- 1Department of Otolaryngology, The Institute of The First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
- 2Guangzhou University of Chinese Medicine, The First Clinical Medical College, Guangzhou, China
- 3Department of Allergy, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
- 4Department of Pathology, Nanfang Hospital, Southern Medical University, Guangzhou, Guangdong, China
As a relatively rare malignant entity, follicular dendritic cell sarcoma (FDCS) of the mediastinum currently suffers from insufficient clinical characterization, warranting more comprehensive studies to optimize therapeutic strategies. The initial symptoms of FDCS are often related to the respiratory system. It is particularly rare for the disease to present initially as recurrent ulcers in the oropharynx, which eventually led to a diagnosis of paraneoplastic pemphigus. Here, we report a case of a young female patient diagnosed with mediastinal FDCS associated with paraneoplastic pemphigus. The patient presented with recurrent pharyngeal ulcers and was diagnosed with mediastinal FDCS associated with paraneoplastic pemphigus 10 months later through imaging and pathological examinations.
Introduction
Follicular dendritic cell sarcoma was first reported in 1986 by Monda in the form of a case report (1). It is a rare tumor associated with paraneoplastic pemphigus. Due to its distinctive growth pattern and cytological variability, it may be confused with a variety of tumors and even inflammatory processes (2–4). The initial symptoms of FDCS are diverse, and may manifest as myasthenia gravis (5), back pain, intestinal polyps (6, 7), recurrent oral ulcers (8–10), and other early symptoms (11–14). Paraneoplastic pemphigus is usually induced by lymphoproliferative diseases, and its classic clinical features include mucosal painful erosions appearing on the basis of latent or diagnosed tumors, as well as polymorphic skin lesions on the limbs and trunk. The diagnostic Criteria for Paraneoplastic Pemphigus (PNP) mainly present as: 1) Mucous membrane lesions with or without cutaneous involvement; 2) Concomitant internal neoplasm; 3) Evidence of anti-plakin autoantibodies, including various tests on transitional epithelium; 4) Acantholysis and/or lichenoid interface observed on histopathology, +/- necrotic keratinocytes; 5) direct immunofluorescence displaying intercellular and/or basement membrane staining. The diagnosis requires fulfillment of either all three primary criteria or any two of the first three criteria in combination with one criterion from items 4-5 (15). The combination of mediastinal dendritic cell sarcoma and paraneoplastic pemphigus is extremely rare, with only a limited number of studies reporting on this disease internationally (16–21). Our case report describes a patient with recurrent oral ulcers that were difficult to heal, who was ultimately diagnosed with mediastinal FDCS in association with paraneoplastic pemphigus.
Case description
The patient (female, 58 years old) developed oral ulcers in January 2022, which did not improve significantly after self-treatment (oral cefaclor extended-release tablets 375 mg twice daily for one week). In March 2022, due to persistent erosions of the buccal mucosa, the patient sought care at several local hospitals and was initially diagnosed with lichen planus. In September 2022, the oral ulcers worsened, accompanied by hoarseness and tongue pain (Figures 1A, B). On September 6, 2022, the patient developed respiratory symptoms such as coughing, sputum production, and difficulty breathing, along with new-onset ulcers in the external genitalia, vesicles on the hands and feet, and crusted ulcers around the lips (Figures 1C, 2A), as well as worsening conjunctival congestion, significant purulent discharge within the conjunctival sac, and cloudy corneas, indicating eye involvement. Histopathological examination of a blister from the dorsum of the hand demonstrated: suprabasal epidermal clefting (positive Nikolsky sign); mixed inflammatory cell infiltration around superficial dermal blood vessels (Figure 2B); DIF analysis of vesicular hand lesions (Figure 2L), which demonstrated: Immunofluorescence staining of the hand lesions showed IgG (green fluorescence) and C3 (red fluorescence) deposition predominantly within the epidermal compartment. The fluorescence intensity varied regionally, with focal areas of strong positivity alternating with zones of weaker signal, resulting in an overall irregular distribution pattern, confirming the tentative diagnosis of pemphigus vulgaris. The patient was treated for pemphigus vulgaris in the dermatology department from September 20, 2022, to October 17, 2022. Methotrexate combined with intravenous immunoglobulin (IVIG, 0.4 g/kg/day) was administered as treatment.
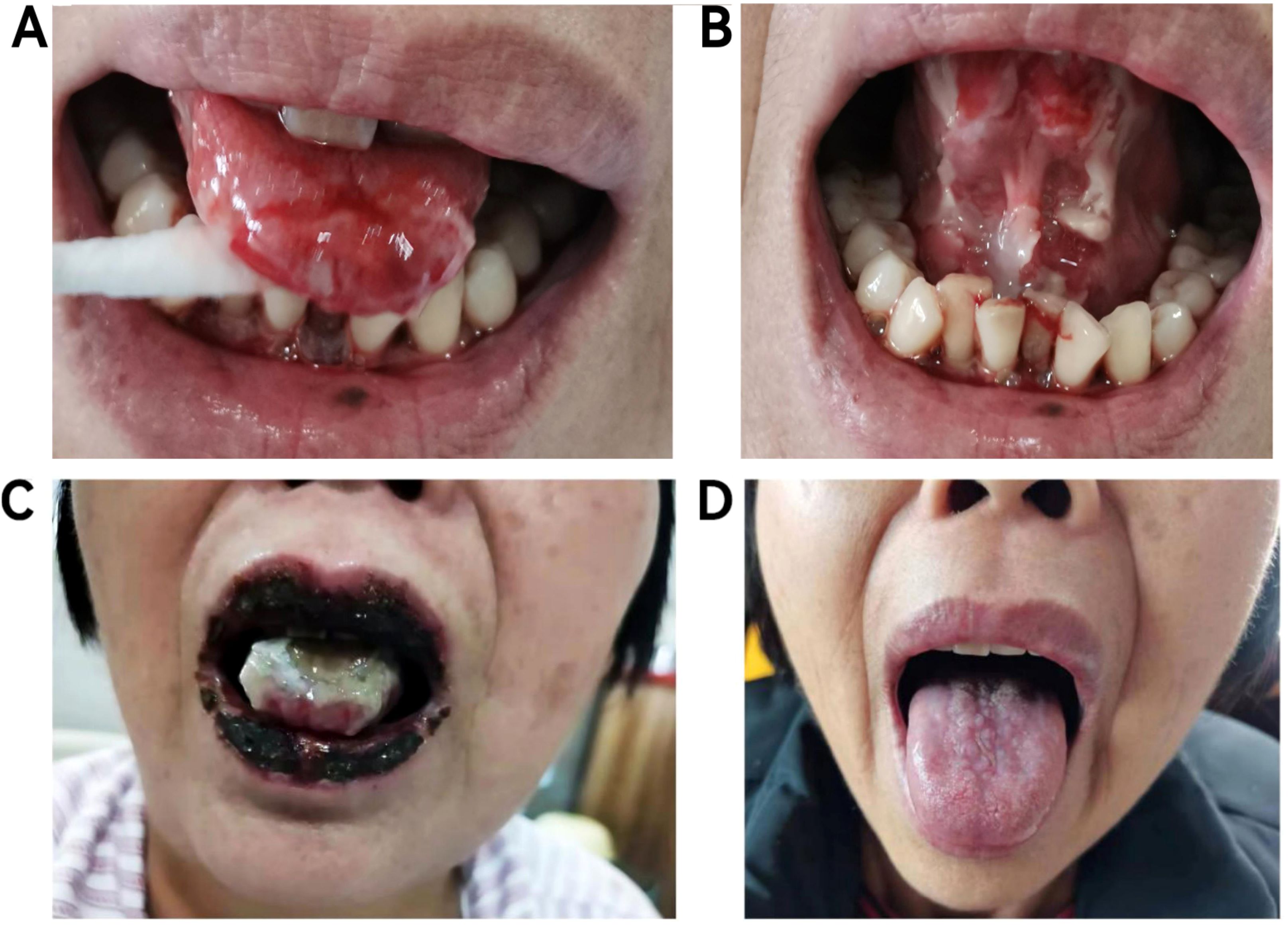
Figure 1. Changes in the patient’s tongue ulcers. (A, B) was taken 10 days earlier than (C), showing that the ulceration of the tongue is evident and tends to bleed easily upon contact. (C) was taken in November 2022 before treatment, showing numerous brown crusts around the lips and multiple ulcers on the tongue, covered with yellowish-white secretions that were difficult to wipe off. (D) was taken in March 2024 after treatment, showing that the tongue had mostly returned to normal, with only minor granulation tissue proliferation.
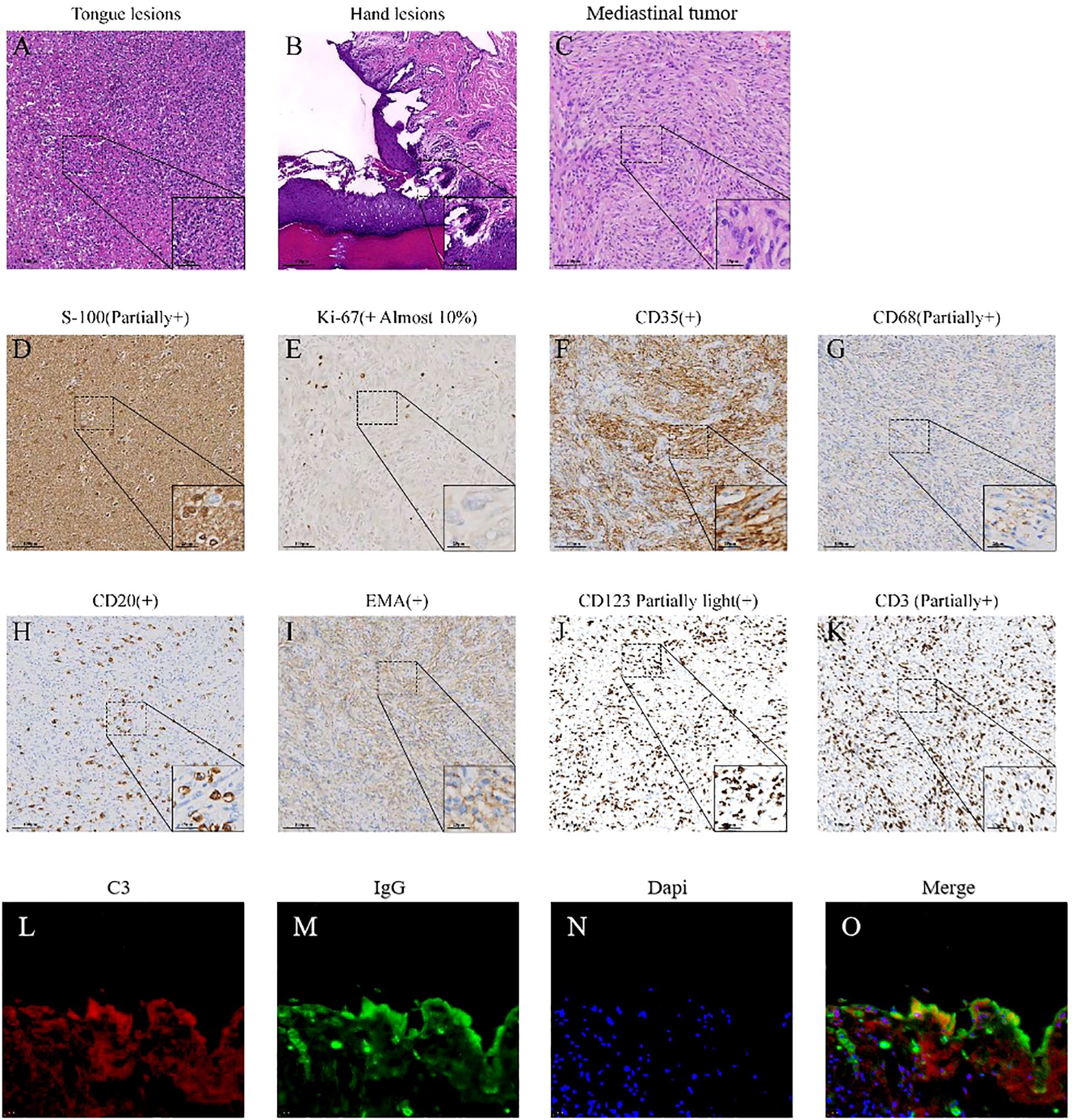
Figure 2. Pathological images of lesions. (A) The HE staining image of tongue lesions. Acute inflammatory exudate and multiple multinucleated giant cells can be seen in the figure. (B) The HE staining image of hand lesions. It shows mild hyperplasia of squamous epithelium with hyperkeratosis and focal hypokeratosis, local elongation of the skin foot, no liquid degeneration of the basal layer, no thickening of the basement membrane, local acantholysis and formation of epidermal bulls, the apex epidermis was generally normal, and local “gravestone” sign is observed, with hyperplasia of dermal papillary collagen fibers. Edema and infiltration of chronic inflammatory cells were observed around small vessels in the superficial dermis, and exudation of acute and chronic inflammatory cells was observed in the blister. (C) The HE staining of mediastinal mass. It can be seen that the tumor cells were arranged in spokes or swirls, and the tumor cells were fusiform and oval. (D–K) Immunohistochemical (IHC) staining of the mediastinal mass. (L–O) Immunofluorescence staining of the hand lesions showed IgG (green fluorescence) and C3 (red fluorescence) deposition predominantly within the epidermal compartment. The fluorescence intensity varied regionally, with focal areas of strong positivity alternating with zones of weaker signal, resulting in an overall irregular distribution pattern.
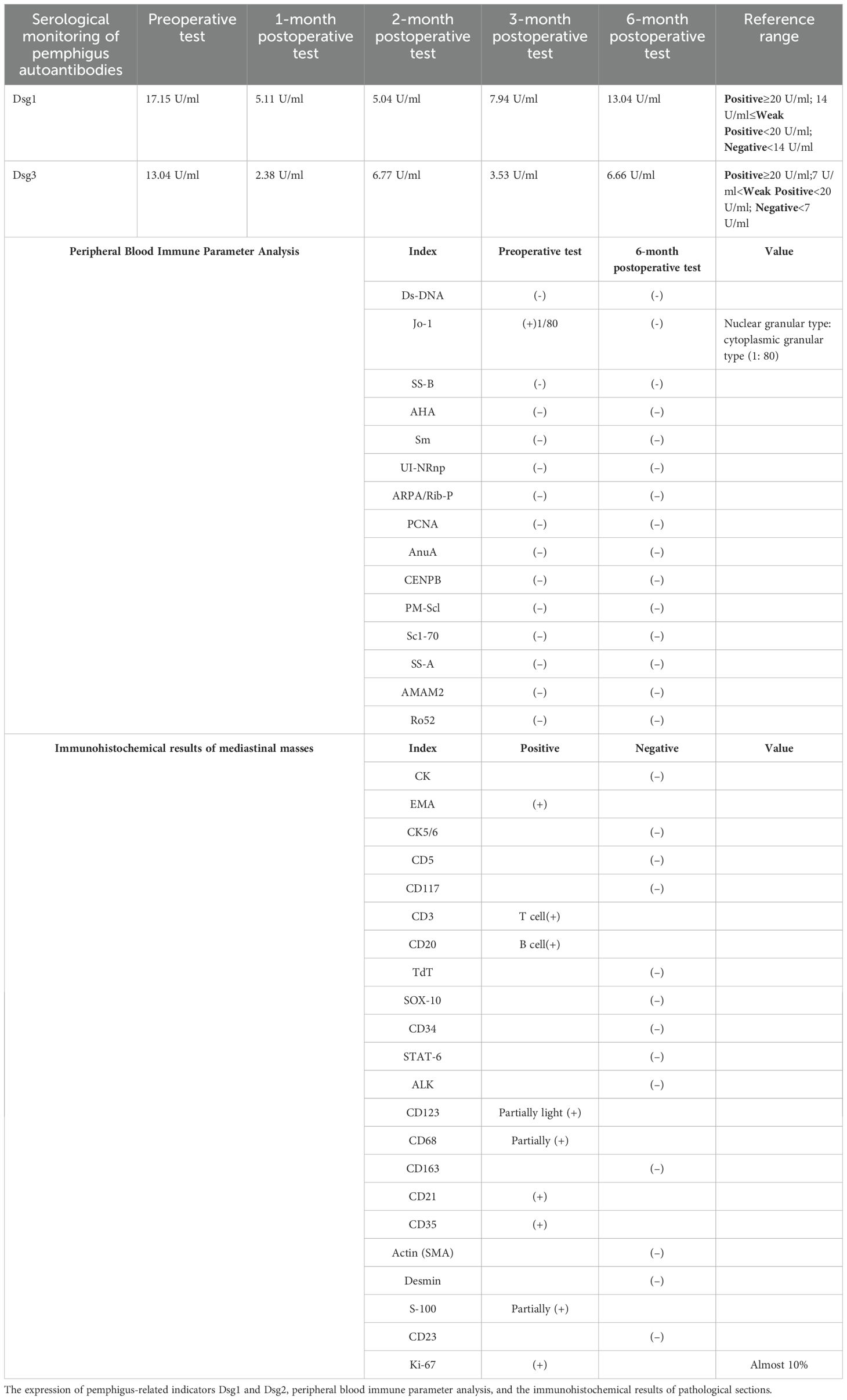
On October 21, 2022, due to worsening cough, sputum production, and lack of improvement in the oral ulcers, the patient had a chest CT scan and the mediastinal mass was revealed. PET-CT revealed a mediastinal soft tissue mass measuring 4.7×2.8×5.9 cm with increased metabolic activity (SUVmax 7.3, SUVave 4.1) (Figure 3). The serological results demonstrated a weakly positive antinuclear antibody (ANA) with a titer of 1:80, exhibiting both nuclear speckled and cytoplasmic speckled patterns, while anti-double stranded DNA (anti-dsDNA) antibodies were negative. Complement 3 levels remained within normal limits without significant reduction. Notably, anti-desmoglein antibody testing revealed elevated anti-Dsg1 levels at 17.51 U/mL and anti-Dsg3 levels at 13.04 U/mL. Additionally, Epstein-Barr virus serology showed strongly positive EBV-VCA-IgG and EBV-NA-IgG titers (>50 AU/mL for both), indicative of past EBV infection.
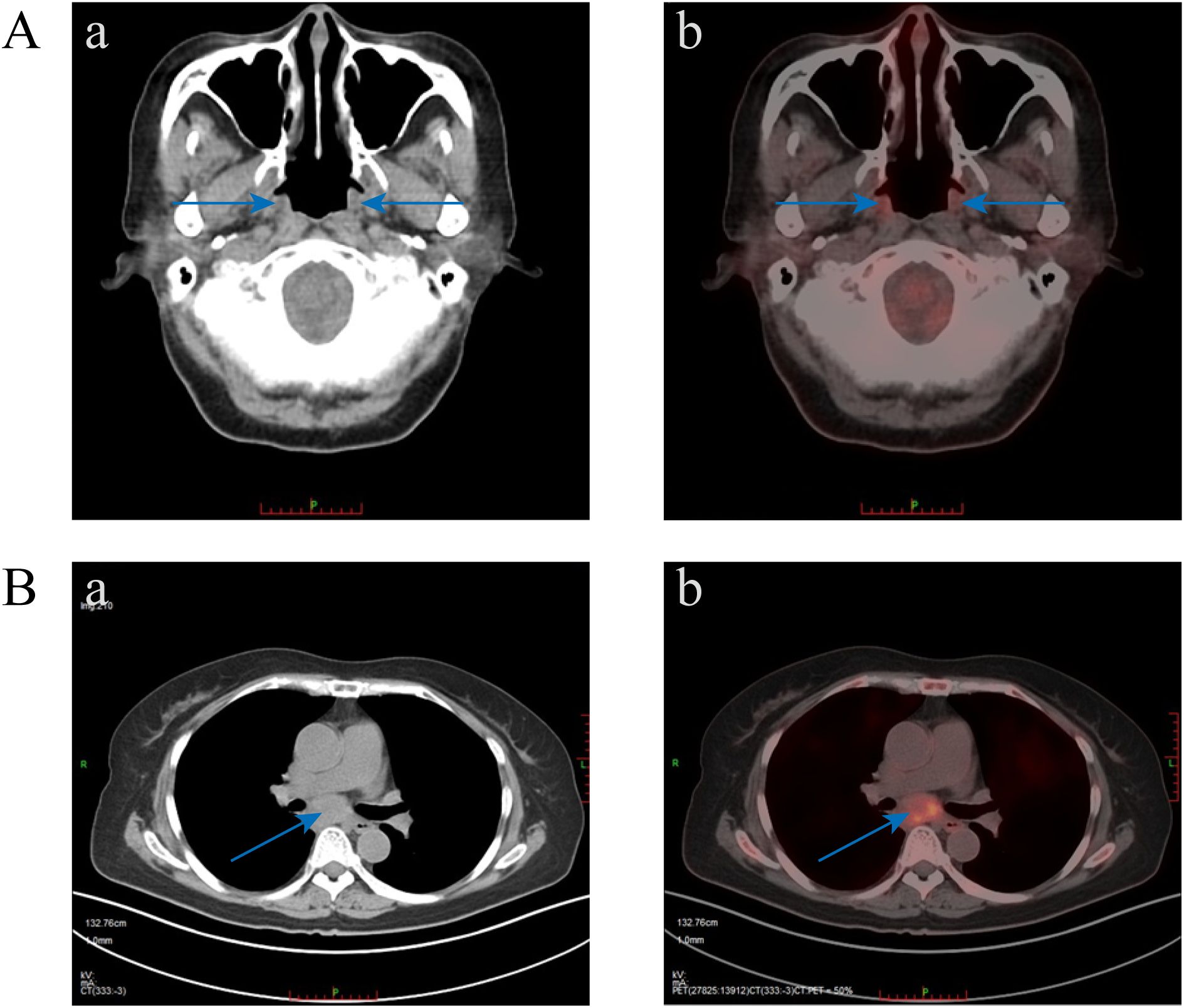
Figure 3. PET-CT images. (A) Diffuse mild increased metabolism of nasal alar, bilateral oral-buccal, bilateral periodontal, upper palate, oropharynx, laryngeal pharynx, and adjacent esophageal cervical mucosa was seen, which was considered to be caused by inflammation (blue arrow indicates increased metabolism area). (B) A mass of soft tissue in the mediastinum with increased metabolic heterogeneity.
Subsequently, on November 18, 2022, the patient underwent general anesthesia for thoracoscopic resection of the mediastinal mass. The final pathological diagnosis of the mediastinal mass was confirmed follicular dendritic cell sarcoma (Figure 2C), staged as pT2N0M0, with characteristic immunohistochemical profile demonstrating positivity for CD21 and CD35, while being negative for CD23, STAT6, and ALK, with the tumor exhibited a low proliferative index (Ki-67, 10%) (Table 1, Figures 2D–K). The mediastinal mass was completely excised with negative surgical margins, and showed no evidence of lymph node involvement in all examined nodal groups. Through multidisciplinary team consultation, the patient was ultimately diagnosed with: 1) follicular dendritic cell sarcoma (FDCS) and 2) paraneoplastic pemphigus (PNP), based on comprehensive evaluation of clinical manifestations (refractory oropharyngeal erosions + mediastinal tumor), laboratory findings (Table 1), histopathological examination of tongue, hand and mediastinal mass lesions, along with corresponding immunohistochemical profiles and immunofluorescence staining results (Figure 2).
Ten days post-surgery, the patient’s pemphigus antibodies (anti-Dsg1 13.04 U/mL, anti-Dsg3 6.66 U/mL) in the blood turned negative, but the oral ulcers worsened with a foul odor. Bacterial microscopy revealed Gram-positive cocci and Gram-negative bacilli, while fungal microscopy showed a small number of fungal spores. Bacterial culture indicated Klebsiella pneumoniae. After multidisciplinary consultation, the patient was treated with itraconazole, methylprednisolone, and symptomatic therapy, the oral ulcers significantly improved 15 days post-surgery. Subsequent postoperative radiotherapy and other related supportive treatments led to substantial healing of the oral ulcers and overall improvement in other symptoms. One year later (March 2024), the patient had no obvious ulceration in the oral cavity, only minimal scarring, and slight gingival swelling and tenderness (Figure 1D). The progression of the disease is shown in Figure 4.
Discussion and conclusion
Diagnosing secondary tumor diseases is extremely complex, as they may present in various forms early on. Some may manifest as paraneoplastic syndromes of the endocrine system, leading to conditions such as inappropriate antidiuretic hormone secretion, hypercalcemia, Cushing’s syndrome, hypoglycemia, while others may present as paraneoplastic syndromes of the nervous system, including limbic encephalitis, paraneoplastic cerebellar degeneration, subacute peripheral sensory neuropathy (22–24). Additionally, they may manifest as paraneoplastic skin diseases and rheumatological syndrome symptoms such as acanthosis nigricans, pemphigus, polymyositis, Sweet’s syndrome, and other paraneoplastic skin conditions and rheumatic syndromes (25, 26).
The initial clinical manifestations of certain tumors can be remarkably insidious: bilateral periorbital cellulitis before lung cancer, dermatomyositis before breast cancer, reactive arthritis before thymoma, Kawasaki disease before early-stage stomach and esophageal cancer, etc. (27–29). This case of mediastinal FDCS complicated by PNP, initially manifesting solely as recurrent oropharyngeal mucosal lesions that presented clinically as nonspecific oral ulcers, underscores both the diagnostic elusiveness of early-stage paraneoplastic syndromes and the critical importance of maintaining high clinical vigilance for seemingly benign presentations.
This paradigmatic case also provides critical clinical insights that when faced with recurrent oral ulcers and mucosal erosion that are unresponsive to conventional treatment, we should initially rule out specific infectious diseases like diphtheria, streptococcal pharyngitis, scarlet fever, scarlet fever, and pharyngeal tuberculosis. Subsequently, non-infectious diseases, particularly immune system disorders, paraneoplastic disorders, and tumor diseases, such as lichen planus, Behçet’s disease, paraneoplastic pemphigus syndrome, systemic lupus erythematosus, should be considered. Given the difficulty of the exclusionary diagnosis in this stage, close monitoring and vigilance for any evolving symptoms are crucial.
The diagnosis of FDCS predominantly relies on pathological confirmation of tumor cells, complemented by the patient’s clinical symptoms and signs (30–32). For this patient, supplementary diagnostic workup is required to substantiate the FDCS diagnosis, with PDGFRB gene analysis being particularly indicative. Early detection and diagnosis of some FDCSs are challenging, often manifesting as paraneoplastic pemphigus syndrome with early symptoms. Biopsy of relevant crucial areas is crucial in early-stage inflammatory diseases. This case presented with dyspnea accompanied by pulmonary function tests demonstrating very severe mixed ventilatory dysfunction. Postoperative thoracic CT revealed pulmonary inflammatory changes with focal bronchial wall thickening, findings consistent with bronchiolitis obliterans. However, definitive diagnosis remains constrained by the absence of histopathological confirmation via bronchial tissue biopsy.
Additionally, FDCS and Castleman disease (CD) are distinct lymphatic disorders with divergent etiologies, pathophysiology, and clinical manifestations. FDCS, a rare malignancy of follicular dendritic cells, typically presents as a localized nodal or extranodal mass (e.g., liver, lung) with metastatic potential and expresses CD21/CD23/CD35 (31, 32). In contrast, CD is a benign lymphoproliferative disorder characterized by systemic symptoms (e.g., fever, weight loss) and IL-6-driven cytokine dysregulation. Accurate differentiation is essential for therapeutic decision-making.
For suspected paraneoplastic syndrome cases, a comprehensive diagnostic approach integrating laboratory tests (e.g., pemphigus antibodies), imaging (PET-CT), and pathological evidence (immunohistochemistry and immunofluorescence results) is essential (33). Future efforts should focus on optimizing early detection protocols for paraneoplastic syndromes through multidisciplinary collaboration and serial monitoring to reduce misdiagnosis of rare tumors and associated paraneoplastic syndromes. In summary, this case provides a diagnostic paradigm for FDCS-associated PNP with mucosal onset, highlighting the critical importance of multisystem evaluation and interdisciplinary cooperation in diagnosing rare paraneoplastic syndromes.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The studies involving humans were approved by The First Affiliated Hospital of Guangzhou University of Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
YW: Data curation, Investigation, Writing – original draft, Writing – review & editing. MZ: Data curation, Investigation, Supervision, Writing – review & editing. FW: Data curation, Formal analysis, Investigation, Supervision, Writing – review & editing. ZH: Data curation, Formal analysis, Investigation, Supervision, Writing – review & editing. YR: Formal analysis, Funding acquisition, Investigation, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (No. 82305325, 82274590) and Young Scientists Fund of the National Natural Science Foundation of China (No. 82401332).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author declares the use of generative AI in the creation of this manuscript. DeepSeek was employed for English grammar correction and text translation.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Monda L, Warnke R, and Rosai J. A primary lymph node Malignancy with features suggestive of dendritic reticulum cell differentiation. A report of 4 cases. Am J Pathol. (1986) 122:562–72.
2. Lopez-Hisijos N, Omman R, Pambuccian S, and Mirza K. Follicular dendritic cell sarcoma or not? A series of 5 diagnostically challenging cases. Clin Med Insights Oncol. (2019) 13:2013427021. doi: 10.1177/1179554919844531
3. Chezar K, Yilmaz A, Auer I, and Trpkov K. Primary follicular dendritic cell sarcoma of urinary bladder. Int J Surg Pathol. (2021) Guangzhou, China. doi: 10.1177/1066896920981622
4. Pagliuca F, Ronchi A, Auricchio A, Lieto E, and Franco R. Inflammatory pseudotumor-like follicular/fibroblastic dendritic cell sarcoma: focus on immunohistochemical profile and association with Epstein-Barr virus. Infect Agent Cancer. (2022) 17:63. doi: 10.1186/s13027-022-00474-8
5. Kaur R, Mehta J, and Borges A. Extranodal follicular dendritic cell sarcoma-A review: “What the mind does not know the eye does not see. Adv Anat Pathol. (2021) 28:21–9. doi: 10.1097/PAP.0000000000000281
6. Xiao N, Xiao S, and Yang W. Follicular dendritic cell sarcoma of the nasopharynx: a case report and literature review. J Int Med Res. (2022) 50:665808706. doi: 10.1177/03000605221097662
7. Sahay A, Bal M, Patil A, Kane S, and Pai P. Follicular dendritic cell sarcoma of the larynx: apropos a rare case with review of the literature. Turk Patoloji Derg. (2019) 35:254–57. doi: 10.5146/tjpath.2017.01408
8. Zhang T, He L, Wang Z, Dong W, Sun W, Zhang P, et al. Follicular dendritic cell sarcoma presenting as a thyroid mass: an unusual case report and literature review. J Int Med Res. (2020) 48:1220719985. doi: 10.1177/0300060520920433
9. Lu X, Wu Y, Gong J, Yu X, Zhang Y, and Yang C. Pancreatic follicular dendritic cell sarcoma: one case report and literature review. J Int Med Res. (2022) 50:665763967. doi: 10.1177/03000605221142401
10. Hanfee AR, Ghazi KR, Ashfaq Z, Kayani N, and Din NU. Primary follicular dendritic cell sarcoma of breast: 2 further cases with review of the literature. Int J Surg Pathol. (2022) 30:55–62. doi: 10.1177/10668969211017317
11. Grosinger A, Garrity JA, and Salomao DR. Follicular dendritic cell sarcoma: a novel orbital tumour. Can J Ophthalmol. (2021) 56:e84–86. doi: 10.1016/j.jcjo.2020.11.009
12. Ke X, He H, Zhang Q, Yuan J, and Ao Q. Epstein-Barr virus-positive inflammatory follicular dendritic cell sarcoma presenting as a solitary colonic mass: two rare cases and a literature review. Histopathology. (2020) 77:832–40. doi: 10.1111/his.14169
13. Carboni F, Covello R, Bertini L, and Valle M. Uncommon retroperitoneal tumour: follicular dendritic cell sarcoma. Acta Chir Belg. (2021) 121:219–21. doi: 10.1080/00015458.2019.1689646
14. Pang J, Mydlarz WK, Gooi Z, Waters KM, Bishop J, Sciubba JJ, et al. Follicular dendritic cell sarcoma of the head and neck: Case report, literature review, and pooled analysis of 97 cases. Head Neck. (2016) 38 Suppl 1:E2241–49. doi: 10.1002/hed.24115
15. Huang S, Anderson HJ, and Lee JB. Paraneoplastic pemphigus/paraneoplastic autoimmune multiorgan syndrome: Part II. Diagnosis and management. J Am Acad Dermatol. (2024) 91:13–22. doi: 10.1016/j.jaad.2023.08.084
16. Chow SC, Yeung EC, Ng CS, Wong RH, Fai TK, and Wan IY. Mediastinal follicular dendritic cell sarcoma with paraneoplastic pemphigus. Asian Cardiovasc Thorac Ann. (2015) 23:732–34. doi: 10.1177/0218492314561501
17. Hu A, Chen T, and Dong J. Promising clinical outcome after body gamma knife radiotherapy for mediastinal follicular dendritic cell sarcoma with thoracic spine invasion and iliac metastasis: A case report and literature review. Front Oncol. (2022) 12:919644. doi: 10.3389/fonc.2022.919644
18. Hu J, Dong D, Jiang Z, and Hu H. Clinicopathological characteristics of mediastinal follicular dendritic cell sarcoma: report of three cases. J Cardiothorac Surg. (2016) 11:56. doi: 10.1186/s13019-016-0464-5
19. Phrathep DD, Healey KD, Anthony S, Fives KR, Boshkos MC, and Galani R. Mediastinal follicular dendritic cell sarcoma with underlying sjogren’s syndrome. Cureus. (2023) 15:e37715. doi: 10.7759/cureus.37715
20. Purkait S, Mallick S, Joshi PP, Mallick S, Murugan NV, Sharma MC, et al. Retroperitoneal and mediastinal follicular dendritic cell sarcoma: report of 3 cases with review of literature. Hematol Oncol. (2017) 35:374–79. doi: 10.1002/hon.2275
21. Wu YL, Wu F, Xu CP, Chen GL, Zhang Y, Chen W, et al. Mediastinal follicular dendritic cell sarcoma: a rare, potentially under-recognized, and often misdiagnosed disease. Diagn Pathol. (2019) 14:5. doi: 10.1186/s13000-019-0779-3
22. Rodrigues C. Thymoma paraneoplastic syndrome awareness. Port J Card Thorac Vasc Surg. (2023) 30:9. doi: 10.48729/pjctvs.375
23. Kang L and Wan C. Paraneoplastic syndrome in neuroophthalmology. J Neurol. (2022) 269:5272–82. doi: 10.1007/s00415-022-11247-z
24. Onyema MC, Drakou EE, and Dimitriadis GK. Endocrine abnormality in paraneoplastic syndrome. Best Pract Res Clin Endocrinol Metab. (2022) 36:101621. doi: 10.1016/j.beem.2022.101621
25. Khan F, Kleppel H, and Meara A. Paraneoplastic musculoskeletal syndromes. Rheum Dis Clin North Am. (2020) 46:577–86. doi: 10.1016/j.rdc.2020.04.002
26. Parrado-Carrillo A, Alcubierre R, Camos-Carreras A, and Sanchez-Dalmau BF. Paraneoplastic syndromes in ophthalmology. Arch Soc Esp Oftalmol (Engl Ed). (2022) 97:350–57. doi: 10.1016/j.oftale.2022.03.006
27. Farina A, Villagran-Garcia M, Vogrig A, Zekeridou A, Muniz-Castrillo S, and Velasco R. Neurological adverse events of immune checkpoint inhibitors and the development of paraneoplastic neurological syndromes. Lancet Neurol. (2024) 23:81–94. doi: 10.1016/S1474-4422(23)00369-1
28. Akagi H and Wada T. A case in which breast cancer developed at the same time as dermatomyositis, and the onset of new cancer was able to be predicted by the exacerbating skin symptoms and parallel increase in the anti-TIF1-gamma antibody levels. Intern Med. (2023) 62:3057–62. doi: 10.2169/internalmedicine.0569-22
29. Liao CH, Lyu SY, Chen HC, Chang DM, and Lu CC. Thymoma-related paraneoplastic syndrome mimicking reactive arthritis. Medicina (Kaunas). (2021) 57(9):932. doi: 10.3390/medicina57090932
30. Dieudonne Y, Silvestrini MA, Dossier A, Meignin V, Jouenne F, Mahevas T, et al. Paraneoplastic pemphigus uncovers distinct clinical and biological phenotypes of western unicentric Castleman disease. Br J Haematol. (2023) 202:267–78. doi: 10.1111/bjh.18847
31. Jain P, Milgrom SA, Patel KP, Nastoupil L, Fayad L, Wang M, et al. Characteristics, management, and outcomes of patients with follicular dendritic cell sarcoma. Br J Haematol. (2017) 178:403–12. doi: 10.1111/bjh.14672
32. Tsunoda S, Harada T, Kikushige Y, Kishimoto T, and Yoshizaki K. Immunology and targeted therapy in Castleman disease. Expert Rev Clin Immunol. (2024) 20:1101–12. doi: 10.1080/1744666X.2024.2357689
Keywords: follicular dendritic cell sarcoma, paraneoplastic pemphigus, dental ulcer, differential diagnosis, multidisciplinary team consultation
Citation: Wang Y, Zhou M, Wang F, Hu Z and Ruan Y (2025) Case Report: A middle-aged woman with mediastinal follicular dendritic cell sarcoma complicated by paraneoplastic pemphigus. Front. Oncol. 15:1512156. doi: 10.3389/fonc.2025.1512156
Received: 16 October 2024; Accepted: 05 June 2025;
Published: 03 July 2025.
Edited by:
Lizza E.L. Hendriks, Maastricht University Medical Centre, NetherlandsReviewed by:
Siquan Wang, Columbia University, United StatesAnanya Datta Mitra, UC Davis Medical Center, United States
David Boutboul, Hôpital Cochin, France
Copyright © 2025 Wang, Zhou, Wang, Hu and Ruan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Ruan, cnVhbnlhbjYzQDE2My5jb20=; Min Zhou, bWluemFpNTM2NkAxMjYuY29t
†ORCID: Yongchun Wang, orcid.org/0000-0002-8479-5028
Min Zhou, orcid.org/0000-0003-2193-7612
Feifei Wang, orcid.org/0000-0002-5962-3136
Zhiyan Hu, orcid.org/0000-0003-1989-705X
Yan Ruan, orcid.org/0000-0002-4224-1289
 Yongchun Wang
Yongchun Wang Min Zhou
Min Zhou Feifei Wang4†
Feifei Wang4† Yan Ruan
Yan Ruan