- 1Department of Hepatopancreatobiliary, Hernia and Vascular Surgery, Caidian District People’s Hospital of Wuhan, Wuhan, China
- 2Department of General Surgery, People’s Liberation Army of China (PLA) Middle Military Command General Hospital, Wuhan, China
- 3Department of Urology, General Hospital of Xinjiang Military Command, Urumqi, China
Background: Percutaneous transhepatic biliary drainage (PTBD) was widely used for bile drainage in hilar cholangiocarcinoma (HCCA) patients, due to its exact effectiveness in relieving obstructive jaundice. However, the potential association between PTBD and increased local tumor spread (including portal vein invasion and lymph node metastasis) remained unclear, as this procedure might prolong the waiting time and lead to potential risks of portal vein injury. This study aimed to investigate whether HCCA patients undergoing PTBD exhibit higher risks of portal vein invasion and lymph node metastasis after radical resection.
Methods: The clinical data of 341 HCCA patients was retrospectively analyzed. PTBD was exclusively used as the preoperative biliary drainage method, excluding patients who underwent endoscopic nasobiliary drainage or endoscopic biliary stenting. Portal vein invasion and lymph node metastasis were verified by postoperative pathological examinations.
Results: In this study, 163 patients (47.8%) received preoperative PTBD. These patients experienced significantly higher risks of portal vein invasion [odds ratio (OR): 1.86, p = 0.027] and lymph node metastasis (OR: 1.94, p = 0.008) compared to those 178 patients (52.2%) in the non-PTBD group. The Kaplan–Meier survival analysis revealed significantly better OS (p = 0.039) in the non-PTBD group. Causal mediation analysis revealed that the effect of PTBD on survival was partly mediated by portal vein invasion and lymph node metastasis. Additionally, the length of hospitalization in PTBD group was obviously longer (26.7 days vs. 21.8 days, p = 0.002).
Conclusion: Preoperative PTBD was associated with increased incidence of portal vein invasion and lymph node metastasis in HCCA patients accepting R0 resection.
Introduction
Hilar cholangiocarcinoma (HCCA) is a rare but highly aggressive malignant tumor originating from the extrahepatic bile duct (1). Preoperative percutaneous transhepatic biliary drainage (PTBD) has been extensively employed to relieve obstructive jaundice in HCCA patients prior to surgery, which can reduce the severity of liver damage, promote the ability of liver regeneration and increase the remaining liver volume (2–4).
Previous investigations have predominantly focused on the benefits of PTBD in reducing jaundice and enhancing hepatic function (5, 6). These studies confirm that PTBD effectively decreases serum bilirubin levels and optimizes the patients’ clinical status, thereby improving eligibility for curative-intent surgery (7). Current evidence regarding PTBD-related oncological complications has primarily focused on implantation metastases, including pleural and peritoneal dissemination (8–10). However, the potential injury of portal vein branches during catheter insertion might lead to portal vein thrombosis, with a reported incidence of 10% in prior research (11–13). Furthermore, the injury of portal vein might lead to the local spread of tumor cells from bile duct to portal veins, causing the invasion of related vessels. Moreover, the prolonged intervals between PTBD and operation might increase the incidence of local tumor invasion, such as vascular invasion and lymph node metastasis (10). Despite these insights, significant knowledge gaps persist regarding PTBD’s specific impact on local tumor invasion patterns, specifically vascular invasion and regional lymph node metastasis. Understanding whether PTBD contributes to local tumor spread is crucial for optimizing preoperative management strategies and improving patient outcomes.
Therefore, we conducted a retrospective analysis of 341 HCCA patients accepting radical resection to investigate the potential associations between PTBD and regional vascular invasion and lymph node metastasis. Also, the potential effects of PTBD on the overall prognosis and survival of HCCA patients were discussed. Through this investigation, our study aimed to inform clinical decision-making and refined the preoperative management of this challenging malignancy.
Methods
Study population
From March 2008 to December 2019, 341 HCCA patients undergoing radical resection at the Southwest Hospital and PLA Middle Military Command General Hospital were enrolled. All procedures were performed according to R0 resection criteria. This study was approved by the Ethical Committee (Approval No. KY20211215), and all patients or their guardians provided written informed consent when informed of potential risks or adverse effects during the clinical process.
Data collection
In this study, preoperative epidemiologic features like age, gender, height, weight, BMI, heart disease and diabetes, laboratory tests like white blood cells, platelet, liver transaminase, bilirubin, albumin, alkaline phosphatase, coagulation and tumor markers, clinic-pathological features like liver cirrhosis, hepatic artery invasion, portal vein invasion, lymph node metastasis, tumor differentiation, and Tumor Node Metastasis (TNM) stage were collected.
The inclusion criteria of this study included the following: patients with pathological confirmation of HCCA; patients received R0 resection; expected survival time longer than 3 months; patients had a clear record of accepting preoperative biliary drainage or not; and PTBD was the unique approach of biliary drainage. This R0 resection implied the histologically negative margins in the operation. Moreover, the proximal end of the bile duct greater than 5 mm from the edge of the tumor and the distal end at the upper edge of the pancreas were resected in the operation. The entire caudate lobe and surrounding liver parenchyma greater than 15 mm surrounding the bile duct axis were removed. For patients with Bismuth–Corlette III or IV HCCA, hemihepatectomy or extended hemihepatectomy and caudate lobectomy were conducted. The lymph nodes of groups 8, 12, and 13 were conventionally dissected.
The criteria of accepting PTBD in this study included the following: the level of total bilirubin was higher than 100 µmol/L or the symptom of jaundice has sustained for more than 4 weeks, or those patients needed to accept large scale hepatectomy (over thee Couinaud segments).
These patients who accepted R0 resection did not routinely accept adjuvant therapy after operation. All individuals were closely followed up in outpatient clinic after discharge. Basic measurements including liver function and blood routine examination, examination of serum tumor markers, and imaging were performed.
Statistical analysis
Continuous variables were presented as the mean and standard deviation or median and interquartile range. Categorical variables were presented as amounts and percentages. Comparisons between clinicopathological factors were made using Student’s t-test or analysis of variance or Kruskal–Wallis test for continuous variables and χ2-test for categorical variables. The log-rank test and t-test were used to compare the differences between those patients accepting PTBD or not.
Univariate and multivariate logistic regression were used to analyze the risk factors associated with portal vein invasion and lymph node metastasis. Odds ratios (OR) derived from logistic regression models represent the likelihood of pathological outcomes per unit increase in exposure, with p-values <0.05, indicating statistically significant associations. The Cox proportional-hazards model was used to identify relationship between clinical characteristics and survival. Kaplan–Meier curves were used to estimate overall survival (OS) and relapse-free survival (RFS), and log-rank test was used to evaluate statistically significant differences. Causal mediation analysis was performed to investigate the mediation effect of PTBD on OS and RFS.
All statistical analyses were performed in the STATA (version 14), and p < 0.05 was considered statistically significant. The figure was re-arranged in the Adobe Illustrator (version 2019).
Results
Basic clinical characteristics of patients accepting PTBD or not
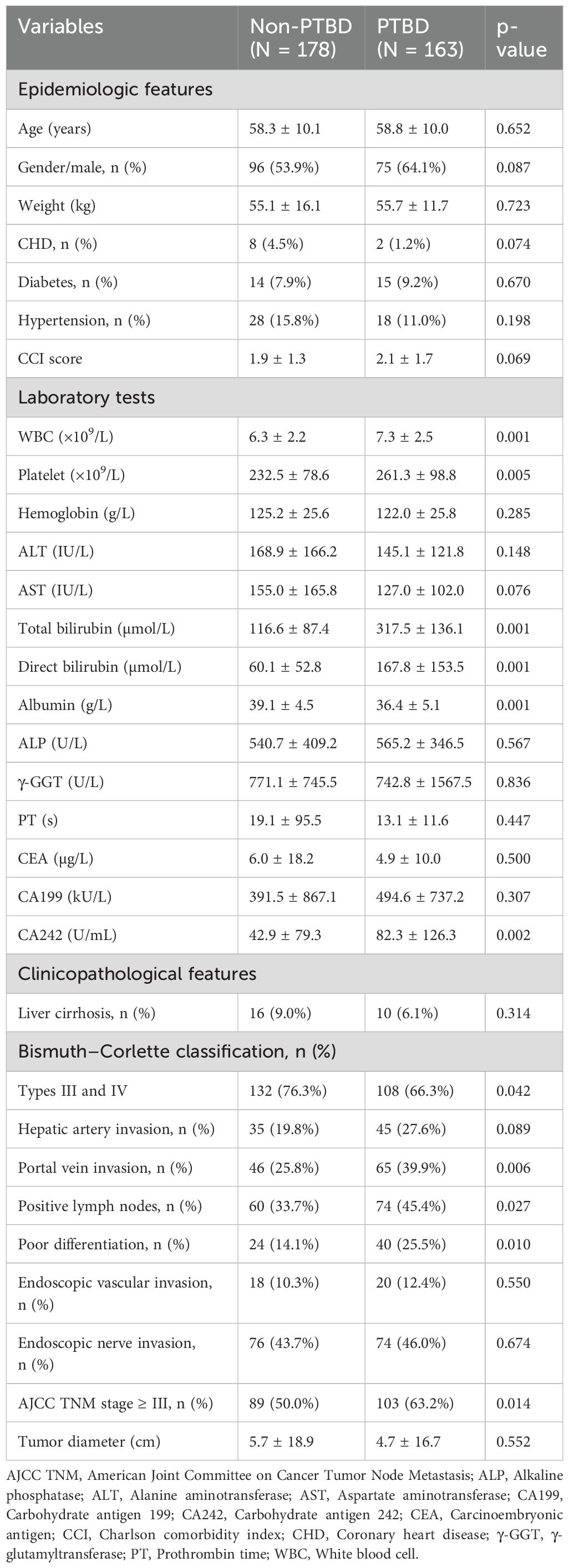
In this study, a total of 341 HCCA patients who accepted radical resection were included. As shown in Table 1, the differences of basic epidemiologic features like age, gender, weight, and chronic diseases between the PTBD group and the non-PTBD group were not statistically significant (p > 0.05). In the PTBD group, the number of white blood cells (7.3 vs. 6.3 × 109/L, p = 0.001) and platelets (261.3 vs. 232.5 × 109/L, p = 0.005) was significantly higher than that in the non-PTBD group. Also, levels of bilirubin, including total bilirubin (317.5 vs. 116.6 µmol/L, p = 0.001) and direct bilirubin (167.8 vs. 60.1 µmol/L, p = 0.001), were significantly elevated in the PTBD group, reflecting the presence of preoperative jaundice as a crucial indication for PTBD acceptance. Furthermore, tumor marker CA242 levels were significantly higher in the PTBD group compared to the non-PTBD group (82.3 vs. 42.9 U/mL, p = 0.002), underscoring its diagnostic relevance in PTBD patients.
Particularly, the proportion of patients with portal vein invasion (39.9% vs. 25.8%, p = 0.006) and lymph node metastasis (45.4% vs. 33.7%, p = 0.027) was significantly higher in the PTBD group compared to that in the non-PTBD group. Additionally, there were more patients with poorly differentiated tumors (25.5% vs. 14.1%, p = 0.010) in the PTBD group than that in the non-PTBD group.
Patients accepting PTBD experienced higher risk for portal vein invasion
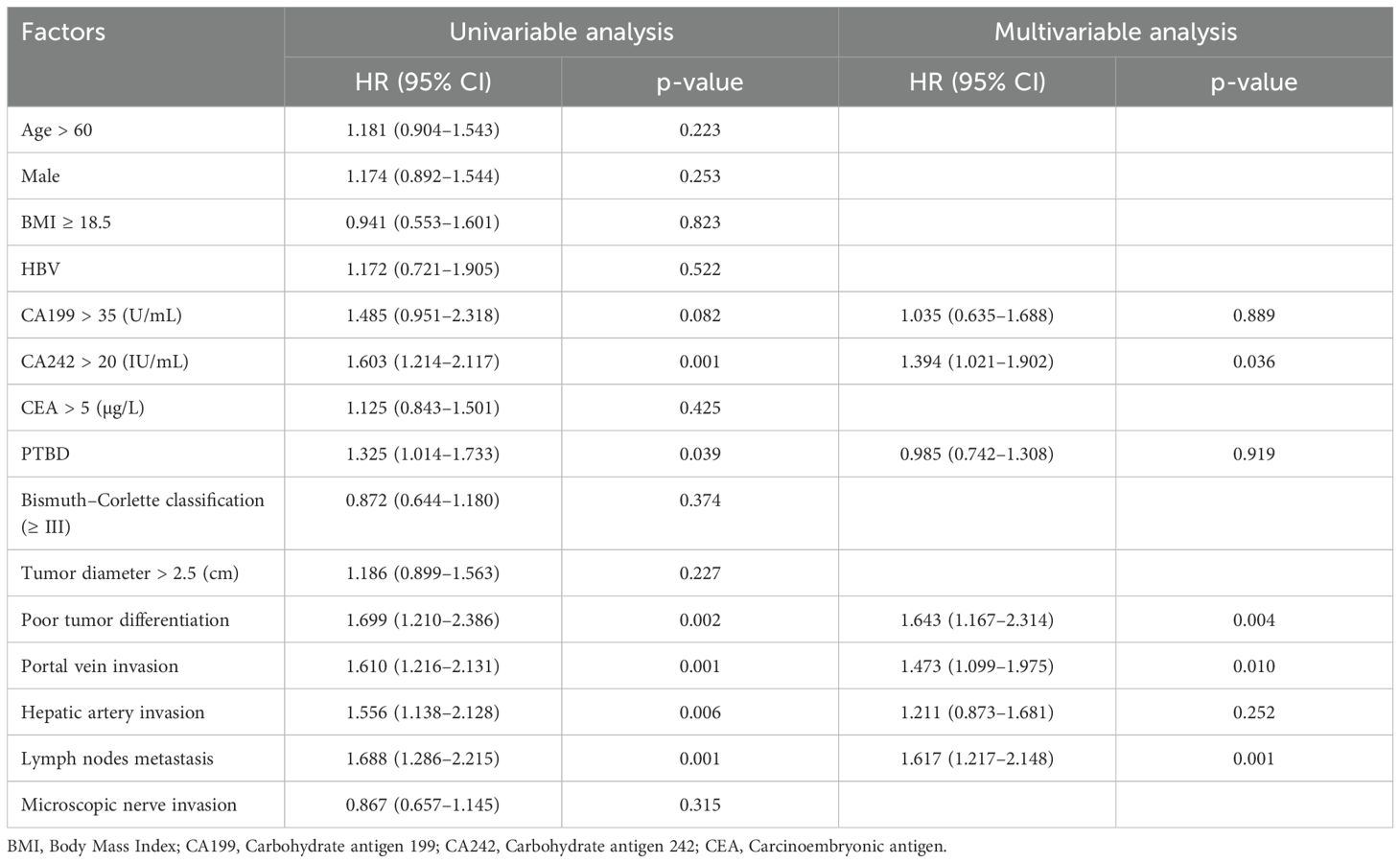
To evaluate potential preoperative risk factors associated with portal vein invasion, univariate analysis was conducted initially (Table 2). Significant factors identified included liver cirrhosis (OR = 4.4, P = 0.001), total bilirubin > 42 µmol/L (OR = 2.1, P = 0.036), Alanine aminotransferase (ALT) > 42 IU/L (OR = 2.2, P = 0.032), Alanine aminotransferase (AST) > 42 IU/L (OR = 2.8, P = 0.018), Alanine aminotransferase (ALP) > 188 U/L (OR = 4.9, P = 0.009), PTBD (OR = 1.9, P = 0.006), and Bismuth–Corlette type III–IV classification (OR = 2.4, P = 0.002). These factors were found to be statistically significant in association with portal vein invasion. Afterward, these factors with p-values less than 0.1 in univariate analysis and those considered clinically important were included in the multivariable logistic regression. As shown in Table 2, previous PTBD (OR = 2.3, P = 0.002) was an independent risk factor for portal vein invasion.
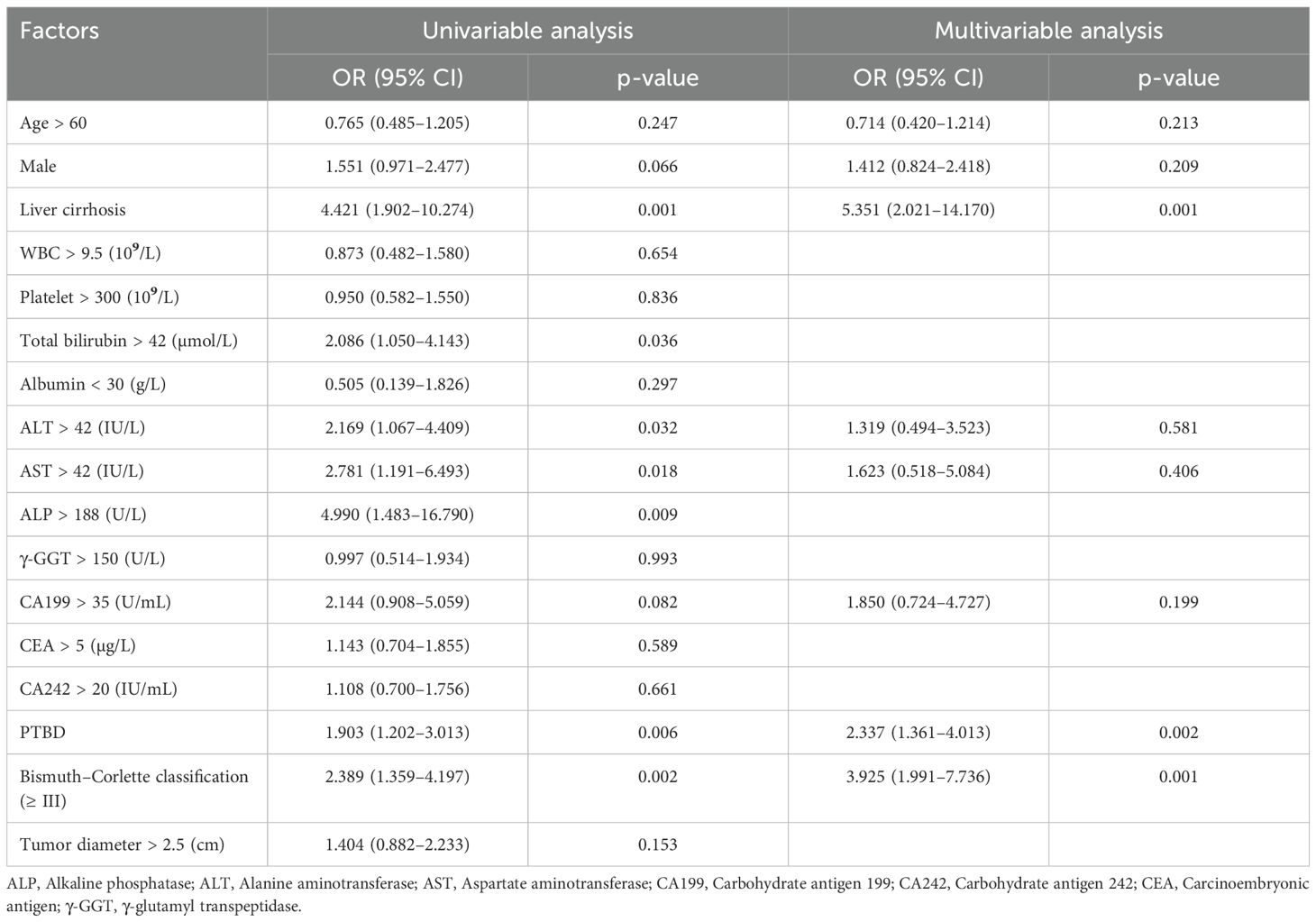
Table 2. Univariable and multivariable analysis of risk factors associated with portal vein invasion.
In the univariate analysis, both total bilirubin and PTBD showed statistically significant differences. However, the level of total bilirubin was excluded from the multivariate analysis because it was a key determinant for deciding PTBD, which was central and critical to this study. Including both variables could introduce confounding effects (14). This approach helped avoid multicollinearity and ensured the clarity of PTBD’s impact on outcomes (15).
Patients accepting PTBD experienced higher risk for lymph node metastasis
Similarly, univariate and multivariate logistic regression analyses were performed to explore the potential relationships between these peri-operative factors and lymph node metastasis, with the results presented in Table 3. In the univariate analysis, PTBD (OR = 1.6, P = 0.028) and tumor diameter > 2.5 cm (OR = 1.7, P = 0.016) were the only two factors significantly associated with lymph node metastasis. Afterward, these factors with p-values less than 0.1 in univariate analysis and those considered clinically important were included in the multivariable logistic regression. Following multivariate analysis, CA242 > 20 IU/mL (OR = 1.9, P = 0.016), PTBD (OR = 1.9, P = 0.008), and tumor diameter > 2.5 cm (OR = 1.8, P = 0.022) emerged as three independent risk factors associated with lymph node metastasis.
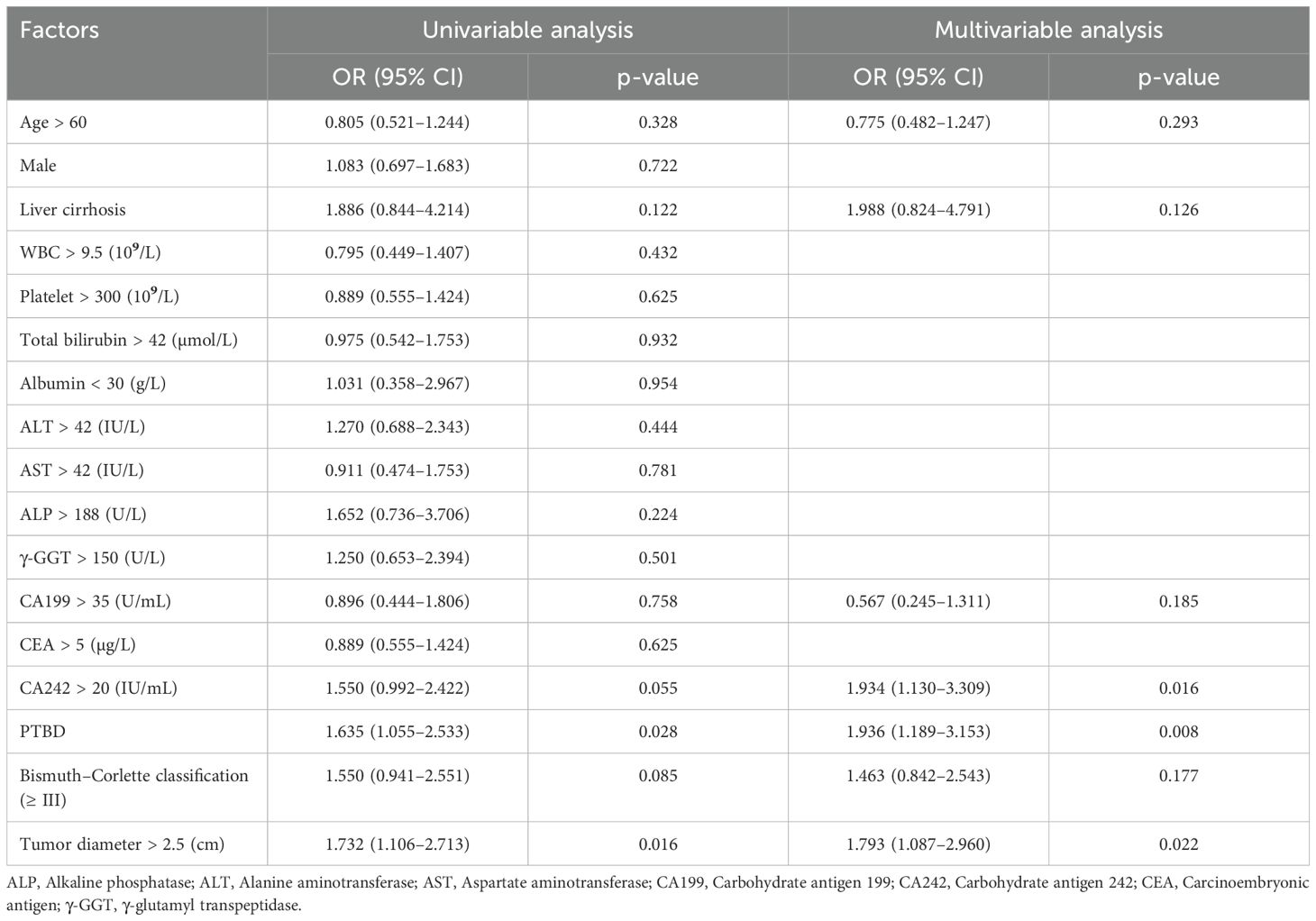
Table 3. Univariable and multivariable analysis of risk factors associated with lymph nodes metastasis.
Survival analysis
This study aimed to investigate the relationship between preoperative percutaneous transhepatic biliary drainage (PTBD), portal vein invasion, lymph node metastasis, and clinical survival in patients with hilar cholangiocarcinoma. The Kaplan–Meier curves were drawn, and the log-rank test was used to evaluate differences between two groups. As presented in Figures 1A, B, these patients who did not accept preoperative PTBD showed a significantly longer OS (p = 0.039) than the PTBD group, whereas the RFS did not show a statistical difference between two groups (p = 0.082). Consistent with other studies, those patients without portal vein invasion and lymph node metastasis had a significantly better prognosis both in OS and RFS (all p < 0.001), which was shown in Figures 1C–F.
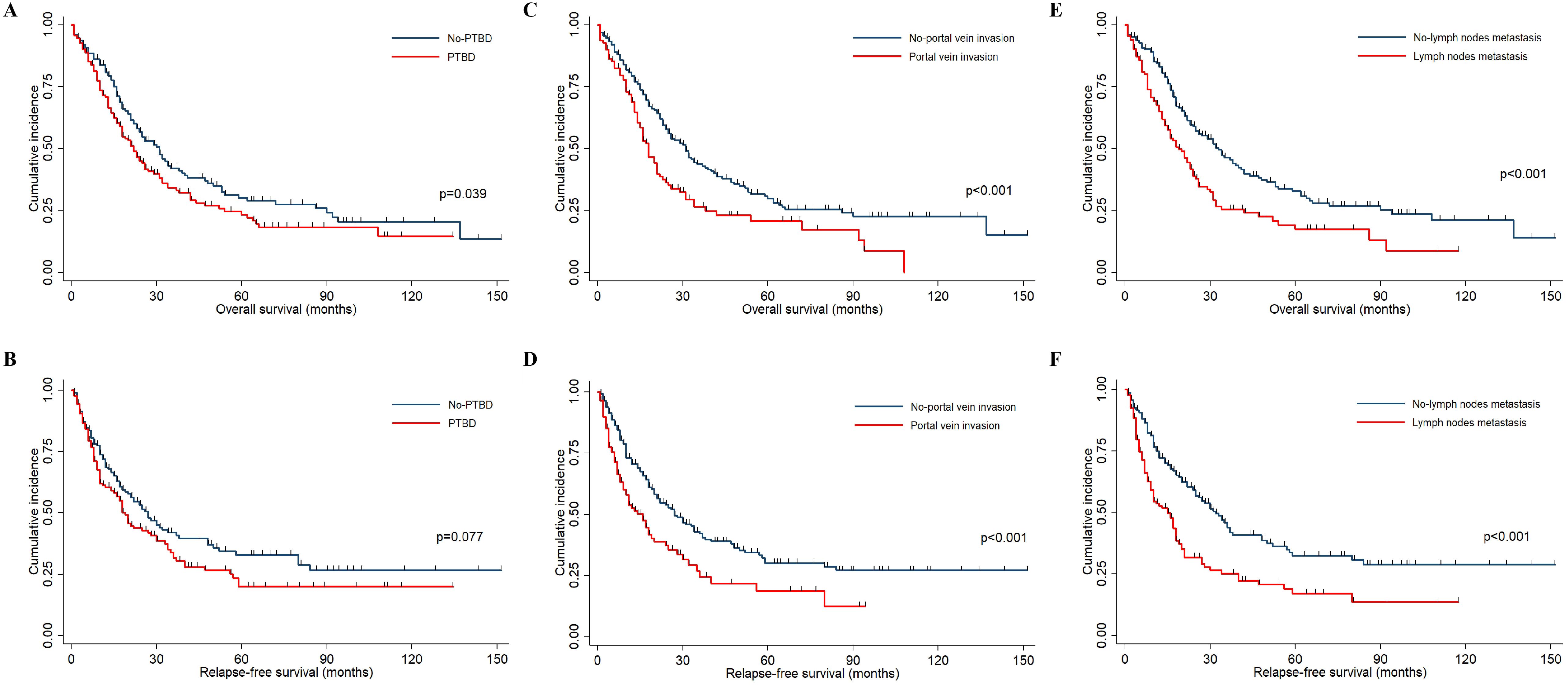
Figure 1. The Kaplan–Meier curves indicated the OS and RFS in HCCA patients. (A, B) The OS and RFS survival curves between patients accepting PTBD or not. (C, D) The OS and RFS survival curves between patients with and without portal vein invasion. (E, F) The OS and RFS survival curves between patients with and without lymph node metastasis.
Furthermore, univariate and multivariate Cox regression models were employed to investigate potential risk factors associated with OS and RFS. Although preoperative PTBD emerged as a significant risk factor for OS (HR = 1.3, P = 0.039) in the univariate analysis, this association did not retain statistical significance (HR = 0.99, P = 0.919) in the multivariate analysis (Table 4). Moreover, the preoperative PTBD was not directly related to RFS in the univariate and multivariate analysis (Table 5). However, the portal vein invasion and lymph node metastasis were two significant risk factors associated with OS and RFS (all p < 0.01). Subsequently, causal mediation analysis was performed to investigate the mediation effect of PTBD on OS and RFS. The analysis revealed that portal vein invasion accounted for 30.1% (p = 0.030) of PTBD’s effect on OS and 36.4% (p = 0.022) on RFS. Similarly, lymph node metastasis mediated 35.9% (p = 0.038) of PTBD’s effect on OS and 18.1% (p = 0.034) on RFS, respectively. These findings indicated that the effect of PTBD on survival was partly mediated by portal vein invasion and lymph node metastasis.
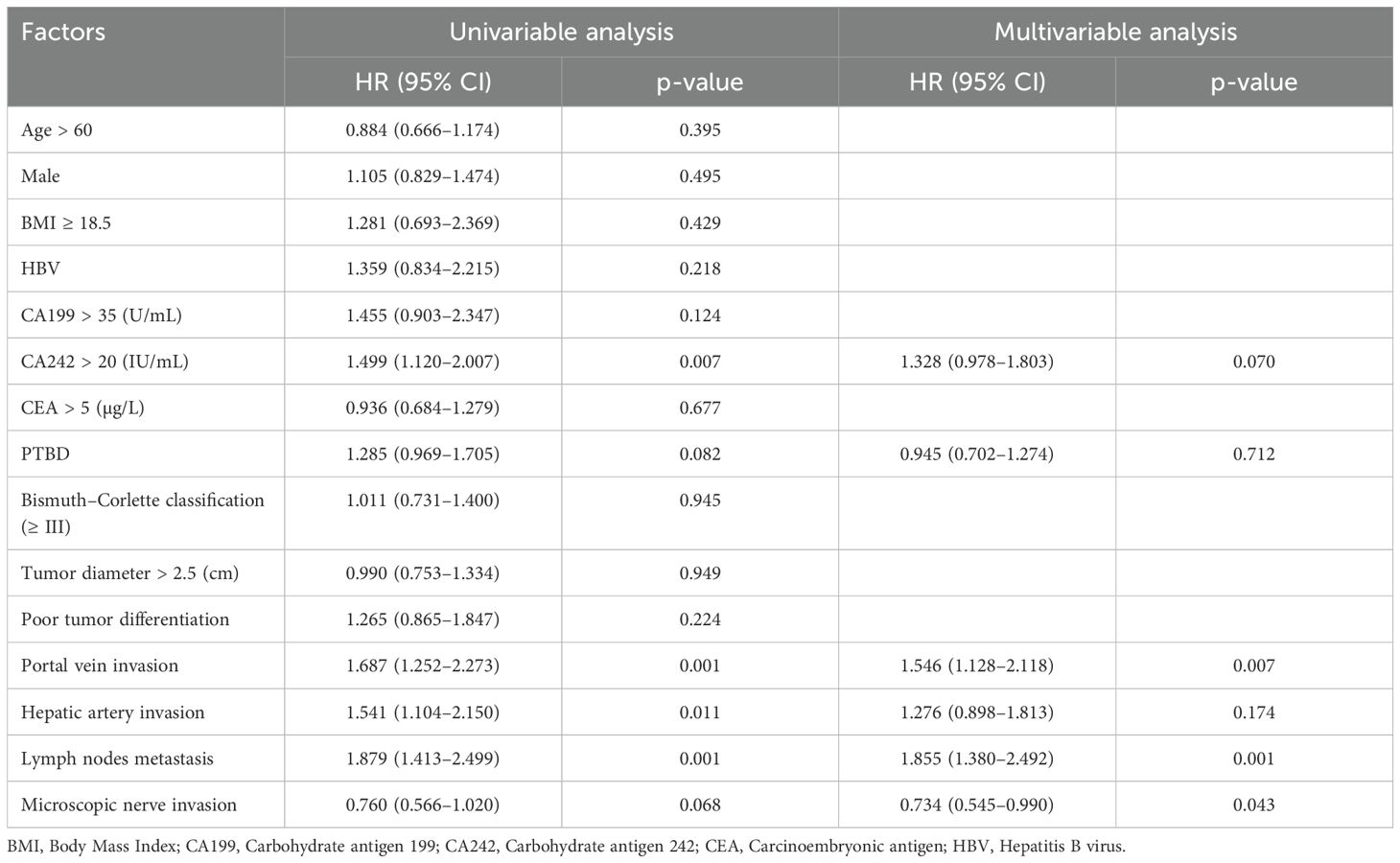
Table 5. Univariable and multivariable analysis of risk factors associated with relapse-free survival.
Postoperative complications in HCCA patients accepting PTBD or not
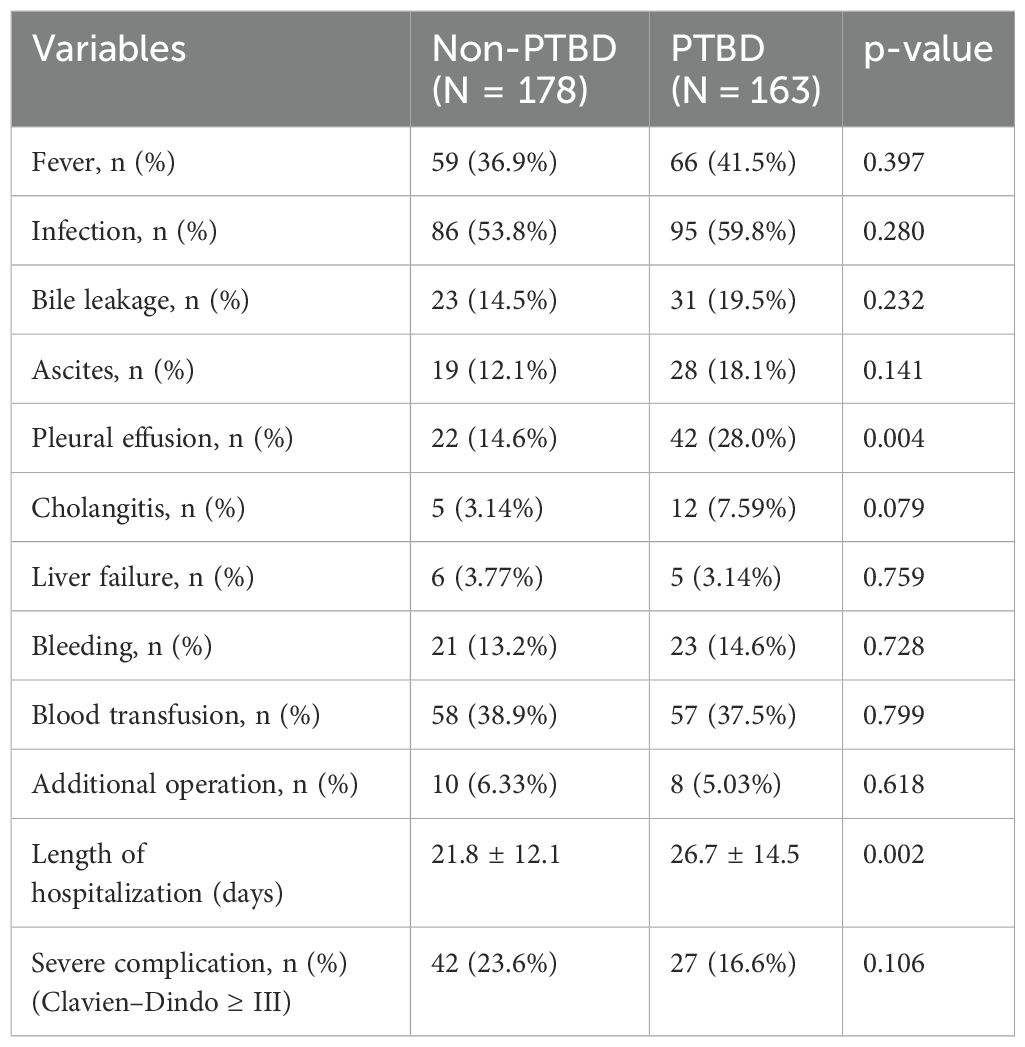
In HCCA patients, the presence of postoperative complications was an important factor affecting hospital mortality and long-term survival (16, 17). In this study, the comparison of postoperative complications is between the PTBD group and the non-PTBD group, and the results were presented in Table 6. The proportion of pleural effusion (28.0% vs. 14.6%, p = 0.004) in the PTBD group was significantly higher than that in the non-PTBD group. Additionally, the mean length of hospitalization was 26.7 days, which was obviously longer than that in the non-PTBD group (21.8 days, p = 0.002). The presence of other complications including fever, infection, bile leakage, cholangitis, liver failure, and bleeding was similar in the PTBD group and the non-PTBD group.
Discussion
In this study, we demonstrated that patients with HCCA who underwent preoperative PTBD exhibited a significantly higher incidence of portal vein invasion and regional lymph node metastasis compared to those who did not undergo PTBD. These differences were statistically significant, highlighting a potential adverse effect of PTBD on tumor local spread.
According to previous studies, several mechanisms might explain why PTBD could lead to increased local tumor metastasis. Firstly, the PTBD procedure itself might cause mechanical injury to the tumor or surrounding tissues like branches of vessels, potentially dislodging tumor cells (18). These dislodged cells could then enter the bloodstream or lymphatic system, facilitating metastasis (19, 20). Moreover, the PTBD might induce a local inflammatory response, which could create a microenvironment that supports tumor growth and spread. Inflammation might suppress local immune responses and promote angiogenesis, providing tumor cells with nutrients and pathways for dissemination (21, 22). Furthermore, prolonged intervals between PTBD and definitive surgery might also increase risks of local tumor progression, including vascular invasion and lymph node metastasis.
Although PTBD in HCCA patients increased the risk of portal vein invasion and lymph node metastasis, the Cox regression model indicated that PTBD was not an independent risk factor for OS and RFS, and the survival curves showed no statistically significant difference in RFS between the PTBD and non-PTBD groups. Notably, patients with portal vein invasion and lymph node metastasis had significantly lower survival rates compared to those without these invasions. This paradoxical finding might be explained through the concept of causal mediation effects. PTBD may indirectly affect survival by increasing the likelihood of portal vein invasion and lymph node metastasis, which were strong prognostic factors for reduced survival. However, other variables like serum elevated markers influencing portal vein invasion and lymph node metastasis may still exist, potentially diluting the direct impact of PTBD on overall survival (23).
The findings of this study might have significant clinical implications for clinicians, as they may need to re-assess the use of PTBD in the preoperative management of HCCA patients. Although PTBD was effective in reducing jaundice and improving liver function, its potential to facilitate local spread of tumor cells must be carefully weighed. Secondly, identifying which patients would most benefit from PTBD and considering the risks of enhanced metastasis were crucial. High-risk patients might need alternative approaches [including endoscopic nasobiliary drainage (ENBD) or endoscopic biliary stenting (EBS)] or closer monitoring for signs of metastasis. Additionally, the safety of the PTBD procedure must be mentioned, as portal vein injury during this intervention was already reported (24). The potential spread of tumor cells from the bile duct into the portal vein system might cause portal vein invasion and micro-metastasis throughout the liver.
However, this study also had several limitations that should be acknowledged. Firstly, as a retrospective clinical study, we could not provide exact mechanisms how the PTBD increased in the incidence of portal vein invasion and lymph node metastasis. The subsequent basic biological studies needed to be conducted. Secondly, the extremely limited number of patients undergoing preoperative ENBD or EBS for biliary drainage in our cohort precluded meaningful comparisons of their effects on lymph node metastasis or portal vein invasion. This limitation narrowed the generalizability of our findings, as the relative safety profiles and tumor progression risks associated with different biliary drainage strategies remained incompletely addressed. Future multicenter studies with larger patient cohorts accepting diverse drainage approaches were needed to verify and extend these findings.
Conclusion
In conclusion, our study indicated that PTBD might be associated with an increased risk of local tumor metastasis including portal vein invasion and lymph node metastasis in HCCA patients. Except for needle tract tumor seeding during the PTBD, its potential to facilitate regional tumor spread warranted careful consideration in clinical decision-making. Further research is necessary to fully understand the implications of PTBD and to develop strategies that optimize patient outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethical Committee of PLA Middle Military Command General Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YC: Funding acquisition, Software, Writing – original draft. YY: Formal Analysis, Investigation, Methodology, Writing – original draft. YD: Methodology, Software, Writing – original draft. DW: Data curation, Methodology, Writing – original draft. JL: Conceptualization, Investigation, Software, Writing – review & editing. GH: Conceptualization, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by foundation research from Caidian District People’s Hospital (XHJBKXYJJJ-ZYCX-08) and Karakoram Talent Fund (22QN066).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
HCCA, hilar cholangiocarcinoma; PTBD, percutaneous transhepatic biliary drainage; ENBD, endoscopic nasobiliary drainage; EBS, endoscopic biliary stenting; OS, overall survival; RFS, relapse-free survival.
References
1. Mansour JC, Aloia TA, Crane CH, Heimbach JK, Nagino M, and Vauthey J-N. Hilar Cholangiocarcinoma: expert consensus statement. Hpb. (2015) 17:691–9. doi: 10.1111/hpb.12450
2. Mehrabi A, Khajeh E, Ghamarnejad O, Nikdad M, Chang D-H, Büchler MW, et al. Meta-analysis of the efficacy of preoperative biliary drainage in patients undergoing liver resection for perihilar cholangiocarcinoma. Eur J Radiol. (2020) 125:108897. doi: 10.1016/j.ejrad.2020.108897
3. Kloek JJ, van der Gaag NA, Aziz Y, Rauws EA, van Delden OM, Lameris JS, et al. Endoscopic and percutaneous preoperative biliary drainage in patients with suspected hilar cholangiocarcinoma. J Gastrointest Surg. (2010) 14:119–25. doi: 10.1007/s11605-009-1009-1
4. Zhao H, Li B, Li X, Lv X, Guo T, Dai Z, et al. Dynamic three-dimensional liver volume assessment of liver regeneration in hilar cholangiocarcinoma patients undergoing hemi-hepatectomy. Front Oncol. (2024) 14:1375648. doi: 10.3389/fonc.2024.1375648
5. Walter T, Ho CS, Horgan AM, Warkentin A, Gallinger S, Greig PD, et al. Endoscopic or percutaneous biliary drainage for klatskin tumors? J Vasc Interventional Radiol. (2013) 24:113–21. doi: 10.1016/j.jvir.2012.09.019
6. Hsu YC, Lee HY, Chang CM, Lin CY, Liu YS, and Huang HS. Clinical outcomes of percutaneous transhepatic biliary drainage at different Couinaud's hepatic entry segments for treating obstructive jaundice. Front Surg. (2023) 10:1039106. doi: 10.3389/fsurg.2023.1039106
7. Pirayvatlou P, Pirayvatlou P, Roushan N, Majidi A, and Khorshidi Z. Comparing the efficacy and complications of Endoscopic Biliary Drainage (EBD) and Percutaneous Transhepatic Biliary Drainage (PTBD) in patients with perihilar cholangiocarcinoma. J Family Med Primary Care. (2022) 11:7720–4. doi: 10.4103/jfmpc.jfmpc_922_22
8. Yamashita H, Ebata T, Yokoyama Y, Igami T, Mizuno T, Yamaguchi J, et al. Pleural dissemination of cholangiocarcinoma caused by percutaneous transhepatic biliary drainage during the management of resectable cholangiocarcinoma. Surgery. (2019) 165:912–7. doi: 10.1016/j.surg.2018.10.015
9. Komaya K, Ebata T, Yokoyama Y, Igami T, Sugawara G, Mizuno T, et al. Verification of the oncologic inferiority of percutaneous biliary drainage to endoscopic drainage: A propensity score matching analysis of resectable perihilar cholangiocarcinoma. Surgery. (2017) 161:394–404. doi: 10.1016/j.surg.2016.08.008
10. Takahashi Y, Nagino M, Nishio H, Ebata T, Igami T, and Nimura Y. Percutaneous transhepatic biliary drainage catheter tract recurrence in cholangiocarcinoma. Br J Surg. (2010) 97:1860–6. doi: 10.1002/bjs.7228
11. Nimura Y, Kamiya J, Kondo S, Nagino M, Uesaka K, Oda K, et al. Aggressive preoperative management and extended surgery for hilar cholangiocarcinoma: Nagoya experience. J Hepatobiliary Pancreat Surg. (2000) 7:155–62. doi: 10.1007/s005340050170
12. Pedersoli F, Schröder A, Zimmermann M, Schulze-Hagen M, Keil S, Ulmer TF, et al. Percutaneous transhepatic biliary drainage (PTBD) in patients with dilated vs. nondilated bile ducts: Tech considerations complications Eur Radiol. (2021) 31:3035–41. doi: 10.1007/s00330-020-07368-6
13. Nennstiel S, Weber A, Frick G, Haller B, Meining A, Schmid RM, et al. Drainage-related complications in percutaneous transhepatic biliary drainage: an analysis over 10 years. J Clin Gastroenterol. (2015) 49:764–70. doi: 10.1097/mcg.0000000000000275
14. Elliott M. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models, Vol. 62. Vittinghoff E, Glidden DV, Shiboski SC, and Mcculloch CE, editors Hoboken, New Jersey, USA: Wiley (on behalf of the International Biometric Society) (2006), pp. 1271–2.
15. Lee H, Herbert RD, and McAuley JH. Mediation analysis. Jama. (2019) 321:697–8. doi: 10.1001/jama.2018.21973
16. Hasegawa S, Ikai I, Fujii H, Hatano E, and Shimahara Y. Surgical resection of hilar cholangiocarcinoma: analysis of survival and postoperative complications. World J Surg. (2007) 31:1256–63. doi: 10.1007/s00268-007-9001-y
17. Liu ZP, Chen WY, Zhang YQ, Jiang Y, Bai J, Pan Y, et al. Postoperative morbidity adversely impacts oncological prognosis after curative resection for hilar cholangiocarcinoma. World J Gastroenterol. (2022) 28:948–60. doi: 10.3748/wjg.v28.i9.948
18. Pankaj P, Kohei M, Kizuki Y, Yuki H, Jun S, and Toshifumi W. Perihilar or (Hilar) Cholangiocarcinoma: Interventional to Surgical Management. In: Luis R, editor. Bile Duct Cancer. IntechOpen, Rijeka (2018). p. 5.
19. Nennstiel S, Weber A, Frick G, Haller B, Meining A, Schmid RM, et al. Drainage-related complications in percutaneous transhepatic biliary drainage: an analysis over 10 years. J Clin Gastroenterol. (2015) 49:764–70. doi: 10.1097/MCG.0000000000000275
20. Stott SL, Hsu CH, Tsukrov DI, Yu M, Miyamoto DT, Waltman BA, et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc Natl Acad Sci U S A. (2010) 107:18392–7. doi: 10.1073/pnas.1012539107
21. Sahu R, Sharma P, and Kumar A. An Insight into Cholangiocarcinoma and Recent Advances in its Treatment. J Gastrointest Cancer. (2023) 54:213–26. doi: 10.1007/s12029-021-00728-5
22. Lazaridis KN and Gores GJ. Cholangiocarcinoma. Gastroenterology. (2005) 128:1655–67. doi: 10.1053/j.gastro.2005.03.040
23. Heng G, Huang B, Shen Y, Wang D, Lan Z, Yao Y, et al. Vascular invasion and lymph node metastasis mediate the effect of CA242 on prognosis in hilar cholangiocarcinoma patients after radical resection. Front Oncol. (2022) 12. doi: 10.3389/fonc.2022.1071439
Keywords: HCCA, PTBD, portal vein invasion, lymph node metastasis, survival
Citation: Cai Y, Yao Y, Dong Y, Wang D, Luo J and Heng G (2025) Hilar cholangiocarcinoma patients accepting preoperative percutaneous transhepatic biliary drainage experienced high incidence of portal vein invasion and lymph node metastasis. Front. Oncol. 15:1504604. doi: 10.3389/fonc.2025.1504604
Received: 01 October 2024; Accepted: 19 May 2025;
Published: 06 June 2025.
Edited by:
Sharon R Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Mario Pacilli, University of Foggia, ItalyJahnvi Dhar, Post Graduate Institute of Medical Education and Research (PGIMER), India
Copyright © 2025 Cai, Yao, Dong, Wang, Luo and Heng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Heng, aGc5MzEwMDRAMTYzLmNvbQ==; Jing Luo, amluZ2x1bzExMjRAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Yingke Cai1†
Yingke Cai1† Jing Luo
Jing Luo Gang Heng
Gang Heng