- Department of Colorectal and Anal Surgery, The First Affiliated Hospital of Guangxi Medical University, Nanning, China
Colorectal cancer is among the most common malignancies of the gastrointestinal tract. Immune checkpoint inhibitors (ICIs) have become a key component in the treatment of locally advanced rectal cancer (LARC), offering promising therapeutic outcomes. However, ICIs can occasionally cause significant adverse effects. Herein, we report a case of rectal cancer with suspected paraneoplastic myasthenia gravis (pMG) induced by immune checkpoint inhibitors (ICIs). Unfortunately, the patient lacked anti-acetylcholine receptor (AChR)/muscle-specific kinase (MuSK) antibody testing and muscle biopsy, which precluded a definitive diagnosis of pMG. Remarkably, following surgical resection, the patient not only achieved complete tumor eradication but also experienced full remission of myasthenia gravis (MG).
Introduction
Colorectal cancer ranks as the third most prevalent malignancy worldwide, with approximately 1.9 million new cases diagnosed annually (1). For patients with locally advanced rectal cancer (LARC), the standard of care includes neoadjuvant chemoradiotherapy followed by total mesorectal excision and adjuvant chemotherapy (2). Recently, immune checkpoint inhibitors (ICIs) have been integrated into the neoadjuvant treatment (NAT) for LARC, yielding promising therapeutic results (3–5). Current evidence on ICIs in LARC predominantly supports their use in microsatellite instability-high (MSI-H) subtypes, where robust responses and guideline-endorsed efficacy have been well documented (5–7). However, since the majority of rectal cancers are microsatellite stable (MSS) (8), and ICIs have not been included as a standard treatment option for MSS rectal cancer in guidelines, ICIs remain investigational for this population. To address this unmet need, our center has initiated a clinical trial (NCT06254521) evaluating neoadjuvant immunotherapy in MSS LARC.
While ICIs have shown significant antitumor efficacy, they are also associated with immune-related neurological complications (9, 10). Myasthenia gravis (MG), an autoimmune neuromuscular disorder primarily caused by autoantibodies targeting acetylcholine receptors at the neuromuscular junction, is commonly observed as a paraneoplastic syndrome (PNS) in patients with thymoma (11), but has also been reported in patients with other malignancies (12–17). However, the occurrence of MG as a PNS in association with rectal cancer has not been reported in the literature.
We present a case of LARC complicated by paraneoplastic myasthenia gravis (pMG), suspected from the symptoms, that developed during NAT with cytotoxic chemotherapy and ICIs. Following curative rectal cancer surgery, the patient achieved complete tumor eradication and full remission of myasthenia gravis.
Case report
A 55-year-old Chinese man presented to our hospital with a one-year history of altered stool characteristics, including hematochezia. His medical history was notable for chronic hepatitis B, with positive HBsAg and anti - HBc. He had no prior history of myasthenia gravis. Moreover, he had no other comorbidities, such as hypertension, diabetes, or coronary artery disease, and no family history of malignancy or autoimmune diseases.
On admission, physical examination revealed a palpable rectal mass, located 7 cm from the anal verge, with limited mobility and fixation on digital rectal examination. Limb muscle strength was intact (grade 5/5), and there was no evidence of eyelid ptosis. Laboratory tests (Table 1) revealed elevated tumor markers, with no significant elevation of muscle injury-related enzymes. Pelvic MRI revealed a rectal tumor staged as T4aN2a, with negative mesorectal fascia (MRF-) and no extramural venous invasion (EMV-) (Figure 1A). Contrast-enhanced CT scans of the chest, abdomen, and pelvis revealed no distant metastasis, and the thymus was unremarkable. Pathological evaluation confirmed mucinous adenocarcinoma. The final diagnosis was locally advanced rectal cancer (LARC), mucinous adenocarcinoma, staged as cT4aN2aM0.
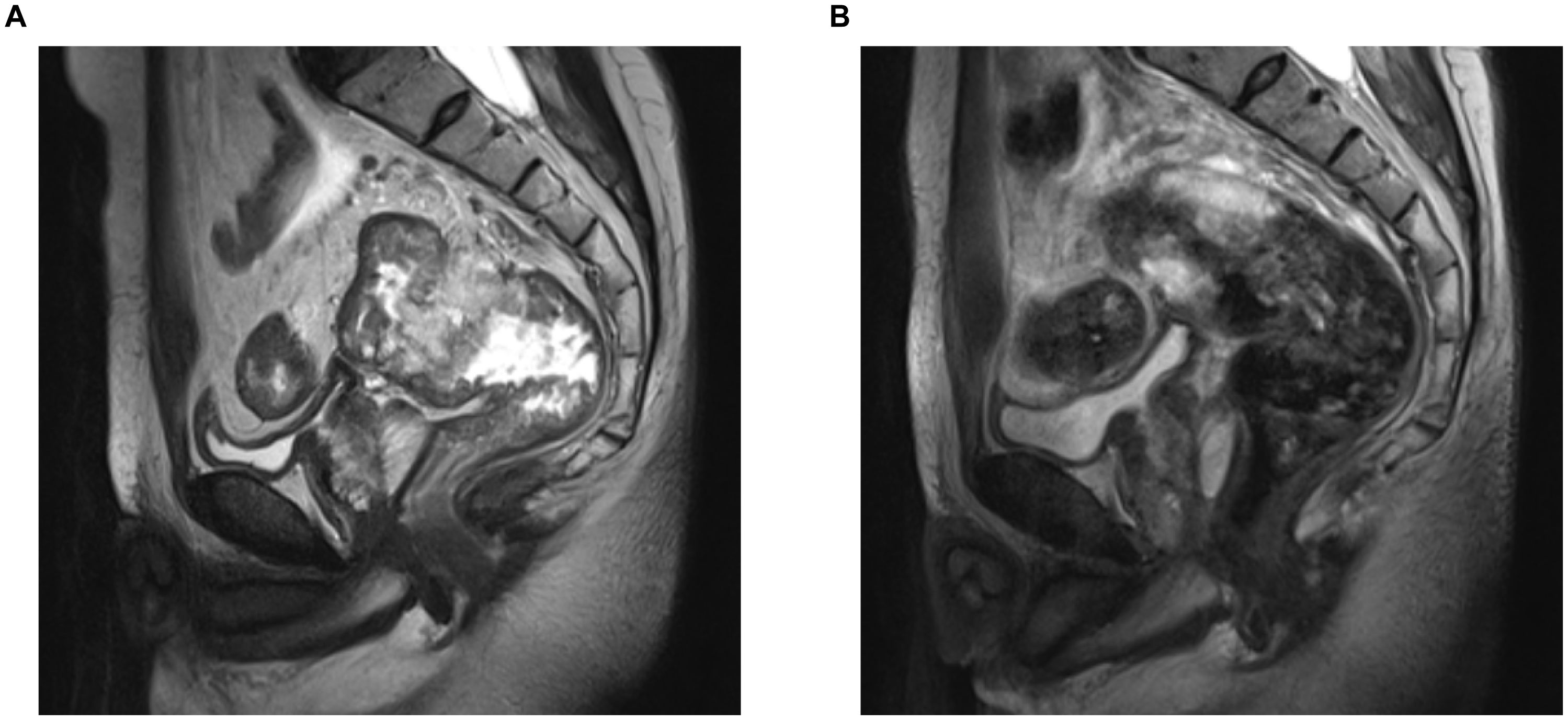
Figure 1. Pelvic MRI of the patient pre- and post-NAT. (A) Pre-NAT. (B) Post-NAT. NAT, neoadjuvant therapy.
Following the diagnosis of LARC, the patient underwent NAT with a combination of CAPOX (oxaliplatin 130 mg/m² intravenously on day 1 and capecitabine 1000 mg/m² orally twice daily from day 1 to 14, every 3 weeks) and tislelizumab (200 mg intravenously on day 1, every 3 weeks). The patient developed right-sided ptosis on day 25 of NAT, demonstrating characteristic diurnal fluctuation (mild morning symptoms worsening by evening). Three days later (day 28), evaluation at a local hospital revealed elevated transaminases (ALT and AST; the exact values are unavailable), prompting hepatoprotective therapy with intravenous magnesium isoglycyrrhizinate and glutathione. NAT was discontinued at this time, though no neurological intervention was initiated.
Neurological progression ensued, with left-sided ptosis and cervical weakness emerging by day 32. By day 40, progressive limb weakness developed, culminating in severe functional impairment by day 60 (including inability to perform activities of daily living and requirement of cervical orthosis). The patient presented to our institution on day 62 post-NAT initiation, with no interim care received between discharge from the initial local hospital and this admission.
On physical examination, the patient exhibited reduced muscle strength in both the limbs and neck, along with bilateral ptosis (Figure 2). Ptosis developed after 2 seconds of upward gaze, with a resting upper and lower eyelid gap measuring 0 mm. In a seated position, he could sustain bilateral upper limb elevation at 90° for 152 seconds. In the supine position, he could maintain head elevation to 45° for 23 seconds and leg elevation to 45° for 42 seconds. Grip strength was recorded at 20 kg.
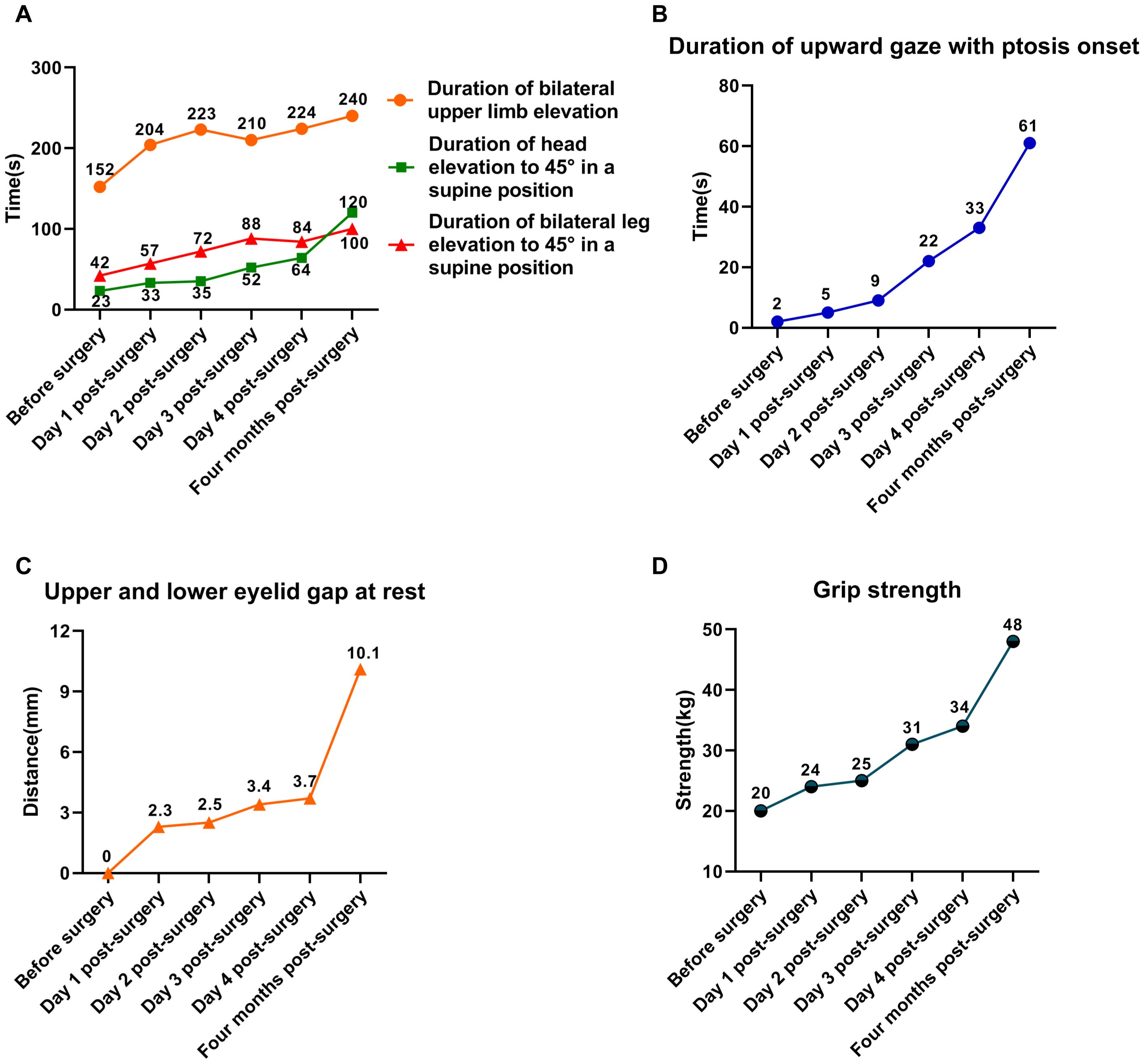
Figure 2. Trends in changes of muscle strength in the patient. (A) Trends in changes of limb and neck muscle strength. (B) Trends in changes of limb and neck muscle strength. (C) Trends in changes of upper and lower eyelid gap at rest. (D) Trends in changes of grip strength.
Laboratory tests (Table 1) showed a significant reduction in tumor markers compared to previous levels, along with an increase in muscle injury-related enzymes. Pelvic MRI and contrast-enhanced CT scans of the chest, abdomen, and pelvis revealed a reduction in the rectal tumor, now staged as T3N0M0 (Figure 1B), demonstrating significant tumor regression compared to prior imaging. No additional tumors, infections, thymic abnormalities, or muscle pathology were observed. The patient had no history of exposure to myotoxic agents. After excluding other causes, the patient's MG was suspected to be a paraneoplastic syndrome (PNS) secondary to rectal cancer.
The patient subsequently underwent laparoscopic radical resection of rectal cancer with a temporary ileostomy. On postoperative day 1, a marked improvement in muscle strength was observed (Figure 2). Ptosis occurred only after 5 seconds of upward gaze, and at rest, the upper and lower eyelid gap was 2 mm. Seated, the patient was able to sustain bilateral upper limb elevation at 90°for 204 seconds. In the supine position, he sustained head elevation to 45°for 33 seconds and leg elevation to 45° for 57 seconds. Grip strength was measured at 24 kg.
By postoperative day 4, the patient had made further gains in muscle strength and was discharged in stable condition, able to ambulate independently (Figure 2). Ptosis developed after 32 seconds of upward gaze, with an eyelid gap of 4 mm at rest. While seated, the patient maintained bilateral upper limb elevation at 90° for 221 seconds. In the supine position, he sustained head elevation to 45°for 64 seconds and leg elevation to 45°for 116 seconds. Grip strength had improved to 34 kg.
Postoperative pathological analysis confirmed an R0 resection, with cancer cells infiltrating the fibrofatty tissue beyond the muscularis propria. Both upper and lower resection margins were free of tumor involvement, and all 19 lymph nodes examined were negative for metastasis. The patient was advised to finish adjuvant chemotherapy. After two cycles of CAPOX, the patient refused to continue. Four months postoperatively, the patient returned for ileostomy closure. By this time, symptoms of MG had fully resolved, with muscle strength completely restored to normal levels (Figure 2).
Discussion
This report presents a case of MG that developed during neoadjuvant chemotherapy combined with ICIs in a patient with LARC. Despite a five-week cessation of neoadjuvant therapy, the patient’s MG symptoms did not improve and showed signs of worsening, raising the suspicion of a PNS. Following surgical resection of the rectal tumor, the patient's MG symptoms began to improve on postoperative day 1, with significant recovery by postoperative day 5. Four months later, during ileostomy closure, the MG symptoms had fully resolved. The complete resolution of MG following radical tumor resection further supports the possibility of a diagnosis of a paraneoplastic etiology.
While MG is commonly associated with thymoma (18, 19), it can also arise as a paraneoplastic syndrome in other malignancies, including lymphoma, breast cancer, and lung cancer (12–17). The diagnosis of pMG is primarily based on a combination of oncological history, neurological manifestations, tumor response to treatment, and the presence of specific serum antibodies (20). In this case, a probable diagnosis of pMG was established based on the patient's oncological history, clinical manifestations, and treatment response. The temporal association between tumor resection and neurological improvement provides compelling clinical support for pMG. However, diagnostic limitations remain, including the lack of autoantibody testing (anti-AChR/MuSK) and confirmatory muscle biopsy, which precludes definitive exclusion of immune-mediated myositis. These gaps reduce the certainty of the pMG diagnosis and underscore the need for future prospective studies incorporating comprehensive serological and histological assessments.
Emerging evidence suggests molecular mimicry between tumor-associated antigens and nicotinic acetylcholine receptors (AChRs) at the neuromuscular junction may drive paraneoplastic MG (pMG) pathogenesis (21–23). Tumor-expressed abnormal antigens are misidentified by the immune system as acetylcholine receptors, thereby activating autoreactive T cells and assisting B cells in secreting anti-AChR antibodies. These antibodies directly block the function of AChR, leading to a significant reduction in the density of AChR at the postsynaptic membrane, disrupting synaptic transmission and ultimately resulting in myasthenia gravis (18, 23). While immune checkpoint inhibitors enhance antitumor immunity, they may break peripheral immune tolerance, activating cross-reactive autoimmune responses against the neuromuscular junction's acetylcholine receptors, thereby contributing to the development and progression of pMG (24). After the patient underwent surgical treatment, the continuous release of cross-reactive antigens was eliminated, interrupting the malignant cycle of the autoimmune response. This allowed for gradual restoration of acetylcholine receptor synthesis and synaptic structure, leading to the rapid alleviation of myasthenia gravis symptoms (11, 25–27). However, the exploration of this mechanism is still in its early stages, and further high-quality research is needed to investigate the underlying mechanisms more deeply.
Studies have shown that when ICIs are administered to tumors frequently associated with paraneoplastic syndromes, the incidence of PNS increases significantly. The underlying mechanism may be related to ICIs stimulating the body to enhance immune responses against potential neural antigens expressed in tumors (28). Yshii et al. (29) confirmed through their research that in mouse models with pre-existing subclinical paraneoplastic autoimmunity, ICIs can block CTLA-4 inhibitory signals, abrogate the suppression of pre-sensitized CD8+ cytotoxic T lymphocytes, enabling them to break through immune tolerance and further attack central nervous system cells expressing the same antigens, ultimately leading to the progression of subclinical lesions to clinical paraneoplastic syndromes.
In recent years, immunotherapy has become a pivotal strategy in cancer treatment (30, 31). While ICIs have demonstrated promising efficacy, they are also associated with a spectrum of immune-related adverse events (irAEs), with neurological irAEs occurring in approximately 1-5% of patients (10, 32). MG, as a neurological irAE, typically improves with discontinuation of the offending immunotherapy (33). However, in this case, the patient’s symptoms persisted and worsened after cessation of treatment, suggesting that this was not a typical irAE. Growing evidence indicates that ICIs can induce or exacerbate paraneoplastic neurological syndromes (34–36). In this patient, tumor resection likely led to antigen removal, resulting in a decrease in autoantibody levels and subsequent improvement in MG symptoms. This case likely represents pMG triggered by immunotherapy.
The current management of pMG typically includes corticosteroids, intravenous immunoglobulin, plasmapheresis, and immunosuppressants (37). Immunosuppressants may elevate the risk of anastomotic fistula by inhibiting fibroblast proliferation, blocking angiogenesis, and slowing the progression of neointimal hyperplasia (38–40). Currently, a significant proportion of rectal cancer patients undergo neoadjuvant chemoradiotherapy prior to surgery (2), and such patients exhibit a further increased risk of anastomotic fistula. When a patient’s myasthenic symptoms fail to resolve and instead exacerbate after discontinuing ICIs, a diagnosis of suspected pMG should be considered. Under such circumstances, radical surgical resection of the tumor may represent an effective therapeutic approach, as it not only achieves radical tumor eradication but also potentially ameliorates myasthenic symptoms. Moreover, given that the patient did not receive immunosuppressant therapy preoperatively, the risk of anastomotic fistula is relatively low. In our case, the patient’s limb muscle strength recovered more rapidly than the muscle strength in the face and neck after surgical intervention. This disparity may be attributed to the varying severity of MG symptoms across different regions during initial onset. However, as this observation is drawn from a single case, further research is necessary to determine whether this pattern is consistent in other patients.
In this case, the marked temporal correlation between tumor resection and resolution of myasthenia gravis symptoms strongly supports the possibility of a diagnosis of pMG. These findings offer novel insights into the management of MG in colorectal cancer, suggesting that surgical resection may represent an effective therapeutic strategy for patients developing MG symptoms during neoadjuvant therapy for LARC potentially avoiding complications associated with immunosuppressive therapies. For non-resectable disease, conventional MG management remains indicated.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by First Affiliated Hospital of Guangxi Medical University Ethical Review Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ZX: Writing – review & editing, Conceptualization, Data curation, Writing – original draft, Investigation, Software. HC: Writing – original draft, Investigation, Software. MQ: Data curation, Investigation, Software, Writing – original draft. WH: Data curation, Software, Writing – original draft. HL: Data curation, Investigation, Writing – original draft. ML: Supervision, Writing – review & editing. SZ: Funding acquisition, Project administration, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (82360371).
Acknowledgments
The authors extend their appreciation to the patient and his family members for their support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Giaquinto AN, and Jemal A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
2. Benson AB, Venook AP, Al-Hawary MM, Azad N, Chen YJ, Ciombor KK, et al. Rectal cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2022) 20:1139–67. doi: 10.6004/jnccn.2022.0051
3. Xiao WW, Chen G, Gao YH, Lin JZ, Wu XJ, Luo HL, et al. Effect of neoadjuvant chemoradiotherapy with or without PD-1 antibody sintilimab in pMMR locally advanced rectal cancer: A randomized clinical trial. Cancer Cell. (2024) 42:1570–81. doi: 10.1016/j.ccell.2024.07.004
4. Li Y, Pan C, Gao Y, Zhang L, Ji D, Cui X, et al. Total neoadjuvant therapy with PD-1 blockade for high-risk proficient mismatch repair Rectal Cancer. JAMA Surg. (2024) 159:529–37. doi: 10.1001/jamasurg.2023.7996
5. Chen G, Jin Y, Guan WL, Zhang RX, Xiao WW, Cai PQ, et al. Neoadjuvant PD-1 blockade with sintilimab in mismatch-repair deficient, locally advanced rectal cancer: an open-label, single-center phase 2 study. Lancet Gastroenterol Hepatol. (2023) 8:422–31. doi: 10.1016/S2468-1253(22)00439-3
6. Yang R, Wu T, Yu J, Cai X, Li G, Li X, et al. Locally advanced rectal cancer with dMMR/MSI-H may be excused from surgery after neoadjuvant anti-PD-1 monotherapy: a multiple-center, cohort study. Front Immunol. (2023) 14:1182299. doi: 10.3389/fimmu.2023.1182299
7. Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, et al. PD-1 Blockade in Mismatch repair–deficient, locally advanced rectal cancer. New Engl J Med. (2022) 386:2363–76. doi: 10.1056/NEJMoa2201445
8. Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, et al. Mismatch repair–deficient rectal cancer and resistance to neoadjuvant chemotherapy. Clin Cancer Res. (2020) 26:3271–9. doi: 10.1158/1078-0432.Ccr-19-3728
9. Duong SL, Barbiero FJ, Nowak RJ, and Baehring JM. Neurotoxicities associated with immune checkpoint inhibitor therapy. J Neurooncol. (2021) 152:265–77. doi: 10.1007/s11060-021-03695-w
10. Rossi S, Gelsomino F, Rinaldi R, Muccioli L, Comito F, Di Federico A, et al. Peripheral nervous system adverse events associated with immune checkpoint inhibitors. J Neurol. (2023) 270:2975–86. doi: 10.1007/s00415-023-11625-1
11. Gilhus NE, Tzartos S, Evoli A, Palace J, Burns TM, and Verschuuren J. Myasthenia gravis. Nat Rev Dis Primers. (2019) 5:30. doi: 10.1038/s41572-019-0079-y
12. Basta I, Pekmezovic T, Peric S, Nikolic A, Rakocevic-Stojanovic V, Stevic Z, et al. Extrathymic Malignancies in a defined cohort of patients with myasthenia gravis. J Neurol Sci. (2014) 346:80–4. doi: 10.1016/j.jns.2014.07.060
13. Dunn C, Nathoo R, Tauer A, and Green L. Alopecia areata and myasthenia gravis presenting as paraneoplastic phenomena of breast cancer. JAAD Case Rep. (2022) 21:35–7. doi: 10.1016/j.jdcr.2021.12.012
14. Khateb M, Zant MA, Bsoul A, Karny T, Yarnitsky D, and Shelly S. The association between myasthenia gravis and higher extrathymic cancer risk. . Brain Behav. (2025) 15:e70143. doi: 10.1002/brb3.70143
15. Levin N, Abramsky O, Lossos A, Karussis D, Siegal T, Argov Z, et al. Extrathymic Malignancies in patients with myasthenia gravis. J Neurol Sci. (2005) 237:39–43. doi: 10.1016/j.jns.2005.05.009
16. Nanni L, Broccoli A, Nanni C, Argnani L, Cavo M, and Zinzani PL. Hodgkin lymphoma presenting with paraneoplastic myasthenia: a case report. Leukemia Lymphoma. (2018) 59:2990–3. doi: 10.1080/10428194.2018.1443336
17. López-Viñas L, Rocío-Martín E, Delis-Gómez S, and Wix-Ramos R. Myasthenia gravis related to small cell lung carcinoma. Cureus. (2021) 13:e13889. doi: 10.7759/cureus.13889
18. Kaminski HJ, Sikorski P, Coronel SI, and Kusner LL. Myasthenia gravis: the future is here. J Clin Invest. (2024) 134:e179742. doi: 10.1172/jci179742
19. Yamada Y, Weis C-A, Thelen J, Sticht C, Schalke B, Ströbel P, et al. Thymoma associated myasthenia gravis (TAMG): differential expression of functional pathways in relation to MG status in different thymoma histotypes. Front Immunol. (2020) 11:664. doi: 10.3389/fimmu.2020.00664
20. Graus F, Vogrig A, Muniz-Castrillo S, Antoine JG, Desestret V, Dubey D, et al. Updated diagnostic criteria for paraneoplastic neurologic syndromes. . Neurol Neuroimmunol Neuroinflamm. (2021) 8:e1014. doi: 10.1212/NXI.0000000000001014
21. Darnell RB and Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. (2003) 349:1543–54. doi: 10.1056/NEJMra023009
22. Marx A, Willcox N, Leite MI, Chuang WY, Schalke B, Nix W, et al. Thymoma and paraneoplastic myasthenia gravis. Autoimmunity. (2010) 43:413–27. doi: 10.3109/08916930903555935
23. Zekeridou A, Griesmann GE, and Lennon VA. Mutated cancer autoantigen implicated cause of paraneoplastic myasthenia gravis. Muscle Nerve. (2018) 58:600–4. doi: 10.1002/mus.26166
24. Sharpe AH and Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. (2018) 18:153–67. doi: 10.1038/nri.2017.108
25. Berrih-Aknin S and Le Panse R. Myasthenia gravis: a comprehensive review of immune dysregulation and etiological mechanisms. J Autoimmun. (2014) 52:90–100. doi: 10.1016/j.jaut.2013.12.011
26. Marx A, Pfister F, Schalke B, Saruhan-Direskeneli G, Melms A, and Strobel P. The different roles of the thymus in the pathogenesis of the various myasthenia gravis subtypes. Autoimmun Rev. (2013) 12:875–84. doi: 10.1016/j.autrev.2013.03.007
27. Okumura M, Ohta M, Takeuchi Y, Shiono H, Inoue M, Fukuhara K, et al. The immunologic role of thymectomy in the treatment of myasthenia gravis: implication of thymus-associated B-lymphocyte subset in reduction of the anti-acetylcholine receptor antibody titer. J Thorac Cardiovasc Surg. (2003) 126:1922–8. doi: 10.1016/s0022-5223(03)00938-3
28. Graus F and Dalmau J. Paraneoplastic neurological syndromes in the era of immune-checkpoint inhibitors. Nat Rev Clin Oncol. (2019) 16:535–48. doi: 10.1038/s41571-019-0194-4
29. Yshii LM, Gebauer CM, Pignolet B, Maure E, Queriault C, Pierau M, et al. CTLA4 blockade elicits paraneoplastic neurological disease in a mouse model. Brain. (2016) 139:2923–34. doi: 10.1093/brain/aww225
30. Robert C. A decade of immune-checkpoint inhibitors in cancer. Ther Nat Commun. (2020) 11:3801. doi: 10.1038/s41467-020-17670-y
31. Bagchi S, Yuan R, and Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. (2021) 16:223–49. doi: 10.1146/annurev-pathol-042020-042741
32. Haugh AM, Probasco JC, and Johnson DB. Neurologic complications of immune checkpoint inhibitors. Expert Opin Drug Saf. (2020) 19:479–88. doi: 10.1080/14740338.2020.1738382
33. Guidon AC, Burton LB, Chwalisz BK, Hillis J, Schaller TH, Amato AA, et al. Consensus disease definitions for neurologic immune-related adverse events of immune checkpoint inhibitors. . J Immunother Cancer. (2021) 9:e002890. doi: 10.1136/jitc-2021-002890
34. Farina A, Villagran-Garcia M, Vogrig A, Zekeridou A, Muniz-Castrillo S, Velasco R, et al. Neurological adverse events of immune checkpoint inhibitors and the development of paraneoplastic neurological syndromes. Lancet Neurol. (2024) 23:81–94. doi: 10.1016/S1474-4422(23)00369-1
35. Sechi E, Markovic SN, McKeon A, Dubey D, Liewluck T, Lennon VA, et al. Neurologic autoimmunity and immune checkpoint inhibitors: Autoantibody profiles and outcomes. Neurology. (2020) 95:e2442–52. doi: 10.1212/WNL.0000000000010632
36. Jean MJ, Samkoff L, and Mohile N. Management of paraneoplastic syndromes in the era of immune checkpoint inhibitors. Curr Treat Options Oncol. (2024) 25:42–65. doi: 10.1007/s11864-023-01157-1
37. Verma N, Jaffer MH, Kolli AS, and Mokhtari S. Updates in the management of paraneoplastic syndrome. Semin Neurol. (2024) 44:36–46. doi: 10.1055/s-0043-1777353
38. Dean PG, Lund WJ, Larson TS, Prieto M, Nyberg SL, Ishitani MB, et al. Wound-healing complications after kidney transplantation: a prospective, randomized comparison of sirolimus and tacrolimus. Transplantation. (2004) 77:1555–61. doi: 10.1097/01.tp.0000123082.31092.53
39. Zakliczynski M, Nozynski J, Kocher A, Lizak MK, Zakliczynska H, Przybylski R, et al. Surgical wound-healing complications in heart transplant recipients treated with rapamycin. Wound Repair Regener. (2007) 15:316–21. doi: 10.1111/j.1524-475X.2007.00232.x
Keywords: paraneoplastic myasthenia gravis, paraneoplastic syndrome, immune checkpoint inhibitors, neoadjuvant treatment, locally advanced rectal cancer
Citation: Xia Z, Chen H, Qiu M, Huang W, Li H, Lin M and Zhang S (2025) Surgical management of suspected paraneoplastic myasthenia gravis in rectal cancer during neoadjuvant immunotherapy: a case report. Front. Oncol. 15:1503362. doi: 10.3389/fonc.2025.1503362
Received: 28 September 2024; Accepted: 18 August 2025;
Published: 02 September 2025.
Edited by:
Mario Trompetto, Clinica Santa Rita, ItalyReviewed by:
Navin K. Chintala, Memorial Sloan Kettering Cancer Center, United StatesStavros P. Papadakos, Laiko General Hospital of Athens, Greece
WenQing Yang, ClinBridge Biotech Co. Ltd, China
Copyright © 2025 Xia, Chen, Qiu, Huang, Li, Lin and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Minglin Lin, bGlubWluZ2xpbkBzci5neG11LmVkdS5jbg==; Sen Zhang, enMwNzcxQDEyNi5jb20=
†These authors have contributed equally to this work
 Zhiyuan Xia
Zhiyuan Xia Haining Chen†
Haining Chen† Ming Qiu
Ming Qiu Hui Li
Hui Li Minglin Lin
Minglin Lin Sen Zhang
Sen Zhang