- Department of Hepatobiliary Surgery, Xianyang Central Hospital, Xianyang, Shaanxi, China
Background: Neoadjuvant systemic therapy has been shown to benefit patients with solid tumors such as breast cancer and colorectal cancer, but its application in hepatocellular carcinoma (HCC) is still in the exploratory stage, with no established effective regimen. This systematic review and meta-analysis aims to investigate the efficacy and safety of neoadjuvant systemic therapy in patients with resectable HCC.
Methods: The clinical trials of resectable HCC neoadjuvant systemic therapy in PubMed, Embase and the Cochrane Library were systematically searched. A meta-analysis was performed using STATA/MP18.0 software, and the effect size was calculated using either a fixed effects model or a random effects model, and 95% confidence intervals (CIs) were calculated. Subgroup analysis was performed according to the neoadjuvant systemic therapy regimen.
Results: This meta-analysis included 328 patients from 15 studies. In patients with resectable HCC, the pooled pathologic complete response (pCR) rate was 15% (95%CI: 10%–21%), the major pathologic response (MPR) rate was 28% (95%CI: 21%–35%), the incidence of grade 3–4 treatment-related adverse events (TRAEs) was 11% (95% CI: 4%–20%), the objective response rate (ORR) was 27% (95% CI: 20%–35%), the surgical resection rate was 84% (95%CI: 75%–92%), and the delay rate was 0.00% (95% CI: 0%–4%). The results of subgroup analysis showed that the efficacy of targeted therapy combined with immunotherapy is superior to dual ICI (immune checkpoint inhibitor) combination therapy and ICI monotherapy, while the safety of the ICI monotherapy was the highest, superior to the dual ICIs and the targeted therapy combined with immunotherapy.
Conclusion: Neoadjuvant systemic therapy shows preliminarily beneficial outcomes in resectable HCC treatment. However, future large-scale and multicenter randomized controlled trials are needed to confirm this conclusion.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42024562257
1 Introduction
Primary liver cancer is one of the most common malignant tumors worldwide, and it is also the main cause of cancer-related death, which seriously threatens human life and health (1, 2). Hepatocellular carcinoma (HCC) is the most common pathological type of primary liver cancer, accounting for about 75%-90% (3, 4). At present, radical surgical resection is still the first choice for HCC treatment, but the postoperative recurrence rate is high, and the 5-year recurrence rate is as high as about 70%; the survival time is short, and the 5-year survival rate of early stage HCC after operation is only 60% (5). Therefore, prolonging the survival period of patients and reducing the risk of postoperative recurrence are urgent problems to be solved.
Neoadjuvant therapy is an effective treatment strategy for patients with resectable malignant tumors with initial surgical opportunities to reduce tumor volume, reduce distant metastasis, increase R0 resection rate and reduce postoperative recurrence, so as to improve the survival of patients (6, 7). At present, it has been proved to be effective in a variety of solid tumors (8–10). Neoadjuvant therapy for HCC mainly includes local therapy represented by vascular intervention and systemic therapy represented by targeted therapy and immunotherapy (11). The role of neoadjuvant therapy in the treatment of HCC is still unclear, and it is still in the exploratory stage. The current guidelines have not clearly recommended any kind of neoadjuvant therapy for HCC (12, 13).
This meta-analysis tries to evaluate the efficacy and safety of neoadjuvant systemic therapy in resectable HCC by collecting existing clinical research data, aiming to provide some reference for preoperative neoadjuvant therapy in clinical patients with HCC.
2 Materials and methods
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (14). The protocol of the systematic review has been prospectively registered on PROSPERO (CRD42024562257).
2.1 Search strategy and eligibility criteria
A comprehensive literature search was conducted for studies published from the inception of databases to 1 Jun 2024, in PubMed, Embase and the Cochrane Library to identify relevant studies. Search subject terms included (“ hepatocellular carcinoma “OR” HCC “OR” liver cancer “OR” primary liver carcinoma “) AND (“ liver resection “OR “surgical resection” OR “hepatectomy” OR “resectable”) AND (“ neoadjuvant “OR” perioperative “[Title] OR “preoperative” [Title]) and related entries (as detailed in the Supplementary Material). The references of the search results were further searched to prevent missing detection.
The inclusion criteria are as follows: (1) clinical studies consist of resectable adult HCC patients (aged > 18 years); (2) at least one form of targeted therapy and immunotherapy should be used as neoadjuvant systemic therapy before surgery; (3) studies to evaluate the efficacy and safety of neoadjuvant systemic therapy include at least one of the following main outcome indicators: pathological complete response (pCR), major pathological response (MPR), grade 3–4 treatment-related adverse event rate (Grade 3–4 TRAEs), objective response rate (ORR), surgical resection rate, and surgical delay rate; (4) studies by the same author only include recent publications or studies with large sample sizes and high quality.
Articles would be excluded if (1) they are duplicate publications; (2) they represent animal experiments, reviews, case reports or meta-analyses; (3) effective outcome indicators could not be obtained in the study(such as pCR, MPR, Grade 3–4 TRAEs, ORR, surgical resection rate, and surgical delay rate); (4) the study included non–primary HCC patients or patients with other malignant neoplastic diseases; (5) patients had received previous systemic therapy (e.g. targeted therapy, immunotherapy).
2.2 Data collection and quality assessment
Two reviewers independently(NL and HD) conducted the identification and extraction of potentially eligible articles. Any discrepancies were resolved by involving a third reviewer(KZ). Subsequently, the identified articles were retrieved, and a comprehensive analysis of their full texts was performed. For each study, a range of data was meticulously recorded, encompassing details such as the first author, publication year, trial number, country, sample size, study stage, intervention, study type, article type, treatment protocol, primary outcome measures (including pCR, MPR, incidence of grade 3–4 TRAEs, ORR, surgical resection rate, surgical delay rate).In cases where this data was unavailable, calculations were made based on the information provided within the articles. Data on these outcomes were collected from each eligible study, and any instances of missing data were noted. When data were incomplete, our team made efforts to contact the corresponding author for clarification. Data were extracted from the included studies using a standardized template developed by the reviewers and maintained in Microsoft Excel (Microsoft, Redmond, WA, USA). Inter-observer agreement was assessed using Cohen’s kappa (κ=0.85).
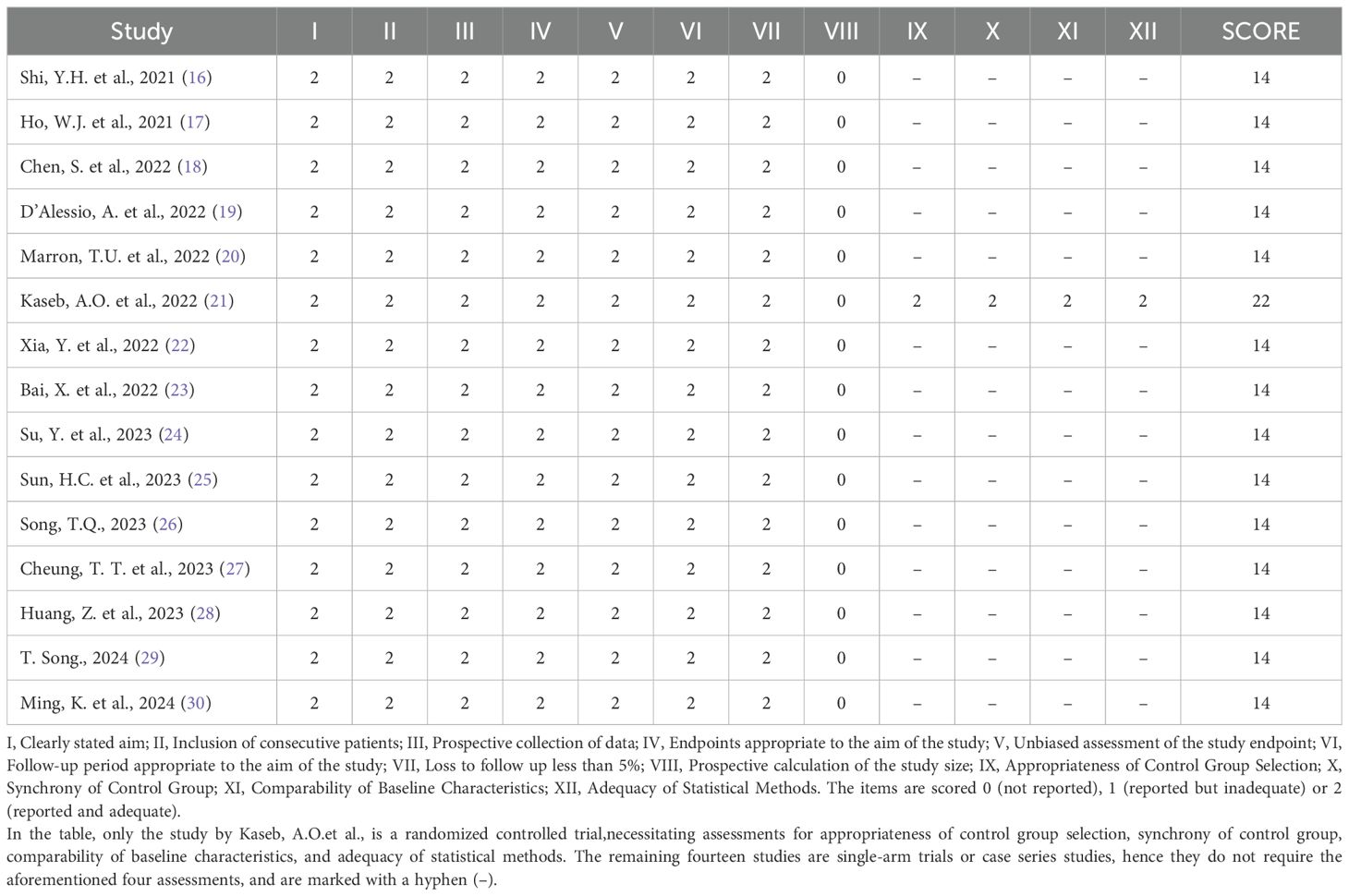
Since all but one of the included studies were single-arm studies with no control group, the quality of the studies was assessed according to the Methodological Index for Non–randomized Studies, (MINORS) (15) scale. Two reviewers conducted the assessment independently and resolved any differences through discussion with the third reviewer. The MINORS scale contains 12 items, of which items 9–12 are used to evaluate studies with control groups. Scores range from 0–2 for each item, 0 indicated unreported, 1 reported but inadequately, and 2 reported and fully detailed. The quality assessment of the included studies is shown in Table 1.
2.3 Statistical analysis
STATA/MP18.0 software (StataCorp LLC, College Station, TX, USA) was used for statistical analysis of all data. Chi-square test and I2 statistic were used for heterogeneity. If there was significant heterogeneity (P<0.1 and I2>50%), a random-effects model was used; otherwise, a fixed-effects model was used. We also performed subgroup analyses to explore the source of heterogeneity and whether there were differences between treatment regiments. Statistical comparison between subgroups was performed using the Cochran’s Q test to assess heterogeneity between groups. For analyses that included more than two subgroups, we determined the significance of the between-group difference by calculating the Q statistic and its corresponding P value. The percent weight was calculated using the inverse variance method, where the weight of each study was the inverse of the variance of its effect size, and the sum of all weights was standardized to 100%. In addition, to assess potential publication bias, funnel plots were used, and Egger’s test was used to evaluate whether the funnel plots were symmetric. P<0.1 indicated statistically significant differences.
3 Results
3.1 Literature search results and basic characteristics
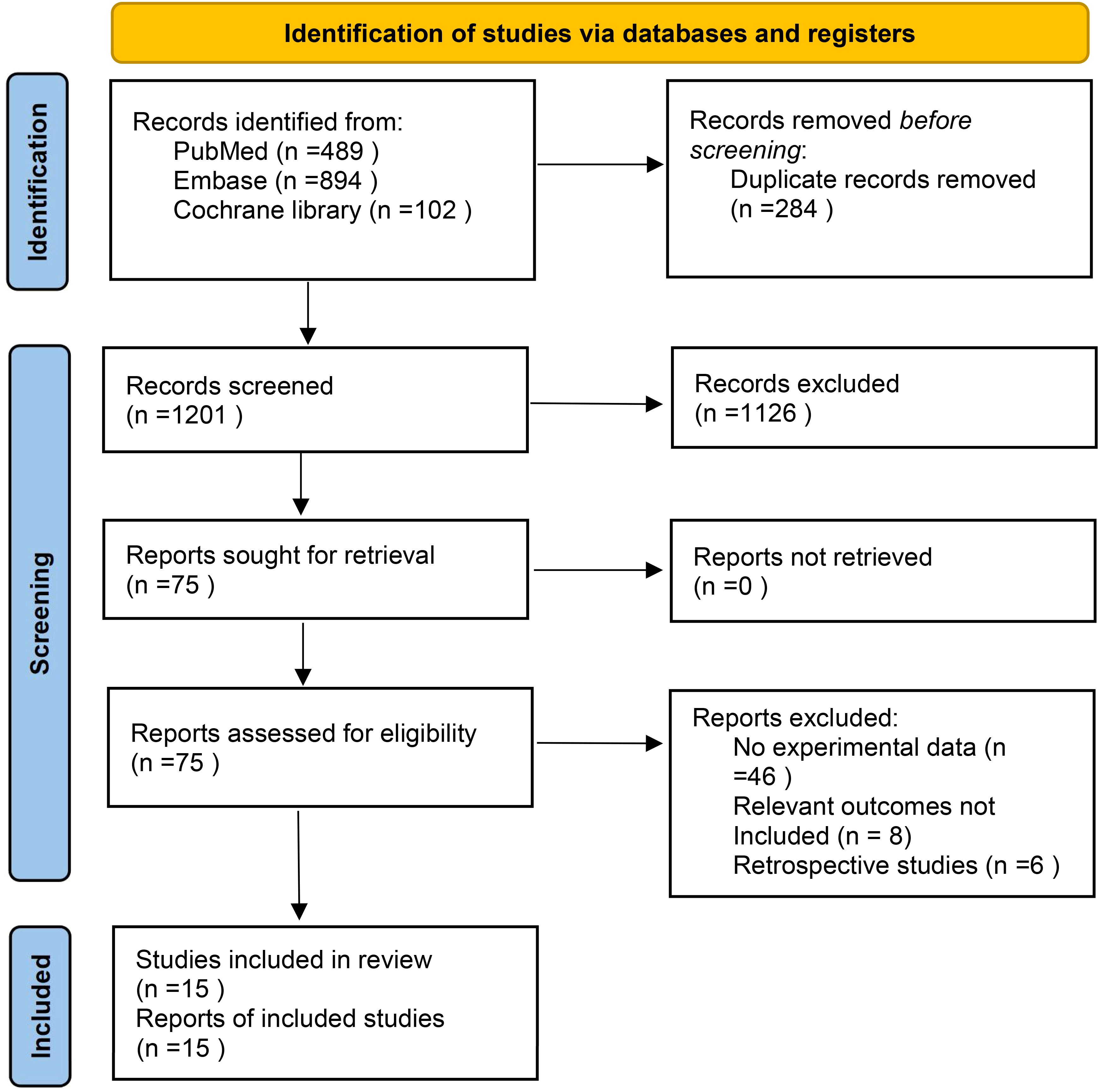
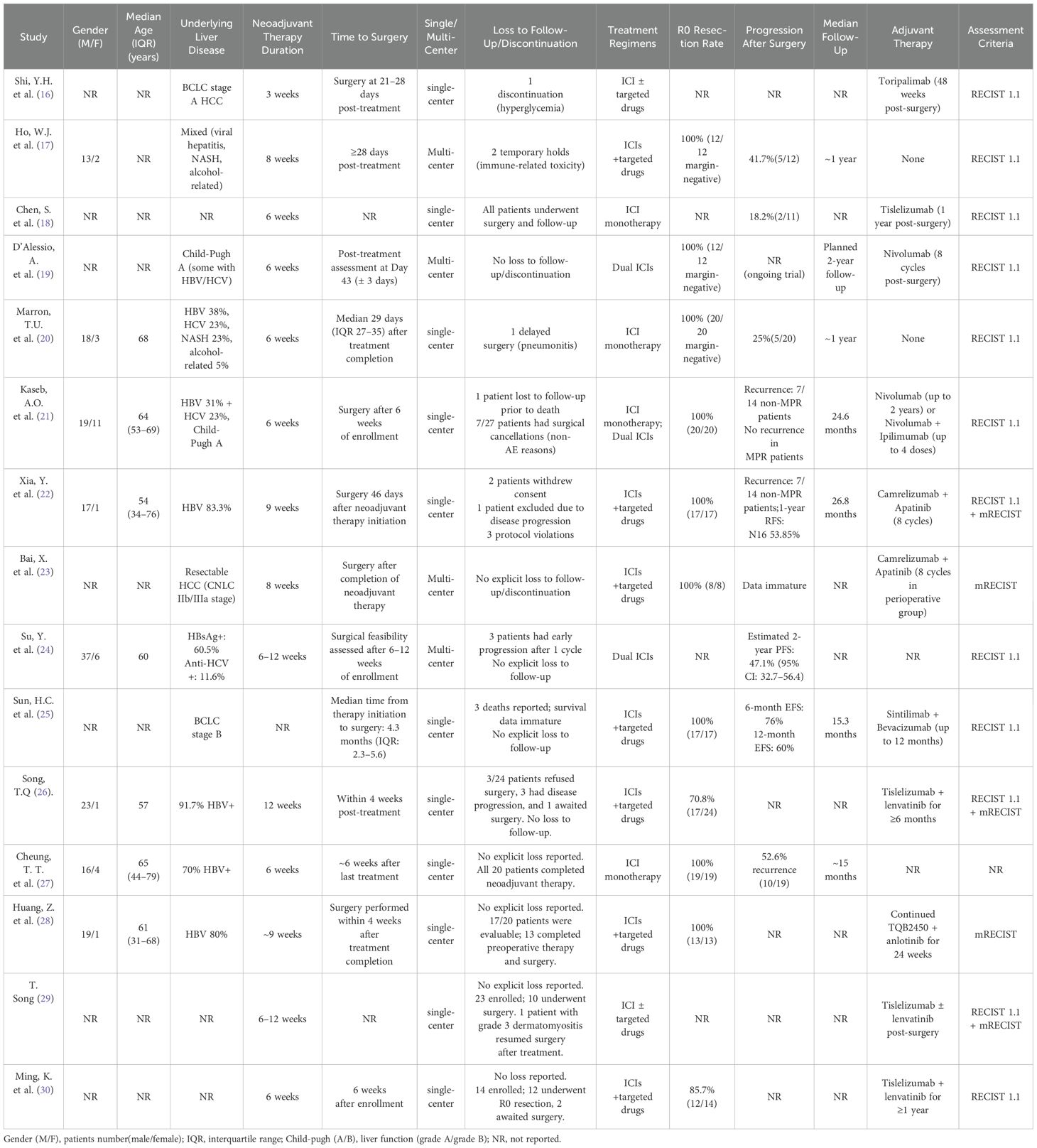
In the selected database, a total of 1485 records were retrieved and screened according to inclusion criteria and exclusion criteria, 284 duplicates were removed, and 1126 records including case reports, animal experiments, reviews, systematic reviews and meta-analyses were excluded by reading titles and abstracts. Finally, 15 articles were included after reading the full-text articles (16–30). The literature screening process is shown in Figure 1. The characteristics and basic information of the included studies are shown in Tables 2, 3.
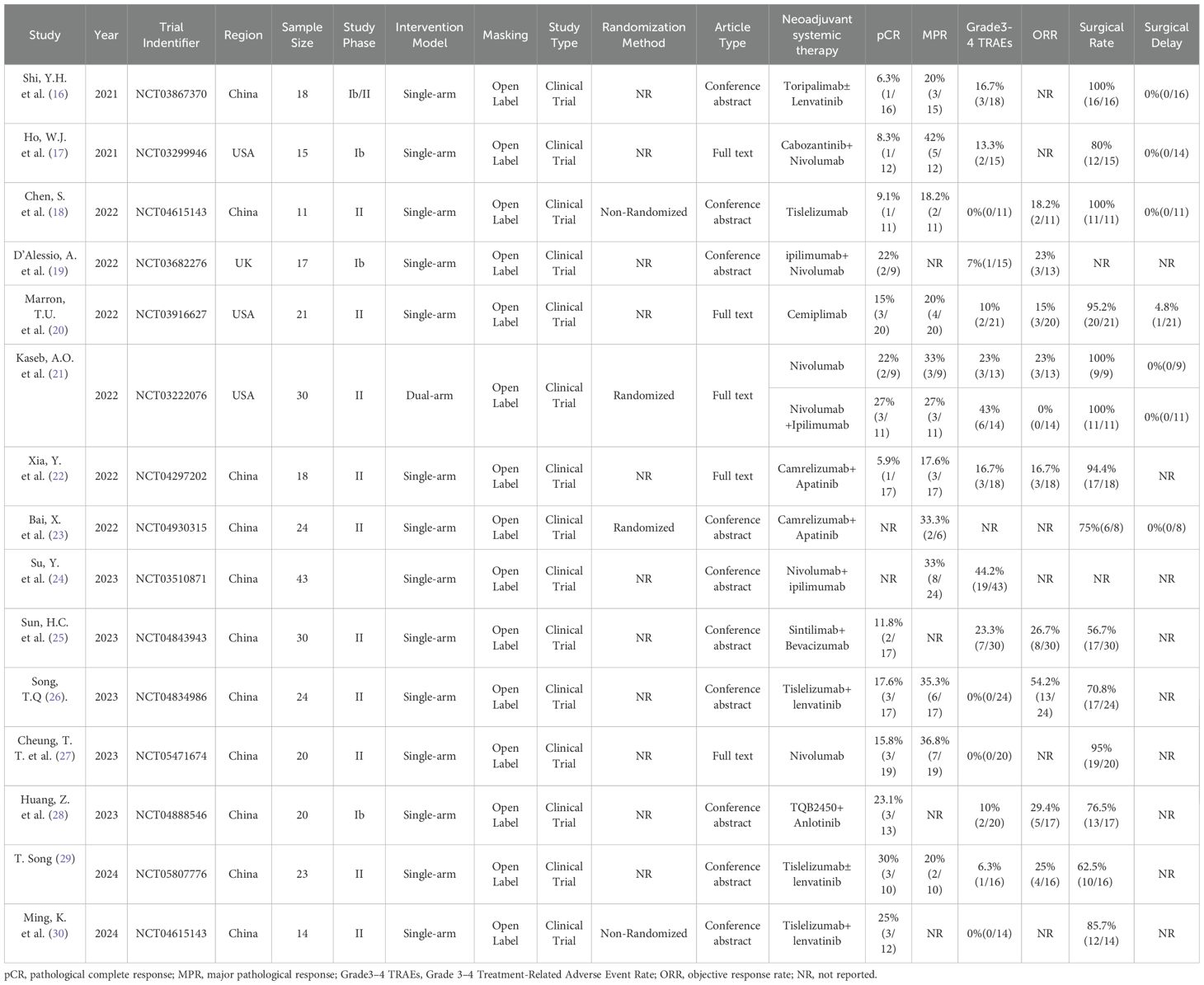
Table 2. Characteristics of neoadjuvant systemic therapy studies in patients with hepatocellular carcinoma.
3.2 Efficacy of neoadjuvant systemic therapy
Pathologic complete response (pCR) was defined as the absence of viable tumor cells in the resected specimen, assessed by histopathological examination. Thirteen studies reported pCR rates ranging from 5.9% to 30%, with no statistical heterogeneity among the studies (P=0.86, I2 = 0.00%), so a meta-analysis using a fixed-effect model showed a pooled pCR rate of 15% (95%CI: 10%–21%) (Figure 2A).
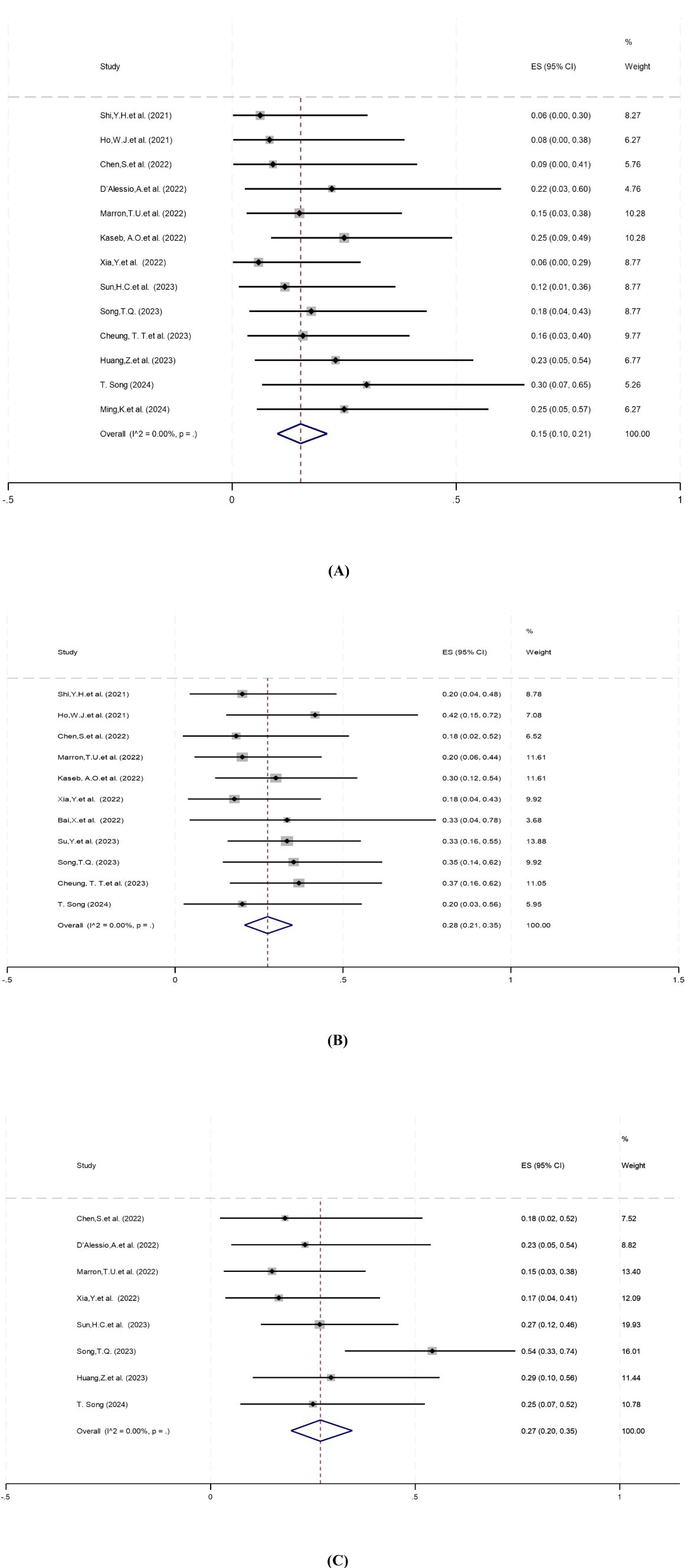
Figure 2. Forest plot of the efficacy of neoadjuvant systemic therapy in resectable hepatocellular carcinoma. (A) pCR, (B) MPR, (C) ORR. pCR, pathological complete response; MPR,major pathological response; ORR,objective response rate. ES, effect size (Proportion).
Major pathologic response (MPR) was defined as ≤10% residual viable tumor in the resected specimen. Eleven studies reported MPR rates ranging from 18% to 42%. There was no statistical heterogeneity among the studies (P=0.89, I2 = 0.00%), and a fixed-effect model was used for meta-analysis. The results showed that the pooled MPR of neoadjuvant systemic therapy for resectable hepatocellular carcinoma was 28% (95% CI: 21%–35%) (Figure 2B).
Objective response rate (ORR) was defined as the proportion of patients achieving complete or partial response per RECIST 1.1 or mRECIST criteria (31, 32). Eight studies reported ORR ranging from 15% to 54%. There was no significant heterogeneity among the studies (P=0.19, I2 = 30.37%), so a fixed-effect model was used for meta-analysis. Results showed that the pooled ORR of neoadjuvant systemic therapy in resectable hepatocellular carcinoma was 27% (95% CI: 20%–35%) (Figure 2C).
3.3 Safety of neoadjuvant systemic therapy
Thirteen studies reported surgical resection rates ranging from 57% to 100%. There was statistical heterogeneity among the studies (P=0.00, I2 = 65.50%), hence a random-effects model was used for meta-analysis. The pooled results showed that the surgical resection rate of neoadjuvant systemic therapy was 84% (95% CI: 75%–92%) (Figure 3A).
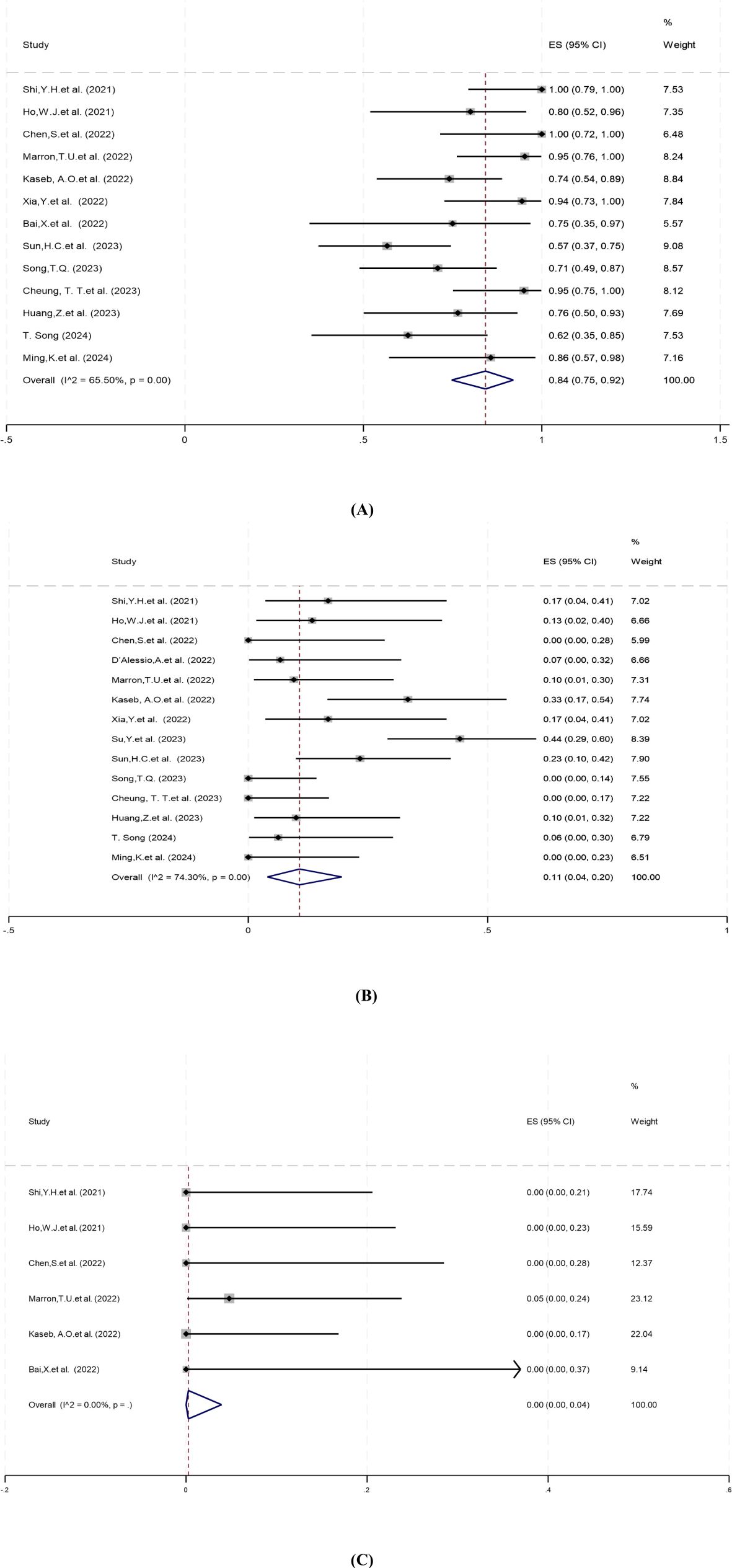
Figure 3. Forest plot of the safety of neoadjuvant systemic therapy in resectable hepatocellular carcinoma. (A) Surgical resection rate, (B) Grade 3–4 TRAEs, (C) Surgery delay rates. TRAEs, treatment-related adverse events. ES, effect size (Proportion).
Treatment-related adverse events (TRAEs), as defined by the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) version 5.0, is associated with the safety of neoadjuvant systemic therapy (33). Fourteen studies reported incidence of grade 3–4 TRAEs ranging from 0% to 44%. There was significant heterogeneity among studies (P=0.00, I2 = 74.30%), and a random-effects model was employed for meta-analysis. The results showed that the pooled incidence of grade 3–4 TRAEs of neoadjuvant systemic therapy for resectable hepatocellular carcinoma was 11% (95% CI: 4%–20%) (Figure 3B).
Surgical delay rate was defined as the ratio of patients whose surgery was delayed due to adverse events caused by neoadjuvant systemic therapy to all patients expected to have surgery. Six studies reported surgery delay rates ranging from 0% to 5%. There was no significant heterogeneity among the studies (P=0.94, I2 = 0.00%), and a fixed-effect model was applied for meta-analysis. The pooled results showed that the surgical delay rate of neoadjuvant systemic therapy was 0.00% (95% CI: 0.00%–4%) (Figure 3C).
3.4 Subgroup analysis
Currently, the neoadjuvant systemic therapy for HCC mainly includes three regimens: ICI monotherapy, dual ICI combination, and immunotherapy combined with targeted therapy. Subgroup analysis was performed to determine the possible sources of heterogeneity, and to find out differences in efficacy and safety among different treatment regimens, and whether they had different effects on clinical outcomes (pCR, MPR, Grade 3–4 TRAEs, ORR, surgical resection rate, surgical delay rate).
For different treatment regimens, the pCR rate for dual ICI therapy was greater than that for ICI monotherapy, which was greater than immunotherapy combined with targeted therapy, but no statistically significant difference was observed among the groups(Q=1.22, df=2, P=0.543) (Figure 4A). The MPR rate for dual ICI therapy was greater than that for immunotherapy combined with targeted therapy, which was greater than that for ICI monotherapy, but no statistically significant difference was observed among the groups(Q=0.25, df=2, P=0.883) (Figure 4B). The ORR for immunotherapy combined with targeted therapy was greater than that for ICI monotherapy, which was greater than that for dual ICI therapy, and showed statistically significant differences among the groups(Q=5.29, df=2, P=0.071) (Figure 4C). The surgical resection rate was greater for dual ICI therapy than for ICI monotherapy, which was greater than that for immunotherapy combined with targeted therapy, and showed statistically significant differences among the groups(Q=14.05, df=2, P=0.001) (Figure 4D). The incidence of grade 3–4 TRAEs was greater for dual ICI therapy than for immunotherapy combined with targeted therapy, which was greater than that for ICI monotherapy, but no statistically significant difference was observed among the groups(Q=4.12, df=2, P=0.128) (Figure 4E). The surgical delay rate was higher for ICI monotherapy than for immunotherapy combined with targeted therapy and dual ICI therapy, but no statistically significant differences were observed among the groups(Q=0.29, df=2, P=0.864) (Figure 4F).

Figure 4. Subgroup analysis based on different neoadjuvant systemic therapy regimens for (A) pCR, (B) MPR, (C) ORR, (D) Surgical resection rate, (E) Grade 3–4 TRAEs, (F) Surgery delay rates. pCR, pathological complete response; MPR, major pathological response; ORR, objective response rate; TRAEs, treatment-related adverse events. ES, effect size (Proportion). Note: Heterogeneity values are not displayed if there are three or fewer studies in the subgroup.
3.5 Publication bias
To assess potential publication bias, we constructed funnel plots and performed Egger’s test for surgical resection rate, pCR, and MPR. The funnel plots showed symmetrical distribution, and the corresponding P values of Egger’s test were 0.157, 0.456 and 0.768, respectively, suggesting that there was no publication bias (Figure 5).
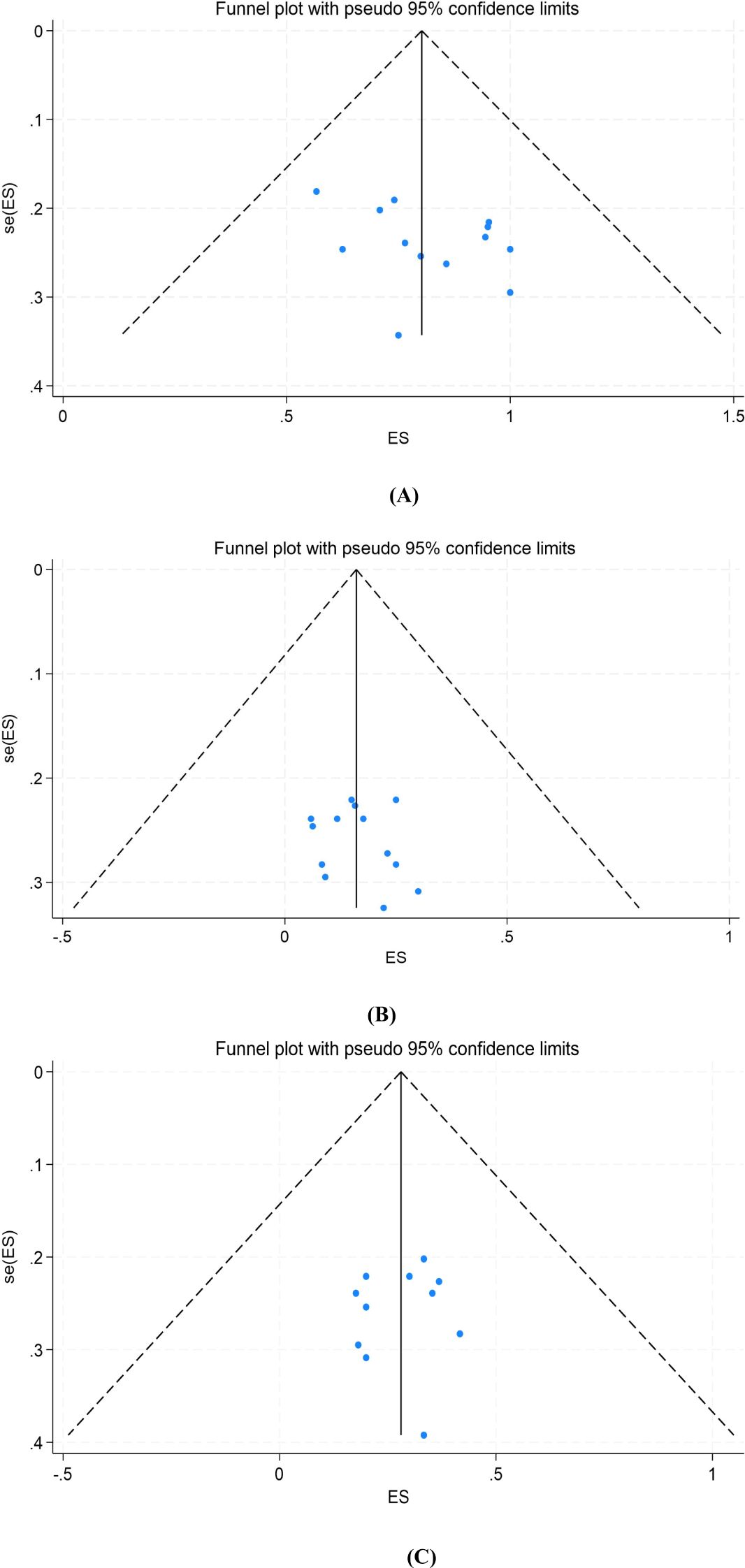
Figure 5. Funnel plot of the meta-analysis. (A) Surgical resection rate, (B) pCR, (C) MPR. pCR, pathological complete response; MPR, major pathological response. ES, effect size (Proportion). se, standard error.
4 Discussion
The results of this meta-analysis preliminarily suggest that neoadjuvant systemic therapy may have an advantage in resectable HCC. First, regarding its efficacy, the pooled results showed an ORR of 27% (95% CI: 20%-35%), with a maximum reported ORR of 54.2% (26). The pooled pCR and MPR results were 15% (95%CI: 10%-21%) and 28% (95%CI: 21%-35%), respectively, with the reported maximum pCR and MPR values of 30% and 42% (17, 29), respectively, a result consistent with neoadjuvant systemic therapy results for other tumor types (34–37). However, given that most clinical studies are ongoing, data on patient survival after tumor resection is limited. Only four articles provided statistical data, among which Kaseb, A.O. et al. (21) reported PFS (Progression-Free Survival) of 9.4 months (95%CI: 1.47-not estimable[NE]) for nivolumab and 19.53 months (95%CI: 2.33-NE) for nivolumab plus ipilimumab. Other studies (22, 25) reported EFS (Event-Free Survival) and RFS (Recurrence-Free Survival) data, with a median EFS of 13.8 months (95% CI: 10.3-17.3) and a 1-year RFS of 53.85% (95% CI: 24.77%-75.99%). However, due to insufficient follow-up time, no studies have reported OS (Overall Survival) data. In other tumors, a significant correlation between pathological response and survival has been demonstrated (38, 39). Statistical analysis was also performed in this study, as Ho, W.J et al. (17) found a correlation between achieving MPR and long-term DFS (Disease-Free Survival), with DFS intervals of more than 230 days in all patients to date. Kaseb, A. O. et al. (21) also reported a significant correlation between pathological response and RFS (P=0.049). Six patients with pathological response did not have recurrence after a median follow-up of 26.8 months, while seven of the other 14 patients without pathological response had recurrence. Xia, Y et al. (22) found that the RFS of patients with MPR/pCR was higher than that of patients non-MPR/pCR.
Postoperative recurrence of HCC is the main obstacle to the improvement of the efficacy of surgical resection, and it is also the common cause of disease progression (12, 40). Neoadjuvant systemic therapy can reduce tumor burden, eliminate intrahepatic micrometastases in advance, and improve the prognosis of patients (41, 42). PD-1/PD-L1 is involved in tumor immune escape. The activation of PD-1/PD-L1 signaling pathway can lead to the formation of immunosuppressive tumor microenvironment, so that tumor cells can escape the body’s immune surveillance and killing (43, 44). In the immune response of tumor cells, the induced apoptosis of tumor antigen-specific T lymphocytes is involved in tumor immune evasion (45). PD-1/PD-L1 plays an important role in inhibiting the activation and proliferation of T lymphocytes. After the anti-tumor immunity of T cells is activated by ICI antibodies, the immune memory effect of T cells is often long-lasting, which can continue to play an immune surveillance role after surgical resection, thereby helping to reduce tumor recurrence (46). Marron, T.U. et al. (20) found that patients with more than 50% tumor necrosis after cemiplimab treatment showed stronger immune infiltration than those with minimal necrosis in surgical samples. However, the incidence of disease progression in PD-1/PD-L1 antibody immune monotherapy is high, generally around 40% (47–50); therefore, immune monotherapy is not an ideal choice for neoadjuvant therapy. Xu J et al. (51) found that apatinib combined with camrelizumab (PD-1 antibody) had a good anti-tumor effect in phase II clinical study, with an ORR of 34.3% and a disease progression rate of 18.6%. Immunotherapy combined with targeted therapy, such as small molecule TKI or bevacizumab, has shown stronger anti-tumor effect and significantly reduced the incidence of disease progression, which is expected to become a more reliable choice of neoadjuvant systemic therapy. In a randomized controlled clinical trial reported by Kaseb, A.O. et al. (21), 30 patients were screened, of whom 27 patients were randomly divided into ICI monotherapy group (receiving nivolumab monotherapy) (n=13) and dual ICI group (receiving nivolumab plus ipilimumab) (n=14). Among the 25 patients who completed the treatment, the median PFS was 9.4 months in the former group and 19.53 months in the latter group. It is suggested that dual ICI therapy may have better long-term survival benefits.
Regarding the safety of neoadjuvant systemic therapy in resectable HCC, the pooled results for grade 3–4 TRAEs were 11% (95% CI: 4% to 20%), with the highest incidence of 44.2% reported by Su, Y. et al. (24). Common adverse events included pneumonia, drug-induced hepatitis, pruritus, maculopapular rash, severe muscle weakness, elevated lipase and leukopenia. Most of the adverse events were manageable, some of which could be resolved by drug withdrawal, and others could be relieved by steroid therapy. Overall, neoadjuvant systemic therapy for HCC has shown a safety profile similar to previous studies in gastrointestinal tumors (52). In addition, the only randomized controlled trial reported by Kaseb, A.O, et al. (21) in this study found a higher incidence of grade 3–4 TRAEs with nivolumab plus ipilimumab than with nivolumab alone (23% vs 43%), indicating that different combinations may lead to different outcomes. Different combination regimens should be optimized to maximize patient benefit. In addition, the pooled surgical resection rate after neoadjuvant systemic therapy was 84% (95% CI: 75%-92%). Although the majority of patients underwent surgery as scheduled, a small number of patients may not tolerate surgery due to toxicity caused by neoadjuvant therapy or lose the opportunity for surgery due to tumor progression. Therefore, neoadjuvant systemic therapy for patients with resectable HCC requires MDT (Multi-Disciplinary Treatment) to develop an individualized and comprehensive treatment plan to maximize patient benefits, ensure treatment safety, and improve treatment outcomes (53).
This study found that there was high heterogeneity in surgical resection rate (I²=65.5%) and grade 3–4 TRAEs (I²=74.3%), which may be related to the study type (most of the included studies were single-arm studies). At the same time, we also conducted a subgroup analysis (according to the three different treatment regimens of ICI monotherapy, dual ICIs and targeted–immunotherapy combination) and found that the reason for high heterogeneity may be related to different treatment regimens. It may also be related to the tumor stage, liver function, surgical standard difference, physical condition and drug dose of patients. The results of subgroup analysis showed that for different neoadjuvant systemic treatment regimens, the surgical resection rate of dual ICIs was better than that of ICI monotherapy, and ICI monotherapy was better than that of targeted–immunotherapy combination. In terms of ORR, targeted–immunotherapy combination was superior to ICI monotherapy, and ICI monotherapy was superior to dual ICIs. Certainly, in terms of the incidence of grade 3–4 TRAEs, the dual ICI combination therapy was the highest and the ICI monotherapy was the lowest. Immunotherapy and targeted therapy have been widely used in the clinical application of HCC (54, 55). Single therapy has limited clinical effect and may also produce drug resistance. Several studies have shown that combination therapy can lead to better treatment outcomes. For example, the IMbrave150 trial (56) showed significant improvement in clinical outcomes with a 1-year survival rate of 67.2% and mPFS of 6.8 months in previously untreated patients with advanced HCC treated with atezolizumab plus bevacizumab. It is currently approved for first-line treatment of advanced HCC. Other combinations such as sintilimab plus bevacizumab and camrelizumab plus apatinib, have also shown promising results (51, 57). In addition, the IMbrave050 (58) phase III clinical study results are expected to show that the combination of ICIs plus antiangiogenic drugs (T+A regimen) can significantly reduce the risk of recurrence, distant metastasis, or death by 28% in patients with HCC after radical treatment (including surgical resection or ablation), showing a clear survival benefit. Mechanistically, immunomodulatory drugs have been found to restore the immune-supporting microenvironment, whereas anti-VEGF agents such as bevacizumab improve immunosuppression and help restore vascular normalization for effective administration, allowing lower doses of ICIs to reduce adverse effects (59). In our subgroup analysis, the ORR of the targeted–immunotherapy combination was significantly superior, but the surgical resection rate was lower than in other subgroups. The long-term survival benefit after surgery still needs to be assessed, which will provide effective clinical evidence for choosing the best treatment strategy in the future.
This meta-analysis has certain limitations. On the one hand, the included studies differed in study design, treatment regimen, and patient inclusion criteria, etc. (including lack of data on tumor size and number at study enrollment and/or at time of post-neoadjuvant evaluation for surgery), resulting in heterogeneity. On the other hand, most of the data came from conference abstracts, no unified standard for R0 resection rate has been reported(as shown in Table 3), and there was also a lack of long-term follow-up survival indicators such as OS, DFS, RFS, and PFS, which may affect the reliability of the results. Because of the lack of these data, we could not perform a comprehensive analysis, and therefore, whether neoadjuvant systemic therapy contributes to the long-term benefit of HCC patients needs to be further confirmed.
5 Conclusion
In conclusion, our meta-analysis demonstrated the efficacy and safety of neoadjuvant systemic therapy in patients with resectable HCC, providing evidence for its future clinical application. However, due to limited clinical data, large-scale and multicenter randomized controlled trials are needed to confirm this conclusion in the future.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
DW: Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. NL: Data curation, Methodology, Writing – review & editing. HD: Conceptualization, Project administration, Supervision, Writing – original draft. KZ: Data curation, Formal analysis, Methodology, Writing – original draft. LD: Conceptualization, Methodology, Software, Supervision, Validation, Visualization, Writing – review & editing. YL: Conceptualization, Project administration, Supervision, Validation, Visualization, Writing – review & editing. YC: Conceptualization, Project administration, Supervision, Writing – review & editing. FM: Conceptualization, Project administration, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1504917/full#supplementary-material
References
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
2. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
3. Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. (2022) 400:1345–62. doi: 10.1016/s0140-6736(22)01200-4
4. Memeo R, Pisani AR, Ammendola M, de’Angelis N, Inchingolo R. A new era for hepatocellular carcinoma. Hepatobil Surg Nutr. (2023) 12:135–6. doi: 10.21037/hbsn-23-10
5. Xu XF, Xing H, Han J, Li ZL, Lau WY, Zhou YH, et al. Risk factors, patterns, and outcomes of late recurrence after liver resection for hepatocellular carcinoma: A multicenter study from China. JAMA Surg. (2019) 154:209–17. doi: 10.1001/jamasurg.2018.4334
6. Wang PH, Yang ST, Liu CH. Neoadjuvant therapy. J Chin Med Assoc. (2023) 86:133–4. doi: 10.1097/jcma.0000000000000855
7. Topalian SL, Forde PM, Emens LA, Yarchoan M, Smith KN, Pardoll DM. Neoadjuvant immune checkpoint blockade: A window of opportunity to advance cancer immunotherapy. Cancer Cell. (2023) 41:1551–66. doi: 10.1016/j.ccell.2023.07.011
8. Takada M, Toi M. Neoadjuvant treatment for her2-positive breast cancer. Chin Clin Oncol. (2020) 9:32. doi: 10.21037/cco-20-123
9. Zhou B, Zhang SR, Chen G, Chen P. Developments and challenges in neoadjuvant therapy for locally advanced pancreatic cancer. World J Gastroenterol. (2023) 29:5094–103. doi: 10.3748/wjg.v29.i35.5094
10. Qin H, Liu F, Zhang Y, Liang Y, Mi Y, Yu F, et al. Comparison of neoadjuvant immunotherapy versus routine neoadjuvant therapy for patients with locally advanced esophageal cancer: A systematic review and meta-analysis. Front Immunol. (2023) 14:1108213. doi: 10.3389/fimmu.2023.1108213
11. Vogel A, Grant RC, Meyer T, Sapisochin G, O’Kane GM, Saborowski A. Adjuvant and neoadjuvant therapies for hepatocellular carcinoma. Hepatology. (2023). doi: 10.1097/hep.0000000000000726
12. Wen N, Cai Y, Li F, Ye H, Tang W, Song P, et al. The clinical management of hepatocellular carcinoma worldwide: A concise review and comparison of current guidelines: 2022 update. Biosci Trends. (2022) 16:20–30. doi: 10.5582/bst.2022.01061
13. Xie D, Shi J, Zhou J, Fan J, Gao Q. Clinical practice guidelines and real-life practice in hepatocellular carcinoma: A chinese perspective. Clin Mol Hepatol. (2023) 29:206–16. doi: 10.3350/cmh.2022.0402
14. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: An updated guideline for reporting systematic reviews. Bmj. (2021) 372:n71. doi: 10.1136/bmj.n71
15. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J Surg. (2003) 73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x
16. Shi Y-H, Ji Y, Liu W-R, Pang Y-R, Ding Z-B, Fu X-T, et al. A phase ib/ii, open-label study evaluating the efficacy and safety of toripalimab injection (js001) or combination with lenvatinib as a neoadjuvant therapy for patients with resectable hepatocellular carcinoma (hcc). Cancer Res. (2021) 81:486. doi: 10.1158/1538-7445.AM2021-486
17. Ho WJ, Zhu Q, Durham J, Popovic A, Xavier S, Leatherman J, et al. Neoadjuvant cabozantinib and nivolumab converts locally advanced hcc into resectable disease with enhanced antitumor immunity. Nat Cancer. (2021) 2:891–903. doi: 10.1038/s43018-021-00234-4
18. Chen S, Wang Y, Xie W, Shen S, Peng S, Kuang M. 710p neoadjuvant tislelizumab for resectable recurrent hepatocellular carcinoma: A non-randomized control, phase ii trial (talent). Ann Oncol. (2022) 33:S867. doi: 10.1016/j.annonc.2022.07.834
19. D’Alessio A, Pai M, Spalding D, Goldin RD, Scheiner B, Korolewicz J, et al. Neoadjuvant immunotherapy with ipilimumab plus nivolumab and radiologically and pathologically quantifiable responses through modulation of the tumor microenvironment in resectable hepatocellular carcinoma. J Clin Oncol. (2022) 41:4129. doi: 10.1200/JCO.2023.41.16_suppl.4129
20. Marron TU, Fiel MI, Hamon P, Fiaschi N, Kim E, Ward SC, et al. Neoadjuvant cemiplimab for resectable hepatocellular carcinoma: A single-arm, open-label, phase 2 trial. Lancet Gastroenterol Hepatol. (2022) 7:219–29. doi: 10.1016/s2468-1253(21)00385-x
21. Kaseb AO, Hasanov E, Cao HST, Xiao L, Vauthey JN, Lee SS, et al. Perioperative nivolumab monotherapy versus nivolumab plus ipilimumab in resectable hepatocellular carcinoma: A randomized, open-label, phase 2 trial. Lancet Gastroenterol Hepatol. (2022) 7:208–18. doi: 10.1016/s2468-1253(21)00427-1
22. Xia Y, Tang W, Qian X, Li X, Cheng F, Wang K, et al. Efficacy and safety of camrelizumab plus apatinib during the perioperative period in resectable hepatocellular carcinoma: A single-arm, open label, phase ii clinical trial. J Immunother Cancer. (2022) 10:e004656. doi: 10.1136/jitc-2022-004656
23. Bai X, Chen Y, Zhang X, Zhang F, Liang X, Zhang C, et al. 712p capt: A multicenter randomized controlled trial of perioperative versus postoperative camrelizumab plus apatinib for resectable hepatocellular carcinoma. Ann Oncol. (2022) 33:S868. doi: 10.1016/j.annonc.2022.07.836
24. Su Y, Lin Y, Hsiao C, Ou D, Chen S, Wu Y, et al. P-124 nivolumab plus ipilimumab as neoadjuvant therapy for potentially resectable hepatocellular carcinoma. Ann Oncol. (2021) 32:S141. doi: 10.1016/j.annonc.2021.05.179
25. Sun H, Zhu XD, Gao Q, Qiu S, Shi Y, Wang XY, et al. Sintilimab combined with bevacizumab biosimilar as a conversion therapy in potentially resectable intermediate-stage hepatocellular carcinoma (hcc): Updated results of a phase ii trial. J Clin Oncol. (2023) 41:e16220. doi: 10.1200/JCO.2023.41.16_suppl.e16220
26. Song TQ. A prospective, single-arm, phase ii clinical study of tislelizumab in combination with lenvatinib for perioperative treatment of resectable primary hepatocellular carcinoma with high risk of recurrence. J Clin Oncol. (2023) 41:e16218. doi: 10.1200/JCO.2023.41.16_suppl.e16218
27. Cheung TT, Ho DW-H, Lyu SX, Zhang Q, Tsui YM, Yu TC-Y, et al. Multimodal integrative genomics and pathology analyses in neoadjuvant nivolumab treatment for intermediate and locally advanced hepatocellular carcinoma. Liver Cancer. (2023) 13:70–88. doi: 10.1159/000531176
28. Huang Z, Zhou J, Yang M, Zhang W, Sun Y, Lu H, et al. 181p tqb2450 (pd-l1 blockade) in combination with anlotinib as a perioperative treatment for patients with hepatocellular carcinoma at high risk of recurrence: Primary results from a prospective, single-arm, phase ib study. Ann Oncol. (2023) 34:S1545. doi: 10.1016/j.annonc.2023.10.760
29. Song T. A prospective, phase ii clinical study of tislelizumab monotherapy or in combination with lenvatinib for neoadjuvant treatment of resectable hepatocellular carcinoma. HPB. (2024) 26:S143. doi: 10.1016/j.hpb.2024.03.258
30. Ming K, Xie W, Chen J, Chen S, Wang Y, Shen S, et al. Neo-adjuvant tislelizumab combined with lenvatinib treatment for resectable, recurrent hepatocellular carcinoma. J Clin Oncol. (2024) 42:517. doi: 10.1200/JCO.2024.42.3_suppl.517
31. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1). Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
32. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. (2010) 30:52–60. doi: 10.1055/s-0030-1247132
33. Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. Using the common terminology criteria for adverse events (CTCAE- version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr (Engl Ed). (2021) 112:90–2. doi: 10.1016/j.ad.2019.05.009
34. Wang H, Li S, Liu T, Chen J, Dang J. Neoadjuvant immune checkpoint inhibitor in combination with chemotherapy or chemoradiotherapy in resectable esophageal cancer: A systematic review and meta-analysis. Front Immunol. (2022) 13:998620. doi: 10.3389/fimmu.2022.998620
35. Petricevic B, Kabiljo J, Zirnbauer R, Walczak H, Laengle J, Bergmann M. Neoadjuvant immunotherapy in gastrointestinal cancers - the new standard of care? Semin Cancer Biol. (2022) 86:834–50. doi: 10.1016/j.semcancer.2022.05.015
36. Kagawa Y, Smith JJ, Fokas E, Watanabe J, Cercek A, Greten FR, et al. Future direction of total neoadjuvant therapy for locally advanced rectal cancer. Nat Rev Gastroenterol Hepatol. (2024) 21:444–55. doi: 10.1038/s41575-024-00900-9
37. Brown ZJ, Heh V, Labiner HE, Brock GN, Ejaz A, Dillhoff M, et al. Surgical resection rates after neoadjuvant therapy for localized pancreatic ductal adenocarcinoma: Meta-analysis. Br J Surg. (2022) 110:34–42. doi: 10.1093/bjs/znac354
38. Tanahashi M, Suzuki E, Yoshii N, Watanabe T, Tsuchida H, Yobita S, et al. Role of fluorodeoxyglucose-positron emission tomography in predicting the pathological response and prognosis after neoadjuvant chemoradiotherapy for locally advanced non-small-cell lung cancer. Interact Cardiovasc Thorac Surg. (2022) 35:ivac113. doi: 10.1093/icvts/ivac113
39. Guo R, Yan W, Wang F, Su H, Meng X, Xie Q, et al. The utility of (18)f-fdg pet/ct for predicting the pathological response and prognosis to neoadjuvant immunochemotherapy in resectable non-small-cell lung cancer. Cancer Imaging. (2024) 24:120. doi: 10.1186/s40644-024-00772-x
40. Alawyia B, Constantinou C. Hepatocellular carcinoma: A narrative review on current knowledge and future prospects. Curr Treat Opt Oncol. (2023) 24:711–24. doi: 10.1007/s11864-023-01098-9
41. Brown L, Naffouje SA, Sam C, Laronga C, Catherine Lee M. Neoadjuvant systemic therapy in geriatric breast cancer patients: A national cancer database (ncdb) analysis. Breast Cancer Res Treat. (2022) 196:441–51. doi: 10.1007/s10549-022-06751-9
42. Chick RC, Ruff SM, Pawlik TM. Neoadjuvant systemic therapy for hepatocellular carcinoma. Front Immunol. (2024) 15:1355812. doi: 10.3389/fimmu.2024.1355812
43. Jiang Y, Zhao X, Fu J, Wang H. Progress and challenges in precise treatment of tumors with pd-1/pd-l1 blockade. Front Immunol. (2020) 11:339. doi: 10.3389/fimmu.2020.00339
44. Jiang Z, Lim SO, Yan M, Hsu JL, Yao J, Wei Y, et al. Tyro3 induces anti-pd-1/pd-l1 therapy resistance by limiting innate immunity and tumoral ferroptosis. J Clin Invest. (2021) 131:e139434. doi: 10.1172/jci139434
45. Laschtowitz A, Roderburg C, Tacke F, Mohr R. Preoperative immunotherapy in hepatocellular carcinoma: Current state of the art. J Hepatocell Carcinoma. (2023) 10:181–91. doi: 10.2147/jhc.S347944
46. Li K, Tandurella JA, Gai J, Zhu Q, Lim SJ, Thomas DL 2nd, et al. Multi-omic analyses of changes in the tumor microenvironment of pancreatic adenocarcinoma following neoadjuvant treatment with anti-pd-1 therapy. Cancer Cell. (2022) 40:1374–91.e7. doi: 10.1016/j.ccell.2022.10.001
47. Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. (2022) 1:EVIDoa2100070. doi: 10.1056/EVIDoa2100070
48. Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (checkmate 459): A randomized, multicenter, open-label, phase 3 trial. Lancet Oncol. (2022) 23:77–90. doi: 10.1016/s1470-2045(21)00604-5
49. Qin S, Kudo M, Meyer T, Finn RS, Vogel A, Bai Y, et al. LBA36 Final analysis of RATIONALE-301: Randomized, phase III study of tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Ann Oncol. (2022) 33:S1402–403. doi: 10.1016/j.annonc.2022.08.033
50. Qin S, Chen Z, Fang W, Ren Z, Xu R, Ryoo BY, et al. Pembrolizumab versus placebo as second-line therapy in patients from Asia with advanced hepatocellular carcinoma: A randomized, double-blind, phase iii trial. J Clin Oncol. (2023) 41:1434–43. doi: 10.1200/jco.22.00620
51. Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (rescue): A nonrandomized, open-label, phase ii trial. Clin Cancer Res. (2021) 27:1003–11. doi: 10.1158/1078-0432.Ccr-20-2571
52. Kou L, Wen Q, Xie X, Chen X, Li J, Li Y. Adverse events of immune checkpoint inhibitors for patients with digestive system cancers: A systematic review and meta-analysis. Front Immunol. (2022) 13:1013186. doi: 10.3389/fimmu.2022.1013186
53. Ng SST, Oehring R, Ramasetti N, Roller R, Thomas P, Chen Y, et al. Concordance of a decision algorithm and multidisciplinary team meetings for patients with liver cancer-a study protocol for a randomized controlled trial. Trials. (2023) 24:577. doi: 10.1186/s13063-023-07610-8
54. Kudo M. Adjuvant atezolizumab-bevacizumab after curative therapy for hepatocellular carcinoma. Hepatobil Surg Nutr. (2023) 12:435–9. doi: 10.21037/hbsn-23-203
55. Ruff SM, Manne A, Cloyd JM, Dillhoff M, Ejaz A, Pawlik TM. Current landscape of immune checkpoint inhibitor therapy for hepatocellular carcinoma. Curr Oncol. (2023) 30:5863–75. doi: 10.3390/curroncol30060439
56. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. (2020) 382:1894–905. doi: 10.1056/NEJMoa1915745
57. Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (ibi305) versus sorafenib in unresectable hepatocellular carcinoma (orient-32): A randomized, open-label, phase 2–3 study. Lancet Oncol. (2021) 22:977–90. doi: 10.1016/s1470-2045(21)00252-7
58. Qin S, Chen M, Cheng AL, Kaseb AO, Kudo M, Lee HC, et al. Atezolizumab plus bevacizumab versus active surveillance in patients with resected or ablated high-risk hepatocellular carcinoma (imbrave050): A randomized, open-label, multicenter, phase 3 trial. Lancet. (2023) 402:1835–47. doi: 10.1016/s0140-6736(23)01796-8
Keywords: hepatocellular carcinoma, neoadjuvant, systemic therapy, resectable, systematic review, meta-analysis
Citation: Wu D, Liu N, Dong H, Zhou K, Du L, Li Y, Chao Y and Ma F (2025) Efficacy and safety of neoadjuvant systemic therapy in resectable hepatocellular carcinoma: a Systematic Review and meta-analysis. Front. Oncol. 15:1504917. doi: 10.3389/fonc.2025.1504917
Received: 01 October 2024; Accepted: 18 April 2025;
Published: 09 May 2025.
Edited by:
Aaron Balasingam Koenig, Celia Scott Weatherhead School of Public Health and Tropical Medicines, United StatesCopyright © 2025 Wu, Liu, Dong, Zhou, Du, Li, Chao and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Du, MjM2ODk5OTA0OEBxcS5jb20=
 Dongdong Wu
Dongdong Wu Ning Liu
Ning Liu