020) 260:118337. doi: 10.1016/J.LFS.2020.118337
89. Mohiuddin IS, Wei S-J, and Kang MH. Role of OCT4 in cancer stem-like cells and chemotherapy resistance. Biochim Biophys Acta Mol Basis Dis. (2020) 1866:165432. doi: 10.1016/j.bbadis.2019.03.005
90. Wang G, Zhou H, Gu Z, Gao Q, and Shen G. Oct4 promotes cancer cell proliferation and migration and leads to poor prognosis associated with the survivin/STAT3 pathway in hepatocellular carcinoma. Oncol Rep. (2018) 40:979–87. doi: 10.3892/or.2018.6491
91. El-Guindy DM, Wasfy RE, Abdel Ghafar MT, Ali DA, and Elkady AM. Oct4 expression in gastric carcinoma: association with tumor proliferation, angiogenesis and survival. J Egypt Natl Canc Inst. (2019) 31:3–3. doi: 10.1186/s43046-019-0005-0
92. Zhang S, Xiong X, and Sun Y. Functional characterization of SOX2 as an anticancer target. Signal Transduct Target Ther. (2020) 5:135. doi: 10.1038/s41392-020-00242-3
93. Al Mamun M, Mannoor K, Cao J, Qadri F, and Song X. SOX2 in cancer stemness: Tumor Malignancy and therapeutic potentials. J Mol Cell Biol. (2020) 12:85–98. doi: 10.1093/JMCB/MJY080
94. Zhu Y, Huang S, Chen S, Chen J, Wang Z, Wang Y, et al. SOX2 promotes chemoresistance, cancer stem cells properties, and epithelial–mesenchymal transition by β-catenin and Beclin1/autophagy signaling in colorectal cancer. Cell Death Dis. (2021) 12:449. doi: 10.1038/s41419-021-03733-5
95. Aiman Mohtar M, Syafruddin SE, Nasir SN, and Yew LT. Revisiting the roles of pro-metastatic epcam in cancer. Biomolecules. (2020) 10:255. doi: 10.3390/biom10020255
96. Schnell U, Cirulli V, and Giepmans BNG. EpCAM: Structure and function in health and disease. Biochim Biophys Acta Biomembr. (2013) 1828:1989–2001. doi: 10.1016/j.bbamem.2013.04.018
97. Xiao D, Xiong M, Wang X, Lyu M, Sun H, Cui Y, et al. Regulation of the function and expression of epCAM. Biomedicines. (2024) 12:1129. doi: 10.3390/biomedicines12051129
98. Liu G, Chen T, Zhang X, Ma X, and Shi H. Small molecule inhibitors targeting the cancers. MedComm (Beijing). (2022) 3:e181. doi: 10.1002/mco2.181
99. Li XS, Xu Q, Fu XY, and Luo WS. Heat shock protein 60 overexpression is associated with the progression and prognosis in gastric cancer. PloS One. (2014) 9:1–8. doi: 10.1371/journal.pone.0107507
100. Yue H, Hu Z, Hu R, Guo Z, Zheng Y, Wang Y, et al. ALDH1A1 in cancers: bidirectional function, drug resistance, and regulatory mechanism. Front Oncol. (2022) 12:918778. doi: 10.3389/fonc.2022.918778
101. Tomita H, Tanaka K, Tanaka T, and Hara A. Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget. (2016) 7:11018–32. doi: 10.18632/oncotarget.6920
102. Xu L, Lin W, Wen L, and Li G. Lgr5 in cancer biology: Functional identification of Lgr5 in cancer progression and potential opportunities for novel therapy. Stem Cell Res Ther. (2019) 10:1–9. doi: 10.1186/s13287-019-1288-8
103. Fujii M and Sato T. Defining the role of Lgr5+ stem cells in colorectal cancer: From basic research to clinical applications. Genome Med. (2017) 9:1–4. doi: 10.1186/s13073-017-0460-y
104. Hadjimichael C. Common stemness regulators of embryonic and cancer stem cells. World J Stem Cells. (2015) 7:1150. doi: 10.4252/wjsc.v7.i9.1150
105. Murar M and Vaidya A. Cancer stem cell markers: premises and prospects. Biomark Med. (2015) 9:1331–42. doi: 10.2217/bmm.15.85
106. Zhao W, Li Y, and Zhang X. Stemness-related markers in cancer. Cancer Transl Med. (2017) 3:87. doi: 10.4103/ctm.ctm_69_16
107. Gopalan V, Islam F, and Lam AK. Surface markers for the identification of cancer stem cells. Methods Mol Biol. (2018) 1692:17–29. doi: 10.1007/978-1-4939-7401-6_2
108. Song S, Li Y, Zhang K, Zhang X, Huang Y, Xu M, et al. Cancer Stem Cells of Diffuse Large B Cell Lymphoma Are Not Enriched in the CD45 + CD19 - cells but in the ALDH high Cells. J Cancer. (2020) 11:142–52. doi: 10.7150/jca.35000
109. Walcher L, Kistenmacher A-K, Suo H, Kitte R, Dluczek S, Strauß A, et al. Cancer stem cells—Origins and biomarkers: perspectives for targeted personalized therapies. Front Immunol. (2020) 11:1280. doi: 10.3389/fimmu.2020.01280
110. Guo W, Wang H, Chen P, Shen X, Zhang B, Liu J, et al. Identification and characterization of multiple myeloma stem cell-like cells. Cancers (Basel). (2021) 13:3523. doi: 10.3390/cancers13143523
111. Huang T-X, Guan X-Y, and Fu L. Therapeutic targeting of the crosstalk between cancer-associated fibroblasts and cancer stem cells. Am J Cancer Res. (2019) 9:1889–904.
112. Yehya A, Youssef J, Hachem S, Ismael J, and Abou-Kheir W. Tissue-specific cancer stem/progenitor cells: Therapeutic implications. World J Stem Cells. (2023) 15:323–41. doi: 10.4252/wjsc.v15.i5.323
113. Bao B, Ahmad A, Azmi AS, Ali S, and Sarkar FH. Overview of cancer stem cells (CSCs) and mechanisms of their regulation: implications for cancer therapy. Curr Protoc Pharmacol. (2013) 61:Chapter 14:Unit 14.25. doi: 10.1002/0471141755.ph1425s61
114. Mayani H, Chávez-González A, Vázquez-Santillan K, Contreras J, and Guzman ML. Cancer stem cells: biology and therapeutic implications. Arch Med Res. (2022) 53:770–84. doi: 10.1016/j.arcmed.2022.11.012
115. Misra R, Kandoi S, Varadaraj S, Vijayalakshmi S, Nanda A, and Verma RS. Nanotheranostics: A tactic for cancer stem cells prognosis and management. J Drug Deliv Sci Technol. (2020) 55:101457. doi: 10.1016/j.jddst.2019.101457
116. Manni W and Min W. Signaling pathways in the regulation of cancer stem cells and associated targeted therapy. MedComm (Beijing). (2022) 3:e176. doi: 10.1002/mco2.176
117. Zeng H, Guo S, Ren X, Wu Z, Liu S, and Yao X. Current strategies for exosome cargo loading and targeting delivery. Cells. (2023) 12:1416. doi: 10.3390/cells12101416
118. Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang X, et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. (2022) 7:3. doi: 10.1038/s41392-021-00762-6
119. Pan Y, Ma S, Cao K, Zhou S, Zhao A, Li M, et al. Therapeutic approaches targeting cancer stem cells. J Cancer Res Ther. (2018) 14:1469. doi: 10.4103/jcrt.JCRT_976_17
120. Futakuchi M, Lami K, Tachibana Y, Yamamoto Y, Furukawa M, and Fukuoka J. The effects of TGF-β Signaling on cancer cells and cancer stem cells in the bone microenvironment. Int J Mol Sci. (2019) 20:5117. doi: 10.3390/ijms20205117
121. Kharkar PS. Cancer stem cell (CSC) inhibitors in oncology—A promise for a better therapeutic outcome: state of the art and future perspectives. J Med Chem. (2020) 63:15279–307. doi: 10.1021/acs.jmedchem.0c01336
122. Kuramoto K, Yamamoto M, Suzuki S, Togashi K, Sanomachi T, Kitanaka C, et al. Inhibition of the lipid droplet–peroxisome proliferator-activated receptor α Axis suppresses cancer stem cell properties. Genes (Basel). (2021) 12:99. doi: 10.3390/genes12010099
123. Doustmihan A, Fathi M, Mazloomi M, Salemi A, Hamblin MR, and Jahanban-Esfahlan R. Molecular targets, therapeutic agents and multitasking nanoparticles to deal with cancer stem cells: A narrative review. J Control Release. (2023) 363:57–83. doi: 10.1016/j.jconrel.2023.09.029
124. Liu Y, Wang Y, Sun S, Chen Z, Xiang S, Ding Z, et al. Understanding the versatile roles and applications of EpCAM in cancers: from bench to bedside. Exp Hematol Oncol. (2022) 11:1–19. doi: 10.1186/s40164-022-00352-4
125. Chatterjee A, Paul S, Bisht B, Bhattacharya S, Sivasubramaniam S, and Paul MK. Advances in targeting the WNT/β-catenin signaling pathway in cancer. Drug Discov Today. (2022) 27:82–101. doi: 10.1016/j.drudis.2021.07.007
126. Banerjee S and Mandal AKA. Role of microRNA modulated wnt pathway in breast cancer and its therapeutic use. Cytol Genet. (2024) 58:326–42. doi: 10.3103/S0095452724040108
127. Madan B and Virshup DM. Targeting wnts at the source—New mechanisms, new biomarkers, new drugs. Mol Cancer Ther. (2015) 14:1087–94. doi: 10.1158/1535-7163.MCT-14-1038
128. Jansson EÅ, Are A, Greicius G, Kuo I-C, Kelly D, Arulampalam V, et al. The Wnt/β-catenin signaling pathway targets PPARγ activity in colon cancer cells. PNAS. (2005) 102:1460–5. doi: 10.1073/pnas.0405928102
129. Zhu C, Cheng K-W, Ouyang N, Huang L, Sun Y, Constantinides P, et al. Phosphosulindac (OXT-328) selectively targets breast cancer stem cells in vitro and in human breast cancer xenografts. Stem Cells. (2012) 30:2065–75. doi: 10.1002/stem.1139
130. Stakheev D, Taborska P, Strizova Z, Podrazil M, Bartunkova J, and Smrz D. The WNT/β-catenin signaling inhibitor XAV939 enhances the elimination of LNCaP and PC-3 prostate cancer cells by prostate cancer patient lymphocytes in vitro. Sci Rep. (2019) 9:4761. doi: 10.1038/s41598-019-41182-5
131. Chen S, Yuan X, Xu H, Yi M, Liu S, and Wen F. WNT974 inhibits proliferation, induces apoptosis, and enhances chemosensitivity to doxorubicin in lymphoma cells by inhibiting wnt/β-catenin signaling. Med Sci Monit. (2020) 26:e939724-1. doi: 10.12659/MSM.923799
132. Zhang Y and Wang X. Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol. (2020) 13:165. doi: 10.1186/s13045-020-00990-3
133. Trujano-Camacho S, Cantú-de León D, Delgado-Waldo I, Coronel-Hernández J, Millan-Catalan O, Hernández-Sotelo D, et al. Inhibition of wnt-β-catenin signaling by ICRT14 drug depends of post-transcriptional regulation by HOTAIR in human cervical cancer heLa cells. Front Oncol. (2021) 11:729228. doi: 10.3389/fonc.2021.729228
134. Choudhary S, Singh MK, Kashyap S, Seth R, and Singh L. Wnt/β-catenin signaling pathway in pediatric tumors: implications for diagnosis and treatment. Children. (2024) 11:700. doi: 10.3390/children11060700
135. Song P, Gao Z, Bao Y, Chen L, Huang Y, Liu Y, et al. Wnt/β-catenin signaling pathway in carcinogenesis and cancer therapy. J Hematol Oncol. (2024) 17:46. doi: 10.1186/s13045-024-01563-4
136. Prabhu VV, Lulla AR, Madhukar NS, Ralff MD, Zhao D, Kline CLB, et al. Cancer stem cell-related gene expression as a potential biomarker of response for first-in-class imipridone ONC201 in solid tumors. PLoS One. (2017) 12:e0180541. doi: 10.1371/journal.pone.0180541
137. Park S-Y, Kim J-Y, Choi J-H, Kim J-H, Lee C-J, Singh P, et al. Inhibition of LEF1-mediated DCLK1 by niclosamide attenuates colorectal cancer stemness. Clin Cancer Res. (2019) 25:1415–29. doi: 10.1158/1078-0432.CCR-18-1232
138. Roy S, Roy S, Kar M, Chakraborty A, Kumar A, Delogu F, et al. Combined treatment with cisplatin and the tankyrase inhibitor XAV-939 increases cytotoxicity, abrogates cancer-stem-like cell phenotype and increases chemosensitivity of head-and-neck squamous-cell carcinoma cells. Mutat Res Genet Toxicol Environ Mutagen. (2019) 846:503084. doi: 10.1016/j.mrgentox.2019.503084
139. Iluta S, Nistor M, Buruiana S, and Dima D. Notch and hedgehog signaling unveiled: crosstalk, roles, and breakthroughs in cancer stem cell research. Life. (2025) 15:228. doi: 10.3390/life15020228
140. Bohl SR, Pircher A, and Hilbe W. Cancer stem cells: characteristics and their potential role for new therapeutic strategies. Onkologie. (2011) 34:269–74. doi: 10.1159/000327815
141. Singh BN, Fu J, Srivastava RK, and Shankar S. Hedgehog signaling antagonist GDC-0449 (Vismodegib) inhibits pancreatic cancer stem cell characteristics: molecular mechanisms. PLoS One. (2011) 6:e27306. doi: 10.1371/journal.pone.0027306
142. Casey D, Demko S, Shord S, Zhao H, Chen H, He K, et al. FDA approval summary: sonidegib for locally advanced basal cell carcinoma. Clin Cancer Res. (2017) 23:2377–81. doi: 10.1158/1078-0432.CCR-16-2051
143. Li W, Yang H, Li X, Han L, Xu N, and Shi A. Signaling pathway inhibitors target breast cancer stem cells in triple-negative breast cancer. Oncol Rep. (2018) 41:437–46. doi: 10.3892/or.2018.6805
144. Xin M, Ji X, de la Cruz LK, Thareja S, and Wang B. Strategies to target the Hedgehog signaling pathway for cancer therapy. Med Res Rev. (2018) 38:870–913. doi: 10.1002/med.21482
145. Choi HS, Kim S-L, Kim J-H, and Lee D-S. The FDA-approved anti-asthma medicine ciclesonide inhibits lung cancer stem cells through hedgehog signaling-mediated SOX2 regulation. Int J Mol Sci. (2020) 21:1014. doi: 10.3390/ijms21031014
146. Siebel C and Lendahl U. Notch signaling in development, tissue homeostasis, and disease. Physiol Rev. (2017) 97:1235–94. doi: 10.1152/physrev.00005.2017
147. Akil A, Gutiérrez-García AK, Guenter R, Rose JB, Beck AW, Chen H, et al. Notch signaling in vascular endothelial cells, angiogenesis, and tumor progression: an update and prospective. Front Cell Dev Biol. (2021) 9:642352. doi: 10.3389/fcell.2021.642352
148. Fasoulakis Z, Koutras A, Ntounis T, Pergialiotis V, Chionis A, Katrachouras A, et al. The prognostic role and significance of dll4 and toll-like receptors in cancer development. Cancers (Basel). (2022) 14:1649. doi: 10.3390/cancers14071649
149. Venkatesh V, Nataraj R, Thangaraj GS, Karthikeyan M, Gnanasekaran A, Kaginelli SB, et al. Targeting Notch signalling pathway of cancer stem cells. Stem Cell Invest. (2018) 5:5–5. doi: 10.21037/sci.2018.02.02
150. Ponnurangam S, Dandawate PR, Dhar A, Tawfik OW, Parab RR, Mishra PD, et al. Quinomycin A targets Notch signaling pathway in pancreatic cancer stem cells. Oncotarget. (2016) 7:3217–32. doi: 10.18632/oncotarget.6560
151. Cook N, Basu B, Smith D-M, Gopinathan A, Evans J, Steward WP, et al. A phase I trial of the γ-secretase inhibitor MK-0752 in combination with gemcitabine in patients with pancreatic ductal adenocarcinoma. Br J Cancer. (2018) 118:793–801. doi: 10.1038/bjc.2017.495
152. Keyghobadi F, Mehdipour M, Nekoukar V, Firouzi J, Kheimeh A, Nobakht Lahrood F, et al. Long-term inhibition of notch in A-375 melanoma cells enhances tumor growth through the enhancement of AXIN1, CSNK2A3, and CEBPA2 as intermediate genes in wnt and notch pathways. Front Oncol. (2020) 10:531. doi: 10.3389/fonc.2020.00531
153. Ju F, Atyah MM, Horstmann N, Gul S, Vago R, Bruns CJ, et al. Characteristics of the cancer stem cell niche and therapeutic strategies. Stem Cell Res Ther. (2022) 13:233. doi: 10.1186/s13287-022-02904-1
154. An SS, Jang G-B, Lee H-Y, Hong I-S, and Nam J-S. Targeting cancer stem cells by using the nanoparticles. Int J Nanomed. (2015) 251. doi: 10.2147/IJN.S88310
155. Ertas YN, Abedi Dorcheh K, Akbari A, and Jabbari E. Nanoparticles for targeted drug delivery to cancer stem cells: A review of recent advances. Nanomaterials. (2021) 11:1755. doi: 10.3390/nano11071755
156. Lahn M, Herbertz S, Sawyer JS, Stauber AJ, Gueorguieva I, Driscoll KE, et al. Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug Des Devel Ther. (2015) 9:4479–99. doi: 10.2147/DDDT.S86621
157. Chan MK-K, Chan EL-Y, Ji ZZ, Chan AS-W, Li C, Leung K-T, et al. Transforming growth factor-β signaling: from tumor microenvironment to anticancer therapy. Explor Target Antitumor Ther. (2023) 4:316–43. doi: 10.37349/etat.2023.00137
158. Yang T, Huang T, Zhang D, Wang M, Wu B, Shang Y, et al. TGF-β receptor inhibitor LY2109761 enhances the radiosensitivity of gastric cancer by inactivating the TGF-β/SMAD4 signaling pathway. Aging. (2019) 11:8892–910. doi: 10.18632/aging.102329
159. Shou M, Zhou H, and Ma L. New advances in cancer therapy targeting TGF-β signaling pathways. Mol Ther Oncolyt. (2023) 31:100755. doi: 10.1016/j.omto.2023.100755
160. Guo Y, Wang Z, Zhou H, Pan H, Han W, Deng Y, et al. First-in-human study of GFH018, a small molecule inhibitor of transforming growth factor-β receptor I inhibitor, in patients with advanced solid tumors. BMC Cancer. (2024) 24:444. doi: 10.1186/s12885-024-12216-7
161. Leonardo-Sousa C, Barriga R, Florindo HF, Acúrcio RC, and Guedes RC. Structural insights and clinical advances in small-molecule inhibitors targeting TGF-β receptor I. Mol Thera Oncol. (2025) 33:200945. doi: 10.1016/j.omton.2025.200945
162. Deng Z, Fan T, Xiao C, Tian H, Zheng Y, Li C, et al. TGF-β signaling in health, disease and therapeutics. Signal Transduct Target Ther. (2024) 9:61. doi: 10.1038/s41392-024-01764-w
163. Mascarenhas J, Migliaccio AR, Kosiorek H, Bhave R, Palmer J, Kuykendall A, et al. A phase ib trial of AVID200, a TGFβ 1/3 trap, in patients with myelofibrosis. Clin Cancer Res. (2023) 29:3622–32. doi: 10.1158/1078-0432.CCR-23-0276
164. Lee H-J. Recent advances in the development of TGF-β Signaling inhibitors for anticancer therapy. J Cancer Prev. (2020) 25:213–22. doi: 10.15430/JCP.2020.25.4.213
165. Welsh BT, Faucette R, Bilic S, Martin CJ, Schürpf T, Chen D, et al. Nonclinical development of SRK-181: an anti-latent TGFβ1 monoclonal antibody for the treatment of locally advanced or metastatic solid tumors. Int J Toxicol. (2021) 40:226–41. doi: 10.1177/1091581821998945
166. Paz-Ares L, Kim TM, Vicente D, Felip E, Lee DH, Lee KH, et al. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in second-line treatment of patients with NSCLC: results from an expansion cohort of a phase 1 trial. J Thorac Oncol. (2020) 15:1210–22. doi: 10.1016/j.jtho.2020.03.003
167. Jaschinski F, Rothhammer T, Jachimczak P, Seitz C, Schneider A, and Schlingensiepen K-H. The antisense oligonucleotide trabedersen (AP 12009) for the targeted inhibition of TGF-&946;2. Curr Pharm Biotechnol. (2011) 12:2203–13. doi: 10.2174/138920111798808266
168. Luangmonkong T, Suriguga S, Adhyatmika A, Adlia A, Oosterhuis D, Suthisisang C, et al. In vitro and ex vivo anti-fibrotic effects of LY2109761, a small molecule inhibitor against TGF-β. Toxicol Appl Pharmacol. (2018) 355:127–37. doi: 10.1016/j.taap.2018.07.001
169. Yingling JM, McMillen WT, Yan L, Huang H, Sawyer JS, Graff J, et al. Preclinical assessment of galunisertib (LY2157299 monohydrate), a first-in-class transforming growth factor-β receptor type I inhibitor. Oncotarget. (2018) 9:6659–77. doi: 10.18632/oncotarget.23795
170. Liu S, Ren J, and ten Dijke P. Targeting TGFβ signal transduction for cancer therapy. Signal Transduct Target Ther. (2021) 6:8. doi: 10.1038/s41392-020-00436-9
171. Tan B, Khattak A, Felip E, Kelly K, Rich P, Wang D, et al. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with esophageal adenocarcinoma: results from a phase 1 cohort. Target Oncol. (2021) 16:435–46. doi: 10.1007/s11523-021-00809-2
172. Choi SH, Myers J, Tomchuck S, Bonner M, Eid S, Kingsley D, et al. Oral TGF-βR1 inhibitor Vactosertib promotes osteosarcoma regression by targeting tumor proliferation and enhancing anti-tumor immunity. Res Sq [Preprint]. doi: 10.21203/rs.3.rs-2709282/v1. Update: Cancer Commun (Lond). (2023) 44:884–8. doi: 10.1002/cac2.12589
173. Naidenow J, Hrgovic I, Doll M, Hailemariam-Jahn T, Lang V, Kleemann J, et al. Peroxisome proliferator-activated receptor (PPAR) α and δ activators induce ICAM-1 expression in quiescent non stimulated endothelial cells. J Inflammation. (2016) 13:27. doi: 10.1186/s12950-016-0135-2
174. Christofides A, Konstantinidou E, Jani C, and Boussiotis VA. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metabolism. (2021) 114:154338. doi: 10.1016/j.metabol.2020.154338
175. Asgharzadeh F, Memarzia A, Alikhani V, Beigoli S, and Boskabady MH. Peroxisome proliferator-activated receptors: Key regulators of tumor progression and growth. Transl Oncol. (2024) 47:102039. doi: 10.1016/j.tranon.2024.102039
176. Pan Y, Li Y, Fan H, Cui H, Chen Z, Wang Y, et al. Roles of the peroxisome proliferator-activated receptors (PPARs) in the pathogenesis of hepatocellular carcinoma (HCC). Biomed Pharmacother. (2024) 177:117089. doi: 10.1016/j.biopha.2024.117089
177. Du S, Wagner N, and Wagner K-D. The emerging role of PPAR beta/delta in tumor angiogenesis. PPAR Res. (2020) 2020:1–16. doi: 10.1155/2020/3608315
178. Talukdar J, Srivastava TP, Sahoo OS, Karmakar A, Rai AK, Sarma A, et al. Cancer stem cells: Signaling pathways and therapeutic targeting. MedComm – Oncol. (2023) 2:e62. doi: 10.1002/mog2.62
179. Sawayama H, Ishimoto T, Watanabe M, Yoshida N, Sugihara H, Kurashige J, et al. Small molecule agonists of PPAR-γ Exert therapeutic effects in esophageal cancer. Cancer Res. (2014) 74:575–85. doi: 10.1158/0008-5472.CAN-13-1836
180. Brown JR, Chan DK, Shank JJ, Griffith KA, Fan H, Szulawski R, et al. Phase II clinical trial of metformin as a cancer stem cell-targeting agent in ovarian cancer. JCI Insight. (2020) 5:e133247. doi: 10.1172/jci.insight.133247
181. Hu Q, Bian Q, Rong D, Wang L, Song J, Huang H-S, et al. JAK/STAT pathway: Extracellular signals, diseases, immunity, and therapeutic regimens. Front Bioeng Biotechnol. (2023) 11:1110765. doi: 10.3389/fbioe.2023.1110765
182. Xue C, Yao Q, Gu X, Shi Q, Yuan X, Chu Q, et al. Evolving cognition of the JAK-STAT signaling pathway: autoimmune disorders and cancer. Signal Transduct Target Ther. (2023) 8:204. doi: 10.1038/s41392-023-01468-7
183. Seif F, Khoshmirsafa M, Aazami H, Mohsenzadegan M, Sedighi G, and Bahar M. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signaling. (2017) 15:23. doi: 10.1186/s12964-017-0177-y
184. Silva VR, Santos L, de S, Dias RB, Quadros CA, and Bezerra DP. Emerging agents that target signaling pathways to eradicate colorectal cancer stem cells. Cancer Commun. (2021) 41:1275–313. doi: 10.1002/cac2.12235
185. Fahmideh H, Shapourian H, Moltafeti R, Tavakol C, Forghaniesfidvajani R, Zalpoor H, et al. The role of natural products as inhibitors of JAK/STAT signaling pathways in glioblastoma treatment. Oxid Med Cell Longev. (2022) 2022:1–17. doi: 10.1155/2022/7838583
186. Diller M, Hülser M-L, Hasseli R, Rehart S, Müller-Ladner U, and Neumann E. AB0492 Jak-inhibition with peficitinib and filgotinib in fibroblast-like synoviocytes in rheumatoid arthritis. Ann Rheum Dis. (2018) 77:1406–7. doi: 10.1136/annrheumdis-2018-eular.2182
187. Arnold R, Vehns E, Randl H, and Djabali K. Baricitinib, a JAK-STAT inhibitor, reduces the cellular toxicity of the farnesyltransferase inhibitor lonafarnib in progeria cells. Int J Mol Sci. (2021) 22:7474. doi: 10.3390/ijms22147474
188. Traves PG, Murray B, Campigotto F, Galien R, Meng A, and Di Paolo JA. JAK selectivity and the implications for clinical inhibition of pharmacodynamic cytokine signalling by filgotinib, upadacitinib, tofacitinib and baricitinib. Ann Rheum Dis. (2021) 80:865–75. doi: 10.1136/annrheumdis-2020-219012
189. Shawky AM, Almalki FA, Abdalla AN, Abdelazeem AH, and Gouda AM. A comprehensive overview of globally approved JAK inhibitors. Pharmaceutics. (2022) 14:1001. doi: 10.3390/pharmaceutics14051001
190. Zhang L, Guo L, Wang L, and Jiang X. The efficacy and safety of tofacitinib, peficitinib, solcitinib, baricitinib, abrocitinib and deucravacitinib in plaque psoriasis – A network meta-analysis. J Eur Acad Dermatol Venereol. (2022) 36:1937–46. doi: 10.1111/jdv.18263
191. Aihie O and Dyer JA. JAK inhibitors: A new weapon in the skin care providers’ Arsenal. Mo Med. (2023) 120:45–8.
192. Roskoski R. Deucravacitinib is an allosteric TYK2 protein kinase inhibitor FDA-approved for the treatment of psoriasis. Pharmacol Res. (2023) 189:106642. doi: 10.1016/j.phrs.2022.106642
193. Taylor PC, Choy E, Baraliakos X, Szekanecz Z, Xavier RM, Isaacs JD, et al. Differential properties of Janus kinase inhibitors in the treatment of immune-mediated inflammatory diseases. Rheumatology. (2024) 63:298–308. doi: 10.1093/rheumatology/kead448
194. Wei X-H and Liu Y-Y. Potential applications of JAK inhibitors, clinically approved drugs against autoimmune diseases, in cancer therapy. Front Pharmacol. (2024) 14:1326281. doi: 10.3389/fphar.2023.1326281
195. Świderska E, Strycharz J, Wróblewski A, Szemraj J, Drzewoski J, and Śliwińska A. Role of PI3K/AKT pathway in insulin-mediated glucose uptake. In: Blood Glucose Levels. IntechOpen (2020). doi: 10.5772/intechopen.80402
196. Kim C-W, Lee JM, and Park SW. Divergent roles of the regulatory subunits of class IA PI3K. Front Endocrinol (Lausanne). (2024) 14:1152579. doi: 10.3389/fendo.2023.1152579
197. Guerau-de-Arellano M, Piedra-Quintero ZL, and Tsichlis PN. Akt isoforms in the immune system. Front Immunol. (2022) 13:990874. doi: 10.3389/fimmu.2022.990874
198. Jhanwar-Uniyal M, Wainwright JV, Mohan AL, Tobias ME, Murali R, Gandhi CD, et al. Diverse signaling mechanisms of mTOR complexes: mTORC1 and mTORC2 in forming a formidable relationship. Adv Biol Regul. (2019) 72:51–62. doi: 10.1016/j.jbior.2019.03.003
199. Panwar V, Singh A, Bhatt M, Tonk RK, Azizov S, Raza AS, et al. Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Signal Transduct Target Ther. (2023) 8:375. doi: 10.1038/s41392-023-01608-z
200. Kim S, Heo S, Brzostowski J, and Kang D. Endosomal mTORC2 is required for phosphoinositide-dependent AKT activation in platelet-derived growth factor-stimulated glioma cells. Cancers (Basel). (2021) 13:2405. doi: 10.3390/cancers13102405
201. Choi E, Duan C, and Bai X. Regulation and function of insulin and insulin-like growth factor receptor signalling. Nature Reviews Molecular Cell Biology. (2025). doi: 10.1038/s41580-025-00826-3
202. Karami fath M, Ebrahimi M, Nourbakhsh E, Zia Hazara A, Mirzaei A, Shafieyari S, et al. PI3K/Akt/mTOR signaling pathway in cancer stem cells. Pathol Res Pract. (2022) 237:154010. doi: 10.1016/j.prp.2022.154010
203. Glaviano A, Foo ASC, Lam HY, Yap KCH, Jacot W, Jones RH, et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol Cancer. (2023) 22:138. doi: 10.1186/s12943-023-01827-6
204. Yang Q, Modi P, Newcomb T, Quéva C, and Gandhi V. Idelalisib: first-in-class PI3K delta inhibitor for the treatment of chronic lymphocytic leukemia, small lymphocytic leukemia, and follicular lymphoma. Clin Cancer Res. (2015) 21:1537–42. doi: 10.1158/1078-0432.CCR-14-2034
205. Suzuki Y, Enokido Y, Yamada K, Inaba M, Kuwata K, Hanada N, et al. The effect of rapamycin, NVP-BEZ235, aspirin, and metformin on PI3K/AKT/mTOR signaling pathway of PIK3CA -related overgrowth spectrum (PROS). Oncotarget. (2017) 8:45470–83. doi: 10.18632/oncotarget.17566
206. Heudel P, Frenel J-S, Dalban C, Bazan F, Joly F, Arnaud A, et al. Safety and efficacy of the mTOR inhibitor, vistusertib, combined with anastrozole in patients with hormone receptor–positive recurrent or metastatic endometrial cancer. JAMA Oncol. (2022) 8:1001. doi: 10.1001/jamaoncol.2022.1047
207. Altundag-Erdogan Ö, Çetinkaya H, Özgür Öteyaka M, and Çelebi-Saltik B. Targeting MDA-MB-231 cancer stem cells with temsirolimus in 3D collagen/PGA/na 2 siO 3 -based bone model. Macromol Mater Eng. (2025) 310:2400360. doi: 10.1002/mame.202400360
208. Sarker D, Ang JE, Baird R, Kristeleit R, Shah K, Moreno V, et al. First-in-human phase I study of pictilisib (GDC-0941), a potent pan–class I phosphatidylinositol-3-kinase (PI3K) inhibitor, in patients with advanced solid tumors. Clin Cancer Res. (2015) 21:77–86. doi: 10.1158/1078-0432.CCR-14-0947
209. Wen PY, Omuro A, Ahluwalia MS, Fathallah-Shaykh HM, Mohile N, Lager JJ, et al. Phase I dose-escalation study of the PI3K/mTOR inhibitor voxtalisib (SAR245409, XL765) plus temozolomide with or without radiotherapy in patients with high-grade glioma. Neuro Oncol. (2015) 17:1275–83. doi: 10.1093/neuonc/nov083
210. Kushner BH, Cheung NV, Modak S, Becher OJ, Basu EM, Roberts SS, et al. A phase I/Ib trial targeting the Pi3k/Akt pathway using perifosine: L ong-term progression-free survival of patients with resistant neuroblastoma. Int J Cancer. (2017) 140:480–4. doi: 10.1002/ijc.30440
211. Wainberg ZA, Alsina M, Soares HP, Braña I, Britten CD, Del Conte G, et al. A multi-arm phase I study of the PI3K/mTOR inhibitors PF-04691502 and gedatolisib (PF-05212384) plus irinotecan or the MEK inhibitor PD-0325901 in advanced cancer. Target Oncol. (2017) 12:775–85. doi: 10.1007/s11523-017-0530-5
212. Banerji U, Dean EJ, Pérez-Fidalgo JA, Batist G, Bedard PL, You B, et al. A phase I open-label study to identify a dosing regimen of the pan-AKT inhibitor AZD5363 for evaluation in solid tumors and in PIK3CA -mutated breast and gynecologic cancers. Clin Cancer Res. (2018) 24:2050–9. doi: 10.1158/1078-0432.CCR-17-2260
213. Garrido-Castro AC, Saura C, Barroso-Sousa R, Guo H, Ciruelos E, Bermejo B, et al. Phase 2 study of buparlisib (BKM120), a pan-class I PI3K inhibitor, in patients with metastatic triple-negative breast cancer. Breast Cancer Res. (2020) 22:120. doi: 10.1186/s13058-020-01354-y
214. Kang BW and Chau I. Molecular target: pan-AKT in gastric cancer. ESMO Open. (2020) 5:e000728. doi: 10.1136/esmoopen-2020-000728
215. Iksen, Pothongsrisit S, and Pongrakhananon V. Targeting the PI3K/AKT/mTOR signaling pathway in lung cancer: an update regarding potential drugs and natural products. Molecules. (2021) 26:4100. doi: 10.3390/molecules26134100
216. Andrikopoulou A, Chatzinikolaou S, Panourgias E, Kaparelou M, Liontos M, Dimopoulos M-A, et al. The emerging role of capivasertib in breast cancer. Breast. (2022) 63:157–67. doi: 10.1016/j.breast.2022.03.018
217. Stanciu S, Ionita-Radu F, Stefani C, Miricescu D, Stanescu-Spinu I-I, Greabu M, et al. Targeting PI3K/AKT/mTOR signaling pathway in pancreatic cancer: from molecular to clinical aspects. Int J Mol Sci. (2022) 23:10132. doi: 10.3390/ijms231710132
218. Bang J, Jun M, Lee S, Moon H, and Ro SW. Targeting EGFR/PI3K/AKT/mTOR signaling in hepatocellular carcinoma. Pharmaceutics. (2023) 15:2130. doi: 10.3390/pharmaceutics15082130
219. Shen S, Safonov A, Bromberg M, Chen Y, Ahmed M, Razavi P, et al. Sequential use of PI3K/AKT/mTOR pathway inhibitors alpelisib and everolimus in patients with hormone receptor-positive (HR+) metastatic breast cancer. J Clin Onco. (2024) 42:1057–7. doi: 10.1200/JCO.2024.42.16_suppl.1057
220. Msweli S, Pakala SB, and Syed K. NF-κB transcription factors: their distribution, family expansion, structural conservation, and evolution in animals. Int J Mol Sci. (2024) 25:9793. doi: 10.3390/ijms25189793
221. Florio TJ, Lokareddy RK, Yeggoni DP, Sankhala RS, Ott CA, Gillilan RE, et al. Differential recognition of canonical NF-κB dimers by Importin α3. Nat Commun. (2022) 13:1207. doi: 10.1038/s41467-022-28846-z
222. Ghosh G and Wang V Y.-F. Origin of the Functional Distinctiveness of NF-κB/p52. Frontiers in Cell and Developmental Biology. (2021) 9. doi: 10.3389/fcell.2021.764164
223. Rinkenbaugh A and Baldwin A. The NF-κB pathway and cancer stem cells. Cells. (2016) 5:16. doi: 10.3390/cells5020016
224. Kubatka P, Mazurakova A, Samec M, Koklesova L, Zhai K, AL-Ishaq R, et al. Flavonoids against non-physiologic inflammation attributed to cancer initiation, development, and progression—3PM pathways. EPMA J. (2021) 12:559–87. doi: 10.1007/s13167-021-00257-y
225. Chekalina N, Burmak Y, Petrov Y, Borisova Z, Manusha Y, Kazakov Y, et al. Quercetin reduces the transcriptional activity of NF-kB in stable coronary artery disease. Indian Heart J. (2018) 70:593–7. doi: 10.1016/j.ihj.2018.04.006
226. Lin Y, Ukaji T, Koide N, and Umezawa K. Inhibition of late and early phases of cancer metastasis by the NF-κB inhibitor DHMEQ derived from microbial bioactive metabolite epoxyquinomicin: A review. Int J Mol Sci. (2018) 19:729. doi: 10.3390/ijms19030729
227. Cione E, La Torre C, Cannataro R, Caroleo MC, Plastina P, and Gallelli L. Quercetin, epigallocatechin gallate, curcumin, and resveratrol: from dietary sources to human microRNA modulation. Molecules. (2019) 25:63. doi: 10.3390/molecules25010063
228. Zhao F, Dang Y, Zhang R, Jing G, Liang W, Xie L, et al. Apigenin attenuates acrylonitrile-induced neuro-inflammation in rats: Involved of inactivation of the TLR4/NF-κB signaling pathway. Int Immunopharmacol. (2019) 75:105697. doi: 10.1016/j.intimp.2019.105697
229. Ziadlou R, Barbero A, Martin I, Wang X, Qin L, Alini M, et al. Anti-inflammatory and chondroprotective effects of vanillic acid and epimedin C in human osteoarthritic chondrocytes. Biomolecules. (2020) 10:932. doi: 10.3390/biom10060932
230. Jia X, Huang C, Hu Y, Wu Q, Liu F, Nie W, et al. Cirsiliol targets tyrosine kinase 2 to inhibit esophageal squamous cell carcinoma growth in vitro and in vivo. J Exp Clin Cancer Res. (2021) 40:105. doi: 10.1186/s13046-021-01903-z
231. Kozłowska A and Dzierżanowski T. Targeting inflammation by anthocyanins as the novel therapeutic potential for chronic diseases: an update. Molecules. (2021) 26:4380. doi: 10.3390/molecules26144380
232. Uddin M, Kabir M, Mamun A, Sarwar M, Nasrin F, Bin ET, et al. Natural small molecules targeting NF-κB signaling in glioblastoma. Front Pharmacol. (2021) 12:703761. doi: 10.3389/fphar.2021.703761
233. Al-Khayri JM, Sahana GR, Nagella P, Joseph BV, Alessa FM, and Al-Mssallem MQ. Flavonoids as potential anti-inflammatory molecules: A review. Molecules. (2022) 27:2901. doi: 10.3390/molecules27092901
234. Chauhan A, Islam AU, Prakash H, and Singh S. Phytochemicals targeting NF-κB signaling: Potential anti-cancer interventions. J Pharm Anal. (2022) 12:394–405. doi: 10.1016/j.jpha.2021.07.002
235. Patel DK. Biological importance and therapeutic potential of Trilobatin in the management of human disorders and associated secondary complications. Pharmacol Res - Mod Chin Med. (2022) 5:100185. doi: 10.1016/j.prmcm.2022.100185
236. Adeyi OE, Somade OT, Ajayi BO, James AS, Adeboye TR, Olufemi DA, et al. The anti-inflammatory effect of ferulic acid is via the modulation of NFκB-TNF-α-IL-6 and STAT1-PIAS1 signaling pathways in 2-methoxyethanol-induced testicular inflammation in rats. Phytomed Plus. (2023) 3:100464. doi: 10.1016/j.phyplu.2023.100464
237. Rojasawasthien T, Usui M, Addison WN, Matsubara T, Shirakawa T, Tsujisawa T, et al. Nobiletin, a NF-κB signaling antagonist, promotes BMP -induced bone formation. FASEB Bioadv. (2023) 5:62–70. doi: 10.1096/fba.2022-00093
238. Krajka-Kuźniak V, Belka M, and Papierska K. Targeting STAT3 and NF-κB signaling pathways in cancer prevention and treatment: the role of chalcones. Cancers (Basel). (2024) 16:1092. doi: 10.3390/cancers16061092
239. Zhang X, Huang F, Liu J, Zhou Z, Yuan S, and Jiang H. Molecular mechanism of ginsenoside rg3 alleviation in osteoporosis via modulation of KPNA2 and the NF-κB signalling pathway. Clin Exp Pharmacol Physiol. (2025) 52:e70019. doi: 10.1111/1440-1681.70019
240. Li T, Yang Y, Qi H, Cui W, Zhang L, Fu X, et al. CRISPR/Cas9 therapeutics: progress and prospects. Signal Transduct Target Ther. (2023) 8:36. doi: 10.1038/s41392-023-01309-7
241. Aljabali AAA, El-Tanani M, and Tambuwala MM. Principles of CRISPR-Cas9 technology: Advancements in genome editing and emerging trends in drug delivery. J Drug Delivery Sci Technol. (2024) 92:105338. doi: 10.1016/j.jddst.2024.105338
242. Xu X, Liu C, Wang Y, Koivisto O, Zhou J, Shu Y, et al. Nanotechnology-based delivery of CRISPR/Cas9 for cancer treatment. Adv Drug Deliv Rev. (2021) 176:113891. doi: 10.1016/j.addr.2021.113891
243. Gao C, Wu P, Yu L, Liu L, Liu H, Tan X, et al. The application of CRISPR/Cas9 system in cervical carcinogenesis. Cancer Gene Ther. (2022) 29:466–74. doi: 10.1038/s41417-021-00366-w
244. Karn V, Sandhya S, Hsu W, Parashar D, Singh HN, Jha NK, et al. CRISPR/Cas9 system in breast cancer therapy: advancement, limitations and future scope. Cancer Cell Int. (2022) 22:234. doi: 10.1186/s12935-022-02654-3
245. Chiu CH. CRISPR/Cas9 genetic screens in hepatocellular carcinoma gene discovery. Curr Res Biotechnol. (2023) 5:100127. doi: 10.1016/j.crbiot.2023.100127
246. Tabibian M, Moghaddam FS, Motevaseli E, and Ghafouri-Fard S. Targeting mRNA-coding genes in prostate cancer using CRISPR/Cas9 technology with a special focus on androgen receptor signaling. Cell Commun Signal. (2024) 22:504. doi: 10.1186/s12964-024-01833-1
247. Chen A, Wen S, Liu F, Zhang Z, Liu M, Wu Y, et al. CRISPR/Cas9 screening identifies a kinetochore-microtubule dependent mechanism for Aurora-A inhibitor resistance in breast cancer. Cancer Commun. (2021) 41:121–39. doi: 10.1002/cac2.12125
248. Zhang J, Li Y, Liu H, Zhang J, Wang J, Xia J, et al. Genome-wide CRISPR/Cas9 library screen identifies PCMT1 as a critical driver of ovarian cancer metastasis. J Exp Clin Cancer Res. (2022) 41:24. doi: 10.1186/s13046-022-02242-3
249. Mirgayazova R, Khadiullina R, Chasov V, Mingaleeva R, Miftakhova R, Rizvanov A, et al. Therapeutic editing of the TP53 gene: is CRISPR/cas9 an option? Genes (Basel). (2020) 11:704. doi: 10.3390/genes11060704
250. Wan T, Chen Y, Pan Q, Xu X, Kang Y, Gao X, et al. Genome editing of mutant KRAS through supramolecular polymer-mediated delivery of Cas9 ribonucleoprotein for colorectal cancer therapy. J Control Release. (2020) 322:236–47. doi: 10.1016/j.jconrel.2020.03.015
251. Xu Y, Chen C, Guo Y, Hu S, and Sun Z. Effect of CRISPR/cas9-edited PD-1/PD-L1 on tumor immunity and immunotherapy. Front Immunol. (2022) 13:848327. doi: 10.3389/fimmu.2022.848327
252. Ju H, Kim D, and Oh Y-K. Lipid nanoparticle-mediated CRISPR/Cas9 gene editing and metabolic engineering for anticancer immunotherapy. Asian J Pharm Sci. (2022) 17:641–52. doi: 10.1016/j.ajps.2022.07.005
253. Radtke L, Majchrzak-Celińska A, Awortwe C, Vater I, Nagel I, Sebens S, et al. CRISPR/Cas9-induced knockout reveals the role of ABCB1 in the response to temozolomide, carmustine and lomustine in glioblastoma multiforme. Pharmacol Res. (2022) 185:106510. doi: 10.1016/j.phrs.2022.106510
254. Zhang D, Liu L, Wang J, Zhang H, Zhang Z, Xing G, et al. Drug-loaded PEG-PLGA nanoparticles for cancer treatment. Front Pharmacol. (2022) 13:990505. doi: 10.3389/fphar.2022.990505
255. Cyranoski D. CRISPR gene-editing tested in a person for the first time. Nature. (2016) 539:479–9. doi: 10.1038/nature.2016.20988
256. Lu Y, Xue J, Deng T, Zhou X, Yu K, Deng L, et al. Safety and feasibility of CRISPR-edited T cells in patients with refractory non-small-cell lung cancer. Nat Med. (2020) 26:732–40. doi: 10.1038/s41591-020-0840-5
257. Jiang T, Gonzalez KM, Cordova LE, and Lu J. Nanotechnology-enabled gene delivery for cancer and other genetic diseases. Expert Opin Drug Deliv. (2023) 20:523–40. doi: 10.1080/17425247.2023.2200246
258. Zhen S, Liu Y, Lu J, Tuo X, Yang X, Chen H, et al. Human papillomavirus oncogene manipulation using clustered regularly interspersed short palindromic repeats/cas9 delivered by pH-sensitive cationic liposomes. Hum Gene Ther. (2020) 31:309–24. doi: 10.1089/hum.2019.312
259. Liu Q, Zhao K, Wang C, Zhang Z, Zheng C, Zhao Y, et al. Multistage delivery nanoparticle facilitates efficient CRISPR/dCas9 activation and tumor growth suppression in vivo. Adv Sci. (2019) 6:1801423. doi: 10.1002/advs.201801423
260. Liu Y, Li F, Lyu Y, Wang F, Lee LTO, He S, et al. A semiconducting polymer nanoCRISPR for near-infrared photoactivatable gene editing and cancer gene therapy. Nano Lett. (2025) 25:4518–25. doi: 10.1021/acs.nanolett.5c00285
261. Fakhr E, Modic Ž, and Cid-Arregui A. Recent developments in immunotherapy of cancers caused by human papillomaviruses. Immunology. (2021) 163(1):33–45. doi: 10.1111/imm.13285
262. Maalej KM, Merhi M, Inchakalody VP, Mestiri S, Alam M, Maccalli C, et al. CAR-cell therapy in the era of solid tumor treatment: current challenges and emerging therapeutic advances. Mol Cancer. (2023) 22:1–54. doi: 10.1186/s12943-023-01723-z
263. Mu W, Zhang M, Hu G, Han Y, Mao X, Chen C, et al. Case report: Differential diagnosis of highly amplified anti-CD5 CAR T cells and relapsed lymphoma cells in a patient with refractory ALK positive anaplastic large cell lymphoma. Front Immunol. (2023) 14:1280007. doi: 10.3389/fimmu.2023.1280007
264. Wang Z, Li N, Feng K, Chen M, Zhang Y, Liu Y, et al. Phase I study of CAR-T cells with PD-1 and TCR disruption in mesothelin-positive solid tumors. Cell Mol Immunol. (2021) 18:2188–98. doi: 10.1038/s41423-021-00749-x
265. Li W, Huang Y, Zhou X, Cheng B, Wang H, and Wang Y. CAR-T therapy for gastrointestinal cancers: current status, challenges, and future directions. Braz J Med Biol Res. (2024) 57:e13640. doi: 10.1590/1414-431X2024e13640
266. Ou X, Ma Q, Yin W, Ma X, and He Z. CRISPR/cas9 gene-editing in cancer immunotherapy: promoting the present revolution in cancer therapy and exploring more. Front Cell Dev Biol. (2021) 9:674467. doi: 10.3389/fcell.2021.674467
267. Meng X, Wu T, Lou Q, Niu K, Jiang L, Xiao Q, et al. Optimization of CRISPR–Cas system for clinical cancer therapy. Bioeng Transl Med. (2023) 8:1–24. doi: 10.1002/btm2.10474
268. McGuirk J, Bachier CR, Bishop MR, Ho PJ, Murthy HS, Dickinson MJ, et al. A phase 1 dose escalation and cohort expansion study of the safety and efficacy of allogeneic CRISPR-Cas9–engineered T cells (CTX110) in patients (Pts) with relapsed or refractory (R/R) B-cell Malignancies (CARBON). J Clin Onco. (2021) 39:TPS7570–TPS7570. doi: 10.1200/JCO.2021.39.15_SUPPL.TPS7570
269. Iyer SP, Sica RA, Ho PJ, Prica A, Zain J, Foss FM, et al. Safety and activity of CTX130, a CD70-targeted allogeneic CRISPR-Cas9-engineered CAR T-cell therapy, in patients with relapsed or refractory T-cell Malignancies (COBALT-LYM): a single-arm, open-label, phase 1, dose-escalation study. Lancet Oncol. (2025) 26:110–22. doi: 10.1016/S1470-2045(24)00508-4
270. Caforio M, Iacovelli S, Quintarelli C, Locatelli F, and Folgiero V. GMP-manufactured CRISPR/Cas9 technology as an advantageous tool to support cancer immunotherapy. J Exp Clin Cancer Res. (2024) 43:1–12. doi: 10.1186/s13046-024-02993-1
271. Bruno B, Wäsch R, Engelhardt M, Gay F, Giaccone L, D’Agostino M, et al. European Myeloma Network perspective on CAR T-cell therapies for multiple myeloma. Haematologica. (2021) 106:2054–65. doi: 10.3324/haematol.2020.276402
272. Pal SK, Tran B, Haanen JBAG, Hurwitz ME, Sacher A, Tannir NM, et al. CD70-targeted allogeneic CAR T-cell therapy for advanced clear cell renal cell carcinoma. Cancer Discov. (2024) 14:1176–89. doi: 10.1158/2159-8290.CD-24-0102
273. Qasim W. Genome-edited allogeneic donor “universal chimeric antigen receptor T cells. Blood. (2023) 141:835–45. doi: 10.1182/blood.2022016204
274. Ghaffari S, Khalili N, and Rezaei N. CRISPR/Cas9 revitalizes adoptive T-cell therapy for cancer immunotherapy. J Exp Clin Cancer Res. (2021) 40:1–18. doi: 10.1186/s13046-021-02076-5
275. Ottaviano G, Georgiadis C, Gkazi SA, Syed F, Zhan H, Etuk A, et al. Phase 1 clinical trial of CRISPR-engineered CAR19 universal T cells for treatment of children with refractory B cell leukemia. Sci Transl Med. (2022) 14:eabq3010. doi: 10.1126/SCITRANSLMED.ABQ3010/SUPPL_FILE/SCITRANSLMED.ABQ3010_MDAR_REPRODUCIBILITY_CHECKLIST.PDF
276. Sharma G, Sharma AR, Bhattacharya M, Lee SS, and Chakraborty C. CRISPR-cas9: A preclinical and clinical perspective for the treatment of human diseases. Mol Ther. (2021) 29:571–86. doi: 10.1016/J.YMTHE.2020.09.028
277. Cook D, Brown D, Alexander R, March R, Morgan P, Satterthwaite G, et al. Lessons learned from the fate of AstraZeneca’s drug pipeline: a five-dimensional framework. Nat Rev Drug Discov. (2014) 13:419–31. doi: 10.1038/nrd4309
278. Thomas J, Kumar V, Sharma N, John N, Umesh M, Kumar Dasarahally Huligowda L, et al. Recent approaches in nanotoxicity assessment for drug delivery applications: Challenges and prospects. Med Drug Discov. (2025) 25:100204. doi: 10.1016/j.medidd.2025.100204
279. Farjadian F, Ghasemi A, Gohari O, Roointan A, Karimi M, and Hamblin MR. Nanopharmaceuticals and nanomedicines currently on the market: challenges and opportunities. Nanomedicine. (2019) 14:93–126. doi: 10.2217/nnm-2018-0120
280. KR S. A brief review on pharmacovigilance. J Clin Med Res. (2024) 5:192–211. doi: 10.37191/Mapsci-2582-4333-5(5)-144
281. Allan J, Belz S, Hoeveler A, Hugas M, Okuda H, Patri A, et al. Regulatory landscape of nanotechnology and nanoplastics from a global perspective. Regul Toxicol Pharmacol. (2021) 122:104885. doi: 10.1016/j.yrtph.2021.104885
282. Đorđević S, Gonzalez MM, Conejos-Sánchez I, Carreira B, Pozzi S, Acúrcio RC, et al. Current hurdles to the translation of nanomedicines from bench to the clinic. Drug Delivery Transl Res. (2022) 12:500–25. doi: 10.1007/s13346-021-01024-2
283. Rodríguez F, Caruana P, de la Fuente N, Español P, Gámez M, Balart J, et al. Nano-based approved pharmaceuticals for cancer treatment: present and future challenges. Biomolecules. (2022) 12:784. doi: 10.3390/biom12060784
284. Alphandéry E, Grand-Dewyse P, Lefèvre R, Mandawala C, and Durand-Dubief M. Cancer therapy using nanoformulated substances: scientific, regulatory and financial aspects. Expert Rev Anticancer Ther. (2015) 15:1233–55. doi: 10.1586/14737140.2015.1086647
285. Mehta M, Bui TA, Yang X, Aksoy Y, Goldys EM, and Deng W. Lipid-based nanoparticles for drug/gene delivery: an overview of the production techniques and difficulties encountered in their industrial development. ACS Mater Au. (2023) 3:600–19. doi: 10.1021/acsmaterialsau.3c00032
286. Koklesova L, Jakubikova J, Cholujova D, Samec M, Mazurakova A, Šudomová M, et al. Phytochemical-based nanodrugs going beyond the state-of-the-art in cancer management—Targeting cancer stem cells in the framework of predictive, preventive, personalized medicine. Front Pharmacol. (2023) 14:1121950. doi: 10.3389/fphar.2023.1121950
287. Alvi M, Yaqoob A, Rehman K, Shoaib SM, and Akash MSH. PLGA-based nanoparticles for the treatment of cancer: current strategies and perspectives. AAPS Open. (2022) 8:12. doi: 10.1186/s41120-022-00060-7
288. López-Royo T, Sebastián V, Moreno-Martínez L, Uson L, Yus C, Alejo T, et al. Encapsulation of large-size plasmids in PLGA nanoparticles for gene editing: Comparison of three different synthesis methods. Nanomaterials. (2021) 11:2723. doi: 10.3390/nano11102723
289. Sharma S, Parmar A, Kori S, and Sandhir R. PLGA-based nanoparticles: A new paradigm in biomedical applications. TrAC Trends Anal Chem. (2016) 80:30–40. doi: 10.1016/j.trac.2015.06.014
290. Perinelli DR, Cespi M, Bonacucina G, and Palmieri GF. PEGylated polylactide (PLA) and poly (lactic-co-glycolic acid) (PLGA) copolymers for the design of drug delivery systems. J Pharm Invest. (2019) 49:443–58. doi: 10.1007/s40005-019-00442-2
291. Salatin S, Barar J, Barzegar-Jalali M, Adibkia K, Kiafar F, and Jelvehgari M. An alternative approach for improved entrapment efficiency of hydrophilic drug substance in PLGA nanoparticles by interfacial polymer deposition following solvent displacement. Jundishapur J Nat Pharm Prod. (2018) 13:e12873. doi: 10.5812/jjnpp.12873
292. Locatelli E and Franchini MC. Biodegradable PLGA-b-PEG polymeric nanoparticles: Synthesis, properties, and nanomedical applications as drug delivery system. J Nanoparticle Res. (2012) 14:1–17. doi: 10.1007/s11051-012-1316-4
293. Afshari M, Derakhshandeh K, and Hosseinzadeh L. Characterisation, cytotoxicity and apoptosis studies of methotrexate-loaded PLGA and PLGA-PEG nanoparticles. J Microencapsul. (2014) 31:239–45. doi: 10.3109/02652048.2013.834991
294. MaChado V, Morais M, and Medeiros R. Hyaluronic acid-based nanomaterials applied to cancer: where are we now? Pharmaceutics. (2022) 14:1–34. doi: 10.3390/pharmaceutics14102092
295. Hou X, Zhong D, Chen H, Gu Z, Gong Q, Ma X, et al. Recent advances in hyaluronic acid-based nanomedicines: Preparation and application in cancer therapy. Carbohydr Polymers. (2022) 292:119662. doi: 10.1016/j.carbpol.2022.119662
296. Zeng X, Wang H, Zhang Y, Xu X, Yuan X, and Li J. pH-responsive hyaluronic acid nanoparticles for enhanced triple negative breast cancer therapy. Int J Nanomed. (2022) 17:1437–57. doi: 10.2147/IJN.S360500
297. Rommasi F and Esfandiari N. Liposomal nanomedicine: applications for drug delivery in cancer therapy. Nanoscale Res Lett. (2021) 16:95. doi: 10.1186/s11671-021-03553-8
298. Raza F, Evans L, Motallebi M, Zafar H, Pereira-Silva M, Saleem K, et al. Liposome-based diagnostic and therapeutic applications for pancreatic cancer. Acta Biomater. (2023) 157:1–23. doi: 10.1016/j.actbio.2022.12.013
299. Gaballu FA, Abbaspour-Ravasjani S, Mansoori B, Yekta R, Hamishehkar H, Mohammadi A, et al. Comparative of in-vitro evaluation between erlotinib loaded nanostructured lipid carriers and liposomes against A549 lung cancer cell line. Iran J Pharm Sci. (2019) 18:1168–79. doi: 10.22037/ijpr.2019.1100775
300. Khan S, Vahdani Y, Hussain A, Haghighat S, Heidari F, Nouri M, et al. Polymeric micelles functionalized with cell penetrating peptides as potential pH-sensitive platforms in drug delivery for cancer therapy: A review. Arab J Chem. (2021) 14:103264. doi: 10.1016/j.arabjc.2021.103264
301. Wang S, Chen Y, Guo J, and Huang Q. Liposomes for tumor targeted therapy: A review. Int J Mol Sci. (2023) 24:1–25. doi: 10.3390/ijms24032643
302. Yafout M, Ousaid A, Khayati Y, and El Otmani IS. Gold nanoparticles as a drug delivery system for standard chemotherapeutics: A new lead for targeted pharmacological cancer treatments. Sci Afr. (2021) 11:e00685. doi: 10.1016/j.sciaf.2020.e00685
303. Yang Y, Zheng X, Chen L, Gong X, Yang H, Duan X, et al. Multifunctional gold nanoparticles in cancer diagnosis and treatment. Int J Nanomed. (2022) 17:2041–67. doi: 10.2147/IJN.S355142
304. Danafar H, Sharaïfi A, Askarlou S, and Manjili HK. Preparation and characterization of PEGylated iron oxide-gold nanoparticles for delivery of sulforaphane and curcumin. Drug Res. (2017) 67:698–704. doi: 10.1055/s-0043-115905
305. Khan Y, Yuan C, Roy M, and Yaqub Khan M. Synthesis, Limitation and Application of Gold Nanoparticles in Treatment of Cancerous Cell Drug Delivery System View project Environment View project Synthesis, Limitation and Application of Gold Nanoparticles in Treatment of Cancerous Cell. Int J Sci Res Multidiscip Stud E. (2019) 5:8–14. doi: 10.26438/ijsrms
306. Kim JH, Moon MJ, Kim DY, Heo SH, and Jeong YY. Hyaluronic acid-based nanomaterials for cancer therapy. Polymers. (2018) 10:1–15. doi: 10.3390/polym10101133
307. Jin GW, Rejinold NS, and Choy JH. Multifunctional polymeric micelles for cancer therapy. Polymers. (2022) 14:1–19. doi: 10.3390/polym14224839
308. Shaikh R, Bhattacharya S, and Saoji SD. Development, optimization, and characterization of polymeric micelles to improve dasatinib oral bioavailability: Hep G2 cell cytotoxicity and in vivo pharmacokinetics for targeted liver cancer therapy. Heliyon. (2024) 10:e39632. doi: 10.1016/j.heliyon.2024.e39632
309. Wei H and Chenming Z. Tuning the size of poly (lactic-co-glycolic acid)(PLGA) nanoparticles fabricated by nanoprecipitation. Biotechnol J. (2018) 13:1–19. doi: 10.1002/biot.201700203
310. Nunes D, Andrade S, Ramalho MJ, Loureiro JA, and Pereira MC. Polymeric nanoparticles-loaded hydrogels for biomedical applications: A systematic review on in vivo findings. Polymers. (2022) 14:1010. doi: 10.3390/polym14051010
311. Gagliardi A, Giuliano E, Venkateswararao E, Fresta M, Bulotta S, Awasthi V, et al. Biodegradable polymeric nanoparticles for drug delivery to solid tumors. Front Pharmacol. (2021) 12:601626. doi: 10.3389/fphar.2021.601626
312. Jain NK, Prabhuraj RS, Bavya MC, Prasad R, Bandyopadhyaya R, Naidu VGM, et al. Niclosamide encapsulated polymeric nanocarriers for targeted cancer therapy. RSC Adv. (2019) 9:26572–81. doi: 10.1039/c9ra03407b
313. Ali I, Alsehli M, Scotti L, Scotti MT, Tsai ST, Yu RS, et al. Progress in polymeric nano-medicines for theranostic cancer treatment. Polymers. (2020) 12:598. doi: 10.3390/polym12030598
314. Kaurav M, Ruhi S, Al-Goshae HA, Jeppu AK, Ramachandran D, Sahu RK, et al. Dendrimer: An update on recent developments and future opportunities for the brain tumors diagnosis and treatment. Front Pharmacol. (2023) 14:1159131. doi: 10.3389/fphar.2023.1159131
315. Zenze M, Daniels A, and Singh M. Dendrimers as modifiers of inorganic nanoparticles for therapeutic delivery in cancer. Pharmaceutics. (2023) 15:398. doi: 10.3390/pharmaceutics15020398
316. Bulent Ozpolata AS, L.-B. G, Martinez SR, Gay MS, and Z. L. 乳鼠心肌提取 HHS public access. Physiol Behav. (2016) 176:139–48. doi: 10.1016/j.apmt.2018.05.002.PAMAM
317. Aurelia Chis A, Dobrea C, Morgovan C, Arseniu AM, Rus LL, Butuca A, et al. Applications and limitations of dendrimers in biomedicine. Molecules. (2020) 25:3982. doi: 10.3390/molecules25173982
318. Tjo K and Varamini P. Nanodiamonds and their potential applications in breast cancer therapy: a narrative review. Drug Delivery Transl Res. (2022) 12:1017–28. doi: 10.1007/s13346-021-00996-5
319. Priyadarshni N, Singh R, and Mishra MK. Nanodiamonds: Next generation nano-theranostics for cancer therapy. Cancer Lett. (2024) 587. doi: 10.1016/j.canlet.2024.216710
320. Singh D and Ray S. A short appraisal of nanodiamonds in drug delivery and targeting: recent advancements. Front Nanotechnol. (2023) 5:1259648. doi: 10.3389/fnano.2023.1259648
321. Naik K, Chaudhary S, Ye L, and Parmar AS. A strategic review on carbon quantum dots for cancer-diagnostics and treatment. Front Bioeng Biotechnol. (2022) 10:882100. doi: 10.3389/fbioe.2022.882100
322. Hamidu A, Pitt WG, and Husseini GA. Recent breakthroughs in using quantum dots for cancer imaging and drug delivery purposes. Nanomaterials. (2023) 13:2566. doi: 10.3390/nano13182566
323. Noel KJ, Umashankar MS, and Narayanasamy D. Exploring research on the drug loading capacity of quantum dots. Cureus. (2024) 16:e67869. doi: 10.7759/cureus.67869
324. Liaqat S, Fatima B, Hussain D, Imran M, Zahra Jawad SE, Saeed A, et al. Doxorubicin encapsulated blend of sitagliptin-lignin polymeric drug delivery system for effective combination therapy against cancer. Int J Biol Macromol. (2024) 269:132146. doi: 10.1016/J.IJBIOMAC.2024.132146
325. Jain N,S, Somanna P, and Patil AB. Application of quantum dots in drug delivery. Nanosci Nanotechnol – Asia. (2021) 12:1–16. doi: 10.2174/2210681211666210211092823
326. Singh R and Kumar S. Cancer targeting and diagnosis: recent trends with carbon nanotubes. Nanomaterials. (2022) 12:2283. doi: 10.3390/nano12132283
327. Elgamal HA, Mohamed SA, Farghali AA, and Hassan AME. PEG at carbon nanotubes composite as an effective nanocarrier of ixazomib for myeloma cancer therapy. Nanoscale Res Lett. (2022) 17:1–14. doi: 10.1186/s11671-022-03707-2
328. Son KH, Hong JH, and Lee JW. Carbon nanotubes as cancer therapeutic carriers and mediators. Int J Nanomed. (2016) 11:5163–85. doi: 10.2147/IJN.S112660
329. Mokhtarzadeh A, Hassanpour S, Vahid ZF, Hejazi M, Hashemi M, Ranjbari J, et al. Nano-delivery system targeting to cancer stem cell cluster of differentiation biomarkers. J Control Release. (2017) 266:166–86. doi: 10.1016/j.jconrel.2017.09.028
330. Mi P, Cabral H, and Kataoka K. Ligand-installed nanocarriers toward precision therapy. Adv Mater. (2020) 32:e1902604. doi: 10.1002/adma.201902604
331. Liu H, Liu M, Zhao Y, and Mo R. Nanomedicine strategies to counteract cancer stemness and chemoresistance. Explor Target Antitumor Ther. (2023) 4:630–56. doi: 10.37349/etat.2023.00157
332. Cao J, Bhatnagar S, Wang J, Qi X, Prabha S, and Panyam J. Cancer stem cells and strategies for targeted drug delivery. Drug Delivery Transl Res. (2021) 11:1779–805. doi: 10.1007/s13346-020-00863-9
333. de Thé H. Differentiation therapy revisited. Nat Rev Cancer. (2018) 18:117–27. doi: 10.1038/nrc.2017.103
334. Clara JA, Monge C, Yang Y, and Takebe N. Targeting signalling pathways and the immune microenvironment of cancer stem cells — a clinical update. Nat Rev Clin Oncol. (2020) 17:204–32. doi: 10.1038/s41571-019-0293-2
335. Sankar Sana S, Vishambhar Kumbhakar D, Thakkar L, Vadde R, Zhang Z, Sillanpää M, et al. Bio-fabrication of tin oxide (SnO2) nanoparticles Capped with rosmarinic acid in Argument with antimicrobial activity and photocatalytic degradation. Inorg Chem Commun. (2024) 170:113337. doi: 10.1016/j.inoche.2024.113337
336. Gavas S, Quazi S, and Karpiński TM. Nanoparticles for cancer therapy: current progress and challenges. Nanoscale Res Lett. (2021) 16:173. doi: 10.1186/s11671-021-03628-6
337. Chehelgerdi M, Chehelgerdi M, Allela OQB, Pecho RDC, Jayasankar N, Rao DP, et al. Progressing nanotechnology to improve targeted cancer treatment: overcoming hurdles in its clinical implementation. Mol Cancer. (2023) 22:169. doi: 10.1186/s12943-023-01865-0
338. Kang MH, Wang J, Makena MR, Lee J-S, Paz N, Hall CP, et al. Activity of MM-398, nanoliposomal irinotecan (nal-IRI), in ewing’s family tumor xenografts is associated with high exposure of tumor to drug and high SLFN11 expression. Clin Cancer Res. (2015) 21:1139–50. doi: 10.1158/1078-0432.CCR-14-1882
339. Liu L, Ye Q, Lu M, Chen S-T, Tseng H-W, Lo Y-C, et al. A new approach to deliver anti-cancer nanodrugs with reduced off-target toxicities and improved efficiency by temporarily blunting the reticuloendothelial system with intralipid. Sci Rep. (2017) 7:16106. doi: 10.1038/s41598-017-16293-6
340. Salehi B, Selamoglu Z, S. Mileski K, Pezzani R, Redaelli M, C. Cho W, et al. Liposomal cytarabine as cancer therapy: from chemistry to medicine. Biomolecules. (2019) 9:773. doi: 10.3390/biom9120773
341. Imantay A, Mashurov N, Zhaisanbayeva BA, and Mun EA. Doxorubicin-conjugated nanoparticles for potential use as drug delivery systems. Nanomaterials. (2025) 15:133. doi: 10.3390/nano15020133
342. SM A and Sheikh S. Bioequivalence study of pegylated doxorubicin hydrochloride liposome (PEGADRIA) and DOXIL® in ovarian cancer patients: physicochemical characterization and pre-clinical studies. J Nanomed Nanotechnol. (2016) 07:1000361. doi: 10.4172/2157-7439.1000361
343. Harrington KJ, Lewanski C, Northcote AD, Whittaker J, Peters AM, Vile RG, et al. Phase II study of pegylated liposomal doxorubicin (CaelyxTM) as induction chemotherapy for patients with squamous cell cancer of the head and neck. Eur J Cancer. (2001) 37:2015–22. doi: 10.1016/S0959-8049(01)00216-7
344. Wang Z, Zheng Q, Zhang H, Bronson RT, Madsen JC, Sachs DH, et al. Ontak-like human IL-2 fusion toxin. J Immunol Methods. (2017) 448:51–8. doi: 10.1016/j.jim.2017.05.008
345. Heo Y-A, Syed YY, and Keam SJ. Pegaspargase: A review in acute lymphoblastic leukaemia. Drugs. (2019) 79:767–77. doi: 10.1007/s40265-019-01120-1
346. Ebbitt L, Johnson E, Herndon B, Karrick K, and Johnson A. Suspected Malignant hyperthermia and the application of a multidisciplinary response. Healthcare. (2020) 8:328. doi: 10.3390/healthcare8030328
347. Chen L-T, Hitre E, Lee W-J, Bai L-Y, Papaï Z, KANG SY, et al. A randomized controlled, open label, adaptive phase III Trial to evaluate safety and efficacy of endoTAG-1 plus gemcitabine versus gemcitabine alone in patients with measurable locally advanced and/or metastatic adenocarcinoma of the pancreas failed on FOLFIRINOX treatment. Ann Oncol. (2019) 30:v321. doi: 10.1093/annonc/mdz247.160
348. Ignatiadis M, Zardavas D, Lemort M, Wilke C, Vanderbeeken M-C, D’Hondt V, et al. Feasibility study of endoTAG-1, a tumor endothelial targeting agent, in combination with paclitaxel followed by FEC as induction therapy in HER2-negative breast cancer. PLoS One. (2016) 11:e0154009. doi: 10.1371/journal.pone.0154009
349. Mitchell P, Thatcher N, Socinski MA, Wasilewska-Tesluk E, Horwood K, Szczesna A, et al. Tecemotide in unresectable stage III non-small-cell lung cancer in the phase III START study: updated overall survival and biomarker analyses. Ann Oncol. (2015) 26:1134–42. doi: 10.1093/annonc/mdv104
350. Munster P, Krop IE, LoRusso P, Ma C, Siegel BA, Shields AF, et al. Safety and pharmacokinetics of MM-302, a HER2-targeted antibody–liposomal doxorubicin conjugate, in patients with advanced HER2-positive breast cancer: a phase 1 dose-escalation study. Br J Cancer. (2018) 119:1086–93. doi: 10.1038/s41416-018-0235-2
351. Duan X, He C, Kron SJ, and Lin W. Nanoparticle formulations of cisplatin for cancer therapy. Wiley Interdiscip Rev: Nanomed Nanobiotechnol. (2016) 8:776–91. doi: 10.1002/wnan.1390
352. Parveen S, Arjmand F, and Tabassum S. Clinical developments of antitumor polymer therapeutics. RSC Adv. (2019) 9:24699–721. doi: 10.1039/C9RA04358F
353. Tian X, Nguyen M, Foote HP, Caster JM, Roche KC, Peters CG, et al. CRLX101, a nanoparticle–drug conjugate containing camptothecin, improves rectal cancer chemoradiotherapy by inhibiting DNA repair and HIF1α. Cancer Res. (2017) 77:112–22. doi: 10.1158/0008-5472.CAN-15-2951
354. Young C, Schluep T, Hwang J, and Eliasof S. CRLX101 (formerly IT-101) – A novel nanopharmaceutical of camptothecin in clinical development. Curr Bioact Compd. (2011) 7:8–14. doi: 10.2174/157340711795163866
355. Fracasso PM, Picus J, Wildi JD, Goodner SA, Creekmore AN, Gao F, et al. Phase 1 and pharmacokinetic study of weekly docosahexaenoic acid-paclitaxel, Taxoprexin®, in resistant solid tumor Malignancies. Cancer Chemother Pharmacol. (2009) 63:451–8. doi: 10.1007/s00280-008-0756-0
356. Bedikian AY, Richards J, Kharkevitch D, Atkins MB, Whitman E, and Gonzalez R. A phase 2 study of high-dose Allovectin-7 in patients with advanced metastatic melanoma. Melanoma Res. (2010) 20:218–26. doi: 10.1097/CMR.0b013e3283390711
357. Perez EA, Awada A, O’Shaughnessy J, Rugo HS, Twelves C, Im S-A, et al. Etirinotecan pegol (NKTR-102) versus treatment of physician’s choice in women with advanced breast cancer previously treated with an anthracycline, a taxane, and capecitabine (BEACON): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. (2015) 16:1556–68. doi: 10.1016/S1470-2045(15)00332-0
358. Dreno B, Thompson JF, Smithers BM, Santinami M, Jouary T, Gutzmer R, et al. MAGE-A3 immunotherapeutic as adjuvant therapy for patients with resected, MAGE-A3-positive, stage III melanoma (DERMA): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. (2018) 19:916–29. doi: 10.1016/S1470-2045(18)30254-7
359. Doi T, Hamaguchi T, Shitara K, Iwasa S, Shimada Y, Harada M, et al. NC-6004 Phase I study in combination with gemcitabine for advanced solid tumors and population PK/PD analysis. Cancer Chemother Pharmacol. (2017) 79:569–78. doi: 10.1007/s00280-017-3254-4
360. Hu H, Petrosyan A, Osna NA, Liu T, Olou AA, Alakhova DY, et al. Pluronic block copolymers enhance the anti-myeloma activity of proteasome inhibitors. J Control Release. (2019) 306:149–64. doi: 10.1016/j.jconrel.2019.05.026
361. Bagley AF, Ludmir EB, Maitra A, Minsky BD, Li Smith G, Das P, et al. NBTXR3, a first-in-class radioenhancer for pancreatic ductal adenocarcinoma: Report of first patient experience. Clin Transl Radiat Oncol. (2022) 33:66–9. doi: 10.1016/j.ctro.2021.12.012
362. Bonvalot S, Rutkowski PL, Thariat J, Carrère S, Ducassou A, Sunyach M-P, et al. NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act.In.Sarc): a multicentre, phase 2–3, randomised, controlled trial. Lancet Oncol. (2019) 20:1148–59. doi: 10.1016/S1470-2045(19)30326-2
363. Fujiwara Y, Mukai H, Saeki T, Ro J, Lin Y-C, Nagai SE, et al. A multi-national, randomised, open-label, parallel, phase III non-inferiority study comparing NK105 and paclitaxel in metastatic or recurrent breast cancer patients. Br J Cancer. (2019) 120:475–80. doi: 10.1038/s41416-019-0391-z
364. Hamaguchi T, Matsumura Y, Suzuki M, Shimizu K, Goda R, Nakamura I, et al. NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel. Br J Cancer. (2005) 92:1240–6. doi: 10.1038/sj.bjc.6602479
365. Mukai H, Kato K, Esaki T, Ohsumi S, Hozomi Y, Matsubara N, et al. Phase I study of NK105, a nanomicellar paclitaxel formulation, administered on a weekly schedule in patients with solid tumors. Invest New Drugs. (2016) 34:750–9. doi: 10.1007/s10637-016-0381-4
366. Mohammadi M, Arabi L, and Alibolandi M. Doxorubicin-loaded composite nanogels for cancer treatment. J Control Release. (2020) 328:171–91. doi: 10.1016/j.jconrel.2020.08.033
367. Gaillard PJ, Appeldoorn CCM, Dorland R, van Kregten J, Manca F, Vugts DJ, et al. Pharmacokinetics, brain delivery, and efficacy in brain tumor-bearing mice of glutathione pegylated liposomal doxorubicin (2B3-101). PLoS One. (2014) 9:e82331. doi: 10.1371/journal.pone.0082331
368. Gibson SJ, Tewari KS, Monk BJ, and Chase DM. Updates on drug discovery in ovarian cancer. Gynecol Oncol Res Pract. (2014) 1:3. doi: 10.1186/2053-6844-1-3
369. Cagel M, Grotz E, Bernabeu E, Moretton MA, and Chiappetta DA. Doxorubicin: nanotechnological overviews from bench to bedside. Drug Discov Today. (2017) 22:270–81. doi: 10.1016/j.drudis.2016.11.005
370. Bozzuto G and Molinari A. Liposomes as nanomedical devices. Int J Nanomed. (2015) 10:975–99. doi: 10.2147/IJN.S68861
371. Beltrán-Gracia E, López-Camacho A, Higuera-Ciapara I, Velázquez-Fernández JB, and Vallejo-Cardona AA. Nanomedicine review: clinical developments in liposomal applications. Cancer Nanotechnol. (2019) 10:11. doi: 10.1186/s12645-019-0055-y
372. Graziani SR, Vital CG, Morikawa AT, Van Eyll BM, Fernandes Junior HJ, Kalil Filho R, et al. Phase II study of paclitaxel associated with lipid core nanoparticles (LDE) as third-line treatment of patients with epithelial ovarian carcinoma. Med Oncol. (2017) 34:151. doi: 10.1007/s12032-017-1009-z
373. Ye L, He J, Hu Z, Dong Q, Wang H, Fu F, et al. Antitumor effect and toxicity of Lipusu in rat ovarian cancer xenografts. Food Chem Toxicol. (2013) 52:200–6. doi: 10.1016/j.fct.2012.11.004
374. Beg MS, Brenner AJ, Sachdev J, Borad M, Kang Y-K, Stoudemire J, et al. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Invest New Drugs. (2017) 35:180–8. doi: 10.1007/s10637-016-0407-y
375. Long X, Zhang X, Chen Q, Liu M, Xiang Y, Yang Y, et al. Nucleus-targeting phototherapy nanodrugs for high-effective anti-cancer treatment. Front Pharmacol. (2022) 13:905375. doi: 10.3389/fphar.2022.905375
376. Attia MF, Anton N, Wallyn J, Omran Z, and Vandamme TF. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J Pharm Pharmacol. (2019) 71:1185–98. doi: 10.1111/jphp.13098
377. Fan D, Cao Y, Cao M, Wang Y, Cao Y, and Gong T. Nanomedicine in cancer therapy. Signal Transduct Target Ther. (2023) 8:293. doi: 10.1038/s41392-023-01536-y
378. Bajracharya R, Song JG, Patil BR, Lee SH, Noh H-M, Kim D-H, et al. Functional ligands for improving anticancer drug therapy: current status and applications to drug delivery systems. Drug Deliv. (2022) 29:1959–70. doi: 10.1080/10717544.2022.2089296
379. Kim D-M, Kim M, Park H-B, Kim K-S, and Kim D-E. Anti-MUC1/CD44 dual-aptamer-conjugated liposomes for cotargeting breast cancer cells and cancer stem cells. ACS Appl Bio Mater. (2019) 2:4622–33. doi: 10.1021/acsabm.9b00705
380. Cho J-H, Kim A-R, Kim S-H, Lee S-J, Chung H, and Yoon M-Y. Development of a novel imaging agent using peptide-coated gold nanoparticles toward brain glioma stem cell marker CD133. Acta Biomater. (2017) 47:182–92. doi: 10.1016/j.actbio.2016.10.009
381. Ning S-T, Lee S-Y, Wei M-F, Peng C-L, Lin SY-F, Tsai M-H, et al. Targeting colorectal cancer stem-like cells with anti-CD133 antibody-conjugated SN-38 nanoparticles. ACS Appl Mater Interf. (2016) 8:17793–804. doi: 10.1021/acsami.6b04403
382. Rao W, Wang H, Han J, Zhao S, Dumbleton J, Agarwal P, et al. Chitosan-decorated doxorubicin-encapsulated nanoparticle targets and eliminates tumor reinitiating cancer stem-like cells. ACS Nano. (2015) 9:5725–40. doi: 10.1021/nn506928p
383. Chiu HI, Samad NA, Fang L, and Lim V. Cytotoxicity of targeted PLGA nanoparticles: a systematic review. RSC Adv. (2021) 11:9433–49. doi: 10.1039/D1RA00074H
384. Yu Z, Ni M, Xiong M, Zhang X, Cai G, Chen H, et al. Poly(lactic-co-glycolic acid) nanoparticles conjugated with CD133 aptamers for targeted salinomycin delivery to CD133+ osteosarcoma cancer stem cells. Int J Nanomed. (2015) 10:2537–54. doi: 10.2147/IJN.S78498
385. Jin H, Pi J, Zhao Y, Jiang J, Li T, Zeng X, et al. EGFR-targeting PLGA-PEG nanoparticles as a curcumin delivery system for breast cancer therapy. Nanoscale. (2017) 9:16365–74. doi: 10.1039/C7NR06898K
386. Verma RK, Yu W, Singh SP, Shankar S, and Srivastava RK. Anthothecol-encapsulated PLGA nanoparticles inhibit pancreatic cancer stem cell growth by modulating sonic hedgehog pathway. Nanomedicine. (2015) 11:2061–70. doi: 10.1016/j.nano.2015.07.001
387. Singh D, Mohapatra P, Kumar S, Behera S, Dixit A, and Sahoo SK. Nimbolide-encapsulated PLGA nanoparticles induces Mesenchymal-to-Epithelial Transition by dual inhibition of AKT and mTOR in pancreatic cancer stem cells. Toxicol In Vitro. (2022) 79:105293. doi: 10.1016/j.tiv.2021.105293
388. Dhar S, Gu FX, Langer R, Farokhzad OC, and Lippard SJ. Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA–PEG nanoparticles. PNAS. (2008) 105:17356–61. doi: 10.1073/pnas.0809154105
389. He Z, Huang J, Xu Y, Zhang X, Teng Y, Huang C, et al. Co-delivery of cisplatin and paclitaxel by folic acid conjugated amphiphilic PEG-PLGA copolymer nanoparticles for the treatment of non-small lung cancer. Oncotarget. (2015) 6:42150–68. doi: 10.18632/oncotarget.6243
390. Bai M-Y and Liu S-Z. A simple and general method for preparing antibody-PEG-PLGA sub-micron particles using electrospray technique: An in vitro study of targeted delivery of cisplatin to ovarian cancer cells. Colloids Surf B Biointerf. (2014) 117:346–53. doi: 10.1016/j.colsurfb.2014.02.051
391. Wang H, Zhao Y, Wu Y, Hu Y, Nan K, Nie G, et al. Enhanced anti-tumor efficacy by co-delivery of doxorubicin and paclitaxel with amphiphilic methoxy PEG-PLGA copolymer nanoparticles. Biomaterials. (2011) 32:8281–90. doi: 10.1016/j.biomaterials.2011.07.032
392. Li B, Li Q, Mo J, and Dai H. Drug-loaded polymeric nanoparticles for cancer stem cell targeting. Front Pharmacol. (2017) 8:51. doi: 10.3389/fphar.2017.00051
393. Anees M, Mehrotra N, Tiwari S, Kumar D, Kharbanda S, and Singh H. Polylactic acid based biodegradable hybrid block copolymeric nanoparticle mediated co-delivery of salinomycin and doxorubicin for cancer therapy. Int J Pharm. (2023) 635:122779. doi: 10.1016/j.ijpharm.2023.122779
394. Desai SA, Manjappa A, and Khulbe P. Drug delivery nanocarriers and recent advances ventured to improve therapeutic efficacy against osteosarcoma: an overview. J Egypt Natl Canc Inst. (2021) 33:4. doi: 10.1186/s43046-021-00059-3
395. Ahmadi-Nouraldinvand F, Bourang S, Azizi S, Noori M, Noruzpour M, and Yaghoubi H. Preparation and characterization of multi-target nanoparticles for co-drug delivery. Med Drug Discov. (2024) 21:100177. doi: 10.1016/j.medidd.2024.100177
396. Medel S, Syrova Z, Kovacik L, Hrdy J, Hornacek M, Jager E, et al. Curcumin-bortezomib loaded polymeric nanoparticles for synergistic cancer therapy. Eur Polym J. (2017) 93:116–31. doi: 10.1016/j.eurpolymj.2017.05.036
397. Vasvani S, Kulkarni P, and Rawtani D. Hyaluronic acid: A review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. Int J Biol Macromol. (2020) 151:1012–29. doi: 10.1016/j.ijbiomac.2019.11.066
398. Xiao B, Han MK, Viennois E, Wang L, Zhang M, Si X, et al. Hyaluronic acid-functionalized polymeric nanoparticles for colon cancer-targeted combination chemotherapy. Nanoscale. (2015) 7:17745–55. doi: 10.1039/C5NR04831A
399. Espinosa-Cano E, Huerta-Madroñal M, Cámara-Sánchez P, Seras-Franzoso J, Schwartz S, Abasolo I, et al. Hyaluronic acid (HA)-coated naproxen-nanoparticles selectively target breast cancer stem cells through COX-independent pathways. Mater Sci Eng C. (2021) 124:112024. doi: 10.1016/j.msec.2021.112024
400. Li Y-F, Zhang H-T, and Xin L. Hyaluronic acid-modified polyamidoamine dendrimer G5-entrapped gold nanoparticles delivering METase gene inhibits gastric tumor growth via targeting CD44+ gastric cancer cells. J Cancer Res Clin Oncol. (2018) 144:1463–73. doi: 10.1007/s00432-018-2678-5
401. Liu P, Chen G, and Zhang J. A review of liposomes as a drug delivery system: current status of approved products, regulatory environments, and future perspectives. Molecules. (2022) 27:1372. doi: 10.3390/molecules27041372
402. Heneweer C, Gendy SE, and Peñate-Medina O. Liposomes and inorganic nanoparticles for drug delivery and cancer imaging. Ther Delivery. (2012) 3:645–56. doi: 10.4155/tde.12.38
403. Wang L, Su W, Liu Z, Zhou M, Chen S, Chen Y, et al. CD44 antibody-targeted liposomal nanoparticles for molecular imaging and therapy of hepatocellular carcinoma. Biomaterials. (2012) 33:5107–14. doi: 10.1016/j.biomaterials.2012.03.067
404. Sun X, Chen Y, Zhao H, Qiao G, Liu M, Zhang C, et al. Dual-modified cationic liposomes loaded with paclitaxel and survivin siRNA for targeted imaging and therapy of cancer stem cells in brain glioma. Drug Deliv. (2018) 25:1718–27. doi: 10.1080/10717544.2018.1494225
405. Huang H, Liu R, Yang J, Dai J, Fan S, Pi J, et al. Gold nanoparticles: construction for drug delivery and application in cancer immunotherapy. Pharmaceutics. (2023) 15:1868. doi: 10.3390/pharmaceutics15071868
406. Mussa Farkhani S, Dehghankelishadi P, Refaat A, Veerasikku Gopal D, Cifuentes-Rius A, and Voelcker NH. Tailoring gold nanocluster properties for biomedical applications: From sensing to bioimaging and theranostics. Prog Mater Sci. (2024) 142:101229. doi: 10.1016/j.pmatsci.2023.101229
407. Cryer AM and Thorley AJ. Nanotechnology in the diagnosis and treatment of lung cancer. Pharmacol Ther. (2019) 198:189–205. doi: 10.1016/j.pharmthera.2019.02.010
408. Mohd-Zahid MH, Mohamud R, Che Abdullah CA, Lim J, Alem H, Wan Hanaffi WN, et al. Colorectal cancer stem cells: a review of targeted drug delivery by gold nanoparticles. RSC Adv. (2020) 10:973–85. doi: 10.1039/C9RA08192E
409. Imanparast A, Attaran N, Eshghi H, and Sazgarnia A. Surface modification of gold nanoparticles with 6-mercapto-1-hexanol to facilitate dual conjugation of protoporphyrin IX and folic acid for improving the targeted photochemical internalization. Iran J Basic Med Sci. (2022) 25:970–9. doi: 10.22038/IJBMS.2022.63622.14033
410. Didamson OC, Chandran R, and Abrahamse H. A gold nanoparticle bioconjugate delivery system for active targeted photodynamic therapy of cancer and cancer stem cells. Cancers (Basel). (2022) 14:4558. doi: 10.3390/cancers14194558
411. Bose A, Roy Burman D, Sikdar B, and Patra P. Nanomicelles: Types, properties and applications in drug delivery. IET Nanobiotechnol. (2021) 15:19–27. doi: 10.1049/nbt2.12018
412. Krishnamurthy S, Ng VWL, Gao S, Tan M-H, and Yang YY. Phenformin-loaded polymeric micelles for targeting both cancer cells and cancer stem cells in vitro and in vivo. Biomaterials. (2014) 35:9177–86. doi: 10.1016/j.biomaterials.2014.07.018
413. Smiley SB, Yun Y, Ayyagari P, Shannon HE, Pollok KE, Vannier MW, et al. Development of CD133 targeting multi-drug polymer micellar nanoparticles for glioblastoma - in vitro evaluation in glioblastoma stem cells. Pharm Res. (2021) 38:1067–79. doi: 10.1007/s11095-021-03050-8
414. Ghosh B and Biswas S. Polymeric micelles in cancer therapy: State of the art. J Control Release. (2021) 332:127–47. doi: 10.1016/j.jconrel.2021.02.016
415. Sun Y, Li B, Cao Q, Liu T, and Li J. Targeting cancer stem cells with polymer nanoparticles for gastrointestinal cancer treatment. Stem Cell Res Ther. (2022) 13:489. doi: 10.1186/s13287-022-03180-9
416. Yu Z, Chen F, Qi X, Dong Y, Zhang Y, Ge Z, et al. Epidermal growth factor receptor aptamer conjugated polymer lipid hybrid nanoparticles enhance salinomycin delivery to osteosarcoma and cancer stem cells. Exp Ther Med. (2017) 15:1247–56. doi: 10.3892/etm.2017.5578
417. Kharwade R, More S, Warokar A, Agrawal P, and Mahajan N. Starburst pamam dendrimers: Synthetic approaches, surface modifications, and biomedical applications. Arab J Chem. (2020) 13:6009–39. doi: 10.1016/j.arabjc.2020.05.002
418. Gogulapati N, Manalan BV, and Nadendla RR. Poly (propylene imine) Dendrimer: Synthesis, characterization and applications in various drug delivery. Asian J Pharm Pharmacol. (2020) 6:190–203. doi: 10.31024/ajpp.2020.6.3.10
419. Borgheti-Cardoso LN, Viegas JSR, Silvestrini AVP, Caron AL, Praça FG, Kravicz M, et al. Nanotechnology approaches in the current therapy of skin cancer. Adv Drug Deliv Rev. (2020) 153:109–36. doi: 10.1016/j.addr.2020.02.005
420. Li Y, Shi S, Ming Y, Wang L, Li C, Luo M, et al. Specific cancer stem cell-therapy by albumin nanoparticles functionalized with CD44-mediated targeting. J Nanobiotechnol. (2018) 16:99. doi: 10.1186/s12951-018-0424-4
421. Kesharwani P, Chadar R, Sheikh A, Rizg WY, and Safhi AY. CD44-targeted nanocarrier for cancer therapy. Front Pharmacol. (2022) 12:800481. doi: 10.3389/fphar.2021.800481
422. Debnath M, Sarkar S, Debnath SK, Dkhar DS, Kumari R, Vaskuri GSSJ, et al. Photothermally active quantum dots in cancer imaging and therapeutics: nanotheranostics perspective. ACS Appl Bio Mater. (2024) 7:8126–48. doi: 10.1021/acsabm.4c01190
423. Rakovich TY, Mahfoud OK, Mohamed BM, Prina-Mello A, Crosbie-Staunton K, Van Den Broeck T, et al. Highly sensitive single domain antibody–quantum dot conjugates for detection of HER2 biomarker in lung and breast cancer cells. ACS Nano. (2014) 8:5682–95. doi: 10.1021/nn500212h
424. Chen H-L, Qu Y-G, Zhang Q, Pan Q, Zhao X-D, Huang Y-H, et al. Quantum dots immunofluorescence histochemical detection of EGFR gene mutations in the non-small cell lung cancers using mutation-specific antibodies. Int J Nanomed. (2014) 9:5771–8. doi: 10.2147/IJN.S71310
425. Zeng Y, Zhu G, Yang X, Cao J, Jing Z, and Zhang C. A quantum dot-based microRNA nanosensor for point mutation assays. ChemComm. (2014) 50:7160. doi: 10.1039/c4cc02034k
426. Nguyen KC, Willmore WG, and Tayabali AF. Cadmium telluride quantum dots cause oxidative stress leading to extrinsic and intrinsic apoptosis in hepatocellular carcinoma HepG2 cells. Toxicol. (2013) 306:114–23. doi: 10.1016/j.tox.2013.02.010
427. Zhao L, Xu Y-H, Akasaka T, Abe S, Komatsu N, Watari F, et al. Polyglycerol-coated nanodiamond as a macrophage-evading platform for selective drug delivery in cancer cells. Biomaterials. (2014) 35:5393–406. doi: 10.1016/j.biomaterials.2014.03.041
428. Pan F, Khan M, Ragab AH, Javed E, Alsalmah HA, Khan I, et al. Recent advances in the structure and biomedical applications of nanodiamonds and their future perspectives. Mater Des. (2023) 233:112179. doi: 10.1016/j.matdes.2023.112179
429. Liu K-K, Wang C-C, Cheng C-L, and Chao J-I. Endocytic carboxylated nanodiamond for the labeling and tracking of cell division and differentiation in cancer and stem cells. Biomaterials. (2009) 30:4249–59. doi: 10.1016/j.biomaterials.2009.04.056
430. Zhang Y, Cui Z, Kong H, Xia K, Pan L, Li J, et al. One-shot immunomodulatory nanodiamond agents for cancer immunotherapy. Adv Mater. (2016) 28:2699–708. doi: 10.1002/adma.201506232
431. Wang X, Low XC, Hou W, Abdullah LN, Toh TB, Mohd Abdul Rashid M, et al. Epirubicin-adsorbed nanodiamonds kill chemoresistant hepatic cancer stem cells. ACS Nano. (2014) 8:12151–66. doi: 10.1021/nn503491e
432. Shao W, Paul A, Zhao B, Lee C, Rodes L, and Prakash S. Carbon nanotube lipid drug approach for targeted delivery of a chemotherapy drug in a human breast cancer xenograft animal model. Biomaterials. (2013) 34:10109–19. doi: 10.1016/j.biomaterials.2013.09.007
433. Gao Y, Mao D, Wu J, Wang X, Wang Z, Zhou G, et al. Carbon nanotubes translocation through a lipid membrane and transporting small hydrophobic and hydrophilic molecules. Appl Sci. (2019) 9:4271. doi: 10.3390/app9204271
434. Sharma S, Naskar S, and Kuotsu K. A review on carbon nanotubes: Influencing toxicity and emerging carrier for platinum based cytotoxic drug application. J Drug Delivery Sci Technol. (2019) 51:708–20. doi: 10.1016/j.jddst.2019.02.028
435. Yan H, Xue Z, Xie J, Dong Y, Ma Z, Sun X, et al. Toxicity of carbon nanotubes as anti-tumor drug carriers. Int J Nanomed Vol. (2019) 14:10179–94. doi: 10.2147/IJN.S220087
436. Jorio A and Saito R. Raman spectroscopy for carbon nanotube applications. J Appl Phys. (2021) 129:021102. doi: 10.1063/5.0030809
437. Nguyen TL, Takai M, Ishihara K, Oyama K, Fujii S, and Yusa S. Facile preparation of water-soluble multiwalled carbon nanotubes bearing phosphorylcholine groups for heat generation under near-infrared irradiation. Polym J. (2021) 53:1001–9. doi: 10.1038/s41428-021-00495-x
438. Bura C, Mocan T, Grapa C, and Mocan L. Carbon nanotubes-based assays for cancer detection and screening. Pharmaceutics. (2022) 14:781. doi: 10.3390/pharmaceutics14040781
439. Burke AR, Singh RN, Carroll DL, Wood JCS, D’Agostino RB, Ajayan PM, et al. The resistance of breast cancer stem cells to conventional hyperthermia and their sensitivity to nanoparticle-mediated photothermal therapy. Biomaterials. (2012) 33:2961–70. doi: 10.1016/j.biomaterials.2011.12.052
440. Al Faraj A, Shaik AS, Al Sayed B, Halwani R, and Al Jammaz I. Specific targeting and noninvasive imaging of breast cancer stem cells using single-walled carbon nanotubes as novel multimodality nanoprobes. Nanomedicine. (2016) 11:31–46. doi: 10.2217/nnm.15.182
441. Yao Y, Zhou Y, Liu L, Xu Y, Chen Q, Wang Y, et al. Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front Mol Biosci. (2020) 7:193. doi: 10.3389/fmolb.2020.00193
442. Vakil D, Doshi R, Mckinnirey F, and Sidhu K. Stem cell-derived exosomes as new horizon for cell-free therapeutic development: current status and prospects. Kitala D In: Possibilities and Limitations in Current Translational Stem Cell Research Biochemistry IntechOpen. (2023). doi: 10.5772/intechopen.108865
443. Dixon CL, Sheller-Miller S, Saade GR, Fortunato SJ, Lai A, Palma C, et al. Amniotic fluid exosome proteomic profile exhibits unique pathways of term and preterm labor. Endocrinology. (2018) 159:2229–40. doi: 10.1210/en.2018-00073
444. Goto T, Fujiya M, Konishi H, Sasajima J, Fujibayashi S, Hayashi A, et al. An elevated expression of serum exosomal microRNA-191, – 21, –451a of pancreatic neoplasm is considered to be efficient diagnostic marker. BMC Cancer. (2018) 18:116. doi: 10.1186/s12885-018-4006-5
445. Liu J, Jiang F, Jiang Y, Wang Y, Li Z, Shi X, et al. Roles of exosomes in ocular diseases. Int J Nanomed Volume. (2020) 15:10519–38. doi: 10.2147/IJN.S277190
446. Yokoi A and Ochiya T. Exosomes and extracellular vesicles: Rethinking the essential values in cancer biology. Semin Cancer Biol. (2021) 74:79–91. doi: 10.1016/j.semcancer.2021.03.032
447. Xu M, Ji J, Jin D, Wu Y, Wu T, Lin R, et al. The biogenesis and secretion of exosomes and multivesicular bodies (MVBs): Intercellular shuttles and implications in human diseases. Genes Dis. (2023) 10:1894–907. doi: 10.1016/j.gendis.2022.03.021
448. Zhang Y, Liang F, Zhang D, Qi S, and Liu Y. Metabolites as extracellular vesicle cargo in health, cancer, pleural effusion, and cardiovascular diseases: An emerging field of study to diagnostic and therapeutic purposes. Biomedicine & Pharmacotherapy. (2023) 157:114046. doi: 10.1016/j.biopha.2022.114046
449. Sunkara SP, Kar NR, Kareemulla S, Sarma KN, Thool KU, Katual MK, et al. Recent advancement in exosome-inspired lipid nanovesicles for cell-specific drug delivery. Int J Pharm Invest. (2024) 14:1085–95. doi: 10.5530/ijpi.14.4.119
450. Ha D, Yang N, and Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. (2016) 6:287–96. doi: 10.1016/j.apsb.2016.02.001
451. Zeng Z, Fu M, Hu Y, Wei Y, Wei X, and Luo M. Regulation and signaling pathways in cancer stem cells: implications for targeted therapy for cancer. Mol Cancer. (2023) 22:172. doi: 10.1186/s12943-023-01877-w
452. Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature. (2015) 527:329–35. doi: 10.1038/nature15756
453. Sen S, Xavier J, Kumar N, Ahmad MZ, and Ranjan OP. Exosomes as natural nanocarrier-based drug delivery system: recent insights and future perspectives. 3 Biotech. (2023) 13:101. doi: 10.1007/s13205-023-03521-2
454. Cheng Z, Lei Z, Yang P, Si A, Xiang D, Tang X, et al. Exosome-transmitted p120-catenin suppresses hepatocellular carcinoma progression via STAT3 pathways. Mol Carcinog. (2019) 58:1389–99. doi: 10.1002/mc.23022
455. Hu Y-B, Yan C, Mu L, Mi YL, Zhao H, Hu H, et al. Exosomal Wnt-induced dedifferentiation of colorectal cancer cells contributes to chemotherapy resistance. Oncogene. (2019) 38:1951–65. doi: 10.1038/s41388-018-0557-9
456. Lan J, Sun L, Xu F, Liu L, Hu F, Song D, et al. M2 macrophage-derived exosomes promote cell migration and invasion in colon cancer. Cancer Res. (2019) 79:146–58. doi: 10.1158/0008-5472.CAN-18-0014
457. Lin F, Yin H, Li X, Zhu G, He W, and Gou X. Bladder cancer cell secreted exosomal miR 21 activates the PI3K/AKT pathway in macrophages to promote cancer progression. Int J Oncol. (2019) 56:151–64. doi: 10.3892/ijo.2019.4933
458. Chang J, Li H, Zhu Z, Mei P, Hu W, Xiong X, et al. microRNA-21-5p from M2 macrophage-derived extracellular vesicles promotes the differentiation and activity of pancreatic cancer stem cells by mediating KLF3. Cell Biol Toxicol. (2022) 38:577–90. doi: 10.1007/s10565-021-09597-x
459. Coradduzza D, Cruciani S, Arru C, Garroni G, Pashchenko A, Jedea M, et al. Role of miRNA-145, 148, and 185 and stem cells in prostate cancer. Int J Mol Sci. (2022) 23:1626. doi: 10.3390/ijms23031626
460. Zabeti Touchaei A, Norollahi SE, Najafizadeh A, Babaei K, Bakhshalipour E, Vahidi S, et al. Therapeutic combinations of exosomes alongside cancer stem cells (CSCs) and of CSC-derived exosomes (CSCEXs) in cancer therapy. Cancer Cell Int. (2024) 24:334. doi: 10.1186/s12935-024-03514-y
461. Ha S-W, Weitzmann MN, and Beck GR. Bioactive Silica Nanoparticles Promote Osteoblast Differentiation through Stimulation of Autophagy and Direct Association with LC3 and p62. ACS Nano. (2014) 8:5898–910. doi: 10.1021/nn5009879
462. Lin J, Huang Z, Wu H, Zhou W, Jin P, Wei P, et al. Inhibition of autophagy enhances the anticancer activity of silver nanoparticles. Autophagy. (2014) 10:2006–20. doi: 10.4161/auto.36293
463. Hessvik NP and Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. (2018) 75:193–208. doi: 10.1007/s00018-017-2595-9
465. Chen L, Guo P, He Y, Chen Z, Chen L, Luo Y, et al. HCC-derived exosomes elicit HCC progression and recurrence by epithelial-mesenchymal transition through MAPK/ERK signalling pathway. Cell Death Dis. (2018) 9:513. doi: 10.1038/s41419-018-0534-9
466. Toffoli G, Hadla M, Corona G, Caligiuri I, Palazzolo S, Semeraro S, et al. Exosomal doxorubicin reduces the cardiac toxicity of doxorubicin. Nanomedicine. (2015) 10:2963–71. doi: 10.2217/nnm.15.118
467. Yong T, Zhang X, Bie N, Zhang H, Zhang X, Li F, et al. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat Commun. (2019) 10:3838. doi: 10.1038/s41467-019-11718-4
468. Asadpour A, Yahaya BH, Bicknell K, Cottrell GS, and Widera D. Uncovering the gray zone: mapping the global landscape of direct-to-consumer businesses offering interventions based on secretomes, extracellular vesicles, and exosomes. Stem Cell Res Ther. (2023) 14:111. doi: 10.1186/s13287-023-03335-2
469. Safaei M, Rajabi SS, Tirgar M, Namdar N, Dalfardi M, Mohammadifar F, et al. Exosome-based approaches in cancer along with unlocking new insights into regeneration of cancer-prone tissues. Regener Ther. (2025) 29:202–16. doi: 10.1016/j.reth.2025.03.005
470. Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, et al. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther. (2008) 16:782–90. doi: 10.1038/mt.2008.1
471. Yoshimura A, Sawada K, and Kimura T. Is the exosome a potential target for cancer immunotherapy? Annals of Translational Medicine. (2017) 5(5):117–117. doi: 10.21037/atm.2017.01.47
472. Samuel M and Gabrielsson S. Personalized medicine and back–allogeneic exosomes for cancer immunotherapy. J Intern Med. (2021) 289:138–46. doi: 10.1111/joim.12963
473. Fritah H, Rovelli R, Chiang CL-L, and Kandalaft LE. The current clinical landscape of personalized cancer vaccines. Cancer Treat Rev. (2022) 106:102383. doi: 10.1016/j.ctrv.2022.102383
474. Escudier B, Dorval T, Chaput N, André F, Caby M-P, Novault S, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J Transl Med. (2005) 3:10. doi: 10.1186/1479-5876-3-10
475. Yao Y, Fu C, Zhou L, Mi Q-S, and Jiang A. DC-derived exosomes for cancer immunotherapy. Cancers. (2021) 13:3667. doi: 10.3390/cancers13153667
476. Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med. (2005) 3:9. doi: 10.1186/1479-5876-3-9
477. Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell derived exosomes. Nat Med. (1998) 4:594–600. doi: 10.1038/nm0598-594
478. Markov O, Oshchepkova A, and Mironova N. Immunotherapy based on dendritic cell-targeted/-derived extracellular vesicles—A novel strategy for enhancement of the anti-tumor immune response. Front Pharmacol. (2019) 10:1152. doi: 10.3389/fphar.2019.01152
479. Meng Y, Yao Z, Ke X, Hu M, Ren H, Gao S, et al. Extracellular vesicles-based vaccines: Emerging immunotherapies against cancer. J Control Release. (2025) 378:438–59. doi: 10.1016/j.jconrel.2024.12.010
480. Viaud S, Terme M, Flament C, Taieb J, André F, Novault S, et al. Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: A role for NKG2D ligands and IL-15Rα. PLoS One. (2009) 4:e4942. doi: 10.1371/journal.pone.0004942
481. Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, Planchard D, et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. OncoImmunology. (2016) 5:e1071008. doi: 10.1080/2162402X.2015.1071008
482. Xia J, Miao Y, Wang X, Huang X, and Dai J. Recent progress of dendritic cell-derived exosomes (Dex) as an anti-cancer nanovaccine. Biomed Pharmacother. (2022) 152:113250. doi: 10.1016/j.biopha.2022.113250ss
483. Näslund TI, Gehrmann U, Qazi KR, Karlsson MCI, and Gabrielsson S. Dendritic cell–derived exosomes need to activate both T and B cells to induce antitumor immunity. J Immunol. (2013) 190:2712–9. doi: 10.4049/jimmunol.1203082
484. Hodge AL, Baxter AA, and Poon IKH. Gift bags from the sentinel cells of the immune system: The diverse role of dendritic cell-derived extracellular vesicles. J Leukoc Biol. (2022) 111:903–20. doi: 10.1002/JLB.3RU1220-801R
485. Tian W, Lei N, Zhou J, Chen M, Guo R, Qin B, et al. Extracellular vesicles in ovarian cancer chemoresistance, metastasis, and immune evasion. Cell Death Dis. (2022) 13:64. doi: 10.1038/s41419-022-04510-8
486. Chen B, Qiu X, and Li Y. Exosomes in ovarian cancer: impact on drug resistance and advances in SERS detection techniques. J Pharmaceut Anal. (2024), 101170. doi: 10.1016/j.jpha.2024.101170
487. Kuang L, Wu L, and Li Y. Extracellular vesicles in tumor immunity: mechanisms and novel insights. Mol Cancer. (2025) 24:45. doi: 10.1186/s12943-025-02233-w
488. Xia W, Tan Y, Liu Y, Xie N, and Zhu H. Prospect of extracellular vesicles in tumor immunotherapy. Front Immunol. (2025) 16:1525052. doi: 10.3389/fimmu.2025.1525052
489. Zeelenberg IS, Ostrowski M, Krumeich S, Bobrie A, Jancic C, Boissonnas A, et al. Targeting tumor antigens to secreted membrane vesicles in vivo induces efficient antitumor immune responses. Cancer Res. (2008) 68:1228–35. doi: 10.1158/0008-5472.CAN-07-3163
490. Sedlik C, Vigneron J, Torrieri-Dramard L, Pitoiset F, Denizeau J, Chesneau C, et al. Different immunogenicity but similar antitumor efficacy of two DNA vaccines coding for an antigen secreted in different membrane vesicle-associated forms. J Extracell Vesicles. (2014) 3:10.3402/jev.v3.24646. doi: 10.3402/jev.v3.24646
491. Lener T, Gimona M, Aigner L, Börger V, Buzas E, Camussi G, et al. Applying extracellular vesicles based therapeutics in clinical trials – an ISEV position paper. J Extracell Vesicles. (2015) 4:30087. doi: 10.3402/jev.v4.30087
492. Gage AA and Baust JG. Cryosurgery for tumors. J Am Coll Surg. (2007) 205:342–56. doi: 10.1016/j.jamcollsurg.2007.03.007
493. Sadhukha T, Niu L, Wiedmann TS, and Panyam J. Effective elimination of cancer stem cells by magnetic hyperthermia. Mol Pharm. (2013) 10:1432–41. doi: 10.1021/mp400015b
494. Huang H, Yu K, Mohammadi A, Karanthanasis E, Godley A, and Yu JS. It’s getting hot in here: targeting cancer stem-like cells with hyperthermia. J Stem Cell Transplant Biol. (2017) 2:113.
495. Oei AL, Vriend LEM, Krawczyk PM, Horsman MR, Franken NAP, and Crezee J. Targeting therapy-resistant cancer stem cells by hyperthermia. Int J Hyperthermia. (2017) 33:419–27. doi: 10.1080/02656736.2017.1279757
496. Li Y, Liu Y, Xing D, Wang J, Zheng L, Wang Z, et al. 2D/2D heterostructure of ultrathin BiVO4/Ti3C2 nanosheets for photocatalytic overall Water splitting. Appl Catal B. (2021) 285:119855. doi: 10.1016/j.apcatb.2020.119855
497. Lobo NA, Shimono Y, Qian D, and Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. (2007) 23:675–99. doi: 10.1146/annurev.cellbio.22.010305.104154
498. Sun T, Wang Y, Wang Y, Xu J, Zhao X, Vangveravong S, et al. Using SV119-gold nanocage conjugates to eradicate cancer stem cells through a combination of photothermal and chemo therapies. Adv Health Mater. (2014) 3:1283–91. doi: 10.1002/adhm.201400026
499. Liang S, Li C, Zhang C, Chen Y, Xu L, Bao C, et al. CD44v6 monoclonal antibody-conjugated gold nanostars for targeted photoacoustic imaging and plasmonic photothermal therapy of gastric cancer stem-like cells. Theranostics. (2015) 5:970–84. doi: 10.7150/thno.11632
500. Yu Z, Zhou P, Pan W, Li N, and Tang B. A biomimetic nanoreactor for synergistic chemiexcited photodynamic therapy and starvation therapy against tumor metastasis. Nat Commun. (2018) 9:5044. doi: 10.1038/s41467-018-07197-8
501. Bu L, Rao L, Yu G, Chen L, Deng W, Liu J, et al. Cancer stem cell-platelet hybrid membrane-coated magnetic nanoparticles for enhanced photothermal therapy of head and neck squamous cell carcinoma. Adv Funct Mater. (2019) 29:1807733. doi: 10.1002/adfm.201807733
502. He Y, Li Z, Cong C, Ye F, Yang J, Zhang X, et al. Pyroelectric catalysis-based “Nano-lymphatic” Reduces tumor interstitial pressure for enhanced penetration and hydrodynamic therapy. ACS Nano. (2021) 15:10488–501. doi: 10.1021/acsnano.1c03048
503. Badir A, Refki S, and Sekkat Z. Utilizing gold nanoparticles in plasmonic photothermal therapy for cancer treatment. Heliyon. (2025) 11:e42738. doi: 10.1016/j.heliyon.2025.e42738
504. Skinner W H, Salimi M, Moran L, Blein-Dezayes I, Mehta M, Mosca S, et al. Plasmonic Nanoparticles for Photothermal Therapy: Benchmarking of Photothermal Properties and Modeling of Heating at Depth in Human Tissues. The Journal of Physical Chemistry C. (2025) 129(3):1864–1872. doi: 10.1021/acs.jpcc.4c06381
505. Atkinson RL, Zhang M, Diagaradjane P, Peddibhotla S, Contreras A, Hilsenbeck SG, et al. Thermal enhancement with optically activated gold nanoshells sensitizes breast cancer stem cells to radiation therapy. Sci Transl Med. (2010) 2:55ra79. doi: 10.1126/scitranslmed.3001447
506. Rastinehad AR, Anastos H, Wajswol E, Winoker JS, Sfakianos JP, Doppalapudi SK, et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. PNAS. (2019) 116:18590–6. doi: 10.1073/pnas.1906929116
507. Tian J, Gu Y, Li Y, and Liu T. CD271 antibody-functionalized HGNs for targeted photothermal therapy of osteosarcoma stem cells. Nanotechnol. (2020) 31:305707. doi: 10.1088/1361-6528/ab8593
508. Pan Y, Ma X, Liu C, Xing J, Zhou S, Parshad B, et al. Retinoic acid-loaded dendritic polyglycerol-conjugated gold nanostars for targeted photothermal therapy in breast cancer stem cells. ACS Nano. (2021) 15:15069–84. doi: 10.1021/acsnano.1c05452
509. Wang X, Li G, Ding Y, and Sun S. Understanding the photothermal effect of gold nanostars and nanorods for biomedical applications. RSC Adv. (2014) 4:30375–83. doi: 10.1039/C4RA02978J
510. Vo-Dinh T. Shining gold nanostars: from cancer diagnostics to photothermal treatment and immunotherapy. J Immunol Sci. (2018) 2:1–8. doi: 10.29245/2578-3009/2018/1.1104
511. Kim C, Song H-M, Cai X, Yao J, Wei A, and Wang LV. In vivo photoacoustic mapping of lymphatic systems with plasmon-resonant nanostars. J Mater Chem. (2011) 21:2841. doi: 10.1039/c0jm04194g
512. Wang J, Sefah K, Altman MB, Chen T, You M, Zhao Z, et al. Aptamer-conjugated nanorods for targeted photothermal therapy of prostate cancer stem cells. Chem Asian J. (2013) 8:2417–22. doi: 10.1002/asia.201300375
513. Peng CA and Wang CH. Cancer stem-like cells photothermolysed by gold nanorod-mediated near-infrared laser irradiation. Int J Nanotechnol. (2014) 11:1157. doi: 10.1504/IJNT.2014.065142
514. Kaushik N, Borkar SB, Nandanwar SK, Panda PK, Choi EH, and Kaushik NK. Nanocarrier cancer therapeutics with functional stimuli-responsive mechanisms. J Nanobiotechnol. (2022) 20:152. doi: 10.1186/s12951-022-01364-2
515. Wang C-H, Chiou S-H, Chou C-P, Chen Y-C, Huang Y-J, and Peng C-A. Photothermolysis of glioblastoma stem-like cells targeted by carbon nanotubes conjugated with CD133 monoclonal antibody. Nanomedicine. (2011) 7:69–79. doi: 10.1016/j.nano.2010.06.010
516. Mei C, Wang N, Zhu X, Wong K, and Chen T. Photothermal-controlled nanotubes with surface charge flipping ability for precise synergistic therapy of triple-negative breast cancer. Adv Funct Mater. (2018) 28:1805225. doi: 10.1002/adfm.201805225
517. Yu Y, Yang X, Reghu S, Kaul SC, Wadhwa R, and Miyako E. Photothermogenetic inhibition of cancer stemness by near-infrared-light-activatable nanocomplexes. Nat Commun. (2020) 11:4117. doi: 10.1038/s41467-020-17768-3
518. Gwak J, Cho M, Gong S-J, Won J, Kim D-E, Kim E-Y, et al. Protein-kinase-C-mediated β-catenin phosphorylation negatively regulates the Wnt/β-catenin pathway. J Cell Sci. (2006) 119:4702–9. doi: 10.1242/jcs.03256
519. Han X, Jing X, Yang D, Lin H, Wang Z, Ran H, et al. Therapeutic mesopore construction on 2D Nb 2 C MXenes for targeted and enhanced chemo-photothermal cancer therapy in NIR-II biowindow. Theranostics. (2018) 8:4491–508. doi: 10.7150/thno.26291
520. Liu Z, Lin H, Zhao M, Dai C, Zhang S, Peng W, et al. 2D superparamagnetic tantalum carbide composite MXenes for efficient breast-cancer theranostics. Theranostics. (2018) 8:1648–64. doi: 10.7150/thno.23369
Keywords: cancer stem cells, signaling pathways, biomarkers, nanocarriers, exosomes, nanoparticle-mediated ablation therapies, targeted therapy
Citation: Kumbhakar DV, Thakkar L, Akhand C, Sharaf S and Vemuganti GK (2025) Nanomaterials targeting cancer stem cells to overcome drug resistance and tumor recurrence. Front. Oncol. 15:1499283. doi: 10.3389/fonc.2025.1499283
Received: 20 September 2024; Accepted: 18 April 2025;
Published: 06 June 2025.
Edited by:
Bilal Bin Hafeez, The University of Texas Rio Grande Valley, United StatesReviewed by:
Junhua Mai, Houston Methodist Research Institute, United StatesPiyush Kumar Gupta, Sharda University, India
Narendra Sankpal, Saint Joseph Hospital Medical Center Phoenix, United States
Copyright © 2025 Kumbhakar, Thakkar, Akhand, Sharaf and Vemuganti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Geeta K. Vemuganti, Z2t2ZW11Z2FudGlAZ21haWwuY29t; Divya Vishambhar Kumbhakar, a3VtYmhha2FyZGl2eWFAZ21haWwuY29t
 Divya Vishambhar Kumbhakar
Divya Vishambhar Kumbhakar Lucky Thakkar
Lucky Thakkar Chetana Akhand
Chetana Akhand Shehna Sharaf
Shehna Sharaf Geeta K. Vemuganti
Geeta K. Vemuganti