- Department of Gastroenterology, Shaoxing People’s Hospital, Shaoxing, China
Background: As a rare condition, sporadic non-ampullary duodenal adenomas (SNADAs) are typically asymptomatic and diagnosed incidentally. It warrants treatment due to the high risk of malignant transformation. However, giant SNADAs, especially those treated using hybrid endoscopic submucosal dissection (ESD), are exceedingly rare, and to our knowledge, this condition has not been reported previously.
Case presentation: A 69-year-old Chinese male patient was diagnosed with an incidentally discovered giant SNADA. Given the size and location and the patient’s non-invasive request, we performed hybrid ESD to completely resect a giant SNADA (4.5*2.8*1.0cm) without postoperative discomfort or complications. Final pathology confirmed a margin-negative tubulovillous adenoma with low-grade intraepithelial neoplasia.
Conclusion: We present a rare case of a giant SNADA that was successfully dissected using hybrid ESD. Further, we provide a brief review, discuss the treatment protocol of this case, and provide a new perspective for the future diagnosis and treatment of a giant SNADA.
Introduction
Duodenal adenomas are uncommon and mostly asymptomatic, often detected incidentally during endoscopic evaluation, with an estimated incidence of 0.03%–0.1% (1–3). It is generally considered that, regardless of their anatomical location and whether they are sporadic or FAP-related, duodenal adenomas carry a high risk of malignant transformation, which follows the adenoma-carcinoma sequence and is similar to the colonic adenoma-carcinoma pattern, and thus deserve treatment (4–7). Wherein, low-grade dysplasia (LGD) lesions show a low risk of progression to adenocarcinoma, while a large initial tumor size and location on the oral side of the papilla of Vater (8) warrant a careful follow-up biopsy due to the risk of progression to high-grade dysplasia (HGD). However, HGD lesions and large non-ampullary Sporadic duodenal adenomas (SDAs) ≥ 20 mm in diameter show a high risk of malignant transformation and thus should be treated immediately (9). In addition, the malignant risk of ampullary adenomas is much higher than that of non-ampullary sporadic duodenal adenomas (10, 11). Current treatment techniques for duodenal adenoma include endoscopy therapy and surgery, of which the former has the advantage of safety and less invasiveness. Endoscopic resection techniques for SDAs include snare polypectomy, argon plasma coagulation (APC) ablation, endoscopic mucosal resection (EMR), underwater EMR, and endoscopic submucosal dissection (ESD), with EMR as the primary treatment technique (1).
Here, we present a very rare case of a giant sporadic non-ampullary duodenal adenoma (SNADA) that was successfully resected using hybrid ESD. Additionally, we present a brief review and case-related discussion.
Case presentation
A 69-year-old male patient, with a history of hypertension, visited our hospital for progressive urinary obstruction. The abdomen contrast computed tomography (CT) scan showed a soft tissue shadow in the descending duodenum, which was significantly enhanced after contrast enhancement. The position of the soft tissue shadow was slightly lowered in the portal phase, and no significant abnormalities were observed in the remaining intestinal lumen (Figure 1). Further transoral enteroscopy identified a giant mass of approximately 5 cm with a wide base approximately 5 to 6 cm below the duodenal papilla, the surface of which was lobulated and the glandular duct was basically present (Figure 2). Biopsy pathology of the mass indicated tubular adenoma, with low-grade intraepithelial neoplasia (LGIN). Combined with the enteroscopy findings and pathological findings, the duodenal mass was considered a case of giant SNADA.
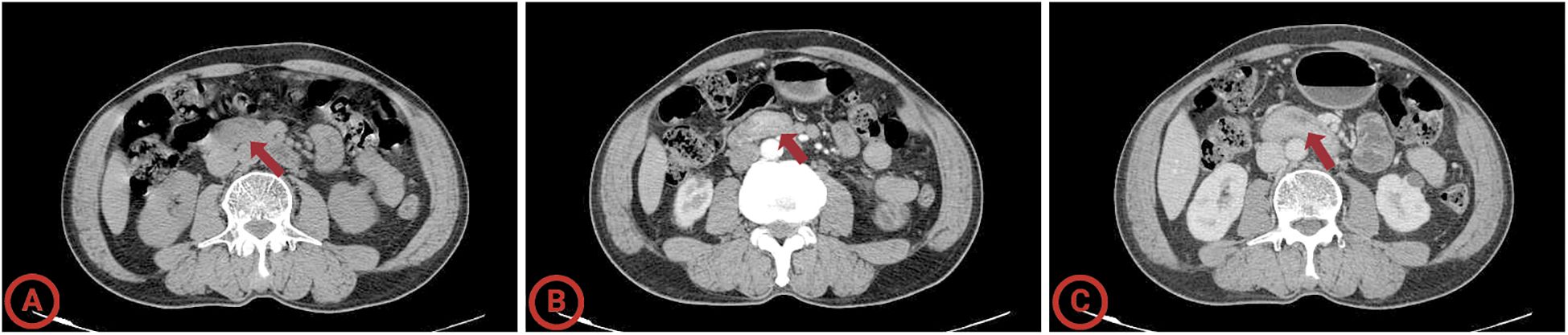
Figure 1. Computed tomography of the giant sporadic non-ampullary duodenal adenoma. (A) Arterial phase; (B) venous phase; (C) delayed scan. The average HU values of the lesion in the plain scan, arterial, and portal venous phases are 63.2 HU, 87.9 HU, and 93.1 HU.
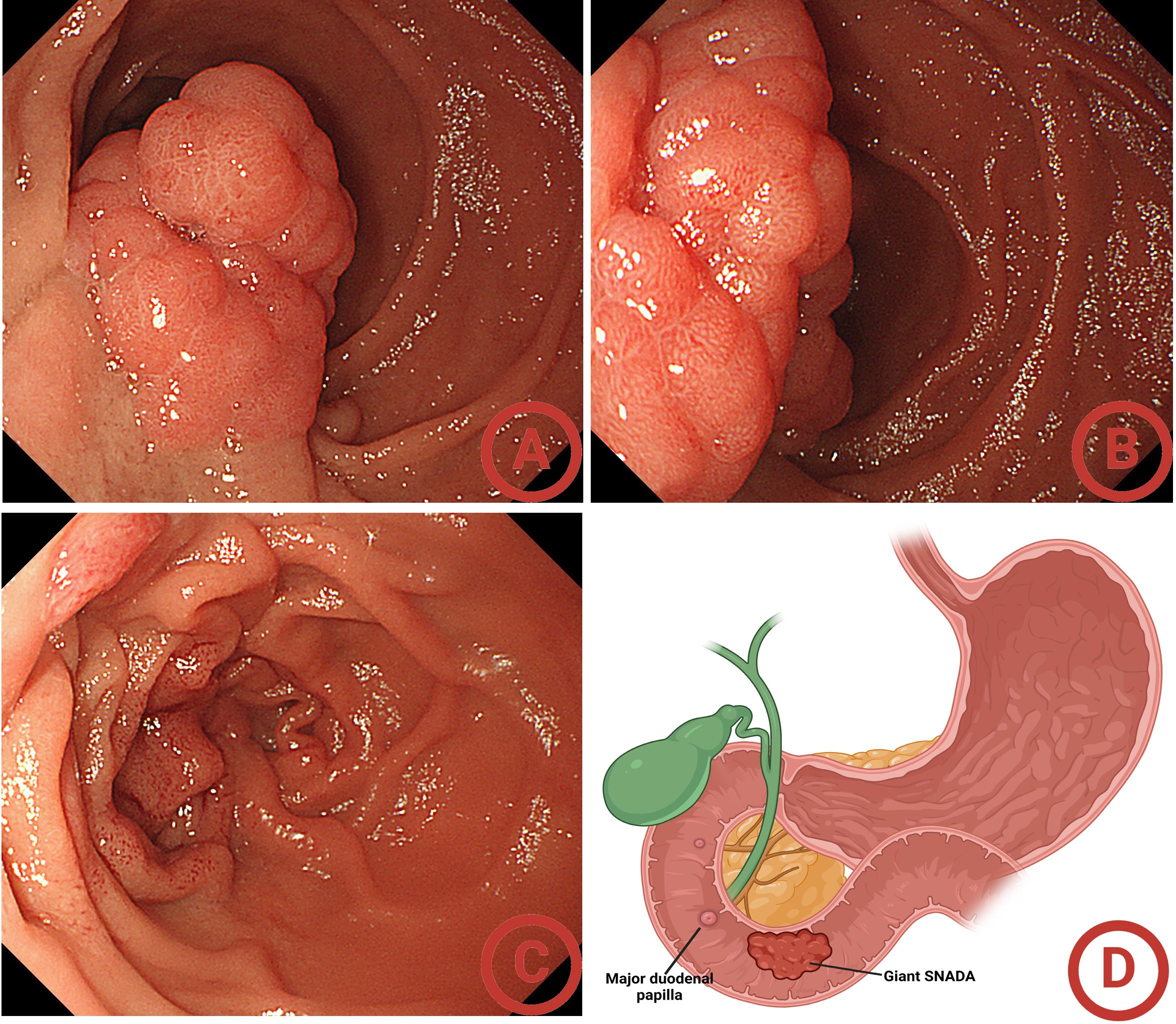
Figure 2. Initial gastroscopy findings. (A) Far-view of the giant sporadic non-ampullary duodenal adenoma (SNADA); (B) near-view of the giant SNADA; (C) confirming the location of the duodenal papilla; (D) the location of the giant SNADA.
Given the characteristics of the lesion and the patient’s request for surgical treatment and minimally invasive resection, we performed hybrid ESD to completely remove the adenoma and reduce the duration of treatment and complications.
Intraoperatively, the descending duodenum was reached using a colonoscope (Olympus CF-H290I) with a transparent cap, and an approximately 5-cm wide-based lobulated adenoma was seen on the medial wall of the greater curvature. After confirming that the adenoma was located below the duodenal papilla and that the anal side of the adenoma had reached the transition zone between the descending and horizontal parts of the duodenum, a mixture of normal saline (NS), methylene blue, and sodium hyaluronate was injected into the submucosa on the anal side of the adenoma, which showed a positive lifting sign. We the used a dual knife to circumferentially dissect the submucosal side from the anal side of the adenoma until the surface of the whole adenoma turned purple and then switched to snare en-bloc resection. Notably, when large blood vessels were exposed during the dissection process, we performed vascular dissection, and then the dual knife was replaced with a hemostatic forceps for preventive electrocoagulation treatment to avoid a massive hemorrhage and achieve the goal of safe and efficient treatment. We then examined the wound and confirmed that the tumor was completely removed, that the wound was intact with no bleeding or perforation, and that the duodenal papilla was intact. The wound was then closed with Boston clips. The whole operation was uneventful and lasted approximately 2 hours (Figure 3). The patient safely returned to the ward and was discharged on postoperative day 5.
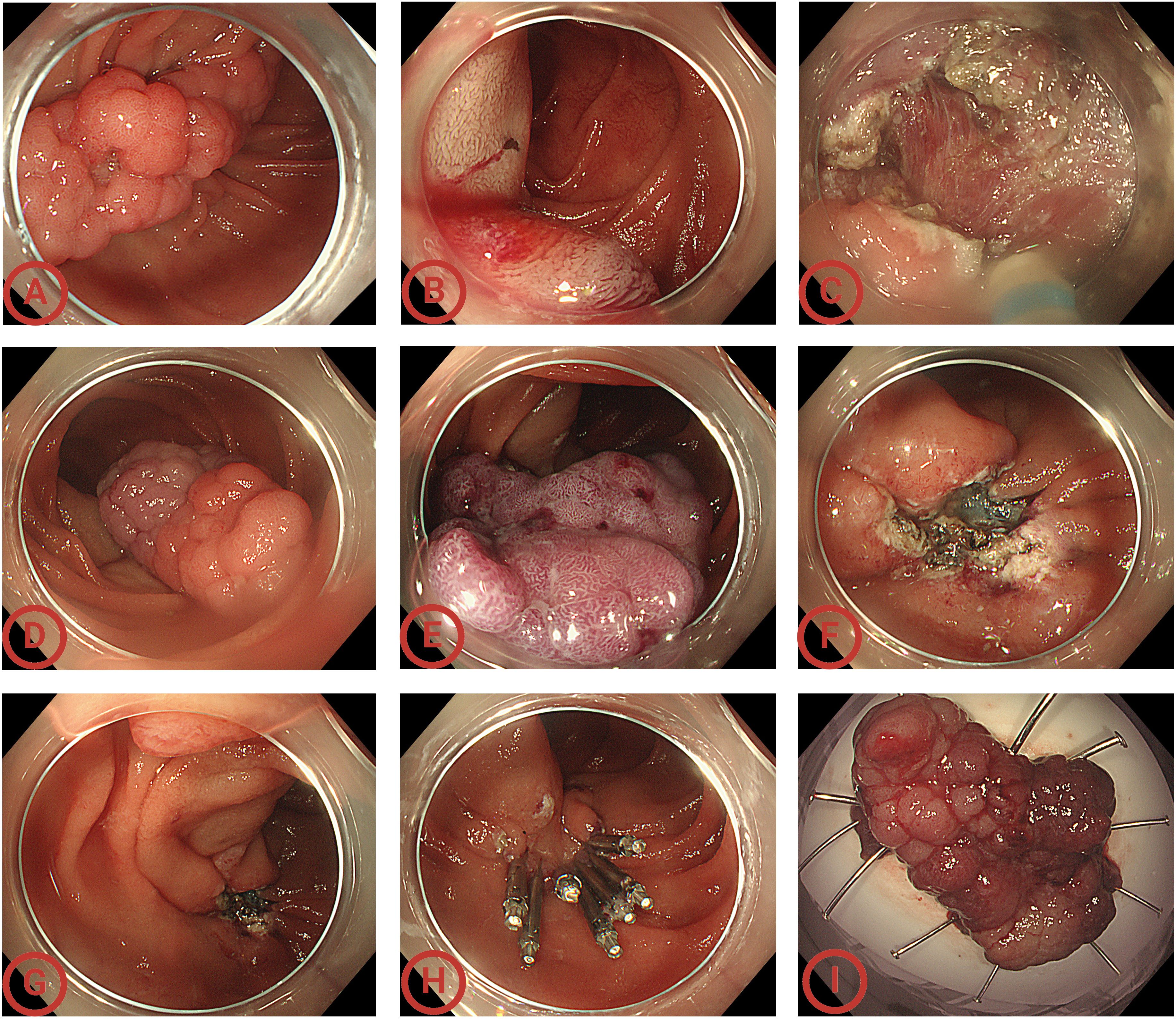
Figure 3. Endoscopic views of the whole operation. (A) Endoscopy showed a sporadic non-ampullary duodenal adenoma (SNADA) at least 5cm in size at the descending part of the duodenum; (B) submucosal injection on the anal side; (C) circumferential incision using the dual knife; (D) during the circumferential incision process, the mass’ color changed gradually; (E) performing submucosal dissection until the surface of the whole adenoma turned purple; (F) the wound surface after dissection; (G) confirming the duodenal papilla was intact and located above the wound; (H) closing the wound; (I) the 4.5 cm × 2.8 cm × 1.0 cm en bloc specimen. Considering that labeling easily causes mucosal damage and leakage after submucosal injection, and the adenoma boundary was clear, the lesion was not labeled.
The completely removed specimen was noted to be 4.5 cm × 2.8 cm × 1.0 cm, which histopathological examinations showed to be a tubulovillous adenoma with low-grade intraepithelial neoplasia, a negative base, and a peripheral incisal margin (Figure 4). Further immunohistochemistry exhibited that both MUC5AC and MUC6 expression were negative and MUC2 expression was partially positive, while the expression of CD10 was positive, which indicated an intestinal-type adenoma. The details are shown in Figure 4. The patient showed no discomfort during the 1-year follow-up after discharge. Unfortunately, the patient refused further endoscopic reinspection after repeated requests by doctors and family members.
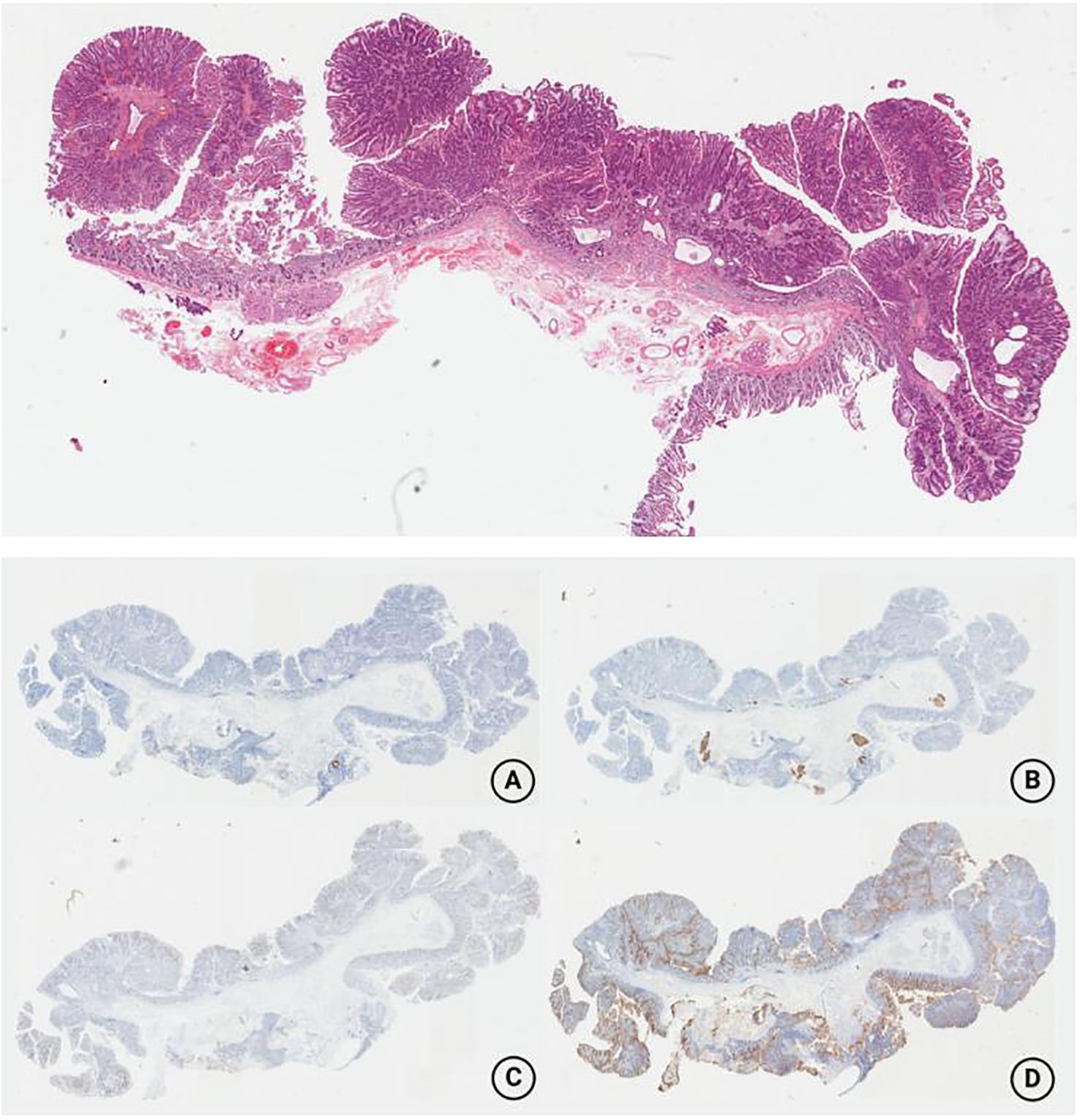
Figure 4. Postoperative pathology revealed the mass to be a tubulovillous adenoma with low-grade intraepithelial neoplasia, a negative base, and a peripheral incisal margin. The immunohistochemistry findings are as follows. (A) The MUC5AC expression was negative; (B) the MUC6 expression was negative; (C) the MUC2 expression was partially positive; (D) the CD10 expression was positive.
Discussion
Herein, we described a very rare case of giant SNADA, which was successfully removed using hybrid ESD. Considering that SNADAs have a risk of malignant transformation comparable to colorectal adenomas, we deemed the giant SNADA in this case to warrant intervention, yet few studies have reported related outcomes. Herein, we will further present a case-related discussion that analyzes the case and explores our selection of treatment strategy.
Surgical operation has previously been the main treatment for duodenal adenoma, with complete resection, yet this results in significant trauma and postoperative complications (2). Endoscopic resection is currently the preferred treatment due to the advantages of being minimally invasive, the low incidence of adverse events, and short hospital stay, and the surgery is usually a cure (1, 12). Although EMR is more commonly used, ESD was selected for the giant SNADA in our case (with a lesion with a length of ≥20mm), as it can completely remove the lesion, allow for a complete histological examination, and reduce the risk of recurrence (1). The overall resection rate of ESD is higher than EMR, which is critical since adenomas >2 cm are associated with a higher recurrence rate (13). Furthermore, the peripapillary anatomical structure, especially the vascular structure, is also clearly displayed during the operation, which enables the pre-treatment of vessels and reduces bleeding (14–16). Notably, no recurrence has been observed following an ESD (15, 16). Furthermore, current evidence suggests that hybrid ESD offers advantages over conventional ESD, including easier applicability, shorter procedural times, and fewer adverse events, without compromising en-bloc resection rates or increasing recurrence rates, which supports the potential of hybrid ESD as a superior alternative to traditional ESD (19–22).
Based on these considerations, we opted for hybrid ESD for this rare case of giant SNADA to achieve efficient and complete resection while minimizing complications.
However, duodenal ESD requires advanced technology and accuracy. Its operative time is long and the incidence of postoperative complications (mainly hemorrhage and perforation) is relatively high (15), which is mainly due to the following characteristics of the special anatomical structure: (1) the high intraluminal pressure in the gastrointestinal tract leads to thin walls and a high perforation rate; (2) the “C”-shaped configuration and relatively narrow lumen complicate endoscopic maneuvers; (3) abundant submucosal blood supply increase the bleeding risk; (4) the presence of numerous Brunner glands complicates submucosal elevation; (5) proximity to the ampulla and the sharing of a common blood supply with the pancreatic head and the bile pancreatic duct may lead to delayed complications due to postoperative wound exposure to pancreatic fluid and bile, affecting regional blood supply and disrupting biliary-pancreatic duct function (9, 17, 18). Thus special focus should be placed as follows: (1) a colonoscope was preferred given its 6 o’clock orientation when located in the working channel, providing vision for submucosal injection and manipulation while ensuring an adequate endoscopic workspace (18); (2) an adequate submucosal injection is needed to separate the mucosal and muscle layers; (3) we considered that most of the tumor blood supply had been blocked when the tumor turned purple, which means the risk of massive hemorrhage was lower, so we switched from the dual knife to snare resection; (4) the position of the duodenal papilla and the lesion were confirmed repeatedly before and after resection to avoid complications; (5) we balanced the electrocoagulation and electrosection to avoid bleeding and perforation; (6) we ensured that the tumor was completely trapped in the snare (the resection in our case is too large, which may partly blind resection) before the snare resection, and examined the wound after resection to avoid incomplete resection or perforation; (7) ESD-associated mucosal defects may increase the risk of delayed bleeding and perforation, especially given the giant size, and special location (it appeared to be influenced by bile and gastric acid) in our case, thus, the wound was firmly closed to minimize adverse events (23, 24).
Fortunately, with the superb technology and cooperation of our endoscopic team, the giant SNADA was completely removed, with no adverse events and postoperative complications. Subsequent pathology confirmed the giant SNADA to be a tubulovillous adenoma with low-grade intraepithelial neoplasia, which emphasized the necessity for endoscopic treatment, as villous adenomas exhibit higher recurrence rates (25). Additionally, based on the available findings, we recommend a follow-up endoscopy within 3–6 months post-resection to monitor for a recurrence; if not, subsequent endoscopic surveillance at 6–12 months is required (26).
The successful treatment of this case led to the following observations. Due to the difficulty of the operation and serious complications, duodenal ESD should be performed with caution. To establish duodenal ESD as a safe and minimally invasive therapeutic strategy for duodenal tumors, a reliable endoscopic procedure strategy should be developed. Our case suggests that hybrid ESD may be the optimal treatment for duodenal adenomas, but this requires further study. Additionally, although the case reports and guidelines on SNADA are currently deficient, based on our team’s experience, we propose the following options for SNADA: 1) EMR for SNADAs<1cm (offering efficient, safe, and complete resection); 2) for SNADAs 1–2 cm, the choice depends on the morphology of the mass, i.e., EMR for semi-pedunculated or pedunculated masses, and traditional or hybrid ESD for wide-based or flat masses; 3) ESD (traditional or hybrid) for SNADAs >2 cm, especially those >3 cm or with wide base, for complete resection. Generally, SNADA treatment requires individualized evaluation according to the size, location, and shape of the tumor; the doctor’s level of experience with the operation; and the hospital’s multidisciplinary capability to achieve safe, efficient, minimally invasive, and economical treatment. Finally, our research does have certain limitations: 1) Due to the rarity of giant SNADAs, our experience in this field remains limited and the successful resection in this case may have been fortuitous despite the extensive analysis and consideration of treatment strategies; 2) Given the variability in doctors’ levels of experience with the operation and although we considered hybrid ESD to be the most suitable option in this case, there may be more optimized approaches. Consequently, the selection of hybrid ESD in this report represents a sharing of experience from a rare case and should not be considered a universal standard.
Conclusion
To conclude, we present a very rare case of a giant SNADA that was successfully completely removed by hybrid ESD, which combines both the advantage of ESD (en-bloc resection) and snare resection (simple, direct, and fast), enabling the safe, rapid, and complete removal of large duodenal lesions. We believe that hybrid ESD serves as an effective bridge between conventional EMR and traditional ESD, potentially offering a superior or alternative approach to traditional ESD. Furthermore, with advancements in endoscopic technology, hybrid ESD may represent a promising and safe treatment for duodenal lesions, particularly those exceeding 2 cm in size.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Academic Ethics Committee of Shaoxing People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by-product of routine care or industry. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
WL: Data curation, Formal Analysis, Funding acquisition, Project administration, Software, Writing – review & editing. JH: Data curation, Writing – original draft, Writing – review & editing. LD: Data curation, Writing – review & editing. YY: Software, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by The Shaoxing City Health Science and Technology Plan Project Foundation of China (NO. 2023SKY025).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Amoyel M, Belle A, Dhooge M, Ali EA, Hallit R, Prat F, et al. Endoscopic management of non-ampullary duodenal adenomas. Endosc Int Open. (2022) 10:E96–e108. doi: 10.1055/a-1723-2847
2. Alkhatib AA. Sporadic nonampullary tubular adenoma of the duodenum: Prevalence and patients’ characteristics. Turk J Gastroenterol. (2019) 30:112–3. doi: 10.5152/tjg.2018.17823
3. Jung SH, Chung WC, Kim EJ, Kim SH, Paik CN, Lee BI, et al. Evaluation of non-ampullary duodenal polyps: comparison of non-neoplastic and neoplastic lesions. World J Gastroenterol. (2010) 16:5474–80. doi: 10.3748/wjg.v16.i43.5474
4. Mitsuishi T, Hamatani S, Hirooka S, Fukasawa N, Aizawa D, Hara Y, et al. Clinicopathological characteristics of duodenal epithelial neoplasms: Focus on tumors with a gastric mucin phenotype (pyloric gland-type tumors). PLoS One. (2017) 12:e0174985. doi: 10.1371/journal.pone.0174985
5. Ahmad NA, Kochman ML, Long WB, Furth EE, and Ginsberg GG. Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointest Endosc. (2002) 55:390–6. doi: 10.1067/mge.2002.121881
6. Johnson MD, Mackey R, Brown N, Church J, Burke C, and Walsh RM. Outcome based on management for duodenal adenomas: sporadic versus familial disease. J Gastrointest Surg. (2010) 14:229–35. doi: 10.1007/s11605-009-1091-4
7. Probst A, Freund S, Neuhaus L, Ebigbo A, Braun G, Goelder S, et al. Complication risk despite preventive endoscopic measures in patients undergoing endoscopic mucosal resection of large duodenal adenomas. Endoscopy. (2020) 52:847–55. doi: 10.1055/a-1144-2767
8. Murray MA, Zimmerman MJ, and Ee HC. Sporadic duodenal adenoma is associated with colorectal neoplasia. Gut. (2004) 53:261–5. doi: 10.1136/gut.2003.025320
9. Ross RK, Hartnett NM, Bernstein L, and Henderson BE. Epidemiology of adenocarcinomas of the small intestine: is bile a small bowel carcinogen? Br J Cancer. (1991) 63:143–5.
10. Seifert E, Schulte F, and Stolte M. Adenoma and carcinoma of the duodenum and papilla of Vater: a clinicopathologic study. Am J Gastroenterol. (1992) 87:37–42.
11. Okada K, Fujisaki J, Kasuga A, Omae M, Kubota M, Hirasawa T, et al. Sporadic nonampullary duodenal adenoma in the natural history of duodenal cancer: a study of follow-up surveillance. Am J Gastroenterol. (2011) 106:357–64.
12. Sakorafas GH, Friess H, and Dervenis CG. Villous tumors of the duodenum: biologic characters and clinical implications. Scand J Gastroenterol. (2000) 35:337–44. doi: 10.3748/wjg.v22.i2.600
13. Spigelman AD, Talbot IC, Penna C, Nugent KP, Phillips RK, Costello C, et al. Evidence for adenoma-carcinoma sequence in the duodenum of patients with familial adenomatous polyposis. The Leeds Castle Polyposis Group (Upper Gastrointestinal Committee). J Clin Pathol. (1994) 47:709–10. doi: 10.1136/jcp.47.8.709
14. Ikenoyama Y, Yoshimizu S, Namikawa K, Tokai Y, Horiuchi Y, Ishiyama A, et al. Sporadic non-ampullary duodenal adenoma with low-grade dysplasia: Natural history and clinical management. Endosc Int Open. (2022) 10:E254–e261. doi: 10.1055/a-1672-3797
15. Lim CH and Cho YS. Nonampullary duodenal adenoma: Current understanding of its diagnosis, pathogenesis, and clinical management. World J Gastroenterol. (2016) 22:853–61. doi: 10.3748/wjg.v22.i2.853
16. Culver EL and McIntyre AS. Sporadic duodenal polyps: classification, investigation, and management. Endoscopy. (2011) 43:144–55. doi: 10.1055/s-0030-1255925
17. Pandolfi M, Martino M, and Gabbrielli A. Endoscopic treatment of ampullary adenomas. Jop. (2008) 9:1–8.
18. Gaspar JP, Stelow EB, and Wang AY. Approach to the endoscopic resection of duodenal lesions. World J Gastroenterol. (2016) 22:600–17. doi: 10.3748/wjg.v22.i2.600
19. Abbass R, Rigaux J, and Al-Kawas FH. Nonampullary duodenal polyps: characteristics and endoscopic management. Gastrointest Endosc. (2010) 71:754–9. doi: 10.1016/j.gie.2009.11.043
20. Hara Y, Goda K, Dobashi A, Ohya TR, Kato M, Sumiyama K, et al. Short- and long-term outcomes of endoscopically treated superficial non-ampullary duodenal epithelial tumors. World J Gastroenterol. (2019) 25:707–18. doi: 10.3748/wjg.v25.i6.707
21. Watanabe D, Hayashi H, Kataoka Y, Hashimoto T, Ichimasa K, Miyachi H, et al. Efficacy and safety of endoscopic submucosal dissection for non-ampullary duodenal polyps: A systematic review and meta-analysis. Dig Liver Dis. (2019) 51:774–81. doi: 10.1016/j.dld.2019.03.021
22. Hoteya S, Kaise M, Iizuka T, Ogawa O, Mitani T, Matsui A, et al. Delayed bleeding after endoscopic submucosal dissection for non-ampullary superficial duodenal neoplasias might be prevented by prophylactic endoscopic closure: analysis of risk factors. Dig Endosc. (2015) 27:323–30. doi: 10.1111/den.2015.27.issue-3
23. Elek G, Gyôri S, Tóth B, and Pap A. Histological evaluation of preoperative biopsies from ampulla vateri. Pathol Oncol Res. (2003) 9:32–41. doi: 10.1007/BF03033712
24. Ma MX and Bourke MJ. Management of duodenal polyps. Best Pract Res Clin Gastroenterol. (2017) 31:389–99. doi: 10.1016/j.bpg.2017.04.015
25. ASGE Technology Committee, Maple JT, Abu Dayyeh BK, Chauhan SS, Hwang JH, Komanduri S, et al. Endoscopic submucosal dissection. Gastrointest Endosc. (2015) 81:1311–25. doi: 10.1016/j.gie.2014.12.010
Keywords: hybrid endoscopic submucosal dissection, sporadic non-ampullary duodenal adenomas, giant duodenal adenoma, endoscopic therapy, case report
Citation: Hou J-J, Ding L, Yang Y-F and Li W-W (2025) Case Report: A rare case of a giant sporadic non-ampullary duodenal adenoma that was safely and effectively resected using hybrid endoscopic submucosal dissection. Front. Oncol. 15:1511454. doi: 10.3389/fonc.2025.1511454
Received: 20 November 2024; Accepted: 30 May 2025;
Published: 23 June 2025.
Edited by:
Zhaohui Jin, Mayo Clinic, United StatesReviewed by:
Rohadi Muhammad Rosyidi, University of Mataram, IndonesiaXiu-He Lv, Sichuan University, China
Copyright © 2025 Hou, Ding, Yang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei-Wei Li, NDY5NzAwODEyQHFxLmNvbQ==
 Jun-Jie Hou
Jun-Jie Hou Liang Ding
Liang Ding Yan-Fei Yang
Yan-Fei Yang Wei-Wei Li
Wei-Wei Li