- 1Department of Urology, Central Hospital Affiliated to Shandong First Medical University, Jinan, China
- 2Department of Pathology, Central Hospital Affiliated to Shandong First Medical University, Jinan, China
- 3Department of Urology, Liaocheng Second People’s Hospital, Liaocheng, China
- 4Department of Health, Shandong Province Hospital, Jinan, China
Background: Papillary renal neoplasm with reverse polarity (PRNRP) is a relatively rare subtype of papillary renal cell carcinoma (PRCC) and is considered to be a tumor of low malignant potential. Membranous nephropathy (MN) is frequently associated with malignant tumors but rarely accompanies renal cell carcinoma. Synchronous papillary renal neoplasm with reverse polarity and membranous nephropathy has not been reported in the current study. (We searched in PubMed, Web of Science databases, Embase, and Medline in the English language from 1970 to October 2024. The keywords used were “papillary renal neoplasm with reverse polarity” and “membranous nephropathy”.)
Case presentation: A 66-year-old man was admitted to the hospital with lower extremity edema and hypertension and presented with nephrotic syndrome including hypoalbuminemia and proteinuria. Enhanced CT scan showed a 3.7*3.0 cm round-like soft tissue density foci at the lower pole of the left kidney, with obvious inhomogeneous enhancement. The patient underwent a laparoscopic partial nephrectomy of the left kidney. Histologic and immunohistochemical results showed typical features of PRNRP, including a papillary structure covered by a single layer of cuboidal cells with finely grained eosinophilic cytoplasm, nuclei that were mostly regular and apically located, and GATA3 (+). The biopsy of pericarcinoma tissue showed membranous nephropathy and glomerular segmental sclerosis. The patient’s nephrotic syndrome resolved and the tumor did not recur or metastasize during 22 months of postoperative follow-up.
Conclusion: We reported a case of synchronous papillary renal neoplasm with reverse polarity with membranous nephropathy. The mechanism of renal tumor-associated nephrotic syndrome is unclear and more medical records are needed for research.
Background
PRNRP is currently classified as a subtype of PRCC. In 2008 Kunju et al. first described PRCC lined by oncocytic cells with peripheralized low-grade nuclei (1). In 2019, Al-Obaidy et al. proposed the new term “ papillary renal neoplasm with reverse polarity “ and subsequent studies identified unique features including papillary structures covered by a single layer of cuboidal cells, granular eosinophilic cytoplasm, nuclei that were mostly regular and apically situated with inconspicuous nucleoli, GATA3 and L1CAM positivity, negative Vimentin staining, frequent KRAS gene mutations (2, 3). Most patients have a good prognosis. Membranous nephropathy was a glomerular disease characterized by thickening of glomerular capillary walls due to immune complex deposition. Patients lost a large amount of protein in the urine and developed nephrotic syndromes such as decreased serum albumin levels and generalized edema (4). Previous studies have shown that some malignant tumors such as gastrointestinal tumors and lung cancer can cause glomerular disease (5, 6). However, there were few reports of nephrotic syndromes caused by renal carcinoma, and the mechanism between renal carcinoma and glomerular disease was unclear. Here we describe for the first time a case of synchronous PRNRP with membranous nephropathy.
Case presentation
A 66-year-old man was hospitalized with symptoms of lower extremity edema and hypertension, and ultrasonography revealed a solid mass in the left kidney. CT plain scan showed a 3.7*3.0 cm round soft tissue density focus at the lower pole of the left kidney (Figure 1A), and the enhancement scan showed the lesion was inhomogeneous enhancement (Figure 1B). Laboratory tests showed serum albumin 23.1 g/L, 24-hour urine protein quantification 3.018 g (reference value <0.140), AFP 7.8 ng/mL, CEA 5.15 ng/mL, NSE 22.62 ng/mL. Tests for PLA2R serology and other autoimmune markers were negative. The patient underwent a laparoscopic partial nephrectomy.
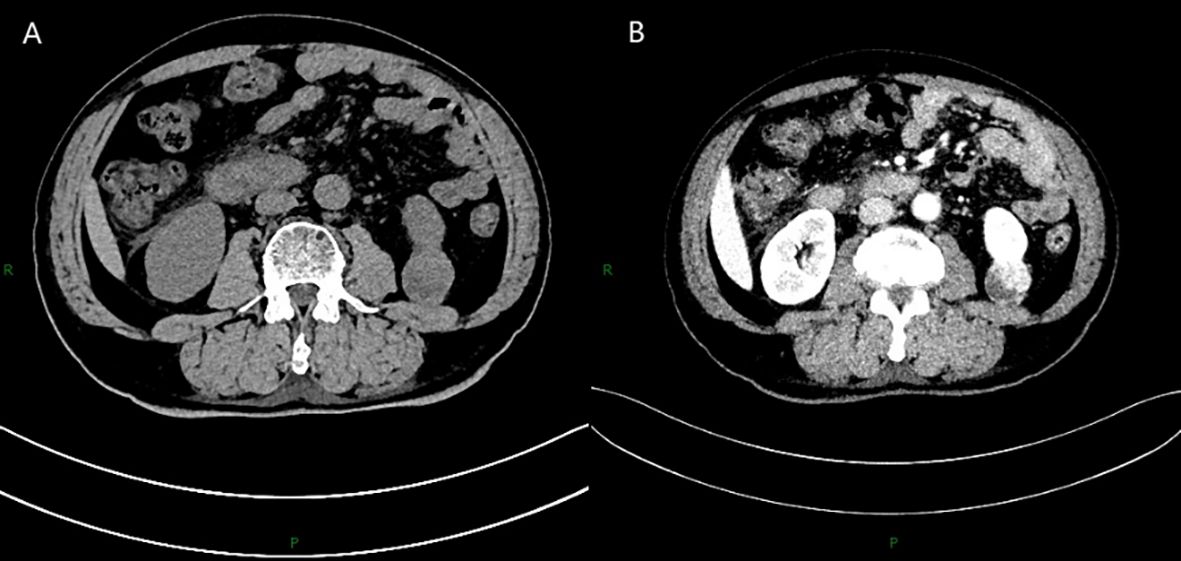
Figure 1. CT plain scan showed a 3.7*3.0 cm round soft tissue density focus at the lower pole of the left kidney (A), and the enhancement scan showed the lesion was inhomogeneous enhancement (B).
Macroscopically, the tumor was dissected with clear boundaries, and a cystic solid mass measuring about 3.5*3.5 cm with gray-red and gray-yellow papillary protrusions in the solid area was seen on the cut surface. Histologically, the tumors were accompanied by a fine fibrovascular core of branching papillae (Figure 2A), and most tumor cells were arranged in a monolayer on the papillary basement membrane. Most of the cells were cuboidal, with fine-grained eosinophilic cytoplasm. The nuclei were located on the apical surface of the cells, mostly small and non-overlapping, with regular nuclear contours and inconspicuous nucleoli (Figure 2B). Tumors tissue immunohistochemistry showed CK7 (+) (Figure 2C), EMA (+), GATA3 (+) (Figure 2D), PAX-8 (+), CD10 (-), CA9 (-), Vimentin (-), CD117 (-), TFE-3 (-), WT-1 (-), weak positivity for P504S and P53, and 5% Ki-67.

Figure 2. Histologically, the tumors were accompanied by a fine fibrovascular core of branching papillae (A), and most tumor cells were arranged in a monolayer on the papillary basement membrane. Most of the cells were cuboidal, with fine-grained eosinophilic cytoplasm. The nuclei were located on the apical surface of the cells, mostly small and non-overlapping, with regular nuclear contours and inconspicuous nucleoli (B). Tumors tissue immunohistochemistry showed CK7 (+) (C), GATA3 (+) (D).
Histopathological analysis of the renal tissue surrounding the tumor showed glomerulosclerosis in 47 out of 284 glomeruli and segmental sclerosis in 10 glomeruli (Figure 3A). PASM staining showed mild hyperplasia of glomerular mesangial cells and stroma, thickening of the basement membrane, numerous spikelike structures and chain-like structures, and the deposition of subepithelial eosinophilic deposit (Figure 3B). Immunofluorescence results showed fine granular deposits of IgG, IgM, and C1q along capillary loops. IgA, C3(-), and C3 in the walls of small arterioles were positive (Figure 3C).
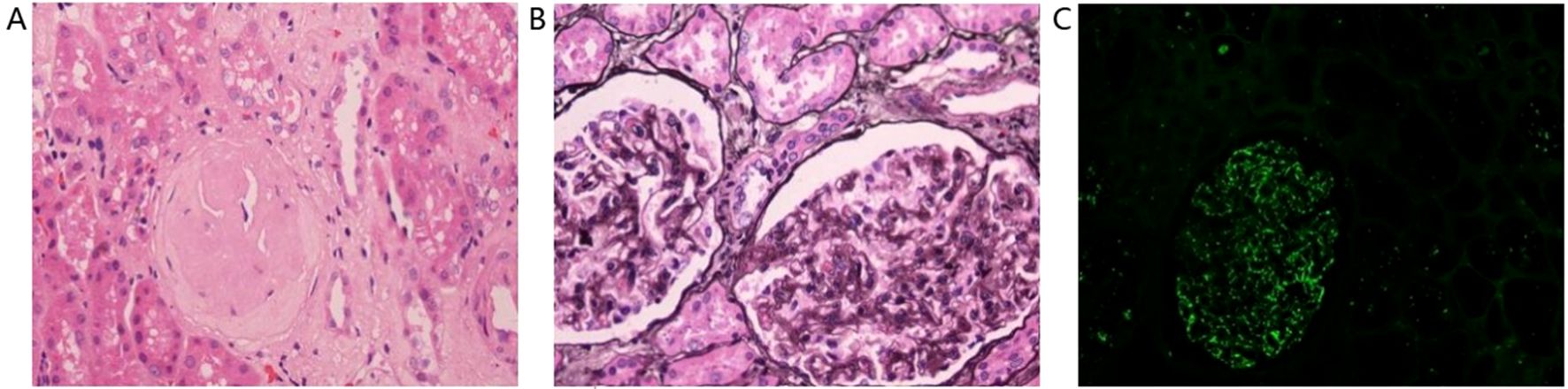
Figure 3. Histopathological analysis of the renal tissue surrounding the tumor showed glomerulosclerosis in 47 out of 284 glomeruli and segmental sclerosis in 10 glomeruli (A). PASM staining showed mild hyperplasia of glomerular mesangial cells and stroma, thickening of the basement membrane, numerous spikelike structures and chain-like structures, and the deposition of subepithelial eosinophilic deposit (B). Immunofluorescence results showed fine granular deposits of IgG, IgM, and C1q along capillary loops. IgA, C3(-), and C3 in the walls of small arterioles were positive (C).
The patient was followed up for 22 months after tumor resection with no recurrence or metastasis of the tumor and normal renal function.
Discussion
PRCC is the second most common renal cell carcinoma and has traditionally been classified into type 1 and type 2 based on histological differences (7). However, in actual clinical practice, a large proportion of tumors, including PRNRP, exhibited overlapping features (8). Studies have shown that PRNRP accounts for approximately 4% of the total number of previously diagnosed PRCCs (9). PRNRP was often considered to have indolent biological behavior due to its papillary growth pattern, low-grade nuclei, fewer chromosomal abnormalities, and good survival outcomes. The study by Wang et al. reported firstly one case of recurrent PRNRP at 6 months postoperatively, and no cases of metastasis and death during the follow-up period (10). Some reported cases of PRNRP showed histologic features of localized invasion of peripheral nerves and renal parenchyma (11).Therefore, the biological behavior of PRNRP needs to be further investigated.
The typical pathological features of membranous nephropathy (MN): abnormal basement membrane, immunofluorescent IgG with or without C3 along the basement membrane deposition, subepithelial electron dense deposition.25% to 30% membrane Nephropathy is secondary to malignant tumors (solid tumors such as lung cancer, bowel cancer, breast cancer), infections (hepatitis B, malaria, etc.), connective tissue diseases (systemic lupus erythematosus, etc.) and drugs/poisons (NSAIDs, Chinese medicines, heavy gold genus etc.).Membranous nephropathy with no secondary factors is called idiopathic membranous nephropathy idiopathic nephropathy (IMN) and also known as primary membranous renal disease nephropathy(PMN).
We reported a patient with PRNRP combined with membranous nephropathy(MN). Previous studies have shown that MN is often associated with malignancy, and the prevalence of cancer in patients with MN has been estimated to be 10% (12). MN often occurred in combination with lung cancer, gastrointestinal tract tumors, and prostate cancer, but less with renal cancer (13). It is difficult to confirm whether MN is primary or secondary in clinical practice, but we can attempt to make inferences based on the available evidence. In our case, the patient’s symptoms of proteinuria improved significantly after surgery. A similar case report described a patient with MN and renal cancer, where MN was cured by resection of the primary tumor (14). On the other hand, tumor development is a relatively slow process, but our patient presented with symptoms of proteinuria and edema for only 2 days, which suggested a late onset of nephrotic syndrome. The above evidence supported to some extent the conclusion that MN is secondary to PRNRP.
The mechanism of tumor-associated nephropathy has not been elucidated. There was experimental evidence that proteinuria could occur in loaded tumor-bearing animals, with diffuse deposition of IgG in the glomeruli and loss of podocyte peduncles (15). The prevailing view was that it was the result of the deposition of antibody complexes to tumor-associated antigens in the glomeruli. It has also been suggested that RCC patients are more susceptible to immune complex damage caused by exogenous or endogenous antigens secondary to altered immune function (16). Our report indicated a relationship between tumors and MN, and further studies are needed to elucidate the mechanism of this association.
The diagnosis of MN usually precedes the diagnosis of the associated cancer, and some clues may suggest the presence of cancer in patients with membranous nephropathy, such as negative anti-PLA2R1 antibodies, prevalent IgG1 and IgG2 deposits, or more than 3 inflammatory cells per glomerulus on renal biopsy (17). With the discovery of anti-phospholipase A2 receptor (PLA2R) antibodies and anti-platelet domain protein type I 7A (THSD7A) antibodies, the study and diagnosis of PMN entered the molecular era (18, 19).The understanding of membranous nephropathy has developed from pathological morphology to molecular level. Serum anti-PLA2R antibody has high specificity in the diagnosis of membranous nephropathy. There is still disagreement on whether serum anti-PLA2R and THSD7A antibody detection can replace kidney biopsy. At the 2019 KDIGO meeting, it was proposed that risk stratification should be performed first for patients with positive anti-PLA2R antibodies, and low-risk patients could not temporarily undergo kidney biopsy and be given supportive treatment first. Renal biopsy is recommended for patients who are at high risk or who are reassessed as high risk during follow-up (20). PLA2R positivity is common in PMN but is not a specific marker of PMN.
At present, the treatment of membrane nephropathy is becoming more and more standardized, and the KDIGO guidelines recommend that the timing and regimen of immunosuppressive therapy be determined according to the risk of kidney disease progression and the risk of complications of immunosuppressive use. For renal function progression, it is possible to benefit from starting immunosuppressive therapy as early as possible in patients with deterioration and no contraindication of immunosuppressive therapy. For patients with mild symptoms and stable renal function, immunosuppressive therapy should be delayed as long as possible (20).For immunosuppressive regimens, hormone combined alkylating agent regimens, rituximab regimens, and Calcineurin inhibitor (CNI) regimens were all first-line regimens. Currently, only hormonal combined alkylating agent regimens have evidence to delay entry to End-stage renal disease (ESRD) (21).
CNI regimens and rituximab regimens for membranous nephropathy evidence of therapeutic effect is limited to induced remission of nephrotic syndrome (21).
Therapeutically, patients with renal tumors combined with MN need to decide on the order of treatment on an individual basis. Treatment of MN may contribute to more rapid progression of preexisting tumors. For example, glucocorticoids can interfere with inflammation, inhibit antigen presentation, and suppress cellular and humoral immunity (18). On the other hand, in patients with renal insufficiency, surgical complications may further impair their renal function. The reason we chose to deal with the renal tumor first in our case was that the patient’s preoperative renal function was essentially normal and the PRNRP was small. Therefore, the patient was suitable for nephron-sparing surgery and the follow-up results showed no deterioration of renal function after surgery. The patient was not treated for MN after surgery, and the postoperative review showed that the symptoms of MN improved. This requires further research and exploration.
Conclusions
In conclusion, we reported a case of synchronous papillary renal neoplasm with reverse polarity with membranous nephropathy. The mechanism of renal tumor-associated nephrotic syndrome is unclear. PRNRP were mostly stage T1 tumors with good prognosis and were more suitable for nephron-sparing surgery considering the damage of MN to renal function.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
QX: Conceptualization, Writing – original draft. MZ: Resources, Software, Writing – review & editing. XXL: Resources, Software, Writing – review & editing. TC: Data curation, Writing – review & editing. LH: Data curation, Writing – review & editing. XTL: Conceptualization, Data curation, Writing – review & editing. FW: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to express their sincere gratitude to the patient and their family for their trust, invaluable assistance, collaborative engagement, and consent to participate in this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kunju LP, Wojno K, Wolf JJ, Cheng L, and Shah RB. Papillary renal cell carcinoma with oncocytic cells and nonoverlapping low grade nuclei: expanding the morphologic spectrum with emphasis on clinicopathologic, immunohistochemical and molecular features. Hum Pathol. (2008) 39:96–101. doi: 10.1016/j.humpath.2007.05.016
2. Al-Obaidy KI, Eble JN, Cheng L, Williamson SR, Sakr WA, Gupta N, et al. Papillary renal neoplasm with reverse polarity: a morphologic, immunohistochemical, and molecular study. Am J Surg Pathol. (2019) 43:1099–111. doi: 10.1097/PAS.0000000000001288
3. Al-Obaidy KI, Eble JN, Nassiri M, Cheng L, Eldomery MK, Williamson SR, et al. Recurrent kras mutations in papillary renal neoplasm with reverse polarity. Mod Pathol. (2020) 33:1157–64. doi: 10.1038/s41379-019-0362-1
4. Ronco P, Beck L, Debiec H, Fervenza FC, Hou FF, Jha V, et al. Membranous nephropathy. Nat Rev Dis Primers. (2021) 7:69. doi: 10.1038/s41572-021-00303-z
5. Lee JC, Yamauchi H, and Hopper JJ. The association of cancer and the nephrotic syndrome. Ann Intern Med. (1966) 64:41–51. doi: 10.7326/0003-4819-64-1-41
6. Bacchetta J, Juillard L, Cochat P, and Droz JP. Paraneoplastic glomerular diseases and Malignancies. Crit Rev Oncol Hematol. (2009) 70:39–58. doi: 10.1016/j.critrevonc.2008.08.003
7. Moch H, Cubilla AL, Humphrey PA, Reuter VE, and Ulbright TM. The 2016 who classification of tumours of the urinary system and male genital organs-part a: renal, penile, and testicular tumours. Eur Urol. (2016) 70:93–105. doi: 10.1016/j.eururo.2016.02.029
8. Saleeb RM, Brimo F, Farag M, Rompre-Brodeur A, Rotondo F, Beharry V, et al. Toward biological subtyping of papillary renal cell carcinoma with clinical implications through histologic, immunohistochemical, and molecular analysis. Am J Surg Pathol. (2017) 41:1618–29. doi: 10.1097/PAS.0000000000000962
9. Chang HY, Hang JF, Wu CY, Lin TP, Chung HJ, Chang YH, et al. Clinicopathological and molecular characterisation of papillary renal neoplasm with reverse polarity and its renal papillary adenoma analogue. Histopathology. (2021) 78:1019–31. doi: 10.1111/his.14320
10. Wang T, Ding X, Huang X, Ye J, Li H, Cao S, et al. Papillary renal neoplasm with reverse polarity-a comparative study with ccprcc, oprcc, and prcc1. Hum Pathol. (2022) 129:60–70. doi: 10.1016/j.humpath.2022.07.010
11. Yang T, Kang E, Zhang L, Zhuang J, Li Y, Jiang Y, et al. Papillary renal neoplasm with reverse polarity may be a novel renal cell tumor entity with low Malignant potential. Diagn Pathol. (2022) 17:66. doi: 10.1186/s13000-022-01235-2
12. Leeaphorn N, Kue-A-Pai P, Thamcharoen N, Ungprasert P, Stokes MB, and Knight EL. Prevalence of cancer in membranous nephropathy: a systematic review and meta-analysis of observational studies. Am J Nephrol. (2014) 40:29–35. doi: 10.1159/000364782
13. Lefaucheur C, Stengel B, Nochy D, Martel P, Hill GS, Jacquot C, et al. Membranous nephropathy and cancer: epidemiologic evidence and determinants of high-risk cancer association. Kidney Int. (2006) 70:1510–17. doi: 10.1038/sj.ki.5001790
14. Fujita Y, Kashiwagi T, Takei H, Takada D, Kitamura H, Iino Y, et al. Membranous nephropathy complicated by renal cell carcinoma. Clin Exp Nephrol. (2004) 8:59–62. doi: 10.1007/s10157-003-0261-z
15. Takeda S, Chinda J, Murakami T, Numata A, Iwazu Y, Akimoto T, et al. Development of features of glomerulopathy in tumor-bearing rats: a potential model for paraneoplastic glomerulopathy. Nephrol Dial Transplant. (2012) 27:1786–92. doi: 10.1093/ndt/gfr565
16. Hoxha E, Wiech T, Stahl PR, Zahner G, Tomas NM, Meyer-Schwesinger C, et al. A mechanism for cancer-associated membranous nephropathy. N Engl J Med. (2016) 374:1995–96. doi: 10.1056/NEJMc1511702
17. Pani A, Porta C, Cosmai L, Melis P, Floris M, Piras D, et al. Glomerular diseases and cancer: evaluation of underlying Malignancy. J Nephrol. (2016) 29:143–52. doi: 10.1007/s40620-015-0234-9
18. Beck LJ, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, et al. M-type phospholipase a2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. (2009) 361:11–21. doi: 10.1056/NEJMoa0810457
19. Tomas NM, Beck LJ, Meyer-Schwesinger C, Seitz-Polski B, Ma H, Zahner G, et al. Thrombospondin type-1 domain-containing 7a in idiopathic membranous nephropathy. N Engl J Med. (2014) 371:2277–87. doi: 10.1056/NEJMoa1409354
20. Floege J, Barbour SJ, Cattran DC, Hogan JJ, Nachman PH, Tang S, et al. Management and treatment of glomerular diseases (part 1): conclusions from a kidney disease: improving global outcomes (kdigo) controversies conference. Kidney Int. (2019) 95:268–80. doi: 10.1016/j.kint.2018.10.018
Keywords: papillary renal cell carcinoma, reverse polarity, membranous nephropathy, nephrotic syndrome, case report
Citation: Xing Q, Zhong M, Li X, Cao T, Liu X, Wang F and He L (2025) Synchronous papillary renal neoplasm with reverse polarity and membranous nephropathy: a rare case report. Front. Oncol. 15:1512036. doi: 10.3389/fonc.2025.1512036
Received: 16 October 2024; Accepted: 21 April 2025;
Published: 16 May 2025.
Edited by:
Anthony Michael Valeri, Columbia University, United StatesReviewed by:
Bo Xu, University at Buffalo, United StatesOvais Shafi, Jinnah Sindh Medical University, Pakistan
Copyright © 2025 Xing, Zhong, Li, Cao, Liu, Wang and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fangfei Wang, MTM0Mzg4NTFAcXEuY29t; Li He, OTkxODA5NTY1QHFxLmNvbQ==
 Qingfei Xing
Qingfei Xing Minglei Zhong1
Minglei Zhong1